Abstract
Background
Infraorbital hollowing can be addressed with hyaluronic acid soft tissue fillers. A prospective, multicenter, evaluator-blinded, randomized, controlled study (NCT03418545) demonstrated the safety and effectiveness of Juvéderm Volbella XC (VYC-15L, Allergan Aesthetics, an AbbVie company, Irvine, CA) in adults seeking correction for infraorbital hollows.
Objective
The objective of the current analysis was to examine patient-reported outcomes from the clinical study.
Methods
Participants were randomly assigned 3:1 to the VYC-15L treatment group or the no-treatment control group. Outcome measurements included: evaluating investigator (EI)- and participant-assessed Global Aesthetic Improvement Scale (GAIS) scores, as well as participant responses to the FACE-Q Appraisal of Lower Eyelids, questions on treatment satisfaction, the extent to which patients were bothered by dark circles under their eyes, and willingness to recommend treatment to a friend.
Results
The modified intent-to-treat population included 135 participants (median age, 47 years; 91.9% female). At Month 3, the majority of VYC-15L-treated participants showed improvements in the EI- and participant-assessed GAIS. The mean change from baseline to Month 3 score (32.7% increase) showed statistically significant improvement (mean [standard deviation], 17.8 [19.8], P < .0001). At Months 3 and 12 posttreatment, most VYC-15L-treated participants reported feeling satisfied with treatment and not feeling moderately or very bothered by dark circles under their eyes, and would recommend treatment to a friend.
Conclusions
The current analysis demonstrated the effectiveness of VYC-15L treatment to reduce infraorbital hollowing and to improve overall satisfaction based on validated patient-reported outcomes. Participant-assessed improvements aligned with EI-assessed outcomes and lasted for 1 year.
Level of Evidence: 2

The aging midface manifests several distinct yet related findings that are commonly described as infraorbital hollowing.1 These include, but are not limited to, the tear trough, which comprises the medial aspect of the infraorbital rim to the midpupillary line, and the palpebromalar groove (or lid-cheek junction), which comprises the lateral aspect of in the infraorbital rim (Figure 1).2,3 These anatomic landmarks contribute to the U-shaped or relative curvilinear depression under the eyes that can result in dark shading, conferring a fatigued appearance.1,4,5 Tear troughs are one of the most common target areas for facial aesthetic treatment.6 Infraorbital rejuvenation techniques range from invasive procedures, such as surgical approaches (eg, fat grafting, lower eyelid blepharoplasty),3,7-9 to more minimally invasive approaches, such as energy-based treatments9,10 and dermal filler injections.4,9,11,12
Figure 1.
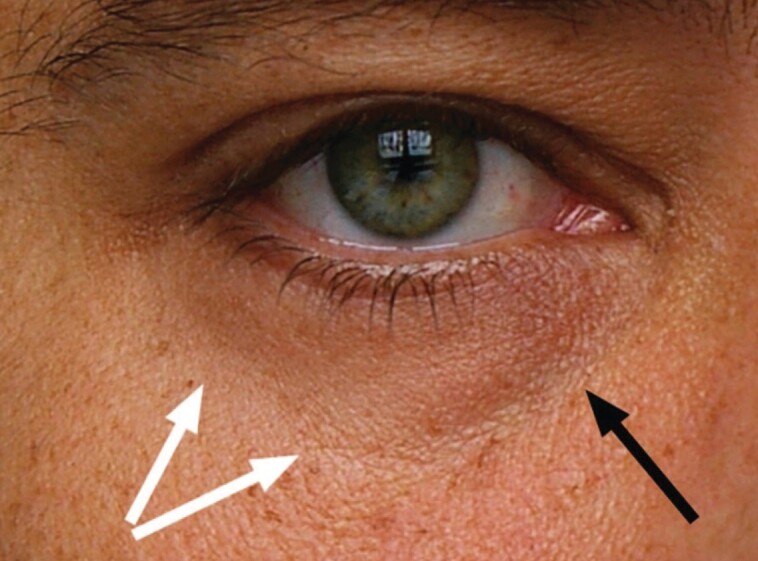
Schematic of infraorbital hollowing. Anatomic landmarks include the tear trough, which extends from the medial canthus to the midpupillary point along the infraorbital rim (black arrow), and the palpebromalar groove or lid-cheek junction, the lateral aspect of the infraorbital rim (white arrows; schematic published with permission from Haddock et al2).
Hyaluronic acid (HA) dermal fillers have become a treatment of choice to address infraorbital hollowing.1,5,9 Due to the thin and in some cases even transparent skin overlying the infraorbital area, this area is especially unforgiving of contour irregularities and requires careful product selection for optimal results.13 Juvéderm Volbella XC (VYC-15L; Allergan Aesthetics, an AbbVie company, Irvine, CA) is a temporary soft tissue filler containing 15 mg/mL HA with 0.3% w/w lidocaine to reduce injection-related pain and to provide consistency in anesthetic dosing.14,15 The proprietary mix of higher- and lower-molecular weight HA and the low elastic modulus (G′ ≈ 160 Pa) of VYC-15L gives this product characteristics suited for treatment of the perioral area, lips, and tear troughs, including improved moldability (ie, spreading, modeling, and shaping) as well as ease of flow during injection.13 VYC-15L was approved by the US FDA in 2016 for lip augmentation and correction of perioral rhytids in adults, and in May 2021 for the improvement of infraorbital hollowing in adults.16,17
The current study was conducted to assess the safety and effectiveness of VYC-15L for correction of infraorbital hollowing. A randomized, evaluator-blinded, controlled study demonstrated the safety and effectiveness of VYC-15L in adults seeking correction for infraorbital hollows.13 This study showed that 83.1% of participants treated with VYC-15L achieved at least a 1-point improvement on the validated Allergan Infraorbital Hollows Scale (AIHS)18 in both infraorbital areas at Month 3, which was the primary effectiveness endpoint.13 Objective calculations from 3-dimensional (3D) imaging also showed that volume increases in the infraorbital area were maintained for 1 year following VYC-15L treatment. The objective of the current analysis was to examine patient-reported outcomes (PROs) from the clinical study.
METHODS
Participants
Eligible male or female adult participants were aged at least 22 years (no age upper limit per protocol), with a baseline grade of 2 (moderate) or 3 (severe) on the 5-point AIHS (range, 0 [none] to 4 [extreme]) for each eye, as assessed by the evaluating investigator (EI). Both eyes had to qualify but did not need to have the same score. The participant's anatomy also needed to be amenable to an AIHS grade improvement of 0 (none) or 1 (minimal). Exclusion criteria included atrophic skin in the tear trough region or large lower lid fat pads that would mask improvement, hyperpigmentation in the infraorbital area (not including dark circles under the eyes), substantial volume loss in the midface, or a cornea that projected farther forward than the most anteriorly projected part of the cheek. Participants were excluded if they had previously received permanent facial implants, blepharoplasty, facelift, or browlift; underwent botulinum toxin injections (within 6 months before enrollment) or fat injections above the subnasale; or underwent volume augmentation with dermal fillers in the malar area, temples, or around the eyes within 12 months before enrollment.
Study Design
This was a prospective, multicenter, evaluator-blinded, randomized, controlled study conducted from January 2018 to August 2019 at 15 US sites to evaluate the safety and effectiveness of VYC-15L for adults seeking correction of infraorbital hollowing (NCT03418545). This study received IRB approval (Copernicus Group IRB, Cary, NC, and Duke University Health Systems IRB for Clinical Investigations, Durham, NC) and was conducted in conformance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use E6 guideline for Good Clinical Practice. Written informed consent was obtained from all participants.
Participants were randomly assigned 3:1 to the VYC-15L treatment group or the no-treatment control group using block randomization stratified by investigational site through an automated interactive web response system. Both the EI and image analysis technician were blinded to the randomization process. Participants from the VYC-15L treatment group had the option to receive a touch-up treatment 30 days after initial treatment if an AIHS score of 0 or 1 was not achieved in either infraorbital hollow or if both the participant and treating investigator agreed that optimal correction had not been achieved. Treatment group participants also had the option for repeat treatment 12 months after initial treatment. Participants from the control group had the option to receive treatment after a 3-month no-treatment control period (including optional touch-up treatment 30 days after initial treatment), but were not offered repeat treatment at 12 months after initial treatment. This is a standard design for an evaluator-blinded, randomized study with a no-treatment control group; this design was utilized because there were no FDA-approved dermal fillers for infraorbital hollowing at the time of the study to use as an active comparator.
Study Procedures
During treatment, VYC-15L was injected in the submuscular/supraperiosteal plane by either a 32G ½-inch needle or a 27G 1½-inch cannula to the infraorbital and adjacent area. Appropriate volumes were determined by the treating investigator, but the maximum volume did not exceed 2.2 mL per side for initial and touch-up treatments combined. Anesthesia (eg, ice, topical, local) was allowed to reduce injection discomfort.
Primary Effectiveness Endpoints
The primary effectiveness endpoint was the AIHS responder rate at Month 3.13 A responder was defined as a participant showing at least a 1-point improvement from baseline on the AIHS in both infraorbital areas based on EI assessment.
Patient-Reported Outcomes (PROs)
Participant Responses on the FACE-Q Appraisal of Lower Eyelids
At baseline (Day 0, before treatment), as well as at Months 1, 3, 6, 9, and 12, participants completed the validated FACE-Q Appraisal of Lower Eyelids questionnaire19 and rated the extent to which they were bothered by their appearance (not at all, a little, moderately, or extremely bothered). The FACE-Q questionnaire consists of the following 7 items: excess fat under your eyes, excess skin under your eyes, puffiness under your eyes, how noticeable the lines under your eyes are, crepey (wrinkled) skin under your eyes, how old the area under your eyes makes you look, and how tired the area under your eyes makes you look. Total scores were converted to a scale score (range, 0-100, with higher scores indicating better outcomes) by means of the conversion table in the Appraisal of Lower Eyelids module of the FACE-Q questionnaire.
EI- and Participant-Assessed Responder Rates at Month 3
Aesthetic improvement in the infraorbital area using the 5-point Global Aesthetic Improvement Scale (GAIS; 2 = much improved, 1 = improved, 0 = no change, −1 = worse, −2 = much worse) was assessed by the EI and participants at Months 1, 3, 6, 9, and 12 after the initial treatment for the treatment group and by the EI at Months 1 and 3 for the control group. To perform the assessment, the EI compared the live participant with a photograph taken at baseline. Responders were defined as a score of “improved” or “much improved” on the GAIS. Grading scales and endpoints at Month 3 were identical between the VYC-15L treatment and the no-treatment control arms. The single difference between groups was, for the control group, only the EI-assessed responder rates determined at Months 1 and 3, whereas for the treatment group, both EI- and participant-assessed responder rates were determined at each visit. The participant-assessed endpoints were only relevant for the treatment group, given that participants knew whether they had received treatment or not.
Other PROs
Other PROs included participant responses to questions on treatment satisfaction based on a 5-point scale, ranging from 1 (definitely dissatisfied) to 5 (definitely satisfied; 1 overall score for both eyes); participant responses on the extent to which they were bothered by dark circles under their eyes using a 4-point scale, ranging from 0 (not at all, or I have no dark circles under my eyes) to 3 (very bothered); and participant's willingness to recommend treatment to a friend (yes/no).
Safety
As described previously,13 participants reported procedural pain at each treatment session, based on a scale of 0 (no pain) to 10 (worst pain imaginable). In addition, 30-day electronic participant diaries were used to capture the incidence, severity, and duration of injection-site responses (ISRs) following each treatment, and adverse events (AEs) were monitored throughout the study.
Statistical Analyses
EI-assessed GAIS analysis was performed on the modified intent-to-treat (mITT) population. The mITT population comprised all randomized participants who received at least 1 VYC-15L treatment (treatment group), as well as a baseline and at least 1 posttreatment assessment of the primary effectiveness endpoint (control and treatment groups). Analyses of participant responses (FACE-Q questionnaire, treatment satisfaction questionnaire, dark circles under eyes, participant willingness to recommend treatment) were performed on the VYC-15L initial treatment (VIT) population. The VIT population comprised all randomized participants who received VYC-15L treatment at the beginning of the 3-month control period. Participant responses on the FACE-Q questionnaire (Month 3 compared with baseline) were analyzed with a 2-sided paired t test at the 5% level. Other participant responses were presented as descriptive statistics. Statistical analyses were conducted with SAS v. 9.3 or newer.
RESULTS
Participant Disposition and Demographics
Of 163 participants screened, 140 were randomly assigned to the groups after screening. A total of 133 participants completed the primary endpoint; 124 participants completed the study. Fifteen participants did not complete the study for the following reasons: study withdrawal (n = 6), lost to follow-up (n = 8), and other reasons (n = 1). One participant who failed screening was randomly assigned to a group in error but did not receive treatment. Of the 15 subjects who discontinued after randomization, 10 were in the VYC-15L treatment group. Subjects' reasons for study withdrawal or loss to follow-up were not recorded. Seven of the 10 VYC-15L–treated participants completed Month ≥3 visits, and therefore had GAIS data available. These subjects all had GAIS scores of “improved” (2 subjects) or “much improved” (5 subjects) at the last time point available (Month 3 for 1 subject and Months 6 and 9 for 3 subjects each). Hence, lack of efficacy was unlikely to be the reason these subjects discontinued the study.
The analysis populations were as follows: mITT (n = 135; n = 103 for treatment group and n = 32 for control group), VIT (n = 105, treatment group only), and safety (n = 139; n = 105 for treatment group and n = 34 for control group). In the treatment group, 105 participants received initial treatment, 37 of whom received repeat treatment. In the control group, 29 participants received optional treatment at the end of the no-treatment control period. One control participant was treated at randomization and was counted as part of the control group for effectiveness analyses.
The overall median age at study entry for the mITT population was 47 years (range, 23-68 years).13 The majority of participants in the mITT group were female (91.9%). Participants had baseline AIHS scores of moderate (40%) or severe (60%).
Injection Volumes
For initial treatment, the median volume of VYC-15L injected was 1.5 mL (0.7 mL each for the left and right infraorbital areas), with a range for total volume of 0.3 to 2.2 mL. For touch-up treatment, the median volume of VYC-15L injected was 1.0 mL (0.5 mL each for the left and right infraorbital areas), ranging from 0.1 to 2.2 mL. Total median volume of VYC-15L injected (initial and touch-up treatments) was 2.1 mL (range, 0.3-4.4 mL), and the median volume injected during the repeat treatment was 1.3 mL (range, 0.3-2.2 mL).
Effectiveness Endpoints
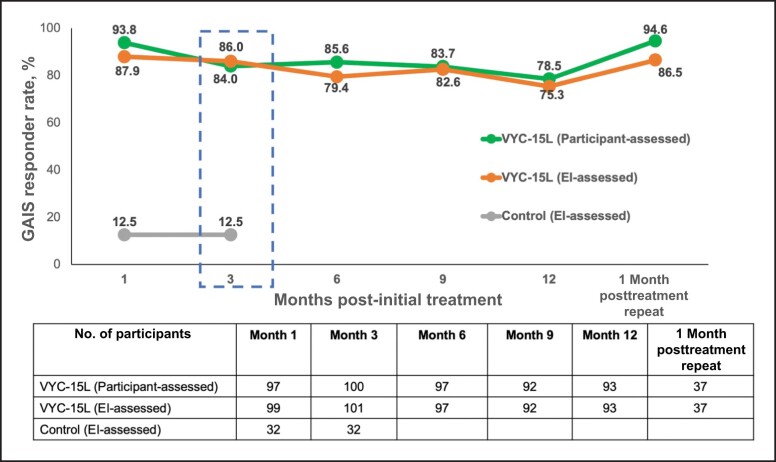
At Month 3, the VYC-15L treatment group had higher EI-rated GAIS responder rates at 86.0% (95% CI, 77.6%-92.1%) compared with the no-treatment control group at 12.5% (95% CI, 3.5%-29.0%; Figure 2). At Month 3, participant-rated GAIS was 84.0% (95% CI, 75.3%-90.6%). Figure 3 illustrates representative results obtained from participants; selected images show participants receiving VYC-15L injection volumes just above the median volumes and are representative of typical results.
Figure 2.
Responders with a score of “improved” or “much improved” on the GAIS. Data for the treatment group are from the VYC-15L initial treatment population (Months 1-12) and the VYC-15L repeat treatment population (1 month posttreatment repeat). Data from the control group are from the modified intent-to-treat population. EI, evaluating investigator; GAIS, Global Aesthetic Improvement Scale.
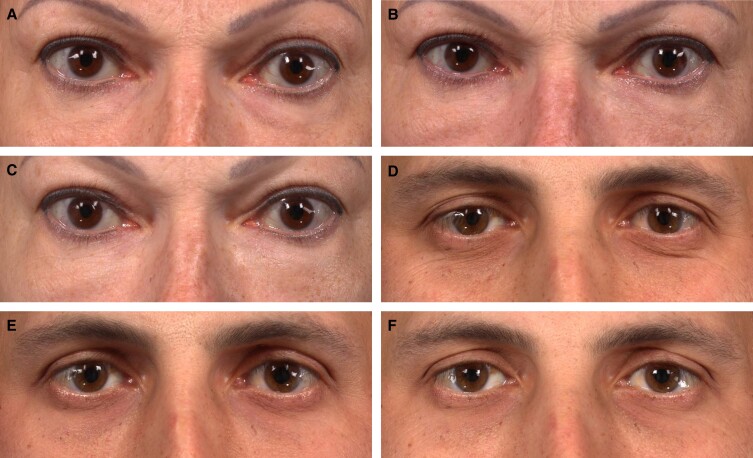
Figure 3.
Participants achieving a 3-point improvement on the AIHS following treatment with VYC-15L. A 53-year-old female participant at (A) baseline with AIHS score = 3 (severe, both sides), at (B) Month 3 with AIHS score = 1 (none, left side) and 0 (none, right side), and at (C) Month 12 with AIHS score = 0 (none, both sides) following initial treatment volume of 0.9 mL (left side) and 0.9 mL (right side) total. A 38-year-old male participant at (D) baseline with AIHS score = 3 (severe, both sides), at (E) Month 3 with AIHS score = 0 (none, both sides), and at (F) Month 12 with AIHS score = 0 (none, both sides) following initial + touch-up treatment volume of 1.1 mL (left) and 1.2 mL (right) total. AIHS, Allergan Infraorbital Hollow Scale.
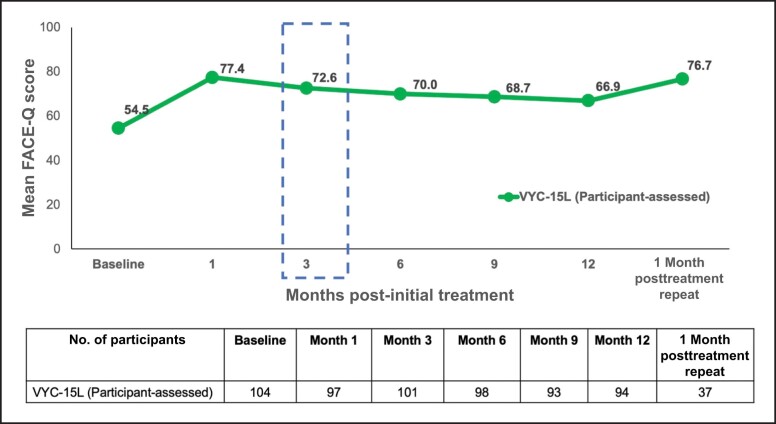
For the VYC-15L treatment group, the mean FACE-Q score at Month 3 was 72.6 (95% CI, 75.3-90.6; Figure 4). The mean change from baseline to Month 3 score (32.7% increase) showed statistically significant improvement (mean [standard deviation], 17.8 [19.8], P < .0001). At Month 12, the FACE-Q score improved by a mean 11.5 [19.6] points from baseline. For participants who received repeat treatment, the FACE-Q score improved by a mean of 19.7 [21.6] points from baseline.
Figure 4.
Participant-assessed FACE-Q appraisal of lower eyelids at baseline and following VYC-15L treatment. Data from baseline and Months 1 to 12 are from the VYC-15L initial treatment population, and data from the 1-month posttreatment repeat are from the VYC-15L repeat treatment population.
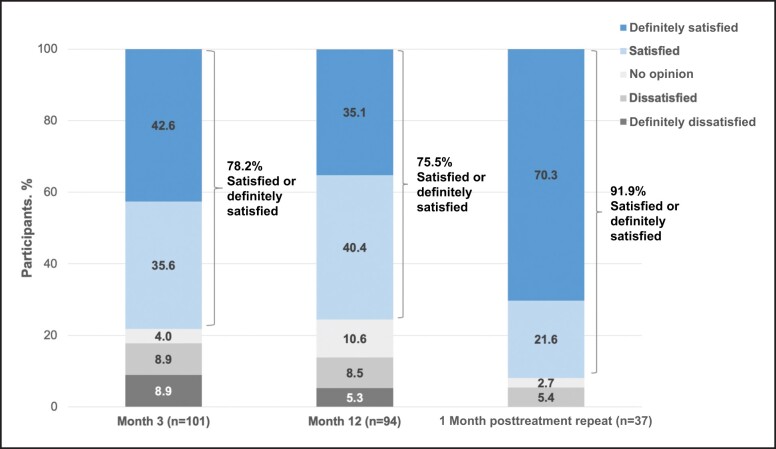
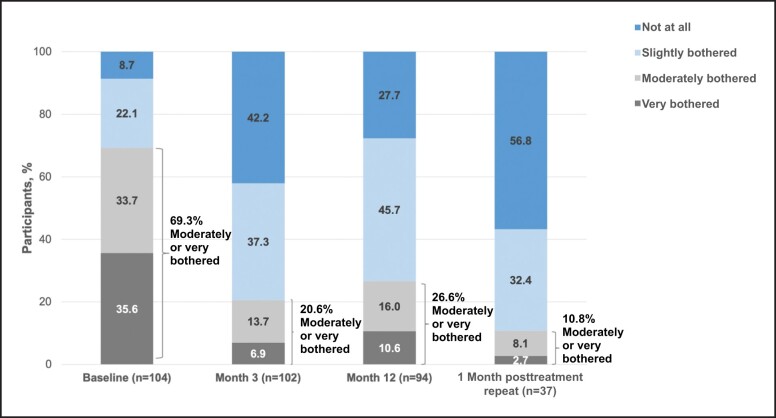
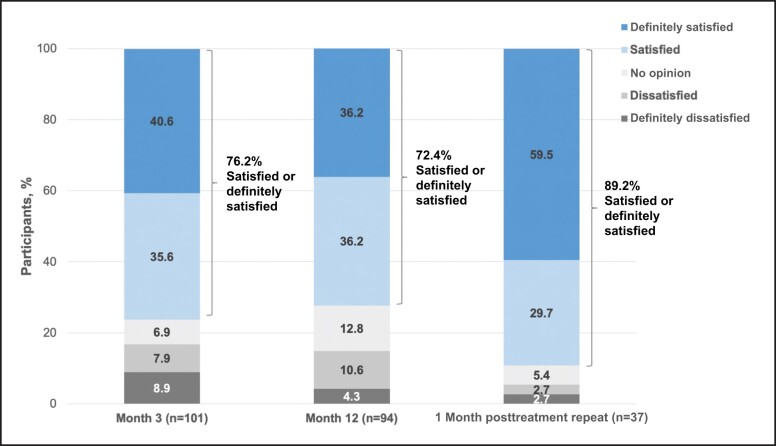
At Month 3 and Month 12 posttreatment, as well as 1 month after repeat treatment, the majority of participants (>72%) reported feeling satisfied with treatment results (Figures 5-7). Compared with baseline, the percentage of participants reporting being “moderately bothered” or “very bothered” by the dark circles under their eyes decreased to <30% at follow-up visits (Figure 8). The majority of participants (>88%) also would recommend treatment to a friend at Month 3 and Month 12 posttreatment, as well as 1 month after repeat treatment. Subgroup analyses of the primary effectiveness endpoint (AIHS responder rate at Month 3) are summarized in Table 1.
Figure 5.
Participant responses to the following question: how satisfied or dissatisfied are you with the overall result of the treatment? Data from Months 3 and 12 are from the VYC-15L initial treatment population, and data from the 1-month posttreatment repeat are from the VYC-15L repeat treatment population.
Figure 7.
Participant responses to the following question: how satisfied or dissatisfied are you with how natural the area under your eyes feels today? Data from Months 3 and 12 are from the VYC-15L initial treatment population, and data from the 1-month posttreatment repeat are from the VYC-15L repeat treatment population.
Figure 8.
Participant responses to the following question: how much have you been bothered by dark circles under your eyes in the last week? Data from baseline, Month 3, and Month 12 are from the VYC-15L initial treatment population, and data from the 1-month posttreatment repeat are from the VYC-15L repeat treatment population.
Table 1.
Subgroup Analyses for AIHS Responder Rates at Month 3 (mITT Population)
| Characteristic | VYC-15L | Control |
|---|---|---|
| Sex, n (%) | ||
| Female | 76/91 (83.5) | 5/31 (16.1) |
| Male | 8/10 (80.0) | 0/1 (0.0) |
| Race, n (%) | ||
| Nonwhite | 20/22 (90.9) | 0/5 (0.0) |
| White | 64/79 (81.0) | 5/27 (18.5) |
| Fitzpatrick skin type subset, n (%) | ||
| I/II | 29/34 (85.3) | 0/11 (0.0) |
| III/IV | 40/50 (80.0) | 5/18 (27.8) |
| V/VI | 15/17 (88.2) | 0/3 (0.0) |
| Baseline AIHS score, n (%) | ||
| Moderate | 34/39 (87.2) | 2/14 (14.3) |
| Severe | 50/62 (80.6) | 3/18 (16.7) |
AIHS, Allergan Infraorbital Hollows Scale; mITT, modified intent-to-treat.
Figure 6.
Participant responses to the following question: how satisfied or dissatisfied are you with how natural the area under your eyes looks today? Data from Months 3 and 12 are from the VYC-15L initial treatment population, and data from the 1-month posttreatment repeat are from the VYC-15L repeat treatment population.
Safety
As reported previously,13 mean procedural pain scores immediately after treatment were ≤1.7 for initial, touch-up, and repeat treatments, indicating minimal pain. ISRs in the VYC-15L treatment group occurred in 56.3% (58/103) and 50.0% (32/64) of participants after initial and touch-up treatments, respectively, with the majority being mild and resolving in ≤1 week. The most frequently reported ISRs were tenderness, bruising, and swelling following initial and touch-up treatments, and tenderness, pain after injection, firmness, and swelling following repeat treatment. Incidence of ISRs in the VYC-15L treatment group was generally higher with cannula injections (n = 58) vs needle (n = 45), particularly for swelling (51.6% vs 28.8%, respectively), tenderness (56.9% vs 40.0%), and firmness (43.1% vs 24.4%).
Treatment-emergent AEs (TEAEs) were reported in 34 participants overall: (28 [26.7%] from the initial VYC-15L treatment group; 6 [20.7%] from the control group receiving optional treatment), with 14 (10.3%) reporting treatment-related TEAEs (mild in intensity, resolving without sequelae). The most common treatment-related TEAEs (occurring in ≥2% of participants) in the initial treatment group were injection site bruising (3.8%) and swelling/edema (2.9%). A total of 3 participants experienced treatment-related late-onset (>30 days posttreatment) TEAEs of swelling or edema, 2 of which resolved within 4 days and 1 of which lasted 45 days (resolving with oral antibiotics; reported as injection site swelling rather than infection based on the medical judgment of the treating investigator). There were no treatment-related serious AEs, AEs of special interest, delayed-onset granulomas, unanticipated adverse device effects, or deaths during the study.
DISCUSSION
The current analysis demonstrates the benefits of VYC-15L for reducing infraorbital hollows/tear troughs. Participants treated with VYC-15L showed improvements in EI- and participant-assessed GAIS, as well as the FACE-Q Appraisal of Lower Eyelids at Month 3. Subgroup analyses by gender, race, Fitzpatrick skin type, and baseline AIHS severity revealed similar improvements in the AIHS at Month 3. Furthermore, the majority of participants were satisfied with treatment results over 1 year and would recommend VYC-15L treatment to a friend. Although the 10.7% discontinuation rate for this study was higher than reported in a previous study of VYC-15L for treatment of infraorbital hollowing (with a discontinuation rate of 6.25%),5 there are few prospective studies of treatment for infraorbital hollowing on which to base a determination of expected discontinuation rates. Because the calculated sample size for this study accounted for a 20% dropout rate, the study was sufficiently powered to provide meaningful results at the observed rate of study completion.
Based on the results of the current clinical study, several considerations may affect treatment outcomes. Full infraorbital hollowing correction is not necessary for high treatment satisfaction. In addition to managing participant expectations that full correction is not usually possible, clinicians must take care not to overfill the area.20 In the current study, the median volume of VYC-15L injected during initial treatment was 0.7 mL per side. Although the maximum allowable volume was 2.2 mL per side, this volume is not what the average person likely needs. Moreover, the AIHS responder rate at Month 3 was lower for participants who received more than the median volume,13 supporting the importance of strategic volume placement over quantity. Despite the AIHS measurements showing a decrease over time in responders,13 satisfaction with treatment was high throughout the study. This observation can be partially attributed to the stricter definition of responder rates for AIHS (both tear troughs need to show at least a 1-point improvement from baseline). Therefore, it is possible that minor changes can have a large impact even on partial responders. The current study underscores how PROs can provide a comprehensive picture of the impact of treatment on participant satisfaction and quality of life.21,22 Prospective studies with dermal fillers (eg, Juvéderm Volite [VYC-12], Juvéderm Voluma XC [VYC-20L]) for other indications have also leveraged PRO instruments (eg, FACE-Q scale and GAIS) to determine treatment effectiveness and participant satisfaction.23,24
Based on EI-assessed responder rates, the duration of VYC-15L effectiveness in the tear troughs is longer compared with more dynamic areas, such as the lips.16,25,26 Glaser et al also found that the duration of effectiveness with another dermal filler (VYC-20L) differed among midface regions, and these variations may be related to the degree of muscle activity.27
Appropriate participant selection is critical to achieving successful outcomes.20,28 For example, potential candidates for injectable filler treatments should possess good skin tone, minimal skin laxity, and mild-to-moderate infraorbital hollowing. In contrast, individuals with orbital fat prominence and pronounced skin laxity may benefit from lower eyelid blepharoplasty.20,28
Patients may have the perception that under-eye rejuvenation with fillers is a more cost-effective alternative to surgery (blepharoplasty). However, it is crucial to counsel patients that fillers are not an alternative if their corrective needs necessitate a blepharoplasty. For instance, infraorbital volume loss in the presence of a herniated infraorbital fat pad differentiates a surgical vs nonsurgical corrective need.3 In this case, restoring volume with a filler alone will not address the herniated fat, excess skin, or laxity of eyelid tissue.3,20 Rather, the ideal candidate for rejuvenation of the infraorbital area with fillers is a patient with infraorbital volume loss, which may or may not include an associated groove or step along the lid-cheek junction. However, if a patient does not wish to undergo surgery or is not a good surgical candidate, under-eye dermal fillers can be considered if the main treatment goal is to improve infraorbital hollowing and not to reduce orbital prominence. Understanding the overall clinical background and motivations of potential patients (eg, preferences for surgical vs nonsurgical procedures) are important considerations for selecting the best treatment option.
In the current study,13 participant-assessed pain during injection was minimal. Participant-reported ISRs, including tenderness to touch, bruising, and swelling, were mild to moderate and resolved within 1 week. The most common treatment-related AEs were bruising and swelling/edema at the injection site. One participant experienced injection site swelling that lasted longer than 30 days, but resolved with oral antibiotic treatment. Therefore, in the clinical setting, physicians should prepare their patients to expect potential bruising and swelling because the infraorbital region is a highly vascular area. Other treatment-related AEs occurred in less than 2% of the participants, were mild in severity, and resolved without sequelae. The safety profile of VYC-15L in the current study was comparable with that of previous studies evaluating this product for the treatment of infraorbital hollowing, as well as previous trials of VYC-15L for the treatment of the lips and perioral area.1,5,16,29
During aging, infraorbital hollows are secondary to midface volume loss.30-32 Treatment paradigms for midface volumizing include injectable fillers.33 A PRO analysis from the pivotal clinical study demonstrating safety and effectiveness of VYC-20L for age-related midface volume deficit reported overall participant satisfaction with facial appearance for up to 2 years.24 More participants also reported being satisfied with adjacent midfacial areas, including the tear troughs. Compared with baseline, there was a 37.5% increase in the proportion of subjects who reported feeling satisfied with their tear troughs at 6 months after treatment.24 These results demonstrated how treatment of the midface alone positively impacted the under-eye area. In the current study, substantial volume loss in the midface was an exclusion criterion. However, some of the participants enrolled in this study may have been candidates for a 2-step approach: midface correction prior to treatment of infraorbital hollows. In clinical practice, the authors suggest that treating the midface first, which can restore structural support to the medial cheek compartment, benefits the tear troughs.24 If comprehensive periorbital filler treatment encompassing the tear troughs, cheeks, and midface is performed, the total volume of under-eye filler needed may be less than used to specifically address the tear troughs.
There were some limitations in the current study. The participants enrolled were predominantly female (91.9%) and white (80%) with Fitzpatrick skin types I/II and III/IV (34.1% and 51.1%, respectively, vs 14.8% for types V/VI). The current study also had limited repeat treatment data (n = 37). However, because tear trough correction was maintained for most participants at 12 months, they were not eligible for repeat treatment at that time and hence the study was concluded for these subjects (Supplemental Table). Additional studies with longer follow-up periods would be required to more clearly show the durability of effect and lasting benefits of repeat treatments. Finally, as this was a pivotal study designed to assess the safety and efficacy of VYC-15L treatment to the infraorbital hollow area in preparation for a label expansion, treatment was limited to this area even though in clinical practice a global approach would normally be considered, correcting volume loss in the adjoining midface and cheek area as needed. As a result, treating investigators may have utilized a larger volume of VYC-15L in the infraorbital area than would be expected in routine clinical practice for moderate to severe infraorbital hollowing.13 Full frontal images were obtained for each participant, but three-quarter or lateral views were not collected in this study as this was not a per-protocol, planned analysis. Nevertheless, the authors do not believe that the clinical examples shown in Figure 3 demonstrate overcorrection.
CONCLUSIONS
Based on validated PRO measurements, the current analysis demonstrates the effectiveness of VYC-15L treatment to reduce infraorbital hollowing and to improve overall satisfaction, with results lasting for 1 year. Improvements from the participant perspective were aligned with EI-assessed improvements.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Supplementary Material
Acknowledgments
The authors acknowledge the additional treating and evaluating investigators who contributed to study conduct: John Fezza and Kalie Kosek (Sarasota, FL); Mary Lupo and Skylar Souyoul (New Orleans, LA); Suzanne Bruce, April Harrison, and Pauline Scott (Houston, TX); Megan Kera (Montclair, NJ); Michael Newman and Richard Zoumalan (Beverly Hills, CA); Leysin Fletcher (San Diego, CA); Melanie Palm and Amanda Abramson Lloyd (Solana Beach, CA); Allison Pontius and Keimun Slaughter (Latham, NY); Sue Ellen Cox and John Soderberg (Chapel Hill, NC); Brian Biesman and Larry Young (Nashville, TN); Suneel Chilukuri and James Boynton (Bellaire, TX); Steven Cohen and Ashley Goodacre (San Diego, CA); Sherly Soleiman (Newport Beach, CA); Julie Woodward, Charles Ryan Woodard, Roshni Ranjit Reeves, and Ilya Leyngold (Durham, NC); and Paul Afrooz (Boca Raton, FL).
Disclosures
Dr Fabi is an investigator for Allergan Aesthetics, an AbbVie Company (Irvine, CA). Dr Zoumalan is a consultant and clinical investigator for Allergan Aesthetics, an AbbVie Company. Dr Fagien is a consultant and clinical investigator for Allergan Aesthetics, an AbbVie Company. Dr Downie is a consultant, clinical investigator, and speaker for Allergan Aesthetics, an AbbVie Company. Dr Yoelin is a consultant and clinical investigator for Allergan Aesthetics, an AbbVie Company. Drs Sartor and Chawla are employees of Allergan Aesthetics, an AbbVie Company, and own stock.
Funding
This study was sponsored by Allergan Aesthetics, an AbbVie Company (Irvine, CA). Writing and editorial assistance was provided to the authors by Maria Lim, PhD of Peloton Advantage, an OPEN Health company (Parsippany, NJ) and was funded by Allergan Aesthetics. Neither honoraria nor other form of payment was made for authorship. The sponsor was involved in the study design, patient enrollment, collection and assembly of data, data analysis and interpretation, manuscript review and revisions, and final approval of manuscript (as noted below in terms of the role of authors Smita Chawla and Marta Sartor).
REFERENCES
- 1. Iera M. Treatment of infraorbital skin depressions using the hyaluronic acid filler VYC-15L based on the MD codes approach: a retrospective analysis. J Clin Exp Dermatol Res. 2020;11(4):526. doi: 10.35248/2155-9554.20.11.526 [DOI] [Google Scholar]
- 2. Haddock NT, Saadeh PB, Boutros S, Thorne CH. The tear trough and lid/cheek junction: anatomy and implications for surgical correction. Plast Reconstr Surg. 2009;123(4):1332–1340. doi: 10.1097/PRS.0b013e31819f2b36 [DOI] [PubMed] [Google Scholar]
- 3. Zoumalan CI, Roostaeian J. Simplifying blepharoplasty. Plast Reconstr Surg. 2016;137(1):196e–213e. doi: 10.1097/prs.0000000000001906 [DOI] [PubMed] [Google Scholar]
- 4. Hall MB, Roy S, Buckingham ED. Novel use of a volumizing hyaluronic acid filler for treatment of infraorbital hollows. JAMA Facial Plast Surg. 2018;20(5):367–372. doi: 10.1001/jamafacial.2018.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niforos F, Acquilla R, Ogilvie P, et al. A prospective, open-label study of hyaluronic acid-based filler with lidocaine (VYC-15L) treatment for the correction of infraorbital skin depressions. Dermatol Surg. 2017;43(10):1271–1280. doi: 10.1097/dss.0000000000001127 [DOI] [PubMed] [Google Scholar]
- 6. Narurkar V, Shamban A, Sissins P, Stonehouse A, Gallagher C. Facial treatment preferences in aesthetically aware women. Dermatol Surg. 2015;41(suppl 1):S153–S160. doi: 10.1097/DSS.0000000000000293 [DOI] [PubMed] [Google Scholar]
- 7. Vrcek I, Ozgur O, Nakra T. Infraorbital dark circles: a review of the pathogenesis, evaluation and treatment. J Cutan Aesthet Surg. 2016;9(2):65–72. doi: 10.4103/0974-2077.184046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fagien S, Fagien S. Lower blepharoplasty: blending the lid/cheek junction with orbicularis muscle and lateral retinacular suspension. In: Putterman's Cosmetic Oculoplastic Surgery, 4th ed.W.B. Saunders; 2008:161–179. [Google Scholar]
- 9. Lipp M, Weiss E. Nonsurgical treatments for infraorbital rejuvenation: a review. Dermatol Surg. 2019;45(5):700–710. doi: 10.1097/dss.0000000000001897 [DOI] [PubMed] [Google Scholar]
- 10. Pak CS, Lee YK, Jeong JH, Kim JH, Seo JD, Heo CY. Safety and efficacy of Ulthera in the rejuvenation of aging lower eyelids: a pivotal clinical trial. Aesthetic Plast Surg. 2014;38(5):861–868. doi: 10.1007/s00266-014-0383-6 [DOI] [PubMed] [Google Scholar]
- 11. Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13(9):1030–1036. doi: S1545961614P1030X [PubMed] [Google Scholar]
- 12. Viana GA, Osaki MH, Cariello AJ, Damasceno RW, Osaki TH. Treatment of the tear trough deformity with hyaluronic acid. Aesthet Surg J. 2011;31(2):225–231. doi: 10.1177/1090820X10395505 [DOI] [PubMed] [Google Scholar]
- 13. Fabi S, Zoumalan C, Fagien S, Yoelin S, Sartor M, Chawla S. A prospective, multicenter, single-blind, randomized, controlled study of VYC-15L, a hyaluronic acid filler, in adults for correction of infraorbital hollowing. Aesthet Surg J. 2021;41(11):NP1675–NP1685. doi: 10.1093/asj/sjab308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(5 suppl):139S–148S. doi: 10.1097/prs.0000000000001734 [DOI] [PubMed] [Google Scholar]
- 15. Weinkle SH, Bank DE, Boyd CM, Gold MH, Thomas JA, Murphy DK. A multi-center, double-blind, randomized controlled study of the safety and effectiveness of Juvéderm injectable gel with and without lidocaine. J Cosmet Dermatol. 2009;8(3):205–210. doi: 10.1111/j.1473-2165.2009.00451.x [DOI] [PubMed] [Google Scholar]
- 16. Geronemus RG, Bank DE, Hardas B, Shamban A, Weichman BM, Murphy DK. Safety and effectiveness of VYC-15L, a hyaluronic acid filler for lip and perioral enhancement: one-year results from a randomized, controlled study. Dermatol Surg. 2017;43(3):396–404. doi: 10.1097/DSS.0000000000001035 [DOI] [PubMed] [Google Scholar]
- 17. Juvéderm Volbella XC [directions for use]. Irvine: Allergan; 2021. [Google Scholar]
- 18. Donofrio L, Carruthers J, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of infraorbital hollows. Dermatol Surg. 2016;42(suppl 1):S251–S258. doi: 10.1097/DSS.0000000000000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klassen AF, Cano SJ, Grotting JC, et al. FACE-Q eye module for measuring patient-reported outcomes following cosmetic eye treatments. JAMA Facial Plast Surg. 2017;19(1):7–14. doi: 10.1001/jamafacial.2016.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125(2):699–708. doi: 10.1097/PRS.0b013e3181c82f90 [DOI] [PubMed] [Google Scholar]
- 21. US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research . Guidance for Industry: Upper Facial Lines: Developing Botulinum Toxin Drug Products. 2014. Accessed May 9, 2022. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm407983.pdf.
- 22. US Food and Drug Administration . Value and Use of Patient-Reported Outcomes (PROs) in Assessing Effects of Medical Devices (CDRH Strategic Priorities 2016-2017). 2019. Accessed May 9, 2022. https://www.fda.gov/files/about%20fda/published/Value-and-Use-of-Patient-Reported-Outcomes-%28PROs%29-in-Assessing-Effects-of-Medical-Devices.pdf.
- 23. Ogilvie P, Safa M, Chantrey J, et al. Improvements in satisfaction with skin after treatment of facial fine lines with VYC-12 injectable gel: patient-reported outcomes from a prospective study. J Cosmet Dermatol. 2020;19(5):1065–1070. doi: 10.1111/jocd.13129 [DOI] [PubMed] [Google Scholar]
- 24. Few J, Cox SE, Paradkar-Mitragotri D, Murphy DK. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35(5):589–599. doi: 10.1093/asj/sjv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eccleston D, Murphy DK. Juvéderm® Volbella in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167–172. doi: 10.2147/CCID.S35800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14(12):1444–1452. doi: S1545961615P1444X [PubMed] [Google Scholar]
- 27. Glaser DA, Kenkel JM, Paradkar-Mitragotri D, Murphy DK, Romagnano L, Drinkwater A. Duration of effect by injection volume and facial subregion for a volumizing hyaluronic acid filler in treating midface volume deficit. Dermatol Surg. 2015;41(8):942–949. doi: 10.1097/DSS.0000000000000416 [DOI] [PubMed] [Google Scholar]
- 28. Zoumalan CI. Managing periocular filler-related syndrome prior to lower blepharoplasty. Aesthetic Plast Surg. 2019;43(1):115–122. doi: 10.1007/s00266-018-1250-7 [DOI] [PubMed] [Google Scholar]
- 29. Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13(2):125–134. doi: 10.1111/jocd.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pontius AT, Chaiet SR, Williams EF III. Midface injectable fillers: have they replaced midface surgery? Facial Plast Surg Clin North Am. 2013;21(2):229–239. doi: 10.1016/j.fsc.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 31. Gierloff M, Stohring C, Buder T, Gassling V, Acil Y, Wiltfang J. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;129(1):263–273. doi: 10.1097/PRS.0b013e3182362b96 [DOI] [PubMed] [Google Scholar]
- 32. Glaser DA, Lambros V, Kolodziejczyk J, Magyar A, Dorries K, Gallagher CJ. Relationship between midface volume deficit and the appearance of tear troughs and nasolabial folds. Dermatol Surg. 2018;44(12):1547–1554. doi: 10.1097/DSS.0000000000001684 [DOI] [PubMed] [Google Scholar]
- 33. Jones D. Volumizing the face with soft tissue fillers. Clin Plast Surg. 2011;38(3):379–390. doi: 10.1016/j.cps.2011.03.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.