Abstract
To help achieve the initial goal of providing universal COVID-19 vaccine access to approximately 258 million adults in 62 US jurisdictions, the federal government launched the Federal Retail Pharmacy Program (FRPP) on February 11, 2021. We describe FRPP’s collaboration among the federal government, US jurisdictions, federal entity partners, and 21 national chain and independent pharmacy networks to provide large-scale access to COVID-19 vaccines, particularly in communities disproportionately affected by COVID-19 (eg, people aged ≥65 years, people from racial and ethnic minority groups). FRPP initially provided 10 000 vaccination sites for people to access COVID-19 vaccines, which was increased to >35 000 vaccination sites by May 2021 and sustained through January 31, 2022. From February 11, 2021, through January 31, 2022, FRPP vaccination sites received 293 million doses and administered 219 million doses, representing 45% of all COVID-19 immunizations provided nationwide (38% of all first doses, 72% of all booster doses). This unprecedented public–private partnership allowed the federal government to rapidly adapt and scale up an equitable vaccination program to reach adults, later expanding access to vaccine-eligible children, during the COVID-19 pandemic. As the largest federal COVID-19 vaccination program, FRPP exemplifies how public–private partnerships can expand access to immunizations during a public health emergency. Pharmacies can help meet critical national public health goals by serving as convenient access points for sustained health services. Lessons learned from this effort—including the importance of strong coordination and communication, efficient reporting systems and data quality, and increasing access to and demand for vaccine, among others—may help improve future immunization programs and support health system resiliency, emphasizing community-level access and health equity during public health emergencies.
Keywords: COVID-19, vaccine distribution, pharmacy, access, equity
The COVID-19 pandemic highlighted and exacerbated long-standing inequities in the social determinants of health in the United States.1-3 Ensuring equitable access to COVID-19 vaccines is essential to reducing health disparities. In December 2020, when rates of COVID-19–related deaths were rising, the US Food and Drug Administration (FDA) authorized use of COVID-19 vaccines; however, vaccine supply was limited, and vaccination efforts focused primarily on health care providers and adults aged ≥65 years.
The initial goal of the federal government’s COVID-19 vaccination program was to provide vaccine access for 258 million US adults. 4 Meeting this goal while ensuring equitable vaccine delivery and administration required the federal government to partner across state and local public health departments and with the private sector.
By the end of January 2021, the need for efficient, equitable vaccine administration was apparent, particularly for people who were at the highest risk of infection and severe adverse health outcomes, many of whom were non-Hispanic Black, non-Hispanic American Indian or Alaska Native, and Hispanic. 5 As the vaccine supply increased and eligibility criteria expanded, additional community-based vaccination sites were needed to expand national immunization capacity; in January 2021, traditional medical vaccine providers were also operating with limited in-person interaction with patients due to COVID-19 protocols, and not all providers had the capacity to meet COVID-19 vaccine storage and handling requirements.
Before the COVID-19 pandemic, planning efforts showed pharmacies to be well positioned to augment vaccine access because they could quickly adjust workflow to support immunizations for a sustained period while continuing to provide routine care.6,7 Most US residents live within 5 miles of a pharmacy, making pharmacists one of the most accessible types of health care professionals in the nation, particularly in low-income communities.8-10 Pharmacists are trusted providers, trained to provide vaccine education and administration.9,11,12 Therefore, pharmacies were well positioned as vaccination sites, given (1) their volume across the country; (2) their ability to handle complex cold storage and large volumes of vaccine, manage patient requirements to receive multiple doses of vaccine, and implement COVID-19 infection control protocols at their vaccination sites; and (3) their capacity to comply with robust data-reporting requirements for COVID-19 beyond collecting and reporting data on routine immunization programs.
Purpose
We describe how the Federal Retail Pharmacy Program (FRPP) was established to be a rapidly scalable federal program that improved COVID-19 vaccine access through use of a network of pharmacies across the United States, with an emphasis on reaching people living in communities disproportionately affected by COVID-19 (eg, people aged ≥65 years, people from racial and ethnic minority groups).
Methods
We used quantitative COVID-19 vaccine distribution and administration data reported to the US Department of Health and Human Services and the Centers for Disease Control and Prevention (CDC) by partners via immunization information systems, the Vaccine Administration Management System, or direct data submission. We used CDC’s Social Vulnerability Index (SVI) to identify communities at high risk of COVID-19 infection. 13 SVI is a summary measure that ranks counties by social vulnerability on a continuous scale from 0 (lowest) to 1 (highest). 13 SVI and percentage of the population aged ≥65 years were the factors used independently to assess high levels of population vulnerability. We defined high SVI as an SVI score >0.66. We obtained SVI data from the 2018 CDC SVI database. 14 County-level SVI characteristics had insufficient resolution to support vaccine equity efforts. Therefore, the federal government developed the Equitable Distribution Index (EDI), a zip code–level proxy for SVI with similar thresholds, as a more granular index to better understand variation in social vulnerability in counties. 15
We summarized the results for FRPP and non-FRPP jurisdictions (ie, state and local health departments and federal entities such as the Bureau of Prisons, US Department of Defense, US Department of State, Indian Health Service, Veterans Health Administration, and Health Resources and Services Administration) by quarter during February 11, 2021, through January 31, 2022, to illustrate program outcomes and adaptations to changing stressors during the COVID-19 pandemic. Quarter 1 was February through April 2021; quarter 2, May through July 2021; quarter 3, August through October 2021; and quarter 4, November 2021 through January 2022. Jurisdictions consisted of the 50 US states, the District of Columbia, 3 cities (Chicago, Illinois; New York City; and Philadelphia, Pennsylvania), 5 US territories (American Samoa, Guam, Northern Mariana Islands, Puerto Rico, and US Virgin Islands), and 3 freely associated states (Federated States of Micronesia, Marshall Islands, and Palau). We used program planning and monitoring documents to capture information on challenges and lessons learned.
CDC reviewed our study, which we conducted consistent with applicable federal law and CDC policy (45 CFR part 46.102[l][2], 21 CFR part 56, 42 USC §241[d], 5 USC §552a, 44 USC §3501 et seq). Because all data were considered surveillance, CDC determined the study to be non–human subjects research and waived institutional review board review.
Outcomes
Launch of FRPP During a Period of Limited Supply and High Demand: Quarter 1 (February–April 2021)
Initiating the program and identifying partners
In February 2021, the federal government launched FRPP through expansion of the US vaccination program to include 21 national chain and independent pharmacy partners. 16 The federal government created the program from more than a decade of pandemic influenza planning and preparing with retail pharmacy chains, with the understanding that pharmacists would expand vaccination capabilities across the United States and aid in public health during an emergency. The federal government designed FRPP to increasingly engage the pharmacy system in its jurisdictions to ensure that the infrastructure would be ready for large-scale implementation when vaccine supply increased. Given that the program was designed to take advantage of the scale of pharmacies nationally, the federal government initially identified large pharmacy chains that had >200 locations to be included in FRPP. Through this process, the federal government also engaged with associations that represented pharmacists, retail chain pharmacies, independent pharmacies, and long-term care pharmacies. With limited initial supply, FRPP started with about 10 000 vaccination sites in February 2021 and expanded to >35 000 by mid-April 2021 (eFigure 1 in Supplemental Material). Through feedback from partners, large networks of independent pharmacies and long-term care pharmacies were also included in the program to improve reach in critical populations, in particular rural communities and long-term care staff and residents. SVI was used to identify communities at high risk of COVID-19 infection to improve access and administration to those disproportionately affected by COVID-19 (vaccine equity). The federal government asked pharmacy partners to prioritize administering vaccine in high-SVI counties. To help jurisdictions select pharmacy partners, the federal government mapped pharmacy locations to identify pharmacy partners with the largest percentage of population living within 10 miles of a pharmacy location and whose population had the highest levels of social vulnerability based on SVI score >0.66.
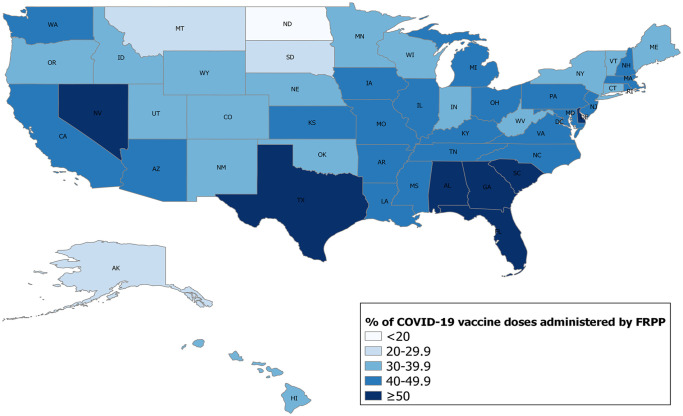
Although the number of pharmacy-based vaccination sites differed across jurisdictions, states with the lowest FRPP administration rates throughout the vaccination program (eg, North Dakota, South Dakota, Montana) often relied on vaccination sites by state and local public health departments and other federal partners, such as the Indian Health Service, for vaccine administration (Figure 1). From February through March 2021, vaccine eligibility criteria were determined on a state-by-state basis, complicating operations and training for pharmacies that had vaccination sites in multiple states. By April 2021, eligibility criteria had expanded nationwide to include all eligible people who wanted to get vaccinated, simplifying FRPP operations. The federal government directly supplied COVID-19 vaccines, which were then provided to eligible people at no cost, with reimbursement policy set by the Centers for Medicare & Medicaid Services and a program covering people without health insurance through the Health Resources and Services Administration COVID-19 Uninsured Program, during the period between February 2021 and January 2022. 17
Figure 1.
Percentage of all COVID-19 vaccine doses administered by the Federal Retail Pharmacy Program (FRPP), by state and excluding territories, United States, February 11, 2021–January 31, 2022. Doses administered by pharmacies varied by state based on demand, jurisdictional characteristics, and geographic reach of pharmacy.
Expansion of Vaccine Distribution During a Period of Decreasing Demand and High Supply: Quarter 2 (May–July 2021)
Vaccine allocation and FRPP expansion
Pharmacy partners received allocations based on number of vaccination sites, including the proximity to the focal populations, and the ability to manage supply and report administration data. The federal government and pharmacy partners tracked activities centrally, leveraging best practices for supply management and minimizing waste and loss due to expired vaccine by ensuring that individual vaccination sites did not hold excessive product at any given time. FRPP assessed pharmacy partners based on whether they achieved high vaccination rates among people from racial and ethnic minority groups and communities with high social vulnerability using an EDI score >0.66. By June 2021, as vaccine supply increased and demand decreased in certain communities, the federal government required pharmacies to have walk-in services in addition to scheduled appointments. With sufficient supply, walk-in services offered increased convenience. However, the shift from scheduled appointments to walk-in services made daily demands unpredictable, complicated staffing, increased patient wait times in high-demand areas, and increased vaccine waste, as doses from multidose vials not used within a certain period had to be discarded. In May 2021, FDA expanded vaccine authorization to include children and adolescents aged 12 to 15 years. In response, pharmacies expanded outreach and planning to vaccinate children and adolescents, administering 53.3% of all doses for those aged 12 to 17 years (eFigure 2 in Supplemental Material).
Distribution adjustment to improve equitable access
FRPP identified 2 key strategies to immediately improve equitable access to vaccines: (1) place vaccine in communities by zip code–level social vulnerability (EDI) and (2) partner with community leaders and employers to increase mobile and on-site vaccination support, bringing access to vaccines closer to eligible recipients.
With 49% of the US population residing in a zip code with a high EDI, increasing vaccination in these communities was critical. Through directed requests from the federal government to each pharmacy partner based on EDI data overlayed with each pharmacy partner’s footprint, as well as through increases of available vaccine, the federal government increased the reach of FRPP sites to people disproportionately affected by COVID-19, increasing the percentage of doses administered in high-EDI areas from 26.6% in quarter 1 to 50.9% in quarter 2, similar to jurisdiction vaccination sites that administered 49.1% of doses in high-EDI areas (Figure 2).
Figure 2.
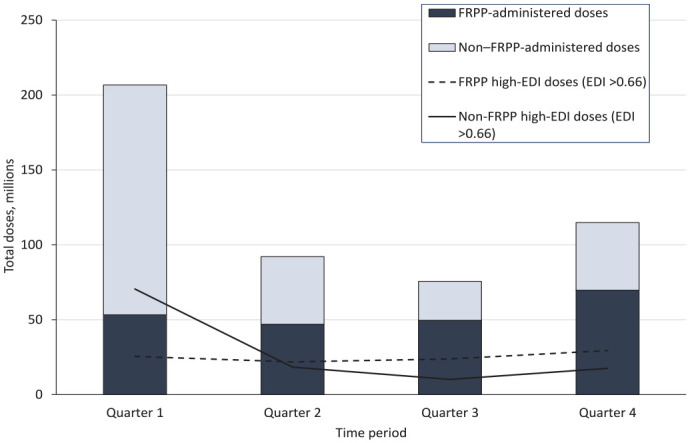
Total number of doses administered by Federal Retail Pharmacy Program (FRPP) sites and non-FRPP jurisdiction and federal entity sites in areas with a high Equitable Distribution Index (EDI), United States, February 11, 2021–January 31, 2022. Because the federal government had included pharmacy-based FRPP sites in high-EDI areas (EDI > 0.66) 15 by April 2021, FRPP provided steady access to vaccine in these areas. The periods were February–April 2021 (quarter 1), May–July 2021 (quarter 2), August–October 2021 (quarter 3), and November 2021–January 2022 (quarter 4).
Beginning in quarter 2, among COVID-19 vaccine recipients who reported their race and ethnicity, FRPP administered >50% of doses to people from racial and ethnic minority groups. By June and July 2021, many jurisdictions began closing state-coordinated mass vaccination sites, increasing national reliance on pharmacies as vaccine providers.18,19
Booster Dose Authorization Leading to Increased Vaccine Demand: Quarter 3 (August–October 2021)
By quarter 3, FDA authorized a third primary series dose of COVID-19 vaccine for people who are immunocompromised and COVID-19 vaccine boosters for other adults. 20 Third dose and booster authorizations, circulation of the Delta variant, and increased hospitalization rates contributed to an increased demand for COVID-19 vaccine, 21 which occurred concurrently with the beginning of the demand for seasonal influenza vaccine. 22 Pharmacies adapted to this surge by promoting coadministration of influenza and COVID-19 vaccination and increasing staffing to support vaccination efforts.
Among Hispanic and Black populations, FRPP administered ≥50% of first and second doses during quarters 2 and 3 (eFigure 3 in Supplemental Material). Federal entity providers, namely the Indian Health Service, and jurisdiction providers administered more doses than pharmacies to American Indian/Alaska Native populations early in the vaccination program, although vaccination of these populations through FRPP increased with second doses and booster doses over time.
Expansion of Vaccine Eligibility and Sustained Demand: Quarter 4 (November 2021–January 2022)
By quarter 4, pharmacies had administered 72% of all booster vaccinations, reflecting the federal government’s reliance on pharmacies as vaccination sites, the ability of pharmacies to scale up vaccination capacity, and the increased public acceptance of receiving immunizations at pharmacies.
On October 29, 2021, the Pfizer-BioNTech COVID-19 vaccine received Emergency Use Authorization for children aged 5 to 11 years. 23 In August 2020, legal authorities implemented an emergency expansion of vaccination criteria to include people aged 3 to 18 years, 24 which enabled pharmacies to vaccinate younger children (aged <3 y), a population that had not traditionally been vaccinated in a pharmacy setting. In 2020 and 2021, only 12.3% of pediatric seasonal influenza vaccine doses were administered to children aged 5 to 12 years in pharmacies.24,25 To facilitate the vaccination of younger children, pharmacies adjusted training and workflow to ensure that children and parents were comfortable with receiving a vaccine in a pharmacy (eg, more time in each appointment, additional privacy areas, kid-friendly waiting spaces). Overall, pharmacies administered >40% of doses to children aged 5 to 11 years and 48% of doses in high-EDI areas. 24
Overall, from February 11, 2021, through January 31, 2022, FRPP received 293 million vaccine doses and administered 219 million doses (eTable 1 in Supplemental Material). FRPP vaccination sites administered 44.5% of the 493 million total doses given nationwide (Figure 3, eTable 2 in Supplemental Material). FRPP sites administered 46.5% of all doses in high-EDI areas and 40.0% to people from racial and ethnic minority groups, including 44.8% of all doses among Hispanic people, 43.5% among Native Hawaiian and other Pacific Islander people, and 40.2% among Black people (eTable 2 in Supplemental Material).
Figure 3.
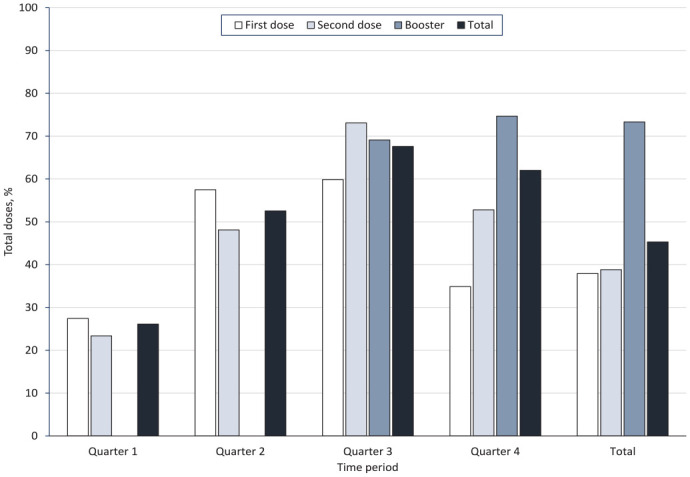
Vaccine doses administered by Federal Retail Pharmacy Program (FRPP) sites as a percentage of total doses given, United States, February 11, 2021–January 31, 2022. The highest volume of immunizations in pharmacies occurred during November 2021–January 2022; 62% of all FRPP doses administered during the surge in vaccination were attributed to the launch of boosters and pediatric vaccines. The periods were February–April 2021 (quarter 1), May–July 2021 (quarter 2), August–October 2021 (quarter 3), and November 2021–January 2022 (quarter 4).
Lessons Learned
Coordination and Communication
Coordination among pharmacy partners, federal agencies, and jurisdictions was a consistent challenge. Frequently updated COVID-19 vaccination recommendations required pharmacies to rapidly update communications, online scheduling tools, and data systems. Because of the initial differences in eligibility criteria across states, the federal government had challenges in implementing the vaccination program across FRPP networks that spanned multiple jurisdictions, requiring processes and systems that quickly adjusted to evolving response needs, such as state-specific screeners and registration processes.
The federal government coordinated FRPP activities with jurisdictions through regular conference calls and Tiberius, 26 a data system platform in which COVID-19 vaccine data (ordering, distribution, administration) are shared among partners and visible to jurisdictions. When FRPP launched, jurisdictions determined which pharmacy partners received vaccine and when they would start receiving it in their jurisdictions, allowing jurisdictions to direct vaccines to locations where they could best serve public health goals. As supply increased, jurisdictions engaged pharmacies to augment vaccination efforts and selected additional pharmacy partners to receive vaccines, allowing jurisdictions and FRPP to move vaccine to high-demand locations and increase community partnerships to reach populations that required additional outreach.
Reporting Systems, Lags, and Data Quality
Pharmacy partners were required to report daily COVID-19 vaccine inventory and document demographic information to state immunization information systems and to the US Department of Health and Human Services to ensure program monitoring and facilitate data-driven program decisions. At the start of FRPP, pharmacy systems and patient interaction steps at pharmacies did not routinely collect demographic data, resulting in missing race and ethnicity data. However, FRPP sites increased reporting of race and ethnicity data from 50% during the first month to 75% by January 2022, with an overall rate of 72.5% (eTable 1 in Supplemental Material). FRPP sites, in reporting of data through state and federal data systems and in weekly telephone calls with CDC FRPP leadership, also reported administration data faster than non-FRPP sites, with 71% of pharmacies versus 22% of non-FRPP sites reporting 80% of their administration data within 3 days to CDC (CDC, internal communications and data analysis in Tiberius dashboards, 2021).
Strategies to Increase Vaccine Uptake and Address Vaccine Confidence
As vaccine demand declined, improving vaccine confidence became a national focus. 27 FRPP sites supported national efforts to reach populations at high risk for developing severe COVID-19 through door-to-door vaccine registration campaigns, partnerships with public health departments and schools, and prioritization of vaccine appointments for teachers.28,29 Pharmacies partnered with organizations (homeless shelters, faith-based organizations, senior centers) already engaging with people who are medically underserved.30,31 Pharmacies also introduced incentive programs to motivate people to get vaccinated (transportation through ride-share company partnerships) 32 and campaigns to build vaccine confidence and address misinformation through education and trusted messengers (community and faith-based leaders).33-35 Although the federal government centralized coordination of FRPP sites, field operations and strategies were decentralized, allowing site flexibility to best address needs locally.
Resource Strain
Pharmacy-supported clinics at community-based locations that were set up to serve high-EDI areas required staff backfilling in stores and increased coordination and staff hiring to sustain those events. Pharmacists and pharmacy technicians had substantially increased work to balance COVID-19 vaccinations while maintaining daily operations. 36 During the COVID-19 pandemic, the pharmacy sector experienced a high rate of staffing shortages that resulted in shorter store hours and closures. 37
Conclusions
FRPP received nearly 300 million COVID-19 vaccine doses and administered >200 million doses, accounting for nearly half the total COVID-19 vaccine doses administered nationwide from February 2021 through January 2022. Not only did FRPP expand access through the broad vaccine distribution and vaccine coverage required in a pandemic, but it was also flexible in its ability to target vaccine to achieve health equity goals, as evidenced by nearly half of all doses administered in high-EDI areas. FRPP, created as part of the COVID-19 emergency response, has had an exceptionally strong start. Recommendations to public health departments and the federal government to sustain and build on FRPP’s momentum include the following:
Maintain momentum created by the COVID-19 pandemic by supporting routine immunizations, trainings, and future pandemic planning with established partners, increasing involvement of pharmacy partners in public health programming.
Evaluate policies required for vaccine providers to execute partnership activities successfully. The Public Readiness and Emergency Preparedness Act and federal reimbursement policies were critical in paving the way for the federal pharmacy program for COVID-19 vaccination.
Identify pharmacy partners that have the greatest potential effects on public health, such as pharmacy presence in communities with high EDI 38 ; articulate how and why partners will be chosen; and communicate what is and is not feasible. Those responsible for implementation may need to develop a sustainability plan for the duration of partnership activities through clear agreements that include plans for reimbursement and program expectations.
Establish clear guidance for pharmacy partners to follow to support collaborative partner planning and implementation efforts. Focus on coordination to leverage private partners’ operational strengths.
Meet regularly with pharmacy partners during the planning and execution phases of a public health collaboration; consistent communication is critical in an environment where information and guidance are constantly evolving.
Supplemental Material
Supplemental material, sj-docx-1-phr-10.1177_00333549231186606 for The US Federal Retail Pharmacy Program: Optimizing COVID-19 Vaccine Delivery Through a Strategic Public–Private Partnership by Christine Kim, Angela Guo, Diana Yassanye, Ruth Link-Gelles, Kirsten Yates, Chris Duggar, Lori Moore, Roua El Kalach, Nkenge Jones-Jack, Chastity Walker, Lynn Gibbs Scharf, Satish K. Pillai and Anita Patel in Public Health Reports
Acknowledgments
The authors acknowledge the following for their support of the US COVID-19 vaccination program: 21 Federal Retail Pharmacy Program partners, 62 jurisdictions, federal entities (Bureau of Prisons, US Department of Defense, US Department of State, Indian Health Service, Veterans Health Administration, Health Resources and Services Administration), CDC COVID-19 Emergency Response Vaccine Task Force, Operation Warp Speed, Countermeasures Acceleration Group, and US Department of Health and Human Services Coordination Operations and Response Element team.
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Dr Anita Patel’s work on this publication was completed prior to her departure from government service.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Christine Kim, PhD, MSPH  https://orcid.org/0000-0001-6211-911X
https://orcid.org/0000-0001-6211-911X
Ruth Link-Gelles, PhD, MPH  https://orcid.org/0000-0002-9617-806X
https://orcid.org/0000-0002-9617-806X
Supplemental Material: Supplemental material for this article is available online. The authors have provided these supplemental materials to give readers additional information about their work. These materials have not been edited or formatted by Public Health Reports’s scientific editors and, thus, may not conform to the guidelines of the AMA Manual of Style, 11th Edition.
References
- 1. Centers for Disease Control and Prevention. What is health equity? Health equity considerations and racial and ethnic minority groups. 2021. Accessed May 28, 2022. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html
- 2. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467. doi: 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilal U, Jemmott JB, Schnake-Mahl A, Murphy K, Momplaisir F. Racial/ethnic and neighbourhood social vulnerability disparities in COVID-19 testing positivity, hospitalization, and in-hospital mortality in a large hospital system in Pennsylvania: a prospective study of electronic health records. Lancet Reg Health Am. 2022;10:100220. doi: 10.1016/j.lana.2022.100220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Department of Health and Human Services. From the factory to the frontlines: the Operation Warp Speed strategy for distributing a COVID-19 vaccine. 2020. Accessed May 24, 2023. https://www.hhs.gov/sites/default/files/strategy-for-distributing-covid-19-vaccine.pdf
- 5. Painter E, Ussery E, Patel A, et al. Demographic characteristics of persons vaccinated during the first month of the COVID-19 vaccination program—United States, December 14, 2020–January 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5):174-177. doi: 10.15585/mmwr.mm7005e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sokolow LZ, Patel A, Koonin LM, Graitcer SB. Scripted surge pharmacy pandemic exercise: testing vaccine administration and antiviral dispensing. Health Secur. 2018;16(4):262-273. doi: 10.1089/hs.2018.0031 [DOI] [PubMed] [Google Scholar]
- 7. Schwerzmann J, Graitcer SB, Jester B, et al. Evaluating the impact of pharmacies on pandemic influenza vaccine administration. Disaster Med Public Health Prep. 2017;11(5):587-593. doi: 10.1017/dmp.2017.1 [DOI] [PubMed] [Google Scholar]
- 8. Bach AT, Goad JA. The role of community pharmacy-based vaccination in the USA: current practice and future directions. Integr Pharm Res Pract. 2015;4:67-77. doi: 10.2147/IPRP.S63822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isenor JE, Edwards NT, Alia TA, et al. Impact of pharmacists as immunizers on vaccination rates: a systematic review and meta-analysis. Vaccine. 2016;34(47):5708-5723. doi: 10.1016/j.vaccine.2016.08.085 [DOI] [PubMed] [Google Scholar]
- 10. Popovian R, Winegarden W, Rivera E, Gavigan K. Accessibility of adult immunizations in pharmacies compared to physician offices in low-income communities. J Am Pharm Assoc (2003). 2022;62(5):1644-1647. doi: 10.1016/j.japh.2022.03.021 [DOI] [PubMed] [Google Scholar]
- 11. Beal JL, Kadakia NN, Reed JB, Illingworth Plake KS. Pharmacists’ impact on older adults’ access to vaccines in the United States. Vaccine. 2020;38(11):2456-2465. doi: 10.1016/j.vaccine.2020.01.061 [DOI] [PubMed] [Google Scholar]
- 12. Burson RC, Buttenheim AM, Armstrong A, Feemster KA. Community pharmacies as sites of adult vaccination: a systematic review. Hum Vaccin Immunother. 2016;12(12):3146-3159. doi: 10.1080/21645515.2016.1215393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flanagan BE, Gregory EW, Hallisey EJ, Heitgerd JL, Lewis B. A social vulnerability index for disaster management. J Homeland Secur Emerg Manage. 2011;8(1):0000102202154773551792. doi: 10.2202/1547-7355.1792 [DOI] [Google Scholar]
- 14. Centers for Disease Control and Prevention. CDC/ATSDR SVI data and documentation. 2018. Accessed May 24, 2023. https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html
- 15. Gold JAW, Kelleher J, Magid J, et al. Dispensing of oral antiviral drugs for treatment of COVID-19 by zip code–level social vulnerability—United States, December 23, 2021–May 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(25):825-829. doi: 10.15585/mmwr.mm7125e1 [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. Understanding the Federal Retail Pharmacy Program for COVID-19 vaccination. 2021. Accessed September 1, 2021. https://www.cdc.gov/vaccines/covid-19/retail-pharmacy-program/index.html
- 17. Health Resources and Services Administration. COVID-19 claims reimbursement to health care providers and facilities for testing, treatment, and vaccine administration for the uninsured. May 2022. Accessed March 29, 2023. https://www.hrsa.gov/provider-relief/about/covid-uninsured-claim
- 18. Hassan A, Weiland N. Some mass vaccination sites in US close as demand begins to fall. The New York Times. April 23, 2021. Accessed May 26, 2023. https://www.nytimes.com/2021/04/23/us/some-mass-vaccination-sites-in-us-close-as-demand-begins-to-fall.html
- 19. Mitchell E. Pentagon closing majority of COVID-19 mass vaccination sites. The Hill. June 8, 2021. Accessed May 15, 2023. https://thehill.com/policy/defense/557432-pentagon-closing-majority-of-covid-19-mass-vaccination-sites
- 20. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA takes additional actions on the use of a booster dose for COVID-19 vaccines. FDA news release. October 20, 2021. Accessed May 15, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-additional-actions-use-booster-dose-covid-19-vaccines [Google Scholar]
- 21. Hamel L, Lopes L, Sparks G, et al. KFF COVID-19 vaccine monitor: October 2021. October 28, 2021. Accessed March 29, 2023. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-october-2021
- 22. Lee C, Palosky C. Surging Delta variant cases, hospitalizations, and deaths are biggest drivers of recent uptick in US COVID-19 vaccination rates. September 28, 2021. Accessed May 23, 2022. https://www.kff.org/coronavirus-covid-19/press-release/surging-delta-variant-cases-hospitalizations-and-deaths-are-biggest-drivers-of-recent-uptick-in-u-s-covid-19-vaccination-rates
- 23. US Food and Drug Administration. FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 years of age. News release. October 29, 2021. Accessed May 15, 2023. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age [Google Scholar]
- 24. Kim C, Yee R, Bhatkoti R, et al. COVID-19 vaccine provider access and vaccination coverage among children aged 5-11 years—United States, November 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(10):378-383. doi: 10.15585/mmwr.mm7110a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2020-21 influenza season. October 7, 2021. Accessed May 24, 2023. https://www.cdc.gov/flu/fluvaxview/coverage-2021estimates.htm
- 26. Simunaci L, US Department of Defense. Tiberius platform aids COVID-19 logistics, delivery. December 16, 2020. Accessed June 20, 2022. https://www.defense.gov/News/News-Stories/Article/Article/2446061/tiberius-platform-aids-covid-19-logistics-delivery
- 27. Hamel L, Kirzinger A, Lopes L, Kearney A, Sparks G, Brodie M. KFF COVID-19 vaccine monitor: January 2021. January 22, 2021. Accessed February 4, 2022. https://www.kff.org/report-section/kff-covid-19-vaccine-monitor-january-2021-vaccine-hesitancy
- 28. The Salinas Californian . Inside look at the first vaccination clinic for 16 and over students in Salinas. April 16, 2021. Accessed June 20, 2022. https://www.thecalifornian.com/picture-gallery/news/2021/04/16/gallery-inside-look-first-vaccination-clinic-high-school-students-salinas-california-monterey-county/7255946002
- 29. Nelson S. Coachella Valley school districts forge effort to get staff vaccinated. News Channel 3. March 10, 2021. Accessed June 20, 2022. https://kesq.com/news/2021/03/10/coachella-valley-school-districts-forge-effort-to-get-staff-vaccinated [Google Scholar]
- 30. Tuggle D. HEB teaming up with local clinic to vaccinate underserved communities. KBTX. April 8, 2021. Accessed June 20, 2022. https://www.kbtx.com/2021/04/08/heb-teaming-up-with-local-clinic-to-vaccinate-underserved-communities [Google Scholar]
- 31. Spoerre A. Rural Kansas pharmacy to deliver 1,000 coronavirus vaccines to KC homeless community. The Kansas City Star. April 13, 2021. Accessed June 20, 2022. https://www.kansascity.com/news/local/article250604484.html#storylink=cpy
- 32. Reuters. Lyft, CVS Health partner to increase access to COVID-19 vaccines. February 19, 2021. Accessed June 20, 2022. https://www.reuters.com/business/healthcare-pharmaceuticals/lyft-cvs-health-partner-increase-access-covid-19-vaccines-2021-02-19
- 33. Belefanti C. Pa NAACP teams up with Rite Aid to provide vaccines in Nicetown. The Philadelphia Tribune. March 26, 2021. Accessed June 20, 2022. https://www.phillytrib.com/news/local_news/pa-naacp-teams-up-with-rite-aid-to-provide-vaccines-in-nicetown/article_5ccd1356-1423-5d0d-a487-336aa8252233.html
- 34. Juarez L. COVID-19 vaccine clinic hosted at Hindu temple in Chino Hills. ABC7. April 17, 2021. Accessed May 24, 2023. https://abc7.com/chino-hills-vaccinations-appointments-hindu-temple-vaccine/10523630
- 35. The Mercury . Registration open for vaccine clinic coming to Pottstown on Friday. March 23, 2021. Accessed May 24, 2023. https://www.pottsmerc.com/2021/03/23/registration-open-for-vaccine-clinic-coming-to-pottstown-on-friday
- 36. Johnston K, O’Reilly CL, Scholz B, Georgousopoulou EN, Mitchell I. Burnout and the challenges facing pharmacists during COVID-19: results of a national survey. Int J Clin Pharm. 2021;43(3):716-725. doi: 10.1007/s11096-021-01268-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaplan A, Springer S, Nguyen V. The latest worker shortage may affect your health: pharmacies don’t have enough staff to keep up with prescriptions. NBC News. December 30, 2021. Accessed June 20, 2022. https://www.nbcnews.com/health/health-news/latest-worker-shortage-may-affect-health-pharmacies-dont-enough-staff-rcna8737
- 38. Berenbrok LA, Tang S, Gabriel N, et al. Access to community pharmacies: a nationwide geographic information systems cross-sectional analysis. J Am Pharm Assoc (2003). 2022;62(6):1816-1822. doi: 10.1016/j.japh.2022.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-phr-10.1177_00333549231186606 for The US Federal Retail Pharmacy Program: Optimizing COVID-19 Vaccine Delivery Through a Strategic Public–Private Partnership by Christine Kim, Angela Guo, Diana Yassanye, Ruth Link-Gelles, Kirsten Yates, Chris Duggar, Lori Moore, Roua El Kalach, Nkenge Jones-Jack, Chastity Walker, Lynn Gibbs Scharf, Satish K. Pillai and Anita Patel in Public Health Reports