Abstract
Introduction:
Epithelioid type inflammatory myofibroblastic sarcoma (EIMS) is a subtype of inflammatory myofibroblastic tumor (IMT). It consists of round or epithelioid cells, and almost all types of EIMS contain rearrangements of the anaplastic lymphoma kinase (ALK) gene.
Case presentation:
We describe a 20-year-old female presenting with abdominal pain and a rapidly growing intraabdominal mass who underwent surgical tumor resection. She was diagnosed with EIMS. ALK and ki-67 expressions were detected in immunohistochemistry assessment. She was started with Crizotinib 200 mg twice a day, and chemotherapy was also initiated due to the recurrence of the disease 4 months after the initial treatment. She was unresponsive to all the medical regimens and died in 8 months.
Conclusion:
Approach to patients with EIMS is really challenging in terms of both diagnosis and treatment. Patients with combined surgical and non-surgical treatment regimen were seen to have a more favorable outcome in some EIMS cases. Therefore, it is essential to implement a multidisciplinary approach to diagnose and treat patients suspicious of EIMS.
Keywords: Sarcoma, ALK inhibitor, Crizotinib
Introduction
Epithelioid type inflammatory myofibroblastic sarcoma (EIMS) is a subtype of inflammatory myofibroblastic tumor (IMT) which mainly consists of round or epithelioid cells. 1 The exact data on the disease epidemiology is not available, but it has been reported that the IMT’s annual incidence is 150 to 200 cases in the USA. These neoplasms may happen at any age however, children, adolescents and young adults are more affected. Most common sites of involvement are the abdomen, pelvic region, lungs, mediastinum and the retroperitoneal region. 2 Trauma, inflammation, viral infections, gene fusion and chromosomal translocations have been proposed as probable etiologies.3,4 An abnormal anaplastic lymphoma kinase (ALK) expression is seen in about half of patients affected with IMT and in almost all EIMS patients, making the disease progression more aggressive.5,6 EIMS is associated with higher local recurrence rate, more inflammatory cell infiltration and poorer prognosis compared to IMT. 7
Furthermore, EIMS has a more aggressive behavior and less survival rate. These data suggest the need for clinical trials and consensus on the best treatment options; surgical resection is the management of choice for local EIMS, and systemic therapies are the preferred option in cases of recurrence or metastasis. Targeted therapies with ALK inhibitor (ALKI) agents such as Crizotinib could be implemented in those patients with EIMS with ALK overexpression. 8
Diagnosis of EIMS could be challenging due to its atypical tumor morphology and nuclear features. The differential diagnoses of the pathological findings are anaplastic large cell lymphoma, malignant mesothelioma, gastrointestinal stromal tumor and epithelioid leiomyosarcoma.
Reports of ALKI-resistant EIMS are very limited. Therefore, in this manuscript we present the case of a 20-year-old female diagnosed with EIMS who failed to respond to surgical treatment along with chemotherapy and ALKIs.
Case Presentation
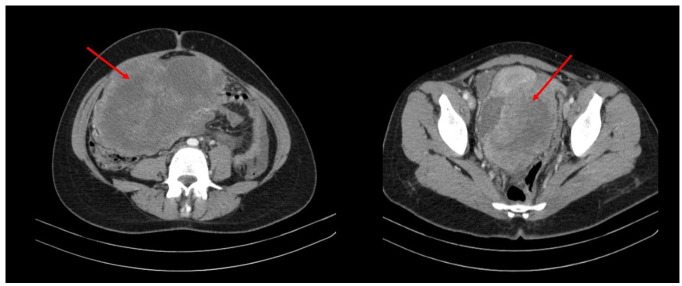
A 20-year-old female was admitted at the Firoozgar hospital in Tehran, Iran, on November 2021, with the complaint of abdominal pain and swelling in the last 2 weeks. Upon admission, an abdominal mass was palpated on the right side. A solid heterogeneous, hypo-echoic mass measuring 280 × 100 mm was visualized by ultrasonography originating from the pelvic region extending to the upper abdomen. Computed Tomography (CT) revealed a solid mass, measuring up to 223 mm in the pelvic cavity and rectouterine pouch, which was extended into the right abdominal cavity, root of the mesentery and mesenteric vessels (Figure 1). Correspondingly, Magnetic Resonance Imaging (MRI) revealed a large T2 intense mass in the pelvic cavity, measuring up to 216 × 134 mm with extensions to the right upper quadrant of the abdomen with adhesions to the uterus fundus (Figure 2). Cancer Antigen 125(CA-125) and Human Epididymis Protein 4 (HE4) levels were 134 IU/mL and 34.6 Pmol/L respectively, and the Risk Of Malignancy Algorithm score was 3.55%.
Figure 1.
Axial CT images showing a huge solid mass (red arrow ) in the pelvic cavity extended into the abdominal cavity.
Figure 2.
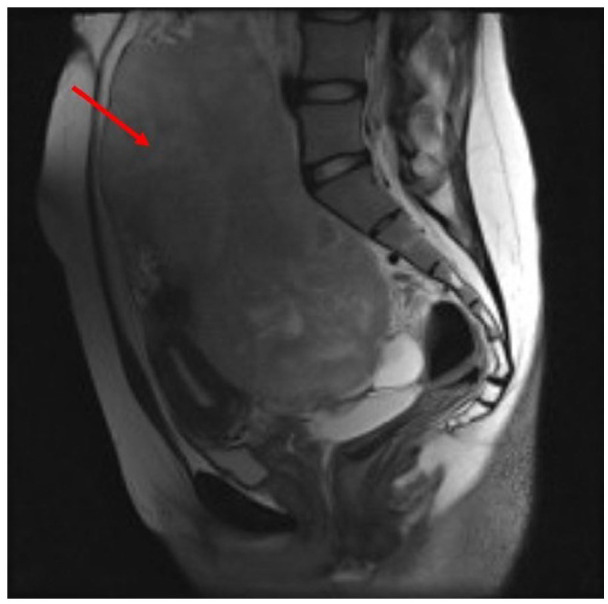
Sagittal MRI T2-weighted images showing a large T2 intense mass (red arrow ) in the pelvic cavity with extensions to the right upper quadrant of the abdomen and adhesions to the uterus fondus .
With suspicion of ovarian germ cell tumor probably dysgerminoma, the patient underwent a staging laparotomy. Intraoperatively, we found a large hemorrhagic mass originating from the rectouterine pouch, which was supplied by mesenteric vessels, associated with 200cc hemorrhagic ascites in the peritoneal cavity. We resected the mass and performed right salpingo-oophorectomy due to tumoral involvement. Two expert pathologists reviewed the specimens, and the mass was diagnosed to be EIMS. involving the right ovary, uterus and peritoneum (Figure 3). Immunohistochemistry study (IHC) revealed positive staining for ALK (Figure 4). Ki-67 proliferatin index was 30%, while no expression of Cluster of Differentiation (CD)34, Caldesmon, Desmin, Myogenic Differentiation 1 (MyoD1), CD117, cytokeratin, and smooth muscle actin (SMA) was detected (Figure 5).
Figure 3.
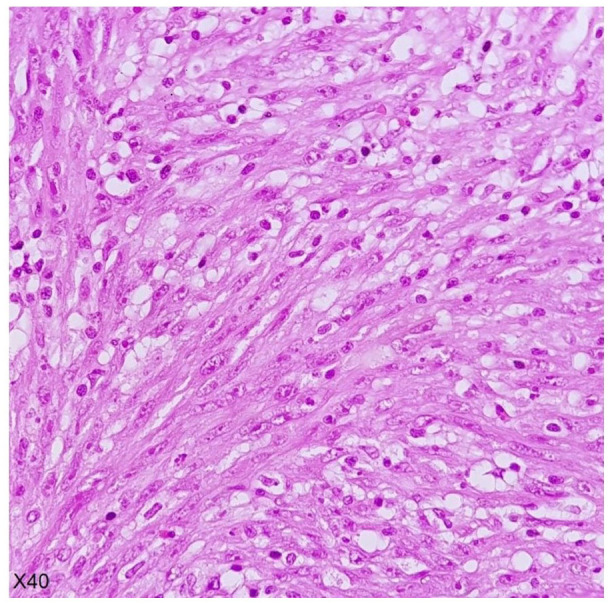
Fibrimyxoid area containing fusiform wavy cells admixed with inflammatory cells.
Figure 4.
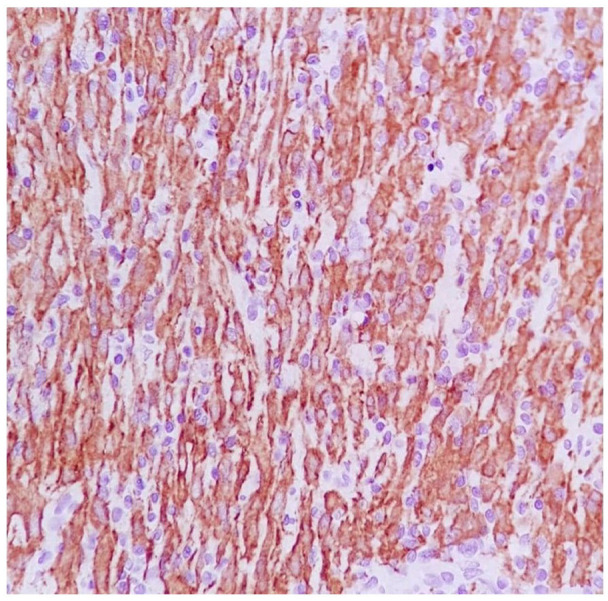
The anaplastic cells show strongly diffuse positivity for ALK and perinuclear accentuation of staining.
Figure 5.
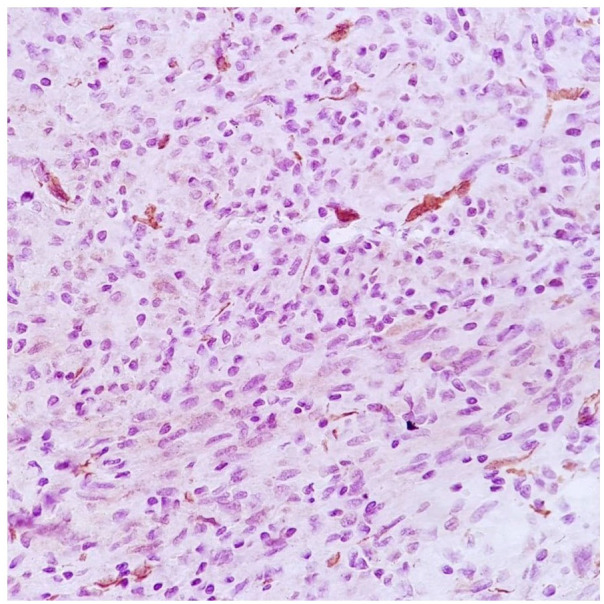
SMA negative expression in neoplastic cells.
A multidisciplinary team reviewed the case at the hospital’s tumor board. After recovering from surgery, she was started on 200 mg of Crizotinib orally once a day. Four months after surgery, patient presented again with abdominal distension and loss of appetite and tumoral recurrence was confirmed on imaging studies. Crizotinib was discontinued and chemotherapy was initiated with Docetaxel 125 mg on day 8 and Gemcitabine 1000 mg on days 1 and 8 every 3 weeks. She had received 4 doses of this regimen and was a candidate for a repeat possible surgery after 2 more chemotherapy sessions. However, no response was attained after this strict management and she passed away after 8 months.
Discussion
EIMS is a rare mesenchymal tumor that usually occurs in children and young adults. In Table 1, we have collected the characteristics of 45 cases of EIMS that have been discussed in English language literature. The age of the patients ranged from 4 months to 72 years (average 34.3 years), and majority of cases were men (60%). This was similar to the article of Du et al that reported majority of cases affected with EIMS to be male, and intraabdominal region as the main tumoral location. 1 However, our case was a young female.
Table 1.
Clinical characteristics of 45 cases of Epithelioid Inflammatory Myofibroblastic Sarcoma.
| Case | Author | Age | Sex | Size (cm); Greatest dimension | Site | Treatment | Follow up/status | Fusion gene |
|---|---|---|---|---|---|---|---|---|
| 1-11 | Enrıquez 7 | 59y | M | 15 | Mesentery of the small bowel | Surgery + CT | 12m/DOD | RANBP2-ALK in 3 cases |
| 41y | M | 26 | Omentum | Surgery + CT +ALKI | 40m/alive | |||
| 6y | M | 10.5 | Omentum | Surgery + CT | 13m/alive | |||
| 28y | M | NA | Mesentery of the small bowel | NA | NA | |||
| 63y | M | 25 | Mesentery of the small bowel | Surgery + CT | 3m/DOD | |||
| 42y | M | NA | Intra-abdominal | Surgery + CT | 13m/alive | |||
| 7m | M | 10 | Peritoneum | Surgery + CT +RT | 36m/DOD | |||
| 40y | M | 8 | Peritoneum | Surgery + CT +RT | 28m/DOD | |||
| 31y | F | 17.5 | Mesentery of the small bowel | Surgery + CT | 11m/DOD | |||
| 6y | M | 14 | Omentum and mesentery | Surgery | NA | |||
| 39y | M | 15 | Mesentery of the small bowel | Surgery | NA | |||
| 12 | Batool et al 9 | 4m | F | 12 | Mesentery of the small bowel | Surgery | 6m | NA |
| 13 | Halllin 10 | NA | M | NA | Intra-abdominal | NA | NA | NA |
| 14 | Du et al 1 | 26y | M | 25 | Intra-abdominal + abdominal wall | Surgery +CT | DOD | RANBP2-ALK |
| 15 | Xu et al 11 | 35y | F | 4 | Stomach | Surgery | 10m/alive | NA |
| 16 | Garg et al 12 | 2y | F | NA | Omentum+mesentry | Surgery | 7m / alive | NA |
| 17 | Xu et al 13 | 28y | M | NA | Intra-abdominal + abdominal wall | ALKI | 12m/alive | RANBP2-ALK/chromosomal ALK-G1269A mutation |
| 18 | Fu et al 14 | 21y | M | 10 | Lung | Surgery + ALKI | 4m/DOD | NA |
| 19 | Liu et al 15 | 22y | M | 13 | Intra-abdominal | Surgery + ALKI | 16m/alive | RANBP2-ALK |
| 20 | Wan et al 16 | 14y | M | 18 | Retroperitoneal | Surgery | 3m/DOD | NA |
| 21-25 | Yu et al 8 | 37y | F | 5 | Rectum | Surgery | 8m/alive | NA |
| 55y | M | NA | Terminal ileum | Surgery +CT | 10m/alive | |||
| 22y | M | 20 | Mesentery of colon | Surgery + ALKI | 14m/alive | |||
| 58y | F | NA | Omentum | Surgery +CT | 8m/DOD | |||
| 15y | F | 12 | Transverse colon | Surgery | 7m/alive | |||
| 26 | Liu et al 17 | NA | F | 10.9 | Sigmoid | Surgery + ALKI | NA | RANBP2-ALK |
| 27 | Wu et al 18 | 47y | F | 7.5 | Mesentery of colon | Surgery +CT | 4m/DOD | RANBP2-ALK |
| 28 | Zhang and Wang 19 | 46y | F | 11 | Intra-abdominal | Surgery + ALKI | DOD | NA |
| 29 | Singh et al 20 | 25y | M | 6.9 | Lung | Surgery + ALKI | 4m/alive | NA |
| 30 | Azad et al 21 | 53y | F | NA | Intrapericardial | Surgery +CT | DOD | No fusion genes |
| 31 | Collins et al 22 | 43y | F | NA | Uterine | Surgery * NA | 22m/alive | RANBP2-ALK |
| 32 | Fang et al 23 | 15y | F | NA | Ovary | Surgery + CT +ALKI | 2y/alive | RANBP2-ALK |
| 33 | Sarmiento et al 24 | 71y | M | 12.5 | Intrapleural | Surgery +ALKI | 1y/alive | NA |
| 34 | Kozu et al 25 | 57y | M | NA | Pleural cavity or chest wall | ALKI | NA | RANBP2-ALK |
| 35 | Kimbara et al 26 | 22y | M | 6 | Intra-abdominal | Surgery + CT +ALKI | 10m/alive | RANBP2-ALK |
| 36 | Wang et al 27 | 42y | F | 19 | Greater omentum | Surgery +ALKI | alive | PRRC2B-ALK |
| 37 | Zhou et al 28 | 8y | M | 18 | Intra-abdominal | Surgery | DOD | NA |
| 38 | Bai et al 29 | 65y | M | 9.3 | Descending colon | Surgery | 18m | NA |
| 39-43 | Lee et al 30 | 34y | M | 8 | Liver | Surgery | DOD | RANBP2-ALK in all cases |
| 62y | M | 25 | Omentum | Surgery+CT | DOD | |||
| 76y | F | 9 | Small bowel serosa | Surgery | DOD | |||
| 30y | M | 10 | Intra-abdominal | Surgery | DOD | |||
| 16y | F | 8 | Lung | Surgery+CT+RT+ALKI | alive | |||
| 44 | Chopra et al 31 | 72y | F | 4.7 | CNS | Surgery +ALKI | alive | VCL–ALK |
| 45 | Aminimoghaddam Current case | 20y | F | 21 | Peritoneum | Surgery+CT+ ALKI | DOD | NA |
Abbreviations: ALKI, ALK inhibitor; cm, centimeter,; CNS, central nervous system; CT, chemotherapy; DOD, dead of disease; F, female; M, male,; m, month; NA, not available; RT, radiotherapy; y, year.
Compared to other inflammatory myofibroblastic tumors, the clinical behavior of EIMS is more aggressive and is accompanied by more local recurrence rate and metastasis. 7 As shown in Table 1, at least 18 out of 38 patients (47%) with a close follow-up period died of this tumor, of which 11 out of 18 were male. Imaging studies cannot definitely make the diagnosis preoperatively, making the diagnosis a big challenge before surgical intervention. Diagnosis is based on the histopathologic evaluation and IHC studies. Even an intraoperative frozen section could not help. In most cases of EIMS, in addition to ALK, desmin, actin, and CD30 may be expressed. 10 In our case, CD34, caldesmon, desmin, MyoD1, CD117, cytokeratin, and smooth muscle actin expressions were not detected.
All EIMS contain rearrangements of the anaplastic lymphoma kinase (ALK) gene, giving rise to ALK inhibitors such as Crizotinib to be an optional drug for this condition. However, their effect on the outcome is unclear. 32 Surgical resection is the cornerstone of treatment in these patients, and ALK inhibitors are used as a part of a combination therapy alongside resection, chemotherapy and radiotherapy.
Enríquez et al. also reported that EIMS has a poorer outcome compared to IMT. He added, tumors with a positive ALK staining are associated with a more aggressive behavior. 7 Butrynski et al had experienced a partial response to Crizotinib in their affected Alk positive IMT patients. Their study emphasized that Crizotinib, along with surgery, could be a good option for the management of local recurrency, however some tumor resistant cases were present. 33
Xu et al also reported a case resistant to Crizotinib. They identified a chromosomal ALK-G1269A mutation in addition to RANBP2-ALK fusion. This secondary acquired mutation is a mechanism of crizotinib-resistance. These patients benefit from second generation ALK inhibitors, such as Brigatinib (AP26113). They observed a significant response to this treatment in their patient. 13 Same with our experience, their case also experienced recurrence of tumor and disease progression despite surgery and administration of Crizotinib.
In our patient, certain ALK gene fusions could not be identified and we did not conduct any specific molecular cytogenetic tests, but, Yu et al in their study, explained that RAN Binding Protein 2 (RANBP2)-ALK fusion genes could lead to rapid growth, recurrence and more aggressive behavior of EIMS. 8 Why does this tumor recur in some population and in another cases responds to the current medical managements making these patient have a longer tumor-free period is a question that needs further investigation? However, the rarity of this potentially lethal tumor is another challenge to face.
Conclusion
The diagnosis and management of EIMS is challenging. A better prognosis has been observed in affected cases who had received a combined approach of surgical and non-surgical management (mainly targeted therapy). Therefore, it is essential to implement a multidisciplinary approach to diagnose and treat patients who are suspicious of EIMS.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contribution: Dr Soheila Aminimoghaddam was responsible for treatment of the patient and edditing the manuscript. Dr Roghayeh Pourali contributed in writing and submission of the paper.
ORCID iD: Roghayeh Pourali  https://orcid.org/0000-0001-7614-0518
https://orcid.org/0000-0001-7614-0518
References
- 1. Du X, Gao Y, Zhao H, Li B, Xue W, Wang D. Clinicopathological analysis of epithelioid inflammatory myofibroblastic sarcoma. Oncol Lett. 2018;15:9317-9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009;9:331-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eilers AL, Nazarullah AN, Shipper ES, Jagirdar JS, Calhoon JH, Husain SA. Cardiac inflammatory myofibroblastic tumor: a comprehensive review of the literature. World J Pediatr Congenit Heart Surg. 2014;5:556-564. [DOI] [PubMed] [Google Scholar]
- 4. Xiang J, Liu X, Wu S, Lv Y, Wang H. Multiple inflammatory myofibroblastic tumor of the duodenum: case report and literature review. J Gastrointest Surg. 2012;16:1442-1445. [DOI] [PubMed] [Google Scholar]
- 5. Zhao JJ, Ling JQ, Fang Y, et al. Intra-abdominal inflammatory myofibroblastic tumor: spontaneous regression. World J Gastroenterol. 2014;20:13625-13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee JC, Li CF, Huang HY, et al. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol. 2017;241:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mariño-Enríquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35:135-144. [DOI] [PubMed] [Google Scholar]
- 8. Yu L, Liu J, Lao IW, Luo Z, Wang J. Epithelioid inflammatory myofibroblastic sarcoma: a clinicopathological, immunohistochemical and molecular cytogenetic analysis of five additional cases and review of the literature. Diagn Pathol. 2016;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Batool S, Ahuja A, Chauhan DS, Bhardwaj M, Meena AK. Epithelioid inflammatory myofibroblastic sarcoma: the youngest case reported. Autops Case Rep. 2021;11:e2021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallin M, Thway K. Epithelioid inflammatory myofibroblastic sarcoma. Int J Surg Pathol. 2019;27:69-71. [DOI] [PubMed] [Google Scholar]
- 11. Xu P, Shen P, Jin Y, Wang L, Wu W. Epithelioid inflammatory myofibroblastic sarcoma of stomach: diagnostic pitfalls and clinical characteristics. Int J Clin Exp Pathol. 2019;12:1738-1744. [PMC free article] [PubMed] [Google Scholar]
- 12. Garg R, Kaul S, Arora D, Kashyap V. Posttransplant epithelioid inflammatory myofibroblastic sarcoma: A case report. Indian J Pathol Microbiol. 2019;62:303-305. [DOI] [PubMed] [Google Scholar]
- 13. Xu X, Li H, Peng K, et al. ALK-G1269A mutation in epithelioid inflammatory myofibroblastic sarcoma after progression on crizotinib: A case report. Oncol Lett. 2019;17:2370-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu X, Jiang J, Tian XY, Li Z. Pulmonary epithelioid inflammatory myofibroblastic sarcoma with multiple bone metastases: case report and review of literature. Diagn Pathol. 2015;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Q, Kan Y, Zhao Y, He H, Kong L. Epithelioid inflammatory myofibroblastic sarcoma treated with ALK inhibitor: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:15328-15332. [PMC free article] [PubMed] [Google Scholar]
- 16. Wan YY, Miao CL, Liu SB, Zhang T, Luo CH. Epithelioid inflammatory myofibroblastic sarcoma with Leukemoid reaction. J Coll Phys Surg. 2022;32:1212-1215. [DOI] [PubMed] [Google Scholar]
- 17. Liu D, Luo R, Tang H, Li T. Sigmoid epithelioid inflammatory myofibroblastic sarcoma with high white blood cell count: A case report. Asian J Surg. 2020;43:838-839. [DOI] [PubMed] [Google Scholar]
- 18. Wu H, Meng YH, Lu P, et al. Epithelioid inflammatory myofibroblastic sarcoma in abdominal cavity: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:4213-4219. [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang S, Wang Z. A case report on epithelioid inflammatory myofibroblastic sarcoma in the abdominal cavity. Int J Clin Exp Pathol. 2019;12:3934-3939. [PMC free article] [PubMed] [Google Scholar]
- 20. Singh P, Nambirajan A, Gaur MK, et al. Primary pulmonary epithelioid inflammatory myofibroblastic sarcoma: a rare entity and a literature review. J Pathol Transl Med. 2022;56:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azad M, Oye M, Torrente N, Mirsaeidi M. Pericardial epithelioid inflammatory myofibroblastic sarcoma: an atypical presentation. Cureus. 2022;14:e26827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins K, Ramalingam P, Euscher ED, Reques Llanos A, García A, Malpica A. Uterine inflammatory myofibroblastic neoplasms with aggressive behavior, including an epithelioid inflammatory myofibroblastic sarcoma: a clinicopathologic study of 9 cases. Am J Surg Pathol. 2022;46:105-117. [DOI] [PubMed] [Google Scholar]
- 23. Fang H, Langstraat CL, Visscher DW, Folpe AL, Schoolmeester JK. Epithelioid inflammatory myofibroblastic sarcoma of the ovary with RANB2-ALK fusion: report of a case. Int J Gynecol Pathol. 2018;37:468-472. [DOI] [PubMed] [Google Scholar]
- 24. Sarmiento DE, Clevenger JA, Masters GA, Bauer TL, Nam BT. Epithelioid inflammatory myofibroblastic sarcoma: a case report. J Thorac Dis. 2015;7:E513-E516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kozu Y, Isaka M, Ohde Y, Takeuchi K, Nakajima T. Epithelioid inflammatory myofibroblastic sarcoma arising in the pleural cavity. Gen Thorac Cardiovasc Surg. 2014;62:191-194. [DOI] [PubMed] [Google Scholar]
- 26. Kimbara S, Takeda K, Fukushima H, et al. A case report of epithelioid inflammatory myofibroblastic sarcoma with RANBP2-ALK fusion gene treated with the ALK inhibitor, crizotinib. Jpn J Clin Oncol. 2014;44:868-871. [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Geng Y, Yuan LY, et al. Durable clinical response to ALK tyrosine kinase inhibitors in epithelioid inflammatory myofibroblastic sarcoma harboring PRRC2B-ALK rearrangement: a case report. Front Oncol. 2022;12:761558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou J, Jiang G, Zhang D, et al. Epithelioid inflammatory myofibroblastic sarcoma with recurrence after extensive resection: significant clinicopathologic characteristics of a rare aggressive soft tissue neoplasm. Int J Clin Exp Pathol. 2015;8:5803-5807. [PMC free article] [PubMed] [Google Scholar]
- 29. Bai Y, Jiang M, Liang W, Chen F. Incomplete intestinal obstruction caused by a rare epithelioid inflammatory myofibroblastic sarcoma of the colon: a case report. Medicine. 2015;94:e2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee JC, Wu JM, Liau JY, et al. Cytopathologic features of epithelioid inflammatory myofibroblastic sarcoma with correlation of histopathology, immunohistochemistry, and molecular cytogenetic analysis. Cancer Cytopathol. 2015;123:495-504. [DOI] [PubMed] [Google Scholar]
- 31. Chopra S, Maloney N, Wang WL. Epithelioid inflammatory myofibroblastic sarcoma with VCL-ALK fusion of central nervous system: case report and brief review of the literature. Brain Tumor Pathol. 2022;39:35-42. [DOI] [PubMed] [Google Scholar]
- 32. Theilen TM, Soerensen J, Bochennek K, et al. Crizotinib in ALK(+) inflammatory myofibroblastic tumors-Current experience and future perspectives. Pediatr Blood Cancer. 2018;65:e26920. [DOI] [PubMed] [Google Scholar]
- 33. Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]