Abstract
Background and Objectives
Stable patients with multiple sclerosis (MS) may discontinue treatment, but the risk of disease activity is unknown. Serum neurofilament light chain (sNfL) and serum glial fibrillary acidic protein (sGFAP) are biomarkers of subclinical disease activity and may help risk stratification. In this study, sNfL and sGFAP levels in stable patients were evaluated before and after treatment discontinuation to determine association with disease activity.
Methods
This observational study included patients enrolled in the Comprehensive Longitudinal Investigation in MS at the Brigham and Women's Hospital who discontinued treatment after >2 years disease activity–free. Two serum samples within 2 years, before and after treatment stop, were sent for sNfL and sGFAP measurements by single-molecule array. Biannual neurologic examinations and yearly MRI scans determined disease activity by 3 time-to-event outcomes: 6-month confirmed disability worsening (CDW), clinical attacks, and MRI activity (new T2 or contrast-enhancing lesions). Associations between each outcome and log-transformed sNfL and sGFAP levels pretreatment stop and posttreatment stop and the percent change were estimated using multivariable Cox regression analysis adjusting for age, disability, disease duration, and duration from attack before treatment stop.
Results
Seventy-eight patients (92% female) discontinued treatment at a median (interquartile range) age of 48.5 years (39.0–55.7) and disease duration of 12.3 years (7.5–18.8) and were followed up for 6.3 years (4.2–8.5). CDW occurred in 27 patients (35%), new attacks in 19 (24%), and new MRI activity in 26 (33%). Higher posttreatment stop sNfL level was associated with CDW (adjusted hazard ratio (aHR) 2.80, 95% CI 1.36–5.76, p = 0.005) and new MRI activity (aHR 3.09, 95% CI 1.42–6.70, p = 0.004). Patients who had >100% increase in sNfL level from pretreatment stop to posttreatment stop had greater risk of CDW (HR 3.87, 95% CI 1.4–10.7, p = 0.009) and developing new MRI activity (HR 4.02, 95% CI 1.51–10.7, p = 0.005). Patients who had >50% increase in sGFAP level also had greater risk of CDW (HR 5.34, 95% CI 1.4–19.9, p = 0.012) and developing new MRI activity (HR 5.16, 95% CI 1.71–15.6, p = 0.004).
Discussion
Stable patients who discontinue treatment may be risk stratified by sNfL and sGFAP levels measured before and after discontinuing treatment. Further studies are needed to validate findings and determine whether resuming treatment in patients with increasing biomarker levels reduces risk of subsequent disease activity.
Introduction
Patients with multiple sclerosis (MS) who have been stable for many years may be interested in discontinuing treatment given the potential adverse effects with prolonged immune suppression1 and associated costs.2-4 However, discontinuing disease-modifying therapies (DMTs) may increase the risk of future disease activity. Currently, there is no guideline or expert consensus on when to discontinue treatment in MS.5 Several cohort studies have identified that future disease activity after stopping treatment was more likely in younger patients and those with recent disease activity.6-13 Three randomized controlled trials, “Discontinuation of DMTs in MS” (DISCOMS, ClinicalTrials.gov Identifier: NCT03073603),14 “Disease Modifying Therapies Withdrawal in Inactive Secondary Progressive Multiple Sclerosis Patients Older Than 50 Years” (STOP-I-SEP ClinicalTrials.gov Identifier: NCT03653273), and “Discontinuing DMTs in Stable Relapsing-Onset Multiple Sclerosis” (DOT-MS, ClinicalTrials.gov Identifier: NCT04260711), are investigating whether patients who meet certain criteria for age and duration without disease activity can safely stop treatment. However, patient outcomes of these trials are planned to be assessed at 24 months after treatment stop, so risk beyond this window will be unanswered, and patients who do not meet inclusion criteria of these trials may still discontinue treatment for other reasons.
Serum biomarkers are emerging tools that may reflect subclinical disease activity and be useful in risk profiling. Two intermediate filaments that are reliably measurable from the serum using single-molecule array (SiMoA) technology are particularly promising and potentially indicate unique disease processes. Serum neurofilament light chain (sNfL) is primarily expressed in neurons, with levels rising in patients with inflammatory damage from new attacks or MRI activity and levels decreasing while stable on treatment.15-22 Serum glial fibrillary acidic protein (sGFAP) is primarily expressed in astrocytes, with levels correlated with brain T2 lesion volume and atrophy and progressive MS.21,23,24 Because elevated levels of these biomarkers have been correlated with future disease activity, they may be useful in identifying patients at risk in the future. However, the dynamics of these biomarkers in patients with stable MS who discontinue treatment is unclear, and whether an increase in these levels after treatment is discontinued has predictive value for future disease activity is unknown.
The Comprehensive Longitudinal Investigation of MS at the Brigham and Women's Hospital (CLIMB, climbstudy.org)25 is a real-world observational cohort that has been following up patients with MS since 2000 with every 6-month clinical examinations to determine Expanded Disability Status Scale (EDSS) scores, MRI scans every year, and blood sample collection every year, even after MS treatment has been stopped. The objective of this study was to evaluate patients in this robust CLIMB cohort who discontinued treatment to assess how sNfL or sGFAP levels before and after treatment discontinuation are associated with future disease activity.
Methods
Study Design and Patients
In this observational cohort study, we analyzed patients enrolled in the CLIMB from January 1, 2000, to December 9, 2021. Data were retrieved through the Oracle-based Brigham MS Patient Database and validated by individual chart review before biomarker analysis. Participants were included if they met the following inclusion criteria: (1) diagnosis of relapsing-remitting MS by 2017 McDonald criteria,26 (2) age older than 18 years, (3) no clinical attacks or MRI activity for >2 years while on treatment who then (4) discontinued treatment after >2 years, and (5) had serum samples obtained both within 2 years before stopping treatment (pretreatment stop) and within 2 years after stopping treatment (posttreatment stop).
Outcomes
We investigated 3 time-to-event outcomes from the treatment stop date. The primary outcome was time-to-confirmed disability worsening (CDW), defined as an increase in EDSS by 1.5 if at treatment discontinuation the EDSS was 0; by 1.0 if the discontinuation EDSS was between 1.0 and 5.0; and 0.5 if the discontinuation EDSS was 5.5 or higher, confirmed at follow-up 6 months later. Secondary outcomes included time-to-attack, defined as new or worsening neurologic symptoms lasting 24 hours or greater without fever or infection, and time-to-MRI activity, defined as new or enlarging T2 lesions, or contrast-enhancing lesions, on brain or spinal cord MRI. We also evaluated probability of retaining no evidence of disease activity, a composite of CDW, clinical attacks, or MRI activity.
Clinical Variables
Patients enrolled in the CLIMB undergo every 6-month clinical assessment by their treating neurologist to complete a Neurostatus-adjudicated EDSS score27 and review attack and medication history. Patients enrolled in the CLIMB who discontinue treatment continue to be followed up at our center with the same assessments. We collected the age, sex, disease duration, and duration from prior attack at the time of treatment stop, and EDSS at each visit.
MRI Variables
Patients enrolled in the CLIMB undergo yearly brain MRI and every 2-year spinal cord MRI scans as part of routine care, even after treatment discontinuation. Scans were performed on 1.5T or 3.0T machines and include brain T1-weighted and T2-weighted fluid-attenuated inversion recovery (FLAIR) images and cervical and thoracic spinal cord sagittal T2-weighted and T1-weighted short-tau inversion recovery images and axial T2-weighted images. Brain and spinal cord T1-weighted sequences were repeated 5 minutes after administration of 0.1 mmol/kg IV gadolinium contrast. MRI activity was reported in the patient's chart by a neuroradiologist as part of routine care. A subset of patients had quantitative processing for T2 lesion volume (T2LV) using previously described in-house pipelines.28,29
Biomarker Measurements
Patients enrolled in the CLIMB donate a serum sample each year, including after treatment discontinuation. Each patient's pretreatment stop and posttreatment stop serum sample was stored at −80 °C immediately after phlebotomy per standardized protocol.25 All samples were shipped to Quanterix (Billerica, USA) for SiMoA analysis (Neurology 4-Plex A) to quantify concentrations of sNfL and sGFAP (pg/mL). For this study, samples were sent in 2 batches: batch 1 with patients who met these criteria up to November 1, 2017, from a prior analysis,6 and batch 2 with patients who met these criteria up to December 9, 2021. Within each batch, patients had their pretreatment stop and posttreatment stop samples randomly distributed among plate runs. Each batch had 2 endogenous quality controls (EQCs) on all plate runs to calculate between-plate (interassay) coefficients of variation (CV). The average EQC CV for batch 1 was 7.8% for sNfL and 5.8% for sGFAP, and for batch 2, the EQC CV was 9.2% for sNfL and 3.8% for sGFAP, indicating reliable measurements.30,31 Tau and UCHL-1 had inappropriately high %CV readings and thus were not included in this analysis.
Statistical Analysis
Patient characteristics and biomarker levels were described as medians with interquartile ranges (IQRs) for continuous data or counts with percentages for categorical data. All available patients meeting inclusion criteria had serum samples sent for analysis; thus, no sample size calculation was performed, and a complete case analysis without imputing missing data was planned. We report the log-transformed levels of sNfL and sGFAP pretreatment stop and posttreatment stop and the percent change in each biomarker, calculated by the difference between pretreatment and posttreatment stop levels and then dividing by the pretreatment stop level.
Primary Analysis: Time-to-Event by Biomarker Level
Each outcome (CDW, clinical attacks, and MRI activity) was reported as the number of patients with the outcome and the number of years from treatment stop date to the event (in patients whom the event occurred). Patients were censored if no event occurred by last follow-up or if treatment was restarted. We used Cox regression models to report hazard ratios (HRs) with 95% CIs and Harrell's C statistic to evaluate the association between log-transformed pretreatment and posttreatment stop levels of sNfL and sGFAP and time-to-event outcomes.32,33 We also assessed the association between the percent change in biomarker levels after stopping treatment and each outcome; thus, a 100% increase, or doubling from pretreatment stop to posttreatment stop, corresponds to the given HR. We also performed multivariable analysis adjusting for the following covariates: age, disease duration, EDSS, duration from last attack, at the treatment stop date, and a term for the 2 batches to account for batch effects. Given the known effect that higher-efficacy cell-depleting infusion treatments have on serum biomarker levels, and their potentially longer treatment effect, we performed a sensitivity analysis excluding patients on rituximab, ocrelizumab, or alemtuzumab pretreatment stop and a supplemental analysis also adjusting for T2LV.
Secondary Analysis: Categorizing Groups by Clinically Applicable Increases in Biomarker Levels
To evaluate the association between outcomes and clinically meaningful percent change increases in biomarker levels after treatment discontinuation, we dichotomized groups above or below clinically applicable cutoffs: for sNfL, this corresponded to a 100% increase, and for sGFAP, this corresponded to a 50% increase. As a supplemental analysis, we conducted area under the receiver operative characteristic curve (AUC) analyses using the Liu method for each outcome to empirically determine optimal cut points for each biomarker assessment.34 We used the Kaplan-Meier survival analysis truncated at the median follow-up available to illustrate how this increase in each biomarker was associated with each outcome and report the relative hazard of each group with χ2 p values and univariate and multivariable Cox regression analyses to report corresponding HR.
We verified that the proportional hazards assumption was not violated for each model and deemed p values <0.05 significant. All statistics were performed using Stata/BE 17.0 (StataCorp LLC).
Data Availability
Anonymized data not published within this article and code will be made available by request from any qualified investigator.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Mass General Brigham Institutional Review Board (2002P001045 and 2017P001169), and written informed consent was obtained from all patients enrolled in the CLIMB study and included retrospective analyses of collected data and serum samples to be used for subsequent studies.
Results
Patients and Biomarker Data
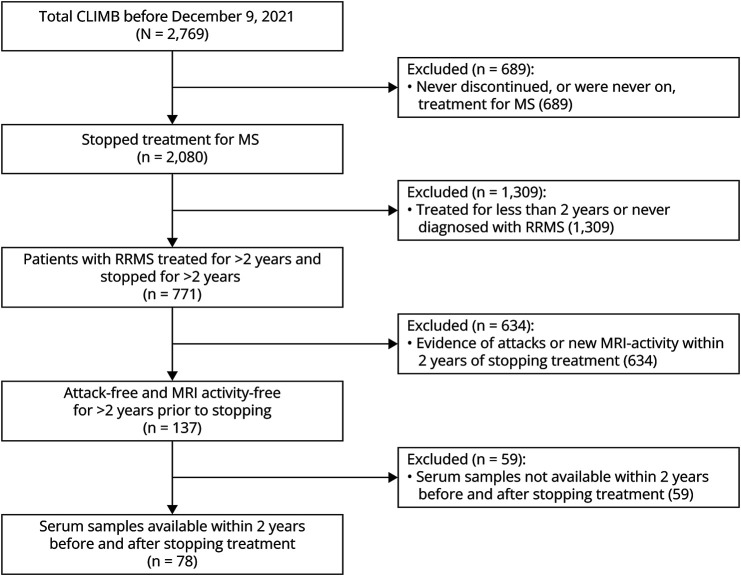
Of 2,769 patients with MS enrolled in the CLIMB as of 9/Dec/2021, 78 were disease activity–free for >2 years on treatment and then discontinued with serum samples obtained within 2 years, both before and after, stopping treatment (Figure 1). Patients were followed up after treatment stop for a median (IQR) 6.3 years (4.2, 8.5), for a total 530.35 patient-years. The median (IQR) age at MS onset was 33.4 years (25.2, 41.5) and at treatment stop was 48.5 years (39.0, 55.7), and 72 (92%) were female (Table 1). The EDSS at treatment stop was 1.5 (0.0, 2.0), and duration from prior attack was 6.1 years (3.7, 11.6). The reason for treatment discontinuation was obtained in 42 patients, with 29 described as patient preference, 11 as intolerance or adverse events, 1 due to pregnancy, and 1 due to protocol completion. The treatment before discontinuation was injectables in 51 (71%), oral DMTs in 13 (18%), and infusion treatments in 10 (14%), where 72 patients (92%) never received cell-depleting infusion treatments.
Figure 1. Patient Flowchart.
CLIMB = Comprehensive Longitudinal Investigation in MS at the Brigham and Women's Hospital.
Table 1.
Patient Characteristics
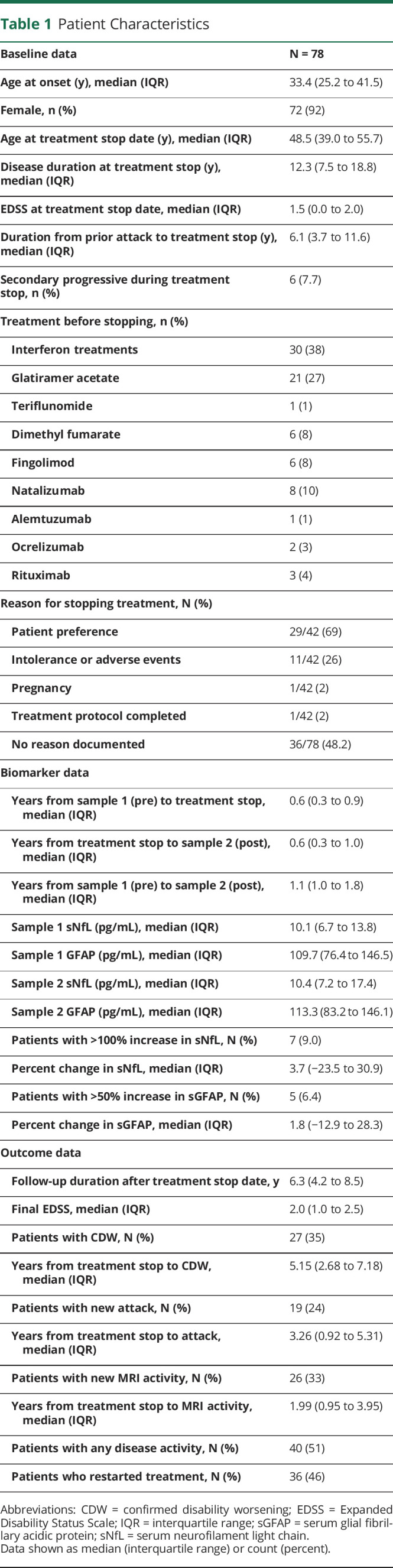
| Baseline data | N = 78 |
| Age at onset (y), median (IQR) | 33.4 (25.2 to 41.5) |
| Female, n (%) | 72 (92) |
| Age at treatment stop date (y), median (IQR) | 48.5 (39.0 to 55.7) |
| Disease duration at treatment stop (y), median (IQR) | 12.3 (7.5 to 18.8) |
| EDSS at treatment stop date, median (IQR) | 1.5 (0.0 to 2.0) |
| Duration from prior attack to treatment stop (y), median (IQR) | 6.1 (3.7 to 11.6) |
| Secondary progressive during treatment stop, n (%) | 6 (7.7) |
| Treatment before stopping, n (%) | |
| Interferon treatments | 30 (38) |
| Glatiramer acetate | 21 (27) |
| Teriflunomide | 1 (1) |
| Dimethyl fumarate | 6 (8) |
| Fingolimod | 6 (8) |
| Natalizumab | 8 (10) |
| Alemtuzumab | 1 (1) |
| Ocrelizumab | 2 (3) |
| Rituximab | 3 (4) |
| Reason for stopping treatment, N (%) | |
| Patient preference | 29/42 (69) |
| Intolerance or adverse events | 11/42 (26) |
| Pregnancy | 1/42 (2) |
| Treatment protocol completed | 1/42 (2) |
| No reason documented | 36/78 (48.2) |
| Biomarker data | |
| Years from sample 1 (pre) to treatment stop, median (IQR) | 0.6 (0.3 to 0.9) |
| Years from treatment stop to sample 2 (post), median (IQR) | 0.6 (0.3 to 1.0) |
| Years from sample 1 (pre) to sample 2 (post), median (IQR) | 1.1 (1.0 to 1.8) |
| Sample 1 sNfL (pg/mL), median (IQR) | 10.1 (6.7 to 13.8) |
| Sample 1 GFAP (pg/mL), median (IQR) | 109.7 (76.4 to 146.5) |
| Sample 2 sNfL (pg/mL), median (IQR) | 10.4 (7.2 to 17.4) |
| Sample 2 GFAP (pg/mL), median (IQR) | 113.3 (83.2 to 146.1) |
| Patients with >100% increase in sNfL, N (%) | 7 (9.0) |
| Percent change in sNfL, median (IQR) | 3.7 (−23.5 to 30.9) |
| Patients with >50% increase in sGFAP, N (%) | 5 (6.4) |
| Percent change in sGFAP, median (IQR) | 1.8 (−12.9 to 28.3) |
| Outcome data | |
| Follow-up duration after treatment stop date, y | 6.3 (4.2 to 8.5) |
| Final EDSS, median (IQR) | 2.0 (1.0 to 2.5) |
| Patients with CDW, N (%) | 27 (35) |
| Years from treatment stop to CDW, median (IQR) | 5.15 (2.68 to 7.18) |
| Patients with new attack, N (%) | 19 (24) |
| Years from treatment stop to attack, median (IQR) | 3.26 (0.92 to 5.31) |
| Patients with new MRI activity, N (%) | 26 (33) |
| Years from treatment stop to MRI activity, median (IQR) | 1.99 (0.95 to 3.95) |
| Patients with any disease activity, N (%) | 40 (51) |
| Patients who restarted treatment, N (%) | 36 (46) |
Abbreviations: CDW = confirmed disability worsening; EDSS = Expanded Disability Status Scale; IQR = interquartile range; sGFAP = serum glial fibrillary acidic protein; sNfL = serum neurofilament light chain.
Data shown as median (interquartile range) or count (percent).
The number of years between the pretreatment stop sample date and the treatment stop date was 0.6 (0.3, 0.9) and that from treatment stop date to posttreatment stop sample date was 0.6 (0.3, 1.0). The median pretreatment stop sNfL level was 10.1 pg/mL (6.7, 13.8) and sGFAP was 109.7 pg/mL (76.4, 146.5), and posttreatment stop sNfL was 10.4 pg/mL (7.2, 17.4) and sGFAP was 113.3 pg/mL (83.2, 146.1). The median percent change from pretreatment stop to posttreatment stop in sNfL was 3.7% (−23.5%, 30.9%) and in sGFAP was 1.8% (−12.9%, 28.3%), and there were 7 patients (9%) with >100% increase in sNfL increase and 5 patients (6.4%) with >50% increase in sGFAP, 3 of whom had an increase in both biomarkers (eFigure 1, links.lww.com/NXI/A912). Biomarker levels and dynamics were not significantly associated with duration from treatment stop.
There were an additional 59 eligible patients who discontinued treatment after >2 years of stability who were without available samples, details of which are summarized in eTable 1 (links.lww.com/NXI/A912). These patients were more likely to have stopped treatment before 2000, at a younger age, with higher EDSS at treatment stop, and after a longer duration since prior attack despite similar disease duration.
Time-to-Confirmed Disability Worsening
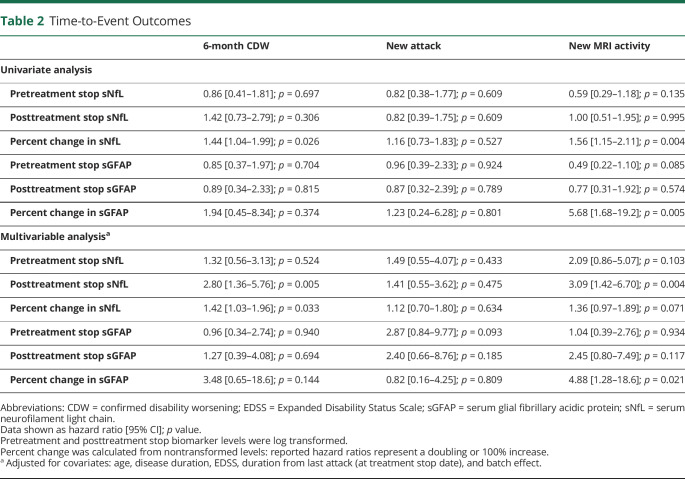
There were 27 patients (35%) who had 6-month CDW at a median 5.15 years (2.7, 7.2) from treatment stop. On univariate analysis, neither the pretreatment stop nor posttreatment stop levels of sNfL were associated with time-to-CDW (Table 2); however, when adjusting for age and other covariates, higher posttreatment stop sNFL was associated with higher risk (aHR: 2.80, 95% CI 1.36–5.76; p = 0.005). Higher percent change in sNfL was associated with higher risk, where a 100% increase (or doubling) in sNfL on univariate analysis accounted for a 44% higher risk (HR: 1.44, 95% CI 1.04–1.99, p = 0.026) and after adjustment (aHR: 1.42, 95% CI 1.03–1.96; p = 0.033). No association between sGFAP levels or dynamics and risk of CDW after treatment stop was identified. When considering only the 72 patients who had not received cell-depleting infusion treatments, high percent change in sNfL was still associated with greater risk of CDW (HR: 1.47, 95% CI 1.06–2.04; p = 0.020), and this association remained significant when adjusting for covariates (aHR: 1.45, 95% CI 1.04–2.01; p = 0.028).
Table 2.
Time-to-Event Outcomes
| 6-month CDW | New attack | New MRI activity | |
| Univariate analysis | |||
| Pretreatment stop sNfL | 0.86 [0.41–1.81]; p = 0.697 | 0.82 [0.38–1.77]; p = 0.609 | 0.59 [0.29–1.18]; p = 0.135 |
| Posttreatment stop sNfL | 1.42 [0.73–2.79]; p = 0.306 | 0.82 [0.39–1.75]; p = 0.609 | 1.00 [0.51–1.95]; p = 0.995 |
| Percent change in sNfL | 1.44 [1.04–1.99]; p = 0.026 | 1.16 [0.73–1.83]; p = 0.527 | 1.56 [1.15–2.11]; p = 0.004 |
| Pretreatment stop sGFAP | 0.85 [0.37–1.97]; p = 0.704 | 0.96 [0.39–2.33]; p = 0.924 | 0.49 [0.22–1.10]; p = 0.085 |
| Posttreatment stop sGFAP | 0.89 [0.34–2.33]; p = 0.815 | 0.87 [0.32–2.39]; p = 0.789 | 0.77 [0.31–1.92]; p = 0.574 |
| Percent change in sGFAP | 1.94 [0.45–8.34]; p = 0.374 | 1.23 [0.24–6.28]; p = 0.801 | 5.68 [1.68–19.2]; p = 0.005 |
| Multivariable analysisa | |||
| Pretreatment stop sNfL | 1.32 [0.56–3.13]; p = 0.524 | 1.49 [0.55–4.07]; p = 0.433 | 2.09 [0.86–5.07]; p = 0.103 |
| Posttreatment stop sNfL | 2.80 [1.36–5.76]; p = 0.005 | 1.41 [0.55–3.62]; p = 0.475 | 3.09 [1.42–6.70]; p = 0.004 |
| Percent change in sNfL | 1.42 [1.03–1.96]; p = 0.033 | 1.12 [0.70–1.80]; p = 0.634 | 1.36 [0.97–1.89]; p = 0.071 |
| Pretreatment stop sGFAP | 0.96 [0.34–2.74]; p = 0.940 | 2.87 [0.84–9.77]; p = 0.093 | 1.04 [0.39–2.76]; p = 0.934 |
| Posttreatment stop sGFAP | 1.27 [0.39–4.08]; p = 0.694 | 2.40 [0.66–8.76]; p = 0.185 | 2.45 [0.80–7.49]; p = 0.117 |
| Percent change in sGFAP | 3.48 [0.65–18.6]; p = 0.144 | 0.82 [0.16–4.25]; p = 0.809 | 4.88 [1.28–18.6]; p = 0.021 |
Abbreviations: CDW = confirmed disability worsening; EDSS = Expanded Disability Status Scale; sGFAP = serum glial fibrillary acidic protein; sNfL = serum neurofilament light chain.
Data shown as hazard ratio [95% CI]; p value.
Pretreatment and posttreatment stop biomarker levels were log transformed.
Percent change was calculated from nontransformed levels: reported hazard ratios represent a doubling or 100% increase.
Adjusted for covariates: age, disease duration, EDSS, duration from last attack (at treatment stop date), and batch effect.
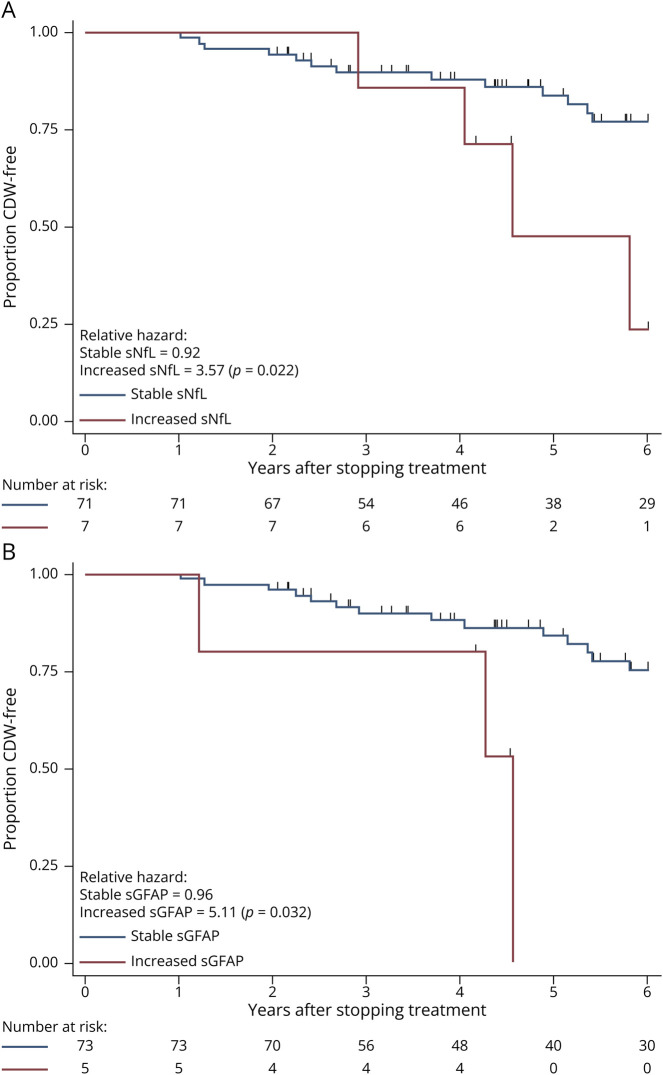
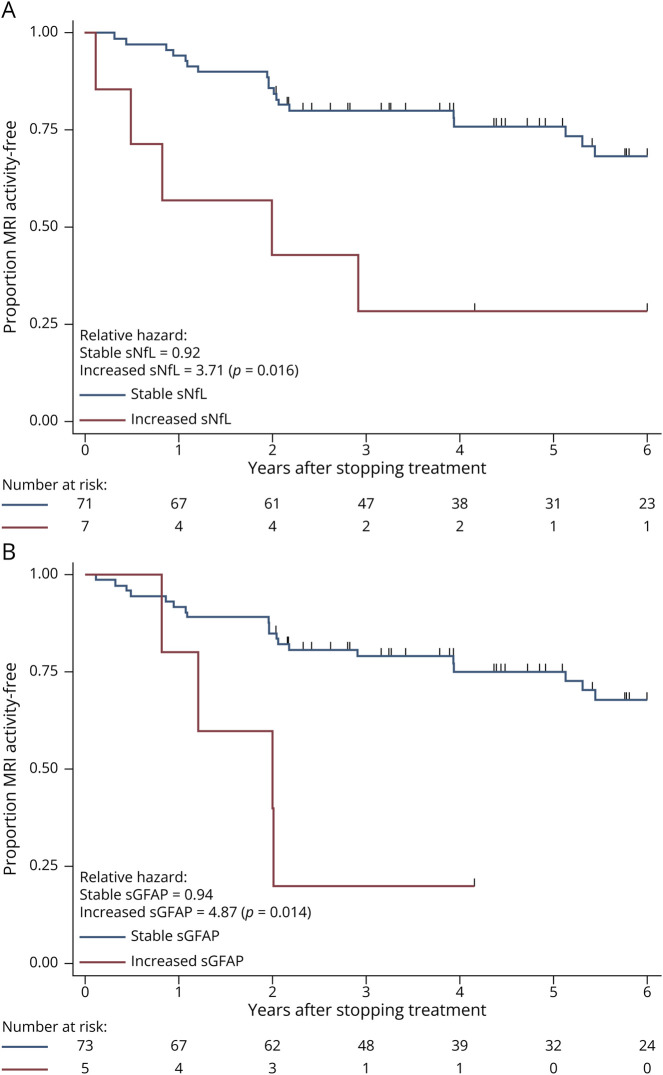
When investigating if a clinically applicable biomarker increase was associated with shorter time-to-CDW, we found that CDW was more likely in patients with a 100% increase in sNfL (HR: 3.87, 95% CI 1.4–10.7; p = 0.009, Figure 2A) and 50% increase in sGFAP (HR: 5.34, 95% CI 1.4–19.9; p = 0.012, Figure 2B). When adjusting for covariates, these associations remained.
Figure 2. Time-to-Confirmed Disability Worsening (CDW).
Survival curves showing proportion of patients CDW-free at each time point. (A) Stratified by patients who had a >100% increase in sNfL values after discontinuation vs those with <100% increase. (B) Stratified by patients who had a >50% increase in sGFAP values after discontinuation vs those with <50% increase. Number at risk shown and patients censored at each stage shown as ticks. Relative hazards for each group with χ2 p values shown. EDSS = Expanded Disability Status Scale; sGFAP = serum glial fibrillary acidic protein; sNfL = serum neurofilament light chain.
Time-to-Attack
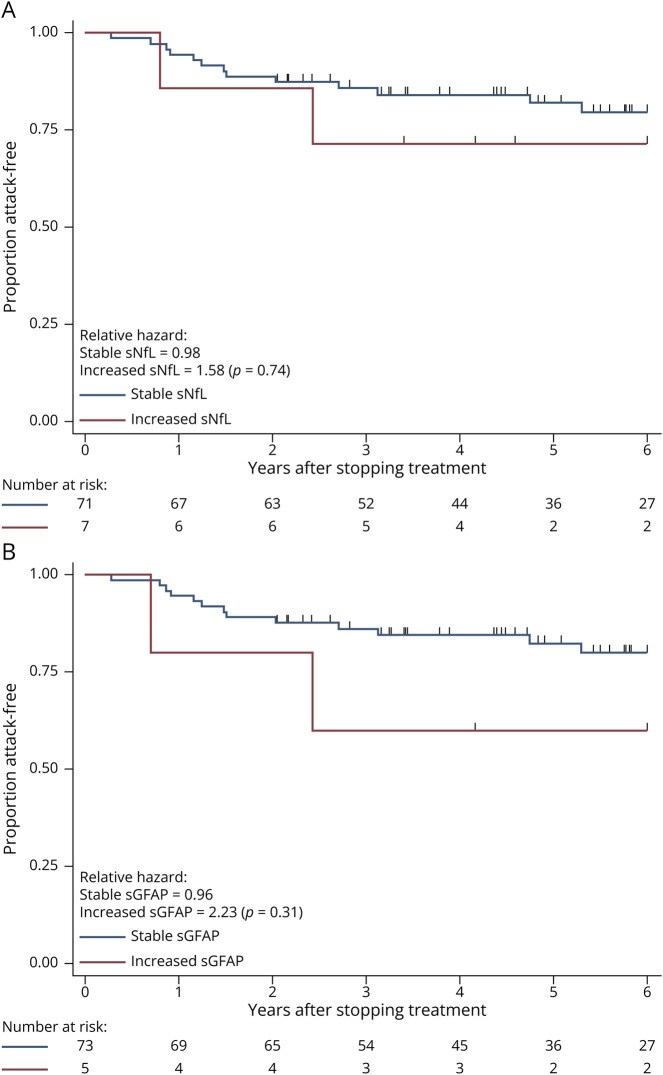
There were 19 patients (24%) who had an attack after stopping treatment, 12 of whom (63.2%) had new lesions on MRI. The median (IQR) time-to-attack was 3.26 (0.92, 5.31) years from treatment stop in patients who had an attack. On either univariate or multivariable analysis, neither biomarker levels nor change in levels were associated with an increased risk of attack (Table 2). Neither biomarker levels nor change in levels were associated with time to attack in patients who did not receive cell-depleting infusion treatments. There was also no increased risk of attack in patients who had a clinically applicable increase in either sNfL or sGFAP (Figure 3).
Figure 3. Time-to-Attack.
Survival curves showing proportion of patients attack-free at each time point. (A) Stratified by patients who had a >100% increase in sNfL values after discontinuation vs those with <100% increase. (B) Stratified by patients who had a >50% increase in sGFAP values after discontinuation vs those with <50% increase. Number at risk shown and patients censored at each stage shown as ticks. Relative hazards for each group with χ2 p values shown. EDSS = Expanded Disability Status Scale; sGFAP = serum glial fibrillary acidic protein; sNfL = serum neurofilament light chain.
Time-to-MRI Activity
New MRI activity occurred in 26 patients (33%) at a median (IQR) 1.99 (0.95, 3.95) years after discontinuing treatment. On univariate analysis, higher risk was associated with higher percent change in both sNfL (HR: 1.56, 95% CI 1.15–2.11; p = 0.004) and sGFAP (HR: 5.68, 95% CI 1.68–19.2; p = 0.005) after stopping treatment (Table 2). After adjusting for baseline factors and biomarker batch effects, higher posttreatment stop sNFL levels were associated with new MRI activity (HR: 3.09, 95% CI 1.42–6.70; p = 0.004), as was higher percent change in sGFAP (HR: 4.88, 95% CI 1.28–18.6; p = 0.021). When considering only the 72 patients who had not received cell-depleting infusion treatments, greater risk was associated with higher percent change in either sNfL (HR: 1.53, 95% CI 1.13–2.08; p = 0.006) or sGFAP (HR: 5.49, 95% CI 1.59–18.9; p = 0.007).
When assessing by biomarker profiles, 5/7 patients who exhibited a >100% increase in sNfL developed new MRI activity compared with 21/71 patients with <100% increase (HR: 4.02, 95% CI 1.51–10.7; p = 0.005, Figure 4A). For sGFAP, 4/5 patients with a >50% increase developed new MRI activity, compared with 22/73 without this increase (HR: 5.16, 95% CI 1.71–15.6; p = 0.004, Figure 4B). When adjusting for covariates, these associations remained.
Figure 4. Time-to-MRI Activity.
Survival curves showing proportion of patients MRI activity-free at each time point. (A) Stratified by patients who had a >100% increase in sNfL values after discontinuation vs those with <100% increase. (B) Stratified by patients who had a >50% increase in sGFAP values after discontinuation vs those with <50% increase. Number at risk shown and patients censored at each stage shown as ticks. Relative hazards for each group with χ2 p values shown. EDSS = Expanded Disability Status Scale; sGFAP = serum glial fibrillary acidic protein; sNfL = serum neurofilament light chain.
Supplemental Analyses
Adjusting T2 Lesion Volume
Volumetric MRI quantitative analysis was available for 76 patients. The images were processed from 3T images for 32 patients and 1.5T for 44 patients. The images were obtained a mean 18.4 days before treatment discontinuation, and the mean T2LV was 4.46 cm3 (range: 0.21–19.75 cm3). When also adjusting for this covariate, there was an association between higher T2LV and shorter time-to-CDW but not time-to attack or new MRI activity. In addition, there was no change in the association between sNfL or sGFAP measurements and any outcome investigated (eTable 2, links.lww.com/NXI/A912).
Investigation of Additional Cut Point Levels
We conducted the Liu method to empirically determine optimal cut points for each biomarker measurement in predicting each outcome (eTable 3, links.lww.com/NXI/A912). The optimal increase in sNfL was 1.22% for CDW, 19% for new attack, and 19.9% for new MRI activity. The AUC for these cut points were poor, at 0.57, 0.52, and 0.55, respectively. The optimal increase for sGFAP was −1.81% for CDW, 3.2% for new attack, and 2.47% for new MRI activity, with similarly poor AUC values of 0.57, 0.60, and 0.65, respectively.
Discussion
Patients with MS who have been stable for years on treatment may wish to discontinue to avoid risks of immune suppression, such as infection or the associated costs of DMT use, if they have a low risk of future disease activity. There is no current consensus on when stopping treatment is safe5; however, prior observational studies have identified that lower risk from stopping may be in older patients with a longer duration without disease activity.6-13 Serum biomarkers may reveal subclinical disease activity through different mechanisms: sNfL through neuroaxonal injury and sGFAP through astrogliosis, and thus, the measurement of both may potentially be useful to help risk stratify patients and help guide decisions regarding discontinuing or reinitiating treatment.15-20,23,24,35 In this study, we evaluated a 20-year observational cohort to report 78 patients who discontinued their treatment with serum obtained before and after stopping to investigate whether biomarker levels or dynamics were associated with eventual disease activity. Overall, there were a limited number of events after patients, who had been stable on treatment, discontinued. We found that biomarker levels obtained while a patient is stable, on treatment, were not predictive of any outcome; however, increasing levels of either sNfL or sGFAP after stopping treatment were associated with a higher risk of 6-month CDW and developing new MRI activity. We also identified that a minority of patients demonstrated a higher increase in these biomarkers, a 100% increase for sNfL or 50% increase for sGFAP, and were at highest risk to ultimately develop this evidence of disease activity. Furthermore, because we obtained biomarker measurements relatively early, within 1 year before and after the treatment stop date, but the average time to CDW and new MRI activity was 5.15 and 1.99 years after discontinuation, respectively, our findings suggest that identifying an early change in biomarker dynamics may indicate that patients who were stable on treatment may have had biomarker levels suppressed, and after discontinuing treatment, those with re-emergent subclinical activity, as evident by increasing sNfL or sGFAP levels, may predict treatment discontinuation failure even years later. The importance of these findings is that they support the concept of measuring biomarker levels before and after treatment stop to potentially allow for actionable resumption of therapy before disease activity occurs. However, further studies investigating whether reinitiating treatment in patients with increasing biomarker levels after discontinuation reduces the risk of eventual disease activity are needed to validate this hypothesis.
Prior studies investigating risk factors of disease activity after discontinuing DMT have focused on clinical risk factors. An earlier study from our group indicated that disease activity was significantly higher in younger patients,6 which was corroborated in a large MSBase registry analysis that also found moderately disabled patients were at higher risk.9 Two other studies investigating clinical predictors also found that longer duration from prior disease activity was associated with lower risk of disease activity.12,13 For these reasons, we included age at treatment stop and disease duration as covariates in our models to evaluate whether sNfL and sGFAP were independently associated with time-to-disease activity, and in a supplemental analysis, we also included T2LV, though this measure may not be available in all centers. These clinical factors are generally accepted and were used in the inclusion criteria for ongoing randomized controlled trials (DISCOMS, STOP-I-SEP, DOT-MS) investigating the risk of subsequent disease reactivation in patients who have been stable for 3–5 years on DMTs. Results of the DISCOMS trial were recently published and found that even in patients who were stable on DMT, the composite endpoint of new MRI activity or relapse was not significantly different between those who continued and those who discontinued (7.5% absolute difference in percentage points, p = 0.52) nor those with confirmed EDSS progression (1.2% absolute difference in percentage points, p = 0.77).14 This suggests that these clinical factors alone may not completely predict which patients are at risk of subsequent disease activity after stopping treatment, and measurement of sNfL and sGFAP levels before and after stopping treatment may provide additional predictive information. Our cohort had a higher rate of disease activity than the DISCOMS trial, potentially due to ours having a younger age at treatment stop and shorter duration from prior disease activity to treatment stop than that of the DISCOMS trial. These trials have outcome assessments planned 2 years after DMT stop, with DISCOMS also having an extension phase planned after an additional 3 years (ClinicalTrials.gov Identifier: NCT04754542). Data from these studies will inform how useful these clinical criteria are for risk of disease activity after discontinuation; however, patient-specific factors and longer-term follow-up will be incompletely investigated by these studies. In our study, we sought to investigate how serum biomarkers may provide additional patient-specific risk information and were able to follow-up patients, albeit in an observational setting, for a median 6.3 years and as much as 13.8 years after treatment stop.
Serum biomarkers are becoming increasingly investigated for risk profiling in MS. Multiple studies have demonstrated that sNfL, an intermediate filament release into the CSF, and nearly proportionally into the blood, during neuroaxonal injury is associated with inflammatory activity, treatment response, and long-term clinical and MRI outcomes.15,17,19,20,36-40 Of importance, patient-specific changes in sNfL have been demonstrated with disease activity, and thus, remeasuring in the same person may reveal subclinical changes in a different way than comparing with age-matched averages.37-39,41,42 Though fewer studies have reported sGFAP, a major intermediate filament within astrocytes that is released during astrogliosis, as demonstrated by higher levels in patients with progressive MS, higher brain T2 lesion volume and atrophy, it too is readily measurable by similar methods as sNfL and reveals different pathology.23,24,43 These biomarkers reflect changes in an individual, and stopping treatment may result in a meaningful change that could preemptively signal impending disease activity. In our study, more than 50% of patients had an increase in at least 1 biomarker shortly after stopping treatment, while disease activity also ensued in more than 50% during the follow-up period available. We demonstrated that biomarker levels obtained before stopping treatment, while patients were stable on DMT, were not associated with disease activity. Rather, we showed that biomarker dynamics, the percent change, after stopping treatment was associated with disability worsening and new MRI activity. Higher sNfL levels after treatment stop was associated with new MRI lesions, as had been previously demonstrated,18 and increasing sNfL was associated with a risk of disability worsening, as had also been demonstrated,39,42 even after adjusting for age and other covariates. Although higher individual sGFAP levels were not associated with any outcome, patients with a higher percent change in sGFAP after stopping treatment were at higher risk of developing new MRI activity, even after adjusting for age, EDSS, disease duration, and duration from prior attack during treatment stop, and patients who had >50% increase in sGFAP were at higher risk of disability worsening. This finding suggests that patients who are stable on treatment may have actively suppressed biomarker levels that can then elevate after stopping treatment and indicate risk of eventual disease activity, and that change in sNfL and sGFAP may represent different risk profiles for patients and thus both be worth measuring in patients who stop treatment. In this cohort, we found risk of clinical attack was not associated with biomarker levels or dynamics. A potential reason for this may by that because yearly MRIs were performed after treatment stop, radiologic disease activity could have been identified and physicians resumed treatment, and hence censoring these patients from our analysis before clinical attacks occurring. Further study is needed to evaluate whether resuming treatment in patients who demonstrate an increase in biomarker values reduces the risk of eventual disease activity.
A strength of this study is the comprehensive longitudinal follow-up in a cohort of patients who discontinued treatment. Indeed, regular clinical visits and MRI examinations for as far as 13 years after MS treatment discontinuation is rare in clinical practice, and serum collection and storage dating back to when treatment was discontinued is also not typically available and provides a unique opportunity to identify events. However, this study has limitations that caution the interpretation of the results. The study was observational, and thus, selection bias may limit the generalizability of the results because the rates of events may be different in different populations, and patients included in this study were 92% female and younger than most patients enrolled in the ongoing randomized trials; thus, ours may have a higher rate of disease activity. Furthermore, retrospective studies are susceptible to ascertainment bias, even with chart validation, and missing information such as reason for treatment discontinuation, or fact that 38% of patients with physician-defined attacks did not have MRI correlate, which raises suspicion for pseudorelapse, may have important implications for interpretation of results; thus, prospective validation is needed. Despite the CLIMB study being such a large observational cohort, the number of patients who discontinued treatment after a period of stability and met inclusion criteria was small and there were relatively few events, which makes the power to detect an association with biomarker levels limited, though stresses the uniqueness of this cohort. Therefore, the findings of this study, especially the clinical relevance of a 100% increase in sNfL and 50% increase in sGFAP after treatment stop, need to be validated in an independent cohort. Finally, most of the patients discontinued lower-efficacy treatments, and postdiscontinuation biomarker dynamics after discontinuing high-efficacy treatments may differ. Rates of disease activity after discontinuing higher-efficacy cell depleting treatments, and the association of biomarker dynamics after treatment stop, will also require further study.
Patients with MS who are stable for many years on treatment may be at low risk of future disease activity and potentially can safely stop treatment. We found increasing sNfL and sGFAP levels after discontinuing treatment may identify patients at risk of future MS disease activity, suggesting patients who wish to stop treatment ought to have biomarker measurements before and after stopping treatment. An independent cohort is needed to validate these findings and determine whether resuming treatment if biomarker levels increase can prevent eventual disease activity.
Acknowledgment
The authors thank the Brigham Multiple Sclerosis Center neurologists and patients for contributing data to the CLIMB study and Tzu-Ying Chung and Taylor Saraceno for their assistance with the project.
Glossary
- aHR
adjusted hazard ratio
- AUC
area under the curve
- CDW
confirmed disability worsening
- CV
coefficients of variation
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- EQC
endogenous quality controls
- IQR
interquartile range
- MS
multiple sclerosis
- sGFAP
glial fibrillary acidic protein
- SiMoA
single-molecule array
- sNfL
serum neurofilament light chain
- T2LV
T2 lesion volume
Appendix. Authors
| Name | Location | Contribution |
| Gauruv Bose, MD | Department of Neurology, Brigham and Women's Hospital, Boston, MA; Harvard Medical School; The University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Brian C. Healy, PhD | Department of Neurology, Brigham and Women's Hospital; Harvard Medical School, Boston, MA | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Shrishti Saxena, MSc | Department of Neurology, Brigham and Women's Hospital, Boston, MA | Analysis or interpretation of data |
| Fermisk Saleh, BS | Department of Neurology, Brigham and Women's Hospital, Boston, MA | Analysis or interpretation of data |
| Bonnie I. Glanz, PhD | Department of Neurology, Brigham and Women's Hospital; Harvard Medical School, Boston, MA | Analysis or interpretation of data |
| Rohit Bakshi, MD | Department of Neurology, Brigham and Women's Hospital; Harvard Medical School, Boston, MA | Analysis or interpretation of data |
| Howard L. Weiner, MD | Department of Neurology, Brigham and Women's Hospital; Harvard Medical School, Boston, MA | Analysis or interpretation of data |
| Tanuja Chitnis, MD | Department of Neurology, Brigham and Women's Hospital; Harvard Medical School, Boston, MA | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
Study Funding
This work was supported by endMS PostDoctoral Fellowship award EG3858 from the Multiple Sclerosis Society of Canada (to G. Bose) and the Department of Defense through the Multiple Sclerosis Research Program under Award No. W81XWH-18-1-0648 (to T. Chitnis); opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Disclosure
G. Bose has received an endMS PostDoctoral Fellowship award from the Multiple Sclerosis Society of Canada. B.C. Healy has received research support from Analysis Group, Celgene (Bristol-Myers Squibb), Verily Life Sciences, Merck-Serono, Novartis, and Genzyme. S. Saxena reports no disclosures. F. Saleh reports no disclosures. B.I. Glanz has received grant support from Merck Serono and Verily Life Sciences. R. Bakshi has received consulting fees from Bristol-Myers Squibb and EMD Serono and research support from Bristol-Myers Squibb, EMD Serono, Novartis, the US Department of Defense, the NIH, and the National Multiple Sclerosis Society. H.L. Weiner has received research support from Cure Alzheimer's Fund, EMD Serono, Inc., Genentech, Inc., NIH, National Multiple Sclerosis Society, Sanofi Genzyme, and Verily Life Sciences. He has received payment for consulting from Genentech, Inc, MedDay Pharmaceuticals, Tiziana Life Sciences, and vTv Therapeutics. T. Chitnis has received compensation for consulting from Biogen, Novartis Pharmaceuticals, Roche Genentech, and Sanofi Genzyme. She has received research support from the NIH, National MS Society, US Department of Defense, EMD Serono, I-Mab Biopharma, Mallinckrodt ARD, Novartis Pharmaceuticals, Octave Bioscience, Roche Genentech, and Tiziana Life Sciences. Go to Neurology.org/NN for full disclosures.
References
- 1.Prosperini L, Haggiag S, Tortorella C, Galgani S, Gasperini C. Age-related adverse events of disease-modifying treatments for multiple sclerosis: a meta-regression. Mult Scler J. 2021;27(9):1391-1402. doi: 10.1177/1352458520964778 [DOI] [PubMed] [Google Scholar]
- 2.Owens GM. Economic burden of multiple sclerosis and the role of managed sare organizations in multiple sclerosis management. Am J Manag Care. 2016;22(6 Suppl):s151-s158. [PubMed] [Google Scholar]
- 3.Hillert J, Magyari M, Soelberg Sørensen P, et al. Treatment switching and discontinuation over 20 years in the big multiple sclerosis data network. Front Neurol. 2021;12:647811. doi: 10.3389/fneur.2021.647811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose G, Freedman MS. Precision medicine in the multiple sclerosis clinic: selecting the right patient for the right treatment. Mult Scler J. 2020;26(5):540-547. doi: 10.1177/1352458519887324 [DOI] [PubMed] [Google Scholar]
- 5.Freedman MS, Devonshire V, Duquette P, et al. Treatment optimization in multiple sclerosis: Canadian MS working group recommendations. Can J Neurol Sci. 2020;47(4):437-455. doi: 10.1017/cjn.2020.66 [DOI] [PubMed] [Google Scholar]
- 6.Yano H, Gonzalez C, Healy BC, Glanz BI, Weiner HL, Chitnis T. Discontinuation of disease-modifying therapy for patients with relapsing-remitting multiple sclerosis: effect on clinical and MRI outcomes. Mult Scler Relat Disord. 2019;35:119-127. doi: 10.1016/j.msard.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 7.McFaul D, Hakopian NN, Smith JB, Nielsen AS, Langer-Gould A. Defining benign/burnt-out MS and discontinuing disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e960. doi: 10.1212/NXI.0000000000000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monschein T, Salhofer-Polanyi S, Altmann P, et al. Should I stop or should I go on? Disease modifying therapy after the first clinical episode of multiple sclerosis. J Neurol. 2021;268(4):1247-1253. doi: 10.1007/s00415-020-10074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. 2018;391:72-76. doi: 10.1016/j.jns.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 10.Kaminsky A-L, Omorou AY, Soudant M, et al. Discontinuation of disease-modifying treatments for multiple sclerosis in patients aged over 50 with disease Inactivity. J Neurol. 2020;267(12):3518-3527. doi: 10.1007/s00415-020-10029-9 [DOI] [PubMed] [Google Scholar]
- 11.Hua LH, Fan TH, Conway D, Thompson N, Kinzy TG. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler J. 2019;25(5):699-708. doi: 10.1177/1352458518765656 [DOI] [PubMed] [Google Scholar]
- 12.Pasca M, Forci B, Mariottini A, et al. Sustained disease remission after discontinuation of disease modifying treatments in relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2021;47:102591. doi: 10.1016/j.msard.2020.102591 [DOI] [PubMed] [Google Scholar]
- 13.Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis—clinical outcome and prognostic factors. Mult Scler J. 2017;23(9):1241-1248. doi: 10.1177/1352458516675751 [DOI] [PubMed] [Google Scholar]
- 14.Corboy JR, Fox RJ, Kister I, et al. Risk of new disease activity in patients with multiple sclerosis who continue or discontinue disease-modifying therapies (DISCOMS): a multicentre, randomised, single-blind, phase 4, non-inferiority trial. Lancet Neurol. 2023;22(7):568-577. doi: 10.1016/s1474-4422(23)00154-0 [DOI] [PubMed] [Google Scholar]
- 15.Thebault S, Bose G, Booth R, Freedman MS. Serum neurofilament light in MS: the first true blood-based biomarker? Mult Scler J. 2021;28(10):1491-1497. doi: 10.1177/1352458521993066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10‐year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol. 2018;5(12):1478-1491. doi: 10.1002/acn3.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosso M, Gonzalez CT, Healy BC, et al. Temporal association of sNfL and gad‐enhancing lesions in multiple sclerosis. Ann Clin Transl Neurol. 2020;7(6):945-955. doi: 10.1002/acn3.51060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delcoigne B, Manouchehrinia A, Barro C, et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology. 2020;94(11):e1201-e1212. doi: 10.1212/WNL.0000000000009097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 2019;76(11):1359. doi: 10.1001/jamaneurol.2019.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bose G, Healy BC, Saxena S, et al. Early neurofilament light and glial fibrillary acidic protein levels improve predictive models of multiple sclerosis outcomes. Mult Scler Relat Disord. 2023;74:104695. doi: 10.1016/j.msard.2023.104695 [DOI] [PubMed] [Google Scholar]
- 22.Lin T-Y, Vitkova V, Asseyer S, et al. Increased Serum neurofilament light and thin ganglion cell–inner plexiform layer are additive risk factors for disease activity in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1051. doi: 10.1212/NXI.0000000000001051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8:14798. doi: 10.1038/s41598-018-33158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayrignac X, Le Bars E, Duflos C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep. 2020;10(1):10923. doi: 10.1038/s41598-020-67934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauthier SA, Glanz BI, Mandel M, Weiner HL. A model for the comprehensive investigation of a chronic autoimmune disease: the multiple sclerosis CLIMB study. Autoimmun Rev. 2006;5(8):532-536. doi: 10.1016/j.autrev.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 26.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/s1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 27.D'Souza M, Yaldizli Ö, John R, et al. Neurostatus e-Scoring improves consistency of Expanded Disability Status Scale assessments: a proof of concept study. Mult Scler J. 2017;23(4):597-603. doi: 10.1177/1352458516657439 [DOI] [PubMed] [Google Scholar]
- 28.Meier DS, Guttmann CRG, Tummala S, et al. Dual-sensitivity multiple sclerosis lesion and CSF segmentation for multichannel 3T brain MRI. J Neuroimaging. 2018;28(1):36-47. doi: 10.1111/jon.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei X, Warfield SK, Zou KH, et al. Quantitative analysis of MRI signal abnormalities of brain white matter with high reproducibility and accuracy. J Magn Reson Imaging. 2002;15(2):203-209. doi: 10.1002/jmri.10053 [DOI] [PubMed] [Google Scholar]
- 30.Wilson DH, Rissin DM, Kan CW, et al. The simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. 2016;21(4):533-547. doi: 10.1177/2211068215589580 [DOI] [PubMed] [Google Scholar]
- 31.Beydoun MA, Noren Hooten N, Beydoun HA, et al. Plasma neurofilament light as a potential biomarker for cognitive decline in a longitudinal study of middle-aged urban adults. Transl Psychiatry. 2021;11(1):436. doi: 10.1038/s41398-021-01563-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105-1117. doi: 10.1002/sim.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longato E, Vettoretti M, Di Camillo B. A practical perspective on the concordance index for the evaluation and selection of prognostic time-to-event models. J Biomed Inform. 2020;108:103496. doi: 10.1016/j.jbi.2020.103496 [DOI] [PubMed] [Google Scholar]
- 34.Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31(23):2676-2686. doi: 10.1002/sim.4509 [DOI] [PubMed] [Google Scholar]
- 35.Barro C, Healy BC, Liu Y, et al. Serum GFAP and NfL levels differentiate subsequent progression and disease activity in patients with progressive multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200052. doi: 10.1212/NXI.0000000000200052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology. 2017;88(9):826-831. doi: 10.1212/wnl.0000000000003653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-2237. doi: 10.1212/WNL.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/s1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 39.Thebault S, Reaume M, Marrie RA, et al. High or increasing serum NfL is predictive of impending multiple sclerosis relapses. Mult Scler Relat Disord. 2022;59:103535. doi: 10.1016/j.msard.2022.103535 [DOI] [PubMed] [Google Scholar]
- 40.Thebault S, R Tessier D, Lee H, et al. High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e598. doi: 10.1212/NXI.0000000000000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentino P, Marnetto F, Martire S, et al. Serum neurofilament light chain levels in healthy individuals: a proposal of cut-off values for use in multiple sclerosis clinical practice. Mult Scler Relat Disord. 2021;54:103090. doi: 10.1016/j.msard.2021.103090 [DOI] [PubMed] [Google Scholar]
- 42.Pauwels A, Van Schependom J, Devolder L, et al. Plasma glial fibrillary acidic protein and neurofilament light chain in relation to disability worsening in multiple sclerosis. Mult Scler J. 2022;28(11):1685-1696. doi: 10.1177/13524585221094224 [DOI] [PubMed] [Google Scholar]
- 43.Saraste M, Bezukladova S, Matilainen M, et al. Increased serum glial fibrillary acidic protein associates with microstructural white matter damage in multiple sclerosis. Mult Scler Relat Disord. 2021;50:102810. doi: 10.1016/j.msard.2021.102810 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article and code will be made available by request from any qualified investigator.