Abstract
Background:
Epidemiologic studies support the hypothesis that reduced microbial exposure in westernized societies promotes atopy. Dichlorophenols are widely used as pesticides and for chlorination of water. They have a strong bactericidal effect that could affect microflora in the environment. However, it is unknown whether their use is associated with a higher prevalence of allergies.
Objective:
To test the association between exposure to environmental pesticides represented by dichlorophenols and allergic sensitization measured by allergen-specific serum IgE levels in a US nationally representative sample of 2,211 persons 6 years and older in the National Health and Nutrition Examination Survey 2005-2006.
Methods:
Exposure to dichlorophenols was defined as high if their levels in urine were present at the 75th percentile and above. Association of the high exposure to dichlorophenols with sensitization to food and environmental allergens was assessed in logistic regression models after adjustment for sample weights and potential confounders.
Results:
Sensitizations to 1 or more food allergens were more common in those with exposure to 2 dichlorophenol metabolites. After multivariable adjustment, urine dichlorophenol levels at the 75th percentile and above were associated with the presence of sensitization to foods (odds ratio, 1.8; 95% confidence interval, 1.2-2.5; P = .003). No significant association was found between dichlorophenol exposure and sensitization to aeroallergens alone.
Conclusion:
High urine levels of dichlorophenols are associated with the presence of sensitization to foods in a US population. Excessive use of dichlorophenols may contribute to the increasing incidence of food allergies in westernized societies.
Introduction
Atopic conditions are highly prevalent in countries with a westernized lifestyle.1 Several hypotheses have been proposed to explain the associations between food and environmental allergies and the westernized lifestyle. Decreased infections or exposure to nonpathogenic microbia due to increased hygiene has been most strongly associated with atopy.2-4 Decreased exposure to environmental microbia can be affected by several factors, including the use of mechanical barriers, such as asphalt pavements,4 living in apartment houses as opposed to living in single-family houses,5 reduced farming,6 use of a large amount of pesticides by industrial agriculture facilities,7 and water and soil treatment practices in westernized countries.4,8,9
Chlorophenol metabolites derived from pesticides and antimicrobials are common in westernized environments.10-12 The major source of 2,4-dichlorophenol in the environment is degradation of 2,4-dichlorophenoxyacetic acid in soil and water.10,13 It can be formed as a byproduct during the manufacturing of various chlorinated chemicals, the chlorination processes involving water treatment, and the incineration or combustion of municipal solid waste.10 In addition, 2,4-dichlorophenol comes from chlorophenoxyacetic acid used as an herbicide.
2,5-Dichlorophenol is a metabolite of 1,4-dichlorobenzene that has been detected in surface water around the world. Surface water detections have been mostly attributed to improper chemical disposal, direct manufacturing sources, chemical waste dumps, and use as urinal block deodorizers that contribute to sewage water contamination.12 It can also be detected in air of households and bathrooms where moth balls and deodorizers are used.14 General population exposure to dichlorophenols occurs through drinking chlorinated water, from breathing of contaminated air, or through skin contact with dichlorophenol-containing pesticides.10,12,15
Chlorophenols present in the environment may cause a decrease in microbial exposure because of their antiseptic action. It is unknown whether their use is associated with a higher prevalence of allergic conditions. To test this association, we examined the relationship between exposure to chlorinated phenols in a national representative sample of the general US population and the prevalence of sensitization to foods and environmental allergens, as measured by serum IgE levels in the US National Health and Nutrition Examination Survey (NHANES) 2005-2006. We hypothesized that higher levels of chlorinated phenols would be positively associated with allergic sensitization.
Methods
Study population
The NHANES 2005-2006 database represents the civilian noninstitutionalized population of the United States. A complex, multistage, clustered, and stratified probability sample design was used to select participants. To obtain accurate estimates in the subpopulations, non-Hispanic blacks and Mexican Americans were oversampled. Data from the survey and details of the plan and operation of NHANES 2005-2006 are publically available on the Centers for Disease Control and Prevention/National Center for Health Statistics’ website at http://www.cdc.gov/nchs/nhanes.htm. All participants and/or their parents signed informed consent forms for participation in NHANES. Data collection for NHANES 2005-2006 was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. Analysis of restricted data through the NCHS Research Data Center was also approved by the NCHS Ethics Review Board. The study was classified as exempt by Albert Einstein College of Medicine Institutional Review Board.
In the 2005-2006 data set, urinary metabolites of chlorinated phenols were measured in a randomly selected one-third subsample of persons 6 years and older. For this analysis, we included all participants who had dichlorophenols and allergen-specific IgE levels measured. Participants with missing covariables included in the multivariable-adjusted model were excluded.
Data collection
Pesticide exposure was determined in 2 ways: (1) a questionnaire about the use of pesticides in the past 7 days in the home to control insects or on the lawn or garden to control weeds and (2) measurement of the chlorinated phenol metabolites in urine. Details of the laboratory protocol and measurements can be found on the NHANES website (http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/lab05_06.htm). Chlorinated phenol levels were measured using gas chromatography or high-performance chromatography coupled with different detection techniques. The detection limits in 100 μL of urine are 0.1 to 2 ng/mL, which is sufficient for measuring urinary levels of phenols in nonoccupationally exposed individuals.
Environmental pesticides measured in NHANES 2005-2006 included 2,4-dichlorophenol, 2,5-dichlorophenol, orthophenylphenol, 2,4,5-trichlorophenol, and 2,4,6-trichlorophenol. We included only the dichlorophenols (2,4-dichlorophenol and 2,5-dichlorophenol) in our analysis because there were a limited number of observations above the limit of detection for trichlorophenols and orthophenylphenol in the NHANES 2005-2006 database. In addition, trichlorophenols are no longer manufactured commercially in the United States.11 Statistical evaluation indicated that the distribution of urine dichlorophenol levels is not well approximated by the normal (gaussian) distribution. For this analysis, we used log-transformed values and dichlorophenol levels dichotomized at the 75th percentile. The levels equal to or above the 75th percentile value were defined as high dichlorophenol levels. We analyzed the associations between high dichlorophenol levels by the presence of either one dichlorophenol metabolite in urine and by having high levels of both metabolites in urine. We attempted to evaluate whether the exposure to both dichlorophenols could have a stronger association with the outcomes.
Allergic sensitization was measured as antigen-specific IgE levels. Participants were tested for allergen-specific serum IgE by using the Pharmacia Diagnostics ImmunoCAP 1000 System (Pharmacia Diagnostics, Kalamazoo, Michigan). Allergen-specific IgE levels were measured for Dermatophagoides farinae, Dermatophagoides pteronyssinus, cat, dog, German cockroach, Alternaria alternata, peanut, egg, milk, short ragweed, ryegrass, Bermuda grass, white oak, birch, shrimp, Aspergillus fumigatus, Russian thistle, mouse, and rat. Positive allergic sensitization was defined as an allergen-specific serum IgE of 0.35 kU/L or higher. Food allergy was defined as the presence of an allergen-specific serum IgE of 0.35 kU/L or higher to at least one of the following food allergens: peanut, egg, milk, or shrimp. Environmental allergy was defined as the presence of an allergen-specific serum IgE level of 0.35 kU/L or greater to at least one of the other listed allergens and excluding foods. Having both food and environmental allergies was defined as having a positive reaction to the combination of at least 1 food and at least 1 environmental allergen. Serum 25-hydroxy-vitamin D levels were measured with a 2-step procedure for detection (25-hydroxy-vitamin D assay; Diasorin, Inc, Stillwater, Minnesota). The assay can detect serum 25-hydroxy-vitamin D values from 5 to 100 ng/mL. Vitamin D levels were categorized into 3 groups: low (<15 ng/mL), insufficient (15-29 ng/mL), and sufficient (≥30 ng/mL). Trained staff collected information on demographics, including age, sex, self-identified race/ethnicity, and the poverty-income ratio, with values of less than 1.00 being below the poverty threshold and defining low socioeconomic status in this analysis.
Geographic variables, including state, county, and tract, were used to merge in US Census 2000 variables, which provided contextual information of the participants residence (urban vs rural). According to the definition of the US Census 2000, urban residence was defined as a densely settled core and the adjacent densely settled surrounding territory (with a density of at least 500 people per square mile) that together has a minimum of 2,500 people or more. Rural residence was defined as all territory, population, and housing units located outside the urban residence.16 State, county, and tract are restricted variables. Therefore, these data were accessed through the Research Data Center at the NCHS (Hyattsville, Maryland).
Statistical analyses
Participants’ characteristics were calculated separately by the presence of sensitization to food and environmental allergies. Nonsensitized individuals served as a control group. Bivariate tests of associations were performed, and nonparametric tests were used when necessary. Potential confounders were entered into the initial model when associations between predictor variables and allergic sensitization had a P ≤ .20. Age, sex, race/ethnicity, vitamin D levels, pesticide use at home, creatinine level, the presence of asthma and hay fever, and residence of the participants (dichotomized as urban and rural) were retained in the adjusted models based on these associations and findings from previous studies.17-19 All terms, including dichlorophenols and creatinine, were entered into the model as separate independent variables to allow for an appropriate adjustment of urinary dichlorophenols and of the other variables in the model by urinary creatinine.20
Multivariable logistic regression models were used to assess the association of dichlorophenol exposure with food allergies, environmental allergies, or both. All analyses used survey command (svy) in Stata statistical software, version 11.2 (StataCorp, College Station, Texas) to account for the complex, multistage probability sample survey design used in NHANES 2005-2006. P <.05 was considered significant.
Results
Participants’ characteristics
Among the 10,348 NHANES participants 2,548 had both measures of dichlorophenols in the urine and allergy testing. Of these, 337 were excluded because of missing data for covariables, resulting in a final sample of 2,211. The 2,211 included in the analysis represent 232,512,264 persons in the United States. Of 2,211 participants, 2,182 had levels of 2,5-dichlorophenol above the limit of detection and 2,020 participants had levels of 2,4-dichlorophenol above the limit of detection (limit of detection for both was 0.14 μg/L). The range of the measured urine concentration was 0.14 to 19,600 μg/L for 2,5-dichlorophenol and 0.14 to 1,230 μg/L for 2,4-dichlorophenol. The crude concentration for the 75th percentile was 53.7 μg/L of 2,5-dichlorophenol and 2.9 μg/L for 2,4-dichlorophenol.
Participant characteristics are listed in Table 1. Of 2,211 participants, 411 were sensitized to at least 1 food allergen and 1,016 to at least 1 environmental allergen.
Table 1.
Participants’ characteristics by food and environmental allergy presence, NHANES 2005-2006a
| Characteristic | All participants (n=2,211) |
Sensitization to food allergens | Sensitization to environmental allergens | ||||
|---|---|---|---|---|---|---|---|
| Yes (n=411) | No (n=1,800) | P value | Yes (n=1,016) | No (n=1,195) | P value | ||
| Age, y | 39.5 (0.9) | 37.2 (1.1) | 39.9 (1.2) | .10 | 37.9 (0.9) | 40.7 (1.3) | .04 |
| Male sex, % | 49 (0.02) | 60.7 (0.03) | 46.7 (0.02) | .003 | 57.0 (0.02) | 42.7 (0.02) | <.001 |
| Race/ethnicity, % | <.001 | .01 | |||||
| Non-Hispanic white | 71.1 (0.03) | 61.7 (0.04) | 72.8 (0.03) | 67.2 (0.04) | 74.0 (0.03) | ||
| Non-Hispanic black | 11.6 (0.2) | 20.0 (0.03) | 10.1 (0.02) | 14.9 (0.03) | 9.2 (0.02) | ||
| Mexican American | 8.9 (0.01) | 8.9 (0.02) | 8.9 (0.01) | 8.8 (0.01) | 9.0 (0.01) | ||
| Other | 8.4 (0.01) | 9.4 (0.02) | 8.2 (0.01) | 9.1 (0.02) | 7.8 (0.01) | ||
| Low SES,b % | 12.8 (0.01) | 14.0 (0.01) | 12.5 (0.01) | .40 | 13.6 (0.01) | 12.1 (0.01) | .30 |
| Presence of dichlorophenol metabolites in urine, %c | <.001 | .047 | |||||
| Low dichlorophenols (<75th percentile) | 79.6 (0.02) | 69.5 (0.03) | 81.4 (0.02) | 77.4 (0.02) | 81.2 (0.02) | ||
| One high dichlorophenol (≥75th percentile) | 9.0 (0.007) | 12.8 (0.02) | 8.3 (0.007) | 10.6 (0.01) | 7.8 (0.007) | ||
| Two high dichlorophenols (≥75th percentile) | 11.4 (0.01) | 17.7 (0.02) | 10.3 (0.01) | 12.0 (0.01) | 11.0 (0.02) | ||
| Vitamin D, % | <.005 | <.04 | |||||
| <15 ng/mL | 15.0 (0.02) | 21.0 (0.03) | 13.9 (0.01) | 16.5 (0.02) | 14.0 (0.01) | ||
| 15-29 ng/mL | 61.5 (0.01) | 60.9 (0.03) | 61.6 (0.01) | 63.7 (0.02) | 59.8 (0.02) | ||
| ≥30 ng/mL | 23.5 (0.02) | 18.1 (0.02) | 24.5 (0.02) | 19.8 (0.02) | 26.2 (0.02) | ||
| Asthma, %d | 13.2 (0.01) | 20.7 (0.03) | 11.8 (0.01) | .004 | 18.5 (0.01) | 9.2 (0.01) | <.001 |
| Allergies, %d | 32.7 (0.01) | 42.0 (0.04) | 31.1 (0.01) | .01 | 42.9 (0.03) | 25.2 (0.01) | <.001 |
| Hay fever, %d | 11.0 (0.01) | 19.3 (0.02) | 9.6 (0.01) | .002 | 18.0 (0.01) | 6.0 (0.01) | <.001 |
| Use products at home to control insects, %e | 9.3 (0.01) | 6.0 (0.02) | 10.0 (0.01) | .03 | 8.7 (0.02) | 10.0 (0.01) | .50 |
| Use products in garden to control weeds, %e | 8.0 (0.01) | 7.3 (0.02) | 8.9 (0.02) | .60 | 8.1 (0.01) | 9.2 (0.01) | .50 |
| Residence, % | .03 | .07 | |||||
| Urbanf | 50.3 (0.08) | 55.1 (0.07) | 48.3 (0.07) | 52.6 (0.07) | 47.0 (0.07) | ||
| Ruralg | 49.7 (0.08) | 44.9 (0.07) | 51.7 (0.07) | 47.4 (0.07) | 53.0 (0.07) | ||
Abbreviations: NHANES, National Health and Nutrition Examination Survey; SES, socioeconomic status.
All estimates are weighted to the sampling weights. All data is presented as mean (SE).
Percentage of participants with a poverty-income ratio of less than 1.0.
Presence of dichlorophenol metabolites in urine was defined as levels equal to or above the 75th percentile value.
Self-reported allergies, asthma, and hay fever as having been told by a physician.
Self-reported use of pesticides in the past 7 days in the home to control insects or on the lawn or garden to control weeds.
Densely settled core and the adjacent densely settled surrounding territory (with a density of at least 500 people per square mile) that together have a minimum of 2,500 people.
All population located outside the urban residence.
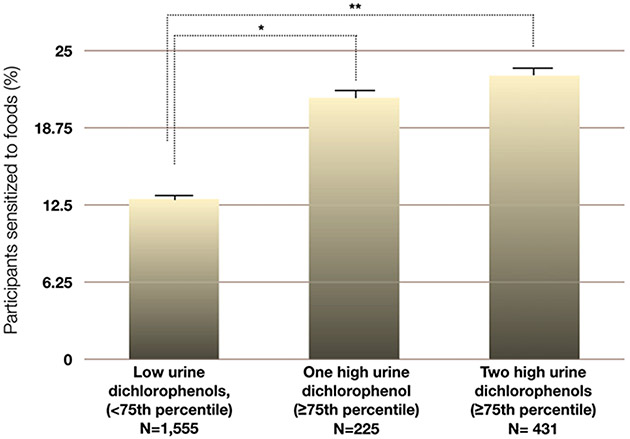
In an unadjusted context, having a high level of 1 or more dichlorophenol metabolites in the urine (at the 75th percentile or above) was significantly associated with sensitization to food (Table 1 and Fig 1) and environmental allergens (Table 1). Food but not environmental allergen sensitivity was positively associated with the use of home pesticides and urban living (Table 1).
Figure 1.
Association between high urine dichlorophenol levels (≥75th percentile) and allergy to foods. *P < .05, weighted χ2 test. **P < .01, weighted χ2 test.
Association of high dichlorophenol levels with food and environmental allergies
A multivariable analysis was performed to further evaluate the allergy association with high dichlorophenol levels using the potential confounders identified in the bivariable analysis. After adjustment for age, sex, race, vitamin D levels, insecticide use at home, presence of hay fever and asthma, and the place of residence, the presence of high levels of the 2 dichlorophenol metabolites in the urine remained significantly associated with sensitization to food allergens (odds ratio, 1.8; 95% confidence interval, 1.2-2.5; P = .003) (Table 2). However, in the adjusted model (as described in Table 2), no significant associations between dichlorophenol exposure and environmental allergies and between the place of residence and sensitization to food or environmental allergens were found. Sensitization to food allergens continued to be associated with male sex, non-Hispanic black ethnicity, low vitamin D levels, self-reported allergies, and home use of insecticide.
Table 2.
Multivariable logistic regression analysis of factors independently associated with allergiesa
| Characteristic | Sensitization to food allergens (n=2,211) |
Sensitization to environmental allergens (n=2,211) |
Sensitization to food and environmental allergens (n=1,500) |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (in 5-year increments) | 0.96 (0.92-1.0) | .10 | 0.96 (0.93-0.99) | .02 | 0.95 (0.91-0.99) | .01 |
| Male sex | 1.8 (1.2-2.7) | .006 | 1.8 (1.4-2.4) | <.001 | 2.6 (1.6-4.5) | .001 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1.0 | 1.0 | 1.0 | |||
| Non-Hispanic black | 1.8 (1.2-2.6) | .007 | 1.7 (1.2-2.5) | .01 | 2.1 (1.2-3.4) | .008 |
| Mexican American | 1.0 (0.7-1.4) | .89 | 1.0 (0.6-1.7) | .99 | 1.0 (0.6-1.7) | .99 |
| Other | 1.3 (0.9-2.3) | .20 | 1.3 (0.8-2.2) | .30 | 1.4 (0.7-2.9) | .30 |
| Presence of dichlorophenol metabolites in urineb | ||||||
| Low dichlorphenols (<75th percentile) | 1.0 | 1.0 | 1.0 | |||
| One high dichlorophenol (≥75th percentile) | 1.5 (0.9-2.4) | .09 | 1.1 (0.8-1.7) | .50 | 1.6 (0.9-2.8) | .08 |
| Two high dichlorophenols (≥75th percentile) | 1.8 (1.2-2.5) | .003 | 0.96 (0.8-1.2) | .70 | 1.61 (1.2-2.2) | .005 |
| Vitamin D | ||||||
| <15 ng/mL | 1.7 (1.05-2.6) | .03 | 1.4 (0.99-2.1) | .05 | 1.9 (1.1-3.2) | .02 |
| 15 to 29 ng/mL | 1.2 (0.9-1.7) | .20 | 1.3 (1.1-1.7) | .02 | 1.2 (0.8-1.8) | .30 |
| ≥30 ng/mL | 1.0 | 1.0 | 1.0 | |||
| Asthmac | 1.6 (1.02-2.5) | .04 | 1.7 (1.1-2.7) | .01 | 1.9 (0.95-3.7) | .07 |
| Hay feverc | 2.5 (1.6-3.8) | .001 | 3.7 (2.5-5.6) | <.001 | 4.9 (2.9-8.1) | <.001 |
| Use products at home to control insectsd | 0.5 (0.3-0.9) | .02 | 0.8 (0.4-1.5) | .50 | 0.5 (0.2-1.1) | .08 |
| Reside in rural arease | 0.9 (0.7-1.2) | .40 | 0.9 (0.7-1.1) | .40 | 0.8 (0.6-1.2) | .20 |
Abbreviations: CI, confidence interval; OR, odds ratio.
All estimates are weighted to the sampling weights. All models were adjusted for urinary creatinine that was added to the models as a separate independent variable.
Presence of dichlorophenol metabolites in urine was defined as levels equal to or above the 75th percentile value.
Self-reported asthma and hay fever as having been told by a physician.
Self-reported use of pesticides in the past 7 days in the home to control insects.
Compared with the residents of urban areas.
Socioeconomic status was not significantly associated with allergic sensitization in the final models.
Associations in participants with both types of allergies
We also examined the association of dichlorophenol exposure in the most atopic group of participants, who were sensitized to both food and to environmental allergens. A total of 1,500 participants met the criteria for this subanalysis. Of these, 358 had both food and environmental allergies.
In the multivariable logistic regression model, having high urine levels of 2 dichlorophenol metabolites was significantly associated with being simultaneously sensitive to both food and environmental allergies (odds ratio, 1.61; 95% confidence interval, 1.2-2.2; P = .005) (Table 2).
Discussion
Our analysis indicates that the presence of high levels of dichlorophenols in the urine was significantly associated with sensitization to food allergens and that this association occurs regardless of cosensitization to common environmental allergens. High levels of urine dichlorophenols were not associated with the sensitization to environmental allergens in the absence of food allergy. To our knowledge, this is the first study demonstrating an association between high dichlorophenol levels and allergic sensitization.
Because food allergies are more common in children,19 there is a question on how clinically relevant these findings are. Our study participants with sensitizations to foods had a mean age of 37.2 years. This is partially because urinary metabolites of chlorinated phenols were not measured in children younger than 6 years in NHANES 2005-2006, and therefore these children were excluded from our analysis. However, in a recently published survey on food allergies in Greece, the age of onset of food allergies in study participants was not during the early childhood but during the second and the third decades of life.21 In addition, certain food allergies, such as seafood, are more prevalent among adults than children as reported in Italy and the United States.22,23 Therefore, it is reasonable to suggest that exposure to prevalent environmental substances, such as dichlorophenols, may affect the development of food allergies in adults, although the age of onset of sensitization to food allergens in our study population is unknown.
Little is known about the association between sensitization to foods and pesticide use in adults. However, previous studies have found that pesticide exposure was associated with respiratory allergic disorders, such as asthma and rhinitis, in both children and adults.24-27 It is not completely understood how pesticides could affect allergic sensitization. Carbaryl increased allergic sensitization to dust mites in rats.28 In addition, biological interactions between allergens and pesticides have been suggested.24
Studies in children have found that healthy commensal flora influence mucosal immune tolerance. These flora are thought to be responsible for the transition from the TH2-predominant profile in early life to a more balanced phenotype in nonallergic individuals.29-31 Pentachlorophenol and its metabolites have a potent antibacterial effect.32 Contamination with pentachlorophenol metabolite 2,4-dichlorophenol has been found in produce, such as cocoa-powder and fruit juices.33,34 Therefore, drinking of chlorinated water and ingestion of crops treated with dichlorophenol-containing pesticides may alter the microbial spectrum to which humans are naturally exposed. In animal studies, a chlorine-containing pesticide that was recently banned by the European Union,35 sodium chlorate, was supplemented to drinking water of cattle stock. Its consumption reduced fecal population of Escherichia coli in cows by more than 100-fold.36 A daily exposure to dichlorophenols through water or food may have an ongoing effect on the bacterial diversity. There are indications that the intestinal microflora of nonallergic children differs from the microflora of allergic children.37 In addition, altered intestinal microbiota is associated with an increase of CD4-mediated inflammation in mice.31 Whether changes in intestinal microflora have a similar effect on allergic sensitization in adults is not known.
Atopic conditions are considered a burden of early childhood.1,27,38,39 However, recent studies suggest that allergic sensitization to environmental and food allergens is also common in adults and may be on the rise.22,40,41 In line with the hygiene hypothesis, rural living had a protective effect on allergic sensitization in adults, regardless of the area of residence during the early childhood.41
Although establishing the mechanism by which dichlorophenols could affect sensitization to foods is beyond the scope of our study, our results suggest that exposure to dichlorophenol-containing pesticides could possibly weaken tolerance to foods in humans. Because one of the major exposure routes to dichlorophenols is oral, this might explain the association of high dichlorophenol levels only with sensitization to foods and not to environmental allergens.
On the other hand, we recognize that there are a number of possible explanations for this association, including that dichlorophenols may have some type of direct effect on the immune system that promotes food allergen sensitization. It is also worth noting that the wide consumption of bottled water in the United States may offset the importance of chlorinated water in supplying dichlorophenols to consumers and leaves other dichlorophenol sources (eg, pesticide-treated crops) a greater role.
This study has several limitations. One is that a causal relationship between allergic sensitization and dichlorophenol exposure cannot be established because of the study’s cross-sectional design. In addition, this analysis did not include children younger than 6 years on whom pesticide levels were not measured in the NHANES 2005-2006 data set. We also attempted to analyze sensitization to foods using higher food-specific IgE levels that have a greater positive predictive value of a clinical response in food-allergic individuals.42 However, only 0.7% of participants met the definitions of the higher IgE cutoffs, and this analysis was not feasible. Taking into account the older age of the subpopulation on whom dichlorophenol levels have been measured in NHANES, this finding is in agreement with the previous reports on clinical food allergy prevalence in the United States.19
Nevertheless, our analysis has many strengths, including the use of a representative sample of the general US population and the analysis of a specific group of chemicals widely used for their bactericidal effect. Moreover, we adjusted for differences in pesticide exposure between urban and rural populations using the restricted data set on geographic variables in the NHANES database.
In this population, we found consistent associations between high levels of dichlorophenol exposure and a higher prevalence of food allergies. Previous research indicated that both environmental pollution43 and the prevalence of food allergies are increasing in the United States.44 The results of this study suggest that these 2 phenomena might be linked. Further prospective studies would be necessary to confirm this link.
Acknowledgments
We thank Nataliya Kravets (National Center for Health Statistics) for her expert assistance in data programming.
Funding Sources:
This study was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, components of the National Institutes of Health, through Clinical and Translational Science Award (grant numbers UL1 RR025750, KL2 RR025749, and TL1 RR025748). Its contents are solely the responsibility of the authors and do not necessary represent the official view of the National Institutes of Health.
Footnotes
Disclosures: Authors have nothing to disclose.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
References
- [1].Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. [DOI] [PubMed] [Google Scholar]
- [2].Umetsu DT, Dekruyff RH. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: microbes, apoptosis and TIM-1 in the development of asthma. Clin Exp Immunol. 2010;160:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].von Mutius E. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: farm lifestyles and the hygiene hypothesis. Clin Exp Immunol. 2010;160:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].von Hertzen L, Haahtela T. Disconnection of man and the soil: reason for the asthma and atopy epidemic? J Allergy Clin Immunol. 2006;117:334–344. [DOI] [PubMed] [Google Scholar]
- [5].Toivola M. Personal Exposure to Micronbial Aerosols. Kuopio, Finland: University of Kuopio; 2004. National Health Institute publication A13/2004. [Google Scholar]
- [6].Demographics. 2009. http://www.epa.gov/agriculture/ag101/demographics.html. Accessed December 20, 2011.
- [7].Industrial Farming vs. Sustainable Farming. 2011. http://www.epa.gov/agriculture/ag101/demographics.html. Accessed December 20, 2011.
- [8].Environmental Protection Agency. The Incorporation of Water Treatment Effects on Pesticide Removal and Transfromations in Food Quality Protection Act (FQPA) Drinking Water Assessments: Science Policy. Washington, DC: Environmental Protection Agency; 2001. [Google Scholar]
- [9].von Hertzen L, Laatikainen T, Pitkanen T, et al. Microbial content of drinking water in Finnish and Russian Karelia: implications for atopy prevalence. Allergy. 2007;62:288–292. [DOI] [PubMed] [Google Scholar]
- [10].Chemical Information. 2,4-Dichlorophenol. http://www.cdc.gov/exposurereport/data_tables/24D_ChemicalInformation.html. Accessed December 25, 2011.
- [11].Chemical Information. Trichlorophenols. http://www.cdc.gov/exposurereport/data_tables/Trichlorophenols_ChemicalInformation.html. Accessed January 30, 2012.
- [12].Chemical Information. 2,5-Dichlorophenol. http://www.cdc.gov/exposurereport/data_tables/25D_ChemicalInformation.html. Accessed February 19, 2012.
- [13].World Health Organization. Chlorophenols in Drinking Water. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- [14].Dodson RE, Houseman EA, Levy JI, Spengler JD, Shine JP, Bennett DH. Measured and modeled personal exposures to and risks from volatile organic compounds. Environ Sci Technol. 2007;41:8498–8505. [DOI] [PubMed] [Google Scholar]
- [15].Glossary of Classes of Non-persistent Pesticides. 2012. http://www.cdc.gov/nceh/clusters/fallon/#glossaries. Accessed February 19, 2012.
- [16].US Census 2000 Urban and Rural Classification 2000. http://www.census.gov/geo/www/ua/ua_2k.html. Accessed March 27, 2012.
- [17].Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2011;127:1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Berns SH, Halm EA, Sampson HA, Sicherer SH, Busse PJ, Wisnivesky JP. Food allergy as a risk factor for asthma morbidity in adults. J Asthma. 2007;44:377–381. [DOI] [PubMed] [Google Scholar]
- [19].Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kalogeromitros D, Makris MP, Chliva C, et al. An internet survey on self-reported food allergy in Greece: clinical aspects and lack of appropriate medical consultation [published online ahead of print]. J Eur Acad Dermatol Venerol. February 21, 2012. 10.1111/j.1468-3083.2012.04482.x. [DOI] [PubMed] [Google Scholar]
- [22].Asero R, Antonicelli L, Arena A, et al. Causes of food-induced anaphylaxis in Italian adults: a multi-centre study. Int Arch Allergy Immunol. 2009;150:271–277. [DOI] [PubMed] [Google Scholar]
- [23].Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159–165. [DOI] [PubMed] [Google Scholar]
- [24].Hoppin JA, Umbach DM, London SJ, et al. Pesticide use and adult-onset asthma among male farmers in the Agricultural Health Study. Eur Respir J. 2009;34:1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chatzi L, Alegakis A, Tzanakis N, Siafakas N, Kogevinas M, Lionis C. Association of allergic rhinitis with pesticide use among grape farmers in Crete, Greece. Occup Environ Med. 2007;64:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Slager RE, Poole JA, LeVan TD, Sandler DP, Alavanja MC, Hoppin JA. Rhinitis associated with pesticide exposure among commercial pesticide applicators in the Agricultural Health Study. Occup Environ Med. 2009;66:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Salam M, Li Y, Langholz B, Gilliland F. Early-life environmental risk factors for asthma: findings from the Children’s Health Study. Environ Health Perspect. 2004;112:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dong W, Gilmour MI, Lambert AL, Selgrade MK. Enhanced allergic responses to house dust mite by oral exposure to carbaryl in rats. Toxicol Sci. 1998;44:63–69. [DOI] [PubMed] [Google Scholar]
- [29].Holt PG, van den Biggelaar AH. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: the role of infections in allergy: atopic asthma as a paradigm. Clin Exp Immunol. 2010;160:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wold AE. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy. 1998;53:20–25. [DOI] [PubMed] [Google Scholar]
- [31].Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bechold H, Ehrlich P. Beziehungen zwischen chemischer Konstitution und Desinfektionswirkung. Z Physiol Chem. 1906;47:173–199. [Google Scholar]
- [33].Jensen N, Whitfield FB. Role of Alicyclobacillus acidoterrestris in the development of a disinfectant taint in shelf-stable fruit juice. Lett Appl Microbiol. 2003;36:9–14. [DOI] [PubMed] [Google Scholar]
- [34].2,4-Dichlorophenol. 2012. http://toxmap.nlm.nih.gov/toxmap/main/chemPage.jsp?chem=2,4-Dichlorophenol. Accessed February 19, 2012.
- [35].Regulatory Update - Products to be withdrawn from the market containing sodium chlorate. Health and Safety Executive, 2009. http://www.pesticides.gov.uk/guidance/industries/pesticides/topics/pesticide-approvals/eu/eu-reviews/regulatory-update-products-to-be-withdrawn-from-the-market-containing-sodium-chlorate. Accessed June 26, 2012. [Google Scholar]
- [36].Callaway TR, Anderson RC, Genovese KJ, et al. Sodium chlorate supplementation reduces E. coli O157:H7 populations in cattle. J Anim Sci. 2002;80:1683–1689. [DOI] [PubMed] [Google Scholar]
- [37].Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–346. [DOI] [PubMed] [Google Scholar]
- [38].Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110:315–322. [DOI] [PubMed] [Google Scholar]
- [39].Almqvist C, Li Q, Britton WJ, et al. Early predictors for developing allergic disease and asthma: examining separate steps in the ‘allergic march’. Clin Exp Allergy. 2007;37:1296–1302. [DOI] [PubMed] [Google Scholar]
- [40].Linneberg A, Gislum M, Johansen N, Husemoen LL, Jorgensen T. Temporal trends of aeroallergen sensitization over twenty-five years. Clin Exp Allergy. 2007;37:1137–1142. [DOI] [PubMed] [Google Scholar]
- [41].Raukas-Kivioja A, Raukas ES, Meren M, Loit HM, Ronmark E, Lundback B. Allergic sensitization to common airborne allergens among adults in Estonia. Int Arch Allergy Immunol. 2007;142:247–254. [DOI] [PubMed] [Google Scholar]
- [42].Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. [DOI] [PubMed] [Google Scholar]
- [43].European Environment Agency. Increasing environmental pollution load. In: The European Environment State and Outlook. Copenhagen, Denmark: European Environment Agency; 2010. [Google Scholar]
- [44].Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549–1555. [DOI] [PubMed] [Google Scholar]