Abstract
Light chain amyloidosis (AL) is a rare disease caused by the generalized deposition of misfolded free light chains. Patients with immunoglobulin M gammopathy (IgM) and indolent B-cell lymphoma such as marginal zone lymphoma (MZL) may in some instances develop AL amyloidosis. So far, CAR T cells for AL amyloidosis have only been reported utilizing the B cell maturation antigen as target, while CD19 has so far not been used in AL amyloidosis.
We report the case of a 71-year-old male, diagnosed with systemic AL kappa amyloidosis and MZL, receiving third-generation CAR T cell therapy targeting CD19. Prior treatment included bendamustine/rituximab and cyclophosphamide/ dexamethasone with subsequent autologous stem cell transplantation. CAR T application was well tolerated despite heart and kidney amyloid manifestations, and only early low-grade procedure-specific toxicities were observed. A continuous decrease in IgM, kappa light chains and kappa-to-lambda light chain difference was observed in the patient from day + 30 on, resulting in a deep hematological response six months after treatment.
In summary, we present a novel case of CAR T cell treatment with third generation CD19 directed infusion for AL amyloidosis with an underlying secretory active B cell lymphoma, showing that this is an effective treatment modality and can be applied to patients with subsequent AL amyloidosis.
Keywords: CAR T cell therapy, Amyloidosis, Free light chains, IgM, Marginal zone lymphoma
To the Editor,
Amyloid light chain amyloidosis (AL amyloidosis) is a rare protein deposition disorder that results in potentially serious organ dysfunction and remains an uncurable disease [1]. In most cases, the underlying disease is a clonal plasma cell disease that produces excess amyloidogenic light chains and in rare instances, patients with immunoglobulin M (IgM) gammopathy and indolent B cell lymphoma like marginal zone lymphoma (MZL) and Waldenstrom's macroglobulinemia may also develop AL amyloidosis [2].
Meanwhile, chimeric antigen receptor (CAR) T cells have emerged as a successful pillar of therapy in the treatment of hematological malignancies. While prior two single-center attempts in AL amyloidosis patients have focused on CAR T cells against the B cell maturation antigen [3, 4], mostly expressed by plasma cells, targeting CD19 could offer a therapeutic option for patients with AL amyloidosis and secretory active indolent B cell lymphoma.
Here we report a first case of systemic AL kappa amyloidosis and MZL treated with our academic third-generation CAR T cell therapy targeting CD19.
Our patient was a 71-year-old white male with an asymptomatic MZL stage IVa (diagnosed with a lymph node biopsy) and subsequent AL amyloidosis (diagnosed two years later with a renal biopsy while becoming nephrotic), with kidney (stage II [5]) and heart (stage IIIa [6]) manifestation (for details Table 1). He received lymphodepletion chemotherapy followed by a single-dose CAR T cell infusion as 3rd-line treatment. Prior to CAR T cell consideration, the patient had received two different treatment regiments with regards to his lymphoma disease, beginning initial treatment with bendamustine/rituximab, and as second line cyclophosphamide/ dexamethasone with subsequent autologous stem cell transplantation. After progressing 33 months later, bridging therapy was applied with bortezomib/rituximab with no response before leukapheresis for CAR T cell manufacturing.
Table 1.
Clinical and laboratory evaluation, including treatment lines
| Month/Year | Clinical course (diagnosis / progress / treatment) | Timepoint laboratory parameters: |
IgM (g/L) |
dFLC (mg/L) | Best achieved hematologic/organ response | NT-proBNP (ng/L) | Creatinine (mg/dL) | Proteinuria (g/day) |
|---|---|---|---|---|---|---|---|---|
| Dec 2016 | initial AL diagnosis | At diagnosis (Dec 2016) | 12.3 | 79.5 | - |
1700 NYHA II |
1.07 | 5.177 |
| Dec 2016 | treatment with R-Bendamustine | After treatment (May 2017) | 3.03 | 27.9 | VGPR | 2642 | 1.03 | 3.209 |
| Nov 2018 | progress | At progress (Nov 2018) | 4.46 | 68.8 | - | 2295 | 1.20 | 0.802 |
| Nov 2018 | mobilisation chemotherapy (cyclophosphamide/dexamethasone) with stem cell collection | After treatment (Dec 2018) | 3.47 | 66.6 | Renal response | 3129 | 1.11 | 0.324 |
| Jan 2019 | consolidation—autologous stem cell transplantation conditioned with HD melphalan (200mg/m2) | After treatment (Apr 2019) | 1.31 | 11.1 | VGPR | 1859 | 1.24 | 0.273 |
| Oct 2021 | progress | At progress (Oct 2021) | 6.36 | 149.6 | - | 2109 | 1.34 | 0.148 |
| Apr 2022 | bridging with R-Bortezomib | After bridging (Jun 2022) | 4.74 | 127.1 | non-response | 1522 | 1.30 | 0.168 |
| Aug 2022 | prior admission for CAR T cell infusion | Prior CAR T cell infusion (Aug 2022) | 3.33 | 105.4 | - | 1916 | 1.20 | 0.156 |
| Aug 2022 | lymphodepleting chemotherapy (cyclophosphamide 500mg/m2/day and fludarabine 30mg/m2/day on days -4, -3 and -2) and CAR T cell infusion | After CAR T cell infusion (Sept 2022) | 1.83 | 83.6 | non-response | 1868 | 1.84 | - |
| Nov 2022 | 3-month CAR T cell follow-up | 3-month follow-up (Nov 2022) | 0.99 | 54.6 | PR | 2047 | 1.43 | - |
| Feb 2023 | 6-month CAR T cell follow-up | 6-month follow-up (Feb 2023) | 0.91 | 30.2 | VGPR | 2544 | 1.23 | 0.171 |
R Rituximab, HD high dose, CAR chimeric antigen receptor, IgM immunoglobulin M, dFLC difference between involved (kappa) and uninvolved (lambda) free light chains, NT-proBNP N-terminal brain natriuretic peptide, VGPR very good partial response
Due to the low toxicity induced by our third-generation product [7] and to not impair efficacy, no changes to the procedure were made; and the patient, after consultation with colleagues from our amyloid center, was cleared for treatment.
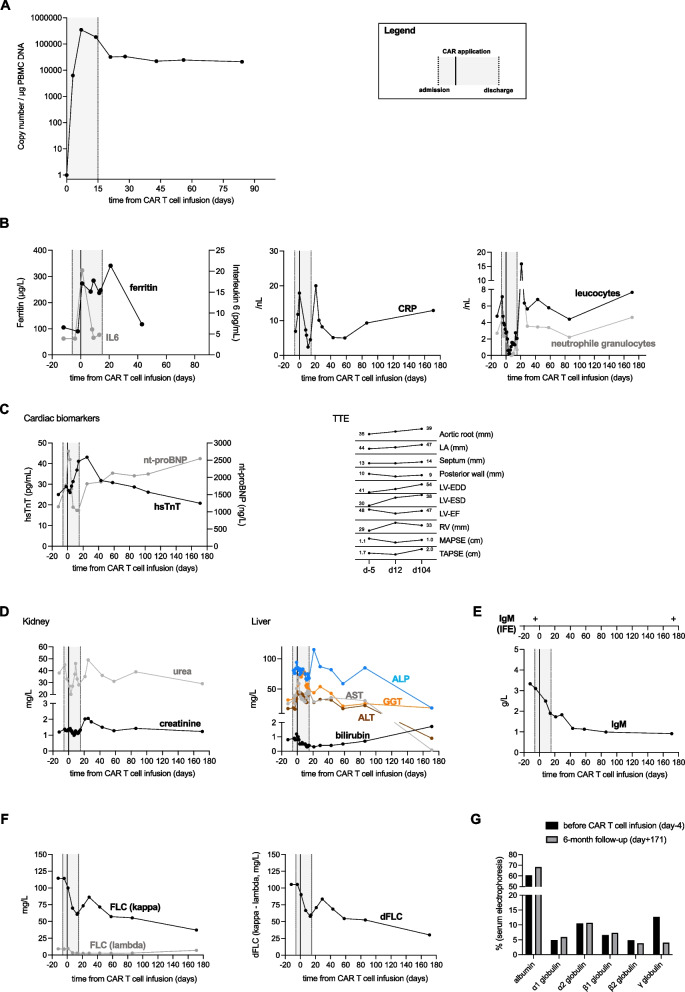
CD19-CAR Ts reached peak expansion at day + 7 with 346,414 copies per µg peripheral blood mononuclear cells (PBMC) DNA assessed by single copy gene duplex quantitative PCR (Fig. 1A), displaying high persistence with > 20,000 copies measured at days + 43 to + 84. The CAR T cell therapy was well tolerated and displayed low grade procedure-specific toxicity with only grade 1 immune effector cell-associated neurotoxicity syndrome (ICANS). Fever during aplasia resolved under antibiotic therapy with initially piperacillin/tazobactam and subsequent escalation to meropenem; with only slightly elevated interleukin 6, ferritin and C reactive protein levels (Fig. 1B). The patient received antibacterial (rifaximin), antiviral (acyclovir) and antifungal (fluconazole) prophylaxis for a month after discharge. Cardiac biomarker returned to pre-infusion values outside of a late N-terminal brain natriuretic peptide rise to 2,544 ng/L at d + 171 (Fig. 1C). No electrocardiogram changes were seen after CAR T cell infusion, with the patient maintaining a priorly diagnosed atrial fibrillation. Most parameters stayed consistent or achieved the pre-infusion level at the later FU, with left ventricle end diastolic and end systolic diameters (LV-EDD and LV-ESD, respectively) as well as tricuspid annular plane systolic excursion (TAPSE) increasing. Renal functional markers as well as liver parameters all returned to pre-treatment levels (Fig. 1D).
Fig. 1.
Overview of treatment- and disease-related parameters
From day + 30 after CAR infusion onwards, the patient experienced a continuous drop of IgM, kappa light chains and kappa-to-lambda light chain difference due to persisting CAR T cell activity (Fig. 1E and F), achieving a deep hematologic response graded as very good partial remission at six months after treatment [8]. Additionally, the gamma-globulin fraction in serum electrophoresis was reduced from 12.7 (before CAR infusion) to 4% (Fig. 1G), with no detectable M-gradient (and in fact the lowest IgM level since diagnosis), however immunofixation stayed positive for IgM kappa (Fig. 1E). This is a direct proof that choosing CD19 as target is appropriate to treat both the FLC producing more plasmacytic differentiated clone) and the IgM producing clon) and strongly suggests third generation CD19 CAR activity towards MZL.
Unfortunately, shortly after the 6-month evaluation, the patient suffered a severe respiratory infection and required hospitalization in a local hospital. Infections are a common complication after CD19 CAR T cells, occurring in 18–60% of patients when assessing approval trial and real-world data, and even 30 days and later after CAR infusion [9]. The condition subsequently worsened, with the patients developing a sepsis caused by haemophilus influenzae. Despite intensive care measures, the patient died of multiorgan failure on day + 195 after CAR T cell infusion.
Limiting the general evaluation of CAR T cell application in this setting is the death of the patient due to infectious complications after six months, preventing long-term response analysis on organ responses. At day + 180, there was no improvement of cardiac and renal biomarkers yet.
In conclusion this case suggests that CD19-directed CAR T cell therapy for MZL with systemic AL amyloidosis is feasible and clinically potent by effectively attacking the IgM and FLC secreting B cell clones, leading to a significant reduction in IgM and amyloidogenic kappa-FLCs levels. Patient selection and monitoring during and after therapy are important cornerstones in this high-risk patient cohort.
Acknowledgements
Not applicable
Authors’ contributions
The study was conceived by FK, SS, MS, and UH. FK and SS wrote the main manuscript text and FK prepared the figure. All authors reviewed and edited the manuscript.
Funding
The study received no funding.
Availability of data and materials
All data and materials can be made available by the authors upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval and approvals from the local and federal competent authorities were granted (Ethics Committee of the Medical Faculty, University Heidelberg; October 2017, AFmu-405/2017) and the patient underwent treatment in analogy with the HD-CAR-1 trial construct as a compassionate use. According to the Declaration of Helsinki, written informed consent from the patient was obtained.
Consent for publication
Not applicable. Data presented in this study has been de-identified and contains no images, videos or individual identifiers.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Merlini G, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4:38. doi: 10.1038/s41572-018-0034-3. [DOI] [PubMed] [Google Scholar]
- 2.Wechalekar AD, Chakraborty R, Lentzsch S. Systemic Amyloidosis due to Low-Grade Lymphoma. Hematol Oncol Clin North Am. 2020;34:1027–1039. doi: 10.1016/j.hoc.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Kfir-Erenfeld S, et al. Feasibility of a Novel Academic BCMA-CART (HBI0101) for the Treatment of Relapsed and Refractory AL Amyloidosis. Clin Cancer Res. 2022;28:5156–5166. doi: 10.1158/1078-0432.CCR-22-0637. [DOI] [PubMed] [Google Scholar]
- 4.Oliver-Caldes A, et al. First report of CART treatment in AL amyloidosis and relapsed/refractory multiple myeloma. J Immunother Cancer. 2021;9. 10.1136/jitc-2021-003783. [DOI] [PMC free article] [PubMed]
- 5.Palladini G, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–2332. doi: 10.1182/blood-2014-04-570010. [DOI] [PubMed] [Google Scholar]
- 6.Wechalekar AD, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121:3420–3427. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 7.Schubert ML, et al. Treatment of adult ALL patients with third-generation CD19-directed CAR T cells: results of a pivotal trial. J Hematol Oncol. 2023;16:79. doi: 10.1186/s13045-023-01470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palladini G, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–4549. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 9.Wudhikarn K, Perales MA. Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2022;57:1477–1488. doi: 10.1038/s41409-022-01756-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials can be made available by the authors upon reasonable request.