Abstract
Background
This is an updated version of the original Cochrane review published in Issue 4, 2008. The role of antipsychotics as adjuvant analgesics is a subject of longstanding controversy. Neuroleptanalgesia (that is a state of quiescence, altered awareness, and analgesia produced by a combination of taking an opioid analgesic and an antipsychotic), an established term for the management of acute pain, was shown to negatively influence disease course and total mortality in unstable angina patients. Nevertheless, antipsychotics are used to treat chronic pain (for example chronic headache, fibromyalgia and diabetic neuropathia). With atypical antipsychotics, a new class of antipsychotics, both fewer extrapyramidal side effects and additional benefits may be available.
Objectives
To assess the analgesic efficacy and adverse effects of antipsychotics in acute or chronic pain in adults.
Search methods
The following databases were searched: CENTRAL, on The Cochrane Library, (Issue 12 of 12, 2012); MEDLINE (1966 to 11/1/2013); EMBASE (1980 to 2013 week 03) and PsycINFO 1806 to Jan week 3 2013. Searches were run originally in 2007 and then again in 2011 and 2013.
Selection criteria
Randomised controlled trials (RCTs) of adults prescribed any dose of an oral antipsychotic for acute or chronic pain, where subjective pain assessment was described as either the primary or a secondary outcome, were included in this review.
Data collection and analysis
Data were extracted by two independent review authors, and results were compared for differences. Discrepancies were resolved by discussion. All trials were quality scored according to the methods set out in section six of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
A total of 770 participants were involved in the 11 included studies. Data from five included randomised double‐blind studies showed beneficial effects of antipsychotics in the treatment of acute and chronic pain. Quantitative analysis of these studies showed a significant reduction of mean pain intensity after administration of the antipsychotic compared to placebo or another active compound, weighted mean difference (WMD) ‐1.78 (95% CI ‐2.71 to ‐0.85) for the continuous data; and relative risk (RR) 0.43 (95% CI 0.25 to 0.73), number needed to treat to benefit (NNT) 2.6 for the dichotomous data. Nevertheless, the test for heterogeneity was significant for both the continuous data (P = 0.0007) and the dichotomous data (P = 0.04). Obviously this makes the calculated NNT less reliable and caution is warranted when interpreting these results.
The most frequently reported adverse effects were extrapyramidal (that is involuntary movements, parkinsonism and akathisia) and sedating effects.
Authors' conclusions
The recent search found five new studies which were all excluded, so the review remains the same as previously.
Antipsychotics might be used as an add‐on therapy in the treatment of painful conditions. Nevertheless, extrapyramidal and sedating side effects have to be considered before using antipsychotics for treating painful conditions.
Results for antipsychotics in the treatment of different painful conditions are mixed and most sample sizes in the reviewed RCTs are small. Further studies on atypical antipsychotics in larger double‐blind placebo‐controlled studies that include standardised pain assessment and documentation are warranted.
Plain language summary
Analgesic effects of antipsychotics in acute and chronic painful states
Medicines called ‘antipsychotics’, which are used to treat some mental health conditions, are sometimes used to treat chronic pain. A new type of these medicines called 'atypical antipsychotics' is available, with fewer side effects and some additional benefits. The review authors assessed the effect of these medicines on pain and their side effects. Based on 5 out of 11 included trials there were some beneficial effects of antipsychotics in the treatment of acute and chronic pain. Analysis of these studies showed a significant reduction in pain after administration of the antipsychotic compared to placebo or another medicine, however these results were based on small studies and therefore they may be unreliable. It is also important to consider the unwanted effects that these medicines might cause.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2008, Issue 4).
Antipsychotics (also called neuroleptics) can be classified according to their chemical structure into tricyclic antipsychotics (phenothiazines, thioxanthenes), butyrophenones, substituted benzamides and other chemical substances (dichlorphenyl‐piperazinyl‐chiloninones, diphenylbutylpiperidines, benzisoxazoles, benzisothiazylpiperazines, phenylpiperidines). Atypical antipsychotics differ from classical antipsychotics, or 'first generation antipsychotics', in the extrapyramidal side effects, effectiveness in negative symptomatology, and lower prolactin elevations with comparable antipsychotic efficacy. Classical antipsychotics have a predominant dopamine D2 antagonism, whereas the atypical antipsychotics also address other neurotransmitter systems, for example the serotonin system. In clinical practice cardiovascular side effects, especially a prolonged QTc, have to be kept in mind when treating patients with antipsychotics.
The therapeutic effects of antipsychotics make them a potential choice as drugs in the treatment of pain. To date the role of classic antipsychotics such as adjuvant analgesics has been a subject of longstanding controversy. Their clinical usefulness in the management of pain is questioned (Patt 1994). Neuroleptanalgesia (that is a state of quiescence, altered awareness, and analgesia produced by a combination of taking an opioid analgesic and an antipsychotic) as an established term for the management of acute pain was shown to negatively influence disease course and total mortality in unstable angina patients (Burduk 2000).
Antipsychotics are also used in a variety of different chronic pain states, from cancer pain (Bloomfield 1964; Breitbart 1998; Khojainova 2002) to chronic non‐cancer pain (Merskey 1997) as in chronic headache or chronic refractory headache (Hakkarainen 1977; Lu 2000; Silberstein 2002), fibromyalgia (Kiser 2001), musculoskeletal pain (Bloomfield 1964), low back pain (Bloomfield 1964; Jermyn 2001), chronic pain in older patients (Feinberg 2000), pain in AIDS (Breitbart 1998), post‐herpes zoster (Gobel 1997; Montilla 1963), chronic facial pain (Lechin 1989; Peschen‐Rosin 2002), and diabetic neuropathia (Gomez‐Perez 1985).
In a meta‐analysis on the analgesic potency of antipsychotics by Nix and colleagues (Nix 1998) only 10 out of 15 studies with a higher statistical power described a possible analgesic effect. None of the studies identified could differentiate between the effects of analgesia and sedation of the drugs used.
The way antipsychotics work to relieve pain is still under debate and may differ between different agents. For some pain syndromes (for example migraine) antidopaminergic properties may mediate the analgesic effects. Also, the serotonin antagonism of some antipsychotic agents is believed to mediate the analgesic effects (Schreiber 1999). Additionally, for some antipsychotics (for example olanzapine) their agonistic activity at alpha2‐adrenoceptors is believed to mediate analgesic effects (Silberstein 2002).
Besides discussions about the potential of antipsychotics to be used as analgesics, a potent antinociceptive effect has been shown for risperidone, an atypical antipsychotic, in an in vivo animal pain model (that is the tail‐flick assay). Further evaluation of risperidone with selective opioid antagonists revealed the involvement of µ1‐, µ2‐, and kappa1‐opioids and, to a lesser extent, delta‐opioid mechanisms. For olanzapine the alpha2‐adrenoceptors and to a lesser extent the opioid and serotonergic receptors are involved in the antinociception (Schreiber 1999).
With the arrival of atypical antipsychotics, a new class of antipsychotics, fewer extrapyramidal side effects and additional benefits are now available. These new treatments were not included in a meta‐analysis of reports on the analgesic effects of antipsychotics performed by Nix 1998. Therefore, a new meta‐analysis is needed to address the question of evidence based pain therapy with antipsychotics.
Objectives
To assess the analgesic efficacy and adverse effects of antipsychotics in acute or chronic pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) which were double blinded and which investigated the analgesic effects of antipsychotics as monotherapy or add‐on treatment in patients with acute or chronic pain, if pain assessment was either the primary or a secondary outcome. Reports were excluded if they were studies which were non‐randomised, studies of experimental pain, case reports, clinical observations (open studies) or studies of antipsychotics used to treat pain produced by other drugs.
Types of participants
Randomised controlled trials (RCTs) of adult patients of either gender who had acute or chronic pain, or both, of all degrees of severity, were included in this review.
Types of interventions
Any form of antipsychotic treatment (at any dose) listed below compared with no treatment, placebo, or other pain relieving treatment (for example non‐steroidal anti‐inflammatory drugs (NSAIDs), antidepressants, anticonvulsants, opiates).
Antipsychotic agents or neuroleptics:
amisulpride,
amoxapine,
chlormethiazole,
clopenthixol,
chlorpiprazine,
chlorpromazine,
chlorprothixene,
cloxazepine,
clozapine,
distraneurine,
dixyrazine,
droperidol
chlorpromazine,
flupentixol decanoate,
fluphenazine,
haloperidol,
levomepromazine,
loxapine,
melperone hydrochloride,
methotrimeprazine,
olanzapine,
oxilapine,
perphenazine,
pimozide,
prochlorperazine
prothipendyl hydrochloride,
quetiapine,
risperidone,
sulpiride,
thioridazine,
tiapride,
tisercin,
trifluoroperazine,
ziprsasidone,
zotepine,
zuclopenthixol.
For the update of this review in January 2013 we included two additional antipsychotics, namely droperidol and prochlorperazine, in our search strategy.
Types of outcome measures
Primary outcomes
The primary outcome measure for this review was the reduction in pain intensity as measured by a visual analogue scale (VAS), self reported global scale, verbal rating scale, numerical rating scale or categorical pain relief scale, and self reported pain relief. We used the effectiveness measures after the longest reported duration of treatment. We excluded studies which did not quantify pain using a scale. We included patient reported pain data, and excluded trials only reporting physician and carer pain assessment.
Secondary outcomes
An assessment of the frequency and severity of the commonly expected adverse effects was undertaken. Adverse effects were classified as minor if they were reported by a participant who continued with the medication and completed the trial. A major adverse effect was defined as one that caused the participant to withdraw from the study. Side effect data are recorded in Table 1.
1. Side effects.
| Author/Year | Substance | Type of side effect | Percentage |
| Ginsberg 1983 | Tiapride | Drowsiness (moderate to mild), mild gastric intolerance | 38.1% |
| Lechin 1989 | Pimozide | Physical and mental retardation, hand tremors, memory impairment, involuntary movements during sleep (jerkings) and slight Parkinson's disease manifestations | 83.3% |
| Langemark 1994 | Sulpiride | Sedation, depression, nausea, sleep disturbance, increased dreaming, uneasiness, weight gain, obstipation, amenorrhoea, galactorrhoea, impotence, restless legs, micturation difficulties, polyuria, accomodation difficulties, dry mouth, orthostatic hypotension | author only provided incidences, at least 34% |
| Roux 1983 | Tiapride | no side effects reported | ‐‐ |
| Johnston 1972 | Thioridazine | No untoward effects were observed or reported at any time during the study | ‐‐ |
| Davidsen 1979 | Levomepromazine | Dry mouth | 59% |
| Graff‐Radford 2000 | Fluphenazine | Sleepiness, dry mouth | ‐‐ |
| Zitman 1991 | Flupentixol | Dry mouth | ‐‐ |
| Judkins 1982 | Haloperidol | No serious side effects were observed, dry mouth | ‐‐ |
| Bussone 1980 | Tiapride | No extrapyramidal, neuroendocrine or neurovegetative side effects were observed | ‐‐ |
| Honkaniemi 2006 | Haloperidol | Motor agitation, sedation, hyperventilation and shortness of breath | 80% |
| Richman 2002 | Droperidol | Sedation, akathisia | Sedation: 6.7% (droperidol) vs. 13.4% (meperidine); akathisia 13.3% (only droperidol reported) |
Additional outcomes
Attrition, the numbers of participants withdrawing before completion of the study in an intervention versus placebo study and an intervention versus other treatment study due to non‐compliance, adverse effects or death
Measures of satisfaction or patient preference (if reported)
Assessment of quality of life (if reported)
Search methods for identification of studies
Electronic searches
This search was run for the original review in October 2007 and subsequent searches were run in 2011 and January 2013.
For the identification of studies to be included or considered for this review, the following databases were searched:
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 12);
MEDLINE (1966 to January 2013);
EMBASE (1989 to January 2013);
PsycINFO (1806 to January 2013).
Detailed search strategies were developed for each database searched and the MEDLINE search strategy from the original 2007 search is given in Appendix 1; for other search strategies please see Appendix 2 and the search strategies used for the 2013 updated searches can be found in Appendix 3. The searches attempted to identify all relevant studies irrespective of language. Non‐English papers would be assessed and translated, if necessary, with the assistance of a native speaker.
Searching other resources
We checked reference lists from retrieved trials for additional studies. We also sought relevant studies cited in reviews identified by searching the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews and Effectiveness (DARE).
We contacted the corresponding authors of the identified articles and experts in the field for additional studies. Furthermore, we sent letters requesting information about published or unpublished trials to pharmaceutical companies which manufacture antipsychotics (Sanofi‐Synthelabo: amisulpride, fluphenazine, sulpiride, tiapride; Lundbeck: chlorprothixene, flupentixol decanoate, melperone, sertindole, zuclopenthixol; Novartis: clozapine, thioridazine; Eli Lilly: olanzapine; AstraZeneca: quetiapine; Janssen‐Cilag: haloperidol, pimozide, risperidone; Asta Medica: prothipendyl hydrochloride; Knoll Ltd: zotepine; Pfizer: ziprasidone; Gerot: levomepromazine; UCB‐Pharma: dixyrazine).
Data collection and analysis
Study selection
Two review authors (MA and MO) independently screened the titles and abstracts of all the references retrieved by the original search strategy. Two other authors (SS and TS) independently screened the titles and abstracts of all references retrieved by the search strategy for this update. The full text versions of relevant studies were retrieved by BW and were assessed independently by the authors (MA, MO, TS and SS) to determine whether they met the inclusion criteria. Disagreements were resolved by discussion among the four authors mentioned above.
Assessment of quality
Trials which met the inclusion criteria were graded independently for methodological quality and assessed for internal validity using the Oxford Quality Scale score (Jadad 1996):
randomisation (1 = yes; 0 = no);
method and description of randomisation (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate);
double blinding (1 = yes; 0 = no);
method and description of double blinding (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate);
sufficient information about loss to follow‐up (1 = yes; 0 = no).
Each study was allocated a score of between one to five points. Because the inclusion criteria for this review required trials to be randomised, the minimum quality score was one. Higher scores indicated a higher quality of conducting or reporting, or both, of the trial. No trial that scored '0' met the inclusion criteria, the minimum score calculated was two.
Data extraction
The following data items were extracted from each of the included studies, where available:
trial characteristics (methods, duration, interventions);
patient characteristics (age, gender, type of pain condition);
trial results (patient reported pain intensity or pain relief);
adverse effects (major and minor);
study withdrawals (due to non‐compliance, adverse effects or death);
measures of satisfaction or patient preference (if reported); and
assessment of quality of life (if reported).
Data were extracted on to a standard form by two review authors working independently. Due to possible carry‐over effects, only the first phase of cross‐over studies was used.
Analysis
Statistical testing of heterogeneity between the trials was carried out by one author (EP) using RevMan Analyses 1.0.3 in Meta‐View 4.2.8 (RevMan 2012). Results from the trials were combined using a fixed‐effect model to calculate relative risks (RR) with 95% confidence intervals (CI) for dichotomous data and weighted mean differences (WMD) for continuous data.
If enough data were available, the number needed to treat to benefit (NNT) was calculated.
Subgroup analyses
The quality of the included trials was used in exploring any significant statistical heterogeneity between them. Cut‐off levels for the subgroup analysis values that were used were 'greater or equal to three' or 'less than or equal to two'.
Results
Description of studies
Study selection
The search for this update (from October 2007 to January 2013) resulted in 2083 hits. After screening of the titles and abstracts five potentially relevant studies were identified. Unfortunately, these studies had to be excluded because of the following reasons.
The study by Hill et al (Hill 2008) compared the efficacy of the antipsychotic to another antipsychotic (that is droperidol).
The study by Friedman et al (Friedman 2008) compared the efficacy of the antipsychotic to metoclopramide.
The study by Miller et al (Miller 2009) compared the efficacy of the antipsychotic to octreotide.
The study by Kostic et al (Kostic 2010) compared a combination of prochlorperazin with diphenhydramine to sumatriptan.
The study by Potvin et al (Potvin 2012) did not use pain as the primary outcome parameter.
The original search strategy, run in October 2007, resulted in 1908 hits. After screening of the titles and abstracts, 56 potentially relevant studies were identified. Forty‐one studies were excluded (see 'Characteristics of excluded studies' table). In short, 39 studies did not meet the quality criteria as assessed by the Oxford Quality Scale. Two high‐quality studies (Brousseau 2004; Weaver 2004;) were excluded because they reported the efficacy of an antipsychotic on headache in children and adolescents (Brousseau 2004) and compared two antipsychotics (droperidol vs. prochlorperazine) without the use of a placebo (Weaver 2004). Hence, 15 studies were considered for inclusion in this review. Two of these studies could not be assessed because a translation into English was not available (Govorin 1990; Lepola 1984). For one further study the authors could not be identified, and it was therefore excluded (Anon 1986).
Overall 12 RCTs of nine different antipsychotics were considered eligible (Bussone 1980; Davidsen 1979; Ginsberg 1983; Graff‐Radford 2000; Honkaniemi 2006; Johnston 1972; Judkins 1982; Langemark 1994; Lechin 1989; Richman 2002a; Roux 1983; Zitman 1991) (n = 772) for inclusion in the review, please see the 'Characteristics of included studies' table for full details of each included study. All included studies were clinic based and single centered, with one (Lechin 1989) explicitly stating the inclusion of outpatients. They were conducted at the departments for neurology (n = 4) (Bussone 1980; Honkaniemi 2006; Langemark 1994; Lechin 1989), anaesthesiology (n = 2) (Graff‐Radford 2000; Judkins 1982), psychiatry (n = 1) (Zitman 1992), neurosurgery (n = 1) (Roux 1983), oncology (n = 1) (Johnston 1972) and the emergency department (n = 2) (Davidsen 1979; Richman 2002a). The site of the remaining study (Ginsberg 1983) could not be specified. The sample size ranged from 29 to 316 participants. Most trials were limited by their small sample size. Only one trial included more than 200 participants (Davidsen 1979). Eight studies were placebo‐controlled and were considered for quantitative analysis. Data from six studies could not be included in the quantitative analysis because of the following reasons.
Two studies compared the efficacy of the antipsychotic to an opioid (Davidsen 1979) or a selective serotonin reuptake inhibitor (SSRI) (Langemark 1994).
One study only reported on the occurrence of headaches following an intervention (Roux 1983).
One study did not provide information about the duration of treatment (Bussone 1980).
The work of Johnston 1972 fulfilled the inclusion criteria, but due to missing data on variability, the study could not be included in the final analyses. Similarly, the study by Judkins 1982 had to be excluded due to missing data on the mean and variability of the selected outcome variables.
Risk of bias is shown in Figure 1 for each included study.
1.
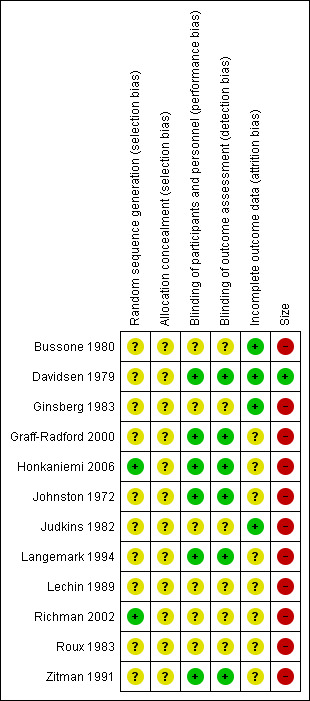
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
For the meta‐analyses, the primary outcome variable was the difference in mean in the treatment and placebo groups (after ‐ baseline) and therefore reflected how much the VAS score could be reduced by the treatment. Additionally, the meta‐analysis was performed for the mean outcome VAS score following treatment in both groups. For the dichotomous event, feeling pain or no pain after treatment, the RR indicated the chance of having pain after receiving the intervention, an absolute risk reduction (ARR) < 1 means that the chance of having pain after the intervention is smaller in the treatment group than in the control group. The study authors were contacted to provide missing data, if necessary. Standard errors were converted into standard deviations. If the standard deviation of the difference in mean was not given, but the standard deviation from baseline and after treatment was given, imputation strategies were used (Higgins 2011). If values for the mean or standard deviation were not mentioned in the text, but were displayed in a figure, values were taken from the figure.
Study design
Nine of the included studies had a parallel design and three had a cross‐over design. Some trials had more than two arms and made more than one comparison.
Outcomes
Pain was patient reported in 12 trials. In one study information on pain was provided by the patients' weekly ratings (Johnston 1972). In six trials pain was reported using a pain diary (Bussone 1980; Ginsberg 1983; Graff‐Radford 2000; Langemark 1994; Lechin 1989; Zitman 1991). Three trials documented the analgesic requirements of the patients on a numeric VAS (Judkins 1982; Richman 2002a; Honkaniemi 2006). Two trials simply reported on the mere occurrence of pain (Davidsen 1979; Roux 1983).
Study methods
All 12 included studies were conducted in a double‐blind fashion. Eight trials compared an antipsychotic or a combination of analgesic and antipsychotic to placebo and three studies compared an antipsychotic or a combination of analgesic and antipsychotic to treatment with an active compound (that is antidepressants, antiepileptics or analgesics).
Antipsychotics
Trials using the following antipsychotics were found:
Five with tricyclic antipsychotics (flupentixol, fluphenazine, thioridazine, levomepromazine);
four with butyrophenones (droperidol, haloperidol);
three with benzamides (sulpiride, tiapride, pimozide).
Patient conditions
The underlying conditions studied were as follows:
somatoform pain disorder, one study;
post‐herpetic neuralgia, one study;
acute (migraine) headache, two studies;
pain in terminal cancer, one study;
postoperative pain, one study;
trigeminal neuralgia, one study;
acute rheumatic pain, one study;
chronic tension‐type headache, two studies;
post‐rachiocentesis headache, one study; and
acute myocardial infarction, one study.
Details of these eligible reports are provided in the 'Characteristics of included studies' table.
Risk of bias in included studies
Each study was scored independently for quality by two of the review authors (MA and MO) using the three‐item Oxford Quality Scale (Jadad 1996). The scores for individual trials are reported in the notes section of the 'Characteristics of included studies' table. The median quality score for the placebo‐controlled studies was three (all trials scored three), and for the active control studies it was also three (range three to four).
Effects of interventions
Overall 12 RCTs of nine different antipsychotics were considered eligible (Bussone 1980; Davidsen 1979; Ginsberg 1983; Graff‐Radford 2000; Honkaniemi 2006; Johnston 1972; Judkins 1982; Langemark 1994; Lechin 1989; Richman 2002a; Roux 1983; Zitman 1991) (total n = 743) for inclusion in the review. Forty studies were excluded and are listed in the 'Characteristics of excluded studies' table.
Tricyclic antipsychotics
Four trials (Davidsen 1979; Graff‐Radford 2000; Johnston 1972; Zitman 1991), with a total of 449 participants, studied the effects of tricyclic antipsychotics in different painful disorders. These trials studied the effect of tricyclic antipsychotics in:
pain following myocardial infarction (Davidsen 1979),
pain due to terminal cancer (Johnston 1972),
somatoform pain disorder (Zitman 1991), and
post‐herpetic neuralgias (Graff‐Radford 2000).
The description of the study results is given below.
Tricyclic antipsychotics versus placebo
One study indicated only a small positive effect of 75 mg thioridazine daily compared to placebo concerning global improvement and pain in terminal cancer patients (P < 0.1) (Johnston 1972). The study of Graff‐Radford et al had four arms (amitriptyline, amitriptyline + fluphenazine, fluphenazine, and placebo). For quantitative analysis we only included data from the fluphenazine and the placebo arms (Graff‐Radford 2000).
Tricyclic antipsychotics versus other active treatment
Three studies compared tricyclic antipsychotics to other active treatments, including amitriptyline (a tricyclic antidepressant) (Graff‐Radford 2000; Zitman 1991) and pethidine (an opioid) (Davidsen 1979).
Administration of levomepromazine proved to significantly reduce the recurrence of pain within the first 72 hours after an acute myocardial infarction compared to treatment with pethidine (P < 0.05) (Davidsen 1979). In the case of post‐herpetic neuralgia the decrease of pain in patients receiving fluphenazine did not reach statistical significance. Combination of the antipsychotic with a tricyclic antidepressant (that is amitriptyline) and comparison with treatment with amitriptyline alone failed to produce a significant advantage using fluphenazine: mean difference (MD) 0.54 (95% CI ‐1.49 to 2.57) (Graff‐Radford 2000). In another study, patients with a somatoform pain disorder receiving 75 mg amitriptyline or 75 mg amitriptyline plus 3 mg flupentixol experienced significantly less pain during the treatment. Yet, the comparison of pain reduction in both groups did not reveal a statistically significant MD (MD ‐0.60, 95% CI ‐2.10 to 0.90) (Zitman 1991).
Butyrophenones
Three placebo‐controlled RCTs (Honkaniemi 2006; Judkins 1982; Richman 2002a), with a total of 110 participants, studied the effects of butyrophenones on postoperative pain (Judkins 1982) and acute migraine headache (Honkaniemi 2006; Richman 2002a).
In the case of postoperative pain two different dosages of haloperidol (5 and 10 mg orally) were compared against placebo as premedication before major abdominal surgery (Judkins 1982). VAS scores for pain at 24 hours after surgery did not differ significantly between the three groups of participants. Only a significant reduction of postoperative emesis was found in both groups treated with haloperidol. In contrast, treatment of acute migraine headache with 5 mg intravenous haloperidol was shown to be significantly superior to placebo (MD ‐4.05, 95% CI ‐5.61 to ‐2.49) (Honkaniemi 2006). Another study on acute migraine compared 2.5mg intramuscular droperidol with 1.5mg/kg meperidine and failed to detect a significant difference regarding post‐treatment pain intensity (VAS) (47 vs. 37 mm, p=.033) (Richman 2002a).
Benzamides
Five double‐blind trials with a total of 240 participants, two of them placebo‐controlled (Bussone 1980; Roux 1983), studied the effects of benzamides on different types of headache (Bussone 1980; Langemark 1994; Roux 1983), trigeminal neuralgia (Lechin 1989) and acute rheumatic pain (Ginsberg 1983).
In the case of chronic tension‐type headache tiapride was compared with placebo (Bussone 1980) and sulpiride was compared with another active component, that is paroxetine an SSRI (Langemark 1994). It seemed noteworthy that five participants in the group receiving sulpiride dropped out during the study due to intolerable side effects (Langemark 1994), see Table 1 for further details.
Benzamides versus placebo
The effect of intravenous tiapride (dosage 200 mg) following rachiocentesis was studied in a small sample (n = 30) (Roux 1983). The authors only reported the percentage of patients who experienced pain within 48 hours after rachiocentesis, but did not provide any further details (for example pain severity or associated symptoms). When compared to the placebo, tiapride led to a reduction in the occurrence of headaches. In detail, 86.7% of patients who had received tiapride before the rachiocentesis and 46.6% of patients who had received placebo did not report headaches within 48 hours following the procedure. These results were statistically significant (P < 0.01).
An Italian group reported on the efficacy of 300 mg tiapride administered orally on chronic tension‐type headache, but failed to provide any information on the duration of the treatment conducted in the study (Bussone 1980). Forty per cent of the patients treated with tiapride were complete responders compared to no responders with placebo (Bussone 1980).
Benzamides versus other active treatment
Sulpiride (400 mg daily) and paroxetine (30 mg daily), an SSRI, were compared in a group of 50 patients suffering from chronic tension‐type headache in a cross‐over conditional‐response design (Langemark 1994). Each treatment period lasted eight weeks. Patients recorded their pain in headache diaries. Total pain scores differed significantly between groups (P = 0.03) favouring treatment with the antipsychotic.
Pimozide (12 mg daily) and carbamazepine (1200 mg daily), antiepileptic drugs established in the treatment of neuralgic pain, were tested in the treatment of pain due to trigeminal neuralgia in 48 participants, using a cross‐over design (Lechin 1989). During both treatment phases pimozide proved to be superior to carbamazepine regarding total trigeminal neuralgia pain scores (P < 0.01) (MD ‐4.11, 95% CI ‐8.08 to ‐0.14).
In the case of acute rheumatic pain tiapride (100 mg daily) and glafenine (200 mg daily), an anthranilic acid derivative with analgesic properties, were compared during a 14‐day double‐blind trial (Ginsberg 1983). Tiapride was significantly superior to glafenine regarding the time delay until the disappearance of the pain (P < 0.05). Concerning pain reduction there was a trend favouring the antipsychotic (RR 0.73, 95% CI 0.37 to 1.44), though it failed to show statistical significance. A separate analysis according to the sex of patients did not reveal any significant differences (Ginsberg 1983).
Five of these trials permitted a quantitative analysis of the study data according to our protocol. The results of these analyses are available in Analysis 1.1, Analysis 1.2 and Analysis 1.3.
1.1. Analysis.
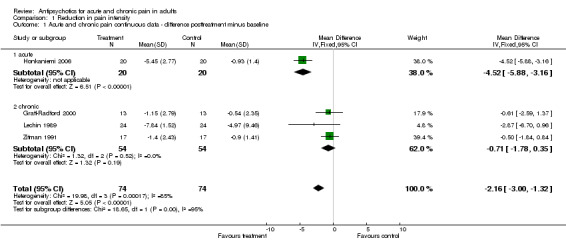
Comparison 1 Reduction in pain intensity, Outcome 1 Acute and chronic pain continuous data ‐ difference posttreatment minus baseline.
1.2. Analysis.
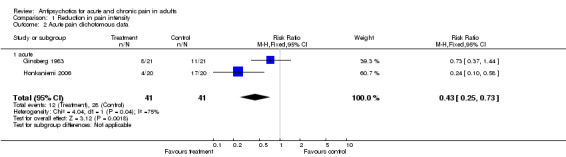
Comparison 1 Reduction in pain intensity, Outcome 2 Acute pain dichotomous data.
1.3. Analysis.
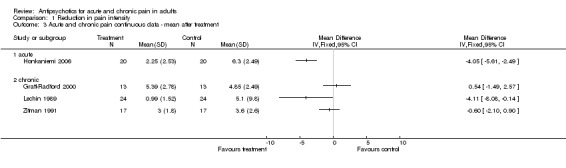
Comparison 1 Reduction in pain intensity, Outcome 3 Acute and chronic pain continuous data ‐ mean after treatment.
Discussion
This systematic review revealed a small number (n = 12) of small‐sized clinical trials (total n = 772) that compared the analgesic effects of antipsychotics to placebo or active compounds in a randomised double‐blind fashion.
There are some preclinical studies in humans that link the dopaminergic system with pain. In one study an inverse correlation of pain threshold and the response criterion with the D2/D3 binding potential in the right putamen was found (Pertovaara 2004). This finding is supported by a number of animal studies which have suggested that dopamine is involved in the regulation of nociception. However, the data are contradictory as dopamine agonists have been shown to produce either antinociception (Shimizu 2004) or hyperalgesia (Paalzow 1992).
It appears reasonable to further investigate the analgesic effect of various antipsychotics in different pain syndromes. Thus, we describe the effects of different antipsychotics in the following painful conditions.
Headaches
The effects of antipsychotics have been studied in the treatment of acute migraine headache (Honkaniemi 2006; Richman 2002a), chronic tension‐type headache (Bussone 1980; Langemark 1994) and headaches following rachiocentesis (Roux 1983) using randomised double‐blind designs. All but one trial (Richman 2002a) demonstrated statistically significant positive results for antipsychotics, that is haloperidol, sulpirid and tiaprid. It seems noteworthy that administration of tiaprid did not lead to a greater reduction of headache intensity, but led to a faster amelioration of headache following rachiocentesis (Roux 1983).
Neuralgic pain
Treatment of patients with neuralgic pain (post‐herpetic neuralgia (Graff‐Radford 2000) and trigeminal neuralgia (Lechin 1989)) delivered both positive and negative results. Fluphenazine, used in the case of post‐herpetic neuralgia, did not prove to be superior to amitriptyline, a tricyclic antidepressant (Graff‐Radford 2000). On the other hand, a study with patients suffering from trigeminal neuralgia appeared to show that pimozide led to a significantly greater reduction of pain in a double‐blind cross‐over study (Lechin 1989).
Other painful conditions
The effects on several other painful conditions have been studied in double‐blind RCTs. We could only identify a single study for each condition, which met our inclusion criteria. This is one of the limitations of the data presented here. In summary, we can say that only one study reported a statistically significant positive effect on pain, that is pain following an acute myocardial infarction (Davidsen 1979). Intriguingly, that study turned out to hold the largest sample (n = 316) of all studies included. Trials on somatoform pain disorders (Zitman 1991), postoperative pain (Judkins 1982) and acute rheumatic pain (Ginsberg 1983) failed to deliver significant results favouring the treatment with antipsychotics.
Antipsychotics and acute pain
Four of six studies on acute painful conditions that were reviewed here (Ginsberg 1983; Judkins 1982; Richman 2002a; Roux 1983) did not find significant positive results for antipsychotics concerning reduction of pain intensity. One study (Davidsen 1979), using a large sample of patients, reported a statistically significant reduction of pain after an acute myocardial infarction. The data of Honkaniemi and colleagues demonstrated an excellent response of acute migraine headache to the administration of haloperidol (Honkaniemi 2006). In addition, after the administration of tiaprid post‐rachiocentesis headache disappeared more quickly when compared to placebo, although pain reduction was not greater than in patients who received placebo (Roux 1983).
Antipsychotics and chronic pain
Six studies included in this review focused on the management of chronic pain conditions. In the case of chronic tension‐type headaches two studies reported beneficial effects for patients treated with antipsychotics when compared to placebo or another active component (Bussone 1980; Langemark 1994). Results concerning neuralgic pain turned out to be both negative (Graff‐Radford 2000) and positive (Lechin 1989). In the latter study the antipsychotic was more efficient than carbamazepine, a well‐established drug in the treatment of neuralgias. Treatment of a somatoform (that is physical symptoms that mimic disease or injury for which there is no identifiable physical cause) pain disorder with flupentixol (Zitman 1991) and pain in terminal cancer patients with thioridazine (Johnston 1972) was not reported to be superior to placebo or another treatment (Zitman 1991).
Summarising the results of all 11 included RCTs we found a positive effect in painful conditions in six trials, whereas five trials failed to report any analgesic effect of the antipsychotics studied. Five trials proved eligible for a meta‐analysis. We next set out to perform quantitative analyses of studies on acute (n = 2) and chronic pain (n = 3) separately, but this does not seem reasonable to us due to the small number and tremendous clinical heterogeneity of the studies. In addition, the data reviewed here have further limitations as most trials studied small samples of patients and only one included more than 200 participants. Moreover, pain assessment varied among the different protocols. Some studies only reported on the (re‐)occurrence of pain, which is, from our point of view, not sufficient to assess the whole effect on painful states.
Thus, in the present review the evidence of analgesic properties of antipsychotics can only be described relying on each single study. From a clinical point of view further research on analgesic properties of antipsychotics is indicated in more RCTs. Since nowadays atypical antipsychotics, which are known to produce lesser extrapyramidal side effects, are available, this new group of antipsychotics clearly needs to be studied regarding their analgesic potency.
In this update we also added antipsychotics (that is prochlorperazine and droperidol) to our search strategy. Nevertheless we could not include any new studies. One of the excluded studies (Hill 2008) concluded that olanzapine, a newer atypical antipsychotic, was equally potent to droperidol, an older antipsychotic, to relieve headaches. This finding should encourage researchers to further study the efficacy of newer antipsychotic agents in the treatment of pain disorders.
Authors' conclusions
Implications for practice.
Antipsychotics could be used as add‐on therapy in the treatment of painful conditions, as a possibility for treatment‐resistant pain. Adverse effects of typical antipsychotics, especially the extrapyramidal and sedating effects, have to be considered in the decision algorithm for the use of antipsychotics in painful conditions. Nevertheless, the significance of these results is limited due to the heterogeneity of the included studies.
Implications for research.
The results for antipsychotics in the treatment of different painful conditions are heterogeneous and most sample sizes in the reviewed randomised double‐blind studies are small. However, further studies are warranted on atypical antipsychotics with fewer side effects than the classical antipsychotics, in larger double‐blind placebo‐controlled studies that include standardised pain assessment.
Feedback
Studies on prochlorperazine and droperidol, 31 October 2013
Summary
Feedback: The methods state that the update included two additional antipsychotics ‐ prochlorperazine and droperidol that were excluded from the original review. This is in contrast with the results text where you state:
Three high‐quality studies (Brousseau 2004; Richman 2002; Weaver 2004) were excluded because the antipsychotics tested (prochlorperazine and droperidol) had not been within the scope of antipsychotics of our protocol and because they were not within the scope of our search strategy.
It is unclear why these studies were not included in the update (even if left out of the original) if part of the purpose was to expand it to include these other drugs. Ultimately, two of the most commonly used antipsychotics in the US (including frequent use for acutely painful conditions) are left out of the review making much less clinically useful to practitioners.
Reply
We are grateful for this clinically relevant feedback and revised our review accordingly. To summarize, the reasons for exclusion of the studies have been corrected (see Brousseau 2004 and Weaver 2004). Another study (Richman 2002a) investigating the effect of droperidol on migraine headaches has now been included.
Contributors
Authors and editors.
What's new
| Date | Event | Description |
|---|---|---|
| 27 November 2015 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 7 November 2013 | Feedback has been incorporated | Inclusion of studies on prochlorperazine and droperidol. See Feedback 1. |
| 4 September 2013 | Amended | Slight amendment to wording of search strategy. |
| 21 August 2013 | New citation required but conclusions have not changed | This search found five new studies which were all excluded, so the review remains the same as previously. |
| 7 February 2013 | New search has been performed | This review is an update of the original review published in The Cochrane Library in 2008. See Published notes. |
| 9 November 2009 | Amended | Contact details updated. |
Notes
This review was first published in Issue 4, 2008. Citation: Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No.: CD004844. DOI: 10.1002/14651858.CD004844.pub2.
At November 2015, the authors and editors have agreed to split this review into separate titles which will serve to update and replace the original. The planned titles are for antipsychotics for headache and migraine, and neuropathic pain. For more information, please contact the PaPaS CRG.
Acknowledgements
We would like to thank Tiina Saarto for data extraction and translation of manuscripts.
Appendices
Appendix 1. MEDLINE search strategy
A , B, C combined with AND
A. Neuroleptic/antipsychotic drugs (combined with OR) Free Term neurolept*, antipsychotic*, Amisulpride*, Chormethiazole*, Clomethiazole*, Distraneurin*, Chlorpromazin*, Aminazine*, Chlorazine*, Chlordelazine*, Contomin*, Fenactil*, Largactil*, Propaphenin*, Thorazine*, Flupenthixol decanoate*, Emergil*, Fluanxol*, Flupentixol*, alphaFlupenthixol*, cisFlupenthixol*, Fluphenazin*, Fluphenazine decanoate*, Flufenazin*, Fluphenazine Hydrochloride*, Lyogen*, Prolixin*, Haloperidol*, Haldol*, Levomepromazin*, Levomeprazin*, Levopromazine*, Tisercin*, Tizercine*, Tizertsin*, Methotrimeprazine*, Loxapine*, Loxapinsuccinate*, Oxilapine*, Cloxazepine*, Loxapine Monohydrochloride*, Loxipine Maleate*, Loxipine Succinate*, Loxitane*, Asendin*, Desmethylloxapine*, Amoxapine*, Olanzapine*, Perphenazine*, Chlorpiprazine*, Perfenazine*, Trilafonor*, Pimozide*, Prothipendyl*, Quetiapine*, Fumarate*, Risperidone*, Risperidal*, Sulpiride*, Dogmatil*, Eglonyl*, Sulperide*, Thioridazine*, Meleril*, Mellaril*, Melleril*, Melleryl*, Sonapax*, Thioridazine Hydrochloride*, Tiaprid*, Tiapridal*, Trifluoperazine Hydrochloride*, Trifluoroperazine*, Triftazin*, Stelazine*, Trifluperazine*, Tripfluoperazine Hydrochloride*, Cisordinol*, Zuclopenthixol*, Clopenthixol*, Clozapine*, Melperone hydrochloride*, Ziprasidone*, Zotemine* MeSH Pimozide, Perphenazine, explode Loxapine, Methotrimeprazine, Haloperidol, Fluphenazine, Flupenthixol, Chlorpromazine, Chlormethiazole, Antipsychotic‐Agents, Clopenthixol, Trifluoperazine, Tiapride, Thioridazine, Sulpiride, Risperidone, Fumarates
B. Pain (combined with OR) Free Term pain* MeSH explode Pain
C. RCT‐Filter See Cochrane Handbook of Systematic Review of Interventions (Higgins 2011)
Appendix 2. Additional search strategies
| Database searched | Search strategy |
| EMBASE | A , B, C combined with AND A. Neuroleptic/antipsychotic drugs (combined with OR) Free Term neurolept*, antipsychotic* EMTREE Neuroleptanalgesia explode neuroleptic‐agent B.Pain (combined with OR) Free Term pain* EMTREE explode Pain C.RCT‐Filter (combined with OR) Free Term random* in ab,ti cross?over* in ab,ti factorial* in ab,ti placebo* in ab,ti volunteer* in ab,ti (singl* or doubl* or trebl* or tripl*) near (blind* or mask*) in ab,ti EMTREE randomized‐controlled‐trial, randomization, controlled‐study, multicenter‐study, phase‐3‐clinical‐trial, phase‐4‐clinical‐trial, double‐blind‐procedure, single‐blind‐procedure |
| PSYNDEX | A , B, C combined with AND A. Neuroleptic/antipsychotic drugs (combined with OR) Free Term neurolept*, antipsychoti*, Amisulpride*, Chormethiazole*, Clomethiazole*, Distraneurin*, Chlorpromazin*, Aminazine*, Chlorazine*, Chlordelazine*, Contomin*, Fenactil*, Largactil*, Propaphenin*, Thorazine*, Flupenthixol decanoate*, Emergil*, Fluanxol*, Flupentixol*, alphaFlupenthixol*, cisFlupenthixol*, Fluphenazin*, Fluphenazine decanoate*, Flufenazin*, Fluphenazine Hydrochloride*, Lyogen*, Prolixin*, Haloperidol*, Haldol*, Levomepromazin*, Levomeprazin*, Levopromazine*, Tisercin*, Tizercine*, Tizertsin*, Methotrimeprazine*, Loxapine*, Loxapinsuccinate*, Oxilapine*, Cloxazepine*, Loxapine Monohydrochloride*, Loxipine Maleate*, Loxipine Succinate*, Loxitane*, Asendin*, Desmethylloxapine*, Amoxapine*, Olanzapine*, Perphenazine*, Chlorpiprazine*, Perfenazine*, Trilafonor*, Pimozide*, Prothipendyl*, Quetiapine*, Fumarate*, Risperidone*, Risperidal*, Sulpiride*, Dogmatil*, Eglonyl*, Sulperide*, Thioridazine*, Meleril*, Mellaril*, Melleril*, Melleryl*, Sonapax*, Thioridazine Hydrochloride*, Tiaprid*, Tiapridal*, Trifluoperazine Hydrochloride*, Trifluoroperazine*, Triftazin*, Stelazine*, Trifluperazine*, Tripfluoperazine Hydrochloride*, Cisordinol*, Zuclopenthixol*, Clopenthixol* Thesaurus Explode Neuroleptic‐Drugs B. Pain (combined with OR) Free Term pain* schmerz* Thesaurus explode Pain C. RCT‐Filter (combined with OR) placebo* random* control* kontroll* prospectiv* prospekti* volunteer* (clin* near trial*) (klin* near fall*) (compar* near stud*) (vergleich* near stud*) (singl* or doubl* or trebl* or tripl*) near (blind* or mask*) |
| PsycINFO | A , B, C combined with AND A. Neuroleptic/antipsychotic drugs (combined with OR) Free Term neurolept*, antipsychotic*, Amisulpride*, Chormethiazole*, Clomethiazole*, Distraneurin*, Chlorpromazin*, Aminazine*, Chlorazine*, Chlordelazine*, Contomin*, Fenactil*, Largactil*, Propaphenin*, Thorazine*, Flupenthixol decanoate*, Emergil*, Fluanxol*, Flupentixol*, alphaFlupenthixol*, cisFlupenthixol*, Fluphenazin*, Fluphenazine decanoate*, Flufenazin*, Fluphenazine Hydrochloride*, Lyogen*, Prolixin*, Haloperidol*, Haldol*, Levomepromazin*, Levomeprazin*, Levopromazine*, Tisercin*, Tizercine*, Tizertsin*, Methotrimeprazine*, Loxapine*, Loxapinsuccinate*, Oxilapine*, Cloxazepine*, Loxapine Monohydrochloride*, Loxipine Maleate*, Loxipine Succinate*, Loxitane*, Asendin*, Desmethylloxapine*, Amoxapine*, Olanzapine*, Perphenazine*, Chlorpiprazine*, Perfenazine*, Trilafonor*, Pimozide*, Prothipendyl*, Quetiapine*, Fumarate*, Risperidone*, Risperidal*, Sulpiride*, Dogmatil*, Eglonyl*, Sulperide*, Thioridazine*, Meleril*, Mellaril*, Melleril*, Melleryl*, Sonapax*, Thioridazine Hydrochloride*, Tiaprid*, Tiapridal*, Trifluoperazine Hydrochloride*, Trifluoroperazine*, Triftazin*, Stelazine*, Trifluperazine*, Tripfluoperazine Hydrochloride*, Cisordinol*, Zuclopenthixol*, Clopenthixol* Thesaurus Explode Neuroleptic‐Drugs B.Pain (combined with OR) Free Term pain* Thesaurus explode Pain C.RCT‐Filter (combined with OR) placebo* random* control* prospectiv* volunteer* (clin* near trial*) (compar* near stud*) (singl* or doubl* or trebl* or tripl*) near (blind* or mask*) |
| The Cochrane LIbrary | (Controlled vocabulary terms (MeSH) are presented in uppercase text; freetext terms in lowercase text.) 1. ANTIPSYCHOTIC AGENTS 2. antipsychotic$ 3. neuroleptic$ 4. (amisulpride or chlormethiazole or (chlorpromazine next hydrochloride) or chlorprothixene or clozapine or dixyrazine or (flupenthixol next decanoate) or (fluphenazine next decanoate) or haloperidol or levomepromazine or loxapine or (melperone next hydrochloride) or methotrimeprazine or olanzapine or perphenazine or pimozide or (prothipendyl next hydrochloride) or (quetiapine next fumarate) or risperidone or sulpiride or thioridazine or (tiapride next hydrochloride) or (trifluoperazine next hydrochloride) or ziprasidone or zotepine or zuclopenthixol) 5. OR/1‐4 6. PAIN (Explode term) 7. VASCULAR HEADACHES (Explode term) 8. PAIN MEASUREMENT 9. neuralgi$ 10 pain$ 11. migrain$ 12. OR/6‐11 13. 5 AND12 |
Appendix 3. Updated searches January 2013
MEDLINE (OVID) Oct 2011 to 11/01/13
1 Antipsychotic Agents/ (38296)
2 (antipsychotic* or neuroleptic*).tw. (36477)
3 (neurolept* or antipsychotic* or Amisulpride* or Chormethiazole* or Clomethiazole* or Distraneurin* or Chlorpromazin* or Aminazine* or Chlorazine* or Chlordelazine* or Contomin* or Fenactil* or Largactil* or Propaphenin* or Thorazine* or "Flupenthixol decanoate* or Emergil*" or Fluanxol* or Flupentixol* or alphaFlupenthixol* or cisFlupenthixol* or Fluphenazin* or "Fluphenazine decanoate*" or Flufenazin* or "Fluphenazine Hydrochloride*" or Lyogen* or Prolixin* or Haloperidol* or Haldol* or Levomepromazin* or Levomeprazin* or Levopromazine* or Tisercin* or Tizercine* or Tizertsin* or Methotrimeprazine* or Loxapine* or Loxapinsuccinate* or Oxilapine* or Cloxazepine* or "Loxapine Monohydrochloride*" or "Loxipine Maleate*" or "Loxipine Succinate*" or Loxitane* or Asendin* or Desmethylloxapine* or Amoxapine* or Olanzapine* or Perphenazine* or Chlorpiprazine* or Perfenazine* or Trilafonor* or Pimozide* or Prothipendyl* or Quetiapine* or Fumarate* or Risperidone* or Risperidal* or Sulpiride* or Dogmatil* or Eglonyl* or Sulperide* or Thioridazine* or Meleril* or Mellaril* or Melleril* or Melleryl* or Sonapax* or "Thioridazine Hydrochloride*" or Tiaprid* or Tiapridal* or "Trifluoperazine Hydrochloride*" or Trifluoroperazine* or Triftazin* or Stelazine* or "Trifluperazine*or Tripfluoperazine Hydrochloride*or Cisordinol*" or "Zuclopenthixol*or Clopenthixol*,or Clozapine*or Melperone hydrochloride*or Ziprasidone*or Zotemine*").mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] (99220)
4 Pimozide/ (1648)
5 Perphenazine/ (1405)
6 exp Loxapine/ (547)
7 Methotrimeprazine/ (670)
8 Haloperidol/ (14331)
9 Fluphenazine/ (2275)
10 Flupenthixol/ (809)
11 Chlorpromazine/ (15304)
12 Chlormethiazole/ (751)
13 Clopenthixol/ (360)
14 Trifluoperazine/ (3342)
15 Tiapride Hydrochloride/ (0)
16 Thioridazine/ (2154)
17 Sulpiride/ (3544)
18 Risperidone/ (4655)
19 Fumarates/ (3100)
20 or/1‐19 (102158)
21 exp Pain/ (282471)
22 pain*.tw. (378943)
23 or/21‐22 (504903)
24 20 and 23 (1768)
25 randomized controlled trial.pt. (336937)
26 controlled clinical trial.pt. (84917)
27 randomized.ab. (240687)
28 placebo.ab. (134089)
29 drug therapy.fs. (1568585)
30 randomly.ab. (172962)
31 trial.ab. (247676)
32 groups.ab. (1131458)
33 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 (2921812)
34 exp animals/ not humans.sh. (3747051)
35 33 not 34 (2482012)
36 24 and 35 (1002)
37 (201110* or 201111* or 201112* or 2012*).ed. (946315)
38 36 and 37 (51)
CENTRAL (The Cochrane Library) Issue 12 of 12 (2012)
#1 MeSH descriptor: [Antipsychotic Agents] this term only
#2 (antipsychotic* or neuroleptic*)
#3 (neurolept* or antipsychotic* or Amisulpride* or Chormethiazole* or Clomethiazole* or Distraneurin* or Chlorpromazin* or Aminazine* or Chlorazine* or Chlordelazine* or Contomin* or Fenactil* or Largactil* or Propaphenin* or Thorazine* or Flupenthixol decanoate* or Emergil* or Fluanxol* or Flupentixol* or alphaFlupenthixol* or cisFlupenthixol* or Fluphenazin* or Fluphenazine decanoate* or Flufenazin* or Fluphenazine Hydrochloride* or Lyogen* or Prolixin* or Haloperidol* or Haldol* or Levomepromazin* or Levomeprazin* or Levopromazine* or Tisercin* or Tizercine* or Tizertsin* or Methotrimeprazine* or Loxapine* or Loxapinsuccinate* or Oxilapine* or Cloxazepine* or Loxapine Monohydrochloride* or Loxipine Maleate* or Loxipine Succinate* or Loxitane* or Asendin* or Desmethylloxapine* or Amoxapine* or Olanzapine* or Perphenazine* or Chlorpiprazine* or Perfenazine* or Trilafonor* or Pimozide* or Prothipendyl* or Quetiapine* or Fumarate* or Risperidone* or Risperidal* or Sulpiride* or Dogmatil* or Eglonyl* or Sulperide* or Thioridazine* or Meleril* or Mellaril* or Melleril* or Melleryl* or Sonapax* or Thioridazine Hydrochloride* or Tiaprid* or Tiapridal* or Trifluoperazine Hydrochloride* or Trifluoroperazine* or Triftazin* or Stelazine* or "Trifluperazine*or Tripfluoperazine Hydrochloride* or Cisordinol*" or Zuclopenthixol* or Clopenthixol* or Clozapine*or Melperone hydrochloride* or Ziprasidone* or Zotemine*)
#4 MeSH descriptor: [Pimozide] this term only
#5 MeSH descriptor: [Perphenazine] this term only
#6 MeSH descriptor: [Loxapine] explode all trees
#7 MeSH descriptor: [Methotrimeprazine] this term only
#8 MeSH descriptor: [Haloperidol] this term only
#9 MeSH descriptor: [Fluphenazine] this term only
#10 MeSH descriptor: [Flupenthixol] this term only
#11 MeSH descriptor: [Chlorpromazine] this term only
#12 MeSH descriptor: [Chlormethiazole] this term only
#13 MeSH descriptor: [Clopenthixol] this term only
#14 MeSH descriptor: [Trifluoperazine] this term only
#15 MeSH descriptor: [Thioridazine] this term only
#16 MeSH descriptor: [Sulpiride] this term only
#17 MeSH descriptor: [Risperidone] this term only
#18 MeSH descriptor: [Fumarates] this term only
#19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
#20 MeSH descriptor: [Pain] explode all trees
#21 pain*
#22 #20 or #21
#23 #19 and #22 from 2011 to 2013
EMBASE (OVID) Oct 2011 to 2013 week 03
1 Antipsychotic Agents/ (58700)
2 (antipsychotic* or neuroleptic*).tw. (55785)
3 (neurolept* or antipsychotic* or Amisulpride* or Chormethiazole* or Clomethiazole* or Distraneurin* or Chlorpromazin* or Aminazine* or Chlorazine* or Chlordelazine* or Contomin* or Fenactil* or Largactil* or Propaphenin* or Thorazine* or "Flupenthixol decanoate* or Emergil*" or Fluanxol* or Flupentixol* or alphaFlupenthixol* or cisFlupenthixol* or Fluphenazin* or "Fluphenazine decanoate*" or Flufenazin* or "Fluphenazine Hydrochloride*" or Lyogen* or Prolixin* or Haloperidol* or Haldol* or Levomepromazin* or Levomeprazin* or Levopromazine* or Tisercin* or Tizercine* or Tizertsin* or Methotrimeprazine* or Loxapine* or Loxapinsuccinate* or Oxilapine* or Cloxazepine* or "Loxapine Monohydrochloride*" or "Loxipine Maleate*" or "Loxipine Succinate*" or Loxitane* or Asendin* or Desmethylloxapine* or Amoxapine* or Olanzapine* or Perphenazine* or Chlorpiprazine* or Perfenazine* or Trilafonor* or Pimozide* or Prothipendyl* or Quetiapine* or Fumarate* or Risperidone* or Risperidal* or Sulpiride* or Dogmatil* or Eglonyl* or Sulperide* or Thioridazine* or Meleril* or Mellaril* or Melleril* or Melleryl* or Sonapax* or "Thioridazine Hydrochloride*" or Tiaprid* or Tiapridal* or "Trifluoperazine Hydrochloride*" or Trifluoroperazine* or Triftazin* or Stelazine* or "Trifluperazine*or Tripfluoperazine Hydrochloride*or Cisordinol*" or "Zuclopenthixol*or Clopenthixol*,or Clozapine*or Melperone hydrochloride*or Ziprasidone*or Zotemine*").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (194441)
4 Pimozide/ (7142)
5 Perphenazine/ (6563)
6 exp Loxapine/ (1943)
7 Methotrimeprazine/ (4973)
8 Haloperidol/ (49400)
9 Fluphenazine/ (8860)
10 Flupenthixol/ (4196)
11 Chlorpromazine/ (42097)
12 Chlormethiazole/ (2771)
13 Clopenthixol/ (767)
14 Trifluoperazine/ (9676)
15 Tiapride Hydrochloride/ (1713)
16 Thioridazine/ (11514)
17 Sulpiride/ (10861)
18 Risperidone/ (24372)
19 Fumarates/ (1849)
20 or/1‐19 (199428)
21 exp Pain/ (727482)
22 pain*.tw. (554811)
23 or/21‐22 (949124)
24 20 and 23 (13310)
25 random$.tw. (790580)
26 factorial$.tw. (20622)
27 crossover$.tw. (46266)
28 cross over$.tw. (21047)
29 cross‐over$.tw. (21047)
30 placebo$.tw. (189432)
31 (doubl$ adj blind$).tw. (140094)
32 (singl$ adj blind$).tw. (13242)
33 assign$.tw. (219299)
34 allocat$.tw. (74370)
35 volunteer$.tw. (169764)
36 Crossover Procedure/ (36012)
37 double‐blind procedure.tw. (224)
38 Randomized Controlled Trial/ (338179)
39 Single Blind Procedure/ (16894)
40 or/25‐39 (1296258)
41 (animal/ or nonhuman/) not human/ (4558955)
42 40 not 41 (1142927)
43 24 and 42 (2327)
44 (201110* or 201111* or 201112* or 2012* or 2013*).dd. (1627558)
45 43 and 44 (276)
PsycINFO (OVID) 2011 to Jan week 3 2013
1 Neuroleptic Drugs/ (13626)
2 (antipsychotic* or neuroleptic*).tw. (24917)
3 (neurolept* or antipsychotic* or Amisulpride* or Chormethiazole* or Clomethiazole* or Distraneurin* or Chlorpromazin* or Aminazine* or Chlorazine* or Chlordelazine* or Contomin* or Fenactil* or Largactil* or Propaphenin* or Thorazine* or "Flupenthixol decanoate* or Emergil*" or Fluanxol* or Flupentixol* or alphaFlupenthixol* or cisFlupenthixol* or Fluphenazin* or "Fluphenazine decanoate*" or Flufenazin* or "Fluphenazine Hydrochloride*" or Lyogen* or Prolixin* or Haloperidol* or Haldol* or Levomepromazin* or Levomeprazin* or Levopromazine* or Tisercin* or Tizercine* or Tizertsin* or Methotrimeprazine* or Loxapine* or Loxapinsuccinate* or Oxilapine* or Cloxazepine* or "Loxapine Monohydrochloride*" or "Loxipine Maleate*" or "Loxipine Succinate*" or Loxitane* or Asendin* or Desmethylloxapine* or Amoxapine* or Olanzapine* or Perphenazine* or Chlorpiprazine* or Perfenazine* or Trilafonor* or Pimozide* or Prothipendyl* or Quetiapine* or Fumarate* or Risperidone* or Risperidal* or Sulpiride* or Dogmatil* or Eglonyl* or Sulperide* or Thioridazine* or Meleril* or Mellaril* or Melleril* or Melleryl* or Sonapax* or "Thioridazine Hydrochloride*" or Tiaprid* or Tiapridal* or "Trifluoperazine Hydrochloride*" or Trifluoroperazine* or Triftazin* or Stelazine* or "Trifluperazine*or Tripfluoperazine Hydrochloride*or Cisordinol*" or "Zuclopenthixol*or Clopenthixol*,or Clozapine*or Melperone hydrochloride*or Ziprasidone*or Zotemine*").mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] (32805)
4 Pimozide/ (196)
5 Perphenazine/ (103)
6 exp Loxapine/ (45)
7 Haloperidol/ (3295)
8 Fluphenazine/ (257)
9 Chlorpromazine/ (363)
10 Trifluoperazine/ (44)
11 Thioridazine/ (145)
12 Sulpiride/ (370)
13 Risperidone/ (2980)
14 exp Pain/ (33805)
15 pain*.tw. (60478)
16 or/14‐15 (67780)
17 or/1‐13 (32819)
18 16 and 17 (485)
19 clinical trials/ (6454)
20 (randomis* or randomiz*).tw. (39468)
21 (random$ adj3 (allocat$ or assign$)).tw. (22565)
22 ((clinic$ or control$) adj trial$).tw. (33593)
23 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (15295)
24 (crossover$ or "cross over$").tw. (5462)
25 random sampling/ (445)
26 Experiment Controls/ (435)
27 Placebo/ (2888)
28 placebo$.tw. (23824)
29 exp program evaluation/ (12478)
30 treatment effectiveness evaluation/ (11837)
31 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. (45024)
32 or/19‐31 (141635)
33 18 and 32 (82)
34 limit 33 to yr="2011 ‐Current" (17)
Data and analyses
Comparison 1. Reduction in pain intensity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute and chronic pain continuous data ‐ difference posttreatment minus baseline | 4 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐1.00, ‐1.32] |
| 1.1 acute | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.52 [‐5.88, ‐3.16] |
| 1.2 chronic | 3 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.78, 0.35] |
| 2 Acute pain dichotomous data | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.25, 0.73] |
| 2.1 acute | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.25, 0.73] |
| 3 Acute and chronic pain continuous data ‐ mean after treatment | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 acute | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 chronic | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bussone 1980.
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | 50 patients, 40 mixed headache, 10 classical migraine Age range between 17 and 68 years | |
| Interventions | Random assignment to either 300 mg tiapride or placebo Duration of treatment: not stated |
|
| Outcomes | Headache intensity, frequency and duration reported by headache diary (including 4 weeks pre‐intervention) | |
| Notes | Type of pain: chronic headache; 1 dropout due to nausea in the treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis, no withdrawals |
| Size | High risk | Fewer than 50 participants/treatment arm |
Davidsen 1979.
| Methods | Double‐blind, controlled, randomised trial | |
| Participants | 316 patients, mean age: levomepromazine group 67 years, pethidine group 68 years. Inclusion criteria: acute myocardial infarction within 24 hours prior to admission
Exclusion criteria: known adverse reactions to narcotics or phenothiazines, treatment with levomepromazine before admission. Comorbidities: previous hypertension (32 (17% versus 21 (15%)) and previous heart insufficiency (73 (39%) versus 63 (44%)) in the levomepromazine and pethidine groups |
|
| Interventions | Patients received one injection upon admission (50 mg pethidine or 12.5 mg levomepromazine) and 100 mg pethidine or 25 mg levomepromazine orally 3 times a day for 3 consecutive days Further injections were given when needed |
|
| Outcomes | Pain intensity was assessed every 30 minutes during the first 6 hours after admission 90% of patients encountered pain during the course of the acute myocardial infarction Recurrences of pain in the first 72 hours were observed in 50% of the levomepromazine‐treated and in 62% of the pethidine‐treated patients (P<0.05) Incidence of nausea was significantly higher in the pethidine‐treated group (P<0.001) Until a one‐year follow‐up mortality rates were significantly lower in the levomepromazine‐treated group |
|
| Notes | Type of pain: pain in myocardial infarction; 3 patients died within 30 min after admission, 5 were not treated according to the protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported ‐ stated to be "on admission the patient was allocated the first available box" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "tablets and vials of identical appearance were dispensed ..." and "...the sealed code was kept in the pharmacy ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as "tablets and vials of identical appearance were dispensed ..." and "...the sealed code was kept in the pharmacy ..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis, no withdrawals |
| Size | Low risk | >100 participants/treatment arm |
Ginsberg 1983.
| Methods | Double‐blind, randomised trial | |
| Participants | 42 patients, 16 male, 26 female. Inclusion criteria: definite soft tissue rheumatism, sole location of pain, continuous pain, pain of non‐specific origin
Exclusion criteria: not stated None had received analgesics for the current affection before entering the study |
|
| Interventions | Randomly assigned to 100 mg tiapride or 200 mg glafenine 3 times daily over a period of 14 days | |
| Outcomes | Pain intensity (VAS) was assessed once daily Initial VAS score: 73 mm (tiapride group) and 73 mm (glafenine group) VAS score on day 14: 18.4 mm (tiapride group) and 30.4 mm (glafenine group) Mild side effects in tiapride group (6 drowsiness, 2 gastric intolerance) No interruption of treatment By the end of treatment, pain had disappeared in 76% of tiapride‐treated and 43% of the glafenine‐treated patients |
|
| Notes | Type of pain: acute rheumatic pain; no differences between sexes regarding the efficacy rating of the drugs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis, no withdrawals |
| Size | High risk | Fewer than 50 participants/treatment arm |
Graff‐Radford 2000.
| Methods | Double‐blind, placebo‐controlled, randomised trial | |
| Participants | 49 patients, mean age 72.9 years Inclusion criteria: post‐herpetic neuralgia, pain duration equal or longer to 6 months Exclusion criteria: not stated | |
| Interventions | Random assignment to 4 groups:
Group 1: amitriptyline
Group 2: amitriptyline + fluphenazine
Group 3: fluphenazine
Group 4: placebo (active to mimic anticholinergic side effects) Starting dose 12.5 mg (amitriptyline) and 1 mg (fluphenazine) Maximum dose 200 mg (amitriptyline) and 3 mg (fluphenazine) 8 weeks, one visit per week |
|
| Outcomes | Visual analogue scale (VAS), McGill Pain questionnaire (MPQ), side effects scale, Minnesota Multiphasic Personality Inventory (MMPI), Beck Depression Inventory (BDI), Spielberger State Trait Anxiety Inventory (SSTAI) VAS: significant changes in Group 1 and 2, but none in Group 3 or 4 Side effects: highest level in Group 3 No evident changes in psychometric measurements |
|
| Notes | Type of pain: post‐herpetic neuralgia; 1 dropout due to heavy sedation effect due to amitriptyline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ states “randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy method. “Active” placebo (glycopyrrolate) to mimic anticholinergic side effects |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy method. “Active” placebo (glycopyrrolate) to mimic anticholinergic side effects |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Fewer than 50 participants/treatment arm |
Honkaniemi 2006.
| Methods | Double‐blind, placebo‐controlled, randomised trial | |
| Participants | 47 patients, 41 female, 6 male, mean age 36 years, mean duration of headache before admission 75 hours. Inclusion criteria: diagnosis of migraine according to the IHSCC. Exclusion criteria: long QT‐interval, usage of drugs prolonging QT‐interval, hepatic disease, epilepsy or history of seizures, hyperthyreosis, parkinsonism, chronic psychiatric disease, other neuroleptic medication, and intoxication | |
| Interventions | Random assignment to either 5 mg haloperidol in 500 ml of normal saline or 500 ml of normal saline alone as a 20 to 30 minute infusion | |
| Outcomes | Pain estimation by a VAS between 1 hour and 3 hours after the infusion Marked or almost total pain relief | |
| Notes | Type of pain: acute migraine headache; 44 patients included in the placebo‐controlled arm of the trial. Of these, 4 were rejected: 2 were included in the study twice, 1 did not fulfil the inclusion criteria and 1 was pain free before the infusion. Of the remaining 40, 36 were female and 4 male. The mean duration of the headache for these 40 patients was 67 h. 80% of patients reported side effects, mainly motor agitation (53%) and sedation (53%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as "...patients were randomized by envelope selection." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "...treatment was carried out by an attending nurse, who prepared and blinded the infusion." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as "...treatment was carried out by an attending nurse, who prepared and blinded the infusion." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Fewer than 50 participants/treatment arm |
Johnston 1972.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | 50 patients, 32 outpatients, 18 inpatients, 6 men, 44 women, mean age 56 years (age range between 31 to 73 years). Inclusion criteria: terminal cancer with a prognosis of a least a 6 week survival Exclusion criteria: not stated | |
| Interventions | Random assignment to either 25 mg thioridazine p.o. or placebo 3 times a day (final dose 75 mg) for 3 weeks | |
| Outcomes | Physician's weekly rating of anxiety‐tension, insomnia, crying spells, fears, anorexia, and withdrawal, overall rating of emotional complaints and pain Statistically significant changes in the thioridazine group for anxiety‐tension, depressive mood, restlessness, insomnia, crying spells, fears, overall rating of emotional complaints and pain compared to placebo |
|
| Notes | Type of pain: cancer pain; 3 dropouts during follow‐up. No untoward effects were observed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "matching placebo capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as "matching placebo capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Fewer than 50 patients/treatment arm |
Judkins 1982.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | 34 patients, 18 to 70 years, both female and male Inclusion criteria: scheduled for elective major upper abdominal surgery, otherwise fit | |
| Interventions | Premedication of either 5 mg, 10 mg haloperidol or placebo | |
| Outcomes | Analgesic requirement (on‐demand system) as measured on a visual analogue scale was assessed every 3 hours within the first 24 hours following surgery No significant differences between all three conditions regarding the analgesic requirements Postoperative emesis reduced in the groups receiving haloperidol |
|
| Notes | Type of pain: postoperative pain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis, no withdrawals |
| Size | High risk | Fewer than 50 patients/treatment arm |
Langemark 1994.
| Methods | Randomised, double‐blind, cross‐over, response‐conditional pilot study | |
| Participants | 50 patients, mean age 42 years, recruited by mailed questionnaire Inclusion criteria: chronic tension‐type headache for at least 6 months and no more than 14 headache‐free days per month | |
| Interventions | Random assignment to either 20 mg paroxetine or 400 mg sulpiride (starting with 200 mg for 1 week) daily for 8 weeks | |
| Outcomes | Headache diary beginning 4 weeks prior to treatment and during 8 weeks of treatment (5‐point verbal scale (no/slight/moderate/very troublesome/worst possible headache) Change in headache score: drug given first, paroxetine (n=18) ‐0.4, sulpiride (n=19) ‐0.7 |
|
| Notes | Type of pain: chronic tension‐type headache; 8 patients dropped out during treatment, 3 headache diaries were incomplete. 1 patient offered paroxetine first never took the drug. Depression was ruled out using the Bech‐Rafaelsen Melancholia rating scale. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy technique described as "identically looking placebo tablets" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy technique described as "identically looking placebo tablets" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Fewer than 50 patients/treatment arm |
Lechin 1989.
| Methods | Double‐blind placebo‐controlled cross‐over trial Four‐centre study | |
| Participants | 68 patients joined the study, final number 48 outpatients, 24 men and 24 women, duration of illness 8 to 17 years Inclusion criteria: severe facial pain for at least 2 years, clinical diagnosis of trigeminal neuralgia Exclusion criteria: placebo responder (improvement of more than 20% during placebo washout period), severe physical illness, history of psychotic episodes, alcohol or drug addiction, epilepsy or any other convulsive disorder | |
| Interventions | 4 weeks of placebo washout, 8 weeks of treatment following random assignment to carbamazepine (final dose of 1200 mg with a 14‐day titration period) or pimozide (final dose of 12 mg with a 14‐day titration period), 4 weeks with abrupt withdrawal and placebo substitution, 8 weeks of cross‐over treatment | |
| Outcomes | Pain intensity using 6‐point registration cards (0 = no pain, 6 = pain present, cannot be ignored, prompt medical advice sought), duration, frequency, basal pain, sensitivity of trigger zones, number of relief tablets Total trigeminal neuralgia score reduction of 78.1 % (pimozide group) compared to 49.7% (carbamazepine group), statistically significant (P<0.001) at week 10 Adverse effects (hand tremors, involuntary movements during sleep, slight Parkinson's symptoms) observed in 40 of 48 patients |
|
| Notes | Type of pain: trigeminal neuralgia; 9 patients were not admitted to the treatment phase, 6 patients were excluded. 11 were not included in the statistical analysis. All patients refused interruption of pimozide treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "identical dark capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "identical dark capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Fewer than 50 patients/treatment arm |
Richman 2002.
| Methods | Double‐blind single‐center randomized controlled trial | |
| Participants | Twenty‐nine patients admitted to emergency department due to acute headaches Inclusion criteria: migraine with or without aura according the criteria of the International Headache Society Exclusion criteria: refusal to give informed consent, known allergy to butyrophenones and/or narcotics, ingestion of antiemetic, antihistamine, phenothiazine, and/or narcotic medication within 24 hours of ED presentation, diagnostic work‐up because headache was not typical in character compared with the patient's prior migraine pattern |
|
| Interventions | 2.5mg intramuscular droperidol (group 1) and 1.5mg/kg intramuscluar meperidine | |
| Outcomes | pain intensity (VAS) after 30 minutes | |
| Notes | none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | only patients with acute migraine, presumably those with higher pain intensity due to presentation in ED |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Very small group size |
Roux 1983.
| Methods | Double‐blind, placebo‐controlled, randomised trial | |
| Participants | 30 patients Headache following rachiocentesis (observation period 48 hours) Inclusion criteria: not stated Exclusion criteria: not stated | |
| Interventions | Random assignment to either 200 mg tiapride i.v. or placebo i.v. after rachiocentesis | |
| Outcomes | Occurrence of headache No headache in 86.7% of patients receiving tiapride and in 46.6% of patients receiving placebo Statistically significant difference |
|
| Notes | Type of pain: post‐rachiocentesis headache. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Fewer than 50 patients/treatment arm |
Zitman 1991.
| Methods | Double‐blind, placebo‐controlled cross‐over trial study | |
| Participants | 34 patients, mean age 34 years Inclusion criteria: pain duration at least 6 months, age between 30 and 60 years Exclusion criteria: severe psychiatric disease, renal, hepatic or cardiac disorders, epilepsy, glaucoma, hypertension, prostate dysfunction | |
| Interventions | Baseline (2 weeks) 75 mg amitriptyline and 3 mg flupentixol; first treatment (5 weeks) 75 mg amitriptyline and 3 mg flupentixol (scheme A) or 75 mg amitriptyline alone (scheme B); washout period (2 weeks) both groups placebo; 2nd treatment (5 weeks) crossing‐over from scheme A to B and vice versa | |
| Outcomes | Zung depression scale, Hamilton depression scale, clinical screening for extrapyramidal side effects, Abnormal Involuntary Movement Scale (AIMS), pain intensities (numeric scale 0 = no pain, 10 = unbearable pain) No differences between the amitriptyline group and the amitriptyline + flupentixol group concerning the pain |
|
| Notes | Type of pain: somatoform pain disorder; 2 refused to participate, 9 dropped out during treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "identical placebo tablets" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as "identical placebo tablets" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not described |
| Size | High risk | Fewer than 50 patients/treatment arm |
i.v. ‐ intravenous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adeloye 1971 | no data |
| Anon 1986 | not able to identify authors |
| Barbier 1989 | haloperidol in combination with buzepide metiodide |
| Brousseau 2004 | pediatric population studied |
| Cerbo 1982a | cross‐over trial, no data on first session given separately |
| Cerbo 1982b | cross‐over trial |
| Clavel 1980 | cross‐over trial |
| Clavel 1984 | cross‐over trial |
| Corazza 1996 | mixed outcome no distinct outcome pain parameter, overall score |
| Friedman 2008 | control group: metoclopramide |
| Girard 1970 | open‐label study, no control condition, no randomisation |
| Gomez‐Perez 1985 | fluphenazine in combination with nortriptyline |
| Gomez‐Perez 1996 | cross‐over design |
| Grillage 1986 | control group: diazepam |
| Hakkarainen 1973 | cross‐over |
| Hakkarainen 1977 | no randomisation |
| Hill 2008 | control group: droperidol |
| Jokinen 1984 | control group: diazepam |
| Kostic 2010 | combination |
| Lam 1978 | pain no outcome |
| Lancaster‐Smith 1982 | combination |
| Le Derff 1982 | open‐label study |
| Lobera 1980 | mix outcome (pain/nausea/emesis) |
| Macarri 1992 | control group: antipsychotic |
| Maina 2002 | single blind |
| Martin 1984 | fluphenazine in combination with nortriptyline no pain outcome |
| Menault 1981 | no randomization, no placebo control |
| Mendel 1986 | combination of amitriptyline and fluphenazine |
| Mereto 1974 | in all three groups perphenazine |
| Miller 2009 | control group: octreotide |
| Pagliarini 1968 | single blind |
| Pisani 1984 | no blinding |
| Potvin 2012 | pain no primary outcome |
| Predescu 1973 | no pain outcome |
| Rennemo 1982 | pain no primary outcome not compared to placebo or other compound in respect to pain |
| Steinbrook 1998 | postoperative pain was treated as needed with fentanyl or morphine no pain outcome |
| Van Kempen 1992 | pain no outcome |
| Weaver 2004 | comparison of two antipsychotics without placebo |
| Weber 1980 | additional pain medication |
| Wohlzogen 1970 | healthy subjects |
| Zitman 1992 | no randomisation |
Contributions of authors
Stefan Seidel: final draft of the manuscript. Thomas Sycha, Martin Aigner, Michael Ossege: search strategy and protocol, conceived and planned the review, quality assessment of identified studies, revision of the manuscript. Elisabeth Pernicka: statistics. Brigitte Wildner: search strategy, delivery of printed versions.
Sources of support
Internal sources
Department of Psychiatry, University of Vienna Medical School, Austria.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bussone 1980 {published data only}
- Bussone G, Boiardi A, Merati B, Recchia M, Salom Pedemonti D, et al. Tiapride in the treatment of chronic headache. Rivista de Patologia Nervosa e Mentale 1981;101:247‐53. [PubMed] [Google Scholar]
Davidsen 1979 {published data only}
- Davidsen O, Lindeneg O, Walsh M. Analgesic treatment with levomepromazine in acute myocardial infarction. A randomized clinical trial. Acta Medica Scandinavica 1979;205:191‐4. [DOI] [PubMed] [Google Scholar]
Ginsberg 1983 {published data only}
- Ginsberg F, Bourguignon RP, Smets P, Famaey JP. Tiapride versus glafenine: a double‐blind comparative study in the management of acute rheumatic pain. Current Medical and Research Opinion 1983;8:562‐9. [DOI] [PubMed] [Google Scholar]
Graff‐Radford 2000 {published data only}
- Graff‐Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clinical Journal of Pain 2000;16:188‐92. [DOI] [PubMed] [Google Scholar]
Honkaniemi 2006 {published data only}
- Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S. Haloperidol in the acute treatment of migraine: a randomized, double‐blind, placebo‐controlled study. Headache 2006;46:781‐7. [DOI] [PubMed] [Google Scholar]
Johnston 1972 {published data only}
- Johnston B. Relief of mixed anxiety‐depression in terminal cancer patients. Effect of thioridazine. New York State Journal of Medicine 1972;72:2315‐7. [PubMed] [Google Scholar]
Judkins 1982 {published data only}
- Judkins KC, Harmer M. Haloperidol as an adjunct analgesic in the management of postoperative pain. Anaesthesia 1982;37:1118‐20. [DOI] [PubMed] [Google Scholar]
Langemark 1994 {published data only}
- Langemark M, Olesen J. Sulpiride and paroxetine in the treatment of chronic tension‐type headache. An explanatory double‐blind trial. Headache 1994;34:20‐4. [DOI] [PubMed] [Google Scholar]
Lechin 1989 {published data only}
- Lechin F, Dijs B, Lechin ME, Amat J, Lechin AE, Cabrera A, et al. Pimozide therapy for trigeminal neuralgia. Archives of Neurology 1989;46:960‐3. [DOI] [PubMed] [Google Scholar]
Richman 2002 {published data only}
- Richman PB, Allegra J, Eskin B, Doran J, Reischel U, Kaiafas C, et al. A randomized clinical trial to assess the efficacy of intramuscular droperidol for the treatment of acute migraine headache. American Journal of Emergency Medicine 2002;20:39‐42. [DOI] [PubMed] [Google Scholar]
Roux 1983 {published data only}
- Roux FX, Mallet A, Meresse S. Prevention of headache following lumbar puncture. A controlled double blind trial of the effectiveness of intravenous tiapride. La Semaine de Hôpitaux 1982;59:319‐21. [PubMed] [Google Scholar]
Zitman 1991 {published data only}
- Zitman FG, Linssen AC, Edelbroek PM, Kempen GM. Does addition of low‐dose flupentixol enhance the analgetic effects of low‐dose amitriptyline in somatoform pain disorder?. Pain 1991;47:25‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adeloye 1971 {published data only}
- Adeloye A. Clinical trial of fluphenazine in the post‐concussional syndrome. Practitioner 1971;206:571‐9. [PubMed] [Google Scholar]
Anon 1986 {published data only}
- Anon. Amitriptyline and fluphenazine for painful diabetic neuropathy. JAMA 1986;256:712‐4. [PubMed] [Google Scholar]
Barbier 1989 {published data only}
- Barbier JP, Dorf G, Gordin J, Krainik F, Neveu D, Parlier H, et al. Effect of a buzepide metiodide‐haloperidol combination in treating functional intestinal disorders. Randomized double‐blind controlled versus placebo study [Effet de l´association métiodure de buzepide‐haloperidol dans le traitement des troubles fonctionnels intestinaux]. Annales de Gastroenterologie et d Hepatologie 1989;25:123‐8. [PubMed] [Google Scholar]
Brousseau 2004 {published data only}
- Brousseau DC, Duffy SJ, Anderson AC, Linakis JG. Treatment of pediatric migraine headaches: A randomized, double‐blind trial of prochlorperazine versus ketorolac. Annals of Emergency Medicine 2004;43:256‐62. [DOI] [PubMed] [Google Scholar]
Cerbo 1982a {published data only}
- Cerbo R, Casacchia M, Boni B, Corona R. Double‐blind trial of tiapride versus placebo in patients with sub‐perennial idiopathic headache. La Semaine de Hôpitaux 1982;58:2839‐44. [PubMed] [Google Scholar]
Cerbo 1982b {published data only}
- Cerbo R, Casacchia M, Boni B, Corona R, Papola S, Lena C. Double‐blind study of tiapride and placebo in patients with subcontinuous essential headache. Rivista di Neurologia 1982;52:51‐64. [PubMed] [Google Scholar]
Clavel 1980 {published data only}
- Clavel M, Pommatau E. Analgesic effects of tiapride in man. A double‐blind comparative clinical trial against placebo. La Semaine de Hôpitaux 1980;56:430‐3. [PubMed] [Google Scholar]
Clavel 1984 {published data only}
- Clavel M, Pommatau E. Controlled study of the analgesic efficacy of 2 drugs: tiapride versus aspirin. La Semaine de Hôpitaux 1984;60:565‐7. [PubMed] [Google Scholar]
Corazza 1996 {published data only}
- Corazza GR, Biagi F, Albano O, Porro GB, Cheli R, Mazzacca G, et al. Levosulpiride in functional dyspepsia: a multicentric, double‐blind, controlled trial. The Italian Journal of Gastroenterology 1996;28:317‐23. [PubMed] [Google Scholar]
Friedman 2008 {published data only}
- Friedman BW, Esses D, Solorzano C, Dua N, Greenwald P, Radulescu R, et al. A randomized controlled trial of prochlorperazine versus metoclopramide for treatment of acute migraine. Annals of Emergency Medicine 2008;52:399‐406. [DOI] [PubMed] [Google Scholar]
Girard 1970 {published data only}
- Girard M, Sambin P, Trepo C. Clinical trial of sulpiride in ulcerous disease. Therapeutique 1970;46:639‐44. [PubMed] [Google Scholar]
Gomez‐Perez 1985 {published data only}
- Gomez‐Perez FJ, Rull JA, Dies H, Rodriquez‐Rivera JG, Gonzalez‐Barranco J, Lozano‐Castaneda O. Nortriptyline and fluphenazine in the symptomatic treatment of diabetic neuropathy. A double‐blind cross‐over study. Pain 1985;23:395‐400. [DOI] [PubMed] [Google Scholar]
Gomez‐Perez 1996 {published data only}
- Gomez‐Perez FJ, Choza R, Rios JM, Reza A, Huerta E, Aguilar CA, et al. Nortriptyline‐fluphenazine vs. carbamazepine in the symptomatic treatment of diabetic neuropathy. Archives of Medical Research 1996;27(4):525‐9. [PubMed] [Google Scholar]
Grillage 1986 {published data only}
- Grillage M. Neurotic depression accompanied by somatic symptoms: a double‐blind comparison of flupenthixol and diazepam in general practice. Pharmatherapeutica 1986;4:561‐70. [PubMed] [Google Scholar]
Hakkarainen 1973 {published data only}
- Hakkarainen H, Viukari M. Sulpiride in the treatment of migraine, tension headache and associated psychosomatic disorders. Duodecim 1973;89:1504‐9. [PubMed] [Google Scholar]
Hakkarainen 1977 {published data only}
- Hakkarainen H. Fluphenazine for tension headache; double‐blind study. Headache 1977;17:216‐8. [DOI] [PubMed] [Google Scholar]
Hill 2008 {published data only}
- Hill CH, Miner JR, Martel ML. Olanzapine versus droperidol for the treatment of primary headache in the emergency department. Academic Emergency Medicine 2008;15:806‐11. [DOI] [PubMed] [Google Scholar]
Jokinen 1984 {published data only}
- Jokinen K, Koskinen T, Selonen R. Flupenthixol versus diazepam in the treatment of psychosomatic disorders: a double‐blind, multi‐centre trial in general practice. Pharmatherapeutica 1984;3:573‐81. [PubMed] [Google Scholar]
Kostic 2010 {published data only}
- Kostic MA, Gutierrez FJ, Rieg TS, Moore TS, Gendron RT. A prospective, randomized trial of intravenous prochlorperazine versus subcutaneous sumatriptan in acute migraine therapy in the emergency. Annals of Emergency Medicine 2010;56:1‐6. [DOI] [PubMed] [Google Scholar]
Lam 1978 {published data only}
- Lam SK, Lam KC, Lai CL, Yeung CK, Yam LY, Wong WS. Treatment of duodenal ulcers with antacid mixtures and sulpiride. A double‐blind controlled study. La Semaine de Hôpitaux 1978;54:1404‐7. [PubMed] [Google Scholar]
Lancaster‐Smith 1982 {published data only}
- Lancaster‐Smith MJ, Prout BJ, Pinto T, Anderson JA, Schiff AA. Influence of drug treatment on the irritable bowel syndrome and its interaction with psychoneurotic morbidity. Acta Psychiatrica Scandinavica 1982;66:33‐41. [DOI] [PubMed] [Google Scholar]
Le Derff 1982 {published data only}
- Derff H. Trial of tiapride as an analgesic in cancer patients. La Semaine de Hôpitaux 1982;58:1708‐11. [PubMed] [Google Scholar]
Lobera 1980 {published data only}
- Lobera A, Avril A, Bonichon F, Maree D. Randomized trial with tiapride in post‐operative care. Annales de Chirurgie 1981;35:62‐4. [PubMed] [Google Scholar]
Macarri 1992 {published data only}
- Macarri G, Biasi L, Brunelli E, Marucci L, Svegliati Baroni G. L‐sulpiride versus metoclopramide in functional dyspepsia: a randomized double‐blind study. Minerva Medica 1992;83:295‐8. [PubMed] [Google Scholar]
Maina 2002 {published data only}
- Maina G, Vitalucci A, Gandolfo S, Bogetto F. Comparative efficacy of SSRIs and amisulpride in burning mouth syndrome: a single‐blind study. Journal of Clinical Psychiatry 2002;63:38‐43. [DOI] [PubMed] [Google Scholar]
Martin 1984 {published data only}
- Martin MR, Schiff AA. Fluphenazine/nortriptyline in the irritable bladder syndrome. A double‐blind placebo controlled study. British Journal of Urology 1984;56:178‐9. [DOI] [PubMed] [Google Scholar]
Menault 1981 {published data only}
- Menault F. Maintenance therapy in cranio‐facial pain: a controlled trial with tiapride. La Semaine de Hôpitaux 1981;57:1015‐6. [PubMed] [Google Scholar]
Mendel 1986 {published data only}
- Mendel CM, Klein RF, Chappell DA, Dere WH, Gertz BJ, Karam JH, et al. A trial of amitriptyline and fluphenazine in the treatment of painful diabetic neuropathy. JAMA 1986;255:637‐9. [PubMed] [Google Scholar]
Mereto 1974 {published data only}
- Mereto GC. Double‐blind controlled trial on treatment of psychosomatic syndromes by a new tranquilizer combination. Minerva Medica 1974;65:923‐35. [PubMed] [Google Scholar]
Miller 2009 {published data only}
- Miller MA, Levsky ME, Enslow W, Rosin A. Randomized evaluation of octreotide vs prochlorperazine for ED treatment of migraine headache. American Journal of Emergency Medicine 2009;27:160‐4. [DOI] [PubMed] [Google Scholar]
Pagliarini 1968 {published data only}
- Pagliarini G, Pollazzon G, Ridolfi C, Rizzi R, Vacondio L. Clinico‐statistical pluricentral study of postoperative pain. Minerva Anestesiologica 1986;34:626‐31. [PubMed] [Google Scholar]
Pisani 1984 {published data only}
- Pisani RA, Lazzari R, Arzilli A, Franzese A, Carfagna L, Cerbo R, et al. Clinical findings and test results in a group of patients with headaches treated with amitriptyline as compared with perphenazine. La Clinica Terapeutica 1985;113:117‐23. [PubMed] [Google Scholar]
Potvin 2012 {published data only}
- Potvin S, Morin M, Cloutier C, Gendron A, Bissonnette A, Marchand S. Add‐on treatment of quetiapine for fibromyalgia: a pilot, randomized, double‐blind, placebo‐controlled 12‐week trial. Journal of Clinical Psychopharmacology 2012;32:684‐7. [DOI] [PubMed] [Google Scholar]
Predescu 1973 {published data only}
- Predescu V, Ciurezu T, Timofte G, Roman I. Symptomatic relief with flupentixol (Fluanxol) of the anxious‐algetic‐depressive syndrome complex in neurotic states. A double‐blind, placebo‐controlled study. Acta Psychiatrica Scandinavica 1973;49:15‐27. [DOI] [PubMed] [Google Scholar]
Rennemo 1982 {published data only}
- Rennemo F, Larsen R, Breivik H. Avoiding psychic adverse effects during induction of neurolept anaesthesia with levomepromazine. A double‐blind study of levomepromazine and droperidol. Acta Anaesthesiologica Scandinavica 1982;26:108‐11. [DOI] [PubMed] [Google Scholar]
Steinbrook 1998 {published data only}
- Steinbrook RA, Gosnell JL, Freiberger D. Prophylactic antiemetics for laparoscopic cholecystectomy: a comparison of perphenazine, droperidol plus ondansetron, and droperidol plus metoclopramide. Journal of Clinical Anaesthesia 1998;10:494‐8. [DOI] [PubMed] [Google Scholar]
Van Kempen 1992 {published data only}
- Kempen GM, Zitman FG, Linssen AC, Edelbroek PM. Biochemical measures in patients with a somatoform pain disorder, before, during, and after treatment with amitriptyline with or without flupentixol. Biological Psychiatry 1992;31:670‐80. [DOI] [PubMed] [Google Scholar]
Weaver 2004 {published data only}
- Weaver CS, Jones JB, Chisholm CD, Foley MJ, Giles BK, Somerville GG, et al. Droperidol versus prochlorperazine for the treatment of acute headache. The Journal of Emergency Medicine 2004;26:145‐50. [DOI] [PubMed] [Google Scholar]
Weber 1980 {published data only}
- Weber H. Comparison of the effect of diazepam and levomepromazine on pain in patients with acute lumbago‐sciatica. Journal of the Oslo City Hospitals 1980;30:65‐80. [PubMed] [Google Scholar]
Wohlzogen 1970 {published data only}
- Wohlzogen FX. Method, design, and analysis of a comparative study of analgetics. Internationale Zeitschrift für klinische Pharmakologie, Therapie und Toxikologie 1970;4:297‐302. [PubMed] [Google Scholar]
Zitman 1992 {published data only}
- Zitman FG, Linssen AC, Edelbroek PM, Kempen GM. Clinical effectiveness of antidepressants and antipsychotics in chronic benign pain. Clinical Neuropharmacology 1992;15 Suppl:377A‐8A. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Govorin 1990 {published data only}
- Govorin AV, Neverov IV, Govorin NV. Evaluation of the effectiveness of using sulpiride in unstable stenocardia. Sovetskaia Meditsina 1990;10:72‐4. [PubMed] [Google Scholar]
Lepola 1984 {published data only}
- Lepola U, Kokko S, Nuutila J, Gordin A. Tiapride and chlordiazepoxide in acute alcohol withdrawal. A controlled clinical trial. International Journal of Clinical Pharmacological Research 1984;4:321‐6. [PubMed] [Google Scholar]
Additional references
Bloomfield 1964
- Bloomfield S, Simard‐Savoir S, Bernier J, Tetrault L. Comparative analgesic activity of levomepromazine and morphine in patients with chronic pain. Canadian Medical Association Journal 1964;90:1156‐9. [PMC free article] [PubMed] [Google Scholar]
Breitbart 1998
- Breitbart W. Psychotropic adjuvant analgesics for pain in cancer and AIDS. Psycho‐oncology 1998;7(4):333‐45. [DOI] [PubMed] [Google Scholar]
Burduk 2000
- Burduk P, Guzik P, Piechocka M, Bronisz M, Rozek A, Jazdon M, et al. Comparison of fentanyl and droperidol mixture (neuroleptanalgesia II) with morphine on clinical outcomes in unstable angina patients. Cardiovascular Drugs and Therapy 2000;14(3):259‐69. [DOI] [PubMed] [Google Scholar]
Feinberg 2000
- Feinberg SD. Prescribing analgesics. How to improve function and avoid toxicity when treating chronic pain. Geriatrics 2000;55(11):49‐50. [PubMed] [Google Scholar]
Gobel 1997
- Gobel H, Stadler T. Treatment of post‐herpes zoster pain with tramadol. Results of an open pilot study versus clomipramine with or without levomepromazine. Drugs 1997;53 Suppl 2:34‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. 2011.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jermyn 2001
- Jermyn RT. A nonsurgical approach to low back pain. The Journal of the American Osteopathic Association 2001;101(4 Suppl Pt2):S6‐11. [PubMed] [Google Scholar]
Khojainova 2002
- Khojainova N, Santiago‐Palma J, Kornick C, Breitbart W, Gonzalez GR. Olanzapine in the management of cancer pain. Journal of Pain and Symptom Management 2002;23:346‐50. [DOI] [PubMed] [Google Scholar]
Kiser 2001
- Kiser RS, Cohen HM, Freedenfeld RN, Jewell C, Fuchs PN. Olanzapine for the treatment of fibromyalgia symptoms. Journal of Pain and Symptom Management 2001;22:704‐8. [DOI] [PubMed] [Google Scholar]
Lu 2000
- Lu SR, Fuh JL, Juang KD, Wang SJ. Repetitive intravenous prochlorperazine treatment of patients with refractory chronic daily headache. Headache 2000;40:724‐9. [DOI] [PubMed] [Google Scholar]
Merskey 1997
- Merskey H. Pharmacological approaches other than opioids in chronic non‐cancer pain management. Acta Anaesthesiologica Scandinavia 1997;41:187‐90. [DOI] [PubMed] [Google Scholar]
Montilla 1963
- Montilla E, Frederik WS, Cass LJ. Analgesic effect of methotrimeprazine and morphine. Archives of Internal Medicine 1963;111:725‐8. [Google Scholar]
Nix 1998
- Nix WA. What is certain in pain therapy? The analgesic potency of neuroleptics in the treatment of chronic pain. A metaanalysis [Was ist gesichert in der Schmerztherapie haben Neuroleptika eine analgetische Potenz?]. Schmerz 1998;12:30‐8. [DOI] [PubMed] [Google Scholar]
Paalzow 1992
- Paalzow GH. L‐dopa induces opposing effects on pain in intact rats: (‐)‐sulpiride, SCH 23390 or alpha‐methyl‐DL‐p‐tyrosine methylester hydrochloride reveals profound hyperalgesia in large antinociceptive doses. The Journal of Pharmacology and Experimental Therapeutics 1992;263:470‐9. [PubMed] [Google Scholar]
Patt 1994
- Patt RB, Proper G, Reddy S. The neuroleptics as adjuvant analgesics. Journal of Pain and Symptom Management 1994;9(7):446‐53. [DOI] [PubMed] [Google Scholar]
Pertovaara 2004
- Pertovaara A, Martikainen IK, Hagelberg N, Mansikka H, Någren K, Hietala J, et al. Striatal dopamine D2/D3 receptor availability correlates with individual response characteristics to pain. European Journal of Neuroscience 2004;20:1587‐92. [DOI] [PubMed] [Google Scholar]
Peschen‐Rosin 2002
- Peschen‐Rosin R. Chronic facial pain from psychiatric point of view ‐ differential diagnosis and therapeutic strategies [Gesichtsschmerzen in der psychiatrie. Differential diagnosen und therapeutische empfehlungen]. Schmerz 2002;16:395‐403. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schreiber 1999
- Schreiber S, Getslev V, Backer MM, Weizman R, Pick CG. The atypical neuroleptics clozapine and olanzapine differ regarding their antinociceptive mechanisms and potency. Pharmacology Biochemistry and Behavior 1999;64(1):75‐80. [DOI] [PubMed] [Google Scholar]
Shimizu 2004
- Shimizu T, Iwata S, Morioka H, Masuyama T, Fukuda T, Nomoto M. Antinociceptive mechanism of L‐DOPA. Pain 2004;110:246‐9. [DOI] [PubMed] [Google Scholar]
Silberstein 2002
- Silberstein SD, Peres MFP, Hopkins MM, Shechter AL, Young WB, Rozen TD. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache 2002;42(6):515‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Seidel 2008
- Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004844.pub2] [DOI] [PubMed] [Google Scholar]


