Abstract
Background
A reported clinical pharmacokinetics and safety study of suspension formulation of ensitrelvir, a therapeutic agent used in severe acute respiratory syndrome coronavirus 2 infection, demonstrated favorable pharmacokinetics and was well tolerated in healthy male Japanese and White participants. Understanding the safety and pharmacokinetic features of ensitrelvir (using the formulation approved for clinical use) in various populations, and the effect of food, is crucial for optimal clinical use.
Objectives
The objectives of this study were to (1) assess the safety, tolerability, and pharmacokinetics of ensitrelvir following multiple-dose administration of ensitrelvir tablets in populations with different races, ages, and sex; and (2) assess the effect of food on the pharmacokinetics of ensitrelvir tablets in the fasted or fed state.
Methods
A phase 1, multicenter, double-blinded, randomized, placebo-controlled study was conducted to evaluate the safety and pharmacokinetics of once-daily ensitrelvir tablets at loading/maintenance doses of 375/125 mg or 750/250 mg for 5 days in healthy Japanese females, Japanese elderly (only 375/125 mg), and White male and female participants. An open-label, two-group, two-period crossover study was also conducted to estimate the effect of food on the pharmacokinetics of ensitrelvir at single dose of 375 mg. The nature, frequency, and severity of treatment-emergent adverse events were evaluated and recorded in safety assessments in both studies.
Results
The maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) were similar within these populations. The geometric mean half-life of ensitrelvir following multiple-dose administration was 48.7–58.9 h across all cohorts. The Cmax and AUC increased in a dose-proportional manner in Japanese female participants, and increased in a less than dose-proportional manner in White participants. Furthermore, there was no clear relationship between the dose and geometric mean half-life of ensitrelvir. The plasma concentration at 24 h (C24) after an initial dose of 375/125 mg exceeded the target plasma concentration (6.09 µg/mL) in all populations. Regarding the effect of food on the pharmacokinetics of ensitrelvir, although time to Cmax in the fed state was delayed, there was no clinically meaningful difference in the exposure levels (Cmax and AUC) of ensitrelvir between the fasted and fed states. Most treatment-emergent adverse events were mild in nature and had resolved.
Conclusion
Ensitrelvir (375/125 mg and 750/250 mg tablet formulation) was well tolerated, without any major safety concerns. The pharmacokinetics of ensitrelvir between all populations in the study were similar and C24 exceeded the target plasma concentration at 375/125 mg. These results suggest that ensitrelvir can be effectively administered with no necessity for dose adjustment for age, sex, and race without food restriction.
Clinical Trial Registration
Japan Registry of Clinical Trials identifier: jRCT2031210202, registered on 16 July 2021.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-023-01309-z.
Key Points
| The pharmacokinetics of ensitrelvir were similar between populations tested. |
| Similar exposure levels (maximum plasma concentration [Cmax] and area under the plasma concentration-time curve [AUC]) of ensitrelvir were observed on days 1 and 5 within each dosing regimen when the ensitrelvir tablet formulation was administered once daily for 5 days as a 375/125 mg or 750/250 mg dosing regimen. |
| There was no clinically meaningful difference in the exposure levels to ensitrelvir between the fasted and fed states except delayed time to maximum plasma concentration. |
| For administering the ensitrelvir tablet formulation (375/125 mg or 750/250 mg), no dose adjustment is necessary for race, age, and sex. |
Introduction
As of 17 May 2023, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a pandemic with 6.93 million deaths worldwide [1]. Ensitrelvir fumaric acid (previously known as S-217622; now referred to as ensitrelvir), a novel 3C-like protease inhibitor of SARS-CoV-2 created through joint research between Hokkaido University and Shionogi & Co., Ltd, suppresses viral replication [2]. In November 2022, ensitrelvir 125 mg tablet obtained emergency regulatory approval from the Ministry of Health, Labour and Welfare, in Japan for the treatment of SARS-CoV-2 infection [3] at a dosage of once-daily ensitrelvir 375 mg on day 1, followed by 125 mg once daily on days 2–5 (375/125 mg) for 5 days.
This bench-to-bedside translation was the result of a rigorous stepwise research and development process. Various preclinical and in vitro studies have demonstrated the antiviral activity of ensitrelvir with therapeutic efficacy against several variants of SARS-CoV-2 [2, 4, 5]. A phase 1 trial of single and multiple ascending doses of once-daily oral ensitrelvir in healthy male adults showed that the ensitrelvir suspension formulation was safe, well tolerated, and had a favorable pharmacokinetic profile with a long half-life, supporting once-daily oral dosing of 375/125 mg and once-daily doses of ensitrelvir 750 mg on day 1 followed by 250 mg on days 2–5 (750/250 mg) [6]. Results of the phase 2a and 2b parts of a phase 2/3 study demonstrated the efficacy, safety, and tolerability of ensitrelvir 375/125 mg and 750/250 mg treatments in mild-to-moderate coronavirus disease 2019 (COVID-19) [7–9].
The safety and pharmacokinetic profiles using the formulation for clinical use (tablet formulation) have also been reported at 375/125 mg and 750/250 mg in drug–drug interaction studies in Japanese healthy male participants [10]. There were no serious treatment-emergent adverse events (TEAEs) and almost all of the TEAEs related to ensitrelvir were mild in severity and had resolved.
Several therapeutic agents exhibit interindividual differences in pharmacokinetics and pharmacodynamics due to a variety of factors, including age, sex, ethnicity, genetic factors, consumption of tobacco or alcohol, presence of comorbidities, food, and concomitant use of medications [11]. Understanding such variability in drug pharmacokinetics and pharmacokinetics/pharmacodynamics is pivotal to ensure that suitable drug exposure is attained to achieve efficacy and avoid toxicity [12]. Specifically, ethnic/racial differences in pharmacokinetics and pharmacokinetics/pharmacodynamics can be further influenced by potential interactions with drug administration through a specific route, dose frequency and/or duration changes, and the use of different dosage forms [13, 14]. Moreover, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Guidelines (ICH) developed guidelines on ethnic factors for evaluating the impact of ethnic factors on safety and efficacy of a drug at a particular dosage and dose regimen [15]. Since some studies indicate that drug clearance is faster in women than in men, differences in sex is one of the influential factors affecting pharmacokinetics [16, 17]. The rational use of medications in the elderly population is of great concern. An in-depth understanding of age-related physiological changes and their impact on the pharmacokinetics of a drug is needed for well tolerated and effective therapy in the elderly population [18, 19]. Furthermore, as some of the drugs show decreased bioavailability and reduced drug efficacy when consumed with food, it is important to know the effect of food on drug absorption [20].
However, previous studies with ensitrelvir suspension [6] and tablet formulations [10] were conducted in healthy Japanese male participants only. Consequently, since Japanese female and elderly patients with SARS-CoV-2 infection were also included in the phase 2/3 study, it was important to obtain the safety and pharmacokinetic information on these populations. In addition, obtaining the safety and pharmacokinetic information for non-Japanese participants is also very important to consider the dose regimen for global clinical studies.
Thus, in the present study, we evaluated the pharmacokinetics and safety of the approved ensitrelvir formulation (tablet). The objectives of this study were to (1) assess the safety, tolerability, and pharmacokinetics of ensitrelvir following multiple-dose administration of ensitrelvir tablets in populations with different races, ages, and sex; and (2) assess the effect of food on the pharmacokinetics of ensitrelvir tablets in the fasted or fed (high-fat/high-calorie meal) state. The results reported here are part of the completed study (jRCT2031210202).
Methods
Study Design
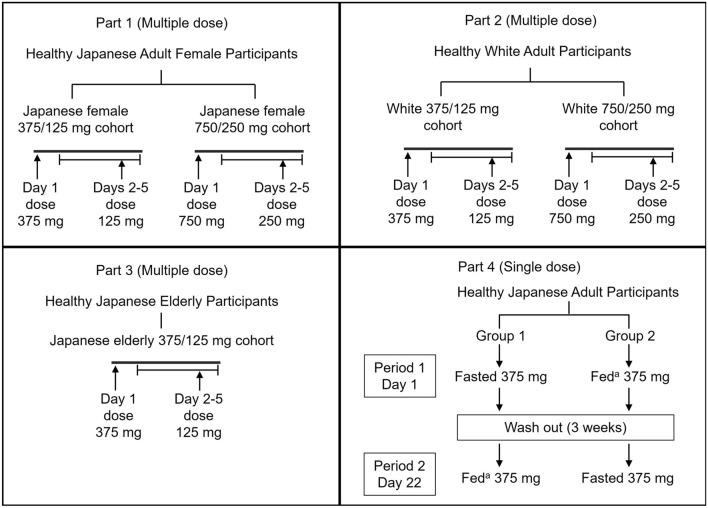
A phase 1, multicenter, double-blinded, randomized, placebo-controlled, four-part study was conducted to assess the safety, tolerability, and pharmacokinetics of single- and multiple-dose administration of ensitrelvir in different populations. Healthy participants were randomized to receive multiple doses (parts 1–3) and a single dose (part 4) of orally administered placebo or ensitrelvir fumaric acid (ensitrelvir) in a tablet formulation. Participants were randomized in pre-decided ratios of approximately 3:1 to ensitrelvir or placebo (parts 1–3), and 1:1 for part 4 (Fig. 1).
Fig. 1.
Study design. aHigh-fat/high-calorie meal (breakfast).
In parts 1–3, healthy Japanese adult female participants were randomized to receive placebo or multiple doses of ensitrelvir in the fasted state. The multiple-dose regimens were once-daily doses of ensitrelvir 375 mg on day 1 followed by 125 mg on days 2–5 (Japanese female 375/125 mg cohort), or 750 mg on day 1 followed by 250 mg on days 2–5 (Japanese female 750/250 mg cohort). Healthy White adult participants were randomized to receive placebo or multiple doses of ensitrelvir in the fasted state. The multiple-dose regimens were once-daily doses of ensitrelvir 375 mg on day 1 followed by 125 mg on days 2–5 (White 375/125 mg cohort), or 750 mg on day 1 followed by 250 mg on days 2–5 (White 750/250 mg cohort). Healthy Japanese elderly participants were randomized to receive placebo or multiple doses of ensitrelvir in the fasted state. The multiple-dose regimens were once-daily doses of ensitrelvir 375 mg on day 1 and 125 mg on days 2–5 (Japanese elderly 375/125 mg cohort).
In part 4, the effect of food on the pharmacokinetics of ensitrelvir was investigated in healthy Japanese participants in a two-group, two-period crossover design using single-dose administration of ensitrelvir 375 mg (three ensitrelvir 125 mg tablets) in the fasted and fed (high-fat/high-calorie) state (Group 1: 375 mg single dose in the fasted state in period 1 followed by 375 mg single dose in the fed state in period 2; Group 2: 375 mg single dose in the fed state in period 1 followed by 375 mg single dose in the fasted state in period 2). Participants in the fasted state were prohibited from eating overnight for at least 10 h predose and at least 4 h postdose, while participants in the fed state also fasted overnight for at least 10 h and were asked to start and finish their breakfast (high calorie, high-fat meal) within 30 min, prior to administration of the study intervention. The intake of beverages, except for 240 mL of water taken with study intervention and 180 mL of beverage provided at breakfast or dinner, was prohibited from 1 h before to 2 h after the study intervention. Thereafter, the intake of beverages was allowed ad libitum.
Eligible participants aged 20–55 years (part 1–3) and ≥65 years (part 4) at the time of signing the informed consent form (ICF), whose body mass index (BMI) ranged between ≥18.5 and ≤30.0 kg/m2 (with weight ≥40 kg for female participants), and not requiring any other medicines for any health conditions were included in this study.
Ethical Compliance
This study (jRCT2031210202) was conducted in compliance with the protocol, the World Medical Association Declaration of Helsinki [21] and Council for International Organizations of Medical Sciences International Ethical Guidelines [22], ICH [23], and other applicable laws and regulations. It was also approved by the Medical Corporation Shinanokai Shinanozaka Clinic Institutional Review Board (Review ID: 757P I; approval date: 14 July 2021). All participants gave their written informed consent for participation in this study.
Bioanalytical Procedure
Plasma concentrations of ensitrelvir were determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS) following protein precipitation with acetonitrile. LC–MS/MS analysis was performed using API4000 (Sciex, Framingham, MA, USA). The method was validated over a range of 200–200,000 ng/mL, with a lower limit of quantification (LLOQ) of 200 ng/mL [10].
Pharmacokinetic Assessments
Blood samples (4 mL at each time point) were collected, centrifuged to obtain plasma samples, and stored at under −70 °C until analysis at several time points to evaluate the pharmacokinetics and determine the concentration of ensitrelvir. For multiple-dose administration, blood samples were collected at predose (0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, and 12 h postdose on day 1; predose (0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 48, 72, and 96 h postdose on day 5, and predose (0 h) on day 13 (at visit). For the effect of food study, blood samples were collected at 0 h predose and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, 24, 48, 72, 120, 168, and 336 h postdose on days 1 and 22.
The pharmacokinetic parameters of ensitrelvir were calculated based on the plasma concentration data of ensitrelvir with non-compartmental analysis. Pharmacokinetic analyses were performed using WinNonlin version 8.3.3 (Certara USA Inc., Princeton, NJ, USA). The pharmacokinetic parameters included maximum plasma concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration-time curve over dosing interval τ (i.e., 24 h) [AUC0-τ], and plasma concentration at 24 h postdose (C24) after days 1 and 5 of multiple-dose administration and also terminal elimination half-life (t½,z) after day 5 of multiple-dose administration. We also examined Cmax, Tmax, AUC to the last measurable concentration (AUC0-last), AUC extrapolated to infinity (AUC0-∞), t½,z, mean residence time (MRT), and apparent volume of distribution in the terminal elimination phase (Vz/F) to evaluate the effect of food on the pharmacokinetics of ensitrelvir.
Safety and Tolerability Analyses
Safety assessments included the analysis of all TEAEs, classified by System Organ Class and Medical Dictionary for Regulatory Activities (MedDRA) version 24.0 Preferred Terms. The nature, frequency, and severity of TEAEs were evaluated and recorded.
Statistical Methods for Pharmacokinetic Analyses
The statistical analyses for pharmacokinetic parameters were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). For summarizing the quantitative variables, mean (standard deviation [SD]) and median (minimum–maximum) values were reported. For qualitative variables, the number (%) data were reported. An analysis of variance (ANOVA) model was used for each cohort to assess dose proportionality by evaluating Cmax and AUC0-τ, dose independency by assessing t½,z, and the exposure ratios of Cmax and AUC0-τ of ensitrelvir, calculated as ratios of the parameters on day 5 to those on day 1 of multiple-dose administration. The effect of food on the pharmacokinetics of ensitrelvir was examined by ANOVA for comparing ln-transformed Cmax, Tmax, AUC0-last, AUC0-∞, t½,z, MRT, and Vz/F between the fasted and fed states. The point estimates and 90% confidence intervals (CIs) were generated for the difference of treatment for ln-transformed Cmax, Tmax, AUC0-last, AUC0-∞, t½,z, MRT, and Vz/F. The point estimates and corresponding 90% CIs were back-transformed to obtain the corresponding geometric least squares (GLS) mean ratios and the corresponding 90% CIs.
Results
Study Participants and Baseline Demographics
A total of 76 healthy adults participated in this study, of whom 22 healthy Japanese adult females participated in part 1 (11 in the Japanese female 375/125 mg cohort and 11 in the Japanese female 750/250 mg cohort, with 8 in the ensitrelvir group and 3 in the placebo group of each cohort [8:3]), 24 healthy White adults participated in part 2 (12 in the White 375/125 mg cohort and 12 in the White 750/250 mg cohort, with 8 in the ensitrelvir group and 4 in the placebo group of each cohort [8:4]), 16 healthy Japanese elderly participants participated in part 3 (16 in the elderly 375/125 mg cohort, with 11 in the ensitrelvir group and 5 in the placebo group [11:5]), and 14 healthy Japanese adults participated in part 4 (7 in each 375 mg single-dose group in the fasted and fed states). The demographic and baseline characteristics of these participants are summarized in Online Resource Table 1. The proportion of females was 100.0%, 50.0%, 0.0%, and 0.0%, in the healthy Japanese adult female cohort, healthy White adult cohort, healthy Japanese elderly cohort, and healthy Japanese adult cohort (fasted vs. fed), respectively. The median age across the Japanese female 375/125 mg, Japanese female 750/250 mg, White 375/125 mg, White 750/250 mg, Japanese elderly 375/125 mg, fasted 375 mg single-dose, and fed 375 mg single-dose cohorts were 25.5, 37.0, 33.0, 37.0, 73.0, 32.0 and 30.0 years, respectively. Correspondingly, the BMI values were 21.95, 21.60, 22.25, 24.15, 22.80, 21.10 and 22.50 kg/m2. Among all participants, a total of two participants discontinued the study; one participant in the Japanese female 375/125 mg cohort (part 1) withdrew from the study, and one participant in the fed 375/125 mg cohort (part 4) discontinued the study due to a TEAE of COVID-19.
Pharmacokinetics
Pharmacokinetics of Ensitrelvir in Healthy Japanese Adult Female Participants
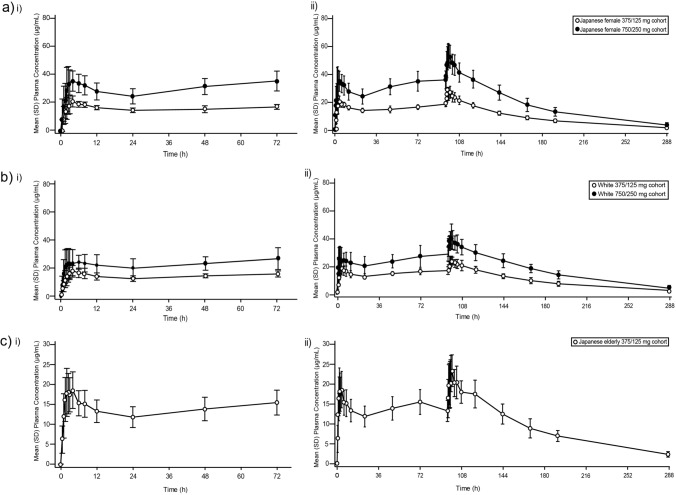
Mean plasma concentration profiles of ensitrelvir following multiple-dose administration of ensitrelvir in healthy Japanese adult female participants in the fasted state until 288 h after the initial administration of ensitrelvir in the Japanese female 375/125 mg and Japanese female 750/250 mg cohorts are shown in Fig. 2a. The corresponding pharmacokinetic parameters are shown in Table 1. The geometric means of Cmax were 22.3 μg/mL on day 1 and 28.1 μg/mL on day 5, and those of AUC0−τ were 372.9 μg·h/mL on day 1 and 518.3 μg·h/mL on day 5, in the Japanese female 375/125 mg cohort. The geometric means of Cmax were 39.9 μg/mL on day 1 and 55.8 μg/mL on day 5, and those of AUC0-τ were 644.4 μg·h/mL on day 1 and 1019.0 μg·h/mL on day 5 in the Japanese female 750/250 mg cohort. The Cmax and AUC0-τ on day 5 versus those on day 1 were 1.25- and 1.39-fold, respectively, in the Japanese female 375/125 mg cohort, while the corresponding values were 1.40- and 1.58-fold, respectively, in the Japanese female 750/250 mg cohort. The median Tmax ranged from 2.00 to 3.50 h between the dose groups on days 1 and 5. The Cmax and AUC0-τ increased in a dose-proportional manner between the dose groups on both day 1 and day 5. However, there was no clear relationship between the dose and t½,z of ensitrelvir in either cohort. The geometric means of C24 in the Japanese female 375/125 mg and 750/250 mg cohorts were 14.0 µg/mL on day 1 and 17.7 µg/mL on day 5, and 23.6 µg/mL on day 1 and 36.1 µg/mL on day 5, respectively.
Fig. 2.
Mean (SD) plasma concentration profile of ensitrelvir following administration of ensitrelvir (tablet formulation) until 288 h after the initial administration of ensitrelvir in (a) Part 1 healthy Japanese adult female participants (Japanese female 375/125 mg cohort and Japanese female 750/250 mg cohort); (b) Part 2 healthy White adult participants (White 375/125 mg cohort and White 750/250 mg cohort); and (c) Part 3 healthy Japanese elderly participants (Japanese elderly 375/125 mg cohort). SD standard deviation
Table 1.
Pharmacokinetic parameters for multiple-dose administration of ensitrelvir in the fasted state for healthy adult female Japanese participants (female 375/125 mg cohort and female 750/250 mg cohort), healthy adult White participants (White 375/125 mg cohort and White 750/250 mg cohort), and healthy elderly Japanese participants (elderly 375/125 mg cohort)a
| Parameters | Ensitrelvir | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Japanese female 375/125 mg | Japanese female 750/250 mg | White 375/125 mg | White 750/250 mg | Japanese elderly 375/125 mg | ||||||
| Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | |
| N | 8 | 7 | 8 | 8 | 8 | 8 | 8 | 8 | 11 | 11 |
| Cmax, μg/mL | 22.3 (14.8) | 28.1 (15.6) | 39.9 (18.3) | 55.8 (15.2) | 19.7 (21.1) | 24.8 (13.4) | 28.9 (21.5) | 42.1 (17.5) | 19.7 (24.0) | 23.8 (17.1) |
| Tmax, h | 2.50 (1.50, 8.00) | 2.00 (1.00, 8.00) | 3.50 (2.00, 8.00) | 2.25 (1.50, 4.00) | 3.25 (1.50, 6.00) | 2.75 (1.50, 12.00) | 2.25 (1.00, 12.00) | 2.75 (1.00, 4.00) | 2.00 (1.00, 4.00) | 3.00 (1.00, 4.00) |
| AUC0-τ, μg·h/mL | 372.9 (12.0) | 518.3 (13.0) | 644.4 (21.0) | 1019.0 (16.3) | 325.0 (15.7) | 475.5 (15.0) | 496.0 (21.3) | 798.3 (16.6) | 319.2 (22.7) | 446.1 (17.4) |
| t½,z, h | NC (NC) | 51.4 (19.0) | NC (NC) | 48.7 (18.6) | NC (NC) | 57.6 (26.2) | NC (NC) | 53.2 (25.6) | NC | 58.9 (16.9) |
| C24, μg/mL | 14.0 (11.3) | 17.7 (10.7) | 23.6 (23.1) | 36.1 (17.3) | 12.2 (16.8) | 16.9 (14.7) | 19.0 (30.1) | 28.9 (20.2) | 11.5 (23.4) | 17.1 (19.7) |
375/125 mg multiple once-daily doses with 375 mg as the loading dose on day 1 and 125 mg as the maintenance dose on days 2–5; 750/250 mg multiple once-daily doses with 750 mg as the loading dose on day 1 and 250 mg as the maintenance dose on days 2–5
AUC0−τ area under the concentration-time curve over dosing interval τ (i.e., 24 h), Cmax maximum plasma concentration, C24 plasma concentration at 24 h after dose, N number of participants, NC not calculated, t½,z, terminal elimination half-life; Tmax time to Cmax
aGeometric means (percentage coefficient of variation) are presented, except for Tmax for which median (minimum, maximum) are presented
Pharmacokinetics of Ensitrelvir in Healthy White Adult Participants
Mean plasma concentration profiles of ensitrelvir following multiple-dose administration of ensitrelvir in healthy White adult participants in the fasted state until 288 h after the initial administration of ensitrelvir in the White 375/125 mg and White 750/250 mg cohorts are shown in Fig. 2b. The corresponding pharmacokinetic parameters are listed in Table 1. The geometric means of Cmax were 19.7 μg/mL on day 1 and 24.8 μg/mL on day 5, and those of AUC0-τ were 325.0 μg·h/mL on day 1 and 475.5 μg·h/mL on day 5 in the White 375/125 mg cohort. The geometric means of Cmax were 28.9 μg/mL on day 1 and 42.1 μg/mL on day 5, and those of AUC0-τ were 496.0 μg·h/mL on day 1 and 798.3 μg·h/mL on day 5 in the White 750/250 mg cohort. The Cmax and AUC0−τ on day 5 versus those on day 1 were 1.26- and 1.46-fold, respectively, in the White 375/125 mg cohort, while the corresponding values were 1.45- and 1.61-fold, respectively, in the White 750/250 mg cohort. The median Tmax ranged from 2.25 to 3.25 h across the dose groups on days 1 and 5. The Cmax and AUC0-τ increased in a less than dose-proportional manner between the dose groups on both day 1 and day 5. Any relationship between the dose and t½,z of ensitrelvir in either cohort was not evident. The geometric means of C24 in the White 375/125 mg and 750/250 mg cohorts were 12.2 µg/mL on day 1 and 16.9 µg/mL on day 5, and 19.0 µg/mL on day 1 and 28.9 µg/mL on day 5, respectively.
Pharmacokinetics of Ensitrelvir in Healthy Japanese Elderly Participants
Mean plasma concentration profiles of ensitrelvir following multiple-dose administration of ensitrelvir in healthy Japanese elderly participants in the fasted state until 288 h after the initial administration of ensitrelvir in the Japanese elderly 375/125 mg cohort are shown in Fig. 2c. The pharmacokinetic parameters are listed in Table 1. The geometric means of Cmax were 19.7 μg/mL on day 1 and 23.8 μg/mL on day 5, and those of AUC0-τ were 319.2 μg·h/mL on day 1 and 446.1 μg·h/mL on day 5 in the elderly 375/125 mg cohort. The Cmax and AUC0-τ on day 5 versus those on day 1 were 1.21- and 1.40-fold, respectively, in the Japanese elderly 375/125 mg cohort. The median Tmax of ensitrelvir was 2 h on day 1 and 3 h on day 5. The geometric means of C24 in the Japanese elderly 375/125 mg cohort were 11.5 µg/mL on day 1 and 17.1 µg/mL on day 5.
Effect of Food on the Pharmacokinetics of Ensitrelvir in Healthy Japanese Adult Participants
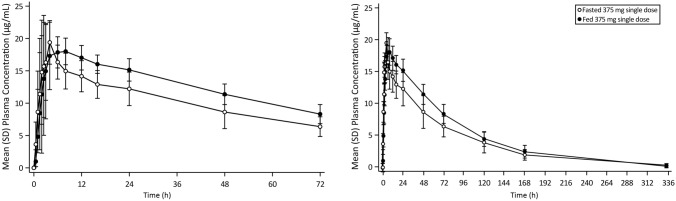
Mean plasma concentration profiles of ensitrelvir until 72 and 336 h after the initial administration of ensitrelvir following single-dose administration of ensitrelvir 375 mg in the fasted and fed states in healthy Japanese adult participants are shown in Fig. 3. The corresponding pharmacokinetic parameters are listed in Table 2. The ensitrelvir (fed/fasted) GLS mean ratios (90% CIs) of Cmax, AUC0-last, and AUC0-∞ were 0.9320 (0.8134–1.0679), 1.2435 (1.1400–1.3564), and 1.2447 (1.1396–1.3596), respectively. The 90% CIs for ensitrelvir Cmax was completely contained within the range of 0.8000–1.2500, suggesting that the Cmax of ensitrelvir was comparable between the fasted and fed states. The AUCs (both AUC0-last and AUC0-∞) of ensitrelvir increased by approximately 25% in the fed state compared with those in the fasted state, with the upper limits of 90% CIs, which were slightly higher than 1.2500, and the Tmax was delayed from 2.50 h in the fasted state to 6.00 h in the fed state. However, the Cmax value remained practically unchanged irrespective of food intake. Although the lower limit of the 90% CI of Vz/F was slightly lower than 0.8000, the GLS mean ratios and those 90% CIs of t½,z and MRT of ensitrelvir (fed/fasted) were completely contained within the range of 0.8000–1.2500.
Fig. 3.
Mean (SD) plasma concentration profiles of ensitrelvir until a 72 h and b 336 h postdose following single-dose administration of ensitrelvir 375 mg in the fasted and fed states for healthy Japanese adult participants. SD standard deviation
Table 2.
The effect of food on the pharmacokinetics of ensitrelvir following single-dose administration of ensitrelvir 375 mg for healthy Japanese adult participants in fasted and fed states
| Parameter | GLS mean with 375 mg single-dose administration | Fed/fasted GLS mean ratio (90% CI) |
|
|---|---|---|---|
| Fasted [n = 13] | Fed [n = 14] | ||
| Cmax , μg/mL | 21.5 | 20.0 | 0.9320 (0.8134–1.0679) |
| AUC0-last, μg·h/mL | 1222 | 1519 | 1.2435 (1.1400–1.3564) |
| AUC0-∞, μg·h/mL | 1235 | 1538 | 1.2447 (1.1396–1.3596) |
| Tmax, ha | 2.50 (1.50, 4.00) | 6.00 (1.50, 16.00) | NA |
| t½,z | 47.7 | 48.5 | 1.0170 (0.9313–1.1106) |
| MRT, h | 74.6 | 76.6 | 1.0267 (0.9704–1.0863) |
| Vz/F, L | 20.9 | 17.1 | 0.8154 (0.7543–0.8815) |
AUC0-∞ area under the concentration-time curve extrapolated to infinity, AUC0-last area under the concentration–time curve to the last measurable concentration, CI confidence intervals, Cmax maximum plasma concentration, GLS geometric least square, MRT mean residence time, NA not applicable, t½,z terminal elimination half-life, Tmax time to Cmax, Vz/F apparent volume of distribution in the terminal elimination phase
a Median (minimum, maximum).
Safety and Tolerability
The major TEAEs observed for ensitrelvir in different cohorts are shown in Table 3. No deaths or other serious TEAEs were reported during this study.
Table 3.
Summary of TEAEs following multiple-dose administration of ensitrelvir in the fasted state in healthy Japanese adult female participants (part 1)a; healthy White adult participants (part 2)a; and healthy Japanese elderly participants (part 3)b and following single-dose administration of ensitrelvir in healthy Japanese adult participants in the fasted and fed states (part 4)c
| System Organ Classd Preferred Term | Ensitrelvir | Placebo | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Japanese female 375/125 mg [n = 8] | Japanese female 750/250 mg [n = 8] | White 375/125 mg [n = 8] | White 750/250 mg [n = 8] | Japanese elderly 375/125 mg [n = 11] | Fasted 375 mg single dose [n = 13] | Fed 375 mg single dose [n = 14] | Japanese female [n = 6] | White [n = 8] | Japanese elderly [n = 5] | |||||||||||
| n (%) | Events | n (%) | Events | n (%) | Events | n (%) | Events | n (%) | Events | n (%) | Events | n (%) | Events | n (%) | Events | n (%) | Events | n (%) | Events | |
| Participants with any TEAE | 5 (62.5) | 11 | 8 (100.0) | 11 | 7 (87.5) | 8 | 7 (87.5) | 17 | 6 (54.5) | 15 | 5 (38.5) | 5 | 8 (57.1) | 11 | 3 (50.0) | 3 | 3 (37.5) | 5 | 2 (40.0) | 4 |
| Infections and infestations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| COVID-19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nervous system disorders | 1 (12.5) | 1 | 1 (12.5) | 1 | 0 | 0 | 3 (37.5) | 5 | 1 (9.1) | 3 | 1 (7.7) | 1 | 2 (14.3) | 2 | 1 (16.7) | 1 | 2 (25.0) | 3 | 0 | 0 |
| Headache | 1 (12.5) | 1 | 1 (12.5) | 1 | 0 | 0 | 3 (37.5) | 5 | 1 (9.1) | 3 | 1 (7.7) | 1 | 2 (14.3) | 2 | 0 | 0 | 2 (25.0) | 3 | 0 | 0 |
| Neuropathy peripheral | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 1 | 0 | 0 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oropharyngeal pain | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | 2 (25.0) | 5 | 2 (25.0) | 2 | 3 (37.5) | 3 | 1 (12.5) | 2 | 1 (9.1) | 5 | 0 | 0 | 1 (7.1) | 1 | 1 (16.7) | 1 | 1 (12.5) | 1 | 1 (20.0) | 1 |
| Diarrhea | 0 | 0 | 1 (12.5) | 1 | 2 (25.0) | 2 | 1 (12.5) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 2 (25.0) | 2 | 1 (12.5) | 1 | 0 | 0 | 0 | 0 | 1 (9.1) | 5 | 0 | 0 | 1 (7.1) | 1 | 1 (16.7) | 1 | 0 | 0 | 0 | 0 |
| Vomiting | 2 (25.0) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 1 (12.5) | 1 | 0 | 0 | 1 (12.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 | 1 (20.0) | 1 |
| Skin and subcutaneous tissue disorders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 1 | 0 | 0 | 0 | 0 |
| Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 1 | 0 | 0 | 0 | 0 |
| Reproductive system and breast disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dysmenorrhea | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| General disorders and administration site conditions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 | 0 | 0 |
| Pyrexia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 | 0 | 0 |
| Investigations | 5 (62.5) | 5 | 8 (100.0) | 8 | 5 (62.5) | 5 | 7 (87.5) | 8 | 6 (54.5) | 7 | 4 (30.8) | 4 | 7 (50.0) | 7 | 0 | 0 | 0 | 0 | 2 (40.0) | 3 |
| High-density lipoprotein decreased | 5 (62.5) | 5 | 8 (100.0) | 8 | 5 (62.5) | 5 | 7 (87.5) | 7 | 5 (45.5) | 5 | 4 (30.8) | 4 | 7 (50.0) | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood triglyceride increased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood creatine phosphokinase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C-reactive protein increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (40.0) | 2 |
| White blood cell count increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20.0) | 1 |
COVID-19 coronavirus disease 2019, Events number of events, MedDRA Medical Dictionary for Regulatory Activities, N number of participants, TEAEs treatment-emergent adverse events
a375/125 mg denotes multiple once-daily doses with 375 mg as the loading dose on day 1 and 125 mg as the maintenance dose on days 2–5; 750/250 mg denotes multiple once-daily doses with 750 mg as the loading dose on day 1 and 250 mg as the maintenance dose on days 2–5
b375/125 mg denotes multiple once-daily doses with 375 mg as the loading dose on day 1 and 125 mg as the maintenance dose on days 2–5
c375 mg single dose with three 125 mg tablets in the fasted or fed (high-fat/high-calorie) state
dSystem Organ Class and Preferred Terms of MedDRA version 24.0
TEAEs observed in part 1 were decreased high-density lipoprotein (5/8 in the female 375/125 mg cohort, 8/8 in the female 750/250 mg cohort), nausea and vomiting (2/8 in the female 375/125 mg cohort, 1/8 in the female 750/250 mg cohort), and headache and constipation (1/8 in the female 375/125 mg cohort, 1/8 in the female 750/250 mg cohort). All these TEAEs were considered related to the study intervention. One participant discontinued the part 1 study due to withdrawal by the participant.
TEAEs observed in part 2 were decreased high-density lipoprotein (5/8 in the White 375/125 mg cohort, 7/8 in the White 750/250 mg cohort), diarrhea (2/8 in the White 375/125 mg cohort, 1/8 in the White 750/250 mg cohort), constipation (1/8 in the White 375/125 mg cohort), headache (3/8 in the White 750/250 mg cohort), and increased oropharyngeal pain, dysmenorrhea, and blood triglycerides (1/8 in the White 750/250 mg cohort). Of these, all 12 events of decreased high-density lipoprotein, three events of headache (which occurred in the same participant in the White 750/250 mg cohort) and one event of increased blood triglycerides (in the White 750/250 mg cohort) were considered related to ensitrelvir.
TEAEs observed in part 3 were decreased high-density lipoprotein (5/11 in the elderly 375/125 mg cohort), increased nausea, headache, and blood creatine phosphokinase, and increased C-reactive protein (1/11 in the elderly 375/125 mg cohort). Except for increased blood creatine phosphokinase, all TEAEs were considered related to ensitrelvir.
TEAEs observed in part 4 were decreased high-density lipoprotein (4/13 in the fasted 375 mg cohort, 7/14 in the fed 375 mg cohort), headache (1/13 in the fasted 375 mg cohort, 2/14 in the fed 375 mg cohort), and COVID-19 and nausea (1/14 in the fed 375 mg cohort). Except for the COVID-19 reported in the fed 375 mg cohort, all TEAEs were considered related to ensitrelvir. In the fed 375/125 mg cohort, one participant discontinued the study due to a TEAE of COVID-19.
Discussion
The pharmacokinetic profile of the drugs may vary depending on ethnic group, sex, age as well as food intake and dietary factors [12, 14, 17]. This study describes the results of the safety, tolerability, and pharmacokinetics of the ensitrelvir tablet formulation at an approved dose of 375/125 mg, and 750/250 mg in extended populations. In addition, we evaluated the effect of food intake on the pharmacokinetics of ensitrelvir 375 mg using the formulation for clinical use.
In this study, generally similar exposure levels of ensitrelvir were observed on days 1 and 5 within each dosing regimen when an ensitrelvir tablet formulation was administered once daily for 5 days, suggesting limited accumulation of ensitrelvir with multiple-dose administration. The results from the Japanese female, White participants, and elderly cohorts in our study were similar to the previously reported results from the Japanese and White healthy adult male participant cohorts who had received multiple-dose administrations of ensitrelvir 375/125 mg and 750/250 mg [6, 10]. Furthermore, a comparison between the 375/125 mg and 750/250 mg dosing regimens across Japanese female participants in the present study demonstrated a dose-proportional change in the Cmax and AUC0−τ of ensitrelvir, although the Cmax and AUC0-τ of ensitrelvir increased in a less than dose-proportional manner in White healthy participants. No clear relationship between the dose and t½,z of ensitrelvir was observed in both parts 1 and 2. Comprehensively considering these results and previous results of dose proportionality in Japanese and White cohorts of healthy adult male participants by suspension formulation [6], the exposure levels of ensitrelvir increased in a dose-proportional manner with multiple-dose administration. Moreover, compared with Japanese female participants, plasma exposure levels were slightly lower for White and elderly Japanese participants. Body weight in Japanese female participants was lighter than those in other populations, which may have resulted in these variations (Online Resource Table 1). Such differences in the pharmacokinetics of drugs may stem from the differences in body weight, one of the major covariates for pharmacokinetics of many drugs [17]. Moreover, the plasma concentration of ensitrelvir obtained during the treatment and at least 24 h after the 5-day administration of ensitrelvir 375/125 mg, the approved dosing regimen in Japan, exceeded 6.09 μg/mL, the estimated target plasma concentration from a nonclinical study [24], in all populations. This result implies that ensitrelvir at the approved dosing regimen provides therapeutic potential across different patient populations. Since there were a number of differences between populations for each background, the statistical analyses were not conducted for comparing the exposure levels in this study. However, a population pharmacokinetic analysis will be planned to find the covariates for the pharmacokinetic profiles on ensitrelvir.
Food intake delayed the Tmax of ensitrelvir in the fed state compared with the fasted state, leading to a relatively smaller increase in AUC, but this did not affect the corresponding Cmax values, indicating no clinically meaningful difference in the exposure of ensitrelvir between the fasted and fed states. Thus, the pharmacokinetics of ensitrelvir were not substantially influenced by food intake. This trend is similar to the effect of food on the ensitrelvir suspension formulation [6], and we speculate that food delays the absorption rate for ensitrelvir.
Overall, the present study assessing the pharmacokinetics of ensitrelvir in tablet formulation in the extended population of healthy Japanese adult females and healthy Japanese elderly participants corroborated the findings of earlier studies. Our results demonstrated no major differences in the pharmacokinetic profile as compared with the previous reports [6, 10] assessing the pharmacokinetics of the ensitrelvir suspension formulation in healthy Japanese male participants.
In general, differences in age, sex, and race would not substantially affect the pharmacokinetics of ensitrelvir, which suggests no need for dose adjustments for age, sex, and race or food restriction while administering ensitrelvir in the oral tablet formulation.
The safety results show that ensitrelvir was well tolerated without any newer safety signals. The observed TEAEs that were more frequent in the ensitrelvir groups than in the placebo groups included increased blood triglycerides, decreased high-density lipoprotein, headache, and nausea and vomiting. Most of the above TEAEs were mild in nature and resolved without any specific treatments.
Conclusions
This study shows the safety and pharmacokinetics of the approved ensitrelvir 375/125 mg tablet formulation in various populations, which demonstrated promising results. The pharmacokinetics of ensitrelvir between all populations in the study were similar, and C24 exceeded the target plasma concentration (6.09 μg/mL) with the ensitrelvir 375/125 mg dosing regimen. The clinical dose of ensitrelvir was well tolerated with no additional safety signals. The findings can be useful as a clinical recommendation for prescribing ensitrelvir with regard to dose adjustment for age, sex, and race, and are considered relevant to other ethnic populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank all the study participants and their families, the investigators, and the study teams. Medical writing support was provided by MedPro Clinical Research. The authors also thank Padma Singu, M.Pharm, PhD; Vidula Bhole, MD, MHSc; and Ivan D’Souza, MS, of MedPro Clinical Research, for providing medical writing support for this manuscript. The plasma concentrations of ensitrelvir were determined at Sumika Chemical Analysis Service, Ltd.
Declarations
Funding
This study was funded by Shionogi & Co., Ltd. The funder of the study was involved in the study design, data collection, data analysis, and data interpretation. Medical writing support by MedPro Clinical Research was funded by Shionogi & Co., Ltd.
Disclosures
R.S., T.S., T.F., A.K., and R.K. are employees of Shionogi & Co., Ltd. and Y.M. was an employee of Shionogi & Co., Ltd. at the time of this research.
Ethics approval
This study was approved by the Medical Corporation Shinanokai Shinanozaka Clinic Institutional Review Board (Review ID: 757P I; approval date: 14 July 2021) and was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation (ICH) guidelines for Good Clinical Practice (GCP).
Consent to participate
All participants gave their written informed consent for participation in this study.
Consent to publish
Not applicable.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
Conceptualization: RS, TS, TF, AK, YM. Methodology: RS, YM, RK. Formal analysis: RS. Investigation: TS. Writing—original draft preparation: RS. Writing—review and editing: RS, TS, TF, AK, YM, RK. All authors have read and agreed to the final version of this manuscript.
References
- 1.World Health Organization. WHO Coronavirus Dashboard. 2022. https://covid19.who.int/. Accessed 28 Feb 2023.
- 2.Unoh Y, Uehara S, Nakahara K, Nobori H, Yamatsu Y, Yamamoto S, et al. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J Med Chem. 2022;65:6499–6512. doi: 10.1021/acs.jmedchem.2c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xocova® (Ensitrelvir Fumaric Acid) tablets 125 mg approved in Japan for the treatment of SARS-CoV-2 infection, under the emergency regulatory approval system [press release, 22 Nov 2022. https://www.shionogi.com/global/en/news/2022/11/e20221122.html. Accessed 17 Dec 2022.
- 4.Uraki R, Kiso M, Iida S, Imai M, Takashita E, Kuroda M, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature. 2022;607(7917):119–127. doi: 10.1038/s41586-022-04856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawashima S, Matsui Y, Adachi T, Morikawa Y, Inoue K, Takebayashi S, et al. Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally. Biochem Biophys Res Commun. 2023;645:132–136. doi: 10.1016/j.bbrc.2023.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu R, Sonoyama T, Fukuhara T, Kuwata A, Matsuo Y, Kubota R. Safety, tolerability, and pharmacokinetics of the novel antiviral agent ensitrelvir fumaric acid, a SARS-CoV-2 3CL protease inhibitor, in healthy adults. Antimicrob Agents Chemother. 2022;66(10):e00632–e722. doi: 10.1128/aac.00632-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukae H, Yotsuyanagi H, Ohmagari N, Doi Y, Imamura T, Sonoyama T, et al. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part. Antimicrob Agents Chemother. 2022;66(10):e00697–e722. doi: 10.1128/aac.00697-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukae H, Yotsuyanagi H, Ohmagari N, Doi Y, Sakaguchi H, Sonoyama T, et al. Efficacy and safety of ensitrelvir in patients with mild-to-moderate Coronoavirus Disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2023;76(8):1403–1411. doi: 10.1093/cid/ciac933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yotsuyanagi H, Ohmagari N, Doi Y, Imamura T, Sonoyama T, Ichihashi G, et al. A phase 2/3 study of S-217622 in participants with SARS-CoV-2 infection (Phase 3 part) Medicine (Baltimore) 2023;102(8):e33024. doi: 10.1097/MD.0000000000033024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu R, Sonoyama T, Fukuhara T, Kuwata A, Matsuzaki T, Matsuo Y, et al. Evaluation of the drug-drug interaction potential of ensitrelvir fumaric acid with cytochrome P450 3A substrates in healthy Japanese adults. Clin Drug Investig. 2023;43(5):335–346. doi: 10.1007/s40261-023-01265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornsson TD, Wagner JA, Donahue SR, Harper D, Karim A, Khouri MS, et al. A review and assessment of potential sources of ethnic differences in drug responsiveness. J Clin Pharmacol. 2003;43(9):943–967. doi: 10.1177/0091270003256065. [DOI] [PubMed] [Google Scholar]
- 12.Gill KL, Machavaram KK, Rose RH, Chetty M. Potential sources of inter-subject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet. 2016;55(7):789–805. doi: 10.1007/s40262-015-0361-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen ML. Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2006;45(10):957–964. doi: 10.2165/00003088-200645100-00001. [DOI] [PubMed] [Google Scholar]
- 14.Arnold FL, Kusama M, Ono S. Exploring differences in drug doses between Japan and Western countries. Clin Pharmacol Ther. 2010;87(6):714–720. doi: 10.1038/clpt.2010.31. [DOI] [PubMed] [Google Scholar]
- 15.International Conference on Harmonization: Guidance on ethnic factors in the acceptability of foreign clinical data. Federal Register 1998;63(111). https://www.govinfo.gov/content/pkg/FR-1998-06-10/pdf/98-15408.pdf. Accessed 13 Apr 2023. [PubMed]
- 16.Flores-Perez C, Flores-Perez J, Moreno-Rocha LA, Chávez-Pacheco JL, Noguez-Mendez NA, Ramirez-Mendiola B, et al. Influence of age and sex on the pharmacokinetics of midazolam and the depth of sedation in pediatric patients undergoing minor surgeries. Pharmaceutics. 2023;15(2):440. doi: 10.3390/pharmaceutics15020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz JB. The influence of sex on pharmacokinetics [published erratum appears in Clin Pharmacokinet. 2004;43(11):732] Clin Pharmacokinet. 2003;42(2):107–121. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- 18.Hutchison LC, O’Brien CE. Changes in pharmacokinetics and pharmacodynamics in the elderly patient. J Pharm Pract. 2007;20(1):4–12. doi: 10.1177/0897190007304657. [DOI] [Google Scholar]
- 19.Shi S, Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab. 2011;12(7):601–610. doi: 10.2174/138920011796504527. [DOI] [PubMed] [Google Scholar]
- 20.Angel JB, Hussey EK, Hall ST, Donn KH, Morris DM, McCormack JP, et al. Pharmacokinetics of 3TC (GR109714X) administered with and without food to HIV-infected patients. Drug Invest. 1993;6:70–74. doi: 10.1007/BF03258455. [DOI] [Google Scholar]
- 21.WMA Declaration of Helsinki. Ethical principles for medical research involving human subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 5 Mar 2023.
- 22.Council for International Organizations of Medical Sciences. International Ethical Guidelines for Health-related Research Involving Humans. https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf. Accessed 5 Mar 2023.
- 23.International Council for Harmonisation. Efficacy guidelines. https://www.ich.org/page/efficacy-guidelines. Accessed 5 Mar 2023.
- 24.Fukao K, Nobori H, Kuroda T, Baba K, Matsumoto K, Tanaka Y, et al. Pharmacokinetic and pharmacodynamic analysis of the 3CL protease inhibitor ensitrelvir in a SARS-CoV-2 infection mouse model. Viruses. 2023;15 (in press). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.