Abstract
Organic dust exposure particularly within hog confinement facilities is a significant cause of airway inflammation and lung disease. In a cohort of Midwestern veterans with COPD and agricultural work exposure we observed reduced zinc intakes which was associated with decreased lung function. Because insufficient zinc intake is common within the U.S. and a potent modulator of innate immune function, we sought to determine whether deficits in zinc intake would impact the airway inflammatory response to hog confinement facility dust extract (HDE). Adult male C57BL/6 mice were randomized to zinc deficient or matched zinc sufficient diets for 3 weeks and subsequently treated with intranasal HDE inhalation or saline once or daily for 3 weeks while maintained on specific diets. Lavage fluid and lung tissue was collected. Conditions of zinc deficiency were also studied in macrophages exposed to HDE. Single and repetitive HDE inhalation exposure resulted in increased influx of total cells and neutrophils, increased mediator hyper-responsiveness (TNFα, IL-6, CXCL1, and amphiregulin), and enhanced tissue pathology that was more pronounced in zinc deficient mice compared to normal dietary counterparts. Airway inflammation was most pronounced in zinc deficient mice treated with repetitive HDE for 3 weeks. Similarly, macrophages maintained in a zinc deficient environment exhibited increased CXCL1 and IL-23 production as a result of increased NF-κB activation.
Conclusion
Given the relatively high incidence of dietary deficiencies in agriculture workers, we anticipate that zinc intake, or a lack thereof, may play an important role in modulating the host response to organic dust exposure.
Keywords: Zinc, Organic Dust, Lung, Innate Immunity, Inflammation
1. Introduction
Agricultural workers in concentrated animal feeding operations (CAFOs) are at an increased risk of developing airway inflammatory diseases (1–3). CAFO workers have increased incidence of cough, dyspnea, and phlegm production with higher rates of chronic bronchitis(4–6). The airway inflammatory response is characterized by neutrophil influx with increased production of inflammatory mediators(7, 8). Initial exposure to organic dust induces an intense proinflammatory response marked by low-grade fevers, airway symptoms, and bronchial hyper-responsiveness that wanes over time. With repeated exposure over time, individuals are at increased risk of lung function decline, airway inflammation, and progressive respiratory impairment, which has been termed the “chronic adaptation inflammatory response.” (9) The factors that determine the magnitude of this response remain unclear, and we propose that dietary factors are important.
Zinc is a candidate dietary factor as approximately one-half of the world’s population consumes diets insufficient in zinc, and it is conservatively estimated that up to 25% are at risk of zinc deficiency(10). Disadvantaged populations around the world subsist primarily on low zinc containing diets due to socioeconomic factors and cereal-based diets, which are high in phytate, a compound that binds zinc and reduces its absorption. The third National Health and Nutrition Examination Survey (NHANES) conducted in the U.S. found that among adults greater than 60 years old, 35–41 % of men and 36–45% of women consumed less dietary zinc than is considered adequate by current standards(11). Zinc absorption and endogenous excretion fall dramatically when zinc intakes are insufficient(12), but those adjustments do not prevent a decline in plasma zinc concentrations because zinc is sequestered in slow metabolizing tissues, such as bone and muscle(13). Sequestration of zinc makes it less available for zinc-demanding functions including maintenance of proper immune function. The response to zinc deficiency can be quite variable among individuals in response to dietary zinc depletion, likely influenced by genetics. Zinc is essential for proper immune function and has been shown to be a key ingredient for cellular activation through a variety of signaling pathways. In particular, zinc is a potent modulator of monocyte and macrophage function in response to harmful stimuli through regulation of PKA, PKC C/EBPβ, NF-κB, and MAPK signaling pathways, all of which are associated with regulation of innate immune function(14–19).
The innate immune response is also important in agriculture exposure induced respiratory disease. Our group has previously shown that human alveolar macrophages are highly sensitive to hog confinement facility dust extract (HDE)(20). Lung mononuclear phagocytes and macrophages are important innate immune lung effectors that serve a critical role in host defense against potentially harmful inhaled environmental particles(21). Alveolar macrophages can orchestrate the innate immune response through the release of mediators that drive inflammation through the activation of resident cells and recruitment of other cells including neutrophils(22). Macrophages produce potent mediators of the innate immune response following exposure to HDE that are associated with agriculturally induced lung disease including, but not limited to, TNF-α, IL-6, and IL-8 (9, 23).
Zinc is essential for proper immune function and has been shown to be a key ingredient for cellular activation through a variety of signaling pathways. In particular, zinc is a potent modulator of monocyte and macrophage function in response to harmful stimuli through regulation of PKA, PKC C/EBPβ, NF-κB, and MAPK signaling pathways, all of which are associated with regulation of innate immune function(14–19).
In this report, we first investigated zinc intake in a subpopulation of Midwestern agricultural workers with chronic obstructive pulmonary disease (COPD). Because the incidence of inadequate zinc intake was high and correlated with lower pulmonary function, we conducted further studies in animals and macrophages with manipulation of zinc exposure. Specifically, we hypothesized that inadequate cellular zinc availability would accentuate the innate immune lung response to HDE and that tissue macrophages would be a driving force that intensifies the inflammatory process.
2. Materials and Methods
2.1. Survey of zinc intake and lung function in a cohort of agricultural workers with COPD
Data was collected from 41 participants (40 male, 1 female) of a cross-sectional, cohort of rural Midwestern veterans with agricultural exposures and COPD at the Veterans Affairs (VA) Nebraska Western Iowa Healthcare system that has been previously described (24). Briefly, all subjects had greater than 2 years’ experience working on a farm. A Food Frequency Questionnaire (FFQ) was mailed to the home address of the participant, with a follow up phone call in 2 weeks if the FFQ had not been returned. FFQs were analyzed by trained personnel in the Harvard University Department of Nutrition. The questionnaire administered was the Willet FFQ, which has been validated in adults of all ages and sexes and among a variety of socio-economic groups(25–27). The FFQ allows for analysis of absolute nutrient intake values from foods and supplements. In addition, studies show that after adjustment for absolute energy intake, the Willett FFQ is robust in validity and reliability in comparison to other validated FFQs(28–30). Zinc intake values were calculated from the completed FFQs and compared to the Dietary Recommended Intake (DRI) values for the appropriate gender and life stage.
2.2. Preparation of Hog Dust Extract
Hog CAFO dust was collected and prepared as HDE as previously described (31, 32).
2.3. Animal Studies
C57BL/6 wild-type (WT) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal studies were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were in accordance with National Institute of Health guidelines for use of rodents. Animals were maintained in micro-isolator cages, on a 12 hr light/dark schedule with free access to water and standard rodent chow (Teklad, Madison, WI).
2.4. Zinc Modified Diets
Ten-week old, adult, male, C57BL/6 mice (~25 g) with fully developed lungs, were randomly placed on a Zn-deficient diet (Harlan Teklad, TD85419; Zn content: 0.5–1.5 ppm; referred to as SZ) or a matched control diet (TD85420; Zn content: 50 ppm; referred to as NZ) for three weeks, a time sufficient to establish subacute Zn-deficiency without requiring pair-feeding. A Zn-free environment was carefully maintained using deionized water in Zn-free containers and stainless steel cages(33). Zinc content was verified by Covance (Princeton, NJ).
2.5. DE Inhalation Model
Mice received either saline or HDE by an intranasal exposure method, as previously established(34). The concentration of 12.5% HDE was previously demonstrated to be an optimal concentration for eliciting lung inflammation in this mouse strain. A minimum of 5 mice were randomly assigned to each treatment group for each experiment. Mice were treated once (acute/single exposure) or daily, 5 days a week for 3 weeks (repetitive exposure) with either saline or HDE.
2.6. Bronchoalveolar Lavage Fluid (BALF)
BALF was collected as previously described (34). The total cell number of pooled lavages (3 × 1 mL lavages) was enumerated, and differential cell counts were determined on cytospin prepared slides (Cytopro Cytocentrifuge; Wescor, Logan UT) stained with Diff-Quick (Dade Behring, Newark, DE). Cell-free supernates for cytokine/chemokine analysis from the first lavage were collected and stored at −20°C.
2.7. Lung Histology
Whole lungs were inflated to 15 cm H2O pressure with 10% formalin (Sigma-Aldrich, St. Louis, MO) to preserve pulmonary architecture. Lungs were processed and embedded in paraffin, and sections (4–5 cm) were cut and stained with hematoxylin and eosin. Slides were reviewed and semi-quantitatively assessed for degree/distribution of bronchiolar compartment inflammation (assigned a value from 0 to 3 with a higher score indicating greater inflammatory changes) by a veterinary pathologist blinded to the treatment conditions, using a previously described lung inflammatory scoring system (35).
2.8. Cytokine/Chemokine Analysis
Murine or human TNFα, IL-6, IL-8 (CXCL8; human neutrophil chemoattractant), KC (CXCL1), IL-23 and amphiregulin concentrations were determined in BAL fluids or culture supernates according to manufacturer’s instructions using commercially available ELISA kits (R&D Systems, Minneapolis, MN).
2.9. Cell Culture Conditions
The murine alveolar macrophage cell line MH-S (ATCC, Manassas, VA) was maintained in standard culture conditions with RPMI-1640 (ATCC) supplemented with 10% normal fetal bovine serum (FBS) or chelex treated FBS (Atlanta Biologics, Flowery Branch, GA) at 37°C in a 5% CO2 incubator for 3 weeks. To deplete zinc from FBS, FBS was incubated overnight at 4°C with Chelex 100 resin beads (Sigma-Aldrich). Media with 2% FBS was used for assays, and cells were stimulated with 1% HDE at indicated time points. For zinc supplementation 10 μM zinc sulphate and 10 μM pyrithione was added to zinc depleted cultures 30 minutes prior to the addition of HDE.
Primary human monocytes were provided by the UNMC elutriation core facility (Omaha, NE). Monocytes were differentiated into macrophage (MDMs) with 50 μg/ml GM-CSF (PeproTech, Rocky Hill, NJ) in RPMI-1640 (HyClone) and supplemented with 10% FBS (Atlanta Biologics) over 6 days. Fresh media containing GM-CSF was changed every 2 days. On day 7 GM-CSF was removed and then MDMs were maintained in standard RPMI-1640 (HyClone) and supplemented with 10% normal FBS or chelex treated FBS for 3 weeks. Media was changed twice-weekly. Media with 2% FBS was used for assays, and cells were treated with 1 % HDE for various time points.
2.10. Cell Signaling Studies
Cell or tissue lysates were obtained with standard lysis buffer (Cell Signaling, Danvers, MA) containing 1 mM PMSF. Cytoplasmic and nuclear fractions were isolated using NE-PER (Thermo-Fisher, Waltham, MA) with Halt protease inhibitors (Thermo-Fisher). The proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with Odyssey Blocking Buffer (Li-Cor, Lincoln, NE) in TBS, followed by probing with antibodies overnight. All antibodies were purchased from Cell Signaling Technology, Inc. Chemiluminescence was detected using WesternSure Premium Chemiluminescent substrate (Li-Cor).
Protein kinase C (PKC) catalytic activity was determined in HDE-treated MH-S cells using a method previously described(36). Supernatant media were decanted and treated macrophage monolayers were washed, snap-frozen in a cell lysis buffer, and stored at −80°C. PKC isoenzyme activity was determined in these cytosolic and particulate fractions using the method of Hannun et al. (37). PKC isoform-specific substrate peptides (200μg/mL) were individually used in a reaction for each cell fraction and treatment condition: alpha (Bachem, Torrance, CA), epsilon and delta (MilliporeSigma, Burlington, MA), and zeta (Enzo, Farmingdale, NY). Cytosolic or particulate fraction samples (20 μl) were prepared and counted in nonaqueous scintillant (National Diagnostics, Atlanta, GA). PKC activity was expressed in relation to the total amount of cellular protein assayed as picomoles of phosphate incorporated per minute per milligram.
2.11. Statistical Analysis
SAS and SPSS were used to calculate descriptive statistics for nutrient intake and the proportion of participants who were below the required dietary allowance RDA. Pearson correlations were used to establish the relationship between zinc intake and lung function, and Student’s t-tests for the comparison of lung function between zinc intake categories above and below the RDA.
For animal studies, data represent mean values ± SEM for results pooled from two independent experiments. There was a minimum of 12 mice studied per condition. For total cell counts and differential counts in BALF, values were averaged from 4 or more slide fields per mouse. For cytokine, chemokine, and AREG measurement in BALF, duplicate measurements were made for each sample on the ELISA (technical replicates: N=26, or 24). Statistical significance was determined using one-way ANOVA with Tukey’s post hoc analysis of all pair-wise comparisons (GraphPad Prism software, San Diego, CA). A 95% confidence level was taken as a significant difference. For cell culture experiments data presented are mean +/− SEM. Data were analyzed for statistical significance using 1-way ANOVA with Tukey post-test for all pair-wise comparisons, using GraphPad Prism. A P-value < 0.05 was considered statistically significant.
3. Results
3.1. Evaluation of zinc intake and lung function in a cohort of agricultural workers with COPD
Organic dust exposure in the agricultural industry and particularly within confinement facilities is a significant cause of airway inflammation and lung disease. Of the 41 completed nutrition surveys of veterans with COPD evaluation of dietary zinc intakes revealed the mean (± SD) zinc intake of the cohort was 15 mg/day. Zinc insufficiency, defined by less than the Recommended Dietary Intake (RDI) of 11 mg/day, was found in 29% (N=12/41) of subjects. This finding was independent of protein as the mean protein intake was 80.1 (±30.0) g/day, which exceeds the DRI of 56 g. In comparing Zn insufficient to Zn sufficient subjects, lower zinc intake was associated with lower pulmonary function (Table 1). Specifically, the FEV1/FVC ratio was significantly lower (p=0.039) with a similar trend (p=0.056) observed with % predicted FEV1 in Zn insufficient COPD subjects (Table 1). There were no significant differences observed between groups relative to other demographic factors of smoking history.
Table 1.
A dietary survey of veterans with agricultural exposure and COPD revealed that participants with a dietary history of insufficient Zinc intake (< 11 mg/day) had reduced pulmonary function as measured by the FEV1/FVC ratio.
| |||||
|---|---|---|---|---|---|
| Zinc Insufficient (n=12) | Zinc Sufficient (n=29) | ||||
|
| |||||
| Variable | Mean | SD | Mean | SD | p-value |
| Age | 66.5 | 6.7 | 66.4 | 8.4 | 0.97 |
|
| |||||
| BMI | 28.2 | 8.1 | 29.6 | 7.4 | 0.61 |
|
| |||||
| FEV1 percent predicted | 60.3 | 25.1 | 75.0 | 20.4 | 0.056 |
|
| |||||
| FEV1/FVC % predicted ratio | 52.9 | 13.4 | 61.0 | 9.9 | 0.039 |
|
| |||||
| FVC % predicted | 82.4 | 23.4 | 90.5 | 15.6 | 0.20 |
|
| |||||
| DLCO | 15.4 | 5.7 | 17.3 | 5.7 | 0.33 |
|
| |||||
| N | % | N | % | ||
|
| |||||
| Smoking status | |||||
| Current | 3 | 25% | 5 | 17% | 0.23 |
| Former | 9 | 75% | 18 | 62% | |
| Never | 0 | 0% | 6 | 21% | |
|
| |||||
| Education | |||||
| High School | 5 | 42% | 13 | 45% | 0.85 |
| More than Hiah School | 7 | 58% | 16 | 55% | |
AII subjects were Caucasian and had COPD.
3.2. Impact of reduced dietary zinc on the airway inflammatory response following single exposure to HDE in mice
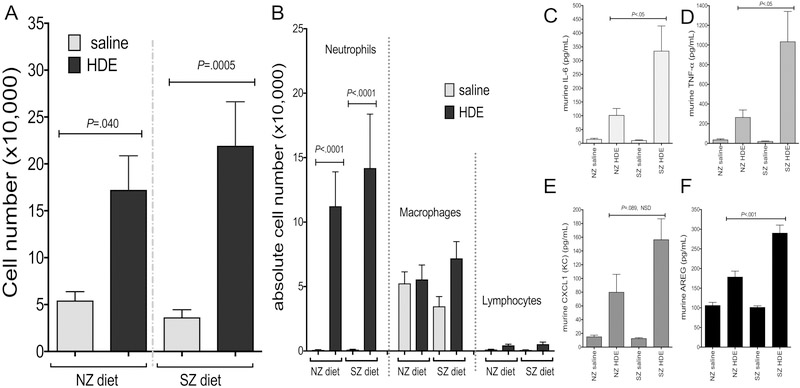
Based on the association between low zinc intake and decreased lung function in veterans with COPD, we next sought to determine whether reduced dietary zinc intake would alter the initial response to HDE. First, mice were maintained on a 3 week “run in” of either a zinc sufficient normal diet (NZ) or a corresponding matched, but zinc deficient diet (SZ) followed by a one-time nasal inhalation of HDE. Of note, for all animal studies, there were no significant differences in weight, appearance, or activity level between mice on NZ or SZ diet with or without HDE (data not shown). At 5 hours following HDE challenge, there were significant (p<0.05) increases in total cell counts, neutrophils and macrophages in BAL fluid of NZ and SZ dietary groups as compared to saline control animal, however, no significant differences in HDE-mediated cell influx were detected when comparing NZ to SZ diet regimens (Figure 1A,B). As compared to saline, a one-time exposure to HDE increased BALF levels of IL-6, TNF-α, CXCL1, and amphiregulin (AREG) in mice regardless of the diet regimen. The response was however significantly augmented in animals fed the SZ diet as compared to the NZ diet (Figure 1C,D,E,F).
Figure 1. Dietary zinc impacted the airway inflammatory mediator response, but not neutrophil response, to acute HDE exposure in mice.
Mice maintained on a zinc deficient diet (SZ) and subjected to a one time HDE exposure did not exhibit significant differences when compared to normal zinc sufficient diet (NZ) mice in (A) BAL cellular influx including (B) macrophages and neutrophils (data pooled from two experiments with 13 mice per treatment group). In contrast, SZ mice did have a significant increase in cytokine, chemokine, and amphiregulin production (C-F) when compared to mice maintained on a normal zinc diet (NZ). (data shown in C-F is representative from two separate experiments). Significance values for indicated comparisons by ANOVA and Tukey’s post test.
3.3. Impact of reduced zinc intake on the airway inflammatory response following repeated exposure to inhaled organic dust extract
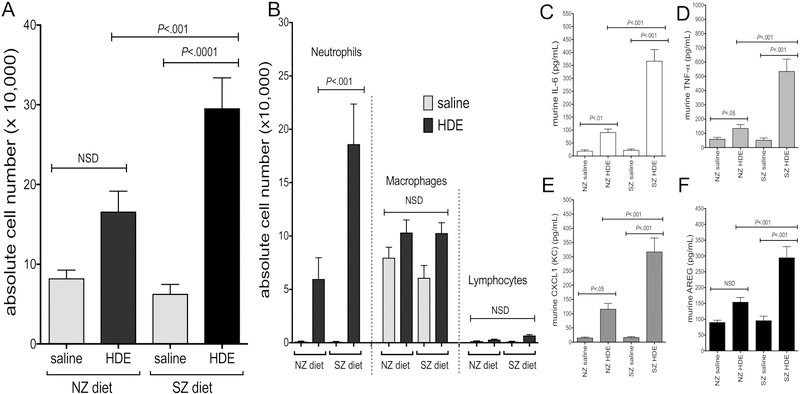
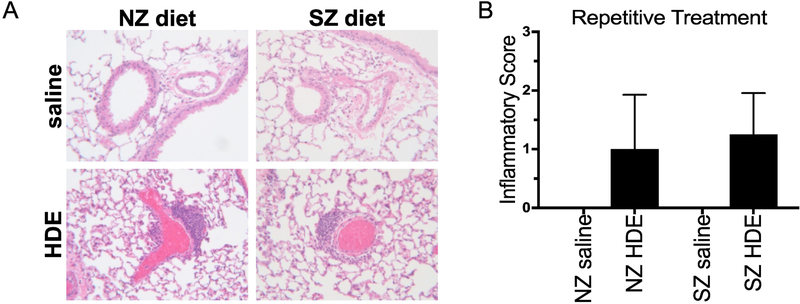
Next, mice were maintained on a three week “run in” of either a zinc sufficient normal diet (NZ) or a corresponding matched, but zinc deficient diet (SZ) followed by a daily, repetitive HDE exposure for 3 additional weeks while being maintained on designated dietary regimes. Following 3 weeks of repeated HDE treatment, there was an increase in total cell counts and neutrophil influx in NZ and SZ dietary groups as compared to saline controls, and the HDE-induced response was significantly augmented in mice fed the SZ diet as compared to the NZ diet (Figure 2A,B). Animals fed the SZ diet and repetitively treated with HDE also demonstrated a significant increase in the production of IL-6, TNFα, CXCL1, and AREG as compared to respective saline-treated controls, and moreover, levels of these mediators were significantly increased as compared to the NZ-HDE treatment group (Figure 2C–F). Microscopic review and semi-quantitative analysis following repetitive HDE exposure resulted in perivascular and peribronchiolar inflammation but there was no significant difference between animals fed the NZ or SZ diet (Figure 3A,B).
Figure 2. Dietary zinc impacted the airway inflammatory cellular and mediator response to repetitive HDE exposure in mice.
Bar graphs depict mean with standard error bars of (A) BAL total cells and (B) cell differential demonstrating increased neutrophils in HDE-treated mice on SZ diet compared to NZ diet. SZ mice also exhibited a significant increase in cytokine, chemokine, and amphiregulin production (C-F) when compared to mice maintained on a normal zinc diet (NZ) following HDE exposure. (data shown in C-F is pooled from two experiments with a minimum of 12 mice per treatment group). Significance values for indicated comparisons by ANOVA with Tukey’s post test. NSD = no significant difference.
Figure 3. Repetitive HDE exposure increased perivascular/bronchiolar lung infiltrates independent of dietary zinc.
Representative lung tissue sections from mice maintained on a zinc deficient (SZ) or sufficient (NZ) diet and exposed to daily HDE or saline for 3 weeks. Bar graph depicts mean ± SEM of the semi-quantitative degree and distribution of bronchiolar inflammation of N=6 mice/group. Although SZ mice did exhibit more inflammation compared to NZ nice this did not result in a statistically significant difference in the inflammation score.
3.3. Impact of zinc deficiency on macrophage function
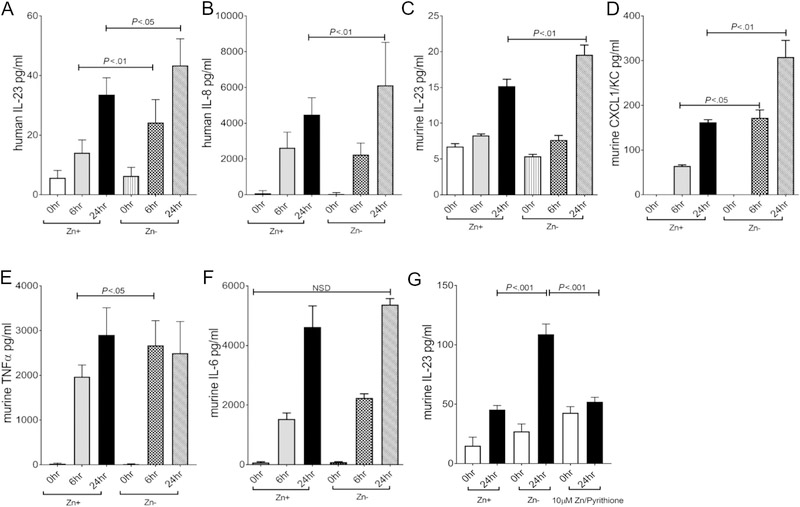
Alveolar macrophages play a key role in directing the host innate immune response to noxious inhaled particulate matter. In these studies we investigated the role of zinc status on HDE-induced neutrophil chemoattractant production (murine CXCL1, human IL-8/CXCL8, and IL-23) by macrophages to determine whether these factors play a potential role in cell recruitment, as previously shown in the lung in response to HDE. Knowing that neutrophil presence was more significant in the lung of mice maintained on a zinc deficient diet, we focused our search on neutrophil chemoattractants that could potentially drive neutrophil recruitment including CXCL1 and IL-23 using monocyte derived macrophages (MDM) obtained from normal human donors and a murine alveolar macrophage cell line (MH-S). In order to achieve a zinc deficient state, cell cultures were maintained in FBS-containing, zinc devoid medium over a 3-week acclimatization period. Comparison of normal medium to conditioned medium demonstrated an expected abundance of zinc in the normal FBS containing medium compared to the Chelexed-FBS containing medium (162.5 reduced to 5.5 ppm respectively). MDM and MH-S cells tolerated extended culture under both conditions well with no evidence of toxicity and a corresponding small decrease in cell-associated zinc when comparing cells incubated in normal versus chelex-treated FBS as determined by ICP-MS (579 vs 560 ppm, respectively). Consistent with animal studies showing increased neutrophil chemotactic factors, human MDMs stimulated with HDE under zinc deficient conditions produced more IL-23, a potent neutrophil chemoattractant that is primarily derived from activated myeloid-lineage cells including macrophages, when compared to cells maintained under normal conditions (Figure 4A). A similar significant increase was observed for IL-8 (Figure 4B). Likewise, MH-S alveolar macrophages maintained in a zinc deficient environment over 3 weeks exhibited a significant increase in IL-23 and CXCL1/KC production in response to HDE when compared to cells maintained in zinc containing medium (Figure 4C,D)(38). Importantly, zinc supplementation of zinc depleted cultures reduced IL-23 production to the level observed in zinc sufficient cultures (Figure 4G). In contrast, macrophages maintained under zinc deficient conditions did not exhibit a further increase in TNFα (Figure 4E) or IL-6 (Figure 4F) compared to cells maintained in zinc sufficient conditions.
Figure 4. Zinc modulates chemokine production in Macrophages following HDE stimulation.
MDMs (N= minimum of 3 donors)(Panels A,B) or MH-S cells (Panels C-G) (N= 5 independent experiments) were maintained in normal or zinc deficient medium for 3 weeks and then stimulated with HDE for 0 (baseline), 6, and 24 hours. Levels of IL-23 and IL-8 in MDMs or IL-23, CXCL1, TNFα, and Amphiregulin in MH-S cells were quantitated in cell-free supernatants by ELISA. Both MDM and MH-S cultures that were maintained in zinc deficient medium exhibited a significant increase in IL-23 and IL-8/CXCL1. Supplementation of zinc pyrithione 30 minutes before HDE exposure significantly decreased IL-23 production in zinc deficient cultures comparable to the response of zinc sufficient culture. (* p < 0.05, ** p < 0.01 ). Significance values for indicated comparisons by ANOVA and Tukey’s post test.
3.4. Impact of zinc deficiency on macrophage cell signaling
Our group and others have previously shown that zinc plays a critical role in regulating cell signal transduction pathways particularly in macrophages(14), and PKC isoforms have been implicated in monocyte HDE-induced monocyte cytokine production(32). Knowing that zinc depletion results in an increase in murine CXCL1, human IL-8 and murine/human IL-23 production, we investigated transcription factors that are known to regulate their transcription. Evaluation of PKC isoforms (Alpha, Epsilon, Delta, Zeta) under zinc sufficient (Zn+) and deficient (Zn−) conditions revealed that a lack of zinc decreased PKC alpha, epsilon, and zeta activity in HDE stimulated MH-S cells whereas PKC delta was unaffected (Figure 5). Next, we evaluated the extent of phosphorylation of p65, ERK, JNK and IκBα under identical conditions (Figure 6A) and revealed a modest increase in phospho-p65 (Figure 6B), but no changes in the phosphorylation of ERK, JNK, or IκBα (Figure 6C,D,E) out to 30 minutes following HDE stimulation. Based on this observation, we then evaluated the extent of p65 DNA binding activity in nuclear extracts and confirmed a significant increase in p65 activity in zinc deprived macrophages in comparison to cells maintained under zinc sufficient conditions (Figure 6F).
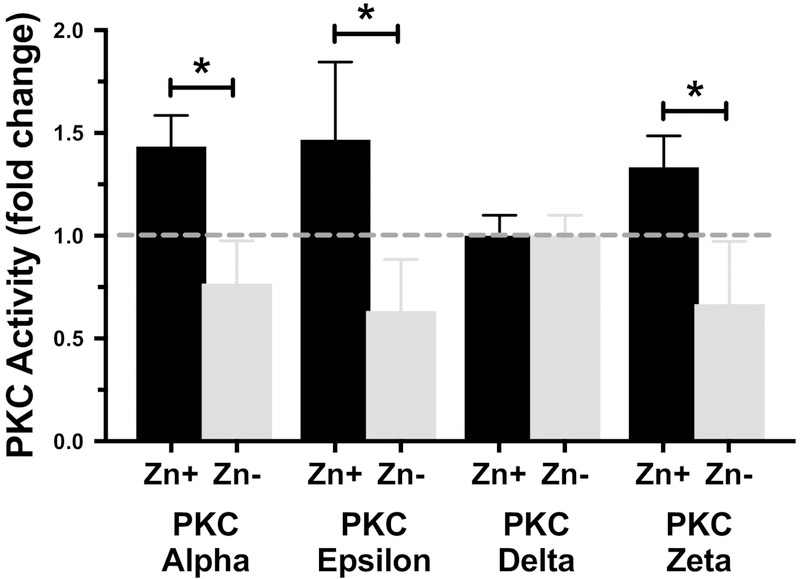
Figure 5. PKC catalytic activity is not increased by zinc deficiency.
PKC catalytic activity was determined in zinc sufficient (Zn+) and zinc deficient (Zn−) MH-S cells at one hour post HDE stimulation. PKC activity for each isoform was was normalized for total cellular protein and expressed as picomoles of phosphate incorporated per min per milligram. Data shown is for all the PKC isoforms in relation to their baseline activity levels showing the fold change in optimum HDE effect. As shown, PKC alpha, epsilon and zeta activity was significantly suppressed in MH-S cells maintained in a zinc deficient environment whereas PKC delta was unaffected. (Results were obtained from a minimum of 3 separate experiments; * p < 0.05). Significance values for indicated comparisons by ANOVA and Tukey’s post test.
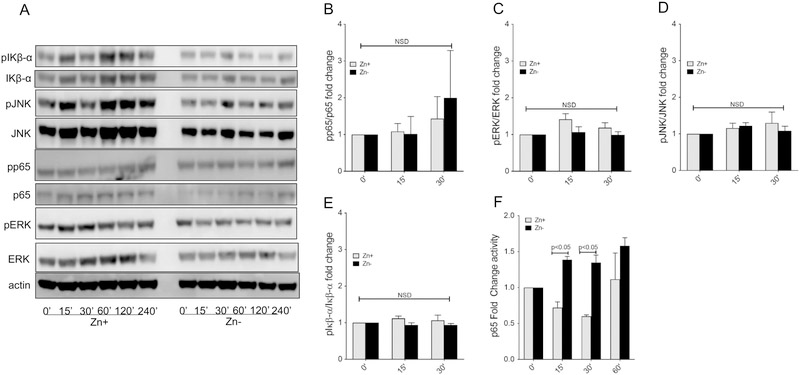
Figure 6. Zinc deprivation increases Macrophage NF-κB activity in response to HDE.
MH-S cultures were maintained in zinc sufficient or zinc deficient conditions for three weeks and then stimulated with HDE for up to 4 hours. Total cell extracts were first harvested between 0 to 240 mins and subject to Western analysis (A) to determine the extent of protein phosphorylation for p65, ERK, JNK and IκBα (B-E) as determined by densitometry and normalized to total protein content. Nuclear extracts were also obtained under similar conditions and the extent of p65 DNA binding activity was evaluated between 0 to 60 minutes (F). Zinc deficient MH-S exhibited an increase in phosphop65 and corresponding p65 DNA binding activity whereas there were no differences in ERK, JNK or IκBα phosphorylation. (Western results are representative of 3 separate experiments; p65 activity is data pooled from 3 separate experiments; * p < 0.05). Significance values for indicated comparisons by ANOVA and Tukey’s post test.
4. Discussion
To our knowledge, this is the first evidence to suggest that insufficient zinc intake contributes to the incidence of adverse pulmonary function within this population. If correct, dietary modification could become a tactic to counter the incidence and severity of organic dust-induced lung disease particularly in CAFO workers with insufficient zinc intake. The present study was designed to determine whether inadequate dietary zinc intake in tandem with single and repetitive lung exposure to hog dust extract (HDE) worsen lung inflammation in a well-established animal model and also begin to identify the mechanisms that may explain the role of zinc and airway disease. Exposure to organic dust in agricultural workplace environments leads to airway inflammation that increases the risk for lung disease (39). Livestock farmers have increased risk for COPD, chronic bronchitis, and asthma when compared with crop farmers (40, 41). Although it is clear that inhalational exposures lead to lung inflammation, it remains unknown why disparity exists with the magnitude of the inflammatory response between individuals. There are likely many factors that contribute to inter-individual variation including food consumption as it is established that military veterans with significant agricultural work exposure have restricted access to adequate nutrition as a consequence of compromised socioeconomic factors(42). In order to understand whether zinc as a specific dietary factor plays a role in susceptibility to lung disease as a consequence of agricultural exposures, our group collected data from 41 participants (40 male, 1 female) in a cross-sectional study of agricultural exposures and COPD in veterans seeking health care at the General Medicine clinics of the Nebraska-Western Iowa Veterans Affairs (VA) Medical Center. Detailed methods for the original study have been described elsewhere(24). Strikingly, within this relatively small cohort of veterans with significant agricultural exposure, COPD, and who completed a diet survey, 29% consumed zinc at a level below the required dietary allowance. Further, individuals with below normal zinc intakes had an increase in obstructive airway disease as evident by a decreased FEVi/FVC ratio. It is important to recognize that following further analysis of this cohort, we observed other intake deficiencies at the respective frequencies: Copper: 7%, Iron: 5%, Selenium: 73%, and Vitamin C: 24%. Taken together, these findings suggest that suboptimal zinc intake, in conjunction with other nutrient deficiencies, may be a predisposing factor for worse outcomes upon exposure to organic dust.
To examine this further, we utilized a well-characterized animal model of HDE exposure to determine whether prolonged, suboptimal zinc intake altered the magnitude of airway inflammation. As previously observed in humans, single and repetitive organic dust exposures in the setting of suboptimal zinc intake lead to a significant increase in BALF neutrophils and immunomodulatory mediators (TNFα, IL-6, CXCL1, and AREG). Unlike previous findings (34), we did not observe a dampening effect in BALF mediators following repetitive exposure, and in fact, observed further increases when compared to animals maintained on a zinc sufficient diet. Repetitive organic dust exposure in hog farmers can cause chronic inflammation, but the robust acute inflammatory response can diminish over time suggestive of an adaptation-like response despite ongoing pulmonary function decline and worsening lung pathology (9, 43). We propose that dietary factors that include zinc, are potential critical factors that modulate the lung inflammatory response to inhalant organic dust exposures. Our findings suggest that suboptimal zinc intake over time in conjunction with repeated exposure to organic dust may enhance the inflammatory response, decrease the apparent adaptation-like response, and increase the risk of further exacerbating pulmonary disease. Furthermore, our data also suggest that zinc may alter repair processes in the airway following HDE in that AREG is augmented in BALF following single and repetitive HDE exposures in mice. AREG is an epidermal growth factor receptor ligand that is known to mediate tissue repair and wound healing within the lung. We have previously reported that AREG is increased after repetitive HDE exposure and increases further during the repair process (44) and importantly, that AREG mediates bronchial epithelial wound repair processes including re-epithelialization of lung scaffolds(45). Our data suggests that zinc insufficiency may also alter AREG mediated processes and requires further investigation.
Zinc is the second most abundant trace element, next to iron, in our bodies and is required for proper immune function and defense against pathogens (46). Nutritional deficits in zinc increase susceptibility to infection (47–49), whereas zinc supplementation can prevent or decrease the extent of infection (49–51). Consistent with our findings, the host response to organic dust, which contains bacterial byproducts, was more intense in a setting of zinc deficiency and could be recapitulated in macrophages. In the case of pathogen invasion, zinc is rapidly transported from the blood compartment to other tissues, including the lung, where it assists in cellular defense (52, 53). Our group previously showed that insufficient zinc intake, using the animal model employed in this study, resulted in a reproducible 2-fold decrease in circulating zinc levels despite no other observable differences in animal appearance or behavior prior to insult (33). In fact, we measured zinc levels in whole lung tissue and found that in comparison to the NZ diet, the SZ diet lead to an approximate two-fold reduction in zinc regardless of saline or HDE exposure respectively (Ave: 18.33 μg/g lung tissue vs. 50 μg/g).
Zinc ions serve as catalytic, structural, and regulatory cofactors for proteins. Indeed, zinc is a component of the PKC regulatory domain, where it is thought to impact enzyme tertiary structure, activity and translocation. Our findings emphasize this because zinc deficient cells exhibited decreased HDE-stimulated PKC activation. Through bioinformatics, it has been estimated that the zinc proteome represents about 9% of the entire proteome in eukaryotes (54). Predictably, zinc plays an important role in immune function, affecting cells of both the innate and adaptive immune systems. Zinc deficiency can also alter the function of neutrophils, monocytes, and macrophages, key cells in mounting a rapid and effective innate immune response. In our investigation, prolonged suboptimal zinc intake lead to a significant increase in BALF neutrophils that corresponded with an increase in KC/CXCL1 production. Further, we demonstrate that a zinc deficient environment increased production of two potent chemoattractants, IL-8 and IL-23, in macrophages indicating that these cells have an important role in enhancing the response to organic dust exposure. Further, the increase in IL-23 production observed in zinc deficient macrophage cultures was reduced to normal zinc sufficient output levels following zinc supplementation thereby demonstrating a zinc-specific effect. Consistent with our previous work, a lack of zinc can result in dysregulation of cell signaling in favor of more activity, enhanced expression of target genes, and an increased inflammatory response that results in more collateral tissue damage(55). Indeed, we observed a corresponding significant increase in p65 activity in zinc-deprived macrophages when compared to macrophages maintained under zinc sufficient conditions. While our studies focused on the impact of zinc on macrophage function, it is likely that zinc deficiency alters other resident and inflammatory cells. For example, previous work has shown that zinc deficiency increases ROS formation (56) and can also inhibit phagocytosis in neutrophils(57). Given the extensive footprint of zinc upon the eukaryotic proteome, it is also likely that other pathways not yet accounted for contribute to immune dysregulation in the lung following repeated exposure to organic dust. Our findings suggest that Zn deficiency is likely one amongst other important variables that may contribute to the heterogeneity of lung disease amongst CFAO workers. Whether alteration in immune function can be readily corrected through dietary supplementation leading to normalization of the host response remains to be studied. Further, zinc is regulated by a network of over 24 zinc transporter proteins, metallothioneins, and other proteins. Based on the complexities of zinc metabolism, it is likely that polymorphic variants that alter protein function also play into the heterogeneity of responses observed across the spectrum of agricultural workers.
Supplementary Material
Nutritional zinc deficits lead to increased lung inflammation in response to inhaled organic dust.
Macrophages maintained in a zinc deficient environment have a greater inflammatory response to organic dust.
The NFkappaB signaling pathway is activated to a greater extent under zinc deficient conditions leading to production of more neutrophil chemoattractants.
Acknowledgements
The authors thank Amy Nelson, Jane Devasure, and Tricia Levan for technical assistance. This work was supported by the National Institutes of Health [HL118268 (DLK)].
Funding Sources: This work was supported by the National Institutes of Health [HL118268 (DLK) and ES019325 (JAP)] and the Central States Center for Agricultural Safety and Health (U54 OH010162). TAW is the recipient of a Research Career Scientist Award (IK6 BX003781) from the Department of Veterans Affairs.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donham KJ. Health effects from work in swine confinement buildings. Am J Ind Med 1990; 17: 17–25. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz DA, Landas SK, Lassise DL, Burmeister LF, Hunninghake GW, Merchant JA. Airway injury in swine confinement workers. Ann Intern Med 1992; 116: 630–635. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz DA, Thorne PS, Jagielo PJ, White GE, Bleuer SA, Frees KL. Endotoxin responsiveness and grain dust-induced inflammation in the lower respiratory tract. Am J Physiol 1994; 267: L609–617. [DOI] [PubMed] [Google Scholar]
- 4.Cormier Y, Boulet LP, Bedard G, Tremblay G. Respiratory health of workers exposed to swine confinement buildings only or to both swine confinement buildings and dairy barns. Scand J Work Environ Health 1991; 17: 269–275. [DOI] [PubMed] [Google Scholar]
- 5.Iversen M, Dahl R, Korsgaard J, Hallas T, Jensen EJ. Respiratory symptoms in Danish farmers: an epidemiological study of risk factors. Thorax 1988; 43: 872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelzang PF, van der Gulden JW, Preller L, Heederik D, Tielen MJ, van Schayck CP. Respiratory morbidity in relationship to farm characteristics in swine confinement work: possible preventive measures. Am J Ind Med 1996; 30: 212–218. [DOI] [PubMed] [Google Scholar]
- 7.Cormier Y, Duchaine C, Israel-Assayag E, Bedard G, Laviolette M, Dosman J. Effects of repeated swine building exposures on normal naive subjects. Eur Respir J 1997; 10: 1516–1522. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J 1997; 10: 381–387. [DOI] [PubMed] [Google Scholar]
- 9.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 2003; 9: 185–196. [DOI] [PubMed] [Google Scholar]
- 10.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 2006; 20: 3–18. [DOI] [PubMed] [Google Scholar]
- 11.Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr 2002; 132: 3422–3427. [DOI] [PubMed] [Google Scholar]
- 12.Baer MT, King JC. Tissue zinc levels and zinc excretion during experimental zinc depletion in young men. Am J Clin Nutr 1984; 39: 556–570. [DOI] [PubMed] [Google Scholar]
- 13.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr 2000; 130: 1360S–1366S. [DOI] [PubMed] [Google Scholar]
- 14.Liu MJ, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, Knoell DL. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep 2013; 3: 386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pyle CJ, Akhter S, Bao S, Dodd CE, Schlesinger LS, Knoell DL. Zinc Modulates Endotoxin-Induced Human Macrophage Inflammation through ZIP8 Induction and C/EBPbeta Inhibition. PLoS One 2017; 12: e0169531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals 2007; 20: 579–585. [DOI] [PubMed] [Google Scholar]
- 17.von Bulow V, Rink L, Haase H . Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-alpha and IL-1 beta production in monocytes by elevation of guanosine 3’,5’-cyclic monophosphate. J Immunol 2005; 175: 4697–4705. [DOI] [PubMed] [Google Scholar]
- 18.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001; 14: 331–341. [DOI] [PubMed] [Google Scholar]
- 19.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J 2003; 376: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, Golden G, West WW, Sisson JH, Romberger DJ. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol 2011; 45: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fels AO, Cohn zA. The alveolar macrophage. J Appl Physiol (1985) 1986; 60: 353–369. [DOI] [PubMed] [Google Scholar]
- 22.Thepen T, Kraal G, Holt PG. The role of alveolar macrophages in regulation of lung inflammation. Ann N Y Acad Sci 1994; 725: 200–206. [DOI] [PubMed] [Google Scholar]
- 23.Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, Larsson L, Allen-Gipson D, Von Essen SG, Romberger DJ. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol 2008; 122: 375–382, 382 e371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissenburger-Moser L, Meza J, Yu F, Shiyanbola O, Romberger DJ, LeVan TD. A principal factor analysis to characterize agricultural exposures among Nebraska veterans. J Expo Sci Environ Epidemiol 2017; 27: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988; 127: 188–199. [DOI] [PubMed] [Google Scholar]
- 26.Longnecker MP, Lissner L, Holden JM, Flack VF, Taylor PR, Stampfer MJ, Willett WC. The reproducibility and validity of a self-administered semiquantitative food frequency questionnaire in subjects from South Dakota and Wyoming. Epidemiology 1993; 4: 356–365. [DOI] [PubMed] [Google Scholar]
- 27.Hunter DJ, Rimm EB, Sacks FM, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 1992; 135: 418–427. [DOI] [PubMed] [Google Scholar]
- 28.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol 2001; 154: 1089–1099. [DOI] [PubMed] [Google Scholar]
- 29.Caan B, Hiatt RA, Owen AM. Mailed dietary surveys: response rates, error rates, and the effect of omitted food items on nutrient values. Epidemiology 1991; 2: 430–436. [PubMed] [Google Scholar]
- 30.Jain M, Howe GR, Rohan T. Dietary assessment in epidemiology: comparison on food frequency and a diet history questionnaire with a 7-day food record. Am J Epidemiol 1996; 143: 953–960. [DOI] [PubMed] [Google Scholar]
- 31.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol (1985) 2002; 93: 289–296. [DOI] [PubMed] [Google Scholar]
- 32.Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, Romberger DJ. Repeat organic dust exposure-induced monocyte inflammation is associated with protein kinase C activity. J Allergy Clin Immunol 2007; 120: 366–373. [DOI] [PubMed] [Google Scholar]
- 33.Knoell Dl, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA, Crouser ED. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 2009; 37: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 2009; 296: L1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson AJ, Roy SK, Warren K, Janike K, Thiele GM, Mikuls TR, Romberger DJ, Wang D, Swanson B, Poole JA. Sex differences impact the lung-bone inflammatory response to repetitive inhalant lipopolysaccharide exposures in mice. J Immunotoxicol 2018; 15: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyatt TA, Slager RE, Heires AJ, Devasure JM, Vonessen SG, Poole JA, Romberger DJ. Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. Am J Respir Cell Mol Biol 2010; 42: 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannun YA, Loomis CR, Bell RM. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem 1985; 260: 10039–10043. [PubMed] [Google Scholar]
- 38.Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol 2001; 166: 7563–7570. [DOI] [PubMed] [Google Scholar]
- 39.Respiratory health hazards in agriculture. Am J Respir Crit Care Med 1998; 158: S1–S76. [DOI] [PubMed] [Google Scholar]
- 40.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest 2009; 136: 716–725. [DOI] [PubMed] [Google Scholar]
- 41.Szczyrek M, Krawczyk P, Milanowski J, Jastrzebska I, Zwolak A, Daniluk J. Chronic obstructive pulmonary disease in farmers and agricultural workers - an overview. Ann Agric Environ Med 2011; 18: 310–313. [PubMed] [Google Scholar]
- 42.Berg I, Hanson C, Sayles H, Romberger D, Nelson A, Meza J, Miller B, Wouters EF, Macnee W, Rutten EP, Romme EA, Vestbo J, Edwards L, Rennard S. Vitamin D, vitamin D binding protein, lung function and structure in COPD. Respir Med 2013; 107: 1578–1588. [DOI] [PubMed] [Google Scholar]
- 43.Palmberg L, Larssson BM, Malmberg P, Larsson K. Airway responses of healthy farmers and nonfarmers to exposure in a swine confinement building. Scand J Work Environ Health 2002; 28: 256–263. [DOI] [PubMed] [Google Scholar]
- 44.Warren KJ, Wyatt TA, Romberger DJ, Ailts I, West WW, Nelson AJ, Nordgren TM, Staab E, Heires AJ, Poole JA. Post-injury and resolution response to repetitive inhalation exposure to agricultural organic dust in mice. Safety (Basel) 2017; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordgren TM, Heires AJ, Bailey KL, Katafiasz DM, Toews ML, Wichman CS, Romberger DJ. Docosahexaenoic acid enhances amphiregulin-mediated bronchial epithelial cell repair processes following organic dust exposure. Am J Physiol Lung Cell Mol Physiol 2018; 314: L421–L431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr 2009; 29: 133–152. [DOI] [PubMed] [Google Scholar]
- 47.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr 2004; 24: 277–298. [DOI] [PubMed] [Google Scholar]
- 48.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998; 68: 447S–463S. [DOI] [PubMed] [Google Scholar]
- 49.Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr 2004; 24: 255–275. [DOI] [PubMed] [Google Scholar]
- 50.Haase H, Mocchegiani E, Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology 2006; 7: 421–428. [DOI] [PubMed] [Google Scholar]
- 51.Kahmann L, Uciechowski P, Warmuth S, Plumakers B, Gressner AM, Malavolta M, Mocchegiani E, Rink L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res 2008; 11: 227–237. [DOI] [PubMed] [Google Scholar]
- 52.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A 2005; 102: 6843–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rofe AM, Philcox JC, Coyle P. Trace metal, acute phase and metabolic response to endotoxin in metallothionein-null mice. Biochem J 1996; 314 ( Pt 3): 793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreini C, Bertini I, Rosato A. Metalloproteomes: a bioinformatic approach. Acc Chem Res 2009; 42: 1471–1479. [DOI] [PubMed] [Google Scholar]
- 55.Liu MJ, Bao S, Napolitano JR, Burris DL, Yu L, Tridandapani S, Knoell DL. Zinc regulates the acute phase response and serum amyloid A production in response to sepsis through jAk-STAT3 signaling. PLoS One 2014; 9: e94934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wessels I, Haase H, Engelhardt G, Rink L, Uciechowski P. Zinc deficiency induces production of the proinflammatory cytokines IL-1beta and TNFalpha in promyeloid cells via epigenetic and redox-dependent mechanisms. J Nutr Biochem 2013; 24: 289–297. [DOI] [PubMed] [Google Scholar]
- 57.Keen CL, Gershwin ME. Zinc deficiency and immune function. Annu Rev Nutr 1990; 10: 415–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.