Abstract
The loss of excitatory synapses is known to underlie the cognitive deficits in Alzheimer’s disease (AD). Although much is known about the mechanisms underlying synaptic loss in AD, how neurons compensate for this loss and whether this provides cognitive benefits remain almost completely unexplored. In this review, we describe two potential compensatory mechanisms implemented following synaptic loss: the enlargement of the surviving neighboring synapses and the regeneration of synapses. Because dendritic spines, the postsynaptic site of excitatory synapses, are easily visualized using light microscopy, we focus on a range of microscopy approaches to monitor synaptic loss and compensation. Here, we stress the importance of longitudinal dendritic spine imaging, as opposed to fixed-tissue imaging, to gain insights into the temporal dynamics of dendritic spine compensation. We believe that understanding the molecular mechanisms behind these and other forms of synaptic compensation and regeneration will be critical for the development of therapeutics aiming at delaying the onset of cognitive deficits in AD.
Introduction
It is well established that the cognitive deficits characteristic of Alzheimer’s disease (AD) are strongly associated with synaptic dysfunction and loss in human patients (DeKosky and Scheff, 1990; Terry et al., 1991; Klein, 2006; Scheff et al., 2006, 2007; Spires-Jones and Knafo, 2012; Tzioras et al., 2023) and in AD animal models (Selkoe, 2002; Penzes et al., 2011; Boehm, 2013; Herms and Dorostkar, 2016). In particular, it has been consistently shown that AD preferentially targets dendritic spines — the postsynaptic sites of excitatory synapses. Toxic amyloid β oligomers (Aβo) colocalize and directly associate with dendritic spines and the postsynaptic density (PSD) both in vitro and in vivo (Lacor et al., 2004; Takahashi et al., 2004; Koffie et al., 2009; Roselli et al., 2009). Mechanistically, Aβo trigger the activation of both NMDARs and metabotropic glutamate receptors (mGluRs), and the activation of calcium-dependent signaling pathways that (1) facilitate LTD and (2) prevent LTP of synaptic transmission (Snyder et al., 2005; Hsieh et al., 2006; Shankar et al., 2007; Renner et al., 2010; Wei et al., 2010). Structurally, the activation of NMDAR- and mGluR-dependent signaling promotes F-actin depolymerization, which in turn results in spine shrinkage and retraction (Zhou et al., 2004; Knobloch and Mansuy, 2008) and ultimately, in the loss of dendritic spines (Hsieh et al., 2006; Shankar et al., 2007).
Because dendritic spine plasticity is strongly correlated with memory formation (Xu et al., 2009; Yang et al., 2009; Fu et al., 2012; Lai et al., 2012; Hayashi-Takagi et al., 2015), the Aβo-mediated disruption of dendritic spine structure and function inevitably leads to cognitive deficits in AD animal models. Crucially, the restoration of dendritic spine density in AD animal models is sufficient to normalize cognitive function (Roy et al., 2016). In humans, the preservation of dendritic spine integrity strongly correlates with cognitive resilience to AD — the ability to remain cognitively normal despite the presence of significant amyloid and tau pathology (Arnold et al., 2013; Boros et al., 2017; King et al., 2023). In this context, a comprehensive understanding of the compensatory and repair mechanisms counteracting synaptic loss will be crucial for restoring cognitive function in AD (Jackson et al., 2019; Neuner et al., 2022; Pham and Dore, 2023).
Synaptic compensation following the loss of dendritic spines in AD
Although much is known about the cellular and molecular mechanisms underlying synaptic loss in AD, the synaptic compensatory and repair mechanisms to counter this loss remain largely unexplored.
Because the early stages of AD are associated with the local loss of synapses — most prominently in the proximity of amyloid plaques (Spires et al., 2005; Bittner et al., 2012) — it is likely that compensation will be initially implemented locally at the synaptic or dendritic level. As the loss of a few local inputs would presumably have no major impact on the neuronal firing rate, it is unlikely that global forms of homeostatic plasticity, such as upscaling, are implemented at this stage (Turrigiano, 2008).
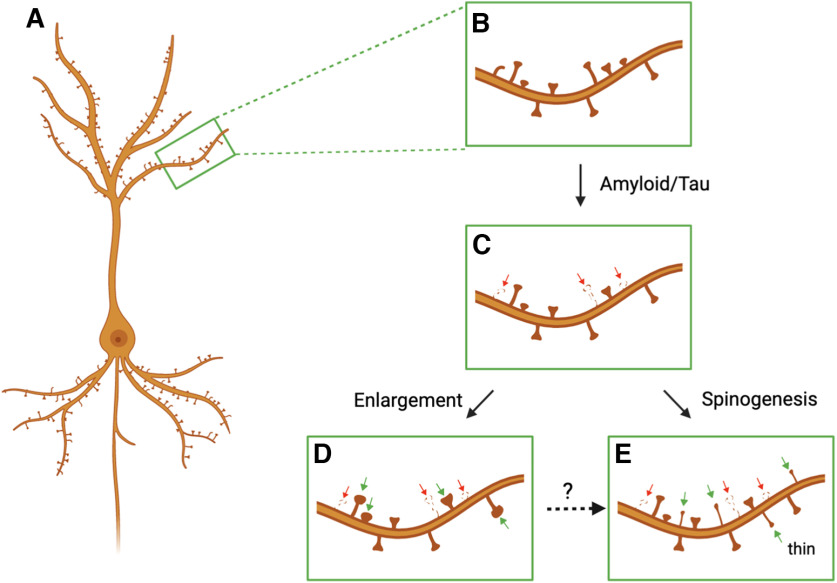
As shown in Figure 1, local synaptic compensation could potentially be implemented (1) via an enlargement of the remaining neighboring dendritic spines in an LTP-like manner or (2) via an increase in the de novo formation of dendritic spines (spinogenesis). If fully integrated into the circuit, either of these compensatory mechanisms would be sufficient to restore the excitatory drive of the affected dendritic region.
Figure 1.
Synaptic compensation following dendritic spine loss in AD. Schematic representation of a hippocampal or cortical pyramidal neuron covered with dendritic spines, the postsynaptic sites of excitatory synapses (A,B). To effectively maintain the excitatory drive of a dendritic branch at a constant level, dendritic spine loss in AD (C; red arrows) could potentially be compensated by either the structural enlargement of surviving preexistent spines (D; green arrows) or by an increase in the generation of new spines (E; green arrows). It is worth noting that the enlargement of dendritic spines might represent an intermediate step for the generation of new dendritic spines (broken line arrow). Figure was created with Biorender.com.
Synaptic compensation via the enlargement of remaining dendritic spines
Although there is no direct evidence that synaptic loss in AD leads to the enlargement of the neighboring dendritic spines, there are several studies pointing in this direction (Table 1). Some of the earlier reports demonstrating loss of synapses in postmortem AD human samples also showed that the remaining synapses were enlarged (Davies et al., 1987; Bertoni-Freddari et al., 1990; DeKosky and Scheff, 1990; Scheff et al., 1990). Because these studies imaged synapses using electron microscopy (EM), it was shown that the degree of PSD enlargement was tightly correlated with the degree of synaptic loss — to such an extent that the total synaptic area in control and AD patients remained the same (DeKosky and Scheff, 1990; Scheff et al, 1990). The enlargement of surviving synapses has been observed in the hippocampus (Bertoni-Freddari et al., 1990; Scheff and Price, 1998, 2006; Scheff et al., 2007; Neuman et al., 2015), entorhinal cortex (Domínguez-Álvaro et al., 2021), and most of the neocortical regions examined. This includes the Brodmann area 9 (BA9) of the frontal cortex (Davies et al., 1987; DeKosky and Scheff, 1990; Scheff et al., 1990) but not BA46 (Boros et al., 2017; Pickett et al., 2018), the temporal cortex (Davies et al., 1987; Scheff and Price, 1993), and the cingulate cortex (Scheff and Price, 2001). The robust and consistent enlargement of the surviving synapses across multiple brain regions suggests that this form of structural plasticity might correspond to a bona fide compensatory mechanism.
Table 1.
Synaptic compensation via the enlargement of surviving dendritic spines
| AD model | Brain region | Microscopy technique | Reference |
|---|---|---|---|
| Human | Hippocampus | EM | Bertoni-Freddari et al., 1990; Neuman et al., 2015; Scheff and Price, 1998, 2006; Scheff et al., 2007 |
| Human | Entorhinal cortex | EM | Domínguez-Álvaro et al., 2021 |
| Human | Frontal cortex | EM | Davies et al., 1987; DeKosky and Scheff, 1990; Scheff et al., 1990 |
| Human | Cingulate cortex | EM | Scheff and Price, 2001 |
| Human | Temporal cortex | EM | Davies et al., 1987; Scheff and Price, 1993 |
| APP/PS1 mouse | Hippocampus | EM | Alonso-Nanclares et al., 2013 |
| APP/PS1 mouse | Hippocampus | Confocal | Smith et al., 2009 |
| APP/PS1 mouse | Hippocampus | Stimulated emission depletion | Viana da Silva et al., 2016 |
| APP/PS1 knock-in mouse | Hippocampus | EM | Androuin et al., 2018 |
| 5xFAD mouse | Hippocampus | EM | Neuman et al., 2015 |
| oAβ injection Rhesus monkey | Frontal cortex | confocal | Beckman et al., 2019 |
| P301S mouse | Somatosensory cortex | In vivo two-photon | Hoffmann et al., 2013 |
In accordance with these human AD studies, several studies in amyloidosis AD mouse models have shown indirect evidence of synaptic compensation (Smith et al., 2009; Viana da Silva et al., 2016). These studies have relied on the imaging of dendritic spines labeled with soluble or membrane-bound fluorophores. For instance, Smith et al. (2009) used confocal microscopy to image dendritic spines labeled with lipophilic DiI dyes in fixed hippocampal sections of the APP/PS1 transgenic mouse model. Similar to human AD, they found that dendritic spine loss was accompanied with an increase in the size of the remaining dendritic spines, both in the spine area and head diameter (Smith et al., 2009). These findings in the APP/PS1 transgenic mouse have been confirmed at the ultrastructural level using EM (Alonso-Nanclares et al., 2013) and more recently, using stimulated emission depletion super-resolution imaging of immunolabelled dendritic spines (Viana da Silva et al., 2016). In addition, the same EM findings have been reported in other amyloidosis AD mice models, including a novel APP/PS1 knock-in mouse (Androuin et al., 2018) and the 5xFAD transgenic mouse (Neuman et al., 2015).
Interestingly, a similar enlargement in the surviving spines have been reported in a recent study using a rhesus monkey model of AD, wherein soluble Aβ oligomers were exogenously administered in the lateral ventricle (Beckman et al., 2019). Using confocal microscopy to image dendritic spines of neurons labeled via intracellular injection of Alexa-568 in fixed sections of the PFC, the authors showed that the loss of spines was also concomitant with an increase in the size of the remaining spines (e.g., spine head diameter).
The fact that the enlargement of surviving dendritic spines is conserved across species, including humans, monkeys, and mice, further highlights the robustness and potential relevance of this putative compensatory mechanism.
It is worth noting that the enlargement of the remaining dendritic spines might also be attributed to a preferential elimination of small spines – while sparing large ones – in AD. Increasing the fraction of large spines might be sufficient to account for the increased average spine size observed at the population level. Although further investigations using longitudinal imaging of the same dendritic region before and after synaptic loss will be necessary to settle this issue (see below), this possibility is inconsistent with a number of studies showing that, in human AD (Scheff and Price, 1993) and in AD animal models (Alonso-Nanclares et al., 2013; Viana da Silva et al., 2016), there is a shift across the entire distributions of synaptic sizes, rather than a selective loss of small spines. In the same line, dendritic spines classified as thin are not selectively vulnerable in human and AD animal models, (Spires et al., 2005; Rozkalne et al., 2011; Boros et al., 2017; but see Beckman et al., 2019), despite belonging to a category of spines characterized by their small sizes (Holtmaat and Svoboda, 2009). All in all, these studies are inconsistent with the notion that the increase in synaptic sizes in AD is because of a preferential loss of small synapses.
In addition to amyloidosis animal models of AD, the compensatory enlargement of surviving spines has also been observed in a tauopathy AD animal model. Using in vivo two-photon microscopy of YFP-expressing neurons in the cortex of the P301S transgenic mouse, it was shown that the loss of synapses is also concomitant with an increase in the size of the remaining spines (Hoffmann et al., 2013).
Together, these findings raise the intriguing possibility that this putative compensatory mechanism might be independent of the original insult (amyloid or tau pathology) leading to synaptic loss. More generally, this compensatory mechanism might potentially be implemented following synaptic loss in other neurodegenerative diseases, such as frontotemporal dementia, Parkinson’s and Huntington’s disease (Herms and Dorostkar, 2016), or neuropsychiatric disorders, such as schizophrenia and depression (Penzes et al., 2011). This concept is also in line with studies showing that synaptic loss driven by insults unrelated to AD, such as deafferentation, ischemia, and malnutrition, also trigger the enlargement of the remaining synapses (Chen and Hillman, 1980, 1982; Hillman and Chen, 1984; Fiala et al., 2002; Barnes et al., 2017). For instance, using EM imaging of excitatory synapses, it was shown that the enlargement of the remaining synapses was sufficient to keep the total PSD area at a constant level following deafferentation (Hillman and Chen, 1984).
Notwithstanding the robustness of local synaptic compensation, the mechanisms by which it is achieved are completely unknown. It is likely that the enlargement of the surviving synapses might be because of (1) the insertion of PSD components from a preexisting extrasynaptic pool in the adjacent dendritic shaft and/or (2) a shift in the normal turnover of PSD components to favor their production (e.g., protein synthesis). Another intriguing possibility corresponds to the relocation or recycling of PSD components from the lost dendritic spine to the compensated spines, which indeed is the most parsimonious explanation for maintaining the constant postsynaptic contact area (Hillman and Chen, 1984).
To be functional, the enlargement of the surviving spine should be accompanied by an increase in AMPARs — the main mediators of synaptic transmission — in an LTP-like manner (Opazo and Choquet, 2011; Huganir and Nicoll, 2013; Nicoll, 2017). Accordingly, AMPARs are significantly increased in the CA1 region of the hippocampus of the 5xFAD transgenic mouse during a period of heightened synaptic compensation as demonstrated using immunogold electron microscopy (Neuman et al., 2015).
Although there is strong indirect evidence for the implementation of synaptic compensation following loss in AD, the majority of studies mentioned above were not intended to investigate synaptic compensation per se but synaptic loss in control versus AD samples. As such, these studies have mostly relied on imaging snapshots of control versus AD samples in fixed preparations. Although these were informative, it is now critical to design longitudinal imaging experiments to monitor the emergence of synaptic compensation over time following synaptic loss in AD models. In this context, microscopy modalities compatible with live imaging — rather than those that rely on fixed-preparations, such as EM or immunofluorescence — are likely to provide further insights into synaptic compensation. In particular, the advent of two-photon laser scanning microscopy has been critical for our current understanding of the temporal dynamics of dendritic spines, both in vitro and in vivo (Denk et al., 1990; Denk and Svoboda, 1997; Helmchen and Denk, 2005; Svoboda and Yasuda, 2006). By imaging the same dendritic branches in vivo before and after different learning paradigms, two-photon microscopy has revealed that dendritic spine turnover (both formation and elimination) strongly correlates with memory formation (Xu et al., 2009; Yang et al., 2009; Fu et al., 2012; Lai et al., 2012). As opposed to conventional confocal microscopy, two-photon microscopy utilizes near infrared wavelengths which provide better penetration in brain tissue. Moreover, by localized excitation of fluorophores in the focal plane, two-photon microscopy significantly reduces tissue photodamage, allowing imaging of the same dendritic branch over weeks and even months.
As expected, two-photon microscopy has also been essential in investigating dendritic spine loss in AD animal models (Subramanian et al., 2020), particularly when double-crossed with the H and M lines of Thy1-YFP transgenic mice. For instance, by simultaneously labeling amyloid plaques with Methoxy-XO4, it has been demonstrated that only dendrites in close proximity of amyloid plaques (<50 μm) showed significant spine loss (Klunk et al., 2002; Spires et al., 2005; Liebscher et al., 2014). In addition to studying the structure of dendritic spines, in vivo two-photon microscopy has been used for investigating the dynamics of fluorescently labeled GluA2 and PSD-95 under basal conditions or following learning paradigms (Gray et al., 2006; Zhang et al., 2015; Roth et al., 2020), and it will also be critical to understand the potential contribution of synaptic protein redistribution to compensation.
Together, two-photon microscopy is the ideal approach to confirm the occurrence and timeline of synaptic enlargement following the loss of synapses near amyloid plaques in the living intact brain; and critically, to investigate the synaptic protein dynamics underlying compensation.
Synaptic compensation via an increase in synaptogenesis
As depicted in Figure 1, an additional mechanism by which synaptic loss in AD may be compensated for is by the de novo generation of new spines. Presumably, the regeneration of dendritic spines might constitute a more robust form of compensation than the enlargement of neighboring dendritic spines, as it would normalize dendritic spine density and restore neuronal connectivity.
In principle, the ability to increase dendritic spine formation could offset the increase in spine elimination in AD and enable continued neuronal function. Although there is no direct evidence that synaptic loss in AD leads to an increase in the rate of spine formation, several studies provide convincing evidence that this is the case in the early stages of AD (Table 2).
Table 2.
Synaptic compensation via an increase in dendritic spinogenesis
| AD model | Brain region | Microscopy technique | Reference |
|---|---|---|---|
| mhAPP mouse | Entorhinal cortex | Bright field | Criscuolo et al., 2017 |
| APP/PS1 mouse | Hippocampus cortex | Bright field | Megill et al., 2015 |
| APP/PS1 mouse | Hippocampus | Confocal | Knafo et al., 2009 |
| APP/PS1 mouse | Motor cortex | In vivo two-photon | Tsai et al., 2004 |
| APP/PS1 mouse | Visual cortex | In vivo two-photon | Liebscher et al., 2014 |
| APP/PS1 mouse | Barrel cortex | In vivo two-photon | Heiss et al., 2017 |
| J20 mouse | Somatosensory cortex | In vivo two-photon | Stephen et al., 2019 |
| rTg4510 mouse | Somatosensory cortex | In vivo two-photon | Jackson et al., 2017 |
In the APP/PS1 transgenic mouse, dendritic spine visualization using Golgi staining revealed that, at a very young age (1 month old), total spine density is increased in both hippocampal CA1 and cortical layers II/III (Megill et al., 2015). Because spine density eventually decreased by 6 months of age, these results suggest that not only was there compensation early on, but also that this homeostatic mechanism might fail at later stages of the disease. Also using Golgi staining, a similar increase in spine density in the entorhinal cortex at a young age (2 month old) was reported in a different AD animal model expressing a mutant form of human APP (mhAPP) (Criscuolo et al., 2017).
Although there is an overall loss of dendritic spines in old APP/PS1 animals (Megill et al., 2015), Knafo et al. (2009) found that dendritic spine density in 12- to 14-month-old transgenics depended on the location relative to amyloid plaques. Using confocal microscopy to image dentate gyrus neurons individually microinjected with Alexa594, they found that, although dendrites passing within an amyloid plaque have a reduced dendritic spine density, those in contact with the plaque periphery have a significantly increase levels of spines, even when compared with free-plaque regions (Knafo et al., 2009). In addition to providing further evidence for increased spinogenesis in AD models, this study suggests that such a putative compensatory mechanism might still be implemented in old animals, though in a spatially restricted manner.
As mentioned above, however, to best assess the presence of compensatory spinogenesis, in vivo two-photon microscopy is the methodology of choice. Using longitudinal imaging of YFP-labeled dendrites in the superficial layers of the cortex in the triple APP/PS1/YFP transgenic animal at 6 months of age, it was reported that dendrites passing near plaques (<15 µm) have an increased rate of spine formation, as well as the expected increase in spine elimination, as opposed to those away from plaques (Tsai et al., 2004), possibly reflecting both the pathologic loss of spines and the effort to compensate via spinogenesis. Similarly, Liebscher et al. (2014) chronically imaged layer V pyramidal neurons in the visual cortex of the APP/PS1/YFP model at 3-4 months of age, and found both an increase in spine elimination, as well as an increase in the rate of spine formation in dendrites close to Aβ plaques (<50 µm). Similarly, longitudinal imaging of layer II/III pyramidal neurons of the barrel cortex in the APP/PS1 model at similar stages (3.5 months old) also revealed that spine elimination is accompanied by significantly increased rates of spine formation (Heiss et al., 2017). Interestingly, in this study, the rate of spine formation in 10-month-old APP/PS1 animals decreased to basal levels while the rate of spine elimination remained elevated. Again, these findings suggest that, at the early stages of disease progression, the upregulation of spinogenesis can compensate for increased losses, but such a putative compensatory mechanism eventually fails as disease pathogenesis continues (Heiss et al., 2017).
Two-photon longitudinal imaging in an alternative mouse model (J20 model, containing a double APP mutation) (Tosh et al., 2018) provides further evidence of compensatory increases in spinogenesis. Pyramidal neurons in the somatosensory cortex of “young” J20 mice were imaged from ∼7 to 10 months old, revealing elevated turnover which was driven by an increase in spine formation, maintaining overall spine density despite plaque deposition (Stephen et al., 2019).
Interestingly, similar to compensation via spine enlargement, there is also evidence of spinogenic compensation in a tauopathy mouse model. Jackson et al. (2017) used the rTg4510 mouse model to demonstrate that spine turnover was significantly elevated versus WT in pyramidal neurons of the somatosensory cortex. This was observed in animals at an earlier “intermediate” stage of tau pathology (5 months old) before an overt decrease in spine density, and reflected both elevated rates of spine formation and loss. Such an increase in spine formation failed to maintain total spine density at later stages (6.5 months old) which was reduced versus controls.
Because spinogenic compensation may be an early compensatory mechanism in disease progression, care needs to be taken when comparing evidence from different mouse models which may develop pathology at different rates. This is further exacerbated when different studies conduct imaging in different cortical areas (as Aβ pathology may be present in certain cortical areas earlier than others) and whether imaging is performed in close proximity of Aβ plaques (and how proximity is defined if so). Together, this can lead to variable reports of spine elimination and formation in AD animal models, and consequently in the dynamics of dendritic spine compensation. For instance, contrary to what has been observed in the visual cortex, Bittner et al. (2012) found that an increase in spine elimination in young APP/PS1 mice was not compensated for by increases in spine formation in the somatosensory cortex. Similarly, Spires-Jones et al. (2007) showed that the increase in dendritic spine elimination in Tg2576 animals (single APP mutation), a slower model of amyloidosis, was not paralleled by increases in dendritic spine formation in the somatosensory cortex. Given these potential sources of variability, to draw clear conclusions about the existence of spinogenic compensatory mechanisms, we need further work attempting a more comprehensive characterization of spine turnover taking all these variables into consideration.
Interestingly, similar to spine size compensation, an increase in spine formation may be a more general mechanism of homeostasis following synapse loss. Evidence from a Huntington’s disease model (Murmu et al., 2013), as well as a mouse model of stroke (Brown et al., 2007) shows that significant increases in spine loss are also compensated for by elevated spinogenesis.
Discussion
Although we have learned a great deal about the mechanisms underlying synaptic loss in AD (Spires-Jones and Hyman, 2014; De Strooper and Karran, 2016; Tzioras et al., 2023), how neurons compensate for this loss remains almost completely unexplored. At the early stages of AD, synaptic loss is local and gradual, so it is unlikely this triggers classical forms of homeostatic plasticity, such as upscaling, which require profound and prolonged decreases in neuronal activity (Turrigiano, 2008). It is more likely that early synaptic loss sets in motion local compensatory mechanisms, such as the ones described in this review: the enlargement of preexistent spines and the de novo formation of spines. In addition, it is possible that, similar to the structural plasticity of dendritic spines occurring during LTP (Engert and Bonhoeffer, 1999; Yuste and Bonhoeffer, 2001; Matsuzaki et al., 2004), these two compensatory mechanisms are implemented at different time scales: spine enlargement might be rapidly implemented following spine loss, while spinogenesis might be a delayed, yet more robust, form of spine compensation. For instance, synaptic loss in the hippocampus triggered by deafferentation is known to promote synaptic enlargement in the short-term but a complete regrowth of synapses 1 month after the insult (Parnavelas et al., 1974; Chen and Hillman, 1982). The temporal dissociation of these two compensatory mechanisms raises the intriguing possibility that they occur in tandem: the synaptic enlargement of surviving synapses precedes and is necessary for the increase in spinogenesis (Fig. 1). As during LTP, the enlargement of activated dendritic spines may generate a “hot spot” for the emergence of new dendritic spines (De Roo et al., 2008). However, although it is clear that synaptic loss coexists with these putative compensatory mechanisms, it remains to be shown whether they are causally related. As mentioned above, longitudinal two-photon microscopy will be critical for starting to assess this question. Ideally, one could longitudinally image the same dendritic region in an animal model of AD before and after the appearance of an amyloid plaque using in vivo two-photon microscopy, as in Bittner et al. (2012). In such a scenario, spine loss as a result of proximity to a nascent amyloid plaque should promote the enlargement of neighboring surviving spines and/or an increase in spinogenesis over time. Because the stochastic nature of amyloid deposition makes this experiment challenging, a more feasible approach would be to image the same dendritic branch before and after the local injection of Aβo directly into the cortex (Zott et al., 2019), to monitor both dendritic spine loss and the subsequent emergence of compensation over time. In addition, this longitudinal experiment will provide a definitive answer to whether the enlargement of the surviving dendritic spines observed in AD corresponds to a bona fide compensatory mechanism or is simply an artifact because of the preferential loss of small spines.
Although we have provided examples of structural spine compensation in AD, it will be critical to assess whether these are accompanied by functional changes. Because of the tight correlation between the structure and function of dendritic spines (Matsuzaki et al., 2004), it is most likely that compensation because of the enlargement of remaining spines is paralleled by increases in synaptic transmission in an LTP-like manner. During compensation via spinogenesis, however, it is less evident that the newly formed spines will be connected to a presynaptic partner, particularly because of protracted nature of synaptogenesis (Knott et al., 2006; Nägerl et al., 2007). Simultaneously imaging the structure and function of dendritic spines using genetically encoded calcium indicators and structural markers will ultimately reveal whether these changes are functionally integrated in the circuit.
Most importantly, it will be crucial to determine whether these putative compensatory mechanisms are indeed beneficial to the animal, possibly through restoration of cognitive function in AD. Because small (or thin) spines are much more plastic than large spines and as such, thought to correspond to “learning” spines (Kasai et al., 2003; Matsuzaki et al., 2004; Bourne and Harris, 2007; Holtmaat and Svoboda, 2009), it is difficult to attribute direct cognitive benefits to the enlargement of the surviving spines in AD. This is in line with aging studies showing that large spines in the PFC are associated with lower cognitive function, both in humans (Boros et al., 2019) and nonhuman primates (Dumitriu et al., 2010; Morrison and Baxter, 2012). It is possible that the enlargement of surviving dendritic spines corresponds to a protective mechanism to simply preserve spines, as large spines tend to be more stable and long-lasting than small spines (Holtmaat et al., 2005; Majewska et al., 2006; Holtmaat and Svoboda, 2009), in the face of continuous spine loss. In other words, the enlargement of surviving spines may correspond to a trade-off between preserving synaptic connectivity at the expenses of synaptic plasticity. As mentioned above, another possibility is that the enlargement of dendritic spines represents a transitory and intermediate step necessary for the generation of new dendritic spines. Because newly formed spines tend to be small (or thin) and thus highly plastic (Holtmaat et al., 2005; Holtmaat and Svoboda, 2009), spinogenesis may correspond to the cognitively relevant compensatory mechanism. This is consistent with recent studies showing that cognitive resilience to AD — the ability to remain cognitively normal despite the presence of significant amyloid and tau pathology — is associated with an increase in the population of thin spines (Boros et al., 2017), which might correspond to the population of newly formed spines. Interestingly, the same study showed that spines in the cognitive resilient population displayed an elongated morphology (Boros et al., 2017, 2019). This finding raises the possibility that these elongated spines may signify newly generated spines actively seeking out presynaptic connections (Holtmaat et al., 2005; Holtmaat and Svoboda, 2009).
In conclusion, because in AD the accumulation of amyloid pathology precedes by several decades the onset of clinical dementia, it is possible that a range of compensatory mechanisms, including the ones described in this review and potentially others like the formation of multisynapses (Martínez-Serra et al., 2022), are implemented to delay the onset of cognitive deficits. This is particularly relevant when considering that synaptic plasticity corresponds to the cellular basis of cognition; and that synaptic dysfunction and loss are the main correlate of cognitive deficits in AD. Consistent with this notion, the preservation of dendritic spine integrity and key synaptic proteins, such as PSD95, strongly correlates with cognitive resilience to AD (Arnold et al., 2013; Perez-Nievas et al., 2013; Scheff et al., 2016; Boros et al., 2017; Walker and Herskowitz, 2021). In this context, a comprehensive understanding of the compensatory and repair mechanisms counteracting synaptic loss will be crucial for the development of therapeutics aiming to delay the onset of cognitive deficits in AD.
Footnotes
This work was supported by UK Dementia Research Institute partner funders (Medical Research Council, Alzheimer’s Research UK, and the Alzheimer’s Society) to P.O.; Dementia Australia Research Foundation Back Block Bards Project Grant to P.O.; and National Health and Medical Research Council Project Grant to P.O.
The authors declare no competing financial interests.
References
- Alonso-Nanclares L, Merino-Serrais P, Gonzalez S, DeFelipe J (2013) Synaptic changes in the dentate gyrus of APP/PS1 transgenic mice revealed by electron microscopy. J Neuropathol Exp Neurol 72:386–395. 10.1097/NEN.0b013e31828d41ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androuin A, Potier B, Nägerl UV, Cattaert D, Danglot L, Thierry M, Youssef I, Triller A, Duyckaerts C, El Hachimi KH, Dutar P, Delatour B, Marty S (2018) Evidence for altered dendritic spine compartmentalization in Alzheimer’s disease and functional effects in a mouse model. Acta Neuropathol 135:839–854. 10.1007/s00401-018-1847-6 [DOI] [PubMed] [Google Scholar]
- Arnold SE, Louneva N, Cao K, Wang LS, Han LY, Wolk DA, Negash S, Leurgans SE, Schneider JA, Buchman AS, Wilson RS, Bennett DA (2013) Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer’s disease. Neurobiol Aging 34:157–168. 10.1016/j.neurobiolaging.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SJ, Franzoni E, Jacobsen RI, Erdelyi F, Szabo G, Clopath C, Keller GB, Keck T (2017) Deprivation-induced homeostatic spine scaling in vivo is localized to dendritic branches that have undergone recent spine loss. Neuron 96:871–882. e5. 10.1016/j.neuron.2017.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D, Ott S, Donis-Cox K, Janssen WG, Bliss-Moreau E, Rudebeck PH, Baxter MG, Morrison JH (2019) Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc Natl Acad Sci USA 116:26239–26246. 10.1073/pnas.1902301116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Casoli T, Meier-Ruge W, Ulrich J (1990) Morphological adaptive response of the synaptic junctional zones in the human dentate gyrus during aging and Alzheimer’s disease. Brain Res 517:69–75. 10.1016/0006-8993(90)91009-6 [DOI] [PubMed] [Google Scholar]
- Bittner T, Burgold S, Dorostkar MM, Fuhrmann M, Wegenast-Braun BM, Schmidt B, Kretzschmar H, Herms J (2012) Amyloid plaque formation precedes dendritic spine loss. Acta Neuropathol 124:797–807. 10.1007/s00401-012-1047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J (2013) A ‘danse macabre’: tau and Fyn in STEP with amyloid beta to facilitate induction of synaptic depression and excitotoxicity. Eur J Neurosci 37:1925–1930. 10.1111/ejn.12251 [DOI] [PubMed] [Google Scholar]
- Boros BD, Greathouse KM, Gentry EG, Curtis KA, Birchall EL, Gearing M, Herskowitz JH (2017) Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann Neurol 82:602–614. 10.1002/ana.25049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros BD, Greathouse KM, Gearing M, Herskowitz JH (2019) Dendritic spine remodeling accompanies Alzheimer’s disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol Aging 73:92–103. 10.1016/j.neurobiolaging.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J, Harris KM (2007) Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17:381–386. 10.1016/j.conb.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH (2007) Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci 27:4101–4109. 10.1523/JNEUROSCI.4295-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hillman DE (1980) Giant spines and enlarged synapses induced in Purkinje cells by malnutrition. Brain Res 187:487–493. 10.1016/0006-8993(80)90221-8 [DOI] [PubMed] [Google Scholar]
- Chen S, Hillman DE (1982) Plasticity of the parallel fiber-Purkinje cell synapse by spine takeover and new synapse formation in the adult rat. Brain Res 240:205–220. 10.1016/0006-8993(82)90217-7 [DOI] [PubMed] [Google Scholar]
- Criscuolo C, Fontebasso V, Middei S, Stazi M, Ammassari-Teule M, Yan SS, Origlia N (2017) Entorhinal cortex dysfunction can be rescued by inhibition of microglial RAGE in an Alzheimer’s disease mouse model. Sci Rep 7:42370. 10.1038/srep42370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO (1987) A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J Neurol Sci 78:151–164. 10.1016/0022-510x(87)90057-8 [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Muller D (2008) LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol 6:e219. 10.1371/journal.pbio.0060219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Karran E (2016) The cellular phase of Alzheimer’s disease. Cell 164:603–615. 10.1016/j.cell.2015.12.056 [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27:457–464. 10.1002/ana.410270502 [DOI] [PubMed] [Google Scholar]
- Denk W, Svoboda K (1997) Photon upmanship: why multiphoton imaging is more than a gimmick. Neuron 18:351–357. 10.1016/s0896-6273(00)81237-4 [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW (1990) Two-photon laser scanning fluorescence microscopy. Science 248:73–76. 10.1126/science.2321027 [DOI] [PubMed] [Google Scholar]
- Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, Plaza-Alonso S, Cano-Astorga N, DeFelipe J, Alonso-Nanclares L (2021) 3D analysis of the synaptic organization in the entorhinal cortex in Alzheimer’s disease. eNeuro 8:ENEURO.0504-20.2021. 10.1523/ENEURO.0504-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH (2010) Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci 30:7507–7515. 10.1523/JNEUROSCI.6410-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399:66–70. 10.1038/19978 [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM (2002) Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev 39:29–54. 10.1016/s0165-0173(02)00158-3 [DOI] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y (2012) Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483:92–95. 10.1038/nature10844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K (2006) Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol 4:e370. 10.1371/journal.pbio.0040370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H (2015) Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525:333–338. 10.1038/nature15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss JK, Barrett J, Yu Z, Haas LT, Kostylev MA, Strittmatter SM (2017) Early activation of experience-independent dendritic spine turnover in a mouse model of Alzheimer’s disease. Cereb Cortex 27:3660–3674. 10.1093/cercor/bhw188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Denk W (2005) Deep tissue two-photon microscopy. Nat Methods 2:932–940. 10.1038/nmeth818 [DOI] [PubMed] [Google Scholar]
- Herms J, Dorostkar MM (2016) Dendritic spine pathology in neurodegenerative diseases. Annu Rev Pathol 11:221–250. 10.1146/annurev-pathol-012615-044216 [DOI] [PubMed] [Google Scholar]
- Hillman DE, Chen S (1984) Reciprocal relationship between size of postsynaptic densities and their number: constancy in contact area. Brain Res 295:325–343. 10.1016/0006-8993(84)90981-8 [DOI] [PubMed] [Google Scholar]
- Hoffmann NA, Dorostkar MM, Blumenstock S, Goedert M, Herms J (2013) Impaired plasticity of cortical dendritic spines in P301S tau transgenic mice. Acta Neuropathol Commun 1:82. 10.1186/2051-5960-1-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K (2009) Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10:647–658. 10.1038/nrn2699 [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K (2005) Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45:279–291. 10.1016/j.neuron.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R (2006) AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron 52:831–843. 10.1016/j.neuron.2006.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA (2013) AMPARs and synaptic plasticity: the last 25 years. Neuron 80:704–717. 10.1016/j.neuron.2013.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Witton J, Johnson JD, Ahmed Z, Ward M, Randall AD, Hutton ML, Isaac JT, O’Neill MJ, Ashby MC (2017) Altered synapse stability in the early stages of tauopathy. Cell Rep 18:3063–3068. 10.1016/j.celrep.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Jambrina E, Li J, Marston H, Menzies F, Phillips K, Gilmour G (2019) Targeting the synapse in Alzheimer’s disease. Front Neurosci 13:735. 10.3389/fnins.2019.00735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H (2003) Structure-stability-function relationships of dendritic spines. Trends Neurosci 26:360–368. 10.1016/S0166-2236(03)00162-0 [DOI] [PubMed] [Google Scholar]
- King D, et al. (2023) Synaptic resilience is associated with maintained cognition during ageing. Alzheimers Dement 19:2560–2574. 10.1002/alz.12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL (2006) Synaptic targeting by Aβ oligomers (ADDLS) as a basis for memory loss in early Alzheimer’s disease. Alzheimers Dement 2:43–55. 10.1016/j.jalz.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT (2002) Imaging Aβ plaques in living transgenic mice with multiphoton microscopy and Methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol 61:797–805. 10.1093/jnen/61.9.797 [DOI] [PubMed] [Google Scholar]
- Knafo S, Alonso-Nanclares L, Gonzalez-Soriano J, Merino-Serrais P, Fernaud-Espinosa I, Ferrer I, DeFelipe J (2009) Widespread changes in dendritic spines in a model of Alzheimer’s disease. Cereb Cortex 19:586–592. 10.1093/cercor/bhn111 [DOI] [PubMed] [Google Scholar]
- Knobloch M, Mansuy IM (2008) Dendritic spine loss and synaptic alterations in Alzheimer’s disease. Mol Neurobiol 37:73–82. 10.1007/s12035-008-8018-z [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K (2006) Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci 9:1117–1124. 10.1038/nn1747 [DOI] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL (2009) Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA 106:4012–4017. 10.1073/pnas.0811698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL (2004) Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci 24:10191–10200. 10.1523/JNEUROSCI.3432-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB (2012) Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 483:87–91. 10.1038/nature10792 [DOI] [PubMed] [Google Scholar]
- Liebscher S, Page RM, Käfer K, Winkler E, Quinn K, Goldbach E, Brigham EF, Quincy D, Basi GS, Schenk DB, Steiner H, Bonhoeffer T, Haass C, Meyer-Luehmann M, Hübener M (2014) Chronic γ-secretase inhibition reduces amyloid plaque-associated instability of pre- and postsynaptic structures. Mol Psychiatry 19:937–946. 10.1038/mp.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska AK, Newton JR, Sur M (2006) Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci 26:3021–3029. 10.1523/JNEUROSCI.4454-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Serra R, Alonso-Nanclares L, Cho K, Giese KP (2022) Emerging insights into synapse dysregulation in Alzheimer’s disease. Brain Commun 4:fcac083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429:761–766. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megill A, Tran T, Eldred K, Lee NJ, Wong PC, Hoe HS, Kirkwood A, Lee HK (2015) Defective age-dependent metaplasticity in a mouse model of Alzheimer’s disease. J Neurosci 35:11346–11357. 10.1523/JNEUROSCI.5289-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG (2012) The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13:240–250. 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murmu RP, Li W, Holtmaat A, Li JY (2013) Dendritic spine instability leads to progressive neocortical spine loss in a mouse model of Huntington’s disease. J Neurosci 33:12997–13009. 10.1523/JNEUROSCI.5284-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägerl UV, Köstinger G, Anderson JC, Martin KA, Bonhoeffer T (2007) Protracted synaptogenesis after activity-dependent spinogenesis in hippocampal neurons. J Neurosci 27:8149–8156. 10.1523/JNEUROSCI.0511-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman KM, Molina-Campos E, Musial TF, Price AL, Oh KJ, Wolke ML, Buss EW, Scheff SW, Mufson EJ, Nicholson DA (2015) Evidence for Alzheimer’s disease-linked synapse loss and compensation in mouse and human hippocampal CA1 pyramidal neurons. Brain Struct Funct 220:3143–3165. 10.1007/s00429-014-0848-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner SM, Telpoukhovskaia M, Menon V, O’Connell KM, Hohman TJ, Kaczorowski CC (2022) Translational approaches to understanding resilience to Alzheimer’s disease. Trends Neurosci 45:369–383. 10.1016/j.tins.2022.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA (2017) A brief history of long-term potentiation. Neuron 93:281–290. 10.1016/j.neuron.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Opazo P, Choquet D (2011) A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci 46:1–8. 10.1016/j.mcn.2010.08.014 [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Lynch G, Brecha N, Cotman CW, Globus A (1974) Spine loss and regrowth in hippocampus following deafferentation. Nature 248:71–73. 10.1038/248071a0 [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14:285–293. 10.1038/nn.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, Fernandez-Carballo L, de Munain EL, Perez J, Marquie M, Serrano-Pozo A, Frosch MP, Lowe V, Parisi JE, Petersen RC, Ikonomovic MD, López OL, Klunk W, Hyman BT, Gómez-Isla T (2013) Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 136:2510–2526. 10.1093/brain/awt171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham AQ, Dore K (2023) Novel approaches to increase synaptic resilience as potential treatments for Alzheimer’s disease. Semin Cell Dev Biol 139:84–92. 10.1016/j.semcdb.2022.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett EK, Rose J, McCrory C, McKenzie CA, King D, Smith C, Gillingwater TH, Henstridge CM, Spires-Jones TL (2018) Region-specific depletion of synaptic mitochondria in the brains of patients with Alzheimer’s disease. Acta Neuropathol 136:747–757. 10.1007/s00401-018-1903-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A (2010) Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron 66:739–754. 10.1016/j.neuron.2010.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli F, Hutzler P, Wegerich Y, Livrea P, Almeida OFX (2009) Disassembly of shank and homer synaptic clusters is driven by soluble β-amyloid1-40 through divergent NMDAR-dependent signalling pathways. PLoS One 4:e6011. 10.1371/journal.pone.0006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RH, Cudmore RH, Tan HL, Hong I, Zhang Y, Correspondence RL, Huganir RL (2020) Cortical synaptic AMPA receptor plasticity during motor learning. Neuron 105:895–908. e5. 10.1016/j.neuron.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S (2016) Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531:508–512. 10.1038/nature17172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozkalne A, Hyman BT, Spires-Jones TL (2011) Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis 41:650–654. 10.1016/j.nbd.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA (1993) Synapse loss in the temporal lobe in Alzheimer’s disease. Ann Neurol 33:190–199. 10.1002/ana.410330209 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA (1998) Synaptic density in the inner molecular layer of the hippocampal dentate gyrus in Alzheimer disease. J Neuropathol Exp Neurol 57:1146–1153. 10.1097/00005072-199812000-00006 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA (2001) Alzheimer’s disease-related synapse loss in the cingulate cortex. J Alzheimers Dis 3:495–505. 10.3233/jad-2001-3509 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA (2006) Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis 9:101–115. 10.3233/jad-2006-9s312 [DOI] [PubMed] [Google Scholar]
- Scheff SW, DeKosky ST, Price DA (1990) Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 11:29–37. 10.1016/0197-4580(90)90059-9 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384. 10.1016/j.neurobiolaging.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68:1501–1508. 10.1212/01.wnl.0000260698.46517.8f [DOI] [PubMed] [Google Scholar]
- Scheff SW, Ansari MA, Mufson EJ (2016) Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol Aging 42:1–12. 10.1016/j.neurobiolaging.2016.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791. 10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27:2866–2875. 10.1523/JNEUROSCI.4970-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M (2009) Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci USA 106:16877–16882. 10.1073/pnas.0908706106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P (2005) Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci 8:1051–1058. 10.1038/nn1503 [DOI] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT (2005) Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci 25:7278–7287. 10.1523/JNEUROSCI.1879-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Knafo S (2012) Spines, plasticity, and cognition in Alzheimer’s model mice. Neural Plast 2012:319836. 10.1155/2012/319836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT (2014) The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82:756–771. 10.1016/j.neuron.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT (2007) Impaired spine stability underlies plaque-related spine loss in an Alzheimer’s disease mouse model. Am J Pathol 171:1304–1311. 10.2353/ajpath.2007.070055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen TL, Tamagnini F, Piegsa J, Sung K, Harvey J, Oliver-Evans A, Murray TK, Ahmed Z, Hutton ML, Randall A, O’Neill MJ, Jackson JS (2019) Imbalance in the response of pre- and post-synaptic components to amyloidopathy. Sci Rep 9:1–11. 10.1038/s41598-019-50781-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian J, Savage JC, Tremblay MÈ (2020) Synaptic loss in Alzheimer’s disease: mechanistic insights provided by two-photon in vivo imaging of transgenic mouse models. Front Cell Neurosci 14:592607. 10.3389/fncel.2020.592607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R (2006) Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 50:823–839. 10.1016/j.neuron.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK (2004) Oligomerization of Alzheimer’s beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci 24:3592–3599. 10.1523/JNEUROSCI.5167-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580. 10.1002/ana.410300410 [DOI] [PubMed] [Google Scholar]
- Tosh JL, Rickman M, Rhymes E, Norona FE, Clayton E, Mucke L, Isaacs AM, Fisher EM, Wiseman FK (2018) The integration site of the APP transgene in the J20 mouse model of Alzheimer’s disease. Wellcome Open Res 2:84–13. 10.12688/wellcomeopenres.12237.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB (2004) Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci 7:1181–1183. 10.1038/nn1335 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG (2008) The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135:422–435. 10.1016/j.cell.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzioras M, McGeachan RI, Durrant CS, Spires-Jones TL (2023) Synaptic degeneration in Alzheimer disease. Nat Rev Neurol 19:19–38. 10.1038/s41582-022-00749-z [DOI] [PubMed] [Google Scholar]
- Viana da Silva S, Haberl MG, Zhang P, Bethge P, Lemos C, Gonçalves N, Gorlewicz A, Malezieux M, Gonçalves FQ, Grosjean N, Blanchet C, Frick A, Nägerl UV, Cunha RA, Mulle C (2016) Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat Commun 7:1–11. 10.1038/ncomms11915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CK, Herskowitz JH (2021) Dendritic spines: mediators of cognitive resilience in aging and Alzheimer’s disease. Neuroscientist 27:487–505. 10.1177/1073858420945964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R (2010) Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci 13:190–196. 10.1038/nn.2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y (2009) Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462:915–919. 10.1038/nature08389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924. 10.1038/nature08577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T (2001) Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 24:1071–1089. 10.1146/annurev.neuro.24.1.1071 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cudmore RH, Lin DT, Linden DJ, Huganir RL (2015) Visualization of NMDA receptor-dependent AMPA receptor synaptic plasticity in vivo. Nat Neurosci 18:402–407. 10.1038/nn.3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM (2004) Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44:749–757. 10.1016/j.neuron.2004.11.011 [DOI] [PubMed] [Google Scholar]
- Zott B, Simon MM, Hong W, Unger F, Chen-Engerer HJ, Frosch MP, Sakmann B, Walsh DM, Konnerth A (2019) A vicious cycle of β amyloid-dependent neuronal hyperactivation. Science 365:559–565. 10.1126/science.aay0198 [DOI] [PMC free article] [PubMed] [Google Scholar]