Abstract
Alveolar echinococcosis, caused by the larval (metacestode) stage of the tapeworm Echinococcus multilocularis, is a lethal parasitosis of the liver prevalent in the Northern Hemisphere. For chemotherapy the benzimidazole derivatives mebendazole and albendazole were introduced, and their use has resulted in a significant improvement in the survival rates. However, data from experiments with animals and clinical observations indicate that these drugs elicit only parasitostatic activity and in most cases are not able to completely eliminate the parasitic metacestode tissue. In the present study, we applied a culture system for the in vitro growth and proliferation of E. multilocularis metacestodes to analyze the parasitostatic and parasitocidal potential of mebendazole. Here, we demonstrate for the first time that at concentrations of >0.1 μM, i.e., at concentrations used for therapy of human alveolar echinococcosis, this antihelminth drug is parasitocidal in vitro. Viability assessment was performed by infection experiments with Meriones unguiculatus and mebendazole-treated metacestode tissue and by reverse transcription-PCR for the detection of E. multilocularis mRNA. The E. multilocularis in vitro model proved to be a valuable tool for the analysis of the potential of antihelminth drugs.
The larval stage of the small fox tapeworm Echinococcus multilocularis, a parasite prevalent in the Northern Hemisphere, is the causative agent of human alveolar echinococcosis (AE), which is considered to be the most lethal helminthic infection in humans (20). The metacestode stage of this parasite has characteristics of a malignant tumor, including infiltrative growth and metastasis formation (7). AE usually affects the livers of the intermediate hosts, i.e., small rodents, and, accidentally, the livers of humans. In humans the parasitic mass increases by proliferation of parasitic vesicles in the periphery of the lesion. Hepatic tissue is replaced by collagen in which large numbers of parasitic vesicles with sizes of 1 to 20 mm are embedded. With impaired vascularity, the central part of the lesion becomes necrotic and liquefied (7). The growth rate of this parasitic tissue in the human host is usually slow, and it is calculated that it takes 10 to 15 years until clinical symptoms occur (7). If no specific therapy is initiated, in 94% of patients the disease is fatal within 10 years following diagnosis (25).
Surgery is the basic form of treatment of early AE, but a prediction of radical resection is almost impossible and recurrences therefore cannot be excluded (7, 31). Therefore, preoperative chemotherapy and postoperative drug administration (3, 7) have been introduced to improve the outcome of surgical therapy. In advanced stages of AE radical resection is impossible and long-term chemotherapy with benzimidazoles (BZs) and palliative surgical measurements, e.g., hepaticojejunostomy, drainage of necrotic cavities, or portocaval shunts, may prevent complications and thus are considered to increase the survival rate.
Two BZ derivatives, mebendazole (MBZ) and albendazole (ABZ), have been introduced for chemotherapy for humans. Data from experimental studies of echinococcosis in rodents have shown that long-term treatment with MBZ and ABZ resulted in an 85 to 99% reduction of the E. multilocularis metacestode masses, but complete erradication could not be achieved (7). Several studies performed with AE patients have shown the benefit of chemotherapy with MBZ, increasing the survival time of the patients, providing an improved clinical condition, and decreasing the size of the parasitic mass (1, 2, 4, 17). Limited data on experience with therapy with ABZ are available (11). However, compared to MBZ, ABZ is absorbed at a higher rate (5). The hepatic metabolite albendazole sulfoxide exhibits antiparasitic activity, whereas metabolization of MBZ results in the loss of antiparasitic activity (19, 23).
Despite the improvements in the chemotherapy of AE with BZ derivatives, complete erradication of the parasitic mass cannot be achieved in the majority of patients (17, 18), although one Alaskan study was more favorable, indicating that long-term application of MBZ may cause the death of the parasite (30). In summary, there is circumstantial evidence from the clinical outcomes of treated patients that BZs should be considered parasitostatic in vivo. However, because until now no assay system has been available for the testing of the susceptibility of the parasite to antiparasitic drugs in vitro, it has remained uncertain whether the BZ derivatives have parasitocidal or only parasitostatic potential against E. multilocularis metacestodes. Furthermore, the possibility of the development of resistance to these chemotherapeutic agents as a reason for the failure of BZ treatment could not be excluded. To address these problems we used a recently established in vitro model of AE to analyze the activity of MBZ against E. multilocularis (12). This model depends on the use of primary hepatocytes of rat or human origin. In the presence of these host cells, E. multilocularis metacestodes proliferate and differentiate in vitro. In the present study we used this model to analyze the activity of MBZ in vitro, and we were able to demonstrate for the first time that at concentrations found in the plasma of MBZ-treated patients (17, 18), this BZ derivative displays parasitocidal effects on E. multilocularis larvae.
MATERIALS AND METHODS
Chemotherapeutic agents.
MBZ (Vermox forte) was supplied by Jansen GmbH, Neuss, Germany. For each experiment stock solutions were freshly prepared by dissolving 50 mg of MBZ in 10 ml of dimethyl sulfoxide (DMSO). Following appropriate dilutions of the stock solutions in DMSO, MBZ was added daily to prewarmed complete medium (CM) at concentrations of 0.01, 0.1, 1, and 10 μM; CM without MBZ was used as a control. The pH of the cultures (pH 7.4) was controlled and did not change after the addition of the drug solutions. The final DMSO concentration in all cultures was 0.5%. DMSO at the same concentration was added to the control cultures grown in the absence of MBZ to exclude the possibility that this solvent has an influence on hepatocyte or parasite viability.
In vitro culture of metacestodes with rat primary hepatocytes.
Rat primary hepatocytes were isolated as described previously (26). Briefly, the livers of female Lewis rats (body weight, 200 to 250 g) were perfused by a modified double-collagenase perfusion technique and isolated hepatocytes were plated onto collagen-coated dishes (35 mm in diameter) as described elsewhere (6). E. multilocularis metacestodes were maintained in Mongolian gerbils (Meriones unguiculatus) by intraperitoneal infection of minced metacestode tissue as described previously (9). After 6 to 8 weeks E. multilocularis metacestodes were isolated for infection of cell cultures. The parasitic tissue was homogenized, and 50 to 150 metacestode vesicles were added to the hepatocyte cultures and covered with a second collagenous layer. Cultures were supplemented with medium (Williams’ E medium; GIBCO-BRL, Eggenstein, Germany) containing 10% (vol/vol) fetal bovine serum, 9.6 μg of prednisolone per ml, 0.014 μg of glucagon (Novo, Mainz, Germany) per ml, 0.16 U of insulin (Hoechst, Frankfurt, Germany) per ml, penicillin (200 U/ml), and streptomycin (200 μg/ml; Biochrom). The medium was changed daily, and cultures were monitored by light microscopy for growth of the parasitic vesicles and the integrity of the hepatocyte layer.
Experiments with animals.
To analyze the infectivity of MBZ-treated metacestodes, the parasitic vesicles from one of the in vitro culture dishes were injected intraperitoneally into M. unguiculatus gerbils. The animals were killed after 4 to 5 months, and the peritoneal cavities and livers were monitored macroscopically and microscopically for the development of parasitic masses. Experiments with animals were performed in accordance with the guidelines of and with the permission of local authorities.
RT-PCR.
Reverse transcription (RT) of the EM10 gene from metacestodes was used to determine the viability of the metacestode vesicles. After termination of the experiment, medium was removed from the culture dishes and metacestodes from treated and untreated in vitro cultures were isolated by digestion with 1 mg of collagenase type II per ml, dissolved in Williams medium (Biochrom, Berlin, Germany), for 10 min at 37°C. Parasitic tissue and hepatocytes from one culture dish were completely transferred to an Eppendorf tube and washed twice with 1 ml of ice-cold phosphate-buffered saline (pH 7.4). Isolation of total RNA from metacestodes was performed with oligo(dT)18-coupled paramagnetic beads (Dynal, Hamburg, Germany) following the instructions of the manufacturer. Bound mRNA was eluted and transferred to a fresh RNase-free tube. After ethanol precipitation the pellet was suspended in 7 μl of diethyl pyrocarbonate-treated H2O and completely mixed with 100 pmol of oligo(dT)18 primer. After denaturation at 65°C for 5 min, the tube was set on ice and first-strand synthesis was carried out by the addition of first-strand buffer (GIBCO-BRL), desoxynucleotides (0.4 mM each), 8 U of RNase inhibitor (RNasin; GIBCO-BRL), and 100 U of Superscript reverse transcriptase (GIBCO-BRL) in a total volume of 10 μl. cDNA synthesis was performed at 37°C for 60 min. Following inactivation of the enzyme by heating at 94°C for 3 min, PCR with Echinococcus-specific primers was performed. For amplification of E. multilocularis cDNA, oligonucleotide primers PF9 (5′-CAAGACGGCAATCCAA-3′) and PF18 (5′-CTACATCGACTCAAACTGTT-3′) were used; these primers are complementary to the E. multilocularis EM10 gene (9), which encodes a protein of the parasite’s cytoskeleton. Amplification of the cDNA with these primers results in the generation of a 1.5-kb DNA fragment (13). Due to the presence of several introns, a fragment of 2.7 kb is amplified from the chromosomal EM10 locus. Therefore, amplification of EM10 cDNA can clearly be distinguished from amplification of chromosomal DNA, but chromosomal DNA amplification was never observed by application of the described mRNA preparation protocol. The generated DNA fragments were separated on agarose gels, visualized by ethidium bromide staining, and blotted onto nylon membranes (22). Hybridization was performed at 42°C overnight with the cloned EM10 cDNA fragment, which was randomly labeled with α-32P by using the Multiprime DNA Labelling Kit (Amersham, Braunschweig, Germany).
RESULTS
Effect of MBZ on in vitro proliferation of E. multilocularis metacestodes.
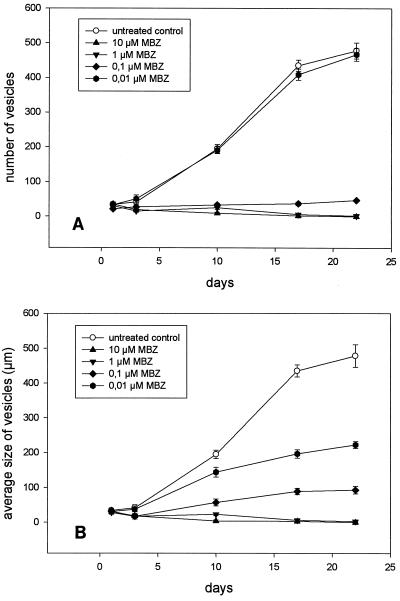
Cultures of E. multilocularis metacestodes with primary rat hepatocytes were treated with different concentrations of MBZ, ranging from 0.01 to 10 μM. Thus, the concentrations of 0.25 to 1 μM that are therapeutic for human AE (17) were included in this experimental setting. The addition of MBZ had no effect on the morphology of the hepatocyte layer, which is consistent with previous observations that BZs have a much higher affinity for helminth tubulin than for the tubulin of mammalian cells (14, 15). Medium containing the chemotherapeutic drugs was changed daily, and proliferation of the metacestodes was monitored microscopically by determination of the parasitic vesicle numbers per culture dish and by measurement of the sizes of individual vesicles. Without the addition of MBZ the number of vesicles increased ninefold during a cultivation period of 3 weeks (Fig. 1A). In accordance with previous observations, in parasite cultures without hepatocytes (12), no proliferation of the echinococcal larvae was detectable (data not shown). The addition of 0.01 μM MBZ to the culture did not inhibit the proliferation of the parasitic tissue during the same incubation period (Fig. 1A). The number of vesicles increased to the same extent as it did in the untreated control cultures. In contrast, a dramatic reduction in the cyst number was achieved in cultures treated with 0.1 μM MBZ compared to the cyst number in the control cultures (Fig. 1A), and at the end of the experiment the number of cysts had doubled. In cultures treated with 1 and 10 μM MBZ, the proliferation of the parasite was completely inhibited. The parasitic vesicles developed a dark stain after 4 to 6 days of treatment with 1 and 10 μM MBZ, and many granules could be detected inside the vesicles. After 18 days of treatment, the vesicles in these cultures collapsed and no intact cysts were present (data not shown). The loss of vesicle turgidity is a widely used criterion for parasite viability (10).
FIG. 1.
(A) Proliferation of E. multilocularis metacestode vesicles in vitro during incubation with MBZ over a period of 3 weeks. (B) Growth of the vesicles measured as the increase in vesicle diameter. The average diameter of all vesicles within a culture is given. All experiments were performed in duplicate and were repeated at least three times. The results of one representative experiment are presented here. The mean and range for two cultures infected with the same parasite suspension are given.
The size of individual vesicles increased 11-fold within 3 weeks in the control cultures to which no BZ was added (Fig. 1B). Growth of the vesicles was affected at a concentration of 0.01 μM MBZ. After 3 weeks the average vesicle size increased only sixfold. Application of higher concentrations of MBZ to the cultures (0.1, 1, and 10 μM) resulted in the complete arrest of vesicle growth (Fig. 1B).
The data described above were obtained by use of rat hepatocyte cultures. However, treatment of the parasitic tissue with BZs in the presence of human hepatocytes revealed no significant differences in parasitic proliferation and growth of individual vesicles (data not shown).
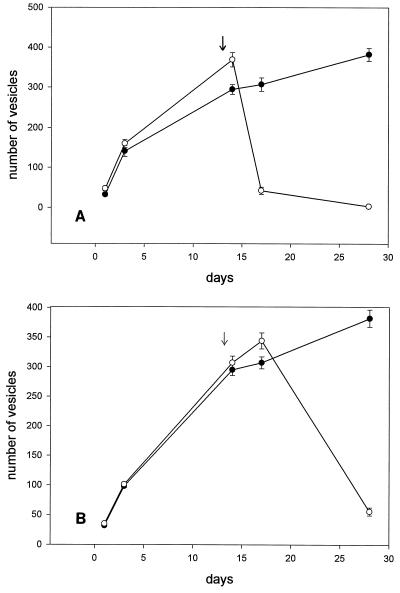
We further investigated the influence of the treatment schedule on the metacestode vesicles. In a second experimental setting, the parasitic infection of the hepatocyte tissue culture was established by in vitro culture for 14 days without the addition of MBZ. During this time the parasitic vesicles proliferated ninefold and the average size of the vesicles increased from 122 to 496 μm (data not shown). After 2 weeks, MBZ (0.1 and 1 μM) was added to the cultures for another 14 days. The addition of 1 μM MBZ had an immediate effect on parasite proliferation. Three days after starting treatment, the number of cysts was reduced by 85% (Fig. 2A). Further treatment inhibited the proliferation completely, and no intact cysts could be observed by light microscopy 14 days after the start of MBZ treatment. After the addition of 0.1 μM MBZ, the inhibition was delayed, but the number of vesicles decreased by 85% (Fig. 2B).
FIG. 2.
Proliferation of E. multilocularis metacestode vesicle in the presence of 1 μM (A) and 0.1 μM (B) MBZ. The parasitic tissue was established in vitro for 13 days. At day 14 (indicated by an arrow) MBZ was added to the culture daily for a further 2 weeks. Open circles, growth curve of the vesicles in the presence of MBZ; closed circles, control experiment without MBZ treatment over a period of 4 weeks. All experiments were performed in duplicate and were repeated twice. The results of one representative experiment are presented here. The mean and range for two cultures infected with the same parasite suspension are given.
Examination of metacestode viability after MBZ treatment.
Determination of the parasitostatic or parasitocidal effects after treatment of in vitro cultures of E. multilocularis in the presence of primary rat hepatocytes with MBZ was performed (i) by intraperitonal infection of M. unguiculatus gerbils with metacestodes isolated from treated and untreated cultures and (ii) by the detection of EM10-specific mRNA by RT-PCR (13).
To analyze the parasitocidal effect of MBZ in vivo, M. unguiculatus gerbils were infected with metacestode tissue treated with MBZ at the different concentrations. The experiments with animals were performed in triplicate. As indicated in Table 1, after 5 months metacestode tissue developed in all animals infected with parasites from cultures treated with 0.01 and 0.1 μM MBZ. In contrast, in all animals infected with metacestodes from cultures treated with 1 and 10 μM MBZ, parasitic growth was absent. This indicates that MBZ at concentrations of >0.1 μM (the concentration achieved in the plasma of humans as therapy for AE [17, 18]) damages the parasitic tissue, resulting in an irreversible loss of the proliferative capacity.
TABLE 1.
Assessment of E. multilocularis metacestode viability after MBZ treatment by infection of M. unguiculatus gerbils and RT-PCR for EM10
| MBZ concn (μM) | Growth of treated metacestodes in infected animals | Detection of EM10 mRNA by RT-PCR |
|---|---|---|
| 0.01 | + | + |
| 0.1 | + | + |
| 1 | − | + |
| 10 | − | − |
For detection of Echinococcus-specific mRNA, vesicles were isolated by digestion of the collagenase from the cultures. By RT-PCR with EM10-specific primers, a transcript of the expected size of 1.5 kb could be amplified from cultures treated with 0.01, 0.1, and 1 μM MBZ (Table 1). In contrast, no EM10-specific mRNA could be amplified from cultures treated with 10 μM MBZ, demonstrating a parasitocidal effect of the drug on the parasitic tissue at a concentration of >1 μM. Detection of EM10-specific mRNA by RT-PCR in parasites treated with 1 μM MBZ therefore indicates that although the cells were not replicating, at least some parasitic cells still have some metabolic activity. However, the half-life of the EM10 transcript is not known, and we therefore cannot exclude the possibility that the persistence of EM10 mRNA after cell death is responsible for the positive RT-PCR result for cultures treated with 1 μM MBZ. RT-PCR of samples from cultures was performed in three independent experiments, all of which gave consistent results.
DISCUSSION
Infection with the larval stage of the tapeworm E. multilocularis is a life-threatening disease in humans. Surgical resection of the involved liver segment and of metacestode lesions from other infected organs is indicated, but radical surgery can be performed on only about 20 to 40% of symptomatic patients (7, 23). Even after complete resection, recurrences have frequently been described (7, 31). AE was therefore considered to be an incurable parasitic disease before the BZ derivatives MBZ and ABZ became available as chemotherapeutic agents. However, data from experiments with animals (8, 24) and clinical observations indicate that these substances are only parasitostatic and that the parasite is not killed and eliminated. Consequently, long-term chemotherapy over several years is necessary. In a German study, with long-term MBZ chemotherapy with a mean duration of 3.9 years, the general clinical condition improved for 57% of the patients but remained unchanged for 22% of the patients. A progression of disease was found for 21% of the patients (16). Similar results were obtained in a Swiss study (1), and a survey of Alaskan patients (30) demonstrated that long-term chemotherapy with MBZ significantly increased the survival rate, from 25% for untreated patients to 90% for treated patients over a 10-year follow-up. However, from these studies it became evident that BZs inhibit the progression of metacestode growth but are not able to completely erradicate the parasite.
In contrast to the observations from these clinical studies, we could demonstrate here that at a concentration of 1 μM MBZ exhibits within a few days parasitocidal activities against E. multilocularis metacestodes in vitro. At a lower concentration of 0.1 μM, MBZ inhibits parasite proliferation and results in a reduction of parasitic masses but has no absolute parasitocidal effect. For in vitro susceptibility testing, we used the recently described coculture system of primary hepatocytes and E. multilocularis metacestode tissue, which mimicks the organotropism of the parasite toward the liver of the intermediate hosts (12). In this system proliferation and differentiation of the parasitic tissue depend on soluble and as yet undefined growth factors secreted by the hepatocytes. The metacestode vesicles can be monitored by light microscopy and appear transparent surrounded by a thick laminated layer. After the addition of MBZ at parasitocidal concentrations, the vesicles became dark and collapsed. In the experiments performed for this study, the parasitocidal effect of MBZ was further demonstrated (i) by infection of M. unguiculatus gerbils with MBZ-treated parasitic tissue and (ii) by RT-PCR with the echinococcal EM10 transcript as the target. By application of this RT-PCR with EM10-specific mRNA, 2 ng of total echinococcal RNA, corresponding to 46 parasitic cells, can be detected (13). In cultures treated with 10 μM MBZ, this EM10-specific DNA fragment could not be amplified, indicating the absence of viable parasitic tissue after MBZ treatment.
The parasitocidal concentration of MBZ in vitro is in the range of 0.1 to 1 μM. It is important to emphasize that the levels in plasma thought to be effective for the treatment of AE (0.25 to 1 μM) are exactly within this range (17, 18). Thus, the parasitocidal effects of BZs in vitro and the parasitostatic effect in vivo may reflect differences in the bioavailabilities of these compounds. However, factors influencing the efficiency of MBZ have not been defined, but it is conceivable that the size and the age of the parasitic mass, calcifications, and fibrosis correlate with the outcome of therapy, as was described for the BZ therapy of cystic echinococcosis, caused by the close relative Echinococcus granulosus (27).
Besides the availability of BZs in sufficient concentrations within the metacestode tissue, the development of drug resistance may also influence the efficiency of chemotherapy. BZs inhibit the polymerization of the cytoskeletal tubulin (15), induce the blockage of glucose absorption, and lead to glycogen depletion (28). BZ resistance has been observed in other parasites (14, 21, 29), and it depends on point mutations within the β-tubulin structural genes, resulting in a reduced binding affinity of the target molecule (21). Development of BZ resistance in E. multilocularis has not yet been described due to the lack of appropriate in vitro culture techniques. However, it is conceivable that during long-term treatment over several years, parasitic cells with mutations in the β-tubulin gene are selected, resulting in failure of the BZ therapy. The in vitro model of AE used in this study to analyze the parasitocidal potentials of BZs should be useful for addressing questions regarding the development of BZ resistance during therapy.
Due to the limited therapeutic success, the development of new agents for the treatment of AE is warranted. The in vitro system described here should be appropriate for analyzing the efficiency of newly developed anthelminthic drugs for the improvement of therapy for AE.
ACKNOWLEDGMENTS
This work was supported by grant Fr689/9-2 from the Deutsche Forschungsgemeinschaft (to M.F.).
We are grateful to E. Lüneberg, F. Mühlschlegel, and U. Vogel for critical comments on the manuscript.
REFERENCES
- 1.Ammann R, Tschudi K, von Ziegler M, Meister F, Cotting J, Eckert J, Witassek F. Langzeitverlauf bei 60 Patienten mit alveolärer Echinokokkose unter Dauertherapie mit Mebendazol. Klin Wochenschr. 1988;66:1060–1073. doi: 10.1007/BF01711918. [DOI] [PubMed] [Google Scholar]
- 2.Ammann R, Ilitsch N, Marincek B, Freiburghaus A U. Effect of chemotherapy on the larval mass and on the long-term course of alveolar echinococcosis. Hepatology. 1993;19:735–742. doi: 10.1002/hep.1840190328. [DOI] [PubMed] [Google Scholar]
- 3.Ammann R W. Improvement of liver resectional therapy by adjuvant chemotherapy in alveolar hydatid disease. Parasitol Res. 1991;77:290–293. doi: 10.1007/BF00930903. [DOI] [PubMed] [Google Scholar]
- 4.Ammann R W, Hirsbrunner R, Cotting J, Steiger U, Jaquier P, Eckert J. Recurrence rate after discontinuation of long-term mebendazole therapy in alveolar echinococcosis (preliminary results) Am J Trop Med Hyg. 1990;43:506–515. doi: 10.4269/ajtmh.1990.43.506. [DOI] [PubMed] [Google Scholar]
- 5.Cook G C. Use of benzimidazole chemotherapy in human helminthiases: indications and efficacy. Parasitol Today. 1990;6:133–136. doi: 10.1016/0169-4758(90)90232-s. [DOI] [PubMed] [Google Scholar]
- 6.Dunn J C Y, Tompkins R G, Yarmush M L. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 7.Eckert J, Ammann R W. Clinical diagnosis and treatment of echinococcosis in humans. In: Thompson R C A, Lymbery A J, editors. Echinococcus and hydatid disease. Wallingford, England: CAB International; 1995. pp. 411–463. [Google Scholar]
- 8.Eckert J, Pohlenz J. Zur Wirkung von Mebendazol auf Metazestoden von Mesocestoides corti und Echinococcus multilocularis. Tropenmed Parasitol. 1976;27:247–262. [PubMed] [Google Scholar]
- 9.Frosch P M, Frosch M, Pfister T, Schaad V, Bitter-Suermann D. Cloning and characterisation of an immunodominant major surface antigen of Echinococcus multilocularis. Mol Biol Parasitol. 1991;48:121–130. doi: 10.1016/0166-6851(91)90108-i. [DOI] [PubMed] [Google Scholar]
- 10.Heath D D, Christie M J, Chevis R A F. The lethal effect of mebendazole on secondary Echinococcus granulosus cysticerci and Taenis pisiformis and tetrathyridia of Mesocestoides corti. Parasitology. 1975;70:273–285. doi: 10.1017/s0031182000049738. [DOI] [PubMed] [Google Scholar]
- 11.Horton R J. Chemotherapy of Echinococcus infection in man with albendazole. Trans R Soc Trop Med Hyg. 1989;83:97–102. doi: 10.1016/0035-9203(89)90724-4. [DOI] [PubMed] [Google Scholar]
- 12.Jura H, Bader A, Hartmann M, Maschek H J, Frosch M. Hepatic tissue culture model for study of host-parasite interactions in alveolar echinococcosis. Infect Immun. 1996;64:3484–3490. doi: 10.1128/iai.64.9.3484-3490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern P, Frosch P, Helbig M, Wechsler J G, Usadel S, Beckh K, Kunz R, Lucius R, Frosch M. Diagnosis of Echinococcus multilocularis infection by reverse-transcription polymerase chain reaction. Gastroenterology. 1995;109:596–600. doi: 10.1016/0016-5085(95)90350-x. [DOI] [PubMed] [Google Scholar]
- 14.Lacey E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of the drug resistance to benzimidazoles. Int J Parasitol. 1988;18:885–936. doi: 10.1016/0020-7519(88)90175-0. [DOI] [PubMed] [Google Scholar]
- 15.Lacey E. Mode of action of benzimidazoles. Parasitol Today. 1990;6:112–115. doi: 10.1016/0169-4758(90)90227-u. [DOI] [PubMed] [Google Scholar]
- 16.Löscher T, von Sonnenburg F, Nothdurft H D. 8th International Congress for Tropical Medicine and Malaria. 1992. Epidemiology and therapy of human echinococcosis in central Europe. [Google Scholar]
- 17.Luder P J, Robotti G, Meister F P, Bircher J. High oral doses of mebendazole interfere with growth of larval Echinococcus multilocularis lesions. J Hepatol. 1985;1:369–377. doi: 10.1016/s0168-8278(85)80774-1. [DOI] [PubMed] [Google Scholar]
- 18.Luder P J, Siffert B, Wittassek F, Meister F, Bircher J. Treatment of hydatid disease with high oral doses of mebendazole. Long-term follow-up of plasma mebendazole levels and drug interactions. Eur J Pharmacol. 1986;31:443–448. doi: 10.1007/BF00613522. [DOI] [PubMed] [Google Scholar]
- 19.Marriner S E, Morris D L, Dickson B, Bogan J A. Pharmacokinetics of albendazole in man. Eur J Clin Pharmacol. 1986;30:705–708. doi: 10.1007/BF00608219. [DOI] [PubMed] [Google Scholar]
- 20.Rausch R L. Life cycle patterns and geographic distribution of Echinococcus spezies. In: Thompson R C A, Lymbery A J, editors. Echinococcus and hydatid disease. Wallingford, England: CAB International; 1995. pp. 89–134. [Google Scholar]
- 21.Roos M H. The molecular nature of benzimidazole resistance. Parasitol Today. 1990;6:125–127. doi: 10.1016/0169-4758(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Schantz P M, Van den Bossche H, Eckert J. Chemotherapy for larval echinococcosis in animals and humans: report of a workshop. Z Parasitenkunde. 1982;53:5–26. doi: 10.1007/BF00929509. [DOI] [PubMed] [Google Scholar]
- 24.Schantz P M, Brandt F H, Dickinson C M, Allen C R, Roberts J M, Eberhard M L. Effects of albendazole on Echinococcus multilocularis infection in the Mongolian jird. J Infect Dis. 1990;162:1403–1407. doi: 10.1093/infdis/162.6.1403. [DOI] [PubMed] [Google Scholar]
- 25.Schicker H J. Die Echinokokkose des Menschen. Stand der Diagnostik, Therapie und Prognose bei Echinokokkenerkrankungen in Baden-Württemberg in den Jahren 1960–72. Medical dissertation. Tübingen, Germany: University of Tübingen; 1976. [Google Scholar]
- 26.Seglen P O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 27.Teggi A, Lastilla M G, De Rosa F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother. 1993;37:1679–1684. doi: 10.1128/aac.37.8.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Bossche H. Mode of action of anticestodal agents. In: Campbell W C, Rew R S, editors. Chemotherapy of parasitic diseases. New York, N.Y: Plenum Press; 1986. pp. 139–157. [Google Scholar]
- 29.Waller P J. Resistence in nematode parasites of livestock to the benzimidazole antihelmintics. Parasitol Today. 1990;6:127–129. doi: 10.1016/0169-4758(90)90230-2. [DOI] [PubMed] [Google Scholar]
- 30.Wilson J F, Rausch R L, McMahon B J, Schantz P M. Parasitocidal effect of chemotherapy in alveolar hydatid disease: review of experience with mebendazole and albendazole in Alaskan Eskimos. Clin Infect Dis. 1992;15:234–249. doi: 10.1093/clinids/15.2.234. [DOI] [PubMed] [Google Scholar]
- 31.Wilson J F, Rausch R L, Wilson F R. Alveolar hydatide disease. Review of the surgical experience in 42 cases of active disease among Alaskan eskimos. Ann Surg. 1995;221:315–323. doi: 10.1097/00000658-199503000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]