Abstract
The 3,4-dihydrocoumarin derivatives were obtained from 2-alkyl phenols and oxazolones via C–H oxidation and cyclization cascade in the presence of silver oxide (Ag2O) and p-toluenesulfonic acid as a Brønsted acid catalyst. This approach provides a one-pot strategy to synthesize the multisubstituted 3,4-dihydrocoumarins with moderate to high yields (64–81%) and excellent diastereoselectivity (>20:1).
Keywords: organocatalysis, one-pot reaction, ortho-quinone methides, dihydrocoumarins, oxazolones
1. Introduction
The dihydrocoumarin core is present as a characteristic structural motif to possess a broad range of biological activities [1,2]. Particularly, 3,4-dihydrocoumarins have attracted considerable attention due to their various pharmacological properties, such as antiviral, anti-inflammatory, and anticancer effects [3,4]. Therefore, various novel methods for the synthesis of 3,4-dihydrocoumarins have been developed [5,6,7,8,9,10,11]. The most general protocol for the synthesis of 3,4-dihydrocoumarins is the [4 + 2] cycloaddition of ortho-quinone methides (o-QMs) [12,13,14,15,16]. ortho-Quinone methides are synthetic intermediates widely used in organic synthesis [17,18]. A variety of useful reactions of ortho-quinone methides with several dipoles have been reported using transition metal catalysts [19,20] or organic catalysts [21,22]. Oxazolone derivatives have been recognized as well-known precursors of α-amino acids, are versatile building blocks in organic synthesis, and are often used in the synthesis of α,α-disubstituted α-amino acids [23].
Many studies have been reported to synthesize 6-membered oxacyclic compounds through [4 + 2] cycloaddition by reacting o-QM with various 1,2-dipoles [24,25]. Recently, the synthesis of 3,4-dihydrocoumarin derivatives by cycloaddition reaction between o-QM and oxazolones using a metal complex or hydrogen bonding organocatalyst has been reported (Scheme 1a) [26,27]. Additionally, the [4 + 2] ring addition of o-QM precursor and oxazolone using an organic catalyst has been reported (Scheme 1b) [28,29]. Despite this progress, the development of new and efficient synthetic methods for the synthesis of 3,4-dihydrocoumarin is still highly desirable. Cascade cyclization reactions have attracted much attention in the past few decades, providing practical protocols for the synthesis of heterocyclic molecules with the convenience afforded by shortening the reaction steps [29,30]. To the best of our knowledge, the single-pot synthesis of dihydrocoumarins from the in situ generation of o-QMs via C–H oxidation of benzyl phenol has not been reported. Therefore, we envisioned a one-pot synthesis of 3,4-dihydrocoumarin derivatives via C–H oxidation and ring closure cascade of 2-benzyl phenol with oxazolone under Brønsted acid conditions (Scheme 1c). In connection with our work on conjugate addition reactions, [31,32], we reported the Michael-type addition/ring closure sequences of o-QM with dipoles [33]. Herein, we describe the one-pot synthesis of 3,4-dihydrocoumarin derivatives from 2-benzyl phenol via C–H oxidation and acid-catalyzed ring closure cascade.
Scheme 1.
Methods for the asymmetric synthesis of dihydrocoumarins.
2. Results and Discussion
We commenced our study with 6-(4-methoxybenzyl)benzo[d][1,3]dioxol-5-ol (1a) and 4-benzyl-2-phenyloxazol-5(4H)-one (2a) as model substrates in the presence of oxidant and Brønsted acid catalyst, which was previously validated as the suitable catalysts for conjugate addition reactions [34,35].
The initial study was conducted with Ag2CO3 as an oxidant for the in situ generation of ortho-quinone methide intermediate via C-H oxidation. The desired 3,4-dihydrocoumarin (3a) was obtained in a 35% yield with 10 mol% of p-toluenesulfonic acid (Table 1, entry 1). To further improve the yield, organic and metallic oxidants, such as Ag2O, AgOAc, AgOTf, AgBF4, AgNO3, MnO2, Mn(OAc)3, Mn(acac)3, K2S2O8, Oxone, TBHP (tert-butyl hydroperoxide), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), (diacetoxyiodo)benzene, and PIFA ([bis(trifluoroacetoxy)iodo]benzene) were evaluated for their potential impact on this reaction. Silver oxide (Ag2O) proved to be a suitable oxidant with regard to yield (Table 1, entry 2). Ag2O has the advantage of excellent thermal stability and is commercially available in various forms (e.g., powder, pellets, flakes, etc.). Next, we examined the evaluation of the efficiency of selected Brønsted acids, including methanesulfonic acid, 2,4-dinitroebenzene sulfonic acid, acetic acid, trifluoroacetic acid, and diphenyl phosphate (DPP) (Table 1, entries 2 and 16–20). Diphenyl phosphate was the optimal catalyst for the oxidative cyclization cascade reaction (81% yield, Table 1, entry 20). Then, other common solvents such as methylene chloride, 1,2-dichloroethane (DCE), ethyl acetate, diethyl ether, tetrahydrofuran (THF), dioxane, benzene, toluene, and p-xylene were tested in the reaction (Table 1, entries 2 and 21–29). Chloroform was confirmed as the optimum solvent in terms of yield (Table 1, entry 20).
Table 1.
Optimization of the reaction conditions [a,c].

| Entry | Oxidant | Catalyst | Solvent | Yield [b] (%) |
|---|---|---|---|---|
| 1 | Ag2CO3 | p-TsOH | CHCl3 | 35 |
| 2 | Ag2O | p-TsOH | CHCl3 | 63 |
| 3 | AgOAc | p-TsOH | CHCl3 | 25 |
| 4 | AgOTf | p-TsOH | CHCl3 | 33 |
| 5 | AgBF4 | p-TsOH | CHCl3 | 28 |
| 6 | AgNO3 | p-TsOH | CHCl3 | 18 |
| 7 | MnO2 | p-TsOH | CHCl3 | 41 |
| 8 | Mn(OAc)3 | p-TsOH | CHCl3 | 29 |
| 9 | Mn(acac)3 | p-TsOH | CHCl3 | 61 |
| 10 | K2S2O8 | p-TsOH | CHCl3 | trace |
| 11 | Oxone | p-TsOH | CHCl3 | trace |
| 12 | TBHP | p-TsOH | CHCl3 | trace |
| 13 | DDQ | p-TsOH | CHCl3 | trace |
| 14 | PhI(OAc)2 | p-TsOH | CHCl3 | trace |
| 15 | PIFA | p-TsOH | CHCl3 | trace |
| 16 | Ag2O | MsOH | CHCl3 | 61 |
| 17 | Ag2O | DNBS | CHCl3 | 57 |
| 18 | Ag2O | AcOH | CHCl3 | 15 |
| 19 | Ag2O | TFA | CHCl3 | 45 |
| 20 | Ag2O | DPP | CHCl3 | 81 |
| 21 | Ag2O | DPP | CH2Cl2 | 72 |
| 22 | Ag2O | DPP | DCE | 70 |
| 23 | Ag2O | DPP | EtOAc | 46 |
| 24 | Ag2O | DPP | EtOEt | 38 |
| 25 | Ag2O | DPP | THF | 58 |
| 26 | Ag2O | DPP | dioxane | 47 |
| 27 | Ag2O | DPP | benzene | 38 |
| 28 | Ag2O | DPP | toluene | 45 |
| 29 | Ag2O | DPP | p-xylene | 42 |
[a] Reaction conditions: benzyl phenol 1a (0.2 mmol), oxazolone 2a (0.2 mmol), oxidant (0.24 mmol), and catalyst (0.02 mmol) in solvent (3 mL) at room temperature for 24 h under N2 atmosphere. [b] Isolated yield. [c] The relative configuration of the indicated diastereomer was determined by 1H-NMR analysis. PMP = para-methoxyphenyl, TBHP = tert-butyl hydroperoxide, DDQ = 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, Hacac = acetylacetone, PIFA = [bis(trifluoroacetoxy)iodo]benzene.
After establishing the optimized conditions, the substrate range of oxazolone derivative 2 was investigated. In reactions with various oxazolone derivatives (2), the corresponding 3,4-dihydrocoumarin derivatives 3 were obtained in reasonable yields (Scheme 2). The reactions of 5-(4H)oxazolones (2) with either electron-donating (4-OMe, 4-Me, 3-Me, and 3,5-di-Me2) or electron-withdrawing (4-CF3, 4-Cl, and 2-Cl) substituents on the phenyl ring in the C2 position yielded the corresponding 3,4-dihydrocoumarins (3b–3h) in high yields (Table 2, 68–77% yields). In addition, when there are p-halo-substituted (F, Cl) benzyl and (methylthio)ethyl groups instead of the benzyl group in the C4 position of 5-(4H)oxazolone (2), the corresponding 3,4-dihydrocoumarins (3i–3k) were processed in reasonable yields and (Table 2, 64–72 yields). In all cases, the diastereoselectivity of the products (3) was excellent (over 20:1). The relative configuration of the indicated diastereomer was determined by 1H-NMR analysis compared with reported works [26,27].
Scheme 2.
The gram-scale synthesis of dihydrocoumarin 3a.
Table 2.
Substrate scope [a,b,c].

|

|
[a] Reaction conditions: benzyl phenol 1 (0.2 mmol), oxazolone 2 (0.2 mmol), Ag2O (0.24 mmol), and diphenyl phosphate (0.01 mmol) in chloroform (3 mL) at room temperature for 24–36 h under N2 atmosphere. [b] Isolated yield. [c] The relative configuration of the indicated diastereomer was determined by 1H-NMR analysis.
In order to demonstrate the synthetic utility of this transformation, the gram-scale synthesis of 3,4-dihydrocoumarin (3a) was conducted. As shown in Scheme 2, when 2-benzyl phenol (1a) was treated with 4-benzyl-2-phenyloxazol-5(4H)-one (2a) under optimal reaction conditions, the reaction proceeded well to afford the chiral 3,4-dihydrocoumarin (3a) with a 78% yield. To achieve the asymmetric version of this reaction, the reaction was conducted with a chiral phosphoric acid catalyst (4) instead of diphenyl phosphate under standard reaction conditions (Scheme 3). The chiral 3,4-dihydrocoumarin (3a) obtained a 63% yield with 86% enantioselectivity. It is necessary to further investigate the structure of the catalyst and optimize reaction conditions suitable for asymmetric reactions, and further research is still being carried out in our laboratory.
Scheme 3.
Asymmetric synthesis of 3,4-dihydrocoumarins 3a.
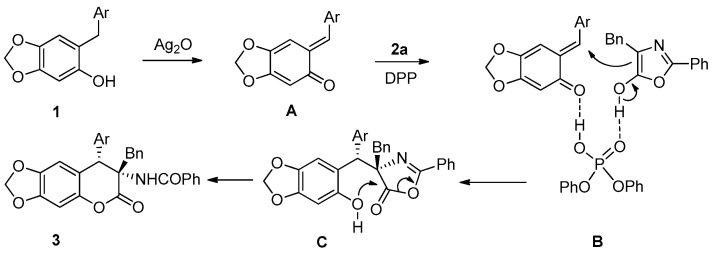
Based on the experimental results, a plausible reaction pathway and activation mode were proposed (Figure 1). The ortho-quinone methide intermediate A was generated in situ from benzylphenol (1) in the presence of Ag2O as an oxidant. Then, diphenyl phosphate simultaneously generated two hydrogen bonds with both the ortho-quinone methide intermediate and enol-form of oxazolone (2a). The subsequent conjugate addition of the C4 of oxazolone enolate to ortho-quinone methide leads to the Michal-type adduct (C). The intermediate (C) would undergo an annulation (lactonization) reaction with a concomitant opening of the azlactone ring to produce cyclic α-acylaminolactone (3).
Figure 1.
Proposed reaction mechanism.
3. Materials and Methods
All chemicals were purchased from commercial suppliers and used without further purification unless otherwise specified. Solvents for extractions and chromatography were of technical grade and were distilled prior to use. Extracts were dried over technical-grade anhydrous Na2SO4. Anhydrous solvents were deoxygenated by sparging with N2 and dried by passing through activated alumina columns of a Pure Solv solvent purification system (Innovative Technology). Reactions were monitored by analytical thin-layer chromatography (TLC) using silica gel 60 F254 pre-coated glass plates (0.25 mm thickness) and visualized using UV light (254 nm and 365 nm), I2, p-anisaldehyde, ninhydrin, and phosphomolybdic acid solution as an indicator. Flash chromatography was carried out on E. Merck silica gel (230–400 mesh). 1H NMR, 13C NMR, and 19F NMR spectra were recorded at 400 MHz, 100 MHz, and 376 MHz, respectively, on a Jeol ECS 400 MHz NMR spectrometer. Chemicals shift values (δ) are reported in parts per million and referenced in relation to the following standards: Me4Si as the internal references for the 1H- and 13C-NMR signals in chloroform and PhCF3 as the external references for the 19F-NMR signal. The peak information is described as s = singlet, d = doublet, t = triplet, q = quartet, and m = multiplet. Mass spectra (MS-EI, 70 eV) were conducted on a GC-MS Shimadzu QP2010. High-resolution mass spectra were measured on a Jeol HX110/110A using the electrospray ionization technique. The enantiomeric excesses (EEs) were determined by HPLC. HPLC analysis was performed on a Shimadzu prominence 20, measured at 254 nm using the indicated chiral column. Optical rotations were measured on a JASCO-DIP-1000 digital polarimeter with a sodium lamp. Infrared spectra were recorded on a Thermo Fisher Scientific Nicolet iS5 FT-IR spectrometer. The elemental analysis was carried out on a Perkin-Elmer 2400 Series II Elemental Analyzer.
General procedure for the one-pot synthesis of 3,4-dihydrocoumarins 3: To a stirred solution of 2-benzylphenol 1 (0.2 mmol), oxazolone 2 (0.2 mmol), and Ag2O (0.24 mmol) in chloroform (3 mL), diphenyl phosphate (0.02 mmol) was added at room temperature under an N2 atmosphere. The reaction mixture was stirred for 24–36 h at room temperature. After completion of the reaction (monitored by TLC), the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (EtOAc/hexane = 1:5) to afford 3,4-dihydrocoumarins 3.
N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide (3a), 81% yield; 1H NMR (400 MHz, CDCl3) δ 7.45–7.41 (m, 2H), 7.20–7.16 (m, 3H), 7.05–7.02 (m, 4H), 6.82 (d, J = 8.8 Hz, 2H), 6.80 (s, 1H), 6.69–6.68 (m, 3H), 6.64 (s, 1H), 5.99 (dd, J = 16.0, 1.6 Hz, 2H), 5.27 (s, 1H), 4.13 (d, J = 14.0 Hz, 1H), 3.67 (s, 3H), 3.13 (d, J = 14.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.5, 168.0, 159.0, 148.1, 144.4, 135.2, 135.0, 131.7, 130.2, 129.9, 129.0, 128.7, 128.5, 127.4, 126.8, 117.8, 114.2, 108.7, 102.1, 98.5, 77.4, 77.1, 76.8, 66.0, 55.2, 49.2, 38.2; IR: 3356, 2970, 1764, 1666, 1502, 1481, 1324, 1128 cm−1; HRMS (ESI): m/z calcd for C31H25NNaO6 [M + Na]+: 530.1580; found 530.1585; Elemental Analysis for C31H25NO6: C, 73.36; H, 4.96; N, 2.76; O, 18.91. Found: C, 73.34; H, 4.98; N, 2.75; O, 18.93.
N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)-4-methoxybenzamide (3b), 74% yield; 1H NMR (400 MHz, CDCl3) δ 7.46–7.40 (m, 2H), 7.23–7.16 (m, 3H), 7.05–6.98 (m, 4H), 6.52 (d, J = 8.8 Hz, 2H), 6.71–6.69 (m, 3H), 6.64 (s, 1H), 5.99 (dd, J = 16.0, 1.6 Hz, 2H), 5.28 (s, 1H), 4.35 (d, J = 14.0 Hz, 1H), 3.80 (s, 3H), 3.67 (s, 3H), 3.14 (d, J = 14.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.6, 167.4, 162.4, 159.0, 148.0, 145.3, 144.4, 135.1, 130.3, 130.0, 129.0, 128.7, 128.4, 127.4, 127.4, 117.9, 114.1, 113.8, 108.7, 102.1, 98.5, 65.9, 55.5, 55.2, 49.2, 38.2; IR: 3342, 2971, 1762, 1675, 1501, 1491, 1324, 1129 cm−1; HRMS (ESI): m/z calcd for C32H27NNaO7 [M + Na]+: 560.1685; found 560.1681; Elemental Analysis for C32H27NO7: C, 71.50; H, 5.06; N, 2.61; O, 20.83. Found: C, 71.47; H, 5.08; N, 2.60; O, 20.85.
N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)-4-methylbenzamide (3c), 77% yield; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 6 Hz, 2H), 7.20–7.10 (m, 3H), 7.05 (d, J = 8.7 Hz, 4H), 6.80 (s, 1H), 6.70 (s, 4H), 5.98 (s, 2H), 6.02 (d, J = 17.2, 4H) 5.30 (s, 1H), 4.15 (d, J = 14.0 Hz, 1H), 3.68 (s, 3H), 3.17 (d, J = 14.4 Hz, 1H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.5, 167.9, 159.0, 148.0, 145.3, 144.4, 142.1, 135.1, 132.4, 130.2, 130.0, 129.3, 129.0, 128.4, 127.4, 126.8, 117.9, 114.2, 108.7, 102.1, 98.5, 65.9, 55.2, 49.2, 38.2, 21.5; IR: 3352, 2971, 1764, 1668, 1502, 1481, 1332, 1130 cm−1; HRMS (ESI): m/z calcd for C32H27NNaO6 [M + Na]+: 544.1736; found 544.1733; Elemental Analysis for C32H27NO6: C, 73.69; H, 5.22; N, 2.69; O, 18.41. Found: C, 73.67; H, 5.23; N, 2.68; O, 18.42.
N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)-4-(trifluoromethyl)benzamide (3d), 71% yield; 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 8.1 Hz, 2H), 7.46 (d, J = 8.0 Hz, 2H), 7.23–7.21 (m, 3H), 7.05–7.03 (m, 4H), 6.82 (s, 1H), 6.62–6.70 (m, 4H), 5.97 (d, J = 16.8 Hz, 2H), 5.23 (s, 1H), 4.11 (d, J = 16 Hz, 1H), 3.70 (s, 3H), 3.20 (d, J = 14 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.4, 166.7, 159.1, 148.2, 145.5, 144.2, 138.3, 134.8, 130.1, 129.9, 128.9, 128.6, 127.6–, 127.2, 125.8, 125.8, 117.5, 114.2, 108.6, 102.1, 98.6, 66.0, 55.3, 49.3, 38.3; 19F NMR (376 MHz, CDCl3) δ -62.9; IR: 3347, 2981, 1758, 1667, 1502, 1481, 1326, 1130 cm−1; HRMS (ESI): m/z calcd for C32H24F3NNaO6 [M + Na]+: 598.1453; found 598.1455; Elemental Analysis for C32H24FNO6: C, 66.78; H, 4.20; N, 2.43; O, 16.68. Found: C, 66.76; H, 4.21; N, 2.43; O, 16.67.
N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)-4-chlorobenzamide (3e), 76% yield; 1H NMR (400 MHz, CDCl3) δ 7.40–7.37 (m, J = 1.8, 4H), 7.20–7.19 (m, J = 3.2, 3H), 7.04 (dd, J = 8.4, 4H), 6.81 (s, 1H), 6.70–6.86 (m, 4H), 6.02 (dd, J = 14.8, 2H), 5.24 (s, 1H), 4.15 (d, J = 14.0, 1H), 3.69 (s, 3H), 3.20 (d, J = 13.6, 1H); 13C NMR (100 MHz, CDCl3) δ 168.4, 166.8, 159.1, 148.1, 145.4, 144.3, 138.0, 134.9, 133.4, 130.1, 129.9, 129.0, 128.5, 128.2, 127.5, 117.7, 114.2, 108.7, 102.1, 98.6, 66.0, 55.2, 49.2, 38.3, 25.5; IR: 3355, 2972, 1762, 1666, 1502, 1481, 1332, 1129 cm−1; HRMS (ESI): m/z calcd for C31H24ClNNaO6 [M + Na]+: 564.1190; found 564.1196; Elemental Analysis for C31H24ClNO6: C, 68.70; H, 4.46; N, 2.58; O, 17.71. Found: C, 68.69; H, 4.46; N, 2.58; O, 17.72.
N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)-3-methylbenzamide (3f), 75% yield; 1H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 10.4 Hz, 2H), 7.21– 7.19 (m, 5H), 7.06 (dd, J = 14, 6.8 Hz, 4H), 6.80 (s, 1H), 6.70 (dd, J = 6.8, 3.2 Hz, 4H), 5.98 (dd, J = 16.8, 1.2 Hz, 2H), 5.28 (s, 1H), 4.15 (d, J = 15.2 Hz, 1H), 3.68 (s, 3H), 3.17 (d, J = 14.0 Hz, 1H), 2.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.5, 168.2, 159.0, 148.1, 145.4, 144.3, 138.6, 135.2, 135.1, 132.4, 130.3, 130.0, 129.0, 128.5, 128.5, 127.4, 123.9, 117.9, 114.2, 108.7, 102.1, 98.5, 65.9, 55.2, 49.2, 38.2, 21.4; IR: 3358, 2972, 1774, 1666, 1514, 1476, 1320, 1128 cm−1; HRMS (ESI): m/z calcd for C32H27NNaO6 [M + Na]+: 544.1736; found 544.1734; Elemental Analysis for C32H27NO6: C, 73.69; H, 5.22; N, 2.69; O, 18.41. Found: C, 73.68; H, 5.23; N, 2.68; O, 18.42.
N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)-2-chlorobenzamide (3g), 68% yield; 1H NMR (400 MHz, CDCl3) δ 7.45–7.40 (m, 2H), 7.20–7.17 (m, 3H), 7.05–7.01 (m, 4H), 6.82 (d, J = 8.8 Hz, 2H), 6.80 (s, 1H), 6.69–6.68 (m, 3H), 6.64 (s, 1H), 5.99 (dd, J = 16.0, 1.6 Hz, 2H), 5.27 (s, 1H), 4.13 (d, J = 14.0 Hz, 1H), 3.80 (s, 3H), 3.67 (s, 3H), 3.13 (d, J = 14.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.3, 166.3, 159.2, 148.1, 145.4, 144.1, 134.8, 134.6, 131.6, 130.7, 130.4, 130.2, 129.6, 129.2, 128.5, 127.5, 126.9, 117.8, 114.4, 108.6, 102.1, 98.6, 66.1, 55.4, 49.6, 38.6; IR: 3359, 2971, 1764, 1666, 1502, 1482, 1324, 1130 cm−1; HRMS (ESI): m/z calcd for C31H24ClNNaO6 [M + Na]+: 564.1190; found 564.1187; Elemental Analysis for C31H24ClNO6: C, 68.70; H, 4.46; N, 2.58; O, 17.71. Found: C, 68.69; H, 4.46; N, 2.58; O, 17.72.
N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)-3,5-dimethylbenzamide (3h), 69% yield; 1H NMR (400 MHz, CDCl3) δ 7.30–7.22 (m, 3H), 7.06–6.86 (m, 7H), 6.81 (s, 1H), 6.69 (dd, J = 46.8, 2.8 Hz, 4H), 5.99 (d, J = 15.6 Hz, 2H), 5.28 (s, 1H), 4.12 (d, J = 14.0 Hz, 1H), 3.70 (s, 3H), 3.17 (d, J = 13.6 Hz, 1H), 2.28 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 168.5, 168.3, 159.0, 148.0, 145.4, 144.4, 138.4, 135.2, 135.1, 133.3, 130.3, 130.0, 129.0, 128.4, 127.4, 124.5, 117.9, 114.2, 108.7, 102.1, 98.5, 65.9, 55.2, 49.2, 38.2, 21.3; IR: 3357, 2973, 1764, 1666, 1502, 1481, 1345, 1142 cm−1; HRMS (ESI): m/z calcd for C33H29NNaO6 [M + Na]+: 558.1893; found 558.1895.
N-(7-(4-fluorobenzyl)-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide (3i), 72% yield; 1H NMR (400 MHz, CDCl3) 7.40 (dd, J = 34.6, 6.5 Hz, 5H), 7.16–6.46 (m, 11H), 5.99 (d, J = 15.1 Hz, 2H), 5.26 (s, 1H), 4.12 (d, J = 14.0 Hz, 1H), 3.67 (s, 3H), 3.14 (d, J = 14.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.4, 167.9, 159.0, 148.0, 145.4, 144.2, 134.9, 131.7, 131.4, 131.3, 130.7, 130.0, 128.9, 128.7, 126.7, 117.6, 115.4, 115.2, 114.2, 108.6, 102.0, 98.4, 65.8, 49.1, 37.4, 25.4; 19F NMR (376 MHz, CDCl3) δ -114.9; IR: 3360, 2971, 1758, 1672, 1500, 1480, 1322, 1123 cm−1; HRMS (ESI): m/z calcd for C31H24FNNaO6 [M + Na]+: 548.1485; found 548.1489.
N-(7-(4-chlorobenzyl)-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide (3j), 71% yield; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 6.7 Hz, 3H), 7.36 (d, J = 6.7 Hz, 2H), 7.16 (d, J = 7.4 Hz, 2H), 7.05 (dd, J = 19.2, 8.8 Hz, 4H), 6.80 (s, 1H) 6.68 (m, 4H), 5.98 (d, J = 15.6 Hz, 2H), 5.25 (s, 1H), 4.14 (d, J = 14.4 Hz, 1H), 3.68 (s, 3H), 3.15 (d, J = 13.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.3, 168.0, 159.1, 148.1, 145.5, 144.3, 134.9, 133.5, 133.4, 131.8, 131.2, 130.0, 129.0, 128.8, 128.7, 126.7, 117.6, 114.2, 108.7, 102.1, 98.5, 65.8, 55.2, 49.1, 37.6; IR: 3348, 2976, 1764, 1666, 1502, 1480, 1324, 1102 cm−1; HRMS (ESI): m/z calcd for C31H24ClNNaO6 [M + Na]+: 564.1190; found 564.1187; Elemental Analysis for C31H24ClNO6: C, 68.70; H, 4.46; N, 2.58; O, 17.71. Found: C, 68.69; H, 4.46; N, 2.58; O, 17.72.
N-(8-(4-methoxyphenyl)-7-(2-(methylthio)ethyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide (3k), 65% yield; 1H NMR (400 MHz, CDCl3) δ 7.55–7.53 (m, 2H), 7.51–7.47 (m, 1H), 7.41–6.37 (m, 2H), 7.00–6.97 (m, 2H), 6.92 (s, 1H), 6.71–6.68 (m, 3H),6.63 (s, 1H), 5.99 (d, J = 15.4 Hz, 2H), 5.09 (s, 1H), 3.69 (s, 3H), 3.25–3.17 (m, 1H), 2.51 (td, J = 12.7, 5.0 Hz, 1H), 2.35 (td, J = 12.4, 4.9 Hz, 1H), 2.18–2.09 (m, 1H), 2.01 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.2, 167.4, 159.1, 148.0, 145.4, 144.3, 134.6, 131.9, 130.0, 128.9, 128.8, 126.9, 117.4, 114.2, 108.6, 102.1, 98.5, 64.2, 55.3, 49.1, 32.6, 29.0, 15.7; IR: 3362, 2972, 1724, 1663, 1502, 1481, 1324, 1104 cm−1; HRMS (ESI): m/z calcd for C27H25NNaO6S [M + Na]+: 514.1300; found 514.1305.
N-(7-isobutyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide (3l), 66% yield; 1H NMR (400 MHz, CDCl3) δ 7.48–7.56 (m, 3H), 7.40 (t, J = 7.4 Hz, 2H), 6.98–7.01 (m, 2H), 6.93 (s, 1H), 6.68–6.71 (m, 3H), 6.64 (s, 1H), 6.00 (dd, J = 14.7, 1.4 Hz, 2H), 5.09 (s, 1H), 3.70 (s, 3H), 3.49 (d, J = 4.8 Hz, 2H), 2.20–2.11 (m, 1H), 0.90 (dd, J = 7.2, 20.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 169.5, 167.6, 159.3, 148.3, 145.6, 144.5, 134.9, 132.1, 130.2, 129.2, 129.0, 127.1, 117.6, 114.5, 108.8, 102.3, 98.8, 64.4, 55.5, 41.04, 21.82, 20.18; IR: 3349, 2970, 1766, 1660, 1500, 1485, 1324, 1102 cm−1; HRMS (ESI): m/z calcd for C28H27NNaO6 [M + Na]+: 496.1736; found 496.1740.
N-(8-(4-methoxyphenyl)-6-oxo-7-phenyl-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide (3m), 67% yield; 1H NMR (400 MHz, CDCl3) δ 7.38–7.52 (m, 3H), 7.29–7.34 (m, 2H), 7.13–7.22 (m, 7H), 7.04–7.09 (m, 2H), 6.82 (s, 1H), 6.70 (d, J = 6.4 Hz, 2H), 6.01 (dd, J = 16.7, 1.1 Hz, 2H), 5.32 (s, 1H), 3.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.7, 168.5, 148.5, 145.7, 144.8, 138.6, 135.6, 135.3, 132.0, 130.4, 129.3, 129.0, 128.9, 128.2, 127.8, 127.1, 117.9, 109.1, 102.5, 99.0, 60.1, 50.5, 38.7; IR: 3344, 2975, 1760, 1660, 1500, 1485, 1324, 1092 cm−1; HRMS (ESI): m/z calcd for C30H23NNaO6 [M + Na]+: 516.1423; found 516.1427.
N-(7-benzyl-6-oxo-8-phenyl-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide (3n), 64% yield; 1H NMR (400 MHz, CDCl3) δ 7.47–7.43 (m, 3H), 7.36–7.32 (m, 2H), 7.21–7.19 (m, 3H), 7.07–7.04 (m, 4H), 6.81 (s, 1H), 6.71–6.68 (m, 4H), 6.01 (d, J = 15.4 Hz, 2H), 4.13 (d, J = 14.0 Hz, 1H), 3.16 (d, J = 14 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.3, 168.1, 148.2, 145.4, 144.4, 138.3, 135.2, 134.9, 131.6, 130.0, 128.9, 128.6, 128.5, 127.9, 127.5, 126.7, 117.5, 108.7, 102.1, 98.6, 65.7, 50.1, 38.3; IR: 3354, 2974, 1762, 1626, 1508, 1488, 1324, 1120 cm−1; HRMS (ESI): m/z calcd for C30H23NNaO5 [M + Na]+: 500.1474; found 500.1471.
N-(7-benzyl-8-((E)-4-methoxystyryl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide (3o), 72% yield; 1H NMR (400 MHz, CDCl3) δ 7.65–7.61 (m, 2H), 7.51–7.46 (m, 1H), 7.41–7.37 (m, 2H), 7.22–7.20 (m, 3H), 7.15–7.11 (m, 2H), 7.04–7.00 (m, 2H), 6.77 (d, J = 5.6 Hz, 2H), 6.74–6.72 (m, 2H), 6.35 (d, J = 16 Hz, 1H), 6.04 (d, J = 4.4 Hz, 2H), 5.89 (dd, J = 15.6, 8.0 Hz, 1H), 3.91 (d, J = 14 Hz, 1H), 3.74 (s, 3H), 3.06 (d, J = 14.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.5, 167.4, 159.3, 148.0, 145.2, 144.1, 134.8, 132.8, 131.7, 129.9, 129.1, 128.7, 128.4, 127.7, 127.4, 126.8, 123.0, 116.6, 113.8, 108.6, 102.0, 98.6, 64.9, 53.5, 47.1, 37.7; IR: 3386, 2978, 1784, 1672, 1500, 1481, 1332, 1135 cm−1; HRMS (ESI): m/z calcd for C33H27NNaO6 [M + Na]+: 556.1736; found 556.1731.
The gram-scale synthesis of dihydrocoumarin 3a: To a stirred solution of 2-benzylphenol (1, 0.517 g, 2.0 mmol), 4-benzyl-2-phenyloxazol-5(4H)-one (2, 0.503 g, 2.0 mmol), and Ag2O (0.556 g, 2.4 mmol) in chloroform (30 mL), diphenyl phosphate (50.0 mg, 0.2 mmol) was added at room temperature under an N2 atmosphere. The reaction mixture was stirred for 62 h at room temperature. After completion of the reaction (monitored by TLC), the solvent was removed under reduced pressure. After extraction with ethyl acetate (90 mL) three times and drying with anhydrous sodium sulfate, the resulting solution was concentrated by an evaporator and purified by silica gel column chromatography (EtOAc/hexane = 1:5) to afford N-(7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide 3a (0.721 g, 78%).
The typical procedure for the asymmetric synthesis of 3,4-dihydrocoumarins 3a: To a stirred solution of 2-benzylphenol (1, 25.8 mg, 0.1 mmol), 4-benzyl-2-phenyloxazol-5(4H)-one (2, 25.1 mg, 0.1 mmol), and Ag2O (27.8 mg, 0.12 mmol) in chloroform (2 mL), (11bR)-2,6-di(anthracen-9-yl)-4-hydroxydinaphtho [2,1-d:1’,2’-f][1–3]dioxaphosphepine 4-oxide (4, 7.0 mg, 0.01 mmol) was added at room temperature under an N2 atmosphere. The reaction mixture was stirred for 42 h at room temperature. After completion of the reaction (monitored by TLC), the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (EtOAc/hexane = 1:5) to afford N-((7S,8S)-7-benzyl-8-(4-methoxyphenyl)-6-oxo-7,8-dihydro-6H-[1,3]dioxolo [4,5-g]chromen-7-yl)benzamide 3a (31.9 mg).
63% yield; [α = +8.01 (c = 0.20, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.45–7.41 (m, 2H), 7.20–7.16 (m, 3H), 7.05–7.02 (m, 4H), 6.82 (d, J = 8.8 Hz, 2H), 6.80 (s, 1H), 6.69–6.68 (m, 3H), 6.64 (s, 1H), 5.99 (dd, J = 16.0, 1.6 Hz, 2H), 5.27 (s, 1H), 4.13 (d, J = 14.0 Hz, 1H), 3.67 (s, 3H), 3.13 (d, J = 14.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.5, 168.0, 159.0, 148.1, 144.4, 135.2, 135.0, 131.7, 130.2, 129.9, 129.0, 128.7, 128.5, 127.4, 126.8, 117.8, 114.2, 108.7, 102.1, 98.5, 77.4, 77.1, 76.8, 66.0, 55.2, 49.2, 38.2; IR: 3356, 2970, 1764, 1666, 1502, 1481, 1324, 1128 cm−1; Elemental Analysis for C31H25NO6: C, 73.36; H, 4.96; N, 2.76; O, 18.91. Found: C, 73.34; H, 4.98; N, 2.75; O, 18.93; HRMS (ESI): m/z calcd for C31H25NNaO6 [M + Na]+: 530.1580; found 530.1585; The EE value was 93%, tR (major) = 36.40 min, tR (minor) = 30.45 min (Daicel chiralcel IA, λ = 254 nm, n-hexane/i-PrOH 97/3, 1.0 mL/min).
4. Conclusions
In summary, we have developed an efficient synthesis of 3,4-dihydrocoumarin derivatives via C–H oxidation between 2-alkyl substituted phenol derivatives and 5-(4H)oxazolones and cyclization cascade under acid-catalyzed conditions. This approach provides a one-pot strategy to synthesize multisubstituted 3,4-dihydrocoumarins in moderate to high yields (64–81%) and excellent diastereoselectivity (>20:1).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28196853/s1. The experimental procedures and 1H NMR, and 13C NMR spectra of products can be found in the supporting information. References are cited from [36,37,38,39,40,41,42].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021-R1I1A3044807, 2021R1A6A1A03039503) and Soonchunhyang University Research Fund (2023-4).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kamat D.P., Tilve S.G., Kamat V.P., Kirtany J.K. Syntheses and Biological Activities of Chroman-2-ones. A Review. Org. Prep. Proced. Int. 2015;47:1. doi: 10.1080/00304948.2015.983805. [DOI] [Google Scholar]
- 2.Musa M.A., Cooperwood J.S., Khan M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008;15:1664. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Kennedy R., Thornes R.D. Coumarins: Biology, Applications, and Mode of Action. 1st ed. Wiley; Chichester, UK: 1997. [Google Scholar]
- 4.Kontogiorgis C.A., Hadjipavlou-Litina D.J. Synthesis and Antiinflammatory Activity of Coumarin Derivatives. J. Med. Chem. 2005;48:6400. doi: 10.1021/jm0580149. [DOI] [PubMed] [Google Scholar]
- 5.Lu D.F., Li Y.J., Gong Y.F. Organocatalytic Asymmetric Tandem Michael Addition−Hemiacetalization: A Route to Chiral Dihydrocoumarins, Chromanes, and 4H-Chromenes. J. Org. Chem. 2010;75:6900. doi: 10.1021/jo101446d. [DOI] [PubMed] [Google Scholar]
- 6.Li J.L., Zhou S.L., Han B., Wu L., Chen Y.C. Aminocatalytic asymmetric inverse-electron-demand aza-Diels–Alder reaction of N-Ts-1-aza-1,3-butadienes based on coumarin cores. Chem. Commun. 2010;46:2665. doi: 10.1039/b925424b. [DOI] [PubMed] [Google Scholar]
- 7.Kuang Y.L., Liu X.H., Chang L., Wang M., Lin L.L., Feng X.M. Catalytic Asymmetric Conjugate Allylation of Coumarins. Org. Lett. 2011;13:3814. doi: 10.1021/ol201312y. [DOI] [PubMed] [Google Scholar]
- 8.Hong B.C., Kotame P., Lee G.H. Asymmetric synthesis of 3,4-dihydrocoumarin motif with an all-carbon quaternary stereocenter via a Michael-acetalization sequence with bifunctional amine-thiourea organocatalysts. Org. Lett. 2011;13:5758. doi: 10.1021/ol202331j. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen C.B., Albrecht Ł., Udmark J., Jorgensen K.A. Enantioselective Formation of Substituted 3,4-Dihydrocoumarins by a Multicatalytic One-Pot Process. Org. Lett. 2012;14:5526. doi: 10.1021/ol302627u. [DOI] [PubMed] [Google Scholar]
- 10.Niharika P., Ramulu B.V., Satyanarayana G. Lewis acid promoted dual bond formation: Facile synthesis of dihydrocoumarins and spiro-tetracyclic dihydrocoumarins. Org. Biomol. Chem. 2014;12:4347. doi: 10.1039/C4OB00490F. [DOI] [PubMed] [Google Scholar]
- 11.Engl O.D., Fritz S.P., Kaslin A., Wennemers H. Organocatalytic Route to Dihydrocoumarins and Dihydroquinolinones in All Stereochemical Configurations. Org. Lett. 2014;16:5454. doi: 10.1021/ol502697s. [DOI] [PubMed] [Google Scholar]
- 12.Zhou D., Mao K., Zhang J., Yan B., Wang W., Xie H. Organocatalytic annulation of aldehydes and o-quinone methides: A facile access to dihydrocoumarins. Tetrahedron Lett. 2016;57:5649. doi: 10.1016/j.tetlet.2016.11.006. [DOI] [Google Scholar]
- 13.Wu B., Yu Z., Gao X., Lan Y., Zhou Y.-G. Regioselective α-Addition of Deconjugated Butenolides: Enantioselective Synthesis of Dihydrocoumarins. Angew. Chem. Int. Ed. 2017;56:4006. doi: 10.1002/anie.201700437. [DOI] [PubMed] [Google Scholar]
- 14.Chen X., Song R., Liu Y., Ooi C.Y., Jin Z., Zhu T., Wang H., Hao L., Chi Y.R. Carbene and Acid Cooperative Catalytic Reactions of Aldehydes and o-Hydroxybenzhydryl Amines for Highly Enantioselective Access to Dihydrocoumarins. Org. Lett. 2017;19:5892. doi: 10.1021/acs.orglett.7b02883. [DOI] [PubMed] [Google Scholar]
- 15.Alden-Danforth E., Scerba M.T., Lectka T. Asymmetric cycloadditions of o-quinone methides employing chiral ammonium fluoride precatalysts. Org. Lett. 2008;10:4951. doi: 10.1021/ol802029e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv D., Zhao M., Wang Y., Zhou Z. 3-Nitro-3,4-dihydrocoumarins: Valuable precursors for the synthesis of enantiomerically enriched masked quaternary α-amino acid derivatives with a 3,4-dihydrocoumarin scaffold. Org. Biomol. Chem. 2019;17:9636. doi: 10.1039/C9OB02089F. [DOI] [PubMed] [Google Scholar]
- 17.Caruana L., Fochi M., Bernardi L. The Emergence of Quinone Methides in Asymmetric Organocatalysis. Molecules. 2015;20:11733. doi: 10.3390/molecules200711733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaworski A.A., Scheidt K.A. Emerging Roles of in Situ Generated Quinone Methides in Metal-Free Catalysis. J. Org. Chem. 2016;81:10145. doi: 10.1021/acs.joc.6b01367. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y., Hayashi T. Asymmetric Synthesis of Triarylmethanes by Rhodium-Catalyzed Enantioselective Arylation of Diarylmethylamines with Arylboroxines. J. Am. Chem. Soc. 2015;137:7556. doi: 10.1021/jacs.5b03277. [DOI] [PubMed] [Google Scholar]
- 20.Zheng J., Lin L., Dai L., Yuan X., Liu X., Feng X. Chiral N,N′-Dioxide–Scandium(III) Complex-Catalyzed Asymmetric Friedel–Crafts Alkylation Reaction of ortho-Hydroxybenzyl Alcohols with C3-Substituted N-Protected Indoles. Chem. Eur. J. 2016;22:18254. doi: 10.1002/chem.201604088. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao C.-C., Raja S., Liao H.-H., Atodiresei L., Rueping M. Ortho-Quinone Methides as Reactive Intermediates in Asymmetric Brønsted Acid Catalyzed Cycloadditions with Unactivated Alkenes by Exclusive Activation of the Electrophile. Angew. Chem. Int. Ed. 2015;54:5762. doi: 10.1002/anie.201409850. [DOI] [PubMed] [Google Scholar]
- 22.Xu M.-M., Wang H.-Q., Wan Y., He G., Yan J., Zhang S., Wang S.-L., Shi F. Catalytic asymmetric substitution of ortho-hydroxybenzyl alcohols with tetronic acid-derived enamines: Enantioselective synthesis of tetronic acid-derived diarylmethanes. Org. Chem. Front. 2017;4:358. doi: 10.1039/C6QO00549G. [DOI] [Google Scholar]
- 23.de Castro P.P., Carpanez A.G., Amarante G.W. Azlactone Reaction Developments. Chem. Eur. J. 2016;22:10294. doi: 10.1002/chem.201600071. [DOI] [PubMed] [Google Scholar]
- 24.Uyanik M., Nishioka K., Kondo R., Ishihara K. Chemoselective oxidative generation of ortho-quinone methides and tandem transformations. Nat. Chem. 2020;12:353. doi: 10.1038/s41557-020-0433-4. [DOI] [PubMed] [Google Scholar]
- 25.You Y., Li T.-T., Yuan S.-P., Xie K.-X., Wang Z.-H., Zhao J.-Q., Zhou M.-Q., Yuan W.C. Catalytic asymmetric [4+2] cycloaddition of 1-((2-aryl)vinyl)naphthalen-2-ols with in situ generated ortho-quinone methides for the synthesis of polysubstituted chromanes. Chem. Commun. 2020;56:439. doi: 10.1039/C9CC08316B. [DOI] [PubMed] [Google Scholar]
- 26.Hu H., Liu Y., Guo J., Lin L., Xu Y., Liu X., Feng X. Enantioselective synthesis of dihydrocoumarin derivatives by chiral scandium(iii)-complex catalyzed inverse-electron-demand hetero-Diels–Alder reaction. Chem. Commun. 2015;51:3835. doi: 10.1039/C4CC10343B. [DOI] [PubMed] [Google Scholar]
- 27.Kim K.S., Jang J., Kim D.Y. Organocatalytic Enantioselective Cycloaddition of o-Quinone Methides with Oxazolones: Asymmetric Synthesis of Dihydrocoumarins. ChemistrySelect. 2020;5:13259. doi: 10.1002/slct.202003817. [DOI] [Google Scholar]
- 28.Zhou J., Wang M.-L., Gao X., Jiang G.-F., Zhou Y.-G. Bifunctional squaramide-catalyzed synthesis of chiral dihydrocoumarins via ortho-quinone methides generated from 2-(1-tosylalkyl)phenols. Chem. Commun. 2017;53:3531. doi: 10.1039/C7CC01072A. [DOI] [PubMed] [Google Scholar]
- 29.Parvin T., Yadav R., Choudhury L.H. Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs) Org. Biomol. Chem. 2020;18:5513. doi: 10.1039/D0OB00595A. [DOI] [PubMed] [Google Scholar]
- 30.Patel D.B., Parmar J.A., Patel S.S., Nail U.J., Patel H.D. Recent Advances in Ester Synthesis by Multi-Component Reactions (MCRs): A Review. Curr. Org. Chem. 2021;25:539. doi: 10.2174/1385272825666210111111805. [DOI] [Google Scholar]
- 31.Jung H.I., Kim D.Y. Synthesis of β-Selenylated Cyclopentanones via Photoredox-Catalyzed Selenylation/Ring-Expansion Cascades of Alkenyl Cyclobutanols. Synlett. 2019;30:1361. doi: 10.1055/s-0037-1611841. [DOI] [Google Scholar]
- 32.Park J.W., Jang S., Choo M.H., Kim D.Y. Enantioselective Organocatalytic Michael Addition and Ring Closure Cascade Reaction of o-Quinone Methides with Nitriles. Bull. Korean Chem. Soc. 2020;41:570. doi: 10.1002/bkcs.12011. [DOI] [Google Scholar]
- 33.Kim K.S., Kim D.Y. Electrochemical C−H Oxidation/Conjugate Addition/Cyclization Sequences of 2-Alkyl Phenols: One-Pot Synthesis of 2-Amino-4H-chromenes. Asian J. Org. Chem. 2022;11:e202200486. doi: 10.1002/ajoc.202200486. [DOI] [Google Scholar]
- 34.Jeong H.J., Kim D.Y. Enantioselective Decarboxylative Alkylation of β-Keto Acids to ortho-Quinone Methides as Reactive Intermediates: Asymmetric Synthesis of 2,4-Diaryl-1-benzopyrans. Org. Lett. 2018;20:2944. doi: 10.1021/acs.orglett.8b00993. [DOI] [PubMed] [Google Scholar]
- 35.Bu H.-Z., Li H.-H., Luo W.-F., Quin P.-C., Ye L.-W. Synthesis of 2 H-Chromenes via Unexpected [4 + 2] Annulation of Alkynyl Thioethers with o-Hydroxybenzyl Alcohols. Org. Lett. 2020;22:648. doi: 10.1021/acs.orglett.9b04421. [DOI] [PubMed] [Google Scholar]
- 36.Lam H., Qureshi Z., Wegmann M., Lautens M. Transition-Metal-Free [4+3]-Cycloaddition of ortho-Quinone Methides and Isomünchnones: Catalytic and Diastereoselective Assembly of Oxa-bridged Oxazocine Scaffolds. Angew. Chem. Int. Ed. 2018;57:16185. doi: 10.1002/anie.201810760. [DOI] [PubMed] [Google Scholar]
- 37.Wong Y.F., Wang Z., Hong W.X., Sun J.J. A one-pot oxidation/cycloaddition cascade synthesis of 2,4-diaryl chromans via ortho-quinone methides. Tetrahedron. 2016;72:2748. doi: 10.1016/j.tet.2015.12.011. [DOI] [Google Scholar]
- 38.Wu B., Gao X., Yan Z., Chen M.W., Zhou Y.Z. C–H Oxidation/Michael Addition/Cyclization Cascade for Enantioselective Synthesis of Functionalized 2-Amino-4H-chromenes. Org. Lett. 2015;17:6134. doi: 10.1021/acs.orglett.5b03148. [DOI] [PubMed] [Google Scholar]
- 39.Xiao W., Mo Y., Guo J., Su Z., Dong S., Feng X. Catalytic enantioselective synthesis of macrodiolides and their application in chiral recognition. Chem. Sci. 2021;12:2940. doi: 10.1039/D0SC06162J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adili A., Tao Z.L., Chen D.F., Han Z.Y. Quinine-catalyzed highly enantioselective cycloannulation of o-quinone methides with malononitrile. Org. Biomol. Chem. 2015;13:2247. doi: 10.1039/c4ob02602k. [DOI] [PubMed] [Google Scholar]
- 41.Alba R., Rios R. Oxazolones in Organocatalysis, New Tricks for an Old Reagent. Chem.-Asian J. 2011;6:720. doi: 10.1002/asia.201000636. [DOI] [PubMed] [Google Scholar]
- 42.Kim S.M., Lee J.H., Kim D.Y. Enantioselective Direct Amination of α-Cyanoketones Catalyzed by Bifunctional Organocatalysts. Synlett. 2008;2008:2659. doi: 10.1055/s-0028-1083510. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.