Abstract
Background
We aimed to study adherence to cardiac screening in long-term childhood cancer survivors (CCS) at high risk of cardiomyopathy.
Methods
This study involved 976 5-year CCS at high risk for cardiomyopathy from the French Childhood Cancer Survivor Study. Determinants of adherence to recommended surveillance were studied using multivariable logistic regression models. Association of attendance to a long-term follow-up (LTFU) visit with completion of an echocardiogram was estimated using a Cox regression model.
Results
Among participants, 32% had an echocardiogram within the 5 previous years. Males (adjusted RR [aRR] 0.71, 95% CI 0.58–0.86), survivors aged 36–49 (aRR 0.79, 95% CI 0.64–0.98), Neuroblastoma (aRR 0.53, 95% CI 0.30–0.91) and CNS tumour survivors (aRR 0.43, 95% CI 0.21–0.89) were less likely to adhere to recommended surveillance. Attendance to an LTFU visit was associated with completion of an echocardiogram in patients who were not previously adherent to recommendations (HR 8.20, 95% CI 5.64–11.93).
Conclusions
The majority of long-term survivors at high risk of cardiomyopathy did not adhere to the recommended surveillance. Attendance to an LTFU visit greatly enhanced the completion of echocardiograms, but further interventions need to be developed to reach more survivors.
Subject terms: Paediatric cancer, Paediatric cancer, Cancer epidemiology
Introduction
Over the past decades, advances in treatments have substantially improved childhood cancer survival. In Europe, the 5-year survival rate is over 80% [1]. However, childhood cancer survivors (CCS) have to face a large spectrum of health conditions related to the iatrogenic effects of cancer therapies: the prevalence of severe, disabling, life-threatening, or fatal conditions at age 45 years is 80% [2].
Cardiac mortality is higher among CCS than in the general population. A study including survivors from France the and United Kingdom found that CCS were sixfold more likely to die as a result of cardiac diseases [3]. Anthracyclines and radiation therapy to the heart area are well-known causes of cardiotoxicity, following a dose-dependent pattern [4]. CCS exposed to these treatments had an increased risk of cardiomyopathy and congestive heart failure (CHF) [5–7]. For survivors who have received these treatments above a certain dose, several international guidelines [8–11] recommend the completion of lifelong regular echocardiograms to allow earlier detection of asymptomatic cardiomyopathy, and thus reduce or delay sequelae by treating it. The recommended frequency of echocardiography ranges from every year to every 5 years, depending on the guideline.
Several studies from the Childhood Cancer Survivor Study (CCSS) have documented the adherence to recommended surveillance in North American CCS at high risk of cardiomyopathy [12–14], showing that only 28% of them reported an echocardiogram within the prior 2 years [12]. Another study from North America followed CCS for an average of 8 years and reported that patients were adherent to cardiac screening recommendations for only 9% of their period of follow-up [13], confirming that the majority of high-risk survivors do not adhere to recommendations.
Increasing adherence to recommended cardiac surveillance in the growing and ageing population of CCS is crucial, but there is limited knowledge about how to enhance it [14]. The effectiveness of interventions such as mailed or web-based health-risk information, motivational telephone counselling, Survivorship Care Plan (SCP), or long-term follow-up clinic attendance is unclear and poorly documented [15], although these interventions are likely to increase adherence to recommended surveillance [16].
In this context, the aim of this work was (1) to describe adherence to recommended surveillance in CCS at high risk of cardiomyopathy, (2) to identify the sociodemographic and clinical predictors of adherence, and (3) to assess whether attending a long-term follow-up visit influences the subsequent completion of an echocardiogram in survivors who were not previously adherent to recommendations.
Methods
Study design and data source
The FCCSS (French Childhood Cancer Survivor Study) cohort includes 7670 5-year CCS treated before age 18 for a solid malignant tumour or lymphoma in five French centers between 1945 and 2000 [17]. The FCCSS aims to explore the long-term outcomes of children/adolescents treated for solid tumors or lymphoma (for more information, see https://fccss.fr/?lang=en).
Data on childhood cancer were extracted from patients’ medical records. Data on echocardiograms were obtained through data on outpatient claims and hospital discharge summaries extracted from the French National health data system (SNDS) [18]. These data were available from January 1, 2006, to December 31, 2018. Vital status was obtained from the national registry of causes of death (CépiDC). Second cancers and cardiac diseases (including myocardial infarction, angina, heart failure, valvular diseases, cardiac arrhythmia, conduction disorder, and pericardial disease) were identified using medical records, long-term follow-up visit reports, and data from the SNDS and CépiDC databases. Cardiac disease cases were confirmed by the medical doctors and/or the cardiologists following patients [7].
The FCCSS study was approved by the INSERM Institutional review board (CEEI n°12-077) and the French National Agency regulating Data Protection (CNIL N°902287). Patients, parents, or guardians’ consent was obtained according to national research ethics requirements.
Study population
As of January 1, 2006, 5592 of the 7670 subjects from the FCCSS cohort (72.9%) were still alive and had available data on echocardiograms from the French National Health data system (Fig. 1). Subjects who have been diagnosed with cardiac disease before 2006 (n = 215) and those with incomplete data regarding childhood cancer and sociodemographic characteristics (n = 618) were excluded. Of the 4759 remaining subjects, the 976 (20.5%) survivors at high risk of cardiomyopathy were included in the present study. Survivors were considered at high risk of cardiomyopathy because of:
cumulative anthracycline dose >250 mg/m2, according to the International Guideline Harmonization Group [8],
radiation dose ≥30 Gy to more than 10% of the left ventricular volume, according to previous findings from the FCCSS cohort [7],
or cumulative anthracycline dose >100 mg/m² and radiation ≥30 Gy to any volume of the left ventricular (Supplementary Fig. S1).
Fig. 1. Flow-chart of the patients from the FCCSS cohort included in the present study.
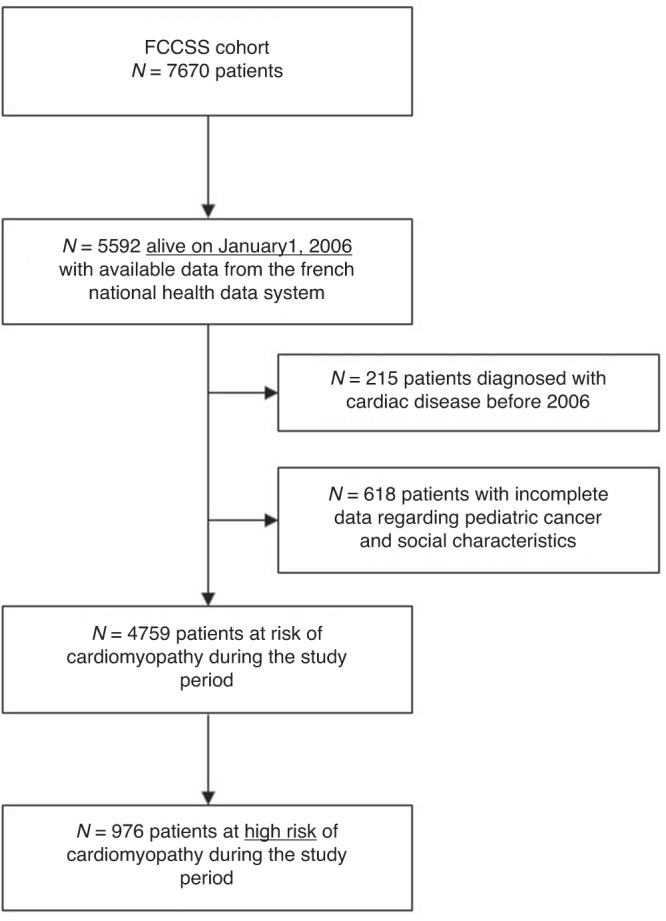
FCCSS French Childhood Cancer Survivor Study.
Outcome measure: adherence to recommended surveillance
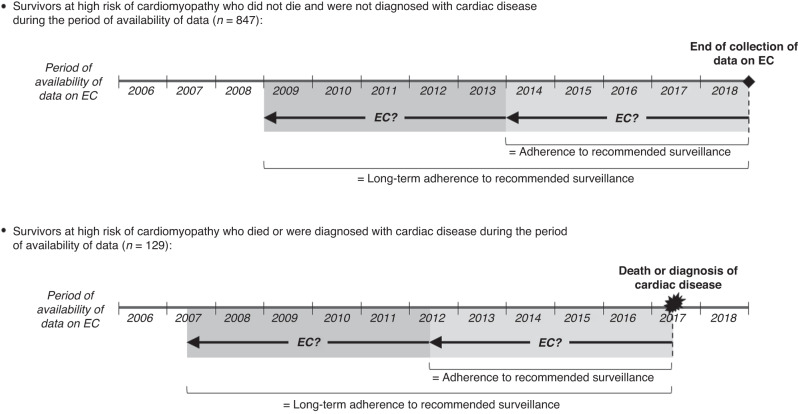
Recommended surveillance for patients at high risk of cardiomyopathy was at least one echocardiogram every 5 years [8]. Data on echocardiograms were collected from January 1, 2006, to the date of the diagnosis of cardiac disease (n = 92), the date of death (n = 37), or December 31, 2018 (n = 847). Being adherent to recommended surveillance was defined as having an echocardiogram over the last 5 years of the data collection period (Fig. 2), which required that patients had a data collection period of at least 5 years (n = 930). Being long-term adherent was defined as receiving an echocardiogram over the last 5 years and another one between 5 and 10 years before the end of the data collection period (Fig. 2), which required that patients had a data collection period of at least 10 years (n = 873).
Fig. 2. Definition of adherence and long-term adherence to recommended cardiac surveillance for survivors at high risk of cardiomyopathy.
EC echocardiogram.
Long-term follow-up (LTFU) visit
Since 2012, FCCSS survivors were contacted to have an LTFU visit. The visit included a medical check-up and a review of the patient’s medical history with a specialized clinician, who then informed the patient of the long-term health risks related to treatments received during childhood/adolescence and recommended a cardiac follow-up if necessary. Data on LTFU visits were only available for the patients treated at Gustave Roussy, one of the five cancer centers from which patients were enrolled. Among the 654 patients at high risk of cardiomyopathy from this center that were contacted for LTFU visit; 153 had at least one LTFU visit between 2012 and 2018.
Predictors of adherence to recommended surveillance
Sociodemographic predictors were sex, age at the end of the data collection period, and level of deprivation estimated using the French deprivation index (FDep). Briefly, FDep was calculated using median household income, percentage of graduates in the population, percentage of blue-collar workers and unemployment rate within the area of the home address [19].
Clinical predictors included childhood cancer type (classified according to the International Classification of Childhood Cancer and divided into 8 categories: Wilms Tumour, neuroblastoma, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, soft tissue sarcoma, bone sarcoma, CNS tumour, or other solid cancers), age at diagnosis, cumulative anthracycline dose >250 mg/m2, Cardiac radiation ≥30 Gy to ≥10% of the LV volume, and diagnosis of second cancer.
Statistical analysis
First, sociodemographic and childhood cancer characteristics of patients at high risk for cardiomyopathy were described, as well as those of all the patients of the FCCSS. Treatment exposures of patients at high risk for cardiomyopathy were described according to childhood cancer type. The proportion of patients at high risk for cardiomyopathy who were adherent to recommended surveillance, i.e., who had an echocardiogram over the last 5 years of their data collection period, and the proportion of patients at high risk for cardiomyopathy who were long-term adherent to recommended surveillance, i.e., who had two echocardiograms over the last 10 years of their data collection period, were calculated.
Then, the etiological part of the statistical analysis consisted of two separate analyses:
Associations between sociodemographic/clinical factors and adherence/long-term adherence to recommended surveillance among patients at high risk for cardiomyopathy were studied using multivariable modified Poisson regression models with robust errors. Using odds ratio estimates from logistic regression would have exaggerated the relative risk (RR) estimates as the prevalence of adherence to recommended surveillance (>30%) was relatively high [20, 21]. Treatment exposures were not included in the multivariable models due to overlap with childhood cancer diagnosis (Fig. 3). Results were reported as crude and adjusted RRs with their 95% confidence intervals (CI).
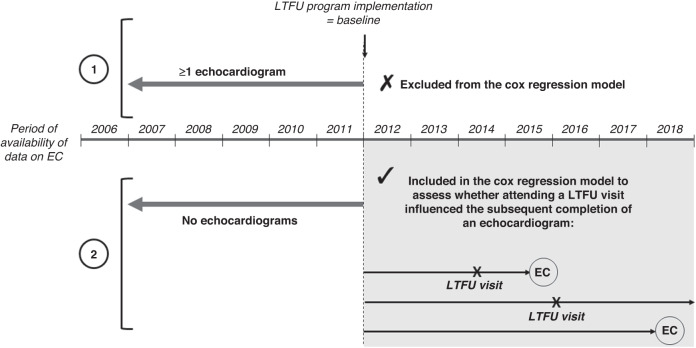
To assess whether attending a long-term follow-up visit influenced the subsequent completion of an echocardiogram in survivors who were not adherent to recommended surveillance at the time of the LTFU program implementation, we isolated the subgroup of survivors at high risk of cardiomyopathy who had not had any echocardiograms during the 5 years preceding the date of the LTFU program implementation, i.e., from January 1, 2007, to January 1, 2012 (Fig. 4). In this subgroup (n = 512), the association between attendance to an LTFU visit and the completion of an echocardiogram from 2012 until the end of the study period was studied using a Cox regression model. We used time-on-study time scale, with time 0 (baseline) corresponding to the date of the implementation of the LTFU visits program (January 1, 2012). Having an LTFU visit was modelled as a time-dependent variable. Survival was censored at the date of echocardiography or at the end of their data collection period. The model was adjusted for sociodemographic and clinical factors, except for treatment exposures due to overlap with pediatric cancer diagnosis (Fig. 3). Results were expressed as hazard ratios (HRs) with their 95% confidence intervals (CI). To better estimate the causal effect of LTFU visit on completion of echocardiograms, the Cox regression model was weighted by inverse probability of treatment weighting (IPTW) to minimize the effects of confounding due to the differences in baseline characteristics between patients who had an LTFU visit and those who did not [20]. IPTW uses the propensity score which was calculated by running a logit model including having an LTFU visit as the outcome and a set of baseline characteristics (sex, age, level of deprivation, childhood cancer type, age at diagnosis, and second cancer) as covariates.
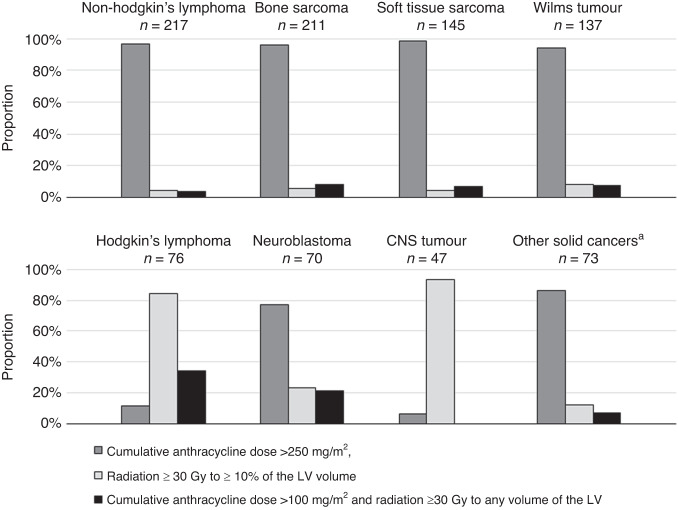
Fig. 3. Description of treatment exposures according to the childhood cancer diagnosed in patients at high risk for cardiomyopathy.
CNS central nervous system, LV left ventricular. a Gonadal tumour, thyroid tumour, retinoblastoma and other types of carcinoma.
Fig. 4. Survivors at high risk of cardiomyopathy included in the Cox regression model to assess whether attending a long-term follow-up visit influenced the subsequent completion of an echocardiogram.
Subjects who were not adherent to recommended surveillance at the time of the LTFU program implementation (2) were included (n = 512), whereas subjects who were not adherent to recommended surveillance at the time of the LTFU program implementation (1) were excluded. EC echocardiogram, LTFU long-term follow-up.
All analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). All P values were two-sided; P values < 0.05 were considered statistically significant.
Results
Sociodemographic and childhood cancer characteristics of CSS at high risk of cardiomyopathy
Among the 976 CSS at high risk of cardiomyopathy, 421 (43.1%) were females and the mean age was 39.2 years (range: 18–72). The most prevalent diagnoses were non-Hodgkin’s lymphoma (22.2%), bone sarcoma (21.6%), soft tissue sarcoma (14.9%) and Wilms tumour (14.0%) (Table 1). More than 80% of the high-risk survivors of CNS tumour and Hodgkin’s lymphoma were exposed to radiation ≥30 Gy to more than 10% of the left ventricular volume, whereas high-risk survivors of other cancers were mainly exposed to cumulative anthracycline dose >250 mg/m2 (Fig. 3).
Table 1.
Sociodemographic and childhood cancer characteristics of all FCCSS patients and of FCCSS patients at high risk for cardiomyopathy.
| All patients | Patients at high risk of cardiomyopathy | |
|---|---|---|
| N = 4759 | N = 976 | |
| Sociodemographic characteristics | ||
| Sex, n (%) | ||
| Females | 2143 (45.0) | 421 (43.1) |
| Males | 2616 (55.0) | 555 (56.9) |
| Age, n (%) | ||
| <36 years | 1869 (39.3) | 295 (30.2) |
| 36–49 years | 2280 (47.1) | 580 (59.4) |
| ≥50 years | 610 (12.8) | 101 (10.4) |
| Deprivation score, mean ± s.d. | −0.4 ± 1.8 | −0.5 ± 1.8 |
| Childhood cancer characteristics | ||
| Childhood cancer, n (%) | ||
| Wilms tumour | 756 (15.9) | 137 (14.0) |
| Neuroblastoma | 674 (14.2) | 70 (7.2) |
| CNS tumour | 646 (13.6) | 47 (4.8) |
| Non-Hodgkin’s lymphoma | 534 (11.2) | 217 (22.2) |
| Soft tissue sarcoma | 510 (10.7) | 145 (14.9) |
| Bone sarcoma | 412 (8.7) | 211 (21.6) |
| Hodgkin’s lymphoma | 271 (5.7) | 76 (7.8) |
| Other solid cancersa | 956 (20.1) | 73 (7.5) |
| Age at diagnosis, n (%) | ||
| <5 years | 2367 (49.7) | 310 (31.8) |
| ≥5 years | 2392 (50.6) | 666 (68.2) |
| Anthracycline dose >250 mg/m2, n (%) | ||
| No | 3945 (82.9) | 162 (16.6) |
| Yes | 814 (17.1) | 814 (83.4) |
| Cardiac radiation ≥30 Gy to ≥10% of the LV volume, n (%) | ||
| No | 4588 (96.4) | 805 (82.5) |
| Yes | 171 (3.6) | 171 (17.5) |
| Second cancer, n (%) | ||
| No | 4412 (92.7) | 881 (90.3) |
| Yes | 347 (33.0) | 95 (9.7) |
CNS central nervous system, LV left ventricular, s.d. standard deviation.
aGonadal tumour, thyroid tumour, retinoblastoma and other types of carcinoma.
Adherence to recommended surveillance
Overall, 31.6% of the patients at high risk of cardiomyopathy were adherent (i.e., they had performed an echocardiogram over the last 5 years of their data collection period), and 15.2% were long-term adherent (i.e., they had performed two echocardiograms over the last 10 years). Among those who had an LTFU visit (n = 153), these proportions were 52.8% and 32.9%, respectively.
Predictors of adherence to recommended surveillance
Males and patients aged 36–49 (vs. patients aged <36) were less likely to have an echocardiogram over the last 5 years (adjusted RR [aRR] 0.71, 95% CI 0.58–0.86, and aRR 0.79, 95% CI 0.64–0.98, respectively) (Table 2). Neuroblastoma and CNS tumour survivors (vs. Wilms tumors survivors) were also less adherent to recommended surveillance (aRR 0.53, 95% CI 0.30–0.91, and aRR 0.43, 95% CI 0.21–0.89, respectively). CCS diagnosed after 5 years of age and those who had a second cancer were more likely to be adherent (aRR 1.27, 95% CI 1.01–1.62 and aRR 1.43, 95% CI 1.13–1.82, respectively).
Table 2.
Factors associated with adherence to recommended surveillance (i.e., completion of an echocardiogram over the last 5 years) among survivors at high risk for cardiomyopathy (n = 930).
| Total number | Adherence, n (%) | Crude RR (95% CI) | Adjusted RRa (95% CI) | |
|---|---|---|---|---|
| Sex | ||||
| Females (ref) | 406 | 161 (39.7) | 1 | 1 |
| Males | 524 | 133 (25.4) | 0.64 (0.53–0.77)*** | 0.71 (0.58–0.86)*** |
| Age (years) | ||||
| <36 | 275 | 98 (35.6) | 1 | 1 |
| 36–49 | 555 | 153 (27.6) | 0.77 (0.63–0.95)* | 0.79 (0.64–0.98)* |
| ≥50 | 100 | 43 (43.0) | 1.21 (0.92–1.59) | 1.09 (0.82–1.46) |
| Deprivation score | ||||
| Quintiles 1 and 2 (ref) | 364 | 131 (36.0) | 1 | 1 |
| Quintiles 3 | 216 | 70 (32.4) | 0.90 (0.71–1.14) | 0.96 (0.76 –1.21) |
| Quintile 4 | 187 | 51 (27.3) | 0.76 (0.58–0.99)* | 0.84 (0.64–1.10) |
| Quintile 5 (most deprived) | 163 | 42 (25.8) | 0.72 (0.53–0.96)* | 0.79 (0.59–1.05) |
| Childhood cancer | ||||
| Wilms tumour (ref) | 133 | 51 (38.4) | 1 | 1 |
| Neuroblastoma | 66 | 12 (18.2) | 0.47 (0.27–0.83)** | 0.53 (0.30–0.91)* |
| Hodgkin’s lymphoma | 64 | 20 (31.3) | 0.82 (0.53–1.24) | 0.72 (0.47–1.11) |
| Non-Hodgkin’s lymphoma | 212 | 56 (22.8) | 0.69 (0.50–0.94)* | 0.72 (0.52–1.00) |
| Soft tissue sarcoma | 139 | 40 (28.8) | 0.75 (0.53–1.05) | 0.75 (0.53–1.05) |
| Bone sarcoma | 204 | 82 (40.2) | 1.05 (0.80–1.38) | 1.01 (0.75–1.35) |
| CNS tumour | 45 | 7 (15.6) | 0.41 (0.20–0.83)* | 0.43 (0.21–0.89)** |
| Other solid cancersb | 67 | 26 (38.8) | 1.00 (0.70–1.47) | 0.99 (0.69–1.44) |
| Age at diagnosis | ||||
| <5 years (ref) | 298 | 80 (26.9) | 1 | 1 |
| ≥5 years | 632 | 214 (33.9) | 1.26 (1.02–1.57)* | 1.27 (1.01–1.62)* |
| Anthracycline dose >250 mg/m2 | ||||
| No (ref) | 144 | 41 (28.5) | 1 | – |
| Yes | 786 | 253 (32.2) | 1.13 (0.86–1.49) | – |
| Cardiac radiation ≥30 Gy to ≥10% of the LV volume | ||||
| No (ref) | 778 | 246 (31.6) | 1 | – |
| Yes | 152 | 48 (31.6) | 1.00 (0.79–1.29) | – |
| Second cancer | ||||
| No (ref) | 844 | 252 (29.9) | 1 | 1 |
| Yes | 86 | 42 (48.8) | 1.65 (1.29–2.09)*** | 1.43 (1.13–1.82)** |
CI confidence interval, CNS central nervous system, FDep French deprivation index, NA non-available, RR relative risk, SD standard deviation.
aAdjusted for all other variables in the multivariable modified Poisson regression model.
bGonadal tumour, thyroid tumour, retinoblastoma and other types of carcinoma.
*P value < 0.05; **P value < 0.01; ***P value < 0.001.
Similar trends were reported when studying factors associated with long-term adherence to recommended surveillance, but only associations with sex and age at cancer diagnosis remained significant (Supplementary Table S1).
Impact of the LTFU visit on completion of echocardiograms
Of the survivors at high risk of cardiomyopathy, 512 were not adherent to recommended surveillance at the time of the LTFU program implementation, i.e., had not had any echocardiograms during the 5 years preceding January 1, 2012. Of them, 79 attended an LTFU visit; 46 (58.2%) subsequently performed an echocardiogram. In this subgroup of non-adherent survivors at the time of the LTFU program implementation, attendance to an LTFU visit was positively associated with the completion of an echocardiogram from 2012 until the end of the study period; the adjusted HR was 8.20 (95% CI 5.64–11.93) in the Cox regression model weighted by IPTW.
Discussion
Using data from a national health administrative database, we reported that the majority of survivors at high risk for cardiomyopathy were not adherent to recommended surveillance. This finding was consistent with those previously reported in North American studies, in which echocardiograms were self-reported [12, 15, 22, 23]. Nathan et al. [12] reported that only 28% of CCS at high risk of cardiomyopathy were adherent to recommended surveillance in the CCSS, while this proportion was 32% in the present study. These proportions were similar, but it should be noted that recommendations were different between the US and France [9]. Thus, the definitions of adherence were different: an echocardiogram within the last year in CCSS [12], and an echocardiogram within the last 5 years in the present study.
We identified several predictors of non-adherence to recommended cardiac surveillance among patients at high risk for cardiomyopathy. Males were less likely to adhere than females, supporting that gender is a well-known predictor of healthcare utilization [13, 24]. This may be due to stereotypical health-related beliefs, with men not seeing themselves vulnerable to disease or considering that asking for help is less acceptable for men [25]. Subjects aged 36–49 were less likely be adherent than those aged <36, but no differences were found between those aged ≥50 and those aged <36. This result suggested a temporary drop in adherence during adulthood, at age 36–49 years. Such a pattern has not been documented in other studies, in which age was generally entered as a continuous variable in models [12, 23]. Patients in this age group may not have discussed their childhood cancer with a care provider for a long time, and they may not feel concerned about cardiac outcomes, as they are still young individuals.
Regarding clinical predictors, we found that CNS tumour and neuroblastoma survivors were less likely to adhere to cardiac surveillance. As the most prevalent long-term outcomes in these survivors are endocrine, neurological, cognitive, sensory, psychiatric, or musculoskeletal disorders [26, 27], cardiac screening may have been somewhat overlooked among them. High-risk patients diagnosed during infancy or early childhood (<5 years) were less adherent than others. Marr et al. [13] reported a similar trend, but associations were not significant, and Nathan et al. [12] did not find any association between adherence and age at diagnosis. Finally, the diagnosis of a second cancer was associated with better adherence to recommended surveillance, as reported in the CCSS [12, 23]. Recurrence of cancer may be the occasion for an oncologist to review the patient’s treatment history and eventually to encourage the patient to undergo an echocardiogram.
Beyond demographic and clinical predictors, there are probably psychological barriers to adherence [28]. Some survivors may avoid medical care in an attempt to forget their childhood cancer; they may feel that the long-term follow-up they have to undergo is a source of anxiety and prevents them from living a normal life [29]. However, psychological barriers remain poorly documented [15] since patients who avoid healthcare professionals are not likely to involve themselves in qualitative studies that investigate how CSS manage their long-term medical follow-up. Another reason for poor adherence could be a lack of survivor knowledge of their treatment exposures and associated surveillance recommendations [15]. Several studies have shown that having frequent clinic visits increased adherence to recommendations in high-risk patients [15] and thus may address this unawareness. Among patients who were not adherent to recommendations, we reported that attendance to the LTFU visit strongly increased the probability of performing an echocardiogram afterwards (HR = 8.2). This result, in line with findings from previous studies [13, 30], demonstrates the effectiveness of LTFU visits in referring non-adherent survivors to the screening process. Nevertheless, LTFU visits are not sufficient to reach full adherence. In our study, less than one quarter of high-risk patients had an LTFU visit, and 42% of those who attended did not have an echocardiogram afterwards. Marr et al. reported that among survivors at high risk for cardiomyopathy with at least one clinic attendance, 45% did not have an echocardiogram afterwards [13]. Furthermore, there is evidence that attendance at LTFU clinics is poor [15, 31]. Further interventions, including implementation of survivorship care plans (SCP), phone counselling, and mailed/web-based information regarding treatment, risks, and screening instructions, have the potential to either increase attendance at LTFU clinics [15, 32, 33] or complement LTFU visits [15, 16, 34, 35]. Moreover, primary care physicians’ knowledge of long-term risks and surveillance recommendations for patients treated for childhood cancer is often lacking, which needs to be addressed [36–39]. Giving CCS as much opportunity as possible to receive recommendations is crucial to increase adherence.
This study has several strengths. It is the first study on adherence to recommended surveillance in CCS at high risk for cardiomyopathy to deal with data from a country outside of North America, and it includes almost 1,000 high-risk survivors. Unlike some other studies [12, 22, 23], data on echocardiograms were not self-reported by patients, but extracted from a health administrative database. The studies with self-reported data included survivors who had agreed to respond to a survey and who were probably more concerned about their long-term follow-up, which may have overestimated the proportions of adherence. In our study, 32% of survivors at high-risk for cardiomyopathy adhered to the recommended surveillance. When limited to those who had responded to a questionnaire for other studies conducted in the FCCSS, this proportion was 36%. Cardiac diseases were also identified using a health administrative database and validated with medical reports; survivors with cardiac diseases were followed-up until the year of the cardiac disease diagnosis to avoid including echocardiograms undergone for reasons other than surveillance (e.g., symptoms or monitoring).
This study has some limitations. None of the high-risk patients were leukemia survivors since the FCCSS only includes patients treated for a solid malignant tumour or lymphoma. We estimated the effect of having an LTFU visit on the completion of an echocardiogram with observational data, whereas an interventional study such as a randomized controlled trial would have been more appropriate for this purpose. Therefore, our model was weighted using the inverse probability of treatment weighting (IPTW) method to simulate a random allocation of the LTFU visit to survivors [40]. Finally, data on LTFU visits were only available for the patients from Gustave Roussy, one of the five cancer centers included in this study. Nevertheless, the majority (67%) of the survivors at high risk for cardiomyopathy were followed-up in this center.
Most CCS at high risk of cardiomyopathy did not adhere to the recommended surveillance despite their risk of treatment-related cardiac outcomes. Thus, there is an absolute need to improve opportunities for high-risk patients to receive surveillance. LTFU visits greatly enhanced the completion of echocardiography, but only a limited number of survivors access such visits. Further interventions based on innovative tools that can reach a large number of patients, such as web-based platforms and communication technologies, should be developed to increase awareness of late effects in survivors and inform them that tertiary prevention strategies can be set up to reduce the risk of cardiomyopathy. Better information and coordination between the actors involved in the patient’s follow-up is crucial—for instance, explicit information about the dose of treatment received and the recommended follow-up should be systematically given in a Survivorship Care Plan. The identification of social, clinical, and psychological predictors of non-adherence to recommended surveillance should help to tailor interventions targeting survivors at high risk for cardiomyopathy, to better design interventions, and to reach full adherence to recommended surveillance.
Supplementary information
Acknowledgements
We thank the patients and all the clinicians and research staff who participated in the study. We are grateful to Carole Rubino, Odile Oberlin, Amel Boumaraf, Amel Belhout and Isao Kobayashi for their contribution to this work.
Author contributions
AD, RSA, BF, NH, NB and FDV designed the study. All authors participated to the collection and assembly of data. NB performed the statistical analysis and drafted the manuscript with the help of AD, BF, RSA and FDV. All authors revised the work, approved its final version and agreed to be accountable for all its aspects.
Funding
This work was supported by the INCa/ARC foundation (CHART project). The FCCSS cohort is supported and funded by the French Society of Cancer in Children and Adolescents (SFCE), the Gustave Roussy Foundation (Pediatric Program “Guérir le Cancer de l’Enfant”), the Foundation ARC (POPHarC program) and The French National Research Agency (ANR, HOPE-EPI project), the ‘Ligue Nationale Contre le Cancer’, and the ‘Programme Hospitalier de Recherche Clinique’. The funders had no role in the conduct of the research.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The FCCSS study was approved by the INSERM Institutional review board (CEEI N°12-077) and the French National Agency regulating Data Protection (CNIL N°902287). Patients, parents, or guardians’ consent was obtained according to national research ethics requirements. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02400-0.
References
- 1.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–85. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. J Am Med Assoc. 2013;309:2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–15. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 4.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–95. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–20. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 6.Yeh ETH, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–31. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 7.Mansouri I, Allodji RS, Hill C, El-Fayech C, Pein F, Diallo S, et al. The role of irradiated heart and left ventricular volumes in heart failure occurrence after childhood cancer. Eur J Heart Fail. 2019;21:509–18. doi: 10.1002/ejhf.1376. [DOI] [PubMed] [Google Scholar]
- 8.Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Children’s Oncology Group [Internet]. [cited 2021 Jul 13]. Available from: http://www.survivorshipguidelines.org/.
- 10.Sieswerda E, Postma A, van Dalen EC, van der Pal HJ, Tissing WJ, Rammeloo LA, et al. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol. 2012;23:2191–8. [DOI] [PubMed]
- 11.Wallace WHB, Thompson L, Anderson RA. Long term follow-up of survivors of childhood cancer: summary of updated SIGN guidance. BMJ. 2013;346:f1190. doi: 10.1136/bmj.f1190. [DOI] [PubMed] [Google Scholar]
- 12.Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401–9. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marr KC, Agha M, Sutradhar R, Pole JD, Hodgson D, Guttmann A, et al. Specialized survivor clinic attendance increases adherence to cardiomyopathy screening guidelines in adult survivors of childhood cancer. J Cancer Surviv Res Pract. 2017;11:614–23. doi: 10.1007/s11764-017-0634-z. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins M, Bhatia S, Henderson TO, Nathan PC, Yan A, Teepen JC, et al. Subsequent primary neoplasms: risks, risk factors, surveillance, and future research. Pediatr Clin North Am. 2020;67:1135–54. doi: 10.1016/j.pcl.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Zabih V, Kahane A, O’Neill NE, Ivers N, Nathan PC. Interventions to improve adherence to surveillance guidelines in survivors of childhood cancer: a systematic review. J Cancer Surviv Res Pract. 2019;13:713–29. doi: 10.1007/s11764-019-00790-w. [DOI] [PubMed] [Google Scholar]
- 16.Hudson MM, Leisenring W, Stratton KK, Tinner N, Steen BD, Ogg S, et al. Increasing cardiomyopathy screening in at-risk adult survivors of pediatric malignancies: a randomized controlled trial. J Clin Oncol. 2014;32:3974–81. doi: 10.1200/JCO.2014.57.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demoor-Goldschmidt C, Allodji RS, Journy N, Rubino C, Zrafi WS, Debiche G, et al. Risk factors for small adult height in childhood cancer survivors. J Clin Oncol. 2020;38:1785–96. doi: 10.1200/JCO.19.02361. [DOI] [PubMed] [Google Scholar]
- 18.Bezin J, Duong M, Lassalle R, Droz C, Pariente A, Blin P, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26:954–62. doi: 10.1002/pds.4233. [DOI] [PubMed] [Google Scholar]
- 19.Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997 - 2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33. doi: 10.1186/1471-2458-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou G. A modified Poisson regression approach to prospective binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RHH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. Can Med Assoc J. 2012;184:895–9. doi: 10.1503/cmaj.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox CL, Hudson MM, Mertens A, Oeffinger K, Whitton J, Montgomery M, et al. Medical screening participation among childhood cancer survivors. Arch Intern Med. 2009;169:454–62. doi: 10.1001/archinternmed.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steele JR, Wall M, Salkowski N, Mitby P, Kawashima T, Yeazel MW, et al. Predictors of risk-based medical follow-up: a report from the childhood cancer survivor study. J Cancer Surviv Res Pract. 2013;7:379–91. doi: 10.1007/s11764-013-0280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumas A. Gender inequalities in health over the life course. Attitudes towards III-health in men and women treated for childhood cancer. Swiss J Sociol. 2018;44:281–300. doi: 10.1515/sjs-2018-0013. [DOI] [Google Scholar]
- 25.Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med. 2000;50:1385–401. doi: 10.1016/S0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]
- 26.Laverdière C, Liu Q, Yasui Y, Nathan PC, Gurney JG, Stovall M, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:1131–40. doi: 10.1093/jnci/djp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunn ME, Lähdesmäki T, Malila N, Arola M, Grönroos M, Matomäki J, et al. Late morbidity in long-term survivors of childhood brain tumors: a nationwide registry-based study in Finland. Neuro-Oncol. 2015;17:747–56. doi: 10.1093/neuonc/nou321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zebrack BJ, Eshelman DA, Hudson MM, Mertens AC, Cotter KL, Foster BM, et al. Health care for childhood cancer survivors: insights and perspectives from a Delphi panel of young adult survivors of childhood cancer. Cancer. 2004;100:843–50. doi: 10.1002/cncr.20033. [DOI] [PubMed] [Google Scholar]
- 29.Howard AF, Goddard K, Tan de Bibiana J, Pritchard S, Olson R, Kazanjian A. Adult childhood cancer survivors’ narratives of managing their health: the unexpected and the unresolved. J Cancer Surviv Res Pract. 2016;10:711–25. doi: 10.1007/s11764-016-0517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baxstrom K, Peterson BA, Lee C, Vogel RI, Blaes AH. A pilot investigation on impact of participation in a long-term follow-up clinic (LTFU) on breast cancer and cardiovascular screening among women who received chest radiation for Hodgkin lymphoma. Support Care Cancer. 2018;26:2361–8. doi: 10.1007/s00520-018-4072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumas A, Milcent K, Bougas N, Bejarano-Quisoboni D, El Fayech C, Charreire H, et al. Predictive factors of long-term follow-up attendance in very long-term childhood cancer survivors. Cancer. 2023; [DOI] [PubMed]
- 32.Jabson JM. Treatment summaries, follow-up care instructions, and patient navigation: could they be combined to improve cancer survivor’s receipt of follow-up care? J Cancer Surviv Res Pract. 2015;9:692–8. doi: 10.1007/s11764-015-0444-0. [DOI] [PubMed] [Google Scholar]
- 33.Edgar AB, Borthwick S, Duffin K, Marciniak-Stepak P, Wallace WHB. Survivors of childhood cancer lost to follow-up can be re-engaged into active long-term follow-up by a postal health questionnaire intervention. Eur J Cancer. 2012;48:1066–73. doi: 10.1016/j.ejca.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Oeffinger KC, Hudson MM, Mertens AC, Smith SM, Mitby PA, Eshelman-Kent DA, et al. Increasing rates of breast cancer and cardiac surveillance among high risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatr Blood Cancer. 2011;56:818–24. doi: 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadan-Lottick NS, Ross WL, Mitchell HR, Rotatori J, Gross CP, Ma X. Randomized trial of the impact of empowering childhood cancer survivors with survivorship care plans. J Natl Cancer Inst. 2018;110:1352–9. doi: 10.1093/jnci/djy057. [DOI] [PubMed] [Google Scholar]
- 36.Landier W, Bhatia S. Cancer survivorship: a pediatric perspective. The Oncologist. 2008;13:1181–92. doi: 10.1634/theoncologist.2008-0104. [DOI] [PubMed] [Google Scholar]
- 37.Nathan PC, Daugherty CK, Wroblewski KE, Kigin ML, Stewart TV, Hlubocky FJ, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv Res Pract. 2013;7:275–82. doi: 10.1007/s11764-013-0271-0. [DOI] [PubMed] [Google Scholar]
- 38.Klosky JL, Cash DK, Buscemi J, Lensing S, Garces-Webb DM, Zhao W, et al. Factors influencing long-term follow-up clinic attendance among survivors of childhood cancer. J Cancer Surviv Res Pract. 2008;2:225–32. doi: 10.1007/s11764-008-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oeffinger KC, Ford JS, Moskowitz CS, Diller LR, Hudson MM, Chou JF, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. J Am Med Assoc. 2009;301:404–14. doi: 10.1001/jama.2008.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.