Abstract
The carbocyclic analog of 2′-deoxyguanosine (CdG) has broad-spectrum antiviral activity. Because of recent observations with other nucleoside analogs that biological activity may be associated the l enantiomer rather than, as expected, with the d enantiomer, we have studied the metabolism of both enantiomers of CdG to identify the enzymes responsible for the phosphorylation of CdG in noninfected and virally infected human and duck cells. We have examined the enantiomers as substrates for each of the cellular enzymes known to catalyze phosphorylation of deoxyguanosine. Both enantiomers of CdG were substrates for deoxycytidine kinase (EC 2.7.1.74) from MOLT-4 cells, 5′-nucleotidase (EC 3.1.3.5) from HEp-2 cells, and mitochondrial deoxyguanosine kinase (EC 2.7.1.113) from human platelets and CEM cells. For both deoxycytidine kinase and mitochondrial deoxyguanosine kinase, the l enantiomer was the better substrate. Even though the d enantiomer was the preferred substrate with 5′-nucleotidase, the rate of phosphorylation of the l enantiomer was substantial. The phosphorylation of d-CdG in MRC-5 cells was greatly stimulated by infection with human cytomegalovirus. The fact that the phosphorylation of d-CdG was stimulated by mycophenolic acid and was not affected by deoxycytidine suggested that 5′-nucleotidase was the enzyme primarily responsible for its metabolism in virally infected cells. d-CdG was extensively phosphorylated in duck hepatocytes, and its phosphorylation was not affected by infection with duck hepatitis B virus. These results are of importance in understanding the mode of action of d-CdG and related analogs and in the design of new biologically active analogs.
d-CdG is an analog of CdG that has broad-spectrum antiviral activity (27–29). Until recently, the biological activity of a nucleoside analog was assumed to be due only to the “natural” β-d form. However, there are now numerous observations of antiviral activity associated with nucleosides in l configurations (for reviews, see references 10 and 26) and one report of antitumor activity associated with a β-l enantiomer (11). Even though l-CdG is much less active against HSV (4) and HCMV (unpublished results), it is essential that the metabolism of both enantiomers be examined to thoroughly understand the mechanism of action of a nucleoside analog. We have reported earlier that both enantiomers of CdG are extensively phosphorylated in cells infected with HSV-1 and that the enantiomers had equal activities as substrates for the virus-encoded kinase (4, 5). It was also observed that d-CdG was converted to its triphosphate (but to a much lesser extent) in noninfected cells and that more than one enzyme appeared to be involved in the initial phosphorylation. Since cellular enzymes presumably are responsible for the activation of CdG in cells infected with HBV (which is not known to code for a kinase) and may also be important for the activation of CdG in cells infected with HCMV (which induces cellular kinases [6, 8, 21, 23, 32, 44], in addition to encoding a ganciclovir-phosphorylating enzyme [22, 35]), we have evaluated the enantiomers of CdG as substrates for the three cellular enzymes known to catalyze phosphorylation of deoxyguanosine (2): dCyd kinase, mitochondrial dGuo kinase, and 5′-nucleotidase. We also report here observations on the metabolism of d-CdG in cells infected with HCMV and in duck hepatocytes infected with DHBV.
(Some of these results have been presented elsewhere in preliminary form [1].)
MATERIALS AND METHODS
Abbreviations.
Ado, adenosine; araHyp, 9-(β-d-arabinofuranosyl)hypoxanthine; BDG, 9-benzyl-9-deazaguanine; CdG, the carbocyclic analog of 2′-deoxyguanosine; CMV, cytomegalovirus; dCyd, 2′-deoxycytidine; dGuo, 2′-deoxyguanosine; DHBV, duck hepatitis B virus, dThd, thymidine; HBV, hepatitis B virus; HCMV, human cytomegalovirus; HPLC, high-performance liquid chromatography; HSV, herpes simplex virus; HSV-1, herpes simplex virus type 1; IC50, 50% inhibitory concentration; IMP, inosinic acid; Ino, inosine; MPA, mycophenolic acid; SAX, strong anion exchange.
Materials.
The preparation of tritiated [8-3H]d-CdG (1,600 mCi/mmol) and [8-3H]l-CdG (900 mCi/mmol) has been described previously (5). BDG, an inhibitor of purine nucleoside phosphorylase (EC 2.4.2.1), was prepared in our laboratories (25). araHyp was obtained from Pfanstiehl Laboratories, Waukegan, Ill.; all other nucleosides and MPA were obtained from Sigma Chemical Co., St. Louis, Mo. HEp-2 cells were grown in Eagle’s minimum essential medium supplemented with bovine calf serum. CEM cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum. Human diploid embryonic lung cells (MRC-5 cells) were obtained from BioWhitaker (Walkersville, Md.) and were cultured in modified Eagle’s medium containing 9% heat-inactivated fetal bovine serum. Human platelets were obtained from the Birmingham Chapter of the American Red Cross.
Enzyme isolations and assays.
dCyd kinase was purified 24,000-fold from MOLT-4 cells to greater than 95% purity; the Vmax with dGuo as the substrate was 720 μmol/h/mg of protein. The procedures for the isolation and for assay of nucleosides as substrates have been described elsewhere (30). 5′-Nucleotidase was isolated from HEp-2 cells essentially as described by Itoh (14) and Worku and Newby (41) except that the original homogenate was applied directly to a column of phosphocellulose. The enzyme was purified 520-fold; the Vmax with Ino as the substrate was 1,600 μmol/h/mg of protein. For assay of nucleosides as substrates, the incubation mixture contained 100 mM imidazole (pH 6.5), 500 mM NaCl, 50 mM MgCl2, 5 mM ATP, and 10 mM IMP. Mitochondrial dGuo kinase was prepared from mitochondria isolated from CEM cells by the no-gradient procedure described by Bogenhagen and Clayton (7). Briefly, cells were resuspended in low-ionic-strength buffer and were disrupted by Dounce homogenization. The nuclei were removed by centrifugation at 1,300 × g, and the supernatant was centrifuged at 22,000 × g to pellet the mitochondria. The mitochondrial pellet was incubated with DNase I for 30 min at 37°C as described by Higuchi and Linn (13) and was washed three times with mannitol-sucrose buffer. The proteins from the mitochondrial pellet were extracted as described by Kosovsky and Soslau (19). dGuo kinase activity was measured in reaction volumes containing 100 mM Tris (pH 8.0), 40 mM ATP, 48 mM MgCl2, 50 mM dithiothreitol, 1 mg of bovine serum albumin per ml, and the desired concentration of nucleoside substrate. The Vmax of this preparation of dGuo kinase with dGuo as the substrate was 31 nmol/h/mg of protein. Experiments were also done with dGuo kinase from mitochondria isolated from human platelets as described by Kosovsky and Soslau (19); the Vmax with dGuo as the substrate was 9 nmol/h/mg of protein. The experiments with dGuo kinase from both sources gave similar results. Nucleoside substrates were separated from the nucleotide products by using DE-81 filters as described previously (5). In addition, product formation with dGuo kinase was confirmed by SAX HPLC, as described below.
The Michaelis-Menten parameters were determined from linear double-reciprocal plots of 1/velocity versus 1/concentration of the substrate. The best line was determined by linear regression from at least five datum points, and the Km and Vmax were determined from the x and the y intercepts.
Metabolism of CdG in intact mammalian cells.
Either [3H]d-CdG or [3H]l-CdG was added to exponentially growing cultures of CEM or HEp-2 cells. After various periods of time the cells were harvested and cold 0.5 N perchloric acid extracts were prepared and subjected to analysis for nucleotides by SAX HPLC (5). Two-milliliter fractions were collected and assayed for radioactivity. Nucleosides that are known to be substrates for one of the kinases acting on deoxynucleosides were added 30 min prior to the addition of labeled compound. Reduction of the conversion of CdG to CdG phosphates was taken as an indication of competition between CdG and the added nucleoside for the same phosphorylating enzyme. To determine the possible involvement of 5′-nucleotidase, experiments were performed with MPA, which has been shown to increase 5′-nucleotidase activity by causing a buildup of IMP consequent to MPA’s inhibition of IMP dehydrogenase (17). An increase in phosphorylation of CdG in MPA-treated cells was taken as evidence that CdG was phosphorylated by 5′-nucleotidase.
Similar experiments were performed with confluent monolayers of MRC-5 cells (75-cm2 flasks) and with HCMV-infected MRC-5 cells (except that these cells were not proliferating). The phosphorylation of [3H]CdG was evaluated in confluent MRC-5 cell cultures at approximately the 26th passage. For evaluation of the effects of HCMV on the phosphorylation of [3H]CdG, MRC-5 cells were inoculated with strain AD169 at a multiplicity of infection of 10. After a 1-h period for virus absorption the medium was replaced with fresh medium and the cells were incubated at 37°C until they were treated with agents. The analysis for nucleotides was performed as described above for uninfected cells.
Metabolism of d-CdG in duck hepatocytes.
Primary hepatocytes were prepared from 2- to 3-week-old Pekin ducks that were chronically infected with DHBV and from ducks that were not infected. The procedures of liver perfusion and hepatocyte isolation and the culture conditions were described previously (36, 42). Hepatocytes were seeded at confluence (approximately 5 × 106 cells) onto 60-mm petri dishes, and the serum-free growth medium was changed daily. The experiments with [3H]d-CdG were done 4 days after initiation of the culture.
RESULTS
d- and l-CdG as substrates for dCyd kinase, 5′-nucleotidase, and mitochondrial dGuo kinase.
Both enantiomers of CdG were substrates for dCyd kinase from MOLT-4 cells (Table 1). l-CdG was clearly the preferred substrate, since the Km for l-CdG was about one-third that for d-CdG and the Vmax for l-CdG was a little higher and about the same as that for the natural substrate dGuo. Both enantiomers were also substrates for 5′-nucleotidase from HEp-2 cells. The Km for l-CdG was lower than that for d-CdG, but the Vmax was also lower. The Vmax/Km ratio indicates that the d-enantiomer is the preferred substrate. The Km for l-CdG was 1.4-fold that for the natural substrate Ino.
TABLE 1.
Kinetic constants for d-CdG and l-CdG with dCyd kinase, 5′-nucleotidase, and dGuo kinasea
| Substrate | dCyd kinase
|
5′-Nucleotidase
|
Mitochondrial dGuo kinase
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Km (mM) | Vmaxb | Vmax/Km | Km (mM) | Vmax | Vmax/Km | Km (mM) | Vmax | Vmax/Km | |
| d-CdG | 1.98 | 0.6 | 0.3 | 31 | 1.6 | 0.05 | 4.9c | 0.2c | 0.04 |
| l-CdG | 0.63 | 1.0 | 1.6 | 13 | 0.2 | 0.02 | 1.2 | 1.7 | 1.4 |
| dGuo | 0.22 | 1.0 | 4.5 | —d | — | — | 0.04 | 1.0 | 25 |
| Ino | — | — | — | 9 | 1.0 | 0.1 | — | — | — |
Each value is the average of at least three separate determinations.
Relative Vmax; the Vmax for the natural substrate is equal to 1 (see Materials and Methods).
Estimates of Km and Vmax values due to the poor activity of this enzyme with d-CdG.
—, not done.
The interaction of the enantiomers of CdG with mitochondrial dGuo kinase was first evaluated by determining their effectiveness in inhibiting the phosphorylation of dGuo in preparations of the enzyme from both CEM cells and human platelets. l-CdG was more effective than d-CdG in inhibiting the formation of dGMP from dGuo: the IC50s of the l- and d-enantiomers were 1.05 and 4.3 mM, respectively (the concentration of dGuo was 30 μM; data not shown). Both enantiomers were substrates for the kinase (Table 1). The Km for l-CdG was approximately 1 mM. Because the activity of mitochondrial dGuo kinase with d-CdG was so low, we were not able to directly measure a Km or a Vmax. However, we estimated a Km for d-CdG of 4.9 mM by multiplying the Km for l-CdG (1.2 mM) by the IC50 ratio (4.3/1.05). The Vmax for d-CdG was calculated by using this estimate of the Km value with the Michaelis-Menten equation and a measurement of the velocity of the reaction with 150 μM d-CdG. In some of the enzyme assays the course of the reaction was also monitored by HPLC. These assays showed that the major products were the triphosphates, indicating the presence in the preparation of nucleotide kinases. The presence of these enzymes should not influence the results determined by the disc assay, because the discs would retain mono-, di-, and triphosphates. Because the mitochondrial dGuo kinase preparations were not highly purified, we performed additional experiments to ascertain that the phosphorylation was catalyzed by mitochondrial dGuo kinase. These experiments involved the addition to the incubation mixture of nucleosides that were good substrates for dCyd kinase, 5′-nucleotidase, or mitochondrial dGuo kinase and the determination of their effects on the rate of phosphorylation of dGuo or CdG. The presence of dCyd or Ino, substrates for dCyd kinase and 5′-nucleotidase, respectively, did not decrease the rate of phosphorylation, whereas the presence of dIno, one of the best substrates for mitochondrial dGuo kinase (2), inhibited the phosphorylation by 54% (data not shown).
Effects of added nucleosides or MPA on the phosphorylation of d-CdG in CEM and HEp-2 cells.
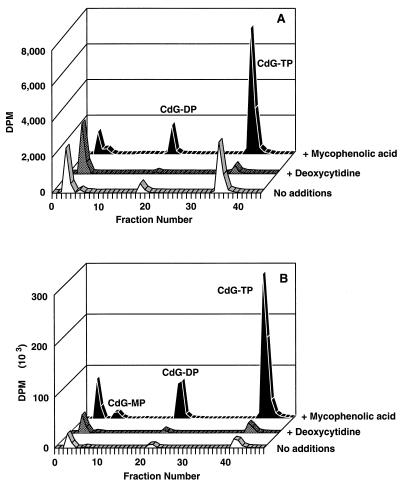
The addition of dCyd strongly reduced the phosphorylation of d-CdG in CEM cells but had little effect on its phosphorylation in HEp-2 cells (Fig. 1). Conversely, the addition of MPA produced a strong increase in phosphorylation in HEp-2 cells (approximately 13-fold) and increased phosphorylation in CEM cells by only about 2-fold. Other experiments (data not shown) were performed with dCyd in HEp-2 cells; in these cells the concentrations of dCyd were varied and multiple additions were made over a period of time. Under none of these conditions did dCyd significantly reduce the phosphorylation of d-CdG. araHyp and dAdo, known substrates of mitochondrial dGuo kinase (2), were also ineffective in reducing phosphorylation (data not shown).
FIG. 1.
Effects of dCyd and MPA on the phosphorylation of d-CdG in intact human cells. CEM cells (A) or HEp-2 cells (B) were incubated with 8 μM [3H]d-CdG alone or in combination with 5 μM MPA or 200 μM dCyd. After 8 h the acid-soluble metabolites of d-CdG were separated by SAX HPLC, and the radioactivity of each fraction was determined. TP, triphosphate; DP, diphosphate; MP, monophosphate.
Studies on the phosphorylation of d-CdG in MRC-5 cells and in MRC-5 cells infected with HCMV.
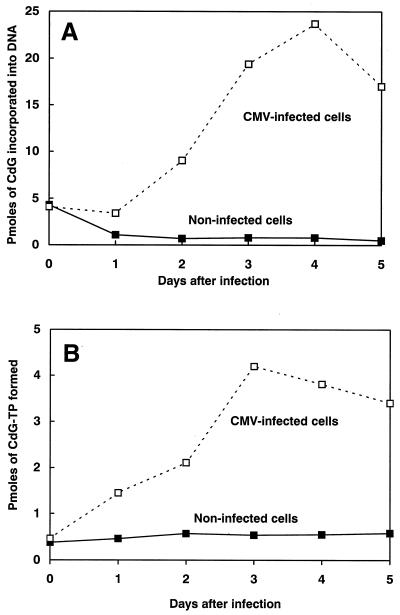
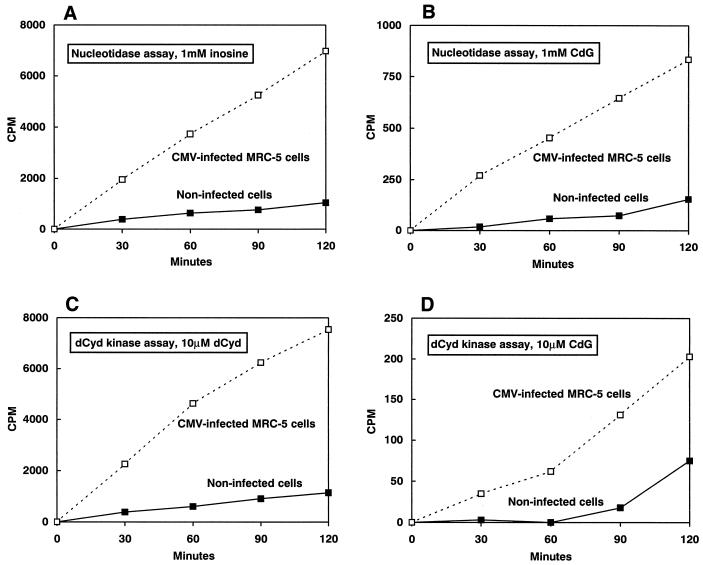
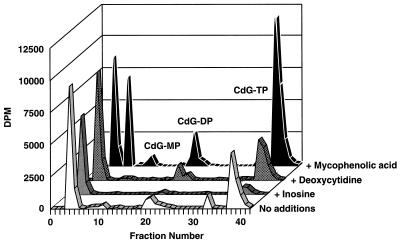
As indicated in Table 2 and Fig. 2, uninfected MRC-5 cells incubated with [3H]d-CdG contained very little radioactivity either in the soluble phosphates or in the acid-insoluble fraction (DNA). Infection with HCMV caused a substantial increase in the phosphorylation of d-CdG. The effects of infection with HCMV on the activities of dCyd kinase and 5′-nucleotidase were also determined by using natural substrates (dCyd and Ino) and d-CdG (Fig. 3). The activities of both of these enzymes were low in noninfected cells and increased markedly in cells infected with HCMV. The extents of increase of the activities of dCyd kinase and 5′-nucleotidase were about the same. On the basis of the kinetic values presented in Table 1 and the data presented in Fig. 3, the amount of d-CdG-phosphorylating activity in these extracts with 5 μM d-CdG that was due to 5′-nucleotidase (0.92 pmol/h/40 μl of assay mixture) was about fivefold that due to dCyd kinase (0.18 pmol/h/40 μl of assay mixture). In both noninfected and infected cells, neither dThd nor dCyd was effective in reducing the level of phosphorylation, but phosphorylation was sharply reduced by dGuo and Ino and was stimulated severalfold by MPA (Table 2; Fig. 4). Qualitatively similar results were obtained with [3H]l-CdG (data not shown).
TABLE 2.
Effect of deoxynucleosides on phosphorylation of d-CdG in MRC-5 cellsa
| Fraction | Total amt of phosphorylation (pmol [% of that for control])
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Noninfected MRC-5 cells
|
CMV-infected MRC-5 cells
|
|||||||
| Alone | With dCyd | With dThd | With dGuo | Alone | With dCyd | With dThd | With dGuo | |
| CdG triphosphate | 0.5 | 0.6 (118) | 0.3 (60) | 0.05 (10) | 4.8 | 4.7 (98) | 3.6 (74) | 0.8 (16) |
| DNA | 1.1 | 1.3 (118) | 1.4 (127) | 0.3 (23) | 26 | 21 (80) | 23 (90) | 4 (15) |
Three days after infection with HCMV, virus-infected and mock-infected MRC-5 cells (confluent monolayer of MRC-5 cells in 75-cm2 flasks) were incubated for 8 h with 5 μM [3H]d-CdG alone, 5 μM [3H]d-CdG plus 10 μM dCyd, 5 μM [3H]d-CdG plus 10 μM dThd, or 5 μM [3H]d-CdG plus 100 μM dGuo. The deoxynucleosides were added 30 min prior to the addition of [3H]d-CdG. The acid-soluble extract was prepared from all of the cells of each treatment and analyzed by SAX HPLC, and the numbers of picomoles of d-CdG incorporated into the triphosphate peak and acid-insoluble fractions (DNA) were determined. The data are from one representative experiment.
FIG. 2.
Effect of HCMV infection on the metabolism of d-CdG. HCMV-infected and noninfected MRC-5 cells (confluent monolayer of MRC-5 cells in 75-cm2 flasks) were treated with 5 μM [3H]d-CdG for 8 h 0, 1, 2, 3, 4, and 5 days after virus infection. After incubation with [3H]d-CdG, the cells were collected and the incorporation of d-CdG into DNA (A) and the incorporation of d-CdG triphosphate (CdG-TP) (B) into all of the cells receiving each treatment were determined.
FIG. 3.
Activities of dCyd kinase and 5′-nucleotidase in noninfected and HCMV-infected cells. Cell extracts were prepared from noninfected and HCMV-infected MRC-5 cells 5 days after infection. The phosphorylating activities of Ino (A) and d-CdG (B) were determined in these extracts by using conditions optimal for the measurement of 5′-nucleotidase activity (100 mM imidazole [pH 6.5], 500 mM NaCl, 50 mM MgCl2, 5 mM ATP, and 10 mM IMP). The phosphorylating activities of dCyd (C) and d-CdG (D) were determined in these extracts by using conditions optimal for the measurement of dCyd kinase activity (50 mM imidazole [pH 7.4], 25 mM dithiothreitol, 2 mM ATP, 2.5 mM MgCl2, 1 mg of bovine serum albumin per ml, and 5% glycerol).
FIG. 4.
Effects of dCyd, Ino, and MPA on the phosphorylation of d-CdG. HCMV-infected MRC-5 cells were incubated with 8 μM [3H]d-CdG alone or in combination with 5 μM MPA, 200 μM dCyd, or 100 μM Ino plus a potent inhibitor of purine nucleoside phosphorylase (BDG). After 8 h the acid-soluble metabolites of d-CdG were separated by SAX HPLC, and the radioactivity of each fraction was determined. TP, triphosphate; DP, diphosphate; MP, monophosphate.
DISCUSSION
Three cellular enzymes that catalyze the phosphorylation of dGuo and certain dGuo analogs are known: dCyd kinase, mitochondrial dGuo kinase, and 5′-nucleotidase (2). In the present work we found both enantiomers of CdG to be substrates for all three of these phosphorylating enzymes. For dCyd kinase the l enantiomer was the preferred substrate, a finding in accord with the already observed preference of this enzyme for the l forms of certain nucleoside analogs (20, 31, 37). For mitochondrial dGuo kinase, l-CdG was also the preferred substrate. The preference for the l enantiomer (relative to the d enantiomer) by the mitochondrial dGuo kinase was even greater than that observed for dCyd kinase. Although the d enantiomer was the preferred substrate for 5′-nucleotidase, the l enantiomer was nonetheless a good substrate. The Km values for the CdG enantiomers for 5′-nucleotidase were high but not greatly different from those for the natural substrate Ino (2). Despite the high Km values, phosphorylation by 5′-nucleotidase has been shown for a number of cytotoxic nucleosides, for example, carbovir, dideoxyinosine, and acyclovir (2).
This report is the first study of the enantiomers of CdG as substrates for these enzymes, but Eriksson’s laboratory (2, 39) has reported results of studies in which racemic CdG was used as a substrate for recombinant dCyd kinase and for mitochondrial dGuo kinase from bovine brain (2, 39). Those workers found that racemic CdG is not a substrate for recombinant dCyd kinase. The disagreement between this finding and our results could reflect the relatively high Km for d-CdG and, possibly, differences in the properties of the recombinant enzyme and those of our preparation from MOLT-4 cells. Eriksson’s laboratory (2) found racemic CdG to have 60% of the activity of dGuo as a substrate for bovine mitochondrial dGuo kinase, which was better activity than we saw with l-CdG. With regard to mitochondrial dGuo kinase, it is to be noted that the Km for dGuo for our preparation from human platelets or CEM cells (ca. 40 μM) is higher than those reported for mitochondrial dGuo kinase isolated from other types of mammalian cells (2). The difference is not due to differences between human cells on the one hand and other mammalian species on the other, because Yamada et al. (43) found a value of 2.5 μM for mitochondrial dGuo kinase from human placenta. The presence of competing enzymes in our relatively crude kinase preparation is a possible explanation, the most likely candidates being other phosphorylating enzymes and purine nucleoside phosphorylase. However, the effects of the addition of various nucleosides to the incubation mixture are consistent with the phosphorylation being catalyzed by mitochondrial dGuo kinase alone. Thus, neither dCyd nor Ino inhibited the phosphorylation of dGuo or CdG (indicating that dCyd kinase and 5′-nucleotidase were not responsible), whereas dIno, a substrate for mitochondrial dGuo kinase (2), was a strong inhibitor. We tested each preparation for the presence of purine nucleoside phosphorylase and found none. These results, together with the fact that mitochondrial dGuo kinase preparations from sources as diverse as platelets and cultured CEM cells had similar Kms, make it probable that the observed values are characteristic of mitochondrial dGuo kinase from these sources.
Our earlier report (5) suggested that the enzymes primarily responsible for phosphorylation of CdG were not the same in CEM cells and HEp-2 cells; the present results confirm this finding and indicate the identities of the enzymes primarily responsible. Since dCyd is not a substrate for mitochondrial dGuo kinase or 5′-nucleotidase (2) and since it markedly inhibited phosphorylation of CdG in CEM cells, dCyd kinase probably is responsible for the major part of the phosphorylation of CdG observed in these cells. However, since dCyd-induced reduction of phosphorylation reached a plateau at about 25% of that of the controls, it is likely that in these cells another enzyme is responsible for some of the phosphorylation. In HEp-2 cells dCyd kinase appears not to be significant in the phosphorylation of CdG since dCyd under no conditions produced a decrease in phosphorylation. The fact that the addition of MPA produced a strong stimulation of CdG phosphorylation in HEp-2 cells suggests (but does not in itself prove) that 5′-nucleotidase may play a major role in the phosphorylation; in contrast, a much lower level of stimulation was observed in CEM cells. From the data at hand, the participation of mitochondrial dGuo kinase in the phosphorylation of CdG in intact cells cannot be completely excluded; however, the fact that the addition of Ino (a natural substrate for the 5′-nucleotidase and a poor one for mitochondrial dGuo kinase [2]) essentially abolished phosphorylation suggests that 5′-nucleotidase is primarily responsible for the phosphorylation of CdG in HEp-2 cells. The ineffectiveness of araHyp and dAdo, both of which are known to be substrates for mitochondrial dGuo kinase (2), in inhibiting phosphorylation of CdG is further evidence that mitochondrial dGuo kinase plays at best a minor role. Taken all together, these results indicate that the major enzymes involved in the phosphorylation of CdG are dCyd kinase in CEM cells and 5′-nucleotidase in HEp-2 cells.
Because d-CdG has anti-HCMV activity, we also performed experiments with HCMV-infected cells. A number of observations on the induction of cellular kinases in HCMV-infected cells have been made (6, 8, 21, 23, 32, 44). In addition to the cellular kinases, a virus-specific phosphorylating activity must also be taken into consideration. It has been shown that an enzyme catalyzing the phosphorylation of ganciclovir is encoded by the UL97 reading frame of HCMV and that the virus-encoded enzyme is essential for the phosphorylation of ganciclovir (22, 35). In our study with MRC-5 cells, infection with HCMV increased the activities of dCyd kinase and 5′-nucleotidase 5- to 10-fold and increased the phosphorylation of d-CdG to about the same extent (Fig. 2 and 3). Competition studies on the effectiveness of various agents in inhibiting the phosphorylation of d-CdG yielded results similar to those obtained with HEp-2 cells: dCyd had little or no effect, MPA gave a large stimulation of phosphorylation, and Ino and dGuo essentially abolished phosphorylation (Table 2 and Fig. 4). These results suggest that dCyd kinase does not contribute significantly to the phosphorylation of d-CdG in HCMV-infected MRC-5 cells and point to 5′-nucleotidase as being the major phosphorylating enzyme. To obtain information on the possible contribution of the UL97-coded enzyme to the phosphorylation of d-CdG, we attempted to measure the phosphorylation of ganciclovir in these extracts but were not successful (data not shown). Therefore, we were not able to evaluate the ability of UL97 to use d-CdG as a substrate. However, our results indicate that the increase in 5′-nucleotidase activity in HCMV-infected cells is sufficient to explain the increased phosphorylation of d-CdG seen in HCMV-infected cells.
Because d-CdG has activity against DHBV, we also performed experiments with duck hepatocytes infected with DHBV (data not shown). Noninfected hepatocytes extensively converted d-CdG to phosphates, and virus infection did not increase the rate of phosphorylation. The rate of phosphorylation was many fold greater than that observed in mammalian cells and was similar to that in HSV-infected mammalian cells. The half-life of d-CdG triphosphate was approximately 8 h and was not affected by infection with DHBV. The high levels of CdG triphosphate formed and the half-life associated with d-CdG triphosphate are sufficient to explain the extended anti-HBV activity seen with d-CdG in ducks (9). The rapid rate of phosphorylation suggests the involvement of 5′-nucleotidase, which is much more abundant in avian liver than in mammalian cells (15). However, competition studies failed to reveal much about the identity of the enzyme responsible. Neither dThd, dGuo, nor dCyd had any effect on the phosphorylation in either infected or noninfected cells. Possibly pertinent to the interpretation of this finding is the observation of Kitos and Tyrrell (18) that of a number of nucleosides assayed, only Ado was highly effective in reducing the phosphorylation of 2′,3′-dideoxyguanosine in duck hepatocytes, which suggested the participation of Ado kinase (EC 2.7.1.20) in the phosphorylation of dGuo analogs.
It is of some interest that two of the three subject enzymes catalyzed the phosphorylation of the unnatural l-CdG better than they did that of d-CdG and that for the other enzyme (5′-nucleotidase) the l enantiomer was a good substrate, even though it was not as effective as the d enantiomer. The apparent lack of stereospecificity of dCyd kinase for CdG enantiomers was noted in our earlier paper of studies with intact cells (5), and high substrate activity for l enantiomers has also been observed with 1-β-d-arabinofuranosylcytosine (20), 3′-thia-2′-deoxycytidine (31), certain dideoxynucleosides (37), and the natural substrate dCyd (34, 38). The fact that HSV-1 dThd kinase (3, 5, 33), mammalian dCyd kinase, and mammalian mitochondrial dGuo kinase are similar with respect to the phosphorylation of both d and l enantiomers may reflect the fact there are regions of homology among these enzymes (12, 16, 40). 5′-Nucleotidase showed a high preference for a single enantiomer of carbovir (24), in contrast to its moderate selectivity for the d enantiomer of CdG. We are unaware of any reported studies of enantiomeric specificity with mitochondrial dGuo kinase. As far as CdG is concerned, mitochondrial dGuo kinase was similar to dCyd kinase in showing a preference for the l enantiomer.
The active forms of most nucleoside analogs are the triphosphates, and therefore, for an l-nucleoside to have biological activity, not only must the nucleoside be phosphorylated but the resulting monophosphate must be converted to the triphosphate. The enantiomeric specificities of the nucleotide kinases therefore are additional determinants of the biological activities of nucleoside analogs. The activities of the monophosphates of d- and l-CdG as substrates for GMP (dGMP) kinase (EC 2.7.4.8) remain to be determined, but from our results with whole cells (5) showing that the monophosphate is the principal metabolite of l-CdG, it appears that l-CdG monophosphate is at best a very poor substrate, which can explain the relatively low antiviral activity of l-CdG.
ACKNOWLEDGMENTS
This study was supported by NCI grant CA34200, NIAID grant AI-18641, a grant from ViraChem, Inc., and a fellowship from the French Association for Research on Cancer.
REFERENCES
- 1.Allan P W, Parker W B, Arnett G, Rose L M, Shaddix S C, Shewach D S, Fourel I, Secrist III J A, Montgomery J A, Shealy Y F, Bennett L L., Jr Metabolism of the carbocyclic analog of 2′-deoxyguanosine (CdG) in human cells. Antivir Res. 1995;26:A266. [Google Scholar]
- 2.Arner E S J, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini J, De Clercq E, Baumgartner H, Bodenteich M, Griengl H. Carbocyclic 5-iodo-2′-deoxyuridine (C-IDU) and carbocyclic (E)-5-(2-bromovinyl)-2′-deoxyuridine (C-BVDU) as unique examples of chiral molecules where the two enantiomeric forms are biologically active: interaction of the (+)- and (−)-enantiomers of C-IDU and C-BVDU with the thymidine kinase of herpes simplex virus type 1. Mol Pharmacol. 1990;37:395–401. [PubMed] [Google Scholar]
- 4.Bennett L L, Jr, Shealy Y F, Allan P W, Rose L M, Shannon W M, Arnett G. Phosphorylation of the carbocyclic analog of 2′-deoxyguanosine in cells infected with herpes viruses. Biochem Pharmacol. 1990;40:1515–1522. doi: 10.1016/0006-2952(90)90448-t. [DOI] [PubMed] [Google Scholar]
- 5.Bennett L L, Jr, Parker W B, Allan P W, Rose L M, Shealy Y F, Secrist III J A, Montgomery J A, Arnett G, Kirkman R L, Shannon W M. Phosphorylation of the enantiomers of the carbocyclic analog of 2′-deoxyguanosine in cells infected with herpes simplex virus type 1 and in uninfected cells. Lack of enantiomeric selectivity with the viral thymidine kinase. Mol Pharmacol. 1993;44:1258–1266. [PubMed] [Google Scholar]
- 6.Biron K K, Stanat S C, Sorrell J B, Fyfe J A, Keller P M, Lambe C U, Nelson D J. Metabolic activation of the nucleoside analog 9-{[2-hydroxy-1-(hydroxymethyl)ethoxy]-methyl}guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci USA. 1985;82:2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogenhagen D, Clayton D A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974;249:7991–7995. [PubMed] [Google Scholar]
- 8.Estes J E, Huang E-S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1997;24:13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fourel I, Saputelli J, Schaffer P, Mason W S. The carbocyclic analog of 2′-deoxyguanosine induces a prolonged inhibition of duck hepatitis B virus DNA synthesis in primary hepatocyte cultures and in the liver. J Virol. 1994;68:1059–1065. doi: 10.1128/jvi.68.2.1059-1065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furman P A, Wilson J E, Reardon J E, Painter G R. The effect of absolute configuration on the anti-HIV and anti-HBV activity of nucleoside analogues. Antivir Chem Chemother. 1995;6:345–355. [Google Scholar]
- 11.Grove K L, Guo X, Liu S-H, Gao Z, Chu C K, Cheng Y C. Anticancer activity of β-l-dioxolane-cytidine, a novel nucleoside analogue with the unnatural l-configuration. Cancer Res. 1995;55:3008–3011. [PubMed] [Google Scholar]
- 12.Harrison P T, Thompson R, Davison A J. Evolution of herpesvirus thymidine kinases from cellular deoxycytidine kinase. J Gen Virol. 1991;72:2583–2586. doi: 10.1099/0022-1317-72-10-2583. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi Y, Linn S. Purification of all forms of HeLa cell mitochondrial DNA and assessment of damage to it caused by hydrogen peroxide treatment of mitochondria or cells. J Biol Chem. 1995;270:7950–7956. doi: 10.1074/jbc.270.14.7950. [DOI] [PubMed] [Google Scholar]
- 14.Itoh R. Purification and some properties of cytosol 5′-nucleotidase from rat liver. Biochim Biophys Acta. 1981;657:402–410. doi: 10.1016/0005-2744(81)90326-0. [DOI] [PubMed] [Google Scholar]
- 15.Itoh R. IMP-GMP 5′-nucleotidase. Comp Biochem Physiol. 1993;105B:13–19. doi: 10.1016/0305-0491(93)90163-y. [DOI] [PubMed] [Google Scholar]
- 16.Johansson M, Karlsson A. Cloning and expression of human deoxyguanosine kinase cDNA. Proc Natl Acad Sci USA. 1996;93:7258–7262. doi: 10.1073/pnas.93.14.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson M A, Fridland A. Phosphorylation of 2′,3′-dideoxyinosine by cytosolic 5′-nucleotidase of human lymphoid cells. Mol Pharmacol. 1989;36:291–295. [PubMed] [Google Scholar]
- 18.Kitos T E, Tyrrell D L J. Intracellular metabolism of 2′,3′-dideoxynucleosides in duck hepatocyte primary cultures. Biochem Pharmacol. 1995;49:1291–1302. doi: 10.1016/0006-2952(95)00052-2. [DOI] [PubMed] [Google Scholar]
- 19.Kosovsky M J, Soslau G. Mitochondrial DNA topoisomerase I from human platelets. Biochim Biophys Acta. 1991;1078:56–62. doi: 10.1016/0167-4838(91)90092-e. [DOI] [PubMed] [Google Scholar]
- 20.Krenitsky T A, Tuttle J V, Koszalka G W, Chen I S, Beacham III L M, Rideout J L, Elion G B. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J Biol Chem. 1976;251:4055–4061. [PubMed] [Google Scholar]
- 21.Lewis R A, Watkins L, St. Jeor S. Enhancement of deoxyguanosine kinase activity in human lung fibroblast cells infected with human cytomegalovirus. Mol Cell Biochem. 1985;65:67–71. doi: 10.1007/BF00226020. [DOI] [PubMed] [Google Scholar]
- 22.Littler E, Stuart A D, Chee M S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 23.Meijer H, Bruggeman C A, Dormans P H J, van Boven C P A. Human cytomegalovirus induces a cellular deoxyguanosine kinase, also interacting with acyclovir. FEMS Microbiol Lett. 1984;25:283–287. [Google Scholar]
- 24.Miller W H, Daluge S M, Garvey E P, Hopkins S, Reardon J E, Boyd F L, Miller R L. Phosphorylation of carbovir enantiomers by cellular enzymes determines the stereoselectivity of antiviral activity. J Biol Chem. 1992;267:21220–21224. [PubMed] [Google Scholar]
- 25.Montgomery J A, Niwas S, Rose J D, Secrist III J A, Babu Y S, Bugg C E, Erion M D, Guida W C, Ealick S E. Structure-based design of inhibitors of purine nucleoside phosphorylase. I. 9-(Arylmethyl) derivatives of 9-deazaguanine. J Med Chem. 1993;36:55–69. doi: 10.1021/jm00053a008. [DOI] [PubMed] [Google Scholar]
- 26.Nair V, Jahnke T S. Antiviral activities of isomeric dideoxynucleosides of d- and l-related stereochemistry. Antimicrob Agents Chemother. 1995;39:1017–1029. doi: 10.1128/aac.39.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price P M, Banerjee R, Acs G. Inhibition of the replication of hepatitis B virus by the carbocyclic analogue of 2′-deoxyguanosine. Proc Natl Acad Sci USA. 1989;86:8541–8544. doi: 10.1073/pnas.86.21.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon W M. Antiretroviral activity of carbocyclic nucleoside analogs. In: Diasio R B, Sommadossi J-P, editors. Advances in chemotherapy of AIDS. New York, N.Y: Pergamon Press, Inc.; 1990. pp. 75–95. [Google Scholar]
- 29.Shealy Y F, O’Dell C A, Shannon W M, Arnett G. Synthesis and antiviral activity of carbocyclic analogues of 2′-deoxyribofuranosides of 2-amino-6-substituted purines and of 2-amino-6-substituted-8-azapurines. J Med Chem. 1984;27:1416–1421. doi: 10.1021/jm00377a007. [DOI] [PubMed] [Google Scholar]
- 30.Shewach D S, Reynolds K K, Hertel L. Nucleotide specificity of human deoxycytidine kinase. Mol Pharmacol. 1992;42:518–524. [PubMed] [Google Scholar]
- 31.Shewach D S, Liotta D C, Schinazi R F. Affinity of the antiviral enantiomers of oxathiolane cytosine nucleosides for human 2′-deoxycytidine kinase. Biochem Pharmacol. 1993;45:1540–1543. doi: 10.1016/0006-2952(93)90058-5. [DOI] [PubMed] [Google Scholar]
- 32.Smee D F. Interaction of 9-(1,3-dihydroxy-2-propoxymethyl)guanine with cytosol and mitochondrial deoxyguanosine kinases: possible role in anti-cytomegalovirus activity. Mol Cell Biochem. 1985;69:75–81. doi: 10.1007/BF00225929. [DOI] [PubMed] [Google Scholar]
- 33.Spadari S, Maga G, Focher F, Ciarrocchi G, Manservigi R, Arcamone F, Capobianco M, Carcuro A, Colonna F, Iotti S, Garbesi A. l-Thymidine is phosphorylated by herpes simplex virus type 1 thymidine kinase and inhibits viral growth. J Med Chem. 1992;35:4214–4220. doi: 10.1021/jm00100a029. [DOI] [PubMed] [Google Scholar]
- 34.Spadari S, Maga G, Verri A, Bendiscioli A, Tondelli L, Capobianco M, Colonna F, Garbesi A, Focher F. Lack of stereospecificity of some cellular and viral enzymes involved in the synthesis of deoxyribonucleotides and DNA: molecular basis for the antiviral activity of unnatural l-β-nucleosides. Biochimie. 1995;77:861–867. doi: 10.1016/0300-9084(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan V, Talarico C L, Stanat S C, Davis M, Coen D M, Biron K K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358:162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- 36.Tuttleman J S, Pugh J C, Summers J W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986;58:17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Draanen N A, Tisdale M, Parry N G, Jansen R, Dornsife R E, Tuttle J V, Averett D R, Koszalka G. Influence of stereochemistry on antiviral activities and resistance profiles of dideoxycytidine nucleosides. Antimicrob Agents Chemother. 1994;38:868–871. doi: 10.1128/aac.38.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verri A, Focher F, Priori G, Gosselin G, Imbach J-L, Capobianco M, Garbesi A, Spadari S. Lack of enantiospecificity of human 2′-deoxycytidine kinase: relevance for the activation of β-l-deoxycytidine analogs as antineoplastic and antiviral agents. Mol Pharmacol. 1997;51:132–138. doi: 10.1124/mol.51.1.132. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Karlsson A, Arner E S J, Eriksson S. Substrate specificity of mitochondrial 2′-deoxyguanosine kinase. J Biol Chem. 1993;268:22847–22852. [PubMed] [Google Scholar]
- 40.Wang L, Hellman U, Eriksson S. Cloning and expression of human mitochondrial deoxyguanosine kinase cDNA. FEBS Lett. 1996;390:39–43. doi: 10.1016/0014-5793(96)00623-0. [DOI] [PubMed] [Google Scholar]
- 41.Worku Y, Newby A C. Nucleoside exchange catalyzed by the cytoplasmic 5′-nucleotidase. Biochem J. 1982;205:503–510. doi: 10.1042/bj2050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu T-T, Coates L, Aldrich C E, Summers J, Mason W S. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- 43.Yamada Y, Goto H, Ogasawara N. Deoxyguanosine kinase from human placenta. Biochim Biophys Acta. 1982;709:265–272. doi: 10.1016/0167-4838(82)90469-1. [DOI] [PubMed] [Google Scholar]
- 44.Zavada V, Erban V, Rezacova D, Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52:333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]