Abstract
Objective:
To characterize patterns of failure using prostate-specific membrane antigen positron emission tomography (PSMA PET) after radical prostatectomy (RP) and salvage radiotherapy (SRT).
Methods:
Patients with rising PSA post-RP+SRT underwent 68Ga-HBED-iPSMA PET/CT on a single-arm, prospective imaging trial (NCT03204123). Scans were centrally reviewed with pattern-of-failure analysis by involved site. Positive scans were classified using three failure categories: pelvic nodal, extra-pelvic nodal or distant non-nodal. Associations with failure categories were analyzed using cumulative incidence and generalized logits regression.
Results:
We included 133 men who received SRT a median of 20 months post-RP; 56% received SRT to the prostatic fossa alone, while 44% received pelvic SRT. PSMA PET/CT was performed a median of 48 months post-SRT. Overall, 31% of PSMA PET/CT scans were negative, 2% equivocal and 67% had at least one positive site. Scan detection was significantly associated with PSA level prior to PSMA PET/CT. Analysis of 89 positive scans demonstrated pelvic nodal (53%) was the most common relapse and fossa relapse was low (9%). Overall, positive scans were pelvic (n=35, 26%), extra-pelvic nodal (n=26, 20%) or distant non-nodal failure (n=28, 21%), and 70% of positive scans were oligorecurrent. We observed similar cumulative incidence for all failure categories and relatively few clinicodemographic associations. Men treated with pelvic SRT had reduced odds of pelvic failure versus exclusive fossa treatment.
Conclusion:
Pelvic, extra-pelvic nodal and distant non-nodal failures occur with similar incidence post-SRT. Regional nodal relapse is relatively common, especially with fossa-only SRT. A high oligorecurrence rate suggests a potentially important role for PSMA-guided focal therapies.
Keywords: PSMA, Prostate Cancer, Biochemical recurrence, Oligometastatic, PSMA PET, Radiotherapy
Introduction
Up to one third with localized prostate cancer (PC) will develop detectable prostate specific antigen (PSA) levels after a radical prostatectomy (RP) [1]. Adjuvant radiation improves biochemical relapse-free survival by 20–25% [2–4] and early salvage radiotherapy (SRT) is a suitable alterative with comparable outcomes and improved toxicity [5,6]. However, 10-year incidence of further biochemical recurrence (BCR) post-SRT remains as high as 60% [7]. Understanding patterns of failure post-SRT is critical to optimizing treatment options. Given the significant heterogeneity in the SRT patient population and their treatment characteristics, patterns of failure remain poorly defined.
Advanced imaging, notably positron emission tomography (PET) utilizing tracers targeting prostate-specific membrane antigen (PSMA), are revolutionizing the diagnostic capabilities for biochemically-recurrent prostate cancer [8,9]. With superior specificity and sensitivity to other available imaging modalities, PSMA PET is now recommended to evaluate BCR by several guidelines, including the National Comprehensive Cancer Network [10–12]. Importantly, PSMA PET can meaningfully change management in over half of patients with BCR [13]. While the diagnostic performance of PSMA PET has been prospectively confirmed for BCR post-SRT, the reported patterns of detection were blended across populations including BCR post-RP or definitive RT alone [14]. There has been limited characterization of patterns of failure solely for BCR post-RP+SRT using modern imaging.
Patients and Methods
Patient Population
Patients were participants of an IRB-approved, single-arm, single-institution, prospective imaging trial. Per protocol, patients with PSA >0.2 ng/mL on at least two consecutive tests were eligible to undergo 68Ga-HBED-iPSMA PET/CT imaging. We focused on patients scanned post-RP+SRT to the prostate fossa and/or pelvis. Additional androgen deprivation therapy (ADT) for subsequent BCR post-SRT was permissible; however, none had documented evidence of any prior metastatic disease. Patients receiving ADT were eligible if they met PSA criteria.
Scan parameters
Participants were injected with 100–300 Mbq of 68Ga-HBED-iPSMA tracer, followed by PET/CT imaging of the skull vertex to mid-thigh for a median of 68 minutes (range: 55–130 min) post injection per standard institutional practice (see Supplemental Materials for detailed scan parameters). Patients also underwent an additional spot view of the pelvis after the torso scan and bladder emptying.
Scan Interpretation
Each PSMA PET/CT underwent a centralized review by a single dual-trained body and nuclear medicine radiologist. Areas with increased radiotracer uptake were classified by site, which included prostate bed, nodal and osseous/other. Nodal sites were further classified by laterality, station (e.g., external iliac) and number of avid nodes within the involved station. If a patient had several areas of increased nodal uptake, each station was classified as a separate site. Within each involved nodal station, the largest unidirectional short axis of an affected node (measured on CT) was recorded. Involved nodal stations were classified as “pelvic” or “non-pelvic” where pelvic was defined as obturator, internal/external iliac, common iliac or mesorectal. Osseous and other sites were classified by location and number of lesions with increased uptake.
For sites with increased radiotracer uptake, the radiologist assigned a level of certainty that the avidity was attributable to recurrent/metastatic prostate cancer using a 5-point Likert scale: 5 (“consistent with”) was >90% confidence, 4 (“suspicious for”) was 75% confidence, 3 (“possibly”) was 50% confidence, 2 (“less likely”) was 25% confidence and 1 (“unlikely”) was <10% confidence.
We then performed a detailed pattern-of-failure analysis by patient and by involved site. At the patient level, scans were “positive” if at least one site scored 4 or greater. To be conservative, any lesions with <75% certainty were considered negative. Positive scans were also classified as one of three broad categories: pelvic, extra-pelvic nodal or distant non-nodal. The PSMA PET/CT scans were defined as “pelvic” if positive findings were limited only to the prostate bed or pelvic nodes. They were defined as “extra-pelvic nodal” if positive findings were limited to non-pelvic adenopathy alone or pelvic and non-pelvic adenopathy. Scans were classified as “distant non-nodal” if they included at least one osseous or visceral metastatic site, with or without extra-pelvic adenopathy or pelvic disease. Scans were considered oligorecurrent if they had five or fewer discrete lesions.
Statistical Analysis
Descriptive statistics were utilized to characterize the cohort and scan positivity rates. Associations between PSMA PET/CT failure patterns (“pelvic” versus “extra-pelvic” versus “distant non-nodal” failure) and baseline clinicodemographic variables of interest were analyzed using two approaches: treating failure as a time-to-event outcome, and as a multinomial outcome.
We first examined cumulative incidence of each failure pattern considering the other patterns as competing events. There were no deaths without failure. Patients with “equivocal” or “negative” results were censored. This approach used univariable (UVA) Fine-Gray competing risk regression and baseline time was defined as the end of SRT. The second approach used UVA generalized logits regression to examine associations with failure type as a multinomial outcome (“pelvic” versus “extra-pelvic” versus “distant non-nodal” versus “no failure”). The “no failure” group included patients with “equivocal” or “negative” PET/CT results. The incremental utility of logits regression over cumulative incidence enabled analysis of time-dependent, non-baseline factors (i.e., time from SRT to PSMA) and quantification of differences between the four patterns using odds ratios. Statistical computations were performed using SAS V.9.4 (The SAS Institute, Cary, NC) and R V.4.1.2.
Results
One hundred and thirty-three men were included with the initial clinicodemographic characteristics shown in Table 1. The cohort was distributed among risk groups with 42% having high-risk or very high-risk disease at diagnosis. At RP, a median of 8 nodes were sampled and 40% of the men had >10 removed. Overall, 20% of the men had pathologically confirmed pelvic nodal disease. These patients received SRT a median of 20 months post-RP to a median dose of 72Gy to the prostate fossa (range 58–81Gy) and 45Gy to the lymph nodes when pelvic treatment was delivered.
Table 1:
Patient clinicodemographic characteristics (n=133).
| Characteristic | n* |
|---|---|
| Median age at PSMA scan (range) | 69 (49–91) |
| Initial Gleason Grade Group | |
| Group 1 (3+3=6) | 4 (3%) |
| Group 2 (3+4=7) | 36 (27%) |
| Group 3 (4+3=7) | 47 (35%) |
| Group 4 (Gleason 8) | 19 (14%) |
| Group 5 (Gleason 9–10) | 27 (20%) |
| Pathologic T stage | |
| T2 | 33 (25%) |
| T3 | 95 (71%) |
| T4 | 5 (4%) |
| Lymph nodal sampling at time of prostatectomy | |
| Median nodes sampled (range) | 8 (0–128) |
| No sampled nodes | 12 (9%) |
| 1–5 nodes | 36 (28%) |
| 6–10 nodes | 31 (24%) |
| 11–20 nodes | 31 (24%) |
| >20 nodes | 21 (16%) |
| Unknown number | 2 |
| Pathologic N stage | |
| N0 | 97 (78%) |
| N1 | 27 (22%) |
| Unknown | 9 |
| Presence of ECE | 99 (74%) |
| Presence of seminal vesicle invasion | 46 (35%) |
| Presence of PNI | 115 (87%) |
| Presence of LVSI | 52 (39%) |
| Positive surgical margin at prostatectomy | 44 (33%) |
| Initial risk level | |
| Low | 9 (7%) |
| Favorable intermediate | 31 (24%) |
| Unfavorable intermediate | 34 (27%) |
| High or very high | 54 (42%) |
| Unknown | 5 |
| Median months from prostatectomy to SRT (range) | 20.3 (3.7–235.2) |
| Median SRT dose in Gy (range) | 72 (57.6–81) |
| Patient received ADT+SRT | 76 (57%) |
| SRT treatment fields | |
| Prostate bed alone | 74 (56%) |
| Prostate bed plus pelvic nodes | 58 (44%) |
| Unknown field | 1 |
| Median months from SRT to PSMA scan (range) | 49.0 (7.2–264) |
Note: Data are presented as number (%) unless indicated otherwise.
Abbreviations: PSMA = prostate specific membrane antigen; ECE = extracapsular extension; PNI = perineural invasion; LVI = lymphovascular invasion; SRT = salvage radiotherapy; ADT = androgen deprivation therapy
Just over half (56%) of the men received SRT to the fossa alone, while the remainder were treated to the fossa and pelvis. Concurrent ADT was given with the SRT in 57%. PSMA PET/CT was performed a median of 48 months (range 7–264 months) post-SRT.
PSMA PET/CT detection rate
Of the 133 PSMA PET/CT scans, 41 (31%) were negative, revealing no sites of recurrence. An additional 3 scans (2%) revealed only sites deemed to be equivocal for prostate cancer. Thus, 89 scans (67%) were positive for at least one site with increased PSMA radiotracer uptake, as shown in Supplemental Table 1. In total, we identified 151 sites of recurrence and the median number of positive sites per positive scan was 1 (range: 1–5).
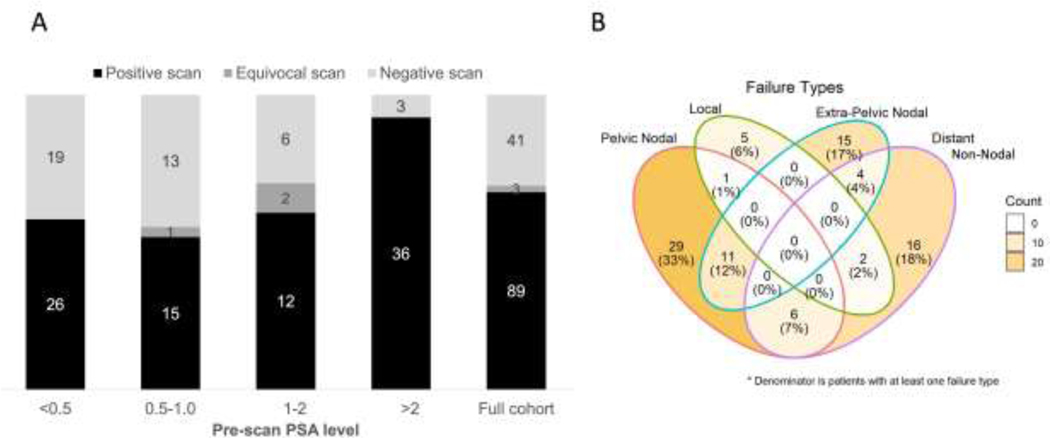
Across the cohort, the median pre-PET PSA level was 0.75 ng/mL (interquartile range: 0.40–2.7). There was a statistically significant association between scan detection (positive versus negative or equivocal) and pre-PET PSA strata (Figure 1a and Supplemental Table 1, Fisher’s exact test p=0.0003). A modest proportion with negative scans had PSA levels of <0.5 (n=45, 42% negative) or 0.5–1 (n=29, 45% negative), which declined to 8% for PSA level >2 (n=39). While the proportion of scan positivity was similar across PSA subgroups <2, the detection rate increased to 92% above that threshold.
Figure 1:
A) Stacked bar chart showing the detection rates across different pre-scan PSA strata. The numbers in the bars reflect the total number of scans which were classified as positive, equivocal or negative. The distributions were significantly different by Fisher’s exact test, p=0.0003 B) Venn diagram showing granular anatomic breakdown of the PSMA PET pattern of failure at the individual patient level for scans that contain at least one positive scan finding (n=89)
Granular patterns of failure
Figure 1b shows a granular pattern-of-failure breakdown. From the 89 positive scans, only 8 (9%) had evidence of prostate bed recurrence. These fossa recurrences were detected long after SRT; PET/CT occurred a median of 98 months post-SRT (range 25–128 months). Of these 8 suspected local recurrences, 4 had undergone a recent prostate MRI and all were concordant. Six of 8 were biopsied, of which two were definitively positive, three were benign and one was non-diagnostic.
Nodal recurrence was more common as 66/89 total scans (74%) had at least one nodal region with increased tracer uptake. Of these, 36/66 scans (55%) had only pelvic nodal uptake, 19/66 (29%) only had non-pelvic nodes and the remaining 11/66 (17%) had at least one involved pelvic and non-pelvic nodal site. In total, we identified 112 positive nodal sites, with a median unidimensional diameter of a representative node of 6 mm.
In total, 28/89 scans (31%) had at least one osseous or non-nodal involved site with increased radiotracer uptake. Fifteen scans (17%) had at least one osseous lesion. Thirteen scans (15%) had a non-osseous, distant non-nodal site of disease, which included pulmonary nodules (n=6), pelvis/peritoneum (n=3), anal canal (n=2) and rectal wall, penile urethra, spermatic cord, liver nodules and urachal remnant (each n=1). Overall, 62/89 scans (70%) were classified as oligorecurrent with <6 suspected discrete lesions.
Cumulative incidence of specific failure patterns
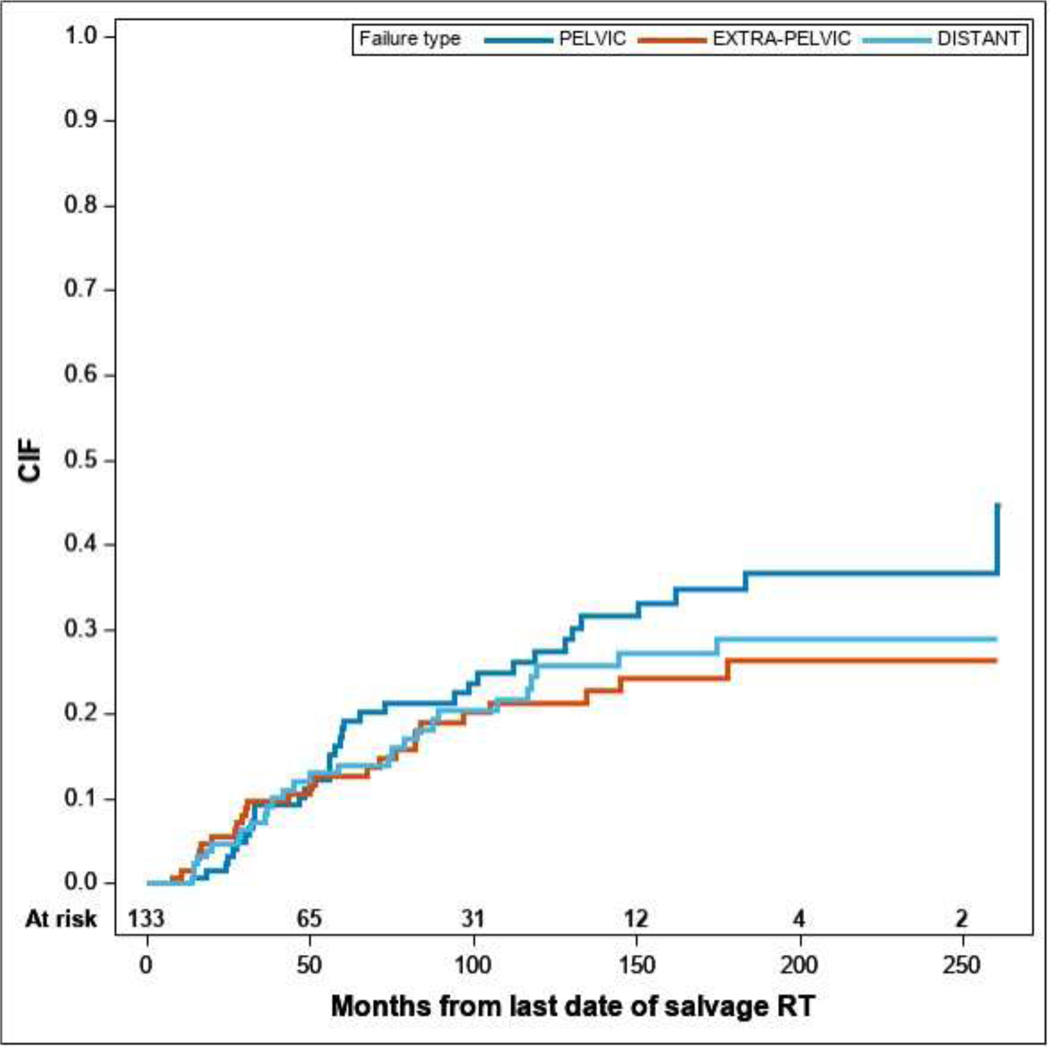
Patterns of failure were then classified as pelvic (n=35, 26%), extra-pelvic (n=26, 20%), distant non-nodal (n=28, 21%) or no failure (n=44, 33%). For positive scans, we observed similar cumulative incidence for the three patterns of failure, as shown in Figure 2. Four-year cumulative incidence of pelvic, extra-pelvic and distant non-nodal patterns were 10.3% (95% CI: 4.7–15.8), 10.7% (5.1–16.2) and 12.0% (6.0–18.0), respectively.
Figure 2.
Cumulative incidence function of different PSMA PET patterns of failure from date of completion of SRT considering the other patterns as competing events.
UVA competing risk regression (Table 2) identified relatively few clinical-demographic factors associated with patterns of failure. Higher Gleason score was associated with greater cumulative incidence of distant non-nodal failure (HR 1.6, 95% CI: 1.1–2.3, p=0.008) as was T4 surgical stage (HR 4.2, 95% CI: 1.3–14.3, p=0.004). Extracapsular extension (ECE) had lower hazard of pelvic failure (HR 0.5, 95% CI: 0.3–0.99, p=0.045). Pathologic nodal positivity and the percentage of positive nodes were associated with increased risk of extra-pelvic nodal failure (HR 2.6, 95% CI: 1.2–5.8, p=0.02). Greater duration of time from RP to SRT was non-significantly protective against distant non-nodal failure (HR 0.98, 95% CI: 0.96–1.0, p=0.06). Patients whose SRT included the pelvis had borderline significantly reduced risk of pelvic failure (HR 0.5, 95% CI: 0.24–1.00, p=0.05). Other factors including initial risk group, SRT dose and usage of concurrent ADT were not associated with a particular failure pattern. Multivariable risk regression was not performed given limited events.
Table 2:
Clinicodemographic associations with each PSMA failure pattern using univariable Fine-Gray competing risks regression.
| PELVIC failure | EXTRA-PELVIC failure | DISTANT NON-NODAL failure | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | N (#Events) | HR (95% CI) | p-value1 | N (#Events) | HR (95% CI) | p-value1 | N (#Events) | HR (95% CI) | p-value1 |
| Age at surgery | 133 (35) | 1.01 (0.98, 1.05) | 0.44 | 133 (26) | 1.01 (0.96, 1.06) | 0.77 | 133 (28) | 1.04 (0.99, 1.09) | 0.14 |
| Pre-op PSA | 129 (34) | 0.98 (0.94, 1.01) | 0.17 | 129 (26) | 1.01 (0.99, 1.04) | 0.28 | 129 (28) | 0.99 (0.94, 1.04) | 0.60 |
| Initial risk level | 128 (33) | 0.65 | 128 (26) | 0.51 | 128 (27) | ||||
| High + | 54 (14) | Ref. | 54 (12) | Ref. | 54 (13) | Ref. | |||
| Fav Int | 31 (10) | 1.16 (0.52, 2.58) | 31 (4) | 0.53 (0.17, 1.66) | 31 (5) | Not reported | |||
| Low risk | 9 (3) | 1.09 (0.39, 3.03) | 9 (3) | 1.63 (0.48, 5.51) | 9 (0) | Not reported | |||
| Unf Int | 34 (6) | 0.62 (0.24, 1.60) | 34 (7) | 0.92 (0.37, 2.30) | 34 (9) | Not reported | |||
| Gleason Score | 129 (34) | 0.99 (0.74, 1.32) | 0.95 | 129 (26) | 1.18 (0.84, 1.65) | 0.34 | 129 (27) | 1.61 (1.13, 2.28) | 0.008 |
| Surgical T Stage | 133 (35) | 133 (26) | 133 (28) | 0.004 | |||||
| T3 | 95 (23) | Ref. | 95 (19) | Ref. | 95 (23) | Ref. | |||
| T2 | 33 (12) | Not reported | 33 (6) | Not reported | 33 (2) | 0.21 (0.05, 0.87) | |||
| T4 | 5 (0) | Not reported | 5 (1) | Not reported | 5 (3) | 4.24 (1.25, 14.32) | |||
| Presence of ECE | 133 (35) | 0.045 | 133 (26) | 0.73 | 133 (28) | ||||
| No | 34 (13) | Ref. | 34 (7) | Ref. | 34 (1) | Ref. | |||
| Yes | 99 (22) | 0.51 (0.27, 0.98) | 99 (19) | 0.86 (0.35, 2.07) | 99 (27) | Not reported | |||
| Presence of SVI | 133 (35) | 0.49 | 133 (26) | 0.58 | 133 (28) | 0.47 | |||
| No | 87 (25) | Ref. | 87 (16) | Ref. | 87 (17) | Ref. | |||
| Yes | 46 (10) | 0.77 (0.37, 1.62) | 46 (10) | 1.24 (0.57, 2.73) | 46 (11) | 1.32 (0.62, 2.80) | |||
| Presence of PNI | 133 (35) | 0.78 | 133 (26) | 0.38 | 133 (28) | 0.93 | |||
| No | 18 (5) | Ref. | 18 (2) | Ref. | 18 (4) | Ref. | |||
| Yes | 115 (30) | 0.87 (0.32, 2.34) | 115 (24) | 1.94 (0.45, 8.49) | 115 (24) | 0.96 (0.35, 2.64) | |||
| Presence of LVSI | 133 (35) | 0.37 | 133 (26) | 0.95 | 133 (28) | 0.79 | |||
| No | 81 (20) | Ref. | 81 (16) | Ref. | 81 (17) | Ref. | |||
| Yes | 52 (15) | 1.35 (0.70, 2.61) | 52 (10) | 1.03 (0.47, 2.24) | 52 (11) | 1.11 (0.52, 2.36) | |||
| Surgical Margin Status | 133 (35) | 0.13 | 133 (26) | 0.72 | 133 (28) | 0.09 | |||
| Negative | 89 (26) | Ref. | 89 (16) | Ref. | 89 (14) | Ref. | |||
| Positive | 44 (9) | 0.56 (0.26, 1.19) | 44 (10) | 1.16 (0.52, 2.56) | 44 (14) | 1.88 (0.91, 3.88) | |||
| Surgical N Stage | 124 (31) | 0.10 | 124 (25) | 0.03 | 124 (26) | 0.87 | |||
| 0 | 97 (28) | Ref. | 97 (16) | Ref. | 97 (21) | Ref. | |||
| 1 | 27 (3) | 0.37 (0.11, 1.22) | 27 (9) | 2.51 (1.12, 5.62) | 27 (5) | 0.92 (0.34, 2.51) | |||
| Total Number of Nodes Removed | 131 (35) | 0.45 | 131 (24) | 0.07 | 131(28) | 0.40 | |||
| 0 | 12 (4) | Ref. | 12 (2) | Ref. | 12 (2) | Ref. | |||
| 1-5 | 36 (11) | 0.94 (0.31, 2.81) | 36 (7) | 1.22 (0.24, 6.20) | 36 (9) | 1.69 (0.39, 7.29) | |||
| 11-20 | 31 (4) | 0.41 (0.11, 1.52) | 31 (4) | 0.90 (0.16, 5.17) | 31 (9) | 2.55 (0.59, 11.07) | |||
| 6-10 | 31 (11) | 1.17 (0.39, 3.48) | 31 (3) | 0.58 (0.09, 3.61) | 31 (5) | 1.03 (0.21, 4.95) | |||
| >20 | 21 (5) | 0.75 (0.22, 2.61) | 21 (8) | 2.98 (0.59, 14.96) | 21 (3) | 1.02 (0.17, 6.05) | |||
| Positive Nodes | 133 (35) | 0.09 | 133 (26) | 0.02 | 133 (28) | 0.91 | |||
| No | 106 (32) | Ref. | 106 (17) | Ref. | 106 (23) | Ref. | |||
| Yes | 27 (3) | 0.36 (0.11, 1.17) | 27 (9) | 2.60 (1.17, 5.79) | 27 (5) | 0.94 (0.35, 2.56) | |||
| Percent Positive Nodes | 119 (31) | 0.98 (0.93, 1.03) | 0.41 | 119 (22) | 1.03 (1.01, 1.06) | 0.01 | 119 (26) | 0.98 (0.94, 1.02) | 0.25 |
| Time from prostatect omy to SRT (months) | 133 (35) | 1.01 (1.00, 1.01) | 0.15 | 133 (26) | 1.00 (0.99, 1.01) | 0.79 | 133 (28) | 0.98 (0.96, 1.00) | 0.06 |
| SRT Dose | 129 (35) | 1.00 (1.00, 1.00) | 0.61 | 129 (25) | 1.00 (1.00, 1.00) | 0.44 | 129 (28) | 1.00 (1.00, 1.00) | 0.75 |
| SRT Treatment Area | 132 (35) | 0.05 | 132 (25) | 0.12 | 132 (28) | 0.13 | |||
| Pros tate | 74 (25) | Ref. | 74 (11) | Ref. | 74 (13) | Ref. | |||
| Pros tate+ nodes | 58 (10) | 0.49 (0.24, 1.00) | 58 (14) | 1.86 (0.85, 4.07) | 58 (15) | 1.75 (0.84, 3.62) | |||
| ADT Given with SRT | 133 (35) | 0.85 | 133 (26) | 0.10 | 133 (28) | 0.93 | |||
| No | 57 (18) | Ref. | 57 (9) | Ref. | 57 (14) | Ref. | |||
| Yes | 76 (17) | 0.94 (0.50, 1.78) | 76 (17) | 1.92 (0.88, 4.20) | 76 (14) | 0.97 (0.47, 2.00) | |||
p-values are from UVA Fine-Gray competing risks regression
Abbreviations: PSA = prostate-specific antigen; ECE = extracapsular extension; SVI = seminal vesicle invasion; PNI = perineural invasion; LVSI = lymphovascular space invasion; SRT = salvage radiotherapy; ADT = androgen deprivation therapy.
We further explored potential associations using generalized logits regression modeling (Supplemental Table 2) using the pelvic failure pattern as the reference state given these men remained theoretically curable. When a distant failure was compared to a pelvic failure, patients with ECE had far higher odds of distant non-nodal failure than patients without ECE (odds ratio, OR: 16.0; 95% CI: 1.9–131.7, overall p=0.08). Again, patients who were pathologically node positive had greater odds of extra-pelvic nodal failure (OR: 5.7, 95% CI: 1.4–23.7, overall p=0.11) when an extra-pelvic failure was compared to a pelvic failure. Compared to pelvic patterns of failure, men whose SRT included the pelvic nodes had greater odds of both extra-pelvic (OR: 3.2, 95% CI: 1.1–9.4) and distant non-nodal failures (OR=2.9, 95% CI: 1.0–8.2, overall p=0.13) compared to men treated just to the prostate bed. In this analysis, the PSA level prior to the PSMA PET/CT and the duration from SRT to PSMA PET/CT were not clearly associated with any recurrence pattern.
Comments
PSMA PET/CT is rapidly emerging as the standard-of-care imaging modality for recurrent prostate cancer given excellent sensitivity at lower PSA levels [14]. It is increasingly vital to understand and anticipate likely patterns of failure appreciated with this modality after common treatments. Overall, 67% of scans in our study revealed recurrence; when stratified by pre-PET/CT PSA level, diagnostic performance was largely similar to other prospective reports [14,15]. Compared to these studies, we found a higher detection rate for the lowest PSA stratum (<0.5), which is likely due to a relatively small sample size. Likely reflective of cohort heterogeneity, we did not observe a dominant or temporally-associated pattern of failure with similar incidence of pelvic, extra-pelvic nodal and distant non-nodal relapses. Despite consideration of numerous clinicodemographic factors, relatively few associations surfaced including the pre-scan PSA level.
Nevertheless, two important observations emerged. First, these data generally support the early effectiveness of SRT given our observation of very few prostatic fossa recurrences. The relative infrequency of local failures was also reported in a large multicenter analysis of anatomic patterns of recurrence post-SRT utilizing conventional imaging [16]. It is important to contextualize that our population had median PSA level at PSMA PET of 0.75 ng/mL, which is relatively high and might be enriched for patients less likely to have local failure. However, for our population, fossa failure did not seem to be a significant pattern of relapse. Durable local control after SRT may be due to fact that the median SRT dose was a relatively “dose-intensified” 72Gy. In light of the SAKK 09/10 study demonstrating no clear control benefits with SRT dose intensification, we suspect our findings may also be relevant for lower SRT doses [17]. This study is not designed to identify men who may have been able to forego prostate bed SRT given propensity for different failure patterns since the full cohort received fossa radiation.
In our cohort, SRT field design was influential. Only 44% of patients’ SRT included the pelvis, and these patients had reduced risk of pelvic failure. This is further supported by the fact that N1 patients (all but three received pelvic SRT) had increased risk of extra-pelvic nodal failure, suggesting a propensity for further nodal disease with control of irradiated sites.
Second, regional nodal failure was the most common relapse site with one third of positive scans showing just pelvic disease. This contrasts with the aforementioned Jackson et al. multi-center analysis [16], which observed significantly higher rates of distant nodal and non-nodal failure compared to regional nodal disease and questioned the necessity of elective pelvic coverage. While our cohort is smaller, our findings suggest pelvic failure risk may have been underrecognized. The difference is likely reflective of PSMA sensitivity; the previous multi-center effort used predominantly CT and considered a node positive if >8 mm in the pelvis, >1 cm in the retroperitoneum or if atypical features. The median size of a positive node in our study was 6 mm, thus PSMA is likely detecting pelvic recurrences earlier. Overall, our findings offer support for pelvic inclusion when considering SRT, as suggested by RTOG 0534 [18]. Integration of PSMA PET to guide SRT fields is also sensible [19–21].
Prospective series have previously studied PSMA PET/CT for this population, but typically combined with other treatment histories including definitive RT or RP alone. A large single-arm diagnostic study found that pelvic nodal relapse was most common at the lowest PSA echelon, while positivity in multiple compartments increased with PSA above 1 ng/mL [14]. This analysis included 204 men with previous RP+SRT, which reflected about one third of their participants. Similarly, the CONDOR study using 18F-DCFPyL PET tracer included 74 (36%) men with prior RP+SRT but reported nonstratified patterns of anatomic localization [15].
Given that patterns of failure using conventional imaging suggest predominantly locoregional recurrence after RP or definitive RT, it is imperative to consider localization results specifically for the post-SRT population. To date, these studies are limited, with fewer patients than the current study. Byrne et al. reported 81 patients who received PSMA PET after RP+SRT with negative conventional imaging [22]. They likewise found very infrequent local failure (4–6%), a relatively high rate of pelvic nodal positivity in patients whose SRT included just the fossa, and enrichment of distant relapses when SRT included the pelvis. Similar to our findings, they also endorsed prophylactic pelvic coverage. A small study of 34 patients imaged using 18F-PSMA PET/CT (18F-DCFBC or 18F-DCFPyl PET tracers) with concurrently performed multiparametric MRI post-SRT found 17/32 (53%) participants had metastatic disease, 8/32 (25%) had locoregional recurrences and 7/32 (22%) had local prostate fossa failure. Nearly all local relapses fell within the prior SRT field; the unexpectedly high local failure rate was attributed to the advantages of MRI localization over CT and/or superior performance of fluorinated tracers [23]. Superior diagnostic performance of PSMA PET/MRI versus MRI alone has been demonstrated [24].
There is growing evidence that PSMA PET/CT can strongly influence and often modify treatment plans [15,25,26]. We found that oligorecurrence was relatively frequent, with over two thirds of positive scans showing <6 lesions. This finding suggests that PSMA-guided integration of stereotactic body radiotherapy (SBRT) may be an important treatment option [27,28]. SBRT has been associated with improved outcomes for oligometastatic prostate cancer [29,30] and potentially as a strategy to delay ADT [28,29]. Furthermore, comprehensive SBRT to PSMA-detected disease is superior to SBRT guided by conventional imaging [30]. Although PSMA PET might improve restaging, SBRT alone is unlikely to be curative given occult microscopic disease. To address this limitation, it may be sensible to combine SBRT with rationally-selected systemic therapies for oligorecurrence. One approach is integration of SBRT with targeted PSMA radioligands; for example, we are conducting a pilot study of a PSMA-based theranostic strategy, combining SBRT with two cycles of 177Lu-PSMA-617 (NCT05079698). We anticipate further PSMA-guided strategies to emerge.
We acknowledge several limitations including the inherent limitation to any pattern of failure study in that results are most applicable to the specific patient group analyzed. Our institutional prospective imaging trial only had PSA inclusion criteria, therefore it enrolled a heterogeneous population with post-SRT PSA recurrence. Thus, the cohort analyzed is heterogeneous with variability in the duration from the end of SRT to PSMA imaging and in the duration and types of ADT utilized, which could influence patterns of failure. Furthermore, some patients received additional ADT post-SRT (though none had documented metastatic disease). We suspect these factors likely affected our ability to detect anatomic associations with ADT usage during SRT which has been shown to influence post-SRT outcomes. We did not routinely assess the validity of positive findings given non-standardized availability of comparison to conventional imaging and/or biopsies. Thus, assessment of PSMA PET positivity was based principally on expert radiologist judgement with available clinical information, which is inherently subjective.
Conclusion
PSMA PET imaging is an important component of the management of patients after RP+SRT. Pelvic, extra-pelvic nodal and distant non-nodal patterns of failure occurred with similar incidence post-SRT. Regional nodal relapse was relatively common, especially for patients whose SRT did not include the pelvis. Many positive PSMA PET scans revealed relatively few lesions with increased radiotracer uptake, suggesting a potential important role for PSMA-guided focal therapies such as SBRT following SRT failure.
Supplementary Material
Acknowledgment
The authors thank Jennifer S Huber, PhD, for scientific editing of the article.
Funding:
This research was funded in part through the National Institute of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure/COI: BI has received an honorarium from GT Medical Technologies. EO, EH, SL, ZZ, RG, DG and MK report no commercial interests or potential conflicts of interest. LP reports consulting agreements with Blackstone Investments / Clarus Ventures, Third Rock Ventures, Galera Therapeutics, Dynamo Therapeutics, Myst Therapeutics, Monte Rosa Therapeutics, Best Doctors / Teladoc Inc and equity ownership in Schrödinger, Novavax and Clovis Oncology. BM reports that he is a Member of the Medical Advisory Board, Radiation Oncology for eviCore Healthcare. SM reports research funding from Janssen and Genentech and honoraria from Astra-Zeneca. MZ receives research funding from Ferring Pharmaceuticals, Novartis, and Bayer.
Data Sharing: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural History of Progression After PSA Elevation Following Radical Prostatectomy. JAMA 1999;281:1591–7. 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- [2].Swanson GP, Thompson IM, Tangen C, Miller G, Lucia MS, Troyer DA, et al. Phase III Randomized Study of Adjuvant Radiation Therapy versus Observation in Patients with Pathologic T3 Prostate Cancer (SWOG 8794). International Journal of Radiation Oncology*Biology*Physics 2005;63:S1. 10.1016/j.ijrobp.2005.07.007.16265758 [DOI] [Google Scholar]
- [3].Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). The Lancet 2012;380:2018–27. 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- [4].Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, et al. Adjuvant Radiotherapy Versus Wait-and-See After Radical Prostatectomy: 10-year Follow-up of the ARO 96–02/AUO AP 09/95 Trial. European Urology 2014;66:243–50. 10.1016/j.eururo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- [5].Kneebone A, Fraser-Browne C, Delprado W, Duchesne G, Fisher R, Frydenberg M, et al. A Phase III Multi-Centre Randomised Trial comparing adjuvant versus early salvage Radiotherapy following a Radical Prostatectomy: Results of the TROG 08.03 and ANZUP “RAVES” Trial. International Journal of Radiation Oncology*Biology*Physics 2019;105:S37–8. 10.1016/j.ijrobp.2019.06.456. [DOI] [Google Scholar]
- [6].Parker CC, Clarke NW, Cook AD, Kynaston HG, Petersen PM, Catton C, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. The Lancet 2020;396:1413–21. 10.1016/S0140-6736(20)31553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jackson WC, Suresh K, Tumati V, Dess RT, Soni PD, Zhao SG, et al. Impact of Biochemical Failure After Salvage Radiation Therapy on Prostate Cancer-specific Mortality: Competition Between Age and Time to Biochemical Failure. Eur Urol Oncol 2018;1:276–82. 10.1016/j.euo.2018.04.014. [DOI] [PubMed] [Google Scholar]
- [8].Lawhn-Heath C, Salavati A, Behr SC, Rowe SP, Calais J, Fendler WP, et al. Prostate-specific Membrane Antigen PET in Prostate Cancer. Radiology 2021;299:248–60. 10.1148/radiol.2021202771. [DOI] [PubMed] [Google Scholar]
- [9].Manfredi C, Fernández-Pascual E, Arcaniolo D, Emberton M, Sanchez-Salas R, Artigas Guix C, et al. The Role of Prostate-specific Membrane Antigen Positron Emission Tomography/Magnetic Resonance Imaging in Primary and Recurrent Prostate Cancer: A Systematic Review of the Literature. Eur Urol Focus 2021:S2405–4569(21)00228–5. 10.1016/j.euf.2021.08.013. [DOI] [PubMed] [Google Scholar]
- [10].Crawford ED, Koo PJ, Shore N, Slovin SF, Concepcion RS, Freedland SJ, et al. A Clinician’s Guide to Next Generation Imaging in Patients With Advanced Prostate Cancer (RADAR III). Journal of Urology 2019;201:682–92. 10.1016/j.juro.2018.05.164. [DOI] [PubMed] [Google Scholar]
- [11].Trabulsi EJ, Rumble RB, Jadvar H, Hope T, Pomper M, Turkbey B, et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. JCO 2020:JCO.19.02757. 10.1200/JCO.19.02757. [DOI] [Google Scholar]
- [12].NCCN. National Comprehensive Cancer Network. Prostate Cancer; (v. 4.2022). n.d. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed July 15, 2022). [Google Scholar]
- [13].Fendler WP, Ferdinandus J, Czernin J, Eiber M, Flavell RR, Behr SC, et al. Impact of 68Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. Journal of Nuclear Medicine 2020;61:1793–9. 10.2967/jnumed.120.242180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 2019;5:856–63. 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res 2021;27:3674–82. 10.1158/1078-0432.CCR-20-4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jackson WC, Desai NB, Abugharib AE, Tumati V, Dess RT, Lee JY, et al. Anatomical patterns of recurrence following biochemical relapse after post-prostatectomy salvage radiation therapy: a multi-institutional study. BJU International 2017;120:351–7. 10.1111/bju.13792. [DOI] [PubMed] [Google Scholar]
- [17].Ghadjar P, Hayoz S, Bernhard J, Zwahlen DR, Hoelscher T, Gut P, et al. Dose-intensified versus conventional dose-salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy: Six-year outcomes of the SAKK 09/10 randomized phase III trial. JCO 2021;39:194–194. 10.1200/JCO.2021.39.6_suppl.194. [DOI] [PubMed] [Google Scholar]
- [18].Pollack A, Karrison TG, Balogh AG, Low D, Bruner DW, Wefel JS, et al. Short Term Androgen Deprivation Therapy Without or With Pelvic Lymph Node Treatment Added to Prostate Bed Only Salvage Radiotherapy: The NRG Oncology/RTOG 0534 SPPORT Trial. International Journal of Radiation Oncology, Biology, Physics 2018;102:1605. 10.1016/j.ijrobp.2018.08.052. [DOI] [Google Scholar]
- [19].Schmidt-Hegemann N-S, Stief C, Kim T-H, Eze C, Kirste S, Strouthos I, et al. Outcome after PSMA PET/CT based salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy: a bi-institutional retrospective analysis. J Nucl Med 2019;60:227–33. 10.2967/jnumed.118.212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Calais J, Armstrong WR, Kishan AU, Booker KM, Elashoff D, Fendler WP, et al. Impact of PSMA PET/CT on SRT planning: Preliminary results from the randomized phase III trial NCT03582774. JCO 2021;39:30–30. 10.1200/JCO.2021.39.6_suppl.30. [DOI] [Google Scholar]
- [21].Vogel MME, Dewes S, Sage EK, Devecka M, Eitz KA, Gschwend JE, et al. Feasibility and Outcome of PSMA-PET-Based Dose-Escalated Salvage Radiotherapy Versus Conventional Salvage Radiotherapy for Patients With Recurrent Prostate Cancer. Frontiers in Oncology 2021;11:2881. 10.3389/fonc.2021.715020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Byrne K, Eade T, Kneebone A, Guo L, Hsiao E, Schembri G, et al. Delineating sites of failure following post-prostatectomy radiation treatment using 68Ga-PSMA-PET. Radiotherapy and Oncology 2018;126:244–8. 10.1016/j.radonc.2017.10.022. [DOI] [PubMed] [Google Scholar]
- [23].Rowe LS, Harmon S, Horn A, Shankavaram U, Roy S, Ning H, et al. Pattern of failure in prostate cancer previously treated with radical prostatectomy and post-operative radiotherapy: a secondary analysis of two prospective studies using novel molecular imaging techniques. Radiat Oncol 2021;16:32. 10.1186/s13014-020-01733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martinez J, Subramanian K, Margolis D, O’Dwyer E, Osborne J, Jhanwar Y, et al. 68Ga-PSMA-HBED-CC PET/MRI is superior to multiparametric magnetic resonance imaging in men with biochemical recurrent prostate cancer: A prospective single-institutional study. Translational Oncology 2022;15:101242. 10.1016/j.tranon.2021.101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ekmekcioglu Ö, Busstra M, Klass ND, Verzijlbergen F. Bridging the Imaging Gap: PSMA PET/CT Has a High Impact on Treatment Planning in Prostate Cancer Patients with Biochemical Recurrence-A Narrative Review of the Literature. J Nucl Med 2019;60:1394–8. 10.2967/jnumed.118.222885. [DOI] [PubMed] [Google Scholar]
- [26].Hope TA, Aggarwal R, Chee B, Tao D, Greene KL, Cooperberg MR, et al. Impact of 68Ga-PSMA-11 PET on Management in Patients with Biochemically Recurrent Prostate Cancer. J Nucl Med 2017;58:1956–61. 10.2967/jnumed.117.192476. [DOI] [PubMed] [Google Scholar]
- [27].Farolfi A, Hadaschik B, Hamdy FC, Herrmann K, Hofman MS, Murphy DG, et al. Positron Emission Tomography and Whole-body Magnetic Resonance Imaging for Metastasis-directed Therapy in Hormone-sensitive Oligometastatic Prostate Cancer After Primary Radical Treatment: A Systematic Review. Eur Urol Oncol 2021;4:714–30. 10.1016/j.euo.2021.02.003. [DOI] [PubMed] [Google Scholar]
- [28].Oehus A-K, Kroeze SGC, Schmidt-Hegemann N-S, Vogel MME, Kirste S, Becker J, et al. Efficacy of PSMA ligand PET-based radiotherapy for recurrent prostate cancer after radical prostatectomy and salvage radiotherapy. BMC Cancer 2020;20:362. 10.1186/s12885-020-06883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446–53. 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- [30].Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020;6:650–9. 10.1001/jamaoncol.2020.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.