Abstract
Background:
Epidemiological studies have reported associations of anti-androgenic phthalate metabolite concentrations with later onset of male puberty, but few have assessed associations with progression.
Objectives:
We examined the association of prepubertal urinary phthalate metabolite concentrations with trajectories of pubertal progression among Russian boys.
Methods:
At enrollment (ages 8–9 years), medical history, dietary, and demographic information were collected. At entry and annually to age 19 years, physical examinations including testicular volume (TV) were performed and spot urines collected. Each boy’s prepubertal urine samples were pooled, and 15 phthalate metabolites were quantified by isotope dilution LC-MS/MS at Moscow State University. Metabolites of anti-androgenic parent phthalates were included: butylbenzyl (BBzP), di-n-butyl (DnBP), diisobutyl (DiBP), di(2-ethylhexyl) (DEHP) and diisononyl (DiNP) phthalates. We calculated the molar sums of DEHP, DiNP, and all AAP metabolites. We used group-based trajectory models (GBTMs) to identify subgroups of boys who followed similar pubertal trajectories from ages 8 to19 years based on annual TV. We used multinomial and ordinal regression models to evaluate whether prepubertal log-transformed phthalate metabolite concentrations were associated with slower or faster pubertal progression trajectories, adjusting for covariates.
Results:
304 boys contributed a total of 752 prepubertal urine samples (median 2, range: 1–6) for creation of individual pools. The median length of follow-up was 10.0 years; 79% of boys were followed beyond age 15. We identified three pubertal progression groups: slower (34%), moderate (43%), and faster (23%) progression. A standard deviation increase in urinary log-monobenzyl phthalate (MBzP) concentrations was associated with higher adjusted odds of being in the slow versus faster pubertal progression trajectory (aOR 1.47, 95% CI 1.06–2.04). None of the other phthalate metabolites were associated with pubertal progression.
Conclusions:
On average, boys with higher concentrations of prepubertal urinary MBzP had a slower tempo of pubertal progression, perhaps attributable to the disruption of androgen-dependent biological pathways.
Keywords: phthalates, endocrine disrupting chemicals, environment, boys, puberty, Tanner staging, pubertal progression
1. Introduction1
Phthalates are a family of synthetic chemicals widely used as plasticizers in vinyl and other consumer products, (Heudorf, Mersch-Sundermann et al. 2007) as well as in many personal care products (Heudorf, Mersch-Sundermann et al. 2007, Wallner, Kundi et al. 2016).
Consequently, human exposure to phthalates is ubiquitous, and a range of phthalate metabolites have been detected in urine samples worldwide at all ages (Koch and Calafat 2009, Wang, Zhu et al. 2019). Although phthalates have short biological half-lives of hours and are rapidly excreted in urine, daily exposure occurs because of their presence in many common household products (Frederiksen, Skakkebaek et al. 2007). Moreover, newly developed phthalates are incorporated in household and personal care products, leading to on-going exposure to new phthalates (Koch, Ruther et al. 2017, Wang, Zhu et al. 2019).
Data in rodent have demonstrated that gestational exposure to anti-androgenic phthalates adversely affects male reproductive development. Specifically, the “phthalate syndrome,” consisting of reproductive tract malformations and reduced anogenital distance (a sign of undervirilization) has been linked to gestational dosing with di-n-butyl, di(2-ethylhexyl) (DEHP) and butylbenzyl (BBzP) phthalate (Fisher, Macpherson et al. 2003, Foster 2006). This syndrome is a result of reduced fetal testicular testosterone production during a critical window for male reproductive tract development (Howdeshell, Rider et al. 2008). Phthalate exposure during the postnatal juvenile period has been linked to later pubertal onset and poorer semen quality (Kay, Bloom et al. 2014). These experimental results contributed to phthalates being classified as “endocrine disrupting chemicals” (Gore, Chappell et al. 2015) and the subsequent identification of additional phthalates with anti-androgenic properties (Howdeshell, Hotchkiss et al. 2017).
Although there is epidemiological evidence that prenatal and peri-pubertal exposure to anti-androgenic phthalate (AAP) metabolites affects male reproductive endpoints (Radke, Braun et al. 2018), including pubertal milestones (Mouritsen, Frederiksen et al. 2013, Ferguson, Peterson et al. 2014, Zhang, Cao et al. 2015, Kasper-Sonnenberg, Wittsiepe et al. 2017, Berger, Eskenazi et al. 2018, Cathey, Watkins et al. 2020), few studies have assessed the tempo of pubertal progression (Zhang, Cao et al. 2015, Kasper-Sonnenberg, Wittsiepe et al. 2017, Cathey, Watkins et al. 2020).
In prior analyses of our prospective cohort of Russian boys, the Russian Children’s Study (RCS), we found that higher prepubertal urinary phthalate metabolite concentrations, particularly AAP metabolites, were associated with later pubertal onset, (Burns, Sergeyev et al. 2022). However, whether AAP metabolites also affect the tempo of pubertal progression remains unknown. This could have long-term impacts on male reproductive health, affecting sexual function and fertility (Radke, Braun et al. 2018). To address this gap, in the same RCS cohort we evaluated whether the aforementioned prepubertal urinary AAP metabolite concentrations and were associated with trajectories of pubertal progression as reflected by testicular volume (TV) as a key physical indicator of pubertal maturity.
2. Materials and Methods
2.1. Study population
The study population included 516 boys from the RCS, a prospective study designed to evaluate the long-term effects of environmental contaminants on male growth and pubertal development (Hauser, Williams et al. 2005). As described previously (Sergeyev, Burns et al. 2017, Burns, Williams et al. 2020), boys ages 8–9 years were recruited from a Samara region city in southwestern Russia (approximately 950 km southeast of Moscow) from 2003 to 2005 and were ages 18–19 at the end of follow-up. Of the 506 boys with at least one urinary phthalate measurement, 320 (63%) provided a prepubertal urine for phthalate metabolite determination. Exclusion criteria were a history of a chronic condition that could impact puberty (n=3) and/or incomplete birth and parental data (n=13). The final sample consisted of 304 boys. A series of baseline questionnaires were completed by each parent/guardian, regarding early childhood and medical history, sociodemographic factors, and food frequency quantification (Rockett and Colditz 1997, Martinchik, Baturin et al. 1998, Hauser, Williams et al. 2005). Birth data for each boy was obtained through medical records. Institutional Review Boards at the Chapaevsk Medical Association, Harvard T.H. Chan School of Public Health, Nemours Children’s Health, and Brigham and Women’s Hospital reviewed and approved the study protocol. Written informed consent was obtained from each boy’s parent or legal guardian, with signed assent from each boy. At age 18 years, each boys signed an informed consent.
2.2. Physical examination and pubertal assessment
A reproductive endocrinologist (O.S.) conducted a standardized anthropometric examination and pubertal staging at study enrollment for each boy. For annual follow-ups, physical measurements were obtained by a nurse and O.S. assessed pubertal staging. Genitalia (G) and pubic hair (P) staging were graded on a scale from 1 (immature) to 5 (sexually mature), according to the Tanner and Whitehouse method (Tanner and Whitehouse 1976). O.S. assessed testicular volume (TV) for each testis separately by both palpation and measurement, and comparison to a Prader orchidometer (Prader 1966; Genentech, Inc., San Francisco, CA, USA). The closest bead volume was used in cases where the TV was between bead sizes. TVs larger than 25 mL were assigned a volume of 30 mL. Each annual TV assessment was conducted without reference to values from previous years. Pubertal onset and sexual maturity were defined as TV>3 mL and TV≥20 mL, respectively, for either testis (Joustra, van der Plas et al. 2015).
2.3. Urinary phthalate metabolite assessment
Each boy provided a spot urine sample at baseline and annual study visits. The samples were collected, aliquoted, and frozen as described previously (Burns, Sergeyev et al. 2022). Pubertal classifications were based on pre-specified G and TV criteria, where either TV=1, 2 and G=1, 2 or TV=3 and G=1 determined prepuberty. The urine samples ascertained within the first ten months of enrollment for 215) boys were unavailable for analysis. However, 304 boys provided 752 prepubertal urine samples (median=2, range 1–6) for pooling. Annual prepubertal aliquots were combined to create pools for each boy, then measured for specific gravity (sg), and aliquoted into 1.8 ml polypropylene cryovials for storage at −35°C. Frozen samples were shipped on dry ice to Moscow State University (MSU) for quantification of urinary phthalate metabolite concentrations using online liquid chromatography tandem mass spectrometry (LC-MS/MS) according to the methods of Koch et al. (Koch, Ruther et al. 2017). Fifteen urinary phthalate metabolites were measured, including monoethyl phthalate (MEP), mono-isobutyl phthalate (MiBP), monobenzyl phthalate (MBzP), mono-n-butyl phthalate (MnBP), mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-(hydroxy-isononyl) phthalate (MHiNP), mono-(oxo-isononyl) phthalate (MOiNP), mono-(carboxy-isooctyl) phthalate (MCOP), mono-(hydroxy-isodecyl) phthalate (MHiDP), mono-(oxo-isodecyl) phthalate (MOiDP), mono-(carboxy-isononyl) phthalate (MCNP), and mono-(3-carboxypropyl) phthalate (MCPP) (see Table 1 for parent phthalates and their metabolites). Details on how calibrations were performed can be found in Minguez-Alarcón et al (2022) and Burns et al (2022).
Table 1.
Descriptive statistics on 304 boys participating in the Russian Children’s Study with prepubertal phthalate measurements
| n = 304 boys | |
|---|---|
|
| |
| Median Assessments [IQR] | 9 [8, 11] |
|
| |
| Baseline Demographics | |
|
| |
| Age at enrollment, mean (SD) | 8.23 (0.42) |
| WHO age-adjusted BMI z-score, mean (SD) | −0.36 (1.24) |
| Total calories consumed per day (kcal), median [IQR] | 2551 [2033, 3258] |
| Birthweight (kg), mean (SD) | 3.26 (0.52) |
| Duration of breast feeding (weeks), median [IQR] | 13 [4, 26] |
| Monthly family income ≤250 USD, n (%) | 184 (60.5) |
| Highest parental education, n (%) High school or less | 27 (9.0) |
| Some college or junior college | 184 (61.1) |
| College graduate | 90 (29.9) |
|
| |
| Prepubertal Phthalate Concentrations | Median [IQR] |
|
| |
| Diethyl (DEP) Metabolite, (ng/mL) | |
|
| |
| Monoethyl, MEP | 91.18 [51.38, 177.30] |
|
| |
| Di-n-butyl (DnBP) Metabolite, (ng/mL) | |
|
| |
| Mono-n-butyl, MnBP | 196.47 [125.38, 301.56] |
|
| |
| Diisobutyl (DiBP) Metabolite, (ng/mL) | |
|
| |
| Mono-isobutyl, MiBP | 57.07 [34.33, 87.66] |
|
| |
| Butyl Benzyl (BBzP) Metabolite, (ng/mL) | |
|
| |
| Monobenzyl, MBzP | 6.19 [2.89, 15.15] |
|
| |
| Di(2-ethylhexyl) (DEHP) Metabolites, (ng/mL) | |
|
| |
| Mono-(2-ethylhexyl), MEHP | 13.21 [7.23, 23.02] |
| Mono-(2-ethyl-5-hydroxyhexyl), MEHHP | 79.12 [51.00, 121.34] |
| Mono-(2-ethyl-5-oxohexyl), MEOHP | 65.83 [41.67, 105.30] |
| Mono-(2-ethyl-5-carboxypentyl), MCEPP | 159.44 [104.85, 260.10] |
|
| |
| Di-iso-nonylphthalate (DiNP) Metabolites, (ng/mL) | |
|
| |
| Mono-hydroxyisononyl, MHiNP | 8.21 [4.93, 14.81] |
| Mono-oxo-isononyl, MOiNP | 2.90 [1.73, 5.88] |
| Mono-carboxy-isononyl, MCOP | 6.41 [3.28, 11.86] |
|
| |
| Diisodecyl (DiDP) Metabolites, (ng/mL) | |
|
| |
| Mono-hydroxy-isodecyl, MHiDP | 4.09 [2.09, 8.24] |
| Mono-oxo-isodecyl, MOiDP | 0.55 [0.30, 0.91] |
| Mono-carboxy-isodecyl, MCNP | 0.80 [0.52, 1.39] |
|
| |
| Other Metabolite, (ng/mL) | |
|
| |
| Mono-(3-carboxypropyl), MCPP | 11.02 [6.56, 18.89] |
|
| |
| Molar Sums, (μmol/L) | |
|
| |
| DEHP Metabolites, ΣDEHP | 1.08 [0.71, 1.75] |
| DiNP Metabolites, ΣDiNP | 0.06 [0.03, 0.10] |
| DiDP Metabolites, ΣDiDP | 0.02 [0.01, 0.03] |
| Anti-androgenic phthalates, ΣAA | 2.49 [1.59, 3.60] |
Means and standard deviations are shown for normally distributed variables. Medians and IQRs are shown for nonnormally distributed variables. SD: Standard Deviation; WHO: World Health Organization; BMI: Body Mass Index; IQR: Interquartile Range; kg: kilograms; ng/mL: Nanograms per milliliter; μmol/L: micromole per liter; Data for some characteristics were not available for all boys: Total calories consumed per day (0.7%), Birthweight (0.3%), Duration of breast feeding (1.6%), and Highest parental education (1%); percentages are calculated based on those with available data.
Analyses were performed in 34 batches of 50 samples. Each batch included two randomly selected repeated samples, two quality control (QC) samples (established low and high concentrations of each metabolite), and 1 field blank. A value of zero was assigned for a peak that was missing or could not be determined. Limits of detection (LOD) were set as a signal-to-noise (S/N) ratio of 3 in a urine matrix. The between batch relative SD percent (RSD%: (SD/mean)*100) across the 34 batches for QChigh ranged from 8.1–19.1%, except for MEHP (24.3%), whereas RSD% for QClow were ≤20%, except for MEHP (41.6%) and DiDP metabolites (23.6 – 57.8%) one of which had 23% of values below the LOD.
2.4. Statistical Analysis
We used group-based trajectory models (GBTMs) to identify subgroups of boys who followed similar pubertal progression trajectories from ages 8 to19 years using all TV measurements of the larger testis (Jones, Nagin et al. 2001, Nagin 2009). GBTMs assume that the population consists of individuals following distinct trajectories and assigns a posterior probability for each trajectory to every individual (Nagin 2009). A posterior probability represents the likelihood that an individual belongs to a specific trajectory given their observed outcomes (Nagin 2009). The posterior probabilities for each individual sum to one, with the highest posterior probability deciding final trajectory group membership (Nagin 2009). We estimated trajectories of pubertal progression as a polynomial function of age using a censored normal distribution with lower and upper limits at 1 and 30 mL, excluding covariates that may have predicted group membership. Our estimation of trajectories included all available TV measurements, which meant that measures before pubertal onset and/or after sexual maturity may have been included; this approach was anticipated to provide the most accurate characterization of trajectories by maximizing the number of observations and providing the widest age range.
Selecting the final GBTM is an iterative process that involves two stages: determining the maximum number of groups and the shape of each group’s trajectory over time (Nagin 2009). In the first stage, we assumed a quadratic function of age and used the Bayesian information criterion (BIC) to identify the optimal number of groups, starting with the smallest number of groups and adding one group at a time. Once we selected the optimal number of groups, we used the BIC to determine the optimal polynomial function of the trajectory within each group. This process yields a predicted probability of belonging to each group for each boy, and we assigned each boy to the group with maximum probability. In both stages of the process, the BIC values are used as an approximation to the log Bayes Factor (2ΔBIC), with the best fitting model having the smallest negative value (Nagin 2009). In our application of GBTMs, this iterative procedure resulted in a five-group model that we believed overfit the data, and yielded some implausible trajectories based on our observed data. We therefore selected a three-group model as the most plausible based on subject matter knowledge and consistency with data from our cohort. A cubic shape was identified for each trajectory group, which is concordant with earlier research that identified a sigmoid shape for pubertal progression (Marceau, Ram et al. 2011).
These group assignments were then used as outcomes in unadjusted multinomial and ordinal regression models to evaluate whether prepubertal concentrations of AAP metabolites were associated with slower trajectories of pubertal progression. The five AAP diesters evaluated were: DnBP [metabolite MnBP (ng/mL)], DiBP [metabolite MiBP (ng/mL)], BBzP [metabolite MBzP (ng/mL)], the molar sum of DEHP metabolites (∑DEHP, μmol/L), the molar sum of DiNP metabolites (∑DiNP, μmol/L), and the molar sum of all metabolites of the five AAPs with DiNP metabolites weighted 0.43 because of its weaker relative impact on fetal testosterone production (∑AAP, μmol/L) (Hannas, Lambright et al. 2011). In addition, we performed analyses examining the associations between prepubertal metabolites of phthalates without recognized anti-androgenic activity: MEP (ng/mL), MCPP (ng/mL), and ∑DiDP (μmol/L). Of note, MCPP is a metabolite of multiple parent phthalates, both HMW and LMW phthalates, e.g., DnBP, DiNP, DiDP, thus representing potential exposures to both AAPs and non-AAPs. Because prepubertal phthalates concentrations were skewed we log transformed them then standardized by dividing this value by the standard deviation of the natural log-transformed prepubertal phthalate concentration. Adjusted models controlled for baseline age, WHO age-adjusted BMI z-score, daily kilocalorie intake, birthweight, breastfeeding history, monthly family income, and highest parental education; these covariates were selected based on a directed acyclic graph (DAG) considering prior literature including previous findings in this cohort (Burns, Lee et al. 2016, Burns, Sergeyev et al. 2022). For ordinal models, we tested whether the proportional odds assumption was met (Brant 1990). For sensitivity analyses, we fit unadjusted and adjusted models for each of the DEHP, DiNP, and DiDP metabolites.
We tabulated descriptive statistics for the study population as well as the GBTM-generated groups. We also summarized the predicted GBTM pubertal trajectories graphically as estimated mean TV from ages 8 to 19 for each subgroup, with 95% confidence intervals. Tabulated results from multinomial and ordinal analyses included odds ratios with corresponding 95% confidence intervals. Statistical analyses were conducted using Stata (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.) and R (R Core Team, 2019) software.
3. Results
3.1. Study population
Table 1 presents a summary of participants’ birthweight and breast-feeding history, and baseline anthropometric measurements, diet and household characteristics, and missing data, which was minimal. At study entry (age 8–9 years), the majority of boys were within the normal range for height and BMI, with 13% overweight (de Onis, Onyango et al. 2007). Approximately 50% of the boys had prenatal exposure to household and/or maternal tobacco smoke, and for 13% maternal alcohol consumption during pregnancy had been reported. Most parents had more than a secondary education (91%), and approximately a third of households were in the lowest income category at the time of study entry. The boys’ dietary intake of total calories was within recommended ranges for boys in this age group (Food and Nutrition Board 2006). At ages 8–9 years, pubarche (P2) had occurred in 7%, 8% were in G2, and none had TV>3 mL. The median length of follow-up was 10.0 years (25th-75th percentile: 8.0–10.9 years), and 79% of boys were followed beyond age 15. Based on fitting interval censored regression models to each pubertal outcome, the estimated mean (95% CI) age of pubertal onset by P2, G2, and TV>3mL was 12.1 (11.9, 12.3), 10.3 (10.1, 10.5), and 11.1 (11.0, 11.3) years, respectively. The estimated mean (95% CI) age of sexual maturity by P5, G5, and TV≥20 mL was 16.3 (16.0, 16.5), 15.0 (14.8, 15.1), and 14.2 (14.0, 14.3) years, respectively.
3.2. Urinary phthalate metabolites
The age range of the boys who contributed to the prepubertal urine pools were 8 to 13 (median 9) years, and the time period spanned 2004–2009 (median 2005). Most of the phthalate metabolite measurements were >LOD, except for the DiDP metabolite MOiDP (23% <LOD) (Table 1). Urinary concentrations of MBzP, DiNP, and DiDP metabolites, were at lower concentrations than other metabolites, with medians <10 ng/mL, <10 ng/mL, and <4 ng/mL, respectively. Most of the phthalate metabolites were moderately positively correlated [Spearman r (rs) 0.30 to 0.64], with higher correlations among the metabolites of DEHP (rs 0.71 to 0.96) and DiNP (rs 0.88 to 0.92). Phthalate metabolites were weakly correlated with organochlorine chemicals and blood lead, as previously reported (Burns, Sergeyev et al. 2022).
We previously reported that urinary phthalates in the RCS cohort differed from European (German and Polish) and U.S. cohorts of similar ages and temporal assessments (Burns, Sergeyev et al. 2022). Briefly, the urinary concentrations of MEP, MnBP, and DEHP metabolites were at least two-fold higher than in European (Kasper-Sonnenberg, Koch et al. 2014, Gari, Koch et al. 2019) and U.S. (CDC 2019) cohorts. Although urinary MBzP concentrations in the RCS were comparable to European childhood concentrations (Kasper-Sonnenberg, Koch et al. 2014, Gari, Koch et al. 2019), they were three-fold lower than in the US NHANES (CDC 2019) (geometric means (GM) 6.6 versus 21.3 ng/mL, respectively). Urinary concentrations of MCOP and MCNP were low across all cohorts, including the RCS, GM <10 ng/mL and ≤4.5 ng/mL, respectively (Kasper-Sonnenberg, Koch et al. 2014, Gari, Koch et al. 2019) (CDC 2019).
3.3. Pubertal progression
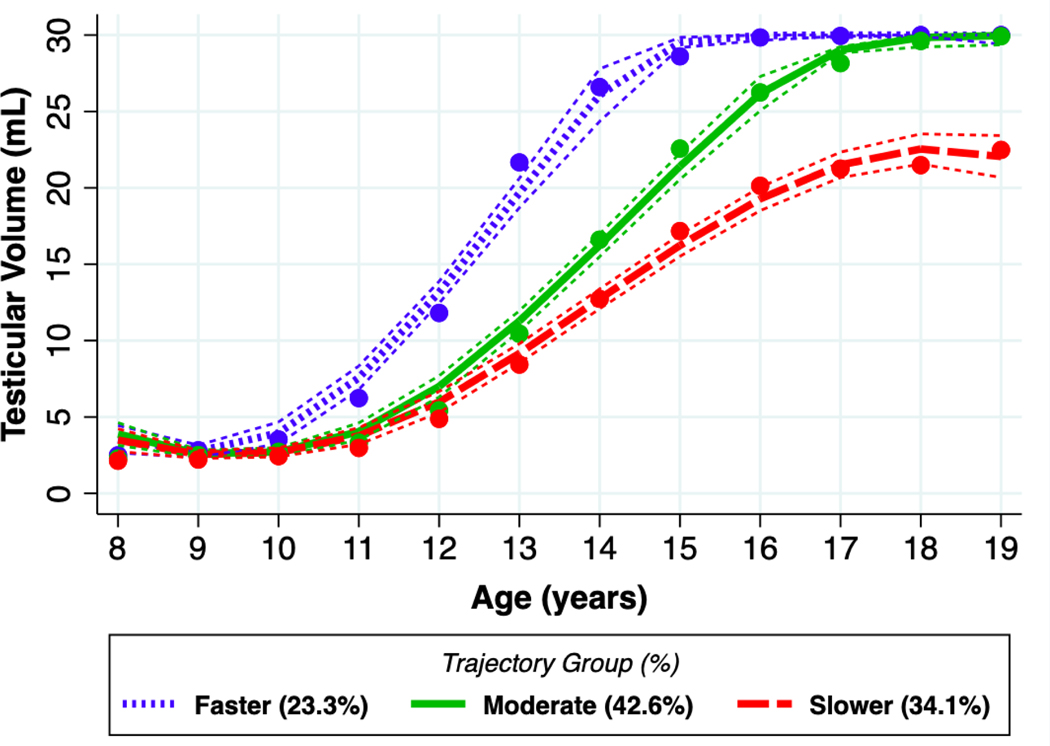
The final GBTM identified three pubertal trajectory groups, with 23% of the boys classified in the ‘faster’ group (average age at sexual maturity was approximately 13 years old). The ‘moderate’ group accounted for 43% of the study population, with an average age at sexual maturity between 14–15 years old. Lastly, the ‘slower’ group, accounting for 34% of the study population, had an average age at sexual maturity of approximately 16 years old (Figure 1). At age 9 years, the trajectories were similar, but by age 10 years, a faster trajectory group diverged from the moderate and slower groups. The moderate and slower groups diverged at 12 years, with the slower group lagging in TV enlargement. At age 18 years, both the faster and moderate trajectory groups achieved a TV of greater 25 mL. By age 19 years, the mean TV for the slower trajectory group remained less than 25 mL.
Figure 1. Mean testicular volume as a function of age for three groups produced by the group-based trajectory model (GBTM).
Points reflect the observed mean testicular volume for each trajectory group through ages 8–19. Smooth curves represent fitted lines with 95% confidence intervals extracted from the GBTM.
The average posterior probability was high across groups: faster (0.96), moderate (0.80), and slower (0.92) (Table 2). Birthweight was lower in slower group (3.1 kg) in comparison with moderate (3.3 kg) and faster (3.4) groups. Age at enrollment was similar for all three groups, but lower baseline BMI-Z was found among those in the slower group (−0.76) versus those in the moderate (−0.20) or faster (−0.15) groups (Table 2). There were differences in baseline indicators of SES (monthly income, parental education) between groups. On average, educational level and income was higher in the families of the faster group than those of the moderate and slower groups. Although there were differences in the median concentration of phthalate metabolites among the three groups, the interquartile ranges (IQR) overlapped (Table 2).
Table 2.
Descriptive statistics by trajectory groups produced from the three-group analysis
| Trajectory Group |
|||
|---|---|---|---|
| Characteristic | Faster | Moderate | Slower |
|
| |||
| Observed n (%) | 63 (20.7) | 151 (49.7) | 90 (29.6) |
| Predicted n (%) | 71 (23.3) | 130 (42.6) | 103 (34.1) |
| Average Posterior Probability | 95.7 | 79.7 | 92.2 |
|
| |||
| Baseline Demographics | |||
|
| |||
| Age at enrollment, mean (SD) | 8.13 (0.34) | 8.25 (0.43) | 8.29 (0.46) |
| WHO age-adjusted BMI z-score, mean (SD) | −0.15 (1.18) | −0.20 (1.21) | −0.76 (1.25) |
| Total calories consumed per day (kcal), median [IQR] | 2632 [2000, 3047] | 2488 [2040, 3239] | 2551 [2036, 3306] |
| Birthweight (kg), mean (SD) | 3.38 (0.45) | 3.29 (0.51) | 3.14 (0.55) |
| Duration of breast feeding (weeks), median [IQR] | 13 [4, 26] | 13 [4, 30] | 13 [4, 26] |
| Monthly family income ≤ 250 USD, n (%) | 30 (47.6) | 98 (64.9) | 56 (62.2) |
| Highest parental education, n (%) | |||
| High school or less | 4 (6.3) | 13 (8.7) | 10 (11.4) |
| Some college or junior college | 36 (57.1) | 97 (64.7) | 51 (58.0) |
| College graduate | 23 (36.5) | 40 (26.7) | 27 (30.7) |
|
| |||
| Prepubertal Phthalate Concentrations | Median [IQR] | Median [IQR] | Median [IQR] |
|
| |||
| MEP, (ng/mL) | 92.61 [45.87, 170.75] | 92.67 [50.24, 181.23] | 82.37 [54.94, 164.29] |
| MnBP, (ng/mL) | 211.50 [126.71, 278.60] | 185.88 [120.29, 322.99] | 198.47 [138.81, 291.37] |
| MiBP, (ng/mL) | 53.16 [30.79, 88.89] | 61.39 [34.74, 87.00] | 55.48 [38.77, 86.08] |
| MBzP, (ng/mL) | 5.81 [2.48, 13.73] | 5.64 [2.74, 15.13] | 7.51 [4.11, 16.83] |
| MCPP, (ng/mL) | 10.49 [6.68, 16.74] | 10.51 [6.02, 19.45] | 11.26 [7.93, 19.64] |
| ΣDEHP, (μmol/L) | 1.02 [0.58, 1.63] | 1.02 [0.66, 1.99] | 1.14 [0.83, 1.64] |
| ΣDiNP, (μmol/L) | 0.06 [0.03, 0.09] | 0.05 [0.03, 0.10] | 0.06 [0.04, 0.12] |
| ΣDiDP, (μmol/L) | 0.02 [0.01, 0.03] | 0.02 [0.01, 0.03] | 0.02 [0.01, 0.03] |
| ΣAA, (μmol/L) | 2.55 [1.44, 3.49] | 2.48 [1.54, 3.73] | 2.46 [1.86, 3.47] |
Means and standard deviations are shown for normally distributed variables. Medians and IQRs are shown for non-normally distributed variables. SD: Standard Deviation; WHO: World Health Organization; BMI: Body Mass Index; IQR: Interquartile Range; kg: kilograms; USD: United States Dollar; ng/mL: Nanograms per milliliter; μmol/L: micromole per liter; MEP: Monoethyl; MnBP: Mono-n-butyl; MiBP: Mono-isobutyl; MBzP: Monobenzyl; MCPP: Mono-(3-carboxypropyl); DEHP: Di(2-ethylhexyl) Metabolites; DiNP: Di-iso-nonylphthalate Metabolites; DiDP: Diisodecyl Metabolites; AA: Anti-androgenic phthalates
3.4. Phthalate associations with pubertal progression
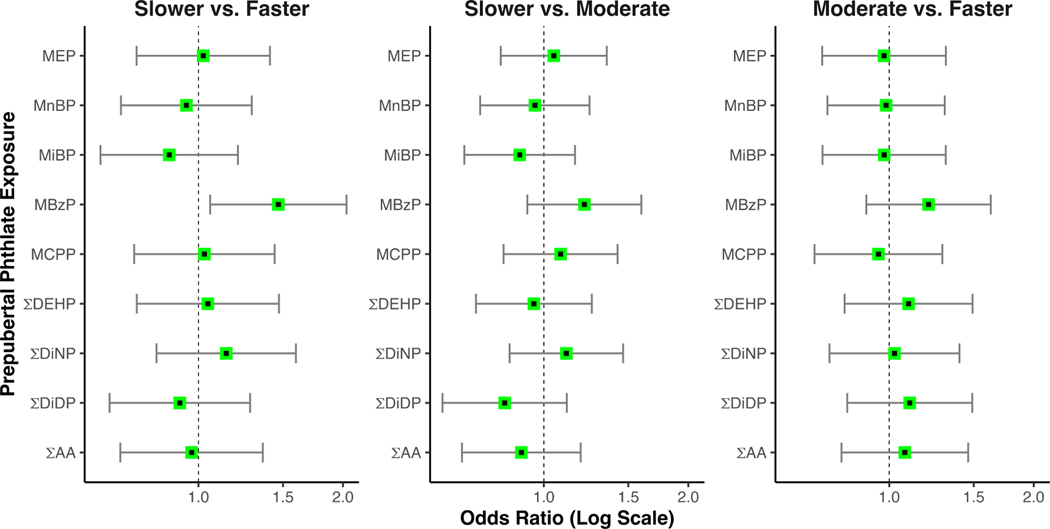
In adjusted models, higher prepubertal urinary MBzP concentrations were associated with slower pubertal progression. For every one SD increase in log(MBzP), the odds of being classified in the slower trajectory versus the faster trajectory increased by 47% (95% CI 1.06, 2.04) (Table 3 and Figure 2). Similarly, each 1 SD increase in log(MBzP) was associated with higher odds of being classified in the slower trajectory than the moderate trajectory (adjusted OR (aOR): 1.21, 95% CI: 0.92, 1.60). While not statistically significant, this finding provides additional support for a trend of slower progression with increasing MBzP concentrations. An ordinal logistic model supported a significant association between increases in log(MBzP) and slower pubertal progression (adjusted OR (aOR) 1.29, 95% CI 1.03, 1.60) (Table 3). There were no associations between other individual or summed phthalate metabolites and pubertal progression (Table 3).
Table 3.
Associations of prepubertal phthalate concentrations and trajectory group membership
| Multinomial Logistic Regression Analysis |
Ordinal Logistic Regression Analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Slower vs. Faster OR (95% CI) | Slower vs. Moderate OR (95% CI) | Moderate vs. Faster OR (95% CI) | Slower vs. Moderate vs. Faster OR (95% CI) | |||||
|
| ||||||||
| Phthalate | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| MEP | 1.05 (0.76, 1.44) | 1.02 (0.74, 1.41) | 1.06 (0.82, 1.37) | 1.05 (0.81, 1.35) | 0.99 (0.74, 1.33) | 0.98 (0.73, 1.31) | 1.04 (0.84, 1.28) | 1.04 (0.84, 1.28) |
| MnBP | 1.02 (0.74, 1.40) | 0.94 (0.69, 1.29) | 1.01 (0.78, 1.31) | 0.96 (0.74, 1.25) | 1.01 (0.75, 1.35) | 0.99 (0.74, 1.31) | 1.01 (0.82, 1.25) | 0.96 (0.78, 1.19) |
| MiBP | 1.00 (0.73, 1.38) | 0.87 (0.62, 1.21) | 1.03 (0.79, 1.33) | 0.89 (0.68, 1.16) | 0.98 (0.73, 1.31) | 0.98 (0.73, 1.31) | 1.01 (0.81, 1.25) | 0.91 (0.73, 1.13) |
| MBzP | * 1.32 (0.95, 1.84) | 1.47 (1.06, 2.04) | 1.22 (0.94, 1.59) | 1.21 (0.92, 1.60) | 1.08 (0.80, 1.47) | 1.21 (0.90, 1.63) | * 1.21 (0.98, 1.49) | 1.29 (1.03, 1.60) |
| MCPP | 1.15 (0.83, 1.58) | 1.03 (0.73, 1.44) | 1.18 (0.91, 1.53) | 1.08 (0.82, 1.42) | 0.97 (0.72, 1.31) | 0.95 (0.70, 1.29) | 1.11 (0.90, 1.37) | 1.01 (0.81, 1.26) |
| ΣDEHP | 1.14 (0.82, 1.59) | 1.05 (0.74, 1.47) | 1.03 (0.79, 1.33) | 0.95 (0.72, 1.26) | 1.11 (0.82, 1.51) | 1.10 (0.81, 1.49) | 1.08 (0.88, 1.33) | 1.01 (0.81, 1.26) |
| ΣDiNP | 1.19 (0.86, 1.65) | 1.14 (0.82, 1.60) | 1.20 (0.92, 1.55) | 1.11 (0.85, 1.46) | 1.00 (0.74, 1.35) | 1.03 (0.75, 1.40) | 1.15 (0.92, 1.43) | 1.09 (0.87, 1.37) |
| ΣDiDP | 0.87 (0.63, 1.21) | 0.91 (0.65, 1.28) | 0.86 (0.66, 1.13) | 0.83 (0.62, 1.12) | 1.01 (0.75, 1.36) | 1.10 (0.82, 1.49) | 0.90 (0.73, 1.12) | 0.92 (0.73, 1.16) |
| ΣAA | 1.06 (0.77, 1.47) | 0.97 (0.69, 1.36) | 0.98 (0.75, 1.27) | 0.90 (0.68, 1.19) | 1.09 (0.81, 1.47) | 1.08 (0.80, 1.46) | 1.03 (0.84, 1.27) | 0.97 (0.77, 1.21) |
Bold indicates that the confidence interval does not include 1; italic and
indicates p-value < 0.10
Phthalates were log-transformed and standardized by the standard deviation of their log transformation; OR: Odds Ratio; CI: Confidence Interval; Adjusted confounders included boys’ age at enrollment, WHO age-adjusted BMI z-score, total calories consumed per day, birthweight, duration of breast feeding, monthly family income, and highest parental education; MEP: Monoethyl; MnBP: Mono-n-butyl; MiBP: Mono-isobutyl; MBzP: Monobenzyl; MCPP: Mono-(3-carboxypropyl); DEHP: Di(2-ethylhexyl) Metabolites; DiNP: Di-iso-nonylphthalate Metabolites; DiDP: Diisodecyl Metabolites; AA: Anti-androgenic phthalates; The proportional odds assumption held for all ordinal models (p > 0.05)
Figure 2. Adjusted associations of prepubertal phthalate concentrations and trajectory group membership.
Points reflect the adjusted odds ratio, with the corresponding 95% confidence interval extracted from the multinomial logistic regression models.
4. Discussion
In this cohort of Russian boys, we found that higher versus lower prepubertal urinary concentrations of MBzP, the metabolite of anti-androgenic BBzP, were associated with a slower trajectory of pubertal progression. In prior analyses we found that higher versus lower prepubertal urinary MBzP concentrations was associated with later pubertal onset, ranging from 5 to 14 months later for TV>3 mL and P2, respectively (Burns, Sergeyev et al. 2022). Overall, these results suggest that MBzP may affect both pubertal onset and the tempo of pubertal development. Although previously we reported that higher prepubertal urinary ∑DiNP concentrations were associated with later gonadarche (Burns, Sergeyev et al. 2022), we did not find an association with pubertal progression in this analysis.
The 304 boys in the prepubertal phthalate and progression analysis were classified into three pubertal progression trajectory groups. The demographic characteristics across the three trajectory groups were similar, except for BMI and SES indicators at study entry. Not surprisingly, we observed higher BMIs in those with the faster trajectory of pubertal progression compared to the other two trajectories as reported by others (Buyken, Karaolis-Danckert et al. 2009, Sorensen, Aksglaede et al. 2010, Williams, Bellavia et al. 2019, Brix, Ernst et al. 2020). Indicators of higher SES, i.e. monthly family income and parental education, were also linked to faster pubertal development in the RCS. Conversely, lower household income has been linked to slower physical growth and later puberty in other observational studies (Facchini, Fiori et al. 2008, Bodzsar and Zsakai 2015, Acacio-Claro, Koivusilta et al. 2019). We found that neither birthweight nor baseline childhood caloric intake were associated with the tempo of pubertal development.
Several epidemiological studies have suggested that higher levels of prenatal and peripubertal AAPs were associated with later pubertal onset by pubic hair stage (Ferguson, Peterson et al. 2014, Kasper-Sonnenberg, Wittsiepe et al. 2017, Berger, Eskenazi et al. 2018, Cathey, Watkins et al. 2020), but not with gonadarche (G2) or testicular growth (TV>3mL). There are fewer studies that have examined the associations of prenatal and peripubertal AAPs with pubertal progression (Zhang, Cao et al. 2015, Kasper-Sonnenberg, Wittsiepe et al. 2017, Cathey, Watkins et al. 2020).
The Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) study reported that among 91 boys followed for an average of 3 years from approximately ages 10 to 13 years, a doubling of maternal urinary MnBP concentrations was associated with faster progression of pubic hair development (aOR=1.28, 95% CI 0.97, 1.69) (Cathey, Watkins et al. 2020). However, they found no associations between urinary AAP metabolites and genitalia development. It is difficult to draw inferences comparing ELEMENT to the RCS because of different developmental processes occurring during the prenatal versus the prepubertal phthalate exposure window after birth. Moreover, prenatal and childhood prepubertal urinary phthalate metabolites are typically not highly correlated (Shoaff, Papandonatos et al. 2017).
An analysis among children in the Puberty Timing and Health Effects in Chinese Children (PTHEC) study measured peripubertal urinary phthalate metabolites among 430 (222 boys) children aged 6–14 years and assessed associations with pubertal progression using TV and pubic hair over 18 months of follow-up (Zhang, Cao et al. 2015). This study reported an increase in urinary peripubertal MnBP was associated with slower progression of pubic hair development during follow-up (aOR=0.61, 95% CI 0.37, 1.00), with no associations observed between other AAP metabolites and genitalia development. There were several key differences between the PTHEC study design versus the RCS. First, the PTHEC only followed the cohort for 18 months for a short window on pubertal development. In contrast, the RCS follow-up of up to 11 years spanned the developmental window from prepuberty to sexual maturity, enabling evaluation of the relationship between prepubertal urinary phthalate metabolites and complete pubertal progression. Second, at study entry in PTHEC, 15% of the boys had TV ≥12 mL, considered mid to late puberty (Biro, Lucky et al. 1995), which increased to only 31% at the end of follow-up. Once TV > 3 mL is achieved, the pace of TV enlargement accelerates, as is illustrated by the typical sigmoid curve associated with TV development (Koskenniemi, Virtanen et al. 2017). Therefore, a sizable proportion of PTHEC boys may have already completed the most rapid stage of TV development, thus truncating observation of progression. In contrast, 87% of the boys among the PTHEC study were prepubertal for pubarche (P1) at study entry, with only 5% advancing to P3 or 4, limiting the ability to assess pubarche progression.
Second, the wide range in age and pubertal stage at entry into the PTHEC study resulted in a mixed cohort in terms of both the timing of exposure assessment and pubertal stage at entry. In contrast, the RCS urinary phthalate measurement was limited to a well-defined prepubertal window. By design, the RCS cohort was prepubertal at initial assessment thereby allowing for a comprehensive prospective analysis of phthalates and TV development, without bias by potential misclassification due to nonlinearity in TV growth over puberty (Koskenniemi, Virtanen et al. 2017). Third, the PTHEC study did not measure MBzP, the only phthalate that we found to be associated with pubertal progression. Finally, the urinary concentrations of AAPs measured in the PTHEC study were lower by 10-fold or greater than that observed in the RCS.
Exposure assessment was comparable to the RCS in a German study evaluating the associations between prepubertal urinary AAPs and pubertal progression among 472 children (250 boys), ages 8–14 years and followed for three years (Kasper-Sonnenberg, Wittsiepe et al. 2017). In this study, higher versus lower urinary ∑DEHP was associated with accelerated pubertal progression (Kasper-Sonnenberg, Wittsiepe et al. 2017). Whereas the RCS used annual physician assessed TV measurements to ascertain pubertal progression, the German study used annual composite Puberty Development Scales (PDS) based on self-reported pubic and facial hair development and voice change as indicators of puberty for boys. They did not examine genitalia nor measure TV. Subanalyses of the individual PDS components showed conflicting associations; for instance, ∑DEHP metabolites were associated with earlier facial hair growth but later and slower pubic hair development.
The discrepancies between the findings in the RCS and these prior observational studies may reflect differences in study design and study populations (Zhang, Cao et al. 2015, Kasper-Sonnenberg, Wittsiepe et al. 2017, Cathey, Watkins et al. 2020). A key difference was our long-term follow-up of up to 11 years which enabled us to follow TV development from prepuberty to sexual maturity as compared with 1.5– 3 years of follow-up in other studies. Moreover, only the German study had a comparable exposure window to ours. Although we did observe that higher versus lower prepubertal urinary MBzP concentrations were associated with slower pubertal progression, we did not find any other phthalate metabolites associated with pubertal progression. In our previous report of the associations between prepubertal urinary AAP metabolites and pubertal onset, we reported that the most robust association between these chemicals and later age of gonadarche was observed for MBzP, where the highest compared to the lowest quartile was 5.6 months later (95% CI 0.6, 10.7) for TV>3mL (Burns, Sergeyev et al. 2022). However, it is unknown whether there is a biological mechanism that might account for the apparent specificity of MBzP, rather than other AAP metabolites, in our observed associations with TV development.
Puberty is a complex and dynamic process that is initiated by activation of the hypothalamic-pituitary-gonadal (HPG) axis, with the pulsatile release of pituitary follicle stimulating hormone (FSH) and luteinizing hormone (LH) (Wood, Lane et al. 2019). In boys, FSH and LH stimulate maturation of the testes (Spaziani, Tarantino et al. 2021), with the earliest reliable physical indicator of pubertal onset being TV enlargement to >3 mL (Koskenniemi, Virtanen et al. 2017). Throughout the typical 5–6-year course of puberty in boys (Spaziani, Tarantino et al. 2021), there is increased secretion of FSH, LH and testosterone. Testosterone action depends on gonadotropin-releasing hormone (GnRH) regulated steroidogenesis and induction of androgen receptor expression, local androgen activation, metabolism, and receptor binding and signaling. These complex pathways are susceptible to disruption by AAPs (Walker, Garza et al. 2021).
Our analysis evaluated the relationship between prepubertal urinary AAP metabolites and TV development, the external physical manifestation of complex physiological pubertal developmental processes. In the RCS, participants in the slower TV trajectory associated with MBzP did achieve sexual maturation as defined by TV≥20 mL during our follow-up period, albeit later than those in the moderate and faster trajectories. However, the flattened slope of the slower trajectory, and the smaller TV at the last assessment could reflect either delay in achieving full sexual maturation, or disruption of complete testicular maturation, ie perturbation of the seminiferous tubules or spermatogenesis, the principle contributors to testicular size. For example, in a cross-sectional survey of 652 young, healthy male Danish conscripts, mean age 19 years, those who recalled later penile and pubic hair growth compared to the rest of the cohort had, on average, smaller TV, poorer semen quality, lower serum testosterone, and more often reported sexual problems (Jensen, Finne et al. 2016). This cross-sectional survey does not confirm a causal relationship, but it suggests that lags in pubertal timing and development may affect adult male sexual function.
This RCS analysis focused on TV and therefore, may have missed an opportunity to detect relationships between prepubertal urinary phthalates and pubertal tempo as indicated by pubic hair development as has been observed in previous studies (Zhang, Cao et al. 2015, Kasper-Sonnenberg, Wittsiepe et al. 2017, Cathey, Watkins et al. 2020). Animal data has provided evidence that prenatal phthalate exposure may have deleterious effects on male pubertal development (Kay, Bloom et al. 2014); however, we did not have maternal prenatal urine samples to evaluate potential associations. Although urinary MBzP concentrations in the RCS were similar to reported European childhood concentrations (Kasper-Sonnenberg, Koch et al. 2014, Gari, Koch et al. 2019), they were much lower than in the US NHANES (CDC 2019). Differences between RCS urinary phthalate metabolite concentrations compared to other populations may impact generalizability. Other potentially correlated endocrine-disrupting chemicals – e.g. parabens, bisphenol A – were not measured in the RCS, therefore we were unable to adjust for possible confounding by these other exposures. Additionally, although our GBTM produced trajectories that conformed to our expectations of pubertal TV development, i.e. sigmoid curves, this method classifies individuals by their maximum posterior probability of being in a group, given their outcome measures over time (Nagin 1999). Because the tempo of development can vary over time across study participants, individuals might be selected into the other trajectory groups. Therefore, the final group assignments we used for multinomial and ordinal models did not account for the uncertainty in group assignments. Another limitation of the GBTM procedure is that it may overfit the number of trajectory groups, despite using the objective BIC measure. Thus, selection of final models must combine subject matter knowledge with the results of the GBTM approach (Roeder, Lynch, and Nagin 1999; Jones, Nagin, and Roeder 2001). Finally, the trajectories observed in the RCS are specific to this cohort and may not be generalizable to other populations.
Our study design had several important strengths, primary among them was the ability to prospectively assess the temporal relationship between prepubertal urinary phthalate concentrations and pubertal progression. We used both TV and G staging to a priori assign each boy’s urine samples to their prepubertal urine pool, with the majority contributing at least two samples. This pooling method provides a better biomarker of exposure than only one sample (Johns, Cooper et al. 2015). In prior studies of pubertal development, boys were followed for only 1.5–3 years (Zhang, Cao et al. 2015, Kasper-Sonnenberg, Wittsiepe et al. 2017, Cathey, Watkins et al. 2020), much shorter than the typical pubertal progression duration of 5 to 6 years (Spaziani, Tarantino et al. 2021). Our analysis utilized up to eleven years of follow-up from prepuberty to sexual maturity, allowing us to assess pubertal development over the entirety of puberty. The RCS use of physician measurements of TV to evaluate pubertal progression is a more objective assessment of pubertal development than physician visual inspections or self-report (Biro, Lucky et al. 1995). The same physician (O.S.) assessed TV, without knowledge of an individual’s prepubertal phthalate urinary concentrations or reference to testicular size at prior visits, which controlled for both inter-observer variability and information bias. The RCS is a well-characterized population, with extensive information collected on potential confounders, and previous publications have documented the relationship between other chemicals and pubertal onset, sexual maturity and semen quality (Minguez-Alarcon, Sergeyev et al. 2017, Sergeyev, Burns et al. 2017, Williams, Bellavia et al. 2019, Minguez-Alarcon, Burns et al. 2022, Williams, Minguez-Alarcon et al. 2022), including phthalates (Burns, Sergeyev et al. 2022). The choice of GBTM models allowed us to identify overall patterns of pubertal progression based on TV and to determine the association of prepubertal urinary AAPs with trajectory membership. In contrast, previous studies have commonly used mixed effects or marginal models where pubertal progression is modeled as an overall mean level and does not reflect the typical sigmoid-shaped patterns observed in other literature on puberty (Koskenniemi, Virtanen et al. 2017).
5. Conclusions
The timely completion of male puberty has been linked with reproductive health, including appropriate social behavior, sexual function, and fertility (Romeo, Richardson et al. 2002, Graber 2013, Jensen, Finne et al. 2016, Hansen, Wojdemann et al. 2021). Anti-androgenic phthalates have been associated with deleterious effects on serum testosterone, semen quality, and fertility (Radke 2018). In our unique examination of the relationship between prepubertal urinary AAPs and pubertal progression, we have expanded the potential impact of urinary MBzP to include slower progression, and a smaller TV at age 19 years. Future analyses in the RCS will assess the impact of AAP phthalates on the hormonal drivers of male sexual maturation, such as LH, FSH, testosterone, other androgens and reproductive hormones.
Highlights:
We measured phthalate metabolites among boys in a well-characterized Russian cohort
Some phthalate metabolite levels were higher in our cohort than in Europe or the US
Pubertal progression by testicular volume had fast, moderate, or slow trajectories
Higher monobenzyl phthalate levels were associated with slower pubertal progression
Other prepubertal anti-androgenic phthalates were not associated with progression
Funding:
This work was funded by the U.S. Environmental Protection Agency (EPA grant R82943701) and the National Institute of Environmental Health Sciences (NIEHS grants R01 ES014370 and P30 ES000002). The funding sources played no role in the study design, the collection, analysis and interpretation of the data, or in the writing or decision to submit this manuscript for publication.
1. Abbreviations:
- AAP
anti-androgenic phthalate
- DEHP
di(2-ethylhexyl) phthalate
- DEP
diethyl phthalate
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- DiBP
di-isobutyl phthalate
- DiDP
di-isodecyl phthalate
- DiNP
di-isononyl phthalate
- DnBP
di-n-butyl phthalate
- EDC
endocrine disrupting chemicals
- G
Tanner stage genitalia
- G2
Tanner stage pubertal onset by genitalia development
- GM
geometric mean
- HMWP
high molecular weight phthalate
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- LMWP
low molecular weight phthalate
- MBzP
monobenzyl phthalate
- MCNP
mono-(carboxy-iso-nonyl) phthalate
- MCOP
mono-carboxyl-iso-octyl phthalate
- MCPP
mono-(3-carboxypropyl) phthalate
- MECPP
mono(2-ethyl-5-carboxy-pentyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxy-hexyl) phthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxo-hexyl) phthalate
- MEP
mono-ethyl phthalate
- MHiDP
mono-(hydroxy-iso-decyl) phthalate
- MHiNP
mono-hydroxy-iso-nonyl phthalate
- MiBP
mono-isobutyl phthalate
- MnBP
mono-n-butyl phthalate
- MOiDP
mono-(oxo-iso-decyl) phthalate
- MOiNP
mono-oxo-iso-nonyl phthalate
- P
Tanner stage pubic hair
- P2
Tanner stage pubertal onset by pubic hair development
- RCS
Russian Children’s Study
- Rs
Spearman correlation
- RSD%
inter-assay relative standard deviation percent
- SES
socioeconomic status
- SG
specific gravity
- S/N
signal-to-noise ratio
- ΣAAP
molar sum of all anti-androgenic phthalate metabolites (μmol/L)
- ΣDEHP
molar sum of DEHP metabolites (μmol/L)
- ΣDiDP
molar sum of DiDP metabolites (μmol/L)
- ΣDiNP
molar sum of DiNP metabolites (μmol/L)
- TV
testicular volume
Footnotes
Author Credit:
JS Burns: Data curation, Writing – the original draft
JR Bather: Methodology, Software, Formal analysis Writing – review and editing, Visualization
O Sergeyev: Conceptualization, Investigation, Resources, Writing – review and editing, Supervision, Project administration
MM Lee: Conceptualization, Methodology, Writing – review and editing
SA Korrick: Conceptualization, Methodology, Writing – review and editing
S Sokolov: Investigation, Validation
S Kovalev: Investigation, Validation
HM Koch: Methodology, Validation, Resources, Writing – review and editing
AT Lebedev: Methodology, Validation, Resources, Writing – review and editing
B Plaku: Methodology, Software
L Minguez-Alarcón: Writing – review and editing
R Hauser: Conceptualization, Methodology, Writing – review and editing, Supervision, Funding acquisition
PL Williams: Conceptualization, Methodology, Writing - review and editing, Supervision
Disclaimers: The opinions in this article are those of the authors and do not necessarily reflect the official opinion of the U.S. EPA or NIEHS.
The study was approved by the Human Studies Institutional Review Boards of the Chapaevsk Medical Association, Harvard T.H. Chan School of Public Health, Nemours Children’s Health, and Brigham and Women’s Hospital. Before participation, the parent/guardian provided informed consent and the boys signed assent forms. At ages 18 and above, each boy signed informed consent.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Acacio-Claro PJ, Koivusilta LK, Doku DT and Rimpela AH (2019). “Timing of puberty and reserve capacity in adolescence as pathways to educational level in adulthood-a longitudinal study.” Ann Hum Biol 46(1): 35–45. [DOI] [PubMed] [Google Scholar]
- Berger K, Eskenazi B, Kogut K, Parra K, Lustig RH, Greenspan LC, Holland N, Calafat AM, Ye X. and Harley KG (2018). “Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and Pubertal Timing in Boys and Girls.” Environ Health Perspect 126(9): 97004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Lucky AW, Huster GA and Morrison JA (1995). “Pubertal staging in boys.” J Pediatr 127(1): 100–102. [DOI] [PubMed] [Google Scholar]
- Bodzsar EB and Zsakai A. (2015). “Sexual maturation pattern in the mirror of socioeconomic background.” Anthropol Anz 72(1): 1–12. [DOI] [PubMed] [Google Scholar]
- Brant R. (1990). “Assessing proportionality in the proportional odds model for ordinal logistic regression.” Biometrics 46(4): 1171–1178. [PubMed] [Google Scholar]
- Brix N, Ernst A, Lauridsen LLB, Parner ET, Arah OA, Olsen J, Henriksen TB and Ramlau-Hansena CH (2020). “Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses.” Int J Epidemiol 49(3): 834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JS, Lee MM, Williams PL, Korrick SA, Sergeyev O, Lam T, Revich B. and Hauser R. (2016). “Associations of Peripubertal Serum Dioxin and Polychlorinated Biphenyl Concentrations with Pubertal Timing among Russian Boys.” Environ Health Perspect 124(11): 1801–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JS, Sergeyev O, Lee MM, Williams PL, Minguez-Alarcon L, Plaku-Alakbarova B, Sokolov S, Kovalev S, Koch HM, Lebedev AT, Hauser R, Korrick SA and Russian Children’s S. (2022). “Associations of prepubertal urinary phthalate metabolite concentrations with pubertal onset among a longitudinal cohort of boys.” Environ Res 212(Pt A): 113218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JS, Williams PL, Sergeyev O, Korrick SA, Rudnev S, Plaku-Alakbarova B, Revich B, Hauser R. and Lee MM (2020). “Associations of peri-pubertal serum dioxins and polychlorinated biphenyls with growth and body composition among Russian boys in a longitudinal cohort.” Int J Hyg Environ Health 223(1): 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyken AE, Karaolis-Danckert N. and Remer T. (2009). “Association of prepubertal body composition in healthy girls and boys with the timing of early and late pubertal markers.” Am J Clin Nutr 89(1): 221–230. [DOI] [PubMed] [Google Scholar]
- Cathey A, Watkins DJ, Sanchez BN, Tamayo-Ortiz M, Solano-Gonzalez M, Torres-Olascoaga L, Tellez-Rojo MM, Peterson KE and Meeker JD (2020). “Onset and tempo of sexual maturation is differentially associated with gestational phthalate exposure between boys and girls in a Mexico City birth cohort.” Environ Int 136: 105469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC). (2019). Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. Health and Human Services. One: 437–513. [Google Scholar]
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C. and Siekmann J. (2007). “Development of a WHO growth reference for school-aged children and adolescents.” Bull World Health Organ 85(9): 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini F, Fiori G, Bedogni G, Galletti L, Ismagulov O, Ismagulova A, Sharmanov T, Tsoy I, Belcastro MG, Rizzoli S. and Goldoni M. (2008). “Puberty in modernizing Kazakhstan: a comparison of rural and urban children.” Ann Hum Biol 35(1): 50–64. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Peterson KE, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, Tellez-Rojo MM and Meeker JD (2014). “Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys.” Reprod Toxicol 47: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N. and Sharpe RM (2003). “Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate.” Hum Reprod 18(7): 1383–1394. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board, Institute of Medicine. Macronutrients and Healthful Diets. In: Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D.C.: National Academy Press; 2005. p. 769–879. [Google Scholar]
- Foster PM (2006). “Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters.” Int J Androl 29(1): 140–147; discussion 181–145. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE and Andersson AM (2007). “Metabolism of phthalates in humans.” Mol Nutr Food Res 51(7): 899–911. [DOI] [PubMed] [Google Scholar]
- Gari M, Koch HM, Palmke C, Jankowska A, Wesolowska E, Hanke W, Nowak D, Bose-O’Reilly S. and Polanska K. (2019). “Determinants of phthalate exposure and risk assessment in children from Poland.” Environ Int 127: 742–753. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J. and Zoeller RT (2015). “Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals.” Endocr Rev 36(6): 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA (2013). “Pubertal timing and the development of psychopathology in adolescence and beyond.” Horm Behav 64(2): 262–269. [DOI] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS and Gray LE Jr. (2011). “Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate.” Toxicol Sci 123(1): 206–216. [DOI] [PubMed] [Google Scholar]
- Hansen AB, Wojdemann D, Renault CH, Pedersen AT, Main KM, Raket LL, Jensen RB and Juul A. (2021). “DIAGNOSIS OF ENDOCRINE DISEASE: Sex steroid action in adolescence: too much, too little; too early, too late.” Eur J Endocrinol 184(1): R17–R28. [DOI] [PubMed] [Google Scholar]
- Hauser R, Williams P, Altshul L, Korrick S, Peeples L, Patterson DG Jr., Turner WE, Lee MM, Revich B. and Sergeyev O. (2005). “Predictors of serum dioxin levels among adolescent boys in Chapaevsk, Russia: a cross-sectional pilot study.” Environ Health 4(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V. and Angerer J. (2007). “Phthalates: toxicology and exposure.” Int J Hyg Environ Health 210(5): 623–634. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK and Gray LE Jr. (2017). “Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment.” Int J Hyg Environ Health 220(2 Pt A): 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS and Gray LE Jr. (2008). “Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats.” Environ Res 108(2): 168–176. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Finne KF, Skakkebaek NE, Andersson AM, Olesen IA, Joensen UN, Bang AK, Nordkap L, Priskorn L, Krause M, Jorgensen N. and Juul A. (2016). “Self-reported onset of puberty and subsequent semen quality and reproductive hormones in healthy young men.” Hum Reprod 31(8): 1886–1894. [DOI] [PubMed] [Google Scholar]
- Johns LE, Cooper GS, Galizia A. and Meeker JD (2015). “Exposure assessment issues in epidemiology studies of phthalates.” Environ Int 85: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol Methods Res. 2001;29(3):374–393. doi: 10.1177/0049124101029003005 [DOI] [Google Scholar]
- Joustra SD, van der Plas EM, Goede J, Oostdijk W, Delemarre-van de Waal HA, Hack WW, van Buuren S. and Wit JM (2015). “New reference charts for testicular volume in Dutch children and adolescents allow the calculation of standard deviation scores.” Acta Paediatr 104(6): e271–278. [DOI] [PubMed] [Google Scholar]
- Kasper-Sonnenberg M, Koch HM, Wittsiepe J, Bruning T. and Wilhelm M. (2014). “Phthalate metabolites and bisphenol A in urines from German school-aged children: results of the Duisburg birth cohort and Bochum cohort studies.” Int J Hyg Environ Health 217(8): 830–838. [DOI] [PubMed] [Google Scholar]
- Kasper-Sonnenberg M, Wittsiepe J, Wald K, Koch HM and Wilhelm M. (2017). “Pre-pubertal exposure with phthalates and bisphenol A and pubertal development.” PLoS One 12(11): e0187922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay VR, Bloom MS and Foster WG (2014). “Reproductive and developmental effects of phthalate diesters in males.” Crit Rev Toxicol 44(6): 467–498. [DOI] [PubMed] [Google Scholar]
- Koch HM and Calafat AM (2009). “Human body burdens of chemicals used in plastic manufacture.” Philos Trans R Soc Lond B Biol Sci 364(1526): 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Ruther M, Schutze A, Conrad A, Palmke C, Apel P, Bruning T. and Kolossa-Gehring M. (2017). “Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012.” Int J Hyg Environ Health 220(2 Pt A): 130–141. [DOI] [PubMed] [Google Scholar]
- Koskenniemi JJ, Virtanen HE and Toppari J. (2017). “Testicular growth and development in puberty.” Curr Opin Endocrinol Diabetes Obes 24(3): 215–224. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ and Susman EJ (2011). “Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models.” Dev Psychol 47(5): 1389–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinchik AN, Baturin AK, Baeva VS, Feoktistova AI, Piatnitskaia IN, Azizbekian GA, Peskova EV and Bormacheva EA (1998). “[Development of a method of studying actual nutrition according to analysis of the frequency of consumption of food products: creation of a questionnaire and general evaluation of the reliability of the method].” Vopr Pitan(3): 8–13. [PubMed] [Google Scholar]
- Minguez-Alarcon L, Burns J, Williams PL, Korrick SA, Lee MM, Bather JR, Kovalev SV, Sokolov SA, Lebedev AT, Smigulina L, Ghayda RA, Koch HM, Sergeyev O. and Hauser R. (2022). “Urinary phthalate metabolite concentrations during four windows spanning puberty (prepuberty through sexual maturity) and association with semen quality among young Russian men.” Int J Hyg Environ Health 243: 113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Sergeyev O, Burns JS, Williams PL, Lee MM, Korrick SA, Smigulina L, Revich B. and Hauser R. (2017). “A Longitudinal Study of Peripubertal Serum Organochlorine Concentrations and Semen Parameters in Young Men: The Russian Children’s Study.” Environ Health Perspect 125(3): 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen A, Frederiksen H, Sorensen K, Aksglaede L, Hagen C, Skakkebaek NE, Main KM, Andersson AM and Juul A. (2013). “Urinary phthalates from 168 girls and boys measured twice a year during a 5-year period: associations with adrenal androgen levels and puberty.” J Clin Endocrinol Metab 98(9): 3755–3764. [DOI] [PubMed] [Google Scholar]
- Nagin D. (2009). Group-based modeling of development. Harvard University Press. [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Radke EG, Braun JM, Meeker JD and Cooper GS (2018). “Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence.” Environ Int 121(Pt 1): 764–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett HR and Colditz GA (1997). “Assessing diets of children and adolescents.” Am J Clin Nutr 65(4 Suppl): 1116S–1122S. [DOI] [PubMed] [Google Scholar]
- Roeder K, Lynch KG, & Nagin DS (1999). Modeling uncertainty in latent class membership: A case study in criminology. Journal of the American Statistical Association, 94(447), 766–776. [Google Scholar]
- Romeo RD, Richardson HN and Sisk CL (2002). “Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential.” Neurosci Biobehav Rev 26(3): 381–391. [DOI] [PubMed] [Google Scholar]
- Sergeyev O, Burns JS, Williams PL, Korrick SA, Lee MM, Revich B. and Hauser R. (2017). “The association of peripubertal serum concentrations of organochlorine chemicals and blood lead with growth and pubertal development in a longitudinal cohort of boys: a review of published results from the Russian Children’s Study.” Rev Environ Health 32(1–2): 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaff J, Papandonatos GD, Calafat AM, Ye X, Chen A, Lanphear BP, Yolton K. and Braun JM (2017). “Early-Life Phthalate Exposure and Adiposity at 8 Years of Age.” Environ Health Perspect 125(9): 097008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen K, Aksglaede L, Petersen JH and Juul A. (2010). “Recent changes in pubertal timing in healthy Danish boys: associations with body mass index.” J Clin Endocrinol Metab 95(1): 263–270. [DOI] [PubMed] [Google Scholar]
- Spaziani M, Tarantino C, Tahani N, Gianfrilli D, Sbardella E, Lenzi A. and Radicioni AF (2021). “Hypothalamo-Pituitary axis and puberty.” Mol Cell Endocrinol 520: 111094. [DOI] [PubMed] [Google Scholar]
- Tanner JM and Whitehouse RH (1976). “Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty.” Arch Dis Child 51(3): 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C, Garza S, Papadopoulos V. and Culty M. (2021). “Impact of endocrine-disrupting chemicals on steroidogenesis and consequences on testicular function.” Mol Cell Endocrinol 527: 111215. [DOI] [PubMed] [Google Scholar]
- Wallner P, Kundi M, Hohenblum P, Scharf S. and Hutter HP (2016). “Phthalate Metabolites, Consumer Habits and Health Effects.” Int J Environ Res Public Health 13(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu H. and Kannan K. (2019). “A Review of Biomonitoring of Phthalate Exposures.” Toxics 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Bellavia A, Korrick SA, Burns JS, Lee MM, Sergeyev O, Hauser R. and Russian Children’s Study T. (2019). “Blood lead levels and timing of male sexual maturity: A longitudinal study of Russian boys.” Environ Int 125: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Minguez-Alarcon L, Korrick SA, Lee MM, Plaku-Alakbarova B, Burns JS, Smigulina L, Dikov Y, Abou Ghayda R, Hauser R, Sergeyev O. and Russian Children’s S. (2022). “Association of peripubertal blood lead levels with reproductive hormones and semen parameters in a longitudinal cohort of Russian men.” Hum Reprod 37(4): 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CL, Lane LC and Cheetham T. (2019). “Puberty: Normal physiology (brief overview).” Best Pract Res Clin Endocrinol Metab 33(3): 101265. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao Y, Shi H, Jiang X, Zhao Y, Fang X. and Xie C. (2015). “Could exposure to phthalates speed up or delay pubertal onset and development? A 1.5-year follow-up of a schoolbased population.” Environ Int 83: 41–49. [DOI] [PubMed] [Google Scholar]