Abstract
Population pharmacokinetic modeling is a useful approach to obtaining estimates of both population and individual pharmacokinetic parameter values. The potential for relating pharmacokinetic parameters to pharmacodynamic outcome variables, such as efficacy and toxicity, exists. A logistic regression relationship between the probability of a successful clinical and microbiological outcome and the peak concentration-to-MIC ratio (and also the area under the plasma concentration-time curve [AUC]/MIC ratio) has previously been developed for levofloxacin; however, levofloxacin assays for determination of the concentration in plasma are not readily available. We attempted to derive and validate demographic variable models to allow prediction of the peak concentration in plasma and clearance (CL) from plasma for levofloxacin. Two hundred seventy-two patients received levofloxacin intravenously for the treatment of community-acquired infection of the respiratory tract, skin or soft tissue, or urinary tract, and concentrations in plasma, guided by optimal sampling theory, were obtained. Patient data were analyzed by the Non-Parametric Expectation Maximization approach. Maximum a posteriori probability Bayesian estimation was used to generate individual parameter values, including CL. Peak concentrations were simulated from these estimates. The first 172 patients were used to produce demographic models for the prediction of CL and the peak concentration. The remaining 100 patients served as the validation group for the model. A median bias and median precision were calculated. A two-compartment model was used for the population pharmacokinetic analysis. The mean CL and the mean volume of distribution of the central compartment (V1) were 9.27 liters/h and 0.836 liter/kg, respectively. The mean values for the intercompartmental rate constants, the rate constant from the central compartment to the peripheral compartment (Kcp) and the rate constant from the peripheral compartment to the central compartment (Kpc), were 0.487 and 0.647 h−1, respectively. The mean peak concentration and the mean AUC values normalized to a dosage of 500 mg every 24 h were 8.67 μg/ml and 72.53 μg · h/ml, respectively. The variables included in the final model for the prediction of CL were creatinine clearance (CLCR), race, and age. The median bias and median precision were 0.5 and 18.3%, respectively. Peak concentrations were predicted by using the demographic model-predicted parameters of CL, V1, Kcp, and Kpc, in the simulation. The median bias and the median precision were 3.3 and 21.8%, respectively. A population model of the disposition of levofloxacin has been developed. Population demographic models for the prediction of peak concentration and CL from plasma have also been successfully developed. However, the performance of the model for the prediction of peak concentration was likely insufficient to be of adequate clinical utility. The model for the prediction of CL was relatively robust, with acceptable bias and precision, and explained a reasonable amount of the variance in the CL of levofloxacin from plasma in the population (r2 = 0.396). Estimated CLCR, age, and race were the final model covariates, with CLCR explaining most of the population variance in the CL of levofloxacin from plasma. This model can potentially optimize the benefit derived from the pharmacodynamic relationships previously developed for levofloxacin.
Population pharmacokinetic modeling has become a well-accepted method of obtaining information about the disposition of a drug. Several approaches to such modeling are being used, including NON-Linear Mixed Effects Modeling (NONMEM), Iterative Two Stage (IT25) population modeling, Non-Parametric Expectation Maximization (NPEM2) population modeling, and Non-Parametric Maximum Likelihood population modeling (NPML) (5, 7, 9, 10). While these programs provide information about the pharmacokinetics of drugs with regard to the entire population, it would be useful for one to be able to use the population pharmacokinetic data to obtain information about the pharmacokinetics for individual subjects. If pharmacodynamic relationships linking measures of plasma exposures to measures of outcome or toxicity were known, one could then use the individual patient estimates to optimize drug therapy.
Levofloxacin, the optical l isomer of ofloxacin, is a fluoroquinolone antimicrobial agent for which pharmacodynamic relationships between both the peak concentration in serum to the MIC (peak/MIC) ratio and the area under the concentration-time curve (AUC)/MIC ratio and both clinical and microbiological outcomes have been developed (8). It would be helpful to be able to use these relationships to maximize the efficacies of drugs in patients. Unfortunately, assays for the concentrations of fluoroquinolones in serum must be performed by the high-performance liquid chromatographic (HPLC) method or a microbiologically based assay. Such assays are labor- and technician time-intensive and HPLC is expensive, making them impractical for routine performance in the clinical setting. Given these constraints, it would be desirable to have a model based on patient demographics to obtain an estimate of the individual pharmacokinetic parameters. Peak concentrations in serum or AUC (approximated by the ratio of dose/clearance [CL]) can be estimated in order to calculate a peak/MIC ratio or AUC/MIC ratio and use the known pharmacodynamic relationships to achieve maximal benefit for a particular patient.
Our objective was to produce a population pharmacokinetic model for levofloxacin using data from an ill, infected, target population, generate maximum a posteriori probability (MAP) Bayesian estimates of individual patient parameters, and derive and validate a demographic variable model which would allow prediction of the peak concentration in plasma and CL from plasma for levofloxacin without the necessity of measuring the concentrations in plasma.
MATERIALS AND METHODS
Patient population.
Two hundred seventy-two patients were prospectively evaluated for model generation and model validation. Patients were 18 years of age or older and were being treated for a community-acquired infection of the skin or skin structure, respiratory tract, or urinary tract.
Drug dosage and administration.
Patients received levofloxacin at a dosage of 500 mg every 24 h by a 1-h intravenous infusion for the treatment of skin or skin structure or respiratory infection. A dosage of 250 mg intravenously (1-h infusion) every 24 h was used for the treatment of urinary tract infection. At least three doses of the drug was administered intravenously. After three doses of levofloxacin had been administered, multiple serum samples were obtained for determination of drug concentrations. Then, if clinically appropriate, the patients could be switched to an equivalent oral dose. For patients who were receiving the 500-mg dose and who had a calculated creatinine clearance (CLCR) of 20 to 50 ml/min (determined by the method of Cockcroft and Gault [2]), the dosing interval was extended to every 48 h. No dosage changes were made for patients receiving the 250-mg dose. Patients whose CLCR was <20 ml/min were excluded from participation in the clinical protocol.
Pharmacokinetic sampling schedule.
Pharmacokinetic parameter means and standard deviations for the volume of distribution of the central compartment (V1), CL, and the intercompartmental transfer rate constants (the rate constant from the central compartment to the peripheral compartment [Kcp] and the rate constant from the peripheral compartment to the central compartment [Kpc]) were obtained from a phase I clinical study conducted by the sponsor (6) and were used in designing the sampling schedule. The mean value and the values of the mean ± 1 standard deviation were then used to generate a model ensemble. The model ensemble was generated by using every possible combination of values for the four parameters (81 possible combinations; three possible values for each of four parameters). Each set of four parameter values was entered into the ADAPT II (4) SAMPLE module to obtain optimal sampling times. The optimal sampling times generated from the model were weighted by the probability of occurrence in the population (e.g., the “subject” whose parameter values were all the mean values for the population would have a higher probability of occurrence than a subject with parameter values which all differed by 1 standard deviation from the mean). The mean ± 1 standard deviation constituted 68.3% of the population. Each “tail” of the population (>1 standard deviation) accounted for 15.8% of the population. All calculations were performed with a D-optimal design criterion, which, when used, minimizes the determinant of the inverse Fisher information matrix (3). A histogram was used to plot the probabilities of information for the population at various time points. After evaluation of the histogram it was felt that a six-sample design (samples obtained at the time of the trough concentration, at the end of infusion, and at 2.0, 6.75, 7.75, and 9.25 h postdosing) represented the best compromise between limiting sample acquisition in the clinical setting and having enough data from informative time points to obtain robust individual parameter estimates in the MAP Bayesian step.
Assays for concentrations in plasma.
Plasma samples were analyzed by a validated reversed-phase HPLC method with UV (330 nm) detection (1). Briefly, the procedure used a single-step liquid-liquid extraction with methyl t-butyl ether. A reversed-phase C18 column was used to separate levofloxacin and the internal standard (ciprofloxacin). Elution was accomplished isocratically with a mobile phase consisting of 0.005 M copper (II) sulfate pentahydrate in 0.01 M isoleucine–methanol (87.5:12.5 [vol/vol]) at a flow rate of 1.0 ml/min. The interassay precision values (as percent coefficient of variation) were less than 10%. The quantification range in plasma was 0.08 to 5.12 μg/ml.
Population pharmacokinetic analysis.
Patient data were analyzed by the NPEM2 approach with a one- and a two-compartment open model with first-order elimination from the central compartment and a zero-order intravenous infusion. Akaike’s information criterion (11) was used to discriminate among models. Population pharmacokinetic parameters including CL (in liters per hour), V1 (in liters per kilogram), and Kcp and Kpc (in hours−1) were estimated. The assay variance was estimated by regression modeling on the basis of the observed variance at four different points throughout the range. The inverse of the estimated assay variance was used as the weighting in the pharmacokinetic model. By this approach, total observation variance is approximated by the assay variance. A change of less than 0.001% in the likelihood function was taken as the convergence criterion for NPEM2.
MAP Bayesian estimation was used to generate Bayesian posterior parameter values by using the population of one utility in the NPEM2 package of programs. The individual patient parameter estimates were then used in the simulation module of ADAPT II to obtain estimates of peak concentrations in plasma.
Demographic model generation.
Data for the first 172 patients were arbitrarily set aside, and these data were used to generate demographic models for prediction of pharmacokinetic parameter values. The general linear model module of SYSTAT (Evanston, Ill.) was used to develop the relationship, with peak concentration, CL, V1, Kcp, and Kpc serving as the dependent variables and site of infection, gender, race, age, body weight, serum creatinine level, inverse serum creatinine level, and CLCR serving as the independent variables (CLCR was estimated by the method of Cockcroft and Gault [2]). Of these, site of infection, gender, and race were treated as categorical variables, with the others being treated as continuous variables. All analyses were performed by a stepwise backward procedure. Criteria for model inclusion and removal were significance levels of 0.05 and 0.10, respectively. For the peak concentration analysis, Bayesian posterior estimated peak concentrations were normalized to a dose of 500 mg and a dosing interval of every 24 h. The covariates included in the final model were then used to predict parameter values and peak concentrations in the remaining 100 patients (an example of the method is presented in the Appendix). The bias and precision of these estimates were calculated for the predicted values for the 100 patients by using the values predicted by the model and the actual values obtained from MAP Bayesian estimation. Bias was calculated as percent error: {[observed parameter value (obs) − predicted parameter value (pred)]/obs value} × 100. Precision was calculated as percent absolute error: [(|obs − pred|)/obs] × 100. Median values of bias and precision are presented to describe the performance of the model with the 100-patient validation sample.
RESULTS
Pharmacokinetic analysis.
The two-compartment model was chosen as most appropriate on the basis of Akaike’s information criterion and visual inspection of the population scatter plots (data not shown) for one- and two-compartment models. The population pharmacokinetic parameter values obtained from the NPEM2 analysis are listed in Table 1. Mean peak concentration and AUC normalized to a dose of 500 mg and a dosing interval of every 24 h were 8.67 ± 3.99 μg/ml and 72.53 ± 51.17 μg · h/ml, respectively.
TABLE 1.
Levofloxacin population pharmacokinetic parametersa
| Value | Kcp (h−1) | Kpc (h−1) | VS (liter/kg)b | CL (liters/h) |
|---|---|---|---|---|
| Mean | 0.487 | 0.647 | 0.836 | 9.27 |
| Median | 0.384 | 0.596 | 0.795 | 9.01 |
| SD | 0.378 | 0.391 | 0.429 | 4.31 |
Data are for 272 patients.
VS, slope of V1 to body weight.
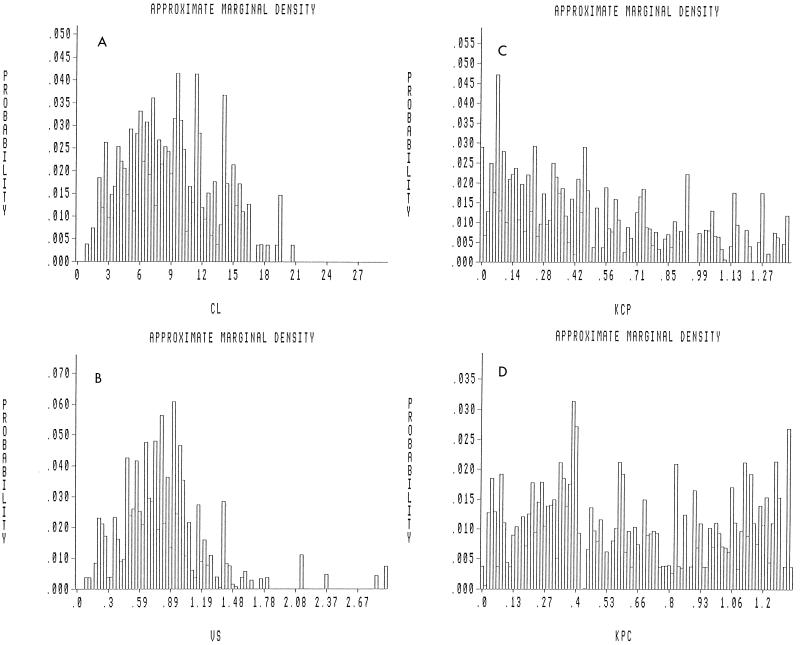
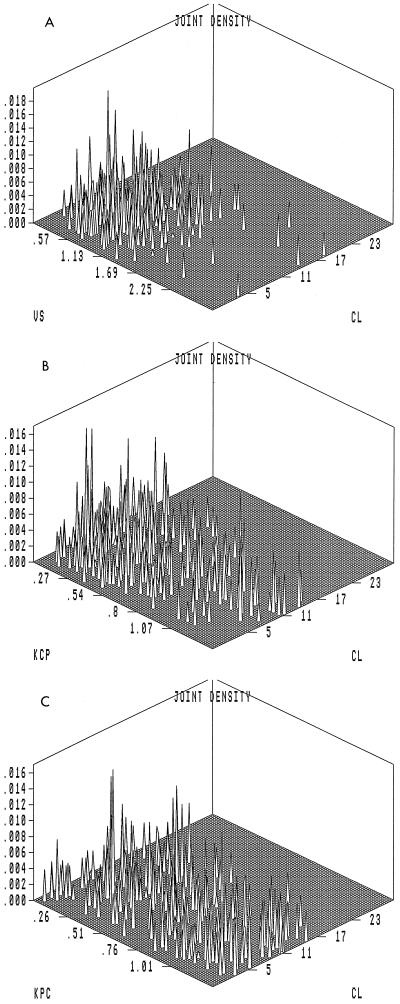
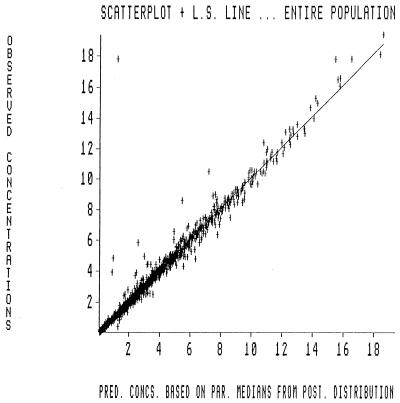
Approximate marginal density plots depicting the probability of occurrence of a value for a particular parameter in the population are displayed in Fig. 1A to D. The probability distributions of CL and V1 tend to follow a Gaussian pattern, while the distributions of the intercompartmental transfer rate constants Kcp and Kpc are flatter with clearance. Three-dimensional joint density plots for all the parameters are illustrated in Fig. 2A to C. These plots demonstrate how two parameters covary with each other. The performance of the MAP Bayesian estimation step is displayed in the plot of the observed versus predicted concentration in plasma in Fig. 3. The Bayesian step used the median values for each parameter as the measure of central tendency. This has been found to improve predictive performance (9a). The coefficient of determination (r2) for the line of best fit was 0.966. The slope was not significantly different from 1.0, and the intercept was not significantly different from 0.0.
FIG. 1.
Probability distributions of pharmacokinetic parameters in the population. The marginal distributions, which indicate the probability of occurrence of pharmacokinetic parameter values in the population, are displayed. (A) Slope of V1 to body weight (VS; in liters/kilogram). (B) CL (in liters/hour). (C and D) Kcp (KCP) and Kpc (KPC), (in hours−1), respectively.
FIG. 2.
Three-dimensional probability distributions of pharmacokinetic parameters. (A to C) Three-dimensional probability distribution plots of the population for pharmacokinetic parameters. Parameters and units are as described in the legend to Fig. 1.
FIG. 3.
Observed versus MAP Bayesian-predicted concentrations in 272 patients. A scatter plot of observed versus MAP Bayesian-predicted concentrations based on pharmacokinetic parameter medians is shown. The slope and the intercept of the line are 1.01 and 0.0054, respectively. The slope is not significantly different from 1.0, and the intercept is not significantly different from 0.0. The r2 value is 0.966.
Demographic model data.
The descriptive statistics for the total group (n = 272), the model generation group (n = 172), and the validation group (n = 100) are listed in Table 2. The covariates included in the final model for the prediction of the peak concentration by a stepwise backward approach were gender, weight, and age, with a coefficient of determination of 0.105. The covariates included in the final model for the prediction of CL were age, race, and CLCR (r2 = 0.396) (Table 3). The covariates included in the stepwise backward analysis for the prediction of V1 included age, gender, and race, with an r2 value of 0.132 (Table 4). Prediction of Kpc included age and site (r2 = 0.087) (Table 5). Prediction of Kcp included weight and race (r2 = 0.065) (Table 6). By using data for all 272 patients, we generated for CL a stepwise backward model which yielded the same covariates in the final model as the covariates for the group of 172 patients (age, race, and CLCR).
TABLE 2.
Descriptive statistics for total group and subgroups used for demographic model generation and validation
| Characteristic | Total groupa | Model generation groupb | Validation groupc |
|---|---|---|---|
| Gender (no. [%]) | |||
| Male | 162 (59.6) | 105 (61.0) | 57 (57.0) |
| Female | 110 (40.4) | 67 (39.8) | 43 (43.0) |
| Race (no. [%]) | |||
| Caucasian | 162 (59.6) | 114 (66.3) | 48 (48.0) |
| Black | 80 (29.4) | 40 (23.2) | 40 (40.0) |
| Hispanic | 28 (10.3) | 17 (9.9) | 11 (11.0) |
| Other | 2 (0.74) | 1 (0.58) | 1 (1.0) |
| Age (yr)d | 46.9 ± 18.6 | 48.4 ± 19.3 | 44.2 ± 17.2 |
| Wt (kg)d | 77.5 ± 18.2 | 76.1 ± 18.6 | 79.8 ± 17.4 |
| CLCR (ml/min)d | 86.4 ± 31.3 | 82.9 ± 31.6 | 92.6 ± 29.8 |
| Site of infection (no. [%]) | |||
| Pulmonary | 189 (69.5) | 118 (68.6) | 71 (71.0) |
| Skin or soft tissue | 52 (19.1) | 32 (18.6) | 20 (20.0) |
| Urinary tract | 31 (11.4) | 22 (12.8) | 9 (9.0) |
n = 272.
n = 172.
n = 100.
Represented as means ± standard deviations.
TABLE 3.
Final model for prediction of CL by a general linear model procedurea
| Covariate | Coefficient | Standard error | Significanceb | r2 |
|---|---|---|---|---|
| Constant | 5.945 | 0.396 | ||
| Race | ||||
| Caucasian | −1.486 | 0.332 | 0.017 | |
| Black | −0.484 | 0.579 | ||
| Hispanic | −3.167 | 0.855 | ||
| Other | 5.137 | 3.504 | ||
| CLCRc | 0.070 | 0.012 | <0.001 | |
| Age | −0.032 | 0.019 | 0.095 |
Data are for 172 patients.
P values were obtained by analysis of variance.
CLCR was predicted by the method of Cockcroft and Gault (2).
TABLE 4.
Final model for prediction of V1 by a general linear model procedurea
| Covariate | Coefficient | Standard error | Significanceb | r2 |
|---|---|---|---|---|
| Constant | 72.096 | 0.132 | ||
| Age (yr) | −0.332 | 0.124 | 0.008 | |
| Gender | ||||
| Male | 6.482 | 8.067 | 0.008 | |
| Female | −6.482 | 8.434 | ||
| Race | ||||
| Caucasian | 10.027 | 2.944 | 0.022 | |
| Black | −3.421 | 4.892 | ||
| Hispanic | −10.45 | 7.431 | ||
| Other | 3.844 | 30.121 |
Data are for 172 patients.
P values were obtained by analysis of variance.
TABLE 5.
Final model for prediction of Kpc by a general linear model procedurea
| Covariate | Coefficient | Standard error | Significanceb | r2 |
|---|---|---|---|---|
| Constant | 0.430 | 0.087 | ||
| Age (yr) | 0.005 | 0.002 | 0.002 | |
| Site | ||||
| Pulmonary | −0.066 | 0.035 | 0.064 | |
| Skin or skin structure | 0.112 | 0.067 | ||
| Urinary tract | −0.046 | 0.082 |
Data are for 172 patients.
P values were obtained by analysis of variance.
TABLE 6.
Final model for prediction of Kcp by a general linear model procedurea
| Covariate | Coefficient | Standard error | Significanceb | r2 |
|---|---|---|---|---|
| Constant | 0.308 | 0.065 | ||
| Wt | 0.003 | 0.002 | 0.029 | |
| Race | ||||
| Caucasian | −0.127 | 0.034 | 0.044 | |
| Black | 0.061 | 0.057 | ||
| Hispanic | −0.074 | 0.087 | ||
| Other | 0.140 | 0.360 |
Data are for 172 patients.
P values were obtained by analysis of variance.
By using the developed models for the prediction of peak concentrations for the validation group of 100 patients, the median bias and median precision were −17.1 and 28.9%, respectively. The median bias and the median precision for the prediction of CL were 0.5 and 18.3%, respectively; those for the prediction of V1 were −4.6 and 31.5%, respectively; those for the prediction of Kcp were −6.6 and 48.9%, respectively; and those for the prediction of Kpc were 4.6 and 44.2%, respectively.
Because of the relatively poor bias and precision for the prediction of peak concentration, the four predicted model parameters (CL, V1, Kpc, and Kcp) were used to simulate predicted peak concentrations by using ADAPT II for each patient. The median bias and the median precision were 3.3 and 21.8%, respectively. For the prediction of AUC, the median bias and the median precision were −1.0 and 19.5%, respectively.
The coefficients of determination for all models except CL were low (r2 = 0.396). A scattergram of measured versus predicted (from the demographics model) CL of levofloxacin from plasma for the validation population is presented in Fig. 4.
FIG. 4.
Measured versus predicted CL obtained with the demographic model. The measured versus predicted CL for the validation group (n = 100) obtained with the demographic model developed in this study is shown. Note that when the measured CL reaches approximately 13 liters/h, the predictive performance decreases (the model underpredicts CL).
DISCUSSION
Determination of the pharmacokinetic profile of a drug in the population of interest is of particular importance for the evaluation of a new agent. The limited ability of ill patients to undergo the rigors of a classical pharmacokinetic study has resulted in the availability of few data on the dispositions of agents in such populations.
The advent of optimal sampling theory and population pharmacokinetic analysis has had a positive effect in this area. In this study, we have combined optimal sampling theory and population pharmacokinetic analysis, as implemented in the ADAPT II program of D’Argenio and Schumitzky (3), as well as the NPEM2 package of programs of Schumitzky and Jelliffe (9a), to allow the study of the disposition of the new fluoroquinolone antimicrobial agent levofloxacin in a phase II multicenter clinical trial.
Our pharmacokinetic findings were highly consistent with those of earlier studies of levofloxacin (7). The V1 value of 0.836 liter/kg and the CL from plasma of 9.27 liters/h are quite close to the mean values determined for levofloxacin in earlier trials, especially when corrected for the older age of the patient population studied in our analysis. This is understandable, because the population that we studied had a mean age of 46.8 years and the clinical study protocol accepted patients with serum creatinine levels of as high as 3.4 mg/dl. For a drug like levofloxacin, which is mainly cleared renally, the older age and the slightly higher average serum creatinine level could reflect glomerular filtration rates which are lower than those seen in healthy volunteers, causing a decreased level of CL by the kidneys and therefore total CL of levofloxacin.
One conclusion which can be drawn is that a relatively sparse sampling strategy, driven by optimal sampling theory, can be implemented in a medium-sized (circa 300-patient) multicenter clinical trial and, when analyzed by population modeling techniques, can provide excellent point estimates of the mean values and their dispersions for important pharmacokinetic parameter values.
Examination of the marginal density plots for the pharmacokinetic parameter values is also of interest. V1 and the CL of levofloxacin from plasma are approximately normal in distribution, while Kcp and Kpc are flatter in their distributions. The Gaussian nature of V1 and CL imply that they conform to our distributional expectations for a predictive model. The relative flatness of the distributions for Kcp and Kpc may be an artifact related to model misspecification. That is, the overall structural model choice for a population analysis may not apply to some patients, who may have a drug disposition profile which differs in nature from that required for a two-compartment model. For the case of a two-compartment model being forced onto a patient’s data which are one compartment in nature, the estimator will, by definition, give more probability of a very low Kcp and a high Kpc.
The three-dimensional joint density plots which are a standard part of the NPEM2 output also provide useful insight into the data. V1 and CL are poorly correlated, as can be seen by examining Fig. 2A. This is to be expected, given the physiologic independence of V1 and CL. The marginal density plots and the joint density plots are also of interest as a way of identifying outlier subpopulations (e.g., rapid acetylators for isoniazid). When examined with this purpose in mind, it is clear that levofloxacin is a very well behaved drug with respect to V1 and CL, with no substantial outlier subpopulations.
A central reason for undertaking this study was to develop a demographic information-based population model which would allow estimates of the peak concentration and the CL of levofloxacin from plasma to be obtained for individual patients if basic demographic patient information is available without having to directly measure the concentrations in plasma. Peak concentration and CL from plasma were chosen because peak concentration and AUC, when placed in a ratio to the MIC for the patient’s infecting pathogen (peak/MIC or AUC/MIC ratio), could be directly linked to the probability of a successful clinical or microbiological outcome in a logistic regression model (8). We also felt it important that we have an independent measure of the predictive performance of the population pharmacokinetic relationship that we developed.
To attain both these ends, we used the data for the first 172 patients in our data set to develop our demographic model, and for the subsequent 100 patients the peak concentrations and AUCs were predicted from our relationships and the bias and precision of estimation determined against the measured values.
For peak concentration estimation, the general linear modeling procedure resulted in a model which produced relatively biased and imprecise predicted peak concentrations. Because of this finding, we attempted to predict the peak concentration by predicting all four model parameters and simulating a peak from those values. Much more acceptable results were obtained, with a median bias of 3.3% and a median precision of 21.8%. However, even this attempt did not explain an acceptable amount of the variance in peak concentrations seen in the population. Consequently, we doubt its utility for optimizing therapy.
For V1 estimation, the model included the covariates of age, race, and gender. An increased V1 was noted for females, but V1 decreased with age.
For the model for estimation of CL from plasma, stepwise backward analyses indicated that CL was influenced by CLCR, age, and race. This relationship was better than that for estimation of the peak concentration, with an r2 value of 0.396. Certainly, the direct correlation of the CLCR estimate with CL from plasma is understandable for a drug which is mainly cleared renally. Also, patients of younger ages would tend to have better CL processes and therefore a higher total CL. The influence of race, however, is difficult for us to understand. We can give no physiological explanation for this finding. It is noteworthy, however, that the race category of “other” included only two patients and the coefficient generated may not be a valid reflection of this particular patient subpopulation.
For the prediction of CL, the median bias was 0.5% and the median precision was 18.3%. Only this relationship predicting CL explains enough of the variance to warrant its use clinically. Of note, when examining the plot of measured CL versus predicted CL (Fig. 4), it appears that the predictive ability of the model decreases above a measured CL of approximately 13 liters/h. This would imply that if the predicted CL was greater than 12 liters/h, there is a reasonably high possibility that it could indeed be an underestimation of the measured CL. This has therapeutic implications in that patients with higher CLs will have a lower peak and a lower AUC and, hence, lower peak/MIC and AUC/MIC ratios, with a consequence lower probability of the successful outcome that the clinician would be expecting on the basis of the predicted CL.
The ability to reasonably estimate CL without the necessity of measuring concentrations in plasma is important. As we have developed pharmacodynamic relationships linking the peak/MIC ratio or the AUC/MIC ratio to the probability that levofloxacin will produce a successful clinical or microbiological outcome, the clinician can now obtain guidance as to the expectation of successful therapy with this agent in the empirical setting (using an MIC for the most resistant pathogen that they are willing to treat) or after the sensitivity result returns from the clinical microbiology laboratory. The predicted result, if it is felt to be potentially suboptimal, could lead to dose escalation, the addition of a second agent, or a switch to a more appropriate agent.
This also has potential economic impact. Patients experiencing therapeutic failure use more hospital resources (longer stays, residence in an intensive care unit, etc.). Optimizing outcome could potentially lead to substantial cost savings as well as the obvious and most important benefit of improved patient care. Whether or not the optimization of therapy can affect length of stay is an interesting question which needs to be addressed in a prospective trial.
In summary, we have developed a population model of the disposition of levofloxacin in a target population for the use of this drug. We have further developed a population demographic model for the prediction of CL from plasma. This relationship was developed to allow patients to directly benefit from the pharmacodynamic relationships that we have developed for this agent. It will be important to validate prospectively the use of this relationship along with our pharmacodynamic relationships with a similar patient population and document the impact (if any) on patient outcome and the expenditure of hospital resources.
ACKNOWLEDGMENT
This work was supported in part by a grant from R. W. Johnson Pharmaceutical Research Institute.
Appendix
An example of the mathematical prediction of levofloxacin parameters for a 50-year-old Caucasian male weighing 70 kg with a skin structure infection and a CLCR of 75 ml/min is as follows: CL = constant + race + (age · −0.032) + (CLCR · 0.070) CL = 5.945 − 1.486 − 1.6 + 5.25 CL = 8.12 liters/h V1 = constant + gender + race + (age · −0.332) V1 = 72.096 + 6.482 + 10.027 − 16.6 V1 = 88.60 liters
REFERENCES
- 1.Anonymous. HPLC assays for the stereospecific determination of levofloxacin in human plasma and urine. Data on file. The R. W. Johnson Pharmaceutical Research Institute, Raritan, N.J.
- 2.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 3.D’Argenio D Z, Schumitzky A. ADAPT II. A program for simulation, identification, and optimal experimental design. User manual. Biomedical Simulations Resource. Los Angeles: University of Southern California; 1992. [Google Scholar]
- 4.Drusano G L. Optimal sampling theory and population modeling: application to determination of the influence of the microgravity environment on drug distribution and elimination. J Clin Pharmacol. 1991;31:962–967. doi: 10.1002/j.1552-4604.1991.tb03657.x. [DOI] [PubMed] [Google Scholar]
- 5.Forrest A, Ballow C H, Nix D E, Birmingham M C, Schentag J J. Development of a population pharmacokinetic model and optimal sampling strategies for intravenous ciprofloxacin. Antimicrob Agents Chemother. 1993;37:1065–1072. doi: 10.1128/aac.37.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodwin A D, Gallis H A, Chow A T, Wong F A, Flor A C, Bartlett J A. Pharmacokinetics and safety of levofloxacin in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1994;38:799–804. doi: 10.1128/aac.38.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallet A. A maximum likelihood estimation method for random coefficient regression models. Biometrika. 1986;73:645–656. [Google Scholar]
- 8.Preston S L, Drusano G L, Berman A L, Fowle C L, Chow A, Dornseif B, Reichl V, Natarajan J, Corrado M. Prospective development of pharmacodynamic relationships between measures of levofloxacin exposure and measures of patient outcome: a new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 9.Schumitzky A. Nonparametric EM algorithms for estimating prior distributions. Appl Math Comput. 1991;45:143–157. [Google Scholar]
- 9a.Schumitzky, A., and R. Jelliffe. Personal communication.
- 10.Sheiner L B, Rosenberg B, Marathe V V. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977;5:445–479. doi: 10.1007/BF01061728. [DOI] [PubMed] [Google Scholar]
- 11.Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]