Abstract
Background
This randomised controlled trial (RCT) assessed the effect of a 1-year, partially supervised, physical activity (PA) intervention on a cardiovascular disease (CVD) risk score in adult survivors of childhood cancer.
Methods
We included childhood cancer survivors ≥16 y at enrolment, <16 y at diagnosis and ≥5 y in remission. The intervention group was asked to perform an additional ≥2.5 h of intense physical activity/week, controls continued exercise as usual; assessments were performed at baseline, 6 months (T6) and 12 months (T12). The primary endpoint was change in a CVD risk score (average z-score of waist circumference, blood pressure, fasting glucose, inverted high-density lipoprotein cholesterol, triglycerides, and inverted cardiorespiratory fitness) from baseline to T12. We performed intention-to-treat (ITT, primary) and 3 per protocol analyses.
Results
We randomised 151 survivors (44% females, 30.4 ± 8.6 years). We found a significant and robust reduction of the CVD risk score in the intervention compared to the control group at T6 and T12 across all analyses; with a difference in the reduction of the CVD risk z-score of −0.18 (95% confidence interval −0.29 to −0.06, P = 0.003) at T12 in favour of the intervention group (ITT analysis).
Conclusions
This RCT showed that a long-term PA intervention can reduce CVD risk in long-term survivors of childhood cancer.
Trial registration
Clinicaltrials.gov: NCT02730767.
Subject terms: Paediatric cancer, Randomized controlled trials, Lifestyle modification
Introduction
Five-year survival rates of childhood cancer have improved drastically, exceeding 80% [1]. This has led to a growing population of childhood cancer survivors (CCS) at risk of late effects [2]; where an estimated 96% suffer from a chronic health condition and 81% from a serious or life-threatening disease by age 45 [3]. Cardiovascular diseases (CVD) are amongst the most frequent late effects in CCS with a sevenfold increased risk for cardiac mortality compared to the general population [4, 5]. Other late effects include obesity and metabolic syndrome [6, 7], reduced physical fitness [2], second cancers [8], reduced bone mineral density [9], and psychological challenges [10, 11], all compromising health-related quality of life [12, 13].
Fortunately, most of these conditions are potentially responsive to physical activity (PA) interventions, as shown in the general population and adult cancer survivors [14–23]. However, the literature suggests that CCS may respond differently to exercise and might not experience the same benefits than healthy populations [24]. For example, exposure to anthracyclines and radiation to the heart can lead to abnormal hypertrophic response to exercise and interfere with cardiac and vascular adaptive mechanisms [24]. Results from interventional and observational studies in CCS suggest a positive effect of PA on cardiorespiratory fitness, muscle strength, fatigue and body composition [25–30]. However, evidence is hampered by a lack of large, high-quality randomised controlled trials (RCTs) [31, 32]. Results from 22 RCTs with PA interventions on various childhood cancer populations have been published [33–54], of which 10 [40–49] were during cancer treatment and the remaining 12 [33–39, 50–54] included mostly short-term survivors [34–39, 51–54], only one included adult participants [50], and none of them investigated the effect of regular PA on cardiovascular health [29, 31].
PA behaviour is difficult to change due to perceived barriers such as lack of time or motivation, tiredness, low physical fitness, physical limitations or overprotection [55–57]. Multi-component interventions including motivational interviews, personal counselling, activity plans, and regular feedback were found to be effective strategies to increase PA [58–61]. There is, however, a lack of evidence for strategies to promote PA among CCS [31].
Thus, the overall aim of the current RCT was to investigate the effect of a partially supervised, personalised, 1-year PA intervention, on cardiovascular health (CVD risk score) in long-term survivors of childhood cancer. Secondary endpoints included single CVD risk factors, physical fitness and PA.
Methods
Trial design
The SURfit study (clinicaltrials.gov: NCT02730767) is a registry-based, single-centre, parallel-arm, 1:1 superiority RCT in adolescent and adult survivors of childhood cancer in Switzerland, performed 2015–2019. A detailed description of the methodology is available in the eMethods of the Online Appendix and published study protocol [62]. There were no changes to the design after trial initiation. The study was approved by the Ethics Committee of Northwestern and Central Switzerland (EKNZ-2015-169) and all participants gave written informed consent.
Study population
We included CCS diagnosed according to the International Classification of Childhood Cancer (ICCC-3) [63] or Langerhans Cell Histiocytosis, treated at a Swiss Pediatric Oncology Group clinic, aged ≥16 y at enrolment, <16 y at diagnosis and ≥5 y since the last cancer event (first cancer diagnosis or potential relapse(s) or second cancer(s), whatever came last). Eligible participants were identified through the Swiss Childhood Cancer Registry (SCCR) [64]. Eligible participants who reported >4 h of intense physical activities per week at baseline were excluded, because adding the target PA of our intervention on top of that might not be beneficial and difficult to implement. We aimed to include a general population of childhood cancer survivors to increase external validity of this preventive trial. Participants and non-participants did not differ by demographic and cancer-related characteristics available from the SCCR [65].
Study outcomes
The current publication reports the pre-specified primary endpoint and secondary endpoints related to CVD, physical fitness and PA. Bone health and psychosocial health outcomes are reported in additional publications [66], as specified in the protocol and clinicaltrials.gov [62]. eTable 1 in the Online Appendix describes all outcomes reported in this paper and any changes after trial commencement. Outcome alterations were adopted before calculating study results and derived outcomes were pre-defined in the statistical analysis plan (SAP) [67].
Our primary outcome was change in a composite CVD risk score [68] from baseline to 12 months post-randomisation, calculated by averaging z-scores of waist circumference (cm), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), fasting glucose (mmol/l), inverted high-density lipoprotein (HDL) cholesterol (mmol/l), triglycerides (mmol/l) and inverted cardiorespiratory fitness (absolute peak performance, watt). Scores with <3 components available were set as missing (only one person at T6 had <3 components; at baseline, n = 140 had 7 components, n = 5 had 6 components, n = 4 had 5 components and n = 1 had 4 and 3 components, respectively). Z-scores were calculated based on age- and sex-stratified reference values [69, 70]. We chose a composite score because we expected prevalence of single CVD risk factors or manifest diseases to be low in a young population of CCS. A longitudinal study showed that a clustered CVD risk score in adolescents predicted metabolic syndrome in adulthood [71] and was sensitive to change by a PA intervention in youth [72].
Secondary outcomes were change in the CVD risk score from baseline to 6 months, and changes from baseline to 6 (if available) and 12 months for waist circumference, systolic and diastolic blood pressure, fasting glucose, HbA1c, insulin resistance based on an oral glucose tolerance test (oGTT), HDL cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, body mass index (BMI), absolute and relative body fat mass (dual-energy X-ray absorptiometry (DXA)), absolute and relative peak performance (cardiopulmonary exercise test (CPET)), hand grip strength (hand grip dynamometer), leg strength and endurance (1-min sit-to-stand (STS) test), total PA, moderate-to-vigorous physical activities (MVPA), sedentary time (all from accelerometer) and the number of steps per day (pedometer; eTable 1 in Online Appendix). Dichotomised outcomes were calculated where standardised cut-offs existed (eTable 1 in Online Appendix).
Assessments
The current study used data collected at baseline (T0), 6 (T6) and 12 (T12) months post-randomisation at the University Children’s Hospital Basel by trained study nurses, exercise scientists or medical doctors [62, 65]. Participants were clinically examined, performed a CPET and strength test, gave fasting blood samples and wore an accelerometer and pedometer for 1–2 weeks at all time points. In addition, oGTT and DXA assessments were performed at T0 and T12. See eMethods in the Online Appendix for a detailed description of assessments.
Covariates, baseline demographics and safety
We collected sex, age, and cancer-related characteristics from medical records (eMethods in Online Appendix) [65]. We recorded any adverse events (AE) at each time point, and classified them according to the Common Terminology Criteria for Adverse Events (CTCAE) 4th edition [73].
Randomisation and sample size
A 1-year PA intervention in children and adolescents found a 14% reduction in the same CVD risk score used in our study [72]. We therefore required 60 survivors in each arm to detect a 15% difference between intervention and control group at T12 (power = 0.80, α = 0.05) [62]. An external and independent collaborator used a web-based minimisation randomisation (www.randomizer.at) for the 1:1 allocation, stratified by sex and initial cancer diagnosis (leukaemia/lymphoma; CNS tumours; bone tumours/soft tissue sarcomas; other diagnoses).
Blinding
It was not possible to blind study participants, project physiotherapists, project physicians and some assessors. Other members of the project team were blinded for group allocation, i.e., those performing DXA, CPET, blood analysis, database quality check and statistical analysis. The SAP was written before unblinding of the data.
Intervention and control conditions
The 12-month intervention aimed at adding ≥2.5 h of intense PA/week, whereof 30 min strength building and 2 h of aerobic exercises (more detailed description in eMethods of the Online Appendix). Intense PA was defined for the participants as activities that lead to increased breathing, sweating, and markedly increased heart rate, where one can only talk in short sentences. The SURfit PA intervention was detailed in a standard operating procedure (SOP) and the coaches (hospital physiotherapists) were trained prior to the study start by a sports physician. At baseline, the responsible coach received a report from the study physician on relevant medical information about the survivor, assessed the current PA level and activities together with the survivor and performed a motivational interview to assess preferences, motivation and barriers towards PA (original forms used in chapter 5 of the Online Appendix) [62]. Participants were informed about their target training heart rate and target PA hours per week (current level +2.5 h) and received a personal PA plan implemented into e normal week. Types of activities were individualised based on the participant’s preferences, weekly schedule, and environment. Motivational tools included regular contact with the coach (face-to-face at 0, 3, 6 and 12 months, phone calls at 1, 2, 4, 5, 8 and 10 months), a pedometer, and a daily self-administered web-based activity diary. Participants were reminded weekly if diary entries were missing. Control group participants were asked to keep their activity levels constant (no PA recommendations) which reflects the current standard of care during follow-up care in Switzerland.
Compliance to the intervention/control group was pre-defined as: (a) assumed compliance allocating those with ≥5% increase in peak performance (watt by CPET) from T0–T12 to the intervention group and those with <5% increase to the control group, independent of original allocation (per protocol allocation 1, PP1); (b) reported compliance including only intervention group participants who reached ≥2/3 of their intense PA goal based on daily web-diary entries with missing days set as zero PA (per protocol allocation 2, PP2); (c) same as (b) but missing days imputed with the participant’s yearly mean PA (per protocol allocation 3, PP3). For both reported compliance protocols (b and c), control group participants reporting ≤30 min increase in weekly intense PA based on interviews at baseline and T12 were included (= compliant controls).
Statistical analysis
Statistical analyses were pre-specified in the SAP [67] and performed in STATA v17; P < 0.05 was considered statistically significant. Analyses were performed on intention-to-treat allocation (as randomised, ITT) and three per protocol allocations (PP1, PP2, PP3). In addition, a PA dose variable was generated based on the proportion of personal activity goal reached, divided into <50%, 50–99% and ≥100%. Compliant controls were set to 0%, non-compliant controls were excluded.
Treatment effects on continuous outcomes, were estimated using generalised linear mixed models (GLMM) with random intercept and slope, treatment-time interaction, and adjusted for sex, initial cancer diagnosis and baseline value of the respective outcome, for each of the above-mentioned group allocations (ITT, PP1, PP2, PP3). Participants with valid baseline values of the respective outcome were included in the analysis (no missing baseline information for the primary CVD risk score) and missing follow-up values were imputed by the mixed model. We report marginal mean changes with 95% confidence intervals (95% CI) from baseline to T6 (if available) and T12 (derived by the delta method). The primary effect estimate was pre-defined as difference between intervention and control group in the CVD risk score marginal mean change from the GLMM at T12 using ITT allocation [67]. Because of small numbers in the binary outcomes, we only present them descriptively.
Additional dose–response analysis was performed on all continuous outcomes using multivariable linear regression models, adjusted for age, sex and initial cancer diagnosis, including only complete cases. Outcomes were difference from T0–T12 in each variable, exposure was the PA dose variable.
We performed a post hoc subgroup analysis on the primary CVD risk score with ITT allocation to investigate whether the intervention effect differed for participants who received versus not received anthracyclines as part of their cancer therapy. This was done by adding anthracyclines (yes/no) to the interaction term in the GLMM described for the primary analysis.
Treatment emerging AEs are reported descriptively by ITT group allocation.
Results
Study population
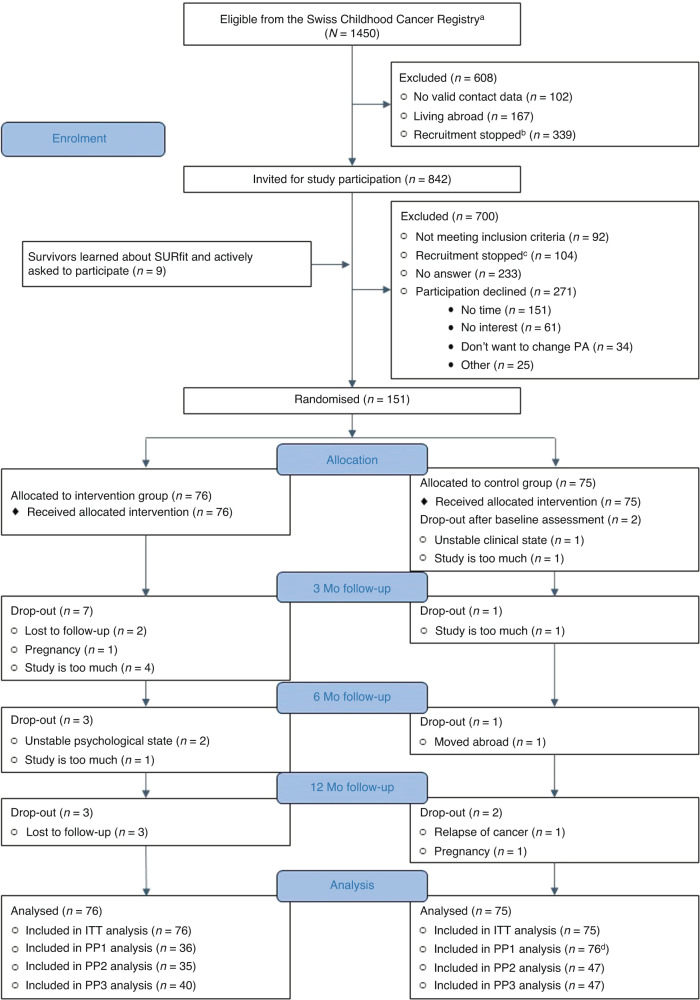
Of 1450 eligible survivors identified in the SCCR, 842 were invited and 151 randomised (18% of 842 invited; 76 intervention, 75 control; Fig. 1). Baseline characteristics were balanced between arms (Table 1). A total of 19 participants dropped out of the study (13 intervention, 6 control). Models to estimate treatment effect on the primary outcome included 151 participants for the ITT allocation (75 control, 76 intervention), 112 for PP1 (76 assumed control, 36 assumed intervention), 82 for PP2 (47 compliant control, 35 compliant intervention) and 87 for PP3 (47 compliant control, 40 compliant intervention; eTable 2 in Online Appendix).
Fig. 1. Flow diagram of the SURfit study.
This figure shows the flow diagram of the SURfit study participants from those identified in the Swiss Childhood Cancer Registry to those included in the analysis. ITT intention-to-treat, Mo months, n number, PA physical activity, PP1 per protocol analysis 1 (assumed allocation to intervention and control group, independent of original randomisation), PP2 per protocol analysis 2 (including compliant participants based on reported compliance with missing data set to 0), PP3 per protocol analysis 3 (including compliant participants based on reported compliance with missing data set to the yearly mean). Footnotes: aEligible survivors were identified in the population-based Swiss Childhood Cancer Registry. bAt some point, recruitment was stopped because of participant saturation. cParticipants who received an initial invitation were no longer followed-up by phone because of participant saturation. dThis group includes more participants than originally randomised because we re-allocated participants based on assumed compliance independent of the initial randomisation (see “Methods” section for a detailed description of the different per protocol group allocations).
Table 1.
Baseline characteristics of participants by study arm, the SURfit study (N = 151).
| Control group | Intervention group | |
|---|---|---|
| (N = 75) | (N = 76) | |
| Age at baseline, mean years (SD) | 29.3 (8.7) | 31.5 (8.3) |
| Sex | ||
| Male | 42 (56%) | 43 (57%) |
| Female | 33 (44%) | 33 (43%) |
| Diagnosis | ||
| Leukaemias | 31 (41%) | 24 (32%) |
| Lymphomas | 14 (19%) | 18 (24%) |
| CNS tumours | 6 (8%) | 11 (14%) |
| Bone tumours & soft tissue sarcomas | 6 (8%) | 10 (13%) |
| Other tumoursa | 18 (24%) | 13 (17%) |
| Age at first diagnosis, mean years (SD) | 7.3 (4.6) | 7.6 (5.1) |
| Time since first diagnosis, mean years (SD) | 22.0 (9.2) | 24.0 (8.6) |
| Relapse of primary cancer | 6 (8%) | 8 (11%) |
| Subsequent primary cancer | 3 (4%) | 4 (5%) |
| Received surgery | ||
| No surgery | 35 (47%) | 30 (39%) |
| 1 surgery | 30 (40%) | 36 (47%) |
| ≥2 surgeries | 10 (13%) | 10 (13%) |
| Received chemotherapy | 67 (89%) | 69 (91%) |
| Received anthracyclines | 51 (68%) | 45 (59%) |
| Total cumulative dose (mg/m2), mean (SD)b | 193.9 (97.8) | 191.7 (89.3) |
| Received steroids | 45 (60%) | 42 (55%) |
| Total cumulative dose (mg/m2), mean (SD) | 4442.5 (3171.9) | 4045.8 (3431.1) |
| Received radiotherapy | ||
| No radiotherapy | 45 (60%) | 45 (59%) |
| Total body irradiation | 2 (3%) | 3 (4%) |
| Cranial radiotherapy | 15 (20%) | 12 (16%) |
| Abdominal radiotherapy | 7 (9%) | 5 (7%) |
| Other location | 6 (8%) | 11 (14%) |
| Received cranial irradiation ≥24 Gy | 10 (13%) | 10 (13%) |
| Received stem cell transplantation | 4 (5%) | 5 (7%) |
CNS central nervous system, Gy grey, N/n number, SD standard deviation.
There was no missing information for all baseline characteristics, except 4 participants who received steroids had missing information on cumulative steroid dose (3 participants of the control group and 1 participant of the intervention group).
aOther tumours included: neuroblastoma (n = 6), retinoblastoma (n = 4), renal tumours (n = 8), hepatic tumours (n = 1), germ cell tumours (n = 3), other and unspecified malignant neoplasms (n = 2), Langerhans cell histiocytosis (n = 7).
bDoxorubicin isotoxic equivalent dose.
Compliance to PA intervention
Seventeen (23%) participants reached ≥100% of their personalised goal (+2.5 h/week), 35 (48%) were considered compliant (≥2/3 of individual goal reached) with missing diary entries set to zero PA and 40 (53%) with missing entries set to the annual PA mean. On average, 16% of all intervention days had missing PA entries.
Primary outcome
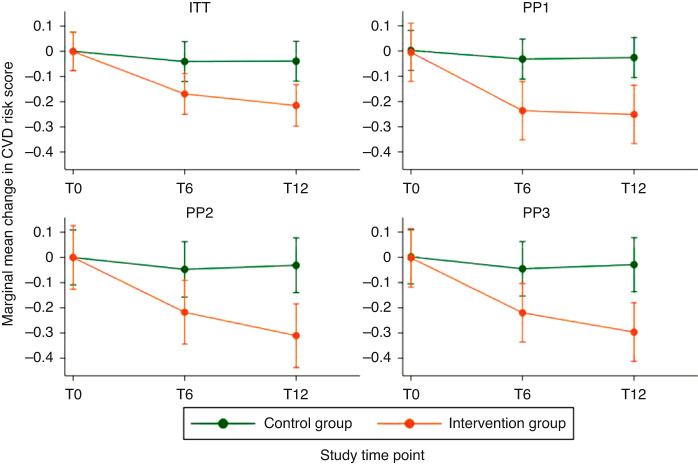
Mean CVD z-score at T0, T6 and T12 was 0.4 (SD = 0.8), 0.4 (SD = 0.9) and 0.3 (SD = 0.8), respectively, for the control group (Table 2), and changed from 0.6 (SD = 0.7) to 0.5 (SD = 0.8) and 0.4 (SD = 0.9) for the intervention group. eTables 3–5 in the Online Appendix show similar results for the 3 per protocol allocations. Individual trajectories showed some variability, but generally little change in controls and a decreasing trend in the intervention group (eFigure 1 in Online Appendix). We found a robust and significant effect of our intervention in the ITT and all per protocol analyses, showing a larger decrease in the composite CVD risk score in the intervention compared to the control group at T6 and T12 (Fig. 2 and Table 3). Our primary effect estimate showed a larger decrease in the CVD risk score at T12 of −0.18 z-scores (95% CI −0.29 to −0.06, P = 0.003) comparing the intervention to control group (Table 3). Proportion of participants with elevated CVD risk (z-score ≥1) decreased in both intervention and control participants from T0–T12 by 7% and 8%, respectively (Table 2 and eFigure 3 in Online Appendix).
Table 2.
Description of all outcomes by study time point and randomised group allocation (ITT).
| Control group | Intervention group | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| (N = 75) | (N = 71) | (N = 70) | (N = 76) | (N = 66) | (N = 63) | |
| Continuous study outcomes | ||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| CVD risk score (z-score), primary outcome | 0.4 (0.8) | 0.4 (0.9) | 0.3 (0.8) | 0.6 (0.7) | 0.5 (0.8) | 0.4 (0.9) |
| Na | 75 | 70 | 70 | 76 | 66 | 63 |
| Waist circumference (cm) | 82.7 (12.7) | 83.4 (12.8) | 82.7 (11.7) | 84.1 (11.9) | 83.5 (11.8) | 83.9 (12.2) |
| N | 74 | 70 | 69 | 76 | 65 | 63 |
| Systolic BP (mmHg) | 120.7 (15.2) | 116.1 (14.8) | 114.9 (14.2) | 123.1 (15.2) | 118.4 (15.4) | 116.9 (14.3) |
| N | 75 | 70 | 70 | 76 | 66 | 63 |
| Diastolic BP (mmHg) | 72.8 (10.0) | 69.9 (9.3) | 71.1 (9.2) | 77.2 (9.9) | 74.5 (10.4) | 74.1 (10.6) |
| N | 75 | 70 | 70 | 76 | 66 | 63 |
| Fasting glucose (mmol/l) | 4.7 (0.8) | 4.7 (1.0) | 4.6 (0.4) | 4.7 (0.4) | 4.8 (0.6) | 4.8 (0.9) |
| N | 69 | 65 | 66 | 73 | 57 | 61 |
| HDL cholesterol (mmol/l) | 1.5 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.4 (0.3) | 1.5 (0.4) | 1.5 (0.4) |
| N | 73 | 68 | 69 | 76 | 64 | 62 |
| Triglycerides (mmol/l) | 1.1 (0.5) | 1.3 (1.4) | 1.3 (1.5) | 1.4 (1.0) | 1.3 (1.2) | 1.3 (1.2) |
| N | 72 | 68 | 67 | 74 | 63 | 61 |
| Absolute peak power (Watt) | 188.6 (46.3) | 186.7 (51.6) | 186.3 (49.3) | 187.4 (50.7) | 194.7 (54.5) | 194.0 (52.7) |
| N | 74 | 68 | 69 | 76 | 63 | 60 |
| HbA1c (%) | 5.2 (0.6) | 5.2 (0.6) | 5.2 (0.4) | 5.2 (0.4) | 5.3 (0.5) | 5.3 (0.6) |
| N | 72 | 64 | 66 | 73 | 57 | 61 |
| Glucose, 2 h oGTTb (mmol/l) | 4.6 (1.5) | na | 4.4 (1.2) | 5.0 (1.5) | na | 4.9 (1.5) |
| N | 66 | 65 | 71 | 59 | ||
| Glucose change, 0 to 2 h oGTTb (mmol/l) | 0.1 (1.5) | na | -0.3 (1.2) | 0.3 (1.4) | na | 0.2 (1.3) |
| N | 66 | 64 | 71 | 59 | ||
| LDL cholesterol (mmol/l) | 2.5 (0.9) | 2.7 (0.9) | 2.6 (0.9) | 2.6 (0.8) | 2.6 (0.8) | 2.6 (0.8) |
| N | 72 | 68 | 69 | 75 | 63 | 60 |
| BMI (kg/m2) | 23.7 (4.0) | 23.7 (4.2) | 23.6 (3.8) | 24.4 (4.3) | 24.2 (3.9) | 24.4 (4.1) |
| N | 75 | 70 | 70 | 76 | 66 | 63 |
| Total body fat mass (kg)b | 21.9 (6.8) | na | 22.0 (7.2) | 22.6 (6.6) | na | 22.5 (7.1) |
| N | 73 | 68 | 74 | 61 | ||
| Total body fat proportion (%)b | 31.0 (7.4) | na | 30.9 (7.3) | 31.4 (6.4) | na | 30.8 (6.4) |
| N | 73 | 68 | 74 | 61 | ||
| % predicted abs. peak power | 70.1 (14.8) | 70.4 (16.0) | 69.7 (15.5) | 70.0 (15.9) | 72.8 (17.3) | 72.4 (17.3) |
| N | 74 | 68 | 69 | 76 | 63 | 60 |
| Relative peak power (Watt/kg) | 2.8 (0.7) | 2.7 (0.7) | 2.7 (0.7) | 2.7 (0.6) | 2.8 (0.7) | 2.7 (0.6) |
| N | 74 | 68 | 69 | 76 | 63 | 60 |
| % predicted rel. peak power | 77.5 (18.9) | 78.0 (19.6) | 77.5 (19.9) | 75.8 (15.5) | 79.1 (18.4) | 78.1 (17.1) |
| N | 74 | 68 | 69 | 76 | 63 | 60 |
| Grip strength dominant (kg) | 42.2 (12.8) | 43.1 (12.6) | 42.6 (12.5) | 43.3 (11.9) | 43.9 (12.8) | 45.0 (12.4) |
| N | 73 | 69 | 70 | 75 | 66 | 63 |
| Grip strength non-dominant (kg) | 39.0 (12.1) | 39.8 (11.8) | 40.5 (11.8) | 39.7 (12.2) | 40.7 (12.4) | 40.7 (12.1) |
| N | 73 | 69 | 70 | 75 | 66 | 63 |
| Repetitions 1-min STS | 49.9 (13.8) | 52.9 (14.4) | 54.9 (14.5) | 49.8 (11.2) | 56.2 (11.8) | 58.0 (12.1) |
| N | 75 | 70 | 69 | 76 | 66 | 62 |
| Overall counts per minute | 416.1 (128.0) | 404.7 (123.6) | 405.9 (129.4) | 386.9 (139.2) | 400.1 (138.2) | 375.2 (127.2) |
| N | 72 | 68 | 67 | 75 | 63 | 62 |
| Time in MVPA (min/day) | 43.4 (20.1) | 42.1 (21.9) | 42.1 (21.1) | 38.4 (20.2) | 38.0 (19.2) | 36.1 (16.9) |
| N | 72 | 68 | 67 | 75 | 63 | 62 |
| Time sedentary (min/day) | 530.5 (71.4) | 523.0 (80.5) | 525.5 (76.7) | 532.5 (92.3) | 514.6 (79.3) | 537.1 (79.8) |
| N | 72 | 68 | 67 | 75 | 63 | 62 |
| Average steps/day | 7637.5 (2685.3) | 7386.7 (2996.9) | 7437.0 (3030.2) | 7343.2 (2543.7) | 7705.3 (2724.8) | 6903.3 (2771.3) |
| N | 71 | 69 | 69 | 74 | 62 | 59 |
| Average aerobic steps/day | 1471.9 (1397.4) | 1427.8 (1784.6) | 1358.8 (1347.4) | 1272.0 (1243.7) | 1656.5 (1516.0) | 1170.3 (1040.6) |
| N | 71 | 69 | 69 | 74 | 62 | 59 |
| Binary study outcomesc | ||||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| CVD risk | ||||||
| Normal CVD risk | 61 (81%) | 60 (86%) | 62 (89%) | 54 (71%) | 52 (79%) | 49 (78%) |
| Elevated CVD risk (z-score ≥1) | 14 (19%) | 10 (14%) | 8 (11%) | 22 (29%) | 14 (21%) | 14 (22%) |
| Waist circumference | ||||||
| Normal | 58 (78%) | 51 (73%) | 52 (75%) | 50 (66%) | 44 (68%) | 45 (71%) |
| High (≥ 94 cm in male, ≥80 cm in female) | 16 (22%) | 19 (27%) | 17 (25%) | 26 (34%) | 21 (32%) | 18 (29%) |
| Blood pressure | ||||||
| Normotensive | 55 (73%) | 58 (83%) | 59 (84%) | 50 (66%) | 45 (68%) | 52 (83%) |
| Hypertensive (≥130 systolic and/or ≥85 diastolic bp) | 20 (27%) | 12 (17%) | 11 (16%) | 26 (34%) | 21 (32%) | 11 (17%) |
| Fasting glucose | ||||||
| Normal | 68 (97%) | 63 (95%) | 68 (99%) | 72 (97%) | 54 (95%) | 59 (95%) |
| Elevated (≥5.6 mmol/l) | 2 (3%) | 3 (5%) | 1 (1%) | 2 (3%) | 3 (5%) | 3 (5%) |
| HbA1c | ||||||
| Normal | 69 (96%) | 60 (94%) | 60 (91%) | 69 (95%) | 54 (95%) | 53 (87%) |
| Elevated (≥5.7%) | 3 (4%) | 4 (6%) | 6 (9%) | 4 (5%) | 3 (5%) | 8 (13%) |
| Insulin resistance (oGTT)b | ||||||
| No | 63 (95%) | na | 64 (98%) | 68 (96%) | na | 56 (95%) |
| Yes (glucose ≥7.8 mmol/l at 2 h of oGTT) | 3 (5%) | na | 1 (2%) | 3 (4%) | na | 3 (5%) |
| HDL cholesterol | ||||||
| Normal | 64 (88%) | 57 (84%) | 58 (84%) | 63 (83%) | 56 (88%) | 53 (85%) |
| Low (< 1.03 mmol/L in male, <1.30 mmol/L in female) | 9 (12%) | 11 (16%) | 11 (16%) | 13 (17%) | 8 (13%) | 9 (15%) |
| LDL cholesterol | ||||||
| Normal | 70 (97%) | 65 (96%) | 67 (97%) | 75 (100%) | 62 (98%) | 60 (100%) |
| Elevated (≥4.9 mmol/l) | 2 (3%) | 3 (4%) | 2 (3%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Triglycerides | ||||||
| Normal | 64 (89%) | 57 (84%) | 60 (90%) | 58 (78%) | 52 (83%) | 51 (84%) |
| Elevated (≥1.7 mmol/L) | 8 (11%) | 11 (16%) | 7 (10%) | 16 (22%) | 11 (17%) | 10 (16%) |
| BMI | ||||||
| BMI < 25 kg/m2 | 55 (73%) | 56 (80%) | 52 (74%) | 45 (59%) | 40 (61%) | 36 (57%) |
| BMI ≥ 25 kg/m2 | 20 (27%) | 14 (20%) | 18 (26%) | 31 (41%) | 26 (39%) | 27 (43%) |
| Total body fat proportionb | ||||||
| Normal | 56 (77%) | na | 53 (78%) | 69 (93%) | na | 54 (89%) |
| High (≥20% in male, ≥33% in female) | 17 (23%) | na | 15 (22%) | 5 (7%) | na | 7 (11%) |
| Reaching CDC recommendations | ||||||
| <150 min MVPA/week | 9 (13%) | 12 (18%) | 13 (19%) | 13 (17%) | 13 (21%) | 11 (18%) |
| ≥150 min MVPA/week | 63 (88%) | 56 (82%) | 54 (81%) | 62 (83%) | 50 (79%) | 51 (82%) |
| Total steps/day | ||||||
| <10,000 steps/day | 55 (77%) | 59 (86%) | 58 (84%) | 62 (84%) | 47 (76%) | 50 (85%) |
| ≥10,000 steps/day | 16 (23%) | 10 (14%) | 11 (16%) | 12 (16%) | 15 (24%) | 9 (15%) |
abs. absolute, BP blood pressure, CDC Centres for Disease Control and Prevention, CVD cardiovascular disease, HbA1c glycosylated haemoglobin, HDL high-density lipoprotein, ITT intention-to-treat, LDL low-density lipoprotein, MVPA moderate-to-vigorous physical activities, n/N number, na not available, oGTT oral glucose tolerance test, rel. relative, SD standard deviation, STS sit-to-stand.
The bold texts cover the variables that were part of the primary CVD risk score.
aNumber of participants with information on the specific outcome at the specific time point (N non-missing).
boGTT and body composition measured by dual-energy X-ray absorptiometry was not performed at time point 6 months.
cColumn proportions were calculated based on available numbers for each variable.
Fig. 2. Marginal mean changes of the CVD risk score (primary outcome) over time and by study group for the intention-to-treat (ITT) and three per protocol (PP) allocations.
This figure shows the marginal mean changes and 95% confidence intervals of the cardiovascular disease (CVD) risk score [z-score, primary outcome] from baseline to 6 and 12 months post-randomisation for the intervention and control group. Estimates from mixed effects generalised linear models adjusted for sex, main diagnostic group and baseline CVD risk score. T0, T6 and T12 denote the study time points: T0 = baseline; T6 = 6 months post-randomisation; T12 = 12 months post-randomisation. The group allocation differed for each of the plots. ITT (intention-to-treat): allocation as randomised (N = 151); PP1 (per protocol analysis 1): control group included participants with <5% increase in maximal power, intervention group included participants with ≥5% increase in maximal power (N = 112); PP2 (per protocol analysis 2): included compliant intervention and control participants only, based on daily self-reported physical activity data with missing days set to 0 activities (N = 82); PP3 (per protocol analysis 3): included compliant intervention and control participants only, based on daily self-reported physical activity data with missing days set to the annual average of activities (N = 87). See Table 3 for the exact point estimates. CVD cardiovascular disease.
Table 3.
Intervention effect (marginal mean difference from randomisation) of the primary outcome (CVD risk score, z-score) at each study time point and for different study group allocations; from mixed effects generalised linear model.
| Time point 6 months (change in CVD risk score from baseline to 6 months post-randomisation) | Time point 12 months (change in CVD risk score from baseline to 12 months post-randomisation) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Difference intervention − control | Intervention group | Control group | Difference intervention − control | |||||||||
| Marginal estimate | 95% CI | Marginal estimate | 95% CI | Marginal estimate | 95% CI | P value | Marginal estimate | 95% CI | Marginal estimate | 95% CI | Marginal estimate | 95% CI | P value | |
| ITT model | −0.17 | −0.25 to −0.09 | −0.04 | −0.12 to 0.04 | −0.13 | −0.24 to −0.01 | 0.027 | −0.22 | −0.30 to −0.13 | −0.04 | −0.12 to 0.04 | −0.18 | −0.29 to −0.06 | 0.003 |
| PP1 model | −0.24 | −0.35 to −0.12 | −0.03 | −0.11 to 0.05 | −0.20 | −0.35 to −0.06 | 0.004 | −0.25 | −0.37 to −0.14 | −0.03 | −0.11 to 0.05 | −0.23 | −0.37 to −0.09 | 0.002 |
| PP2 model | −0.22 | −0.34 to −0.09 | −0.05 | −0.16 to 0.06 | −0.17 | −0.34 to 0.00 | 0.048 | −0.31 | −0.44 to −0.18 | −0.03 | −0.14 to 0.08 | −0.28 | −0.45 to −0.11 | 0.001 |
| PP3 model | −0.22 | −0.34 to −0.10 | −0.05 | −0.15 to 0.06 | −0.17 | −0.33 to −0.01 | 0.033 | −0.30 | −0.41 to −0.18 | −0.03 | −0.14 to 0.08 | −0.27 | −0.43 to −0.11 | 0.001 |
CI confidence interval, CVD cardiovascular disease, ITT intention-to-treat, PP1 per protocol analysis 1, PP2 per protocol analysis 2, PP3 per protocol analysis 3.
The estimates represent the change in the CVD risk score [z-score] from baseline to 6 and 12 months post-randomisation for the intervention and control group. A negative score represents a reduction in the CVD risk and therefore a favourable outcome. The group allocation differed for each of the models. ITT (intention-to-treat): allocation as randomised (N = 151); PP1 (per protocol analyses 1): control group included participants with <5% increase in maximal power, intervention group included participants with ≥5% increase in maximal power (N = 112); PP2 (per protocol analysis 2): included compliant intervention and control participants only, based on daily self-reported physical activity data with missing days set to 0 activities (N = 82); PP3 (per protocol analysis 3): included compliant intervention and control participants only, based on daily self-reported physical activity data with missing days set to the annual average of activities (N = 87). The models were adjusted for sex, main diagnostic group and baseline CVD risk score. See Fig. 2 for a graphical display of the same results reported in Table 3.
Bold value means a significant p-value based on the significance level of α < 0.05.
Secondary outcomes
Descriptive results of all secondary outcomes are given in Table 2 (ITT) and eTables 3–5 in the Online Appendix (per protocol) with individual trajectories in eFigure 1 (Online Appendix).
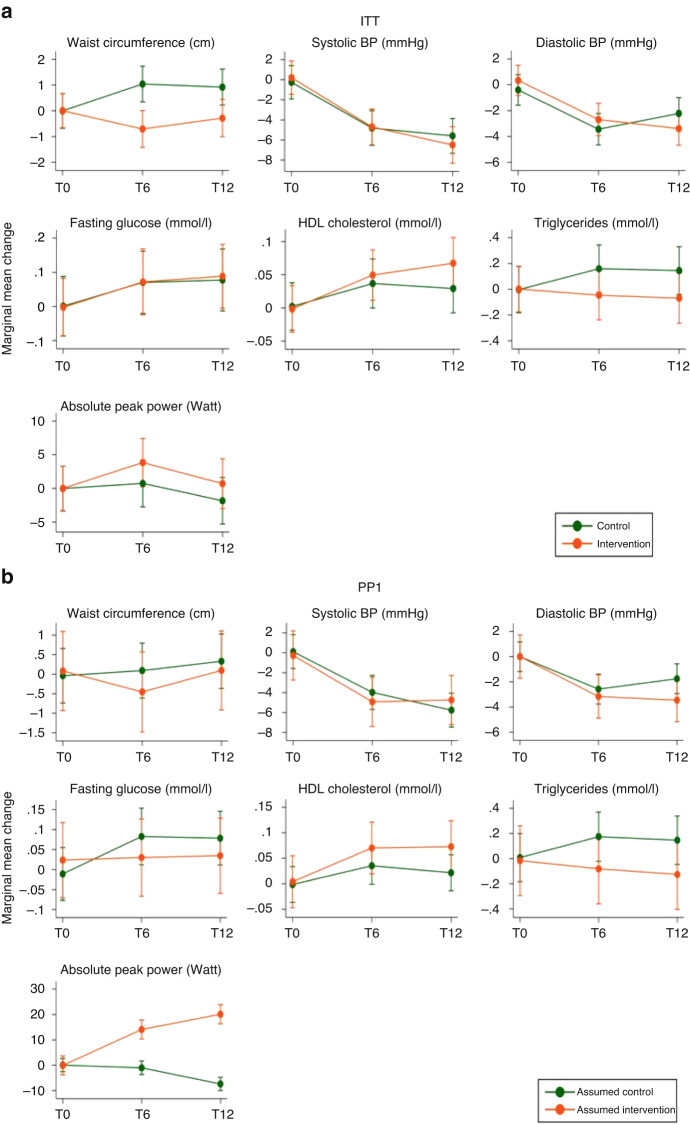
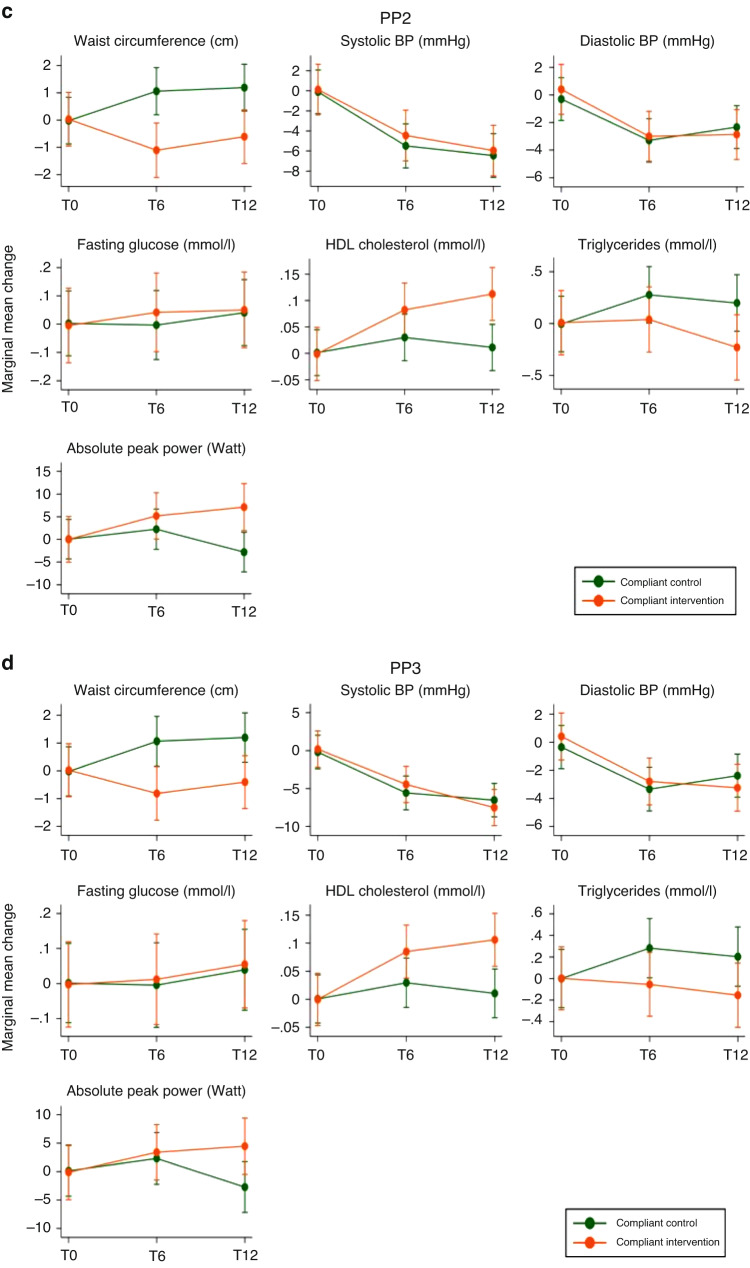
Figure 3 and eTables 6–9 in the Online Appendix (grey-shaded areas) show the intervention effect on single parameters of the CVD risk score. There was a positive, but mostly non-significant intervention effect on waist circumference, HDL cholesterol, triglycerides and absolute peak power and no effect on blood pressure and fasting glucose.
Fig. 3. Marginal mean changes of the single outcomes included in the CVD risk score for the intention-to-treat (ITT) and three per protocol (PP) allocations.
This figure shows the marginal mean changes and 95% confidence intervals of the of the single secondary outcomes that were part of the primary cardiovascular disease (CVD) risk score, from baseline to 6 and 12 months post-randomisation for the intervention and control group. Estimates from mixed effects generalised linear models adjusted for sex, main diagnostic group and baseline value of the respective outcome. T0, T6 and T12 denote the study time points: T0 = baseline; T6 = 6 months post-randomisation; T12 = 12 months post-randomisation. The group allocation differed for each panel. a ITT (intention-to-treat): allocation as randomised; b PP1 (per protocol analysis 1): control group included participants with <5% increase in maximal power, intervention group included participants with ≥5% increase in maximal power; c PP2 (per protocol analysis 2): included compliant intervention and control participants only, based on daily self-reported physical activity data with missing days set to 0 activities. d PP3 (per protocol analysis 3): included compliant intervention and control participants only, based on daily self-reported physical activity data with missing days set to the annual average of activities. See eTables 6–9 (grey-shaded area) for the exact point estimates. Cave: the intervals of the y axes differ depending on the unit of the respective outcome. The unit is provided in brackets after the outcome, in the title of each graph. BP blood pressure.
The intervention effect on further secondary outcomes are provided in eFigure 2 and eTables 6–9 in the Online Appendix. For binary outcomes, larger proportion of intervention participants than controls experienced reduction in high waist circumference, hypertension, low HDL cholesterol and high triglycerides from T0–T12 (Table 2 and eFigure 3 in Online Appendix).
Dose–response analysis
Higher proportion of PA goal reached was significantly associated with a larger reduction in the CVD risk score and larger increase in fasting glucose, HbA1c, LDL cholesterol, peak performance, and dominant hand grip strength from T0–T12 (eFigure 4 and eTables 10 and 11 in Online Appendix).
Post hoc subgroup analysis
We found no evidence of a differential effect of our intervention on the CVD risk score comparing survivors who received versus not received anthracyclines as part of their cancer therapy (Pinteraction=0.984; eFigure 5 and eTable 12 in Online Appendix).
Safety
A total of 170 AEs were registered, 78 in the control and 91 in the intervention group; 4 serious adverse events were reported in each group (eTable 13 in Online Appendix). At least one AE was reported by 22 intervention and 21 control participants. Eleven events were judged likely related to the PA intervention; 2 events (one psychiatric disorder [intervention group] and one neoplasm [control group]) resulted in withdrawal from the study. The most frequent AEs (≥5% of all events) were back pain, depression, flu like symptoms, gastrointestinal problems, injuries, and musculoskeletal and connective tissue disorders (eTable 14 in Online Appendix).
Discussion
Main findings
This is the first RCT to investigate the effect of a PA intervention on CVD risk in long-term CCS. Our intervention was effective at significantly reducing the CVD risk in the intervention compared to the control group at 6 and 12 months post-randomisation across all analyses. We further found positive effects on some single CVD risk factors and physical fitness. The intervention was safe with few and equally distributed serious adverse events; only 11 AEs were likely related to the exercise intervention.
Comparison with other studies
We are not aware of any other RCT in long-term CCS investigating the effect of PA on CVD risk. The existing 12 randomised, controlled PA interventions in CCS including child and adolescent survivors reported mostly no effect on PA [33, 37, 38, 50, 53], fitness [33, 39, 50], and cardiometabolic outcomes [34, 39, 50]; 4 trials, of which 3 are based on the same intervention, reported a positive effect on PA [35, 36, 51, 54], 1 on fitness [52] and 2 on body weight [34, 54].
Previous exercise RCTs with CCS used various intervention approaches with a maximum 6-month intervention period compared to 12 months in our study: a 4-day adventure-based training programme [35, 36, 51], PA camps [53], distant delivered web-, phone- or video-based interventions [33, 34, 52, 54], activity tracker and virtual peer support group [37], active video gaming [38], group-based sessions and app [50], or a home-based exercise plan with follow-up calls [39]. Our intervention was novel with a personalised exercise programme embedded in each survivor’s daily life and included various motivational tools. Such a tailored long-term intervention might have a higher potential for lasting behaviour change [58–60] and might be the reason why we observed a positive effect on CVD risk and physical fitness in our study, opposite to most of the above-mentioned trials in young survivors of childhood cancer.
Interpretation of results and clinical implications
Our main finding, showing a robust and protective effect of a long-term PA intervention on CVD risk factors in adult CCS, is novel and important for this growing population with many years ahead of them [1, 2]. Cardiovascular diseases are amongst the most common late effects in this population where >50% survivors report any cardiac disease [3], and is a leading cause of early mortality [4]. The Framingham Heart Study showed that standard CVD risk factors like the ones used in our score, were strong predictors of CVD incidence after 10 and 30 years of follow-up [74]. Moreover, our composite score was more stable when tracking CVD risk over time than single risk factors [75]. Thus, the implementation of a cheap and universal treatment like PA can reduce CVD risk factors in CCS and may ultimately improve the cardiac disease burden in this population. We also found a clear dose–response relationship of our PA intervention on CVD risk, which is an important message for survivors: a bit of PA is better than no activity and more activity is better than little activity.
The driving factors that led to a lower CVD risk score were a reduction in waist circumference and triglycerides as well as an increase in HDL cholesterol and physical fitness. Fasting glucose might have been less responsive to the intervention because it is well regulated in young people, even if they are insulin resistant, since their β-cells can produce enough insulin. In contrast to body composition, glucose and blood lipids, blood pressure is not “metabolically” regulated but rather reduced by less sympathetic activity and upregulated nitric oxide-signalling of peripheral arteries [76]. This might be the reason why our intervention, in accordance with other exercise interventions [77], was not effective in lowering blood pressure. While most individual factors of the CVD risk score did not reach statistically significant intervention effects, our sum score was sensitive to the subtle, but cumulative changes expected by regular PA. It could therefore be used as a screening tool in follow-up care to identify CCS that could profit most from targeted PA counselling.
Interestingly, we found no effect of our intervention on the PA parameters measured by accelerometer and pedometer. This contrasts with the positive effect we observed on physical fitness and the CVD parameters, assuming an increase in PA in the intervention group compared to the control group. Our PA assessments at arbitrary weeks over a 12-month period may thus not be representative, vulnerable to random behaviour changes and subject to social desirability while being measured. Changes in fitness and CVD risk was on the other hand result of cumulative differences in PA between intervention and control group over 1 year [78–80]. We therefore recommend the use of long-term and real-life tracking of PA for future studies, which is nowadays feasible with affordable, wearable activity-tracking devices [81]. Such activity tracker can provide both, intrinsic motivation for the patient and feedback to the health professionals to monitor and motivate patients. Another explanation might be that even though the total number of minutes spent in MVPA did not change, the quality of the exercise performed was better and more targeted to increase cardiovascular health, because it was individualised and prescribed by exercise professionals.
While our intervention was effective in reducing CVD risk and increasing fitness in CCS, the question remains whether such a programme can be implemented in clinical practice. Compared to traditional supervised exercise interventions, where participants meet for structured exercise programmes 2–3 times per week, our partially supervised intervention is less resource intense. The initial motivational interviews with personalised PA recommendations have low resource requirements in clinical settings. However, the long-term coaching and follow-up are critical but more resource-demanding. In our study, more participants of the intervention group dropped out (13 compared to 6 in the control group) despite several motivational tools and individual follow-up. Future studies should therefore find optimal levels of support for CCS to become more active long-term, improve fitness, and decrease their risk of late effects by testing PA interventions with various degrees of coaching, follow-up and motivational tools.
Our PA intervention can be considered safe, which is reassuring for professionals who want to recommend PA for CCS. Our intervention did not result in an increased risk of AEs in participants of the intervention group and only 11 of the registered, mostly mild or moderate events, were likely related to the intervention. This is in line with a recent German study that found an incidence of 2800 for grade 1 AEs and 17 for grade 2 AEs per 100,000 exercise interventions in childhood cancer patients and survivors [82].
Strengths and limitations
This is a high-quality RCT with appropriate power, many repeated high-quality assessments, following strict guidelines for confirmatory trials and with rigorous and detailed statistical analyses and SAP prior to unblinding the data. We used a novel intervention with personalised PA recommendations and multiple motivational tools with a long intervention period to observe long-term and sustainable changes [61]. The study used a composite CVD risk score similar to the Framingham score who used the sum of dichotomised parameters [74]. We explicitly did not reduce information of our parameters through dichotomisation as all the risk factors are linearly related to CVD with no biological cut-points.
A limitation of the comprehensive intervention might be the difficulty of implementation into clinical practice because of the resources needed. However, on the long-term, it would probably be less resource-demanding than traditional treatment of chronic late effects in this population. Due to technical problems of the gas analyser of the CPET, we could not use peak oxygen uptake (VO2max) to assess cardiorespiratory fitness, which is the current gold standard. However, we used maximal power in watt instead, which is a valid marker of cardiorespiratory fitness [83]. Another limitation could be potential selection bias towards survivors interested in PA, resulting in an on-average active population at baseline. We explicitly recruited CCS from the general survivor population and independent of their baseline CVD risk. This could have led to a ceiling effect, i.e., that survivors with a healthy CVD profile at baseline had no room for improvement, which leads to an underestimation of our intervention effect. However, our CVD risk score was sensitive enough to detect an improvement. Also, such a non-selected population in terms of CVD risk makes our results generalisable to the general population of CCS. By chance, controls started with slightly lower levels of CVD compared to participants of the intervention group, potentially giving them less room for improvement. However, we accounted for this difference by using mixed models for repeated measures and including the baseline value of the outcome as fixed effect in the models [84]. Finally, randomisation to a PA intervention could lead to other healthy behaviour changes (healthy diet, quit smoking), which could have contributed to the observed positive effect.
Conclusion
This high-quality RCT showed a robust and significant effect of a 1-year PA intervention to reduce CVD risk in long-term survivors of childhood cancer. We showed that PA can be a safe intervention against the high burden of cardiovascular late effects in this population and should be routinely recommended and implemented in the follow-up care of CCS.
Supplementary information
Acknowledgements
We thank all participants for taking part in our study. We thank the study nurses, assistants, master students and physiotherapists for their great work within the study.
Author contributions
Conceptualisation: SK, NXvdW and CSR; data curation: CSR, RJ and SJZ; formal analysis: CSR; funding acquisition: SK, CSR and NXvdW; investigation: SJZ, CS, RJ, IB and SK; methodology: CSR, SK, SJZ, CS, RJ and NXvdW; project administration: CSR, SJZ, CS, RJ, IB, NXvdW and SK; resources: NXvdW; software: JS, RJ, CSR and SJZ; supervision: CSR, NXvdW and SK; validation: SK, CSR, IB and RJ; visualisation: CSR; writing—original draft: CSR; writing—review and editing: all authors.
Funding
This work was supported by the Swiss Cancer League (KLS-3175-02-2013), the “Stiftung für krebskranke Kinder, Regio Basiliensis”, “Gedächtnis-Stiftung Susy Rückert zur Krebsbekämpfung”, “Taecker-Stiftung für Krebsforschung”, “Stiftung Henriette & Hans-Rudolf Dubach-Bucher”, “Stiftung zur Krebsbekämpfung”, “Stiftung Krebs-Hilfe Zürich”, “Fondation Recherche sur le Cancer de l’Enfant (FORCE)”, and Fond’Action contre le Cancer. CSR has received funding from the European Union Seventh Framework Programme (FP7-PEOPLE-2013-COFUND) under grant agreement n°609020-Scientia Fellows. WHD is paid by a research grant from the South-Eastern Norway Regional Health Authority (grant number 2019039, to CSR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
De-identified individual participant data that underlie the results reported in this article, statistical programmes (Stata do-files) and data dictionary are available upon request to the corresponding author, immediately following publication and without end date, to anyone who provides a sound proposal. The study protocol, statistical analysis plan, patient information and informed consent forms are published at the Open Science Framework platform: https://osf.io/w6j4y/.
Code availability
Statistical programmes (Stata do-files) and data dictionary are available upon request to the corresponding author, immediately following publication and without end date, to anyone who provides a sound proposal. The study protocol, statistical analysis plan, patient information and informed consent forms are published at the Open Science Framework platform: https://osf.io/w6j4y/.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and approved by the Swiss Ethics Committee on research involving humans (Ethikkommision Nordwest-und Zentralschweiz [EKNZ], EKNZ-2015-169). Informed consent as documented by signature, was obtained from each survivor prior to participation in the study. Data protection is assured by pseudonymization and secure storage of sensitive data.
Consent for publication
Not applicable.
Data access, responsibility and analysis
CSR had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nicolas X. von der Weid, Susi Kriemler.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02410-y.
References
- 1.Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, et al. Survival of European children and young adults with cancer diagnosed 1995-2002. Eur J Cancer. 2009;45:992–1005. [DOI] [PubMed]
- 2.Ness KK, Armstrong GT, Kundu M, Wilson CL, Tchkonia T, Kirkland JL. Frailty in childhood cancer survivors. Cancer. 2015;121:1540–7. doi: 10.1002/cncr.29211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. J Am Med Assoc. 2013;309:2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Ross JD. Late cardiotoxicity in aging adult survivors of childhood cancer. Prog Pediatr Cardiol. 2014;36:19–26. doi: 10.1016/j.ppedcard.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–80. doi: 10.1200/jco.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DM, Cox CL, Zhu L, Krull KR, Srivastava DK, Stovall M, et al. Risk factors for obesity in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2012;30:246–55. [DOI] [PMC free article] [PubMed]
- 8.Olsen JH, Möller T, Anderson H, Langmark F, Sankila R, Tryggvadóttír L, et al. Lifelong cancer incidence in 47 697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst. 2009;101:806–13. doi: 10.1093/jnci/djp104. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CL, Ness KK. Bone mineral density deficits and fractures in survivors of childhood cancer. Curr Osteoporos Rep. 2013;11:329–37. doi: 10.1007/s11914-013-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clanton NR, Klosky JL, Li C, Jain N, Srivastava DK, Mulrooney D, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood. Cancer Cancer. 2011;117:2559–68. doi: 10.1002/cncr.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianinazzi ME, Rueegg CS, Wengenroth L, Bergstraesser E, Rischewski J, Ammann RA, et al. Adolescent survivors of childhood cancer: are they vulnerable for psychological distress? Psycho-Oncol. 2013;22:2051–8. doi: 10.1002/pon.3249. [DOI] [PubMed] [Google Scholar]
- 12.Rueegg CS, Gianinazzi ME, Rischewski J, Beck Popovic M, Von der Weid NX, Michel G, et al. Health-related quality of life in survivors of childhood cancer: the role of chronic health problems. J Cancer Survivorship. 2013;7:511–22. doi: 10.1007/s11764-013-0288-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang FF, Hudson MM, Huang IC, Bhakta N, Ness KK, Brinkman TM, et al. Lifestyle factors and health-related quality of life in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. 2018. 10.1002/cncr.31647. [DOI] [PMC free article] [PubMed]
- 14.Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175:959–67. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurdana M. Physical activity and cancer risk. Actual knowledge and possible biological mechanisms. Radiol Oncol. 000010247820200063. 2021. 10.2478/raon-2020-0063. [DOI] [PMC free article] [PubMed]
- 16.Rimer J, Dwan K, Lawlor DA, Greig CA, McMurdo M, Morley W, et al. Exercise for depression. Cochrane Database Syst Rev. 2012;11. 10.1002/14651858.CD004366.pub5. [DOI] [PubMed]
- 17.Leon AS, Connett J, Jacobs DRJ, Rauramaa R. Leisure-time physical activity levels and the risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. J Am Med Assoc. 1987;258:2388–95. doi: 10.1001/jama.1987.03400170074026. [DOI] [PubMed] [Google Scholar]
- 18.Paffenbarger RS, Hyde RT, Wing AL, Lee I-M, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 19.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ Can Med Assoc J = J de l’Assoc Med Canadienne. 2006;174:801–9. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;1:3–63. [DOI] [PubMed]
- 21.Wolff I, van Croonenborg JJ, Kemper HCG, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9:1–12. doi: 10.1007/s001980050109. [DOI] [PubMed] [Google Scholar]
- 22.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA A Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 23.Walker KZ, O’Dea K, Gomez M, Girgis S, Colagiuri R. Diet and exercise in the prevention of diabetes. J Hum Nutr Diet. 2010;23:344–52. doi: 10.1111/j.1365-277X.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 24.Wogksch MD, Goodenough CG, Finch ER, Partin RE, Ness KK. Physical activity and fitness in childhood cancer survivors: a scoping review. Aging Cancer. 2021;2:112–28. doi: 10.1002/aac2.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales JS, Valenzuela PL, Herrera-Olivares AM, Rincón-Castanedo C, Martín-Ruiz A, Castillo-García A, et al. What are the effects of exercise training in childhood cancer survivors? A systematic review. Cancer Metastasis Rev. 2020;39:115–25. doi: 10.1007/s10555-020-09852-3. [DOI] [PubMed] [Google Scholar]
- 26.Scott JM, Li N, Liu Q, Yasui Y, Leisenring W, Nathan PC, et al., Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018. 10.1001/jamaoncol.2018.2254. [DOI] [PMC free article] [PubMed]
- 27.Mizrahi D, Wakefield CE, Fardell JE, Quinn VF, Lim Q, Clifford BK, et al. Distance-delivered physical activity interventions for childhood cancer survivors: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;118:27–41. doi: 10.1016/j.critrevonc.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Bourdon A, Grandy SA, Keats MR. Aerobic exercise and cardiopulmonary fitness in childhood cancer survivors treated with a cardiotoxic agent: a meta-analysis. Support Care Cancer. 2018;26:2113–23. doi: 10.1007/s00520-018-4208-z. [DOI] [PubMed] [Google Scholar]
- 29.Morales JS, Valenzuela PL, Herrera-Olivares AM, Baño-Rodrigo A, Castillo-García A, Rincón-Castanedo C, et al. Exercise interventions and cardiovascular health in childhood cancer: a meta-analysis. Int J Sports Med. 2020;41:141–53. doi: 10.1055/a-1073-8104. [DOI] [PubMed] [Google Scholar]
- 30.Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;3:Cd008796. doi: 10.1002/14651858.CD008796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esbenshade AJ, Ness KK. Dietary and exercise interventions for pediatric oncology patients: the way forward. J Natl Cancer Inst Monogr. 2019;2019:157–62. doi: 10.1093/jncimonographs/lgz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung AT, Li WHC, Ho LLK, Ho KY, Chan GCF, Chung JOK. Physical activity for pediatric cancer survivors: a systematic review of randomized controlled trials. J Cancer Survivorship. 2021. 10.1007/s11764-020-00981-w. [DOI] [PMC free article] [PubMed]
- 33.Howell CR, Krull KR, Partin RE, Kadan-Lottick NS, Robison LL, Hudson MM, et al. Randomized web-based physical activity intervention in adolescent survivors of childhood cancer. Pediatr Blood Cancer. 2018;65:e27216. doi: 10.1002/pbc.27216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang JS, Dillon L, Terrones L, Schubert L, Roberts W, Finklestein J, et al. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatr Blood Cancer. 2014;61:894–900. doi: 10.1002/pbc.24937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li HC, Chung OK, Ho KY, Chiu SY, Lopez V. Effectiveness of an integrated adventure-based training and health education program in promoting regular physical activity among childhood cancer survivors. Psychooncology. 2013;22:2601–10. doi: 10.1002/pon.3326. [DOI] [PubMed] [Google Scholar]
- 36.Li WHC, Ho KY, Lam KKW, Lam HS, Chui SY, Chan GCF, et al. Adventure-based training to promote physical activity and reduce fatigue among childhood cancer survivors: a randomized controlled trial. Int J Nurs Stud. 2018;83:65–74. doi: 10.1016/j.ijnurstu.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Mendoza JA, Baker KS, Moreno MA, Whitlock K, Abbey-Lambertz M, Waite A, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: a pilot study. Pediatr Blood Cancer. 2017;64. 10.1002/pbc.26660. [DOI] [PubMed]
- 38.Sabel M, Sjölund A, Broeren J, Arvidsson D, Saury JM, Blomgren K, et al. Active video gaming improves body coordination in survivors of childhood brain tumours. Disabil Rehabil. 2016;38:2073–84. doi: 10.3109/09638288.2015.1116619. [DOI] [PubMed] [Google Scholar]
- 39.Tanir MK, Kuguoglu S. Impact of exercise on lower activity levels in children with acute lymphoblastic leukemia: a randomized controlled trial from Turkey. Rehabil Nurs. 2013;38:48–59. doi: 10.1002/rnj.58. [DOI] [PubMed] [Google Scholar]
- 40.Braam KI, van Dijk-Lokkart EM, Kaspers GJL, Takken T, Huisman J, Buffart LM, et al. Effects of a combined physical and psychosocial training for children with cancer: a randomized controlled trial. BMC Cancer. 2018;18:1289. doi: 10.1186/s12885-018-5181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox CL, Zhu L, Kaste SC, Srivastava K, Barnes L, Nathan PC, et al. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65:e26929. doi: 10.1002/pbc.26929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Macedo TMF, Oliveira KMC, Melo J, De Medeiros MG, De Medeiros Filho WC, Ferreira GMH. Inspiratory muscle training in patients with acute leukemia: preliminary results. Rev Paul de Pediatr. 2010;28:352–8. doi: 10.1590/S0103-05822010000400011. [DOI] [Google Scholar]
- 43.Fiuza-Luces C, Padilla JR, Soares-Miranda L, Santana-Sosa E, Quiroga JV, Santos-Lozano A, et al. Exercise intervention in pediatric patients with solid tumors: the physical activity in pediatric cancer trial. Med Sci Sports Exerc. 2017;49:223–30. doi: 10.1249/mss.0000000000001094. [DOI] [PubMed] [Google Scholar]
- 44.Hartman A, Winkel MLT, Beek RDV, Keizer-Schrama SMPFDM, Kemper HCG, Hop WCJ, et al. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53:64–71. doi: 10.1002/pbc.21942. [DOI] [PubMed] [Google Scholar]
- 45.Hinds PS, Hockenberry M, Rai SN. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. J Pain Symptom Manag. 2007;33:686–97. doi: 10.1016/j.jpainsymman.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42:127–33. doi: 10.1002/pbc.10481. [DOI] [PubMed] [Google Scholar]
- 47.Moyer-Mileur LJPRD, Ransdell LPF, Bruggers CSMD. Fitness of children with standard-risk acute lymphoblastic leukemia during maintenance therapy: response to a home-based exercise and nutrition program. J Pediatr Hematol Oncol. 2009;31:259–66. doi: 10.1097/MPH.0b013e3181978fd4. [DOI] [PubMed] [Google Scholar]
- 48.Speyer E, Herbinet A, Vuillemin A, Briancon S, Chastagner P. Effect of adapted physical activity sessions in the hospital on health-related quality of life for children with cancer: a cross-over randomized trial. Pediatr Blood Cancer. 2010;55:1160–6. doi: 10.1002/pbc.22698. [DOI] [PubMed] [Google Scholar]
- 49.Stössel S, Neu MA, Wingerter A, Bloch W, Zimmer P, Paret C, et al. Benefits of exercise training for children and adolescents undergoing cancer treatment: results from the randomized controlled MUCKI trial. Front Pediatrics. 2020;8:243–243. doi: 10.3389/fped.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devine KA, Viola A, Levonyan-Radloff K, Mackowski N, Bozzini B, Chandler A, et al. Feasibility of FitSurvivor: a technology-enhanced group-based fitness intervention for adolescent and young adult survivors of childhood cancer. Pediatr Blood Cancer. 2020;67:e28530. doi: 10.1002/pbc.28530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li WHC, Chan GCF, Lam MHS, Chung JOK, Chiu SY, Fong DYT. Integrated adventure-based training and health education programme in promoting regular physical activity among childhood cancer survivors. Hong Kong Med J. 2019;25:40–43. [PubMed] [Google Scholar]
- 52.Manchola-González JD, Bagur-Calafat C, Girabent-Farrés M, Serra-Grima JR, Pérez RÁ, Garnacho-Castaño MV, et al. Effects of a home-exercise programme in childhood survivors of acute lymphoblastic leukaemia on physical fitness and physical functioning: results of a randomised clinical trial. Support Care Cancer. 2020;28:3171–8. doi: 10.1007/s00520-019-05131-2. [DOI] [PubMed] [Google Scholar]
- 53.Ruble K, Scarvalone S, Gallicchio L, Davis C, Wells D. Group physical activity intervention for childhood cancer survivors: a pilot study. J Phys Act Health. 2016;13:352–9. doi: 10.1123/jpah.2015-0050. [DOI] [PubMed] [Google Scholar]
- 54.Stern M, Bleck J, Ewing LJ, Davila E, Lynn C, Hale G, et al. NOURISH-T: targeting caregivers to improve health behaviors in pediatric cancer survivors with obesity. Pediatr Blood Cancer. 2018. 10.1002/pbc.26941. [DOI] [PMC free article] [PubMed]
- 55.Rueegg CS, Gianinazzi ME, Michel G, von der Weid NX, Bergstraesser E, Kuehni CE. Do childhood cancer survivors with physical performance limitations reach healthy activity levels? Pediatr Blood Cancer. 2013;60:1714–20. doi: 10.1002/pbc.24595. [DOI] [PubMed] [Google Scholar]
- 56.Mizrahi D, Wakefield CE, Simar D, Ha L, McBride J, Field P, et al. Barriers and enablers to physical activity and aerobic fitness deficits among childhood cancer survivors. Pediatr Blood Cancer. 2020;67:e28339. doi: 10.1002/pbc.28339. [DOI] [PubMed] [Google Scholar]
- 57.Ross WL, Le A, Zheng DJ, Mitchell HR, Rotatori J, Li F, et al. Physical activity barriers, preferences, and beliefs in childhood cancer patients. Support Care Cancer. 2018;26:2177–84. doi: 10.1007/s00520-017-4041-9. [DOI] [PubMed] [Google Scholar]
- 58.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. J Am Med Assoc. 2007;298:2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 59.Miller WR, Rollnick S. The effectiveness and ineffectiveness of complex behavioral interventions: impact of treatment fidelity. Contemp Clin Trials. 2014;37:234–41. doi: 10.1016/j.cct.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Morgan F, Battersby A, Weightman AL, Searchfield L, Turley R, Morgan H, et al. Adherence to exercise referral schemes by participants—what do providers and commissioners need to know? A systematic review of barriers and facilitators. BMC Public Health. 2016;16:227. doi: 10.1186/s12889-016-2882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kettle VE, Madigan CD, Coombe A, Graham H, Thomas JJC, Chalkley AE, et al. Effectiveness of physical activity interventions delivered or prompted by health professionals in primary care settings: systematic review and meta-analysis of randomised controlled trials. BMJ. 2022;376:e068465. doi: 10.1136/bmj-2021-068465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rueegg CS, Kriemler S, Zuercher SJ, Schindera C, Renner A, Hebestreit H, et al. A partially supervised physical activity program for adult and adolescent survivors of childhood cancer (SURfit): study design of a randomized controlled trial [ NCT02730767] BMC Cancer. 2017;17:822. doi: 10.1186/s12885-017-3801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103:1457–67. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 64.Michel G, von der Weid NX, Zwahlen M, Adam M, Rebholz CE, Kuehni CE. The Swiss Childhood Cancer Registry: rationale, organisation and results for the years 2001–2005. Swiss Med Wkly. 2007;137:502–9. doi: 10.4414/smw.2007.11875. [DOI] [PubMed] [Google Scholar]
- 65.Schindera C, Zürcher SJ, Jung R, Boehringer S, Balder JW, Rueegg CS, et al. Physical fitness and modifiable cardiovascular disease risk factors in survivors of childhood cancer: a report from the SURfit study. Cancer. 2021. 10.1002/cncr.33351. [DOI] [PubMed]
- 66.Jung R, Zürcher SJ, Schindera C, Eser P, Meier C, Schai A, et al. Effect of a physical activity intervention on lower body bone health in childhood cancer survivors: a randomized controlled trial (SURfit) Int J Cancer. 2023;152:162–71. doi: 10.1002/ijc.34234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rueegg CS, Kriemler S, von der Weid NX, Zürcher SJ, Schindera C, Jung R. The SURfit study — an RCT to investigate the effects of a one-year physical activity intervention in long-term survivors of childhood cancer. (2021, October 19) Retrieved from osf.io/w6j4y. 10.17605/OSF.IO/W6J4Y.
- 68.Andersen LB, Lauersen JB, Brond JC, Anderssen SA, Sardinha LB, Steene-Johannessen J, et al. A new approach to define and diagnose cardiometabolic disorder in children. J Diabetes Res. 2015;2015:539835. doi: 10.1155/2015/539835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slagter SN, van Waateringe RP, van Beek AP, van der Klauw MM, Wolffenbuttel BHR, van Vliet-Ostaptchouk JV. Sex, BMI and age differences in metabolic syndrome: the Dutch Lifelines Cohort Study. Endocr Connect. 2017;6:278–88. doi: 10.1530/ec-17-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van de Poppe DJ, Hulzebos E, Takken T. Reference values for maximum work rate in apparently healthy Dutch/Flemish adults: data from the LowLands fitness registry. Acta Cardiol. 2019;74:223–30. doi: 10.1080/00015385.2018.1478763. [DOI] [PubMed] [Google Scholar]
- 71.Kelly AS, Steinberger J, Jacobs DR, Hong CP, Moran A, Sinaiko AR. Predicting cardiovascular risk in young adulthood from the metabolic syndrome, its component risk factors, and a cluster score in childhood. Int J Pediatr Obes. 2011;6:e283–289. doi: 10.3109/17477166.2010.528765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kriemler S, Zahner L, Schindler C, Meyer U, Hartmann T, Hebestreit H, et al. Effect of school based physical activity programme (KISS) on fitness and adiposity in primary schoolchildren: cluster randomised controlled trial. BMJ. 2010;340:c785. doi: 10.1136/bmj.c785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.CTCAE. The Common Terminology Criteria for Adverse Events (CTCAE). November 2017. Retrieved from https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- 74.Pencina MJ, D’Agostino RB, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease. Circulation. 2009;119:3078–84. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersen LB, Hasselstrøm H, Grønfeldt V, Hansen SE, Karsten F. The relationship between physical fitness and clustered risk, and tracking of clustered risk from adolescence to young adulthood: eight years follow-up in the Danish Youth and Sport Study. Int J Behav Nutr Phys Act. 2004;1:6. doi: 10.1186/1479-5868-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koller A, Laughlin MH, Cenko E, de Wit C, Tóth K, Bugiardini R, et al. Functional and structural adaptations of the coronary macro- and micro-vasculature to regular aerobic exercise by activation of physiological, cellular and molecular mechanisms: Esc Working Group on Coronary Pathophysiology & Microcirculation Position Paper. Cardiovasc Res. 2021 doi: 10.1093/cvr/cvab246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, Elliott WJ, et al. Beyond medications and diet: alternative approaches to lowering blood pressure. Hypertension. 2013;61:1360–83. doi: 10.1161/HYP.0b013e318293645f. [DOI] [PubMed] [Google Scholar]
- 78.Nordengen S, Andersen LB, Solbraa AK, Riiser A. Cycling and cardiovascular disease risk factors including body composition, blood lipids and cardiorespiratory fitness analysed as continuous variables: part 2—systematic review with meta-analysis. Br J Sports Med. 2019;53:879–85. doi: 10.1136/bjsports-2018-099778. [DOI] [PubMed] [Google Scholar]
- 79.Salchow J, Mann J, Koch B, von Grundherr J, Jensen W, Elmers S, et al. Comprehensive assessments and related interventions to enhance the long-term outcomes of child, adolescent and young adult cancer survivors—presentation of the CARE for CAYA-Program study protocol and associated literature review. BMC Cancer. 2020;20:16. doi: 10.1186/s12885-019-6492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orrow G, Kinmonth A-L, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012;344:e1389. doi: 10.1136/bmj.e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015;12:159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gauß G, Beller R, Boos J, Däggelmann J, Stalf H, Wiskemann J, et al. Adverse events during supervised exercise interventions in pediatric oncology—a nationwide survey. Front Pediatr. 2021;9. 10.3389/fped.2021.682496. [DOI] [PMC free article] [PubMed]
- 83.Balady GJ, Berra KA, Golding LA, Gordon NF, Mahler DA, Myers JN, Sheldahl LM. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription, 6th edition. Ed, Franklin BA. Philadelphia: Lippincott Williams & Wilkins; 2000.
- 84.Detry MA, Ma Y. Analyzing repeated measurements using mixed models. J Am Med Assoc. 2016;315:407–8. doi: 10.1001/jama.2015.19394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data that underlie the results reported in this article, statistical programmes (Stata do-files) and data dictionary are available upon request to the corresponding author, immediately following publication and without end date, to anyone who provides a sound proposal. The study protocol, statistical analysis plan, patient information and informed consent forms are published at the Open Science Framework platform: https://osf.io/w6j4y/.
Statistical programmes (Stata do-files) and data dictionary are available upon request to the corresponding author, immediately following publication and without end date, to anyone who provides a sound proposal. The study protocol, statistical analysis plan, patient information and informed consent forms are published at the Open Science Framework platform: https://osf.io/w6j4y/.