Abstract
Background:
The antimicrobial resistance catastrophe is a growing global health threat and predicted to be worse in developing countries. Phages for Global Health (PGH) is training scientists in these regions to isolate relevant therapeutic phages for pathogenic bacteria within their locality, and thus contributing to making phage technology universally available.
Materials and Methods:
During the inaugural PGH workshop in East Africa, samples from Ugandan municipal sewage facilities were collected and two novel Escherichia coli lytic phages were isolated and characterized.
Results:
The phages, UP19 (capsid diameter ∼100 nm, contractile tail ∼120/20 nm) and UP30 (capsid diameter ∼70 nm, noncontractile tail of ∼170/20 nm), lysed ∼82% and ∼36% of the 11 clinical isolates examined, respectively. The genomes of UP19 (171.402 kb, 282 CDS) and UP30 (49.834 kb, 75 CDS) closely match the genera Dhakavirus and Tunavirus, respectively.
Conclusion:
The phages isolated have therapeutic potential for further development against E. coli infections.
Keywords: Phages for Global Health, Escherichia coli phage, phage therapy, antimicrobial resistance
Introduction
Antimicrobial resistance (AMR) is a growing global problem in agriculture and human medicine.1,2 A UK government commissioned review estimated that the continual rise in AMR will lead to 10 million deaths worldwide and 100 trillion USD in cost by the year 2050.3,4 Although AMR is a universal health threat, its impact is predicted to be particularly severe in countries within Africa, particularly in sub-Saharan countries.4,5 This is due to the exponential population growth, economic decline, abject antibiotic stewardship, and the historical impact of other serious infections such as HIV, tuberculosis, and malaria.4–6
Bacteriophages (phages) specifically target and kill bacteria and are inexpensive to isolate, characterize, and develop as therapeutics, and they are much safer to the environment and public health than antibiotics.7,8 Other advantages of phage therapy in these parts of the world are the single-dose potential, short production times, and relatively decreased probability of developing resistance compared with antibiotics.9,10 Therefore, therapeutic phage products could be developed as viable alternatives or complementary approaches to antibiotics as part of a control strategy for bacterial infections and would help to mitigate the growing impending threat of AMR.
Despite the obvious compelling threats of AMR, research into new antibiotics or alternative innovations in Africa are grossly inadequate due to limited expertise, research funding, infrastructures, and awareness. This calls for urgent sustainable strategies to empower scientists in these parts of the world so that they are better equipped to combat AMR not only at a local scale, but also internationally, thereby contributing to global infection control alongside their Western counterparts.11
Phages for Global Health (PGH), a U.S.-based nonprofit organization, took the initiative to develop a coherent international collaboration between researchers in developed countries and those in lower- and middle-income countries to combat global AMR using phage technology.12,13 This is being achieved through training workshops that aim to transfer knowledge and skills in phage biology, genomics, therapeutic development, writing of research grant proposals and scientific articles, strategies for engaging stakeholders, and establishing international collaborations.12,14
Scientists in more than thirteen African countries have started to isolate and study phages that target diverse human, animal, and plant pathogenic bacteria.11,12,15 Most of the phages, however, are poorly characterized and research into their therapeutic development are still in rudimentary stages.11 In East Africa, to the best of our knowledge, very little phage therapy research was conducted before July 2017 when the inaugural PGH phage workshop was conducted for scientists from that region hosted at Makerere University in Uganda.12
The workshop involved lectures covering all aspects of phage biology, genomics and applications, as well as hands-on practical sessions on phage isolation, characterization, and bioinformatics. This workshop and other subsequent ones in East Africa (Kenya, June/July 2018; Tanzania, January 2020), West Africa (July 2019), and Southeast Asia (March 2022 and May 2023) were funded by a variety of foundations, scientific research grants, and individual donations.12–14
During the Ugandan workshop, 20 phage isolates were identified that could lyse Escherichia coli strains from Africa and the United Kingdom. They were isolated from local sewage sources using standard phage isolation procedures. Further isolation steps and characterization of clonal phages from these crude isolates were conducted at the Centre for Phage Research, University of Leicester, UK. In this study, we present the morphology and genome characteristics of two of these phages alongside their host range properties (the number of bacterial strains the phages can lyse), which highlight their potential for future therapeutic application for E. coli infections in Africa and other parts of the world.
Materials and Methods
Bacterial strains and culture conditions
Eleven human clinical E. coli isolates were examined in this study. Six of the isolates (isolated from fecal samples of patients with diarrhea) were kindly donated by Dr. Jesca Nakavuma from Makerere University in Uganda. Three additional fecal isolates (from patients with gastroenteritis) were kindly donated by Dr. Ngalla Jillani and Dr. Atunga Nyachieo from the Institute of Primate Research in Kenya. The remaining two isolates (from swabs from diabetic foot patients) were kindly provided by Prof. Martha Clokie from the University of Leicester in the United Kingdom (Table 1). E. coli PA5 and EA2 were used in the initial screening of the sewage samples to isolate phages.
Table 1.
Table Showing the Sources of Bacterial Isolates Examined in this Study and Their Susceptibility to Lysis by the Two Newly Isolated Phages, UP19 and UP30

|
All bacterial isolates were used for host range analyses and stored in Protect bacterial preservers (Abtek Biologicals Ltd., Liverpool, United Kingdom) at −80°C and recovered by streaking out on Tryptone Soy Agar (TSA) (ThermoFisher Scientific, Hampshire, UK) and incubating aerobically at 37°C for 24 h. Bacterial liquid cultures were prepared by inoculating two colonies of 24 h-bacterial cultures from TSA into 5 mL of Tryptone Soy Broth (TSB; ThermoFisher Scientific) and incubated with shaking at 50 rpm for 3 h.
Phage isolation and purification
All ethical measures relating to collecting, handling of sewage material, and disposing of waste were observed (Uganda National Water and Sewerage Corporation Reference: BSS/R&D/17-02). Approximately 40 mL of sewage samples from the Kampala city municipal treatment facilities were collected in 50 mL Falcon tubes, transported on ice, and processed within 2 h of collection. The samples were centrifuged at 5000 g for 10 min to remove debris and other undissolved particles, and the resultant ∼20 mL filtrates (filtered using 0.45 μm filters) were each divided into two equal 10 mL aliquots. Phages were isolated directly from the first set of aliquots. The second set of filtered sample portions were subjected to an enrichment procedure in Erlenmeyer flasks containing 10 mL double strength TSB supplemented with 2 mM CaCl2 and 100 μL of overnight TSB culture of PA5, which is a locally sourced E. coli strain from Uganda (Table 1). After incubating with shaking at 50 rpm at 37°C for 24 h, the cultures were centrifuged and filtered as aforementioned.
The resultant filtrates from both the first (direct isolation process) and second (enrichment method) aliquots were serially diluted in phage buffer (50 mM Tris-HCl pH7. 5, 100 mM NaCl, and 8 mM MgSO4) and subjected to initial phage screening using a double agar overlay plaque assay technique. The top semisolid overlays of 4 mL TSB with 0.75% agar contained 100 μL of TSB cultures of either E. coli locally sourced strains PA5 or EA2, cast and set on TSA 90 mm plates (Table 1).
Afterward, 10 μL aliquots of the diluted filtrates were applied to the confluent bacterial cultures in the top agar overlays. After incubation overnight at 37°C, individual clear plaques were randomly selected, added to 1 mL of phage buffer and transported to the United Kingdom for further phage purification and screening. Crude phage isolates were further subjected to five rounds of single plaque purification using plaque assay technique on the corresponding E. coli host strains as described earlier and previously.16,17
In the final purification step, five plates containing semi-confluent plaques of each purified phage were selected and the top semisolid agar was scraped and collected into 5 mL phage buffer and incubated at 4°C for 1 h to allow the phages to diffuse out of the agar. This was followed by centrifuging as earlier and filtering through 0.22 μm filters. Supernatants containing 108 PFU/mL of the phages (titered using plaque assay as previously described) were subjected to further analyses as described hereunder.16,17
Phage host range analysis and morphology screening
We determined the host range properties of the phages using a spot test technique.16 In brief, confluent bacterial cultures (Table 1) in semisolid overlays were prepared by adding 100 μL broth cultures to 4 mL of warm (at 50–55°C) semisolid TSB containing 0.75% agar and mixed by inversion. This was poured on TSA agar plates and allowed to set at room temperature for ∼10 min. Afterward, 10 μL volumes of the phages were applied in duplicates on each agar plate and allowed to dry for ∼5 min under a flow hood with the lid slightly opened followed by incubation overnight under aerobic conditions.
The morphologies of the phages were determined using transmission electron microscopy (TEM) analyses as described previously and the images were edited using ImageJ.16
DNA extraction and genome characterization of phages
DNA was extracted from 2 mL of the purified phage lysates using previously described methods.16 A total of ∼2 ng of the resulting DNA in 5 μL Tris-HCl were subjected to genomic sequencing using the Illumina sequencing technology with two × 250 bp paired-end reads and 30 × coverage (MicrobesNG, Birmingham, UK). The raw data were trimmed using Fastp and assembled using SPAdes v3.13.0.18 The genome quality was assessed using FastQC (v0.11.7).19 Assembly statistics were generated using BBMap (v38.88), SAMtools (v0.1.8) and QUAST (v5.2.0).19–21 The data for this study have been deposited in the European Nucleotide Archive (ENA) (https://www.ebi.ac.uk/ena/browser/view/PRJEB61472) under the project accession number PRJEB61472.
Afterward, BACterioPHage LIfestyle Predictor (BACPHLIP v0.9.6) was used to predict the lifestyle (temperate or virulent) of each phage based on a local data set of 634 phage genomes.22 BACPHLIP detects the presence of conserved domains and utilizes these data to predict lifestyle using a random forest classifier. Genome annotation was performed using Prokka v1.14.6 with PHROGS (Prokaryotic Virus Remote Homologous Groups database) using a previously described method and visualized with Proksee.23–25 The genome relatedness was determine using BLAST and the genome sequence with the highest similarity percentage was used for downstream analyses and to determine if the phages were unique. Phage genomes with varying degree of similarity were analyzed on the VIRIDIC web-based version with default settings.26
Results
Two morphologically distinct E. coli phages were isolated from the samples
We aimed to isolate E. coli phages from sewage samples collected from Kampala city municipal treatment plants during the PGH workshop using methods described in other studies.27 To ensure this was achieved during the 2-week workshop, the sewage samples were divided into two equal aliquots and phages were isolated either directly from the first aliquot or through enrichment procedures in the second.16 Owing to limited resources we characterized just two phages that we would potentially explore for therapeutic purposes against E. coli in the future.
To isolate distinct phages we attempted to base our initial phage selection criteria on the differences in the plaque morphology and size produced by each phage.16,17 However, the plaques produced by the phages were essentially identical, about 2 mm in diameter; hence, it was extremely difficult to identify distinct phages and differentiate them through this method. Therefore, we turned our focus to the phage primary host specificity, ensuring the two phages lysed different hosts.16,28 We selected two E. coli phages, UP19 and UP30, which individually lysed strains EA2 and PA5, respectively. Phage UP19 was isolated directly from a sewage sample, whereas UP30 was isolated from the enrichment procedure using the culture of host PA5.
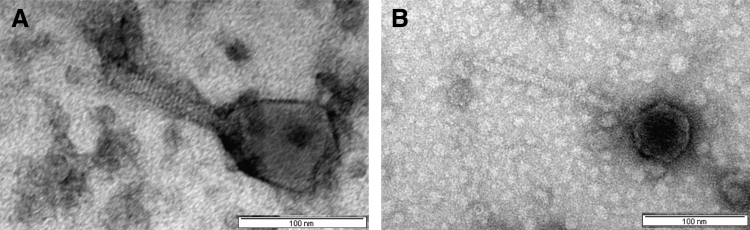
Our TEM analysis showed that both phages are tailed viruses with phage UP19 having a particularly large capsid of ∼100 nm in diameter and a contractile tail of ∼120 nm length and ∼20 nm diameter. The second phage, UP30, has a capsid of ∼70 nm in diameter and a noncontractile tail of ∼170 nm length and ∼20 nm diameter (Fig. 1). The dimensions of both phages were estimated after measuring at least ten viral particles.
FIG. 1.
Morphologies of the two newly isolated Escherichia coli phages (A) UP19 and (B) UP30 from Uganda during the PGH workshop held at Makerere University in 2017. PGH, Phages for Global Health. Both phages belonged to Class Caudoviricetes with UP19 isolated directly from a sewage sample and phage UP30, isolated from an overnight enrichment procedure with the liquid culture of host, PA5. Phage dimension was based on measuring at least 10 particles (standard deviation, 0.47) on ImageJ. Bar ∼100 nm.
The two phages isolated have different host range properties
To gain insight into the therapeutic potential of the phages, we determined their ability to lyse relevant strains through host range analysis using spot test on eleven human clinical E. coli isolates from three different geographical locations and sources.16 The bacterial strains from Uganda (six isolates) and Kenya (three isolates) were extracted from human fecal samples, whereas the remaining two UK strains were isolated from diabetic foot infection (Table 1).
From our data, it was clear that the two phages had distinct host range properties, with UP19 having a broader host target specificity (clinical coverage), infecting nine of the eleven strains (∼82%) of the isolates from the three countries. In contrast, phage UP30 had a narrower host range, targeting only four of the eleven isolates (∼36%) from East Africa and none from the United Kingdom (Table 1).
Genome analysis and taxonomy studies revealed that the two isolated phages are distinct and belonged to novel species
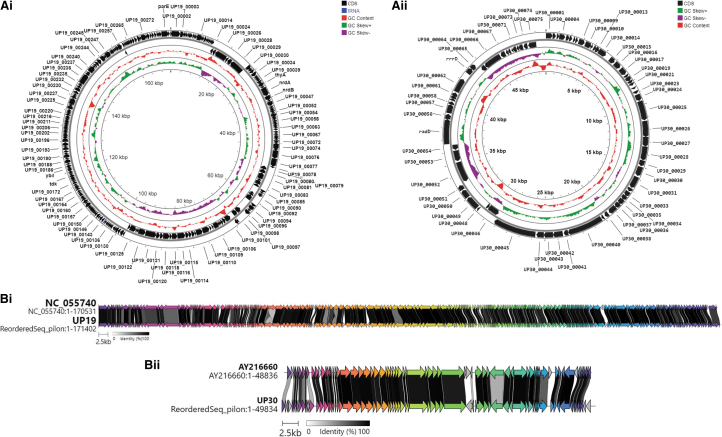
Analysis of the genomes of both phages UP19 and UP30 revealed that they are double-stranded linear DNA viruses. Phage UP19 had a larger genome size of 171.402 kb, comprising 282 CDS and 3 tRNAs compared with UP30, which had a 49.834 kb genome and encoded only 75 CDS and no observed tRNAs (Fig. 2Ai, Aii and Supplementary Tables S1–S3). Further BLAST searches showed that both phages belonged to Class Caudoviricetes and genome annotation showed that the phages are strictly lytic as no lysogeny genes were detected in the genomes (Fig. 2Ai, Aii and Supplementary Tables S1–S3).
FIG. 2.
Genome maps of E. coli phages (Ai) UP19 and (Aii) UP30 isolated in Uganda during the inaugural PGH workshop in 2017 and alignments of (Bi) UP19 and (Bii) UP30 to type phages showing regions of gene similarities. Genomes were annotated with PHROGS database and visualized using Proksee. The phages were isolated from sewage samples collected in Kampala municipal area. Genomes were annotated with PHROGS database and alignments conducted using Clinker. Accession numbers of phages used in the alignment are shown in Supplementary Table S2. The data for this study have been deposited in the ENA PRJEB61472.
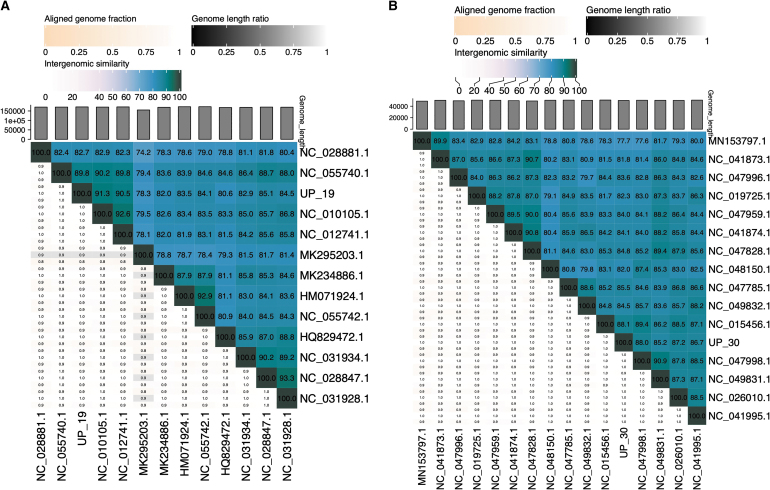
Genome alignment of phage UP19 against closely related phages showed high similarities to an Enterobacteria phage vB_EcoM_IME281 (Fig. 2Bi) within the genus Dhakavirus, subfamily Tevenvirinae, family Straboviridae. The Tunavirus UP30 belongs to the family Drexlerviridae, subfamily, Tunavirinae with similarities to Escherichia_phage T1 (Fig. 2Bii). Based on pairwise intergenomic similarities at the nucleotide level among related phages analyzed using the VIRIDIC tool, we observed that both Escherichia_phage UP19 and UP30 are unique and confirmed the identification of novel species (Fig. 3A, B) in both cases.
FIG. 3.
Images of VIRIDIC generated heatmap showing intergenomic similarity values and alignment indicators for phage UP19 (A) and UP30 (B) isolates from in this study and other closely related phages. Based on the ICTV established thresholds for the demarcation of viruses into species (95%) and into genera (∼70%) we could infer both phages UP19 and UP30 as novel species based on the intergenomic similarities shown on the heatmaps. International Committee on Taxonomy of Viruses.
Discussion
The current global AMR health threat is quickly developing into a future catastrophe due to declining antibiotic innovations.3,4,29,30 The Western world is leading research into alternative strategies for infection control and so many prospective anti-infectives are currently under different stages of clinical investigations.11 However, contributions to AMR control must extend beyond the West, and lower- and middle-income countries need to gain greater autonomy to develop or at least contribute to the identification of innovative technologies for global pathogen control.11,12
In recent years, phage therapy has gained worldwide successes in the treatment of human, animal, and plant bacterial infections, including those caused by AMR strains.31,32 For Africa, the advantages of phage therapy are far-reaching.9,10,33 Thus, PGH has undertaken the initiative to engage and empower scientists in the region to identify, characterize, and develop therapeutic phages for pathogenic bacteria in the region and globally.13
Studies have shown that phages that were isolated from one geographical region are capable of lysing bacterial strains from a different location.9,34–36 However, due to the specificity of phages, it is still unclear if phages isolated in a particular geographical location would have maximum therapeutic efficacy on bacterial strains from elsewhere. Clearly, phages can evolve with their host bacteria with associated costs and benefits that may affect overall therapeutic efficacy.37,38 It is, therefore, of paramount importance to develop phages that can effectively target and lyse bacterial strains from Africa, in addition to the many phage products that are being developed in the West. This has been a key focus of the work presented here.
The phages isolated in this study can be further developed locally for the treatment of pathogens found in the African niche.9,10,35 Alternatively, the characterized phages can be deposited in phage banks/repositories and may thereby contribute to important worldwide therapeutic resources to aid in the control of difficult-to-treat bacterial infections.11
Although AMR has been reported for many pathogenic bacteria in Africa, we focused on E. coli, which is one of the leading causes of diarrhea and other serious infections with higher morbidity and mortality both regionally and globally. Hence, it is an important candidate before the investigation here.5,6,9 Generally, E. coli strains are amenable to culturing and a number of specific phages have been isolated from Africa.39 Thus, the data and knowledge gained during PGH workshop provided vital training for the workshop participants, as well as those they might eventually train. The knowledge is also transferable to any pathogenic bacteria or model systems. Therefore, there is significant potential for phage research diversification among the participants.
With these goals in mind, we ventured into the environments of Africa, in the municipal area of Kampala, Uganda, focusing on the local sewage treatment plants as our main source for isolating phages. Sewage sites are recognized as an important reservoir for E. coli and also, for their specific phages, which can be isolated directly from the samples as previously reported.40–43 Both the direct and enrichment procedures yielded phages, and as expected, the phage UP30 was biased toward the bacterial strain (PA5) used for its enrichment procedure. This concurs with previous work on Salmonella and Shigella and may have notable benefits for the targeted selection toward specific bacterial strains.44,45 However, this could also limit the diversity of phages that are isolated.
The phages UP19 and UP30 have different levels of therapeutic potential since they can target and lyse multiple strains with UP19 showing a wider clinical coverage, lysing more strains (∼82%) than UP30 (∼36%) from diverse origins and geographical locations, including the United Kingdom. It is essential to ascertain the diversity of strains a phage can kill to determine how it might be therapeutically applied.16,17,28,41 Also, the host range properties of phages can help establish how their activity could be enhanced, either through cocktail optimizations or genetic engineering to improve lysis efficacy or clinical coverage.16,28,32
Our observed variability in the genome sizes with UP19 having a relatively larger genome size compared with UP30, concurs with reports on other E. coli phages.46 The fact that UP30 has no tRNA genes but UP19 encodes 3 tRNA genes has been observed in E. coli phages with comparable genome sizes.46,47 tRNA genes are retained in phages during infection if they correspond to the codons found in the phages rather than the host and have been shown to sustain phage translation, growth, or host range.48,49 This could potentially support the presence of three tRNA genes in UP19, which infects more hosts from more sources than UP30. However, more studies are required to further explore this in our phages.
Interestingly, the high intergenomic similarities (>70%) among the two observed genera within the Caudoviricetes have been reported in many E. coli phages that infect closely related Enterobacteriaceae, E. coli, and Shigella.46 Our analyses showed that the majority of the genes from the two phages do not have identifiable functions, this may be attributed to the fact that the phages are novel and, therefore, more studies will be required to determine the functions of these genes. Furthermore, from our analyses none of the phages encode any toxigenic or lysogeny genes. Thus, they have a potential to be developed for therapeutic applications in humans and agriculture.50
Conclusion
In this study, two morphologically and genetically distinct novel species of lytic E. coli phages were isolated from sewage obtained from Kampala city municipal treatment facilities during the inaugural PGH workshop in 2017. The phages have therapeutic potential, lysing human clinical isolates from East Africa and the United Kingdom. Further work will be required to ascertain their potential clinical utility in relevant therapeutic settings. To our knowledge, these data are the first account of phages of clinical importance that were isolated from Uganda. These findings may bolster phage research in Africa and other developing countries, thereby contributing to global infection control and mitigating the impending AMR crisis.
Supplementary Material
Acknowledgments
This work is published in memory and honor of Prof. Rudovick Kazwala. We thank Natalie Allcock and Anna Straatman-Iwanowska (Electron Microscopy Laboratory, Core Biotechnology Services, College of Medicine, Biological Sciences and Psychology, University of Leicester, Leicester, United Kingdom) for their technical assistance with the electron microscopy work.
Authors' Contributions
J.Y.N., B.J., T.E.N. and M.R.J.C. conceived and designed the experiments. J.Y.N., A.S.H., S.P.W., I.I., A.N.M., A.A.A., V.T.M., N.M., G.W.M., I.A., A.N., P.M., D.K., R.N., I.N., R.R.K., E.K., A.A.N., A.K.W., N.B., G.M., N.L.M., N.J., A.N., G.N., J.N., T.E.N., and M.R.J.C. collected the samples and conducted the initial phage isolation procedures. J.Y.N., J.K.J.C., S.M., L.C., H.B., and R.A.E.N. purified the phages, conducted the host range work and isolated the phage DNA for sequencing. N.N.-A. and C.T. carried out the TEM analyses. J.Y.N., N.E.N., and A.D.M. analyzed the host range data and phage genomes. J.Y.N. drafted the article. All authors edited and agreed to be accountable for all aspects of the article and approved the final version to be published.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by Biotechnology and Biological Sciences Research Council (BBSRC, grant number RM38G0140) awarded to Prof. M.R.J.C., Leicester Microbial Sciences and Infectious Diseases (LeMID) pump priming grant (grant number M38DF66) awarded to Dr. J.Y.N. and donations to PGH.
Supplementary Material
References
- 1. Velazquez-Meza ME, Galarde-López M, Carrillo-Quiróz B, et al. Antimicrobial resistance: One Health approach. Vet World 2022;15(3):743–749; doi: 10.14202/vetworld.2022.743-749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog Glob Health 2015;109(7):309–318; doi: 10.1179/2047773215y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Neill J. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Philos Trans R Soc Lond B Biol Sci 2015;370(1670):20140082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Neill J. Tackling drug-resistant infections globally: Final report and recommendations. The review on antimicrobial resistance. 2016. Available from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf [Last accessed: March 14, 2023].
- 5. Kariuki S, Kering K, Wairimu C, et al. Antimicrobial resistance rates and surveillance in sub-Saharan Africa: Where are we now? Infect Drug Resist 2022;15:3589–3609; doi: 10.2147/idr.S342753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moyo P, Moyo E, Mangoya D, et al. Prevention of antimicrobial resistance in sub-Saharan Africa: What has worked? What still needs to be done? J Infect Public Health 2023;16(4):632–639; doi: 10.1016/j.jiph.2023.02.020 [DOI] [PubMed] [Google Scholar]
- 7. El-Shibiny A, El-Sahhar S. Bacteriophages: The possible solution to treat infections caused by pathogenic bacteria. Can J Microbiol 2017;63(11):865–879; doi: 10.1139/cjm-2017-0030 [DOI] [PubMed] [Google Scholar]
- 8. Principi N, Silvestri E, Esposito S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front Pharmacol 2019;10:513; doi: 10.3389/fphar.2019.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makumi A, Mhone AL, Odaba J, et al. Phages for Africa: The potential benefit and challenges of phage therapy for the livestock sector in sub-Saharan Africa. Antibiotics (Basel) 2021;10(9):1085; doi: 10.3390/antibiotics10091085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nagel TE, Chan BK, De Vos D, et al. The developing world urgently needs phages to combat pathogenic bacteria. Front Microbiol 2016;7:882; doi: 10.3389/fmicb.2016.00882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagel T, Musila L, Muthoni M, et al. Phage banks as potential tools to rapidly and cost-effectively manage antimicrobial resistance in the developing world. Curr Opin Virol 2022;53:101208; doi: 10.1016/j.coviro.2022.101208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagel TE, Mutai IJ, Josephs T, et al. A brief history of phage research and teaching in Africa. PHAGE 2022;3(4):184–193; doi: 10.1089/phage.2022.29037.inp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. PfGH. Phages for Global Health. Available from: https://www.phagesforglobalhealth.org [Last accessed: June 6, 2023].
- 14. Nagel TE, Chan BK, Nale JY, et al. Phages as antibacterial agents: Laboratory training in developing countries. In: AMR Control 2018 Overcoming Global Antimicrobial Resistance (Carlet J, Upham G. eds.). Tim Probart; 2018:89–93. Available from: http://resistancecontrol.info/wp-content/uploads/2018/05/89-93.pdf [Last accessed: September 13, 2023].
- 15. van Zyl LJ, Abrahams Y, Stander EA, et al. Novel phages of healthy skin metaviromes from South Africa. Sci Rep 2018;8(1):12265; doi: 10.1038/s41598-018-30705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nale JY, Spencer J, Hargreaves KR, et al. Bacteriophage combinations significantly reduce clostridium difficile growth in vitro and proliferation in vivo. Antimicrob Agents Chemother 2016;60(2):968–981; doi: 10.1128/aac.01774-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montso PK, Mlambo V, Ateba CN. Characterization of lytic bacteriophages infecting multidrug-resistant shiga toxigenic atypical Escherichia coli O177 strains isolated from Cattle Feces. Front Public Health 2019;7:355; doi: 10.3389/fpubh.2019.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen S, Zhou Y, Chen Y, et al. FASTP: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018;34(17):i884–i890; doi: 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bushnell B. BBMap: A Fast, Accurate, Splice-Aware Aligner. Berkeley, CA; 2014. Available from: https://www.osti.gov/biblio/1241166 [Last accessed: September 13, 2023].
- 20. Bankevich A, Nurk S, Antipov D, et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012;19(5):455–477; doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gurevich A, Saveliev V, Vyahhi N, et al. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013;29(8):1072–1075; doi: 10.1093/bioinformatics/btt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hockenberry AJ, Wilke CO. BACPHLIP: Predicting bacteriophage lifestyle from conserved protein domains. PeerJ 2021;9:e11396; doi: 10.7717/peerj.11396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terzian P, Olo Ndela E, Galiez C, et al. PHROG: Families of prokaryotic virus proteins clustered using remote homology. NAR Genom Bioinform 2021;3(3):lqab067; doi: 10.1093/nargab/lqab067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014;30(14):2068–2069; doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 25. Shen A, Millard A. Phage genome annotation: Where to begin and end. Phage (New Rochelle) 2021;2(4):183–193; doi: 10.1089/phage.2021.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moraru C, Varsani A, Kropinski AM. VIRIDIC-a novel tool to calculate the intergenomic similarities of prokaryote-infecting viruses. Viruses 2020;12(11):1268; doi: 10.3390/v12111268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ribeiro KVG, Ribeiro C, Dias RS, et al. Bacteriophage isolated from sewage eliminates and prevents the establishment of Escherichia coli Biofilm. Adv Pharm Bull 2018;8(1):85–95; doi: 10.15171/apb.2018.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ross A, Ward S, Hyman P. More is better: Selecting for broad host range bacteriophages. Front Microbiol 2016;7:1352; doi: 10.3389/fmicb.2016.01352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022. 2022. Available from: https://www.who.int/publications/i/item/9789240062702 [Last accessed: March 14, 2023].
- 30. Rogers Van Katwyk S, Hoffman SJ, Mendelson M, et al. Strengthening the science of addressing antimicrobial resistance: A framework for planning, conducting and disseminating antimicrobial resistance intervention research. Health Res Policy Systems 2020;18(1):60; doi: 10.1186/s12961-020-00549-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Doffkay Z, Dömötör D, Kovács T, et al. Bacteriophage therapy against plant, animal and human pathogens. Acta Biol Szegedien 2015;59:291–302. [Google Scholar]
- 32. Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019;25(5):730–733; doi: 10.1038/s41591-019-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kassa T. Bacteriophages against pathogenic bacteria and possibilities for future application in Africa. Infect Drug Resist 2021;14:17–31; doi: 10.2147/idr.S284331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kakabadze E, Makalatia K, Grdzelishvili N, et al. Selection of potential therapeutic bacteriophages that lyse a CTX-M-15 extended spectrum β-lactamase producing Salmonella enterica serovar typhi strain from the Democratic Republic of the Congo. Viruses 2018;10(4):172; doi: 10.3390/v10040172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhvania P, Hoyle NS, Nadareishvili L, et al. Phage therapy in a 16-year-old boy with netherton syndrome. Front Med 2017;4:94; doi: 10.3389/fmed.2017.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nale JY, Ahmed B, Haigh R, et al. Activity of a bacteriophage cocktail to control Salmonella growth ex vivo in Avian, Porcine, and human epithelial cell cultures. PHAGE 2023;4(1):11–25; doi: 10.1089/phage.2023.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koskella B, Brockhurst MA. Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol Rev 2014;38(5):916–931; doi: 10.1111/1574-6976.12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bull JJ, Levin BR, Molineux IJ. Promises and pitfalls of in vivo evolution to improve phage therapy. Viruses 2019;11(12):1083; doi: 10.3390/v11121083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bumunang EW, McAllister TA, Polo RO, et al. Genomic profiling of non-O157 shiga toxigenic Escherichia coli-infecting bacteriophages from South Africa. PHAGE 2022;3(4):221–230; doi: 10.1089/phage.2022.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Topka G, Bloch S, Nejman-Faleńczyk B, et al. Characterization of bacteriophage vB-EcoS-95, isolated from urban sewage and revealing extremely rapid lytic development. Front Microbiol 2019;9:3326; doi: 10.3389/fmicb.2018.03326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aghaee BL, Mirzaei MK, Alikhani MY, et al. Sewage and sewage-contaminated environments are the most prominent sources to isolate phages against Pseudomonas aeruginosa. BMC Microbiol 2021;21(1):132; doi: 10.1186/s12866-021-02197-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hyman P. Phages for phage therapy: Isolation, characterization, and host range breadth. Pharmaceuticals 2019;12(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Debartolomeis J, Cabelli VJ. Evaluation of an Escherichia coli host strain for enumeration of F male-specific bacteriophages. Appl Environ Microbiol 1991;57(5):1301–1305; doi: 10.1128/aem.57.5.1301-1305.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muniesa M, Blanch AR, Lucena F, et al. Bacteriophages may bias outcome of bacterial enrichment cultures. Appl Environ Microbiol 2005;71(8):4269–4275; doi: 10.1128/aem.71.8.4269-4275.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Twest R, Kropinski AM. Bacteriophage enrichment from water and soil. Methods Mol Biol 2009;501:15–21; doi: 10.1007/978-1-60327-164-6_2 [DOI] [PubMed] [Google Scholar]
- 46. Śliwka P, Weber-Dąbrowska B, Żaczek M, et al. Characterization and comparative genomic analysis of three virulent E. coli bacteriophages with the potential to reduce antibiotic-resistant bacteria in the environment. Int J Mol Sci 2023;24(6):5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li S, Lu S, Huang H, et al. Comparative analysis and characterization of Enterobacteria phage SSL-2009a and ‘HK578likevirus' bacteriophages. Virus Res 2019;259:77–84; doi: 10.1016/j.virusres.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 48. Delesalle VA, Tanke NT, Vill AC, et al. Testing hypotheses for the presence of tRNA genes in mycobacteriophage genomes. Bacteriophage 2016;6(3):e1219441; doi: 10.1080/21597081.2016.1219441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang JY, Fang W, Miranda-Sanchez F, et al. Degradation of host translational machinery drives tRNA acquisition in viruses. Cell Systems 2021;12(8):771–779.e5; doi: 10.1016/j.cels.2021.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin DM, Koskella B, Lin HC. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther 2017;8(3):162–173; doi: 10.4292/wjgpt.v8.i3.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.