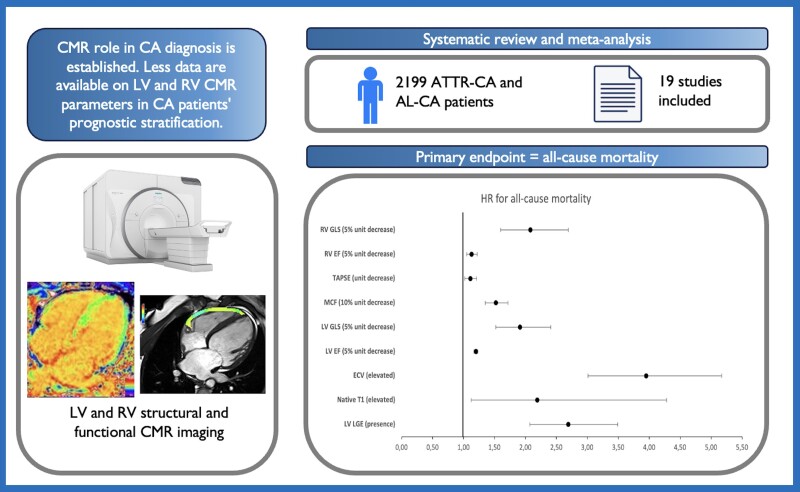
Abstract
Cardiac involvement is the foremost determinant of the clinical progression of amyloidosis. The diagnostic role of cardiac magnetic resonance (CMR) imaging in cardiac amyloidosis has been established, but the prognostic role of various right and left CMR tissue characterization and functional parameters, including global longitudinal strain (GLS), late gadolinium enhancement (LGE), and parametric mapping, is yet to be delineated. We searched EMBASE, PubMed, and MEDLINE for studies analysing the prognostic use of CMR imaging in patients with light chain amyloidosis or transthyretin amyloidosis cardiac amyloidosis. The primary endpoint was all-cause mortality. A random effects model was used to calculate a pooled odds ratio using inverse-variance weighting. Nineteen studies with 2199 patients [66% males, median age 59.7 years, interquartile range (IQR) 58–67] were included. Median follow-up was 24 months (IQR 20–32), during which 40.8% of patients died. Both tissue characterization left heart parameters such as elevated extracellular volume [hazard ratio (HR) 3.95, 95% confidence interval (CI) 3.01–5.17], extension of left ventricular (LV) LGE (HR 2.69, 95% CI 2.07–3.49) elevated native T1 (HR 2.19, 95% CI 1.12–4.28), and functional parameters such as reduced LV GLS (HR 1.91, 95% CI 1.52–2.41) and reduced LV ejection fraction (EF; HR 1.20, 95% CI 1.17–1.23) were associated with increased all-cause mortality. Unlike the presence of right ventricular (RV) LGE (HR 3.40, 95% CI 0.51–22.54), parameters such as RV GLS (HR 2.08, 95% CI 1.6–2.69), RVEF (HR 1.13, 95% CI 1.05–1.22), and tricuspid annular systolic excursion (TAPSE) (HR 1.11, 95% CI 1.02–1.21) were also associated with mortality. In this large meta-analysis of patients with cardiac amyloidosis, CMR parameters assessing RV and LV function and tissue characterization were associated with an increased risk of mortality.
Keywords: Amyloidosis, Cardiac magnetic resonance imaging, MRI, Transthyretin amyloidosis, Light chain
Graphical Abstract
Graphical Abstract.
Introduction
Cardiac amyloidosis (CA) is a rapidly progressive heart disease, with a median survival from diagnosis ranging from <6 months to 5 years for light chain amyloidosis (AL-CA),1 and 3–5 years for transthyretin amyloidosis (ATTR-CA).2 Although reported as an uncommon condition, continuous advancements in cardiac imaging are revealing a non-negligible prevalence of cardiac amyloid deposition in specific populations.3–5 Despite the major differences in precursor proteins, patient demographics, and clinical course, both types of patients with CA should undergo an early diagnostic and prognostic evaluation to initiate treatment and slow disease progression.
In this setting, cardiac magnetic resonance (CMR) imaging deserves a major role in functional and tissue characterization assessment of patients and represents a cornerstone for CA diagnosis with a sensitivity and specificity that approaches 85–90%, with even higher sensitivity using native T1 mapping and extracellular volume (ECV) fraction.6,7 Despite the extensive use of CMR imaging for CA diagnosis, its prognostic value has not been well established, with several studies yielding mixed results. Although widely used CMR parameters such as late gadolinium enhancement (LGE) presence and ECV demonstrated a role in prognostic stratification of patients with CA,8,9 sparse data are available comparing different CMR imaging modalities and biventricular assessment. The aim of this meta-analysis was to summarize and compare comprehensive prognostic information derived from CMR imaging.
Methods
Eligibility criteria
We included studies assessing patients with CA having had CMR with available follow-up data according to all-cause mortality. Studies including mixed cardiomyopathy populations, including patients with ischaemic or non-ischaemic cardiomyopathies, and whose results for patients with CA were not provided separately were excluded. Patient, intervention, control, outcome, timing and setting is presented in Supplementary material online, Table S1.
Search strategy
EMBASE and MEDLINE were searched (March 2023) for studies assessing the prognosis of patients with known or suspected CA. The keywords used in the search were (‘prognosis’ OR ‘outcome’) AND (‘cardiac amyloidosis’ OR ‘systemic amyloidosis’) AND (‘delayed gadolinium enhancement’ OR ‘late gadolinium enhancement’ OR ‘cardiac MRI’ OR ‘CMR’). The literature was systematically reviewed using Rayyan. Literature screening was based on title, abstract, and application of exclusion criteria (i.e. non-English language, cardiomyopathies not included within the scope of our study, case reports, and literature reviews). Full-text screening was performed, and studies that evaluated CMR prognostic information with mortality follow-up data were included. Hazard ratios (HRs) for each parameter were extracted from multivariable analysis if available; if not available, univariate HRs were included. For variables showing different cut-offs in different studies due to different MR protocols or scanners (e.g. native T1 and ECV), we performed a comparison between ‘elevated’ vs. ‘normal’ modality based on specific cut-offs reported in the paper of interest. All excluded studies were selected on the basis of consensus of the authors. We performed our systematic review and meta-analysis in compliance with the guidelines outlined in the Meta-analysis of Observational Studies in Epidemiology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The risk of bias was assessed using the Newcastle Ottawa Scale, and a low risk was defined as a Newcastle Ottawa Scale score ≥7.10
Data extraction was carried out independently (N.H.P., K.P., M.R., and M.S.) in order to populate demographic tables such as author and year study publication, number of participants per study, number of deaths per study, mean participant age, gender distribution, mean follow-up period length, CMR scanner model, amyloid classification (AL, ATTR), cardiac amyloid validation technique, LGE criteria, ejection fraction (EF), New York heart association functional class, mean N-Terminal-pro B-type Natriuretic Peptide (NTproBNP), and CMR data modality [global longitudinal strain (GLS), native T1, and ECV]. The authors were requested access to raw data where unavailable in the study publication. The prognostic value of different modalities of CMR imaging in all-cause mortality was the outcome of interest. The following groups of parameters related to CMR modalities were analysed: left ventricular (LV) parameters [ECV, native T1 mapping, LV LGE, LVEF, LV GLS, and LV myocardial contraction fraction (MCF)] and right ventricular (RV) parameters [tricuspid annular systolic excursion (TAPSE), RVEF, RV LGE, and RV GLS].
Statistical analysis
Continuous variables were reported as mean ± standard deviation or median and interquartile range (IQR) as appropriate. Dichotomous variables were reported as counts and percentages. Due to the observational nature of most included studies, a random effects model was used to calculate a pooled HR using inverse-variance weighting.11 Hazard ratios were included in the analysis as binary HR when available. For studies presenting only continuous HR, we converted continuous HR to homogeneous fold increase HR if different unit increases were used in the included studies. If subgroup data were not available (e.g. ECV and T1 in studies by Wan et al.12 and Kotecha et al.13), pooled binary HRs of the same studies were obtained from meta-analysis by Pan et al.,14 if available.
Heterogeneity between studies was assessed both visually from the forest plots of individual parameters and using Cochran’s Q index and Higgins I2 statistics. Significant heterogeneity was defined as having both a significant Cochran’s Q (P < 0.05) and I2 > 50%. A sensitivity analysis was performed by rerunning the analysis, excluding one study at a time and reassessing heterogeneity to obtain an I2 < 50%. Publication bias was evaluated by funnel plot examination. Statistical significance for hypothesis testing was set at alpha < 0.05, two-tailed level. Statistical analyses were performed using R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Literature search
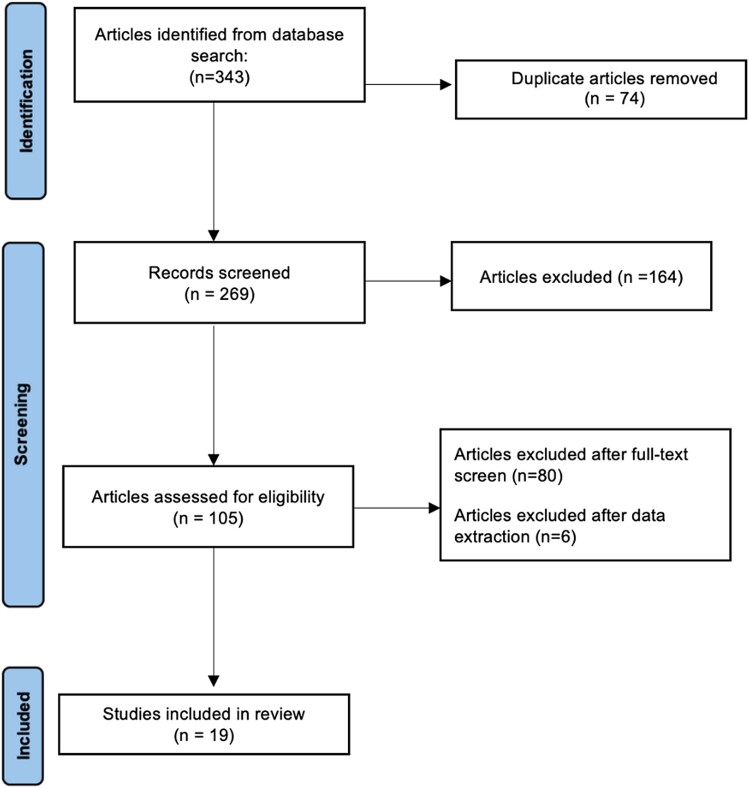
Following our literature search, 269 non-duplicate citations were retrieved. Following title and abstract screening along with the application of exclusion criteria, 164 articles were excluded. An additional 80 articles were excluded due to impertinence of data or primary study outcomes. Of the 25 included articles, 6 were excluded during data extraction because of incomplete data. For one recent study,15 ECV and native T1 data were excluded, as HRs per subgroup analysis were not reported and the data were not comparable with other included studies. Figure 1 shows the study selection. A total of 19 prognostic studies with 2199 patients were included in the final data set. The overall risk of bias assessed by the Newcastle Ottawa Scale was estimated to be low in all the included studies (see Supplementary material online, Table S2).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart and study selection.
Baseline studies and pooled patients’ characteristics
The baseline characteristics for each study are reported in Tables 1 and 2, while Supplementary material online, Table S3 shows the pooled baseline characteristics of included patients. Among 2199 patients, 66% were males, median age was 59.7 (IQR 58–67) years, and median EF was 57.5% (IQR 54.7–61.5). Thirty-six per cent of patients had a NYHA functional class >II, and median NTproBNP was 2020 ng/L (IQR 225–3381). The median follow-up was 24 months (IQR 20–32). The overall death rate was 40.8% (N = 898 deaths). Four studies19–22 included patients with both AL and ATTR amyloidosis, while only the Martinez-Naharro et al.23 population included all patients with ATTR. The remaining studies only included patients with AL amyloidosis. No data on amyloid type were available in two studies.24,25
Table 1.
Summary of patients characteristics, amyloidosis classification, and follow-up
| First author, year | Study type | N | Age (years) | Male (%) | Deaths | Follow-up (months) | Amyloid type | AL included proportion (%) | Cardiac involvement validation |
|---|---|---|---|---|---|---|---|---|---|
| Banypersad, 201416 | Prospective cohort | 100 | 62 | 67 | 25 | 23 | AL | 100 | Echo |
| White, 2014 | Prospective cohort | 90 | 62 | 58 | 50 | 29 | AL and ATTR | — | Echo |
| Fontana, 2015 | Prospective cohort | 250 | 67 | 68 | 67 | 24 | AL and ATTR | 48 | EMB |
| Bhatti, 2016 | Prospective cohort | 251 | 63 | 64 | 97 | 28 | AL | 100 | EMB, CMR |
| Baroni, 2017 | Retrospective cohort | 42 | 58 | 74 | 31 | 37 | AL and ATTR | — | EMB, CMR |
| Ochs, 2017 | Retrospective cohort | 68 | 58 | 59 | 44 | 14 | AL | 100 | EMB |
| Illman, 2018 | Retrospective cohort | 76 | 60 | 67 | 52 | 20 | AL | 100 | CMR |
| Kotecha, 2018 | Prospective cohort | 100 | 64 | 61 | 28 | 23 | AL | 100 | CMR |
| Lin, 2018 | Prospective cohort | 82 | 56 | 63 | 21 | 8 | AL | 100 | CMR |
| Martinez-Naharro, 2018 | Prospective cohort | 227 | 72 | 80 | 95 | 32 | ATTR | 0 | EMB, nuclear |
| Ridouani, 2018 | Prospective cohort | 44 | 69 | 70 | 18 | 27 | AL and ATTR | 54 | EMB |
| Wan, 2018 | Prospective cohort | 78 | 59 | 59 | 54 | 38 | AL | 100 | CMR, echo |
| Arenja, 201917 | Prospective cohort | 74 | 59 | 67 | 29 | 41 | AL | 100 | CMR |
| Knight, 2019 | Prospective cohort | 322 | 71 | 75 | 90 | 22 | AL and ATTR | 96 | EMB, nuclear, Echo |
| Wan, 2019 | Prospective cohort | 77 | 50 | 66 | 46 | 28 | AL | 100 | CMR |
| Aquaro, 2020 | Prospective cohort | 80 | 70 | 69 | 36 | 36 | AL and ATTR | 47 | EMB, nuclear |
| Li, 2020 | Prospective cohort | 87 | 57 | 64 | 34 | 21 | AL | 100 | EMB |
| Liu, 202018 | Prospective cohort | 64 | 58 | 56 | 37 | 20 | AL | 100 | Echo |
| Tan, 2022 | Retrospective cohort | 87 | 58 | 55 | 44 | 12 | AL | 100 | EMB, CMR |
AL, light chain amyloidosis; ATTR, transthyretin amyloidosis; CMR, cardiac magnetic resonance; EMB, endomyocardial biopsy.
Table 2.
Magnetic resonance imaging sequence, cardiac magnetic resonance imaging parameters, and characteristics of individual studies
| First author, year | LGE criteria | T1 cut-off (ms) | ECV cut-off (%) | LVEF (%) | NYHA functional class >II (%) | LA area in AL population (cm2) | LA area in ATTR population (cm2) |
|---|---|---|---|---|---|---|---|
| Banypersad, 201416 | — | 1044 | 45 | 56 | 15 | 13 ± 3 | — |
| White, 2014 | Global | — | — | 58 | >50 | — | — |
| Fontana, 2015 | Transmural | — | — | 66 | — | 26 ± 5 | 32 ± 5 |
| Bhatti, 2016 | Typical | — | — | 60 | — | — | — |
| Baroni, 2017 | Typical | — | — | 61 | — | — | — |
| Ochs, 2017 | Transmural | — | — | 56 | >50 | — | — |
| Ridouani, 2018 | — | 1092 | 59 | 65 | 50 | — | — |
| Illman, 2018 | Global | — | — | 46 | 29 | — | — |
| Kotecha, 2018 | — | — | 45 | 58 | 15 | — | — |
| Lin, 2018 | Global | 1456 | 44 | 57 | 34 | 21.4 ± 5.0 | — |
| Martinez-Naharro, 2018 | — | 1065 | 53 | 63 | — | 26 ± 5 | 31 ± 8 |
| Wan, 2018 | Transmural | — | — | 56 | — | — | — |
| Arenja, 201917 | Transmural | — | — | 63 | 48 | — | — |
| Knight, 2019 | — | — | — | 51 | — | 13 ± 4 | 16 ± 3 |
| Wan, 2019 | — | 1394 | 44 | 54 | 45 | — | — |
| Aquaro, 2020 | — | — | — | 58 | 36 | — | — |
| Li, 2020 | Global | — | — | — | 29 | — | — |
| Liu, 202018 | Global | — | — | 55 | — | — | — |
| Tan, 2022 | Global | — | — | — | 69 | — | — |
CMR, cardiac magnetic resonance; ECV, extracellular volume; EF, ejection fraction; LGE, late gadolinium enhancement; LV, left ventricle.
Cardiac magnetic resonance imaging characteristics
Among the included studies, 5 out of 19 studies20,24,26,27 analysed patients comparing LGE-positive vs. LGE-negative groups, while 6 studies included transmural LGE subgroup analysis.12,15,23,28–31 Cardiac amyloidosis diagnostic criteria for each included study are presented in Supplementary material online, Table S4. Available T1 mapping and ECV cut-offs are described in Table 2. Significant variability was evident in T1 mapping cut-offs in the included studies, while ECV cut-offs were similar in all studies, with the exception of the paper by Ridouani et al.
Left ventricular parameters
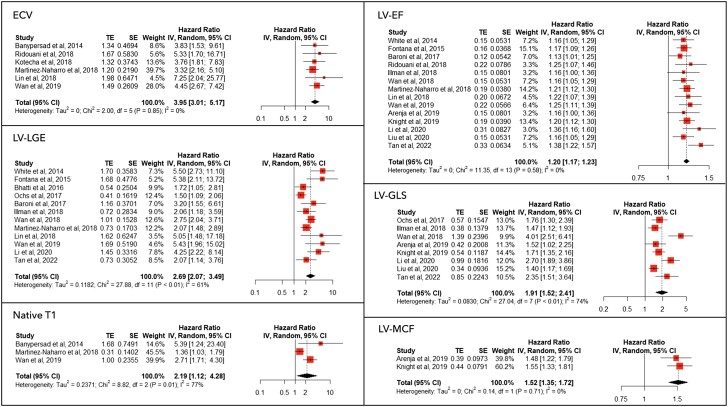
The prognostic meta-analysis results, along with with the forest plots for LV imaging parameters, are shown in Figure 2. Abnormally high ECV, the presence of LV LGE, and elevated native T1 values of HR are presented as binary HR, as reported by the included studies; LVEF and LV-GLS HR are reported as per 5% decrease (less negative values for GLS), while LV-MCF HR is reported as per 10% decrease. On pooled analysis, elevated ECV was associated with an HR of 3.95 [95% confidence interval (CI) 3.01–5.17] for all-cause mortality, without significant heterogeneity, while both the presence of LGE and elevation of native T1 were associated with an increased HR for all-cause death [2.69 (95% CI 2.07–3.49) and 2.19 (95% CI 1.12–4.28), respectively] with moderate-high heterogeneity.
Figure 2.
Meta-analysis results for left ventricular parameters. The forest plots of different anatomical and functional parameters of the left ventricle assessed by cardiac magnetic resonance are shown. Extracellular volume, left ventricular late gadolinium enhancement, and T1 hazard ratio are presented as binary hazard ratios as reported by the included studies; left ventricular ejection fraction and left ventricular global longitudinal strain hazard ratio are reported as per 5% decrease (less negative values for global longitudinal strain); left ventricular myocardial contraction fraction hazard ratio is reported as per 10% decrease.
On sensitivity analysis, the exclusion of the study by Martinez-Naharro et al. significantly reduced native T1 mapping heterogeneity, while the removal of the study by Ochs et al. reduced the heterogeneity of LV LGE (see Supplementary material online, Table S5). Among LV systolic parameters, LVEF, LV GLS, and MCF were associated as continuous variables with increased HRs for all-cause mortality. A sensitivity analysis indicated that the results for LV GLS were not greatly impacted by any one study; HRs were consistent and the lower 95% CI remained >1 when data were reanalysed excluding one study at a time.
A meta-regression was also performed, including age, publication year, follow-up, male sex, LVEF, type of CA, and NTproBNP as covariates. We found that elevated native T1 HR was significantly higher in studies with a higher proportion of AL-type amyloidosis, while a higher prevalence of male sex was associated with lower HR for native T1 (see Supplementary material online, Figures S1 and S2). No significant difference was evident in LV-LGE HR in studies with a higher proportion of AL-type amyloidosis (see Supplementary material online, Figure S3). Also, LV-GLS HRs were lower in studies with higher LVEF (see Supplementary material online, Figure S4). The pooled prognostic performance of LV parameters is summarized in Table 3. A subanalysis of studies including only AL-type amyloid patients was performed, which confirmed the significant association between all the investigated LV parameters and all-cause mortality. The extended results of the AL subanalysis are available in Supplementary material online, Figure S5.
Table 3.
Pooled prognostic performance
| Modality | Studies | n | Hazard ratio (95% CI) |
|---|---|---|---|
| LV LGE (presence) | 12 | 1415 | 2.69 (2.07–3.49) |
| Native T1 (elevated) | 3 | 404 | 2.19 (1.12–4.28) |
| ECV (elevated) | 6 | 630 | 3.95 (3.01–5.17) |
| LVEF (5% unit decrease) | 14 | 1600 | 1.20 (1.17–1.23) |
| LV GLS (5% unit decrease) | 8 | 856 | 1.91 (1.52–2.41) |
| MCF (10% unit decrease) | 2 | 396 | 1.52 (1.35–1.72) |
| TAPSE (unit decrease) | 2 | 390 | 1.11 (1.02–1.21) |
| RVEF (5% unit decrease) | 5 | 671 | 1.13 (1.05–1.22) |
| RV GLS (5% unit decrease) | 2 | 151 | 2.08 (1.60–2.69) |
| RV LGE (presence) | 2 | 151 | 3.40 (0.51–22.54) |
ECV, extracellular volume; EF, ejection fraction; GLS, global longitudinal strain; LGE, late gadolinium enhancement; LV, left ventricle; MCF, myocardial contraction fraction; TAPSE, tricuspid annular systolic excursion; RV, right ventricle.
Right ventricular parameters
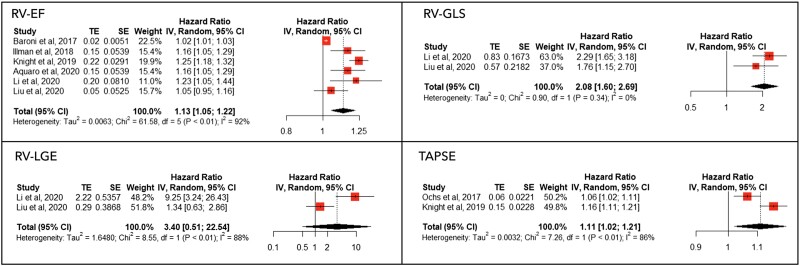
The forest plots of the HR for RV parameters are presented in Figure 3. Right ventricular EF and RV-GLS HR are reported as per 5% decrease (less negative values for GLS); TAPSE HR is reported as per unit decrease. Among RV functional parameters, lower RV GLS [HR 2.08 (95% CI 1.6–2.69)], lower RVEF [HR 1.13 (95% CI 1.05–1.22)] and reduced TAPSE [HR 1.11 (95% CI 1.02–1.21)] were significantly associated with increased all-cause mortality, while the association between RV LGE and mortality was not significant [HR 3.40 (95% CI 0.51–22.54)]. At the meta-regression analysis, no significant differences were found according to AL-type amyloidosis proportion, age, publication year, sex, NTproBNP levels, or LVEF. Significant heterogeneity was present for RVEF, which was significantly reduced by the exclusion of Baroni et al., although not entirely (I2= 0.53). The pooled prognostic performance of RV parameters is summarized in Table 3.
Figure 3.
Meta-analysis results for right ventricular parameters. The forest plots of different anatomical and functional parameters of the right ventricle assessed by cardiac magnetic resonance are shown. Right ventricular ejection fraction and right ventricular global longitudinal strain hazard ratio are reported as per 5% decrease (less negative values for global longitudinal strain); tricuspid annular systolic excursion hazard ratio is reported as per unit decrease.
We performed a sensitivity analysis for both LV and RV CMR parameters, stratifying results accounting for abnormally high or normal NTproBNP levels. All the analysis confirmed the results in both subgroups, except for native T1 mapping, which was not significantly associated with increased all-cause mortality among patients with lower NTproBNP levels [HR 2.22 (95% CI 0.61–8.11)—see Supplementary material online, Figures S6–S15].
Discussion
In this study, we assessed the prognostic role of CMR features associated with CA in a large cohort of patients with both AL and ATTR amyloid type. Our main findings can be summarized as follows: (i) several CMR imaging parameters have prognostic implications in patients with CA; (ii) both anatomical and tissue characterization parameters of LV are associated with worse outcomes in patients with CA; (iii) RV function assessed by CMR predicts mortality in patients with CA.
In this study, we confirmed that in a highly symptomatic CA population (median NTproBNP value of 2020 ng/L and 36% of patients in NYHA Classes III and IV), LV tissue characterization parameters (native T1 and ECV) are significantly associated with a worse prognosis. Our results further support the role of LV LGE by including new evidence when compared with meta-analyses by Pan et al.14 and Raina et al.,8 being the largest study investigating the prognostic implications of LGE in CA to date. Late gadolinium enhancement is a known independent predictor of mortality in a wide range of cardiomyopathies, including CA, with transmural involvement having a worse prognosis than subendocardial involvement alone.20 As demonstrated by Fontana et al., AL and ATTR amyloidosis differ in LGE pattern with transmural involvement being more frequent in ATTR type and subendocardial involvement in AL type. Despite the differences in pattern, in our study, the LGE prognostic impact was not influenced by AL vs. ATTR amyloid-type proportion in meta-regression analysis, while we found that native T1 HRs were significantly higher in studies with a greater prevalence of patients with AL-CA.
Quantitative parameters such as native T1 and ECV have recently been proposed as alternative methods to LGE for prognostic evaluation of CA. Growing evidence supports T1 imaging and ECV prospective variation trend as a marker of disease stage and therapeutic response. In this study, we confirm that in CA, myocardial ECV is a surrogate of extracellular infiltration and may precociously be increased even in the absence of myocardial LGE.32,33 Furthermore, ECV reduction has been associated with treatment response both in ATTR and AL amyloidosis with higher accuracy than other imaging parameters.23,34
Besides anatomical features of the LV, functional assessment with CMR also improved prognostic stratification. In this study, several parameters of LV contractility, such as LVEF, LV GLS, and MCF, were associated with increased mortality. All these parameters can be assessed with CMR with higher reproducibility compared with echocardiography.35 As previously pointed out, high values of echography-derived GLS are associated with poor prognosis in AL-CA,36 showing a good correlation with chemotherapy-induced CA regression. Also, in ATTR-CA, patients’ GLS is an independent predictor of mortality,37 with an incremental prognostic value over cardiac biomarkers such as NTproBNP and troponin. Of note, in this meta-analysis, we found that LV GLS has a continuous association with mortality in patients with both AL-CA and ATTR-CA, with increasing HR for lower values of LVEF. Left ventricular CMR functional imaging demonstrates promising implications for medical treatment and infiltration regression monitoring. In fact, CMR provides more accurate endocardial visualization, better quantification of both volumes and myocardial strain and offers additional information on LGE and ECV, which are also a treatment response target. This allows an extensive anatomical and functional assessment of LV.
In CA, most of the focus of diagnostic and prognostic workup and research studies has been on the LV, whereas the role of the RV has not been extensively studied. Right ventricular dysfunction is a well-known major prognostic predictor in other heart failure models, particularly in ischaemic and dilated cardiomyopathy due to limited therapeutic options for RV failure, leading to reduced survival. In addition, neurohormonal therapy in both ischaemic and non-ischaemic cardiomyopathy has been demonstrated to successfully improve survival, HF hospitalizations, and symptoms mainly by improvement in LV function.38 Compared with these models of heart failure, in CA therapies, targeting neurohormonal axis such as beta-blockers, mineralocorticoid receptor antagonists, angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) and Sodium-GLucose co-Transporter-2 inhibitors is not only lacking efficacy data, but in some cases, it is associated with worse outcomes due to hypotension (for ACE-inhibitors/ARBs) or conduction abnormalities (for beta-blockers).39 In this context, we believe that the relative prognostic role of RV dysfunction compared with LV dysfunction is more complex to analyse. To date, this is the first meta-analysis assessing the prognostic role of RV features measured by CMR imaging in patients with CA, and no systematic reviews are available on this topic. We found that among different RV parameters, TAPSE, RVEF, and RV GLS were significantly associated with increased mortality, while RV LGE was not. Although limited by scarcity of studies on RV CMR features, these findings point out that RV involvement in CA deserves careful examination. Previous studies encourage RV assessment in patients with CA, mainly referring to echocardiographic parameters. Both TAPSE and RV GLS have been associated with worse outcomes40,41 and our findings further support these results by CMR imaging. Of note, in our study, no interaction was found between RV functional parameters and amyloid type, in accordance with the previous results of Palmiero et al.42 using echocardiography. Right ventricular LGE did not predict mortality in patients with CA in contrast with the marked predictive value of LV LGE. This finding may be explained by differences in wall thickness between the right and left ventricles. In fact, while a thicker LV allows a more accurate visualization of LGE, particularly in patients with hypertrophied CA, the physiological reduced RV thickness may affect visual estimation of LGE. These technical limitations result in higher inter- and intra-observer variability and may be of particular interest in AL and early stage ATTR patients, where LV and RV thickening is less prominent.
Study limitations
This meta-analysis has several limitations. Firstly, since most of the included studies are observational with a risk of bias, the inferential power of our results may be limited. Also, since patient-level data were not available, potential effects of patient heterogeneity could not be assessed. Secondly, the included studies presented heterogeneity in several parameters with different cut-offs for determining normal and abnormal native T1 and ECV. Variability in CMR scanners and field strengths could have also influenced our findings, and it is hoped that a progressive diffusion of CMR techniques will lead to a standardization of cut-offs for parametric mapping in future studies. Also, LGE quantification as a percentage of myocardial involvement was not available in most studies included, limiting the possibility to assess the relationship between the extension of fibrosis and patient outcomes beyond the presence or absence of LGE. Thirdly, the included studies had a higher proportion of AL-type patients; therefore, although we investigated amyloid-type interaction with each prognostic parameter we studied, we could not exclude this in a prevalent ATTR population, and it may not be fully reproducible. Thus, these results should be applied mainly to the AL-amyloidosis patient population. Also, the included studies did not provide a disease-stage classification, which would have been useful to stratify prognostic information. Nevertheless, as previously reported, CA is a late diagnosis with reduced median survival for both AL and ATTR type,43 suggesting that most patients are in an advanced disease stage at the time of diagnosis, thereby reducing the disease-stage bias in this analysis. Indeed, most studies did not provide data on pharmacological and chemotherapy treatment, bone marrow transplantation, and serological biomarkers, which may improve prognostic stratification. Fourthly, for native T1 and ECV, we could not find additional useful studies compared with the meta-analysis by Pan et al.,14 although we extrapolated all the prognostic information in the included CMR studies. Fifthly, few studies were available for the anatomical and functional evaluation of the RV. Although most parameters analysed were derived from just two studies, we decided to include these results as a significant association with all-cause mortality was observed in three out of four parameters. The limitations of RV LGE were already discussed above. Furthermore, no data were available for atrial strain, despite the present increasing use of this parameter in the echocardiographic evaluation of patients with suspected CA and as a prognostic marker in patients with established CA. Finally, no data was available on outcomes other than all-cause mortality. Despite all-cause death remaining a strong outcome, we could not extend our findings to other specific causes of death, such as cardiac death and sudden arrhythmic death.
Conclusions
In this study, several CMR imaging parameters were associated with increased all-cause mortality in patients with CA. Among the tissue characterization and functional parameters evaluated, elevated ECV showed the highest association with all-cause mortality without significant heterogeneity. Also, RV evaluation improved prognostic stratification. Further research is needed to investigate prognostic stratification and combined prognostic information from CMR, biomarkers, and additional imaging techniques.
Supplementary Material
Contributor Information
Paolo Boretto, Department of Cardiovascular and Thoracic, Città della Salute e della Scienza Hospital, University of Turin, Corso Bramante, 88, 10126 Turin, Italy.
Neal Hitesh Patel, Research Department of Medical Education, UCL Medical School, 74 Huntley St, WC1E 6DE London, UK.
Keval Patel, Research Department of Medical Education, UCL Medical School, 74 Huntley St, WC1E 6DE London, UK.
Mannat Rana, Research Department of Medical Education, UCL Medical School, 74 Huntley St, WC1E 6DE London, UK.
Andrea Saglietto, Department of Cardiovascular and Thoracic, Città della Salute e della Scienza Hospital, University of Turin, Corso Bramante, 88, 10126 Turin, Italy.
Manas Soni, Research Department of Medical Education, UCL Medical School, 74 Huntley St, WC1E 6DE London, UK.
Mahmood Ahmad, Department of Cardiology, Royal Free Hospital, Royal Free London NHS Foundation Trust, 10 Pond St, NW3 2PS London, UK.
Jamie Sin Ying Ho, Department of Cardiology, Royal Free Hospital, Royal Free London NHS Foundation Trust, 10 Pond St, NW3 2PS London, UK.
Ovidio De Filippo, Department of Cardiovascular and Thoracic, Città della Salute e della Scienza Hospital, University of Turin, Corso Bramante, 88, 10126 Turin, Italy.
Rui Andre Providencia, Institute of Health Informatics Research, University College London, 222 Euston Road, NW1 2DA London, UK.
Jonathan James Hyett Bray, Institute of Health Informatics Research, University College London, 222 Euston Road, NW1 2DA London, UK; Institute of Life Sciences-2, Swansea Bay University Health Board and Swansea University Medical School, Swansea University, 4 Mumbles Rd, Sketty, SA3 5AU Swansea, UK.
Fabrizio D’Ascenzo, Department of Cardiovascular and Thoracic, Città della Salute e della Scienza Hospital, University of Turin, Corso Bramante, 88, 10126 Turin, Italy.
Lead author biography
 Dr Paolo Boretto studied medicine at the University of
Turin. After graduating in 2017, he started his training in cardiology at Città della Salute
e della Scienza Hospital, University of Turin, Italy, where he currently works as a clinical
cardiologist. His activity focuses on heart failure and cardiomyopathies, including CA. He
is also interested in cardiovascular imaging and CMR applications in clinical
decision-making. He is currently training in intensive cardiac care in the cardiac intensive
care unit and advanced heart failure therapies.
Dr Paolo Boretto studied medicine at the University of
Turin. After graduating in 2017, he started his training in cardiology at Città della Salute
e della Scienza Hospital, University of Turin, Italy, where he currently works as a clinical
cardiologist. His activity focuses on heart failure and cardiomyopathies, including CA. He
is also interested in cardiovascular imaging and CMR applications in clinical
decision-making. He is currently training in intensive cardiac care in the cardiac intensive
care unit and advanced heart failure therapies.
Data availability
The data underlying this article will be shared on reasonable request with the corresponding author.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
None declared.
References
- 1. Merlini G, Palladini G. Light chain amyloidosis: the heart of the problem. Haematologica 2013;98:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012;126:1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, George I, Kodali S, Leon MB, Hahn R, Bokhari S, Maurer MS. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017;38:2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA, Shapiro D, Evans PJ, Maschke S, Kilpatrick SE, Tan CD, Rodriguez ER, Monteiro C, Tang WHW, Kelly JW, Seitz WH Jr, Hanna M. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol 2018;72:2040–2050. [DOI] [PubMed] [Google Scholar]
- 5. Castano A, Bokhari S, Maurer MS. Unveiling wild-type transthyretin cardiac amyloidosis as a significant and potentially modifiable cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2595–2597. [DOI] [PubMed] [Google Scholar]
- 6. Zhao L, Tian Z, Fang Q. Diagnostic accuracy of cardiovascular magnetic resonance for patients with suspected cardiac amyloidosis: a systematic review and meta-analysis. BMC Cardiovasc Disord 2016;16:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dungu JN, Valencia O, Pinney JH, Gibbs SD, Rowczenio D, Gilbertson JA, Lachmann HJ, Wechalekar A, Gillmore JD, Whelan CJ, Hawkins PN, Anderson LJ. CMR based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging 2014;7:133–142. [DOI] [PubMed] [Google Scholar]
- 8. Raina S, Lensing SY, Nairooz RS, Pothineni NV, Hakeem A, Bhatti S, Pandey T. Prognostic value of late gadolinium enhancement CMR in systemic amyloidosis. JACC Cardiovasc Imaging 2016;9:1267–1277. [DOI] [PubMed] [Google Scholar]
- 9. Fontana M, Martinez-Naharro A, Hawkins PN. Staging cardiac amyloidosis with CMR: understanding the different phenotypes. JACC Cardiovasc Imaging 2016;9:1278–1279. [DOI] [PubMed] [Google Scholar]
- 10. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses. 2014.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (5 August 2015).
- 11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 12. Wan K, Li W, Sun J, Xu Y, Wang J, Liu H, Dong Y, Cheng W, Zhang Q, Zeng Z, Zhou X, Han Y, Chen Y. Regional amyloid distribution and impact on mortality in light-chain amyloidosis: a T1 mapping cardiac magnetic resonance study. Amyloid 2019;26:45–51. [DOI] [PubMed] [Google Scholar]
- 13. Kotecha T, Martinez-Naharro A, Treibel TA, Francis R, Nordin S, Abdel-Gadir A, Knight DS, Zumbo G, Rosmini S, Maestrini V, Bulluck H, Rakhit RD, Wechalekar AD, Gilbertson J, Sheppard MN, Kellman P, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Myocardial edema and prognosis in amyloidosis. J Am Coll Cardiol 2018;71:2919–2931. [DOI] [PubMed] [Google Scholar]
- 14. Pan JA, Kerwin MJ, Salerno M. Native T1 mapping, extracellular volume mapping, and late gadolinium enhancement in cardiac amyloidosis: a meta-analysis. JACC Cardiovasc Imaging 2020;13:1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan Z, Yang Y, Wu X, Li S, Li L, Zhong L, Lin Q, Fei H, Liao P, Wang W. Left atrial remodeling and the prognostic value of feature tracking derived left atrial strain in patients with light-chain amyloidosis: a cardiovascular magnetic resonance study. Int J Cardiovasc Imaging 2022;38:1519–1532. [DOI] [PubMed] [Google Scholar]
- 16. Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, Piechnik SK, Whelan CJ, Herrey AS, Gillmore JD, Lachmann HJ, Wechalekar AD, Hawkins PN, Moon JC. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J 2015;36:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arenja N, Andre F, Riffel JH, Siepen FAD, Hegenbart U, Schönland S, Kristen AV, Katus HA, Buss SJ. Prognostic value of novel imaging parameters derived from standard cardiovascular magnetic resonance in high risk patients with systemic light chain amyloidosis. J Cardiovasc Magn Reson 2019;21:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu H, Fu H, Guo YK, Yang ZG, Xu HY, Shuai X, Xu R, Li ZL, Xia CC, He Y, Zhou XY. The prognostic value of right ventricular deformation derived from cardiac magnetic resonance tissue tracking for all-cause mortality in light-chain amyloidosis patients. Cardiovasc Diagn Ther 2020;10:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ridouani F, Damy T, Tacher V, Derbel H, Legou F, Sifaoui I, Audureau E, Bodez D, Rahmouni A, Deux JF. Myocardial native T2 measurement to differentiate light-chain and transthyretin cardiac amyloidosis and assess prognosis. J Cardiovasc Magn Reson 2018;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, Maestrini V, Barcella W, Rosmini S, Bulluck H, Sayed RH, Patel K, Mamhood S, Bucciarelli-Ducci C, Whelan CJ, Herrey AS, Lachmann HJ, Wechalekar AD, Manisty CH, Schelbert EB, Kellman P, Gillmore JD, Hawkins PN, Moon JC. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2015;132:1570–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aquaro GD, Morini S, Grigoratos C, Taborchi G, Di Bella G, Martone R, Vignini E, Emdin M, Olivotto I, Perfetto F, Cappelli F. Electromechanical dissociation of left atrium in patients with cardiac amyloidosis by magnetic resonance: prognostic and clinical correlates. Int J Cardiol Heart Vasc 2020;31:100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knight DS, Zumbo G, Barcella W, Steeden JA, Muthurangu V, Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Kotecha T, Francis R, Rezk T, Quarta CC, Whelan CJ, Lachmann HJ, Wechalekar AD, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Cardiac structural and functional consequences of amyloid deposition by cardiac magnetic resonance and echocardiography and their prognostic roles. JACC Cardiovasc Imaging 2019;12:823–833. [DOI] [PubMed] [Google Scholar]
- 23. Martinez-Naharro A, Kotecha T, Norrington K, Boldrini M, Rezk T, Quarta C, Treibel TA. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovasc Imaging 2018;12:810–819. [DOI] [PubMed] [Google Scholar]
- 24. Baroni M, Nava S, Quattrocchi G, Milazzo A, Giannattasio C, Roghi A, Pedrotti P. Role of cardiovascular magnetic resonance in suspected cardiac amyloidosis: late gadolinium enhancement pattern as mortality predictor. Neth Heart J 2017;26:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White JA, Kim HW, Shah D, Fine N, Kim KY, Wendell DC, Al-Jaroudi W, Parker M, Patel M, Gwadry-Sridhar F, Judd RM, Kim RJ. CMR Imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. JACC Cardiovasc Imaging 2014;7:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Illman JE, Arunachalam SP, Arani A, Chang IC, Glockner JF, Dispenzieri A, Grogan M, Araoz PA. MRI feature tracking strain is prognostic for all-cause mortality in AL amyloidosis. Amyloid 2018;25:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wan K, Sun J, Yang D, Liu H, Wang J, Cheng W, Zhang Q, Zeng Z, Zhang T, Greiser A, Jolly MP, Han Y, Chen Y. Left ventricular myocardial deformation on cine MR images: relationship to severity of disease and prognosis in light-chain amyloidosis. Radiology 2018;288:73–80. [DOI] [PubMed] [Google Scholar]
- 28. Lin L, Li X, Feng J, Shen KN, Tian Z, Sun J, Mao YY, Cao J, Jin ZY, Li J, Selvanayagam JB, Wang YN. The prognostic value of T1 mapping and late gadolinium enhancement cardiovascular magnetic resonance imaging in patients with light chain amyloidosis. J Cardiovasc Magn Reson 2018;20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, Li J, Lin L, Shen K, Tian Z, Sun J, Zhang C, An J, Jin Z, Vliegenthart R, Selvanayagam JB, Wang Y. Left and right ventricular myocardial deformation and late gadolinium enhancement: incremental prognostic value in amyloid light-chain amyloidosis. Cardiovasc Diagn Ther 2020;10:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhatti S, Watts E, Syed F, Vallurupalli S, Pandey T, Jambekar K, Mazur W, Hakeem A. Clinical and prognostic utility of cardiovascular magnetic resonance imaging in myeloma patients with suspected cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2016;17:970–977. [DOI] [PubMed] [Google Scholar]
- 31. Ochs MM, Fritz T, Arenja N, Riffel J, Andre F, Mereles D, Siepen FAD, Hegenbart U, Schönland S, Katus HA, Friedrich MGW, Buss SJ. Regional differences in prognostic value of cardiac valve plane displacement in systemic light-chain amyloidosis. J Cardiovasc Magn Reson 2017;19:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mongeon FP, Jerosch-Herold M, Coelho-Filho OR, Blankstein R, Falk RH, Kwong RY. Quantification of extracellular matrix expansion by CMR in infiltrative heart disease. JACC Cardiovasc Imaging 2012;5:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barison A, Aquaro GD, Pugliese NR, Cappelli F, Chiappino S, Vergaro G, Mirizzi G, Todiere G, Passino C, Masci PG, Perfetto F, Emdin M. Measurement of myocardial amyloid deposition in systemic amyloidosis: insights from cardiovascular magnetic resonance imaging. J Intern Med 2015;277:605–614. [DOI] [PubMed] [Google Scholar]
- 34. Fontana M, Martinez-Naharro A, Chacko L, Rowczenio D, Gilbertson JA, Whelan CJ, Strehina S, Lane T, Moon J, Hutt DF, Kellman P, Petrie A, Hawkins PN, Gillmore JD. Measuring cardiac amyloid load in patients on patisiran: evidence from prospective UK early access programme. JACC Cardiovascular Imaging 2021;14:189–199. [DOI] [PubMed] [Google Scholar]
- 35. Feng KY, Loungani RS, Rao VN, Patel CB, Khouri MG, Felker GM, DeVore AD. Best practices for prognostic evaluation of a patient with transthyretin amyloid cardiomyopathy. JACC CardioOncol 2019;1:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barros-Gomes S, Williams B, Nhola LF, Grogan M, Maalouf JF, Dispenzieri A, Pellikka PA, Villarraga HR. Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging 2017;10:398–407. [DOI] [PubMed] [Google Scholar]
- 37. Chacko L, Martone R, Bandera F, Lane T, Martinez-Naharro A, Boldrini M, Rezk T, Whelan C, Quarta C, Rowczenio D, Gilbertson JA, Wongwarawipat T, Lachmann H, Wechalekar A, Sachchithanantham S, Mahmood S, Marcucci R, Knight D, Hutt D, Moon J, Petrie A, Cappelli F, Guazzi M, Hawkins PN, Gillmore JD, Fontana M. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J 2020;41:1439–1447. [DOI] [PubMed] [Google Scholar]
- 38. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 39. Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio ALP, Damy T, Eriksson U, Fontana M, Gillmore JD, Gonzalez-Lopez E, Grogan M, Heymans S, Imazio M, Kindermann I, Kristen AV, Maurer MS, Merlini G, Pantazis A, Pankuweit S, Rigopoulos AG, Linhart A. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2021;42:1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bodez D, Ternacle J, Guellich A, Galat A, Lim P, Radu C, Guendouz S, Bergoend E, Couetil JP, Hittinger L, Dubois-Randé JL, Plante-Bordeneuve V, Deux JF, Mohty D, Damy T. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid 2016;23:158–167. [DOI] [PubMed] [Google Scholar]
- 41. Tjahjadi C, Fortuni F, Stassen J, Debonnaire P, Lustosa RP, Marsan NA, Delgado V, Bax JJ. Prognostic implications of right ventricular systolic dysfunction in cardiac amyloidosis. Am J Cardiol 2022;173:120–127. [DOI] [PubMed] [Google Scholar]
- 42. Palmiero G, Rubino M, Monda E, Caiazza M, Trinchillo MG, Ascione L, Caso P, Limongelli G. The right heart in cardiac amyloidosis: a comparison between subtypes and with other genetic and non-genetic causes of left ventricular hypertrophy. Eur Heart J 2020;41:ehaa946.2148. [Google Scholar]
- 43. Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, Clarke JO, Dember LM, Frantz JG, Hershberger RE, Maurer MS, Nativi-Nicolau J, Sanchorawala V, Sheikh FH. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023;81:1076–1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request with the corresponding author.