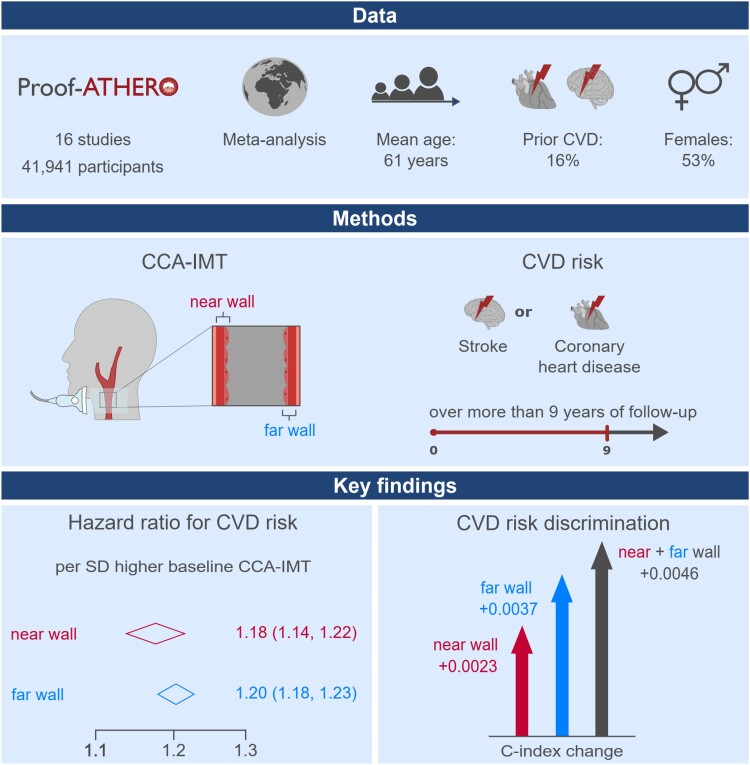
Abstract
Aims
Current guidelines recommend measuring carotid intima-media thickness (IMT) at the far wall of the common carotid artery (CCA). We aimed to precisely quantify associations of near vs. far wall CCA-IMT with the risk for atherosclerotic cardiovascular disease (CVD, defined as coronary heart disease or stroke) and their added predictive values.
Methods and results
We analysed individual records of 41 941 participants from 16 prospective studies in the Proof-ATHERO consortium {mean age 61 years [standard deviation (SD) = 11]; 53% female; 16% prior CVD}. Mean baseline values of near and far wall CCA-IMT were 0.83 (SD = 0.28) and 0.82 (SD = 0.27) mm, differed by a mean of 0.02 mm (95% limits of agreement: −0.40 to 0.43), and were moderately correlated [r = 0.44; 95% confidence interval (CI): 0.39–0.49). Over a median follow-up of 9.3 years, we recorded 10 423 CVD events. We pooled study-specific hazard ratios for CVD using random-effects meta-analysis. Near and far wall CCA-IMT values were approximately linearly associated with CVD risk. The respective hazard ratios per SD higher value were 1.18 (95% CI: 1.14–1.22; I² = 30.7%) and 1.20 (1.18–1.23; I² = 5.3%) when adjusted for age, sex, and prior CVD and 1.09 (1.07–1.12; I² = 8.4%) and 1.14 (1.12–1.16; I²=1.3%) upon multivariable adjustment (all P < 0.001). Assessing CCA-IMT at both walls provided a greater C-index improvement than assessing CCA-IMT at one wall only [+0.0046 vs. +0.0023 for near (P < 0.001), +0.0037 for far wall (P = 0.006)].
Conclusions
The associations of near and far wall CCA-IMT with incident CVD were positive, approximately linear, and similarly strong. Improvement in risk discrimination was highest when CCA-IMT was measured at both walls.
Keywords: Individual-participant-data meta-analysis, Common carotid artery intima-media thickness, Cardiovascular risk
Graphical Abstract
Graphical Abstract.
CCA-IMT, common carotid artery intima-media thickness; CI, confidence interval; CVD, cardiovascular disease; Proof-ATHERO, Prospective Studies of Atherosclerosis.
Introduction
Carotid intima-media thickness (cIMT), the distance from the lumen-intima interface to the media-adventitia interface of the carotid artery wall, is a proposed marker for early atherosclerosis.1 There is a bulk of evidence that individuals with elevated cIMT are at higher risk of developing cardiovascular disease (CVD) later in life. For instance, in the USE-IMT collaboration involving 45 828 individuals from 14 population-based cohorts, a 0.1 mm higher common carotid artery intima-media thickness (CCA-IMT) was associated with a multivariable adjusted hazard ratio of 1.09 for incident myocardial infarction or stroke [95% confidence interval (CI): 1.07–1.12].2 Furthermore, CCA-IMT measurement provides a small yet statistically significant improvement of 10-year CVD prediction when added to a Framingham risk score model.2 Furthermore, we have recently shown that CCA-IMT is also associated with a higher long-term risk of developing a first-ever carotid plaque.3
A challenge when measuring CCA-IMT using carotid ultrasonography is that absolute values are influenced by several factors,4 including the site at which it is measured.5 Guidelines on cIMT assessment recommend measuring cIMT at the far wall of the CCA-IMT1,6 because such measurement is considered to afford higher precision and reproducibility.7–10 However, available evidence from prospective cohort studies on the association with disease risk is conflicting, with one study showing a stronger association of far wall CCA-IMT with coronary heart disease (CHD) risk and another study showing a weaker association of far wall CCA-IMT with CVD risk compared with combined near and far wall measurements.11,12
Against this backdrop, we conducted an individual-participant-data meta-analysis including 16 studies of the Prospective Studies of Atherosclerosis (Proof-ATHERO) consortium. We aimed to (i) reliably quantify and compare associations of near and far wall CCA-IMT with the CVD risk and (ii) determine the added value of such measurements for CVD prediction over and beyond conventional CVD risk factors.
Methods
Study sample
Details on the Proof-ATHERO consortium have been described previously, including the design features, ultrasound methodologies, and outcomes definitions of the contributing studies.13 The current analysis included studies that had (i) provided data on both near and far wall CCA-IMT at baseline, (ii) recorded ≥10 incident CVD events, and (iii) supplied data on age, sex, and prior CVD (defined as CHD or stroke). Studies contributing individual participant data to the present analysis have obtained informed consent from the study participants and ethical approval from their respective institutional review boards.
Assessment of common carotid artery intima-media thickness
Studies assessed CCA-IMT using carotid ultrasonography, as described in Supplementary material online, Table S1. In the analysis, we gave preference to mean CCA-IMT measurement; if unavailable, we analysed the maximum value at the CCA instead. Whenever separate CCA-IMT measurements were available at different sites, we used the arithmetic mean of all values for analysis.
Study endpoints
The primary outcome was a combined CVD endpoint defined as fatal and non-fatal stroke or CHD. Fatal events were classified using International Classification of Diseases codes or study-specific classification systems. Further information on the exact composition of the combined CVD endpoint and the number of events recorded in each contributing study is provided in Supplementary material online, Table S2. In time-to-event analyses, we considered follow-up for participants until they experienced the combined CVD endpoint, died, reached the end of the study period, or were lost to follow-up, whichever came first.
Statistical analyses
We assessed agreement of baseline near and far wall CCA-IMT values within individuals using Bland–Altman plots and their correlation using partial correlation coefficients adjusted for age, sex, prior CVD, and trial arm. Furthermore, we quantified within-person stability of near and far wall CCA-IMT values during follow-up using regression dilution ratios.14 Time trends in regression dilution ratios across repeat measurements were assessed using meta-regression.14 Study-specific mean differences, limits of agreement, Fisher’s z-transformed correlation coefficients, and regression dilution ratios were pooled using random-effects meta-analysis.15,16
In the primary analysis, we estimated hazard ratios (i) across study-specific quintiles of CCA-IMT values with 95% CIs based on floating absolute risks17 and (ii) per study-specific standard deviation (SD) higher level of CCA-IMT. Hazard ratios were first estimated within each study separately using Cox regression stratified by trial arm and adjusted for age, sex, and prior CVD and then pooled using random-effects meta-analysis.16 The I2 statistic was used to quantify between-studies heterogeneity.18 The proportional hazards assumption was checked using Schoenfeld’s residuals and log–log plots of survival. We used bootstrapping with 10 000 repetitions to test whether strengths of associations with CVD risk differed between near and far wall measurements.
We also conducted several sensitivity analyses. First, we tested whether associations differed according to pre-specified study- or participant-level characteristics using random-effects meta-regression19 or by including formal multiplicative interaction terms.20 Second, we progressively adjusted the model for the additional potential confounding variables smoking, history of diabetes, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, intake of lipid-lowering medication, and intake of anti-hypertensive medication. Third, we employed within-study multiple imputation of missing values in the multivariable adjustment model.21 We imputed 80 data sets per study if less than 80% of the observations were missing and combined estimates by Rubin’s rules in each study before meta-analysing them across studies. The imputation model included all variables of the multivariable adjustment model, the Nelson–Aalen estimator of the baseline cumulative hazard, and the outcome indicator. Fourth, we adjusted hazard ratios for near and far wall CCA-IMT for each other. Fifth, we expressed hazard ratios per 100 µm higher CCA-IMT value. Sixth, we used long-term average CCA-IMT values, estimated using regression calibration22 on the basis of repeat CCA-IMT measurements over time. If participants experienced a CVD event during follow-up, any CCA-IMT measurements taken after the event were censored. Seventh, we restricted the study population to participants who are known to be free of carotid plaque assessed at any segment of the carotid artery at baseline. Eighth, we examined associations separately for the endpoints stroke, CHD, and fatal CVD (as defined in Supplementary material online, Table S2) while censoring the individual endpoints against each other to allow estimation of cause-specific hazard ratios. Ninth, we conducted analyses taking into account all-cause mortality as a competing risk.23
Finally, we quantified improvements in risk discrimination and re-classification metrics upon the addition of near and far wall CCA-IMT measurements to a model containing data on age, sex, prior CVD, smoking, history of diabetes, systolic blood pressure, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol. For risk discrimination, we pooled study-specific C-index changes using a meta-analysis weighting the studies by the number of events they had recorded.24 For risk re-classification, we calculated the net re-classification index (NRI) across four categories of predicted 10-year CVD risk (<5%, 5 to <7.5%, 7.5 to <20%, and ≥20%) among participants with 10-year or longer follow-up. We also assessed how well the prediction models were calibrated using methods published previously.25,26 Analyses involved Stata version 15.1,27 two-sided P-values, and a significance level of P ≤ 0.05 in the principal analysis and Bonferroni-corrected P-values in subgroup analyses.
Results
Characteristics of contributing studies
Sixteen studies in the Proof-ATHERO consortium involving 41 941 participants fulfilled the inclusion criteria for the present analysis (see flow chart in Supplementary material online, Figure S1). At baseline, participants had a mean age of 61 years (SD = 11), 53% were female, and 16% had a prior CVD (Table 1). Mean CCA-IMT was 0.83 mm (SD = 0.28) on the near and 0.82 mm (SD = 0.27) on the far wall (see Supplementary material online, Table S3).
Table 1.
Participant characteristics
| Study | No. of participants | Women, n (%) |
Age (years), mean (SD) | Prior CVD, n (%) | No. of CVD eventsa | Duration of follow-up (years), median (5th–95th percentile) |
|---|---|---|---|---|---|---|
| ALLO-IMT | 80 | 34 (43) | 68 (10) | 80 (100) | 11 | 1.0 (0.2–1.1) |
| ARIC | 13 444 | 7315 (54) | 55 (6) | 1505 (11) | 5468 | 22.0 (2.3–30.2) |
| CHS | 5779 | 3317 (57) | 73 (6) | 1300 (22) | 2753 | 9.4 (0.8–22.1) |
| CONTRAST | 146 | 55 (38) | 61 (14) | 66 (45) | 29 | 3.5 (0.3–6.1) |
| CSN | 4674 | 1978 (42) | 59 (10) | 150 (3) | 41 | 2.9 (0.0–11.7) |
| GRACE | 1185 | 430 (36) | 63 (8) | 659 (56) | 321 | 5.8 (0.6–6.9) |
| HART | 924 | 221 (24) | 69 (7) | 787 (85) | 152 | 5.0 (1.1–5.3) |
| JHS | 3656 | 2284 (62) | 55 (13) | 407 (11) | 262 | 11.7 (3.7–13.7) |
| NOMAS-INVEST | 777 | 477 (61) | 69 (8) | 76 (10) | 49 | 5.9 (0.0–8.2) |
| OSACA2 | 291 | 115 (40) | 65 (9) | 109 (37) | 35 | 6.1 (2.2–7.2) |
| PIVUS | 986 | 494 (50) | 70 (1) | 145 (15) | 51 | 5.1 (0.0–5.5) |
| RADIANCE I | 884 | 448 (51) | 46 (13) | 7 (1) | 43 | 2.0 (0.4–2.1) |
| RADIANCE II | 740 | 262 (35) | 57 (8) | 7 (1) | 37 | 2.0 (0.2–2.1) |
| ROTTERDAM | 6325 | 3740 (59) | 70 (9) | 902 (14) | 1039 | 11.3 (1.5–14.1) |
| SECURE | 731 | 174 (24) | 66 (7) | 624 (85) | 103 | 4.4 (0.9–5.2) |
| STARR | 1319 | 727 (55) | 54 (11) | 5 (0) | 29 | 3.9 (2.4–5.3) |
| Total | 41 941 | 22 071 (53) | 61 (11) | 6829 (16) | 10 423 | 9.3 (0.4–29.1) |
aStudy-specific definitions of CVD events are provided in Supplementary material online, Table S2.
Agreement and correlation of near and far wall common carotid artery intima-media thickness
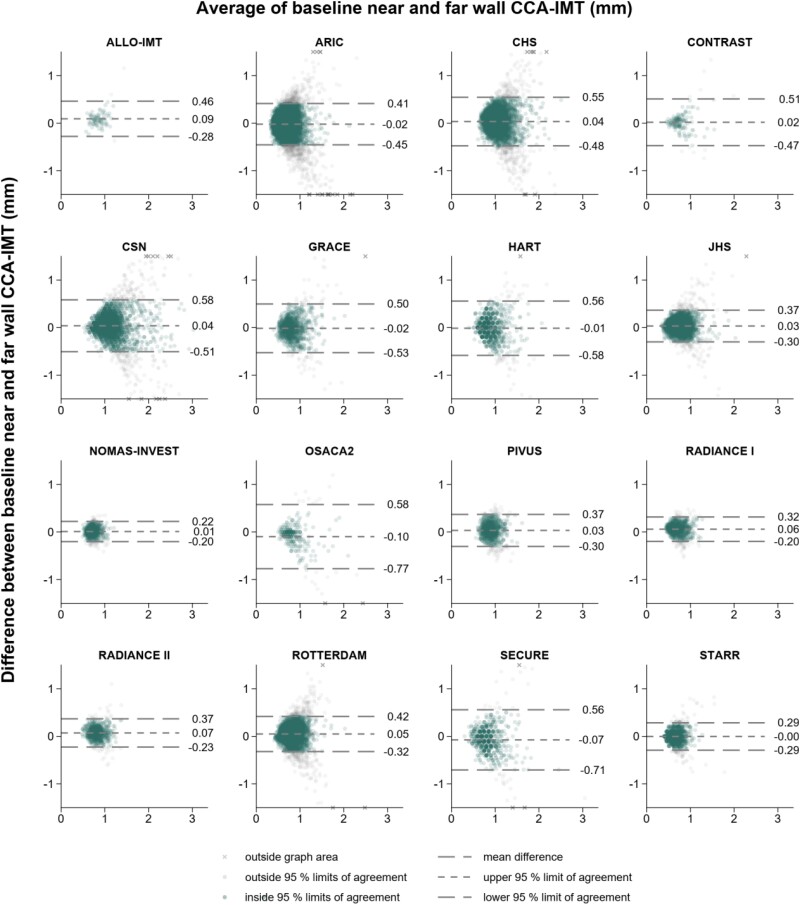
Figure 1 summarizes the agreement of CCA-IMT values measured at the two walls. Overall, the mean difference of near vs. far wall CCA-IMT values was close to 0 (0.02 mm; 95% CI: −0.00 to 0.03 mm; P = 0.095) and pooled 95% limits of agreement ranged from −0.40 (95% CI: −0.45 to −0.34) to 0.43 (0.37–0.48) mm. The pooled partial correlation coefficient between near and far wall CCA-IMT values was 0.44 (95% CI: 0.39–0.49) (see Supplementary material online, Figure S2).
Figure 1.
Agreement of baseline common carotid artery intima-media thickness values measured at the near and far wall. For purpose of presentation, graph areas were limited to −1.5 to 1.5 mm on the vertical axes and values outside this range are marked with the symbol x. CCA-IMT, common carotid artery intima-media thickness.
When assessing within-person stability of CCA-IMT values during follow-up, the overall regression dilution ratio was 0.69 (95% CI: 0.63–0.75) for near and 0.75 (0.68–0.83) for far wall CCA-IMT (see Supplementary material online, Figure S3). The average regression dilution ratio declined with longer follow-up duration by 0.13 (0.03–0.22; P = 0.011) and 0.12 (0.03–0.20; P = 0.007) for every 5 years between repeat measurements, respectively. A direct comparison of study-specific regression dilution ratios confirmed that CCA-IMT measurements taken at the far wall often were more stable over time than measurements taken at the near wall (see Supplementary material online, Figure S4).
Association with the combined cardiovascular disease endpoint
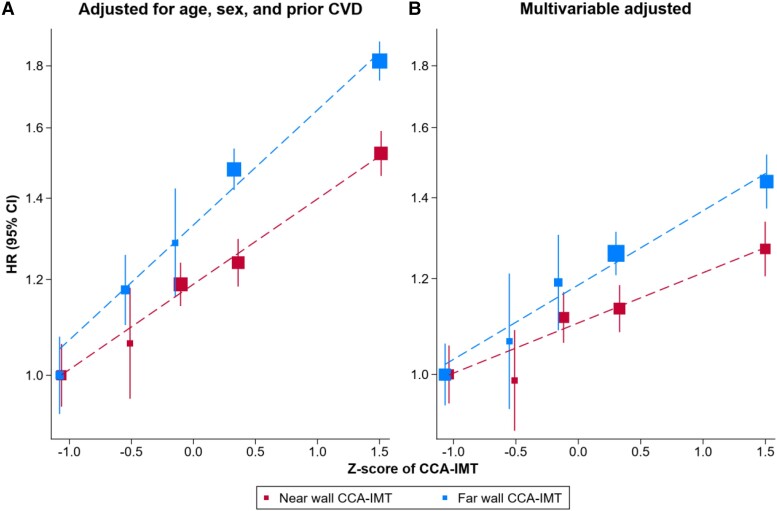
Over a median follow-up of 9.3 years (5th–95th percentile: 0.4–29.1), 10 423 participants reached the combined CVD endpoint (Table 1). Both near and far wall CCA-IMT values were positively and approximately linearly associated with CVD risk (Figure 2). In models adjusted for age, sex, and prior CVD, the pooled hazard ratios per SD higher CCA-IMT were 1.18 (95% CI: 1.14–1.22; I² = 30.7%) for near and 1.20 (1.18–1.23; I² = 5.3%) for far wall CCA-IMT and did not differ significantly between the two walls (P = 0.500). Study-specific hazard ratios are provided in Supplementary material online, Figure S5. In comparison, when considering the arithmetic mean of near and far wall CCA-IMT values as an exposure, the corresponding hazard ratio was 1.23 (1.19–1.28; I² = 35.1%).
Figure 2.
Shapes of associations of baseline near and far wall common carotid artery intima-media thickness with incident cardiovascular disease. Pooled hazard ratios for incident cardiovascular disease were plotted against the mean Z-score of baseline common carotid artery intima-media thickness within each study-specific quintile. (A) Analysis involved data on 16 studies, 41 941 participants, and 10 423 cardiovascular disease events. Cox proportional hazards models were adjusted for age, sex, and prior cardiovascular disease and were stratified by trial arm. (B) Analysis involved data on nine studies, 24 609 participants, and 8587 cardiovascular disease events. Cox proportional hazards models were adjusted for age, sex, prior cardiovascular disease, smoking, history of diabetes, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, intake of lipid-lowering medication, and intake of anti-hypertensive medication and were stratified by trial arm. CCA-IMT, common carotid artery intima-media thickness; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Sensitivity analyses
In the first set of sensitivity analyses, we investigated effect modification by study- and participant-level characteristics (see Supplementary material online, Table S4). After applying the multiplicity-adjusted threshold for statistical significance of 0.0031, associations of both near and far wall CCA-IMT were significantly stronger in younger vs. older participants (near wall: Pinteraction < 0.001; far wall: Pinteraction = 0.003) and among females vs. males (near wall: Pinteraction = 0.001; far wall: Pinteraction = 0.002). The association was also stronger among participants without prior CVD for far wall CCA-IMT (Pinteraction < 0.001) but not for near wall CCA-IMT despite similar point estimates (Pinteraction = 0.062). Notably, there was no evidence for difference in the strength of associations according to type of the ultrasound protocol used in the study, such as measurement by the same sonographer or using multiple scans, ECG-gating, angle control, or edge detection.
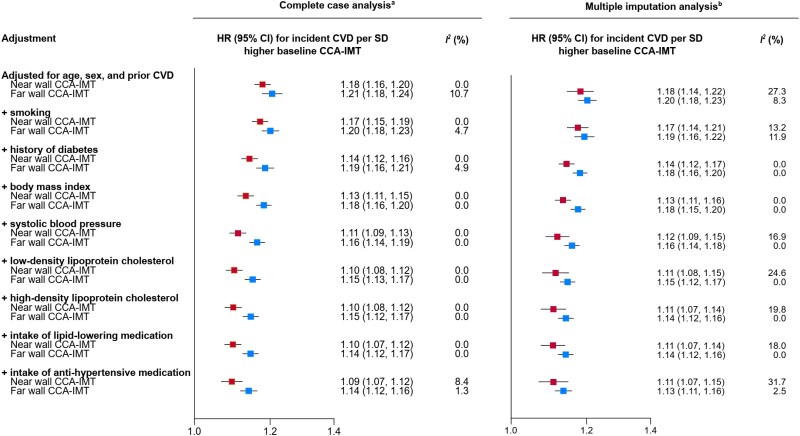
In a second set of sensitivity analyses, we progressively adjusted hazard ratios for established CVD risk factors (Figure 3). Hazard ratios attenuated from 1.18 (1.16–1.20) to 1.09 (1.07–1.12) for near wall CCA-IMT and from 1.21 (1.18–1.24) to 1.14 (1.12–1.16) for far wall CCA-IMT but remained statistically significant (all P < 0.001). We obtained similar results in analyses employing multiple imputations (Figure 3).
Figure 3.
Progressively adjusted hazard ratios for incident cardiovascular disease of near and far wall common carotid artery intima-media thickness in a complete case analysis and a multiple imputation analysis. Analysis included data from ALLO-IMT, ARIC, CHS, CONTRAST, GRACE, JHS, OSACA2, PIVUS, and SECURE. aRestricted to participants with complete data on all variables used in the progressive adjustment (24 609 participants; 8587 cardiovascular disease events). bRestricted to studies included in the complete case analysis (26 150 participants; 9017 cardiovascular disease events). Imputed variables (percentage of missing values that were imputed): smoking (0.18%), history of diabetes (0.41%), body mass index (0.17%), systolic blood pressure (0.10%), low-density lipoprotein cholesterol (4.09%), high-density lipoprotein cholesterol (2.97%), intake of lipid-lowering medication (2.33%), and intake of anti-hypertensive medication (0.97%). CCA-IMT, common carotid artery intima-media thickness; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; SD, standard deviation.
Finally, Supplementary material online, Table S5 summarizes the findings in sensitivity analyses that estimated hazard ratios (i) for near and far wall CCA-IMT adjusted for each other; (ii) per 100 µm higher CCA-IMT value; (iii) based on long-term average CCA-IMT values (rather than baseline values); (iv) in participants known to be free of carotid plaque at baseline; (v) for different types of CVD outcomes, including CHD, stroke, and fatal CVD; and (vi) treating all-cause mortality as a competing risk.
Added value of assessing near vs. far wall common carotid artery intima-media thickness for cardiovascular disease prediction
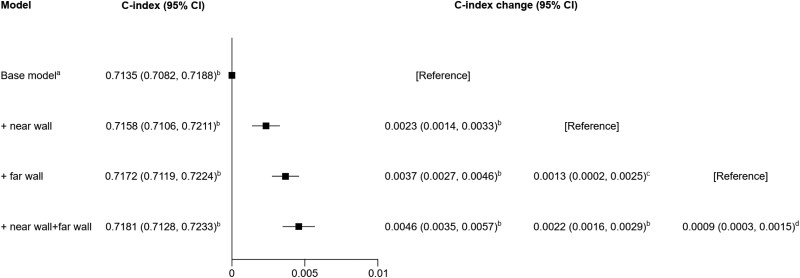
When adding CCA-IMT measurements to a model containing information on conventional risk factors, the C-index improved by 0.0023 (95% CI: 0.0014–0.0033) for near and 0.0037 (0.0027–0.0046) for far wall CCA-IMT (Figure 4). Concomitant assessment of CCA-IMT at both walls improved the C-index by 0.0046 (0.0035–0.0057), which was significantly greater than assessing CCA-IMT at only the far wall (P < 0.001) or only the near wall (P = 0.006). When restricting the analysis to participants without a prior CVD, C-index improvements afforded by wall-specific CCA-IMT information were even higher (see Supplementary material online, Figure S6).
Figure 4.
Improvement of risk discrimination for cardiovascular disease events upon addition of wall-specific common carotid artery intima-media thickness measurements. Analysis included data from ALLO-IMT, ARIC, CHS, CONTRAST, CSN, GRACE, JHS, NOMAS-INVEST, OSACA2, PIVUS, and SECURE (26 901 participants; 8729 cardiovascular disease events). aIncluded information on age, sex, prior cardiovascular disease, smoking, history of diabetes, systolic blood pressure, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol and was stratified by trial arm. bP < 0.001. cP = 0.018. dP = 0.006. CI, confidence interval.
The overall NRI across predicted 10-year risk categories was 0.010 (0.004–0.016) for near, 0.021 (0.014–0.029) for far, and 0.021 (0.013–0.029) for near and far wall CCA-IMT measurement, with higher point estimates for participants that did not develop CVD than those that did (see Supplementary material online, Table S6). There was no significant difference in the NRI when comparing the prediction model including far wall CCA-IMT only with the one including both near and far wall CCA-IMT (NRIoverall: P = 0.903; NRInon-cases: P = 0.602; NRIcases: P = 0.803). Details on the number of participants re-classified across risk categories are provided in Supplementary material online, Table S7. Prediction models were well calibrated as visualized in Supplementary material online, Figure S7 and indicated by non-significant tests for goodness of fit.
Discussion
In this large-scale analysis of individual records on 41 941 participants and 10 423 CVD outcomes, both near and far wall CCA-IMT were positively and approximately linearly associated with CVD risk, and these associations were similarly strong. In models adjusted for age, sex, and prior CVD, the respective pooled hazard ratios per SD higher level were 1.18 (95% CI: 1.14–1.22; I² = 30.7%) and 1.20 (1.18–1.23; I² = 5.3%). Findings were robust across subgroups and in a variety of sensitivity analyses. Improvement in risk discrimination was the highest when CCA-IMT was measured at both walls and any of the CCA-IMT measurements significantly improved risk re-classification.
Evidence supporting assessment of common carotid artery intima-media thickness at the far wall
Current guidelines on measuring cIMT recommend measurement at the far wall of the CCA.1,6 This is supported by the notion that far wall CCA-IMT can be quantified more precisely due to the physics of ultrasound.7–10,28,29 A general recommendation for assessing interfaces between two different tissues using ultrasound is to perform measurements at the leading edge of echoes.9 Measurements at the trailing edge of echoes should be avoided because this demarcation line is below the ‘true’ anatomically interface,9 in the case of CCA-IMT effectively underestimating its ‘true’ value by ∼20%.28 The trailing edge also depends on several non-standardizable factors, such as gain setting and the individual composition of the adventitia.29 While the leading edge of the adventitia-media interface of the far wall of the carotid artery can generally be visualized clearly, the one of the near wall cannot be identified precisely. This is because the adventitia is a more echogenic layer compared with the media and, as a result, echoes from lower parts of the adventitia disturb those from the adventitia-media interface.10 Therefore, near wall cIMT is usually measured on the trailing edge and is consequently an approximation of the ‘true’ cIMT.30 Consistent with these data, in our study, a higher measurement error when assessing near wall CCA-IMT may have contributed to the slightly lower within-person stability we observed for repeat CCA-IMT values at the near wall (see Supplementary material online, Figure S3).
Additional information captured by near wall assessment
Over the last decades, technological advancements have led to better visualization of near wall CCA-IMT and higher reproducibility of such measurement.30,31 Underestimation of ‘true’ CCA-IMT at the near wall due to the physics of ultrasound is likely systematic rather than random and therefore does not lead to an attenuation of reproducibility, correlation with far wall CCA-IMT, nor associations with cardiovascular outcomes.32 Furthermore, near wall CCA-IMT measurement is hypothesized to entail additional information relevant to CVD risk assessment and stratification30 because atherosclerotic wall thickening is not a uniform process and cIMT has an asymmetrical helix-like distribution.33,34
While some previous studies suggested that near wall CCA-IMT may be thicker than far wall CCA-IMT,11,35 our analysis shows that, on average, near and far wall CCA-IMT values were similar. However, the 95% limits of agreement between absolute values of near and far wall CCA-IMT were wide (Figure 1), and, in a subset of individuals, a considerably higher CCA-IMT value was observed only at one of the two walls. While dispersion of CCA-IMT values at the near vs. the far wall could be related to carotid plaque, the majority of studies had measured CCA-IMT at the segment of 0–10 mm below the bulbar widening, which is less prone to carotid plaque.33 Interestingly, isolated elevations of CCA-IMT at one of the walls do not appear to be related to specific cardiovascular risk factors, although the variation collectively explained by conventional cardiovascular risk factors appears to be slightly higher for near wall CCA-IMT than for far wall CCA-IMT.11 Despite affecting different subsets of individuals, in our analysis, elevated values of CCA-IMT at the near or the far wall were equally and independently associated with a higher risk of experiencing a CVD event. This finding was robust in an analysis restricted to participants known to be free of carotid plaque at baseline or in analyses comparing studies with differing ultrasound methodologies.
Previous studies on the associations with cardiovascular disease outcomes
There are only two prospective cohort studies, the Multi-Ethnic Study of Atherosclerosis (MESA)11 and the Cardiovascular Health Study (CHS),12 which have previously investigated and directly compared associations of near and far wall CCA-IMT with CVD outcomes. Both studies used the same ultrasound acquisition protocol and quantified the maximum CCA-IMT values averaged over the left and right carotid artery.11 In the MESA study, the respective multivariable adjusted hazard ratios of near, far, and average of near and far wall CCA-IMT values with CHD risk were 1.01 (95% CI: 0.92–1.11), 1.21 (1.13–1.30), and 1.17 (1.08–1.28) per SD higher level, leading the investigators to conclude that far wall CCA-IMT showed the strongest association.11 The CHS study reported that far wall CCA-IMT was more weakly associated with incident CVD than the average of near and far wall CCA-IMT.12 Our study builds upon available evidence, provides new insights, and enhances the generalizability of the results by analysing a ∼15-fold larger number of CHD events and a ∼20-fold larger number of CVD events compared with previously published data. Analogous to our result for the primary CVD outcome, associations of near and far wall CCA-IMT were similarly strong for the outcomes of CHD, stroke, and fatal CVD (see Supplementary material online, Table S5).
Subgroup analyses
Subgroup analyses revealed significantly higher hazard ratios for near and far wall CCA-IMT in females and in younger participants. Effect modification by sex has previously been described in the USE-IMT collaboration2 and in the Atherosclerosis Risk in Communities (ARIC) study,2,36,37 for overall CCA-IMT, and it is now also demonstrated for wall-specific measurements in our study. Effect modification by age has previously been described by the Carotid Atherosclerosis Progression Study (CAPS) reporting a considerably higher hazard ratio for CVD events in individuals <50 years of age compared with individuals ≥50 years of age for far wall CCA-IMT.38 In addition, the USE-IMT collaboration showed a hazard ratio for CVD of 1.40 (95% CI: 1.11–1.76) per SD higher CCA-IMT in individuals aged <45 years39 and therefore stronger than in the principal analysis that included participants of all ages.2 Furthermore, CCA-IMT was more strongly associated with CVD risk in individuals free from symptomatic CVD at baseline. It may be speculated that this is related to less ‘competition’ with other cardiovascular risk factors (e.g. history of diabetes, hypertension, or high cholesterol) predominant in high-risk subgroups, fewer medical visits, and a lower prevalence of medication intake.
Assessment of common carotid artery intima-media thickness at the near and far wall improves risk discrimination
Besides being independently associated with incident CVD, our analyses also demonstrate that CVD risk prediction is improved by additional assessment of wall-specific CCA-IMT. Both the near and the far wall CCA-IMT independently provided added predictive value, with the improvement in the C-index being the largest when information on CCA-IMT measured at both walls was taken into account. Also, the NRI improved after adding wall-specific CCA-IMT information, although improvement was similar when adding far wall CCA-IMT only and both near and far wall CCA-IMT to the base model. However, the NRI is criticized to be strongly dependent on the thresholds of risk categories. Only the MESA study has previously investigated the added value of wall-specific CCA-IMT for prediction of ‘hard’ cardiovascular events and reported non-significant C-index changes when assessing far wall CCA-IMT.11 Our study therefore crucially extends current evidence on this topic. Moreover, since near and far wall CCA-IMT can be measured simultaneously with little additional effort and costs, future studies may consider using both near and far wall CCA-IMT to assess cardiovascular risk.
Strengths and limitations
Strengths of our study are the access to large-scale participant-level data, enabling harmonization of outcome definitions, a consistent approach to statistical analyses across studies, and estimation of effect estimates with high precision. Furthermore, our analysis showed broadly similar strengths of associations across different types of study populations, thereby supporting generalizability of our findings to different settings. A limitation of our analysis is that only 16 out of 74 studies from the Proof-ATHERO consortium were included (nine studies in the multivariable adjusted analysis). This was mainly because over 70% of the Proof-ATHERO studies lacked CCA-IMT measurements at the near wall. However, our analysis included more than 15 times the number of events than in previously published studies.11,12 A further limitation of our study is that there were some differences in ultrasound protocols between studies; however, they did not impact the strengths of associations as shown in the subgroup analyses. Furthermore, it is possible that measuring far wall CCA-IMT at different insonation angles could be superior to measuring near and far wall CCA-IMT due to a higher reproducibility,1 but angle-specific data were too scarce to analyse them with adequate statistical power. Moreover, study baseline dates back to 1980 when ultrasound resolution was lower than nowadays. However, we did not detect differences in wall-specific CCA-IMT values depending on the date of baseline or other ultrasound methodologies. Other limitations include inherent between-study differences in the adjudication of incident events and the majority of study participants in this analysis originate from Europe and North America, thereby limiting the generalizability of our findings to other continents.
Conclusions
In conclusion, this large-scale individual-participant-data meta-analysis showed that the association of near and far wall CCA-IMT with incident CVD was positive, approximately linear, and similarly strong. Improvement in risk discrimination was highest when CCA-IMT was measured at both walls.
Supplementary Material
Acknowledgements
This paper was prepared using data of the Atherosclerosis Risk in Communities Study (ARIC), the Cardiovascular Health Study (CHS), and the Jackson Heart Study (JHS) obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) and does not necessarily reflect the opinions or views of the ARIC, CHS, JHS, or NHLBI. The work of AC is supported in part by Ministero della Salute ricerca corrente. Results of this paper are included in the first author’s (L.S.) PhD thesis submitted at the Medical University of Innsbruck.
Contributor Information
Lisa Seekircher, Institute of Health Economics, Department of Medical Statistics, Informatics, and Health Economics, Medical University of Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria.
Lena Tschiderer, Institute of Health Economics, Department of Medical Statistics, Informatics, and Health Economics, Medical University of Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria.
Lars Lind, Department of Medicine, Uppsala University, Uppsala, Sweden.
Maya S Safarova, Division of Cardiovascular Medicine, Department of Medicine, Froedtert and Medical College of Wisconsin, Milwaukee, WI, USA.
Maryam Kavousi, Department of Epidemiology, Erasmus University Medical Center, Rotterdam, The Netherlands.
M Arfan Ikram, Department of Epidemiology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Eva Lonn, Department of Medicine and Population Health Research Institute, McMaster University, Hamilton, Ontario, Canada; Hamilton General Hospital, Hamilton, Ontario, Canada.
Salim Yusuf, Department of Medicine and Population Health Research Institute, McMaster University, Hamilton, Ontario, Canada; Hamilton General Hospital, Hamilton, Ontario, Canada.
Diederick E Grobbee, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands.
John J P Kastelein, Department of Vascular Medicine, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands.
Frank L J Visseren, Department of Vascular Medicine, University Medical Center Utrecht, Utrecht, The Netherlands.
Matthew Walters, School of Medicine, Dentistry and Nursing, University of Glasgow, Glasgow, UK.
Jesse Dawson, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Peter Higgins, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Stefan Agewall, Department of Clinical Sciences, Division of Cardiology, Danderyd Hospital, Karolinska Institutet, Stockholm, Sweden; Institute of Clinical Sciences, University of Oslo, Oslo, Norway.
Alberico Catapano, Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy; IRCCS Multimedica, Milan, Italy.
Eric de Groot, Imagelabonline & Cardiovascular, Erichem, The Netherlands; Department of Gastroenterology and Hepatology, Amsterdam UMC-Academic Medical Centre, Amsterdam, The Netherlands.
Mark A Espeland, Department of Biostatistics and Data Science, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Gerhard Klingenschmid, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Dianna Magliano, Department of Epidemiology and Preventive Medicine, Monash University, Alfred Hospital, Melbourne, Australia.
Michael H Olsen, Department of Internal Medicine, Holbaek Hospital, University of Southern Denmark, Odense, Denmark.
David Preiss, Nuffield Department of Population Health, MRC Population Health Research Unit, Clinical Trial Service Unit, University of Oxford, Oxford, UK.
Dirk Sander, Department of Neurology, Benedictus Hospital Tutzing & Feldafing, Feldafing, Germany; Department of Neurology, Technische Universität München, Munich, Germany.
Michael Skilton, Faculty of Medicine and Health, Charles Perkins Centre, University of Sydney, Sydney, NSW, Australia.
Dorota A Zozulińska-Ziółkiewicz, Department of Internal Medicine and Diabetology, Poznan University of Medical Sciences, Poznan, Poland.
Muriel P C Grooteman, Department of Nephrology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, Amsterdam, The Netherlands.
Peter J Blankestijn, Department of Nephrology, University Medical Center Utrecht, Utrecht, The Netherlands.
Kazuo Kitagawa, Department of Neurology, Tokyo Women’s Medical University, Tokyo, Japan.
Shuhei Okazaki, Department of Neurology, Osaka University Graduate School of Medicine, Osaka, Japan.
Maria V Manzi, Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy.
Costantino Mancusi, Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy.
Raffaele Izzo, Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy.
Moise Desvarieux, Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA; METHODS Core, Centre de Recherche Epidémiologie et Statistique Paris Sorbonne Cité (CRESS), Institut National de la Santé et de la Recherche Médicale (INSERM) UMR 1153, Paris, France.
Tatjana Rundek, Department of Neurology, University of Miami Miller School of Medicine, Miami, FL, USA.
Hertzel C Gerstein, Department of Medicine and Population Health Research Institute, McMaster University, Hamilton, Ontario, Canada; Hamilton General Hospital, Hamilton, Ontario, Canada.
Michiel L Bots, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands.
Michael J Sweeting, Department of Health Sciences, University of Leicester, Leicester, UK; British Heart Foundation Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Papworth Road, Cambridge CB2 0BB, UK.
Matthias W Lorenz, Department of Neurology, Goethe University, Frankfurt am Main, Germany; Klinik für Neurologie, Krankenhaus Nordwest, Frankfurt am Main, Germany.
Peter Willeit, Institute of Health Economics, Department of Medical Statistics, Informatics, and Health Economics, Medical University of Innsbruck, Anichstraße 35, 6020 Innsbruck, Austria; British Heart Foundation Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Papworth Road, Cambridge CB2 0BB, UK.
Lead author biography
 Dr Lisa Seekircher works as a Postdoc at the Department of Medical Statistics, Informatics and Health Economics of the Medical University of Innsbruck, Austria. She has a background in mathematics and epidemiology. Her research mainly focuses on large-scale studies on cardiovascular and infectious disease outcomes.
Dr Lisa Seekircher works as a Postdoc at the Department of Medical Statistics, Informatics and Health Economics of the Medical University of Innsbruck, Austria. She has a background in mathematics and epidemiology. Her research mainly focuses on large-scale studies on cardiovascular and infectious disease outcomes.
Data availability
The data sets supporting the conclusions of this article are not made publicly available because of legal restrictions arising from the data distribution policy of the Proof-ATHERO consortium and from the bilateral agreements between the consortium’s coordinating centre and participating studies, but they may be requested directly from individual study investigators.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
This work was supported by the Austrian Science Fund (FWF; P 32488) and the Dr. Johannes-and-Hertha-Tuba Foundation.
Ethical approval
Studies that shared individual-participant data have obtained informed consent from the study participants and ethical approval from their respective institutional review boards.
References
- 1. Touboul P-J, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif J-C, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012;34:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Den Ruijter HM, Peters SAE, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CDA, Witteman JC, Moons KG, Bots ML. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA 2012;308:796–803. [DOI] [PubMed] [Google Scholar]
- 3. Tschiderer L, Seekircher L, Izzo R, Mancusi C, Manzi MV, Baldassarre D, Amato M, Tremoli E, Veglia F, Tuomainen T-P, Kauhanen J, Voutilainen A, Iglseder B, Lind L, Rundek T, Desvarieux M, Kato A, de Groot E, Aşçi G, Ok E, Agewall S, Beulens JWJ, Byrne CD, Calder PC, Gerstein HC, Gresele P, Klingenschmid G, Nagai M, Olsen MH, Parraga G, Safarova MS, Sattar N, Skilton M, Stehouwer CDA, Uthoff H, van Agtmael MA, van der Heijden AA, Zozulińska-Ziółkiewicz DA, Park H-W, Lee M-S, Bae J-H, Beloqui O, Landecho MF, Plichart M, Ducimetiere P, Empana JP, Bokemark L, Bergström G, Schmidt C, Castelnuovo S, Calabresi L, Norata GD, Grigore L, Catapano A, Zhao D, Wang M, Liu J, Ikram MA, Kavousi Ma, Bots ML, Sweeting MJ, Lorenz MW, Willeit P. Association of intima-media thickness measured at the common carotid artery with incident carotid plaque: individual participant data meta-analysis of 20 prospective studies. J Am Heart Assoc 2023;12:e027657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liao X, Norata GD, Polak JF, Stehouwer CDA, Catapano A, Rundek T, Ezhov M, Sander D, Thompson SG, Lorenz MW, Balakhonova T, Safarova M, Grigore L, Empana J-P, Lin H-J, McLachlan S, Bokemark L, Ronkainen K, Schminke U, Lind L, Willeit P, Yanez DN, Steinmetz H, Poppert H, Desvarieux M, Ikram MA, Johnsen SH, Iglseder B, Friera A, Xie W, Plichart M, Su T-C, Srinivasan SR, Schmidt C, Tuomainen T-P, Völzke H, Nijpels G, Willeit J, Franco OH, Suarez C, Zhao D, Ducimetiere P, Chien K-L, Robertson C, Bergström G, Kauhanen J, Dörr M, Dekker JM, Kiechl S, Sitzer M, Bickel H, Sacco RL, Hofman A, Mathiesen EB, Gabriel R, Liu J, Berenson G, Kavousi M, Price JF. Normative values for carotid intima media thickness and its progression: are they transferrable outside of their cohort of origin? Eur J Prev Cardiol 2016;23:1165–1173. [DOI] [PubMed] [Google Scholar]
- 5. Bots ML, Evans GW, Tegeler CH, Meijer R. Carotid intima-media thickness measurements: relations with atherosclerosis, risk of cardiovascular disease and application in randomized controlled trials. Chin Med J 2016;129:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS; American Society of Echocardiography Carotid Intima-Media Thickness Task Force . Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography carotid intima-media thickness task force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93–111. [DOI] [PubMed] [Google Scholar]
- 7. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986;74:1399–1406. [DOI] [PubMed] [Google Scholar]
- 8. Swijndregt AD vM, The SH, Gussenhoven EJ, Lancee CT, Rijsterborgh H, de Groot E, van der Steen AFW, Bom N, Ackerstaff RGA. An in vitro evaluation of the line pattern of the near and far walls of carotid arteries using B-mode ultrasound. Ultrasound Med Biol 1996;22:1007–1015. [DOI] [PubMed] [Google Scholar]
- 9. Wendelhag I, Gustavsson T, Suurküla M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol 1991;11:565–577. [DOI] [PubMed] [Google Scholar]
- 10. Wikstrand J. Methodological considerations of ultrasound measurement of carotid artery intima-media thickness and lumen diameter. Clin Physiol Funct Imaging 2007;27:341–345. [DOI] [PubMed] [Google Scholar]
- 11. Polak JF, Szklo M, O'Leary DH. Associations of coronary heart disease with common carotid artery near and far wall intima-media thickness: the multi-ethnic study of atherosclerosis. J Am Soc Echocardiogr 2015;28:1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Cardiovascular Health Study Collaborative Research Group . Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 13. Tschiderer L, Seekircher L, Klingenschmid G, Izzo R, Baldassarre D, Iglseder B, Calabresi L, Liu J, Price JF, Bae J-H, Brouwers FP, de Groot E, Schmidt C, Bergström G, Aşçi G, Gresele P, Okazaki S, Kapellas K, Landecho MF, Sattar N, Agewall S, Zou Z-Y, Byrne CD, Nanayakkara PWB, Papagianni A, Witham MD, Bernal E, Ekart R, van Agtmael MA, Neves MF, Sato E, Ezhov M, Walters M, Olsen MH, Stolić R, Zozulińska-Ziółkiewicz DA, Hanefeld M, Staub D, Nagai M, Nieuwkerk PT, Huisman MV, Kato A, Honda H, Parraga G, Magliano D, Gabriel R, Rundek T, Espeland MA, Kiechl S, Willeit J, Lind L, Empana JP, Lonn E, Tuomainen T-P, Catapano A, Chien K-L, Sander D, Kavousi M, Beulens JWJ, Bots ML, Sweeting MJ, Lorenz MW, Willeit P. The Prospective Studies of Atherosclerosis (Proof-ATHERO) consortium: design and rationale. Gerontology 2020;66:447–459. [DOI] [PubMed] [Google Scholar]
- 14. Wood AM, White I, Thompson SG, Lewington S, Danesh J. Regression dilution methods for meta-analysis: assessing long-term variability in plasma fibrinogen among 27,247 adults in 15 prospective studies. Int J Epidemiol 2006;35:1570–1578. [DOI] [PubMed] [Google Scholar]
- 15. Tipton E, Shuster J. A framework for the meta-analysis of Bland–Altman studies based on a limits of agreement approach. Stat Med 2017;36:3621–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 17. Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med 1991;10:1025–1035. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693–2708. [DOI] [PubMed] [Google Scholar]
- 20. Riley RD, Price MJ, Jackson D, Wardle M, Gueyffier F, Wang J, Staessen JA, White IR. Multivariate meta-analysis using individual participant data. Res Synth Methods 2015;6:157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burgess S, White IR, Resche-Rigon M, Wood AM. Combining multiple imputation and meta-analysis with individual participant data. Stat Med 2013;32:4499–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fibrinogen Studies Collaboration . Correcting for multivariate measurement error by regression calibration in meta-analyses of epidemiological studies. Stat Med 2009;28:1067–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 24. Pennells L, Kaptoge S, White IR, Thompson SG, Wood AM, Tipping RW, Folsom AR, Couper DJ, Ballantyne CM, Coresh J, Goya Wannamethee S, Morris RW, Kiechl S, Willeit J, Willeit P, Schett G, Ebrahim S, Lawlor DA, Yarnell JW, Gallacher J, Cushman M, Psaty BM, Tracy R, Tybjærg-Hansen A, Price JF, Lee AJ, McLachlan S, Khaw K-T, Wareham NJ, Brenner H, Schöttker B, Müller H, Jansson J-H, Wennberg P, Salomaa V, Harald K, Jousilahti P, Vartiainen E, Woodward M, D'Agostino RB, Bladbjerg Ee-M, Jørgensen T, Kiyohara Y, Arima H, Doi Y, Ninomiya T, Dekker JM, Nijpels G, Stehouwer CDA, Kauhanen J, Salonen JT, Meade TW, Cooper JA, Cushman M, Folsom AR, Psaty BM, Shea S, Döring A, Kuller LH, Grandits G, Gillum RF, Mussolino M, Rimm EB, Hankinson SE, Manson JE, Pai JK, Kirkland S, Shaffer JA, Shimbo D, Bakker SJL, Gansevoort RT, Hillege HL, Amouyel P, Arveiler D, Evans A, Ferrières J, Sattar N, Westendorp RG, Buckley BM, Cantin B, Lamarche B, Barrett-Connor E, Wingard DL, Bettencourt R, Gudnason V, Aspelund T, Sigurdsson G, Thorsson B, Kavousi M, Witteman JC, Hofman A, Franco OH, Howard BV, Zhang Y, Best L, Umans JG, Onat A, Sundström J, Michael Gaziano J, Stampfer M, Ridker PM, Michael Gaziano J, Ridker PM, Marmot M, Clarke R, Collins R, Fletcher A, Brunner E, Shipley M, Kivimäki M, Ridker PM, Buring J, Cook N, Ford I, Shepherd J, Cobbe SM, Robertson M, Walker M, Watson S, Alexander M, Butterworth AS, Angelantonio ED, Gao P, Haycock P, Kaptoge S, Pennells L, Thompson SG, Walker M, Watson S, White IR, Wood AM, Wormser D, Danesh J. Assessing risk prediction models using individual participant data from multiple studies. Am J Epidemiol 2014;179:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parzen M, Lipsitz SR. A global goodness-of-fit statistic for Cox regression models. Biometrics 1999;55:580–584. [DOI] [PubMed] [Google Scholar]
- 26. Pennells L, Kaptoge S, Wood A, Sweeting M, Zhao X, White I, Burgess S, Willeit P, Bolton T, Moons KGM, van der Schouw YT, Selmer R, Khaw K-T, Gudnason V, Assmann G, Amouyel P, Salomaa V, Kivimaki M, Nordestgaard BG, Blaha MJ, Kuller LH, Brenner H, Gillum RF, Meisinger C, Ford I, Knuiman MW, Rosengren A, Lawlor DA, Völzke H, Cooper C, Marín Ibañez A, Casiglia E, Kauhanen J, Cooper JA, Rodriguez B, Sundström J, Barrett-Connor E, Dankner R, Nietert PJ, Davidson KW, Wallace RB, Blazer DG, Björkelund C, Donfrancesco C, Krumholz HM, Nissinen A, Davis BR, Coady S, Whincup PH, Jørgensen T, Ducimetiere P, Trevisan M, Engström G, Crespo CJ, Meade TW, Visser M, Kromhout D, Kiechl S, Daimon M, Price JF, Gómez de la Cámara A, Wouter Jukema J, Lamarche B, Onat A, Simons LA, Kavousi M, Ben-Shlomo Y, Gallacher J, Dekker JM, Arima H, Shara N, Tipping RW, Roussel R, Brunner EJ, Koenig W, Sakurai M, Pavlovic J, Gansevoort RT, Nagel D, Goldbourt U, Barr ELM, Palmieri L, Njølstad I, Sato S, Monique Verschuren WM, Varghese CV, Graham I, Onuma O, Greenland P, Woodward M, Ezzati M, Psaty BM, Sattar N, Jackson R, Ridker PM, Cook NR, D'Agostino RB, Thompson SG, Danesh J, Di Angelantonio E, Tipping RW, Simpson LM, Pressel SL, Couper DJ, Nambi V, Matsushita K, Folsom AR, Shaw JE, Magliano DJ, Zimmet PZ, Knuiman MW, Whincup PH, Wannamethee SG, Willeit J, Santer P, Egger G, Casas JP, Amuzu A, Ben-Shlomo Y, Gallacher J, Tikhonoff V, Casiglia E, Sutherland SE, Nietert PJ, Cushman M, Psaty BM, Søgaard AJ, Håheim LL, Ariansen I, Tybjærg-Hansen A, Jensen GB, Schnohr P, Giampaoli S, Vanuzzo D, Panico S, Palmieri L, Balkau B, Bonnet F, Marre M, de la Cámara AG, Rubio Herrera MA, Friedlander Y, McCallum J, McLachlan S, Guralnik J, Phillips CL, Guralnik J, Guralnik J, Guralnik J, Khaw K-T, Wareham N, Schöttker B, Saum K-U, Holleczek B, Nissinen A, Tolonen H, Giampaoli S, Donfrancesco C, Vartiainen E, Jousilahti P, Harald K, D’Agostino RB, Massaro JM, Pencina M, Vasan R, D’Agostino RB, Massaro JM, Pencina M, Vasan R, Kayama T, Kato T, Oizumi T, Jespersen J, Møller L, Bladbjerg EM, Chetrit A, Rosengren A, Wilhelmsen L, Björkelund C, Lissner L, Nagel D, Dennison E, Kiyohara Y, Ninomiya T, Doi Y, Rodriguez B, Nijpels G, Stehouwer CDA, Sato S, Kazumasa Y, Iso H, Goldbourt U, Salomaa V, Vartiainen E, Kurl S, Tuomainen T-P, Salonen JT, Visser M, Deeg DJH, Meade TW, Nilsson PM, Hedblad B, Melander O, De Boer IH, DeFilippis AP, Verschuren WMM, Sattar N, Watt G, Meisinger C, Koenig W, Koenig W, Meisinger C, Verschuren WMM, Rosengren A, Kuller LH, Tverdal A, Gillum RF, Cooper JA, Kirkland S, Shimbo D, Shaffer J, Sato S, Kazumasa Y, Iso H, Ducimetiere P, Bakker SJL, van der Harst P, Hillege HL, Crespo CJ, Amouyel P, Dallongeville J, Assmann G, Schulte H, Trompet S, Smit RAJ, Stott DJ, van der Schouw YT, Després J-P, Cantin B, Dagenais GR, Laughlin G, Wingard D, Khaw K-T, Trevisan M, Aspelund T, Eiriksdottir G, Gudmundsson EF, Ikram A, van Rooij FJA, Franco OH, Rueda-Ochoa OL, Muka T, Glisic M, Tunstall-Pedoe H, Völzke H, Howard BV, Zhang Y, Jolly S, Gallacher J, Davey-Smith G, Can G, Yüksel H, Nakagawa H, Morikawa Y, Miura K, Njølstad I, Ingelsson M, Giedraitis V, Ridker PM, Gaziano JM, Kivimaki M, Shipley M, Brunner EJ, Shipley M, Arndt V, Brenner H, Cook N, Ridker PM, Ford I, Sattar N, Ibañez AM, Geleijnse JM. Equalization of four cardiovascular risk algorithms after systematic recalibration: individual-participant meta-analysis of 86 prospective studies. Eur Heart J 2019;40:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. StataCorp LLC . Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 28. Wong M, Edelstein J, Wollman J, Bond MG. Ultrasonic-pathological comparison of the human arterial wall. Verification of intima-media thickness. Arterioscler Thromb 1993;13:482–486. [DOI] [PubMed] [Google Scholar]
- 29. Gamble G, Beaumont B, Smith H, Zorn J, Sanders G, Merrilees M, MacMahon S, Sharpe N. B-mode ultrasound images of the carotid artery wall: correlation of ultrasound with histological measurements. Atherosclerosis 1993;102:163–173. [DOI] [PubMed] [Google Scholar]
- 30. Bots ML, Evans GW, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke 2003;34:2985–2994. [DOI] [PubMed] [Google Scholar]
- 31. Dogan S, Duivenvoorden R, Grobbee DE, Kastelein JJP, Shear CL, Evans GW, Visseren FL, Bots ML. Completeness of carotid intima media thickness measurements depends on body composition: the RADIANCE 1 and 2 trials. J Atheroscler Thromb 2010;17:526–535. [DOI] [PubMed] [Google Scholar]
- 32. Bots ML, de Jong PT, Hofman A, Grobbee DE. Left, right, near or far wall common carotid intima-media thickness measurements: associations with cardiovascular disease and lower extremity arterial atherosclerosis. J Clin Epidemiol 1997;50:801–807. [DOI] [PubMed] [Google Scholar]
- 33. Masawa N, Glagov S, Zarins CK. Quantitative morphologic study of intimal thickening at the human carotid bifurcation: I. Axial and circumferential distribution of maximum intimal thickening in asymptomatic, uncomplicated plaques. Atherosclerosis 1994;107:137–146. [DOI] [PubMed] [Google Scholar]
- 34. Tajik P, Meijer R, Duivenvoorden R, Peters SAE, Kastelein JJ, Visseren FJ, Crouse JR, Palmer MK, Raichlen JS, Grobbee DE, Bots ML. Asymmetrical distribution of atherosclerosis in the carotid artery: identical patterns across age, race, and gender. Eur J Prev Cardiol 2012;19:687–697. [DOI] [PubMed] [Google Scholar]
- 35. Stensland-Bugge E, Bønaa KH, Joakimsen O. Reproducibility of ultrasonographically determined intima-media thickness is dependent on arterial wall thickness. The Tromsø study. Stroke 1997;28:1972–1980. [DOI] [PubMed] [Google Scholar]
- 36. Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2000;151:478–487. [DOI] [PubMed] [Google Scholar]
- 37. Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) study, 1987–1993. Am J Epidemiol 1997;146:483–494. [DOI] [PubMed] [Google Scholar]
- 38. Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006;37:87–92. [DOI] [PubMed] [Google Scholar]
- 39. Eikendal ALM, Groenewegen KA, Anderson TJ, Britton AR, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Holewijn S, Ikeda A, Kitagawa K, Kitamura A, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Dekker JM, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CDA, Hoefer IE, Peters SAE, Bots ML, den Ruijter HM; USE-IMT Project Group . Common carotid intima-media thickness relates to cardiovascular events in adults aged <45 years. Hypertension 2015;65:707–713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets supporting the conclusions of this article are not made publicly available because of legal restrictions arising from the data distribution policy of the Proof-ATHERO consortium and from the bilateral agreements between the consortium’s coordinating centre and participating studies, but they may be requested directly from individual study investigators.