Abstract
Background
Invasive fungal infections are associated with significant morbidity and mortality in children. Optimal treatment strategies are yet to be defined.
Objectives
This review aims to systematically identify and summarise the effects of different antifungal therapies in children with proven, probable or suspected invasive fungal infections.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, Issue 3), MEDLINE (1966 to September 2008), EMBASE (1980 to September 2008) and CINAHL (1988 to September 2008) without language restrictions. We also handsearched reference lists and abstracts of conference proceedings and scientific meetings, and contacted authors of included studies and pharmaceutical manufacturers.
Selection criteria
We included randomised clinical trials (RCTs) comparing a systemic antifungal agent with a comparator (including placebo) in children (one month to 16 years) with proven, probable or suspected invasive fungal infection.
Data collection and analysis
Two review authors independently applied selection criteria, performed quality assessment, and extracted data using an intention‐to‐treat approach. We synthesised data using the random‐effects model and expressed results as relative risks (RR) with 95% confidence intervals (CIs).
Main results
We included seven trials of antifungal agents in children with prolonged fever and neutropenia (suspected fungal infection) and candidaemia or invasive candidiasis (proven fungal infection). Four trials compared a lipid preparation of amphotericin B with conventional amphotericin B (395 participants), one trial compared an echinocandin with a lipid preparation of amphotericin B (82 participants) in suspected infection; one trial compared an echinocandin with a lipid preparation of amphotericin B in children with candidaemia or invasive candidiasis (109 participants) and one trial compared different azole antifungals in children with candidaemia (43 participants). No difference in all‐cause mortality and other primary endpoints (mortality related to fungal infection or complete resolution of fungal infections) were observed. No difference in breakthrough fungal infection was observed in children with prolonged fever and neutropenia.
When lipid preparations and conventional amphotericin B were compared in children with prolonged fever and neutropenia, nephrotoxicity was less frequently observed with a lipid preparation (RR 0.43, 95% CI 0.21 to 0.90, P = 0.02) however substantial heterogeneity was observed (I2 = 59%, P = 0.06). Children receiving liposomal amphotericin B were less likely to develop infusion‐related reactions compared with conventional amphotericin B (chills: RR 0.37, 95% CI 0.21 to 0.64, P = 0.0005). Children receiving a colloidal dispersion were more likely to develop such reactions than with liposomal amphotericin B (chills: RR 1.76, 95% CI 1.09 to 2.85, P = 0.02). The rate of other clinically significant adverse reactions attributed to the antifungal agent (total reactions; total reactions leading to treatment discontinuation, dose reduction or change in therapy; hypokalaemia and hepatotoxicity) were not significantly different. When echinocandins and lipid preparations were compared, the rate of clinically significant adverse reactions (total reactions; total reactions leading to treatment discontinuation, dose reduction or change in therapy) were not significantly different.
Authors' conclusions
Limited paediatric data are available comparing antifungal agents in children with proven, probable or suspected invasive fungal infection. No differences in mortality or treatment efficacy were observed when antifungal agents were compared. Children are less likely to develop nephrotoxicity with a lipid preparation of amphotericin B compared with conventional amphotericin B. Further comparative paediatric antifungal drug trials and epidemiological and pharmacological studies are required highlighting the differences between neonates, children and adults with invasive fungal infections.
Plain language summary
Antifungal agents for infants and children with invasive fungal infections
Invasive fungal infections are a significant problem for children whose immune system is not functioning properly. The majority of the children have cancer. Antifungal medications can be given when these children develop a fever (for example a fever occurring when the white cells or neutrophils are low during chemotherapy) or when an infection has been formally identified (as in candidaemia, candidiasis and invasive aspergillosis). The antifungal agents that were compared appear equally efficacious. Pooling the data from the few studies that were available suggest kidney damage was less likely with a lipid preparation of amphotericin B compared with conventional amphotericin B. It is reasonable to recommend a lipid preparation of amphotericin B, if cost permits. No significant differences have been observed in children when other antifungal agents have been compared. More studies in children evaluating available antifungal are required to further clarify any benefits with regard to the risk of dying, prospects of complete recovery and drug toxicities.
Background
Invasive fungal infections (IFI) cause significant morbidity and mortality. The incidence of IFI is increasing (Groll 1996; Marr 2002; Martin 2003). Most children who develop fungal infections have received chemotherapy for a malignancy or have undergone a haematopoietic stem cell or solid organ transplant. The incidence of IFI in these groups is between 5% and 25% (Groll 1999; Rosen 2005). A minority of children developing IFI have a congenital or acquired immunodeficiency such as chronic granulomatous disease or HIV/AIDS (Dvorak 2005; Steinbach 2005a). Mortality is high in those who develop IFI and is influenced by the type of fungus, site of disease and presence of ongoing immunosuppression (Dvorak 2005; Lin 2001).
Definitive diagnosis by tissue microscopy and culture is difficult in the early stages of infection. Using host factor and microbiological and clinical criteria, current definitions exist for proven, probable and possible invasive fungal infection (Ascioglu 2002; de Pauw 2008). In practice, IFI is often suspected in those with persistent fever and neutropenia who fail to respond to broad‐spectrum antibacterial agents, within three to five days, and in whom a reasonable attempt has been made to exclude bacterial and viral infections. As mortality is high, early and aggressive treatment of suspected infections with empiric antifungal therapy is common (Hughes 2002). Empirical antifungal therapy is associated with a lower incidence of IFI yet no demonstrable survival benefit (Goldberg 2008). As many of the patients with persistent fever and neutropenia who receive empiric therapy do not have a fungal infection, there is increasing interest in biological markers and radiological imaging to identify those at highest risk of IFI (Cordonnier 2009; Maertens 2005). There are currently insufficient data to support this approach in children.
Conventional amphotericin B deoxycholate is a broad spectrum antifungal agent that has previously been the treatment of choice for most IFI. Its use is complicated by nephrotoxicity and infusion‐related reactions (Gallis 1990). More recently, lipid formulations of amphotericin B have become available and have less toxicity. Triazole derivatives (fluconazole, itraconazole, voriconazole, posaconazole and ravuconazole) and echinocandins (caspofungin, micafungin and anidulafungin) have increased the number of available agents. The role of biological agents (for example antiHSP90 monoclonal antibodies, Mycograb®) is being explored (Pachl 2006). The increasing use of combination antifungal therapy adds further complexity to decision making about treatment (Marr 2004; Mukherjee 2005).
Numerous meta‐analyses and randomised controlled trials (RCTs) have compared different antifungal agents in immunocompromised adults, as empirical treatment in patients with persistent fever and neutropenia (Johansen 2000; Johansen 2002; Prentice 1997; Walsh 1999; Walsh 2002; Walsh 2004a; Winston 2000), treatment of invasive aspergillosis (Cornely 2007; Herbrecht 2002) and invasive candidal infections (Kuse 2007; Mora‐Duarte 2002; Reboli 2007; Rex 1994). Many differences, particularly with respect to antifungal toxicity and cost, have been demonstrated. As significant variation exists between adult and paediatric antifungal pharmacokinetics (Benson 1989; Brammer 1994; Martin 2003; Walsh 2004b; Walsh 2005) cautious interpretation of the efficacy, toxicity and economic endpoints from these trials is required when managing children with IFI. It remains unclear which is the most appropriate antifungal agent to treat children with proven, probable or suspected IFI.
Objectives
To assess different antifungal agents or combinations of agents in children with proven, probable or suspected invasive fungal infections (including empiric antifungal therapy in children with persistent fever and neutropenia) with respect to comparative:
effectiveness;
toxicity;
cost.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised and quasi‐randomised (using a method of allocating participants to a treatment that is not strictly random, for example by date of birth or hospital record number) clinical trials.
Types of participants
Infants and children (age older than 28 days and up to 16 years) with proven, probable or suspected invasive fungal infection were included. Neonates were excluded given the numerous epidemiological differences (Blyth 2009) and the publication of a previous meta‐analysis assessing systemic antifungal drugs for invasive fungal infection in this population (Clerihew 2004). Proven or probable invasive fungal infection was defined as a clinical illness consistent with infection plus either radiological, histopathological or microbiological evidence of invasive fungal disease (Ascioglu 2002; de Pauw 2008). Suspected invasive fungal infection was defined pragmatically as an individual clinician's choice to prescribe a systemic antifungal agent based on the clinical suspicion of invasive fungal infection in the absence of a confirmed diagnosis. This definition was inclusive of empiric, systemic antifungal therapy in patients with persistent fever and neutropenia despite appropriate antibacterial agents.
Where insufficient information was available to classify infections, we contacted the study authors.
Types of interventions
Trials including any of the following agents were reviewed: conventional amphotericin B deoxycholate; lipid preparations of amphotericin (for example liposomal amphotericin B, amphotericin B colloidal dispersion (ABCD), amphotericin B lipid complex (ABLC)); 5‐fluorocytosine; azoles (for example fluconazole, itraconazole, voriconazole, posaconazole and ravuconazole); echinocandins (for example caspofungin, micafungin and anidulafungin) or monoclonal antibodies (for example antiHSP90 monoclonal antibody (Mycograb®). We considered any dose designed to produce a therapeutic effect. We accepted trials that compared different systemic antifungal agents or combination of agents, no treatment or an inactive placebo.
We specifically excluded trials assessing the use of antifungal therapy to prevent invasive fungal infection (that is antifungal prophylaxis).
Types of outcome measures
Studies were eligible for inclusion if they reported any of the following outcome measures.
Primary outcome
1. All‐cause mortality (defined as the death of any randomised patient regardless of cause). 2. Invasive fungal infection‐related mortality (defined as death of a patient diagnosed with invasive fungal infection (IFI) whose death is attributable to IFI). 3. Complete resolution of invasive fungal infection (defined as complete resolution of clinical symptoms, with or without radiological or microbiological findings that led to diagnosis of IFI, enabling cessation of anti‐fungal medication).
Secondary outcomes
4. Partial resolution of invasive fungal infection (defined as any improvement in clinical symptoms with or without radiological findings that led to diagnosis of IFI yet not fulfilling criteria for complete resolution).
5. Resolution of fever in suspected fungal infection (defined as absence of temperature > 38 °C for more than 72 hours or another closely‐related definition of resolution of fever).
6. Progression of disease requiring change or addition of an antifungal agent (defined as progression of existing symptoms with or without radiological or microbiological findings that are of enough concern for a clinician to change the antifungal dose or add a new antifungal agent).
7. Breakthrough fungal infection requiring change or addition of an antifungal agent (defined as new clinical symptoms or signs with or without radiological or microbiological findings that are of enough concern for clinicians to change antifungal dose or add a new antifungal agent).
8. Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in the therapy.
9. Any adverse reactions attributed to the antifungal agent including:
a. abnormal renal function (a number of definitions were accepted including a raised creatinine, a reduced glomerular filtration rate or creatinine clearance as compared with normal values or baseline values);
b. electrolyte imbalance such as hypokalaemia;
c. abnormal hepatic function (a number of definitions were accepted including raised transaminases or bilirubin greater than normal or baseline values for age);
d. infusion‐related reactions such as chills, rigors or anaphylaxis;
e. gastrointestinal disturbances such as nausea, vomiting or diarrhoea;
f. neurological disturbances such as blurred vision or dizziness;
g. haematological disturbances such as anaemia, granulocytopenia or thrombocytopenia.
10. Length of stay (days). 11. Quality of life (QOL). 12. Cost.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, Issue 3), MEDLINE (1966 to September 2008), EMBASE (1980 to September 2008) and CINAHL (1988 to September 2008).
A sensitive subject search strategy was combined with the optimum trial search strategy for use in MEDLINE (Appendix 1), CENTRAL (Appendix 2), EMBASE (Appendix 3) and CINAHL (Appendix 4). All languages were considered. The search strategies were devised and executed by the author team.
Searching other resources
We considered letters, abstracts and unpublished trials in an attempt to minimise the impact of publication bias. In addition, we made a systematic search for relevant controlled trials in this area by:
searching citation lists of articles identified in primary searches;
contacting experts in the field and leading authors (as identified by personal communication and searching both the articles found and the Internet) to ask for additional relevant data in terms of published or unpublished RCTs;
handsearching relevant textbooks (Feigin RD, Cherry J, Demmler GJ, Kaplan S. Textbook of Pediatric Infectious Diseases, 5th edition; Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases, 6th edition);
handsearching conference proceedings, where available: Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC) (1995 to 2008); General Meeting of the America Society of Microbiology (ASM) (1999 to 2008); Annual Meeting of the Infectious Diseases Society of America (IDSA) (1995 to1997, 2001 to 2008); European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (2001 to 2008); General Meeting of the American Society of Hematology (ASH) (1990 to 2007); European Society of Paediatric Infectious Diseases Conference (ESPID) (2001 to 2008).
contacting relevant drug manufacturers: Astellas/Gilead (contacted 15th December 2008; reply received 16th April 2009); Merck Sharp & Dohme (contacted 15th December 2008, reply received 7th January 2009); Pfizer (contacted 20th January 2009, reply received 22nd January 2009); and Schering‐Plough (contacted 15th December 2008, reply received 5th May 2009).
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic and hand searching were downloaded to a reference management database and duplicates removed. Two review authors (CCB, KH) were responsible for handsearching, examination of electronic search results and identification of potentially eligible studies. Full reports of these studies were retrieved and reviewed independently for inclusion by the same two review authors. Any differences were resolved by discussion with and referral to two further review authors (PP, TO). We contacted authors for clarification of trial methods and to request individual patient data when required. The reasons for exclusion of studies that were reviewed as full texts were recorded.
Data extraction and management
Data were independently extracted from included trials by two review authors (CCB, KH). A standardised data extraction sheet was used (Appendix 5; Appendix 6) that covered all outcomes specified above.
Paediatric data from trials inclusive of children and adults were extracted, when available. Where paediatric data were not extractable, authors were contacted for paediatric subgroup analysis. If paediatric analyses were not available, data were excluded from our analysis.
Actual numbers were extracted, where possible. When required, we calculated the actual numbers of patients with specified outcomes from percentages given in the report of a trial.
Assessment of risk of bias in included studies
Two review authors (CCB, KH) graded the selected studies independently. Every study was assessed following the criterion grading system described in the Cochrane Handbook for Systematic Reviews of Interventions version 5.0.0 (updated September 2009) (Higgins 2009). The results were summarised in the risk of bias tables for each included study. The grading was compared and any inconsistencies between the review authors in the interpretation of inclusion criteria and their significance to the selected studies were discussed and resolved. The methodological quality of included studies was assessed using the following six questions.
(1) Was the allocation sequence adequately generated?
Each study was graded as yes (that is allocation sequence was adequately generated with a subsequent low risk of bias), no (that is allocation sequence was not adequately generated with a subsequent high risk of bias) or unclear. Adequate allocation sequence generation included: random number tables, computer random number generators, coin tossing, shuffling cards or envelopes, throwing dice or drawing of lots.
(2) Was allocation adequately concealed?
Each study was graded as yes (that is allocation sequence was adequately concealed with a subsequent low risk of bias), no (that is allocation sequence was not adequately concealed with a subsequent high risk of bias) or unclear. Adequate allocation concealment included: central allocation methods (for example telephone, web‐based and pharmacy‐controlled randomisation), sequential numbered drug containers of identical appearance or sequentially numbered, opaque or sealed envelopes.
(3) Was knowledge of the allocation interventions adequately prevented during the study?
Each study was graded as yes (that is knowledge of the allocation interventions was adequately prevented with a subsequent low risk of bias), no (that is knowledge of the allocation interventions was not adequately prevented with a subsequent high risk of bias) or unclear. Knowledge of the allocation interventions was adequately prevented when blinding of participants and outcome assessors were maintained for objective outcomes, and both of these groups plus the healthcare workers involved were blinded for subjective outcomes.
(4) Were incomplete outcome data adequately addressed?
Each study was graded as yes (that is incomplete outcome data were adequately address with a subsequent low risk of bias), no (that is incomplete outcome data were not adequately address with a subsequent high risk of bias) or unclear. Incomplete outcome data were adequate addressed if: outcome data were not missing, the reasons for missing outcome data were unlikely to be related to true outcomes, missing outcome data were balanced in numbers across the intervention groups with similar reasons for missing data across groups, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate (dichotomous outcome data), the plausible effect size among missing outcomes was not enough to have a clinically relevant impact on the observed effect sizes (continuous outcome data) or missing data were imputed using appropriate methods.
(5) Were reports of the study free of suggestion of selective outcome reporting?
Each study was graded as yes (that is the reports of the study were free of suggestion of selective outcome reporting with a subsequent low risk of bias), no (that is the reports of the study were not free of suggestion of selective outcome reporting with a subsequent high risk of bias) or unclear. The reports of the study were free of suggestion of selective outcome reporting if: the study protocol was available and all of the study's pre‐specified outcomes that were of interest in the review had been reported in the pre‐specified way; or if the study protocol was not available, it was clear that the published reports included all expected outcomes including those that were pre‐specified.
(6) Was the study apparently free of other problems that could put it at a risk of bias?
We recorded any such problems. Specifically we looked for potential sources of bias related to the study design, early trial cessation due to some data‐dependent process (for example formal‐stopping rule), extreme baseline imbalance between comparator groups and financial considerations (for example withdrawal of participants because they could not pay for drugs). Each study was graded as yes (that is the study was apparently free of other problems that could put it at a risk of bias with a subsequent low risk of bias), no (that is the study was not apparently free of other problems that could put it at a risk of bias with a subsequent high risk of bias) or unclear.
Data synthesis
All analyses were performed using the RevMan 5.0 software. As an estimate of the statistical significance of a difference in proportional outcomes between experimental and control interventions, we calculated the relative risk (RR) for benefit using antifungal therapy with 95% confidence intervals (CI). We assumed a statistically significant difference between the experimental intervention and control intervention if the 95% CI of the RR did not include the value 1.0. As an estimate of the clinical relevance of any difference between experimental and control interventions, we calculated the number needed to treat to benefit (NNTB) and number needed to treat to harm (NNTH) with 95% CI, as appropriate. For continuous variables, we converted data to the mean difference (MD) using the inverse variance method and calculated an overall MD. We used a fixed‐effect model where there was no evidence of significant heterogeneity between studies (see below), and employed a random‐effects model when such heterogeneity was likely.
We pooled data for individual agents as applied to specific fungal infections or described clinical syndromes (that is candidaemia or invasive candidiasis, prolonged fever and neutropenia). We considered the appropriateness of groups of both agents and fungal types for pooled analysis based on the clinical interpretation of the comparisons made. Below are details of each outcome considered, with any specific variation to this approach.
Primary outcomes
1. All‐cause mortality (defined as death of any randomised patient regardless of cause): we intended to extract the hazard ratio (HR) and variance from trial reports, however only absolute numbers of case fatalities were reported in the included studies. We therefore compared the case fatality rates at specific times (end of therapy, 12 weeks and six months) to estimate the RR between groups.
2. IFI‐related mortality (defined as death of a patient diagnosed with IFI whose death was attributable to IFI). IFI‐related mortality was analysed in the same way as for all‐cause mortality.
3. Complete resolution of invasive fungal infection (defined as complete resolution of clinical symptoms, with or without radiological or microbiological findings that led to diagnosis of IFI, enabling cessation of antifungal medication). We dichotomised participants into complete resolution and not resolved.
Secondary outcomes
4. Partial resolution of invasive fungal infection (defined as any improvement in clinical symptoms with or without radiological findings that led to diagnosis of IFI yet not fulfilling criteria for complete resolution).
5. Resolution of fever in suspected fungal infection (defined as absence of temperature > 38 °C for more than 72 hours or another closely‐related definition of resolution of fever).
6. Progression of disease requiring change or addition of an antifungal agent (defined as progression of existing symptoms, with or without radiological or microbiological findings, that were of enough concern for a clinician to change antifungal dose or add a new antifungal agent).
7. Breakthrough fungal infection requiring change or addition of an antifungal agent (defined as new clinical symptoms or signs, with or without radiological or microbiological findings, that were of enough concern for clinicians to change antifungal dose or add a new antifungal agent).
8. Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in the therapy.
9. Any adverse reactions attributed to the antifungal agent including: a. abnormal renal function (a number of definitions were accepted including a raised creatinine, a reduced glomerular filtration rate or creatinine clearance as compared with normal or baseline values); b. electrolyte imbalance such as hypokalaemia; c. abnormal hepatic function (a number of definitions were accepted including raised transaminases or bilirubin greater than normal or baseline values for age); d. infusion‐related reactions such as chills, rigors or anaphylaxis; e. gastrointestinal disturbances such as nausea, vomiting or diarrhoea; f. neurological disturbances such as blurred vision or dizziness; g. haematological disturbances such as anaemia, granulocytopenia or thrombocytopenia.
10. Length of stay (days). We intended to compare mean length of stay between the groups.
11. Quality of life (QOL). We intended to compare mean QOL scores between the groups.
12. Cost. If available, we intended to discuss the results and implications of any cost analyses reported in any of the included trials.
Subgroup analysis and investigation of heterogeneity
We examined the data for statistical heterogeneity using the I2 statistic. We considered an I2 greater than 30% as an indication that an important proportion of the variability between studies could be due to heterogeneity between studies. In that case, or when we suspected significant clinical heterogeneity, we considered the appropriateness of meta‐analysis and employed a random‐effects model. In the absence of any such indication, we used a fixed‐effect model. In the presence of important statistical or clinical heterogeneity we also considered the appropriateness of subgroup analysis for the following factors.
1. Age (infant versus older child). 2. Underlying immunosuppressive condition (e.g. hematopoietic stem cell transplant, neutropenia, HIV/AIDS, congenital immunodeficiency, immunosuppressive medication). 3. Concomitant or pre‐existing organ dysfunctions (e.g. renal and hepatic impairment). 4. Purpose of therapy (antifungal therapy for proven or probable infection or empiric therapy for suspected infection). 5. Site of infection. 6. Dose of medication. 7. Single agent versus combination therapy.
Sensitivity analysis
We planned to perform sensitivity analyses investigating the effects of study quality and missing data.
Study quality
If appropriate, we intended to conduct a sensitivity analysis by study quality based on our estimate of the risk of bias in the 'Risk of bias' tables and an assessment of adequate sample size to detect the clinically important difference in outcome for which the study was designed.
Missing data
We employed sensitivity analyses using different approaches to imputing missing data. The best‐case scenario assumed that none of the originally enrolled patients missing from the primary analysis in the treatment group had the negative outcome of interest whilst all those missing from the control group did. The worst‐case scenario was the reverse.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
Trials were selected for inclusion by two review authors (CCB and KH). There were no disagreements regarding trial inclusion. A total of 3305 potentially relevant references were reviewed (Figure 1). A total of 30 trials were retrieved for full text review of which seven were performed in children or had sufficient paediatric subgroup analysis, published or available from authors, to satisfy our inclusion criteria.
1.
Included studies
Four RCTs enrolling 395 children compared a lipid preparation of amphotericin B (liposomal amphotericin B or ABCD) with conventional amphotericin B in the setting of neutropenic fever persisting for > 72 to 120 hours (that is suspected fungal infection) (Sandler 2000; Prentice 1997; Walsh 1999; White 1998). Three of these trials included both adults and children (Prentice 1997; Walsh 1999; White 1998). A further trial published in abstract form only and enrolling 82 children compared an echinocandin (caspofungin) and a lipid preparation of amphotericin B (liposomal amphotericin B) in children with neutropenic fever persistent for > 96 hours (that is suspected fungal infection) (Maertens 2007). A single trial enrolling 109 neonates and children compared an echinocandin (micafungin) with a lipid preparation of amphotericin B (liposomal amphotericin B) in children with invasive candidiasis (that is proven invasive fungal infection) (Queiroz‐Telles 2008). Lastly, a trial enrolling 43 children compared two azole agents (enteral itraconazole and enteral fluconazole) in children with candidaemia (that is proven invasive fungal infection) (Mondal 2004).
Excluded studies
Studies were excluded if they were not randomised (n = 4) (Ellis 1995; Khayat 2005; Wheat 2001; Yao 2000) and enrolled only neonates or adults (n = 2) (Driessen 1996; Rex 1994). Two studies were excluded as they were duplicate publications of Queiroz‐Telles (Arrietta 2006; Arrietta 2007). Studies which enrolled both children and adults were further excluded if specific paediatric information was not available from the text, authors or drug companies (n = 15) (Bodhe 2002; Bowden 2002; Cornely 2007; Ellis 1998; Galgiani 2000; Herbrecht 2002; Kim 2007; Kullberg 2005; Pizzo 1982; Shikanai‐Yasuda 2002; Viscoli 1996; Walsh 2002; Wang 2007; Wingard 2000; Winston 2000).
Risk of bias in included studies
There was substantial variation in the risk of bias of studies included. The risk of bias was low in RCTs performed by Walsh 1999, White 1998, Queiroz‐Telles 2008 and Mondal 2004. Prentice 1997, the largest of the paediatric trials published, conducted an open‐label RCT. The outcome data were difficult to extract from the published paper as whole numbers were not included in the text and were not available from authors. Furthermore, it was not stated in the report why all patients were not included in the analysis of all safety endpoints. Sandler 2000 conducted a double‐blind RCT, however insufficient data on the methods of sequence generation and allocation concealment could be extracted from the report. Maertens 2007 has only been published in abstract form and, as a result, details about the trial methods and results (including absolute numbers) were limited.
Effects of interventions
Primary outcomes
1. Mortality (all cause)
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 1)
See Analysis 1.1; Table 1
1.1. Analysis.

Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 1 Mortality (all cause).
1. Effects of interventions.
| Indication | Prolonged fever and neutropenia (suspected IFI) |
Candidaemia / Invasive Candidiasis (proven IFI) |
||
| Comparisons | 1. Lipid preparations of amphotericin B compared with conventional amphotericin B | 2. Echinocandin compared with lipid preparations of amphotericin B | 3. Echinocandin compared with lipid preparations of amphotericin B | 4. Fluconazole compared with itraconazole |
| 1. Mortality (all cause) | RR = 0.73 (95% CI 0.17 to 3.11), P = 0.67 (Analysis 1.1) | ‐ | RR = 1.05 (95% CI 0.53 to 2.02), P = 0.91 (Analysis 3.1) | RR = 0.52 (95% CI 0.11 to 2.56), P = 0.42 (Analysis 4.1) |
| 2. Mortality (related to fungal infection) | RR = 0.20 (95% CI 0.01 to 3.98), P = 0.29 (Analysis 1.2) | ‐ | RR = 1.38 (95% CI 0.33 to 5.89), P = 0.66 (Analysis 3.2) | RR = 1.05 (95% CI 0.07 to 15.69), P = 0.97). (Analysis 4.2) |
| 3. Complete resolution of fungal infection | RR = 1.50 (95% CI 0.18 to 12.46), P = 0.71 (Analysis 1.3) | ‐ | RR = 0.88 (95% CI 0.68 to 1.13), P = 0.49 (Analysis 3.3) | RR = 0.99 (95% CI 0.74 to 1.32), P = 0.94 (Analysis 4.3) |
| 4. Partial resolution of fungal infection | ‐ | ‐ | ‐ | ‐ |
| 5. Resolution of fever in suspected fungal infection | RR = 1.23 (95% CI 1.00 to 1.52), P = 0.05, I 2= 0% (Analysis 1.5) | RR = 1.34 (95% CI 0.70 to 2.56), P = 0.38. (Analysis 2.5) | ‐ | ‐ |
| 6. Progression of disease requiring change or addition of an antifungal agent | ‐ | ‐ | ‐ | ‐ |
| 7. Breakthrough fungal infection requiring change or addition of an antifungal agent | RR = 0.67 (95% CI 0.24 to 1.84), P = 0.43, I2 = 0% (Analysis 1.7) | RR = 0.15 (95% CI 0.01 to 3.61), P = 0.24 (Analysis 2.7) | ‐ | ‐ |
| 8. Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in the therapy | ‐ | ‐ | RR = 0.35 (95% CI 0.04 to 3.22), P = 0.35 (Analysis 3.8) | ‐ |
| 9. Any adverse reactions attributed to the antifungal agent (total) | RR = 0.49 (95% CI 0.21 to 1.17), P = 0.11, I2 = 86% (Analysis 1.9) | ‐ | RR = 0.86 (95% CI 0.53 to 1.38), P = 0.53 (Analysis 3.9) | ‐ |
| 9a. Abnormal renal function | RR = 0.43 (95% CI 0.21 to 0.90), P = 0.02, I2 = 59% (Analysis 1.10) | ‐ | ‐ | RR = 0.71 (95% CI 0.13 to 3.72), P = 0.68 (Analysis 4.10) |
| 9b. Electrolyte imbalance such as hypokalaemia | RR = 0.71 (95% CI 0.45 to 1.14), P = 0.16, I2 = 54% (Analysis 1.11) | ‐ | RR = 0.47 (95% CI 0.18 to 1.27), P = 0.14 (Analysis 3.11) | RR = 1.06 [95% CI 0.25 to 4.54], P = 0.94 (Analysis 4.11) |
| 9c. Abnormal hepatic function | RR = 1.12 (95% CI 0.74 to 1.71), P = 0.59, I2= 0% (Analysis 1.12) | ‐ | RR = 1.93 (95% CI 0.19 to 20.12), P = 0.58 (Analysis 3.12) | RR = 1.06 (95% CI 0.07 to 15.62), P = 0.97 (Analysis 4.12) |
| 9d. Infusion‐related reactions such as chills, rigors or anaphylaxis | see text; Effects of interventions | ‐ | RR = 0.73 (95% CI 0.30 to 1.77), P = 0.48 (Analysis 3.13) | ‐ |
| 9e. Gastrointestinal disturbances such as nausea, vomiting or diarrhoea | ‐ | ‐ | Vomiting: RR = 1.19 (95% CI 0.46 to 3.04), P = 0.72 (Analysis 3.14) Diarrhoea: RR = 0.83 (95% CI 0.24 to 2.92), P = 0.77 (Analysis 3.14) |
‐ |
| 9f. Neurological disturbances such as blurred vision or dizziness | ‐ | ‐ | ‐ | ‐ |
| 9g. Haematological disturbances such as anaemia, granulocytopaenia or thrombocytopaenia | ‐ | ‐ | RR = 1.56 (95% CI 0.60 to 4.07), P = 0.37 (Analysis 3.16) | ‐ |
| 10. Length of stay | ‐ | ‐ | ‐ | ‐ |
| 11. Quality of life | ‐ | ‐ | ‐ | ‐ |
| 12. Cost | ‐ | ‐ | ‐ | ‐ |
Four trials compared a lipid preparation of amphotericin B with conventional amphotericin B in febrile neutropenic children (Prentice 1997; Sandler 2000; Walsh 1999; White 1998). Attempts were made to obtain these data from all authors, only data from Walsh 1999 were available for analysis of all‐cause mortality. This trial reported that seven children died during the course of the study; 3 of 48 children (6.3%) in the liposomal amphotericin group (3 mg/kg/day) and 4 of 47 children (8.5%) in the conventional amphotericin group (0.6 mg/kg/day). This difference was not statistically significant (RR of dying with a lipid preparation was 0.73, 95% CI 0.17 to 3.11, P = 0.67).
Prentice 1997 reported that three children died during the trial but gave no details regarding which antifungal each patient received. White 1998 and Sandler 2000 did not report mortality endpoints.
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 1)
See Analysis 2.1
2.1. Analysis.
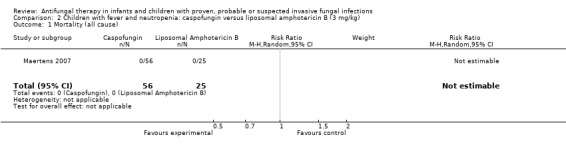
Comparison 2 Children with fever and neutropenia: caspofungin versus liposomal amphotericin B (3 mg/kg), Outcome 1 Mortality (all cause).
One trial, reported in abstract form, compared an echinocandin (caspofungin 70 mg/m2 on day one followed by 50 mg/m2/day) with a lipid preparation of amphotericin B (liposomal amphotericin B 3 mg/kg/day) in febrile neutropenic children (Maertens 2007). No deaths were reported in children receiving either drug therapy up to seven days post‐treatment.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 1)
See Analysis 3.1; Table 1
3.1. Analysis.
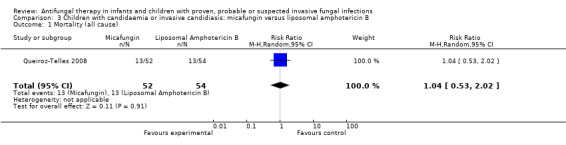
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 1 Mortality (all cause).
One trial compared an echinocandin (micafungin 2 mg/kg/day) with a lipid preparation of amphotericin B (liposomal amphotericin B 3 mg/kg/day) in neonates, infants and children with invasive candidiasis (Queiroz‐Telles 2008). Queiroz‐Telles reported that whilst receiving treatment, all‐cause mortality was 1 of 52 (1.9%) in neonates and children receiving micafungin and 6 of 54 (11.1%) in neonates and children receiving liposomal amphotericin B. During the entire study period, including the 12‐week follow up, all‐cause mortality was 13 of 52 (25%) in neonates and children receiving micafungin and 13 of 54 (24.1%) in those receiving liposomal amphotericin. This difference was not statistically significant (RR of dying with micafungin was 1.05, 95% CI 0.53 to 2.02, P = 0.91). Outcome data in neonates could not be separated from outcome data in infants and children and thus are included in the analysis.
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 1)
See Analysis 4.1; Table 1
4.1. Analysis.
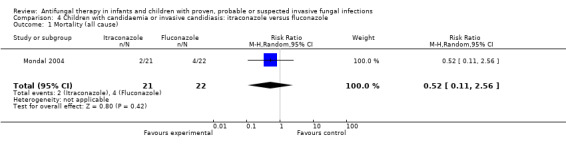
Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 1 Mortality (all cause).
One trial compared enteral itraconazole (approximately 10 mg/kg/day) and fluconazole (approximately 10 mg/kg/day) in children aged one month to 12 years who were admitted to an intensive care unit with candidaemia (Mondal 2004). Two of 21 children (9.5%) who received itraconazole and 4 of 22 children (18.2%) who received fluconazole died during treatment and follow up. This difference was not statistically significant (RR of dying with itraconazole was 0.52, 95% CI 0.11 to 2.56, P = 0.42).
2. Mortality (related to fungal infection)
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 2)
See Analysis 1.2; Table 1
1.2. Analysis.
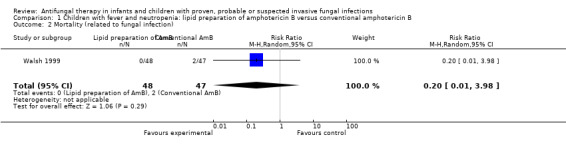
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 2 Mortality (related to fungal infection).
Two trials reported this outcome (Prentice 1997; Walsh 1999), however only Walsh 1999 reported IFI‐related mortality in all groups. In this trial, 2 of 48 children (4.2%) died with IFI (the leading cause of death in the conventional amphotericin B group). No IFI‐related deaths were observed in children receiving liposomal amphotericin B. This difference was not statistically significant (RR of dying with a lipid preparation was 0.20, 95% CI 0.01 to 3.98, P = 0.29).
Prentice 1997 reported that 1 of 71 children (1.4%) receiving liposomal amphotericin B died with Candidemia that developed one day following enrolment. An autopsy revealed Pneumocystis carinii lung infection. IFI‐related mortality was not reported for the other groups.
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 2)
No deaths were reported in children receiving either caspofungin or liposomal amphotericin B up to seven days post‐treatment (Maertens 2007).
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 2)
See Analysis 3.2; Table 1
3.2. Analysis.
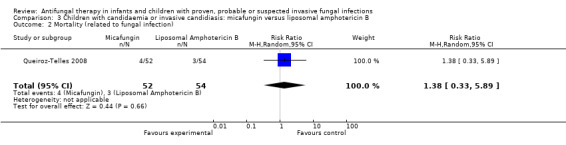
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 2 Mortality (related to fungal infection).
Queiroz‐Telles 2008 reported that fungal infection was considered by the investigators to have contributed to death in 4 of 52 children (7.7%) receiving micafungin and 3 of 54 children (5.6%) receiving liposomal amphotericin B. This difference was not statistically significant (RR of dying with micafungin was 1.38, 95% CI 0.33 to 5.89, P = 0.66).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 2)
See Analysis 4.2; Table 1
4.2. Analysis.
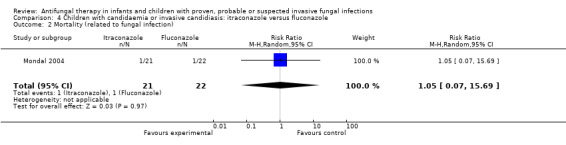
Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 2 Mortality (related to fungal infection).
Mondal 2004 reported that IFI‐related mortality was observed in 1 of 21 children (4.8%) receiving itraconazole and 1 of 22 children (4.5%) receiving fluconazole. This different was not statistically significant (RR of dying with itraconazole was 1.05, 95% CI 0.07 to 15.69, P = 0.97).
3. Complete resolution of fungal infection
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 3)
See Analysis 1.3; Table 1
1.3. Analysis.
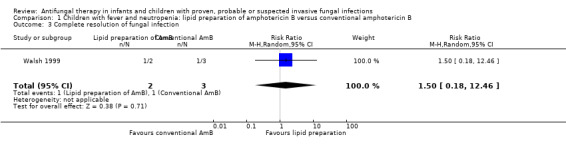
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 3 Complete resolution of fungal infection.
Resolution of a baseline IFI in febrile neutropenic children was considered relevant to this outcome. Resolution of fever in neutropenic children was included in outcome 5. Resolution of baseline IFI was reported in two trials (Prentice 1997; Walsh 1999).
Baseline fungal infection was reported in 2 of 48 children (4.2%) receiving liposomal amphotericin B compared with 3 of 47 children (6.4%) receiving conventional amphotericin B (Walsh 1999). Resolution of baseline fungal infection was observed in one child in each treatment group (2.0% and 2.1% respectively). This difference was not statistically significant (RR of resolution of a baseline IFI with a lipid preparation was 1.50, 95% CI 0.18 to 12.46, P = 0.71).
No children in Prentice 1997 had a baseline IFI.
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 3)
The number of children with baseline IFI was not reported in Maertens 2007.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 3)
See Analysis 3.3; Table 1
3.3. Analysis.
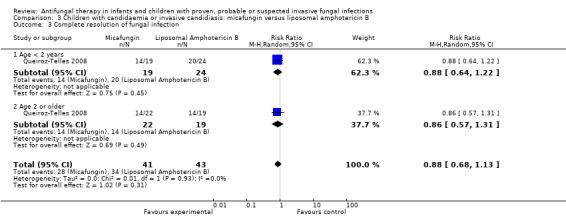
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 3 Complete resolution of fungal infection.
The primary endpoint of the study performed by Queiroz‐Telles 2008 was treatment success (defined by both clinical and mycological responses). Clinical response was defined as a complete or partial resolution of signs and symptoms. Mycologic response was defined as eradication or presumed eradication of positive cultures. Data on the number of children with complete resolution of signs and symptoms were not available. Treatment success in infants and children was similar in both groups (micafungin: 28/41 children, 68.3%; liposomal amphotericin B: 34/43 children, 79.1%). This difference was not statistically significant (RR of treatment success with micafungin was 0.88, 95% CI 0.68 to 1.13, P = 0.49).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 3)
See Analysis 4.3; Table 1
4.3. Analysis.
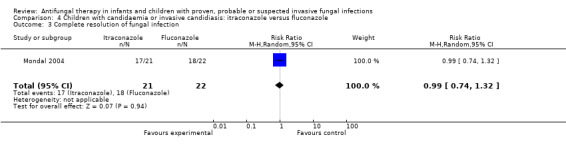
Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 3 Complete resolution of fungal infection.
The primary endpoint of the study performed by Mondal 2004 was treatment success (defined by both clinical and mycological responses). Treatment success in children was similar in both groups (itraconazole: 17/21, 81%; fluconazole: 18/22, 82%). This difference was not statistically significant (RR of treatment success with itraconazole was 0.99, 95% CI 0.74 to 1.32, P = 0.94).
Secondary outcomes
4. Partial resolution of invasive fungal infection
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 4)
Partial resolution of a baseline IFI in febrile neutropenia studies was considered relevant to this outcome.
No children in Prentice 1997 had a baseline IFI. Walsh 1999, White 1998 and Sandler 2000 did not report this outcome.
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 4)
The number of children with baseline IFI was not reported by Maertens 2007.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 4)
Queiroz‐Telles 2008 did not separately report this outcome. They combined complete and partial clinical response with mycologic response into treatment success (see Analysis 3.3).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 4)
Mondal 2004 did not report on this outcome.
5. Resolution of fever in suspected fungal infection
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 5)
See Analysis 1.5; Table 1
1.5. Analysis.
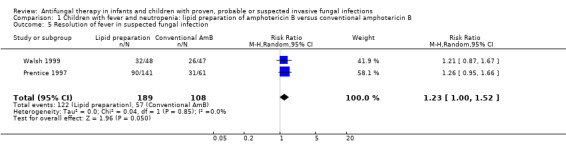
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 5 Resolution of fever in suspected fungal infection.
Two studies reported resolution of fever in children with prolonged fever and neutropenia (Prentice 1997; Walsh 1999). Prentice 1997 considered treatment response as at least three consecutive days without fever (at 38 °C). Forty‐five of 70 children (64.3%) who received liposomal amphotericin B (1 mg/kg/day) and 45 of 71 children (63.4%) who received liposomal amphotericin B (3 mg/kg/day) responded to treatment. Thirty‐one of 61 children (50.8%) randomised to conventional amphotericin B (1 mg/kg/day) responded to treatment. Walsh 1999 included fever resolution in the composite endpoint used to define treatment success. Thirty‐two of 48 children (66.7%) who received liposomal amphotericin B and 26 of 47 children (55.3%) who received conventional amphotericin B had resolution of their fever during neutropenia.
Pooled analysis using paediatric data from Prentice 1997 and Walsh 1999 was performed. The probability of fever resolution with a lipid preparation compared with conventional amphotericin B was of borderline significance (RR of fever resolution with a lipid preparation was 1.23, 95% CI 1.00 to 1.52, P = 0.05) (Analysis 1.5). No significant heterogeneity was observed (I 2= 0%).
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 5)
See Analysis 2.5; Table 1
2.5. Analysis.
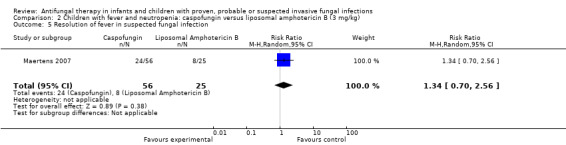
Comparison 2 Children with fever and neutropenia: caspofungin versus liposomal amphotericin B (3 mg/kg), Outcome 5 Resolution of fever in suspected fungal infection.
Resolution of fever in suspected fungal infection (defined as a temperature below 38 °C for at least 48 hours) was observed in 24 of 56 children (43%) receiving caspofungin and 8 of 25 children (32%) receiving liposomal amphotericin B (Maertens 2007). This difference was not statistically significant. The RR of fever resolution with caspofungin was 1.34 (95% CI 0.70 to 2.56, P = 0.38).
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 5)
Queiroz‐Telles 2008 did not report on resolution of fever.
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 5)
Mondal 2004 reported time to clinical cure (defined as complete resolution of fever and improvement in signs and symptoms present at time of enrolment). Mean time to clinical cure was not significantly different: 7.9 ± 1.3 days (itraconazole) compared with 7.0 ± 2.6 days (fluconazole).
6. Progression of disease requiring change or addition of an antifungal agent
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 6)
This outcome was not reported in sufficient detail in any paper (Prentice 1997; Sandler 2000; Walsh 1999; White 1998).
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 6)
This outcome was not reported in Maertens 2007.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 6)
Mycologic persistence at the end of therapy was observed in 7 of 45 (15.6%) of neonates, infants and children receiving both micafungin and liposomal amphotericin B (Queiroz‐Telles 2008). Insufficient published data were available to exclude neonates from these analyses. Antifungal drugs administered following trial therapy were not reported on.
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 6)
No children had progression of disease requiring change or addition of an antifungal agents (Mondal 2004).
7. Breakthrough fungal infection requiring change or addition of an antifungal agent
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 7)
See Analysis 1.7; Table 1
1.7. Analysis.
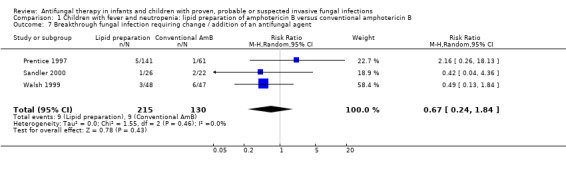
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 7 Breakthrough fungal infection requiring change / addition of an antifungal agent.
Three trials reported breakthrough fungal infection in febrile neutropenic children (Prentice 1997; Sandler 2000; Walsh 1999). One of 61 children (1.6%) who received conventional amphotericin B, 3 of 71 children (4.2%) who received liposomal amphotericin B (1 mg/kg/day) and 2 of 71 children (2.8%) who received liposomal amphotericin B (3 mg/kg/day) developed a breakthrough fungal infection (Prentice 1997). Six of 47 (12.8%) children in the conventional amphotericin B arm and 3 of 48 (6.3%) who received liposomal amphotericin B developed a breakthrough IFI (Walsh 1999). One of 26 (3.8%) children who received ABCD (4 mg/kg/day) developed IFI compared with 2 of 22 (9.1%) who received conventional amphotericin B (0.8 mg/kg/day) (Sandler 2000).
Pooled analyses of data from Prentice 1997, Walsh 1999 and Sandler 2000 were performed. No significant differences in breakthrough fungal infection requiring change or addition of an antifungal agent were observed when lipid preparations and conventional amphotericin B were compared in febrile neutropenic children (RR 0.67, 95% CI 0.24 to 1.84, P = 0.43) (Analysis 1.7). No heterogeneity was observed (I2 = 0%, P = 0.46).
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 7)
See Analysis 2.7; Table 1
2.7. Analysis.
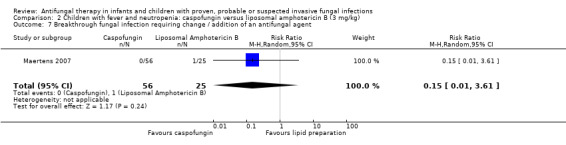
Comparison 2 Children with fever and neutropenia: caspofungin versus liposomal amphotericin B (3 mg/kg), Outcome 7 Breakthrough fungal infection requiring change / addition of an antifungal agent.
Breakthrough fungal infection was not observed in children receiving caspofungin. One of 25 children (4%) receiving liposomal amphotericin had a breakthrough fungal infection (Maertens 2007). This difference was not statistically significant (RR of breakthrough fungal infection caspofungin was 0.15, 95% CI 0.01 to 3.61, P = 0.24).
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 7)
No breakthrough fungal infection was observed whilst on therapy in infants and children receiving either treatment (Queiroz‐Telles 2008).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 7)
No breakthrough fungal infection was observed whilst on therapy in infants and children receiving either treatment (Mondal 2004).
8. Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in the therapy
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 8)
Eight of 48 children (16.7%) receiving liposomal amphotericin B and 7 of 47 children (14.9%) receiving conventional amphotericin B discontinued therapy for reasons of toxicity or lack of efficacy (Walsh 1999). This difference was not statistically significant (RR of therapy discontinuation was 1.12, 95% CI 0.44 to 2.84, P = 0.81). The proportion of children who discontinued therapy or had a dose reduction or change in therapy due to clinically significant adverse reactions was not reported. This outcome was not reported by Prentice 1997, White 1998 or Sandler 2000.
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 8)
Discontinuation due to drug‐related adverse events was noted in 4% of children receiving caspofungin compared with 12% receiving liposomal amphotericin B (Maertens 2007). Absolute numbers were not published.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 8)
See Analysis 3.8; Table 1
3.8. Analysis.

Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy.
One of 52 neonates, infants and children (1.9%) receiving micafungin and 3 of 54 (5.6%) neonates, infants and children receiving liposomal amphotericin B had a treatment‐related adverse event that led to treatment discontinuation (Queiroz‐Telles 2008). This difference was not statistically significant. The RR of a treatment‐related adverse event that led to treatment discontinuation with micafungin was 0.35 (95% CI 0.04 to 3.22, P = 0.35). Treatment discontinuation (regardless of causality) occurred less frequently with micafungin (2/52, 3.8%) compared with liposomal amphotericin B (9/54, 16.7%, P = 0.05 as reported).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 8)
No drug discontinuation was observed secondary to adverse reactions in children receiving either therapy (Mondal 2004).
Echinocandin compared with lipid preparations of amphotericin B in children (any indication; Comparison 5, Outcome 8)
See Analysis 5.8
5.8. Analysis.
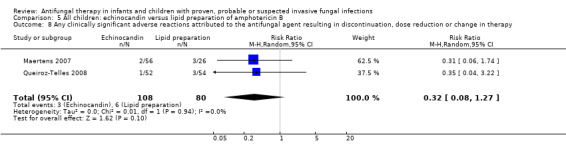
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy.
Clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy were assessed using data from children with fever and neutropenia (Maertens 2007) and candidaemia or invasive candidiasis (Queiroz‐Telles 2008) and receiving either echinocandins or lipid preparations. Absolute numbers were not published by Maertens 2007 so, for pooled analyses, absolute numbers have been calculated from percentages. There was no significant heterogeneity (I 2= 0%) and no significant differences were observed (RR 0.32, 95% CI 0.08 to 1.27, P = 0.1) (Analysis 5.8).
9. Any adverse reactions attributed to the antifungal agent (total)
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 9)
See Analysis 1.9; Table 1
1.9. Analysis.
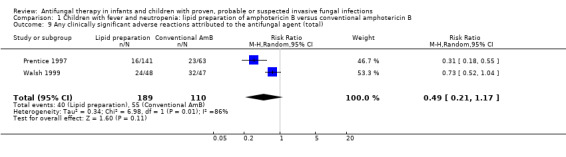
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 9 Any clinically significant adverse reactions attributed to the antifungal agent (total).
Walsh 1999 and Prentice 1997 reported total adverse reactions attributed to the antifungal agents. Twenty‐four of 48 (50%) children who received liposomal amphotericin B and 32 of 47 (68.1%) children who received conventional amphotericin B experienced a drug‐related adverse event (Walsh 1999). Serious drug‐related adverse events were reported in 12 children in each treatment group. Six per cent of children receiving liposomal amphotericin (1 mg/kg/day), 17% of children receiving liposomal amphotericin (3 mg/kg/day) and 36% receiving conventional amphotericin B groups had an adverse reaction attributable to the antifungal agent (Prentice 1997). Severe drug‐related adverse events were reported in 1% of both cohorts receiving liposomal amphotericin B and 8% receiving conventional amphotericin B. Absolute numbers were not published by Prentice 1997 so, for pooled analyses, absolute numbers have been calculated from percentages.
Pooled analysis of Prentice 1997 and Walsh 1999 was performed. No significant differences in total adverse reactions attributed to the antifungal agent were observed when lipid preparations and conventional amphotericin B were compared in febrile neutropenic children (RR 0.49, 95% CI 0.21 to 1.17, P = 0.11) (Analysis 1.9). Significant heterogeneity was observed (I2 = 86%, P = 0.008).
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 9)
Drug‐related adverse events were noted in 48% of children receiving caspofungin compared with 46% receiving liposomal amphotericin B (Maertens 2007). Serious treatment‐related adverse events were reported in 2% of children receiving caspofungin compared with 11% receiving liposomal amphotericin B. Absolute numbers were not published.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 9)
See Analysis 3.9; Table 1
3.9. Analysis.

Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 9 Any clinically significant adverse reactions attributed to the antifungal agent (total).
Nineteen of 52 (36.5%) children who received micafungin compared with 23 of 54 (42.6%) receiving liposomal amphotericin B reported a treatment‐related adverse event (Queiroz‐Telles 2008). This difference was not statistically significant (RR of any treatment related adverse event with micafungin was 0.86, 95% CI 0.53 to 1.38, P = 0.53). Serious treatment‐related adverse events were reported in 2 of 52 children (3.8%) who receiving micafungin compared with 5 of 54 children (9.3%) who received liposomal amphotericin B.
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 9)
One patient developed gastrointestinal side effects in the trial (Mondal 2004). The therapy received by the patient was not reported. The total number of treatment‐related adverse events with either therapy was not reported.
Echinocandin compared with lipid preparations of amphotericin B in children (any indication: Comparison 5, Outcome 9)
See Analysis 5.9
5.9. Analysis.
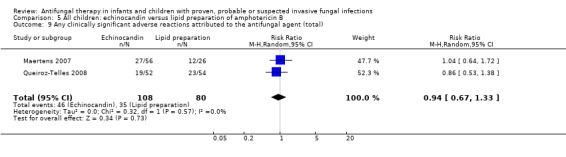
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 9 Any clinically significant adverse reactions attributed to the antifungal agent (total).
Using data from children with fever and neutropenia (Maertens 2007) and candidaemia or invasive candidiasis (Queiroz‐Telles 2008), echinocandins were compared with lipid preparations with regard to clinically significant adverse reactions attributed to the antifungal agent. Absolute numbers were not published by Maertens 2007 so, for pooled analyses, absolute numbers have been calculated from percentages. No significant differences were observed (RR of a clinically significant adverse reaction with an echinocandin was 0.94, 95% CI 0.67 to 1.33, P = 0.73) (Analysis 5.9). There was no significant heterogeneity (I 2= 0%).
a. Abnormal renal function
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcomes 10)
See Analysis 1.10; Table 1; Analysis 6.1; Analysis 7.1
1.10. Analysis.
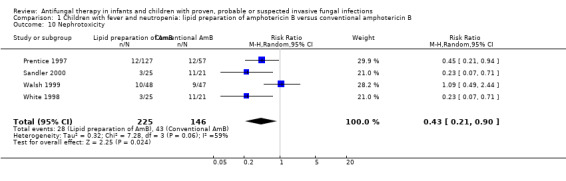
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 10 Nephrotoxicity.
6.1. Analysis.
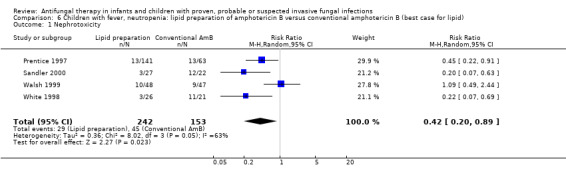
Comparison 6 Children with fever, neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B (best case for lipid), Outcome 1 Nephrotoxicity.
7.1. Analysis.
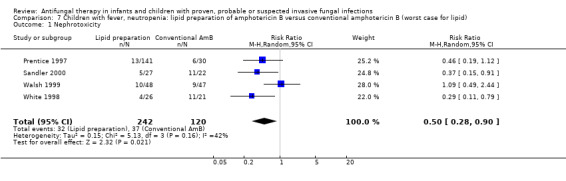
Comparison 7 Children with fever, neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B (worst case for lipid), Outcome 1 Nephrotoxicity.
All four trials comparing lipid preparations of amphotericin B and conventional amphotericin B in children with fever and neutropenia reported nephrotoxicity endpoints (Prentice 1997; Sandler 2000; Walsh 1999; White 1998). Nephrotoxicity (defined as ≥ 100% rise in serum creatinine) occurred in 10 of 48 children (20.1%) receiving liposomal amphotericin and 9 of 47 children (19.1%) receiving conventional amphotericin B (Walsh 1999). Nephrotoxicity (defined as ≥ 100% rise in serum creatinine) occurred in 8% of children receiving liposomal amphotericin B (1 mg/kg/day), 11% receiving liposomal amphotericin B (3 mg/kg/day) and 21% of children receiving conventional amphotericin B (Prentice 1997). Absolute numbers were not published by Prentice 1997 so, for pooled analyses, absolute numbers have been calculated from percentages. Three of 25 children (12.0%) receiving ABCD (4 mg/kg/day in both trials) and 11 of 21 (52.4%) receiving conventional amphotericin B (0.8 mg/kg/day in both trials) developed nephrotoxicity (Sandler 2000; White 1998). Nephrotoxicity was defined in both trials as doubling in serum creatinine level from baseline, an increase of 88 mmol/L (1.0 mg/dL) in the serum creatinine level from baseline, or a ≥ 50% decrease in the calculated creatinine clearance from baseline during study drug administration. It should be noted that rates of nephrotoxicity in White 1998 and Sandler 2000 were the same. As many children enrolled in the study were treated at the same institutions during similar time periods, it is possible that data have been duplicated in these publications. Clarification from the authors was not forthcoming.
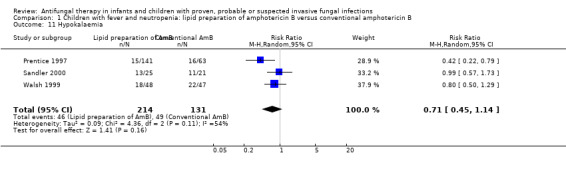
Pooled analysis of Prentice 1997, Walsh 1999, White 1998 and Sandler 2000 was performed. Treatment with a lipid preparation decreased the risk of nephrotoxicity by 57% compared with conventional amphotericin B (RR 0.43, 95% CI 0.21 to 0.90, P = 0.02) (Analysis 1.10). Substantial heterogeneity was observed (I2 = 59%, P = 0.06). The number of children needing to receive a lipid preparation of amphotericin B to avoid one child developing nephrotoxicity was 6 (95% CI 3.9 to 11.8). A sensitivity analysis was performed to investigate the effects of missing data. Lipid preparations were significantly less toxic in both best‐case (RR 0.42, 95% CI 0.20 to 0.89, P = 0.02) (Analysis 6.1) and worst‐case scenarios (RR 0.50, 95% CI 0.28 to 0.90, P = 0.02) (Analysis 7.1). If White 1998 or Sandler 2000 were excluded from the analysis (allowing for the possibility of duplicated reporting) the treatment effect was not preserved (RR 0.51, 95% CI 0.22 to 1.18, P = 0.12).
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 10)
Maertens 2007 did not report on abnormal renal function.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 10)
No children receiving micafungin or liposomal amphotericin B developed nephrotoxicity (defined as ≥ 100% rise in serum creatinine) (Queiroz‐Telles 2008).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparion 4, Outcome 10)
See Analysis 4.10; Table 1
4.10. Analysis.
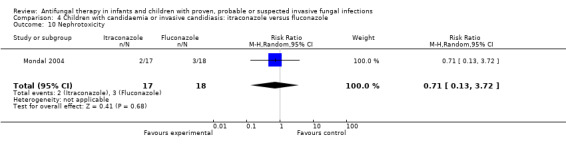
Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 10 Nephrotoxicity.
Two of 17 children (11.8%) receiving itraconazole group and 3 of 18 (16.7%) receiving fluconazole experienced a serum creatinine > 1 mg/dL on therapy. This difference was not statistically significant (RR of nephrotoxicity with itraconazole was 0.71, 95% CI 0.13 to 3.72, P = 0.68). Doubling of serum creatinine was not reported (Mondal 2004).
b. Electrolyte imbalance such as hypokalaemia
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 11)
See Analysis 1.11; Table 1;
1.11. Analysis.
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 11 Hypokalaemia.
Three trials comparing a lipid preparation to conventional amphotericin B in children with fever and neutropenia reported electrolyte disturbances (Prentice 1997; Walsh 1999; White 1998). Eighteen of 48 children (37.5%) in the liposomal amphotericin group and 22 of 47 children (46.8%) receiving conventional amphotericin B developed hypokalaemia < 3.0 mmol/L (Walsh 1999). Thirteen of 25 children (52.0%) receiving ABCD and 11 of 21 children (52.4%) receiving conventional amphotericin B developed hypokalemia (Sandler 2000). Hypokalemia was not defined in this paper. Ten pe rcent of children in the liposomal amphotericin B (1 mg/kg/day) group, 11% in the 3 mg/kg/d liposomal amphotericin B group and 26% in the conventional amphotericin B group developed hypokalemia < 2.5 mmol/L (Prentice 1997). Absolute numbers were not published so, for pooled analyses, absolute numbers have been calculated from percentages.
Pooled analysis of Prentice 1997, Walsh 1999 and Sandler 2000 were performed. No differences in the rate of hypokalaemia were observed with lipid preparations compared with conventional amphotericin B (RR 0.71, 95% CI 0.45 to 1.14, P = 0.16) (Analysis 1.11). Substantial heterogeneity was observed (I2 = 54%, P = 0.11). These pooled data need to be interpreted with caution given the variable case definition used in different trials.
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 11)
Maertens 2007 did not report on electrolyte imbalance.
Echinocandin compared with lipid preparations of amphotericin B in children with candidemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 11)
See Analysis 3.11; Table 1;
3.11. Analysis.
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 11 Hypokalaemia.
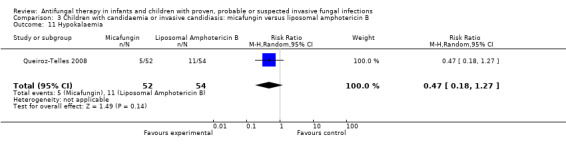
FIve of 52 neonates, infants and children (9.6%) receiving micafungin developed hypokalaemia (not defined) compared with 11 of 54 (20.4%) receiving liposomal amphotericin B (Queiroz‐Telles 2008). This difference was not statistically significant (RR of hypokalaemia with micafungin was 0.47, 95% CI 0.18 to 1.27, P = 0.14).
Fluconazole compared with itraconazole in children with candidemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 11)
See Analysis 4.11; Table 1;
4.11. Analysis.
Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 11 Hypokalaemia.
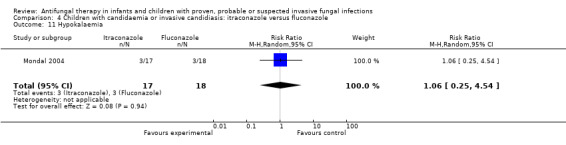
Three of 17 (17.6%) children receiving itraconazole developed hypokalaemia (< 3.5 mmol/L) compared with 3 of 18 (16.7%) receiving fluconazole (Mondal 2004). This difference was not statistically significant (RR of hypokalaemia with itraconazole was 1.06, 95% CI 0.25 to 4.54, P = 0.94).
c. Abnormal hepatic function
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 12)
See Analysis 1.12; Table 1
1.12. Analysis.
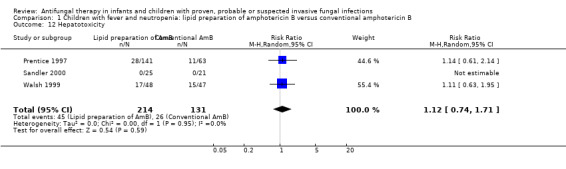
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 12 Hepatotoxicity.
Three trials comparing a lipid preparation to conventional amphotericin B in children with fever and neutropenia reported disturbances in hepatic function (Prentice 1997; Walsh 1999; White 1998). Seventeen of 48 (16.7%) in the liposomal amphotericin group and 15 of 47 (14.9%) in the conventional amphotericin B group developed hepatotoxicity, defined as transaminases twice the normal values for age (Walsh 1999). No children receiving either ABCD or conventional amphotericin B developed hepatotoxicity (not defined) (Sandler 2000). Seventeen per cent of children receiving liposomal amphotericin B (1 mg/kg/day), 22% receiving liposomal amphotericin B and 17% receiving conventional amphotericin B group developed transaminitis (≥ 110 U/L) (Prentice 1997). Eleven per cent of children receiving liposomal amphotericin B (1 mg/kg/day), 12% receiving liposomal amphotericin B and 10% receiving conventional amphotericin B group developed hyperbilirubinaemia (≥ 35 µmol/L). Absolute numbers were not published by Prentice 1997 so, for pooled analyses, absolute numbers have been calculated from percentages.
Pooled analysis of Prentice 1997, Walsh 1999 and Sandler 2000 was performed. No significant differences in the rate of hepatotoxicity were observed with lipid preparations when compared with conventional amphotericin B (RR 1.12, 95% CI 0.74 to 1.71, P = 0.59) (Analysis 1.12). There was no significant heterogeneity (I2 = 0%). These pooled data need to be interpreted with caution given the variable case definition used in the different trials.
Echinocandin compared with Lipid preparations of Amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 12)
Maertens 2007 did not report on abnormal hepatic function.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 12)
See Analysis 3.12; Table 1
3.12. Analysis.
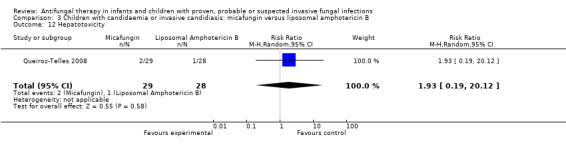
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 12 Hepatotoxicity.
Two of 29 (6.9%) neonates, infants and children receiving micafungin developed a raised alanine aminotransferase (ALT) (> 2.5 times upper limit of normal) compared with 1 of 28 (3.6%) receiving liposomal amphotericin B. This difference was not statistically significant (RR of developing hepatotoxicity with micafungin was 1.93, 95% CI 0.19 to 20.12, P = 0.58). Two of 30 (6.7%) neonates, infants and children receiving micafungin developed a raised aspartate aminotransferase (AST) (> 2.5 times upper limit of normal) compared with 1 of 25 (4.0%) receiving liposomal amphotericin B. No children receiving either therapy developed hyperbilirubinaemia (> 2.5 times upper limit of normal) (Queiroz‐Telles 2008).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 12)
See Analysis 4.12; Table 1
4.12. Analysis.
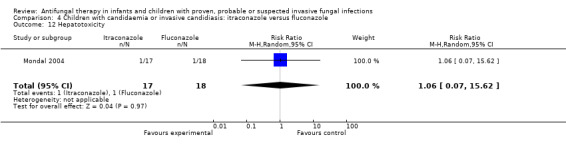
Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 12 Hepatotoxicity.
One of 17 (5.9%) children receiving itraconazole had a serum bilirubin < 2mg/dL following therapy compared with none receiving fluconazole. One of 18 (5.6%) children receiving fluconazole had a serum glutamic‐oxaloacetic transaminase (SGOT) > 40 IU/L following fluconazole compared with none receiving itraconazole (Mondal 2004). This difference was not statistically significant (RR of developing hepatotoxicity with itraconazole was 1.06, 95% CI 0.07 to 15.62, P = 0.97).
d. Infusion‐related reactions such as chills, rigors or anaphylaxis
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 13)
See Analysis 1.13; Table 1
1.13. Analysis.
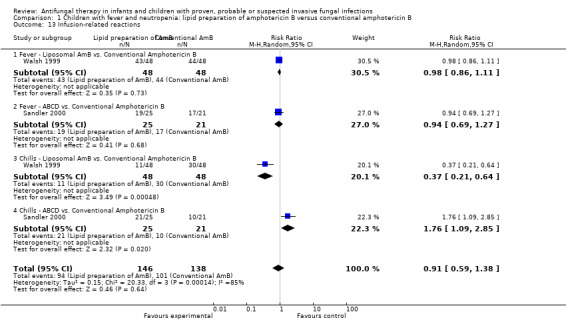
Comparison 1 Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B, Outcome 13 Infusion‐related reactions.
Three trials comparing a lipid preparation to conventional amphotericin B in children with fever and neutropenia reported infusion‐related reactions (Prentice 1997; Walsh 1999; White 1998).
Forty‐six of 48 children (95.8%) receiving liposomal amphotericin and 45 of 47 children (95.7%) receiving conventional amphotericin B had an infusion‐related reaction (Walsh 1999). Eleven of 48 children (22.9%) receiving liposomal amphotericin B and 30 of 47 children (63.8%) receiving conventional amphotericin B developed infusion‐related chills (Walsh 1999). Forty‐three of 48 children (89.6%) receiving liposomal amphotericin and 44 of 47 children (93.6%) receiving conventional amphotericin B developed infusion‐related fevers (Walsh 1999). Two of 25 children (8.0%) receiving ABCD and 0 of 21 children (0%) receiving conventional amphotericin B developed chills and rigors (Sandler 2000). Nineteen of 25 children (76.0%) receiving ABCD and 10 of 21 children (47.6%) children receiving conventional amphotericin B developed chills (Sandler 2000). Nineteen of 25 children (76.0%) receiving ABCD and 17 of 21 children (80.1%) receiving conventional amphotericin B developed fevers (Sandler 2000). One per cent of children receiving liposomal amphotericin B (1 mg/kg/day), 1% of children receiving liposomal amphotericin B (3 mg/kg/day) and 2% of children receiving conventional amphotericin B developed an allergic reaction following drug administration (Prentice 1997). "Allergic reaction" was not further defined by the authors.
Given the variation in endpoints reported, definitions used and the previously demonstrated differences in infusion‐related reactions between liposomal amphotericin B and ABCD, these data have not been pooled. Analysis of individual studies (Sandler 2000) identified an increased risk of chills with ABCD compared with conventional amphotericin (RR 1.76, 95% CI 1.09 to 2.85, P = 0.02). Liposomal amphotericin B was associated with a decreased risk of chills compared with conventional amphotericin B (RR 0.37, 95% CI 0.21 to 0.64, P = 0.0005) (Walsh 1999). No other significant differences were identified.
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 13)
Maertens 2007 did not report on infusion‐related reactions.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 13)
See Analysis 3.13; Table 1
3.13. Analysis.
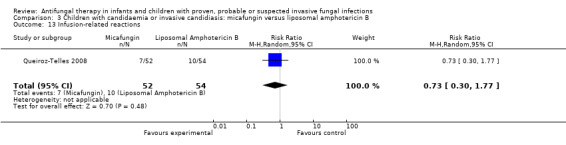
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 13 Infusion‐related reactions.
An infusion‐related reaction was reported in 7 of 52 (13.5%) neonates, infants and children receiving micafungin compared with 10 of 54 (18.5%) receiving liposomal amphotericin B (Queiroz‐Telles 2008). This difference was not statistically significant (RR of an infusional‐related reaction with micafungin was 0.73, 95% CI 0.30 to 1.77, P = 0.48).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 13)
Chills, fever and dyspnoea did not complicate therapy in any children receiving itraconazole or fluconazole (Mondal 2004).
e. Gastrointestinal disturbances such as nausea, vomiting or diarrhoea
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 14)
Only one trial comparing a lipid preparation to conventional amphotericin B in children with fever and neutropenia reported gastrointestinal disturbances (Sandler 2000). Vomiting was reported in 14.8% of children who received ABCD compared with 18.2% who received conventional amphotericin B. Nausea was reported in 11.1% of children who received ABCD and 9.1% who received conventional amphotericin B. Absolute numbers were not reported.
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 14)
Maertens 2007 did not report on gastrointestinal disturbances.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 14)
See Analysis 3.14; Table 1
3.14. Analysis.
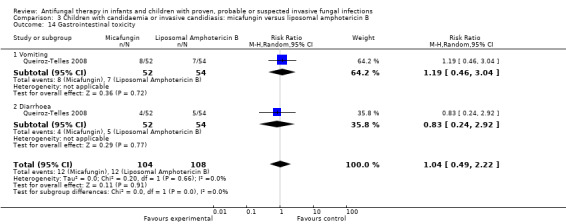
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 14 Gastrointestinal toxicity.
Vomiting was reported in 8/52 (15.4%) of neonates, infants and children receiving micafungin compared with 7/54 (13%) receiving liposomal amphotericin B (Queiroz‐Telles 2008). This different was not statistically significant. The RR of vomiting with micafungin was 1.19 (95% CI 0.46 to 3.04, P = 0.72). Diarrhoea was reported in 4/52 (7.7%) of neonates, infants and children receiving micafungin compared with 5/54 (9.3%) receiving liposomal amphotericin B. This difference was not statistically significant (RR of diarrhoea with micafungin was 0.83 (95% CI 0.24 to 2.92, P = 0.77).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 14)
One patient developed gastrointestinal side effects however it was not reported whether they received itraconazole or fluconazole (Mondal 2004).
f. Neurological disturbances such as blurred vision or dizziness
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 15)
No study reported this outcome (Prentice 1997; Sandler 2000; Walsh 1999; White 1998).
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 15)
Maertens 2007 did not report on neurological disturbances.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 15)
No infants or children with neurological disturbances were reported in either treatment arms (Queiroz‐Telles 2008).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 15)
Mondal 2004 did not report on neurological disturbances.
g. Haematological disturbances such as anaemia, granulocytopaenia or thrombocytopaenia
Lipid preparations of amphotericin B compared with conventional amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 1, Outcome 16)
No study reported this outcome (Prentice 1997; Sandler 2000; Walsh 1999; White 1998).
Echinocandin compared with lipid preparations of amphotericin B in children with prolonged fever and neutropenia (suspected fungal infection; Comparison 2, Outcome 16)
Maertens 2007 did not report on haematological disturbances.
Echinocandin compared with lipid preparations of amphotericin B in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 3, Outcome 16)
See Analysis 3.16; Table 1
3.16. Analysis.
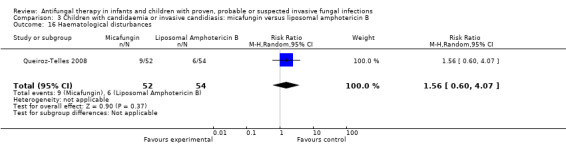
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 16 Haematological disturbances.
Anaemia (not defined) was reported in 9/52 (17.3%) of neonates, infants and children receiving micafungin compared with 6/54 (11.1%) receiving liposomal amphotericin B (Queiroz‐Telles 2008). This difference was not statistically significant (RR of anaemia with micafungin was 1.56, 95% CI 0.60 to 4.07, P = 0.37).
Fluconazole compared with itraconazole in children with candidaemia or invasive candidiasis (proven fungal infection; Comparison 4, Outcome 16)
Mondal 2004 did not report on haematological disturbances.
10. Length of stay (days)
No trials reported on length of stay.
11. Quality of life (QOL)
No trials reported on quality of life.
12. Cost (Outcome 19)
No trials reported on cost of antifungal therapy.
Discussion
Using data from RCTs comparing antifungal agents in adult populations is a challenge for paediatricians due to differences in comparators, conditions, populations, power and duration of follow up. The paucity of paediatric studies compounds this. Limited data are therefore available to guide paediatricians when managing neonates and children with invasive fungal infection.
Data were available from seven randomised controlled trials enrolling children from two distinct populations, children with candidaemia and invasive candidiasis (that is proven invasive fungal infection) and children with prolonged fever and neutropenia (that is suspected invasive fungal infection). Lipid preparations of amphotericin B (liposomal amphotericin B or amphotericin B colloidal dispersion (ABCD)) were compared with conventional amphotericin B in children with prolonged neutropenic fever; echinocandins (caspofungin and micafungin) were compared with liposomal amphotericin B in children with prolonged neutropenic fever and candidaemia or invasive candidiasis respectively; and itraconazole was compared with fluconazole in children with candidaemia and invasive candidiasis. No differences in all‐cause mortality and other primary endpoints (invasive fungal infection (IFI) related mortality or complete resolution of fungal infections) were observed in indiividual trials or pooled analyses.
The only significant differences in secondary endpoints identified in children with prolonged fever and neutropenia were i) reduced nephrotoxicity with lipid preparations of amphotericin B (liposomal amphotericin B or ABCD) compared with conventional amphotericin B; ii) reduced chills with liposomal amphotericin B when compared with conventional amphotericin B; and iii) increased chills with ABCD compared with conventional amphotericin B. No significant differences were identified in other analyses. The finding of reduced nephrotoxicity with lipid preparations of amphotericin B needs to be interpreted with caution as significant heterogeneity was observed (I 2 = 59%) and it is possible that duplicated reporting (Sandler 2000; White 1998) occurred.
This is the first attempt to use a meta‐analytical approach to guide antifungal use in paediatric patients. The comprehensive search for published trials, rigorous attempts to obtain unpublished paediatric data, use of strict outcome definitions and analysis of both individual drugs and drug classes strengthen the findings of the analysis.The studies included were of moderate to high quality as sequence generation was judged to be adequate in five studies, allocation concealment was present in five and blinding used in six of seven included trials.
Numerous limitations exist. In pooling data from the seven trials, low numbers limited power to detect real differences between comparators. Despite attempts to obtain unpublished data, included in combined adult and paediatric trials, this was not always possible. Paediatric patients were enrolled in studies comparing conventional amphotericin B with placebo in the setting of prolonged fever and neutropenia (Pizzo 1982); and numerous studies comparing amphotericin B based products with triazoles in the setting of prolonged fever and neutropenia (Kim 2007; Viscoli 1996; Walsh 2002; Winston 2000), candidaemia or invasive candidiasis (Kullberg 2005) and invasive aspergillosis (Herbrecht 2002). Trials comparing amphotericin B preparations and echinocandins in prolonged fever and neutropenia (Wang 2007) and different formulations or dosages of amphotericin B products in prolonged fever and neutropenia (Wingard 2000) and invasive aspergillosis (Bowden 2002; Cornely 2007; Ellis 1998) also enrolled children. As paediatric subgroup analysis was not made available for these trials, it is possible that only significant results have been reported. Furthermore, as unpublished data were only obtained from one study in febrile neutropenic participants (Walsh 1999) and one study in children with candidaemia (Mondal 2004), selective reporting may have influenced results. Attempts to overcome this by comparing mixed studies with children‐only studies (data not shown) were confounded by the different percentages of children and age ranges in each study. Requests for further data from the relevant companies, not available at the time of publication, may be forthcoming and will be included in any further amendments.
Definitions of treatment success and toxicity varied in different trials making comparison difficult. Differences in duration of follow up in children with fever and neutropenia (seven days in Sandler 2000; 30 days in Prentice 1997 and Walsh 1999) and candidaemia or invasive candidiasis (12 weeks in Queiroz‐Telles 2008; median eight weeks in Mondal 2004) are another source of potential bias. The shorter studies may under‐report important outcomes such as invasive fungal infection, death or toxicity. In most studies in febrile neutropenic participants (including Maertens 2007; Prentice 1997; Sandler 2000; Walsh 1999; White 1998) treatment success was measured by a composite endpoint. Key features of the composite endpoints include survival, resolution of fever and the absence of breakthrough fungal infection. Definitions varied however between trials. The relevance of the composite endpoints is debatable, particularly when resolution of fever is a main contributor (Bennett 2003; de Pauw 2006). Minor differences in definition of fever may be relevant, and the resolution of fever is not specific for fungal infection. The power needed to detect real differences in individual endpoints remains a problem as most studies are not of adequate size to do this. Pooling data goes some way to overcoming this. Using data from three trials of children with febrile neutropenia (Prentice 1997; Sandler 2000; Walsh 1999) greater treatment success was observed with lipid preparations of amphotericin compared with conventional amphotericin B deoxycholate (RR 0.72, 95% CI 0.56 to 0.93, P = 0.01, data not shown). This was not defined a priori in our protocol and, therefore, we have not presented it in the results. Cautious interpretation of this finding is necessary as only three trials were included in the analysis, treatment success was defined differently in the different trials, different doses of conventional amphotericin and liposomal amphotericin B were used and no other difference in efficacy endpoints including mortality and breakthrough fungal infection were observed. Moreover, as initial placebo‐controlled trials of conventional amphotericin B in prolonged fever and neutropenia were underpowered (EORTC 1989; Pizzo 1982) and recent meta‐analyses have demonstrated no survival benefit when using empiric antifungal therapy (Goldberg 2008), some authors question the role of empiric antifungal therapy in the setting of prolonged fever and neutropenia. A pre‐emptive approach to diagnosis of IFI using biological marks and radiological imaging has been studied as an alternative to empiric antifungal therapy in prolonged febrile neutropenia in adults (Cordonnier 2009; Maertens 2005). In the absence of any paediatric data, it is likely that empiric antifungal therapy for suspected antifungal infection will continue to be used in the future.
The analysis of paediatric data yields very similar results to previous adult studies and meta‐analyses. No difference in mortality has been demonstrated when lipid preparations of amphotericin B and conventional amphotericin B (Johansen 2000) or amphotericin B based products and echinocandins (Walsh 2004a) are compared in the setting of prolonged fever and neutropenia. No difference in mortality has been demonstrated in the setting of candidaemia or invasive candidiasis when amphotericin B based products and echinocandins (Kuse 2007; Mora‐Duarte 2002) or different triazole preparations (Kullberg 2005) have been compared. A reduced rate of nephrotoxicity with lipid preparations in children is consistent with previous adult studies and meta‐analyses (Barrett 2003; Girois 2006; Walsh 1999). Children generally tolerate the renal effects of amphotericin B better than adults due to increased nephronal reserve (Steinbach 2005b). The relevance of a doubling of serum creatinine equating to nephrotoxicity is debatable, however it was the most commonly used definition. Less extreme changes in serum creatinine may also be clinically relevant in certain populations, particularly high‐risk patients with multiple co‐morbidities receiving concurrent nephrotoxins (for example haematopoietic stem cell recipients).
There are numerous deficiencies in the paediatric literature. Paediatric data are insufficient to determine the role of triazole drugs particularly in children with prolonged fever and neutropenia and candidaemia or invasive candidiasis. Despite the increasing use of mould‐active triazoles and echinocandins, particularly in children with confirmed or suspected invasive mould infections, little comparative paediatric data exists to guide therapy. The costs of antifungal agents are real considerations for many centres when choosing antifungal therapy. No studies specifically addressed this.
Authors' conclusions
Implications for practice.
Few significant differences were observed in paediatric antifungal trials in children with prolonged fever and neutropenia and candidaemia or invasive candidiasis. No differences in mortality and efficacy were observed. Conventional amphotericin B was more likely to be associated with nephrotoxicity in children than a lipid preparation. Given the paucity of data and the findings of previous meta‐analyses, it is reasonable to recommend a lipid preparation of amphotericin B in this population if cost permits. In studies comparing children receiving an echinocandin and liposomal amphotericin B, no significant differences in efficacy and toxicity were observed. Echinocandins may be considered for use in children with fever with neutropenia and candidaemia or invasive candidiasis as efficacy and safety compared with amphotericin B based products have been demonstrated.
Implications for research.
Numerous questions remain unanswered when managing invasive fungal infection. Further epidemiological studies highlighting differences between neonates, children and adults with invasive fungal infection are needed to allow accurate interpretation of the published literature. Further randomised controlled antifungal trials enrolling children are required. The two recent paediatric studies (Maertens 2007; Queiroz‐Telles 2008) have given hope that more RCTs in paediatric mycology will be undertaken, enabling evidence‐based choice when selecting an antifungal agent.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 2, 2010
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
| 21 February 2011 | Amended | Assessed as Up‐to‐date date amended. |
Acknowledgements
The authors wish to acknowledge Professor Tania Sorrell, Associate Professor Katrina Williams and Danielle Wheeler for their critical review of the protocol.
Appendices
Appendix 1. MEDLINE search strategy
Antifungal Agents/
exp amphotericin B/
fungizon$.tw
ampho$.tw.
anfo$.tw.
candipres$.tw.
ABLC$.tw.
ABCD$.tw.
abelcet$.tw.
ambisome$.tw.
exp triazoles/
triazole$.tw.
exp fluconazole/
flucon$.tw.
diflucan$.tw.
exp Itraconazole/
itracon$.tw.
sporanox$.tw.
voriconazol$.tw.
vfend$.tw.
posaconazol$.tw.
posafilin$.tw.
posanin$.tw.
ravuconazol$.tw.
exp Flucytosine/
flucytosin$.tw.
ancotil$.tw.
5FC$.tw.
5‐fluorocytosin$.tw.
ancobon$.tw.
ancotil$.tw.
alcobon$.tw.
echinocandin$.tw.
caspofungin$.tw.
cancidas$.tw.
micafungin$.tw.
funguard$.tw.
mycamine$.tw.
anidulafungin$.tw.
anidrasona$.tw.
anidrosan$.tw.
HSP90$.tw.
mycograb$.tw.
antibodies, Monoclonal/tu
or/1‐44
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
or/46‐52
humans.sh.
53 and 54
boy$.tw.
girl$.tw.
infant.tw.
pediatr$.tw.
paediatr$.tw.
infant/
exp child/
exp adolescent/
or/56‐63
45 and 55 and 64
Appendix 2. CENTRAL search strategy
Antifungal Agents/
exp amphotericin B/
fungizon$.tw
ampho$.tw.
anfo$.tw.
candipres$.tw.
ABLC$.tw.
ABCD$.tw.
abelcet$.tw.
ambisome$.tw.
exp triazoles/
triazole$.tw.
exp fluconazole/
flucon$.tw.
diflucan$.tw.
exp Itraconazole/
itracon$.tw.
sporanox$.tw.
voriconazol$.tw.
vfend$.tw.
posaconazol$.tw.
posafilin$.tw.
posanin$.tw.
ravuconazol$.tw.
exp Flucytosine/
flucytosin$.tw.
ancotil$.tw.
5FC$.tw.
5‐fluorocytosin$.tw.
ancobon$.tw.
ancotil$.tw.
alcobon$.tw.
echinocandin$.tw.
caspofungin$.tw.
cancidas$.tw.
micafungin$.tw.
funguard$.tw.
mycamine$.tw.
anidulafungin$.tw.
anidrasona$.tw.
anidrosan$.tw.
HSP90$.tw.
mycograb$.tw.
antibodies, Monoclonal/tu
or/1‐44
boy$.tw.
girl$.tw.
infant.tw.
child.tw
pediatr.tw.
chill$.tw
pediatr$.tw
paediatr$.tw.
infant/
exp child
or/46‐55
45 and 56
Appendix 3. EMBASE search strategy
Antifungal Agents/
exp amphotericin B/
fungizon$.tw
ampho$.tw.
anfo$.tw.
candipres$.tw.
ABLC$.tw.
ABCD$.tw.
abelcet$.tw.
ambisome$.tw.
exp triazoles/
triazole$.tw.
exp fluconazole/
flucon$.tw.
diflucan$.tw.
exp Itraconazole/
itracon$.tw.
sporanox$.tw.
voriconazol$.tw.
vfend$.tw.
posaconazol$.tw.
posafilin$.tw.
posanin$.tw.
ravuconazol$.tw.
exp Flucytosine/
flucytosin$.tw.
ancotil$.tw.
5FC$.tw.
5‐fluorocytosin$.tw.
ancobon$.tw.
ancotil$.tw.
alcobon$.tw.
echinocandin$.tw.
caspofungin$.tw.
cancidas$.tw.
micafungin$.tw.
funguard$.tw.
mycamine$.tw.
anidulafungin$.tw.
anidrasona$.tw.
anidrosan$.tw.
HSP90$.tw.
mycograb$.tw.
antibodies, Monoclonal/tu
or/1‐44
exp controlled study/ or controlled study.ti,ab,hw,tn,mf.
exp statistical analysis/ or clinical study.ti,ab,hw,tn,mf.
exp major clinical study/ or major clinical study.ti,ab,hw,tn,mf.
exp randomised controlled trial/ or randomised controlled study.ti,ab,hw,tn,mf.
exp randomized controlled trial/ or randomized controlled study.ti,ab,hw,tn,mf.
random$.ti,ab,hw,tn,mf.
exp double blind procedure/ or double blind procedure.ti,ab,hw,tn,mf.
exp single blind procedure/ or single blind procedure.ti,ab,hw,tn,mf.
exp multicenter study/ or multicenter study.ti,ab,hw,tn,mf.
exp placebo/ or placebo.ti,ab,hw,tn,mf.
or/46‐55
boy$.tw.
girl$.tw.
infant.tw.
child.tw
pediatr.tw.
child$.tw.
pediatr$.tw.
paediatr$.tw.
infant/
exp child/
exp adolescent/
or/57‐67
45 and 56 and 68
Appendix 4. CINAHL search strategy
Antifungal agents/
Triazoles
Amphotericin B
Fluconazole
Itraconazole
Flucytosine
or/1‐6
Clinical trials
7 and 8
Limit to Infant (1 to 23 month) or Child (preschool 2 to 5) or Child (6 to 12) or Adolescent (13 to18)
Appendix 5. Data extraction form 1
| Reference | |||
| First author | Journal | ||
| Year | Source (medline, cochrane etc) | ||
| Title | |||
| Language | |||
| Reference available (Yes or No) | Additional data provided (Yes/No) | ||
| Authors contacted (date 1) | Additional data received (date) | ||
| Authors contacted (date 2) | |||
| Disease (e.g. candidaemia) | |||
| Age limits (years) | |||
| Comparator A | Comparator B | ||
| Drug | Drug | ||
| Formulation | Formulation | ||
| Dose | Dose | ||
| Assessment of risk of bias (yes, no or unclear) | |||
| (1) Was the allocation sequence adequately generated? | |||
| (2) Was allocation adequately concealed? | |||
| (3) Was knowledge of the allocation interventions adequately prevented during the study? | |||
| (4) Were incomplete outcome data adequately addressed? | |||
| (5) Are reports of the study free of suggestion of selective outcome reporting? | |||
| (6) Was the study apparently free of other problems that could put it at a risk of bias? | |||
| Participants (characteristics of patient population) | |||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Follow up period | |||
| Flow diagram (children only) | Total | Comparator A | Comparator B |
| Number eligible | |||
| Excluded pre‐allocation | |||
| Numbers entered | |||
| Excluded post‐allocation | |||
| Number analysed | |||
Appendix 6. Data extraction form 2
| Reference | ||||||
| Dichotomous variables | Definition | Time point | Comparator A | Comparator B | ||
| Number assessed | Number with outcome | Number assessed | Number with outcome | |||
| Primary outcomes | ||||||
| (1) All cause mortality | Death of any randomised patient regardless of cause | End of therapy | ||||
| Other time point ______________ | ||||||
| (2) IFI related mortality | Death of a patient diagnosed with IFI whose death is attributable to IFI | End of therapy | ||||
| Other time point ______________ | ||||||
| (3) Complete resolution of fungal infection | Complete resolution of clinical symptoms +/‐ radiological or microbiological findings that led to diagnosis of IFI and enabling cessation of anti‐fungal medication | End of therapy | ||||
| Secondary outcomes | ||||||
| (4) Complete or partial resolution of fungal infection | Any improvement in clinical symptoms +/‐ radiological findings that led to diagnosis of IFI yet not fulfilling criteria for complete resolution | End of therapy | ||||
| (5) Resolution of fever in suspected fungal infection | Absence of temperature > 38°C for more than 72 hours | |||||
| (6) Progression of disease requiring change / addition of an antifungal agent | Progression of existing symptoms +/‐ radiological or microbiological findings that are of enough concern for a clinician to change anti‐fungal dose or add a new anti‐fungal agent | |||||
| (7) Breakthrough fungal infection requiring change / addition of an antifungal agent | New clinical symptoms or signs +/‐ radiological or microbiological findings that are of enough concern for clinicians to change antifungal dose or add a new anti‐fungal agent | |||||
| (8) Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy | ||||||
| (9) Any adverse reaction attributed to the antifungal agent including | ||||||
| (9a) Abnormal renal function | ≥ 100% rise in serum creatinine | |||||
| Other definition of nephrotoxicity ________________________ | ||||||
| (9b) electrolyte imbalance | hypokalaemia < 3.0mmol/L | |||||
| other definition of hypokalaemia | ||||||
| other definitions of electrolyte imbalance | ||||||
| (9c) abnormal hepatic function | transaminases > 2x normal values for age | |||||
| Other definition of hepatotoxicity ________________________ | ||||||
| (9d) infusion related reactions | Any infusion related reactions | |||||
| Chills (definition ______________________________________) | ||||||
| Fevers (definition ______________________________________) | ||||||
| Other infusion related reaction (definition _______________) | ||||||
| (9e) gastrointestinal disturbances such as nausea, vomiting or diarrhoea | Any gastrointestinal disturbances | |||||
| Nausea | ||||||
| Vomiting | ||||||
| Diarrhoea | ||||||
| (9f) neurological disturbances such as blurred vision or dizziness | Any neurological disturbances | |||||
| Blurred vision | ||||||
| Dizziness | ||||||
| Other neurological disturbances | ||||||
| (9g) Haematological disturbances such as anaemia, granulocytopaenia or thrombocytopaenia | Any haematological disturbances | |||||
| Anaemia (definition ___________________________________) | ||||||
| Granulocytopaenia (definition __________________________) | ||||||
| Thrombocytopaenia (definition _________________________) | ||||||
| Dichotomous variables | Definition | Time point | Comparator A | Comparator B | ||
| Number assessed | Mean (SD) | Number assessed | Mean (SD) | |||
| Secondary outcomes | ||||||
| (10) Length of stay | number of days | |||||
| (11) Quality of life | QOL (definition________________________________________) | |||||
| (12) Cost | $US | |||||
Data and analyses
Comparison 1. Children with fever and neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all cause) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.17, 3.11] |
| 2 Mortality (related to fungal infection) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.98] |
| 3 Complete resolution of fungal infection | 1 | 5 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.18, 12.46] |
| 4 Partial resolution of fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Resolution of fever in suspected fungal infection | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.00, 1.52] |
| 6 Progression of disease requiring change / addition of an antifungal agent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Breakthrough fungal infection requiring change / addition of an antifungal agent | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.24, 1.84] |
| 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Any clinically significant adverse reactions attributed to the antifungal agent (total) | 2 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.17] |
| 10 Nephrotoxicity | 4 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.21, 0.90] |
| 11 Hypokalaemia | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.14] |
| 12 Hepatotoxicity | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.74, 1.71] |
| 13 Infusion‐related reactions | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.38] |
| 13.1 Fever ‐ Liposomal AmB vs. Conventional Amphotericin B | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.86, 1.11] |
| 13.2 Fever ‐ ABCD vs. Conventional Amphotericin B | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 13.3 Chills ‐ Liposomal AmB vs. Conventional Amphotericin B | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.21, 0.64] |
| 13.4 Chills ‐ ABCD vs. Conventional Amphotericin B | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.09, 2.85] |
| 14 Haematological disturbances | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Neurological disturbances | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Gastrointesitnal toxicity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Length of Stay | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Quality of Life | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Cost | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Children with fever and neutropenia: caspofungin versus liposomal amphotericin B (3 mg/kg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all cause) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Mortality (related to fungal infection) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Complete resolution of fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Partial resolution of fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Resolution of fever in suspected fungal infection | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.70, 2.56] |
| 6 Progression of disease requiring change / addition of an antifungal agent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Breakthrough fungal infection requiring change / addition of an antifungal agent | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 3.61] |
| 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.06, 1.74] |
| 9 Any clinically significant adverse reactions attributed to the antifungal agent (total) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.64, 1.72] |
| 10 Nephrotoxicity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Hypokalaemia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Hepatotoxicity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Infusion‐related reactions | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Gastrointestinal toxicity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Neurological disturbances | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Haematological disturbances | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Length of Stay | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Quality of Life | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Cost | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.2. Analysis.
Comparison 2 Children with fever and neutropenia: caspofungin versus liposomal amphotericin B (3 mg/kg), Outcome 2 Mortality (related to fungal infection).
2.8. Analysis.
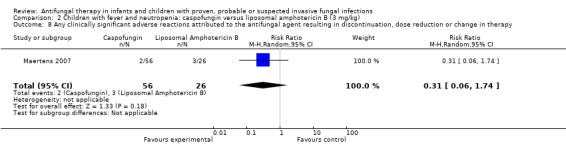
Comparison 2 Children with fever and neutropenia: caspofungin versus liposomal amphotericin B (3 mg/kg), Outcome 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy.
2.9. Analysis.

Comparison 2 Children with fever and neutropenia: caspofungin versus liposomal amphotericin B (3 mg/kg), Outcome 9 Any clinically significant adverse reactions attributed to the antifungal agent (total).
Comparison 3. Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all cause) | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.53, 2.02] |
| 2 Mortality (related to fungal infection) | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.33, 5.89] |
| 3 Complete resolution of fungal infection | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.68, 1.13] |
| 3.1 Age < 2 years | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.22] |
| 3.2 Age 2 or older | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.57, 1.31] |
| 4 Partial resolution of fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Resolution of fever in suspected fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Progression of disease requiring change / addition of an antifungal agent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Breakthrough fungal infection requiring change / addition of an antifungal agent | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.22] |
| 9 Any clinically significant adverse reactions attributed to the antifungal agent (total) | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.38] |
| 10 Nephrotoxicity | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Hypokalaemia | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.27] |
| 12 Hepatotoxicity | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.19, 20.12] |
| 13 Infusion‐related reactions | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.30, 1.77] |
| 14 Gastrointestinal toxicity | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.49, 2.22] |
| 14.1 Vomiting | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.46, 3.04] |
| 14.2 Diarrhoea | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.24, 2.92] |
| 15 Neurological disturbances | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Haematological disturbances | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.60, 4.07] |
| 17 Length of stay | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Quality of life | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Cost | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.7. Analysis.

Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 7 Breakthrough fungal infection requiring change / addition of an antifungal agent.
3.10. Analysis.
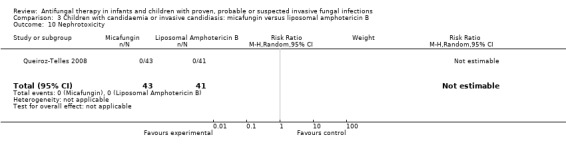
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 10 Nephrotoxicity.
3.15. Analysis.
Comparison 3 Children with candidaemia or invasive candidiasis: micafungin versus liposomal amphotericin B, Outcome 15 Neurological disturbances.
Comparison 4. Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all cause) | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.11, 2.56] |
| 2 Mortality (related to fungal infection) | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.07, 15.69] |
| 3 Complete resolution of fungal infection | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.32] |
| 4 Partial resolution of fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Resolution of fever in suspected fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Progression of disease requiring change / addition of an antifungal agent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Breakthrough fungal infection requiring change / addition of an antifungal agent | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Any clinically significant adverse reactions attributed to the antifungal agent (total) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Nephrotoxicity | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.13, 3.72] |
| 11 Hypokalaemia | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.25, 4.54] |
| 12 Hepatotoxicity | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.07, 15.62] |
| 13 Infusion‐related reactions | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Gastrointesintal toxicity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Neurological disturbances | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Haematological disturbances | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Length of stay | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Quality of life | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Cost | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.7. Analysis.
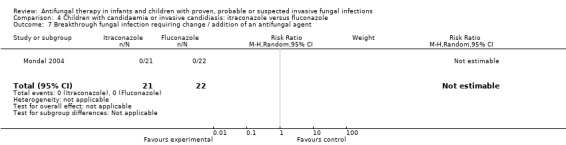
Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 7 Breakthrough fungal infection requiring change / addition of an antifungal agent.
4.8. Analysis.

Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy.
4.13. Analysis.
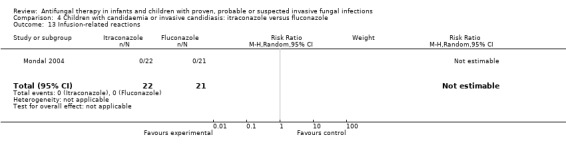
Comparison 4 Children with candidaemia or invasive candidiasis: itraconazole versus fluconazole, Outcome 13 Infusion‐related reactions.
Comparison 5. All children: echinocandin versus lipid preparation of amphotericin B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all cause) | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.76, 4.17] |
| 2 Mortality (related to fungal infection) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Complete resolution of fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Partial resolution of fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Resolution of fever in suspected fungal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Progression of disease requiring change / addition of an antifungal agent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Breakthrough fungal infection requiring change / addition of an antifungal agent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Any clinically significant adverse reactions attributed to the antifungal agent resulting in discontinuation, dose reduction or change in therapy | 2 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.08, 1.27] |
| 9 Any clinically significant adverse reactions attributed to the antifungal agent (total) | 2 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.33] |
| 10 Nephrotoxicity | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Hypokalaemia | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.27] |
| 12 Hepatotoxicity (>2.5 X ULN) | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.16, 17.32] |
| 13 Infusion‐related reactions | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.30, 1.77] |
| 14 Gastrointesintal toxicity | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Neurological disturbances | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Haematological disturbances | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.60, 4.07] |
| 17 Length of stay | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Quality of life | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Cost | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
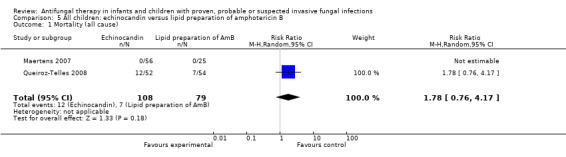
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 1 Mortality (all cause).
5.10. Analysis.
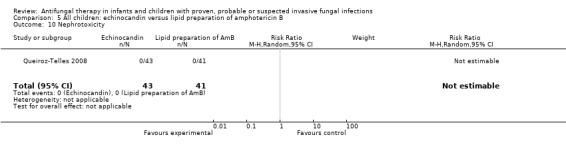
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 10 Nephrotoxicity.
5.11. Analysis.
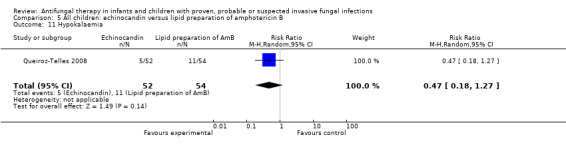
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 11 Hypokalaemia.
5.12. Analysis.
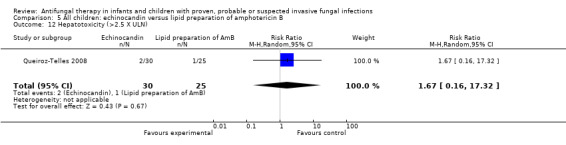
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 12 Hepatotoxicity (>2.5 X ULN).
5.13. Analysis.
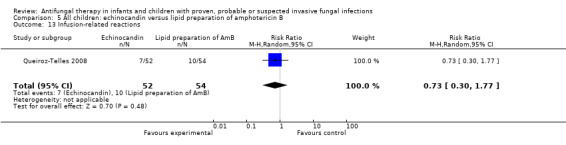
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 13 Infusion‐related reactions.
5.15. Analysis.
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 15 Neurological disturbances.
5.16. Analysis.
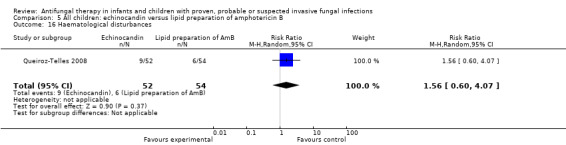
Comparison 5 All children: echinocandin versus lipid preparation of amphotericin B, Outcome 16 Haematological disturbances.
Comparison 6. Children with fever, neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B (best case for lipid).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nephrotoxicity | 4 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.20, 0.89] |
Comparison 7. Children with fever, neutropenia: lipid preparation of amphotericin B versus conventional amphotericin B (worst case for lipid).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nephrotoxicity | 4 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Maertens 2007.
| Methods | Multicentred, international (Belgium, Germany, Spain, US), doubled blind, randomised trial | |
| Participants | Paediatric patients (2 ‐ 17 years) with fever and neutropenia. Neutropenia was defined as ANC < 0.5x109/L. Pyrexia of unknown origin was defined as 96 hours of fever > 38°C not responding to broad‐spectrum antibacterial therapy | |
| Interventions | Caspofungin versus liposomal amphotericin B Patients were enrolled in a 2:1 fashion comparing caspofungin (70mg/m2 on day 1 followed by 50mg/m2) with liposomal amphotericin B (Ambisome® 3mg/kg/day). An increase to caspofungin 70 mg/m2 (maximum 70 mg daily) or liposomal amphotericin B 5 mg/kg was allowed after 5 days. The period of follow up was 7 days following completion of therapy |
|
| Outcomes | Primary endpoint: the proportion of patients with more than one drug‐related adverse event during the study period and 14 days post‐therapy Secondary endpoints: drug‐related serious adverse events, discontinuation due to drug‐related adverse events and proportion of patients with a favourable overall efficacy outcome based on a five‐part composite endpoint (Walsh 2004a). Treatment was judged to be successful if all of the following criteria were met: i) successful treatment of any baseline fungal infection, ii) absence of any breakthrough fungal infection during therapy or within seven days after the completion of therapy, iii) survival for seven days after the completion of therapy, iv) no premature discontinuation of study therapy because of drug related toxicity or lack of efficacy, and v) resolution of fever (defined as a temperature below 38°C for at least 48 hours) during neutropenia. |
|
| Notes | Paediatric population only (range: 2 to 17 years). Authors contacted for further data (3/12/2008). Data was only available in abstract form. Some absolute numbers are not yet published so therefore for pooled analyses, some absolute numbers were calculated from percentages. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear from the abstract although it is likely to be adequate as the study was modelled on Walsh 2004a. |
| Allocation concealment? | Unclear risk | Unclear from the abstract although it is likely to be adequate as the study was modelled on Walsh 2004a. |
| Blinding? All outcomes | Low risk | Patients and investigators were blinded to the treatment administered. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Patients enrolled: 82 patients. One patient had no screening temperature above 38°C. Modified Intention to treat population: 81 patients. Five patients received < 4 days of study therapy, 1 patient received concomitant antifungal therapy and 14 patients had other protocol violations. Evaluable‐patient population: 65 patients. |
| Free of selective reporting? | Unclear risk | All expected outcomes are included in the abstract however full data was not available. |
| Free of other bias? | Unclear risk | The study appears to be free from other sources of bias although only published in an abstract form. |
Mondal 2004.
| Methods | Single‐centre (India) double‐blind randomised trial conducted in a paediatric intensive care unit | |
| Participants | Pediatric patients (1 month ‐ 12 years) with candidaemia | |
| Interventions | Itraconazole versus fluconazole. Itraconazole (10mg/kg daily either orally or via gastric tube) or fluconazole (10mg/kg daily either orally or via gastric tube) were administered. The therapy was continued ≥1 wk after the blood culture become negative. Minimum duration of therapy was 2 weeks. Patients were followed for a median of 8 weeks | |
| Outcomes | Primary endpoint: cure (treatment success) included both clinical and mycological endpoints. Clinical cure was defined as complete resolution of fever and improvement in signs and symptoms present at the time of enrolment. Mycological cure was defined as no growth of yeast in blood culture on two consecutive occasions 48 hrs apart Secondary endpoints: all‐cause mortality, IFI related mortality (candida‐attributable mortality was defined as death of a patient who had documented candidaemia (positive blood culture for Candida) within the previous 48 hrs), toxicities |
|
| Notes | Paediatric population only (range: 0 to 12 years). Authors contacted for further data (17/11/2008). Further data was received (11/12/2008). The study was conducted during an outbreak ofC. pelliculosa with 62% of cases caused by this unusual species. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was achieved by using random tables. |
| Allocation concealment? | Low risk | A person not directly involved in the study made case allocation as per random number. The envelopes containing drug were stored with a member of staff who was responsible for case allocation. |
| Blinding? All outcomes | Low risk | Patients and investigators were blinded to the treatment administered. |
| Incomplete outcome data addressed? All outcomes | Low risk | Patients enrolled: 43 patients. Six patients received less than 1 week of therapy (3 patients each receiving itraconazole and fluconazole): Itraconazole; 1 patient died, two discontinued therapy. fluconazole; 2 patients died, one discontinued therapy. |
| Free of selective reporting? | Low risk | All expected outcomes are included in the report. |
| Free of other bias? | High risk | One potential bias given the small numbers in the study is the discontinuation of treatment due to social and financial reasons. Two patients receiving itraconazole, one patient receiving fluconazole and a further patient in whom the therapy is unclear left hospital due to financial and social reasons. Three patients discontinued all therapy (itraconazole; 2 patients, fluconazole; 1 patient) whereas the forth patients continued azole therapy at home. |
Prentice 1997.
| Methods | Multicentred, international (UK, Europe) open‐label randomised trial. Adult studies (104‐10) and paediatric studies (104‐14) are presented in the same paper | |
| Participants | Neutropenic children and adults (ANC < 0.5x109/L) with pyrexia of unknown origin (96 hours of fever defined as a temperature ≥ 38°C not responding to broad‐spectrum antibacterial therapy) | |
| Interventions | Liposomal amphotericin B versus conventional amphotericin B Liposomal amphotericin B (Ambisome® 1 and 3mg/kg/day) was compared with conventional amphotericin B (1mg/kg/day) Therapy was continued until resolution of fever (<38°C), recovery of neutrophils to above or equal to 0.5x109/L for 3 consecutive days, patient death, unresolved toxicity, patient or physician request to withdraw |
|
| Outcomes | Primary endpoint::safety Secondary endpoints: efficacy or treatment response which was defined by a minimum of 3 consecutive days without fever (<38°C) that continued until study end, indicated by recovery of neutrophils to 0.5x109/L. Failure was defined as patients who remained febrile at the of study, patients who developed a systemic fungal infection on study and patients in whom a further systemic fungal agent was added |
|
| Notes | Paediatric and adult population. Authors contacted for further data (5/12/2008). No further data were available The study contains two difference concentrations of liposomal amphotericin B (1mg/kg and 3mg/kg). These have been pooled for some comparisons. Toxicity endpoints are only expressed as percentages without clear denominators. It was therefore necessary to calculate numbers from percentages. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was done centrally and each participating centre was provided with a set of blinded, numbered envelopes which required sequential opening. The arms were balanced per centre and per block of six patients. |
| Allocation concealment? | Low risk | Blinded, numbered envelopes were provided by study investigators preventing allocation concealment. |
| Blinding? All outcomes | High risk | The trial was open labelled. |
| Incomplete outcome data addressed? All outcomes | High risk | Patients enrolled: 204 children and 134 adults. Of 204 randomised children, 202 reported with 2 charts not available for review. Toxicity endpoints were expressed as percentages in both adults and children. Nephrotoxicity data was calculated from 305 adults and children however 338 subjects were evaluable for safety. It was not clear from the report why these patients were not included in the analysis. |
| Free of selective reporting? | Unclear risk | Given the incomplete outcome data, selective reporting is potentially a problem. |
| Free of other bias? | Low risk | The study appeared to be free from other sources of bias. |
Queiroz‐Telles 2008.
| Methods | Multicentred, international (Europe, North and South America, Thailand, India) double‐blind randomised trial (a substudy of the larger study inclusive of adults and children) | |
| Participants | Paediatric patients (0 ‐ 16 years) with candidaemia or Invasive candidiasis | |
| Interventions | Micafungin versus liposomal amphotericin B. Micafungin was administered at a daily dose of 2 mg/kg of body weight for patients who weighed ≤40 kg and 100 mg for patients who weighed >40 kg. Liposomal amphotericin B was administered at a daily dose of 3 mg/kg of body weight. The recommended minimum duration of study drug therapy was 14 days. The period of follow up was 12 weeks | |
| Outcomes | Primary endpoint: treatment success which included both clinical and mycological endpoints. Clinical response was defined as a complete or partial resolution of signs and symptoms, and mycologic response was defined as eradication or presumed eradication Secondary endpoints: all‐cause mortality, IFI‐related mortality, toxicities |
|
| Notes | Paediatric population only (range: 0 to 16 years). Neonates could not be excluded for mortality and toxicity endpoints and so have been included in the analysis. Authors contacted for further data (17/11/2008). No further data were available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was achieved by computer generated system and stratified by centre and baseline neutropenic status. |
| Allocation concealment? | Low risk | Central allocation prevented patients and investigators from identifying assignment. |
| Blinding? All outcomes | Low risk | Patients and investigators were blinded to the treatment administered. Preparation of the study drug infusion bottle and changes in dose were managed by an unblinded staff member of the centre, and an unblinded study monitor reviewed drug accountability records. |
| Incomplete outcome data addressed? All outcomes | Low risk | Patients enrolled: 109 patients. Three patients did not receive any drug. Intention to treat population: 106 patients. Eight patients did not have candidaemia infection confirmed. Modified intention to treat population: 98 patients. |
| Free of selective reporting? | Low risk | All expected outcomes were included in the report. |
| Free of other bias? | Low risk | The study appeared to be free from other sources of bias. |
Sandler 2000.
| Methods | Multicentred, double‐blind randomised trial | |
| Participants | Patients were eligible for the study if they were neutropenic following chemotherapy or hematopoietic stem cell transplantation. Neutropenic children (ANC ≤ 0.5x109/L) were enrolled if they had pyrexia of unknown origin (72 hours of fever not responding to broad‐spectrum antibacterial therapy) | |
| Interventions | Amphotericin B colloidal dispersion (ABCD) versus conventional amphotericin B ABCD (4mg/kg/day) was compared with conventional amphotericin B (0.8mg/kg/day). A patient remained in this study until one of these events occurred: i) the patient was no longer neutropenic (absolute neutrophil count >500/mm3), ii) the patient's cause of fever was identified, iii) the patient experienced a grade 3 drug‐related toxicity, iv) the patient received study drug for 14 days. To be evaluable, a patient had to receive therapy for a minimum of 7 days and not use another systemic antifungal medication. The period of follow up was 7 days after the last dose of trial drug |
|
| Outcomes | Endpoint 1: treatment success which included: i) survival of at least 7 days after the last dose of study drug; ii) no documented or suspected fungal infection during the study and within 7 days post study; iii) not prematurely terminated from the study because of any toxicities; and iv) either afebrile or with infusion‐associated fever only on the last day of therapy Endpoint 2: toxicity |
|
| Notes | Paediatric population only (range: 0 to 15 years). Nephrotoxicity data is reported in the paper. Other toxicity data is not reliable as denominator is not specified and number of patients not calculable from percentage. It is possible that Sandler et al (2000) and White et al (1998) may contain the same patients as the sponsor is the same, the methods are very similar, many of the institutions contributed data to both studies and number of paediatric patients enrolled are similar. Authors contacted for further data (4/12/2008). No further data were available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not specified. |
| Allocation concealment? | Unclear risk | Insufficient methodological information about method of concealment to permit judgement. |
| Blinding? All outcomes | Low risk | Patients and investigators were blinded to the treatment administered. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Patients enrolled: 49 patients. Evaluable population: 46 patients. |
| Free of selective reporting? | Low risk | All expected outcomes were included in the report.. |
| Free of other bias? | Low risk | The study appeared to be free from other sources of bias. |
Walsh 1999.
| Methods | Multicentred, national (US), doubled‐blind, randomised trial | |
| Participants | Paediatric and adult patients with fever and neutropenia. Neutropenia was defined as ANC < 0.5x109/L. Pyrexia of unknown origin was defined as 120 hours of fever > 38°C not responding to broad‐spectrum antibacterial therapy | |
| Interventions | Liposomal amphotericin B versus conventional amphotericin B Liposomal amphotericin B (Ambisome® 3mg/kg/day) was compared with conventional amphotericin B (1mg/kg/day). Patients received study drug until recovery from neutropenia. The period of follow up was 7 days following after completion of therapy |
|
| Outcomes | Primary endpoint: the proportion of patients with favourable overall efficacy outcome based on a five‐part composite endpoint: i) survival for seven days after initiation of the study drug, ii) resolution of fever during the period of neutropenia, iii) successful treatment of baseline fungal infection, iv) the absence of breakthrough fungal infection during therapy or within seven days after the completion of therapy and v) absence of premature discontinuation of the study drug because of toxicity or lack of efficacy Secondary endpoints: all‐cause mortality and adverse events including severe adverse events and drug related adverse events |
|
| Notes | Paediatric and adult population (age 2 to 80 years). Authors contacted for further data (17/11/2008). Further data was received (13/3/2008). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was achieved by computer generated system. |
| Allocation concealment? | Low risk | Central allocation prevented patients and investigators from identifying assignment. |
| Blinding? All outcomes | Low risk | All patients, investigators, industrial sponsors and study coordinators were blinded to the treatment administered. |
| Incomplete outcome data addressed? All outcomes | Low risk | Patients enrolled: 702 patients, intention‐to‐treat population: 687 patients. The reason for the exclusion of 15 patients from the intention to treat population is not clear. All patients in the intention to treat population were included in the assessment of efficacy and toxicity. |
| Free of selective reporting? | Low risk | All expected outcomes were included in the report. |
| Free of other bias? | Low risk | The study appeared to be free from other sources of bias. |
White 1998.
| Methods | Multicentred, double‐blind randomised trial | |
| Participants | Patients were eligible for the study if they were neutropenic following chemotherapy for hematologic malignancy or had undergone marrow or stem cell transplantation in the previous 3 months. Neutropenic children and adults (ANC ≤ 0.5x109/L or ANC ≤ 1x109/L and expected to decline to ≤ 0.5x109/L within 2 days ) were enrolled if they had pyrexia of unknown origin (72 hours of fever defined as a temperature ≥ 38°C not responding to broad‐spectrum antibacterial therapy) or recrudescent fever (temperature ≥ 38°C on two or more occasions after initial defervescence with antibacterial treatment) | |
| Interventions | Amphotericin B colloidal dispersion (ABCD) versus conventional amphotericin B ABCD (4mg/kg/day) was compared with conventional amphotericin B (0.8mg/kg/day). Therapy was continued until recovery of neutrophils to > 0.5x109/L for 2 consecutive days, identification of an infection thought to be the cause of the fever, toxicity leading to study drug discontinuation or a maximum of 14 days. The period of follow up was 28 days |
|
| Outcomes | Endpoint 1: treatment success which included survival for ≥ 7 days after the last study drug, lack of suspected or documented fungal infection during the study and within 7 days of the last dose of the study drug, lack of study drug discontinuation because of adverse events and lack of fever on the day of discontinuation of therapy Endpoint 2: toxicity |
|
| Notes | Paediatric and adult population. Authors contacted for further data (5/12/2008). No further data were available. The authors stated that no difference between groups (i.e. children and adults administered conventional amphotericin B and ABCD) in efficacy endpoints, breakthrough fungal infection and drug discontinuation however data not available in the report. Rates of infusional toxicity more frequent with ABCD but subgroup analysis not reported. It is possible that Sandler et al (2000) and White et al (1998) may contain the same patients as the sponsor is the same, the methods were very similar, many of the institutions contributed data to both studies and number of paediatric patients enrolled were similar. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was performed centrally with randomisation tables. |
| Allocation concealment? | Low risk | Central allocation prevented patients and investigators from identifying assignment. |
| Blinding? All outcomes | Low risk | Patients and investigators were blinded to the treatment administered. Preparation of the study drug was performed by an unblinded site pharmacist. |
| Incomplete outcome data addressed? All outcomes | Low risk | Patients enrolled: 213 patients. Seventeen patients were not evaluable because of study drug discontinuation before reaching a study endpoint or because of receipt of concomitant systemic antifungal therapy. Analysis performed on 196 patients. Three patients lost to follow up so response was assessed on 193 patients. |
| Free of selective reporting? | Low risk | All expected outcomes were included in the report however efficacy and infusional toxicity for children were not reported separately. |
| Free of other bias? | Low risk | The study appeared to be free from other sources of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arrietta 2006 | Published as Queiroz‐Telles 2008. |
| Arrietta 2007 | Published as Queiroz‐Telles 2008. |
| Bodhe 2002 | The authors were contacted for data from the included paediatric cases (11th May 2009). Data were not available. |
| Bowden 2002 | The authors were contacted for data from the included paediatric cases (18th November 2008). Data were not available. |
| Cornely 2007 | The authors were contacted for data from the included paediatric cases (18th November 2008). Data were not available. |
| Driessen 1996 | Neonatal study: Age limit of those included was 0‐30 days. |
| Ellis 1995 | Not a randomised controlled trial. |
| Ellis 1998 | The authors were contacted for data from the included paediatric cases (18th November 2008). Data were not available. |
| Galgiani 2000 | The authors were contacted for data from the included paediatric cases (18th November 2008). Data were not available. |
| Herbrecht 2002 | Authors contacted for data from included neonatal and paediatric cases (18th November 2008). Data was not available. |
| Khayat 2005 | Retrospective comparison therefore excluded. |
| Kim 2007 | No contact details were available for authors. Insufficient data in abstract for analysis. |
| Kullberg 2005 | The authors were contacted for data from the included paediatric cases (18th November 2008). Data were not available. |
| Pizzo 1982 | The authors were contacted for data from the included paediatric cases (18th November 2008). Data were not available. |
| Rex 1994 | The authors were contacted (18th November 2008). No children included in study. |
| Shikanai‐Yasuda 2002 | The authors were contacted for data from the included paediatric cases (11th May 2009). Data were not available. |
| Viscoli 1996 | The authors were contacted for data from the included paediatric cases (5th December 2008). Data were not available. |
| Walsh 2002 | The authors and pharmaceutical company were contacted for data from the included paediatric cases (11th March 2009). Data were not available. |
| Wang 2007 | Manuscript was translated from Chinese to English. Paediatric subgroup analysis was not reported in manuscript. |
| Wheat 2001 | Not a randomised controlled trial. |
| Wingard 2000 | The authors were contacted for data from the included paediatric cases (4th December 2008). Data was not available. |
| Winston 2000 | The authors were contacted for data from the included paediatric cases (5th December 2008). Data were not available. |
| Yao 2000 | Not a randomised controlled trial. |
Differences between protocol and review
The age range published in the original protocol was between 28 days and 18 years. This has been amended to between 28 days and 16 years as this reflects the more frequently used age range in clinical trials.
The sensitive search strategy was modified as per the Cochrane Handbook of Systematic Reviews of Intervention, Version 5.0.1, September 2008, Chapter 6.4.11.
Contributions of authors
All authors were involved in writing and reviewing the review.
Declarations of interest
Dr Christopher Blyth and Dr Katherine Hale are members of the Australian and New Zealand Mycology Interest Group, which is sponsored by Gilead Science, Pfizer, Merck Sharp & Dohme and Schering‐Plough.
Dr Katherine Hale has received investigator initiated research funding from Gilead Sciences and Pfizer for other research projects.
Edited (no change to conclusions)
References
References to studies included in this review
Maertens 2007 {published and unpublished data}
- Maertens J, Madero L, Reilly A, Lehrnbecher T, Groll A, Jafri H, et al. A randomized, double blind, multicenter trial of caspofungin (CAS) versus (vs) liposomal amphotericin B (LAMB) for empirical antifungal therapy (EAFRx) of pediatric patients (pts) with persistent fever & neutropenia (PFN). Program and abstracts of the 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, USA [Abstract M‐621]. 2007 Sept.
Mondal 2004 {published and unpublished data}
- Mondal RK, Singhi SC, Chakrabarti A, Jayashree M. Randomized comparison between fluconazole and itraconazole for the treatment of candidemia in a pediatric intensive care unit: a preliminary study. Pediatric Critical Care Medicine 2004;5(6):561‐5. [DOI] [PubMed] [Google Scholar]
Prentice 1997 {published data only (unpublished sought but not used)}
- Prentice HG, Hann IM, Herbrecht R, Aoun M, Kvaloy S, Catovsky D, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. British Journal of Haematology 1997;98(3):711‐8. [DOI] [PubMed] [Google Scholar]
Queiroz‐Telles 2008 {published data only (unpublished sought but not used)}
- Queiroz‐Telles F, Berezin E, Leverger G, Freire A, Vyver A, Chotpitayasunondh T et al for the Micafungin Invasive Candidiasis Study Group. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: Substudy of a randomized double‐blind trial. Pediatric Infectious Disease Journal 2008;27(9):820–6. [DOI] [PubMed] [Google Scholar]
Sandler 2000 {published data only (unpublished sought but not used)}
- Sandler ES, Mustafa MM, Tkaczewski I, Graham ML, Morrison VA, Green M, et al. Use of amphotericin B colloidal dispersion in children. Journal of Pediatric Hematology/Oncology 2000;22(3):242‐6. [DOI] [PubMed] [Google Scholar]
Walsh 1999 {published and unpublished data}
- Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. New England Journal of Medicine 1999;340(10):764‐71. [DOI] [PubMed] [Google Scholar]
White 1998 {published data only (unpublished sought but not used)}
- White MH, Bowden RA, Sandler ES, Graham ML, Noskin GA, Wingard JR, et al. Randomized, double‐blind clinical trial of amphotericin B colloidal dispersion vs. amphotericin B in the empirical treatment of fever and neutropenia. Clinical Infectious Diseases 1998;27(2):296‐302. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arrietta 2006 {published data only}
- Arrieta AC, Telles Filho F, Berezin E, Freire A, Diekmann‐Berndt H. A randomized, double‐blind trial comparing micafungin (MCFG) and liposomal amphotericin B (L‐AMB) in pediatric patients with invasive candidiasis.. Program and abstracts of the 46th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco USA [Abstract M‐1308b] . 2006 Sept.
Arrietta 2007 {published data only}
- Arrieta A, Queiroz‐Telles F, Berezin E, Freire A, Diekmann S, Koblinger S. Micafungin versus liposomal amphotericin B (AmBisome®) in paediatric patients with invasive candidiasis or candidaemia. Program and abstracts of the 17th European Congress of Clinical Microbiology and Infectious Diseases. Munich, Germany [Abstract O141]. 2007 April.
Bodhe 2002 {published data only}
- Bodhe PV, Kotwani RN, Kirodian BG, Kshirsagar NA, Pandya SK. Open label, randomised, comparative phase III safety and efficacy study with conventional amphotericin B and liposomal amphotericin B in patients with systemic fungal infection. Journal of the Association of Physicians India 2002;50(5):662‐70. [PubMed] [Google Scholar]
Bowden 2002 {published data only}
- Bowden R, Chandrasekar P, White MH, Li X, Pietrelli L, Gurwith M, Burik JA, et al. A double‐blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clinical Infectious Diseases 2002;35(4):359‐66. [DOI] [PubMed] [Google Scholar]
Cornely 2007 {published data only}
- Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, et al. AmBiLoad Trial Study Group. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high‐loading dose regimen with standard dosing (AmBiLoad trial). Clinical Infectious Diseases 2007;44(10):1289‐97. [DOI] [PubMed] [Google Scholar]
Driessen 1996 {published data only}
- Driessen M, Ellis JB, Cooper PA, Wainer S, Muwazi F, Hahn D, et al. Fluconazole vs. amphotericin B for the treatment of neonatal fungal septicemia: a prospective randomized trial. Pediatric Infectious Disease Journal 1996;15(12):1107‐12. [DOI] [PubMed] [Google Scholar]
Ellis 1995 {published data only}
- Ellis ME, Halim MA, Spence D, Ernst P, Clink H, Kalin M, et al. Systemic amphotericin B versus fluconazole in the management of antibiotic resistant neutropenic fever‐preliminary observations from a pilot, exploratory study. Journal of Infection 1995;30(2):141‐6. [DOI] [PubMed] [Google Scholar]
Ellis 1998 {published data only}
- Ellis M, Spence D, Pauw B, Meunier F, Marinus A, Collette L, et al. An EORTC international multicenter randomized trial (EORTC number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosis. Clinical Infectious Diseases 1998;27(6):1406‐12. [DOI] [PubMed] [Google Scholar]
Galgiani 2000 {published data only}
- Galgiani JN, Catanzaro A, Cloud GA, Johnson RH, Williams PL, Mirels LF, et al. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double‐blind trial. Mycoses Study Group. Annals of Internal Medicine 2000;133(9):676‐86. [DOI] [PubMed] [Google Scholar]
Herbrecht 2002 {published data only}
- Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. New England Journal of Medicine 2002;347(6):408‐15. [DOI] [PubMed] [Google Scholar]
Khayat 2005 {published data only}
- Khayat N, Amsallem D, Nerich V, Coquet F, Leroy J, Plouvier E, et al. Caspofungin versus liposomal amphotericin B for empirical therapy in children with persistently febrile neutropenia. Program and abstracts of the 45th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC, USA [Abstract G‐939] . 2005 Dec.
Kim 2007 {published data only}
- Kim SY, Park JS, Jeong NG, Jeong DC, Cho B, Kim HW. Amphotericin B and itraconazole as empirical antifungal therapy in children with acute leukemia with neutropenic fever. Program and abstracts of the 47th American Society of Hematology Meeting. Atlanta. USA [abstract ‐ not selected for publication]. 2005 Dec.
Kullberg 2005 {published data only}
- Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non‐neutropenic patients: a randomised non‐inferiority trial. Lancet 2005;366(9495):1435‐42. [DOI] [PubMed] [Google Scholar]
Pizzo 1982 {published data only}
- Pizzo PA, Robichaud KJ, Gill FA, Witebsky FG. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. American Journal of Medicine 1982;72(1):101‐11. [DOI] [PubMed] [Google Scholar]
Rex 1994 {published and unpublished data}
- Rex JH, Bennett JE, Sugar AM, Pappas PG, Horst CM, Edwards JE, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. New England Journal of Medicine 1994;331(20):1325‐30. [DOI] [PubMed] [Google Scholar]
Shikanai‐Yasuda 2002 {published data only}
- Shikanai‐Yasuda MA, Benard G, Higaki Y, Negro GM, Hoo S, Vaccari EH, et al. Randomized trial with itraconazole, ketoconazole and sulfadiazine in paracoccidioidomycosis. Medical Mycology 2002;40(4):411‐7. [DOI] [PubMed] [Google Scholar]
Viscoli 1996 {published data only}
- Viscoli C, Castagnola E, Lint MT, Moroni C, Garaventa A, Rossi MR, et al. Fluconazole versus amphotericin B as empirical antifungal therapy of unexplained fever in granulocytopenic cancer patients: a pragmatic, multicentre, prospective and randomised clinical trial. European Journal of Cancer 1996;32A(5):814‐20. [DOI] [PubMed] [Google Scholar]
Walsh 2002 {published data only}
- Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. New England Journal of Medicine 2002;346(4):225‐34. [DOI] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang J, Sun A‐N, Wu D‐P, Chen S‐N, Qiu H‐Y, Jin Z‐M, et al. Comparison of caspofungin and liposomal amphotericin B for empirical antifungal therapy in patients with hematologic disease. Chinese Journal of Antibiotics 2007;32(5):316‐9. [Google Scholar]
Wheat 2001 {published data only}
- Wheat LJ, Cloud G, Johnson PC, Connolly P, Goldman M, Monte A, et al. AIDS Clinical Trials Group, Mycoses Study Group of NIAID. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrobial Agents and Chemotherapy 2001;45(8):2354‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wingard 2000 {published data only}
- Wingard JR, White MH, Anaissie E, Raffalli J, Goodman J, Arrieta A, L Amph/ABLC Collaborative Study Group. A randomized, double‐blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clinical infectious Diseases 2000;31(5):1155‐63. [DOI] [PubMed] [Google Scholar]
Winston 2000 {published data only}
- Winston DJ, Hathorn JW, Schuster MG, Schiller GJ, Territo MC. A multicenter, randomized trial of fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients with cancer. American Journal of Medicine 2000;108(4):282‐9. [DOI] [PubMed] [Google Scholar]
Yao 2000 {published data only}
- Yao Z, Liao W, Wen H. Antifungal therapy for treatment of cryptococcal meningitis. Chinese Medical Journal 2000;113(2):178‐80. [PubMed] [Google Scholar]
Additional references
Ascioglu 2002
- Ascioglu S, Rex JH, Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clinical Infectious Diseases 2002;34(1):7‐14. [DOI] [PubMed] [Google Scholar]
Barrett 2003
- Barrett JP, Vardulaki KA, Conlon C, Cooke J, Daza‐Ramirez P, Evans EG, et al. A systematic review of the antifungal effectiveness and tolerability of amphotericin B formulations. Clinical Therapeutics 2003;25(5):1295‐320. [DOI] [PubMed] [Google Scholar]
Bennett 2003
- Bennett JE, Powers J, Walsh T, Viscoli C, Pauw B, Dismukes W, et al. Forum report: issues in clinical trials of empirical antifungal therapy in treating febrile neutropenic patients. Clinical Infectious Diseases 2003;36 Suppl 3:117‐22. [DOI] [PubMed] [Google Scholar]
Benson 1989
- Benson JM, Nahata MC. Pharmacokinetics of amphotericin B in children. Antimicrobial Agents and Chemotherapy 1989;33(11):1989‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blyth 2009
- Blyth CC, Chen SC, Slavin MA, Serena C, Nguyen Q, Marriott D, et al. on behalf of members of the Australian Candidemia Study. Not just little adults: candidemia epidemiology, molecular characterization and antifungal susceptibility in neonatal and pediatric patients. Paediatrics 2009;123(5):1360‐8. [DOI] [PubMed] [Google Scholar]
Brammer 1994
- Brammer KW, Coates PE. Pharmacokinetics of fluconazole in pediatric patients. European Journal of Clinical Microbiology & Infectious Diseases 1994;13(4):325‐9. [DOI] [PubMed] [Google Scholar]
Clerihew 2004
- Clerihew L, McGuire W. Systemic antifungal drugs for invasive fungal infection in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 1. [CD003953.DOI: 10.1002/14651858.CD003953.pub2] [DOI] [PubMed] [Google Scholar]
Cordonnier 2009
- Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, et al. Empirical versus preemptive antifungal therapy for high‐risk, febrile, neutropenic patients: A randomized, controlled trial. Clinical Infectious Diseases 2009;48(8):1042‐51. [DOI] [PubMed] [Google Scholar]
Cornely 2007
- Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E et al for the AmBiLoad Trial Study Group. Liposomal amphotericin B as initial therapy for invasive mold infection: A randomized trial comparing a high–loading dose regimen with standard dosing (AmBiLoad Trial). Clinical Infectious Diseases 2007;44:1289‐97. [DOI] [PubMed] [Google Scholar]
de Pauw 2006
- Pauw BE, Sable CA, Walsh TJ, Lupinacci RJ, Bourque MR, Wise BA, et al. Impact of alternate definitions of fever resolution on the composite endpoint in clinical trials of empirical antifungal therapy for neutropenic patients with persistent fever: analysis of results from the Caspofungin Empirical Therapy Study. Transplant Infectious Disease 2006; 8 ( 1 ):31‐7. [DOI] [PubMed] [Google Scholar]
de Pauw 2008
- Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases 2008;46:1813‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dvorak 2005
- Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation 2005;36(7):621‐9. [DOI] [PubMed] [Google Scholar]
EORTC 1989
- EORTC International Antimicrobial Therapy Cooperative Group. Empiric antifungal therapy in febrile granulocytopenic patients. The American Journal of Medicine 1989;86(6 Pt 1):668‐72. [DOI] [PubMed] [Google Scholar]
Gallis 1990
- Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Reviews of Infectious Diseases 1990;12(2):308‐29. [DOI] [PubMed] [Google Scholar]
Girois 2006
- Girois SB, Chapuis F, Decullier E, Revol BGP. Adverse effects of antifungal therapies in invasive fungal infections: review and meta‐analysis. European Journal of Clinical Microbiology and Infectious Diseases 2006;25:138‐49. [DOI] [PubMed] [Google Scholar]
Goldberg 2008
- Goldberg E, Gafter‐Gvili A, Robenshtok E, Leibovici L, Paul M. Empirical antifungal therapy for patients with neutropenia and persistent fever: Systematic review and meta‐analysis. European Journal of Cancer 2008;44:2192‐203. [DOI] [PubMed] [Google Scholar]
Groll 1996
- Groll AH, Shah PM, Mentzel C, Schneider M, Just‐Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. Journal of Infection 1996;33(1):23‐32. [DOI] [PubMed] [Google Scholar]
Groll 1999
- Groll AH, Kurz M, Schneider W, Witt V, Schmidt H, Schneider M, et al. Five‐year‐survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long‐term survival. Mycoses 1999;42(7‐8):431‐42. [DOI] [PubMed] [Google Scholar]
Herbrecht 2002
- Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. New England Journal of Medicine 2002;347(6):408‐15. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.0.0 (updated September 2009). Cochrane Collaboration 2009; Vol. Available from www.cochrane‐handbook.org.
Hughes 2002
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clinical Infectious Diseases 2002;34(6):730‐51. [DOI] [PubMed] [Google Scholar]
Johansen 2000
- Johansen HK, Gotzsche PC. Amphotericin B lipid soluble formulations versus amphotericin B in cancer patients with neutropenia. Cochrane Database of Systematic Reviews 2000, Issue 2. Art No.: CD000969. DOI: 10.1002/14651858.CD000969. [Google Scholar]
Johansen 2002
- Johansen HK, Gotzsche PC. Amphotericin B versus fluconazole for controlling fungal infections in neutropenic cancer patients. Cochrane Database of Systematic Reviews 2002, Issue 2 Art No.: CD000239. DOI: 10.1002/14651858.CD000239. [2 Art No.: CD000239. DOI: 10.1002/14651858.CD000239] [DOI] [PubMed] [Google Scholar]
Kuse 2007
- Kuse ER, Chetchotisakd P, Arns da Cunha C, Ruhnke M, Barrios C, Raghunadharao D et al for the Micafungin Invasive Candidiasis Working Group. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double‐blind trial. Lancet 2007;369:1519‐27. [DOI] [PubMed] [Google Scholar]
Lin 2001
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis case‐fatality rate: systematic review of the literature. Clinical Infectious Diseases 2001;32(3):358‐66. [DOI] [PubMed] [Google Scholar]
Maertens 2005
- Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, et al. Galactomannan and computed tomography–based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: A prospective feasibility study. Clinical Infectious Diseases 2005;41(9):1242‐50. [DOI] [PubMed] [Google Scholar]
Marr 2002
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clinical Infectious Diseases 2002;34(7):909‐17. [DOI] [PubMed] [Google Scholar]
Marr 2004
- Marr KA, Boeckh M, Carter RA, Kim HW. Combination antifungal therapy for invasive aspergillosis. Clinical Infectious Diseases 2004;39(6):797‐802. [DOI] [PubMed] [Google Scholar]
Martin 2003
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine 2003;348(16):1546‐54. [DOI] [PubMed] [Google Scholar]
Mora‐Duarte 2002
- Mora‐Duarte J, Betts R, Rotstein C, Colombo AL, Thompson‐Moya L, Smietana J, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. New England Journal of Medicine 2002;347(25):2020‐9. [DOI] [PubMed] [Google Scholar]
Mukherjee 2005
- Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. Combination treatment of invasive fungal infections. Clinical Microbiology Reviews 2005;18(1):163‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pachl 2006
- Pachl J, Svoboda P, Jacobs F, Vandewoude K, Hoven B, Spronk P, et al. A randomized, blinded, multicenter trial of lipid‐associated amphotericin B alone versus in combination with an antibody‐based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clinical Infectious Diseases 2006;42(10):1404‐13. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Prentice 1997
- Prentice HG, Hann IM, Herbrecht R, Aoun M, Kvaloy S, Catovsky D, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. British Journal of Haematology 1997;98(3):711‐8. [DOI] [PubMed] [Google Scholar]
Reboli 2007
- Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D et al for the Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. New England Journal of Medicine 2007;356:2472‐82. [DOI] [PubMed] [Google Scholar]
Rex 1994
- Rex JH, Bennett JE, Sugar AM, Pappas PG, Horst CM, Edwards JE, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. New England Journal of Medicine 1994;331(20):1325‐30. [DOI] [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31(1):150‐3. [DOI] [PubMed] [Google Scholar]
Rosen 2005
- Rosen GP, Nielsen K, Glenn S, Abelson J, Deville J, Moore TB. Invasive fungal infections in pediatric oncology patients: 11‐year experience at a single institution. Journal of Pediatric Hematology/Oncology 2005;27(3):135‐40. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Steinbach 2005a
- Steinbach WJ. Pediatric aspergillosis: disease and treatment differences in children. Pediatric Infectious Disease Journal 2005;24(4):358‐64. [DOI] [PubMed] [Google Scholar]
Steinbach 2005b
- Steinbach WJ, Benjamin DK. New antifungal agents under development in children and neonates. Current Opinion in Infectious Diseases 2005;18(6):484‐9. [DOI] [PubMed] [Google Scholar]
Walsh 1999
- Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. New England Journal of Medicine 1999;340(10):764‐71. [DOI] [PubMed] [Google Scholar]
Walsh 2002
- Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. New England Journal of Medicine 2002;346(4):225‐34. [DOI] [PubMed] [Google Scholar]
Walsh 2004a
- Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. New England Journal of Medicine 2004;351(14):1391‐402. [DOI] [PubMed] [Google Scholar]
Walsh 2004b
- Walsh TJ, Karlsson MO, Driscoll T, Arguedas AG, Adamson P, Saez‐Llorens X, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single‐ or multiple‐dose administration. Antimicrobial Agents and Chemotherapy 2004;48(6):2166‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2005
- Walsh TJ, Adamson PC, Seibel NL, Flynn PM, Neely MN, Schwartz C, et al. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrobial Agents and Chemotherapy 2005;49(11):4536‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winston 2000
- Winston DJ, Hathorn JW, Schuster MG, Schiller GJ, Territo MC. A multicenter, randomized trial of fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients with cancer. American Journal of Medicine 2000;108(4):282‐9. [DOI] [PubMed] [Google Scholar]


