Abstract
Peri-implantitis is an important cause of oral implant failure. In the past, TLR4 and TLR2 in the Toll-like family were generally considered as the key immune recognition receptors regulating peri-implantitis. However, under the guidance of this theory, there are still some unexplainable peri-implantitis symptoms. With the discovery of novel intracellular LPS receptor Caspase-11, a new understanding of inflammatory signaling and immune regulation in the development of peri-implantitis has been gained. However, the regulatory role of Caspase-11 in peri-implantitis and its crosstalk with the TLR4 pathway remain unclear. The therapeutic effect of drugs targeting Caspase-11 on peri-implantitis is still in its early stages. In view of this situation, this paper reviews the possible role of Caspase-11 in peri-implant inflammation, elaborated the entry process of LPS and the activation mechanism of Caspase-11, and analyzes the differences in Caspase-11 between commonly studied animals, mice and humans. The current research hotspots and challenges are also analyzed to provide new insights and ideas for researchers.
Keywords: caspase-11, pyroptosis, inflammation, peri-implantitis
Introduction
Peri-implantitis is a leading cause of implant failure, and approximately 30% of implant sites develop peri-implantitis after implantation. Peri-implantitis is strongly associated with pathogens such as Porphyromonas gingivalis. The interplay between pathogens and the host immune system has the potential to induce periodontal tissue inflammation.1 Different from other diseases dominated by bacterial infection, the destruction of soft tissue and hard tissue caused by peri-implantitis is currently believed to be caused by bacteria breaking the implant-host immune balance and mainly mediated by the host’s own immune cells.2 Bacteria activate and continuously stimulate immune cells by releasing endotoxin, exotoxin and immunogenic fragments.3 This weak inflammatory signal is amplified by layers of host immune cells and can show corresponding clinical symptoms after breaking a certain threshold.4,5
Pyroptosis, which shows cell swelling, perforation, dissolving and releasing massive inflammatory factors, is a type of programmed cell death caused by activation of caspase family.6 Hippocrates was the first known person to use the term “pyroptosis” in the literature (c. 460 – c. 370 BC), where “pyro” means fire or fever in ancient Greek and “ptosis” is associated with falling or dying. This description aptly reflects that pyrosis is an inflammatory process of programmed cell death which is dependent on caspase.7 The prefix indicates amounts of endogenous pyrogen released by the process. The root of the word is the same as the root of “apoptosis”, indicating a programmed cell death process. In addition to causing corresponding cell death, this process also causes obvious damage and even death of the surrounding cells. Pyroptosis has a great impact on oral periodontal diseases and is the main culprit leading to rapid amplification of inflammatory signals, degradation of gum collagen fibers, and bone absorption.8 However, caspase cannot be directly activated by extracellular LPS, which requires capture and transmission of signals by immune recognition receptors.
The most common immune recognition receptors are toll-like receptors, which were discovered by Christiane in 1995. It has a variety of subtypes with different functions and locations, so that it is considered as important functional receptors for intercellular communication, signal transductions and recognition.9 Studies of traditional lipopolysaccharides, which are considered to be one of the main pathogens that bacteria stimulate the host’s immune system, and its receptor TLR4 do not explain the full extent of the inflammatory response in peri-implantitis.10 Mohammed found that although TLR4 inhibitor could reduce the expression level of inflammatory factors in LPS-stimulated macrophages,11 it still could not block the macrophages from expressing a considerable amount of TNF-alpha due to the alveolar bone loss. This phenomenon was also demonstrated in NLRP3 and ASC downstream of TLR4: TLR4 receptor inhibitors only partially reduced LPS-induced NLRP3 and ASC expression.12 The former is a multi-protein signal transduction complex involved in inflammatory response, causing the cytokines maturate and secret, which is strongly linked to the immune reaction and tissue damage of peri-implantitis.13 The latter can not only promote cell apoptosis and bone destruction but also be used as an indicator to judge the status of peri-implantitis.14 Therefore, we believe that an independent immune-recognition receptor in addition to TLR4 plays an unparalleled role in the immune reaction and tissue damage progression of peri-implantitis. It provides a new idea for us to further explain the pathogenesis of peri-implantitis and find better therapeutic targets for peri-implantitis.
Caspase-11 is a special member of the Caspase family. Caspase-11 was officially verified to be independent of TLR4 in recognizing bacterial LPS, mediating immune response and specific pyroptosis by Nobuhiko in 2013.15 The specific recognition of Caspase-11 and LPS depends on their unique caspase-activated recruitment domain (CARD protein domain) which binds to lipid A of gram-negative bacteria LPS.16 This binding depends on the electrostatic adsorption of LPS to several positively charged groups in the CARD domain, which is similar to the affinity of LPS and TLR4-MD2 complex.17 Unlike the activation process of TLRs, the activation process of Caspase-11 does not rely on the assistance of NRL-like scaffold and can complete the immune recognition of LPS completely by itself. Binding of LPS to the CARD of caspase-11 not only triggers its oligomerization but also catalyzes its activation, much like the activation process of the caspase family.18 Activation of TLR and activation of Caspase-11 often coexist in inflammatory tissues, and the occurrence of Caspase activation often leads to unexpected serious consequences, such as tissue damage that does not match the stimulus or even life-threatening.19 Combined with the fact that gram-negative anaerobe is the most highly associated microbe in periodontitis, it is not difficult to conclude that the importance of Caspase-11 in the progression of peri-implantitis may be significantly underestimated, and Caspase-11 is a promising therapeutic target candidate.
Unfortunately, the role of Caspase-11 and its regulation of pyroptosis in the oral domain remains unclear, and its regulatory mechanism in peri-implantitis is still indistinct. For the above reasons, there is a growing interest in further exploring the role of Caspase-11 and its regulation of pyroptosis in oral peri-implantitis. In this paper, we review the role and mechanism of Caspase-11 in mediating soft and hard tissue damage and discuss its potential to promote tissue repair. This review aims to provide new ideas for the basic research of oral peri-implantitis and the development of therapeutic drugs for oral peri-implantitis.
Role of Caspase-11 in Peri-Implantitis
The peri-implant supporting tissue can be divided into gingival tissue (soft tissue) dominated by above skin cells and fibroblasts and bone tissue (hard tissue) dominated by cancellous bone and bone cortex. Those two tissues are different in tissue characteristics, cell gene expression and response to inflammatory substances. Therefore, this section will, respectively, describe the damage caused by Caspase-11-mediated immune recognition and pyroptosis to the two tissues.
Soft-Tissue Damage
Peri-implant soft tissue is less vascular and the soft tissue sealing of the implant is weak, only internal basal laminin and hemidesmosome are used to form a connection.20 Due to microbial pressure in oral and potential rejection of foreign bodies (generally Ti particles), the peri-implant soft tissue may be in a mild inflammatory-healthy alternation for a long time.21 It is usually difficult to detect such inflammatory state, and there are no corresponding clinical symptoms. Combined with this special phenomenon, some scholars have proposed the implant-host immune balance theory to explain it. With the increase of bacterial pressure and the change of the flora around the implant, this delicate equilibrium state was broken by the excessive release of pathogenic substances by the bacteria. The first step in this process is the recognition of the LPS predominant pathogenic substance by the immune recognition receptor.
Macrophages are important cells that play a key role in immune recognition. It is deeply involved in initiating the validation process, promoting local inflammatory cell infiltration, increasing local angiogenesis and enhancing vascular permeability, and the emergence of pyroptosis will greatly accelerate this process.22 Studies have now demonstrated that LPS secreted by pathogenic bacteria not only binds to conventional receptors (especially TLR4) but also specifically binds to the intracellular pattern-recognition receptor caspase-11, triggering the inflammasome even more and leading to the release of substantial IL-1β and IL-18, which is also thought to be an important manifestation of inflammatory cell recruitment and amplification of inflammatory signals in peri-implantitis.23 Notably, in Caspase-11-deficient macrophages, the expression and pathology of LPS-induced pyroptosis were significantly reduced.24 In conclusion, Caspase-11 can enhance the TlR4-mediated caspase-1-dominated pyroptosis process.
Metalloproteinase (MMP) family is also a key protein in peri-implantitis, playing an important role in regulating inflammation and promoting collagen fiber degradation, and can also be used as an important indicator to evaluate the degree of periodontal soft tissue inflammation and microbial stress.25 In the state of pyroptosis, cells will release more MMP, thus enhancing the damage to gingival tissue.26 This also explains why Caspase-11-mediated inflammation leads to the effect of oral epithelial connections.27 By inhibiting the immune recognition of Caspase-11, the researchers found that, in addition to the usual reduction in cytokine release, such as interleukin and tumor necrosis factor, MMP expression was significantly reduced, similar to the inhibition of pyroptosis.28 This phenomenon suggests that Caspase-11 may play an exceeding expectational role in soft tissue destruction in peri-implantitis.
Caspase-11 also interacts with other regulators to regulate local levels of inflammation. He et al suggested that the pyroptosis level, which is mediated by caspase-11, could be improved by lack of Differentiated Embryonic Chondrocyte Gene 2 (Dec2).29 Dec2 can protect periodontal membrane and gingival from Caspase-11-mediated inflammation.30 However, the mechanism of Dec2 on Caspase-11 still needs to be further explored, and more evidence needs to be provided. Besides, the role of Caspase-11-mediated focal death in the destruction of peri-implantitis soft tissue remains unclear. More researches and evidences are needed to confirm the significant mechanism of Caspase-11 in gingival tissue, so as to reveal more mysteries about the occurrence and development of peri-implantitis.
Hard-Tissue Damage
Compared to long bones, the alveolar bone has a stronger blood supply, which leads to more active bone activity. It should be clarified that bone resorption around the implant is affected by a variety of conditions that differ from soft tissue in peri-implantitis.31 In this paper, only peri-implant bone resorption induced by microbial stress is discussed. The balance of bone destruction-bone reconstruction, known as bone homeostasis, exists at all times in the bone tissue surrounding healthy implants.32 This metabolic balance constantly reconstructs the supporting bone around the implant, thus making the trabecular distribution more suitable for the biomechanical requirements of mastication and occlusal of the implant.33 The increase of microbial pressure will break this balance, making osteoclasts much more active than osteoblasts, and tilting the bone balance toward bone absorption. In this process, pyroptosis plays an indispensable role in bone damage in peri-implantitis.
The process of pyroptosis can change the cationic flux of osteocytes, induce cell swelling, activate WNT/beta-catenin, induce osteoclast proliferation, and initiate bone resorption.34 This process is due to the release of a large amount of inflammatory signals by cell pyroptosis, causing bone tissue to develop an inflammatory state inconsistent with the amount of disease-causing substances secreted by bacteria it contacts. When the pyroptosis process is inhibited, the inflammatory signal transmission initiated by bacteria is weakened or even interrupted, and the bone resorption process can be significantly reduced.35
It is generally believed that TLR4, MD2 and their downstream caspase-1 are the main regulatory components involved in cell pyroptosis when bacterial bone resorption occurs in alveolar bone.36 In the study of articular cartilage inflammation, we found that the activation of Caspase-11 alone triggers a considerable pyroptosis, which further leads to the invasion and destruction of cartilage edges.37 NLRP3, downstream of Caspase-11 immune recognition receptor, also deeply involved in the regulation of bone state. At present, there is definite evidence that inhibition of NLRP3 can significantly occur in inflammatory resorption of alveolar bone.38 Interestingly, when NLRP3 gene defect was present, the damage of toxin produced by Porphyromonas gingivalis, a major pathogenic bacterium of periodontitis, to bone tissue was significantly reduced, and the expressions of RANKL and IL-1B were significantly decreased.39 Therefore, we speculated that the activation of Caspase-11 is one of the important reasons for the severe host immune response caused by peri-implantitis. However, there is a lack of direct evidence on the role of Caspase-11 in regulating bone balance and promoting the differentiation and proliferation of cells to osteoclasts in peri-implantitis, which requires further exploration by researchers.
Mechanism of Caspase-11 Mediated Pyroptosis
The recognition process of Caspase-11 as an immune receptor is different from TLR-4. Caspase-11 is located in cells, so the immune process and subsequent regulatory mechanism of Caspase-11 are divided into the mechanism of pathogenesis internalization and the mechanism of receptor recognition and induced pyroptosis in this section.
Internalization Mechanisms of LPS
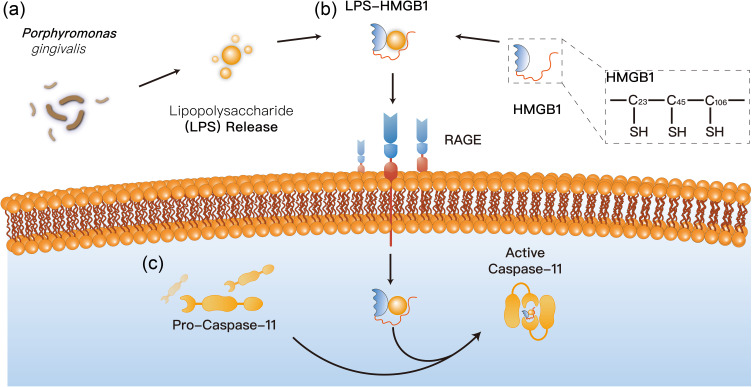
Unlike the immune recognition receptors on the plasma membrane, Caspase-11 requires LPS internalization in order to perform its immune recognition function. So far, this process mainly involves endotoxin internalization through the outer membrane vesicles (OMVs) pathway released by bacteria and endotoxin delivery protein HMGB1.40
OMV is released by gram-negative pathogenic bacteria during growth and proliferation.41 Proteomic and biochemical analyses show that these vesicles have a variety of outer-membrane-components of classical Gram-negative bacteria, including lipopolysaccharide, lipoprotein, DNA, RNA, etc.42 This secretion of OMV is more pronounced when the bacteria are under pressure from the host.43 Both IgG secretion in gingival crevicular fluid and sIgA secretion in saliva may lead to the release of more OMVs, thereby increasing bacterial biotoxicity. This explains why periodontal infections tend to show a more violent inflammatory response than other regional infections. OMV is considered to have a certain function of bacteria-host information transmission. After OMVs are captured by clathrin in the plasma membrane and enter the endocytosis vesicles, OMVs can release LPS and other inflammatory signaling molecules contained in the vesicles into the cytoplasm, thus completing the transport process of LPS into the cytoplasm.44
If OMVs are a carefully wrapped “gift” from bacteria to cells, then HMGB1 is the host cell’s weapon of choice in its search for wandering bacteria. High mobility group box 1 (HMGB1) is an effective inflammatory factor, which can exist in cytoplasm, nucleus and extracellular, and can combine with LPS to form LPS-HMGB1 complex in extracellular.45 The binding process is a physical binding like adsorption. LPS-HMGB1 complex was actively recognized by macrophages, and the complex was phagocytized into lysosome of macrophages by RAGE dependent internalization process.46 After entering the cell, the complex decreases the local pH value, leading to the enhanced penetration of the complex to the lysosome membrane, which is mainly composed of phospholipid biomolecule, leading to the leakage of lysosome contents and cell damage (Figure 1). This phenomenon is entirely attributed to the ph-dependent membrane penetration of HMGB1, that is, the lower the pH value, the higher the penetration of lipid bilayer membrane.46 Combined with the local oral anatomical structure, whether this process involves HMGB1’s penetration into the gingival crevicular barrier needs further study to confirm. If this hypothesis is correct, then the macrophages in the gums may exaggerate the state of infection, which could effectively explain why the periodontal tissue inflammation is chronically low.
Figure 1.
Proposed internalization mechanisms of LPS. (a) OMVs release LPS and other inflammatory signaling molecules contained in the vesicles into the cytoplasm; (b) HMGB1 can combine with LPS to form LPS-HMGB1 complex in extracellular, entering the lysosome membrane of cell; (c) LPS-HMGB1 complex obtains the ability to recruit and activate caspase-11, leading to the leakage of lysosome contents and cell damage.
There is also evidence that P. gingivalis has an obvious invasion effect on gingival epithelial cells and can achieve immune escape function by entering gingival cells.47 Whether this unique invasion process can serve as an alternative pathway for LPS to enter cells remains to be investigated.
Caspase-11 Mediated Pyroptosis Mechanism
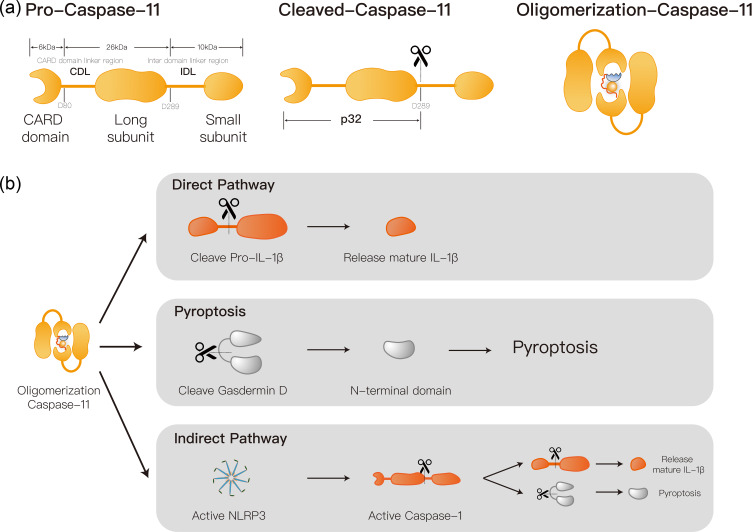
The core of the Caspase-11 activation by LPS is the oligomerization of Caspase-11. This process is mediated by the CARD domain. The initiation of Caspase-11 oligomerization depends on the penta-acylated or hexacylated structure of the A subunit of the lipid in LPS.48 Current studies have demonstrated that the dimerized Caspase-11 has protease activity and D285 site cleavage activity, that is, complete Caspase catalytic activity.49 The combination of caspase and LPS in cells often reached the ratio of 14:1.50 Thus, Caspase-11 is a highly sensitive immune recognition receptor. Activated Caspase-11 can mediate cell pyroptosis through direct and indirect pathways (Figure 2). The former is through direct lysis of Gasdermin D (GSDMD) and the release of its Gasdermin-N domain. This pathological process penetrates the plasma membrane, leading to cellular swelling and lysis, thereby triggering the process of pyroptosis.51 The latter is to activate NLRP3 inflammasome via Caspase-11. The NLRP3 inflammatory vesicle, one of the most studied inflammasome, is distributed in the cytoplasm, composed by the NLRP3, ASC adapter protein and caspase-1. The ASC protein contains the same PYD structure as the caspase recruitment domain (CARD). NLRP3 is a protein coincidence body that is assembled by a central NACHT domain, a Leucine-rich repeat (LRR) domain and an amino-terminal pyrin domain (PYD).51 The activation of NLRP3 inflammasome results in inflammatory cytokines interleukin-1b (IL-1b) and interleukin-18 (IL-18)’s secretion, as well as pyroptosis. NLRP3 and ACS adapter formation complex can further activate caspase-1, thus mediating the traditional pathway of cell pyroptosis.52 ACS spots have two domains: PYRIN domain and Caspase activation recruitment domain (CARD domain).53 The 50 bases at the n-terminal of the former peptide chain are mainly responsible for the recognition and binding of NLRP3, and the NLRP3-ACS complex obtains the ability to recruit and activate caspase-1.54 This process also rapidly amplifies inflammatory signals, resulting in caspase-1-mediated cell pyroptosis.
Figure 2.
Proposed caspase-11 mediated pyroptosis mechanism. (a) Caspase-11 consists of large subunit, small subunits and a CARD domain, which can be activated through oligomerization mediated by cleavage at the D289 site. (b) Activated caspase-11 can mediate cell pyroptosis through direct and indirect pathways: The direct pathway directly mediates pyroptosis by directly cleaving the gasdermin D and releasing the N-terminal; The indirect pathway mediates cellular pyroptosis by promoting caspase-1 activation through activation of the NLRP3 inflammasome.
Interestingly, Ti ion release from the implant enhanced NLRP3 activation and significantly promoted pyroptosis and the release of inflammatory factors.13 The serum and salivary NLRP3 concentrations were demonstrated to be a significant predictor of periodontitis.55 If we can prove that Caspase-11 was deeply involved in the development of peri-implantitis, this phenomenon may also be the underlying cause of the higher inflammation intensity and greater damage in peri-implantitis than in periodontitis.
Human versus Mouse of Caspase-11 Mediated LPS-Sensings
Caspase-11 was first identified as an immune-recognition receptor in mice. Caspase-11 does not exist in the human genome and is replaced by caspase-4 and caspase-5, both highly conserved genes.56 Despite the different names, the structure and function of the three are not much different. In human, caspase-5 is mainly expressed in skin, epithelium, small intestine and other tissues in contact with the external environment, while caspase-4 is mainly expressed in internal organs, but both are highly expressed in monocytes and macrophages.57 Not only that, there are differences in the structures that activate them. Caspase-4 can be activated and oligomerized by tetraacylated LPS to obtain enzyme activity and corresponding function, which Caspase-11 cannot do.58 In addition, unlike in mice, caspase-4/5 expression is maintained in cells without infection. This advantage is that it can capture the relevant information of pathogen invasion more quickly and sensitively and make a response more rapidly.
Unfortunately, the details of how the pathways are regulated vary greatly between human and mice, suggesting that researchers should be more cautious when translating mice data into clinical applications.
Research Prospects and Challenges
The more important role of Caspase-11 and pyroptosis has been verified in different fields, and the more scholars recognize the importance of Caspase-11 in inflammation regulation, tumor therapy and virus prevention and treatment. The role of Caspase-11 and its regulation of pyroptosis studying based on mice provides clues for clinical application. It has been demonstrated that macrophages from Gsdmd(-/-) mice generated by gene targeting also exhibit defective pyroptosis and interleukin-1β secretion induced by cytoplasmic lipopolysaccharide or Gram-negative bacteria. Mechanistically, caspase-11 cleaves gasdermin D, and the resulting amino-terminal fragment promotes both pyroptosis and NLRP3-dependent activation of caspase-1 in a cell-intrinsic manner. Data identify gasdermin D as a critical target of caspase-11 in mice and a key mediator of the host response against Gram-negative bacteria.59
Although currently there is no caspase-11 used for early diagnosis, prevention, or targeted interventions in peri-implantitis, it has been used as an intervention in other human diseases. The significant association between caspase-11 and Ulcerative Colitis (UC) has been proven by some large cohort clinical trials. The activation of C3a/C3aR axis promoted the expression of caspase-11. In early stage of Ulcerative Colitis (UC), application of C3aR inhibitor induced the poor prognosis of UC by upregulating the activation of caspase-11, and treatment of C3aR inhibitor in later stage relieved the symptoms of UC and lead to the favorable prognosis of UC by inhibiting the pathway of caspase-11.60 Whether C3a/C3aR axis could affect the development of peri-implantitis by modulating the expression of caspase-11 is unclear. It has been proved that the function of caspase-11 in rat periodontitis models is similar to that of caspase-4 in clinical periodontitis, IL-1β and TNF-α release in periodontitis depends on the recognition of P. gingivalis LPS by caspase-11/4.61 It has been found that bacterial lipopolysaccharide (LPS), during the active stages of periodontitis, can cause overexpression of serum and salivary NT-proBNP levels through a mechanism involving proteolysis product formation. Reduction of NT-proBNP was influenced by the periodontal treatment and positively influenced the efficacy of periodontal treatment.62
Since higher levels of caspase-11 could induce the pyroptosis and inflammation of cells, we suggested that caspase-11 could promote and suppress the inflammation during the development of peri-implantitis. But the fact is the role of Caspase-11 in oral peri-implantitis, the role of LPS in the activation of Caspase-11 in P. gingivalis, the regulation of the subsequent pyroptosis, early diagnostic markers and development of peri-implant inflammation have not been clearly revealed. The therapeutic drugs targeting Caspase-11 for peri-implantitis also need to be further researched and developed.
Conclusions
The discovery of Caspase-11 as a highly sensitive immune recognition receptor can lead us to solve more mysteries about peri-implantitis, and provide ideas for the development of new drugs and therapies in the future as an important potential therapeutic target.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Song X, Li L, Gou H, Xu Y. Impact of implant location on the prevalence of peri-implantitis: a systematic review and meta-analysis. J Dent. 2020;103:103490. doi: 10.1016/j.jdent.2020.103490 [DOI] [PubMed] [Google Scholar]
- 2.Jamalpoor Z, Asgari A, Lashkari MH, et al. Modulation of macrophage polarization for bone tissue engineering applications. Iran J Allergy Asthma Immunol. 2018;17(5):398–408. doi: 10.18502/ijaai.v17i5.298 [DOI] [PubMed] [Google Scholar]
- 3.Lafaurie GI, Sabogal MA, Castillo DM, et al. Microbiome and microbial biofilm profiles of peri-implantitis: a systematic review. J Periodontol. 2017;88(10):1066–1089. doi: 10.1902/jop.2017.170123 [DOI] [PubMed] [Google Scholar]
- 4.Liu LR, Liu JC, Bao JS, et al. Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. 2020;11:1024. doi: 10.3389/fimmu.2020.01024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu L, Liu X, Nemeth DP, et al. Interleukin-1 causes CNS inflammatory cytokine expression via endothelia-microglia bi-cellular signaling. Brain Behav Immun. 2019;81:292–304. doi: 10.1016/j.bbi.2019.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–1364. doi: 10.1016/j.immuni.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi: 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Zhang C, Yang P, et al. Eldecalcitol Inhibits LPS-Induced NLRP3 inflammasome-dependent pyroptosis in human gingival fibroblasts by activating the Nrf2/HO-1 signaling pathway. Drug Des Devel Ther. 2020;14:4901–4913. doi: 10.2147/DDDT.S269223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anthoney N, Foldi I, Hidalgo A. Toll and Toll-like receptor signalling in development. Development. 2018;145(9):dev156018. doi: 10.1242/dev.156018 [DOI] [PubMed] [Google Scholar]
- 10.Deng S, Hu Y, Zhou J, et al. TLR4 mediates alveolar bone resorption in experimental peri-implantitis through regulation of CD45(+) cell infiltration, RANKL/OPG ratio, and inflammatory cytokine production. J Periodontol. 2020;91(5):671–682. doi: 10.1002/JPER.18-0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AlQranei MS, Senbanjo LT, Aljohani H, et al. Lipopolysaccharide- TLR-4 Axis regulates Osteoclastogenesis independent of RANKL/RANK signaling. BMC Immunol. 2021;22(1):23. doi: 10.1186/s12865-021-00409-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Dai Y, Li Q, et al. Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci Lett. 2020;736:135279. doi: 10.1016/j.neulet.2020.135279 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Tang L, Ye Myat T, Chen D. Titanium ions play a synergistic role in the activation of NLRP3 inflammasome in Jurkat T cells. Inflammation. 2020;43(4):1269–1278. doi: 10.1007/s10753-020-01206-z [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Wu X, Liu Q, et al. Memory B Cell as an indicator of peri-implantitis status: a pilot study. Int J Oral Maxillofac Implants. 2021;36(1):86–93. doi: 10.11607/jomi.8641 [DOI] [PubMed] [Google Scholar]
- 15.Kayagaki N, Wong MT, Stowe IB, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi: 10.1126/science.1240248 [DOI] [PubMed] [Google Scholar]
- 16.Ye J, Zeng B, Zhong M, et al. Scutellarin inhibits Caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling. Acta Pharm Sin B. 2021;11(1):112–126. doi: 10.1016/j.apsb.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagar JA, Powell DA, Aachoui Y, et al. Cytoplasmic LPS activates Caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rathinam VAK, Zhao Y, Shao F. Innate immunity to intracellular LPS. Nat Immunol. 2019;20(5):527–533. doi: 10.1038/s41590-019-0368-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Deng M, Loughran PA, et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and Caspase-11/GSDMD Signaling. Front Immunol. 2020;11:229. doi: 10.3389/fimmu.2020.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Z, Ao X, Xie P, et al. The biological width around implant. J Prosthodont Res. 2021;65(1):11–18. doi: 10.2186/jpr.JPOR_2019_356 [DOI] [PubMed] [Google Scholar]
- 21.Salvi GE, Cosgarea R, Sculean A. Prevalence and Mechanisms of Peri-implant Diseases. J Dent Res. 2017;96(1):31–37. doi: 10.1177/0022034516667484 [DOI] [PubMed] [Google Scholar]
- 22.Zhao P, Yue Z, Nie L, et al. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J Clin Periodontol. 2021;48(10):1379–1392. doi: 10.1111/jcpe.13517 [DOI] [PubMed] [Google Scholar]
- 23.Yi YS. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology. 2017;152(2):207–217. doi: 10.1111/imm.12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Q, Pan J, Zhou ZL, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin. 2021;42(6):954–963. doi: 10.1038/s41401-020-00525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero-Castro NS, Vazquez-Villamar M, Munoz-Valle JF, et al. Relationship between TNF-alpha, MMP-8, and MMP-9 levels in gingival crevicular fluid and the subgingival microbiota in periodontal disease. Odontology. 2020;108(1):25–33. doi: 10.1007/s10266-019-00435-5 [DOI] [PubMed] [Google Scholar]
- 26.Zu Y, Mu Y, Li Q, et al. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J Orthop Surg Res. 2019;14(1):307. doi. doi: 10.1186/s13018-019-1307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Li B, Liu Y, et al. Porphyromonas gingivalis lipopolysaccharide affects oral epithelial connections via pyroptosis. J Dent Sci. 2021;16(4):1255–1263. doi: 10.1016/j.jds.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marquez-Flores YK, Villegas I, Cardeno A, et al. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J Nutr Biochem. 2016;30:143–152. doi: 10.1016/j.jnutbio.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 29.Oka S, Li X, Sato F, et al. A deficiency of Dec2 triggers periodontal inflammation and pyroptosis. J Periodontal Res. 2021;56(3):492–500. doi: 10.1111/jre.12849 [DOI] [PubMed] [Google Scholar]
- 30.He D, Li X, Zhang F, et al. Dec2 inhibits macrophage pyroptosis to promote periodontal homeostasis. J Periodontal Implant Sci. 2022;52(1):28–38. doi: 10.5051/jpis.2101380069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Insua A, Monje A, Wang HL, et al. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J Biomed Mater Res A. 2017;105(7):2075–2089. doi: 10.1002/jbm.a.36060 [DOI] [PubMed] [Google Scholar]
- 32.Gruber R. Osteoimmunology: inflammatory osteolysis and regeneration of the alveolar bone. J Clin Periodontol. 2019;46(21):52–69. doi: 10.1111/jcpe.13056 [DOI] [PubMed] [Google Scholar]
- 33.Huja SS, Fernandez SA, Hill KJ, Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec a Discov Mol Cell Evol Biol. 2006;288(12):1243–1249. doi: 10.1002/ar.a.20396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallman DA, List A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood. 2019;133(10):1039–1048. doi: 10.1182/blood-2018-10-844654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu X, Zhang K, Lu K, et al. Inhibition of pyroptosis attenuates Staphylococcus aureus-induced bone injury in traumatic osteomyelitis. Ann Transl Med. 2019;7(8):170. doi: 10.21037/atm.2019.03.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocha FRG, Delitto AE, de Souza JAC, et al. Relevance of caspase-1 and Nlrp3 inflammasome on inflammatory bone resorption in A murine model of periodontitis. Sci Rep. 2020;10(1):7823. doi: 10.1038/s41598-020-64685-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chadha S, Behl T, Bungau S, et al. Mechanistic insights into the role of pyroptosis in rheumatoid arthritis. Curr Res Transl Med. 2020;68(4):151–158. doi: 10.1016/j.retram.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 38.Zang Y, Song JH, Oh SH, et al. Targeting NLRP3 inflammasome reduces age-related experimental alveolar bone loss. J Dent Res. 2020;99(11):1287–1295. doi: 10.1177/0022034520933533 [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi Y, Kurita-Ochiai T, Kobayashi R, et al. Regulation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated periodontal disease. Inflamm Res. 2017;66(1):59–65. doi: 10.1007/s00011-016-0992-4 [DOI] [PubMed] [Google Scholar]
- 40.Skirecki T, Cavaillon JM. Inner sensors of endotoxin - implications for sepsis research and therapy. FEMS Microbiol Rev. 2019;43(3):239–256. doi: 10.1093/femsre/fuz004 [DOI] [PubMed] [Google Scholar]
- 41.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15(6):375–387. doi: 10.1038/nri3837 [DOI] [PubMed] [Google Scholar]
- 42.Santos JC, Dick MS, Lagrange B, et al. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 2018;37(6):e98089. doi: 10.15252/embj.201798089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giordano NP, Cian MB, Dalebroux ZD. Outer membrane lipid secretion and the innate immune response to gram-negative bacteria. Infect Immun. 2020;88(7):e00920–19. doi: 10.1128/IAI.00920-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanaja SK, Russo AJ, Behl B, et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell. 2016;165(5):1106–1119. doi: 10.1016/j.cell.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Li R, Peng Z, et al. HMGB1 participates in LPS‑induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF‑kappaB signaling pathways. Int J Mol Med. 2020;45(1):61–80. doi: 10.3892/ijmm.2019.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng M, Tang Y, Li W, et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. 2018;49(4):740–753.e7. doi: 10.1016/j.immuni.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi H, Amano A. Invasion of gingival epithelial cells by porphyromonas gingivalis. Methods Mol Biol. 2021;2210:215–224. doi: 10.1007/978-1-0716-0939-2_21 [DOI] [PubMed] [Google Scholar]
- 48.Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- 49.Ross C, Chan AH, Von Pein J, et al. Dimerization and auto-processing induce Caspase-11 protease activation within the non-canonical inflammasome. Life Sci Alliance. 2018;1(6):e201800237. doi: 10.26508/lsa.201800237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wacker MA, Teghanemt A, Weiss JP, et al. High-affinity caspase-4 binding to LPS presented as high molecular mass aggregates or in outer membrane vesicles. Innate Immun. 2017;23(4):336–344. doi: 10.1177/1753425917695446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 52.Downs KP, Nguyen H, Dorfleutner A, Stehlik C. An overview of the non-canonical inflammasome. Mol Aspects Med. 2020;76:100924. doi: 10.1016/j.mam.2020.100924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell L, Raheem I, Malemud CJ, et al. The relationship between NALP3 and autoinflammatory syndromes. Int J Mol Sci. 2016;17(5):725. doi: 10.3390/ijms17050725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magupalli VG, Negro R, Tian Y, et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science. 2020;369(6510):eaas8995. doi: 10.1126/science.aas8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isola G, Polizzi A, Santonocito S, et al. Periodontitis activates the NLRP3 inflammasome in serum and saliva. J Periodontol. 2022;93(1):135–145. doi: 10.1002/JPER.21-0049 [DOI] [PubMed] [Google Scholar]
- 56.Sakamaki K, Satou Y. Caspases: evolutionary aspects of their functions in vertebrates. J Fish Biol. 2009;74(4):727–753. doi: 10.1111/j.1095-8649.2009.02184.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vigano E, Diamond CE, Spreafico R, et al. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun. 2015;6:8761. doi: 10.1038/ncomms9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagrange B, Benaoudia S, Wallet P, et al. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine Caspase-11. Nat Commun. 2018;9(1):242. doi: 10.1038/s41467-017-02682-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang XH, Chen Y, Yu SX, et al. Inhibition of C3a/C3aR axis in diverse stages of ulcerative colitis affected the prognosis of UC by modulating the pyroptosis and expression of caspase-11. Inflammation. 2020;43(6):2128–2136. doi: 10.1007/s10753-020-01280-3 [DOI] [PubMed] [Google Scholar]
- 60.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasomesignalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 61.Li Z, Cai Q, Li B, et al. Caspase-11/4 is involved in bacteria-mediated periodontitis by promoting the release of interleukin-1 β and tumor necrosis factor-α. Arch Oral Biol. 2022;142:105517. doi: 10.1016/j.archoralbio.2022.105517 [DOI] [PubMed] [Google Scholar]
- 62.Isola G, Tartaglia GM, Santonocito S, Polizzi A, Williams RC, Iorio-Siciliano V. Impact of N-terminal pro-B-type natriuretic peptide and related inflammatory biomarkers on periodontal treatment outcomes in patients with periodontitis: an explorative human randomized-controlled clinical trial. J Periodontol. 2023. doi: 10.1002/JPER.23-0063 [DOI] [PubMed] [Google Scholar]