Abstract
Meglumine antimonate (Glucantime), a drug of choice for the treatment of leishmaniasis, is produced by the reaction of pentavalent antimony with N-methyl-d-glucamine, a carbohydrate derivative. We investigated the structure and composition of meglumine antimonate, which remain poorly understood, despite 50 years of use. Measurement of the antimony content of meglumine antimonate powder indicated a 1:1.37 molar ratio of antimony to N-methyl-d-glucamine. Osmolality measurements performed with meglumine antimonate solutions demonstrated an average of 1.43 antimony atoms per molecule of meglumine antimonate. The osmolality of a 1:10 dilution of stock meglumine antimonate increased by 45% over 8 days, suggesting hydrolysis to less complex species. A comparison of the proton nuclear magnetic resonance spectra of N-methyl-d-glucamine and meglumine antimonate revealed an increase in complexity in the latter but with all of the resonances of the former still being evident, consistent with the presence of coordination complexes between antimony and each of the N-methyl-d-glucamine hydroxyls. Fast atom bombardment and electrospray ionization mass spectrometry coupled with several derivatization procedures provided evidence that up to four N-methyl-d-glucamine hydroxyls are coordinated with each antimony. A series of oligomers were observed. The major moiety has a molecular mass of 507 atomic mass units and consists of NMG-Sb-NMG, where Sb represents antimony and NMG represents N-methyl-d-glucamine. Additional species containing up to four antimony atoms and five N-methyl-d-glucamine moieties and corresponding to the general form (NMG-Sb)n-NMG are also present. These results suggest that this agent is a complex mixture that exists in equilibrium in aqueous solution.
The protozoal parasite Leishmania causes a spectrum of clinical diseases afflicting 12 million people worldwide (13). Two pentavalent antimonial drugs, sodium stibogluconate (Pentostam) and meglumine antimonate (Glucantime), are currently the agents of choice for the treatment of leishmaniases and have been for 50 years. In spite of their wide use for half a century, relatively little is known about either their chemical structures or their mechanism(s) of action. Sodium stibogluconate and meglumine antimonate are prepared by the reaction of pentavalent antimony with gluconic acid and N-methyl-d-glucamine, respectively. After the initial reaction, the mixture is allowed to age, a step critical to clinical effectiveness (see reference 9 and references therein). Previous work on sodium stibogluconate suggested that it was a complex mixture of components with apparent molecular masses ranging from 100 to 4,000 Da (2). Efforts to obtain detailed structural information about sodium stibogluconate by proton (12) and carbon-13 (6) nuclear magnetic resonance (NMR) spectroscopy were hampered by its complex nature. The clinical effectiveness of sodium stibogluconate appears to be influenced by its composition. Some lots of the drug have been associated with poor clinical outcomes and have had higher osmolalities than clinically effective lots (2). The higher osmolalities have been presumed to reflect a diminished degree of sodium stibogluconate polymerization. However, when sodium stibogluconate was fractionated by anion-exchange chromatography into 10 fractions, all fractions showed similar activities against Leishmania amastigotes in vitro (8). These data suggest that subtle differences in the composition of sodium stibogluconate (and possibly meglumine antimonate) may be important for clinical antileishmanial activity. The extent of polymerization may influence the pharmacokinetics of drug delivery, uptake by the reticuloendothelial system, and the intracellular distribution of pentavalent antimony.
Equally little is known about the structure and composition of meglumine antimonate. Analogy with sodium stibogluconate suggests that it may also be a complex mixture of carbohydrate-antimony polymers. Data obtained by mass spectrometry have been used to propose a structure in which two molecules of N-methyl-d-glucamine are coordinated with a single antimony atom (4). In this report osmolality measurements, proton NMR, fast atom bombardment (FAB) mass spectrometry (FAB-MS), and electrospray ionization (ES) mass spectrometry (ES-MS) were used to obtain additional structural information on meglumine antimonate. This information may aid in both the elucidation of the mechanism of action of this agent and the rational design of new antileishmanial drugs.
MATERIALS AND METHODS
Deuterium oxide (99.9 atom % deuterium), N-methyl-d-glucamine, sodium cyanoborohydride (95%), and sodium trimethylsilylpropionate were from Aldrich Chemical Co. (Milwaukee, Wis.) and d4-methanol (99.9 atom % deuterium) was from Sigma Chemical Co. (St. Louis, Mo.). Meglumine antimonate (Rhone Poulenc, Paris, France) was provided by J. D. Berman at the Walter Reed Army Institute of Research (Washington, D.C.).
The antimony contents of meglumine antimonate solutions were determined by electrothermal atomic absorption spectroscopy with a Perkin-Elmer 4100 ZL spectrophotometer as described previously (7). The osmolalities of meglumine antimonate solutions were measured by freezing point depression on a One-Ten osmometer (Fiske Associates, Needham, Mass.). The sodium and chloride ion contents of meglumine antimonate solutions were determined with ion-selective electrodes on a Hitachi model 717 automated chemistry analyzer (Boehringer Mannheim, Indianapolis, Ind.). Deuterium exchange on meglumine antimonate or thioglycerol was performed by repeated additions of deuterium oxide to solutions dried on a Speed-Vac vacuum concentrator (Savant Instruments, Farmingdale, N.Y.).
Reductive methylation prior to ES-MS was performed with an aqueous solution of meglumine antimonate (35 mg/ml) by the addition of formaldehyde and sodium cyanoborohydride (final concentrations of each, 0.5 M) followed by incubation at 25°C for 30 min. Insoluble material was removed by centrifugation at 2,000 × g for 5 min. Methylated meglumine antimonate was precipitated from the reaction mixture by the addition of methanol (final concentration, 90%), and the product was redissolved in water.
Acetylation of meglumine antimonate was carried out by mixing equal volumes (100 μl each) of acetic anhydride and a stock solution of meglumine antimonate (10 mg/ml). The reaction was allowed to proceed for 24 to 48 h at room temperature. Peracetylation, the acetylation of all free hydroxyl and amino groups, was performed in a similar fashion, except equal volumes of pyridine, acetic anhydride, and meglumine antimonate stock solution were added. The addition of acetic anhydride and pyridine was repeated several times at 24-h intervals. The course of each reaction was monitored by FAB-MS. Under the reaction conditions used acetylation proceeded slowly.
Periodate oxidation was carried out by a modified procedure (1) with a solution of meglumine antimonate (35 mg/ml) whose pH was adjusted to approximately 5 with acetic acid, using 8 mM sodium periodate followed by incubation for at least 48 h. The extent of the reaction was monitored by serial analysis by FAB-MS.
The proton NMR spectra of N-methyl-d-glucamine and meglumine antimonate dissolved in 500 mM sodium phosphate-buffered deuterium oxide (pH 7) were obtained on a Bruker AM 500-MHz spectrometer. Chemical shifts are reported relative to those of sodium trimethylsilylpropionate, which was added as an internal standard.
FAB mass spectra were recorded on a VG-ZAB-SE spectrometer (Micromass, Manchester, United Kingdom) with a 35-kV cesium ion gun. ES spectra were recorded on a VG-Quattro (Micromass) triple-quadrupole mass spectrometer equipped with an Electrospray source (Analytica, Branford, Conn.). FAB spectra were recorded by using thioglycerol as the matrix. Deuterium exchange spectra were recorded by exchanging thioglycerol with deuterium oxide several times. It was necessary to make several insertions of the probe into the ion source with only deuterated thioglycerol to remove residual protons and prevent the appearance of meglumine antimonate species with incompletely exchanged protons.
ES spectra were recorded by using either the standard mode at flows of 1 μl/min or the pneumatically assisted mode at flows of 5 to 10 μl/min. In either case samples were introduced by direct infusion with a syringe pump (Harvard Apparatus, Inc., South Natick, Mass.). Spectra were recorded and processed with the standard VG software (OPUS and MassLynx; Micromass).
Molecular modeling of meglumine antimonate was performed with the Gaussian 94 suite of ab initio programs (Gaussian, Inc., Pittsburgh, Pa.).
RESULTS
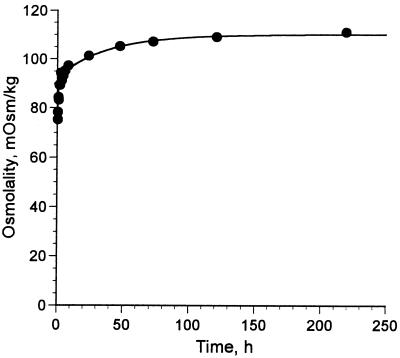
Aqueous solutions containing weighed amounts of meglumine antimonate powder were prepared, and the antimony content of each solution was quantified by electrothermal atomic absorption spectrophotometry. The average antimony content of the powder was 30.3% antimony on a weight basis. A liquid preparation of meglumine antimonate (Glucantime) containing 80 mg of Sb/ml (657 mM) was found to contain 58 mM sodium and 252 mM chloride. On the basis of these analyses the amount of N-methyl-d-glucamine was calculated by subtraction, and the mole ratio of antimony and N-methyl-d-glucamine in meglumine antimonate was estimated to be 1:1.37. Determinations of the osmolality of liquid meglumine antimonate by freezing point depression were not reproducible. Therefore, samples were diluted 1:10 with water and an osmolality of 77 ± 1 mOsm/kg (n = 3) was measured within 1 min after dilution. The extrapolated osmolarity of the original solution was 770 mosM/L, with an average of 1.43 antimony atoms per particle (because this dilution was volumetric, the extrapolated result is in milliosmoles per liter [osmolarity]). When liquid meglumine antimonate was diluted with water the osmolality of a dilute solution increased by 45% over a period of 8 days. Concomitantly, the pH decreased from 7.1 to 5.9 over the 8-day period. A plot of osmolality versus time after a 1:10 dilution with water is shown in Fig. 1. On the basis of an osmolality of 111 mosM/kg measured 8 days after dilution, diluted meglumine antimonate was calculated to consist almost entirely of a 1:1 complex of antimony and N-methyl-d-glucamine, some free N-methyl-d-glucamine, and sodium and chloride ions. The predicted composition is based on the assumption of negligible amounts of uncomplexed antimonic acid [hydrated antimony (V) oxide], which is only very slightly soluble in water (5).
FIG. 1.
Kinetics of increase in osmolality in diluted meglumine antimonate. Stock meglumine antimonate (Glucantime) was diluted 1:10 with water at time zero, and osmolality was measured at intervals. Measurements were made in duplicate at 6, 7, and 8 h and in triplicate from 1 to 8 days. The points were fitted to a function with two accumulation terms, yielding the equation y = 75.4 + 18.0(1 − e−0.027t) + 16.9(1 − e−1.01t) (r2 = 0.987).
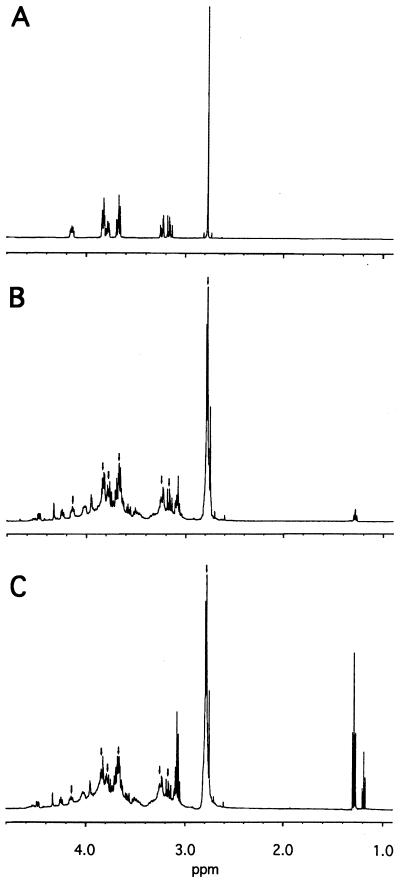
Proton NMR was used to characterize the carbohydrate species present in meglumine antimonate in aqueous solution. A spectrum of N-methyl-d-glucamine is shown in Fig. 2A, and spectra obtained from two different preparations of meglumine antimonate are shown in Figs. 2B and C, respectively. Both meglumine antimonate spectra contain all of the proton resonances seen in N-methyl-d-glucamine, as indicated by the arrows. The relative intensity of these resonances is identical to that observed in uncomplexed N-methyl-d-glucamine. New resonances were seen in meglumine antimonate at 1.3 ppm in one preparation and at 1.2 and 1.3 ppm in another one. The methyl resonance at 2.8 ppm is no longer a singlet but shows evidence of complex coupling. Finally, the region from 3.0 to 4.6 ppm is markedly more complex in the antimonial preparations than in the starting material, with multiple additional resonances of varying complexity present both up- and downfield from those in uncomplexed N-methyl-d-glucamine. A comparison of the spectra obtained on the day of dissolution and 2 weeks later revealed no apparent change in the spectra of either meglumine antimonate preparation (data not shown). These results suggest that meglumine antimonate is a complex mixture of antimony–N-methyl-d-glucamine complexes with protons residing in numerous chemical microenvironments.
FIG. 2.
Proton NMR spectra of N-methyl-d-glucamine and meglumine antimonate. Spectra of N-methyl-d-glucamine (A), dry meglumine antimonate powder (B), and liquid meglumine antimonate dried by vacuum centrifugation (C) were obtained for samples containing 100 mmol of each compound per liter dissolved in deuterium oxide containing 500 mM potassium phosphate (pH 7.0). No resonances were seen outside of the region shown. The arrows in panels B and C indicate resonances in common with panel A.
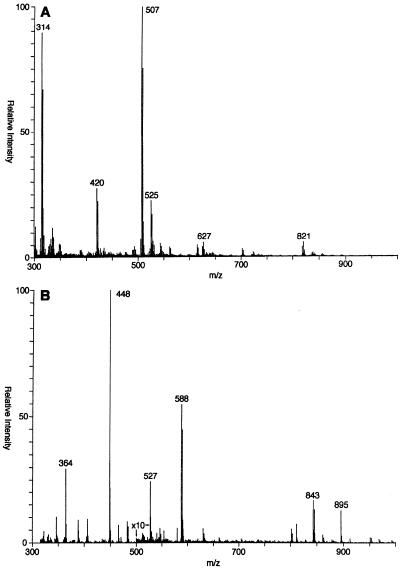
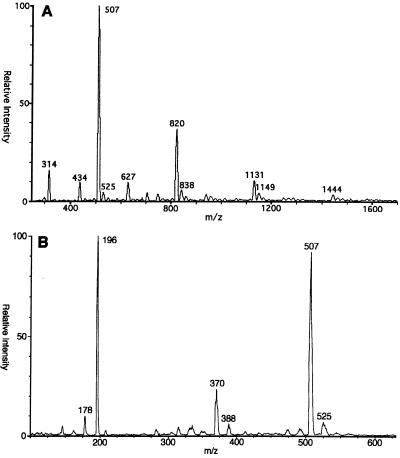
Positive-ion FAB-MS analysis of meglumine antimonate (Fig. 3A) revealed a number of ionized species. On the basis of isotope ratios, most appear to have a charge of +1 such that the mass/charge ratio (m/z) is equal to the molecular weight. A major ion that does not contain antimony and that represents protonated N-methyl-d-glucamine was observed at m/z 196 (data not shown). All of the remaining ions in the spectrum are easily characterized as containing antimony by the distinctive isotope pattern of antimony (ratio of 121Sb:123Sb, 57:43). Ions containing one antimony atom were observed at m/z 227, 314, 420, 507, and 525. Less abundant ion triplets containing two atoms of antimony were observed and were centered on m/z 627 and 821. The compositions and molecular weights of these species are summarized in Table 1.
FIG. 3.
Positive-ion FAB mass spectra of meglumine antimonate and peracetylated meglumine antimonate. The spectra of meglumine antimonate (A) and peracetylated meglumine antimonate (B) were obtained with a thioglycerol matrix. The sensitivity was increased 10-fold, as indicated by the arrows.
TABLE 1.
FAB mass spectral properties of meglumine antimonate componentsa
| Component (molecular formula)b | Mass in H2O (no. of Sb atomsc) | Mass after reductive methylation (no. of methyl groupsd) | Mass after exhaustive acetylation (no. of hydroxyl and amino groupse) | Mass in D2O (no. of exchangeable protonsf) |
|---|---|---|---|---|
| GlcNMe (C7H15O5N) | 196 (0) | 210 (1) | 448 (6) | 203 (7) |
| SbGlcNMe (C7H15O5NSb) | 314 (1) | 326 (1) | 482 (4) | 318 (4) |
| SbGlcNMe-thioglycerol (C10H21O7NSSb) | 420 (1) | 434 (1) | 588 (4) | 425 (5) |
| Sb(GlcNMe)2 (C14H30O10N2Sb) | 507 (1) | 535 (2) | 843 (8) | 515 (8) |
| Sb(GlcNMe)2 · H2O (C14H32O11N2Sb) | 525 (1) | NDg | ND | 536 (11) |
| Sb2(GlcNMe)2 (C14H27O10N2Sb2) | 627 (2) | ND | ND | 632 (5) |
| Sb2(GlcNMe)3 (C21H42O15N3Sb2) | 821 (2) | ND | ND | 830 (9) |
The most abundant singly charged ion observed for each component is indicated. All masses are in amu rounded to the nearest integer.
Glc, glucamine; Me, methyl.
The number of antimony atoms present in each component was determined from the observed isotope distribution pattern.
The mass shift produced by reductive methylation of amino groups with formaldehyde and sodium cyanoborohydride was used to estimate the number of free amino groups present in each component. The single secondary amino group on N-methyl-d-glucamine should incorporate one methyl group, with a consequent mass increase of 14 amu.
Peracetylation was performed with acetic anhydride and pyridine. A mass shift of 42 amu was predicted for each amino and hydroxyl acetylated.
The number of exchangeable protons was estimated from the difference between the mass of each species in water and deuterium oxide. Results are rounded to the nearest integer.
ND, could not be determined.
In order to obtain additional information about meglumine antimonate complexes, several derivatization procedures were performed. First, reductive methylation of amino groups with formaldehyde and sodium cyanoborohydride was performed. For each amino group proton replaced there is an incremental mass increase of 14 atomic mass units (amu). This permitted confirmation of the number of N-methyl-d-glucamine moieties present in each species (Table 1) and also indicated that amino groups are not complexed with antimony.
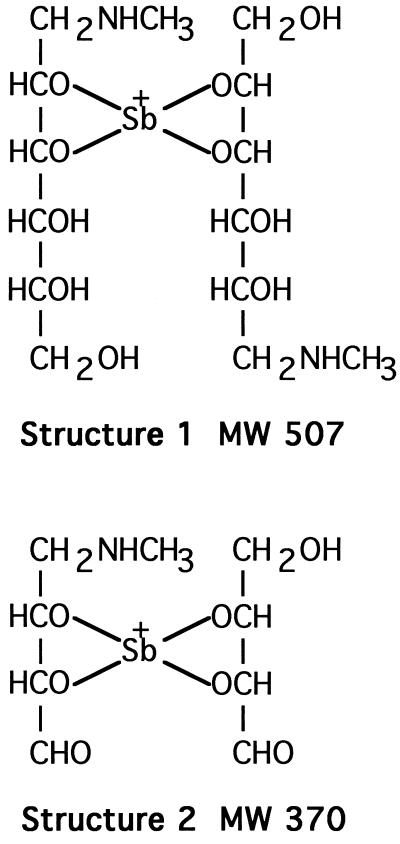
Meglumine antimonate was acetylated with acetic anhydride alone and with a combination of acetic anhydride and pyridine to determine the number of free hydroxyl and amino groups. The FAB spectrum of the product from the reaction with acetic anhydride alone contained peaks at m/z 549 and m/z 591 (data not shown), both with the appropriate antimony isotope ratio, indicating the addition of one and two acetyl groups, respectively, to the m/z 507 ion in unacetylated material. Under these acetylation conditions, acetylation should occur only at an amino group. The FAB spectrum of peracetylated meglumine antimonate (Fig. 3B) has ions at m/z 801 and 843 as a result of the addition of seven and eight acetyl groups, respectively, to m/z 507. These results are compatible with a structure in which four hydroxyl groups from two N-methyl-d-glucamine moieties are covalently bonded to antimony (Fig. 4, structure 1). Several higher-mass ions observed prior to acetylation and reductive methylation were not observed after derivatization. This may have been due to depolymerization during the derivatization process or to the high background resulting from the high concentration of salt in the reaction mixtures.
FIG. 4.
Proposed structures of the m/z 507 ion from meglumine antimonate (structure 1) and the m/z 370 ion from periodate oxidation of meglumine antimonate (structure 2). MW, molecular weight.
Deuterium exchange of meglumine antimonate protons was performed with deuterium oxide to determine the number of exchangeable protons present in the major species observed by FAB-MS. The number of protons exchanged was calculated from the mass increment observed for a given peak after exchange (Table 1). The major ion at m/z 515 indicated the exchange of eight protons from the m/z 507 ion. This finding is again consistent with four N-methyl-d-glucamine hydroxyl groups bonded with antimony and the ability to incorporate a total of eight acetyl groups (e.g., Fig. 4, structure 1). These deuterium exchange data are also compatible with the m/z 507 ion being an M+ ion and not an [M + H]+ ion because the latter would require the incorporation of nine deuterium atoms.
ES-MS was used to obtain additional information about the overall composition of meglumine antimonate. This is a softer ionization technique than FAB-MS and consequently may ionize larger molecular species without fragmentation. When the potential on the skimmer lens in the electrospray ion source was initially set to 150 V, all of the primary peaks were followed by a series of higher m/z peaks at multiples of 18 amu (data not shown). When the skimmer potential was increased to 300 V, the intensities of the higher-mass ions in each group were reduced, presumably as a result of the removal of water molecules, and the spectrum shown in Fig. 5A was produced. Some of the more abundant higher-mass ions include ions of m/z 507, 820, 1131, and 1444. It was difficult to obtain reproducible intensities for the higher-mass components. Satellite ions corresponding to the addition of one or more water molecules were still visible even with the higher skimmer potential.
FIG. 5.
Positive-ion ES mass spectra of meglumine antimonate. The spectra of meglumine antimonate (A) and periodate oxidized meglumine antimonate (B) were obtained.
Additional structural information on the major ions observed by ES-MS was obtained by deuterium exchange and reductive methylation. After deuterium exchange, satellite ions 20 amu larger were observed for ions of m/z 515, 829, and 1141, indicating the formation of water adducts. A summary of the results is presented in Table 2. Several ions observed prior to reductive methylation were not observed after modification, probably due to the high salt content of the reaction mixture. These results for the major individual organoantimonial polymer components of meglumine antimonate detected by ES-MS are compatible with the general formula of (NMG-Sb)n-NMG, with n ranging from one to four and where NMG is N-methyl-d-glucamine. The molecular weight of NMG-Sb of 314 may be predicted from those of N-methyl-d-glucamine and antimony after the loss of two protons during complex formation (195 + 121 − 2 = 314); the addition of another N-methyl-d-glucamine moiety with the loss of two more protons yields a total molecular weight of 507. The major ions observed at m/z 820, 1131, and 1444 correspond to the addition of NMG-Sb units with the loss of protons during the complexation process.
TABLE 2.
Electrospray mass spectral properties of meglumine antimonate componentsa
| Component (molecular formula)b | Mass in H2O (no. of Sb atomsc) | Mass after reductive methylation (no. of methyl groupsd) | Mass in D2O (exchangeable protonse) |
|---|---|---|---|
| SbGlcNMe (C7H15O5NSb) | 314 (1) | 328 (1) | 318 (4) |
| Sb2GlcNMe (C7H12O5NSb2) | 434 (2) | NDf | 436 (2) |
| Sb(GlcNMe)2 (C14H30O10N2Sb) | 507 (1) | 535 (2) | 515 (8) |
| Sb2(GlcNMe)2 (C14H27O10N2Sb2) | 627 (2) | ND | 632 (5) |
| Sb2(GlcNMe)3 (C21H42O15N3Sb2) | 820 (2) | 862 (3) | 829 (9) |
| Sb3(GlcNMe)4 (C28H54O20N4Sb3) | 1,132 (3) | ND | 1,141 (9) |
| Sb4(GlcNMe)5 (C35H66O25N5Sb4) | 1,444 (4) | ND | 1,454 (10) |
The most abundant singly charged ion observed for each component is indicated. All masses are in amu rounded to the nearest integer.
Glc, glucamine; Me, methyl.
The number of antimony atoms present in each component was determined from the observed isotope distribution pattern.
The mass shift produced by reductive methylation of amino groups with formaldehyde and sodium cyanoborohydride was used to estimate the number of free amino groups present in each component. The single secondary amino group on N-methyl-d-glucamine should incorporate one methyl group, with a consequent mass increase of 14 amu.
The number of exchangeable protons was estimated from the difference between the mass of each species in water and deuterium oxide. Results are rounded to the nearest integer.
ND, not determined.
To determine whether specific N-methyl-d-glucamine hydroxyl groups were preferentially complexed with antimony, meglumine antimonate was subjected to periodate oxidation. The ES-MS spectrum of the reaction products (Fig. 5B) contains a new ion at m/z 370 (isotope peaks not resolved), in addition to ions at m/z 196 and 507 that may be ascribed to residual unreacted material. Structure 2 (Fig. 4) is consistent with an ion of m/z 370 that is an oxidation product of an ion of m/z 507 that cannot be further oxidized by periodate and that contains two N-methyl-d-glucamine moieties complexed with antimony in an asymmetric fashion. Specifically, the C-2 and C-3 hydroxyls of one N-methyl-d-glucamine and the C-4 and C-5 hydroxyls of the second N-methyl-d-glucamine form bonds with an antimony atom. Structure 1 (Fig. 4) is proposed for the m/z 507 ion of meglumine antimonate observed by both FAB and ES.
DISCUSSION
Relatively little is known about the composition of pentavalent antileishmanial drugs, despite their many years of use. Textbooks frequently suggest a dimeric structure for sodium stibogluconate in which two antimony atoms are linked via an oxygen and each is coordinated with one molecule of gluconate (11), although no evidence for the structure is presented. (This may have been developed in analogy to a structure for antimony potassium tartrate proposed earlier [9], although the latter is now known to be a cyclic dimer in which two tartrates are linked by coordination to two bridging antimonies [10].) Berman and Grogl (2) have shown, however, that sodium stibogluconate in fact is a mixture of a number of components with apparent molecular weights ranging from 100 to 4,000 and an overall composition of equimolar amounts of antimony and sodium gluconate (2). On the basis of mass spectral data, Headley et al. (4) proposed a structure for meglumine antimonate in which two molecules of N-methyl-d-glucamine are coordinately linked to a single antimony. They observed by FAB-MS species containing up to three antimony atoms, but they suggested that the larger aggregates were formed under the ionization conditions and were not present at significant levels in meglumine antimonate (4).
The studies that we report here suggest that meglumine antimonate consists of a mixture of components with general formulas of (NMG-Sb)n-NMG (major components; molecular weights = 507, 820, 1132, and 1444) and (NMG-Sb)n (minor components; molecular weights = 314 and 627). Antimony and N-methyl-d-glucamine alternate in these chains, with each antimony coordinately linked via two hydroxyl groups from each of the adjacent N-methyl-d-glucamine moieties. N-methyl-d-glucamine that are not in terminal positions are linked to two antimonies (Fig. 6). The (NMG-Sb)2 component (molecular weight = 627) may have a cyclic structure. The linkages are relatively labile, and the relative amounts of the components are in an equilibrium determined by the overall concentration, with a higher concentration favoring an increased extent of oligomerization, possibly following simple mass action. The following evidence supports these hypotheses.
FIG. 6.
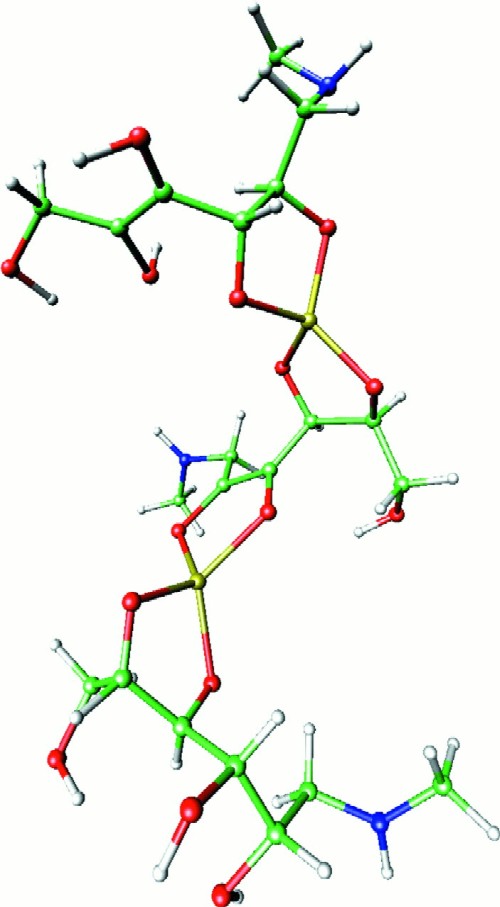
Three-dimensional structure of an m/z 820 ion from meglumine antimonate. The structure shown represents one meglumine antimonate molecule containing two antimony atoms and three N-methyl-d-glucamine moieties. The geometry of structure 1 (Fig. 4) was optimized at the Hartree-Fock level by using the LanL2DZ basis set. The optimized geometry was then used as a subunit to construct the complete structure as shown. Hydrogen atoms are light gray, carbon atoms are green, nitrogen atoms are blue, oxygen atoms are red, and antimony atoms are yellow.
ES-MS data suggest that meglumine antimonate predominantly consists of aggregates of antimony and N-methyl-d-glucamine of the general compositions SbnNMGn and SbnNMGn + 1, with the forms containing one more N-methyl-d-glucamine moiety than antimony being in greater abundance for any value of n (Fig. 5A; Table 2). One must not presume that the mass spectrum accurately reflects the composition of the starting material, since detection efficiency may decrease with mass and some aggregation may occur as an artifact of the electrospray process. The former effect would tend to underestimate the abundance of higher-mass species, while the latter would tend to produce an overestimate. Nonetheless, the overall composition from ES-MS (determined by averaging the compositions of each species, weighted by their relative abundances) agrees reasonably well with the measured composition: The average N-methyl-d-glucamine-to-antimony molar ratio derived from ES-MS was 1.45 and the average antimony-to-particle ratio was 1.65. The measured values were an N-methyl-d-glucamine:antimony mole ratio of 1.37 and a ratio of 1.43 antimony atoms per particle, derived from the antimony content and osmolarity of liquid meglumine antimonate. The concordance suggests that the ES-MS distribution provides at least a qualitative picture of the composition of liquid meglumine antimonate preparations.
Of note, while the general structure proposed by Headley et al. (4) (SbNMG2; mass, 507) is the most abundant form seen by ES-MS, it cannot be the sole species present in N-methyl-d-glucamine, since it requires a 1:2 antimony:N-methyl-d-glucamine ratio and a 1:1 antimony-to-particle ratio.
The proton NMR spectrum of meglumine antimonate had all of the resonances observed for free N-methyl-d-glucamine and a number of unique resonances as well. This pattern is consistent with the presence of some free N-methyl-d-glucamine (<50% of the total) or with a random association of N-methyl-d-glucamine hydroxyl groups with antimony such that each chemically distinct hydroxyl group is found both uncomplexed and complexed with antimony. The former hypothesis is supported by the observation of free N-methyl-d-glucamine in the mass spectra at m/z 196, although this ion could also arise by fragmentation of larger species. Daughter ion analysis of the m/z 507 ion after collision-induced dissociation did not reveal a major ion at m/z 196 (data not shown), which argues against the m/z 196 ion arising principally from fragmentation. The additional variety of proton resonances observed for meglumine antimonate in the region from 4.6 to 2.8 ppm are compatible with multiple unique microenvironments, both shielded and deshielded, for N-methyl-d-glucamine protons. The NMR spectrum is consistent with meglumine antimonate comprising a variety of related structures rather than a single structure.
The spectra obtained by FAB-MS and ES-MS are similar, but some higher-mass species seen in the latter are not seen in the former. This may be a consequence of the inability of FAB to ionize larger components without fragmenting them. A component of m/z 420 was found by FAB-MS but not by ES-MS. This m/z corresponds to an antimony coordinated with one molecule of N-methyl-d-glucamine and one molecule of thioglycerol, the FAB matrix. Headley et al. (4) attributed an m/z 404 ion found by FAB-MS with glycerol as the matrix to fragmentation, but it could also have been the corresponding adduct with glycerol. Since we found no ions at m/z 404 and the previous report noted none at m/z 420, adduct formation with the matrix is the most likely explanation.
The ready formation of adducts with the matrix, probably by exchange for an N-methyl-d-glucamine, suggests that the coordinate links between the antimony and the hydroxyls of the N-methyl-d-glucamine are relatively labile. This was further shown by experiments in which concentrated preparations were diluted 10-fold with water. This resulted in a subsequent increase in osmolality of the solution over time, as well as a decrease in pH (Fig. 1). The probable explanation for this behavior is the hydrolysis of the more polymerized species into simpler ones, with an accompanying increase in the number of particles. Given the relative lability of the coordination linkages, one would expect increasing depolymerization with increasing dilution on the basis of simple mass action effects (the overall reaction is simply replacement of a coordinated organic hydroxyl with a water-derived hydroxyl). Replacement of organic hydroxyl groups with water in the coordination complex with antimony would be expected to result in the generation of acidic protons and to lower the pH. A decrease in pH with time has been observed for arylstibonic acids treated with alkali (3). These observations are fully consistent with the concept that simple N-methyl-d-glucamine complexes with antimony are in reversible equilibrium with more complex forms.
The increase in osmolality showed complex kinetics, which might be expected with the wide variety of species capable of undergoing hydrolysis. The time course of the hydrolysis could be reasonably modeled by the equation y = 75.4 + 18.0(1 − e−0.027t) + 16.9(1 − e−1.01t). This suggests that hydrolysis events could be grouped into two sets, one with a half-life of about 40 min and the other with a half-life of about 26 h.
The methylation and acetylation experiments, as well as deuterium exchange, were carried out to characterize more specifically the way in which the antimony and N-methyl-d-glucamine are linked in the complexes. Previously, when FAB-MS and ES-MS were used to partially characterize meglumine antimonate, the investigators (4) proposed a symmetric structure in which antimony was bonded to the hydroxyls at the C-4 and C-6 positions of two N-methyl-d-glucamine to form six-membered rings. We hypothesize that the chemical reaction to form meglumine antimonate is consistent with the following general reaction scheme:
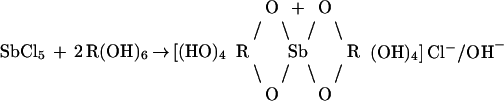 |
One would expect that the basic nitrogen of the N-methyl-d-glucamine would be easily protonated to produce a molecular ion. However, the formation of a protonated amine on meglumine antimonate would be hindered at neutral pH by electrostatic repulsion from the positively charged antimony. This would explain our observation of an M+ ion (based on deuterium exchange and peracetylation experiments) rather than an [M + H]+ ion. The formation of the [M + H]+ ion had previously been observed when hydrochloric acid was added to the glycerol matrix (4).
Our FAB-MS and ES-MS results for meglumine antimonate and its derivatives are most compatible with four oxygen atoms from two N-methyl-d-glucamine molecules being bonded to a pentavalent antimony atom in the most abundant species (m/z 507). Our data do not allow specification of the specific hydroxyls involved in the coordination complexes. However, the data for the m/z 507 species (Fig. 4) do suggest that when specific hydroxyls coordinate with antimony, the resulting complex is more resistant to periodate oxidation. The complexities of the NMR patterns suggest that multiple possible sets of linkages may be present.
We have drawn our proposed structures as involving coordination with vicinal hydroxyls (e.g., structure 1 in Fig. 4). This follows the pattern observed for antimony potassium tartrate and is most consistent with the structure of the major ion at m/z 370 observed after periodate oxidation (structure 2, Fig. 4). On the basis of structure 2, structure 1 is proposed as being most likely for the dominant m/z 507 ion observed by both FAB-MS and ES-MS. Figure 6 provides one possible three-dimensional structure for (NMG-Sb)2-NMG that is representative of oligomeric structures that are expected to be present in meglumine antimonate, based on the data that we have presented.
ACKNOWLEDGMENTS
This work was supported in part by Merck/American Federation for Clinical Research Foundation (postdoctoral fellowship to W.L.R.), by the University of Mississippi Medical Center (Biomedical Research Grant award to W.L.R.), and by the AB Foundation for Medical Research (protocol 92-02 to P.M.R.). NMR instrumentation facilities were provided by NIH (grant RR03475), NSF (grant DMB8610557), and ACS (grant RD259) grants. Mass spectrometer instrumentation facilities were provided by an NIH Shared Instrumentation grant (grant RR05358).
We thank Guiseppe Giordano and Yufang Zheng for technical assistance and Ian Armitage and Jack Faller for helpful discussions and critical review of the manuscript. We gratefully acknowledge Steven Davis for preparing the three-dimensional molecular model for Fig. 6.
REFERENCES
- 1.Angel A-S, Nilsson B. Linkage positions in glycoconjugates by periodate oxidation and fast atom bombardment mass spectrometry. Methods Enzymol. 1990;193:587–607. doi: 10.1016/0076-6879(90)93440-v. [DOI] [PubMed] [Google Scholar]
- 2.Berman J D, Grogl M. Leishmania mexicana: chemistry and biochemistry of sodium stibogluconate (Pentostam) Exp Parasitol. 1988;67:96–103. doi: 10.1016/0014-4894(88)90012-4. [DOI] [PubMed] [Google Scholar]
- 3.Doak G O, Freedman L D. Organometallic compounds of arsenic, antimony and bismuth. New York, N.Y: Wiley-Interscience; 1960. p. 291. [Google Scholar]
- 4.Headley J V, Yong M S, Brooks P W, Phillips A. Fast-atom bombardment mass spectrometry of the organometallic parasiticide, meglumine antimonate. Rapid Commun Mass Spectrom. 1995;9:372–376. [Google Scholar]
- 5.Lide D R, editor. CRC handbook of chemistry and physics. 77th ed. New York, N.Y: CRC Press; 1996. pp. 4–41. [Google Scholar]
- 6.Masamori E, Benitez A, Lim P. Assay of sodium stibogluconate (Pentostam). Report no. 552; contract no. DAMD17-85-C-5141. Washington, D.C: United States Army Medical Research and Development Command, Office of the Surgeon General; 1986. [Google Scholar]
- 7.Roberts W L, Rainey P M. Antimony quantification in Leishmania by electrothermal atomic absorption spectroscopy. Anal Biochem. 1993;211:1–6. doi: 10.1006/abio.1993.1223. [DOI] [PubMed] [Google Scholar]
- 8.Roberts W L, Rainey P M. Antileishmanial activity of sodium stibogluconate fractions. Antimicrob Agents Chemother. 1993;37:1842–1846. doi: 10.1128/aac.37.9.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steck E A. The leishmaniases. Prog Drug Res. 1974;18:289–351. doi: 10.1007/978-3-0348-7087-0_22. [DOI] [PubMed] [Google Scholar]
- 10.Tapscott R E, Belford R L, Paul I C. Stereochemistry of tartrato(4-)-bridged binuclear complexes. Coord Chem Rev. 1969;4:323–359. [Google Scholar]
- 11.Tracy J W, Webster L T. Chemotherapy of parasitic infections. In: Hardman J G, Limbird L E, Molinoff P B, Ruddon R W, Gilman A G, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 9th ed. New York, N.Y: McGraw-Hill Book Co.; 1996. pp. 987–1008. [Google Scholar]
- 12.Watnick, P. I., and P. M. Rainey. Unpublished data.
- 13.World Health Organization. Report of a WHO expert committee. World Health Organization Technical Report Services Report no. 793. Geneva, Switzerland: World Health Organization; 1990. Control of the leishmaniases; pp. 9–15. [PubMed] [Google Scholar]