Abstract
The menopausal transition is often accompanied with distressing manifestations, such as vasomotor symptoms, sleep disruptions, and depressive syndrome. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) have emerged as a potential intervention to alleviate these symptoms. This review aimed to comprehensively assess the impact of n-3 PUFAs supplementation on vasomotor symptoms, sleep quality, and depression among postmenopausal women. We conducted a systematic literature search of randomized controlled trials across the Cochrane Library, Web of Science, PubMed, CINAHL, EMBASE, and SCOPUS databases from inception to August 2023. Among the initial pool of 163 identified studies, nine studies met the inclusion criteria and were incorporated into this systematic review. Notably, four studies detected potential benefits of n-3 PUFAs in improving hot flashes and night sweats. On the contrary, sleep quality outcomes displayed heterogeneity across the studies. Incorporating diverse scales, such as the Hamilton Depression Rating Scale-21, the Patient Health Questionnaire depression scale, and Generalized Anxiety Disorder-7 for depression outcomes, we found inconclusive evidence of n-3 PUFA’s impact on depression. Overall, the combined analysis of these studies did not provide substantial evidence to support the efficacy of n-3 PUFAs in improving vasomotor symptoms, sleep quality, and depression. Further well-designed randomized clinical trials with larger participant groups are crucial to validate and generalize these results. Review Registration: PROSPERO registration no: CRD42023421922.
Keywords: amenorrhea, depression, hot flashes, omega-3 PUFA, post-menopause, sleep quality, vasomotor symptoms
1. Introduction
Menopause, a phase marked by complex physiological changes in women, significantly impacts their well-being [1]. Defined as a cessation of menstruation for approximately one year after the last menstrual cycle, menopause spans 40 to 60 years, with an average age of 52 [2]. Vasomotor symptoms (VMS), encompassing hot flashes (HF), and night sweats, alongside various other manifestations such as sleep disturbances, anxiety, depression, vaginal dryness, muscular discomfort, and sexual dysfunction, collectively impair the quality of life during this period [3,4]. It has been discovered that 30–44% of women who experience moderate to severe VMS meet the diagnostic criteria for persistent insomnia. In contrast, among women who do not have VMS, this number is significantly lower, at only 11% [5]. Furthermore, HF and major depressive disorder (MDD) emerge as prominent symptoms, with HF occurring in up to 80% of cases [6] and MDD affecting over 20% [7] of menopausal women. Consequently, more than 1/3rd of women obtain medical assistance due to the discomfort induced by HF [8]. Menopause-related depression needing medication has a substantial age of onset; it is more prevalent (10–15%) when symptoms begin before 45, but less common (5–6%) when symptoms begin at 48 or later [9].
The origins of HF remain not fully elucidated but are theorized to result from disturbances in temperature regulation, possibly linked to factors such as fluctuations in estrogen levels and alterations in neurotransmitter function [10,11]. The decline in estradiol (E2) levels is known to disrupt both the hypothalamic-pituitary-adrenal (HPA) axis and serotonergic systems, which subsequently trigger VMS, including hot flashes and night sweats. These VMS, in turn, disrupt normal sleep patterns through mechanisms such as stress-induced cortisol elevation and serotonin dysregulation. The intricate interplay between hormonal changes, particularly the decrease in estrogen, and disrupted sleep is thought to contribute to the development of depressive symptoms [12,13].
Estrogen Therapy (ET) is a common therapy used to treat postmenopausal symptoms and age-related sarcopenia. ET works by increasing myogenic gene expression, myogenic regulatory factors (myogenic differentiation factor (MyoD), myogenic factor 5 (Myf5), muscle regulatory factor 4 (MRF4), and myogenin), and muscle satellite activation and proliferation [14,15]. Hormone therapy (HT) remains the prevailing strategy for mitigating VMS, yet it harbors a spectrum of associated risks and potential adverse outcomes [16]. Integral healthcare for menopausal women should prioritize lifestyle assessment and counseling, with a specific emphasis on nutrition, as it impacts overall well-being and quality of life in the postmenopausal period by mitigating the negative effects of estrogen deficiency [17]. Currently, certain vitamins (A, D, E, and B-complex) and omega-3 polyunsaturated fatty acids (n-3 PUFAs) are playing a role in the menopausal transition symptoms, but the evidence to support their definite roles is still inconclusive.
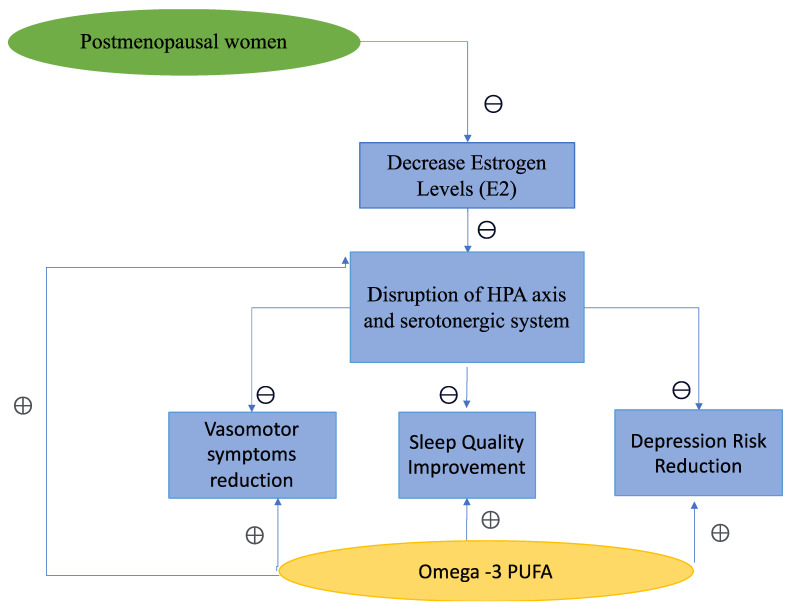
n-3 PUFAs, including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha-linolenic acid (ALA), represent essential dietary components with multiple double bonds [18]. Renowned for their therapeutic potential, these long-chain n-3 PUFAs supplements have been utilized for treating diverse medical conditions such as cardiovascular disease, depression, and cognitive disorders [19,20]. Their efficacy in addressing menopausal symptoms and MDD in perimenopausal and postmenopausal women has also been investigated [21,22,23]. Human and animal investigations elucidating the mechanistic underpinnings of n-3 PUFAs indicate their involvement in the regulation of serotonergic and dopaminergic neurotransmitter systems. However, the definitive favorable impact of n-3 PUFAs on menopausal transition-associated HF, depression, and cognitive symptoms remains inconclusive [24,25,26] (Figure 1).
Figure 1.
n-3 PUFAs’ role in improving vasomotor symptoms sleep quality and depression. Plus (+), and minus (−) signs represent a positive and negative relationship.
In a clinical investigation, supplementation of ethyl EPA resulted in reduced HF and improved HF scores compared to a placebo [27]. However, another study failed to observe any significant alterations in VMS and sleep quality when compared to a placebo [28]. A comprehensive review of 32 studies underscored the vulnerability of menopausal women to depression and anxiety [29]. Exploring the interplay of HF, sleep patterns, and depression in women undergoing menopause induced by a Gonadotropin-releasing hormone (GnRH) agonist medication, a study revealed significant associations between increased sleep interruptions, nocturnal HF, and heightened depression scores [30]. Although certain studies supported the potential of n-3 PUFAs in mitigating depression and HF [31,32], discrepant evidence arises from studies that found no support for the influence of n-3 PUFAs on depression scores, as assessed through diverse rating scales [28].
Presently, the precise impact of n-3 PUFAs supplementation on VMS remains elusive. Convergent research in both animal and human subjects suggests that n-3 PUFAs may modulate neurotransmitter levels, including serotonin and dopamine, within the brain by elevating levels of these fatty acids [25,33]. Consequently, the definitive impact of n-3 PUFAs supplements on VMS, sleep quality, and depression scores lacks empirical validation. As such, this systematic review endeavors to synthesize existing evidence to elucidate the efficacy of n-3 PUFAs supplementation in ameliorating VMS, enhancing sleep quality, and reducing depression scores in the context of postmenopausal women.
2. Materials and Methods
2.1. Study Search Strategy and Selection
The systematic review was performed according to the systematic reviews and metanalysis (PRISMA) guidelines [34]; details are available in Supplementary Table S1. The study’s registration in the Prospective Register of Systematic Reviews (PROSPERO) was completed under registration number CRD42023421922. The PICOS (Patients, Intervention, Comparison, Outcome, Study Design) paradigm was used to develop the search terms (Table 1). The PICO question was “How do n-3 PUFAs affect vasomotor symptoms, sleep quality, and depression in postmenopausal women?”
Table 1.
Study research question (PICOS).
| PICOS Components | Determinants |
|---|---|
| Population (P) | Postmenopausal women |
| Intervention (I) | N-3 PUFAs |
| Comparison (C) | Control group and placebo |
| Outcome (O) | Vasomotor Symptoms, Sleep Quality and Depression |
| Study Design (S) | Randomized Clinical Trials |
Multiple databases, including the Cochrane Library, Web of Science, PubMed, Embase, CINAHL, and SCOPUS, were utilized. The search employed both free text and Medical Subject Headings (MeSH) terms, such as “omega-3”, “fish oils”, “PUFA”, “menopause”, “hot flashes”, “night sweats”, “vasomotor”, “sleep quality”, “insomnia”, and “depression”. Supplementary sources, such as Google Scholar and ClinicalTrials.gov, were also consulted to identify ongoing or unpublished research. The search encompassed studies published in English from inception to the present, without imposing restrictions on publication time or status. A comprehensive search strategy was executed to identify pertinent studies for this systematic review presented in the Supplementary Table S2.
2.2. Included and Excluded Studies
The systematic review applied the following inclusion criteria: (1) Randomized controlled trials (RCTs) featuring a single intervention group receiving n-3 PUFAs supplementation in comparison to a placebo or alternative control group; (2) Encompassing studies involving both naturally postmenopausal women (defined as having experienced more than 12 months since their last amenorrhea) and surgically postmenopausal women (as indicated by follicle-stimulating hormone levels exceeding 40 IU/L); (3) Inclusion of studies regardless of the administered n-3 PUFAs dosage; (4) Studies that reported relevant outcomes pertaining to postmenopausal symptoms, including but not limited to hot flashes, night sweats, mood disturbances, sleep quality, and depression.
Exclusion criteria comprised: (1) Studies lacking the reporting of pertinent outcomes or those without access to full-text publications; (2) Observational and non-human studies; (3) Studies published in languages other than English.
2.3. Study Participants
The study focused on women at both menopausal and post-menopausal stages, who were experiencing VMS and depression due to menopause, or women undergoing surgical menopause who were also experiencing VMS and depression.
2.4. Type of Intervention and Control
Included studies evaluated n-3 PUFAs supplementation at any dosage, frequency, and form (capsule, oil, powder) compared to placebo or other control groups. Studies involving fish consumption, use of antidepressants, hormone replacement therapy, use of anticoagulants, and those lacking placebo or adequate control groups, were excluded.
2.5. Outcome Measures
The primary outcomes targeted VMS, including the frequency and intensity of HF and night sweats, which were assessed through patient-maintained diaries or measured using scales such as the Hot Flash-Related Daily Interference Score Kupermann index, and menopause rating scale. Other primary outcomes included sleep quality and depression, measured using established scales including the Pittsburgh Sleep Quality Index, Insomnia Severity Index, Beck’s Depression Inventory, Montgomery–Asberg Depression Rating Scale, Generalized Anxiety Disorder Questionnaire, 20-item Hopkins Symptom Checklist Depression Scale, 21-item Hamilton Depression Rating Scale, and Physician’s Health Questionnaire depression domains. Secondary outcomes encompass menopause-specific quality of life scores and the monitoring of adverse events.
2.6. Data Extraction and Quality Assessment
Three reviewers (A.Z., S.K., and S.D.L.) screened the titles and abstracts in stage one of the screening. A third member (K.P.S.) resolved disagreements. In stage two, full papers extracted from the previous stage were independently screened by two reviewers (A.Z., and S.K.). To begin, nine papers were chosen to determine reviewer consistency. The quality of the included studies was assessed using the Cochrane Collaboration Risk of Bias Assessment Tool by two reviewers (A.Z. and S.K.) separately [35]. The third member (K.P.S) resolved any apparent discrepancy resulting from the assessment process. To assess the quality of studies, Cochrane’s seven areas of assessment were used: randomization, allocation concealment, blinding of participants, blinding of outcome assessment, inadequate outcome data, selective reporting, and additional bias.
2.7. Statistical Analysis
The initial plan was to perform a meta-analysis to quantify the overall effect of n-3 PUFAs supplementation on VMS reduction, sleep quality improvement, and depression risk reduction as the outcome measures with respect to the control or placebo group. However, the inability to conduct a quantitative synthesis in this study is attributed to the substantial heterogeneity observed among the included studies for several reasons. First, variations in the dosages and frequencies of n-3 PUFAs supplementation were prevalent across these studies, making it challenging to combine their findings in a statistically meaningful way. Secondly, the utilization of diverse assessment methods for measuring outcomes introduced a potential source of bias and measurement error, further complicating the comparability of results between studies.
3. Results
3.1. Selected Studies
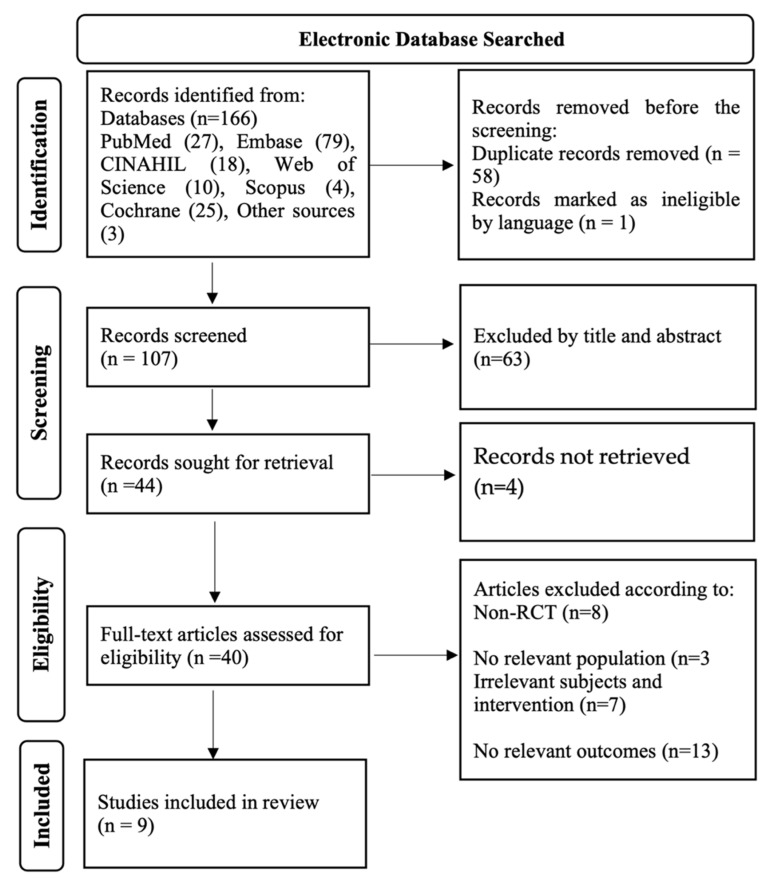
Figure 2 depicts the results of the screening process. The database searches yielded a total of 163 studies; after eliminating 58 duplicate entries, 107 publications were reviewed. After selection based on the title and abstract, 44 publications were selected. Further, information regarding other excluded articles can be found in Supplementary Materials Table S3. Finally, this systematic review identified nine relevant papers that contained RCTs with sample sizes ranging from 60 to 546 people. These studies evaluated how n-3 PUFAs supplements affect menopausal symptoms, sleep quality, and depression in menopausal women. The participants in the RCTs were given varying amounts of EPA and DHA, the n-3 PUFAs of interest. Depending on the trial design, the placebo groups received soybean oil, sunflower oil, or olive oil. Furthermore, some research used interventions other than the aforementioned placebos, extending the types of interventions used.
Figure 2.
PRISMA flowchart for study search and selection strategy.
3.2. The Effect of n-3 PUFAs on VMS
The studies evaluating the impact of n-3 PUFAs supplementation on menopausal symptoms are summarized in Table 2. Two RCTs detected no significant difference in the frequency of vasomotor symptoms (VMS) with n-3 PUFAs supplementation [28,36], whereas others found a decrease in both VMS and HF frequency and intensity [27,37,38]. Moreover, a separate study found a decreased HF frequency but no effect on the intensity [39]. Overall, this systematic review indicates that n-3 PUFAs supplementation may have a variable impact on menopausal symptoms, with some studies showing a decrease in symptoms and others reporting no significant changes.
Table 2.
The main characteristics of nine included studies evaluating the effect of n-3 PUFAs intake on vasomotor symptoms, sleep quality, and depression in postmenopausal women.
| Authors & Year | Study Design | Participants, No. | Intervention | Duration | Vasomotor Symptoms | Sleep Quality | Depression | Other Outcomes |
|---|---|---|---|---|---|---|---|---|
| [40] | Triple-Blind Randomized Controlled Trial | Menopause women, n = 60 | Intervention group: 20 mg citalopram and 1 g of n-3 PUFAs per day Placebo group: 20 mg citalopram along with a placebo per day |
4 weeks | -------- | -------- | BDI-II (p < 0.001) |
------ |
| Intervention group, n = 30 Control group, n = 30 | ||||||||
| [41] | Double-blind placebo-controlled, RCT | Postmenopausal women, n = 188 | Intervention group: 1.8 g n-3 PUFAs per day Placebo group: 3 capsules per day containing olive oil |
12 weeks | -------- | PSQ-I (p = 0.0933) ISI (p = 0.729) |
------ | ------ |
| Intervention group, n = 95 Control group, n = 93 | ||||||||
| [28] | Double-Blind, Randomized Clinical Trial | Menopause women, n = 355 | Intervention group: 615 mg n-3 PUFAs (EPA = 425 mg, DHA = 100 mg) 3 capsules per day Placebo group: 3 capsules per day containing olive oil |
12 weeks | VMS frequency (p = 0.283) | PSQ-I (p = 0.0933) ISI (p = 0.729) |
PHQ-8 (p = 0.097) GAD-7 (p = 0.191) |
No Adverse Effect |
| Intervention group, n = 177; Placebo group, n = 178 | ||||||||
| [31] | Double-blind placebo-controlled, RCT | Menopause women, n = 120 | Intervention group: 500 mg n-3 PUFAs (EPA= 350 mg and DHA= 50 mg in ethyl esters form)/day Placebo group: 500 mg capsule containing sunflower oil per day 0.2% of regular fish oil (18% EPA/12% DHA) 3 times daily |
8 weeks | ------- | ----- | PGWB (p = 0.034) HSCL-D-20 (p = 0.040) HAM-D-21 (p = 0.030) |
------ |
| Intervention group, n = 59; Placebo group, n = 61 | ||||||||
| [27] | Double-blind placebo-controlled, RCT | Menopause women, n = 120 Intervention group, n = 59; Placebo group, n = 61 |
Intervention group: 500 mg n-3 PUFAs (EPA = 350 mg and DHA = 50 mg in ethyl esters form)/day Placebo group: 500 mg capsule containing sunflower oil per day 0.2% of regular fish oil (18% EPA/12% DHA) 3 times daily |
8 weeks | HF and night sweats Frequency (p = 0.005) and Intensity (p = 0.64) |
------ | ------- | MENQOL (p = 0.2) No Adverse Effect |
| [36] | Randomized control trial | Menopause women, n = 355; | Intervention group: n-3 PUFAs supplement contained 425 mg ethyl EPA, 100 mg DHA acid per day Placebo group: 90 mg placebo containing olive oil per day |
12 weeks | VMS frequency (p = 0.06) | PSQ-I (p = 0.0933) ISI (p = 0.729) PSS (p = 0.08) |
PHQ-8 (p = 0.097) GAD-7 (p = 0.191) |
MENQOL (p = 0.12) |
| Intervention group, n = 177; Placebo group, n = 178 | ||||||||
| [37] | Randomized, Prospective, Two-Arm Study | Menopause women, n = 76; | Intervention group: n-3 PUFAs (425 mg of n-3 PUFAs/capsule), 2 capsules per day Placebo group: Soybean isoflavones (54.4 mg of isoflavones/tablet), 2 tablets per day |
16 weeks | VMS Frequency and HF (p < 0.001) |
----- | ------ | No Adverse Effect |
|
n-3 PUFAs group, n = 40; Isoflavone group, n = 36 | ||||||||
| [38] | Double-blind, Placebo-Controlled, Randomized Clinical Trial | Menopause women, n = 180; | Intervention group: 1000 mg Omega-rex soft gel Soygan 500 mg capsule Placebo group: placebo |
3 months | MRS (p = 0.03) |
----- | ----- | No Adverse Effect |
| Soy group, n = 60; n-3 PUFAs group, n = 60; Placebo group, n = 60 | ||||||||
| [39] | Double-blind, randomized controlled clinical trial | Menopause women, n = 68; | Intervention group: 300 mg (contain EPA = 120 mg and DHA = 180 mg) per day Placebo group: Placebo containing paraffin |
8 weeks | HF frequency (p = 0.003) but no intensity (p = 0.2) | ----- | ------- | No Adverse Effect |
|
n-3 PUFAs group, n = 38; Control, n = 38 |
Abbreviations: PUFAs, Polyunsaturated Fatty Acids; BDI, Beck’s Depression Inventory; PSQI, Pittsburgh Sleep Quality Index; EPA, Eicosapentaenoic Acid; DHA, Docosahexaenoic acid; VMS, Vasomotor Symptoms; ISI, Insomnia Severity Index; PHQ-8, Physician’s Health Questionnaire depression domains; GAD-7, Generalized Anxiety Disorder questionnaire; PGWB, Psychological General Well-Being Schedule; HSCL-D-20, 20-item Hopkins Symptom Checklist Depression Scale; HAM-D- 21, 21-item Hamilton Depression Rating Scale; HF, Hot flashes; MENQOL, Menopause-specific quality of life score; MRS, Menopause Rating Score.
3.3. The Effect of n-3 PUFAs on Sleep Quality
Three studies examined the effect of n-3 PUFAs on the sleep quality of postmenopausal women. Sleep quality was evaluated using the PSQI and ISI measures in all the studies. Specifically, Reed et al. found that n-3 PUFA supplementation had no influence on sleep quality among 355 menopausal women compared to the placebo group [36]. Similar to this, a double-blind, randomized clinical trial by Cohen et al. found no effect in menopausal women taking 615 mg of n-3 PUFAs daily for 12 weeks [28]. Moreover, an increased daily intake of 1.8 g of n-3 PUFAs also had no impact on sleep quality, according to Guthrie et al. In the study, n-3 PUFA supplements did not appear to have a substantial impact on postmenopausal women’s sleep quality compared with placebo [41]. Based on the existing research, this systematic review concludes that n-3 PUFA supplementation does not appear to have a substantial impact on sleep quality in postmenopausal women.
3.4. The Effect of n-3 PUFAs on Depression
This systematic review included four studies investigating the effects of n-3 PUFA supplementation on depression in menopausal women. Masoumi et al.’s triple-blind, randomized controlled trial demonstrated that menopausal women who received a combination of 20 mg citalopram and 1 g of n-3 PUFAs showed a decrease in depression as measured by the BDI-II [40]. However, the double-blind, randomized clinical trial conducted by Cohen et al. and Reed et al. observed no statistically significant alterations in depression levels, as evaluated through the PHQ-8 and GAD-7 scales, following a 12-week regimen of 1.8 g/day n-3 PUFAs supplementation (425 mg of EPA, 100 mg DHA and 90 mg of other n-3 PUFAs, 3 pills/day) [28,36]. In contrast, the double-blind, placebo-controlled study conducted by Lucas et al. revealed a reduction in depression scores (measured using PGWB, HSCL-D-20, and HAM-D-21) among menopausal women administered with 500 mg n-3 PUFAs capsules (350 mg EPA and 50 mg DHA) thrice daily over 8 weeks [31]. Overall, our systematic review reveals that n-3 PUFA supplementation may improve depressive symptoms in postmenopausal women, as indicated by several of the included studies, even though not all studies showed meaningful changes.
3.5. Other Outcomes
Out of the nine studies, the majority of the studies found no adverse effects associated with n-3 PUFA supplementation [27,28,37,38,39]. In addition, as indicated by the menopause-specific quality of life score (MENQOL), two studies found an increase in quality of life specifically related to menopause, suggesting a potential positive impact of n-3 PUFAs supplementation [27,36]. These findings highlight the safety and potential benefits of omega-3 supplementation for menopausal women.
3.6. Risk of Bias Assessment (RoB)
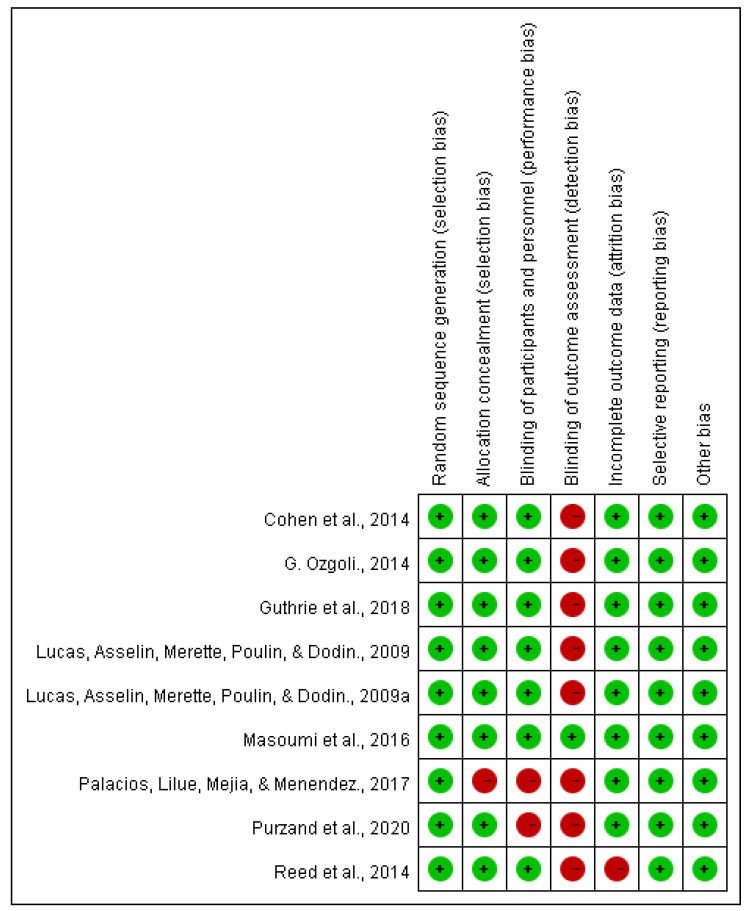
Figure 3 provides a comprehensive assessment of the methodological quality across the various studies included in our systematic review, employing a diverse set of criteria to gauge potential biases present in their research design and reporting. Each study underwent a meticulous evaluation process, resulting in categorizations of “low”, “high”, or “unclear” to signify the extent of perceived bias. Notably, the analysis uncovers that only a single study manages to demonstrate a low level of bias across multiple domains, encompassing random sequence generation, allocation concealment, blinding of participants, blinding of personnel, management of incomplete outcome data, selective reporting, and other potential sources of bias [40]. However, the majority of the reviewed studies raise concerns, primarily about a high level of bias in the blinding of participants [37,38] and personnel [27,28,31,36,37,38,39,41]. Additionally, there is a somewhat lesser but still noteworthy level of bias observed in the attrition bias [36]. These findings collectively suggest that, while many of the studies exhibit robust methodologies in areas such as randomization and participant blinding, there exists a notable vulnerability to performance and detection biases that could potentially impact the validity and reliability of their reported results. Thus, critical consideration of these biases is imperative when interpreting and drawing conclusions from the body of research examined.
Figure 3.
Risk of bias for studies with n-3 PUFAs and premenopausal women. Green (+), a low risk of bias; red (−), a high risk of bias. [27,28,31,35,36,37,38,39,40,41].
4. Discussion
This systematic review aimed to evaluate the impact of n-3 PUFAs supplementation on menopausal symptoms in postmenopausal women. The review encompassed nine pertinent randomized controlled trials that exhibited diversity in terms of sample sizes and treatment approaches. The trials were comprehensive in investigating a range of menopause-related issues, including VMS, sleep quality, depression, and various indicators of quality of life.
The menopausal transition signifies a profound period of transformation for women. This natural progression involves a decline in E2 (10–20 pg/mL) levels [42], potentially leading to modifications in brain neurochemicals and instability within the hypothalamus—the brain region responsible for regulating body temperature. These changes are often attributed to the emergence of VMS, encompassing HF and night sweats [43]. HT stands as the foremost efficacious treatment for HFs and holds the sole FDA-approved indication for symptom relief. Nevertheless, a significant number of women currently exhibit reluctance to pursue HT, primarily attributed to apprehensions regarding associated risks [44]. Non-hormonal treatments, such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs), offer a promising approach to addressing menopausal symptoms in women [45]. Extensive research has confirmed the effectiveness of non-hormonal treatments in significantly diminishing the intensity and severity of HF, with reported decreases of up to 70–80% [46]. However, it is crucial to acknowledge that their suitability for women undergoing tamoxifen therapy is subject to specific constraints. More precisely, particular SSRIs and SNRIs, notably paroxetine and fluoxetine, have demonstrated the ability to inhibit the enzyme CYP2D6. This inhibition can lead to a reduction in the levels of the active tamoxifen metabolite, endoxifen [47]. Simultaneously, the consumption of a diet rich in n-3 PUFAs has shown the potential to alleviate vasomotor symptoms (VMS), thereby suggesting the possible utility of n-3 PUFAs in addressing such symptoms [29]. Within the scope of this study, the trials included demonstrated a heterogeneous nature, with certain trials indicating a decrease in VMS (including HF and night sweats) following n-3 PUFAs intervention, while others did not exhibit such effects. For example, Lucas et al. documented a reduction in both frequency and intensity of HF and night sweats in menopausal women who consumed n-3 PUFAs capsules [27]. In contrast, Cohen et al. and Reed et al. did not observe substantial effects on VMS through n-3 PUFAs treatment. The divergent outcomes might potentially be attributed to variations in dosages, treatment durations, and participant characteristics [28,36].
Sleep disturbances frequently afflict postmenopausal women, often linked to the presence of HF and night sweats [48]. Epidemiologic studies have commonly reported increased sleep disturbances during menopause [49], but laboratory research presents differing insights. One specific study in a laboratory context revealed no notable variations in sleep metrics, performance assessments, or questionnaire responses among premenopausal women, symptomatic postmenopausal women, and asymptomatic postmenopausal women [50]. Perimenopause, a phase characterized by a lack of precise boundaries, encompasses the concluding years of a female’s reproductive cycle with an average of 15–30 pg/mL E2 levels [51,52]. Furthermore, when considering whole-night data, hot flashes were not found to be immediate triggers for awakenings or arousal [50]. Another study involving women aged 44–56 experiencing poor sleep quality highlighted that objective sleep quality primarily correlated with factors such as apneas, periodic limb movements, and arousal, while subjective sleep quality was linked to anxiety levels and the number of hot flashes in the initial half of the night. These findings suggest that anxiety may mediate some reported instances of poor sleep during menopause, underscoring the significance of identifying primary sleep disorders like apnea and periodic limb movements, which can significantly disrupt sleep and hold important medical implications [53]. Several other studies have also revealed a graduated correlation between the frequency and severity of HF and the intensity of insomnia symptoms, accompanied by quantifiable measures of disrupted sleep patterns [54,55]. An intriguing randomized controlled trial displayed noteworthy results; where n-3 PUFAs supplementation was employed alongside conventional medication, it improved outcomes spanning depression symptoms, anxiety, sleep dimensions, and emotional self-regulation, surpassing placebo effects [56]. However, our comprehensive systematic analysis did not yield robust evidence supporting the notion that n-3 PUFAs supplementation significantly enhances sleep quality in postmenopausal women. In line with this, Guthrie et al., Cohen et al., and Reed et al. all concurred by reporting no substantial impact on sleep quality through diverse sleep assessment scales, including the PSQI and ISI [28,36,41]. Despite the common occurrence of sleep issues in menopausal women, it appears that n-3 PUFAs supplementation does not offer discernible efficacy in augmenting sleep quality within this cohort. In contrast, a distinct study highlighted that DHA/EPA supplementation did enhance sleep quality in middle-aged and elderly individuals, even at the lower doses employed in earlier investigations [57]. These disparities in outcomes could potentially be attributed to suboptimal n-3 PUFAs doses or an imbalance in the optimal quantities of individual components needed for a comprehensive effect.
Depression, characterized by persistent low mood and reduced interest in daily activities for more than two weeks, is notably more prevalent among females, with 1.5 to 3 times higher incidence rates compared to males [58,59]. A significant predisposing factor for depression during menopause was a prior history of depressive disorder, indicating a recurrence of pre-existing depression. Notably, VMS like insomnia and HF exhibited strong associations with the onset of new depressive episodes, increased anxiety, and the recurrence of pre-existing depressive conditions [60]. It has been postulated that alterations in hormonal profiles throughout the menopausal transition could potentially impact the central nervous system by modulating hypothalamic and hippocampal functions. Steroid hormones can exert an influence on serotonin and gamma-aminobutyric acid (GABA) signaling pathways [61,62]. Additionally, in conjunction with the fluctuations in neuronal opioids observed during menopause, these hormonal changes have been linked to the manifestation of symptoms such as depression, irritability, and anxiety [63]. In seeking relief from depressive symptoms, individuals often turn to antidepressants, particularly SSRIs, despite potential side effects such as sexual dysfunction and weight gain if used over extended periods [64,65]. A recent network meta-analysis (NMA) was conducted to determine the effect of pharmacological interventions and hormone therapies on depressive symptoms in peri and post-menopause women. The NMA presented evidence that fluoxetine plus HRT may be beneficial to menopausal women with a definite diagnosis of depression but not to those without depression, or post-menopausal women [66]. In contrast, emerging research has spotlighted the role of polyunsaturated fatty acids, including n-3 PUFAs, in mitigating depressive symptoms [67,68,69]. An intriguing study underscored the clinical efficacy of endocannabinoids derived from n-3 PUFAs in the treatment of MDD, opening avenues for innovative therapeutic approaches [70]. However, the impact of n-3 PUFAs supplementation on depression among postmenopausal women remains equivocal. Masoumi et al. demonstrated reduced depression scores through combined citalopram and n-3 PUFAs supplementation [40]. In contrast, Cohen et al. and Reed et al. did not observe significant changes in depression scores with n-3 PUFAs supplementation alone [28,36]. Lucas et al., on the other hand, reported lowered depression scores in women who received n-3 PUFAs capsules, suggesting potential benefits in alleviating depressive symptoms [31]. Notably, due to the diverse range of outcomes, prudence is necessary when drawing definitive conclusions about the antidepressant effects of omega-3 supplementation in menopausal women.
VMS, which can significantly compromise women’s quality of life, has often been linked to the menopausal transition. Although prior epidemiological studies have primarily associated this transition with somatic symptoms, the connection to other areas of quality of life remains unclear [71]. A study found that menopausal symptoms significantly reduced the quality of life in affected individuals, leading to decreased productivity and economic implications [72]. Also, a study within this review demonstrated improvements in the MENQOL score among the n-3 PUFAs-supplemented group, indicating a potential positive impact on overall well-being during menopause [27]. Additionally, our comprehensive analysis affirms the general safety of n-3 PUFAs supplementation in menopausal women, as adverse effects were not prominently noted. This observation aligns with findings from another systematic review conducted to assess the impact of n-3 PUFAs supplementation during the menopausal transition [26].
5. Strengths and Limitations
Firstly, our systematic review provides a thorough compilation of included studies, all of which were randomized and had a homogenous control group. Secondly, by focusing specifically on postmenopausal women, it addresses a critical demographic group with unique health concerns. Thirdly, its quality assessment enhances the reliability of the conclusions drawn. This systematic review has several important limitations that require attention. Firstly, there were differences in the dosages and frequencies of n-3 PUFAs supplementation among the included studies. Secondly, the use of various assessment methods for outcomes introduces the possibility of bias and measurement error. Thirdly, the exclusion of non-English papers may have led to the omission of pertinent data. Lastly, the relatively small sample size and the study’s relatively short duration restrict its ability to capture long-term effects or rare outcomes. These limitations should be considered when interpreting the findings and can serve as guidance for future research efforts aimed at addressing these constraints more thoroughly.
6. Conclusions
In summary, the outcomes of our investigation indicate that the impact of n-3 PUFA supplementation on menopausal symptoms in postmenopausal women is varied. While certain studies highlight benefits for VMS and mood disturbances, others do not corroborate these effects. The data suggesting a positive influence of n-3 PUFA supplementation on sleep quality in menopausal women are limited. Nonetheless, the safety profile of such supplementation remains promising. Therefore, we propose that future research should entail extended follow-up periods, encompass larger cohorts, and explore combined therapeutic approaches with other medications aimed at enhancing the management of menopausal symptoms.
Abbreviations
| ALA | ALPHA-LINOLENIC ACID |
| BDI | Beck’s Depression Inventory |
| CINAHL | Cumulated Index to Nursing and Allied Health Literature |
| DHA | Deocosahexaenoic acid |
| EMBASE | Excerpta Medica Database |
| EPA | Eicosapentaenoic acid |
| ET | Estrogen Therapy |
| FDA | Food and Drug administration |
| GABA | Gamma-Aminobutyric acid |
| GAD-7 | Generalized Anxiety Disorder scale |
| GNRH | Gonadotropin-releasing hormone |
| HAM-D- | Hamilton Rating Scale for Depression |
| HF | Hot flashes |
| HPA | Hypothalamic-pituitary-adrenal |
| HRT | Hormone replacement therapy |
| HSCL-D-20 | Hopkins Symptom Checklist Depression Scale |
| HT | Hormone therapy |
| ISI | Insomnia Severity Index |
| IU | International Unit |
| MDD | Major depressive disorder |
| MENQOL | Menopause-specific quality of life score |
| MESH | Medical Subject Headings |
| MRF4 | Muscle regulatory factor 4 |
| MRS | Menopause rating scale |
| MYOD | Myogenic differentiation factor |
| NMA | Network meta-analysis |
| PGWB | Psychological General Well-Being Schedule |
| PHQ-8 | Physician’s Health Questionnaire depression domains |
| PICO | Population, Intervention, Comparison and Outcome |
| PICOS | Population, Intervention, Comparison, Outcome and Study |
| PRISMA | performed according to the systematic reviews and metanalysis |
| PROSPERO | Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
| PSQI | Pittsburgh Sleep Quality Index |
| PSS | Perceived Stress Score |
| PUBMED | Player Unknown Battle Mantra Ending In Domination |
| PUFA | Polyunsaturated fatty acid |
| RCT | Randomized controlled trial |
| ROB | Risk of bias assessment |
| SNRIS | Serotonin-norepinephrine reuptake inhibitors |
| SSRIS | Selective serotonin reuptake inhibitors |
| VMS | Vasomotor symptoms |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15194231/s1, Table S1. PRISMA 2020 Main Checklist; Table S2. Search strategy; Table S3. List of excluded studies.
Author Contributions
The authors responsibilities were as follows: Conceptualization, A.Z.I. and S.-D.L.; methodology, A.Z.I. and S.-D.L.; investigation, A.Z.I., S.-D.L. and S.-K.W.; writing—original draft preparation, A.Z.I. and S.-D.L.; writing—review and editing, A.Z.I., S.-D.L., S.-K.W., H.Z., W.-C.L., W.-C.C. and K.-P.S.; supervision, S.-D.L. and K.-P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
The authors of this work were supported by the following grants: NSTC 109-2320-B-038-057-MY3, 110-2321-B-006-004, 110-2811-B-039-507, 110-2320-B-039-048-MY2, 110-2320-B-039-047-MY3, 110-2813-C-039-327-B, 110-2314-B-039-029-MY3, 111-2321-B-006-008, 111-2314-B-039-041-MY3, and 113-2923-B-039-001-MY3 from the National Science and Technology Council, Taiwan; ANHRF 109-31, 109-40, 110-13, 110-26, 110-44, 110-45, 111-27, 111-28, 111-47, 111-48, and 111-52 from An-Nan Hospital, China Medical University, Tainan, Taiwan; CMRC-CMA-2 from Higher Education Sprout Project by the Ministry of Education (MOE), Taiwan; CMU 110-AWARD-02, 110-N-17, 1110-SR-73 from the China Medical University, Taichung, Taiwan; and DMR-106-101, 106-227, 109-102, 109-244, 110-124, 111-245, 112-097, 112-086, 112-109, 112-232 and DMR-HHC-109-11, HHC-109-12, HHC-110-10, and HHC-111-8 from the China Medical University Hospital, Taichung, Taiwan.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dalal P.K., Agarwal M. Postmenopausal syndrome. Indian J. Psychiatry. 2015;57:S222. doi: 10.4103/0019-5545.161483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. 2011;38:425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson H.D., Haney E., Humphrey L., Miller J., Nedrow A., Nicolaidis C., Vesco K., Walker M., Bou-gatsos C., Nygren P. Management of menopause-related symptoms. Evid. Rep. Technol. Assess. (Summ.) 2005:1–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Noll P.R.E.S., Nascimento M.G., Bayer L.H.C.M., Zangirolami-Raimundo J., Turri J.A.O., Noll M., Baracat E.C., Soares Junior J.M., Sorpreso I.C.E. Changes in Food Consumption in Postmenopausal Women during the COVID-19 Pandemic: A Longitudinal Study. Nutrients. 2023;15:3494. doi: 10.3390/nu15153494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant L.K., Coborn J.E., Cohn A., Nathan M.D., Scheer F., Klerman E.B., Kaiser U.B., Harder J., Abramson M., Elguenaoui E., et al. Sleep Fragmentation and Estradiol Suppression Decrease Fat Oxidation in Premenopausal Women. J. Clin. Endocrinol. Metab. 2022;107:e3167–e3176. doi: 10.1210/clinem/dgac313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim N.Y., Yoo S.-K., Jin J.-C., Yoon Y.J., Han D.H., Kim S.M. Latent Profile Analysis for Classification of Psychosomatic Symptoms in Perimenopausal Women. J. Acad. Consult.-Liaison Psychiatry. 2023;64:136–146. doi: 10.1016/j.jaclp.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Han Y., Gu S., Li Y., Qian X., Wang F., Huang J.H. Neuroendocrine pathogenesis of perimenopausal depression. Front. Psychiatry. 2023;14:1162501. doi: 10.3389/fpsyt.2023.1162501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessler R.C. Epidemiology of women and depression. J. Affect. Disord. 2003;74:5–13. doi: 10.1016/S0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 9.Kułak-Bejda A., Krajewska-Ferishah K., Szyszko-Perłowska A., Waszkiewicz N. Risk Assessment of Depression amongst Women during Menopause before and during the COVID-19 Pandemic. Int. J. Environ. Res. Public. Health. 2022;20:596. doi: 10.3390/ijerph20010596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill S., Eden J. The pathophysiology of menopausal symptoms. Obstet. Gynaecol. Reprod. Med. 2017;27:303–310. doi: 10.1016/j.ogrm.2017.07.002. [DOI] [Google Scholar]
- 11.Clayton A.H., Ninan P.T. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim. Care Companion J. Clin. Psychiatry. 2010;12:26233. doi: 10.4088/PCC.08r00747blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannini A., Caretto M., Genazzani A.R., Simoncini T. Neuroendocrine Changes during Menopausal Transition. Endocrines. 2021;2:405–416. doi: 10.3390/endocrines2040036. [DOI] [Google Scholar]
- 13.Weber M.T., Maki P.M., McDermott M.P. Cognition and mood in perimenopause: A systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 2014;142:90–98. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieli-Conwright C.M., Spektor T.M., Rice J.C., Sattler F.R., Schroeder E.T. Influence of hormone replacement therapy on eccentric exercise induced myogenic gene expression in postmenopausal women. J. Appl. Physiol. 2009;107:1381–1388. doi: 10.1152/japplphysiol.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dam T.V., Dalgaard L.B., Johansen F.T., Bengtsen M.B., Mose M., Lauritsen K.M., Gravholt C.H., Hansen M. Effects of transdermal estrogen therapy on satellite cell number and molecular markers for muscle hypertrophy in response to resistance training in early postmenopausal women. Eur. J. Appl. Physiol. 2023;123:667–681. doi: 10.1007/s00421-022-05093-0. [DOI] [PubMed] [Google Scholar]
- 16.Mehta J., Kling J.M., Manson J.E. Risks, Benefits, and Treatment Modalities of Menopausal Hormone Therapy: Current Concepts. Front. Endocrinol. 2021;12:564781. doi: 10.3389/fendo.2021.564781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva T.R., Oppermann K., Reis F.M., Spritzer P.M. Nutrition in Menopausal Women: A Narrative Review. Nutrients. 2021;13:2149. doi: 10.3390/nu13072149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zailani H., Satyanarayanan S.K., Liao W.C., Liao H.F., Huang S.Y., Gałecki P., Su K.P., Chang J.P. Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review. J. Clin. Med. 2023;12:2653. doi: 10.3390/jcm12072653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper L., Thompson R.L., Harrison R.A., Summerbell C.D., Ness A.R., Moore H.J., Worthington H.V., Durrington P.N., Higgins J.P., Capps N.E. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu S.-K., Chen W.-J., Chang J.P.-C., Guu T.-W., Hsin M.-C., Huang C.-K., Mischoulon D., Capuron L., Su K.-P. Personalized Medicine of Omega-3 Fatty Acids in Depression Treatment in Obese and Metabolically Dysregulated Patients. J. Pers. Med. 2023;13:1003. doi: 10.3390/jpm13061003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes P.M., Bloom B., Nahin R.L. Complementary and Alternative Medicine Use among Adults and Children: United States, 2007. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2008. [PubMed] [Google Scholar]
- 22.Appleton K.M., Hayward R.C., Gunnell D., Peters T.J., Rogers P.J., Kessler D., Ness A.R. Effects of n–3 long-chain polyunsaturated fatty acids on depressed mood: Systematic review of published trials. Am. J. Clin. Nutr. 2006;84:1308–1316. doi: 10.1093/ajcn/84.6.1308. [DOI] [PubMed] [Google Scholar]
- 23.Chae M., Park K. Association between dietary omega-3 fatty acid intake and depression in postmenopausal women. Nutr. Res. Pract. 2021;15:468. doi: 10.4162/nrp.2021.15.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbeln J.R., Linnoila M., Umhau J.C., Rawlings R., George D.T., Salem N., Jr. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early-and late-onset alcoholics. Biol. Psychiatry. 1998;44:235–242. doi: 10.1016/S0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 25.Carlezon W.A., Jr., Mague S.D., Parow A.M., Stoll A.L., Cohen B.M., Renshaw P.F. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol. Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Ciappolino V., Mazzocchi A., Enrico P., Syrén M.-L., Delvecchio G., Agostoni C., Brambilla P. N-3 Polyunsatured Fatty Acids in Menopausal Transition: A Systematic Review of Depressive and Cognitive Disorders with Accompanying Vasomotor Symptoms. Int. J. Mol. Sci. 2018;19:1849. doi: 10.3390/ijms19071849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas M., Asselin G., Mérette C., Poulin M.-J., Dodin S. Effects of ethyl-eicosapentaenoic acid omega-3 fatty acid supplementation on hot flashes and quality of life among middle-aged women: A double-blind, placebo-controlled, randomized clinical trial. Menopause. 2009;16:357–366. doi: 10.1097/gme.0b013e3181865386. [DOI] [PubMed] [Google Scholar]
- 28.Cohen L.S., Joffe H., Guthrie K.A., Ensrud K.E., Freeman M., Carpenter J.S., Learman L.A., Newton K.M., Reed S.D., Manson J.E., et al. Efficacy of omega-3 for vasomotor symptoms treatment: A randomized controlled trial. Menopause. 2014;21:347–354. doi: 10.1097/GME.0b013e31829e40b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grigolon R.B., Ceolin G., Deng Y., Bambokian A., Koning E., Fabe J., Lima M., Gerchman F., Soares C.N., Brietzke E., et al. Effects of nutritional interventions on the severity of depressive and anxiety symptoms of women in the menopausal transition and menopause: A systematic review, meta-analysis, and meta-regression. Menopause. 2023;30:95–107. doi: 10.1097/GME.0000000000002098. [DOI] [PubMed] [Google Scholar]
- 30.Joffe H., Crawford S.L., Freeman M.P., White D.P., Bianchi M.T., Kim S., Economou N., Camuso J., Hall J.E., Cohen L.S. Independent Contributions of Nocturnal Hot Flashes and Sleep Disturbance to Depression in Estrogen-Deprived Women. J. Clin. Endocrinol. Metab. 2016;101:3847–3855. doi: 10.1210/jc.2016-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas M., Asselin G., Mérette C., Poulin M.-J., Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: A double-blind, placebo-controlled, randomized clinical trial2. Am. J. Clin. Nutr. 2009;89:641–651. doi: 10.3945/ajcn.2008.26749. [DOI] [PubMed] [Google Scholar]
- 32.Freeman M.P., Hibbeln J.R., Silver M., Hirschberg A.M., Wang B., Yule A.M., Petrillo L.F., Pascuillo E., Economou N.I., Joffe H., et al. Omega-3 fatty acids for major depressive disorder associated with the menopausal transition: A preliminary open trial. Menopause. 2011;18:279–284. doi: 10.1097/gme.0b013e3181f2ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patrick R.P., Ames B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29:2207–2222. doi: 10.1096/fj.14-268342. [DOI] [PubMed] [Google Scholar]
- 34.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed S.D., Guthrie K.A., Newton K.M., Anderson G.L., Booth-LaForce C., Caan B., Carpenter J.S., Cohen L.S., Dunn A.L., Ensrud K.E., et al. Menopausal quality of life: RCT of yoga, exercise, and omega-3 supplements. Am. J. Obstet. Gynecol. 2014;210 doi: 10.1016/j.ajog.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palacios S., Lilue M., Mejia A., Menendez C. Omega-3 versus isoflavones in the control of vasomotor symptoms in postmenopausal women. Gynecol. Endocrinol. 2017;33:951–957. doi: 10.1080/09513590.2017.1332588. [DOI] [PubMed] [Google Scholar]
- 38.Purzand B., Rokhgireh S., Shabani Zanjani M., Eshraghi N., Mohamadianamiri M., Esmailzadeh A., Alkatout I., Gitas G., Allahqoli L. The comparison of the effect of soybean and fish oil on supplementation on menopausal symptoms in postmenopausal women: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Clin. Pract. 2020;41:101239. doi: 10.1016/j.ctcp.2020.101239. [DOI] [PubMed] [Google Scholar]
- 39.Ozgoli R.M.G., Molaei B., Hajifaraji M., Soori H., Najafi S. Effect of Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) Supplementation on Hot Flashes in Menopausal Women: A Randomized, Double—Blind, Placebo-Controlled Clinical Trial. N. Y. Sci. J. 2014;7:37–42. [Google Scholar]
- 40.Masoumi S.Z., Kazemi F., Tavakolian S., Rahimi A., Oshvandi K., Soltanian A., Shobeiri F. Effect of citalopram in combination with omega-3 on depression in post-menopausal women: A triple blind randomized controlled trial. J. Clin. Diagn. Res. 2016;10:QC01–QC05. doi: 10.7860/JCDR/2016/19487.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guthrie K.A., Larson J.C., Ensrud K.E., Anderson G.L., Carpenter J.S., Freeman E.W., Joffe H., LaCroix A.Z., Manson J.E., Morin C.M., et al. Effects of Pharmacologic and Nonpharmacologic Interventions on Insomnia Symptoms and Self-reported Sleep Quality in Women with Hot Flashes: A Pooled Analysis of Individual Participant Data from Four MsFLASH Trials. Sleep. 2018;41:zsx190. doi: 10.1093/sleep/zsx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmina E., Lobo R.A. CHAPTER 32—Evaluation of Hormonal Status. In: Strauss J.F., Barbieri R.L., editors. Yen & Jaffe’s Reproductive Endocrinology. 6th ed. W.B. Saunders; Philadelphia, PA, USA: 2009. pp. 801–823. [Google Scholar]
- 43.Freedman R.R., Norton D., Woodward S., Cornélissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J. Clin. Endocrinol. Metab. 1995;80:2354–2358. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 44.Freedman R.R. Menopausal hot flashes: Mechanisms, endocrinology, treatment. J. Steroid Biochem. Mol. Biol. 2014;142:115–120. doi: 10.1016/j.jsbmb.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feduniw S., Korczyńska L., Górski K., Zgliczyńska M., Bączkowska M., Byrczak M., Kociuba J., Ali M., Ciebiera M. The Effect of Vitamin E Supplementation in Postmenopausal Women—A Systematic Review. Nutrients. 2023;15:160. doi: 10.3390/nu15010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson H.D. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 47.Stubbs C., Mattingly L., Crawford S.A., Wickersham E.A., Brockhaus J.L., McCarthy L.H. Do SSRIs and SNRIs reduce the frequency and/or severity of hot flashes in menopausal women. J. Okla. State Med. Assoc. 2017;110:272–274. [PMC free article] [PubMed] [Google Scholar]
- 48.Shaver J.L., Woods N.F. Sleep and menopause: A narrative review. Menopause. 2015;22:899–915. doi: 10.1097/GME.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 49.Zolfaghari S., Yao C., Thompson C., Gosselin N., Desautels A., Dang-Vu T.T., Postuma R., Carrier J. Effects of menopause on sleep quality and sleep disorders: Canadian Longitudinal Study on Aging. Menopause. 2019;27:295–304. doi: 10.1097/GME.0000000000001462. [DOI] [PubMed] [Google Scholar]
- 50.Freedman R.R., Roehrs T.A. Lack of sleep disturbance from menopausal hot flashes. Fertil. Steril. 2004;82:138–144. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 51.Santoro N. Perimenopause: From Research to Practice. J. Womens Health. 2016;25:332–339. doi: 10.1089/jwh.2015.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson H., Ho V., Pasquet R., Singh R.J., Goetz M.P., Tu D., Goss P.E., Ingle J.N. Baseline estrogen levels in postmenopausal women participating in the MAP.3 breast cancer chemoprevention trial. Menopause. 2020;27:693–700. doi: 10.1097/GME.0000000000001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedman R.R., Roehrs T.A. Sleep disturbance in menopause. Menopause. 2007;14:826–829. doi: 10.1097/gme.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 54.Ensrud K.E., Stone K.L., Blackwell T.L., Sawaya G.F., Tagliaferri M., Diem S.J., Grady D. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009;16:286–292. doi: 10.1097/gme.0b013e31818c0485. [DOI] [PubMed] [Google Scholar]
- 55.Ohayon M.M. Severe hot flashes are associated with chronic insomnia. Arch. Intern. Med. 2006;166:1262–1268. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 56.Jahangard L., Sadeghi A., Ahmadpanah M., Holsboer-Trachsler E., Sadeghi Bahmani D., Haghighi M., Brand S. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders—Results from a double-blind, randomized and placebo-controlled clinical trial. J. Psychiatr. Res. 2018;107:48–56. doi: 10.1016/j.jpsychires.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Yokoi-Shimizu K., Yanagimoto K., Hayamizu K. Effect of Docosahexaenoic Acid and Eicosapentaenoic Acid Supplementation on Sleep Quality in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients. 2022;14:4136. doi: 10.3390/nu14194136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kessler R.C., McGonagle K.A., Swartz M., Blazer D.G., Nelson C.B. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-G. [DOI] [PubMed] [Google Scholar]
- 59.Judd F.K., Hickey M., Bryant C. Depression and midlife: Are we overpathologising the menopause? J. Affect. Disord. 2012;136:199–211. doi: 10.1016/j.jad.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Alblooshi S., Taylor M., Gill N. Does menopause elevate the risk for developing depression and anxiety? Results from a systematic review. Australas. Psychiatry. 2023;31:165–173. doi: 10.1177/10398562231165439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gava G., Orsili I., Alvisi S., Mancini I., Seracchioli R., Meriggiola M.C. Cognition, Mood and Sleep in Menopausal Transition: The Role of Menopause Hormone Therapy. Medicine. 2019;55:668. doi: 10.3390/medicina55100668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schweizer-Schubert S., Gordon J.L., Eisenlohr-Moul T.A., Meltzer-Brody S., Schmalenberger K.M., Slopien R., Zietlow A.L., Ehlert U., Ditzen B. Steroid Hormone Sensitivity in Reproductive Mood Disorders: On the Role of the GABA(A) Receptor Complex and Stress During Hormonal Transitions. Front. Med. 2020;7:479646. doi: 10.3389/fmed.2020.479646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herson M., Kulkarni J. Hormonal Agents for the Treatment of Depression Associated with the Menopause. Drugs Aging. 2022;39:607–618. doi: 10.1007/s40266-022-00962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su K.P. Nutrition, psychoneuroimmunology and depression: The therapeutic implications of omega-3 fatty acids in interferon-α-induced depression. Biomedicine. 2015;5:21. doi: 10.7603/s40681-015-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirschfeld R.M. Long-term side effects of SSRIs: Sexual dysfunction and weight gain. J. Clin. Psychiatry. 2003;64((Suppl. S18)):20–24. [PubMed] [Google Scholar]
- 66.Tseng P.-T., Chiu H.-J., Suen M.-W., Zeng B.-S., Wu M.-K., Tu Y.-K., Hung K.-C., Wu Y.-C., Su K.-P., Li D.-J., et al. Pharmacological interventions and hormonal therapies for depressive symptoms in peri- and post-menopausal women: A network meta-analysis of randomized controlled trials. Psychiatry Res. 2023;326:115316. doi: 10.1016/j.psychres.2023.115316. [DOI] [PubMed] [Google Scholar]
- 67.Nemets B., Stahl Z., Belmaker R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatry. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 68.Peet M., Horrobin D.F. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 69.Su K.P., Huang S.Y., Chiu C.C., Shen W.W. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 2003;13:267–271. doi: 10.1016/S0924-977X(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 70.Yang B., Lin L., Bazinet R.P., Chien Y.-C., Chang J.P.-C., Satyanarayanan S.K., Su H., Su K.-P. Clinical Efficacy and Biological Regulations of ω–3 PUFA-Derived Endocannabinoids in Major Depressive Disorder. Psychother. Psychosom. 2019;88:215–224. doi: 10.1159/000501158. [DOI] [PubMed] [Google Scholar]
- 71.Matthews K.A., Bromberger J.T. Does the menopausal transition affect health-related quality of life? Am. J. Med. 2005;118:25–36. doi: 10.1016/j.amjmed.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 72.Whiteley J., DiBonaventura M., Wagner J.S., Alvir J., Shah S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J. Womens Health. 2013;22:983–990. doi: 10.1089/jwh.2012.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.