Abstract
Active nuclear importation of the human immunodeficiency virus (HIV) type 1 (HIV-1) preintegration complex (PIC) is required for the productive infection of nondividing cells, but it is believed to be dispensable for the infection of proliferating cells, such as activated T lymphocytes. To investigate this question, we exploited the properties of the small arylene bis (methyl ketone) compound CNI-H0294. We have previously shown that this compound associated with the HIV-1 matrix protein nuclear localization sequence and blocked binding of the HIV-1 PIC to yeast karyopherin α. CNI-H0294 abrogated nuclear importation of the HIV-1 genome in macrophages and effectively inhibited infection of nondividing cells. In this study we demonstrate that CNI-H0294 inhibits binding of the HIV-1 PIC to human karyopherin α and reduces nuclear importation of the viral genome in primary peripheral blood mononuclear cells (PBMCs). We also demonstrate that CNI-H0294 inhibits acute infection of PBMC cultures in vitro with a primary isolate of HIV-1 and reduces virus replication and virus load in cultures of endogenously infected PBMCs from seropositive individuals. Thus, as for infection of nondividing, terminally differentiated macrophages, HIV-1 uses active nuclear importation of the virus genome to infect activated CD4+ T cells. These results support nuclear importation as a novel target and CNI-H0294 and its derivatives as novel compounds for therapeutic intervention in HIV infection and AIDS.
Human immunodeficiency virus (HIV) type 1 (HIV-1) and other lentiviruses infect nondividing, terminally differentiated cells such as primary macrophages (17, 18), primary blood dendritic cells (27), and epidermal Langerhans’ cells (43). This is primarily due to the active importation of the HIV-1 preintegration complex, which incorporates the viral genome, across the intact nuclear envelope of the nondividing cell (5, 6, 50). This active process obviates the requirement for cell division, thus allowing HIV-1 to infect nonproliferating as well as proliferating cells (28, 29, 50), the usual targets of retroviruses (29, 44).
It was recently shown (16, 41) that the preintegration complex (PIC) of HIV-1 associates with karyopherins, the cellular proteins involved in active nuclear import (for a review, see reference 37). Karyopherin α binds to target proteins via their nuclear localization sequence (NLS), while karyopherin β mediates docking of the karyopherin α-target protein complex to nuclear pore structures (1, 19, 20, 25, 35, 42). Two forms of karyopherin α have been identified in human cells; these forms have been designated hSRP1α (51), Rch1 (10), or K2 (36) and hSRP1 (51), NPI-1 (38), or K1 (36), and they share approximately 45 to 50% sequence homology. The human karyopherin α has sequence homology similar to that of the yeast karyopherin α (9, 38, 51). In addition to karyopherins, nuclear translocation involves several other small proteins such as the GTPase Ran/TC4 (8, 31–33) and p10/NTF2 (39), as well as the nuclear pore proteins, nucleoporins (for a review, see reference 25).
The HIV-1 matrix protein (MA) contains one defined (K26KKYK) and one putative (K110SKKK) NLS and represents a major karyophilic structure within the PIC (5, 7, 16, 50). Synthetic peptides encompassing either of the two MA NLSs bound to both hSRP1α (K2) and hSRP1 (K1) present in B-cell and T-cell lysates (36). Mutations in the KKKYK NLS of MA, alone or in combination with the deletion of Vpr, reduced the levels of nuclear importation of the PIC and inhibited infection of primary macrophage cultures (24, 50), as well as growth-arrested T cells (6) and CD4+-HeLa cell cultures (13). Amino acid substitutions within the KKKYK NLS also reduced the level of binding of the PIC to yeast karyopherin α in vitro (12), thus providing a link between the binding of PIC to karyopherin α, nuclear import, and viral replication in nondividing cells.
In this study, we explored the role of active, receptor-mediated nuclear importation in the infection of peripheral blood mononuclear cell (PBMC) cultures using the arylene bis (methyl ketone) compound CNI-H0294. This compound interacts primarily with the HIV-1 PIC by forming Schiff-base adducts with lysine residues in the MA NLS (12). We have previously shown that CNI-H0294 interferes with the association of the PIC with the yeast karyopherin α (41) and effectively inhibits infection of primary macrophage cultures with the macrophage-tropic isolate HIVADA (50% inhibitory concentration [IC50], = 10 to 50 nM) (12). We now demonstrate that CNI-H0294 blocks the interaction between HIV-1 PIC and human karyopherin α and reduces the level of nuclear importation of nascent HIV-1 cDNA in activated T lymphocytes, resulting in substantial inhibition of viral replication in both acute and chronic infection. These results provide evidence for the critical role of nuclear importation in HIV-1 infection of primary T lymphocytes.
MATERIALS AND METHODS
HIV-1 strains.
The primary isolate HIV-1M1 was described previously (26). HIV-1LAI is a T-cell line-adapted virus isolate.
Cells.
PBMCs were collected from either seronegative or seropositive individuals and were depleted of CD8+ T cells by negative selection with a CD8-specific monoclonal antibody (MAb) and rabbit complement as described previously (47).
Production of hSRP1α fusion protein.
The cDNA for hSRP1α was amplified by PCR from a phytohemagglutinin-activated human T-cell cDNA library. The product was inserted into the pCDM8 expression vector 3′ to the coding sequence for the CD5 signal peptide and 5′ to the sequence coding for the human Fc portion of immunoglobulin G1 (hinge-CH2-CH3). The hSRP1α-immunoglobulin fusion protein was derived from COS cell lysates (lysis buffer, 0.1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol in phosphate-buffered saline [PBS]) following transient transfection with the new construct. The glutathione-S-transferase hSRP1α (gst-hSRP1α) was derived from Escherichia coli DH5α (Gibco-BRL, Grand Island, N.Y.) transformed with a construct containing the hSRP1α gene inserted into the BamHI-EcoRI sites of pGEX-2T. The fusion protein was purified from disrupted bacteria by a one-step purification with a glutathione column, followed by elution with 10 mM reduced glutathione in PBS and subsequent dialysis against PBS.
Analysis of PIC-hSRP1α interaction.
Jurkat cells were incubated with HIV-1LAI (200 ng of p24/106 cells) for 1 h, and then the cells were washed free of the virus inoculum and were allowed to incubate for an additional 3 h. The cells were lysed in ice-cold hypotonic buffer (10 mM Tris [pH 7.5], 10 mM KCl, 0.5 mM MgCl2, 1 μg each of leupeptin and aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride, and 1 U of RNasin per μl when needed) with a Dounce homogenizer. After removal of the nuclei by centrifugation, the cytoplasmic fractions containing PICs were adjusted to 150 mM NaCl and were then incubated with increasing concentrations of compounds (0, 0.1, 1, and 10 μM) for 2 h prior to the addition of the gst-hSRP1α fusion protein immobilized on glutathione-coated beads. The beads were then assayed for the presence of HIV-1-specific DNA by a standard PCR method as described previously (47). Briefly, isolated cDNA was suspended in 10× PCR buffer (200 mM Tris-HCl [pH 8.4], 500 mM KCl, 1.5 mM MgCl2), 250 mM 5′-oligonucleotide primer, and 2.5 U of Taq polymerase (Boehringer Mannheim, Indianapolis, Ind.) for a final reaction volume of 100 μl, and then the reaction was cycled at 95°C for 0.5 min, 60°C for 1 min, and 72°C, for 1 min for 30 cycles. The amplified DNA products were transferred to a nylon membrane and were hybridized to a [γ-32P]ATP-labeled gag-specific oligonucleotide probe as described previously (22).
Analysis of HIV-1 nuclear import.
CD8+ T-cell-depleted PBMCs from a seronegative individual were activated for 1 h with an anti-CD3 MAb (MAb G19-4; 1 μg/ml) (11, 26, 34, 46, 47) in the presence of various concentrations of CNI-H0294 (0, 1, and 10 μM) and were then infected with the primary isolate, HIV-1M1, (20 ng of p24/106 cells; six replicates/treatment condition). In a similar experiment, Jurkat cells were preincubated with CNI-H0294 (0 and 10 μM) for 1 h and were then infected with HIV-1LAI (50 ng of p24/106 cells; six replicates/treatment condition). After a 1-h adsorption period, the virus inocula were washed out and the culture media were supplemented with the appropriate concentrations of CNI-H0294. The infections were allowed to proceed for either 40 h (PBMC cultures) or 20 h (Jurkat cells cultures), which allows a single replication cycle. The cells from the replicates were then pooled and lysed as described previously (47), and the cell lysates were evaluated for the presence of two long terminal repeat (2-LTR) circles as described elsewhere (5); 2-LTR circles were used as indicators of successful nuclear import (5, 12, 24, 50). Cell lysates were also evaluated for the presence of HIV-1 gag sequences and β-globin sequences by PCR (47); these data were used as indicators of virus entry, reverse transcription, and input DNA levels in the PCR mixtures.
Analysis of HIV-1 replication.
CD8+ T-cell-depleted cultures of PBMCs from a seronegative individual were activated with an anti-CD3 MAb (1 μg/ml) in the presence of various concentrations of CNI-H0294 (0, 1, and 10 μM; six replicates/treatment condition) for 2 h and then infected with HIV-1M1 (20 ng of p24/106 cells). After a 1-h adsorption period, the virus was washed away and the cultures were supplemented with an anti-CD3 MAb (1 μg/ml) and the appropriate concentration of CNI-H0294. Virus release was determined 6 days after infection by measuring p24 levels in the culture supernatants by a p24-specific enzyme-linked immunosorbent assay (34). In parallel, cells were evaluated for viral DNA content by measuring the levels of gag sequences by PCR techniques (47). DNA levels were quantified by densitometric analysis of phosphor screens with a Phosphorimager (Molecular Dynamics Inc., Sunnyvale, Calif.). β-Globin sequences were evaluated as an indicator of input DNA levels in the PCRs.
To determine the effects of CNI-H0294 on virus replication in endogenously infected PBMC cultures, PBMCs were collected from seropositive individuals, depleted of CD8+ T cells, and activated with an anti-CD3 MAb (1 μg/ml) in the presence of CNI-H0294 (six replicates/treatment condition). Virus production and virus load were evaluated as described above.
Cell activation was measured by evaluating the incorporation of [3H]thymidine as described previously (47).
RESULTS
CNI-H0294 inhibits binding of the HIV-1 PIC to human karyopherin α.
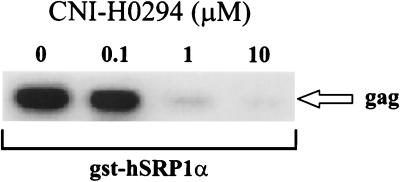
Since CNI-H0294 was previously shown to inhibit the interaction of the HIV-1 PIC with the yeast karyopherin α (41), we tested the activity on binding of the HIV-1 PIC to human karyopherin α. Jurkat cells were infected with HIV-1LAI and lysed, and the postnuclear cytosolic fractions containing the PIC were incubated with increasing concentrations of CNI-H0294 for 2 h prior to binding to gst-hSRP1α fusion protein. The gst-hSRP1α-PIC complexes were sedimented with glutathione-coated Sepharose beads, and the presence of viral cDNA in the sedimented fractions was evaluated by PCR. As indicated in Fig. 1, CNI-H0294 treatment reduced the levels of HIV-1 gag sequences that sedimented with gst-hSRP1α in a dose-dependent fashion, suggesting that binding of the HIV-1 PIC to the human karyopherin α was specifically blocked.
FIG. 1.
Binding of the HIV-1 preintegration complex to human karyopherin α. Jurkat cells were infected with HIV-1LAI (200 ng of p24/106 cells). After a 20-h infection period the cells were solubilized and the cell lysates were preincubated with increasing concentrations of CNI-H0294, as indicated, prior to the addition of gst-hSRP1α. The PIC-gst-hSRP1α complexes were sedimented with glutathione-coated beads. The levels of HIV-1 DNA in the sedimented fractions were evaluated by PCR.
CNI-H0294 inhibits nuclear importation of HIV-1 PIC in T cells.
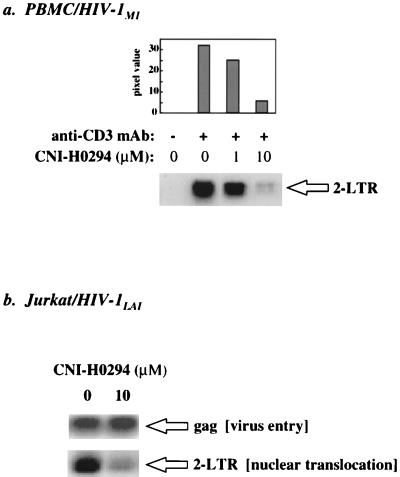
Since CNI-H0294 blocks the binding of the viral PIC to human karyopherin α in vitro (Fig. 1), we wanted to determine if this inhibition had a measurable effect on nuclear importation during acute infection of T cells. This question was addressed by assaying for the presence of 2-LTR circles, a specific nuclear form of the HIV-1 genome (5, 6), in cell lysates from infected cells. PBMCs from a seronegative donor were depleted of CD8+ T cells and were activated with a soluble anti-CD3 MAb in the presence of various concentrations of CNI-H0294 (as indicated in the figure legends). Activated cells were incubated with a primary isolate, HIV-1M1 (47), for 1 h, and then the virus inoculum was washed out and the cultures were supplemented with an anti-CD3 MAb and the appropriate concentrations of CNI-H0294. After 40 h, the cells were lysed and the presence of 2-LTR circles was evaluated by PCR analysis with specific primers that span the LTR junction. Figure 2a (autoradiograph) shows that CNI-H0294 inhibited the formation of 2-LTR circles in a dose-dependent fashion. The bar graph in Fig. 2a represents densitometric quantitation of the various radiolabeled bands. This effect was not unique for primary cells or primary isolates of HIV-1, since CNI-H0294 also inhibited 2-LTR circle formation in Jurkat cells acutely infected with HIV-1LAI (Fig. 2b). As for the PBMC cultures, the Jurkat cells were infected for a short time (20 h), which allows limited virus life cycles. The presence of equivalent levels of total viral DNA, as measured by the amount of gag sequences in the Jurkat cell lysates (Fig. 2b), suggested that CNI-H0294 specifically altered nuclear importation but not virus entry or reverse transcription. In support of the in vitro binding results (Fig. 1), this reduced nuclear importation suggests that the association of the incoming PIC with endogenous karyopherin α is essential for nuclear localization of the HIV-1 genome in activated primary T lymphocytes and dividing T cells from a T-cell line.
FIG. 2.
CNI-H0294 inhibits 2-LTR circle formation in infected cells. Anti-CD3 MAb-activated PBMC cultures (a) or Jurkat cell cultures (b) were preincubated with CNI-H0294 and were infected with either HIV-1M1 (20 ng of p24/106 cells) for 40 h (a) or HIV-1LAI (50 ng of p24/106 cells) for 20 h (b) in the presence of the compound. The presence of 2-LTR circle forms of the HIV-1 DNA was a measure of nuclear translocation, while PCR analysis of gag sequences was used as a measure of the overall HIV-1 DNA content, reflecting viral entry into the cells and reverse transcription.
CNI-H0294 inhibits virus replication in activated PBMCs acutely infected with a primary HIV-1 isolate.
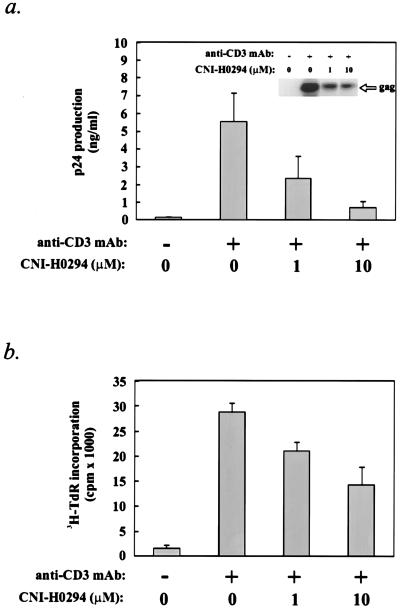
To determine if inhibition of the PIC-karyopherin α interaction and nuclear importation influence HIV-1 replication in T cells, cultures of CD8− PBMCs from a seronegative donor were activated with an anti-CD3 MAb in the presence of various concentrations of CNI-H0294 for 2 h and were then acutely infected with HIV-1M1. The virus was allowed to adsorb for 1 h and was then washed out, and the cultures were again supplemented with an anti-CD3 MAb and the appropriate concentrations of CNI-H0294. Virus production and virus load were determined 6 to 7 days after infection, while cell proliferation was evaluated on day 4 after infection (see Materials and Methods). The data indicated that CNI-H0294 substantially reduced the level of virus infection in these cultures, as demonstrated by the reduction in the p24 levels in the supernatants of treated cultures, as well as by the reduction of the viral DNA content in cell lysates (Fig. 3a, histogram and inset autoradiograph, respectively). Whereas CNI-H0294 effectively reduced the level of virus replication (IC50, ≤1 μM), cell proliferation, as measured by the level of [3H]thymidine incorporation, was much less affected (IC50, 10 μM) (Fig. 3b). These results indicate that inhibition of the nuclear importation of the HIV-1 PIC (Fig. 2a) correlates with a reduced levels of infection of activated T cells.
FIG. 3.
CNI-H0294 inhibits de novo infection of activated PBMCs. PBMCs from a seronegative individual were activated with an anti-CD3 MAb in the presence of increasing concentrations of CNI-H0294, as indicated, and were then infected with HIV-1M1 (20 ng of p24/106 cells). (a) Virus production was evaluated on day 6 after activation by a p24-specific enzyme-linked immunosorbent assay; the virus DNA content in pooled cell lysates was evaluated by PCR (inset autoradiograph). (b) Cell proliferation was evaluated on day 4 after activation by measuring the level of incorporation of [3H]thymidine.
CNI-H0294 inhibits virus replication in activated PBMCs from seropositive individuals.
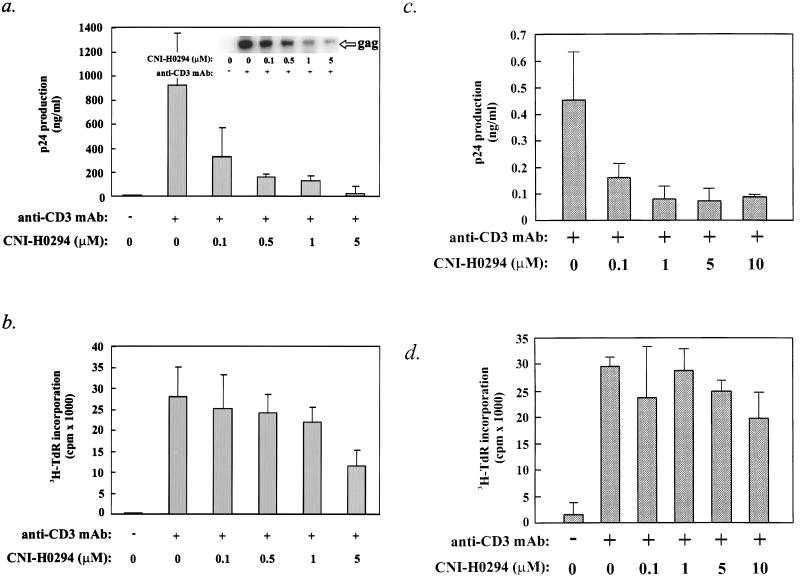
To determine if nuclear importation plays a similar role in virus replication in the background of a preexisting infection, we used freshly isolated PBMCs from HIV-1-infected individuals. Infected individuals have integrated provirus in their CD4+ T cells, and this provirus can be induced in vitro by specific activation of the PBMCs with an anti-CD3 MAb (11, 34, 46, 47). The number of infected cells and the levels of expression of the endogenous virus in the PBMCs are usually low, so the measured virus output in these cultures is primarily a result of amplification by virus spread to new targets (11, 34, 46, 47). Cultures of CD8− PBMCs from two seropositive individuals (cultures Z78 and Z44), who we had previously shown were responsive to anti-CD3 MAb-induced virus replication (46), were activated in the presence of increasing concentrations of CNI-H0294. Virus production (Z78 and Z44 cultures) and virus load (Z78 cultures) were measured as described in Materials and Methods. Figure 4a (inset autoradiograph) indicates that the level of viral DNA in the Z78 cultures treated with CNI-H0294 was substantially lower (by more than 95% at 5 μM) than that in the untreated control cultures, suggesting that the compound effectively reduced virus spread in the activated PBMC cultures. Consistent with the observed reduction in the virus load, CNI-H0294 also reduced the level of virus production, as measured by the levels of HIV-1 p24 in the supernatants of treated versus untreated cultures (Fig. 4a, histogram). As with the seronegative PBMCs acutely infected with HIV-1 (Fig. 3), the proliferation of endogenously infected PBMCs was only moderately affected by CNI-H0294 (for Z78 cultures, IC50 = 5 μM; for Z44 cultures, IC50 > 10 μM) (Fig. 4b, and d, respectively) compared to the effect of CNI-H0294 on virus replication (IC50, <0.1 μM for both donor cultures) (Fig. 4a and c, respectively). Moreover, CNI-H0294 was not toxic to either primary cells or T-cell lines at the highest concentration tested, as determined by exclusion of trypan blue (data not shown). These results indicate that CNI-H0294 can effectively inhibit virus replication in freshly isolated PBMCs from HIV-1-infected individuals without significantly altering cell metabolism.
FIG. 4.
CNI-H0294 limits virus replication in cultures of activated PBMCs from seropositive individuals. PBMCs collected from two seropositive individuals (PBMC culture Z78 [a and b]; PBMC culture Z44 [c and d]) were activated with anti-CD3 MAb in the presence of increasing concentrations of CNI-H0294 (as indicated). (a and c) Virus production was evaluated on day 6 after activation as described in the legend to Fig. 3. Viral DNA content (a, inset autoradiograph) in pooled cell lysates was evaluated by PCR. (b and d) Cell proliferation was evaluated on day 4 after activation.
DISCUSSION
Inhibitors of nuclear importation, such as high concentrations of prototypic NLS peptides (22) which bind to karyopherin α or chemical entities such as CNI-H0294 that associate with NLS motifs on the HIV-1 MA (12, 41), reduce the level of HIV-1 infection of growth-arrested T cells (22) and primary macrophage cultures (12).
In this study we explored the role of nuclear importation in HIV-1 infection of activated T cells by using the compound CNI-H0294. Given that the nuclear importation machinery is extremely well conserved in different cell types, it is reasonable to assume that the mechanisms of action of CNI-H0294 are similar in T cells and macrophages. Therefore, this compound provides us with an opportunity to investigate the nuclear importation of HIV-1 in the context of primary viral isolates and primary cells from HIV-infected persons, something not possible by other methods. We now show that CNI-H0294 inhibited the association of HIV-1 PIC with human karyopherin α and that this association was necessary for infection of activated PBMCs. Inhibition of nuclear importation by CNI-H0294 was achievable in activated PBMCs acutely infected in vitro with a primary isolate of HIV-1, as well as in Jurkat cells infected with a laboratory isolate of the virus. Furthermore, CNI-H0294 effectively inhibited virus replication following infection of PBMCs with a large virus inoculum (Fig. 2 and 3). Activated PBMCs were less sensitive to the effects of CNI-H0294 on a molar basis than primary macrophage cultures (100 versus 10 nM, respectively) (Fig. 3) (12). This difference may be due in part to lower level of uptake of the compound by activated T cells compared to that by macrophages (4).
Similar to infection in vivo, the HIV-1 load in vitro is maintained largely by recruitment of newly infected cells (11, 34, 46, 47). Thus, compounds that slow the rate of viral infection, as CNI-H0294 does in T cells and macrophages, would be expected to reduce the viral load significantly. This notion is supported by our observations of a significant reduction of viral load by CNI-H0294 in freshly isolated PBMCs from seropositive individuals (Fig. 4a and c). The HIV-1 DNA detected in the treated cultures (Fig. 4a) is derived primarily from integrated provirus in proliferating infected cells not susceptible to the effects of the compound and from PIC-associated cDNA sequestered by CNI-H0294 in the cytoplasm of newly infected cells. The data presented for the PBMCs in the Z78 culture (Fig. 4a), which exhibit relatively high levels of virus replication (approximately 1 μg of p24/ml of culture supernatant), and the PBMCs in the Z44 culture (Fig. 4c), which replicate virus to a lesser degree in response to anti-CD3 activation, confirms the effectiveness of CNI-H0294 in the presence of an existing virus infection. In addition, these results indicate that the inhibitory effect of CNI-H0294 is not restricted to any particular HIV-1 strain since cells from HIV-infected individuals harbor an uncloned mixture of virus quasispecies. Our results therefore suggest that HIV-1 uses active nuclear importation during infection of activated T cells.
The [3H]thymidine incorporation data presented in Fig. 4b and d predict a therapeutic index for CNI-H0294 in HIV-1-infected donor PBMCs of between ≥50 (Z78 culture) and ≥100 (Z44 culture). This represents an underestimate since cell toxicity analysis by trypan blue exclusion in PBMC cultures, release of lactate dehydrogenase from primary macrophages cultures, or 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide metabolism in immortalized cell lines treated with the drug showed an average cytotoxic concentration equivalent to >200 μM (data not shown), which significantly increases the therapeutic index. Furthermore, CNI-H0294 was well tolerated by mice when it was administered as single high-dose intraperitoneal injection or by daily subchronic (28 days) intraperitoneal administration. For example, the 50% lethal dose for the drug in mice was determined to be equivalent to 560 mg/kg (3).
We and others have previously shown that virus replication in PBMC cultures was dependent on cell activation (11, 34, 46, 47). However, no consistent correlation between the magnitude of cell proliferation and virus infection had been observed (11, 34, 46, 47). In fact, nonmitogenic stimulation was shown to induce de novo transcription of the HIV-1 message (21, 52), as well as virus production and amplification in PBMCs from seropositive individuals (2). Moreover, it has been shown that activated T cells arrested in the G1-S (5) or G2 (45) phase of the cell cycle could be infected with HIV-1, while G0-phase T cells were refractory to productive infection (49, 53). These results suggest that T-cell activation is both necessary and sufficient for productive infection with HIV-1. Cell cycle analysis of freshly isolated PBMCs from HIV-1-infected individuals indicated that CD4+ T cells are either quiescent or resident in the early cell cycle phases, phases G1 and S (48). In our experimental system, freshly isolated PBMCs would be synchronously activated by an anti-CD3 MAb and would require up to 4 days of culture before entering mitosis. Thus, the effects of CNI-H0294 on infection of activated PBMCs are most likely exerted during cell cycle phases that precede mitosis (G1-S and G2 phases) and which represent the bulk of the cell cycle time. Given the defined mechanism of action of CNI-H0294, it would be predicted that rapidly dividing cells in vitro, such as PBMCs following mitogen stimulation (e.g., stimulation with phytohemagglutinin and interleukin 2), or immortalized T-cell lines would be less sensitive to the antiviral effect of the drug. This is consistent with our observation of the greatly reduced activity of CNI-H0294 in infected MT-4 and CEM cultures. However, given that in vivo the major proportion of T cells are quiescent, that T-cell activation is tightly regulated, and that cell division requires long periods of time (22 weeks for memory T cells) (30), CNI-H0294 and related compounds should significantly limit virus spread and the number of infection cycles and thus effectively reduce the viral load in vivo.
The mechanism of action of CNI-H0294 may include inhibition of other HIV-1 NLSs, in addition to the major MA NLS (K26KKYK) (41). Recently published reports (14, 15) suggest that this MA NLS is not the only karyophilic determinant in the HIV-1 PIC. In fact, additional NLS motifs have been identified in the C terminus of MA (K110SKKK) (23, 36) and in integrase (15). It is feasible to speculate that CNI-H0294 can form Schiff-base adducts with these lysine-rich NLSs, similar to the formation of such adducts with the N-terminal MA NLS, thus effectively inactivating all identified NLSs in the HIV-1 PIC. The Vpr protein also seems to play a role in HIV-1 nuclear import (24). However, Vpr, which lacks a classical NLS, binds to karyopherin α in an NLS-independent manner (16, 40) and appears to increase the affinity of karyopherin α for NLS-containing proteins (40). Therefore, the karyophilic properties of Vpr rely on the presence of other NLSs within the PIC. Under such a scenario, even a Vpr-positive PIC would be left deficient in nuclear import when the NLSs are inactivated. This is exactly the result observed with CNI-H0294 (12; this study).
In conclusion, our study indicates that active nuclear importation plays an important role in the infection of activated T cells and supports the introduction of inhibitors of nuclear importation, such as CNI-H0294, as novel therapeutic agents for intervention against HIV-1 infection.
ACKNOWLEDGMENTS
We thank Kathleen Critchett and Doug Tritschler for technical assistance.
M.I.B. is supported in part by NIH grants RO1AI40386 and RO1AI33776. This work was supported by Bristol-Myers Squibb Pharmaceutical Research Institute.
REFERENCES
- 1.Adam S A, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- 2.Åsjö B, Cefai D, Debré P, Dudoit Y, Autran B. A novel mode of human immunodeficiency virus type 1 (HIV-1) activation: ligation of CD28, activates the long terminal repeat. J Virol. 1993;67:4395–4398. doi: 10.1128/jvi.67.7.4395-4398.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukrinsky, M. I. Unpublished data.
- 4.Bukrinsky, M. I. Data not shown.
- 5.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubei A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett A H, Koepp D M, Schlenstedt G, Lee M S, Hopper A K, Silver P A. Rna 1p, a Ran/TC4 GTPase activating protein, is required for nuclear import. J Cell Biol. 1995;130:1017–1026. doi: 10.1083/jcb.130.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes P, Ye Z-S, Baltimore D. RAG-1 interacts with the repeated amino acid motif of the human homologue of the yeast protein SRP1. Proc Natl Acad Sci USA. 1994;91:7633–7637. doi: 10.1073/pnas.91.16.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuomo C A, Kirch S A, Gyuris J, Brent R, Ottinger M A. Rch1, a protein that specifically interacts with the RAG-1 recombination-activating protein. Proc Natl Acad Sci USA. 1994;91:6156–6160. doi: 10.1073/pnas.91.13.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diegel M L, Moran P A, Gilliland L K, Damle N K, Hayden M S, Zarling J M, Ledbetter J A. Regulation of HIV-1 production by blood mononuclear cells from HIV-infected donors: HIV-1 production depends on T cell-monocyte interaction. AIDS Res Hum Retroviruses. 1993;9:465–473. doi: 10.1089/aid.1993.9.465. [DOI] [PubMed] [Google Scholar]
- 12.Dubrovsky L, Ulrich P, Nuovo G J, Manogue K R, Cerami A, Bukrinsky M. Nuclear localization signal of HIV-1 as a novel target for therapeutic intervention. Mol Med. 1995;1:217–230. [PMC free article] [PubMed] [Google Scholar]
- 13.Emerman M, Bukrinsky M, Stevenson M. HIV-1 infection of nondividing cells. Nature (London) 1994;369:107–108. [Google Scholar]
- 14.Fouchier R A M, Meyer B E, Simon J H M, Fischer U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of non-dividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 18.Gendelman H E, Narayan O, Kennedy-Stoskopf S, Kennedy P G E, Ghotbi Z, Clements J E, Stanley J, Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986;58:67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Görlich D, Mattaj J W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 20.Görlich D, Vogel F, Mills A D, Hartmann E, Laskey R A. Distinct functions for the two importin subunits in nuclear protein import. Nature (London) 1995;377:246–248. doi: 10.1038/377246a0. [DOI] [PubMed] [Google Scholar]
- 21.Gruters R A, Otto S A, Al B J M, Verhoeven A J, Van Lier R A W, Miedema F. Non-mitogenic T cell activation signals are sufficient for induction of human immunodeficiency virus transcription. Eur J Immunol. 1991;21:167–172. doi: 10.1002/eji.1830210125. [DOI] [PubMed] [Google Scholar]
- 22.Gulizia J, Dempsey M P, Sharova N, Bukrinsky M I, Spitz L, Goldfarb D, Stevenson M. Reduced nuclear import of human immunodeficiency virus type 1 preintegration complexes in the presence of a prototypic nuclear targeting signal. J Virol. 1994;68:2021–2025. doi: 10.1128/jvi.68.3.2021-2025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haffar, O. K., and M. I. Bukrinsky. Unpublished data.
- 24.Heizinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of HIV-1 influences nuclear localization of viral nucleic acids in non-dividing host cells. Proc Natl Acad Sci USA. 1992;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurt E C. Importins/karyopherins meet nucleoporins. Cell. 1996;84:509–515. doi: 10.1016/s0092-8674(00)81026-0. [DOI] [PubMed] [Google Scholar]
- 26.Kanner S B, Haffar O K. HIV-1 down-regulates CD4 costimulation of TCR/CD3-directed tyrosine phosphorylation through CD4/p56lck dissociation. J Immunol. 1995;154:2996–3005. [PubMed] [Google Scholar]
- 27.Langhoff E, Terwilliger E F, Bos H J, Kalland K H, Poznasky M C, Bacon O M L, Haseltine W A. Replication of human immunodeficiency virus type in primary dendritic cell cultures. Proc Natl Acad Sci USA. 1991;88:7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLean A R, Michie C A. In vivo estimates of division and death rates of human T lymphocytes. Proc Natl Acad Sci USA. 1995;92:3707–3711. doi: 10.1073/pnas.92.9.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melchior F, Guan T, Yokoyama N, Nishimoto T, Gerace L. GTP hydrolysis by Ran occurs at the nuclear pore complex in an early step of protein import. J Cell Biol. 1995;131:571–581. doi: 10.1083/jcb.131.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore M S, Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- 33.Moore M S, Blobel G. Purification of Ran-interacting protein that is required for protein import into the nucleus. Proc Natl Acad Sci USA. 1994;91:10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran P A, Diegel M L, Sias J C, Ledbetter J A, Zarling J M. Regulation of HIV-1 production by blood mononuclear cells from HIV-infected donors. I. Lack of correlation between HIV-1 production and cell activation. AIDS Res Hum Retroviruses. 1993;9:455–464. doi: 10.1089/aid.1993.9.455. [DOI] [PubMed] [Google Scholar]
- 35.Moroianu J, Blobel G, Radu A. Previously identified protein of uncertain function is karyopherin α and together with karyopherin β docks import substrate at the nuclear pore complex. Proc Natl Acad Sci USA. 1995;92:2008–2011. doi: 10.1073/pnas.92.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadler S G, Tritschler D, Haffar O K, Blake J, Bruce A G, Cleaveland J S. Differential expression and sequence-specific interaction of karyopherin α with nuclear localization sequences. J Biol Chem. 1997;272:4310–4315. doi: 10.1074/jbc.272.7.4310. [DOI] [PubMed] [Google Scholar]
- 37.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms, and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill R E, Palese P. NPI-1, the human homologue of SRP-1, interacts with influenza virus nucleoprotein. Virology. 1995;206:116–125. doi: 10.1016/s0042-6822(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 39.Paschal B M, Gerace L. Identification of NTF2, a cytosolic factor for nuclear protein import that interacts with nuclear pore complex protein p62. J Cell Biol. 1995;129:925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popov S, Rexach M, Zybarth G, Reiling N, Lee M-A, Ratner L, Lane C M, Moore M S, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popov S, Dubrovsky L, Lee M-A, Pennathur S, Haffar O, Al-Abed Y, Tonge P, Ulrich P, Rexach M, Blobel G, Cerami A, Bukrinsky M. Critical role of reverse transcriptase in the inhibitory mechanism of CNI-H0294 on HIV-1 nuclear translocation. Proc Natl Acad Sci USA. 1996;93:11859–11864. doi: 10.1073/pnas.93.21.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radu A, Blobel G, Moore M S. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci USA. 1995;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramazzotti E, Marconi A, Re M C, Girolomoni G, Cenacchi G, Vignoli M, Zambruno G, Furlini G, La Placa M, Giannetti A. In vitro infection of human epidermal Langerhans’ cells with HIV-1. Immunology. 1995;85:94–98. [PMC free article] [PubMed] [Google Scholar]
- 44.Roe T Y, Reynolds T C, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smithgall M D, Wong J G P, Critchett K E, Haffar O K. IL-7 upregulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J Immunol. 1996;156:2324–2330. [PubMed] [Google Scholar]
- 47.Smithgall M D, Wong J G P, Linsley P S, Haffar O K. Costimulation of CD4+ T cells via CD28 modulates human immunodeficiency virus type 1 infection and replication in vitro. AIDS Res Hum Retroviruses. 1995;11:885–892. doi: 10.1089/aid.1995.11.885. [DOI] [PubMed] [Google Scholar]
- 48.Spina C A, Guatelli J C, Richman D D. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weis K, Mattaj I W, Lamond A I. Identification of hSRP1 α as a functional receptor for nuclear localization sequences. Science. 1995;268:1049–1053. doi: 10.1126/science.7754385. [DOI] [PubMed] [Google Scholar]
- 52.Wong J G P, Smithgall M D, Haffar O K. TCR-independent CD28-mediated gene expression in peripheral blood lymphocytes from donors chronically infected with HIV-1. Immunology. 1997;90:281–285. doi: 10.1046/j.1365-2567.1997.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]