Abstract
Hair shafts from three trichothiodystrophy (TTD) patients with mutations in the ERCC2 (XPD) gene were examined by transmission electron microscopy. TTD is a rare, recessive disorder with mutations in several genes in the DNA repair/transcription pathway, including ERCC2. Unlike previous studies, the hair shafts were examined after relaxation of their structure by partial disulphide bond reduction in the presence of sodium dodecyl sulphate, permitting improved visualization. Compared with hair shafts of normal phenotype, TTD cuticle cells displayed aberrant marginal bands and exocuticle layers. Clusters of cells stained differently (light versus dark) in the cortex of aberrant shafts, and the keratin macrofibrils appeared much shorter in the cytoplasm. Considerable heterogeneity in these properties was evident among samples and even along the length of single hair shafts. The results are consistent with not only a paucity of high sulphur components, such as keratin-associated proteins, but also a profound imbalance in protein content and organization.
Keywords: DNA repair/transcription disease, hair cortex, hair exocuticle, keratin macrofibrils, marginal band, neuroectodermal genodermatosis, transmission electron microscopy
1 |. BACKGROUND
Trichothiodystrophy (TTD) is a rare, autosomal recessive multisystem disorder characterized by short brittle hair, failure to thrive, ichthyosis and developmental delays.1 In addition, TTD patients have brain dysmyelination, bone and dental abnormalities, congenital cataracts, immune deficiencies and a 20-fold increased risk of death before age 10 years.2 TTD patients are often acutely sun sensitive and experience blistering burns after minimal exposure. TTD children are frequently born with a collodion membrane, and 70% are low birth weight (<2500 g) requiring admission to the neonatal intensive care unit (NICU). In addition, the mothers of TTD children experience a high frequency of pregnancy complications including preterm labour, pre-eclampsia and HELLP (haemolysis, low platelets and elevated liver enzymes) syndrome.3–5
Two diagnostic features of the hair are alternating light/dark banding visible in polarized light, which is termed “tiger tail banding,” and low cysteine content, typically about half of normal, revealed by amino acid analysis.6–8 Visible by light microscopy, unusual morphological features are evident, including narrowing of the shaft, ribboning, trichorrhexis nodosa-like fractures and trichoschisis. With higher resolution, scanning electron microscopy reveals surface irregularities and indicates loose attachment of cuticle cells to the shaft. The degree of departure of TTD hair from normal parallels the degree of cysteine deficiency in the shaft.7 Understanding the basis for this low cysteine content and hair fragility may help elucidate the pathogenesis of this neuroectodermal disease.2,8
Since a high degree of disulphide bonding occurs in the mature hair shaft, and likely contributes greatly to its cohesiveness, considerable effort has focused on explaining the brittleness of TTD hair as a consequence of its lower cysteine content. Ordinarily, cuticle cells exhibit a high overall cysteine content (estimated 17.5%) concentrated especially in the marginal band (estimated 37%) and to a lesser extent in the exocuticle.9 However, the marginal band in TTD hair is deficient in sulphur, consistent with loss of cuticle cells by weathering along the length of the shaft, not only at the distal end.10 This deficiency has been attributed to a lack of ultrahigh and certain high sulphur proteins.11
2 |. QUESTIONS ADDRESSED
A genetic basis for TTD has now been established. Causative mutations have been reported in 10 genes: DNA repair/transcription genes XPB, XPD and TTDA2,12; a M-phase-specific MLK1 interacting protein TTDN1 whose function is not clear13–15; GTF2E2 which has a role in the initiation of RNA transcription16,17; CARS1, TARS1, AARS1, MARS1, whose function is to load each amino acid onto its specific cognate tRNA through aminoacylation reactions18; and RNF113A which has a role in the transcriptional process.19,20 Since the original work characterizing the ultrastructure of TTD hair preceded the genetic findings, we have examined sections of TTD hair with known defects in the XPD gene (ERCC2) by transmission electron microscopy to find whether they differ from previous findings and from each other. To permit improved visualization, the hairs were processed after relaxation of the structure by partial disulphide bond reduction in the presence of sodium dodecyl sulphate.
3 |. EXPERIMENTAL DESIGN
3.1 |. Hair samples and genotypes
Hair shafts were obtained with informed consent from three unrelated TTD patients, a (clinically normal) parent of each patient, and an unaffected individual. In all cases, the parental and unaffected individual control hair shaft samples were visibly indistinguishable. The genotypes of the samples examined were all compound heterozygotes in the XPD (ERCC2) gene as indicated below. For convenience, the samples were numbered T1-T3 (NIH cell/Patient ID number in parentheses) and the parental samples were numbered P1-P3, respectively.
3.2 |. Patients
T1 (TTD568BE) was a 2 year 11-month-old female patient who has heterozygous mutations in the ERCC2 gene: one allele c.1867dupG (p.V623Gf*26) resulted in a premature stop mutation and a novel second allele, c.2158 T>C (p. F720L), resulted in a missense mutation. She was born at 31 weeks gestation following a pregnancy complicated by severe maternal pre-eclampsia; extremely low birth weight (1452 g) and had a collodion membrane. She was admitted to the NICU for 5 weeks. She had recurrent infections with multiple hospitalizations, poor linear growth and weight gain, and delayed development. Due to the feeding difficulties and poor weight gain, she had a gastrostomy tube placed at age 2 ½ yrs (Figure S1A,B).
T2 (TTD397BE) was a 19-month-old male patient who has heterozygous mutations in the ERCC2 gene: one allele, c. 2176 C>G (p.Q726E) was missense, and the other allele was an in-frame large deletion (g.16603_18447del; c.1666_2190del; p.G556_R730del) expected to result in a truncated XPD protein missing the amino acids encoded by exons 18–22. Hair sulphur content was 2.30% [normal 5.0%]. He was born at 35 weeks gestation following a pregnancy complicated by preterm labour and abnormal maternal serum screening values. He had low birthweight (2313 g) and a collodion membrane. He was admitted to the NICU for 19 days. He had 2 older siblings with TTD. A hair sample was obtained while he was hospitalized and found to have tiger tail banding on polarized microscopy, and he was diagnosed with TTD at 1 week of age. His developmental and motor milestones were delayed, and he did not begin walking until age 18 months. He had multiple otitis media infections and pneumonias which did not require hospitalization. He has been found to be neutropenic with mildly abnormal immunoglobulins (Figure S1C,D).
T3 (TTD475BE) was a 14-month-old male patient who has heterozygous mutations in the ERCC 2 gene: one allele has an insertion of “T” at the exon 15 splice donor site (c.1479 + 2insT) and the other allele has a deletion of ‘G’ at exon 22 splice donor site (c.2190 + 1delG). Hair sulphur content was 2.2% (normal 4.9%). The patient was born at 36 ½ weeks following a relatively uncomplicated pregnancy; however, the mother had mildly elevated blood pressure towards the end of the pregnancy. He had a collodion membrane at birth and was admitted to the NICU for 9 days. He was diagnosed with cryptorchidism, congenital cataracts and a patent foramen ovale in the immediate neonatal period. He had feeding difficulties in the neonatal period with gastrointestinal reflux. His cataracts were removed at age 6 weeks, and he wears soft contact lenses. He was diagnosed with TTD by assessing his hair for tiger tail banding and sulphur content (Figure S1E,F).
3.3 |. Transmission electron microscopy (TEM)
Samples were cut into 5 cm lengths and incubated at room temperature for 1 h or 3 h in 2 ml of 0.1 M sodium phosphate buffer (pH 7.8) containing 2% sodium dodecyl sulphate (SDS) and 25 mM dithiothreitol. These conditions induced swelling of the hair shaft10,21 and better embedding for TEM.22 The different incubation times gave equivalent results, although in some cases, the features of interest were slightly more prominent at 3 h. Processed by standard methods,23 samples were then immersed in Karnovsky’s fixative for several days, rinsed with 0.1 M phosphate buffer and post-fixed for 2 h in 1% buffered osmium tetroxide. Samples were dehydrated with graded ethanol, 50% propylene oxide in ethanol and finally two changes of pure propylene oxide. Samples were infiltrated with Spurr’s resin in three ascending concentrations in propylene oxide. Infiltration was accomplished with microwave assistance (Pelco 34 700 BioWave, Ted Pella Inc.). Following three resin changes, samples were allowed to polymerize overnight in fresh resin solution. Ultrathin sections (60–80 nm) of the polymerized blocks were cut on an ultramicrotome (Leica Ultracut UCT) using a Diatome diamond knife (Switzerland, Electron Microscopy Sciences USA distributor). Sections were placed on formvar/carbon coated slotted copper grids and poststained using uranyl acetate and lead citrate. The prepared sections were viewed in a FEI Talos 120C TEM (ThermoScientific Hillsboro, OR, USA, made in Eindhoven) and images were acquired using an integrated FEI Ceta CMOS camera.
4 |. RESULTS
Despite having the same average thickness, TTD cuticle cells exhibited dramatic differences in appearance under electron microscopy from those in phenotypically normal hair. The fine structure of cuticle cells in the normal hair shaft can be visualized readily after treatment with detergent under reducing conditions, as shown in Figure 1A. Three prominent features are (a) an intensely stained layer at the superficial edge of the cell (called the marginal band or A-layer), (b) a layer of fine-grained, nearly homogeneous material beneath the marginal band (called the exocuticle) and (c) a layer of more coarsely grained material (called the endocuticle) in which fragments or remnants of intracellular organelles frequently appear embedded. Ordinarily little material appears to be extracted from the cuticle even by harsh detergent treatment except for some small features in the endocuticle.
FIGURE 1.
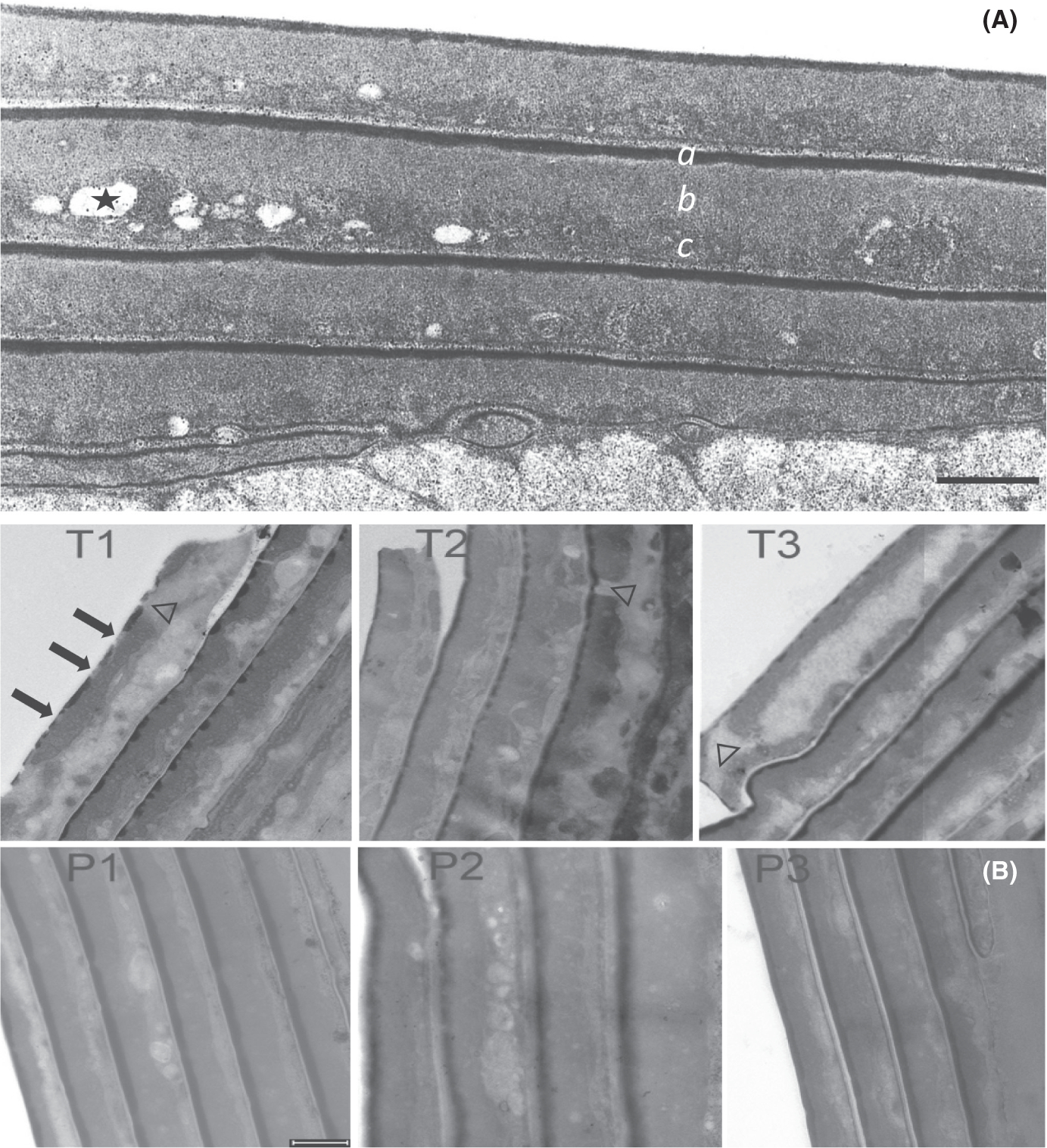
Transmission electron micrographs. (A) Cuticle of normal hair incubated in 2% SDS – 25 mM DTT at pH 7.8 for 6 h. The shaft was fixed, stained, sectioned and examined by transmission electron microscopy as described.10 The dark layer on the outer edge of each cell (a) is called the marginal band or A-layer. The fine-grained layer immediately beneath the marginal band is called the exocuticle (b). The coarse-grained layer at the bottom edge of the cell is the endocuticle (c), in which remnants of intracellular organelles frequently appear embedded, some of which are extracted by the harsh detergent treatment (one indicated by *). (B) Hair shaft cuticle from TTD patients (T1, T2, T3), their respective parents (P1, P2, P3). Arrows in T1 point to discontinuities in the marginal band of the outermost cell; note the clear discontinuities in the next two inner cells. Discontinuities in the exocuticle layer in the outermost cell in T1 and T3 and an inner cell in T2 are denoted by Δ. Note the disorganized appearance of the endocuticle in cells in the TTD samples. Scale bars = 0.5 μm
The cuticle seen in TTD hair shafts exhibited distinct differences from that in normal parental hair (Figure 1B). First, the marginal band (A layer) often appeared incomplete or discontinuous and even absent in some cells. Although mostly fine grained as in the normal samples, the exocuticle in TTD hair was less uniform in width and sometimes contained large features similar to those in the endocuticle. The boundary between exo- and endo-cuticle was often quite variable, appearing disorganized along the length of the cuticle cell. Considerable variation in the integrity of the marginal band and the boundaries of the exo- and endo-cuticle was noted even in adjoining cells in the same hair shaft.
In the cortex, longitudinal sections frequently revealed distinctive regions of differential staining intensity in TTD hair that were uncommon in the parental normal hair. As seen in Figure 2, these regions at lower magnification appeared as an intermingling of small clusters of cells staining light or dark (panels T1, T2), or elongated structures that were more tightly arranged (panel T3). By contrast, the parental control samples were much more uniformly stained (panels P1, P2, P3).
FIGURE 2.
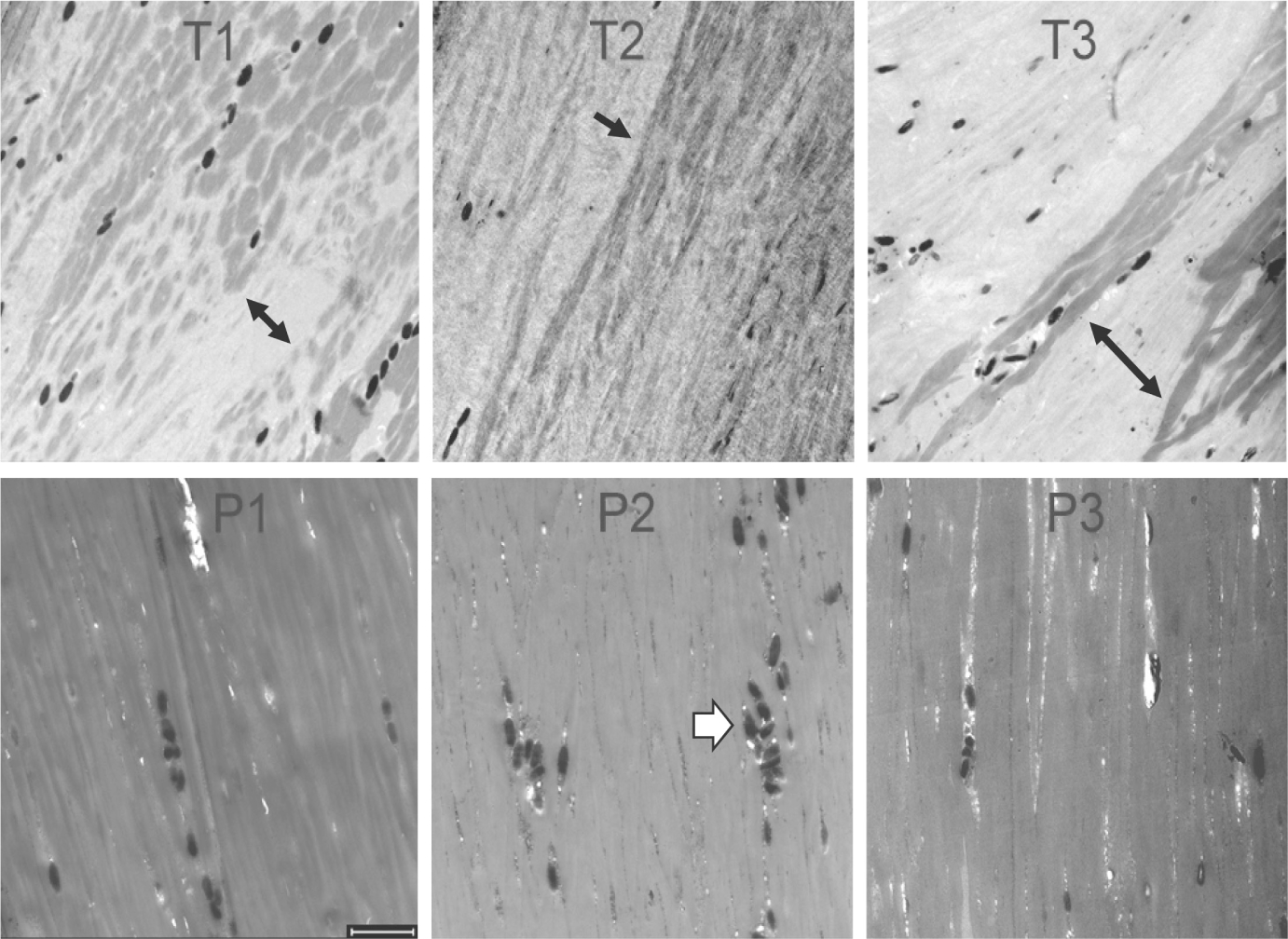
Longitudinal sections of hair shafts (3 h incubation) from TTD patients (T1, T2, T3) and their parents (P1, P2, P3). Note the variegated staining patterns in T1, T2 and T3 (black arrows), which differ from the parental samples and from each other. The dark ellipsoids seen in all the samples (white arrowhead in P2) are melanosomes (0.4–0.9 μm diameter).28 Scale bar 2 μm
At higher magnification, the dark stain in the TTD cortex appeared to delineate islands of curved short macrofibrils (Figure 3). By contrast, the macrofibrils in parental hair appeared to extend along the length of the cells. The parental cells also exhibited more electron dense fine granules among individual macrofibrils and at the edges of cortical cells (the latter also illustrated in Figure S2).
FIGURE 3.
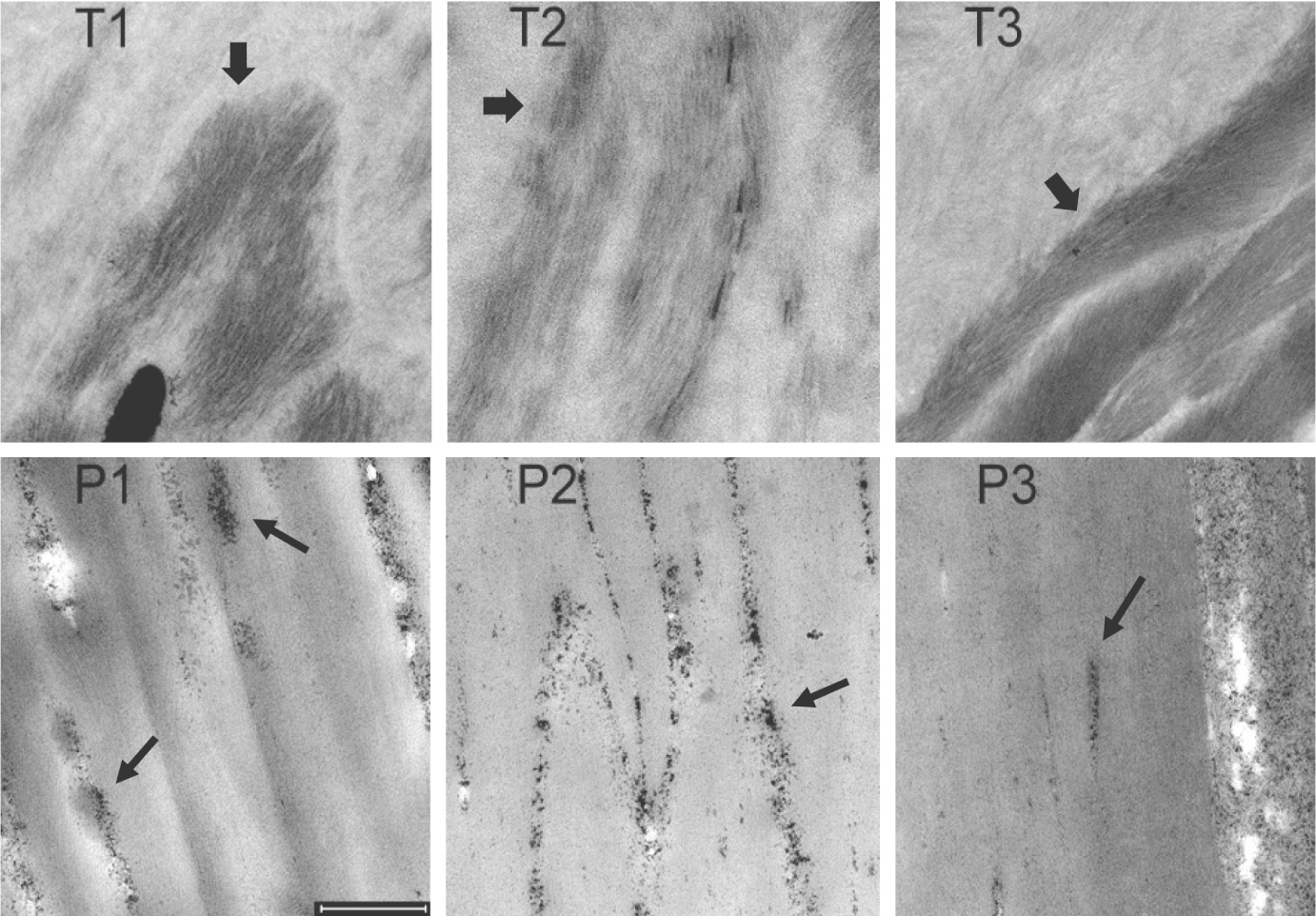
Sections of hair cortex from TTD patients (T1, T2, T3) and their parents (P1, P2, P3). Thick arrows in panels T1, T2 and T3 point to concentrations of darkly stained short fibrils. Thin arrows in panels P1, P2 and P3 draw attention to clusters of darkly staining particles appearing in intercellular spaces. Scale bar = 0.5 μm
5 |. CONCLUSIONS AND PERSPECTIVES
Present findings of aberrant features in the TTD hair cuticle and cortex resemble those in previous work of unknown genotype.10 A striking observation is the intra-individual variability in each sample. As previously reported, the marginal band can be discontinuous or absent, the exocuticle can appear disturbed and staining of the cortex can show regional variation.10,24 Analogous to findings with defects in TFIIEβ,17 we hypothesize that such defects result from transcriptional deficiencies of certain components, including ultrahigh sulphur proteins that normally assist in proper intracellular protein distribution during the final stages of development in the follicle. Since the deficiencies permit near normal cell growth, deficiencies in the hair keratinocytes could occur primarily in transcription or translation of components needed in relatively high amount for terminal differentiation. If it occurred in epidermal keratinocytes, this phenomenon could help rationalize the frequent finding of an ichthyosis phenotype and onychodystrophy in epidermis and nail plate in TTD, respectively.
Lack of expression of proteins with high cysteine content participating in disulphide bonding is unlikely to be the only factor bestowing fragility on TTD hair shafts. Some TTD hair samples also display a near-total lack of isopeptide-stabilized protein in the exocuticle, judging by its complete detergent extraction.25 A deficiency in such protein in the cuticle could be responsible for the absence or irregular appearance of a marginal band in present samples after extraction with detergent and reducing agent. The different alleles of XPD are known to affect the phenotype in the related condition xeroderma pigmentosum.26 Possibly even cellular mosaicism in hair shaft formation27 may play a role in the TTD phenotype. Moreover, the wide spectrum of clinical abnormalities observed in TTD patients,2 affecting multiple organ systems, points to transcriptional defects in numerous cell types and thus beyond those participating in keratinocyte differentiation.
Supplementary Material
Figure S2 Electron dense granules at the edges of cortical cells in hair from Parent 1.
Figure S1 TTD patients studied and polarized light micrography of their hair. (A) Patient T1 (TTD568BE) a 2 years 11 month-old girl with unruly brittle hair, loss of lateral eyebrows and typical TTD features as described in the text. (B) Hair from patient T1 with alternating dark and light (tiger tail) bands (white arrows), trichoschisis (horizontal split in hair strand) (black arrow), abrupt change in diameter of hair shaft (*) and shafts of different diameters. Overlapping hair strands cause isolated bands that is not true tiger tail banding. (40× original magnification). (C) Patient T2 (TTD397BE) a 19-month-old boy with short brittle hair and an outgoing smile typical of TTD patients. (D) Hairs from patient T2 with tiger tail banding and irregularities of hair shaft surface on the middle hair strand in vertical orientation. (100× original magnification). Note that the banding may not be visible in all hairs at the same time. However, rotation of the hairs or the stage will show this banding by changing the relative orientation of the hair shaft and the polarized light. (E) Patient T3 (TTD475BE) a 14-month- old boy with brittle, unruly hair, sparse eyebrows and typical TTD features as described in the text. (F) Hairs from patient T3 with tiger tail banding and hair shafts of varying diameters. (40× original magnification).
ACKNOWLEDGEMENTS
We thank Drs. Paradi Mirmirani and Kent E. Pinkerton for valuable advice, Patricia Kysar for expert technical assistance in electron microscopy and the USDA(NIFA)/University of California Agricultural Experiment Station (CA-D-ETX-2152-H) for financial support of this work. This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors state that they have no conflict of interest.
PHOTOGRAPH CONSENT STATEMENT
The signed forms are on file at NIH. A redacted copy with the identifiers blacked out can be provided on request. We are not able to send the form signed by the patients’ family members since that would violate patient privacy regulations. Other journals have accepted our written statements that we have signed copies of the photograph permission forms.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.DiGiovanna JJ, Kraemer KH. Shining a light on xeroderma pigmentosum. J Invest Dermatol. 2012;132:785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faghri S, Tamura D, Kraemer KH, Digiovanna JJ. Trichothiodystrophy: a systematic review of 112 published cases characterises a wide spectrum of clinical manifestations. J Med Genet. 2008;45:609–621. doi: 10.1136/jmg.2008.058743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moslehi R, Signore C, Tamura D, et al. Adverse effects of trichothiodystrophy DNA repair and transcription gene disorder on human fetal development. Clin Genet. 2010;77:365–373. doi: 10.1111/j.1399-0004.2009.01336.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura D, Khan SG, Merideth M, et al. Effect of mutations in XPD(ERCC2) on pregnancy and prenatal development in mothers of patients with trichothiodystrophy or xeroderma pigmentosum. Eur J Human Genet. 2012;20:1308–1310. doi: 10.1038/ejhg.2012.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura D, Merideth M, DiGiovanna JJ, et al. High-risk pregnancy and neonatal complications in the DNA repair and transcription disorder trichothiodystrophy: report of 27 affected pregnancies. Prenat Diagn. 2011;31:1046–1053. doi: 10.1002/pd.2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang C, Kraemer KH, Morris A, et al. Characterization of tiger-tail banding and hair shaft abnormalities in trichothiodystrophy. J Am Acad Dermatol. 2005;52:224–232. doi: 10.1016/j.jaad.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 7.Liang C, Morris A, Schlücker S, et al. Structural and molecular hair abnormalities in trichothiodystrophy. J Invest Dermatol. 2006;126:2210–2216. doi: 10.1038/sj.jid.5700384 [DOI] [PubMed] [Google Scholar]
- 8.Price VH, Odom RB, Ward WH, Jones FT. Trichothiodystrophy. Sulfur deficient brittle hair as a marker for a neuro-ectodermal symptom complex. Arch Dermatol. 1980;116:1375–1384. [DOI] [PubMed] [Google Scholar]
- 9.Swift JA. Minimum depth electron probe X-ray microanalysis as a means for determining the Sulphur content of the human hair surface. Scanning. 1979;2:83–88. [Google Scholar]
- 10.Gummer CL, Dawber RP, Price VH. Trichothiodystrophy: an electron-histochemical study of the hair shaft. Br J Dermatol. 1984;110:439–449. doi: 10.1111/j.1365-2133.1984.tb04659.x [DOI] [PubMed] [Google Scholar]
- 11.Gillespie JM, Marshall RC. A comparison of the proteins of normal and trichothiodystrophic human hair. J Invest Dermatol. 1983;80:195–202. doi: 10.1111/1523-1747.ep12534032 [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Khan SG, Tamura D, et al. Abnormal XPD-induced nuclear receptor transactivation in DNA repair disorders: trichothiodystrophy and xeroderma pigmentosum. Eur J Human Genet. 2013;21:831–837. doi: 10.1038/ejhg.2012.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller ER, Khan SG, Kuschal C, Tamura D, DiGiovanna JJ, Kraemer KH. Mutations in the TTDN1 gene are associated with a distinct trichothiodystrophy phenotype. J Invest Dermatol. 2015;135:734–741. doi: 10.1038/jid.2014.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakabayashi K, Amann D, Ren Y, et al. Identification of C7orf11 (TTDN1) gene mutations and genetic heterogeneity in nonphoto-sensitive trichothiodystrophy. Am J Hum Genet. 2005;76:510–516. doi: 10.1086/428141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou YK, Yang XC, Cao Y, et al. A homozygous G insertion in MPLKIP leads to TTDN1 with the hypergonadotropic hypogonadism symptom. BMC Med Genet. 2018;19(Suppl 1):214. doi: 10.1186/s12881-018-0723-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuschal C, Botta E, Orioli D, et al. GTF2E2 mutations destabilize the general transcription factor complex TFIIE in individuals with DNA repair-proficient tichothiodystrophy. Am J Hum Genet. 2016;98:627–642. doi: 10.1016/j.ajhg.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theil AF, Mandemaker IK, van den Akker E, et al. Trichothiodystrophy causative TFIIEβ mutation affects transcription in highly differentiated tissue. Hum Molec Genet. 2017;26:4689–4698. doi: 10.1093/hmg/ddx351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botta E, Theil A, Raams A, et al. Protein instability associated with AARS1 and MARS1 mutations causes trichothiodystrophy. Hum Molec Genet. 2021;30:1711–1720. doi: 10.1093/hmg/ddab123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu NY, Chung CS, Cheng SC. Role of Cwc24 in the first catalytic step of splicing and fidelity of 5′ splice site selection. Molec Cell Biol. 2017;37(6):e00580–16. doi: 10.1128/MCB.00580-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shostak K, Jiang Z, Charloteaux B, et al. The X-linked trichothiodystrophy-causing gene RNF113A links the spliceosome to cell survival upon DNA damage. Nat Commun. 2020;11(1):1270. doi: 10.1038/s41467-020-15003-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice RH. Proteomic analysis of hair shaft and nail plate. Int J Cosmetic Sci. 2011;62:229–236. [PMC free article] [PubMed] [Google Scholar]
- 22.Rice RH, Rocke DM, Tsai H-S, Lee YJ, Silva KA, Sundberg JP. Distinguishing mouse strains by proteomic analysis of pelage hair. J Invest Dermatol. 2009;129:2120–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suvarna KS, Layton C, Bancroft JD. Bancroft’s Theory and Practice of Histological Techniques. 7th ed. Churchill Livingston Elsevier; 2013. [Google Scholar]
- 24.Khumalo NP, Dawber RP, Ferguson DJ. Apparent fragility of African hair is unrelated to the cystine-rich protein distribution: a cyto-chemical electron microscopic study. Exp Dermatol. 2005;14:311–314. doi: 10.1111/j.0906-6705.2005.00288.x [DOI] [PubMed] [Google Scholar]
- 25.Rice RH, Wong VJ, Price VH, Hohl D, Pinkerton KE. Cuticle cell defects in lamellar ichthyosis hair and anomalous hair shaft syndromes visualized after detergent extraction. Anatomic Rec. 1996;246:433–440. [DOI] [PubMed] [Google Scholar]
- 26.Ueda T, Compe E, Catez P. Both XPD alleles contribute to the phenotype of compound heterozygote xeroderma pigmentosum patients. J Exp Med. 2009;206:3031–3046. doi: 10.1084/jem.20091892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Masaki T, Khan SG, et al. Four-dimensional, dynamic mosaicism is a hallmark of normal human skin that permits mapping of the organization and patterning of human epidermis during terminal differentiation. PLoS One. 2018;13(6):e0198011. doi: 10.1371/journal.pone.0198011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi A, Valencia JC, Hu Z-Z, et al. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J Proteome Res. 2006;5:3135–3144. doi: 10.1021/pr060363j [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2 Electron dense granules at the edges of cortical cells in hair from Parent 1.
Figure S1 TTD patients studied and polarized light micrography of their hair. (A) Patient T1 (TTD568BE) a 2 years 11 month-old girl with unruly brittle hair, loss of lateral eyebrows and typical TTD features as described in the text. (B) Hair from patient T1 with alternating dark and light (tiger tail) bands (white arrows), trichoschisis (horizontal split in hair strand) (black arrow), abrupt change in diameter of hair shaft (*) and shafts of different diameters. Overlapping hair strands cause isolated bands that is not true tiger tail banding. (40× original magnification). (C) Patient T2 (TTD397BE) a 19-month-old boy with short brittle hair and an outgoing smile typical of TTD patients. (D) Hairs from patient T2 with tiger tail banding and irregularities of hair shaft surface on the middle hair strand in vertical orientation. (100× original magnification). Note that the banding may not be visible in all hairs at the same time. However, rotation of the hairs or the stage will show this banding by changing the relative orientation of the hair shaft and the polarized light. (E) Patient T3 (TTD475BE) a 14-month- old boy with brittle, unruly hair, sparse eyebrows and typical TTD features as described in the text. (F) Hairs from patient T3 with tiger tail banding and hair shafts of varying diameters. (40× original magnification).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


