Abstract
Purpose of review:
Ending the HIV epidemic will require the development of additional effective immune-mediated and non-immune-mediated means of HIV prevention. Evaluating novel interventions requires large, controlled trials demonstrating efficacy. Recent advances in the field of HIV prevention necessitate new approaches to efficacy trial design.
Recent findings:
Three classes of efficacy trial designs are possible: standard of prevention-controlled trials, active-controlled trials, and active-controlled trials augmented with external control data. Recent experience with these approaches provides lessons on considerations around and success of the designs. Additional experience and development is needed for the augmented active-controlled trial design.
Summary:
Efficacy trials of new HIV prevention interventions are feasible but require careful consideration, given the complexity and dynamic state of the prevention field. While standard of prevention-controlled efficacy trials are reasonable approaches for HIV vaccine and monoclonal antibody efficacy trials, trials of new antiretroviral agents may require active-controlled designs.
Keywords: HIV prevention, randomized controlled trial, placebo, active control, efficacy trial
Introduction
Major advances in biomedical HIV prevention have occurred in the last two decades: research demonstrated that completely virally suppressed persons living with HIV cannot transmit HIV to their sexual partners,1–3 (“U = U”),4 and oral antiretrovirals (ARVs), when taken as pre-exposure prophylaxis (PrEP), are effective.5–7 More recently, PrEP with long-acting injectable cabotegravir (CAB-LA) was found highly effective8,9 and may circumvent some of the challenges associated with pill-taking adherence.
These advances bode well for reducing population HIV incidence,10,11 but create challenges testing new interventions. Individual choice is critical in HIV prevention uptake,12,13 thus a portfolio of prevention options is required. However, an effective HIV vaccine, which may be necessary for ending the HIV epidemic, remains elusive.14–16
A standard of prevention-controlled efficacy trial including a placebo arm has traditionally been required for regulatory approval of new HIV prevention modalities. Although there are settings and populations in which this design remains appropriate, there are other settings where alternative control groups are needed.
We overview three main study design options for future HIV prevention efficacy trials: 1) standard of prevention-controlled, 2) active-controlled, and 3) active-controlled augmented with external control data.
1. Standard of prevention-controlled trial design
In this study design, individuals without HIV are enrolled and have access to HIV prevention standard of care. To maintain blinding, participants are randomized to the experimental intervention or placebo on top of the standard of prevention, and HIV acquisition rates are compared between arms (Figure 1). Only individuals behaviorally vulnerable to HIV are recruited; those who are using or intend to use highly effective biomedical prevention may not be considered vulnerable to HIV. The Mosaico vaccine trial17,18 (HPX3002/HVTN 706, ClinicalTrials.gov # NCT03964415) illustrates this design. Because it is unlikely that early generations of HIV vaccines will be as efficacious as PrEP, and because an active-controlled design would require witholding known effective prevention from the vaccine group, the Mosaico trial utilized a standard of prevention-controlled design. Potential participants were first counseled about and navigated to low/no-cost PrEP services. Those who declined PrEP were then screened for trial enrollment. Current use of oral PrEP or prior use of CAB-LA precluded enrollment in the trial. However, once enrolled, participants were counseled throughout follow-up about HIV prevention including PrEP and were linked to low/no-cost PrEP services or offered PrEP by the study site, if interested. Each site had a written PrEP plan delineating access for potential and enrolled participants which were reviewed and approved by local and centralized investigators and community. The standard of prevention-controlled design addressed the question critical to advancing an early-generation vaccine: Did the vaccine provide any efficacy? This is in contrast with the question addressed by an active-controlled design: Was the vaccine as effective as highly-effective ARV-based PrEP?
Figure 1.
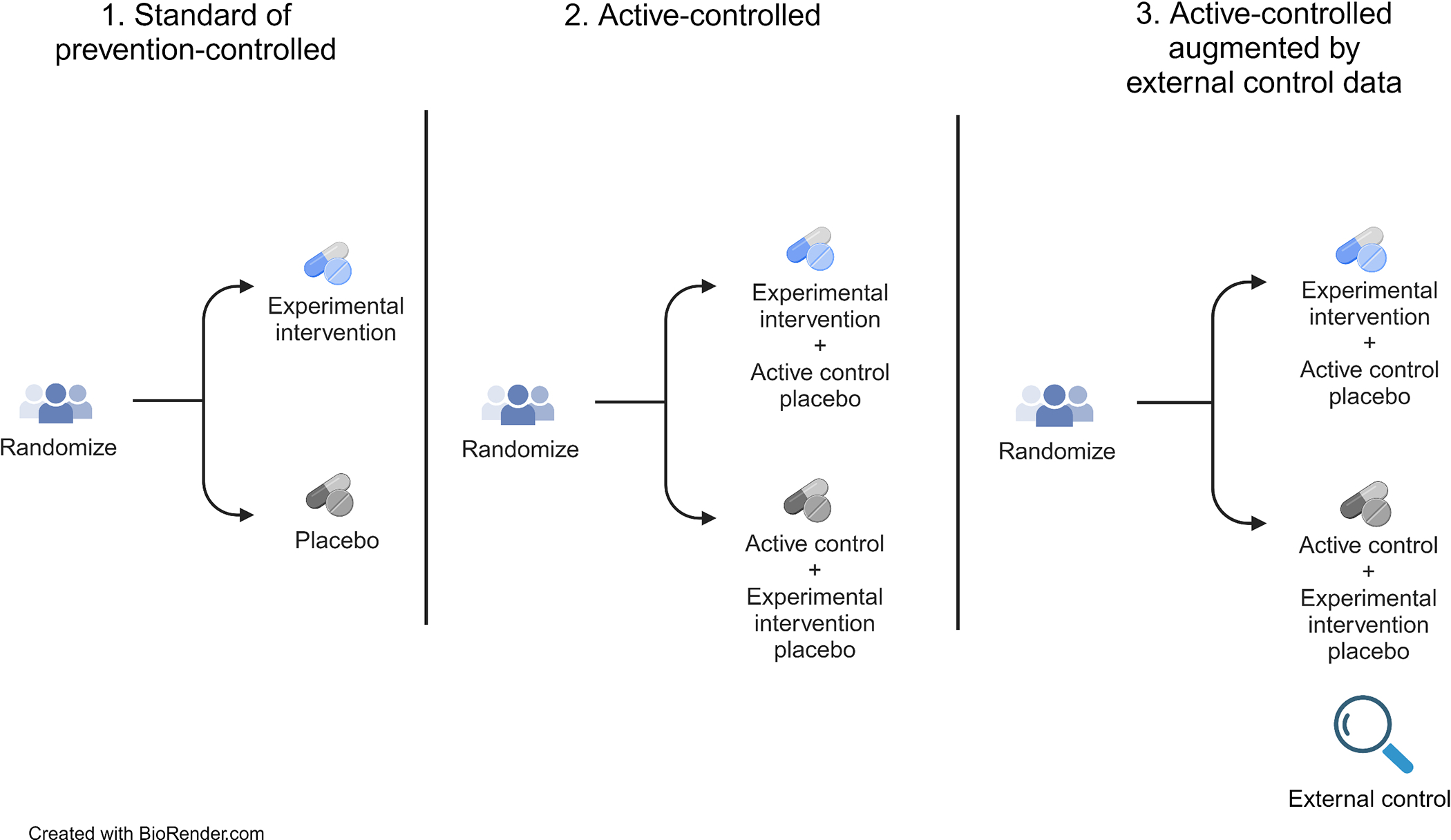
Main study design options for future HIV prevention efficacy trials
Mosaico successfully enrolled more than 3800 cisgender men who have sex with men and transgender (MSM/TG) persons across the Americas and Europe, randomized 1:1 to vaccine or placebo. After enrollment, fewer than 15% self-reported PrEP uptake. The study was closed early due to lack of efficacy in preventing HIV acquisition.19
There were several lessons learned from the Mosaico trial. Community engagement was critical for designing the trial, as it was community members who suggested that participants be offered PrEP prior to enrollment. Ethicist input was also important: offering low/no-cost PrEP prior to screening ensured that participants’ decision not to take PrEP was an “authenticity of expressions of undesirability.”20 Low PrEP uptake during follow-up despite having access suggests that PrEP was not of interest for this population.
However, as PrEP use becomes more widespread and options expand, more people may desire PrEP, and the strategy of enrolling people not desiring PrEP, who are willing to be randomized to an experimental intervention or placebo, may become more challenging. If this strategy is pursued in future trials, we must ensure that authentic choice determines enrollment. Otherwise, we run the risk of enrolling vulnerable populations who do not have adequate knowledge of or access to PrEP. Multiple studies showed the populations without adequate knowledge of or access to PrEP are more likely to include people of color in the U.S., and younger people and women globally.21,22 Authentic choice may also become more challenging to achieve the more similar the participant experiences are across existing PrEP options and the experimental intervention under study. With these caveats, this trial design remains an important and ethical option, particularly when assessing the efficacy of novel non-ARV agents, where an active-controlled trial would create the ethical dilemma that persons receiving the active product (e.g., an experimental HIV vaccine of unknown efficacy) would not have access to ARV-based PrEP.
2. Active-controlled
In an active-controlled trial design, individuals without HIV are enrolled and randomized to the experimental intervention or an active control (Figure 1). Blinding is maintained with use of a placebo or ‘dummy’ for each arm, i.e., participants receive the experimental intervention plus a placebo version of the active control, or the active control plus a placebo version of the experimental intervention. The success of the experimental intervention is evaluated by comparing HIV incidence between the two arms. Pre-specified success criteria may entail establishing superiority or non-inferiority of the experimental intervention relative to the active control. Superiority of the intervention means that the HIV incidence under the experimental intervention is lower than that under the active control, non-inferiority means that the HIV incidence is ‘not meaningfully higher’ under the experimental intervention vs. active control.
HPTN 0838 and HPTN 0849 (ClinicalTrials.gov #NCT02720094 and NCT03164564, respectively) evaluating CAB-LA for HIV prevention serve as useful illustrations: HPTN 083 was a randomized double blind, double dummy, non-inferiority trial, while HPTN 084 was similar but with a superiority objective. HPTN 083 enrolled more than 4500 cisgender MSM and transgender women (TGW) and HPTN 084 enrolled more than 3200 cisgender women. In both trials, CAB-LA was compared to oral tenofovir disoproxil fumarate plus emtricitabine (TDF-FTC) (i.e., active control). The active-controlled design compared a highly effective ARV PrEP agent (TDF-FTC) with a new ARV PrEP agent (CAB-LA). The HPTN 083 protocol cites the UNAIDS/WHO on Ethical Considerations in Biomedical HIV Prevention Trials (Guidance Point 15): “The use of a placebo control arm is ethically acceptable in a biomedical HIV prevention trial only when there is no HIV prevention modality of the type being studied that has been shown to be effective in comparable populations.” TDF-FTC PrEP was proven effective in cisgender MSM and cis- and transgender women at the time these two trials were planned.
Investigators from both trials incorporated feedback from community groups and ethicists early in study design. HPTN 083 was designed as a non-inferiority trial because there was substantial evidence from multiple trials that TDF-FTC was highly effective in MSM/TGW. Non-inferiority trials require a reliable estimate of active control efficacy because efficacy of the experimental intervention is established through comparison with the active control. Supporting the assumed efficacy of TDF-FTC, it was important to select populations similar to those enrolled in previous placebo-controlled TDF-FTC trials that demonstrated efficacy.5,23 HPTN 084 was designed as a superiority trial because trials of TDF-FTC in women had both positive24 and negative25,26 results.
The high efficacy of CAB-LA has implications for future active-controlled PrEP trials. For example, will the most appropriate comparator be CAB-LA, or will it be acceptable to use TDF-FTC? It will be difficult to demonstrate superiority over CAB-LA, as clinic administration minimizes the adherence challenges that appear to influence oral PrEP efficacy.27–30 Its high cost has restricted CAB-LA adoption as standard of care,31,32 thus providing it as part of a clinical trial raises questions around post-study access. Daily oral PrEP was the comparator in both PURPOSE-133 and PURPOSE-234 studies (ClinicalTrials.gov #NCT04994509 and NCT04925752, respectively) of semi-annual lenacapavir compared with oral PrEP, and the prematurely terminated Merck studies (MK- 8591–02235 and MK-8591–02436) of oral monthly islatravir (ClinicalTrials.gov #NCT04644029 and NCT04652700, respectively). However, all of these studies were launched prior to U.S. Food & Drug Administration approval of CAB-LA for PrEP. Community, ethical, and regulatory engagement will be required in selecting the appropriate comparator for future PrEP trials. A key limitation of the active-controlled design is that it can only address relative efficacy of the experimental intervention.
3. Active-controlled augmented by external control
To overcome the key limitation of the active-controlled design, the trial may be augmented by external control data to evaluate absolute prevention efficacy.37 Several types of external control data have been put forward, which we discuss in turn.
a). Historical placebo arm
Historical placebo arm HIV incidence from clinical trials conducted in the same geographic area as the active-controlled trial, may serve as external control. We illustrate this approach using data from the antibody mediated prevention (AMP) trials (HVTN 703/HPTN 081 and HVTN 704/HPTN 085;38 ClinicalTrials.gov #NCT02568215 and NCT02716675, respectively). Specifically, we compare the placebo arm incidence in the AMP trial among women in sub-Saharan Africa [(SSA) HVTN 703/HPTN 081] with a ‘counterfactual placebo’ HIV incidence estimate based on blinded placebo arm data from historical HIV prevention trials conducted among women in SSA in the preceding 10 years (Figure 2).14,39–42 We also compare the placebo arm incidence among MSM/TG persons in the U.S., Peru, Brazil, and Switzerland (HVTN 704/HPTN 805) with a counterfactual placebo HIV incidence estimate based on blinded placebo arm data from historical HIV prevention trials conducted among MSM in the preceding 10 years (Figure 3).5,15,23,43,44 This analysis illustrates how a historical external control would have performed, had it been used (see Supplemental Materials).
Figure 2.
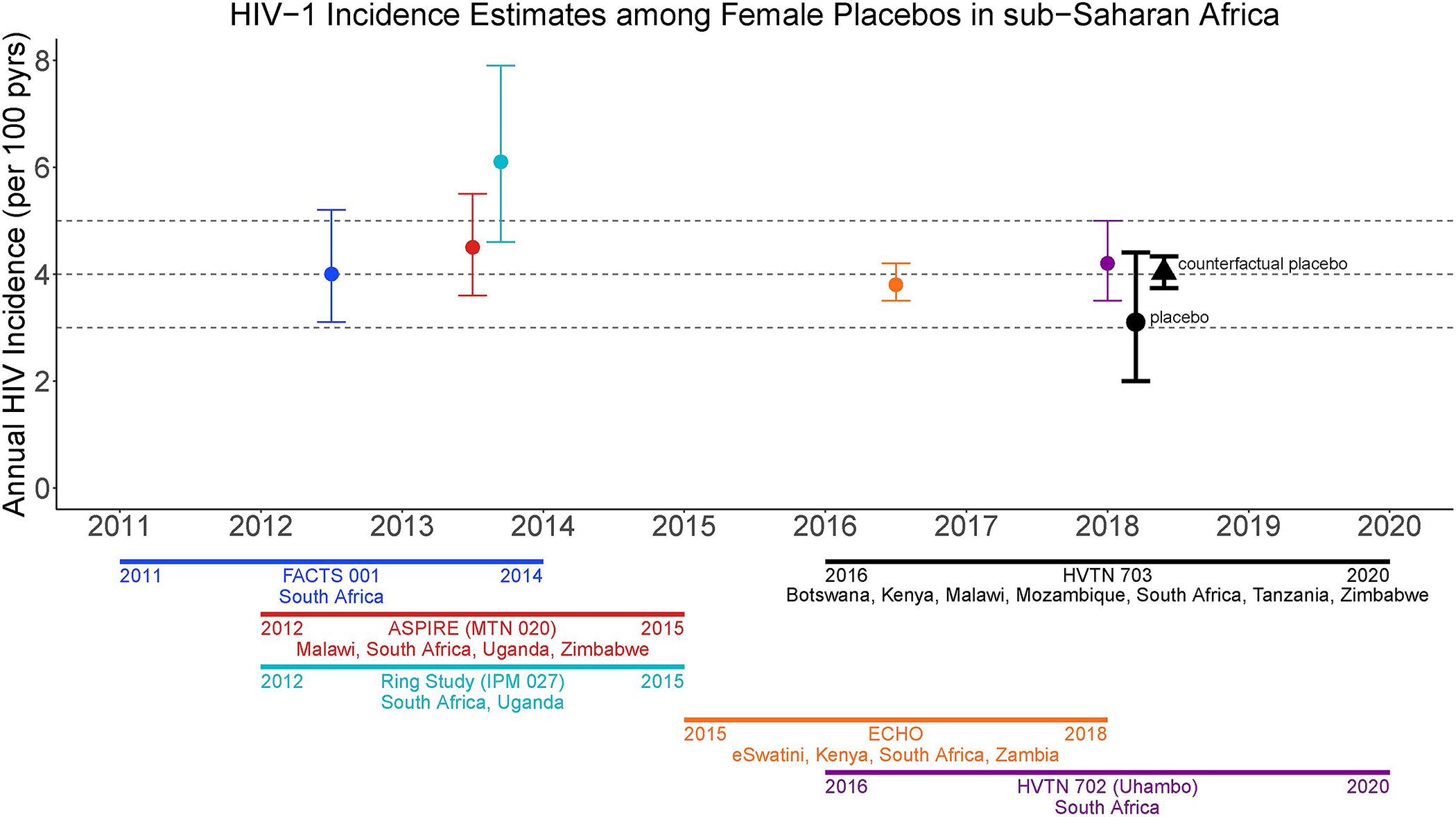
Historical placebo-arm HIV incidence for HIV prevention efficacy trials in women in SSA, 2011 to 2020 spanning eSwatini, Kenya, Malawi, South Africa, Uganda, Zambia, Zimbabwe, and a counterfactual placebo HIV incidence estimate based on the historical incidence data. The placebo-arm HIV incidence for HVTN 703/HPTN 081 (2016–2020; Botswana, Kenya, Malawi, Mozambique, South Africa, Tanzania, and Zimbabwe) is shown for comparison.
FACTS 001 (ClinicalTrials.gov #NCT01386294)
ASPIRE (ClinicalTrials.gov #NCT01617096)
HVTN 703 (ClinicalTrials.gov #NCT02568215)
Ring Study (IPM 027) (ClinicalTrials.gov #NCT01539226
ECHO (ClinicalTrials.gov #NCT02550067)
HVTN 702 (ClinicalTrials.gov #NCT02968849)
Figure 3.
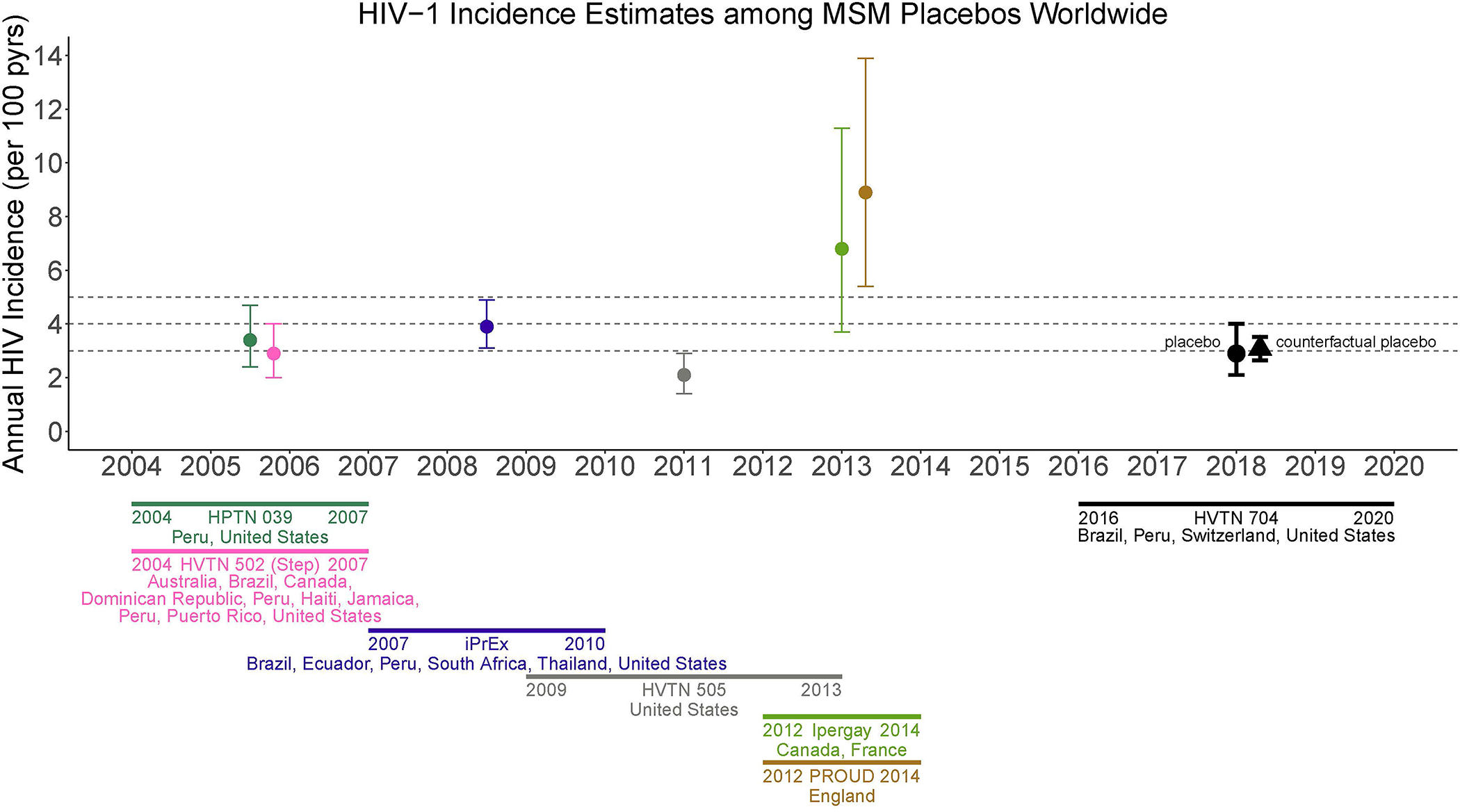
Historical placebo-arm HIV incidence for HIV prevention efficacy trials in men who have sex with men (MSM), 2004 to 2014 spanning Australia, Brazil, Canada, Dominican Republic, Ecuador, England, France, Haiti, Jamaica, Peru, Puerto Rico, South Africa, Thailand, United States, and a counterfactual placebo HIV incidence estimate based on the historical incidence data. The placebo-arm HIV incidence for HVTN 704/HPTN 085 (2016–2020; United States, Peru, Switzerland, Brazil) is shown for comparison.
HPTN 039 (ClinicalTrials.gov #NCT00076232)
HVTN 502 (ClinicalTrials.gov #NCT00865566)
HVTN 704 (ClinicalTrials.gov #NCT02716675)iPrEx (ClinicalTrials.gov #NCT00458393)
Ipergay (ClinicalTrials.gov #NCT01473472)
PROUD (ClinicalTrials.gov #NCT02065986)
Placebo-group HIV incidence estimates for women in SSA have been relatively stable in recent years. The counterfactual placebo HIV incidence is 4.0% (95% CI: 3.7%, 4.3%) (Figure 2 and Table 1), higher than the observed placebo-group HIV incidence in HVTN 703/HPTN 081 of 3.1% (95% CI: 2.0%, 4.4%).
Table 1.
Comparison of observed and counterfactual placebo-group HIV incidence rates in the AMP studies, HVTN 703/HPTN 081 and HVTN 704/HPTN 085.
| Trial | Subset | Observed placebo HIV incidence (95% CI) | Placebo HIV incidence accounting for PrEP (95% CI) | Historical data-based counterfactual placebo HIV incidence (95% CI) | RGC-based counterfactual placebo HIV incidence (95% CI) |
|---|---|---|---|---|---|
| HVTN 703/HPTN 081 | Overall | 3.1% (2.0%, 4.4%) | 3.1% (2.0%, 4.2%) | 4.0% (3.7%, 4.3%) | -- |
| South Africa | 3.2% (1.8%, 5.1%) | 3.3% (1.8%, 5.1%) | 4.6% (4.2%, 4.9%) | -- | |
| HVTN 704/HPTN 085 | Overall | 2.9% (2.1%, 4.0%) | 3.9% (2.6%, 5.5%) | 3.1% (2.6%, 3.5%) | 4.5% (3.5%, 5.8%) |
| United Stated | 1.2% (0.5%, 2.3%) | 2.2% (0.7%, 4.2%) | 2.3% (1.7%, 2.9%) | 4.4% (3.5%, 5.7%) | |
| Peru | 5.2% (3.4%, 7.6%) | 5.4% (3.4%, 7.7%) | 3.0% (2.3%, 3.7%) | 4.6% (3.5%, 6.0%) |
PrEP=pre-exposure prophylaxis
RGC=rectal gonorrhea
AMP= antibody mediated prevention
HVTN 703/HPTN 081 (ClinicalTrials.gov #NCT02568215)
HVTN 704/HPTN 085 (ClinicalTrials.gov #NCT02716675)
Historical HIV incidence estimates from studies enrolling MSM have been more variable (Figure 3). The counterfactual placebo HIV incidence is 3.1% (95% CI: 2.6%, 3.5%), similar to the observed placebo-group HIV incidence in HVTN 704/HPTN 085 of 2.9% (95% CI: 2.1%, 4.0%) (Table 1).
The limitations of historical placebo arm data as external control are numerous45 including the possibility of lack of available historical data for some regions that may be included in a future trial. Another is the numerous demographic, behavioral, epidemiologic, and socio-cultural factors that influence HIV incidence, only a small fraction of which are measured in HIV prevention trials. Furthermore, there is considerable heterogeneity in whether and how HIV behavioral vulnerability is measured, making adjustment for these factors difficult. A third major limitation is potential bias due to calendar time trends in HIV incidence, associated with an improving standard of HIV prevention, increased penetration of HIV treatment, and other epidemiologic and societal factors that influence transmission.
b. Incidence of non-HIV sexually transmitted infections (STIs)
Another approach leverages the historical correlation between incidence of HIV and other STIs to estimate counterfactual placebo HIV incidence.46–48 The historical studies must have been conducted in contexts without HIV or STI biomedical interventions that would change their correlation, e.g., without HIV PrEP or doxycycline postexposure prophylaxis (PEP) to prevent bacterial STIs. There are three other strong assumptions underlying the method:48 1. There is a common correlation between HIV incidence and STI incidence across the historical studies and the active-controlled trial; 2. The common correlation can be estimated with historical data; and 3. At least one intervention in the active-controlled trial does not influence acquisition of the STI. This approach is described using data from HVTN 704/HPTN 085 in Supplemental Materials.
The STI approach has major limitations. Few, if any, future studies are expected to measure HIV and STI incidence in the absence of PrEP and effective bacterial STI prevention;49 the set of historical data with which to estimate the HIV vs. STI correlation may be fixed. Additionally, individual-level factors associated with the correlation between HIV and STI outcomes differ among historical studies and adjusting for these factors is necessary for accurate inference.
c. Additional approaches
Several other external control data sources are being developed. HIV recency assays have long been used to estimate HIV incidence in a population given a cross-sectional sample; they can identify individuals infected in the recent past.50–54 HIV prevention trials routinely test individuals at trial screening, and recency testing could be applied to estimate past HIV incidence in the screened population. For this to work, recruitment must target individuals not recently tested for HIV, yet willing to enroll in an HIV prevention trial; finding such populations may be difficult in areas with high HIV testing and PrEP uptake. Also, individuals screened for a trial may differ from individuals enrolled, and these differences will induce bias if they are associated with HIV. These challenges notwithstanding, two ongoing trials are utilizing the recency assay approach: the PURPOSE-1 and PURPOSE-2 trials compare HIV incidence in the lenacapavir arm with HIV incidence in the screened population estimated using HIV recency assays.33,34
Another approach is to leverage a biomarker that predicts the efficacy of the active control to infer efficacy of the experimental intervention relative to a counterfactual placebo. For trials utilizing oral PrEP as the active control, plasma ARV drug levels may be used as the biomarker given historical evidence oral PrEP efficacy is strongly associated with plasma drug level.55,56 This approach has been used to estimate efficacy of oral emtricitabine and tenofovir alafenamide (TAF-FTC) based on the active-controlled DISCOVER trial (ClinicalTrials.gov #NCT02842086).6,57 The approach requires multiple historical placebo-controlled trials of an intervention to establish that a biomarker predicts efficacy, with standardized specimen collection and biomarker assays. Identifying a biomarker that predicts efficacy of the active control is challenging for interventions such as vaccines that may have multiple mechanisms of action. An important simple and special case occurs, however, if active control efficacy is believed to be constant, based on similar efficacy estimates across diverse historical settings. In this case, the assumed active control efficacy may be used to infer efficacy of the experimental intervention, without use of a biomarker.
A final approach is to use a ‘baseline control,’ whereby HIV incidence in a run-in period, or registrational cohort, is the external control. Participants remaining without HIV at the end of the run-in period and satisfying other trial eligibility criteria are eligible for enrollment. This approach is being used to evaluate efficacy of TAF-FTC and FTC-TDF in the ongoing PrEPVacc trial58 (ClinicalTrials.gov # NCT04066881). Its application requires that individuals behaviorally vulnerable to HIV be followed without effective prevention intervention prior to trial enrollment. Accounting for differences between individuals enrolling in the run-in period vs. the trial is important. The approach is also sensitive to bias due to secular trends in HIV incidence.
Study designs and analytical approaches combining multiple sources of external controls, for increasing precision and robustness, are compelling and require investigation.
Conclusion
Success in HIV prevention poses challenges in designing clinical trials evaluating new preventive interventions. While traditional randomized, standard of prevention-controlled trials with a placebo arm are appropriate and feasible to evaluate novel non-ARV HIV prevention methods, such as vaccines, careful conduct is critical to ensure that standard of care is a consequence of authentic individual choice. Active-controlled designs are now the norm for evaluating new PrEP agents, with oral PrEP as the active control. With excellent efficacy of injectable PrEP, the appropriate active control is an open question and demonstrating superiority or non-inferiority to injectable PrEP is a challenge. Active-controlled designs augmented with external control data hold promise for evaluating absolute efficacy of the intervention, yet further methodological development and experience with these approaches is needed.
Supplementary Material
Key points.
There are 3 main classes of designs of future HIV prevention efficacy trials: standard of prevention designs, active-controlled designs, and active-controlled designs augmented with external controls.
The potential and ideal design depends on the class of experimental intervention, the target population, the criterion for success of the intervention.
Considerations around study design are dynamic, and influenced by scientific evidence and availability, regulatory status, ethical considerations, community input, and acceptability and uptake of other preventive interventions.
Acknowledgements.
The authors gratefully acknowledge the analyses contributed by Jia Jin Kee and Ethan Ashby of the Fred Hutchinson Cancer Center and University of Washington, and technical editing by Heather Angier at the Fred Hutchinson Cancer Center.
Financial support and sponsorship.
This work was supported by the National Institutes of Health Grant #R01CA152089 and National Institute of Allergy and Infectious Diseases of the National Institutes of Health grant #UM1AI068635 for HJ and #5U01AI068614 for SB.
Footnotes
Conflicts of interest.
None
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med 2016; 375(9): 830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365(6): 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis 2014; 14(4): 281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS. Prevailing Against Pandemics by Putting People at the Centre, 2020.
- 5.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363(27): 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020; 396(10246): 239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. Aids 2016; 30(12): 1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **8. Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. N Engl J Med 2021; 385(7): 595–608. -Reports on a pivotal phase 3 trial of long-acting injectable Cabotegravir as PrEP in cisgender men and transgender women in the United States, Latin America, Asia, and Africa. The results demonstrated that CAB-LA was not only non-inferior, but was superior to the active control, daily oral tenofovir disoproxil fumarate–emtricitabine (TDF–FTC), at preventing HIV infection. The study supported licensure of CAB-LA in many countries around the globe.
- **9. Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022; 399(10337): 1779–89. -Reports on a pivotal phase 3 trial of long-acting injectable Cabotegravir as PrEP in women living in sub-Saharan Africa. Results demonstrated that CAB-LA was superior to the active control, daily oral tenofovir disoproxil fumarate–emtricitabine (TDF–FTC), at preventing HIV infection. The study supported licensure of CAB-LA in many countries around the globe.
- 10.Smith DK, Sullivan PS, Cadwell B, et al. Evidence of an Association of Increases in Pre-exposure Prophylaxis Coverage With Decreases in Human Immunodeficiency Virus Diagnosis Rates in the United States, 2012–2016. Clin Infect Dis 2020; 71(12): 3144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson LF, Meyer-Rath G, Dorrington RE, et al. The Effect of HIV Programs in South Africa on National HIV Incidence Trends, 2000–2019. J Acquir Immune Defic Syndr 2022; 90(2): 115–23. [DOI] [PubMed] [Google Scholar]
- *12. Bekker LG, Pike C, Hillier SL. HIV prevention: better choice for better coverage. J Int AIDS Soc 2022; 25(1): e25872. -Reviews ‘first generation’ and ‘second generation’ PrEP products for HIV prevention, and the barriers and challenges with their usage; and argues for recognition of the importance of individual choice in achieving broad coverage of effective HIV prevention.
- 13.Beymer MR, Holloway IW, Pulsipher C, Landovitz RJ. Current and Future PrEP Medications and Modalities: On-demand, Injectables, and Topicals. Curr HIV/AIDS Rep 2019; 16(4): 349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **14. Gray GE, Bekker LG, Laher F, et al. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C gp120-MF59 in Adults. N Engl J Med 2021; 384(12): 1089–100. -Reports on the results of a phase 2b trial evaluating a candidate HIV vaccine for preventing acquisition of HIV among at-risk men and women in South Africa. The vaccine, a canarypox ALVAC-HIV + bivalent subtype C gp120-MF59 vaccine, was modified from the ALVAC-HIV + AIDSVAX B/E found to partially protect in a previous trial in Thailand. However, the results demonstrated a lack of efficacy of the clade C vaccine and raise questions about the future of non-neutralizing-antibody-based HIV vaccines.
- 15.Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013; 369(22): 2083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson & Johnson. Johnson & Johnson and Global Partners Announce Results from Phase 2b Imbokodo HIV Vaccine Clinical Trial in Young Women in Sub-Saharan Africa. 2021. https://www.jnj.com/johnson-johnson-and-global-partners-announce-results-from-phase-2b-imbokodo-hiv-vaccine-clinical-trial-in-young-women-in-sub-saharan-africa (accessed Mar 3 2023).
- 17.Janssen Vaccines & Prevention B.V. A Study of Heterologous Vaccine Regimen of Adenovirus Serotype 26 Mosaic4 Human Immunodeficiency Virus(Ad26.Mos4.HIV), Adjuvanted Clade C gp140 and Mosaic gp140 to Prevent HIV-1 Infection Among Cis-gender Men and Transgender Individuals Who Have Sex With Cis-gender Men and/or Transgender Individuals (MOSAICO). 2019. https://clinicaltrials.gov/ct2/show/NCT03964415 (accessed Mar 9 2023).
- 18.National Institute of Allergy and Infectious Diseases. NIH and Partners to Launch HIV Vaccine Efficacy Trial in the Americas and Europe. 2019. https://www.niaid.nih.gov/news-events/nih-and-partners-launch-hiv-vaccine-efficacy-trial-americas-and-europe (accessed Mar 9 2023).
- 19.Johnson & Johnson. Janssen and Global Partners to Discontinue Phase 3 Mosaico HIV Vaccine Clinical Trial. Leiden, The Netherlands; 2023. [Google Scholar]
- 20.Sugarman J, Celum CL, Donnell D, Mayer KH. Ethical considerations for new HIV prevention trials. Lancet HIV 2019; 6(8): e489–e91. [DOI] [PubMed] [Google Scholar]
- 21.Finlayson T, Cha S, Xia M, et al. Changes in HIV Preexposure Prophylaxis Awareness and Use Among Men Who Have Sex with Men - 20 Urban Areas, 2014 and 2017. MMWR Morb Mortal Wkly Rep 2019; 68(27): 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. HIV and Women. 2023. https://www.cdc.gov/hiv/group/gender/women/index.html (accessed March 10 2023).
- 23.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387(10013): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367(5): 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372(6): 509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367(5): 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogunbajo A, Storholm ED, Ober AJ, et al. Multilevel Barriers to HIV PrEP Uptake and Adherence Among Black and Hispanic/Latinx Transgender Women in Southern California. AIDS Behav 2021; 25(7): 2301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang M, Scheim AI, Teti M, et al. Barriers and Facilitators to HIV Pre-Exposure Prophylaxis Uptake, Adherence, and Persistence Among Transgender Populations in the United States: A Systematic Review. AIDS Patient Care STDS 2022; 36(6): 236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pyra M, Johnson AK, Devlin S, et al. HIV Pre-exposure Prophylaxis Use and Persistence among Black Ciswomen: “Women Need to Protect Themselves, Period”. J Racial Ethn Health Disparities 2022; 9(3): 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayesu I, Mayanja Y, Nakirijja C, et al. Uptake of and adherence to oral pre-exposure prophylaxis among adolescent girls and young women at high risk of HIV-infection in Kampala, Uganda: A qualitative study of experiences, facilitators and barriers. BMC Womens Health 2022; 22(1): 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepperrell T, Cross S, Hill A. Cabotegravir—Global Access to Long-Acting Pre-exposure Prophylaxis for HIV. Open Forum Infectious Diseases 2022; 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamieson L, Johnson LF, Nichols BE, et al. Relative cost-effectiveness of long-acting injectable cabotegravir versus oral pre-exposure prophylaxis in South Africa based on the HPTN 083 and HPTN 084 trials: a modelled economic evaluation and threshold analysis. Lancet HIV 2022; 9(12): e857–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov. Pre-Exposure Prophylaxis Study of Lenacapavir and Emtricitabine/Tenofovir Alafenamide in Adolescent Girls and Young Women at Risk of HIV Infection (PURPOSE 1). 2023. https://clinicaltrials.gov/ct2/show/NCT04994509 (accessed May 9 2023).
- 34.ClinicalTrials.gov. Study of Lenacapavir for HIV Pre-Exposure Prophylaxis in People Who Are at Risk for HIV Infection (PURPOSE 2). 2023. https://clinicaltrials.gov/ct2/show/NCT04925752 (accessed May 9, 2023 2023).
- 35.ClinicalTrials.gov. Oral ISL QM as PrEP in Cisgender Women at High Risk for HIV-1 Infection (MK-8591–022) (Impower-022). 2022. https://classic.clinicaltrials.gov/ct2/show/NCT04644029 (accessed July 3 2023).
- 36.ClinicalTrials.gov. Oral Islatravir (MK-8591) Once-Monthly as Preexposure Prophylaxis (PrEP) in Men and Transgender Women Who Are at High Risk for HIV-1 Infection (MK-8591–024) (Impower-024). 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04652700 (accessed July 3 2023).
- 37.Janes H, Donnell D, Gilbert PB, Brown ER, Nason M. Taking stock of the present and looking ahead: envisioning challenges in the design of future HIV prevention efficacy trials. Lancet HIV 2019; 6(7): e475–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **38. Corey L, Gilbert PB, Juraska M, et al. Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N Engl J Med 2021; 384(11): 1003–14. -Reports on the results of two proof-of-concept trials evaluating passive infusion of the VRC01 monoclonal antibody for prevention of HIV, in at-risk women in sub-Saharan Africa and cisgender men and transgender persons in the Americas and Europe. The results established that a monoclonal antibody can prevent acquisition of HIV infection with antibody-susceptible viruses, and motivate consideration of broader and more potent antibodies/combination antibodies for HIV prevention.
- 39.Delany-Moretlwe S, Lombard C, Baron D, et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2018; 18(11): 1241–50. [DOI] [PubMed] [Google Scholar]
- 40.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. New England Journal of Medicine 2016; 375(22): 2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nel A, van Niekerk N, Kapiga S, et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N Engl J Med 2016; 375(22): 2133–43. [DOI] [PubMed] [Google Scholar]
- 42.Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet 2019; Jul 27(303–313). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molina JM, Capitant C, Spire B, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med 2015; 373(23): 2237–46. [DOI] [PubMed] [Google Scholar]
- 44.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371(9630): 2109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnell D, Kansiime S, Glidden DV, et al. Study Design Approaches for Future HIV Prevention Trials: A virtual workshop series. Statistical Communications in Infectious Diseases Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullick C, Murray J. Correlations Between Human Immunodeficiency Virus (HIV) Infection and Rectal Gonorrhea Incidence in Men Who Have Sex With Men: Implications for Future HIV Preexposure Prophylaxis Trials. J Infect Dis 2020; 221(2): 214–7. [DOI] [PubMed] [Google Scholar]
- 47.Murray JS. Regulatory Perspectives for Streamlining HIV Prevention Trials. Statistical Communications in Infectious Diseases 2019; 11(1). [Google Scholar]
- 48.Zhu Y, Gao F, Glidden DV, Donnell D, Janes H. Estimating Counterfactual Placebo HIV Incidence in HIV Prevention Trials Without Placebo Arms Based on Markers of HIV Exposure. Clinical Trials Revised and Resubmit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luetkemeyer AF, Donnell D, Dombrowski JC, et al. Postexposure Doxycycline to Prevent Bacterial Sexually Transmitted Infections. N Engl J Med 2023; 388(14): 1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim AA, Behel S, Northbrook S, Parekh BS. Tracking with recency assays to control the epidemic: real-time HIV surveillance and public health response. Aids 2019; 33(9): 1527–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brookmeyer R Measuring the HIV/AIDS epidemic: approaches and challenges. Epidemiol Rev 2010; 32: 26–37. [DOI] [PubMed] [Google Scholar]
- 52.Busch MP, Pilcher CD, Mastro TD, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. Aids 2010; 24(18): 2763–71. [DOI] [PubMed] [Google Scholar]
- 53.Konikoff J, Brookmeyer R, Longosz AF, et al. Performance of a limiting-antigen avidity enzyme immunoassay for cross-sectional estimation of HIV incidence in the United States. PLoS One 2013; 8(12): e82772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaplan EH, Brookmeyer R. Snapshot Estimators of Recent HIV Incidence Rates. Operations Research 1999; 47(1): 29–37. [Google Scholar]
- 55.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66(3): 340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4(151): 151ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glidden DV, Das M, Dunn DT, et al. Using the adherence-efficacy relationship of emtricitabine and tenofovir disoproxil fumarate to calculate background hiv incidence: a secondary analysis of a randomized, controlled trial. J Int AIDS Soc 2021; 24(5): e25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ClinicalTrials.gov. A Combination Efficacy Study in Africa of Two DNA-MVA-Env Protein or DNA-Env Protein HIV-1 Vaccine Regimens With PrEP (PrEPVacc) 2022. https://clinicaltrials.gov/ct2/show/NCT04066881 (accessed May 9 2023). [Google Scholar]
- 59.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29(2): 384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother 2018; 62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. The Lancet Infectious Diseases 2014; 14(9): 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


