Abstract
Purpose of Study:
This scoping review explored research literature on the integration and coordination of services for high-need, high-cost (HNHC) patients in an attempt to answer the following questions: What models of transitional care are utilized to manage HNHC patients in the United States? and How effective are they in reducing low-value utilization and in improving continuity?
Primary Practice Settings:
U.S. urban, suburban, and rural health care sites within primary care, veterans’ services, behavioral health, and palliative care.
Methodology and Sample:
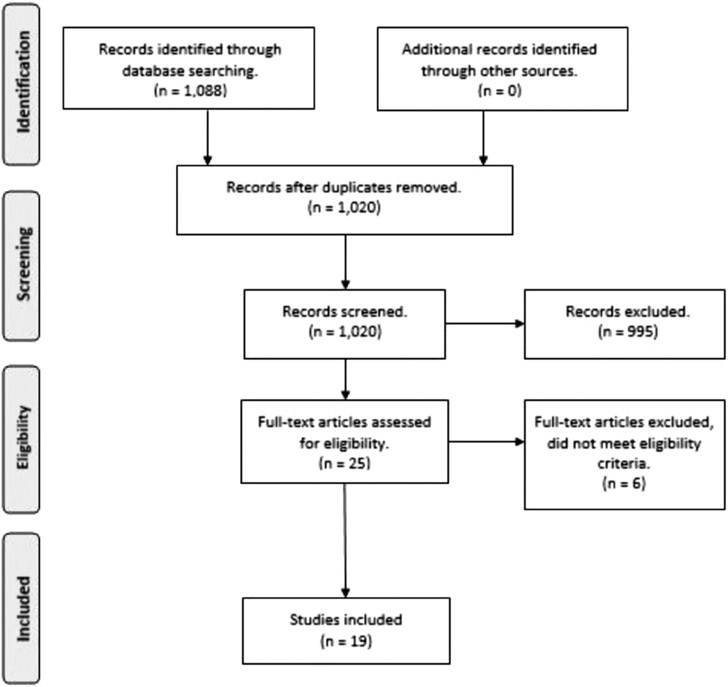
Utilizing the Joanna Briggs Institute and PRISMA guidelines for scoping reviews, a stepwise method was applied to search multiple databases for peer-reviewed published research on transitional care models serving HNHC adult patients in the United States from 2008 to 2018. All eligible studies were included regardless of quality rating. Exclusions were foreign models, studies published prior to 2008, review articles, care reports, and studies with participants younger than 18 years. The search returned 1,088 studies, of which 19 were included.
Results:
Four studies were randomized controlled trials and other designs included case reports and observational, quasi-experimental, cohort, and descriptive studies. Studies focused on Medicaid, Medicare, dual-eligible patients, veterans, and the uninsured or underinsured. High-need, high-cost patients were identified on the basis of prior utilization patterns of inpatient and emergency department visits, high cost, multiple chronic medical diagnoses, or a combination of these factors. Tools used to identify these patients included the hierarchical condition category predictive model, the Elder Risk Assessment, and the 4-year prognostic index score. The majority of studies combined characteristics of multiple case management models with varying levels of impact.
Implications for Case Management Practice:
Care coordination and case management were the primary strategies used to address the care needs of HNHC patients;
Interventions must reflect a strategy to efficiently identify and direct HNHC patients to the most appropriate resources;
The full potential of current technological offerings has not been realized in the science of care coordination;
Care management interventions must evolve to bridge multiple health care settings and community-based organizations through communication and collaboration; and
Continuity of care is vital during the immediate post discharge period,; however, tracking of continuity as an outcome remains poorly defined and is not reflective of actual practice.
Keywords: case management, cohort studies, patient discharge, primary health care, transitional care
High-need patients, the 5% of persons who account for 50% of health care spending (Mitchell, 2016), require post-acute care designed to address their unique combination of behavioral, social, functional, and clinical complexities. The American Hospital Association defines high-need, high-cost (HNHC) patients “… as adults who have three or more chronic diseases and functional limitations in their ability to care for themselves or perform routine daily tasks” (American Hospital Association, 2017). Indeed, taxonomy defines six segments of the high-need population based on its clinical/functional and social/behavioral characteristics (Long et al., 2017). The segments range from children with complex needs to adults with advancing illness; however, this review focuses on transitional care management (TCM) for adults in four of the six segments: those with multiple chronic or major complex chronic conditions and those with functional limitations, including frail elderly and nonelderly persons with disability. Recently, value-based care management models have been designed to reduce unnecessary hospital utilization during transitions from the hospital (both inpatient and emergency departments) to posthospital care in facilities and the community by addressing the complex needs of these segments (Kripalani, Theobald, Anctil, & Vasilevskis, 2014). Critical components of these programs include segmentation of high-need patients to match services with needs, team-based care, health information exchange (HIE), and payment for non-medical treatment (Blumenthal & Abrams, 2016).
The models in the United States range from post-acute programs that attempt to reduce length of stay and prevent readmissions (Burke et al., 2015; Cross & Adler-Milstein, 2016) to complex case management embedded in primary care settings (Hochman & Asch, 2017) that often include integrated behavioral health services (Desmedt et al., 2016). However, the care management activities are often setting-specific and result in poor continuity of care even when decreasing hospital (low-value) utilization. Questions about how programs hand off responsibility to the next care providers remain, and the decisions are often influenced by eligibility criteria (Bowles et al., 2019; Buntin, Colla, & Escarce, 2009). Providers question the necessity of using professional staff to provide transitional care services. The purpose of this scoping review is to focus on the integration and coordination of services for HNHC patients as they transition between the acute, post-acute, and outpatient settings.
Background
Transitional Care for High-Need Cases
Two seminal transitional care programs highlight the controversy about appropriate staffing for transitional care. The transitional care model as it has evolved uses advanced practice registered nurses (APRNs) to provide patient-centered, comprehensive holistic care and to provide oversight to other team members (Hirschman, Shaid, McCauley, Pauly, & Naylor, 2015; Naylor et al., 2018). The model includes seven components, including engaging elderly persons and their caregivers in care planning, assessing symptoms and risks, preparation for self-management, collaboration and communication with team members, and coordination of services (Naylor et al., 2018). In contrast, in the care transitions intervention, a transitions coach trained in a 4-week program develops a close relationship with the patient and the caregiver and coaches the older adult in self-management. The model focuses on medication self-management, dynamic care plan shared across settings, patient/family support for physician follow-up, and knowledge of red flags specific to the medical condition (Coleman, n.d.). Both models are considered best practice (Rochester-Eyeguokan, Pincus, Patel, & Reitz, 2016) because they included a multimodal intervention, a multidisciplinary team, bridged across settings, were tested in multiple settings or for multiple conditions, and reported positive patient outcomes. Only two other models met Rochester-Eyeguokan et al.’s criteria for best practice: Project RED (reengineered discharge) (Jack et al., 2009) and Project BOOST (Hansen et al., 2013). In all of these programs, the concepts of bridging care across the acute and post-acute settings and using a care plan to coordinate care among team members were essential to success in reducing readmissions (Burke, Kripalani, Vasilevskis, & Schnipper, 2013).
Complex care management targets continuity of care and aims to replace low-value inpatient and emergency utilization with coordinated care outside the hospital setting (Blumenthal & Abrams, 2016; Hong, Siegel, & Ferris, 2014). Successful complex care management promotes value-based care, segments the high-need population into cohorts with similar needs, aligns the care team to the specific needs, and exchanges electronic health information (Blumenthal & Abrams, 2016). In addition, continuity requires consideration of the relationship between the patient and providers as well as connections between providers (Hong et al., 2014). Because of the focus on avoiding readmissions, transitional care tends to concentrate on improving the hospital discharge to post-acute care whereas complex care management focuses on stabilizing the person in the community. Increasingly, technology is playing an important role in complex care management through HIE (Cross & Adler-Milstein, 2016; Hewner, Sullivan, & Yu, 2018) and in development of algorithms to identify cases requiring post-acute services (Keim & Bowles, 2018). These data-driven approaches have the potential to improve continuity between the hospital and post-acute settings.
Integrated care programs provide comprehensive, team-based care for specific segments of the high-need population, especially those with comorbid behavioral conditions and chronic disease (Rosenberg et al., 2014). Integrated care focuses on the continuum of preventive and restorative services needed by those with multiple chronic conditions delivered by a range of health professionals with coordination of these services across the continuum (Desmedt et al., 2016). Collaborative care management includes systematic psychiatric assessment, nonphysician symptom monitoring, specialist recommendations, and care coordination (Huffman, Niazi, Rundell, Sharpe, & Katon, 2014). The TEAMcare trial treated patients with depression that complicated the management of poorly controlled diabetes within the primary care setting and improved adherence to diet and exercise recommendations (Rosenberg et al., 2014). Depressed patients had faster remission and shorter duration of persistent depressive symptoms in collaborative care that included a registered nurse (RN) care manager and an integrated behavioral health team (Garrison, Angstman, O’Connor, Williams, & Lineberry, 2016). Although these programs did not evaluate the impact on low-value utilization, colocating behavioral health providers within the primary care setting improved continuity for high-need patients.
Patient-centered medical homes are becoming increasingly involved in developing care management models for high-need patients because of the emphasis on value-based payment (Hewner et al., 2017; Hochman & Asch, 2017). The practice-based approach embeds complex case management within the primary care setting. However, the need to have multidisciplinary resources to coordinate services for high-need patients can be burdensome for office-based practices. In contrast, the centralized approach to care management for high-need, high-cost patients uses an RN or social worker to lead a multidisciplinary team but is located outside of the practice (Holtrop, Potworowski, Fitzpatrick, Kowalk, & Green, 2016; Luo et al., 2016). Finally, a number of primary care practices are experimenting with embedding community outreach workers in primary care to address social determinants of health (Freund et al., 2016) Goldman, 2018). Embedded complex care management may have additional benefits because of the opportunity for face-to-face interaction between team members (interactional workability), enhanced skill sets, organizational support (contextual integration), and long-term relationship with the patient (relational integration) that result in improved continuity for the patient (Holtrop et al., 2016).
The research questions that guided this review are as follows: “What models of transitional care are being used to manage HNHC patients in the United States?” and “How effective are they in reducing low-value utilization and in improving continuity?” We hypothesized there was an evolution from models that focused solely on transitions to reduce readmissions to ones that used care management strategies to keep people with complex chronic conditions, social needs, and functional decline out of the hospital.
Methods
Protocol
The methodology for this scoping review was based on the Joanna Briggs Institute (JBI) Reviewers’ Manual 2017 Methodology for JBI Scoping Reviews (Peters et al., 2017). In keeping with the JBI methodology, the reviewers developed a protocol to define objectives, methods, and inclusion/exclusion criteria prior to study selection and data extraction.
Information Sources
A methodical search was conducted in December 2018 in PubMed, CINAHL, Web of Science, and EMBASE databases using key words and medical subject headings (MeSH) listed in Table 1. The categories of concepts were models of transitional care, HNHC patients and outcomes (utilization and continuity).
TABLE 1.
Key Words and Medical Subject Headings (MeSH)
| Category 1 (Care Management Models) |
Category 2 (High- Need, High-Cost) |
Category 3 (Outcomes) |
|---|---|---|
| Care management | Disabled | Emergency department visits |
| Integrated care | Multiple chronic illness | Hospital admissions |
| Transitions of care | Frail elderly | Continuity |
| Transitional care model | Advancing illness | |
| Care transitions | Chronic illness | |
| Care coordination | Super utilizer | |
| Post-acute care | High-need, high-cost | |
| Managed long-term care | Multimorbidity | |
| Aging in place |
Search Strategy
A stepwise method to search the databases was used by exploring and combining three concepts from the MeSH key words with assistance of a Health Sciences librarian who had expertise in systematic review searching. An example of full Web of Science search strategy is provided in Table 2. This search strategy was adapted to the syntax and consistently applied to the rest of the database.
TABLE 2.
Web of Science Search Strategy–Clarivate Analytics Interface
| Number | Query | Results |
|---|---|---|
| 1 | (TS = (care model OR integrated care OR transitions of care OR transitional care model OR care transitions OR care coordination OR managed long term care OR aging in place)) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article) | 198,903 |
| Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan = 2008–2019 | ||
| 2 | (TS = (disabled OR multiple chronic illness OR frail elderly OR advancing illness OR super utilizer OR high-need high-cost OR multimorbidity)) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article) | 32,287 |
| Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan = 2008–2019 | ||
| 3 | (TS = (emergency department visits OR hospital admissions OR continuity)) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article) | 106,788 |
| Indexes = SCI-EXPANDED, SSCI, A8HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan = 2008–2019 | ||
| 4 | #3 AND #2 AND #1 | 431 |
Eligibility Criteria
Qualitative, quantitative, and mixed-methods research articles were searched and selected using specific inclusion and exclusion criteria (see Table 3). To keep the scope of the review as broad as possible, the reviewers did not restrict study inclusion to a particular research methodology, intervention, or target population with the exception of excluding patients younger than 18 years. Because of the influence of government-based payer systems and policies on models of care, studies were restricted to those conducted in the United States within the past 10 years.
TABLE 3.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Primary, original research | Pediatric participants (<18 years) |
| Published in a peer-reviewed journal | Review articles |
| Published in English language | Care report |
| Published between 2008 and 2018 | Care model outside the United States |
| Adult patients (≥18 years) | |
| Care model in the United States |
Literature Search Results
The search strategy yielded an initial 1,088 references (see Figure 1), and no additional new references were identified from other web-based sources or manual searching. Duplicate references (n = 68) were discarded from across the four bibliographic sources (CINAHL, EMBASE, PubMed, and Web of Science). Based on title and abstract review for eligibility criteria of original research articles, studies with an adult focus and articles incorporating transitional care model in the United States, 121 abstracts were then retrieved and read. Of these, 995 references were removed and 25 were extracted for full-text review. Six articles were excluded at this stage because they did not meet the original inclusion criteria, which were identified when reading the full text in detail. The excluded studies related to long-term care (Hicks & Cimarolli, 2018; Temkin-Greener, Bajorska, & Mukamel, 2008; Weaver et al., 2008), narrowly defined populations (Bandy et al., 2014), had the wrong outcome (Berg, Donnelly, Miller, Medina, & Warnick, 2012), or focused on electronic health record (EHR) integration (Graetz et al., 2014). Thus, 19 references were selected for this scoping review following critique of the full-text articles.
FIGUER 1.
PRISMA diagram.
Study Selection
Results of the database search were imported into the Covidence systematic review management system (Covidence, n.d.). Duplicates were removed, and two reviewers independently screened the titles and abstracts of the remaining studies while a third reviewer screened discrepancies. Two reviewers then reviewed the remaining potentially relevant articles in full text, with disagreements again resolved by a third reviewer or by group consensus. The team of reviewers met routinely through videoconferencing throughout the scoping review process. Consistent with the JBI scoping review methodology, the inclusion and exclusion criteria were refined in an iterative fashion as the reviewers became more familiar with the available evidence.
Data Extraction
A preliminary data collection tool was developed in Excel, and fields were agreed upon by all reviewers prior to data extraction. Each article was read in full, and data were extracted and charted by one author and reviewed/confirmed by a second author. Extracted fields included study design, sample, population, model, interventions, utilization outcomes, and continuity outcomes.
Quality Appraisal and Risk of Bias
The quality of each study was assessed using the applicable JBI Critical Appraisal Tool for each (i.e., randomized control trial [RCT], cohort study, quasi-experimental, and case series/report). Studies ranged from fair to very high in quality and impact. No study was determined to have poor quality. Because of the specificity of the review criteria and paucity of research on the HNHC population, all eligible studies were included regardless of quality rating.
Results
Study Characteristics
The initial search strategy returned 1,088 studies for screening. After removal of duplicates and application of inclusion and exclusion criteria, 19 studies were selected for this review. The heterogeneity of the methods, target populations, care models, interventions, and outcomes made study comparisons challenging. Of the 19 articles, four were RCTs (Boult et al., 2011; Brown, Peikes, Peterson, Schore, & Razafindrakoto, 2012; Hanrahan, Solomon, & Hurford, 2014 ; Zulman et al., 2017) and three were case studies (Fleming & Haney, 2013; Kitzman, Hudson, Sylvia, Feltner, & Lovins, 2017; Waxmonsky et al., 2011). Table 4 highlights the design, sample size, and outcomes of the studies. The remaining articles were a mix of observational, quasi-experimental, cohort, and descriptive studies. Sample sizes ranged from 18 (Hanrahan et al., 2014) to 22,000 in an 11-site study (Brown et al., 2012), for a total of 35,939 participants over all studies. One study was conducted in a rural setting (Kitzman et al., 2017), five studies used multiple sites or the setting was not specified (Baldwin, Zook, & Sanford, 2018; Brown et al., 2012; Fleming & Haney, 2013; Kim, Higgins, Esposito, & Hamblin, 2017; Waxmonsky et al., 2011), and the remaining studies were conducted in urban or suburban areas.
TABLE 4.
Study Characteristics and Outcomes
| Author, Year | Design | Target Population | Sample Size | Utilization Outcomes | Continuity Outcomes |
|---|---|---|---|---|---|
| Baldwin et al., 2018 | Single group compared with national benchmarks | IP referral for patients with high readmission risk | N = 75 compared with national benchmarks | Reduced 30-day readmission rate (17.3%–2.7%; statistical significance not stated) | Not measured |
| Block et al., 2013 | Quasi-experimental time-series analysis of treat and release ED visits | Uninsured or underinsured patients | Pre/posttest analysis; intervention n = 374; comparison n = 249 | ED rates increased in both groups (intervention 3.0–3.6; comparison 4.5–7.1); rates of ED sig. lower in the intervention group (p = .04) | Not measured |
| Boult et al., 2011 | Clustered randomized controlled trial; Bayesian analysis | Medicare elders (65+ years) at risk for future utilization (older w/multiple chronic conditions) | Sample n = 446; control n = 404 | Reduced IP admissions; reduced IP days; reduced SNF admissions; reduced HH episodes (significant; p = .99/control 1.9; 95.9% probability of lower use) | Not measured |
| Brown et al., 2012 | Multisite randomized controlled trial | Fee-for-service beneficiaries with chronic conditions | Sample comparison of four of seven successful programs (seven total programs N = 22,000; study n = 4,290) | Reduced hospitalizations; treatment 1.376, difference from control −0.147 (significant; p = .001) | Not measured |
| Chen et al., 2015 | Retrospective cohort pilot | Homebound patients with life-limiting illness | Observational (sample: intervention n = 54; comparison n = 108) | Reduced hospitalizations (0.35/1.36); p < .001); reduced ED visits (NS); reduced IP days (1.46 days/6.59; p < .001) | Not measured |
| Chu et al., 2017 | Retrospective propensity-matched cohort | Medicaid under age 65 years with disabilities; subsample Group 3 with average age 34 years for the intervention group, 37 years for the control group | Observational (sample: intervention n = 1,283; comparison n = 1,283) | PCMH reduced rate of patients with more than two ED visits (15.8% compared with 18.9%; significant p < .05); reduced IP admissions (NS); reduced 30-day readmissions (NS) | Not measured |
| Fleming & Hanley, 2013 | Single group, case study | Inpatients requiring home health postdischarge | Observational (single group n = 1,907) | Reduced hospitalizations (significance not stated) | Not measured |
| Graham et al., 2018 | Repeat surveys | Dual-eligible managed care compared with fee-for-service | Intervention (n = 488; comparison n = 474) | Intervention group increased overnight hospitalization (NS); reduced ED (intervention 0.58/comparison 0.84; NS) | Repeat care coordination surveys without improvement |
| Hanrahan et al., 2014 | Randomized controlled trial | Patients discharged from IP psychiatric care | Control n = 18; intervention n=17; 868 encounters analyzed | Significantly higher rehospitalization (intervention n = 10/control n = 4; p = .054); lower ED (intervention n = 5/control n = 6; NS) | Not measured |
| Hardin et al., 2017 | Descriptive analysis and outcomes pre- and postintervention | HNHC referrals with more than three IP or ED visits | Sample n = 355 | Decreased IP (1.3 pre, 0.7 post; p < .001); decreased ED (10.2 pre, 5.9 post; p < .001); decreased 30-day readmissions (p < .001); Wilcox signed ranks | Higher continuity (p = .023; χ2) |
| Kim et al., 2017 | Mixed methods: focus groups and outcome analysis using Medicaid claims | Medicaid recipients with SMI and chronic disease (high need) | Intervention n = 4,788; comparison n = 7,039 | Reduced inpatient; decreased ED use by 4%/comparison increased 6% (p = .036) | Not measured |
| Kitzman et al., 2017 | Case study of patients with stroke | Patients discharged from IP rehabilitation | Participant n = 30; control n = 12 | Decreased 30-day readmits (3.3% intervention/42% control); decreased 30-day ED (0% intervention/83% control) | Not measured |
| Ohar et al., 2018 | Retrospective chart review with a propensity-matched control group | Adults (aged 40+ years) discharged after treatment for COPD exacerbation | Intervention n = 597; comparison n = 677 | 16% reduction in odds of all-cause readmission (p = .04); 37% lower odds of 30-day mortality (p = .007); 22% lower odds of 30-day mortality or readmit (p = .003) | Not measured |
| Sander et al., 2018 | Descriptive outcomes analysis pre- and postintervention including the patient | Medicaid adults (aged 18+ years) with more than three ED or more than two IP (high risk) | Pre/posttest single group, n = 86 | 65% reduction in IP; 67% reduction in ED visits (statistical significance not reported); ROI ~$1.6M | Patient activation measure measured only once |
| Steele et al., 2017 | Retrospective longitudinal review pre- and postintervention | Medicaid adults with SMI + no PCP | Pre/posttest single group; n=343 | Postdecrease in ED visits from 2.39 visits per person (prior 1.88) | Not measured |
| Watkins et al., 2012 | Descriptive, one group, nonexperimental | Medicare frail elderly (aged 65+ years) + more than two criteria for readmission prediction (high risk) | Pre/posttest design (single group n = 292) | 67% readmit rate decrease; 28% w/postdischarge ED visits (statistical significance not reported) | Not measured |
| Waxmonsky et al., 2011 | Implementation case study randomly assigning top 20% of health risk or cost | Medicaid HNHC adults (21–64 years) + chronic disease + pilot enrollee | Observational study (intervention n = 703; comparison n = 2,611) | 22% decrease in ED visits; 9% decreased IP use; increased OP; statistical significance not reported; qualitative analysis | Not measured |
| Zulman et al., 2014 | Preliminary observational partnered research study in health system redesign | HNHC veterans (top 5% cost) | Random sample, n = 150 eligible patients | 80% with ED use; high IP use | Not measured |
| Zulman et al., 2017 | Randomized controlled trial; difference-in-difference analysis | Randomly assigned PACT/ImPACT HNHC veterans; risk based on care assessment scores | ImPACT n = 96; PACT n = 405 | Significant difference in PCP (p < .001); decreased ED visits (NS); decreased IP use in both (NS); failed to align service intensity with need; regression to mean in both groups | Not measured |
Note. COPD = chronic obstructive pulmonary disease; ED = emergency department; HH = home health care; HNHC = high-need, high-cost; IP = inpatient; NS = not significant; OP = outpatient; PCP = primary care provider; PCMH = patient-centered medical home; ROI = return on investment; SMI = serious mental illness; SNF = skilled nursing facility.
Outcomes
The most frequently measured outcomes concerned health services utilization. Conversely, very few measured continuity of care. All 19 studies measured hospital admissions or readmissions as the primary outcome. Fifteen of the 19 studies looked at emergency department utilization (Block et al., 2013; Boult et al., 2011; Chen et al., 2015; Chu et al., 2017; Graham, Liu, Hollister, Kaye, & Harrington, 2018; Hanrahan et al., 2014; Hardin, Kilian, Muller, Callison, & Olgren, 2017; Kim et al., 2017; Kitzman et al., 2017; Sander et al., 2018; Steele, Ungemack, Mormile-Mehler, & Rabitaille, 2017; Watkins, Hall, & Kring, 2012; Waxmonsky et al., 2011; Zulman et al., 2014, 2017). Additional service utilization measures included hospital days (Boult et al., 2011; Hardin et al., 2017; Ohar, Loh, Lenoir, Wells, & Peters, 2018; Steele et al., 2017), primary or specialty care visits (Boult et al., 2011; Graham et al., 2018; Ohar et al., 2018; Steele et al., 2017; Waxmonsky et al., 2011; Zulman et al., 2017), home health visits (Boult et al., 2011; Watkins et al., 2012, and skilled nursing utilization (Boult et al., 2011). Nine studies measured financial outcomes including costs or savings (Baldwin et al., 2018; Brown et al., 2012; Hardin et al., 2017; Sander et al., 2018; Steele et al., 2017; Watkins et al., 2012; Waxmonsky et al., 2011; Zulman et al., 2014, 2017). Other outcome measures included patient satisfaction (Graham et al., 2018; Sander et al., 2018; Watkins et al., 2012; Zulman et al., 2017), health-related quality of life (Hanrahan et al., 2014; Watkins et al., 2012, care continuity (Hanrahan et al., 2014), and mortality (Chen et al., 2015; Ohar et al., 2018).
Target Population
The studies varied in target populations. Some studies incorporated specific payer groups into their study inclusion criteria including four studies that focused on Medicaid patients (Chu et al., 2017; Kim et al., 2017; Sander et al., 2018; Waxmonsky et al., 2011), one on Medicare patients (Brown et al., 2012), one on dual-eligible patients (Graham et al., 2018), two on veterans (Zulman et al., 2014, 2017), and one on the uninsured or underinsured (Block et al., 2013. The remaining studies included multiple payer groups or did not address payer group. In addition, one study’s inclusion criteria included specific medical conditions including three on behavioral health (Hanrahan et al., 2014; Kim et al., 2017; Steele et al., 2017) and one study each on stroke (Kitzman et al., 2017) and chronic obstructive pulmonary disease (Ohar et al., 2018).
The remaining 10 studies targeted populations deemed HNHC; however, there were inconsistent criteria for defining HNHC patients across studies. Patients deemed HNHC included people with disabilities (Chu et al., 2017, chronic medical conditions (Brown et al., 2012), requiring specialty care (Block et al., 2013), and requiring home health (Fleming & Haney, 2013). Two studies defined HNHC based on the patient’s prior health care utilization patterns including inpatient and emergency department visits (Hardin et al., 2017; Sander et al., 2018). Three studies defined HNHC as a combination of chronic medical conditions and prior health care utilization (Brown et al., 2012; Watkins et al., 2012; Zulman et al., 2014). Three studies used specific tools to define patients as HNHC. Boult et al. (2011) used the claims-based hierarchical condition category predictive model (Pope et al., 2004) to estimate a patient’s health expenditure risk. Chen et al. (2015) used the Elder Risk Assessment score (Takahashi, Chandra, Cha, & Borrud, 2011; Centers for Medicare & Medicaid Services, 2014), and the 4-year prognostic index score identified homebound frail patients with life-limiting illness (Lee, Lindquist, Segal, & Covinsky, 2006). The VA Care Assessment Need risk prediction algorithm (Wang et al., 2013) was used by Zulman et al. (2017) to identify patients at high risk of hospitalization. Finally, Waxmonsky et al. (2011) defined HNHC as those patients in the top 20% highest cost in the prior year or highest risk by case-mix index or Kronick score (Kronick, Gilmer, Dreyfus, & Lee, 2000).
Care Management Models
The synthesis clustered 18 studies into four categories of care management (excluding the Brown et al. meta-synthesis of demonstration projects): integrated behavioral health and primary care; embedded interprofessional care management teams; centralized care management; and TCM models. Three studies (Kim et al., 2017; Sander et al., 2018; Steele et al., 2017) employed integrated and colocated teams that included both behavioral health and primary care providers. Care management teams included non-nurse navigators (Block et al., 2013; Watkins et al., 2012) and nurses in the role of care coordinator (Boult et al., 2011; Chu et al., 2017; Zulman et al., 2014, 2017. Centralized care management models provided care management remotely (Chen et al., 2015; Graham et al., 2018; Hanrahan et al., 2014; Hardin et al., 2017; Waxmonsky et al., 2011), and the remaining studies focused on transitional care from the hospital to another setting (Baldwin et al., 2018; Fleming & Haney, 2013; Hanrahan et al., 2014; Kitzman et al., 2017; Ohar et al., 2018). However, many studies combined characteristics of multiple models (Boult et al., 2011; Hanrahan et al., 2014; Kim et al., 2017; Watkins et al., 2012; Waxmonsky et al., 2011).
Intervention Characteristics
Regardless of the model employed, studies delivered complex interventions that used multiple components as described in Table 5. The majority of studies used an identified individual (APRN, RN, social worker, or navigator) to guide the transition intervention, usually in combination with multidisciplinary team-based care, Other components of the transitional care interventions were service based and included comprehensive assessment of discharge needs, comprehensive education about disease self-management and behavior change, behavioral mental health interventions, increased number of contacts, medication review/management, increased access to community resources, and quality outcome tracking.
TABLE 5.
Interventions
| Interventions | Baldwin et al., 2018 | Block et al., 2013 | Boult et al., 2011 | Brown et al., 2012 | Chen et al., 2015 | Chu et al., 2017 | Fleming & Haney, 2013 | Graham et al., 2018 | Hanrahan et al., 2014 | Hardin et al., 2017 | Kim et al., 2017 | Kitzman et al., 2017 | Ohar et al., 2018 | Sander et al., 2018 | Steele et al., 2017 | Watkins et al., 2012 | Waxmonsky et al., 2011 | Zulman et al., 2014 | Zulman et al., 2017 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modela | TCM | ECM | ECM | NA | CCM | ECM | TCM | CCM | CCM | CCM | IBH | TCM | TCM | IBH | IBH | ECM | CCM | ECM | ECM |
| Multidisciplinary team-based care | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Nurse practitioner/APRN-led team | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Care coordinators/RN | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Navigators/case managers, not RNs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Social workers | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| EHR featured | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Increased number of contacts | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Early clinic/home visit follow-up after discharge | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Medication review/management | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Comprehensive education self-management/behavior change | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Care plan/maps | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Comprehensive assessment of discharge needs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Behavioral/mental health | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Community resource access | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| Quality outcomes tracked | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Note. APRN = advanced practice registered nurse; EHR = electronic health record; RN = registered nurse.
Abbreviations for care management models are IBH = integrated behavioral health and primary care; ECM = embedded care management; CCM = centralized care management; and TCM = transitional care management NA = not applicable because the study compared multiple models.
Impact of the Studies
The 19 studies ranged in impact from fair (2) to very high (5) on a 5-point scale that considered the quality of study design, the complexity of the target population, the intensity of the intervention, and the significance of the results. Table 6 displays the studies and their care management model, ranked according to impact. It is important to note that all studies reported outcomes for a regional rather than national impact, and this limits the ability to generalize the results. Studies with the highest impact (Boult et al., 2011; Brown et al., 2012; Zulman et al., 2017) used multidisciplinary team-based care, with an RN or APRN as communication hub, and included comprehensive assessment of discharge needs and comprehensive education about self-management and behavior change, among other high-intensity interventions. The highest impact studies based their target populations on both chronic disease history and risk for utilization for Medicare and veteran patients.
TABLE 6.
Impact of the Study Ranked From Highest to Lowest
| Study | Care Management Model | Impact |
|---|---|---|
| Brown et al., 2012 | Multiple models | Highest: large RCT, large sample size, multisite; significant reduction in IP use in the six successful studies |
| Boult et al., 2011 | Embedded CM team | Highest: RCT with adequate sample size; identifies potential reasons for modest results |
| Zulman et al., 2017 | Embedded CM team | Highest: RCT with moderate sample size; compared two models of team-based care; intensive intervention in study and comparison |
| Kim et al., 2017 | Integrated behavioral health | Very good: observational study with significant reduction in ED visits, a large sample size and a comparison group, intensive intervention |
| Waxmonsky et al., 2011 | Embedded or centralized CM team | Very good: observational study with a large sample and a comparison population, intensive intervention, improved utilization value |
| Hardin et al., 2017 | Centralized CM team | Very good: good sample, very intensive intervention, very high-risk group, excellent results and care plan employed |
| Hanrahan et al., 2014 | Transitional CM or integrated behavioral health | Very good: RTC with adequate sample of encounters, very intensive intervention for the high-risk population, nonsignificant reduced ED use, higher rehospitalization rates |
| Ohar et al., 2018 | Transitional CM | Very good: observational study with comparison, very intensive intervention, statistically significant reduction in readmission and mortality; care plan employed |
| Steele et al., 2017 | Integrated behavioral health | Good: observational study with pre/posttest design with statistically significant reduction in ED, strong intervention, good sample size, but no control |
| Block et al., 2013 | Centralized CM team (navigator) | Good: observational study with pre/posttest design with an intervention group and a comparison group; good sample size, but weak intervention and ED increased in both groups, but significantly less than that in the intervention group |
| Watkins et al., 2012 | Transitional CM (navigator) | Good: pre/posttest design, good sample size with no comparison group, reduction in readmission |
| Chu et al., 2017 | Embedded CM team | Good: observational study with propensity-matched groups, significant reduction in multiple ED visits, large sample size, weak intervention; PCMH staff not specified |
| Graham et al., 2018 | Centralized CM | Good: observational study with a large sample and a comparison population, weak intervention, positive but nonsignificant results |
| Chen et al., 2015 | Centralized CM | Good: observational study with a small sample for the intervention group, strong intervention, multiple significant results |
| Sander et al., 2018 | Integrated behavioral health | Good: large reduction in both IP and ED, a small sample without control, moderately intensive program; pre/posttest design |
| Zulman et al., 2014 | Embedded CM team | Fair: preliminary study to Zulman, et al., 2017, which developed partnered research to redesign care; utilization tracking, and a strong interdisciplinary team |
| Baldwin et al., 2018 | Transitional | Fair: observational study with a small sample no control, good intervention, positive results |
| Fleming & Haney, 2013 | Transitional | Fair: case study with no control, large sample size, positive results |
| Kitzman et al., 2017 | Transitional | Fair: small sample, case study of patients with stroke, strong intervention using outreach workers, positive results |
Note. CM = care management; ED = emergency department; PCMH = patient-centered medical home; RCT = randomized controlled trial.
One RCT examining patients discharged from inpatient psychiatric care (Hanrahan et al., 2014) was rated as very good because the outcomes were not statistically significant. The other integrated behavioral health models were rated very good (Kim et al., 2017) or good (Sander et al., 2018; Steele et al., 2017) based on if there was a control population. Interventions varied in models targeting behavioral health populations; however, all but Kim et al. (2017) included APRNs or social workers or both as part of the team.
Three other observational studies with control groups and positive outcomes were rated as very good (Hardin et al., 2017; Ohar et al., 2018; Waxmonsky et al., 2011). Although the studies targeted different populations, a common feature of the studies was the inclusion of a care plan or care map (Hardin et al., 2017). Three transitional care models were rated as having a fair impact because they were case studies without controls, but they reported positive outcomes (Baldwin et al., 2018; Fleming & Haney, 2013; Kitzman et al., 2017). Zulman et al. (2014) also rated their study as fair, but it was a preliminary study to Zulman et al. (2017).
Discussion
Similar to past reviews on the care of HNHC patients during transition (Bleich et al., 2015), care coordination and case management remain the primary strategy of the studies included in this review. The review found the following themes: successful interventions featured models that target specific population segments; current interventions rely on high investment in labor with low technology integration or optimization; interventions focus on a single organization, system, or type of care; and structured measurement of continuity is lacking.
The importance and prevalence of targeted interventions aimed to address unique population segments are clear from the studies in this review. Population segments of HNHC patients exhibit varying levels of complexity that require triage to determine appropriate intervention type and content. Although primary and comorbid conditions, utilization habits, and health insurers serve as proxies for identification of need and cost, studies in this review failed to pinpoint a clear, evidence-based, replicable method of targeting population segments for greatest impact. Unique programs with a variety of interventions, such as embedded behavioral health (Steele et al., 2017), complex care management (Boult et al., 2011), and the imPACT model (Zulman et al., 2017), proved successful, but strategies to triage populations to programs that match their complexity remain unclear. Without triaging tool or strategy, targeted interventions may not be appropriately customized for population segments in need and resources can be misapplied or misspent. For example, in a primary care practice with medically complex patients, addressing behavioral health needs within the practice may not be economically feasible, but with targeted screening, specific patients in need could be referred to an integrated behavioral health model.
Second, the studies in this review largely comprised interventions that require substantial investment in human resources, with scant investment in the optimization or use of health information technology. Few interventions were based in technology, only one (Hardin et al., 2017) referenced an HIE, and none were automated on the basis of an algorithm. Although clinical work is refined through technology and technology could be used to make the work of clinicians more efficient and measurable, the full potential of current technological offerings has not been realized in the science of care coordination. According to Popejoy et al., the quantification of care coordination activities is currently dependent on care coordinators documenting their activities (Popejoy, Galambos, et al., 2015a; Popejoy, Jaddoo, et al., 2015b) and there is little, if any, technological integration to enhance efficiency of practice. Without understanding what care coordinators do, how much is done, and at what cost, care coordination as an intervention remains poorly understood in terms of how much effort is needed to produce positive patient outcomes (Popejoy, Galambos, et al., 2015a; Popejoy, Jaddoo, et al., 2015b; Popejoy, Khalilia, et al., 2015c). Further limitations to a science dependent upon personnel and lacking in automation are the financial contribution required to sustain care coordination teams. In addition, although the social needs of many HNHC patients require extensive human interaction, interventions are difficult to measure, track productivity, and variance can ensue. Two studies speak directly to the potential for EHR and information exchange optimization via the use of a care plan or care map for medically complex patients (Hardin et al., 2017; Ohar et al., 2018), demonstrating the potential for automation.
The third theme found in the analysis of these studies was that interventions were place-based and did not effectively cross the continuum of care, nor was technology used to increase the capacity to cross the continuum of care. Only two interventions bridged multiple settings (Watkins et al., 2012; Kitzman et al., 2017), and care coordinators were primarily focused on outcomes relevant to their organization. Cross-sector (including community-based organizations) communication and collaboration are of vital importance and extend beyond simply attending outpatient appointments or embedding behavioral health services in a primary care setting. Communication about patients consumes nearly 50% of care coordination time, followed by 22% of time spent in assessing needs and goals (Popejoy, Galambos, et al., 2015a; Popejoy, Jaddoo, et al., 2015b). Care coordinators have information that needs to be efficiently communicated across all health care sectors to manage increasing social complexity of patients. Despite ample evidence that social complexity contributes to poor outcomes, there is very little programming for managing collaboration with community-based organizations in the studies in this review. Hardin et al.’s (2017) cross-continuum tool included social service information; however, sharing was primarily contained to the providers in the hospitals of care.
Finally, this review set out to describe current care coordination models and to query whether they decreased low-value utilization and improved continuity of care. Although utilization was measured extensively, this review found that there was no systematic, structured, or even defined measurement of continuity. Utilization is likely the primary outcome measured because of availability of data found in claims data and value-based regulatory requirements. Although continuity tracking is possible through Medicare TCM billing, TCM remains underutilized and its impact unclear (Huckfeldt, Neprash, & Nuckols, 2018). The Agency for Healthcare Research and Quality (2013) Continuity of Care Practices survey is a lengthy alternative, dependent upon patient self-report. The ways to measure continuity are poorly defined and inconsistently applied and may not be a measure of actual practice. Furthermore, the question remains whether improved continuity correlates with improved patient outcomes, quality of life, or decreased burden of illness. Patient experience of care, adherence to postdischarge appointments, and outpatient utilization have the potential to serve as proxies for elements of a measure of continuity; however, few studies in this review measured these outcomes or discussed continuity as a measurable goal.
Future Research
In tandem with described themes, future research must address the growing need for a systematic identification of population segments best suited for targeted interventions, such as in the PACT/imPACT model (Zulman et al., 2017), and do so by utilizing data mining and structured algorithms for standardized patient selection. It is clear from recent complex care coordination research that interventions applied across diagnoses and social determinants have the potential to show substandard results (Finklestein, Zhou, Taubman, & Doyle, 2020). With costly care coordination resources, it is vitally important to risk stratify in a targeted manner and consider that HNHC patients require high-intensity treatment in the immediate postdischarge window. Whatever the level of care, this costly, time-intensive service must be specifically applied and the method for successfully targeting populations should be further studied.
Future research should test automated information exchange platforms and protocols using HIEs, comprehensive shared care plans (Baker et al., 2016; Dykes et al., 2014; Cipriano et al., 2013), and EHR modifications that span organizations and settings of care and touch on the importance of evidence-based decision-making and accountability. For example, clinical decision support for discharged patients with multiple chronic conditions, delivered using HIE, facilitated nurse care coordinator outreach and care planning and resulted in a significant reduction in emergency visits (Hewner et al., 2018). In addition, studies testing the improvement in team member accountability when HIE notifications are limited to the HNHC population should be pursued.
Finally, and perhaps most importantly, future studies must outline the parameters for successful achievement of continuity as an outcome. After receiving disappointing results from their recent complex care coordination RCT (Finklestein et al., 2020), Camden Coalition CEO Kathleen Noonan confirmed the need for new metrics, saying, “While the RCT used the 180-day hospital readmission rates as a proxy for improved health, systems-level interventions cannot be effectively appraised using a single quantitative metric” (Noonan, 2020). In addition to process and productivity metrics, a structured understanding of continuity and its quantitative parameters is a necessary step in diversifying the measurement of this often-varying field of care.
Limitations
This review is limited by the design of many of the studies; only three represent true RCTs. As quality assessment is not a requirement of scoping reviews, articles were not excluded on this basis; this may be a source of bias in this review. Also, limiting our selection to U.S.-based studies removes many innovative international models for this population.
Implications for Case Management Practice
It is essential that care coordination practices serve as the bridge between multiple health care settings and community-based service organizations. One way to do this is to improve the use of HIE across care settings in both integrated and unintegrated health care systems, so all providers receive health care information about the patients they serve in a timely and usable way. For care coordination to bridge multiple settings, there must be shared care plans that cross settings and providers that identify who is responsible and accountable for interventions and activities within those plans and set key short- and long-term care outcomes. It is essential that emerging current and emerging technologies that add efficiency to care management be used as current shortages in health care professional are expected to worsen in the upcoming decades. There is need for care coordinators to have access to technology to provide data that support early illness and adverse event recognition so that timely and effective interventions can be put in place before serious illness and injuries occur (Rantz et al., 2017).
Conclusion
Reflecting on the characteristics of HNHC patients, we conclude by continuing to ask the following questions: How are we to move beyond the barriers of resource-heavy interventions and begin to design HNHC care coordination in a way that is agile, measurable, structured, replicable, and standardized? How do we efficiently deploy resources where they are needed, in measured ways, using innovative health information technology, to influence diverse outcomes?
This review has demonstrated that significant gaps in research and practice remain with regard to clearly aligning the HNHC population with the most effective interventions that (a) strategically utilize health information technology to amplify existing care coordination resources, and (b) look across sectors of the continuum of care by including community-based organizations that specialize in addressing social determinants of health. In addition, the nearly complete void of a standardized measurement of continuity of care as an outcome is striking, and this profound gap should be the focus of immediate future research for this population. The importance of continuity as a measure that directly improves patient experience and lessens the burden of care is of significant interest to patients, clinicians, and scientists alike. Care coordination seems to work in a variety of settings, with different populations, requiring a wide variety of team members. The challenge remains to move beyond utilization outcomes to studies that impact patient experience of care, continuity, and overall burden of illness.
The literature continues to show that care coordination of HNHC patients is impactful in its ability to decrease low-value health care utilization in single settings of care, but this impact comes at a price. Care coordination is a labor-heavy, therefore expensive, intervention that does not have outcomes that can be readily measured upon which to judge effectiveness. With the diversification of value-based payment moving alongside the increasing acuity of an aging patient population, we must understand and begin to standardize the use of technology and data that are difficult to extract or not typically used, such as nursing notes and ancillary clinician narrative, to pinpoint appropriate patients and tailor interventions toward standardization and scalable outcomes.
Biographies
Sharon Hewner, PhD, RN, FAAN, is a faculty in the Department of the Family, Community and Health Systems Science Department in the University at Buffalo School of Nursing. Her research focuses on implementing technology-supported care management interventions to improve transitional care for persons with social needs and multiple chronic conditions.
Chiahui Chen, MS, RN, FNP-BC, is a University at Buffalo School of Nursing PhD candidate. Her research interests are concerned with the development of a comprehensive understanding of end-of-life care in the intensive care unit and the improvement of nursing care to enhance the quality of end of life.
Linda Anderson, BSN, RN, is a PhD student in Sinclair School of Nursing at the University of Missouri–Columbia. Her doctoral research focuses on exploring functional status, health care experiences, and health-related quality of life in older women with chronic illness and disability.
Lana Pasek, EdM, MSN, ANP-BC, CCRN, CNRN, is a University at Buffalo Nursing doctoral student. She is an adult nurse practitioner with experience managing high-need, high-cost patients in a county hospital and an inner-city clinic. Her research interest is the development of patient-reported outcome measures for chronic diseases.
Amanda Anderson, MSN, MPA, RN, is a University at Buffalo Nursing doctoral student. Amanda develops care transitions programs utilizing nurses and telehealth, and she is a contributing editor for the American Journal of Nursing. Her research looks at gaps homeless patients face when transitioning between community-based and acute care institutions.
Lori Popejoy, PhD, RN, FAAN, is the Associate Dean for Innovation and Partnerships in Sinclair School of Nursing at the University of Missouri. She is a health system researcher focused on understanding the complex issues surrounding care to older adults across the continuum and implementation of evidence-based approaches to care coordination.
Footnotes
The authors report no conflicts of interest.
Contributor Information
Sharon Hewner, Department of the Family, Community and Health Systems Science Department in the University at Buffalo School of Nursing..
Chiahui Chen, University at Buffalo School of Nursing PhD candidate..
Linda Anderson, Sinclair School of Nursing at the University of Missouri–Columbia..
Lana Pasek, University at Buffalo Nursing doctoral student..
Amanda Anderson, University at Buffalo Nursing doctoral student..
Lori Popejoy, Sinclair School of Nursing at the University of Missouri..
References
- Agency for Healthcare Research and Quality. (2013). Clinical–community relationships measures (CCRM) atlas. Retrieved from https://www.ahrq.gov/prevention/resources/chronic-care/clinical-community-relationships-measures-atlas/ccrm-atlasapd5.html [Google Scholar]
- American Hospital Association. (2017). Improving care for high-need, high-cost patients. Retrieved from https://www.aha.org/system/files/hpoe/Reports-HPOE/2017/improving-care-for-high-need-high-cost-patients.pdf [Google Scholar]
- Baker A, Cronin K, Conway P, DeSalvo K, Rajkumar R, & Press M (2016). Making the comprehensive shared care plan a reality. NEJM Catalyst. Retrieved from http://catalyst.nejm.org/making-the-comprehensive-shared-care-plan-a-reality/ [Google Scholar]
- Baldwin SM, Zook S, & Sanford J (2018). Implementing posthospital interprofessional care team visits to improve care transitions and decrease hospital readmission rates. Professional Case Management, 23(5), 264–271. doi: 10.1097/ncm.0000000000000284 [DOI] [PubMed] [Google Scholar]
- Bandy R, Sachs GA, Montz K, Inger L, Bandy RW, & Torke AM (2014). Wishard Volunteer Advocates Program: An intervention for at-risk, incapacitated, unbefriended adults. Journal of the American Geriatrics Society, 62(11), 2171–2179. doi: 10.1111/jgs.13096 [DOI] [PubMed] [Google Scholar]
- Berg GD, Donnelly S, Miller M, Medina W, & Warnick K (2012). Dose–response effects for disease management programs on hospital utilization in Illinois Medicaid. Population Health Management, 15(6), 352–357. doi: 10.1089/pop.2011.0091 [DOI] [PubMed] [Google Scholar]
- Bleich S, Sherrod C, Chiang A, Boyd C, Wolff J, DuGoff E, … Anderson G (2015). Systematic review of programs treating high-need and high-cost people with multiple chronic diseases or disabilities in the United States, 2008–2014. Preventing Chronic Disease, 12, E197. doi: 10.5888/pcd12.150275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block L, Ma S, Emerson M, Langley A, de Torre DL, & Noronha G (2013). Does access to comprehensive outpatient care alter patterns of emergency department utilization among uninsured patients in East Baltimore? Journal of Primary Care & Community Health, 4(2), 143–147. doi: 10.1177/2150131913477116 [DOI] [PubMed] [Google Scholar]
- Blumenthal D, & Abrams MK (2016). Tailoring complex care management for high-need, high-cost patients. JAMA, 316(16), 1657–1658. doi: 10.1001/jama.2016.12388 [DOI] [PubMed] [Google Scholar]
- Boult C, Reider L, Leff B, Frick KD, Boyd CM, Wolff JL, … Scharfstein DO (2011). The effect of guided care teams on the use of health services: Results from a cluster-randomized controlled trial. Archives of Internal Medicine, 171(5), 460–466. doi: 10.1001/archinternmed.2010.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles KH, Ratcliffe SJ, Holmes JH, Keim S, Potashnik S, Flores E, … Naylor MD (2019). Using a decision support algorithm for referrals to post-acute care. Journal of the American Medical Directors Association, 20(4), 408–413. doi: 10.1016/j.jamda.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RS, Peikes D, Peterson G, Schore J, & Razafindrakoto CM (2012). Six features of Medicare coordinated care demonstration programs that cut hospital admissions of high-risk patients. Health Affairs (Millwood), 31(6), 1156–1166. doi: 10.1377/hlthaff.2012.0393 [DOI] [PubMed] [Google Scholar]
- Buntin MB, Colla CH, & Escarce JJ (2009). Effects of payment changes on trends in post-acute care. Health Services Research, 44(4), 1188–1210. doi: 10.1111/j.1475-6773.2009.00968.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, & Ginde AA (2015). Rise of post–acute care facilities as a discharge destination of US hospitalizations. JAMA Internal Medicine, 175(2), 295–296. doi: 10.1001/jamainternmed.2014.6383 [DOI] [PubMed] [Google Scholar]
- Burke RE, Kripalani S, Vasilevskis EE, & Schnipper JL (2013). Moving beyond readmission penalties: Creating an ideal process to improve transitional care. Journal of Hospital Medicine, 8(2), 102–109. doi: 10.1002/jhm.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. (2014). CMS manual system—Pub 100-02 Medicare benefit policy. Baltimore, MD: Centers for Medicare & Medicaid Services. Retrieved from https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R192BP.pdf [Google Scholar]
- Chen CY, Thorsteinsdottir B, Cha SS, Hanson GJ, Peterson SM, Rahman PA, … Takahashi PY (2015). Health care outcomes and advance care planning in older adults who receive home-based palliative care: A pilot cohort study. Journal of Palliative Medicine, 18(1), 38–44. doi: 10.1089/jpm.2014.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L, Sood N, Tu M, Miller K, Ray L, & Sayles JN (2017). Reduction of emergency department use in people with disabilities. The American Journal of Managed Care, 23(12), e409–e415. Retrieved from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L623516172 [PubMed] [Google Scholar]
- Cipriano PF, Bowles KH, Dailey M, Dykes PC, Lamb G, & Naylor MD (2013). The importance of health information technology in care coordination and transitional care. Nursing Outlook, 61(6), 475–489. doi: 10.1016/j.outlook.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Coleman EA (n.d.). The Care Transitions Program®. Retrieved from https://caretransitions.org/contact-us [Google Scholar]
- Covidence. (n.d.). World-class systematic review management. Retrieved from https://www.covidence.org/home [Google Scholar]
- Cross DA, & Adler-Milstein J (2016). Investing in post-acute care transitions: Electronic information exchange between hospitals and long-term care facilities. Journal of the American Medical Directors Association, 18(1), 30–34. doi: 10.1016/j.jamda.2016.07.024 [DOI] [PubMed] [Google Scholar]
- Desmedt M, Vertriest S, Hellings J, Bergs J, Dessers E, Vankrunkelsven P, … Vandijck D (2016). Economic impact of integrated care models for patients with chronic diseases: A systematic review. Value in Health, 19(6), 892–902. doi: 10.1016/j.jval.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Dykes PC, Samal L, Donahue M, Greenberg JO, Hurley AC, Hasan O, … Bates DW (2014). A patient-centered longitudinal care plan: Vision versus reality. Journal of the American Medical Informatics Association, 21(6), 1082–1090. doi: 10.1136/amiajnl-2013-002454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finklestein A, Zhou A, Taubman S, & Doyle J (2020). Health care hotspotting—A randomized controlled trial. The New England Journal of Medicine, 382(2), 152–162. doi: 10.1056/NEJMsa1906848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MO, & Haney TT (2013). Improving patient outcomes with better care transitions: The role for home health. Cleveland Clinic Journal of Medicine, 80(Electronic Suppl. 1), eS2–eS6. doi: 10.3949/ccjm.80.e-s1.02 [DOI] [PubMed] [Google Scholar]
- Freund T, Peters-Klimm F, Boyd CM, Mahler C, Gensichen J, Erler A, … Szecsenyi J (2016). Medical assistant-based care management for high-risk patients in small primary care practices: A cluster randomized clinical trial. Annals of Internal Medicine, 164(5), 323–330. doi: 10.7326/M14-2403 [DOI] [PubMed] [Google Scholar]
- Garrison GM, Angstman KB, O’Connor SS, Williams MD, & Lineberry TW (2016). Time to remission for depression with collaborative care management (CCM) in primary care. The Journal of the American Board of Family Medicine, 29(1), 10–17. doi: 10.3122/jabfm.2016.01.150128 [DOI] [PubMed] [Google Scholar]
- Goldman TR (2018). Charting a pathway to better health. Health Affairs (Millwood), 37(12), 1918–1922. doi: 10.1377/hlthaff.2018.05166 [DOI] [PubMed] [Google Scholar]
- Graetz I, Reed M, Shortell SM, Rundall TG, Bellows J, & Hsu J (2014). The next step towards making use meaningful electronic information exchange and care coordination across clinicians and delivery sites. Medical Care, 52(12), 1037–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CL, Liu PJ, Hollister BA, Kaye HS, & Harrington C (2018). Beneficiaries respond to California’s program to integrate Medicare, Medicaid, and long-term services. Health Affairs (Millwood), 37(9), 1432–1441. doi: 10.1377/hlthaff.2018.0452 [DOI] [PubMed] [Google Scholar]
- Hanrahan NP, Solomon P, & Hurford MO (2014). A pilot randomized control trial: Testing a transitional care model for acute psychiatric conditions. Journal of the American Psychiatric Nurses Association, 20(5), 315–327. doi: 10.1177/1078390314552190 [DOI] [PubMed] [Google Scholar]
- Hansen LO, Greenwald JL, Budnitz T, Howell E, Halasyamani L, Maynard G, … Williams MV (2013). Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization. Journal of Hospital Medicine, 8(8), 421–427. doi: 10.1002/jhm.2054 [DOI] [PubMed] [Google Scholar]
- Hardin L, Kilian A, Muller L, Callison K, & Olgren M (2017). Cross-continuum tool is associated with reduced utilization and cost for frequent high-need users. The Western Journal of Emergency Medicine, 18(2), 189–200. doi: 10.5811/westjem.2016.11.31916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewner S, Casucci S, Sullivan S, Mistretta F, Xue Y, Johnson BJ, … Fox C (2017). Integrating social determinants of health into primary care clinical and informational workflow during care transitions. EGEMS (Washington, DC), 5(2), 2. doi: 10.13063/2327-9214.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewner S, Sullivan SS, & Yu G (2018). Reducing emergency room visits and in hospitalizations by implementing best practice for transitional care using innovative technology and big data. Worldviews on Evidence Based Nursing, 15(3), 170–177. doi: 10.1111/wvn.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SA, & Cimarolli VR (2018). The effects of telehealth use for post-acute rehabilitation patient outcomes. Journal of Telemedicine and Telecare, 24(3), 179–184. doi: 10.1177/1357633x16686771 [DOI] [PubMed] [Google Scholar]
- Hirschman KB, Shaid E, McCauley K, Pauly MV, & Naylor MD (2015). Continuity of care: The transitional care model. Online Journal of Issues in Nursing, 20(3), 1. doi: 10.3912/OJIN.Vol20No03Man01 [DOI] [PubMed] [Google Scholar]
- Hochman M, & Asch SM (2017). Disruptive models in primary care: Caring for high-needs, high-cost populations. Journal of General Internal Medicine, 32(4), 392–397. doi: 10.1007/s11606-016-3945-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtrop JS, Potworowski G, Fitzpatrick L, Kowalk A, & Green LA (2016). Effect of care management program structure on implementation: A normalization process theory analysis. BMC Health Services Research, 16(1), 386. doi: 10.1186/s12913-016-1613-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Siegel AL, & Ferris TG (2014). Caring for high-need, high-cost patients: What makes for a successful care management program? (Vol. 19, pp. 1–19) (Issue Brief No. 1764). New York, NY: Commonwealth Fund. [PubMed] [Google Scholar]
- Huckfeldt P, Neprash H, & Nuckols T (2018). Transitional care management services for Medicare beneficiaries—Better quality and lower cost but rarely used. JAMA Internal Medicine, 178(9), 1171–1173. doi: 10.1001/jamainternmed.2018.2545 [DOI] [PubMed] [Google Scholar]
- Huffman JC, Niazi SK, Rundell JR, Sharpe M, & Katon WJ (2014). Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: A publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. PsycHosomatics, 55(2), 109–122. doi: 10.1016/j.psym.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, … Culpepper L (2009). A reengineered hospital discharge program to decrease rehospitalization: A randomized trial. Annals of Internal Medicine, 150(3), 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim SK, & Bowles KH (2018). Comparison of algorithm advice for post-acute care referral to usual clinical decision-making: Examination of 30-day acute healthcare utilization. AMIA Annual Symposium Proceedings, 2017, 1051–1059. [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Higgins TC, Esposito D, & Hamblin A (2017). Integrating health care for high-need Medicaid beneficiaries with serious mental illness and chronic physical health conditions at managed care, provider, and consumer levels. Psychiatric Rehabilitation Journal, 40(2), 207–215. doi: 10.1037/prj0000231 [DOI] [PubMed] [Google Scholar]
- Kitzman P, Hudson K, Sylvia V, Feltner F, & Lovins J (2017). Care coordination for community transitions for individuals post-stroke returning to low-resource rural communities. Journal of Community Health, 42(3), 565–572. doi: 10.1007/s10900-016-0289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripalani S, Theobald C, Anctil B, & Vasilevskis EE (2014). Reducing hospital readmission rates: Current strategies and future directions. Annual Review of Medicine, 65(1), 471–485. doi: 10.1146/annurev-med-022613-090415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronick R, Gilmer T, Dreyfus T, & Lee L (2000). Improving health-based payment for Medicaid beneficiaries: CDPS. Health Care Financing Review, 21(3), 29–64. [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Lindquist K, Segal MR, & Covinsky KE (2006). Development and validation of a prognostic index for 4-year mortality in older adults. JAMA, 295(7), 801–808. doi: 10.1001/jama.295.7.801 [DOI] [PubMed] [Google Scholar]
- Long P, Abrams M, Milstein A, Anderson G, Lewis Apton K, Lund Dahlberg M, & Whicher D (2017). Effective care for high-need patients: Opportunities for improving outcomes, value, and Health. Washington, DC: National Academy of Medicine. [PubMed] [Google Scholar]
- Luo Z, Chen Q, Annis AM, Piatt G, Green LA, Tao M, & Holtrop JS (2016). A comparison of health plan- and provider-delivered chronic care management models on patient clinical outcomes. Journal of General Internal Medicine, 31(7), 762–770. doi: 10.1007/s11606-016-3617-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EM (2016). Concentration of health expenditures in the U.S. civilian noninstitionalized population, 2014. Rockville, MD: Agency for Healthcare Research and Quality. Retrieved from http://meps.ahrq.gov/mepsweb/data_files/publications/st497/stat497.pdf [PubMed] [Google Scholar]
- Naylor MD, Hirschman KB, Toles MP, Jarrín OF, Shaid E, & Pauly MV (2018). Adaptations of the evidence-based transitional care model in the U.S. Social Science & Medicine, 213, 28–36. doi: 10.1016/j.socscimed.2018.07.023 [DOI] [PubMed] [Google Scholar]
- Noonan K (2020, January 9). Disappointing randomized controlled trial results show a way forward on complex care in Camden and beyond. Health Affairs Blog. Retrieved from https://www.healthaffairs.org/do/10.1377/hblog20200102.864819/full/?af=R&content=blog&mi=3egtxy&sortBy=Earliest&target=do-blog&MessageRunDetailID=1169147057&PostID=10498785&utm_medium=email&utm_source=rasa_io [Google Scholar]
- Ohar JA, Loh CH, Lenoir KM, Wells BJ, & Peters SP (2018). A comprehensive care plan that reduces readmissions after acute exacerbations of COPD. Respiratory Medicine, 141, 20–25. doi: 10.1016/j.rmed.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M, Godfrey C, McLnerney P, Baldini SC, Khalil H, & Parker D (2017). Scoping reviews. In Aromataris E & Munn Z (Eds.), Joanna Briggs Institute reviewer’s manual (chap. 11). Retrieved from https://reviewersmanual.joannabriggs.org [Google Scholar]
- Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, … Robst J (2004). Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financing Review, 25(4), 119–141. [PMC free article] [PubMed] [Google Scholar]
- Popejoy LL, Galambos C, Stetzer F, Popescu M, Hicks L, Khalilia MA, … Marek KD (2015a). Comparing aging in place to home health care: Impact of nurse care coordination on utilization and costs. Nursing Economics, 33(6), 306–313. [PMC free article] [PubMed] [Google Scholar]
- Popejoy LL, Jaddoo J, Sherman J, Howk C, Nguyen R, & Parker JC (2015b). Monitoring resource utilization in a healthcare coordination program. Professional Case Management, 20(6), 310–320. [DOI] [PubMed] [Google Scholar]
- Popejoy LL, Khalilia M, Popescu M, Galambos C, Lyons V, Rantz M, … Stetzer F (2015c). Quantifying care coordination dose using natural language processing and domain-specific ontology. Journal of the American Informatics Association, 22(e1), e93–e103. doi: 10.1136/amia-jnl-2014-002702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantz M, Phillips LJ, Galambos C, Lane K, Alexander GL, Despins L, … Deroche CB (2017). Randomized trial of intelligent sensor systems for early alerts in senior housing. Journal of the American Medical Directors Association, 18(10), 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester-Eyeguokan CD, Pincus KJ, Patel RS, & Reitz SJ (2016). The current landscape of transitions of care practice models: A scoping review. Pharmacotherapy, 36(1), 117–133. doi: 10.1002/phar.1685 [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Lin E, Peterson D, Ludman E, Von Korff M, & Katon W (2014). Integrated medical care management and behavioral risk factor reduction for multicondition patients: Behavioral outcomes of the TEAMcare trial. General Hospital Psychiatry, 36(2), 129–134. doi: 10.1016/j.genhosppsych.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander LD, Albert M, Okeke N, Kravet S, Rediger K, Conway SJ, & McGuire MJ (2018). Building a Medicaid ambulatory complex care program within an urban medical home. Population Health Management, 21(6), 446–453. doi: 10.1089/pop.2017.0200 [DOI] [PubMed] [Google Scholar]
- Steele C, Ungemack J, Mormile-Mehler M, & Rabitaille W (2017). Changes in hospital utilization among seriously mentally ill patients following enrollment in an integrated primary and behavioral health care program. Connecticut Medicine, 81(5), 271–279. [PubMed] [Google Scholar]
- Takahashi PY, Chandra A, Cha S, & Borrud A (2011). The relationship between Elder Risk Assessment Index score and 30-day readmission from the nursing home. Hospital Practice (1995), 39(1), 91–96. doi: 10.3810/hp.2011.02.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin-Greener H, Bajorska A, & Mukamel DB (2008). Variations in service use in the Program of All-Inclusive Care for the Elderly (PACE): Is more better? Journals of Gerontology Series A Biological Sciences and Medical Sciences, 63(7), 731–738. doi: 10.1093/gerona/63.7.731 [DOI] [PubMed] [Google Scholar]
- Wang L, Porter B, Maynard C, Evans G, Bryson C, Sun H, … Fihn SD (2013). Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Medical Care, 51(4), 368–373. doi: 10.1097/MLR.0b013e31827da95a [DOI] [PubMed] [Google Scholar]
- Watkins L, Hall C, & Kring D (2012). Hospital to home: A transition program for frail older adults. Professional Case Management, 17(3), 117–123; quiz 124. doi: 10.1097/NCM.0b013e318243d6a7 [DOI] [PubMed] [Google Scholar]
- Waxmonsky JA, Giese AA, McGinnis GF, Reynolds RT, Abrahamson A, McKitterick ML, … Thomas MR (2011). Colorado access’ enhanced care management for high-cost, high-need Medicaid members: Preliminary outcomes and lessons learned. The Journal of Ambulatory Care Management, 34(2), 183–191. doi: 10.1097/JAC.0b013e31820f64be [DOI] [PubMed] [Google Scholar]
- Weaver FM, Hickey EC, Hughes SL, Parker V, Fortunato D, Rose J, … Baskins J (2008). Providing all-inclusive care for frail elderly veterans: Evaluation of three models of care. Journal of the American Geriatrics Society, 56(2), 345–353. doi: 10.1111/j.1532-5415.2007.01538.x [DOI] [PubMed] [Google Scholar]
- Zulman DM, Chee CP, Ezeji-Okoye SC, Shaw JG, Holmes TH, Kahn JS, … Asch SM (2017). Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs patients: A randomized clinical trial. JAMA Internal Medicine, 177(2), 166–175. doi: 10.1001/jamain-ternmed.2016.8021 [DOI] [PubMed] [Google Scholar]
- Zulman DM, Ezeji-Okoye SC, Shaw JG, Hummel DL, Holloway KS, Smither SF, … Asch SM (2014). Partnered research in healthcare delivery redesign for high-need, high-cost patients: Development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). Journal of General Internal Medicine, 29(Suppl. 4), 861–869. doi: 10.1007/s11606-014-3022-7 [DOI] [PMC free article] [PubMed] [Google Scholar]