Abstract
To identify genes that can confer resistance to antimalarial drugs in yeast, we transformed the quinidine-sensitive strain CYX247-9A of Saccharomyces cerevisiae with a yeast genomic library and selected for transformants that grow in the presence of elevated levels of antimalarial drugs. Plasmids were rescued from such clones and were analyzed for the presence of individual open reading frames that can confer drug resistance. Using quinidine as the selective drug, we were able to identify three genes that can cause resistance to antimalarial drugs. Overexpression of the yeast genes CIN5 (a member of the family of bZIP transcription factors), STI1 (a Hsp90 cochaperone), and YOR273c (a member of the major facilitator superfamily of transmembrane transporters) conferred 3.9-, 7.0-, and 4.3-fold resistance to quinidine, respectively, over that of control yeast. Cross-resistance assays determined that STI1 also conferred resistance to mefloquine (3.4-fold), while CIN5 also conferred resistance to mefloquine (9.6-fold) and chloroquine (5.4-fold). Using mefloquine as the selective drug, we determined that overexpression of YBR233w, a member of the hnRNPK family of nuclear RNA binding proteins, conferred resistance to mefloquine (13.5-fold). Expression of the human hnRNPK homolog of YBR233w in S. cerevisiae also conferred mefloquine resistance, suggesting that homologs of the identified resistance genes may perform similar functions in species other than yeast. Our experiments have identified heretofore unknown pathways of resistance to quinoline ring-containing antimalarial drugs in S. cerevisiae.
Malaria is a major infectious disease, with 200 million to 300 million cases worldwide causing an estimated 2 million deaths annually. The emergence of drug-resistant strains of Plasmodium falciparum, the parasite that causes the most severe form of human malaria, represents one of the predominant problems in malaria control. Since its first manifestation in the 1960s, resistance to quinoline-containing antimalarial drugs, such as chloroquine (CQ) and quinine (QN), has spread to all regions where malaria is endemic (6, 51). One plasmodial gene which may function in resistance to quinoline-containing antimalarial agents is pfmdr1 (P. falciparum multidrug resistance [21]). Recent data have suggested a role for pfmdr1 in the resistance to mefloquine (MQ), QN, and halofantrine in field isolates and cultured lines of P. falciparum (15, 33, 50). Likewise, heterologous expression of different allelic forms of pfmdr1 has been shown to modulate cellular resistance to quinoline-containing drugs (35, 43, 47). A gene localized to chromosome VII of P. falciparum has been shown to have a major role in CQ resistance on the basis of linkage and haplotype analyses with resistant and susceptible parasites (40). However, it is clear that these two genes cannot explain the full spectrum of quinoline drug resistance observed in regions where malaria is endemic. Given the low amount of genetic information available and the severity of the problem of antimalarial drug resistance, the identification of additional drug resistance genes is crucial for obtaining a better understanding of drug resistance mechanisms in order to develop more specific and more efficient therapeutic tools.
Transfection of P. falciparum is still in its beginning, and functional analysis of plasmodial genes through gene expression experiments has largely been done with heterologous expression systems such as mammalian cell cultures, the yeast Saccharomyces cerevisiae, or Escherichia coli. To identify novel genes that can give rise to quinoline drug resistance, we have decided to use a screening approach that is based on the overexpression of yeast genes in S. cerevisiae. This choice was influenced by the ease with which genes can be expressed in S. cerevisiae, the availability of extensive genetic tools for yeast and the ready access to the entire sequence of the S. cerevisiae genome which allows easy identification of open reading frames (ORFs) through the sequencing of small DNA segments followed by database searches. A possible drawback of this approach is that S. cerevisiae and P. falciparum are two fundamentally different organisms. Hence, the mechanisms of quinoline drug resistance in yeast may not apply to Plasmodium. On the other hand, experimental evidence suggesting that the principles of drug resistance mechanisms have been conserved among phylogenetically distant organisms has recently accumulated (6). In this report, we describe the use of S. cerevisiae as a screening system for the isolation of genes involved in the resistance to antimalarial compounds and the identification of four genes that confer resistance to quinidine (QD), CQ, and MQ.
MATERIALS AND METHODS
Yeast strain and media.
S. cerevisiae CYX247-9A (qds1-1 ura3-52 leu1 MATa; a kind gift from D. Conklin, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.) (14) was used for all drug screening assays. Yeast transformations were performed either by the modified lithium acetate yeast transformation method (27) or by electroporation (3). Yeast cells were propagated on complete yeast-peptone-dextrose (YPD) medium or on synthetic complete (SC) medium minus uracil (SC −ura) (37).
Determination of the MICs of the drugs.
Overnight cultures of S. cerevisiae CYX247-9A transformed with the YEp24 vector were adjusted to an optical density at 600 nm (OD600) of 1.0 and were then diluted 104-fold. One hundred microliters of this dilution was spread onto SC −ura plates containing increasing concentrations of the drug to be tested. For each drug, the lowest concentration which blocked growth completely was designated the MIC. Drug-containing plates were prepared by adding drugs from a stock solution into SC −ura agar that had been cooled to 50°C. The quinoline-containing compounds were obtained from Sigma. Concentrated stock solutions were prepared in 50% ethanol at 100 mg/ml for QD and QN and at 25 mg/ml for MQ. CQ was prepared at 50 mg/ml in 25% ethanol.
Library screen.
A YEp24-based genomic S. cerevisiae library (10) was transformed by electroporation into S. cerevisiae CYX247-9A (14), and approximately 4.8 × 104 Ura+ transformants were recovered. The titer of the library was determined, and 104, 105, or 106 transformants/plate were spread onto selective medium containing one, two, or three times the MIC of either MQ or QD. To determine the plasmid dependency of the resistant phenotype, plasmids from resistant primary transformants were isolated (25) and retransformed into CYX247-9A. These secondary transformants were plated onto selective medium containing the appropriate drug, and resistant colonies were further analyzed in drug resistance assays.
Subcloning of ORFs conferring drug resistance.
A search of the complete yeast genome was performed with the Basic Local Alignment Search Tool (BLAST) by using sequence information obtained from the 3′ and 5′ ends of the YEp24 genomic inserts which conferred drug resistance. By using the information available at the Saccharomyces Genome Database (SGD), ORFs present within the insert were identified and amplified by PCR, digested with the appropriate restriction enzymes that had been introduced at the 5′ ends of the PCR primers, and subcloned into yeast vector pVT-U or YEp24 (7, 46). Both vectors are URA3-based high-copy-number plasmids. Plasmid pVT-U also contains the strong constitutive alcohol dehydrogenase (ADH) promoter to achieve high levels of expression. For subcloning into YEp24, 500 to 800 bp of 5′ and 3′ noncoding sequences was included in the amplification. For subcloning into pVT-U, only the coding region was amplified. The ORFs to be analyzed were subcloned in the sense and the antisense orientations into pVT-U. The cDNAs for the human cytidine-binding protein PCBP-1 and different splice variants of hnRNPK (hnRNPKA, -B, -C, and -D [18]) were obtained as a gift from H. Leffers (Department of Growth and Reproduction, Rigshospitalet, Copenhagen, Denmark). A cDNA clone for hnRNPK was obtained from G. Dreyfuss (Howard Hughes Medical Institute, University of Pennsylvania School of Medicine, Philadelphia). Different splice variants of hnRNPK and PCBP-1 were amplified by PCR and were subcloned into pVT-U. For each ORF conferring drug resistance, two to five independent subclones were analyzed in the drug resistance assay.
Drug resistance assay.
Resistance to antimalarial drugs was assessed by streaking 5 μl of an overnight culture of yeast adjusted to an OD600 of 0.1 onto SC −ura plates containing different drug concentrations. The growth of the primary transformants was compared to the growth of YEp24 control transformants or that of antisense control transformants. Drug resistance in liquid medium was determined by a modification of a previously published growth inhibition assay (34). Briefly, overnight yeast cultures were adjusted to an OD600 of 0.1 in YPD followed by a second 1:20 dilution. Fifty microliters of this dilution was added to 50 μl of a 2×-concentrated drug solution in a 96-well microtiter plate. Incubation was for 24 h at 30°C in a wet chamber. The fold resistance of each clone was determined by calculating the ratio of growth between yeast cells transformed with an insertless vector (YEp24 or pVT-U) and secondary transformants or yeasts transformed with pVT-U containing specific ORFs in the sense or antisense orientation. The significance of drug resistance was assessed for individual ORFs by using the t test to compare the mean fold drug resistance conferred by ORFs cloned into pVT-U in the sense and antisense orientations.
Sequence analysis.
Sequence information obtained from YEp24 genomic library clones was compared to the whole yeast genome sequence at SGD by using the BLAST program at SGD. Other BLAST searches (2) were done with the BLAST server at the National Center for Biotechnology Information. Amino acid sequence alignments were performed with the program “pileup,” which is part of the Genetics Computer Group (GCG) package of DNA analysis software (GCG version 8). Similar or identical residues in the alignments were shaded by using the program “boxshade” (version 2.7) run under UNIX on a Sparc station 20. Boxshade output files were further edited in “xfig.”
RESULTS
Determination of the MICs of quinoline-containing antimalarial compounds.
The aim of this study was to evaluate the use of S. cerevisiae as a screening system for the detection of genes involved in resistance to quinoline-based antimalarial drugs. Since wild-type yeasts generally tolerate high concentrations of quinoline-ring-containing drugs, we decided to use for our experiments the recently generated yeast strain CYX247-9A (14). This strain displays hypersensitivity to the quinoline ring-containing drug QD due to a loss-of-function mutation in the KEX2 gene which encodes a neutral serine protease (14). We determined the MICs of four quinoline-based antimalarial drugs for strain CYX247-9A. CYX247-9A cells displayed detectable sensitivity to CQ (MIC, 250 mg/ml), QD (MIC, 6 mg/ml), and MQ (MIC, 200 μg/ml). Due to the precipitation of QN in the agar below concentrations required for complete growth inhibition we could not define the corresponding MIC but classified it as >6 mg/ml. Since plating of a yeast library did not allow isolation of CQ-resistant clones, we focused in subsequent experiments on the identification of genes that confer resistance to MQ and QD when the genes are overexpressed in CYX247-9A.
Identification of ORFs that confer resistance to mefloquine.
Strain CYX247-9A was transformed with a YEp24-based genomic S. cerevisiae library and screened for MQ resistance, yielding a total of 22 colonies with increased MQ resistance compared to that of the YEp24 vector control. After determining the plasmid dependency of the resistant phenotype, a total of seven MQ-resistant clones were retained for further analysis. Restriction digest analysis indicated that two different classes of plasmids conferred increased levels of resistance to MQ. Plasmids derived from six of the seven resistant clones showed identical restriction digest patterns. In these clones, MQ resistance was found to be due to the overexpression of a glutamine suppressor tRNA. These clones were not further analyzed.
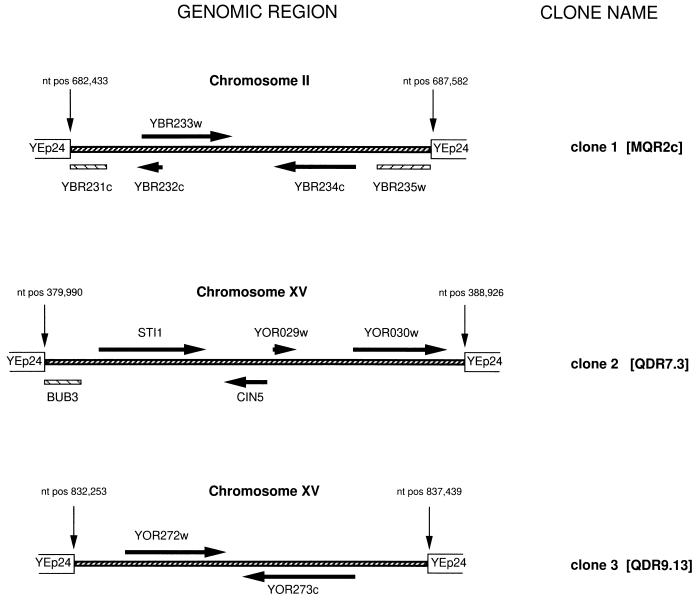
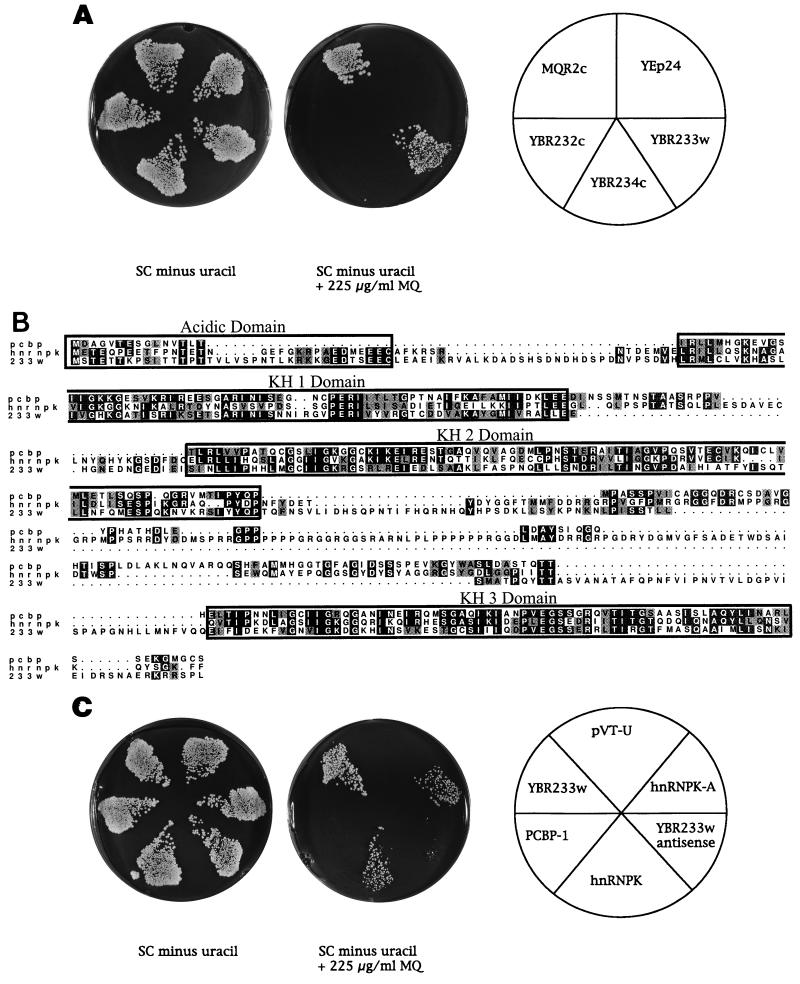
Sequence analysis of the remaining MQ-resistant clone, designated clone 1 (MQR2c), revealed a 5.8-kb insert. The genomic fragment in clone 1 is derived from chromosome II and spans three intact ORFs, YBR232c, YBR233w, and YBR234c, as well as the truncated ORFs YBR231c and YBR235w (Fig. 1). To determine which of these ORFs confers MQ resistance, we isolated YBR232c, YBR233w, and YBR234c by PCR and subcloned them into YEp24. Subsequent drug survival experiments on selective medium containing MQ revealed that only transformants expressing YBR233w but none of the other clones showed increased levels of resistance to MQ (Fig. 2A).
FIG. 1.
Schematic representation of the yeast chromosomal regions contained in the YEp24 genomic DNA clones obtained by direct selection with MQ for clone 1 (MQR2c) or QD for clone 2 (QDR7.3) and clone 3 (QDR9.13). Vector arms are shown as open boxes, while the genomic insert is represented by a black slashed line. ORFs are indicated by black arrows pointing in the direction of transcription, with the name of the ORF given above or below each arrow. Partial ORFs are indicated by slashed lines under the genomic insert line, with the corresponding name given under each ORF. The insert in clone 1 carries two partial ORFs. YBR231c is an ORF of 912 bp, of which 613 bp is contained within the insert, while ORF YBR235w represents a 3.3 kb gene, of which only 726 bp is contained within the insert of clone 1. Likewise, in clone 2 only 210 bp of the 1,025-bp BUB3 gene is contained within the insert. The chromosomal nucleotide positions (nt pos) of the flanking nucleotides for each of the three inserts are indicated above the genomic insert line, with a thin arrow pointing to the insert end.
FIG. 2.
MQ resistance in S. cerevisiae can be conferred by overexpression of yeast and human members of the hnRNPK family. (A) MQ resistance of clone 1 (MQR2c) is caused by the ORF YBR233w. The plate on the left shows growth of yeast transformed with the three ORFs YBR232c, YBR233w, and YBR234w, the YEp24 vector controls, and primary transformant clone 1 (MQR2c) in the absence of MQ. The middle plate shows the growth of the same yeast transformants in the presence of 225 μg of MQ per ml. The right plate depicts the plating scheme used. (B) Multiple amino acid sequence alignment of PCBP-1, hnRNPK, and YBR233w proteins. The extensive sequence homology in the region of the acidic and the KH domains identifies YBR233w as a yeast member of the hnRNPK family. (C) Overexpression of human hnRNPK genes can confer MQ resistance in yeast. The left plate shows the growth of a yeast transformed with the expression vector pVT-U containing ORF YBR233w in the sense and the antisense directions and cDNAs for the human genes PCBP-1, hnRNPK, and hnRNPK-A as an example of a splice variant plated on MQ-free agar. The middle plate shows the growth of the same yeast transformants in the left plate plated in the presence of 225 μg of MQ per ml. The right plate depicts the plating scheme used.
The YBR233w ORF encodes a protein with unknown function. However, a search with the predicted amino acid sequence of the YBR233w-encoded protein with the BLAST program revealed significant homologies to members of the family of heterogeneous nuclear ribonucleoproteins. The human homologs with the highest degree of similarity were hnRNPK (P = 1.8 × 10−17) and PCBP-1 (P = 8.6 × 10−29). hnRNPK, the major nuclear poly(rC)-binding protein, is involved in pre-mRNA metabolism (18, 31). Its RNA binding activity is mediated by three characteristic repetitive sequence elements termed KH (for K homology) domains (39). Multiple sequence alignment analysis revealed that the strongest homology between the products of YBR233w, PCBP-1, and hnRNPK is found in the KH domains, while the YBR233w-encoded protein shares a higher degree of homology with hnRNPK than with PCBP-1 in the amino-terminal acidic domain (Fig. 2B).
To determine if overexpression of the human genes could also confer MQ resistance, the cDNA clones for hnRNPK or PCBP-1 and several splice variants of hnRNPK were subcloned into the yeast expression vector pVT-U and tested in the MQ resistance assay (Fig. 2C). Yeast transformants expressing hnRNPK (and all its tested splice variants) showed an increased level of resistance to MQ; however, the level of resistance was lower than the level of resistance to MQ observed for YBR233w transformants. In contrast, yeast clones expressing the PCBP-1 member of the hnRNPK family did not show any increased resistance to MQ (Fig. 2C).
Identification of ORFs that confer resistance to QD.
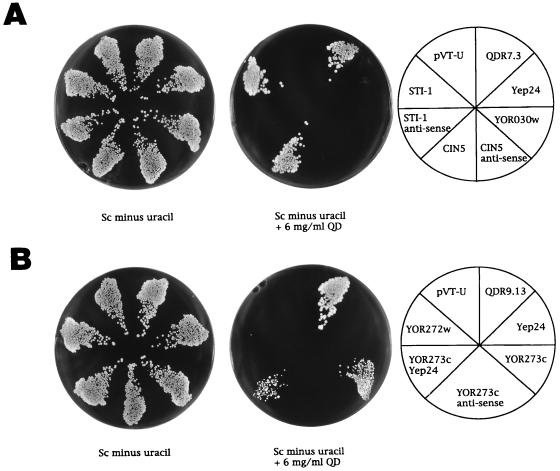
Strain CYX247-9A was transformed with the YEp24-based genomic S. cerevisiae library, and transformants were screened for QD resistance, yielding a total of 15 colonies able to grow in the presence of QD. Retransformation of the recombinant plasmids isolated from the resistant colonies identified two clones displaying a plasmid-dependent QD resistance phenotype. These clones were designated QDR7.3 (clone 2) and QDR9.13 (clone 3). Sequence analysis revealed that clone 2 contains a genomic fragment of 8.9 kb derived from chromosome XV and that clone 3 contains a 5.2-kb fragment derived from chromosome XV (Fig. 1). The chromosome XV-derived fragment in clone 2 spans the complete ORFs YOR027w (STI1), YOR028c (CIN5), YOR029w, and YOR030w and the truncated ORF YOR026w (BUB3). The ORF YOR029w encodes a very short polypeptide (111 amino acids) and was not considered for further analysis. The ORFs encoding the genes STI1, CIN5, and YOR030w were isolated by PCR and were subcloned into a yeast vector, pVT-U or YEp24. Transformants were tested on selective medium containing QD (Fig. 3A). Interestingly, increased resistance to QD was observed for transformants expressing either the STI1 gene or the CIN5 gene; these genes encode a heat shock protein-associated polypeptide and a transcriptional activator, respectively. Control cells transformed with pVT-U vectors containing an antisense construct of CIN5 or STI1 did not show any increased growth in the presence of QD. Likewise, transformants expressing YOR030w showed a growth pattern similar to that of the vector control.
FIG. 3.
Identification of three genes that confer resistance to QD when they are overexpressed in yeast. (A) CIN5 and STI1 are two genes that confer QD resistance and that are contained within the same QD-resistant primary transformant clone 2 (QDR7.3). The right plate depicts the plating scheme and the identification of transformants. The left plate shows equal growth of all yeast transformants in the absence of QD. The middle plate shows only growth of the yeast transformed with primary library clone 2 (QDR7.3) or with CIN5 and STI1 expressed under the control of the ADH promoter in pVT-U. (B) The ORF YOR273c contained in the QD-resistant primary transformant clone 3 (QDR9.13) confers QD resistance when it is overexpressed in yeast. The right plate depicts the plating scheme and the identities of the transformants. The left plate shows equal growth of all yeast transformants in the absence of QD. The middle plate shows only the growth of yeast transformed with primary library clone 3 (QDR9.3) and with YOR273c cloned in YEp24 or pVT-U (YOR273c) in the presence of QD.
The recombinant plasmid in clone 3 carries a genomic fragment from chromosome XV that spans the two ORFs YOR272w (YMT1) and YOR273c (Fig. 1). Both ORFs were isolated by PCR and were subcloned into pVT-U or YEp24. Drug survival assays on QD-containing selective medium demonstrated that transformants expressing YOR273c (pVT-U) or YOR273c (YEp24) but not transformants expressing YOR272w showed increased levels of growth in the presence of QD (Fig. 3B). The somewhat reduced level of growth of the YOR273c (YEp24) transformants relative to that of the YOR273c (pVT-U) yeasts may reflect a lower level of protein expression in this clone. Control cells transformed with antisense constructs of YOR273c did not confer increased resistance to QD. YOR273c is predicted to be a member of the major facilitator superfamily of transmembrane proteins (22).
Cross-resistance analysis.
To determine if the overexpression of gene products identified in our screen confers multidrug resistance, we tested all resistant clones for growth in the presence of QD, MQ, QN, and CQ using a growth inhibition assay in liquid medium (34). This assay requires much lower concentrations of drugs for the inhibition of yeast growth than the drug resistance assay on agar plates. Therefore, we were able to test cross-resistance to QN and CQ, two drugs for which we had not been able to identify resistant clones by selection on agar plates (Table 1). Cross-resistance was assayed for secondary YEp24 clones for which gene expression is independent of clone orientation (data not shown) and for individual ORFs cloned in the sense and antisense orientations into the pVT-U expression vector. Resistance was measured as fold resistance over that of control yeast transformed with insertless YEp24 or pVT-U vectors (Table 1). Clone 2 showed a pleiotropic resistance phenotype with a 6.5-fold resistance to QD, a 5.6-fold resistance to MQ, and a 5.6-fold resistance to CQ. This clone displayed only a low level of resistance to QN (2.5-fold). The STI1 subclone displayed a level of QD resistance similar to that of clone 2, a low level of resistance to MQ, and nonsignificant resistance to QN and CQ. In contrast, yeasts containing the pVT-U plasmid with STI1 in the antisense orientation did not display any increased level of drug resistance. The overexpression of CIN5, the second drug resistance locus present within the clone 2 insert, confers 3.9-fold resistance to QD, 5.4-fold resistance to CQ, and 9.6-fold resistance to MQ. No significant resistance to QN was detected (Table 1). The QD resistance phenotype conferred by clone 3 and its subclone YOR273c is very specific: YOR273c confers 4.3-fold resistance to QD, but we did not detect cross-resistance to any of the other compounds tested. Control transformants containing an antisense construct of YOR273c did not display increased QD resistance. We next tested clone 1 and its subclone YBR233w for cross-resistance to quinoline-containing drugs (Table 1). Both clones displayed very high levels of resistance to MQ but no significant resistance to QD, QN, or CQ. The difference in resistance between clone 1 and YBR233w is not significant (P = 0.127).
TABLE 1.
Quantitation of resistance to QD, MQ, QN, and CQ
| Yeast clone | Fold resistancea
|
|||
|---|---|---|---|---|
| QD | MQ | QN | CQ | |
| Clone 2b | 6.5 ± 1.0 | 5.6 ± 0.8 | 2.5 ± 0.5 | 5.6 ± 3.8 |
| STI1c | 7.0 ± 0.4d | 3.4 ± 0.5e | 2.0 ± 0.8f | 2.7 ± 1.7f |
| Anti-STI1c | 1.0 ± 0.1 | 1.3 ± 0.6 | 1.3 ± 0.6 | 1.3 ± 0.6 |
| CIN5c | 3.9 ± 0.5d | 9.6 ± 1.4d | 1.2 ± 0.2f | 5.4 ± 0.7d |
| Anti-CIN5c | 1.0 ± 0.1 | 1.5 ± 0.5 | 1.0 ± 0.1 | 1.3 ± 0.5 |
| Clone 3b | 4.8 ± 1.3 | 1.5 ± 0.6 | 1.3 ± 0.5 | 2.7 ± 0.6 |
| YOR273cc | 4.3 ± 1.5g | 1.0 ± 0.0f | 1.4 ± 0.5f | 1.3 ± 0.6f |
| Anti-YOR273cc | 1.5 ± 0.5 | 1.0 ± 0.0 | 1.3 ± 0.6 | 1.5 ± 0.5 |
| Clone 1b | 2.5 ± 1.3 | 22.4 ± 12.4 | 1.6 ± 0.6 | 1.8 ± 1.3 |
| YBR233wc | 1.3 ± 0.6f | 13.5 ± 3.6d | 1.2 ± 0.3f | 2.9 ± 1.7f |
| Anti-YBR233wc | 1.2 ± 0.3 | 1.3 ± 0.6 | 1.2 ± 0.3 | 1.3 ± 0.6 |
Significance of resistance for individual ORFs was assessed by comparison with drug resistance conferred by the same ORF cloned in the antisense orientation in the pVT-U expression vector.
Fold resistance over that of transformants containing insertless vector YEp24.
Fold resistance over that of transformants containing insertless vector pVT-U.
P < 0.001.
P < 0.01.
Not significant.
P < 0.05.
DISCUSSION
The mechanisms of resistance to quinoline-containing antimalarial drugs remain controversial and the underlying molecular patterns are still poorly understood. In order to identify structural and functional features involved in resistance to antimalarial compounds in yeast, we used a strategy that is based on the functional overexpression of genes in S. cerevisiae. This approach seemed reasonable since gene amplification and overexpression are frequent causes of drug resistance among eukaryotic parasites (6). Since yeast generally displays a low level of sensitivity to quinoline ring-containing drugs, we used in our experiments the CYX247-9A S. cerevisiae mutant strain (14). Although the mechanism of QD hypersensitivity of CYX247-9A is not known, results obtained by Conklin et al. (14) make it likely that absence of modification of an unknown Kex2p substrate is essential for mediating QD hypersensitivity. However, we noticed in control experiments that a number of wild-type yeast strains were only slightly more tolerant of quinine-like drugs than CYX247-9A (data not shown). This observation may also explain why we failed to isolate the KEX2 gene by our experiments since the conditions of our screen might have been too stringent for overexpressed KEX2 to mediate the growth of CYX247-9A transformants. Moreover, it is possible that we did not isolate KEX2-carrying clones since either we did not achieve transfection saturation of the yeast used for drug selection or KEX2 was missing from the library.
We show in this report that overexpression of the YOR273c gene product confers approximately fourfold resistance to QD. This activity appears to be specific for QD because we did not detect any resistance to the related compounds QN, CQ, and MQ. YOR273c is a member of the major facilitator superfamily of transmembrane transporters (22). Several proteins of this family have a transport function in drug resistance such as the 3-aminotriazole/4-nitroquinoline-N-oxide (3-AT/4-NQO) resistance protein (ATR1) (23, 28) and the fluconazole resistance protein 1 (FLR1) (1) in S. cerevisiae, the amiloride resistance protein (CAR1) (26) in Schizosaccharomyces pombe, the benomyl and methotrexate resistance protein (BMRP) (20) in Candida albicans, and the cycloheximide resistance protein (CYHR) (36) in Candida maltosa. Of these proteins, BMRP (P = 5.5e−60), FLR1 (P = 1.1e−55), CYHR (P = 9.6e−52), and CAR1 (P = 1.3e−30) show the highest degree of homology with the predicted amino acid sequence of the YOR273c protein. On the basis of the high degree of sequence homology with the sequences of other drug transporters in the major facilitator superfamily, YOR273c appears to encode a transport protein that functions as a drug transporter.
The overexpression of STI1 conferred resistance to QD and MQ (Table 1). Sti1p belongs to the family of heat shock protein 90 (Hsp90)-associated proteins (Hsp90APs), such as the human protein p60, which are thought to modulate Hsp90 activities (12, 32). Hsp90 binds to extremely varied target proteins such as steroid hormone receptors (5), basic helix-loop-helix transcription factors (38), and the oncogenic tyrosine kinase v-Src (8). This association leads to structural and functional stabilization of the target proteins. Recent evidence indicates that Sti1p physically interacts with Hsp90, thereby modulating the activity of the glucocorticoid receptor (11). It is conceivable that Sti1p confers resistance to MQ and QD through its interaction with Hsp90 and its subsequent modulation of the activity of a downstream target(s) involved in drug resistance. Certain elements of the heat shock response pathways are highly conserved throughout phylogeny. The p60 and STI1 homolog in P. falciparum has been cloned and has been shown to interact with several plasmodial proteins, one of which corresponds in size to Hsp90 (16).
The overexpression of CIN5 confers pleiotropic resistance to QD, MQ, and CQ (Table 1). Although we were surprised to find the two drug resistance loci CIN5 and STI1 in very close proximity (Fig. 1), clustering of resistance loci is not without precedent. Recently, three contiguous genes conferring resistance to arsenic compounds were found on a 4.2-kb fragment of yeast chromosome XVI (4). CIN5, also known as YAP4 (19), is a member of the bZIP family of transcriptional activators which share a basic signature motif required for sequence-specific DNA binding (b) and a leucine zipper domain (ZIP) involved in dimerization. Two members of the yeast bZIP family, YAP1 and YAP2, have previously been shown to mediate drug resistance. Overexpression of YAP1 confers multidrug resistance via trans-activation of the YCF1 gene encoding a cadmium transporter (49), FLR1, encoding a fluconazole transporter (1), and ATR1, encoding the 3-AT/4-NQO transporter (13). In contrast, the targets of YAP2 trans-activation are still unknown. The highly pleiotropic resistance phenotype mediated by CIN5 (YAP4) makes it likely that Cin5p, by analogy to Yap1p, upregulates unknown transporters of antimalarial drugs or other types of target proteins conferring drug resistance. The molecular identity and specific function performed by Cin5p targets are under investigation.
Our results indicate that overexpression of the YBR233w gene product conferred resistance to MQ but not resistance to QD, QN, or CQ (Table 1). The YBR233w gene product is the yeast homolog of the hnRNPK family of nuclear RNA binding proteins which are involved in various stages of pre-mRNA metabolism of cytidine-rich transcripts (31). In addition to its function in pre-mRNA metabolism, hnRNPK acts as a transcriptional activator and has been shown to increase the levels of transcription from the P1 promoter of human c-myc (42) and several other polymerase II promoters (29). A common feature of all members of the hnRNPK family is the presence of one or more 65- to 70-amino-acid repeats termed KH domains (39). KH domains mediate the interaction of hnRNPK proteins with RNA and have been identified in a variety of functionally diverse proteins including the human poly(C)-binding proteins PCBP-1 and PCBP-2 (30) that contain three KH domains and that display a high affinity for poly(rC) (17). Together with hnRNPK, they are the major cellular human poly(rC) binding proteins (30). However, in contrast to other hnRNPs, which are predominantly localized to the nucleus, PCBPs appear to be mostly cytoplasmic. The YBR233w protein shows similar sequence homology to both hnRNPK and PCBP-1 over all three KH domains, while in the acidic domain there is a higher degree of homology between the YBR233w protein and hnRNPK (Fig. 2B). It is not known if the sequence divergence between hnRNPK and PCB-1 in the acidic domain explains the differential induction of MQ resistance by both proteins (Fig. 2C). However, it has been shown that the acidic domain is essential for trans-activation of the c-myc promoter by hnRNPK (42). Induction of MQ resistance by the human hnRNPK protein in yeast supports the proposal that basic principles of transcription activation have been conserved among phylogenetically distant species. However, at present we do not know how transcriptional activation or mRNA stabilization by hnRNPK proteins can mediate MQ resistance.
While the results of our experiments provided new insight into the physiology and control of quinoline ring-containing drug resistance in yeast, it is debatable if and to what extent our findings apply to quinoline drug resistance in P. falciparum. S. cerevisiae and P. falciparum are fundamentally distinct organisms that do not share a close evolutionary relationship and that require different extracellular environments to thrive in vitro and in vivo. It is thought that quinoline ring-containing drugs exert their antimalarial effects via inhibition of hemozoin polymerization, a physiological process that does not exist in yeast (41). The molecular basis for the MQ-, QD-, and QN-mediated growth inhibition of yeast and the detailed mechanism of how loss of function of the KEX2 gene can lead to the increased sensitivity of the CYX247-9A strain to drugs are not known. In addition, the concentrations of quinoline ring-containing drugs for inhibition of yeast growth are much higher than those required for killing of P. falciparum cells. These clear differences between yeast and P. falciparum make it possible that the cellular pathways involved in resistance are distinct between the two organisms. On the other hand, drug resistance mechanisms that involve overexpression and upregulation of specific drug transporters may be conserved between yeast and P. falciparum. For example, overexpression of the ldmdr1 gene of Leishmania donovani, like its mammalian mdr homologs, confers resistance to multiple anthracyclic drugs (24), the leishmanial PgpA transporter, in analogy to its mammalian Mrp homologs, confers oxyanion resistance (9), the plasmodial pfmdr1-encoded Pgh1 protein can functionally complement the transport of a mating factor by its yeast homolog Ste6p (35, 48), and the bacterial ABC transporter LmrA is functionally interchangeable with its human P-glycoprotein homolog (44, 45). These findings suggest that despite the differences that exist between yeast and plasmodial cellular physiologies, common mechanistic pathways of drug resistance may exist between the two organisms. Therefore, the cloning and functional characterization of plasmodial transcriptional activators together with their targets may provide a new avenue for the study of drug resistance in P. falciparum.
ACKNOWLEDGMENTS
We are grateful for the expert technical assistance of A. Miller. We thank D. S. Conklin for the gift of yeast strain CYX247-9A and H. Leffers and G. Dreyfuss for the gifts of the hnRNPK and PCBP cDNA clones, respectively.
The work described here was supported by a grant from the Medical Research Council of Canada (MRC) (to E.S.). U.D. was supported by an MRC postdoctoral fellowship, M.R. is a scholar of MRC, and E.S. is supported by a career award from the Fonds de Recherches en Santé du Québec.
REFERENCES
- 1.Alarco A M, Balan I, Talibi D, Mainville N, Raymond M. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J Biol Chem. 1997;272:19304–19313. doi: 10.1074/jbc.272.31.19304. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Becker D M, Guarente L. High-efficiency transformation of yeast by electroporation. In: Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press, Inc.; 1991. pp. 182–186. [DOI] [PubMed] [Google Scholar]
- 4.Bobrowicz P, Wysocki R, Owsianik G, Goffeau A, Ulaszewski S. Isolation of three contiguous genes, ACR1, ACR2, and ACR3 involved in resistance to arsenic compounds in the yeast Saccharomyces cerevisiae. Yeast. 1997;13:819–828. doi: 10.1002/(SICI)1097-0061(199707)13:9<819::AID-YEA142>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Bohen S P, Yamamoto K R. Modulation of steroid receptor signal transduction by heat shock proteins. In: Morimoto R I, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 313–334. [Google Scholar]
- 6.Borst P, Ouellette M. New mechanisms of drug resistance in parasitic protozoa. Annu Rev Microbiol. 1995;49:427–460. doi: 10.1146/annurev.mi.49.100195.002235. [DOI] [PubMed] [Google Scholar]
- 7.Botstein D, Falco S C, Stewart S E, Brennan M, Scherer S, Stinchcomb D T, Struhl K, Davis R W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979;8:17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- 8.Brugge S J. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- 9.Callahan H L, Beverley S M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991;266:18427–18439. [PubMed] [Google Scholar]
- 10.Carlson M, Botstein D. Two differentially regulated mRNAs with different 5′ ends encode secreted intracellular forms of yeast invertase. Cell. 1982;25:145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- 11.Chang H C, Nathan D F, Lindquist S. In vivo analysis of the Hsp90 cochaperone STI1 (p60) Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S V, Prapanich R A, Rimerman R A, Honore B, Smith D. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins Hsp90 and Hsp70. Mol Endocrinol. 1996;10:682–693. doi: 10.1210/mend.10.6.8776728. [DOI] [PubMed] [Google Scholar]
- 13.Coleman S T, Tseng E, Moye-Rowley W S. Saccharomyces cerevisiae basic region-leucine zipper protein regulatory networks converge at the ATR1 structural gene. J Biol Chem. 1997;272:23224–23230. doi: 10.1074/jbc.272.37.23224. [DOI] [PubMed] [Google Scholar]
- 14.Conklin D S, Culbertson M R, Kung C. Saccharomyces cerevisiae mutants sensitive to the antimalarial and antiarrhythmic drug quinidine. FEMS Microbiol Lett. 1994;119:221–228. doi: 10.1111/j.1574-6968.1994.tb06892.x. [DOI] [PubMed] [Google Scholar]
- 15.Cowman A F, Galatis D, Thompson J K. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc Natl Acad Sci USA. 1994;91:1143–1147. doi: 10.1073/pnas.91.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das A, Syin C, Fujioka H, Zheng H, Goldman N, Aikawa M, Kumar N. Molecular characterization and ultrastructural localization of Plasmodium falciparum Hsp60. Mol Biochem Parasitol. 1997;88:95–104. doi: 10.1016/s0166-6851(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 17.Dejgaard H, Leffers H. Characterisation of the nucleic-acid-binding activity of KH domains: different properties of different domains. Eur J Biochem. 1996;241:425–431. doi: 10.1111/j.1432-1033.1996.00425.x. [DOI] [PubMed] [Google Scholar]
- 18.Dejgaard K, Leffers H, Rasmussen H H, Madsen P, Kruse T A, Gesser B, Nielsen H, Celis J E. Identification, molecular cloning, expression and chromosome mapping of a family of transformation upregulated hnRNPK proteins derived by alternative splicing. J Mol Biol. 1994;236:33–48. doi: 10.1006/jmbi.1994.1116. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes L, Rodrigues-Pousada C, Struhl K. Yap, a novel family of eight bZIP proteins in Saccharomyces cerevisiae with distinct biological functions. Mol Cell Biol. 1997;17:6982–6993. doi: 10.1128/mcb.17.12.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fling M E, Kopf J, Tamarkin A, Gorman J A, Smith H A, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991;227:318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- 21.Foote S J, Thompson J K, Cowman A F, Kemp D J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989;57:921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- 22.Goffeau A, Park J, Paulsen I T, Jonniaux J-L, Dinh T, Mordant P, Saier M H., Jr Multidrug-resistant transport proteins in yeast: complete inventory and phylogenetic characterization of yeast open reading frames within the major facilitator superfamily. Yeast. 1997;13:43–54. doi: 10.1002/(SICI)1097-0061(199701)13:1<43::AID-YEA56>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Gompel-Klein P, Brendel M. Allelism of SNQ1 and ATR1, genes of the yeast Saccharomyces cerevisiae required for controlling sensitivity to 4-nitroquinoline-N-oxide and aminotriazole. Curr Genet. 1990;18:93–96. doi: 10.1007/BF00321122. [DOI] [PubMed] [Google Scholar]
- 24.Henderson D M, Sifri C D, Rodgers M, Wirth D F, Hendrickson N, Ullman B. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol Cell Biol. 1992;12:2855–2865. doi: 10.1128/mcb.12.6.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of E. coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 26.Jia Z P, McCullogh N, Wong L, Young P G. The amiloride resistance gene CAR1, of Schizosaccharomyces pombe. Mol Gen Genet. 1993;241:298–304. doi: 10.1007/BF00284681. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 28.Kanazawa S, Driscoll M, Struhl K. ATR1, a Saccharomyces cerevisiae gene encoding a transmembrane protein required for aminotriazole resistance. Mol Cell Biol. 1988;8:664–673. doi: 10.1128/mcb.8.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M H, Mori S, Raychaudhuri P. Trans-activation by the hnRNPK protein involves an increase in RNA synthesis from the reporter genes. J Biol Chem. 1996;271:3420–3427. doi: 10.1074/jbc.271.7.3420. [DOI] [PubMed] [Google Scholar]
- 30.Leffers H, Dejgaard K, Celis J E. Characterisation of two major cellular poly(rC)-binding human proteins each containing three K-homologous (KH) domains. Eur J Biochem. 1995;230:447–453. [PubMed] [Google Scholar]
- 31.Matunis M J, Michael W M, Dreyfuss G. Characterization and primary structure of the poly(C)-binding heterogeneous nuclear ribonucleoprotein complex K protein. Mol Cell Biol. 1992;12:164–171. doi: 10.1128/mcb.12.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolet C M, Craig E A. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:3638–3646. doi: 10.1128/mcb.9.9.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peel S A, Bright P, Yount B, Handy J, Baric R S. A strong association between mefloquine and halofantrine resistance and amplification, overexpression, and mutation in the P-glycoprotein homolog (pfmdr) of Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1994;51:648–658. doi: 10.4269/ajtmh.1994.51.648. [DOI] [PubMed] [Google Scholar]
- 34.Raymond M, Ruetz S, Thomas D Y, Gros P. Functional expression of P-glycoprotein in Saccharomyces cerevisiae confers cellular resistance to the immunosuppressive and antifungal agent FK520. Mol Cell Biol. 1994;14:277–286. doi: 10.1128/mcb.14.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruetz S R, Delling U, Brault M, Schurr E, Gros P. The pfmdr1 gene of Plasmodium falciparum confers cellular resistance to antimalarial drugs in yeast cells. Proc Natl Acad Sci USA. 1996;93:9942–9947. doi: 10.1073/pnas.93.18.9942. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Sasnauskas K, Jomantiene R, Lebediene E, Lebedys J, Januska A, Janulaitis A. Cloning and sequence analysis of a Candida maltosa gene which confers resistance to cycloheximide. Gene. 1992;116:105–108. doi: 10.1016/0378-1119(92)90636-4. [DOI] [PubMed] [Google Scholar]
- 37.Sherman F. Getting started with yeast. In: Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press, Inc.; 1991. pp. 3–21. [Google Scholar]
- 38.Shue G, Kohtz D S. Structural and functional aspects of basic helix-loop-helix protein folding by heat-shock protein 90. J Biol Chem. 1994;269:2707–2711. [PubMed] [Google Scholar]
- 39.Siomi H, Matunis M J, Michael W M, Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993;21:1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su X Z, Kirkman L A, Fujioka H, Wellems T E. Complex polymorphisms in an ∼330kDa protein are linked to chloroquine-resistant P. falciparum in southeast Asia and Africa. Cell. 1997;91:593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan D J, Jr, Gluzman I Y, Russell D G, Goldberg D E. On the molecular mechanism of chloroquine’s antimalarial action. Proc Natl Acad Sci USA. 1996;93:11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomonaga T, Levens D. Heterogenous nuclear ribonucleoprotein K is a DNA-binding transactivator. J Biol Chem. 1995;270:4875–4881. doi: 10.1074/jbc.270.9.4875. [DOI] [PubMed] [Google Scholar]
- 43.Van Es H H G, Karcz S, Chu F, Cowman A, Vidal S, Gros P, Schurr E. Expression of the plasmodial pfmdr1 gene in mammalian cells is associated with increased susceptibility to chloroquine. Mol Cell Biol. 1994;14:2419–2428. doi: 10.1128/mcb.14.4.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Veen H W, Callaghan R, Soceneantu L, Sardini A, Konings W N, Higgins C F. A bacterial antibiotic-resistance gene that complements the human multidrug-resistance P-glycoprotein gene. Nature. 1998;391:291–295. doi: 10.1038/34669. [DOI] [PubMed] [Google Scholar]
- 45.van Veen H W, Venema K, Bolhuis H, Oussenko I, Kok J, Poolman B, Driessen A J, Konings W N. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc Natl Acad Sci USA. 1996;93:10668–10672. doi: 10.1073/pnas.93.20.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vernet T, Dignard D, Thomas D Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 47.Volkman S K, Chow L M C, Woodcock S A, Frank M, Wirth D F. Abstracts of the Molecular Parasitology Meeting. 1997. Saccharomyces cerevisiae as a model system to examine drug resistance in protozoan parasites. [Google Scholar]
- 48.Volkman S K, Cowman A F, Wirth D F. Functional complementation of the ste6 gene of Saccharomyces cerevisiae with the pfmdr1 gene of Plasmodium falciparum. Proc Natl Acad Sci USA. 1995;92:8921–8925. doi: 10.1073/pnas.92.19.8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wemmie J A, Szczypka M S, Thiele D J, Moye-Rowley W S. Cadmium tolerance mediated by the yeast yAP-1 protein requires the presence of an ATP-binding cassette transporter-encoding gene, YCF1. J Biol Chem. 1994;269:32592–32597. [PubMed] [Google Scholar]
- 50.Wilson C M, Volkman S K, Thaithong S, Martin R K, Kyle D E, Milhous W K, Wirth D F. Amplification of pfmdr1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol Biochem Parasitol. 1993;57:151–160. doi: 10.1016/0166-6851(93)90252-s. [DOI] [PubMed] [Google Scholar]
- 51.Wirth D, Cowman A. Mechanisms of drug resistance in protozoan parasites. In: Smith D F, Parsons M, editors. Molecular biology of parasitic protozoa. New York, N.Y: Oxford University Press; 1996. pp. 181–204. [Google Scholar]