Abstract
This randomized controlled trial is aimed at assessing the efficacy of combining time-restricted eating (TRE) with behavioral economic (BE) interventions and comparing it to TRE alone and to the usual care for reducing fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), and other cardiometabolic risk factors among patients with impaired fasting glucose (IFG). Seventy-two IFG patients aged 18–65 years were randomly allocated for TRE with BE interventions (26 patients), TRE alone (24 patients), or usual care (22 patients). Mean FPG, HbA1c, and other cardiometabolic risk factors among the three groups were compared using a mixed-effect linear regression analysis. Mean body weight, FPG, HbA1c, fasting insulin, and lipid profiles did not significantly differ among the three groups. When considering only patients who were able to comply with the TRE protocol, the TRE group showed significantly lower mean FPG, HbA1c, and fasting insulin levels compared to the usual care group. Our results did not show significant differences in body weight, blood sugar, fasting insulin, or lipid profiles between TRE plus BE interventions, TRE alone, and usual care groups. However, TRE might be an effective intervention in lowering blood sugar levels for IFG patients who were able to adhere to the TRE protocol.
Keywords: behavioral economic intervention, time-restricted eating, impaired fasting glucose, cardiometabolic risk, randomized controlled trial
1. Introduction
Diabetes mellitus (DM) is a major health problem in Thailand with a reported prevalence of 9.9% in 2014 [1]. Diabetes is a significant risk factor for cardiovascular diseases (CVDs), chronic kidney disease (CKD), peripheral arterial disease, and diabetic retinopathy which together account for 4% of mortality in the Thai population [2]. Impaired fasting glucose (IFG) is an intermediate stage between normal blood sugar and DM. Individuals with IFG have a 13 times higher risk of DM than individuals with normal blood glucose [3]. Therefore, intensive lifestyle modification by diet control and exercise is an important intervention to reduce the progression towards DM in this population [4]. Caloric restriction (CR) through low and very low-calorie diets is an effective dietary intervention that significantly reduces the risk of DM but the adherence and sustainability of this dietary intervention over the long term are questionable [5]. As a result, other methods of diet control, such as time-restricted eating (TRE), have emerged as potential alternatives.
TRE is one type of dietary approach that limits the daily eating window, commonly to less than 10 h per day, prolonging the fasting time [6,7,8]. Evidence from animal studies found that increased fasting time could reduce free radical production, inhibit inflammation, and increase stress resistance, leading to improved metabolic health and glucose regulation [9,10,11]. To date, there have been limited randomized controlled trials (RCTs) investigating the impact of TRE in individuals with a high risk of DM, such as IFG. One crossover RCT examined the effects of early TRE (where food intake is restricted to the early period of the day) or delayed TRE (where food intake is restricted to the later period of the day) on 15 men who were at risk of type 2 diabetes mellitus. The study revealed that both early and delayed TRE improved the glycemic response, but only the early TRE was able to lower average fasting plasma glucose (FPG) levels [12]. Another RCT explored the effects of early TRE in eight IFG men and found that it could reduce insulin levels, blood pressure, and food appetite and increase insulin sensitivity and beta-cell responsiveness but could not reduce FPG [13]. Consequently, further RCTs are required to investigate the long-term effects of TRE in both male and female patients at risk of type 2 diabetes.
Despite widespread adoption and given the lack of restrictions on food groups, specific macronutrients, or constant calorie monitoring [14,15], there remains uncertainty regarding the long-term adherence to TRE in real-life scenarios. Previously, various behavioral economic interventions have been employed to enhance adherence to dietary control, including the use of financial incentives [16,17] and text reminders [18]. Behavioral economics integrates principles and methods from psychology and economics to better understand human decision-making [19,20] and motivation. Previous research has demonstrated the effectiveness of financial incentives in promoting a healthy lifestyle, such as smoking cessation, physical activity [21], and weight loss [22]. Text reminders that reinforce an individual’s commitment, performance, or goals can serve as immediate prompts to prioritize and maintain adherence to treatment [23]. Consequently, employing behavioral economic (BE) techniques, namely financial incentives and text reminders, could potentially enhance compliance with lifestyle modifications like TRE and even facilitate the long-term maintenance of behavioral changes.
Nevertheless, no studies have assessed the efficacy of combined TRE with BE interventions in patients with IFG. Therefore, this RCT was conducted to compare the efficacy of additional BE combined with TRE against TRE alone and the usual care in patients with IFG. Levels of FPG, HbA1c, and other cardiometabolic risk factors, including body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting insulin, serum triglyceride levels (TG), total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and high sensitivity C-reactive protein (hs-CRP), were compared among the TRE plus BE intervention, TRE alone, and usual care groups.
2. Materials and Methods
2.1. Study Design
This parallel RCT was conducted at the outpatient clinic of the Department of Family Medicine, Faculty of Medicine, Ramathibodi Hospital, from October 2021 to February 2023. This research complies with the Consolidated Standards of Reporting Trials (CONSORT) statement. The trial protocol was registered at the Thai Clinical Trials Registry (TCTR20210520002) and was published elsewhere [24]. The protocol was approved by the Ethics Committee of Ramathibodi Hospital, Mahidol University (MURA 2021/389).
2.2. Participants
This study included patients and staff of Ramathibodi Hospital using the following eligibility criteria: adults aged 18 to 65 years, diagnosed with IFG (i.e., FPG of 100–125 mg/dL and HbA1c less than 6.5%) [25], having BMI ≥ 25 kg/m2, and willing to provide informed consent. Patients were excluded if they met any of the following criteria: (1) followed a ketogenic or vegetarian diet, (2) worked night shift for a minimum of 3 h between 10:00 PM and 5:00 AM on more than one day per week, (3) experienced body weight changes exceeding 5 kg in the three months prior to study enrolment, (4) were in receipt of medication to be consumed with food either before 8:00 AM or after 5:00 PM, (5) were pregnant or breastfeeding, (6) had psychiatric disorders, such as eating or mood disorders (except depression), (7) were taking corticosteroid or anti-diabetic medications, (8) had a history of bariatric surgery, or (9) had impaired nutrient absorption.
2.3. Randomization
Patients were randomly assigned to (1) TRE plus BE, (2) TRE alone, or (3) usual care in a ratio of 1:1:1. A random sequence was generated using stratified-block randomization with varying block sizes (6 and 9) and age stratification (i.e., 18–59 years and 60–65 years).
A random sequence list was concealed in sequential opaque sealed envelopes.
2.4. Study Interventions
This study focused on two specific interventions: TRE and BE-informed interventions. In the TRE groups, patients were instructed to restrict their daily food intake to a 9 h window (between 8:00 AM and 5:00 PM), without any limitation on the types of food and beverages consumed. This approach involved a fasting period of 15 h per day.
The BE interventions included financial incentives and text reminders aimed at increasing adherence to TRE. Participants were eligible to receive a monthly monetary payment of 1000 THB (approximately 85 USD) if they self-reported adherence to the TRE for at least 5 days a week over a 4-week period. In addition, text reminders were sent to participants every 2 days to reinforce their commitment to achieving the TRE goal of 5 days per week, track their personal performance, and remind them of the TRE interval. Participants in all three groups were also requested to maintain a logbook to record their first and last mealtime each day.
2.5. Outcomes
The primary outcomes were FPG and HbA1c levels measured at 12 weeks after randomization. Secondary outcomes included body weight, SBP, DBP, fasting insulin, TG, total cholesterol, LDL-C, HDL-C, and hs-CRP. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by multiplying the fasting insulin (mIU/L) with FPG (mg/dL) and dividing by 405 to measure insulin sensitivity in the participants. FPG was measured using hexokinase glucose-6 phosphate dehydrogenase. HbA1c levels were measured using the turbid metric inhibition immunoassay certified by the National Glycohemoglobin Standardization Program. Serum triglyceride was measured by lipase/glycerol kinase glycerol-3-phosphate oxidase. HDL-C and LDL-C levels were measured by the accelerator selective detergent method. Chemiluminescence and nephelometry methods were used for measuring fasting insulin and hs-CRP, respectively. Body weight was measured without shoes to the nearest 100 g using a Seca 284 Wireless Measuring Station—Weight & Height 2841300109. Blood pressure was measured by the Connex Spot Monitor; Welch Allyn, Inc. (Auburn, NY, USA) with an automatic blood pressure cuff after resting for at least 15 min. Body weight and blood pressure were measured by trained research assistants.
All primary and secondary outcomes were assessed at baseline and weeks 4, 8, and 12 after randomization. Possible adverse events of TRE, such as syncope, dizziness, and light headedness, were monitored and recorded throughout the study period.
2.6. Study Procedure and Data Collection
At the first visit, potential participants were screened for the eligibility criteria (e.g., FPG, HbA1c, age, and BMI) by investigators and research assistants.
One week after enrolment (2nd visit), demographic information (e.g., age, sex, educational level, and marital status), underlying diseases (e.g., hypertension, dyslipidemia, chronic kidney disease, fatty liver disease, and history of gestational diabetes mellitus), and health risk behaviors (i.e., smoking and alcohol consumption) were collected by an interview with trained research assistants. Blood pressure, body weight, and height were measured, and participants were randomly allocated to one of three groups: TRE plus BE, TRE alone, and usual care. Physical examination including blood pressure and body weight, and laboratory measurements (i.e., FPG, HbA1c, TG, total cholesterol, LDL-C, HDL-C, fasting insulin, and hs-CRP) were obtained during the 3rd visit (4th week), 4th visit (8th week), and 5th visit (12th week) after randomization or study end. Dietary intake at baseline and weeks 4, 8, and 12 post randomization were collected using 24 h food recall. Participants were asked to record their food diary for 7 days at each visit. INMUCAL-nutrients version 4.0 (developed by the Institute of Nutrition, Mahidol University, Thailand; https://inmu2.mahidol.ac.th/inmucal/index.php, accessed on 24 May 2023) was used for calculating nutrient intake from dietary data.
2.7. Statistical Analysis
A superiority trial approach utilizing a one-way analysis of variance method was applied for estimating the sample size. The baseline mean and standard deviation (SD) of FPG in the prediabetes cohort were 105 and 9 mg/dL, respectively [26]. We anticipated that receiving TRE plus BE, and TRE alone would lead to a reduction in the FPG of approximately 7% and 5%, respectively, compared to the control group. With type I error and power set to 0.05 and 0.8, respectively, a total of 90 participants, with 30 participants per group, were required to detect these differences. However, given that the recruitment was adversely affected since it fell within COVID-19 restrictions, the final sample size was limited to 72 participants.
Mean (SD) or median (interquartile range, IQR) and frequency and percentages were used to describe continuous data and categorical data, respectively. Means of primary and secondary outcomes among the three groups were compared using a mixed-effect linear regression model by regressing an outcome of intervention and time, considering patients as a random effect (i.e., repeated measure at 4, 8, and 12 weeks after randomization) and the intervention arms (TRE plus BE and TRE alone versus usual care) as a fixed effect. Marginal means and differences between any pair of the three interventions were estimated accordingly. If baseline characteristics differed between the 3 groups, these were adjusted using multivariate regression analysis.
All analyses were performed using three approaches. First, an intention-to-treat analysis (ITT) was applied by considering interventions that were initially assigned regardless of compliance. Second, a per-protocol analysis (PPA) excluded patients who did not comply with TRE (i.e., complied < 5 days per week) in the TRE plus BE and TRE alone and patients in the usual care group who took TRE 5 days or more per week from the analysis. Third, an “as-treated” analysis kept participants in either TRE plus BE or TRE alone if they complied with TRE ≥ 5 days/week; otherwise, they were considered in the usual care group. Likewise, participants in a usual care group were moved to the TRE group if they adhered to TRE ≥ 5 days/week. If a participant missed an appointment in week 4, 8 or 12, the previous outcome measure was carried forward to impute the missing outcome to minimize the lost to follow-up.
All analyses were performed using STATA 18.0. A p-value < 0.05 was considered statistically significant.
3. Results
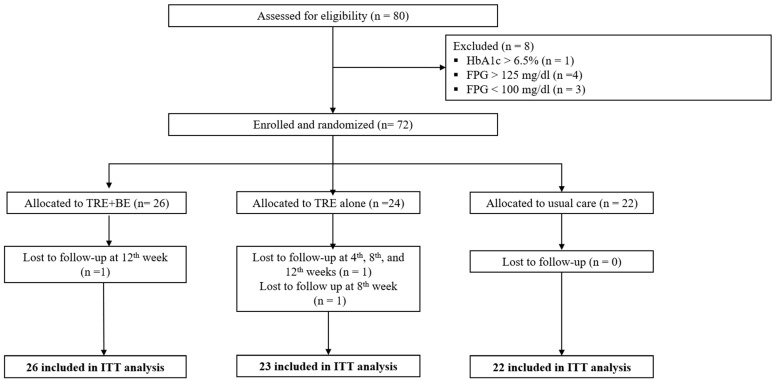
A total of 80 patients with IFG were recruited following eligibility screening from October 2021 to February 2023. Four, three, and one patients were excluded due to FPG > 125 mg/dL, FPG < 100 mg/dL, and HbA1c > 6.5%, respectively. Thus, 72 patients with IFG were randomly allocated to the TRE plus BE (n = 26), TRE alone (n = 24), and usual care (n = 22) groups. One participant in the TRE group was lost to follow-up in weeks 4, 8, and 12; thus, this patient was excluded from the analysis due to having no data available after randomization. One participant in the usual care group was lost to follow-up in week 12, while one patient in the TRE alone group was unable to attend the week 8 follow-up but was followed up again in week 12. Therefore, 71 participants were included in the ITT analysis with sample sizes of 26, 23, and 22 for TRE plus BE, TRE, and usual care, respectively (see Figure 1). Considering adherence to interventions, 12/26 (46%) in the TRE plus BE group and only 2/23 (9%) in the TRE alone, adhered to TRE ≥ 5 days/week, while 1/22 (5%) in the usual care group adhered to TRE ≥ 5 days/week. Therefore, the PPA could not be performed due to the low number of participants in the TRE alone group. For the as-treated analysis, 15 participants (12 from TRE plus BE, 2 from TRE alone, and 1 from the usual care group) were included in the TRE group, while 56 participants (14 from TRE plus BE, 21 from TRE alone, and 21 from usual care groups) were included in the usual care group.
Figure 1.
Participant flow diagram.
Baseline participant characteristics are presented in Table 1. The mean age was 54.6 years (8.1); the majority were female (69.4%) and self-reported as never smokers (86.1%) or alcohol consumers (56.9%). Most participants had dyslipidemia (90.3%) and approximately half had hypertension (55.6%), while none reported a history of depression, gestational diabetes, or coronary artery disease. Demographic characteristics were not significantly different between the three groups. Baseline dietary intakes and laboratory measures were also similar. However, the amount of carbohydrates (mean = 143.3 vs. 179.3 vs. 142.9 g/day), cholesterol intake per day (mean = 189.3 vs. 201.9 vs. 207.0 mg/d), serum triglycerides (mean = 119.5 vs. 134.5 vs. 131.5 mg/dL), and fasting insulin levels (mean = 8.8 vs. 8.6 vs. 11.1 mIU/L) were clinically different among the three groups, likely due to the small sample size. Therefore, these covariates were considered as confounding factors in the multivariate mixed linear regression analysis.
Table 1.
Baseline characteristics of participants.
| Characteristics | Total (N = 72) |
TRE Plus Behavioral Economics (N = 26) |
TRE Alone (N = 24) |
Usual Care (N = 22) |
p-Value |
|---|---|---|---|---|---|
| Age at enrollment, year, mean (SD) | 54.6 (8.1) | 53.2 (9.2) | 55.5 (7.2) | 55.2 (7.9) | 0.558 |
| Sex | |||||
|
50 (69.4) | 18 (69.2) | 16 (66.7) | 16 (72.7) | 0.905 |
|
22 (30.6) | 8 (30.8) | 8 (33.3) | 6 (27.3) | |
| Educational level | |||||
|
14 (19.7) | 6 (24.0) | 4 (16.7) | 4 (18.2) | 0.932 |
|
19 (26.8) | 6 (24.0) | 6 (25.0) | 7 (31.8) | |
|
38 (53.5) | 13 (52.0) | 14 (58.3) | 11 (50.0) | |
| Marital status | |||||
|
11 (15.3) | 6 (23.1) | 3 (12.5) | 2 (9.1) | 0.788 |
|
48 (66.7) | 15 (57.7) | 18 (75.0) | 15 (68.2) | |
|
10 (13.9) | 4 (15.4) | 2 (8.3) | 4 (18.2) | |
|
3 (4.2) | 1 (3.8) | 1 (4.2) | 1 (4.5) | |
| Reimbursement | |||||
|
6 (8.3) | 4 (15.4) | 2 (8.3) | 0 (0.0) | 0.524 |
|
21 (29.2) | 7 (26.9) | 8 (33.3) | 6 (27.3) | |
|
29 (40.3) | 11 (42.3) | 9 (37.5) | 9 (40.9) | |
|
16 (22.2) | 4 (15.4) | 5 (20.8) | 7 (31.8) | |
| Smoking status | |||||
|
1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (4.5) | 0.580 |
|
9 (12.5) | 3 (11.5) | 4 (16.7) | 2 (9.1) | |
|
62 (86.1) | 23 (88.5) | 20 (83.3) | 19 (86.4) | |
| Alcohol consumption | |||||
|
15 (20.8) | 6 (23.1) | 6 (25.0) | 3 (13.6) | 0.831 |
|
16 (22.2) | 6 (23.1) | 4 (16.7) | 6 (27.3) | |
|
41 (56.9) | 14 (53.8) | 14 (58.3) | 13 (59.1) | |
| Family history of DM | |||||
|
38 (52.8) | 17 (65.4) | 12 (50.0) | 9 (40.9) | 0.226 |
|
34 (47.2) | 9 (34.6) | 12 (50.0) | 13 (59.1) | |
| Underlying diseases | |||||
| Hypertension | |||||
|
40 (55.6) | 12 (46.2) | 14 (58.3) | 14 (63.6) | 0.452 |
|
32 (44.4) | 14 (53.8) | 10 (41.7) | 8 (36.4) | |
| Dyslipidemia | |||||
|
65 (90.3) | 23 (88.5) | 23 (95.8) | 19 (86.4) | 0.515 |
|
7 (9.7) | 3 (11.5) | 1 (4.2) | 3 (13.6) | |
| Depression | |||||
|
0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
|
72 (100.0) | 26 (100.0) | 24 (100.0) | 22 (100.0) | |
| CKD | |||||
|
2 (2.8) | 1 (3.8) | 0 (0.0) | 1 (4.5) | 0.592 |
|
70 (97.2) | 25 (96.2) | 24 (100.0) | 21 (95.5) | |
| CAD | |||||
|
0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
|
72 (100.0) | 26 (100.0) | 24 (100.0) | 22 (100.0) | |
| CVA | |||||
|
2 (2.8) | 0 (0.0) | 2 (8.3) | 0 (0.0) | 0.128 |
|
70 (97.2) | 26 (100.0) | 22 (91.7) | 22 (100.0) | |
| NAFLD | |||||
|
8 (11.1) | 2 (7.7) | 2 (8.3) | 4 (18.2) | 0.447 |
|
64 (88.9) | 24 (92.3) | 22 (91.7) | 18 (81.8) | |
| History of GDM | |||||
|
0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
|
72 (100.0) | 26 (100.0) | 24 (100.0) | 22 (100.0) | |
| Cancer | |||||
|
1 (1.4) | 1 (3.8) | 0 (0.0) | 0 (0.0) | 0.408 |
|
71 (98.6) | 25 (96.2) | 24 (100.0) | 22 (100.0) | |
| Physical examination | |||||
|
76.6 (12.9) | 77.3 (15.8) | 75.4 (9.6) | 77.1 (12.7) | 0.856 |
|
29.9 (3.8) | 30.3 (4.9) | 29.2 (2.9) | 30.3 (3.2) | 0.536 |
|
135.1 (15.3) | 134.6 (14.4) | 134.0 (15.3) | 137.0 (16.9) | 0.789 |
|
79.9 (7.7) | 80.0 (7.4) | 79.2 (9.0) | 80.5 (6.9) | 0.835 |
|
95.7 (9.3) | 95.8 (10.4) | 94.8 (7.8) | 96.4 (9.7) | 0.837 |
|
104.4 (7.5) | 104.7 (8.6) | 103.8 (6.5) | 104.9 (7.4) | 0.864 |
|
37.0 (3.3) | 37.3 (3.4) | 37.5 (3.1) | 36.3 (3.3) | 0.427 |
| Dietary intakes | |||||
|
1141.0 (522.1) | 1121.5 (547.9) | 1210.5 (614.1) | 1095.4 (382.5) | 0.746 |
|
154.5 (85.4) | 143.3 (58.0) | 179.3 (129.1) | 142.9 (47.1) | 0.259 |
|
47.4 (25.1) | 47.2 (30) | 46 (20.3) | 49.3 (23.8) | 0.911 |
|
32.9 (19.2–46.4) | 34.7 (18.3–55.1) | 28.0 (21.1–43.1) | 35.0 (19.0–50.0) | 0.744 |
|
205.1 (105.0–335.4) | 158.1 (73.2–406.7) | 205.1 (134.7–283.1) | 248.2 (182.1–340.8) | 0.607 |
|
31.9 (17.1–51.1) | 32.3 (18.5–48.0) | 35.0 (16.5–60.6) | 29.4 (17.3–44.5) | 0.338 |
|
6.7 (3.3–12.2) | 6.7 (3.0–14.5) | 7.0 (3.1–12.2) | 6.8 (3.7–11.8) | 0.419 |
| Laboratory results | |||||
|
107.7 (6.0) | 107.2 (6.3) | 106.8 (5.6) | 109.2 (5.9) | 0.350 |
|
5.9 (0.4) | 5.8 (0.4) | 5.8 (0.3) | 5.9 (0.4) | 0.433 |
|
128.5 (50.0, 430.0) | 119.5 (88, 139) | 134.5 (50.0, 430.0) | 131.5 (66.0, 311.0) | 0.057 |
|
199.1 (41.6) | 189.3 (34.2) | 201.9 (48.2) | 207.0 (41.0) | 0.319 |
|
50.8 (10.0) | 51.9 (10.6) | 49.7 (9.0) | 50.6 (10.6) | 0.738 |
|
131.8 (40.8) | 126.7 (34.0) | 131.6 (54.1) | 138.2 (31.2) | 0.628 |
|
1.8 (0.1, 10.3) | 1.7 (0.96, 3.21) | 1.3 (0.1, 6.1) | 3.2 (0.8, 9.1) | 0.051 |
|
8.7 (3.1, 38.2) | 8.8 (3.1, 38.2) | 8.6 (4.0, 21.7) | 11.1 (3.4, 19.7) | 0.595 |
|
2.29 (1.85, 3.43) | 2.24 (1.83, 3.43) | 2.27 (1.50, 2.93) | 2.95 (1.96, 3.68) | 0.540 |
BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; CVA, cerebrovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus; HC, hip circumference; Hs-CRP, high-sensitivity C-reactive protein; HDL-C, high density lipoprotein cholesterol; HOMA-IR, Homeostatic model assessment of insulin resistance; IQR, interquartile range; LDL-C, low density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease; NC, neck circumference; SBP, systolic blood pressure; TRE, time-restricted eating; WC, waist circumference.
3.1. FPG and HbA1c Levels
3.1.1. Intention-to-Treat Analysis
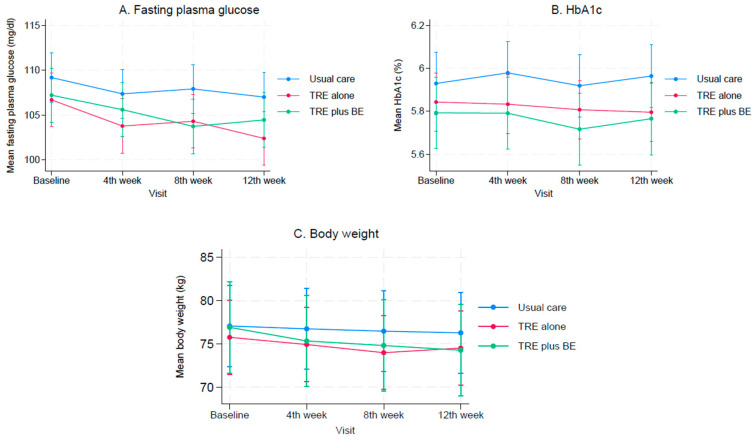
When compared to baseline levels, FPG decreased over time for all three groups (see Figure 2A); however, a significant decrease in FPG was only found in the TRE alone group (p-value = 0.001). TRE plus BE and TRE alone lowered the FPG levels by −1.74 mg/dL (95% CI: −5.60, 2.12) and −3.03 mg/dL (95% CI: −7.00, 0.93), respectively, when compared to the usual care group (see Table 2), although these did not reach statistical significance. Additionally, the effect of lowering the FPG level was not significantly different between TRE plus BE and TRE groups with a mean difference (95% CI) of 1.29 (−2.46, 5.04) mg/dL; see Table 2.
Figure 2.
Mean changes in fasting plasma glucose, HbA1c, and body weight in time-restricted eating plus behavioral economic intervention, time-restricted alone, and usual care groups: (A) Fasting plasma glucose; (B) HbA1c; (C) Body weight.
Table 2.
The mean difference in outcomes among time-restricted eating plus behavioral economic interventions, time-restricted eating alone, and usual care groups: An intention-to-treat analysis.
| Outcomes. | Treatment Comparison | Mean Difference (95% CI) | p-Value |
|---|---|---|---|
| FPG, mg/dL | TRE vs. Usual care | −3.03 (−7.00, 0.93) | 0.134 |
| TRE + BE vs. Usual care | −1.74 (−5.60, 2.12) | 0.376 | |
| TRE + BE vs. TRE | 1.29 (−2.46, 5.04) | 0.500 | |
| HbA1c, mg% | TRE vs. Usual care | −0.15 (−0.36, 0.07) | 0.177 |
| TRE + BE vs. Usual care | −0.17 (−0.38, 0.04) | 0.113 | |
| TRE + BE vs. TRE | −0.02 (−0.21, 0.17) | 0.821 | |
| DBP, mmHg | TRE vs. Usual care | 0.37 (−4.04, 4.78) | 0.871 |
| TRE + BE vs. Usual care | −3.42 (−7.70, 0.87) | 0.118 | |
| TRE + BE vs. TRE | −3.78 (−8.03, 0.47) | 0.081 | |
| SBP, mmHg | TRE vs. Usual care | 0.69 (−7.26, 8.63) | 0.865 |
| TRE + BE vs. Usual care | −9.67 (−17.40, −1.95) | 0.014 | |
| TRE + BE vs. TRE | −10.36 (−17.96, −2.76) | 0.008 | |
| Body weight, kg | TRE vs. Usual care | −2.67 (−8.65, 3.30) | 0.381 |
| TRE + BE vs. Usual care | 0.13 (−5.80, 6.07) | 0.965 | |
| TRE + BE vs. TRE | 2.81 (−0.23, 5.85) | 0.070 | |
| hs-CRP, mg/dL | TRE vs. Usual care | −1.68 (−3.20, −0.14) | 0.032 |
| TRE + BE vs. Usual care | −0.43 (−1.91, 1.05) | 0.569 | |
| TRE + BE vs. TRE | 1.25 (−0.25, 2.74) | 0.103 | |
| Fasting insulin, mIU/L | TRE vs. Usual care | −1.57 (−3.37, 0.23) | 0.087 |
| TRE + BE vs. Usual care | −1.59 (−3.34, 0.16) | 0.076 | |
| TRE + BE vs. TRE | −0.02 (−1.74, 1.70) | 0.985 | |
| HOMA-IR | TRE vs. Usual care | −0.47 (−1.00, 0.05) | 0.079 |
| TRE + BE vs. Usual care | −0.40 (−0.91, 0.11) | 0.127 | |
| TRE + BE vs. TRE | 0.07 (−0.43, 0.58) | 0.773 | |
| TG, mg/dL | TRE vs. Usual care | −6.32 (−25.57, 12.93) | 0.520 |
| TRE + BE vs. Usual care | −9.35 (−28.03, 9.34) | 0.327 | |
| TRE + BE vs. TRE | −3.03 (−21.71, 15.66) | 0.751 | |
| Chol, mg/dL | TRE vs. Usual care | −17.10 (−35.97, 1.78) | 0.076 |
| TRE + BE vs. Usual care | −7.70 (−26.08, 10.69) | 0.412 | |
| TRE + BE vs. TRE | 9.40 (−8.23, 27.02) | 0.296 | |
| LDL-C, mg/dL | TRE vs. Usual care | −12.58 (−29.87, 4.70) | 0.154 |
| TRE + BE vs. Usual care | −3.68 (−20.53, 13.17) | 0.668 | |
| TRE + BE vs. TRE | 8.90 (−7.00, 24.79) | 0.272 | |
| HDL-C, mg/dL | TRE vs. Usual care | −1.55 (−7.01, 3.91) | 0.578 |
| TRE + BE vs. Usual care | −3.82 (−9.16, 1.52) | 0.161 | |
| TRE + BE vs. TRE | −2.27 (−7.00, 2.45) | 0.347 | |
| Total energy intake, kcal/day | TRE vs. Usual care | −72.53 (−241.50, 96.44) | 0.400 |
| TRE + BE vs. Usual care | −75.47 (−239.0, 88.05) | 0.366 | |
| TRE + BE vs. TRE | −2.94 (−166.41, 160.53) | 0.972 | |
| Carbohydrate, g/day | TRE vs. Usual care | −8.36 (−33.45, 16.73) | 0.514 |
| TRE + BE vs. Usual care | −9.09 (−33.36, 15.18) | 0.463 | |
| TRE + BE vs. TRE | −0.73 (−25.03, 23.57) | 0.953 | |
| Protein, g/day | TRE vs. Usual care | −1.74 (−10.51, 7.02) | 0.696 |
| TRE + BE vs. Usual care | −0.67 (−9.15, 7.81) | 0.877 | |
| TRE + BE vs. TRE | 1.07 (−7.41, 9.55) | 0.804 | |
| Total fat, g/day | TRE vs. Usual care | −3.51 (−11.77, 4.74) | 0.404 |
| TRE + BE vs. Usual care | −4.09 (−12.08, 3.90) | 0.316 | |
| TRE + BE vs. TRE | −0.58 (−8.56, 7.41) | 0.888 | |
| Cholesterol, mg/day | TRE vs. Usual care | −34.26 (−95.79, 27.26) | 0.275 |
| TRE + BE vs. Usual care | −12.73 (−72.27, 46.80) | 0.675 | |
| TRE + BE vs. TRE | 21.53 (−38.01, 81.08) | 0.478 | |
| Saturated fat, g/day | TRE vs. Usual care | −2.19 (−5.00, 0.62) | 0.126 |
| TRE + BE vs. Usual care | −2.80 (−5.51, −0.09) | 0.043 | |
| TRE + BE vs. TRE | −0.61 (−3.33, 2.12) | 0.663 | |
| Simple sugar, g/day | TRE vs. Usual care | 2.24 (−11.43, 15.90) | 0.748 |
| TRE + BE vs. Usual care | −4.23 (−17.45, 8.98) | 0.530 | |
| TRE + BE vs. TRE | −6.47 (−19.72, 6.78) | 0.339 |
CI, confidence interval; DBP, diastolic blood pressure; FPG, fasting plasma glucose; hs-CRP, high-sensitivity C-reactive protein; HDL-C, high density lipoprotein cholesterol; HOMA-IR, Homeostatic model assessment of insulin resistance; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure; TRE, time-restricted eating.
The HbA1c levels after receiving interventions did not decrease significantly when compared to baseline levels in all three groups (Figure 2B). Mean differences (95% CI) in TRE plus BE vs. usual care and TRE alone vs. usual care were −0.17% (−0.38, 0.04) and −0.15% (−0.36, 0.07), respectively (see Table 2). This suggested that TRE plus BE and TRE alone did not provide any additional benefit in reducing HbA1c levels. Additionally, the HbA1c levels after receiving the interventions were not significantly different between the TRE plus BE and TRE alone groups with a mean difference of −0.02% (−0.21, 0.17).
3.1.2. As-Treated Analysis
TRE significantly decreased FPG and HbA1c levels, when compared to usual care with mean differences of −4.74 mg/dL (−8.58, −0.90) for FPG and −0.24% (−0.457, −0.03) for HbA1c level (Table 3).
Table 3.
The mean difference in outcomes between time-restricted eating and usual care groups (as-treated analysis).
| Outcomes | Mean Difference (95% CI) | p-Value |
|---|---|---|
| FPG, mg/dL | −4.74 (−8.58, −0.90) | 0.015 |
| HbA1C, mg% | −0.24 (−0.457, −0.03) | 0.026 |
| Body weight, kg | −1.01 (−7.61, 5.59) | 0.765 |
| SBP, mmHg | −7.21 (−15.25, 0.83) | 0.079 |
| DBP, mmHg | −5.28 (−9.57, −0.99) | 0.016 |
| Triglyceride, mg/dL | −18.54 (−37.03, −0.05) | 0.049 |
| Total cholesterol, mg/dL | 6.30 (−13.01, 25.61) | 0.523 |
| LDL-C, mg/dL | 7.77 (−9.87, 25.41) | 0.388 |
| HDL-C, mg/dL | 0.16 (−5.40, 5.72) | 0.954 |
| Fasting insulin, mIU/L | −1.94 (−3.71, −0.18) | 0.031 |
| HOMA-IR | −0.61 (−1.12, −0.10) | 0.020 |
| hs-CRP | −0.92 (−2.45, 0.60) | 0.236 |
| Total energy intake, kcal/day | −182.19 (−341.46, −22.92) | 0.025 |
| Carbohydrate, g/day | −19.86 (−43.81, 4.09) | 0.104 |
| Protein, g/day | −6.81 (−15.16, 1.53) | 0.110 |
| Total fat, g/day | −8.38 (−16.22, -0.55) | 0.036 |
| Cholesterol, mg/day | −38.44 (−98.05, 21.17) | 0.206 |
| Saturated fat, g/day | −3.66 (−6.31, −1.00) | 0.007 |
| Simple sugar, g/day | −15.01 (−27.88, −2.13) | 0.002 |
DBP, diastolic blood pressure; FPG, fasting plasma glucose; hs-CRP, high-sensitivity C-reactive protein; HDL-C, high density lipoprotein cholesterol; HOMA-IR, Homeostatic model assessment of insulin resistance; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure.
3.2. Body Weight, Nutrition Intake, Systolic and Diastolic Blood Pressure
3.2.1. Intention-to-Treat Analysis
Body weight significantly decreased over time when compared to the baseline levels for all three groups (see Figure 2C). Among the intervention groups, both TRE plus BE and TRE did not additionally lower body weight when compared to the usual care, with mean differences of 0.13 kg (−5.80, 6.07) and −2.67 kg (−8.65, 3.30); see Table 2. Mean body weight was also not significantly different between TRE plus BE and TRE alone groups, with a mean difference of 2.81 kg (−0.23, 5.85). In addition, total energy, carbohydrate, protein, fat, cholesterol, and simple sugar intakes were not significantly different among the three groups (Table 2). However, the amount of saturated fat intake was significantly lower in the TRE plus BE group, compared to the usual care group.
TRE plus BE significantly decreased mean SBP by approximately −9.67 mmHg (−17.40, −1.95) and −10.36 mmHg (−17.96, −2.76), when compared to the usual care and TRE alone groups, respectively. However, these effects were not observed in DBP with the mean difference in these corresponding groups of −3.42 mmHg (−7.70, 0.87) and −3.78 mmHg (−8.03, 0.47) vs. usual care. Mean SBP and DBP levels in the TRE alone group were not significantly different from those in the usual care group (Table 2).
3.2.2. As-Treated Analysis
Similar to ITT, the TRE did not decrease body weight when compared to usual care. However, total energy, total fat, saturated fat, and simple sugar intakes were significantly lower in TRE than in the usual care groups (Table 3). DBP was significantly lower in the TRE group than in the usual care group (mean difference = −5.28 (−9.57, −0.99) mmHg) but not SBP (mean difference = −7.21 (−15.25, 0.83) mmHg); see Table 3.
3.3. Lipid Profiles
3.3.1. Intention-to-Treat Analysis
TRE plus BE and TRE alone lowered serum TG, total cholesterol, and LDL-C levels when compared to the usual care group, but these effects did not reach statistical significance (Table 2). When comparing TRE plus BE and TRE alone, serum TG, total cholesterol, and LDL-C levels were not significantly different.
Regarding HDL-C, both TRE plus BE and TRE alone did not significantly improve HDL-C when compared to usual care. Moreover, the mean HDL-C was not significantly different between the TRE plus BE and TRE groups (Table 2).
3.3.2. As-Treated Analysis
Results from the as-treated analysis were similar to the results from ITT analysis: Total cholesterol, LDL-C, and HDL-C levels were not significantly different among TRE and usual care groups (Table 3). However, serum TG in the TRE group was significantly lower than serum TG in the usual care group (mean difference = −18.54 mg/dL; 95% CI: −37.03, −0.05).
3.4. Fasting Insulin and HOMA-IR
3.4.1. Intention-to-Treat Analysis
Mean differences in fasting insulin and HOMA-IR between TRE plus BE vs. usual care and TRE alone vs. usual care were −1.59 (−3.34, 0.16) and −1.57 (−3.37, 0.23) for fasting insulin and −0.40 (−0.91, 0.11) and −0.47 (−1.00, 0.05) for HOMA-IR, respectively. These suggested that both TRE plus BE and TRE alone did not additionally decrease fasting insulin and insulin resistance when compared to usual care. There was also no significant difference in fasting insulin and HOMA-IR levels between TRE plus BE and TRE alone groups (Table 2).
3.4.2. As-Treated Analysis
Fasting insulin and HOMA-IR levels were significantly lower in the TRE group than in the usual care group, with mean differences of −1.94 (95% CI: −3.71, −0.18) and −0.61 (−1.12, −0.10), respectively.
3.5. Hs-CRP
3.5.1. Intention-to-Treat Analysis
There was a significant reduction in hs-CRP in the TRE group, when compared to the usual care group with a mean difference of −1.68 (−3.20, −0.14), while there was no significant difference in hs-CRP levels between TRE plus BE and usual care groups with a mean difference = −0.43 (−1.91, 1.05). In addition, Hs-CRP levels did not differ significantly between TRE plus BE and TRE alone groups (Table 2).
3.5.2. As-Treated Analysis
Contrary to ITT, the hs-CRP level was not significantly different between TRE and usual care groups, with a mean difference of −0.92 (95% CI: −2.45, 0.60).
3.6. Adverse Events
None of the patients in TRE plus BE, TRE alone, and usual care groups reported adverse events.
4. Discussion
Our study investigated the effects of TRE with and without BE on patients with IFG. The results showed that neither TRE plus BE nor TRE alone had a significant impact on FPG, HbA1c, body weight, DBP, lipid profiles, and fasting insulin levels when compared to usual care. However, when comparing TRE plus BE to usual care, there was a significant reduction in SBP. Additionally, when comparing TRE alone to usual care, a significant decrease in hs-CRP levels was also observed. When considering the “as-treated” analysis, complying with the TRE protocol brought significant improvements in FPG, HbA1c, serum TG, fasting insulin, HOMA-IR, and DBP levels when compared to usual care alone.
4.1. Effect of TRE on Blood Sugar Level and Cardiometabolic Risk Factors
The effectiveness of TRE in improving blood glucose levels, weight reduction, and lipid profiles in patients with IFG has been investigated previously. A recent RCT conducted on IFG patients found that TRE with a 16 h fasting period for three weeks led to a significant decrease in FPG levels at the three-month follow-up [27]. However, the findings from multiple studies in patients with obesity/overweight [28,29,30], non-alcoholic fatty liver disease [31], or prediabetes [13], did not show significant benefits of TRE in lowering FPG levels when compared to the designated control group. Our results are consistent with these findings, as both TRE plus BE and TRE alone did not significantly lower FPG and HbA1c levels when compared to usual care. The lack of significant results might be attributed to low adherence to the TRE regimen among our participants, as well as our low sample size.
Our hypothesis was confirmed when analyzing the data considering adherence (as-treated analysis); TRE showed significant improvements in FPG, HbA1c, fasting insulin, and HOMA-IR levels compared to usual care. Similar results were observed previously in a crossover RCT where prediabetes patients who adhered to TRE for five weeks experienced a significant reduction in fasting insulin levels [12]. Findings from both our study and that in prediabetes suggest that adhering to TRE may enhance insulin sensitivity and β-cell responsiveness in prediabetes patients, potentially reducing the risk of progression to DM for these individuals.
Body weight changes in our study were not significantly different among the three groups. However, by this study’s end, both the TRE plus BE and TRE alone groups showed a significant decrease in body weight compared to their baseline measurements. Our results support previous findings from individuals with prediabetes, which showed no significant difference in body weight reduction between the TRE and control groups. However, in both groups, there was a notable loss of body weight compared to their respective baseline measurements [12]. Similar positive outcomes related to body weight were observed in various RCTs involving patients with obesity or overweight conditions. These studies reported a significant decrease in body weight when participants followed a TRE approach compared to their baseline measurements [28,32,33]. The beneficial effect of TRE on weight loss could potentially be attributed to the unintentional reduction in energy intake during prolonged fasting periods [34]. Moreover, extending the fasting window increases the utilization of stored fat, leading to higher levels of ketone bodies, which in turn can suppress appetite and result in reduced energy intake [35].
Weight reduction can reduce future diabetic risk in individuals with prediabetes and obesity. Our study observed a significant reduction in body weight from the baseline in participants who adhered to the TRE protocol.
Consequently, it is essential to consider that the observed benefits of TRE in reducing FPG and HbA1c, as identified from the “as-treated” analysis, might be attributed to mechanisms other than weight loss. While weight reduction is well-accepted for its preventive role in type 2 diabetes onset and progression, it appears that TRE may exert its positive effects on FPG and HbA1c through different pathways, independent of weight reduction. Glucose metabolism is intricately regulated by the circadian clock. Aligning the eating period with the body’s natural circadian rhythms, such as consuming meals earlier in the day and fasting for the remaining time, can have a significant impact on metabolic processes. This eating pattern has been shown to restore cyclic AMP response element-binding protein phosphorylation, which, in turn, leads to a reduction in gluconeogenesis and contributes to improved glucose homeostasis [34].
Our study observed significantly lower SBP in the TRE plus BE group, but no significant change was found in the TRE alone group. An RCT conducted on men with prediabetes also reported significant decreases in both SBP and DBP compared to the control group [13]. However, findings from other RCTs involving diverse population groups showed inconsistent effects of TRE on blood pressure levels [33,36,37,38]. A systematic review analyzing the effects of TRE with different feeding windows suggested that the benefits of TRE might be more pronounced in early TRE, where food intake is restricted to the early period of the day [39], likely due to better alignment with circadian rhythms.
The potential benefits of TRE in suppressing inflammation, identified from animal studies [9,10,11], have yet to be fully recognized in humans. In our study, we observed a significant decrease in hs-CRP levels in the TRE alone group, but not in the TRE plus BE group. This contradicts previous findings [13,40], which reported that TRE did not significantly affect hs-CRP levels. However, the beneficial effects of TRE in improving hs-CRP levels in our study were not observed in the “as-treated” analysis. This raises the possibility that our observed results may have occurred by chance, emphasizing the need for further studies to confirm the potential impact of TRE on the inflammatory process.
4.2. Effect of Behavioral Economic Interventions on Adherence to TRE Protocol
Despite the proposed seemingly easier adoption of the TRE approach compared to other diet-control techniques, adherence to TRE remains challenging as observed by the low adherence rate in the TRE alone group (9%). Applying behavioral-economic informed interventions (i.e., financial incentives and text reminders in this study) could motivate some participants to higher adherence, as observed in the TRE plus BE group (46%) compared to TRE alone (9%). This may be due to the short-term financial incentives that complement future health benefits [17], while reminders make TRE salient to participants every two days [41]. However, we could not disentangle the effects of financial incentives and reminders from each other. A recent feasibility RCT comparing text reminders and text reminders plus financial incentives (in the form of a deposit contract) to increase adherence to TRE in patients with obesity and hypertension showed no difference between both groups [42], although this study was underpowered. Future studies focusing on the effect of each of these behavioral-economic informed interventions might be of interest for clinical and cost-effective intervention design.
4.3. Strengths and Limitations
Given the limited evidence of the efficacy of TRE in individuals with IFG, our study contributes valuable knowledge to this field. The findings from our RCT can inform the recommendation of appropriate dietary interventions to reduce future diabetic risk. Furthermore, our study assessed the efficacy of BE-informed interventions to promote intervention adherence. This evidence is valuable for encouraging adherence not only to dietary but other interventional approaches.
However, our study had several limitations. First, ongoing COVID-19 pandemic restrictions during the recruitment period resulted in a smaller sample size than anticipated which reduced the statistical power of our findings. Second, the participant adherence rate to the TRE protocol in our study was quite low. Consequently, the non-significant effectiveness of TRE in our study should be interpreted with caution given the poor intervention compliance. This is supported by the results from the “as-treated” analysis that point to potentially significant benefits of TRE for improving blood sugar and fasting insulin levels. Third, our interventions could not be blinded; hence, the findings may be subject to observer and information bias. However, the majority of our outcomes were objectively measured, and the assessors were blinded to allocation; thus, measurement error or ascertainment bias was minimized. In addition, our study did not measure oral glucose tolerant test. As a result, we were unable to determine the status of impaired glucose tolerance among the participants in the study. Lastly, only surrogate outcomes such as FPG, HbA1c, body weight, and other laboratory tests were assessed; thus, further RCTs that measure long-term or clinically important outcomes, such as DM incidence, are needed.
5. Conclusions
The current study demonstrated a non-significant benefit of TRE plus BE and TRE alone in improving body weight, blood sugar, fasting insulin, and lipid profiles, when compared to the usual standard of care, albeit subject to reduced statistical power. However, the benefits of TRE in reducing blood sugar, fasting insulin, and HOMA-IR levels were observed specifically among subjects who were able to adhere to the TRE protocol. This suggests that TRE may offer promise as a dietary intervention for high-risk individuals and thereby potentially decrease the associated risk of DM onset and progression. In addition, BE interventions might increase the adherence to TRE.
Author Contributions
U.S. and T.A. are the principal investigators. U.S., T.A., S.B., S.P., S.R. and A.T. designed the study. U.S., T.A., S.B. and S.P. collected the data. U.S., T.A., S.B., S.S. and A.T. analyzed the data. U.S., T.A., S.B., J.A., G.J.M., S.R. and A.T. interpreted the results. U.S., T.A. and S.B. drafted the manuscript. U.S., T.A., S.B., S.P., S.S., S.R., J.A., G.J.M. and A.T. critically appraised and revised the manuscript. The entire project was supervised by T.A., S.R. and A.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Faculty of Medicine, Ramathibodi Hospital (COA. MURA2021/389; date of approval 24 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the National Research Council of Thailand, grant number 186/2564.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Aekplakorn W., Chariyalertsak S., Kessomboon P., Assanangkornchai S., Taneepanichskul S., Putwatana P. Prevalence of Diabetes and Relationship with Socioeconomic Status in the Thai Population: National Health Examination Survey, 2004–2014. J. Diabetes Res. 2018;2018:1654530. doi: 10.1155/2018/1654530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porapakkham Y., Rao C., Pattaraarchachai J., Polprasert W., Vos T., Adair T., Lopez A.D. Estimated causes of death in Thailand, 2005: Implications for health policy. Popul. Health Metr. 2010;8:14. doi: 10.1186/1478-7954-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeboah J., Bertoni A.G., Herrington D.M., Post W.S., Burke G.L. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2011;58:140–146. doi: 10.1016/j.jacc.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toi P.L., Anothaisintawee T., Chaikledkaew U., Briones J.R., Reutrakul S., Thakkinstian A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients. 2020;12:2722. doi: 10.3390/nu12092722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das S.K., Gilhooly C.H., Golden J.K., Pittas A.G., Fuss P.J., Cheatham R.A., Tyler S., Tsay M., McCrory M.A., Lichtenstein A.H., et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: A 1-y randomized controlled trial. Am. J. Clin. Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 6.Varady K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011;12:e593–e601. doi: 10.1111/j.1467-789X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 7.Schuppelius B., Peters B., Ottawa A., Pivovarova-Ramich O. Time Restricted Eating: A Dietary Strategy to Prevent and Treat Metabolic Disturbances. Front. Endocrinol. 2021;12:683140. doi: 10.3389/fendo.2021.683140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manoogian E.N.C., Zadourian A., Lo H.C., Gutierrez N.R., Shoghi A., Rosander A., Pazargadi A., Wang X., Fleischer J.G., Golshan S., et al. Protocol for a randomised controlled trial on the feasibility and effects of 10-hour time-restricted eating on cardiometabolic disease risk among career firefighters doing 24-hour shift work: The Healthy Heroes Study. BMJ Open. 2021;11:e045537. doi: 10.1136/bmjopen-2020-045537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkacemi L., Selselet-Attou G., Hupkens E., Nguidjoe E., Louchami K., Sener A., Malaisse W.J. Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats. Int. J. Endocrinol. 2012;2012:962012. doi: 10.1155/2012/962012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung H., Chou W., Sears D.D., Patterson R.E., Webster N.J., Ellies L.G. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism. 2016;65:1743–1754. doi: 10.1016/j.metabol.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan M.J., Smith J.T., Narbaiza J., Mueez F., Bustle L.B., Qureshi S., Fieseler C., Legan S.J. Restricting feeding to the active phase in middle-aged mice attenuates adverse metabolic effects of a high-fat diet. Physiol. Behav. 2016;167:1–9. doi: 10.1016/j.physbeh.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Hutchison A.T., Regmi P., Manoogian E.N.C., Fleischer J.G., Wittert G.A., Panda S., Heilbronn L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity. 2019;27:724–732. doi: 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- 13.Sutton E.F., Beyl R., Early K.S., Cefalu W.T., Ravussin E., Peterson C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27:1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesztyüs D., Cermak P., Gulich M., Kesztyüs T. Adherence to Time-Restricted Feeding and Impact on Abdominal Obesity in Primary Care Patients: Results of a Pilot Study in a Pre-Post Design. Nutrients. 2019;11:2854. doi: 10.3390/nu11122854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.A., Sypniewski C., Bensadon B.A., McLaren C., Donahoo W.T., Sibille K.T., Anton S. Determinants of Adherence in Time-Restricted Feeding in Older Adults: Lessons from a Pilot Study. Nutrients. 2020;12:874. doi: 10.3390/nu12030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlaev I., King D., Darzi A., Dolan P. Changing health behaviors using financial incentives: A review from behavioral economics. BMC Public Health. 2019;19:1059. doi: 10.1186/s12889-019-7407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boonmanunt S., Pattanaprateep O., Ongphiphadhanakul B., McKay G., Attia J., Vlaev I., Thakkinstian A. Evaluation of the Effectiveness of Behavioral Economic Incentive Programs for Goal Achievement on Healthy Diet, Weight Control and Physical Activity: A Systematic Review and Network Meta-analysis. Ann. Behav. Med. 2022;57:277–287. doi: 10.1093/abm/kaac066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bickel W.K., Moody L., Higgins S.T. Some current dimensions of the behavioral economics of health-related behavior change. Prev. Med. 2016;92:16–23. doi: 10.1016/j.ypmed.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thaler R.H., Sunstein C.R. Nudge: Improving Decisions about Health, Wealth, and Happiness. Yale University Press; New Haven, CT, USA: 2008. [Google Scholar]
- 20.Camerer C. Behavioral economics: Reunifying psychology and economics. Proc. Natl. Acad. Sci. USA. 1999;96:10575. doi: 10.1073/pnas.96.19.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giles E.L., Robalino S., McColl E., Sniehotta F.F., Adams J. The effectiveness of financial incentives for health behaviour change: Systematic review and meta-analysis. PLoS ONE. 2014;9:e90347. doi: 10.1371/journal.pone.0090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volpp K.G., John L.K., Troxel A.B., Norton L., Fassbender J., Loewenstein G. Financial incentive-based approaches for weight loss: A randomized trial. JAMA. 2008;300:2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foreman K.F., Stockl K.M., Le L.B., Fisk E., Shah S.M., Lew H.C., Solow B.K., Curtis B.S. Impact of a text messaging pilot program on patient medication adherence. Clin. Ther. 2012;34:1084–1091. doi: 10.1016/j.clinthera.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Suthutvoravut U., Anothaisintawee T., Boonmanunt S., Pramyothin S., Chaithanasarn A., Reutrakul S., Thakkinstian A. Efficacy of time-restricted eating and behavioural economic interventions in reducing fasting plasma glucose, HbA1c and cardiometabolic risk factors compared with time-restricted eating alone or usual care in patients with impaired fasting glucose: Protocol for an open-label randomised controlled trial. BMJ Open. 2022;12:e058954. doi: 10.1136/bmjopen-2021-058954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ElSayed N.A., Aleppo G., Aroda V.R., Bannuru R.R., Brown F.M., Bruemmer D., Collins B.S., Hilliard M.E., Isaacs D., Johnson E.L., et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19–S40. doi: 10.2337/dc23-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thamakaison S., Anothaisintawee T., Sukhato K., Unwanatham N., Rattanasiri S., Reutrakul S., Thakkinstian A. Hemoglobin A1c in combination with fasting plasma glucose trumps fasting plasma glucose alone as predictive indicators for diabetes mellitus: An ambidirectional cohort study of Thai people with impaired fasting glucose. BMJ Open Diabetes Res. Care. 2021;9:e002427. doi: 10.1136/bmjdrc-2021-002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chair S.Y., Cai H., Cao X., Qin Y., Cheng H.Y., Ng M.T. Intermittent Fasting in Weight Loss and Cardiometabolic Risk Reduction: A Randomized Controlled Trial. J. Nurs. Res. 2022;30:e185. doi: 10.1097/jnr.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 28.Jamshed H., Steger F.L., Bryan D.R., Richman J.S., Warriner A.H., Hanick C.J., Martin C.K., Salvy S.J., Peterson C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults with Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022;182:953–962. doi: 10.1001/jamainternmed.2022.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu D., Huang Y., Huang C., Yang S., Wei X., Zhang P., Guo D., Lin J., Xu B., Li C., et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022;386:1495–1504. doi: 10.1056/NEJMoa2114833. [DOI] [PubMed] [Google Scholar]
- 30.Lowe D.A., Wu N., Rohdin-Bibby L., Moore A.H., Kelly N., Liu Y.E., Philip E., Vittinghoff E., Heymsfield S.B., Olgin J.E., et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men with Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020;180:1491–1499. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H., Qin Y.L., Shi Z.Y., Chen J.H., Zeng M.J., Zhou W., Chen R.Q., Chen Z.Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019;19:219. doi: 10.1186/s12876-019-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow L.S., Manoogian E.N.C., Alvear A., Fleischer J.G., Thor H., Dietsche K., Wang Q., Hodges J.S., Esch N., Malaeb S., et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity. 2020;28:860–869. doi: 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cienfuegos S., Gabel K., Kalam F., Ezpeleta M., Wiseman E., Pavlou V., Lin S., Oliveira M.L., Varady K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020;32:366–378.e3. doi: 10.1016/j.cmet.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cienfuegos S., McStay M., Gabel K., Varady K.A. Time restricted eating for the prevention of type 2 diabetes. J. Physiol. 2022;600:1253–1264. doi: 10.1113/JP281101. [DOI] [PubMed] [Google Scholar]
- 35.Roekenes J., Martins C. Ketogenic diets and appetite regulation. Curr. Opin. Clin. Nutr. Metab. Care. 2021;24:359–363. doi: 10.1097/MCO.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 36.Gabel K., Hoddy K.K., Haggerty N., Song J., Kroeger C.M., Trepanowski J.F., Panda S., Varady K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging. 2018;4:345–353. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parr E., Devlin B., Lim K., Moresi L., Geils C., Brennan L., Hawley J. Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: A feasibility study. Nutrients. 2020;12:3228. doi: 10.3390/nu12113228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens C.R., Rossman M.J., Mazzo M.R., Jankowski L.R., Nagy E.E., Denman B.A., Richey J.J., Johnson S.A., Ziemba B.P., Wang Y., et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience. 2020;42:667–686. doi: 10.1007/s11357-020-00156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Z., He Z., Ye Y., Mao Y. Effects of time-restricted feeding with different feeding windows on metabolic health: A systematic review of human studies. Nutrition. 2022;102:111764. doi: 10.1016/j.nut.2022.111764. [DOI] [PubMed] [Google Scholar]
- 40.Schroder J.D., Falqueto H., Mânica A., Zanini D., de Oliveira T., de Sá C.A., Cardoso A.M., Manfredi L.H. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J. Transl. Med. 2021;19:3. doi: 10.1186/s12967-020-02687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolan P., Hallsworth M., Halpern D., King D., Metcalfe R., Vlaev I. Influencing behaviour: The mindspace way. J. Econ. Psychol. 2012;33:264–277. doi: 10.1016/j.joep.2011.10.009. [DOI] [Google Scholar]
- 42.Fanaroff A.C., Coratti S., Halaby R., Sanghavi M., O'Quinn R.P., Krishnan S., Glassberg H., Bajaj A., Adusumalli S., Chokshi N., et al. Feasibility and outcomes from using a commitment device and text message reminders to increase adherence to time-restricted eating: A randomized trial. Am. Heart J. 2023;258:85–95. doi: 10.1016/j.ahj.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending.