Abstract
We have previously shown the immunological mimicry of human sialyl-Lewisx (CD15s) by a surface antigen of Streptococcus pyogenes. This mimicking surface antigen may act as a ligand to the selectin family and may induce antibody production against CD15s on host cells, suggesting a possible role in the pathogenesis of S. pyogenes. In this study, the effects of antibiotics on the CD15s-related antigen expression of S. pyogenes were examined at a concentration below the MIC (sub-MIC). The amounts of CD15s on the surfaces of S. pyogenes cells and on the surfaces of S. pyogenes biofilms were determined by a whole-cell enzyme-linked immunosorbent assay and by laser scanning fluorescence microscopy, respectively, by using an anti-CD15s monoclonal antibody. At the sub-MICs, fosfomycin (1R,2S-1,2-epoxypropyl phosphonic acid), its enantiomer (1S,2R-1,2-epoxypropyl phosphonic acid), and benzylpenicillin significantly inhibited the CD15s expression of all strains studied. The effects of fosfomycin and its enantiomer on biofilms were also observed by scanning electron microscopy. Incubation of S. pyogenes with the sub-MIC of fosfomycin or its enantiomer, which has no antibacterial activity, reduced the amount of CD15s on the biofilm surface and made it smooth. These results suggest that fosfomycin or its enantiomer might be useful for preventing S. pyogenes adherence to human CD15s receptors and the resulting immunological pathogenicity.
Sialyl-Lewisx (CD15s; Neu5Ac alpha 2-3 Gal beta 1-4 [Fuc alpha 1-3] GlcNAc beta 1-R; a receptor for the selectin family) and related antigens are expressed on human neutrophils, monocytes (6, 22, 26), various adenocarcinomas (13–15, 23), Schistosoma mansoni (29), Helicobacter pylori (1, 3), and Streptococcus gallolyticus (12). We previously demonstrated that an anti-CD15s monoclonal antibody (MAb) (26) reacted with a cell surface antigen of Streptococcus pyogenes (11). The role of the CD15s-related antigen in the pathogenesis of S. pyogenes has not been studied in detail. The expression on S. pyogenes of an antigen that mimics the host structure may camouflage S. pyogenes after infection (16, 19), thereby aiding survival and successful colonization. S. pyogenes possesses multiple adhesins: lipoteichoic acid, fibronectin-binding protein, M protein, vitronectin-binding protein, and C carbohydrate (9). In addition, CD15s on the streptococcal surface may act as an adhesin to the selectin family expressed on host cells such as endothelial cells and neutrophils. Infection with a bacterium expressing CD15s-related antigen possibly induces antibody specific for CD15s, which has a potential role in autoimmunity, as suggested for H. pylori and S. mansoni (1, 29).
Brook et al. (2) reported that sub-MICs of penicillin and clindamycin reduce the level of expression of the S. pyogenes capsule. However, the effects of antibiotics on CD15s-related antigen expression have not been studied. In this study, therefore, the effects of sub-MICs of antibiotics on CD15s-related antigen expression by S. pyogenes and on S. pyogenes biofilms were determined by an enzyme-linked immunosorbent assay (ELISA) and laser scanning fluorescence microscopy. The morphological changes in S. pyogenes biofilms as a result of treatment with antibiotics at sub-MICs were studied by scanning electron microscopy.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. pyogenes ATCC 19615 was isolated from a patient with a sore throat. S. pyogenes TDP-1 (M type 1), TDP-3 (M type 3), and TDP-4 (M type 12) are isolates from children with acute glomerulonephritis (11). S. pyogenes TDP-11 (M type 6) is an isolate from an upper pharynx tumor. S. pyogenes A374 (M type 12) is an isolate from a patient with poststreptococcal glomerulonephritis (24). Serotypes M1 and M3 are reported to be particularly associated with invasive disease and fatal infections (4, 17), and M6 and M12 are potentially nephritogenic types (7). All strains were cultured in brain heart infusion broth (BHI; Difco, Detroit, Mich.) supplemented with 0.5% glucose for 18 h at 37°C.
Antibiotics and MIC determination.
The antibiotics used in this study were fosfomycin (1R,2S-1,2-epoxypropyl phosphonic acid), the enantiomer of fosfomycin (1S,2R-1,2-epoxypropyl phosphonic acid), benzylpenicillin, cefditoren, streptomycin (all five antimicrobial agents were from Meiji Seika Kaisha Ltd., Tokyo, Japan), minocycline (Lederle Japan, Tokyo, Japan), ofloxacin (Daiichi Seiyaku, Tokyo, Japan), and erythromycin (Wako Jun-yaku, Osaka, Japan). Antibiotic susceptibility was determined by a broth microdilution method. Briefly, 10 μl of an S. pyogenes whole-cell suspension was added to 100 μl of a serial twofold dilution of antibiotics in Anaerobe Broth MIC (Difco) in wells of a microtiter plate to achieve a final concentration of 106 organisms per ml. The plate was incubated overnight at 37°C in an atmosphere of 5% CO2. The MIC was the lowest concentration of antibiotic which yielded no bacterial growth.
Whole-cell ELISA.
CD15s expression on bacterial surfaces was measured as described previously (11). CD15s-polyacrylamide polymer (CD15s-PA; monosaccharide composition; Neu5Ac, 9.3 mol%; Fuc, 10.1 mol%; Gal, 9.6 mol%; GlcNAc, 9.5 mol%; Seikagaku Kogyo, Tokyo, Japan) was used as a positive control. Briefly, 50 μl of a whole-cell suspension (2.0 × 108 CFU/ml) or CD15s-PA suspension was divided into aliquots, and each aliquot was placed into individual wells of an ELISA plate (MS-8696F; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) and allowed to dry overnight at 37°C. The wells were pretreated with 1% bovine serum albumin in 0.01 M phosphate-buffered saline (PBS; pH 7.2) for 1 h at room temperature. After washing three times with PBS, anti-CD15s MAb SNH-3 (diluted to 1:200; Wako Pure Chemical, Osaka, Japan) (24) was added to each well and the plate was allowed to stand at room temperature for 1 h. The plate was again washed as described above, horseradish peroxidase-labeled goat anti-mouse immunoglobulin M (IgM; diluted to 1:2,000; μ chain; Cappel Research Products, Durham, N.C.) was added to each well, and the plate was stored at room temperature for 1 h. After washing, 50 μl of a mixture of H2O2 and 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulfonate) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added. The absorbance at 414 nm of each well was measured with an immunoreader (model 2550; Bio-Rad Laboratories, Richmond, Calif.). Control wells contained the second antibody and the substrate mixture alone.
To investigate the effects of antibiotics on CD15s expression, bacterial cells were incubated in the presence of sub-MICs of antibiotic at 37°C for 1 h. Bacterial cells were harvested by centrifugation, washed, resuspended, and used for whole-cell ELISA.
Biofilm formation and antibiotic treatment.
We examined the effects of fosfomycin and its enantiomer on an S. pyogenes biofilm with an in vitro system. S. pyogenes was grown overnight in BHI (Difco) at 37°C. Bacterial cells were harvested by centrifugation and washed with PBS. The cells were resuspended at a concentration of 2.0 × 109 CFU/ml, a 100-μl aliquot was inoculated into each well of a 24-well multiplate containing 900 μl of fresh BHI and a plastic coverslip (cell desk; diameter, 13.5 mm; Sumitomo Bakelite Co., Ltd.), and the plates were incubated for 4 days at 37°C. After S. pyogenes formed a biofilm on the cell desk, antibiotics or BHI, as a control, was added to each well and the plates were incubated for an additional 1 h at 37°C. Biofilms were observed by laser scanning fluorescence microscopy and scanning electron microscopy.
Laser scanning fluorescence microscopy.
The S. pyogenes biofilm was observed by laser scanning fluorescence microscopy (ACAS 570; Meridian Instruments, Okemos, Mich.) with MAb SNH-3. Samples were reacted with MAb SNH-3 (200 μg/100 μl, diluted to 1:500; Wako Pure Chemical), followed by a reaction with fluorescein isothiocyanate-labeled goat anti-mouse IgM (diluted to 1:2,000; μ chain; Cappel Research Products) at room temperature for 1 h. The fluorescence was measured at 488 nm by laser scanning fluorescence microscopy. Data were processed by image analysis and line analysis by using the complement data program of the ACAS software system (Meridian Instruments). A complementary image which replicated the real immunohistomorphology was obtained.
Scanning electron microscopy.
S. pyogenes biofilms formed on a plastic coverslip were fixed in 0.1 M cacodylate buffer (pH 7.2) with 2.5% glutaraldehyde for 1 h at room temperature. The samples were dehydrated with a series of ethanol solutions which ranged in 10% increments from 50% (vol/vol) ethanol in distilled water to absolute ethanol. All samples were dried to the critical point with a critical point drier, coated with gold, and examined by scanning electron microscopy (Hitachi S-800; Hitachi, Tokyo, Japan).
RESULTS
The MICs of the antibiotics for the S. pyogenes strains used in this study are presented in Table 1. The MIC of fosfomycin was 32 μg/ml for all strains, and the enantiomer of fosfomycin had no growth-inhibitory activity at 128 μg/ml. No strains were found to be resistant to any of the other antibiotics tested. Fosfomycin, its enantiomer, and benzylpenicillin significantly reduced the amount of CD15s antigen of S. pyogenes at concentrations lower than their MICs (Table 2). The level of reduction of CD15s as a result of treatment with fosfomycin and its enantiomer was greater than that as a result of treatment with benzylpenicillin.
TABLE 1.
Antibiotic susceptibility of S. pyogenes
| Antibiotic | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| ATCC 19615 | TDP-1 | TDP-3 | TDP-4 | A374 | |
| Fosfomycin | 32 | 32 | 32 | 32 | 32 |
| Enantiomer of fosfomycin | >128 | >128 | >128 | >128 | >128 |
| Benzylpenicillin | 0.016 | 0.016 | 0.016 | 0.031 | 0.016 |
| Cefditoren | 0.008 | 0.008 | 0.008 | 0.016 | 0.008 |
| Erythromycin | 0.016 | 0.016 | 0.016 | 0.016 | 0.031 |
| Minocycline | 0.031 | 0.031 | 0.031 | 0.016 | 0.016 |
| Ofloxacin | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 |
| Streptomycin | 0.063 | 0.063 | 0.016 | 0.031 | 0.031 |
TABLE 2.
Effects of antibiotics on CD15s of S. pyogenes
| Antibiotic | Amt of CD15s (absorbance at 414 nm)
|
||||
|---|---|---|---|---|---|
| ATCC 19615 | TDP-1 | TDP-3 | TDP-4 | A374 | |
| Control | 0.870 ± 0.014 | 0.864 ± 0.006 | 0.829 ± 0.016 | 0.852 ± 0.011 | 0.842 ± 0.013 |
| Fosfomycin | 0.623 ± 0.027a | 0.614 ± 0.011a | 0.610 ± 0.009a | 0.631 ± 0.017a | 0.606 ± 0.006a |
| Enantiomer of fosfomycin | 0.625 ± 0.020a | 0.614 ± 0.011a | 0.603 ± 0.010a | 0.606 ± 0.009a | 0.614 ± 0.009a |
| Benzylpenicillin | 0.714 ± 0.009a | 0.690 ± 0.041a | 0.685 ± 0.030a | 0.676 ± 0.022a | 0.713 ± 0.019a |
| Cefditoren | 0.871 ± 0.019 | 0.865 ± 0.007 | 0.804 ± 0.012 | 0.821 ± 0.010 | 0.817 ± 0.011 |
| Erythromycin | 0.822 ± 0.030 | 0.790 ± 0.027 | 0.768 ± 0.038 | 0.801 ± 0.033 | 0.801 ± 0.048 |
| Minocycline | 0.781 ± 0.044 | 0.818 ± 0.014 | 0.795 ± 0.029 | 0.798 ± 0.016 | 0.813 ± 0.010 |
| Ofloxacin | 0.803 ± 0.011 | 0.808 ± 0.020 | 0.799 ± 0.016 | 0.794 ± 0.038 | 0.802 ± 0.024 |
| Streptomycin | 0.803 ± 0.012 | 0.823 ± 0.027 | 0.802 ± 0.018 | 0.797 ± 0.014 | 0.806 ± 0.018 |
P < 0.01.
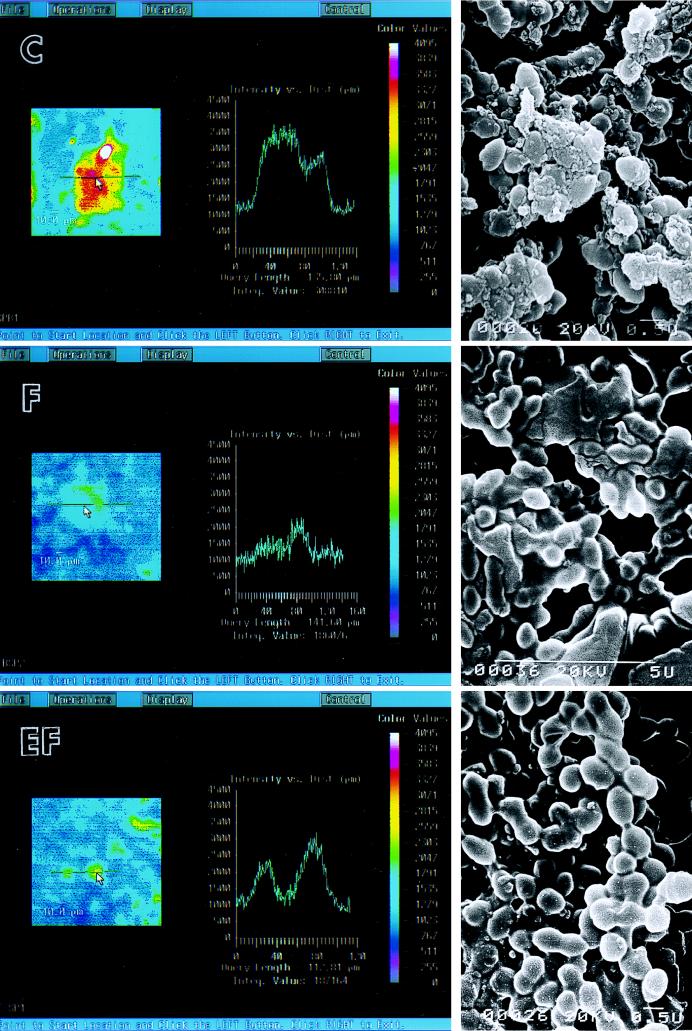
The effect of fosfomycin and its enantiomer on an S. pyogenes biofilm was studied in an in vitro system. S. pyogenes ATCC 19615 formed a biofilm well under the conditions that we used (Fig. 1). CD15s expression on the biofilms detected by laser scanning fluorescence microscopy was shown by image analysis and line analysis (Fig. 2, left and center). Anti-CD15s MAb-reactive sites were demonstrated as white, red, and yellow areas contrasted with a blue background. The scale to the right of the image gives the intensity of fluorescence (color values of 0 to 4,095). Line analysis indicates the staining intensities of the surface of the S. pyogenes biofilm in a cut line. The intensity of the control biofilm was an integrated value of 308,810 with a query length of 135.80 μm, the intensity of the biofilm treated with fosfomycin at the sub-MIC was an integrated value of 186,076 with a query length of 141.60 μm, and the intensity of the biofilm treated with the fosfomycin enantiomer was an integrated value of 187,164 with a query length of 112.81 μm. Strong fluorescence was seen on the surface of the control biofilm. Treatment with fosfomycin or its enantiomer at their sub-MICs significantly reduced the amount of CD15s on the biofilm surface, as shown either by image analysis or by line analysis. The fosfomycin enantiomer demonstrated slightly less of an effect compared with the effect of fosfomycin.
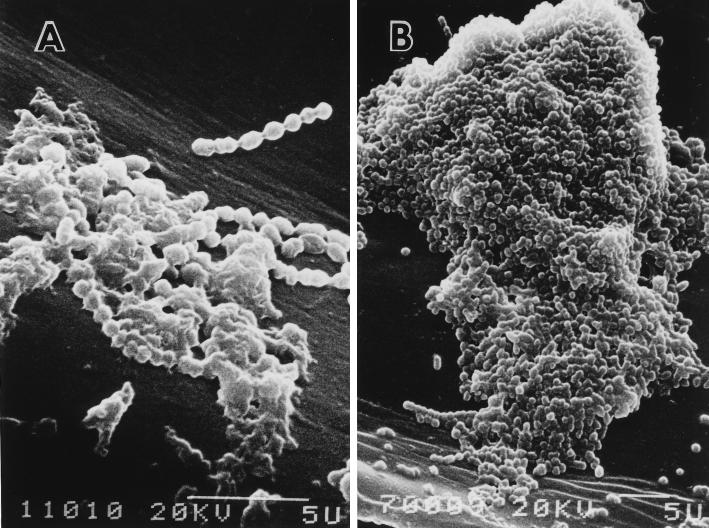
FIG. 1.
S. pyogenes ATCC 19615 biofilm in vitro. (A) One-day culture in antibiotic-free medium. (B) Four-day culture in antibiotic-free medium.
FIG. 2.
Effects of antibiotics on CD15s expression in S. pyogenes biofilms in vitro. (Left) Image analysis by laser scanning fluorescence microscopy; (center) line analysis; (right) scanning electron microscopy. C, control; F, fosfomycin; EF, enantiomer of fosfomycin. The scale to the right of the image gives the intensity of fluorescence (color values of 0 to 4,095). An S. pyogenes biofilm was treated with fosfomycin or its enantiomer at the sub-MIC (5 μg/ml). Biofilms were observed by laser scanning fluorescence microscopy to demonstrate the amount of CD15s and by scanning electron microscopy. A sample for laser scanning fluorescence microscopy reacted with an anti-CD15s MAb and fluorescein isothiocyanate-labeled goat anti-mouse IgM. Bars, 10.0 μm.
When observed by scanning electron microscopy, the surfaces of the bacterial cells in the control biofilm seemed rough and had tiny particle-like substances. The bacterial cells in an antibiotic-treated biofilm appeared to be smoother than those of the control biofilm, but they were still covered with a glycocalyx (Fig. 2, right). Within the antibiotic treatment period used in this study sub-MICs of fosfomycin and its enantiomer significantly reduced the level of expression of CD15s, although they produced no pronounced change in the biofilm structure.
DISCUSSION
Bacterial adherence is an essential step in the initiation of bacterial infection. Many kinds of molecules on the bacterial cell surface mediate bacterial adherence to the host, and these are called adhesins. It is well known that CD15s is a ligand for the human selectin family. The selectin family is mainly expressed on an inflamed endothelium, activated platelets, and lymphocytes. It is therefore possible that CD15s on the streptococcal surface acts as an adhesin to human cells and plays a role in initial adherence and bacterial translocation.
In the present study we demonstrated that fosfomycin, the enantiomer of fosfomycin, and benzylpenicillin reduced the amount of CD15s expressed on the surfaces of S. pyogenes cells. Benzylpenicillin and fosfomycin are cell wall inhibitors that act during different steps of cell wall synthesis. These agents may affect the surface structure by affecting the cell wall structure. Penicillin was reported to inhibit the formation of S. pyogenes capsules, although the effect was not as strong as that of clindamycin (2). Cefditoren, a cephem, however, did not reduce the amount of CD15s, although that agent is also a cell wall inhibitor.
It is of interest that treatment with the enantiomer of fosfomycin, which has no detectable effect on bacterial growth, resulted in a significant change in the amount of CD15s. The fact that the enantiomer of fosfomycin has an effect almost equivalent to that of fosfomycin suggests that their respective activities, other than growth inhibition, play a role in the suppression of CD15s. Fosfomycin is reported to possess a wide variety of biological activities other than bacterial growth inhibition or bactericidal action. These include an effect on human T-lymphocyte function (20), an effect on cytokine production by human monocytes (21), and an effect that reduces the toxicity caused by cisplatin (30). Some of these activities are also shown by the enantiomer of fosfomycin. Fosfomycin is similar in structure to phosphoenol pyruvate, which is involved in several biosynthetic pathways (17). The fact that the enantiomer is not a functional inhibitor of one class of enzymes (such as those involved in cell wall biosynthesis) does not necessarily mean that it cannot inhibit other putative classes (such as those involved in capsular biosynthesis).
Although antibiotic treatment of planktonic cells was assessed by ELISA, bacteria infecting the host usually exist as sessile or biofilm cells. It is therefore of importance to investigate the effects of sessile or biofilm cells on CD15s. Experiments with biofilms focused on the effects of fosfomycin and its enantiomer. When used to treat S. pyogenes biofilms in vitro, fosfomycin and its enantiomer at their sub-MICs reduced the level of antigen expression on the biofilm surface, although these agents did not cause significant morphological changes in the biofilm. High IgM and IgG titers against S. pyogenes surface antigens were observed in the sera of S. pyogenes-infected patients (8, 10). CD15s-bearing glycolipids were quite abundant on neutrophils, with 2 × 107 copies/cell (27) and a calculated density of CD15s-bearing glycolipids on the neutrophil surface of 44,000 molecules/μm (5, 22, 28). Anti-CD15 antibodies are produced in patients with infections caused by S. mansoni, which expresses CD15, and these, together with complement, cause lysis of human neutrophils (29). A potential role of autoimmunity from molecular mimicry of the human Lewis blood group antigen has also been suggested for H. pylori (1). It is probable that human neutrophils and other cells that express CD15s might be recognized by anti-CD15s antibodies produced against S. pyogenes. The effect of fosfomycin and its enantiomer on the streptococcal antigen may alter the immunological pathogenesis of S. pyogenes.
Although further experiments are needed to elucidate the pathogenesis of the streptococcal CD15s antigen, because of their suppressive effects, fosfomycin and its enantiomer could be used to treat streptococcal infections. Specifically, the enantiomer of fosfomycin is a unique agent that reduces the amount of streptococcal CD15s without affecting human commensal bacteria.
ACKNOWLEDGMENT
This work was supported in part by a grant-in-aid for scientific research (grant 09671857) from The Ministry of Education, Science, and Culture of Japan.
REFERENCES
- 1.Appelmelk B J, Simoons-Smit I, Negrini R, Moran A P, Aspinall G O, Forte J G, De Vries T, Quan H, Verboom T, Maaskant J J, Ghiara P, Kuipers E J, Bloemena E, Tadema M M, Townsend R R, Tyagarajan K, Crothers J M, Jr, Monteiro M A, Savio A, De Graaff J. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook I, Gober A E, Leyva F. In vitro and in vivo effects of penicillin and clindamycin on expression of group A beta-hemolytic streptococcal capsule. Antimicrob Agents Chemother. 1995;39:1565–1568. doi: 10.1128/aac.39.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan N W, Stangier K, Sherburne R, Taylor D E, Zhang Y, Dovichi N J, Palcic M M. The biosynthesis of Lewis X in Helicobacter pylori. Glycobiology. 1995;5:683–688. doi: 10.1093/glycob/5.7.683. [DOI] [PubMed] [Google Scholar]
- 4.Colman G, Tanna A, Efstratiou A, Gaworzewska E T. The serotypes of Streptococcus pyogenes present in Britain during 1980–1990 and their association with disease. J Med Microbiol. 1993;39:165–178. doi: 10.1099/00222615-39-3-165. [DOI] [PubMed] [Google Scholar]
- 5.Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56:151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foxall C, Watson S R, Dowbenko D, Fennie C, Lasky L A, Kiso M, Hasegawa A, Asa D, Brandley B K. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewisx oligosaccharide. J Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaworzewska E, Colman G. Changes in the pattern of infection caused by Streptococcus pyogenes. Epidemiol Infect. 1988;100:257–269. doi: 10.1017/s095026880006739x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halperin S A, Ferrieri P, Gray E D, Kaplan E L, Wannamaker L W. Antibody response to bacteriophage hyaluronidase in acute glomerulonephritis after group A streptococcal infection. J Infect Dis. 1987;155:253–261. doi: 10.1093/infdis/155.2.253. [DOI] [PubMed] [Google Scholar]
- 9.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi S, Yokota K, Takizawa Y, Tomizawa I, Nejime T, Oguma K. Development and evaluation of capture enzyme-linked immunosorbent assays for detection of immunoglobulin G and M antibodies to group A streptococcal antigens. Microbiol Immunol. 1993;37:271–279. doi: 10.1111/j.1348-0421.1993.tb03210.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirota K, Kanitani H, Nemoto K, Ono T, Miyake Y. Cross-reactivity between human sialyl Lewisx oligosaccharide and common causative oral bacteria of infective endocarditis. FEMS Immunol Med Microbiol. 1995;12:159–164. doi: 10.1111/j.1574-695X.1995.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirota K, Osawa R, Nemoto K, Ono T, Miyake Y. Highly expressed human sialyl Lewisx antigen on cell surface of Streptococcus gallolyticus. Lancet. 1996;347:760. doi: 10.1016/s0140-6736(96)90109-9. [DOI] [PubMed] [Google Scholar]
- 13.Hoff S D, Matsushita Y, Ota D M, Cleary K R, Yamori T, Hakomori S, Irimura T. Increased expression of sialyl-dimeric Lex antigen in liver metastases of human colorectal carcinoma. Cancer Res. 1989;49:6883–6888. [PubMed] [Google Scholar]
- 14.Itzkowitz S H, Yuan M, Fukushi Y, Palekar A, Phelps P C, Shamsuddin A M, Trump B F, Hakomori S, Kim Y S. Lewisx- and sialylated Lewisx-related antigen expression in human malignant and nonmalignant colonic tissues. Cancer Res. 1986;46:2627–2632. [PubMed] [Google Scholar]
- 15.Izumi Y, Taniuchi Y, Tsuji T, Smith C W, Nakamori S, Fidler I J, Irimura T. Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: liver colonization and adhesion to vascular endothelial cells. Exp Cell Res. 1995;216:215–221. doi: 10.1006/excr.1995.1027. [DOI] [PubMed] [Google Scholar]
- 16.Jann, K., and B. Jann. 1987. Polysaccharide antigens of Escherichia coli. Rev. Infect. Dis. 9(Suppl. 5):S517–S526. [DOI] [PubMed]
- 17.Kahan F M, Kahan J S, Cassidy P J, Kropp H. The mechanism of action of fosfomycin (phosphonomycin) Ann N Y Acad Sci. 1974;235:364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Murai T, Ichiyama S, Saito M, Arakawa Y, Ohta M. Prevalence of the spea2 and spea3 alleles in Streptococcus pyogenes isolated from TSLS patients in Japan. FEMS Microbiol Lett. 1997;150:233–237. doi: 10.1016/s0378-1097(97)00120-1. [DOI] [PubMed] [Google Scholar]
- 19.Moran A P, Prendergast M M, Appelmelk B J. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol. 1996;16:105–115. doi: 10.1111/j.1574-695X.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 20.Morikawa K, Oseko F, Morikawa S, Sawada M. Immunosuppressive activity of fosfomycin on human T-lymphocyte function in vitro. Antimicrob Agents Chemother. 1993;37:2684–2687. doi: 10.1128/aac.37.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro J M, Lo S K, Corless C, Robertson M J, Lee N C, Barnhill R L, Weinberg D S, Bevilacqua M P. Expression of sialyl-Lewis x, an E-selectin ligand, in inflammation, immune processes, and lymphoid tissues. Am J Pathol. 1992;141:1397–1408. [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 24.Ohkuni H, Todome Y, Suzuki H, Mizuse M, Kotani N, Horiuchi K, Shikama N, Tsugita A, Johnston K H. Immunochemical studies and complete amino acid sequence of the streptokinase from Streptococcus pyogenes (group A) M type 12 strain A374. Infect Immun. 1992;60:278–283. doi: 10.1128/iai.60.1.278-283.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohmori K, Takada A, Ohwaki I, Takahashi N, Furukawa Y, Maeda M, Kiso M, Hasegawa A, Kannagi M, Kannagi R. A distinct type of sialyl Lewis X antigen defined by novel monoclonal antibody is selectively expressed on helper memory T cells. Blood. 1993;82:2797–2805. [PubMed] [Google Scholar]
- 26.Phillips M L, Nudelman E, Gaeta F C, Perez M, Singhal A K, Hakomori S, Paulson J C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand sialyl-Lex. Science. 1990;250:1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 27.Symington F W, Hedges D L, Hakomori S. Glycolipid antigens of human polymorphonuclear neutrophils and the inducible HL-60 myeloid leukemia line. J Immunol. 1985;134:2498–2506. [PubMed] [Google Scholar]
- 28.Ting-Beall H P, Needham D, Hochmuth R M. Volume and osmotic properties of human neutrophils. Blood. 1993;81:2774–2780. [PubMed] [Google Scholar]
- 29.Van Dam G J, Bergwerff A A, Thomas-Oates J E, Rotmans J P, Kamerling J P, Vliegenthart J F, Deelder A M. The immunologically reactive O-linked polysaccharide chains derived from circulating cathodic antigen isolated from the human blood fluke Schistosoma mansoni have Lewis x as repeating unit. Eur J Biochem. 1994;225:467–482. doi: 10.1111/j.1432-1033.1994.00467.x. [DOI] [PubMed] [Google Scholar]
- 30.Wagner T, Kreft B, Bohlmann G, Schwieder G. Effect of fosfomycin, mesna, and sodium thiosulfate on the toxicity and antitumor activity of cisplatin. J Cancer Res Clin Oncol. 1988;114:497–501. doi: 10.1007/BF00391499. [DOI] [PMC free article] [PubMed] [Google Scholar]