Abstract
In this study we defined the pharmacodynamic parameter that optimizes outcome in deep-seated Candida albicans infections treated with fluconazole. Using a murine model of systemic candidiasis, we conducted single-dose dose-ranging studies with fluconazole to determine the dosage of this drug that resulted in a 50% reduction in fungal densities (50% effective dose [ED50]) in kidneys versus the fungal densities in the kidneys of untreated controls. We found that the ED50 of fluconazole given intraperitoneally was 4.56 mg/kg of body weight/day (95% confidence interval, 3.60 to 5.53 mg/kg/day), and the dose-response relationship was best described by an inhibitory sigmoid maximal effect (Emax) curve. To define the pharmacodynamics of fluconazole, we gave dosages lower than, approximating, and higher than the ED50 of fluconazole (range, 3.5 to 5.5 mg/kg/day, equivalent to the ED16 to the ED75) to various groups of infected animals using three dose-fractionation schedules. For each total dose of fluconazole examined, the dose-fractionation schedules optimized the ratio of the area under the concentration-time curve (AUC) to the MIC (the AUC/MIC ratio), the ratio of the maximum concentration of drug in serum (Cmax) to the MIC, and the time that the drug remained above the MIC for the infecting C. albicans isolate. Similar reductions in fungal densities in kidneys were seen between groups that received the same total dose of fluconazole in one, two, or four equally divided doses. Thus, dose-fractionation studies demonstrated that the pharmacodynamic parameter of fluconazole that best predicted outcome was the AUC/MIC ratio.
Deep-seated infections due to the fungus Candida albicans are an important cause of nosocomial infection (2, 3, 20, 23). Despite treatment with amphotericin B or fluconazole, the morbidity and mortality associated with Candida infections remain substantial (23, 25).
It has been shown that the use of dosing schedules for antibacterial agents that maximize specific pharmacodynamic parameters can improve the outcomes for infected patients (6, 7, 10, 17, 19, 22). For the aminoglycosides and quinolones, for example, improved outcome is associated with higher ratios of the maximum concentration of drug in serum (Cmax) to the MIC (Cmax/MIC ratios) or ratios of the area under the concentration-time curve (AUC) to the MIC (AUC/MIC ratios) (10, 17, 19). In contrast, the maximum antimicrobial effect of beta-lactams is seen when one optimizes the duration of time that the concentrations of these drugs in serum remain above the MIC (time > MIC) for the infecting bacterium (6, 7, 22). For antifungal drugs, the pharmacodynamic variable most closely linked to outcome in the treatment of Candida infections is unknown for any class of therapeutic agent. However, it is possible that outcome associated with specific antifungal drugs, such as fluconazole, can be improved by delivering the antifungal agents at doses and on dosing schedules that maximize specific pharmacodynamic parameters, e.g., time > MIC for the infecting pathogen, the AUC/MIC ratio, or the Cmax/MIC ratio.
In the current study we conducted single-dose dose-ranging studies with fluconazole to define the relationship between the fluconazole dose and the reduction in C. albicans densities in the kidneys of infected mice. We also conducted dose-fractionation studies, in which selected total doses of fluconazole that were found on the steep portion of the dose-response curve were administered to infected mice in one, two, or four divided doses (to optimize the Cmax/MIC ratio, the AUC/MIC ratio, and time > MIC) to determine which pharmacodynamic variable was most closely linked with outcome in this model system.
MATERIALS AND METHODS
C. albicans isolate.
C. albicans ATCC 36082 (American Type Culture Collection, Rockville, Md.) was used throughout the study. The organism was maintained on Sabouraud dextrose agar (BBL Microbiology Systems, Cockeysville, Md.) at 4°C until use. For each study, two to three colonies of the fungus were subcultured onto fresh potato dextrose agar (BBL), and the plates were incubated at 35°C for 48 h. A fungal suspension was prepared by transferring three to four colonies of C. albicans to 10 ml of sterile, pyrogen-free normal saline (Baxter Inc., Chicago, Ill.) and was quantified by hemocytometry. The suspension was diluted with normal saline to a final concentration of 1.5 × 106 organisms per ml. Morphologic examination revealed that >95% of the organisms were blastoconidia. The viability of the yeast was >90% by trypan blue exclusion analysis.
The MIC of fluconazole was determined on eight separate occasions by the broth macrodilution method described by the National Committee for Clinical Laboratory Standards (18). The median MIC after 48 h of incubation was 0.5 μg/ml (range, 0.25 to 0.5 μg/ml).
Antifungal agent.
Fluconazole powder was supplied by Pfizer Inc. (New York, N.Y.). The drug was dissolved in sterile, pyrogen-free saline to a stock concentration of 4 mg/ml, and the solution was stored at −70°C. For each study, the drug was thawed and further diluted to the desired concentration(s) with sterile normal saline. The drug was used immediately.
Mice.
Female NYLAR mice (weight, 18 to 20 g) were raised at the Animal Research Facility of the Wadsworth Center for Laboratories and Research (Griffin Laboratories, Guilderland, N.Y.). These outbred Swiss mice were housed in plastic boxes at three to four animals per container. They received food and water ad libitum. All animal experimentation procedures were approved by and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the New York State Department of Health, Albany.
Pharmacokinetics of fluconazole in infected mice.
Dose-ranging studies were conducted to determine the pharmacokinetics of fluconazole when it was administered intraperitoneally (i.p.) as a single dose. NYLAR mice were intravenously inoculated with 3 × 105 C. albicans blastoconidia via a lateral tail vein. The organism was administered in 0.2 ml of sterile saline. Five hours later, mice were injected i.p. with one of various doses of fluconazole in 0.2 ml of saline. The doses of fluconazole examined were 0, 0.875, 1, 1.125, 1.25, 1.375, 1.75, 2, 2.25, 2.5, 2.75, 3.5, 4, 4.5, 5, 5.5, and 20 mg/kg. Three animals from each group were sacrificed by CO2 asphyxiation at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, and 8 h after drug administration. Blood was collected by cardiac puncture and was allowed to clot on ice. The serum was separated from the clot by centrifugation and was stored at −70°C.
The concentration of fluconazole in each serum sample was determined by a well diffusion microbiological assay developed by Jorgensen et al. (13) with the modifications described by Madu et al. (16). Candida pseudotropicalis ATCC 46764 was used as the assay organism. Pour plates containing the fungus were prepared with Synthetic Amino Acid Medium Fungal molten agar and were allowed to solidify at room temperature. Four-millimeter-diameter wells were made in the agar. Twenty-microliter aliquots of serum collected from mice or standards were pipetted into the wells, and the plates were kept at 4°C for 1 h and were then incubated overnight for 16 h at 30°C in an ambient-air incubator. The diameters of inhibition for each serum sample and standards were measured to the nearest 0.1 mm with a vernier caliper. Antifungal drug concentrations in serum samples were calculated by using the data from the curves derived from the fluconazole standards. The standard curve was linear for concentrations of fluconazole of between 1 and 100 μg/ml of serum. The intraday and interday coefficients of variation of the microbiological assay were 4.9 and 6.8%, respectively.
Pilot study to determine the ED50 of single-dose fluconazole for systemic candidiasis.
In one dose-ranging study, eight mice per group were given 0, 0.5, 1, 2.5, 5, 7.5, and 10 mg of fluconazole per kg of body weight i.p. 5 h after the animals were infected intravenously (i.v.) with 3 × 105 C. albicans blastoconidia. The drug and fungus were each delivered in 0.2 ml of saline. The drug or saline was given as a single injection. Twenty-four hours later, animals from each group were humanely killed by CO2 asphyxiation and the right kidneys were collected. Each kidney was weighed, homogenized, and serially diluted with saline. Two hundred microliters of each dilution was plated onto potato dextrose agar that was supplemented with 100 IU of penicillin and 100 μg of streptomycin per ml of agar. After 48 h of incubation at 35°C, the colonies were counted and the results between groups were compared. The cultures reproducibly detected ≥50 organisms/g of tissue.
The 50% effective dose (ED50) was calculated by using an inhibitory sigmoid maximal reduction (Emax) dose-response model. Selected total doses of fluconazole that were lower than, equivalent to, and higher than the ED50 and that were likely to lie on the steep portion of the dose-response curve were used in the dose-scheduling study described below.
Expanded fluconazole dose-ranging validation study.
An expanded single-dose dose-ranging validation study was conducted (i) to more completely characterize the relationship between the fluconazole dose and the reduction in fungal density in kidneys and (ii) to verify the ED50 that was calculated with the data derived from the pilot study. Smaller increments in fluconazole doses were used. Otherwise, the study methods were identical to those described for the pilot dose-ranging study. The doses of fluconazole used were 0, 2, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, and 8 mg/kg given i.p. as a single dose. Eight C. albicans-infected mice were in each group.
Effect of scheduling of fluconazole dose on fungal densities in kidneys.
Simultaneously with the expanded dose-ranging validation study described above, we conducted a dose-fractionation study to determine if the pharmacodynamic parameter that best predicts the maximal benefit of fluconazole was defined by the AUC/MIC ratio, the Cmax/MIC ratio, or time > MIC. The dose-fractionation study was conducted simultaneously with the expanded dose-ranging validation study to eliminate the impact of interday variability with (i) fungal inoculum preparation and viability, (ii) fluconazole concentrations in the solutions used for therapy, and (iii) the drift in the ED50 that may occur because of the effects of (i) and (ii) on the study results. The doses selected for the dose-fractionation study were those that, on the basis of the results of the pilot dose-ranging study, were predicted to fall on the steep portion of the sigmoid Emax dose-response curve. It was important to select dosages of fluconazole which lay on the steep portion of the dose-response curve because it is only with these dosages that one can readily detect an improvement or worsening of outcome associated with different schedules of drug administration. Differences in efficacy may be difficult to observe if doses associated with minimal or maximal drug effects are used.
The total dosages of fluconazole examined were 3.5, 4.0, 4.5, 5.0, and 5.5 mg/kg per 24 h. Each total dose of fluconazole selected was given i.p. to groups of infected mice as either a single injection, two equally divided doses given 12 h apart, or four equally divided doses given 6 h apart. An additional group of infected mice received saline and served as controls. There were eight mice per group.
The first dose of fluconazole was administered to each group of animals 5 h after the mice were inoculated i.v. with 3 × 105 C. albicans blastoconidia. Each i.p. and i.v. injection was administered in 0.2 ml of saline. Twenty-four hours after the first dose of fluconazole or saline was given, the animals were humanely killed by CO2 asphyxiation. The right kidney was collected from each mouse, and quantitative cultures were conducted as described above for the pilot dose-ranging study. The results for the groups that received the same total dose of fluconazole were compared with each other and with those for the control group.
Power analysis, conducted with data generated from the preliminary data, determined that six animals/group were needed to have a 90% probability of identifying a 0.3-log10 difference between treatment groups (data not shown).
In preliminary studies, colony counts of C. albicans in kidneys obtained from infected animals were similar to those in the kidneys of identically infected animals who also received 120 mg of fluconazole per kg i.p. 1 to 2 h before they were killed (data not shown). Therefore, antifungal drug carryover did not affect the culture results.
Pharmacokinetic analysis.
Pharmacokinetic analysis of the fluconazole concentration in serum-time relationships were performed with a nonlinear least-square regression program, RSTRIP II (Micromath Scientific Software, Salt Lake City, Utah). The most appropriate pharmacokinetic models were determined by using model selection criteria based on a modified form of Akaike’s information criterion (1). The Cmax was defined as the highest concentration of fluconazole measured in serum after the drug was administered. To determine the AUC, the trapezoidal method was used for data obtained from time zero to the last time point, and the data were then extrapolated to infinity.
Statistical analysis.
The relationship between the dose of fluconazole administered and the fungal density in kidneys of infected mice was evaluated by an inhibitory sigmoid Emax dose-response model by using the identification module of the ADAPT II package of programs of D. D’Argenio and A. Schumitzky (Biomedical Simulations Resource, University of Southern California, Los Angeles). Weighting was performed by obtaining the inverse of the observation variance. The significance of differences between fungal densities in the kidneys of groups that received the same total dose of fluconazole in one, two, or four divided doses was evaluated by analysis of variance. A difference was considered statistically significant if the P value was <0.05. Power analysis to determine the number of kidney samples needed to have a 90% probability of identifying a 0.3-log10 difference between groups that received the various fluconazole doses by different dosing schedules was determined with the software program True Epistat version 5.3 (Epistat Services, Richardson, Tex.).
RESULTS
Single-dose pharmacokinetics of fluconazole in infected mice.
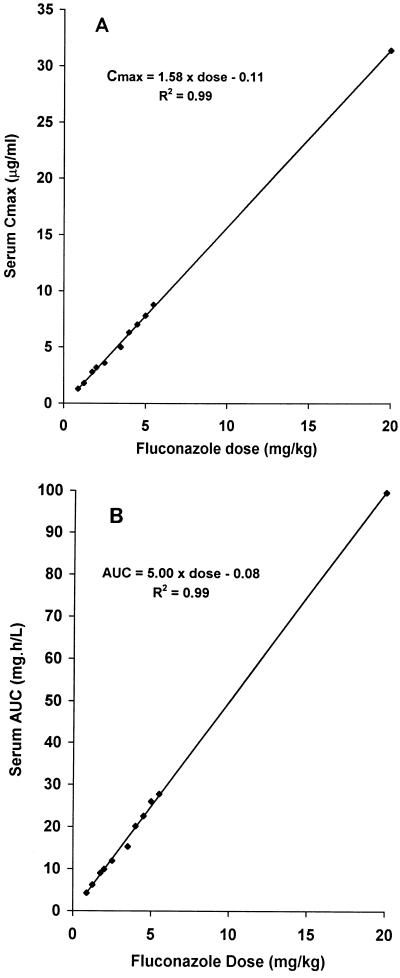
The pharmacokinetics of fluconazole in mice who received a single i.p. injection of drug 5 h after they were inoculated i.v. with C. albicans were determined. The Cmax was observed 1 h after the drug was administered. Both the Cmax and AUC increased in proportion to the dose of fluconazole administered (Fig. 1A and B, respectively). The pharmacokinetics were best described by a two-compartment model with a terminal half-life of 2.4 h. The terminal half-life did not change with the dose of fluconazole administered.
FIG. 1.
Relationship between single-dose fluconazole and Cmax (A) and AUC (B) for selected doses of fluconazole. The drug was given i.p. as a single dose 5 h after the mice were infected i.v. with C. albicans.
Identifying the ED50 of single-dose fluconazole in mice with systemic candidiasis: results of the pilot study.
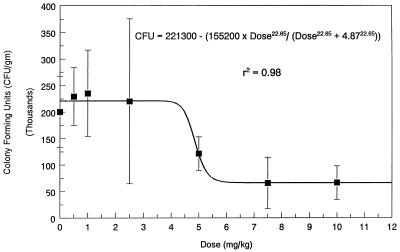
The fungal densities in the kidneys of mice that received a single injection of incremental doses of fluconazole are presented in Fig. 2. These animals received treatment 5 h after fungal inoculation. As demonstrated in Fig. 2, a dose-response relationship existed, and this relationship was well described by an inhibitory sigmoid Emax curve, as follows: the number of CFU per gram = 221,300 − [155,200 × dose22.65/(dose22.65 + 4.8722.65)] (r2 = 0.98; P << 0.001). The lower doses of fluconazole showed no effect relative to that of the controls. For the middle doses, a steep dose-response relationship between the amount of drug administered and the reduction in the fungal counts in the kidneys was found. No further reduction in fungal densities were obtained with doses of ≥7.5 mg/kg.
FIG. 2.
Pilot dose-ranging study demonstrating the dose-response relationship between the dose of fluconazole administered to infected mice and C. albicans density in kidneys (mean ± 1 standard deviation). Fluconazole was given i.p. as a single dose 5 h after the mice were inoculated with fungus i.v., and quantitative cultures of kidneys were conducted 24 h after drug administration. There were eight mice per group.
The ED50 of fluconazole was 4.87 mg/kg. Because only one point fell on the steep portion of the sigmoid Emax curve, the confidence interval (CI) was large (95% CI, −287.7 to 297.5 mg/kg). The slope factor (sigmoidicity) of the dose-response curve was 22.65. The steep portion of the dose-response curve extended only from 4.25 to 5.5 mg/kg.
Confirmation of the ED50 of single-dose fluconazole in the expanded dose-ranging validation study.
An expanded dose-ranging study was conducted (i) to more completely characterize the relationship between the fluconazole dose and the reduction in the fungal density in the kidneys and (ii) to validate the ED50 identified in the pilot dose-ranging study. Smaller increments in fluconazole doses were examined. The doses chosen were those that the results of the pilot study suggested were likely to lie on the steep portion and adjacent plateau sections of the sigmoid Emax dose-response curve.
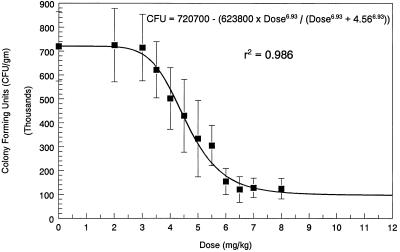
The results of the dose-ranging validation study were similar to those of the pilot study (Fig. 3). An inhibitory sigmoid Emax curve best described the dose-response relationship between the dose of fluconazole administered and the reduction in the fungal load in the kidneys. The inhibitory sigmoid Emax equation was as follows: the number of CFU per gram = 720,700 − [623,800 × dose6.93/(dose6.93 + 4.566.93)] (r2 = 0.986; P << 0.001).
FIG. 3.
Expanded dose-ranging validation study demonstrating a more complete delineation of the inhibitory sigmoid Emax dose-response curve. The study methods are described in the legend to Fig. 2. The ED50 of 4.56 mg/kg was similar to the ED50 of 4.87 mg/kg that was derived from the smaller number of doses of fluconazole used in the pilot dose-ranging study.
The ED50 in the expanded validation study was 4.56 mg/kg/day, which was similar to the ED50 of 4.87 mg/kg/day that was identified in the pilot dose-ranging study. Because nine of the dosages used in the expanded validation study lay on the steep portion of the dose-response curve, the 95% CI for the ED50 in the expanded validation study was small (3.60 to 5.53 mg/kg/day).
Pharmacodynamic variables for three fluconazole regimens.
The relationship between pharmacokinetic parameters and drug activity for fluconazole doses of 3.5, 4.0, 4.5, 5.0, and 5.5 mg/kg is presented in Table 1. When examining the Cmax/MIC ratio, it is clear that once-daily administration of a total dose of fluconazole produced the maximal value. For time > MIC, the most fractionated schedule (e.g., the regimen of administration once every 6 h) produced the longest time > MIC, while once-daily dosing produced the shortest time > MIC. Because fluconazole has linear pharmacokinetics (12, 15; this study), one would not expect that the AUC for a full 24-h period would be altered by the schedule of administration. We have demonstrated this, because the three schedules of administration produced essentially the same 24-h cumulative AUC (and, hence, the same AUC/MIC ratio) for each of the total doses of fluconazole examined. The half-life of fluconazole was 2.4 h in the sera of infected mice for all of the doses of drug examined.
TABLE 1.
Pharmacodynamic variables for five total doses of fluconazole administered in one, two, or four equally divided doses
| Total fluconazole dosage (mg/kg/24 h) | Regimen (no. of doses)a | Cmax/MIC ratiob | AUC/MIC ratio | Time > MIC/24 h |
|---|---|---|---|---|
| 3.5 | 3.5 mg/kg (one) | 11.0 | 34.87 | 9.1 |
| 1.75 mg/kg q 12 h (two) | 5.6 | 35.99 | 13.4 | |
| 0.875 mg/kg q 6 h (four) | 2.6 | 34.23 | 17.2 | |
| 4.0 | 4.0 mg/kg (one) | 12.5 | 40.37 | 9.3 |
| 2.0 mg/kg q 12 h (two) | 6.4 | 39.81 | 13.8 | |
| 1.0 mg/kg q 6 h (four) | 3.2 | 40.98 | 18.0 | |
| 4.5 | 4.5 mg/kg (one) | 14.0 | 45.14 | 9.94 |
| 2.25 mg/kg q 12 h (two) | 7.0 | 44.63 | 15.08 | |
| 1.125 mg/kg q 6 h (four) | 3.6 | 44.97 | 20.26 | |
| 5.0 | 5.0 mg/kg (one) | 15.6 | 49.48 | 10.60 |
| 2.5 mg/kg q 12 h (two) | 7.2 | 47.83 | 15.44 | |
| 1.25 mg/kg q 6 h (four) | 3.4 | 50.02 | 20.80 | |
| 5.5 | 5.5 mg/kg (one) | 17.2 | 55.02 | 11.27 |
| 2.75 mg/kg q 12 h (two) | 8.5 | 54.88 | 16.54 | |
| 1.38 mg/kg q 6 h (four) | 4.4 | 54.93 | 21.58 |
The first dose was administered 5 h after infection. q 6 h and q 12 h, every 6 and 12 h, respectively.
The MIC for C. albicans ATCC 36082 was 0.5 μg/ml by the macrobroth dilution method of the National Committee for Clinical Laboratory Standards.
Dose-fractionation trials.
To determine which pharmacodynamic parameter best predicted outcome, groups of mice were given 3.5, 4.0, 4.5, 5.0, or 5.5 mg of fluconazole per kg in one, two, or four equally divided doses over 24 h, and the fungal densities in the kidneys of each group were compared. A control group of infected mice received saline instead of drug.
Post hoc analysis of the results of the expanded dose-ranging validation study demonstrated that all doses of fluconazole chosen for the dose-fractionation study lay on the steep portion of the inhibitory sigmoid Emax dose-response curve. The total doses of 3.5, 4.0, 4.5, 5.0, and 5.5 mg of fluconazole per kg corresponded to the ED16, ED37, ED49, ED60, and ED75, respectively. As demonstrated in Table 2, the fungal densities were similar for groups that received the same total dose of fluconazole in one, two, or four equally divided doses over 24 h. These results demonstrate that, for fluconazole, the AUC/MIC ratio is the pharmacodynamically linked variable.
TABLE 2.
Fungal densities in kidneys of mice treated with various total doses of fluconazole administered in one, two, or four divided doses
| Total fluconazole dose (mg/kg) | Fungal density (mean ± 1 SD log10 CFU/g) for the following schedule of administration:
|
P valuea | ||
|---|---|---|---|---|
| One dose | Two divided dosesb | Four divided dosesc | ||
| 3.5 | 5.79 ± 0.12 | 5.78 ± 0.11 | 5.79 ± 0.12 | 0.68 |
| 4.0 | 5.70 ± 0.11 | 5.69 ± 0.10 | 5.71 ± 0.10 | 0.59 |
| 4.5 | 5.64 ± 0.15 | 5.56 ± 0.27 | 5.62 ± 0.18 | 0.34 |
| 5.0 | 5.48 ± 0.21 | 5.44 ± 0.19 | 5.41 ± 0.11 | 0.46 |
| 5.5 | 5.46 ± 0.14 | 5.25 ± 0.10 | 5.43 ± 0.19 | 0.32 |
By analysis of variance. A P value of <0.05 was considered statistically significantly different.
The doses were given 12 h apart.
The doses were given 6 h apart.
DISCUSSION
With advances in the treatment of oncologic malignancies with high-dose antineoplastic chemotherapy and in bone marrow transplantation and with improvements in the ability of health care providers to support critically ill patients in intensive care units, the incidence of deep-seated fungal infections is rising (3). Recently, it was reported that 10% of all nosocomial bloodstream infections were due to fungi, particularly C. albicans (3). In a matched, case-control study, Wey et al. (25) reported that the attributable mortality due to systemic fungal disease is 38%, despite antifungal drug therapy.
Amphotericin B has traditionally been considered the cornerstone of therapy for deep-seated fungal infections and fungemia. However, two recent blinded, multicenter, randomized controlled trials suggest that fluconazole is as efficacious as amphotericin B in the treatment of C. albicans fungemia in neutropenic and nonneutropenic hosts (2, 20). However, in those studies, treatment failure was seen in as many as 30% of the patients (2). Thus, further improvement in outcome in association with antifungal therapy is needed.
Part of the optimization of patient outcome is related to administration of the drug on an optimal schedule. This allows the maximal therapeutic benefit to be obtained at the lowest dose, allowing attainment of the goal of maximal therapeutic efficacy with minimal attendant toxicity.
In the areas of antibacterial and antiviral chemotherapy, both in vitro and in vivo studies have demonstrated the ability of the dosing schedule to influence the effect produced by the drug (4, 5, 8, 9, 11, 14, 21, 24). While generally consistent within drug classes, different classes of agents often have different pharmacodynamically linked variables. While the data are convincing that for beta-lactam agents time > MIC is most closely linked to the drug’s effect (9, 21, 24), for classes of drugs whose killing rates are concentration dependent, such as fluoroquinolones or aminoglycosides, either the Cmax/MIC ratio or the AUC/MIC ratio has been linked to the effect (8, 11, 14, 24). The findings obtained with animal models and in vitro findings have been validated in clinical trials (6, 7, 10, 17, 19, 22). This has practical implications because for beta-lactams any daily dose of drug should be given at smaller doses over shorter dosing intervals to produce the optimal effects. In contrast, when the Cmax/MIC or the AUC/MIC ratio is linked to the outcome, the daily dose should be administered on a once-daily basis, if toxicity issues permit, either for improved efficacy (Cmax/MIC ratio linked) or for improved convenience and compliance (AUC/MIC ratio linked).
While antifungal agents have been available for many years, there is virtually no information regarding the pharmacodynamics of any of these drugs. Because azoles in general and fluconazole in particular have gained wide popularity for the therapy of a variety of fungal infections, we thought that it was important to determine which pharmacodynamic variable (Cmax/MIC ratio, AUC/MIC ratio, or time > MIC) was most closely linked to fluconazole’s ability to decrease colony counts in this mouse model. This particular model was chosen because it is simple, inexpensive, quantitative, and reproducible.
It is noteworthy that we used fungal densities in the kidneys and not survival as our study endpoint. Pharmacodynamic studies evaluate the effects of drugs on pathogens, with consideration of the pharmacokinetics of the compound. This evaluation can readily be achieved by comparing the effects of different short-course therapies on the reduction in the number of CFU in tissues. Survival in fungal studies is usually assessed at ≥28 days after the initiation of therapy. However, antifungal drug therapy lasts for between 3 and 14 days. Much of the observation time in survival studies occurs when the animals are not receiving therapy. Survival in antifungal drug trials is determined in part by drug therapy. Other factors, however, are also important, such as the host cytokine response; the host neutrophil, macrophage, and antibody responses; and the potential development of resistance to the anti-infective agent by the pathogen. Consequently, survival is a result of many factors and, therefore, may not be the best endpoint for use in defining which pharmacodynamic variable is most closely associated with optimal drug efficacy in an experimental animal infection model.
The dose-ranging study, in which all doses were given on a once-daily schedule, gave clear-cut results. Over a dose range of from 0 to 10 mg/kg, fluconazole produced a maximal response of approximately 87% in terms of reducing the colony counts from those in the untreated control (Emax = 623,800 colonies/g; control = 720,700 colonies/g). The exposure-response curve was quite steep, with a sigmoidicity of 6.93. This makes it important that a dose quite near the ED50 be chosen for study so that any change in the effect as a result of the treatment schedule can be appreciated.
Likewise, the results of the dose-fractionation study were clear-cut. The pharmacokinetic study demonstrated that each regimen would produce essentially the same 24-h AUC (and the same AUC/MIC ratio). Consequently, if no differences were seen across groups, the AUC/MIC ratio would be the pharmacodynamically linked variable. The group that received a total dose of fluconazole as a single dose would have the highest Cmax/MIC ratio. If this were the dynamically linked variable, then this group would have the lowest number of CFU per gram of tissue at evaluation. Likewise, because the longest time > MIC was found for the group that received a total dose of fluconazole in four equally divided doses, this group should have had the lowest colony counts if this were the linked variable. Table 2 demonstrates that for any total dose of fluconazole there were no statistically significant differences in the reduction in the fungal density among the three groups (receiving doses on different schedules) examined. This indicates that for fluconazole the AUC/MIC ratio is the pharmacodynamically linked variable. Whether this finding can be applied to other azoles with different pharmacokinetic and physical properties is an important question which needs study. However, for fluconazole it is clear that the once-daily dosing schedule produces an antifungal effect equivalent to those of fluconazole given on the other dosing schedules examined. It should be pointed out that this is so even though the half-life of fluconazole in mice is on the order of 2.4 h and that the total time > MIC for mice on the once-daily schedule ranged between 9.1 and 10.6 h for the doses used in the dose-fractionation study, reminiscent of the situation for once-daily aminoglycoside therapy. There is no need to lose any of compliance and convenience advantages of once-daily fluconazole dosing by using a more frequent schedule of administration.
ACKNOWLEDGMENTS
These studies were supported by Pfizer, Inc., New York, N.Y.
We thank the staff of the Animal Resource Facility of the Wadsworth Center for Laboratories and Research, New York State Department of Health, for excellent care of the animals.
REFERENCES
- 1.Akaike H. A new look at the statistical model identification. IEEE Trans Automated Control. 1974;19:716–723. [Google Scholar]
- 2.Anaissie E J, Darouiche R O, Abi-Said D, Uzun O, Mera J, Gentry L O, Williams T, Kontoyiannis D P, Karl C L, Bodey G P. Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clin Infect Dis. 1996;23:964–972. doi: 10.1093/clinids/23.5.964. [DOI] [PubMed] [Google Scholar]
- 3.Beck-Sague C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 4.Billelo J A, Bauer G, Dudley M N, Cole G A, Drusano G L. Effect of 2′,3′-didehydro-3′-desoxythymidine in an in vitro hollow-fiber pharmacodynamic model system correlates with results of dose-ranging clinical studies. Antimicrob Agents Chemother. 1994;38:1386–1391. doi: 10.1128/aac.38.6.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilello J A, Eiseman J L, Standiford H C, Drusano G L. Impact of dosing schedule upon suppression of a retrovirus in a murine model of AIDS. Antimicrob Agents Chemother. 1994;38:628–631. doi: 10.1128/aac.38.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodey G P, Ketchel S J, Rodriguez V. A randomized study of carbenicillin plus cefamandole or tobramycin in the treatment of febrile episodes in cancer patients. Am J Med. 1979;67:608–616. doi: 10.1016/0002-9343(79)90242-0. [DOI] [PubMed] [Google Scholar]
- 7.Drusano G L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drusano G L, Johnson D E, Rosen M, Standiford H C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas aeruginosa sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fluckiger U, Segessenmann C, Gerber A U. Integration of pharmacokinetics and pharmacodynamics of imipenem in a human-adapted mouse model. Antimicrob Agents Chemother. 1991;35:1905–1910. doi: 10.1128/aac.35.9.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber A U, Wiprachtiger P, Stettler-Spichiger U, Lebek G. Constant infusion vs. intermittent doses of gentamicin against Pseudomonas aeruginosa in vitro. J Infect Dis. 1982;145:554–560. doi: 10.1093/infdis/145.4.554. [DOI] [PubMed] [Google Scholar]
- 12.Grant S M, Clissold S P. Fluconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs. 1990;39:877–916. doi: 10.2165/00003495-199039060-00006. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen J H, Alexander G A, Graybill J R, Drutz D J. Sensitive bioassay for ketoconazole in serum and cerebrospinal fluid. Antimicrob Agents Chemother. 1981;20:59–62. doi: 10.1128/aac.20.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leggett J E, Ebert S, Fantin B, Craig W A. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis models. Scand J Infect Dis. 1991;74:179–184. [PubMed] [Google Scholar]
- 15.Louie A, Chan C, Mayers M, Perkins R, Drusano G, Anaissie E, Miller M. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Dosing of fluconazole in rabbits to achieve pharmacokinetic parameters that mimic those of high-dose fluconazole in humans, abstr. A-119; p. 164. [Google Scholar]
- 16.Madu A, Cioffe C, Mian U, Burroughs M, Toumanen E, Mayers M, Schwartz E, Miller M. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum of rabbits: validation of an animal model used to measure drug concentration in cerebrospinal fluid. Antimicrob Agents Chemother. 1994;38:2111–2115. doi: 10.1128/aac.38.9.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore, R. D., P. S. Lietman, and C. R. Smith. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J. Infect. Dis. 155:93–99. [DOI] [PubMed]
- 18.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeast; tentative standard. M27-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 19.Powell S H, Thompson W L, Luthe M A, Stern R C, Grossniklaus D A, Bloxham D D, Groden D L, Jacobs M R, DiScenna A O, Cash H A, Klinger J D. Once-daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. J Infect Dis. 1983;147:918–932. doi: 10.1093/infdis/147.5.918. [DOI] [PubMed] [Google Scholar]
- 20.Rex J H, Bennett J E, Sugar A M, Pappas P G, Van der Horst C M, Edwards J E, Washburn R G, Scheld W M, Karchmer A W, Dine A P, Levenstein M J, Webb C D the Candidemia Study Group; the NIAID Mycoses Study Group. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994;331:1325–1330. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 21.Roosendaal R, Bakker-Woudenberg I A, van den Berghe-van Raffe M, Michel M F. Continuous versus intermittent administration of ceftazidime in experimental Klebsiella pneumoniae pneumonia in normal and leukopenic rats. Antimicrob Agents Chemother. 1986;30:403–408. doi: 10.1128/aac.30.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schentag, J. J., I. L. Smith, D. J. Swanson, C. DeAngelis, J. E. Fracasso, A. Vari, and J. W. Vance. 1984. Role for dual individualization with cefmenoxime. Am. J. Med. 77(Suppl. 6a):43–50. [DOI] [PubMed]
- 23.Solomkin J S, Flohr A M, Simmons R L. Indications for therapy for fungemia in postoperative patients. Arch Surg. 1982;117:1272–1275. doi: 10.1001/archsurg.1982.01380340008003. [DOI] [PubMed] [Google Scholar]
- 24.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 25.Wey S B, Mori M, Pfaller M A, Woolson R F, Wenzel R P. Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]