Abstract
Sea cucumbers have high economic value, and in most forms of trade, their body wall is typically the only part that is harvested and sold. The organs of the sea cucumber, collectively known as the viscera, are frequently discarded, contributing to land and water pollution. However, discarded sea cucumber viscera contain various nutrients that can be used in many applications. Therefore, this review highlights the biological and economic aspects of sea cucumbers, followed by a critical discussion of the nutritional value of their internal organs and possible applications, including as functional feed additives in the aquaculture industry, sources of natural testosterone for application in sex reversal and production of monosex population, of neuroprotective agents against central nervous system disorders and of cosmetic ingredients, especially for skin whitening and anti-ageing products. The review further highlights the valorisation potential of viscera to maximize their economic potential, thus providing an enormous prospect for reusing sea cucumber waste, thereby reducing the negative impact of the sea cucumber fishery sector on the environment.
Keywords: Sea cucumber, Viscera, Waste, Bioactive compound, Valorisation
Introduction
Sea cucumber is a valuable seafood item, particularly in Asia, where it is not only priced as an exotic and costly food but also valued for its medicinal and tonic properties (Rahantoknam, 2015; Hossain et al., 2022). Generally, sea cucumbers are soft-bodied, elongated, worm-shaped echinoderms with a leathery texture and jelly-like body, resembling a cucumber (Conand, 1990; Zhang et al., 2019). The sea cucumber fishery supports the livelihood of coastal communities in Asian countries, and with rising market demand, the global fishery production of sea cucumbers has reached 100,000 tons annually (Ardiansyah et al., 2020). According to the Food and Agriculture Organization (FAO) global statistics reported in 2019, Indonesia is the largest supplier of wild sea cucumbers among all Southeast Asian countries (Southeast Asian Fisheries Development Center (SEAFDEC), 2022).
There are a total of 1,200 known species of sea cucumbers scattered across the world’s oceans (Aydin, 2018). Among them, many species within the genus Holothuria are considered valuable (Samyn, Massin & Vandenspiegel, 2019). In most Southeast Asian countries, the preferred high-value sea cucumber species are Holothuria scabra (sandfish) (Fig. 1A) and Stichopus horrens (Fig. 1B) (dragonfish) (Southeast Asian Fisheries Development Center (SEAFDEC), 2022). Wild populations of these species are declining due to extensive commercial exploitation in coastal waters, driven by high demand from both domestic and international markets (Yaghmour & Whittington-Jones, 2018; Kamaruddin & Rehan, 2015). From a nutritional point of view, sea cucumbers are low in sugar, fat, and cholesterol, but rich in proteins and essential amino acids (Senadheera et al., 2023). Additionally, sea cucumbers contain various essential nutritional components, including vitamins, minerals, collagen, and polyunsaturated fatty acids (Liu et al., 2021). Consequently, they are harvested and used as food and raw materials. Sea cucumbers have been traditionally consumed as tonic food and used to produce tonic medicine, such as ‘gamat’ oil in Indonesia and Malaysia. The international sea cucumber market primarily focuses on Asia and the Indo-Pacific region (Elvevoll et al., 2022).
Figure 1. Sea cucumbers and their products.
(A) Holothuria scabra; (B) Stichopus horrens; (C) dried sea cucumber (Stichopus horrens); (D) smoke sea cucumber (Thelenota ananas); (E) sea cucumber crackers (Holothuria edulis).
Coastal communities have been exploiting various sea cucumber to develop processed products, including dried sea cucumber (Fig. 1C), smoke sea cucumber (Fig. 1D), and sea cucumber crackers (Fig. 1E). Unfortunately, the dwindling supply of sea cucumbers and the ever-increasing demand have forced local fishermen to harvest sea cucumbers of all sizes, which poses a threat to their sustainability (Southeast Asian Fisheries Development Center (SEAFDEC), 2022). In addition, sea cucumbers play important roles in the ecosystem. Over-exploitation of sea cucumbers can have several adverse effects, such as compromising sediment health, reducing the ecosystem’s capacity to recycle nutrients and resist ocean acidification, diminishing the biodiversity of associated symbionts, and impeding the movement of organic matter from higher trophic levels (Purcell et al., 2016a; Pierrat et al., 2022). Owing to their high market demand, sea cucumber aquaculture is gaining momentum. The main techniques used in sea cucumber culture include pond farming, pen culture, marine ranching, and tank culture. Sea cucumber aquaculture serves as a viable alternative to relieve the strong fishing pressure on wild sea cucumber populations. However, efforts are still needed to develop sustainable sea cucumber aquaculture programs.
In most forms of trade, only the body wall and muscle bands of sea cucumbers are harvested and sold off (Senadheera et al., 2023), while the intestines, gonads, and other organs (termed viscera), which can account for up to 50% of the sea cucumber’s total weight, are considered unwanted products and are often discarded (Oktaviani, Mulyani & Rochima, 2015; Hossain et al., 2022). The discarding of sea cucumber viscera results in resource waste and environmental contamination since they may contain heavy metals such as arsenic, cadmium, lead, and mercury, which are well-known to be toxic to the environment and human health when exceeding standard limits (Babji et al., 2020). Sea cucumber viscera have been reported to contain various nutrients such as oligosaccharides, saponins, phenols, flavonoids, lipids, proteins, fatty acids, and amino acids (Zhang & Chang, 2014). They also contain high levels of omega-3 PUFAs and glycine, making them suitable for processing into functional foods, dietary supplements, and pharmaceuticals (Liu et al., 2021). Sea cucumber viscera are considered a delicacy and are consumed raw, dried, and fermented in some countries, such as Samoa and Japan (Eriksson et al., 2007; Charan-Dixon et al., 2019; Nishanthan et al., 2021). According to Babji et al. (2020), consumption of relatively small amounts of sea cucumber viscera hydrolysate may satisfy various vitamin needs in both animal and human nutrition.
As sea cucumber viscera are known to contain various nutrients and bioactive compounds, further research should be conducted to valorise them for the industrial production of high-value nutritional products while addressing the issue of harmful heavy metal content. Even with high heavy metal contents, it is possible to formulate sea cucumber viscera extracts to meet the maximum legally permissible requirements. Additional processing techniques, such as ion-exchange chromatography, can be employed to reduce or eliminate heavy metals during the extraction of sea cucumber viscera (Ayangbenro & Babalola, 2017; Babji et al., 2020). Therefore, this review summarizes and discusses the importance of sea cucumbers by addressing their general biology, the nutritional content of various body parts, and their socioeconomic contribution. The review further focuses on the often-discarded viscera and explores their nutritional aspects. Additionally, future directions are suggested for recycling viscera, aiming to turn waste into wealth.
Survey methodology
Literature searches were conducted on the Web of Science (https://www.webofscicence.com/) and Scopus (https://www.scopus.com) database using the PRISMA method (Page et al., 2021) (Fig. 2). Keywords and phrases such as “distribution of sea cucumber”, “nutritional value of sea cucumber”, “sea cucumber viscera”, and “nutritional value of sea cucumber viscera” were used to search for publications in both databases. All the pertinent articles were thoroughly examined after the initial screening to ensure that they were all relevant to the topic.
Figure 2. PRISMA flow diagram of study selection.
Sea cucumber biology
Sea cucumbers belong to the phylum Echinodermata, along with other marine invertebrates that exhibit radial symmetry (Oh et al., 2017). They are deposit feeders and play a crucial role in coastal mariculture by directly contributing to the recycling of nutrients and the breakdown of detritus and organic matter (Gao et al., 2011; Zamora et al., 2018). Sea cucumbers have numerous shield-like buccal tentacles around the mouth, which are enclosed in the external oral hood (Fig. 3). The body wall of sea cucumbers consists of a thick layer of collagenous connective tissue that envelops and protects their internal organs (Slater & Chen, 2015). Sea cucumbers are found on practically all substrates, including sand, muddy sand, and sandy mud near seagrass, with depths ranging from 1 to 40 m (Lewerissa, Uneputty & Waliulu, 2021; Liubana, Surbakti & Tubo, 2022). They are widely distributed in all oceans (Table S1), inhabiting regions from the Arctic to the tropics, and their habitats vary from the intertidal zone to the deepest seas, such as the bottom of the Mariana Trench (Gonzales-Duran et al., 2021; Liu, Xue & Li, 2022).
Figure 3. Anatomy of sea cucumber (Holothuria scabra).
Abbreviation: a, tentacles; b, pharynx retractor muscles; c, stone canal; d, polian vesicle; e, tube feet (podia); f, gonad (much enlarged at sexual maturity); g, longitudinal muscle bands; h, cloaca; i, anus; j, respiratory tract; k, intestine; l, stomach; m, ring canal; n, pharynx.
Asexual reproduction is possible in sea cucumbers through a process known as fission. They can divide themselves along the median line, where the anterior and posterior ends spin in opposite directions. After a while, the two ends slowly move away from each until they rip the body wall apart, resulting in the division of the organism into two distinct individuals (Al-Rashdi, Eechaut & Claereboudt, 2012). Factors such as failure of sexual reproduction, eutrophication, malnutrition, and environmental stimulation, such as drought during prolonged low tides, can all contribute to the occurrence of asexual reproduction in sea cucumbers (Widianingsih, Hartati & Endrawati, 2014).
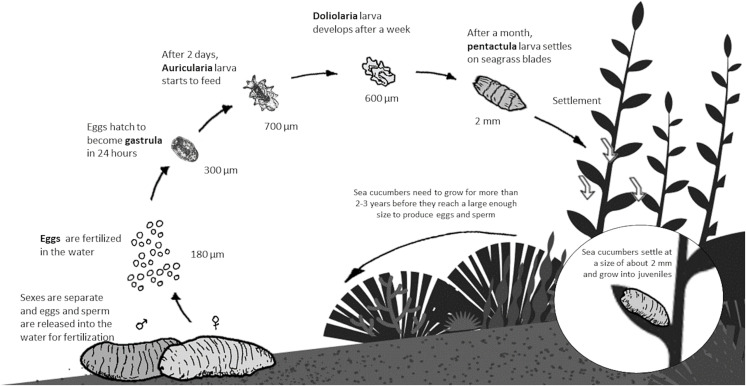
In the wild, sea cucumbers naturally gather in groups consisting of more than ten individuals spaced approximately 5 m apart to perform simultaneous spawning. This synchronized behaviour ensures the highest possible fertilization rates during sexual reproduction (Rahman & Yusoff, 2017). Sea cucumber eggs are externally fertilized when the male and female gametes fuse in the water column, as shown in Fig. 4. Fertilized eggs quickly progress to the blastula stage within an hour after fertilization, and by the end of the day, they reach the typical gastrula stage. After two days, the fertilized eggs transform into planktonic auricularia larvae, which exhibit a pelagic habit and feed on suspended microalgae (Kumara et al., 2013). After approximately two weeks, sea cucumber larvae will reach the doliolaria stage (non-feeding stage). From there, they metamorphose into the pentactula stage and subsequently into early juveniles. At this stage, they begin to feed by grazing on the biological film covering the leaf surface. As they grow and mature into adults over another week or two, then move down into the sediment and begin grazing in deeper areas (Altamirano & Rodriguez, 2022).
Figure 4. Lifecycle of sea cucumber (Holothuria scabra).
The reproductive success of sea cucumbers relies on their social behaviour, population diversity and density, chemical communication, and egg-laying synchronization (Scannella et al., 2022). A reduction in population density can have an impact on fertilization and lead to a decrease in population size, thus affecting their reproductive success (Hasan, 2019; Gonzales-Duran et al., 2021). Holothurians are vulnerable to changes in pH, temperature, and salinity (Liu, 2015; Gonzales-Duran et al., 2021), which can impact their population frequency, growth, and survival rates (Kashenko, 2000; Dong et al., 2011; Liu, 2015; Zhou, Zhang & Li, 2018). Additionally, larval development and survival are highly susceptible to climate change (Asha & Muthiah, 2005; Zamora & Jeffs, 2013; Liu, 2015; Gonzales-Duran et al., 2021). Fluctuations in these environmental variables trigger a variety of biochemical and physiological adaptations that delay larval metamorphosis, especially during the gastrulation stage, which marks the development of digestive tract feeding activity. This results in slower larval development, increases the risk of predation, and adversely affects larval survival (Asha & Muthiah, 2005; Gonzales-Duran et al., 2021).
Nutritional value of sea cucumber
The consumption of sea cucumbers as food products, tonics and aphrodisiacs is only popular in China and other Asian countries (Han, Keesing & Liu, 2016). However, sea cucumbers are very nutritious, and the bioactive components found in them are valuable for both the food and the biomedical industries (Pangestuti & Arifin, 2018). The body wall is the main part of the sea cucumber that is consumed and consists mainly of epithelial and dermal connective tissues, collagenous fibres, proteoglycans, glycoproteins, and amorphous interstitial materials (Yang, Hamel & Mercier, 2015).
The proximate compositions (moisture, protein, lipid, ash, and carbohydrates) of various sea cucumber species are shown in Table 1. Most sea cucumber species exhibit high amounts of saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) (Table 2) and are abundant in essential amino acids (Table 3). SFAs were found to be the dominat fatty acid elements in H. scabra (71.76%), H. leucospilota (69.57%), and H. atra (57.04%) (Ridzwan et al., 2014).
Table 1. Proximate composition of sea cucumber and sea cucumber viscera (%).
| Species | Moisture | Protein | Lipid | Ash | Carbohydrates | References |
|---|---|---|---|---|---|---|
| Sea cucumber | ||||||
| H. arenicola (f) | 93.01 | 4.40 | 0.60 | 2.01 | – | Barzkar, Fariman & Taheri (2017) |
| H. atra (f) | 78.34 | 42.32 | 1.12 | 2.38 | 0.87 | Oedjoe (2017) |
| H. edulis (f) | 78.16 | 41.61 | 1.08 | 2.47 | 1.14 | Oedjoe (2017) |
| H. impatiens (f) | 78.41 | 39.94 | 1.12 | 2.16 | 1.37 | Oedjoe (2017) |
| H. lessoni (d) | 13.47 | 41.18 | 3.02 | 34.51 | 7.86 | Andriamanamisata & Telesphore (2019) |
| H. leucospilota (f) | 81.24 | 45.71 | 4.60 | 4.30 | 44.96 | Omran (2013) |
| H. mammata (f) | 85.24 | 7.88 | 0.09 | 5.13 | – | Aydin et al. (2011) |
| H. nobilis (f) | 76.05 | 42.54 | 1.09 | 2.39 | 0.56 | Oedjoe (2017) |
| H. parva (f) | 67.92 | 17.61 | 2.43 | 32.74 | – | Salarzadeh et al. (2012) |
| H. sanctori (f) | – | 8.02 | 0.55 | – | – | Gocer, Olgunoglu & Olgunoglu (2018) |
| H. scabra (f) | 76.03 | 44.07 | 1.02 | 2.01 | 0.45 | Oedjoe (2017) |
| H. tubulosa (f) | 80.77 | 7.07 | 10.21 | – | – | Zmemlia et al. (2020) |
| P. australis (f) | 74.92 | 20.22 | 1.42 | 2.58 | 0.86 | Widianingsih et al. (2016) |
| P. californicus (d) | 4.03 | 47.03 | 8.19 | 25.73 | 15.02 | Bechtel, Oliveira & Smiley (2012) |
| P. parvimensis (f) | 90.80 | 4.70 | 0.30 | 3.40 | – | Chang-Lee, Price & Lampila (1989) |
| S. herrmanni (d) | 8.00 | 67.90 | 1.09 | 17.24 | 7.34 | Shojaei, Ebrahimi & Nazemi (2020) |
| S. horrens (f) | 92.80 | 3.47 | 0.39 | 3.40 | – | Barzkar, Fariman & Taheri (2017) |
| S. japonicus (f) | 92.00 | 7.70 | 0.20 | 2.70 | – | Tanikawa (1955) |
| S. variegatus (d) | 6.27 | 34.33 | 1.08 | 4.34 | – | Ridhowati et al. (2018) |
| S. vastus (f) | 19.46 | 38.70 | 0.38 | 34.04 | 7.42 | Rasyid (2017) |
| Sea cucumber viscera | ||||||
| A. japonicus (f) | 89.54 | 2.20 | 2.12 | 3.94 | – | Lee et al. (2012) |
| C. frondosa (ad) | 7.84 | 42.20 | 23.68 | 11.51 | – | Liu et al. (2021) |
| C. frondosa (fd) | 1.79 | 46.12 | 22/77 | 12.31 | – | Liu et al. (2021) |
| C. frondosa (f) | 82.07 | 8.65 | 4.68 | 2.14 | – | Liu et al. (2021) |
| C. frondosa (f) | 92.3 | 4.5 | 2.0 | 0.7 | – | Mamelona, Louis & Pelletier (2010) |
| I. japonicus (f) | 86.92 | 6.63 | 0.16 | 3.26 | – | Vergara & Rodriguez (2016) |
| P. californicus (d) | 5.50 | 68.40 | 5.30 | 12.18 | – | Bechtel et al. (2013) |
| - (h) | – | 50.27 | 22.16 | – | – | Babji et al. (2020) |
| - (fr) | 76.5 | 9.3 | 1.3 | 12.4 | – | Kim & Kim (2014) |
Note:
–, Not determine; ad, air-dried; d, dried; f, fresh; fr, fermented; h, hydrolysate.
Table 2. Fatty acid content of sea cucumber and sea cucumber viscera (%).
| Species | SFA | MUFA | PUFA | EPA | DHA | References |
|---|---|---|---|---|---|---|
| Whole sea cucumber | ||||||
| A. mauritiana | 39.62 | 28.27 | 32.12 | – | – | Haider et al. (2015) |
| A. mollis | 23.62 | 22.61 | 53.75 | 10.63 | 4.64 | Liu et al. (2017) |
| A. japonicus | 56.00 | 23.15 | 18.5 | 1.44 | 7.8 | Anisuzzaman et al. (2019) |
| B. marmorata | 47.16 | 19.37 | 33.48 | 3.71 | 4.54 | Nishanthan et al. (2018) |
| Bohadschia sp. | 44.76 | 22.71 | 32.53 | 9.3 | 3.47 | Nishanthan et al. (2018) |
| H. arenicola | 15.91 | 33.17 | 50.92 | – | – | Haider et al. (2015) |
| H. edulis | 83.95 | – | 16.05 | – | – | Al Azad, Shaleh & Siddiquee (2017) |
| H. forskali | 22.95 | 6.44 | 43.64 | 10.49 | 1.01 | Santos et al. (2015) |
| H. mammata | 19.21 | 15.01 | 53.38 | 4.99 | 10.30 | Aydin et al. (2011) |
| H. scabra | 41.61 | 22.14 | 36.25 | 18.55 | 1.27 | Nishanthan et al. (2018) |
| H. spinifera | 40.12 | 30.72 | 29.16 | 10.95 | 0.61 | Nishanthan et al. (2018) |
| H. leucospilota | 34.55 | 30.14 | 35.29 | – | – | Yahyavi et al. (2012) |
| H. tubulosa | 15.48 | 13.29 | 57.76 | 6.18 | 12.37 | Aydin et al. (2011) |
| I. badionotus | 52.62 | 24.35 | 22.98 | 3.02 | 4.32 | Zacarias-Soto & Olvera-Novoa (2015) |
| S. chloronotus | 52.21 | 20.56 | 27.23 | 13.00 | 1.43 | Nishanthan et al. (2018) |
| T. anax | 45.02 | 29.47 | 25.51 | 5.51 | 0.99 | Nishanthan et al. (2018) |
| Sea cucumber viscera | ||||||
| A. chilensis | 50.39 | 23.97 | 6.42 | 2.71 | – | Careaga, Muniain & Maier (2013) |
| C. frondosa | – | – | 29.72 | 28.23 | – | Abuzaytoun et al. (2022) |
| C. frondosa | Liu et al. (2021) | |||||
| Air-dried | 26.46 | 28.73 | 40.14 | 28.71 | 0.87 | |
| Freeze-dried | 26.97 | 28.60 | 39.91 | 27.97 | 0.85 | |
| Fresh | 25.93 | 27.01 | 33.01 | 27.76 | 0.88 | |
| C. frondosa | 26.40 | 28.20 | 45.40 | 17.10 | 0.30 | Mamelona, Louis & Pelletier (2010) |
| C. frondosa | 3.19 | 11.3 | – | – | – | Ramalho et al. (2020) |
| P. californicus | 18.13 | 32.05 | 43.64 | 22.63 | 8.93 | Bechtel et al. (2013) |
| – | 39.76 | 26.86 | 33.39 | – | – | Babji et al. (2020) |
Note:
–, Not determined.
Table 3. Amino acid profiles of sea cucumber and sea cucumber viscera (%).
| Species | Parameters | References | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leu* | His* | Lys* | Arg | Val* | Ile* | Thr* | Phe* | Met* | Tyr | Asp | Ala | Pro | Gly | Gln | Ser | Cys | ||
| Whole sea cucumber | ||||||||||||||||||
| A. mauritiana | 1.58 | 0.65 | 3.52 | 0.99 | 2.13 | 0.43 | 2.19 | 0.99 | 0.42 | 0.33 | 4.48 | 6.45 | 0.24 | 18.80 | 5.25 | 2.11 | – | Omran (2013) |
| H. arenicola | 5.19 | 1.41 | 2.06 | 6.12 | 2.94 | 3.37 | 4.59 | 2.80 | 0.43 | 2.45 | 15.71 | 11.72 | 7.56 | 17.33 | 11.77 | 4.54 | – | Haider et al. (2015) |
| H. leucospilota | 1.86 | 0.36 | 0.73 | 1.71 | 1.51 | 0.49 | 2.73 | 0.75 | 0.19 | 0.49 | 4.65 | 5.80 | 0.14 | 19.17 | 5.64 | 2.33 | – | Omran (2013) |
| H. mammata | 3.70 | 2.00 | 2.20 | 6.80 | 3.70 | 2.00 | 3.30 | 3.30 | 1.00 | 7.10 | 5.30 | 12.20 | 8.20 | 11.80 | 12.10 | 3.20 | 8.20 | Gonzales-Wanguemert et al. (2018) |
| H. polii | 5.40 | – | 1.10 | 13.40 | 5.40 | 2.90 | 3.40 | 8.10 | 0.70 | 3.80 | 4.50 | 15.10 | 10.10 | 10.60 | 8.40 | 3.00 | 2.40 | Gonzales-Wanguemert et al. (2018) |
| H. scabra | 2.33 | 0.46 | 1.91 | 4.37 | 1.85 | 1.36 | 2.52 | 1.02 | 0.66 | 1.13 | 5.46 | 4.52 | 6.29 | 8.22 | 8.32 | 2.20 | 6.47 | Doungpai et al. (2022) |
| H. tubulosa | 5.80 | – | 1.60 | 12.90 | 5.50 | 3.30 | 3.70 | 7.90 | 0.10 | 3.30 | 4.80 | 14.50 | 9.50 | 10.60 | 8.60 | 3.50 | 2.40 | Gonzales-Wanguemert et al. (2018) |
| S. horrens | 1.27 | 0.30 | 0.47 | 3.76 | 1.07 | 0.87 | 1.51 | 0.96 | 0.33 | 0.75 | 2.66 | 3.09 | 3.04 | 8.29 | 4.89 | – | – | Stiani et al. (2021) |
| Sea cucumber viscera | ||||||||||||||||||
| C. frondosa | 0.78 | 1.04 | 0.59 | – | 0.17 | 15.56 | 10.00 | 15.31 | 8.12 | 1.07 | 0.50 | 4.92 | 10.80 | 3.17 | 25.93 | 1.70 | 0.35 | Liu et al. (2021) |
| C. frondosa | 7.20 | 2.30 | 6.60 | 9.10 | 5.40 | 4.70 | 5.00 | 3.50 | 2.30 | 5.00 | 10.00 | 6.60 | 4.00 | 8.00 | 14.30 | 4.30 | 1.60 | Mamelona, Louis & Pelletier (2010) |
| – | 4.24 | 1.24 | 1.11 | 5.24 | 2.36 | 2.15 | 4.19 | 2.23 | 1.08 | 2.06 | 5.64 | 2.18 | 2.75 | 4.22 | 7.36 | 3.59 | 0.01 | Babji et al. (2020) |
Note:
Leu, leucine; His, histidine; Lys, lysine; Arg, arginine; Val, valine; Ile, isoleucine; Thr, threonine; Phe, phenylalanine; Met, methionine; Tyr, tyrosine; Asp, aspartic acid; Pro, proline; Gly, glycine; Gln, glutamine; Ser, serine; Cys, cysteine; *, essential amino acid; –, not determined.
Additionally, sea cucumbers have outstanding vitamin and mineral profiles, including vitamins A (455 µg/100 g), B1 (thiamine) (0.04 mg/kg), B2 (riboflavin) (0.06 mg/kg), B3 (niacin) (0.4 mg/kg), C (3.19 mg/100 g), and E (2.82 mg/100 g), as well as essential minerals, particularly calcium, magnesium, and iron (Bordbar, Anwar & Saari, 2011; Sroyraya et al., 2017; Achmad et al., 2020; Ardiansyah et al., 2020) (Table 4). Notably, Stichopus vastus had the highest mineral content compared to other species. In addition to being valuable marine commodities, sea cucumbers are a significant source of medicine (Zhao et al., 2018). They are used in traditional Chinese medicine and are thought to have therapeutic capabilities, to treat conditions such as arthritis, high blood pressure, asthma, cancer, frequent urination, and impotence (Guo et al., 2015; Pangestuti & Arifin, 2018; Liang et al., 2022). They have extensive uses in the biomedical industry, where they are believed to possess therapeutic capabilities (Zohdi et al., 2011) and numerous active ingredients, including polysaccharides, peptides, proteins, lipids, (Janakiram, Mohammed & Rao, 2015), collagen, gelatine, saponins and acid mucopolysaccharides (Kariya et al., 2004; Lu et al., 2010; Zhou, Wang & Jiang, 2012; Yang, Hamel & Mercier, 2015).
Table 4. Mineral compositions of sea cucumber and sea cucumber viscera (g/100 g).
| Species | Ca | Na | Mg | P | K | Fe | References |
|---|---|---|---|---|---|---|---|
| Sea cucumber | |||||||
| H. arenicola | 0.083 | – | 0.115 | – | – | 0.060 | Barzkar, Fariman & Taheri (2017) |
| H. sanctori | 0.657 | 0.552 | 0.156 | 0.011 | – | – | Gocer, Olgunoglu & Olgunoglu (2018) |
| H. scabra | 1.821 | 0.666 | 0.305 | 0.088 | 0.061 | 0.022 | Rasyid et al. (2020) |
| H. tubulosa | 2.807 | 3.902 | 0.431 | 0.048 | 0.443 | <0.001 | Kunili & Colakoglu (2019) |
| P. californicus | 0.002 | 0.008 | 0.001 | <0.001 | <0.001 | 0.018 | Bechtel, Oliveira & Smiley (2012). |
| P. parvimensis | 0.095 | 0.016 | 0.011 | 0.014 | 0.047 | 0.021 | Chang-Lee, Price & Lampila (1989) |
| S. horrens | 0.106 | – | 0.093 | – | – | 0.521 | Barzkar, Fariman & Taheri (2017) |
| S. japonicus | 0.003 | – | <0.001 | – | – | – | Tanikawa (1955) |
| S. vastus | 2.449 | 8.054 | – | 5.085 | 0.160 | 0.521 | Rasyid (2017) |
| Sea cucumber viscera | |||||||
| C. frondosa | 0.900 | 1.240 | – | – | 1.870 | 0.019 | Mamelona, Louis & Pelletier (2010) |
| C. frondosa | 0.014 | 0.190 | – | 0.110 | 0.200 | 0.009 | Ramalho et al. (2020) |
| P. californicus | – | – | – | – | – | 0.003 | Bechtel et al. (2013) |
| – | 128.3 | 338.3 | – | – | 0.05 | 39.70 | Babji et al. (2020) |
| – | 0.075 | – | – | 0.112 | – | – | Kim & Kim (2014) |
Note:
–, Not determined.
Socio-economic status of sea cucumber
Monetized marine resources, such as sea cucumbers, greatly support the livelihoods of coastal communities in the Indo-Pacific region (Hair et al., 2019). Sea cucumbers are easily processed (gutted, boiled, and dried) using basic, affordable tools (Ram et al., 2017; Hair et al., 2019). They are relatively sedentary, making them easy to collect (Wolfe & Byrne, 2022). They are commonly harvested by fisher in traditional ways, such as collecting from coral reefs at low tide or by diving in shallow waters (Friedman et al., 2008; Plaganyi et al., 2020; Prasada, 2020).
Coastal communities process sea cucumbers and sell the final products at a higher price. The processed and dried body wall of sea cucumbers, known as ‘beche-de-mer’, is a valuable marine export commodity and an important source of income for coastal communities. It is massively exported throughout Asia and is highly regarded as a seafood delicacy. Prices for sea cucumbers can fetch up to US$983.47 per kilogram in China or US$110.78 per kilogram in Japan (Kinch et al., 2007; Purcell, Williamson & Ngaluafe, 2018; Hair et al., 2019; Wolfe & Byrne, 2022). The market price on the market is not only based on the type and species but also on size. There are various grades of processed sea cucumbers in demand, but in general, sellers often grade sea cucumbers into at least three grades: grade 1 (largest size), grade 2 (medium size) and grade 3 (smallest size) (Ochiewo et al., 2010).
Greater quantities of ‘beche-de-mer’ are exported to Asia, where it is a valuable product. Louw & Burgener (2020) reported Madagascar is the top exporter of dried sea cucumbers, sending 40% of its total exports to Asian markets between 2012 and 2019. Hong Kong serves as a major importer and commercial hub for dried seafood, including sea cucumber (Ben-Hasan et al., 2021). Over the past 8 years, Hong Kong has been the greatest importer in Asia, bringing in almost 56 million kg from around the world. However, nearly 70% of its total imports are then re-exported to mainland China (Jun, 2009; Louw & Burgener, 2020; Ben-Hasan et al., 2021). Due to the high demand in the Asian market and the high shipping costs that restrict reef fish exports, sea cucumber fisheries have become the second-most profitable export fishery in the South Pacific, after tuna (Carleton et al., 2013).
Nutritional value of sea cucumber viscera
Novel sulphated polysaccharide
The viscera of sea cucumbers are a good source of nutrients. The purified chondroitin sulphate polysaccharides from the digestive tract of Apostichopus japonicus are postulated to have anti-tumour proliferation properties (Wei, Jian & Mao, 2011; Xin, Jing & Jian, 2016), whereas the purified sulphated polysaccharides from Pattalus mollis have an anticoagulant effect (Zheng et al., 2019). Sea cucumber viscera contain approximately4.9% crude polysaccharides, from which sulphated polysaccharides can be extracted (Yang et al., 2020). Sulphated polysaccharides have a variety of beneficial biological properties, including anticoagulant, antiviral, antioxidant, anticancer, and immuno-inflammatory properties, making them suitable for nutraceutical, cosmeceutical, and pharmaceutical applications (Wijesekara, Pangestuti & Kim, 2011; Jiao et al., 2011; Zhu et al., 2018).
Novel saponins
Sea cucumber viscera are abundant in saponins—secondary metabolites that influence metabolism and enhance the immune system by reducing cholesterol and exhibiting anti-cancer (Shi et al., 2004) and anti-bacterial properties (Sumarto & Karnila, 2022). Recent studies revealed that viscera contain higher saponin content than their body walls (Zhang et al., 2018). Novel saponins with potent fungicidal, antioxidant, anti-viral, and anti-cancer effects have been identified and purified from the viscera of Holothuria scabra, Holothuria lessoni and Apostichopus japonicus, making them promising candidates for cosmeceutical, medical, and pharmaceutical applications (Bahrami, Zhang & Franco, 2014; Zhang et al., 2018; Sumarto & Karnila, 2022).
Novel source of nutrients
Numerous nutrients, including oligosaccharides, phenols, flavonoids, and trace metals, have been found in the viscera of sea cucumbers (Zhang & Chang, 2014). the reported proximate composition of sea cucumber viscera may differ among studies (Table 1) due to species, sample preparation techniques, feed, and environmental factors such as habitat and climate. Sea cucumber viscera contain low lipid but high protein contents. He et al. (2017) demonstrated that protein hydrolysate from sea cucumber viscera had high antifatigue and antioxidant activity. Additionally, due to their high profile of essential amino acids, sea cucumber protein hydrolysate may be a suitable dietary protein source (Senadheera, Dave & Shahidi, 2021).
Babji et al. (2020) reported that sea cucumber viscera contain high levels of vitamins. The consumption of a relatively small amount of sea cucumber viscera may satisfy the nutritional needs for several vitamins such as vitamins A, B, C, and E in both animal and human diets, which support the immune system, strengthen bones, heal wounds, turn food into energy, and repair cellular damage. The vitamin content in sea cucumber viscera is shown in Table 5. According to Table 5, vitamin B3 (nicotinic acid) is the most abundant vitamin in the viscera of both C. frondosa (Mamelona, Louis & Pelletier, 2010) and undetermined species (Babji et al., 2020).
Table 5. Vitamin contents in sea cucumber viscera (mg/kg).
| Parameter | Species | |
|---|---|---|
| Undetermined species | Cucumaria frondosa | |
| Vitamin B1 (Thiamine) | 80 | 0.439 |
| Vitamin B2 (Riboflavin) | 458 | 1.081 |
| Vitamin B3 (Nicotinic acid) | 947 | 6.704 |
| Vitamin B6 (Pyridoxine) | 60 | – |
| Vitamin B5 (Pantothenic acid) | 170 | 3.157 |
| Vitamin B7 (Biotin) | 0.2 | – |
| Vitamin B9 (Folic acid) | 0.107 | 0.193 |
| Vitamin B12 (Cobalamin) | 1.222 | – |
| Vitamin C | 55 | – |
| Vitamin E as a-tocopherol | 1.4 | 1.947 |
| Vitamin A as b-carotene | 23 | – |
| Vitamin A as retinol | – | 0.015 |
| Vitamin D | 0.037 | – |
| Vitamin K | 1.2 | – |
| References | Babji et al. (2020) | Mamelona, Louis & Pelletier (2010) |
Note:
–, Not determine.
Furthermore, sea cucumber viscera contain a significant number of essential minerals (e.g., Cu, Fe, Zn, K, Na, Mn, As, Mg, Se, Ni, and Ca) that play important roles in maintaining general biological systems, along with relatively small amounts of nonessential trace elements (Cd, Co, and Pb) (Mamelona, Louis & Pelletier, 2010). The mineral composition of sea cucumber viscera is shown in Table 4. Babji et al. (2020) reported that sodium is the highest element content in sea cucumber viscera, approximately 338.3 g/100 g, while potassium was found to be the most abundant element in Cucumaria frondosa, at approximately 1.870 g/100 g, as reported by Mamelona, Louis & Pelletier (2010), and 0.200 g/100 g, as reported by Ramalho et al. (2020). Potassium is a micromineral that regulates the activity of blood cells and muscles, especially the heart muscle, maintains fluid balance in the body, regulates blood pressure, and acts as an enzyme activator (Susanti, Sukmawardani & Musfiroh, 2016).
Sea cucumber viscera are rich in fatty acids, especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are essential micronutrients with beneficial health impacts (Shepon et al., 2022). In addition, Mamelona, Louis & Pelletier (2010) reported that sea cucumber viscera are abundant in essential amino acids, which play a crucial role in metabolic pathway regulation and are crucial components in growth, development, and reproduction. The fatty acid and amino acid profiles of sea cucumber viscera reported by several studies are shown in Tables 2 and 3, respectively. Liu et al. (2021) highlighted that EPA was the predominant fatty acid, constituting 27.76% of the total fatty acids found in the viscera of Cucumaria frondosa. This percentage is significantly higher than that of crude oil from other marine byproducts, which typically contain only 4.63–9.54% EPA. EPA, as an essential omega-3 fatty acid, has been linked to numerous positive health effects, including decreased cardiovascular risk, stimulation of foetal development, and improved cognitive function (Swanson, Block & Mousa, 2012). Therefore, sea cucumber viscera can be valorised to produce omega-3 PUFAs (especially EPA) and EPA-enriched nutritional products. Additionally, approximately 25.93% (Liu et al., 2021) and 14.30% (Mamelona, Louis & Pelletier, 2010) of the total amino acids in the viscera of Cucumaria frondosa are composed of glutamine, which is essential for cellular metabolic activities and animal immunity (Binod et al., 2017; Shah, Wang & Ma, 2020). The nutritional content of sea cucumber viscera and the richness of their metabolites indicates their enormous potential for being transformed into high-value products.
Due to their high nutritional value sea cucumber viscera have traditionally been consumed both raw and processed in some countries. In Samoa, Stichopus horrens is the most sought-after species, as the viscera are consumed raw and commercially sold in local markets, while the body parts are returned to the sea alive (Eriksson et al., 2007; Charan-Dixon et al., 2019). In addition, salt-fermented sea cucumber viscera, known as konowata, is one of the three major Japanese delicacies that are commercially consumed in Japan (Nishanthan et al., 2021). Their edibility and nutritional value indicate their potential to be consumed as therapeutic foods.
Future directions
Valorisation of sea cucumber viscera into value-added products
Sea cucumber viscera contain sulphated polysaccharides (e.g., sulphate, fucose, galactosamine) that can be used in pharmacological activities, such as improving gut health, antiviral mechanisms and wound healing (Cao, Surayot & You, 2017; Li et al., 2021). Conventional extraction methods, such as chemical methods can be applied to extract sulphated polysaccharides from sea cucumber viscera. However, the alkaline conditions during the chemical extraction process can influence the conformation of sulphated polysaccharides, leading to potential degradation and desulphation of the polysaccharides. Consequently, this may affect the physicochemical and biological properties of the polysaccharides (Li et al., 2021).
Due to the limitations of conventional extraction methods, microwave-assisted extraction has emerged as a promising technology to minimize degradation and desulphation during the extraction of sulphated polysaccharides from sea cucumber viscera. The use of microwave heating can lead to a reduction in the use of solvents, thereby lowering operating costs. Moreover, microwave radiation provides rapid heating, which, in turn, reduces energy consumption during extraction (Wan Mahari et al., 2022). The microwave radiation generated during heat activation can contribute to cell wall lysis and break the protein bonds to release the cellular contents and polysaccharides into the extraction medium. However, further studies are needed to optimize the operating conditions (e.g., microwave power, duration) during microwave-assisted extraction to enhance the yield and properties of sulphated polysaccharides.
Enzymatic hydrolysis is another emerging technology that can be applied to improve the extraction of sulphated polysaccharides (Wang et al., 2022). The types of proteases (e.g., papain, pancreatin) and duration of hydrolysis are important factors that influence the release of polysaccharides from sea cucumber viscera and their biological properties. Previous study reported that protein hydrolysates produced by pancreatin exhibited greater antioxidant activity compared to that produced by papain (Karamac, Kosińska-Cagnazzo & Kulczyk, 2016). Therefore, further studies should be scrutinized to develop an extraction method that can enhance the production of sulfated polysaccharides with desirable physicochemical and biological properties.
Potential cosmetic ingredients from sea cucumber viscera
The abundance of essential active compounds found in sea cucumber viscera extracts highlights their high potential for use in the cosmeceutical field. Studies have reported that sea cucumber (S. japonicus) viscera extracts promote the expression of tyrosinase, tyrosinase-related protein (TRP-1 and TRP-2), and microphthalmia-associated transcription factor (MITF) protein levels, as well as extracellular-regulated kinase (ERK) activation. These elements are known to be effective in skin whitening and anti-ageing treatments by reducing melanin production and increasing collagen synthesis through ERK signalling (Kwon et al., 2018).
Potential as animal nutrition supplements and feed additives
In aquaculture, good nutrition is essential for the optimal growth and production of high-quality products, and the feed component typically represents approximately 60–80% of the production cost (Ragasa, Osei-Mensah & Amewu, 2022). Feed additives are products used to enhance nutrients and reduce production costs. Functional feed additives in animal food stimulate growth, promote good health, boost the immune system, and provide physiological advantages over conventional feeds (Alemayehu, Geremew & Getahun, 2018).
Sea cucumber viscera are a high-value source of nutrients and bioactive compounds that can be used as functional feed additives in the aquaculture industry. Babji et al. (2020) successfully converted sea cucumber viscera into prospective health supplement products for the agro-based food and health industries using an enzymatic hydrolysis method. This review demonstrates the potential of turning waste materials into high-value products, which not only increases the market value of sea cucumber viscera but also helps reduce waste that could otherwise pollute the environment.
Potential as sex reversal agent for aquatic animal
The males of many species of ornamental fish have highly pigmented bodies and usually more developed fins, making them preferred over female fish by hobbyists (Piferrer & Lim, 1997). Due to the higher demand for these male fish, the use of sex reversal technology for commercial production of an all-male population of these aquatic animals could significantly increase the economic benefits of this type of aquaculture operation. The hormones used for sex reversal to produce all-male fish are testosterone, 17-α-methyltestosterone, and androstenedione. In aquaculture, sex reversal can be triggered by using steroid hormones either through immersion, injection, or oral administration by feeding. However, synthetic steroids have a negative impact on the environment and the fish itself (Emilda, 2015). Therefore, it is essential to explore natural steroid sources that are safe for both humans and the environment.
Testosterone, the steroid hormone used in sex reversal, is naturally produced in sea cucumbers. The potential utilization of sea cucumber extract as a natural source of testosterone is promising. However, the optimal extraction method has yet to be determined. Recently, studies have been conducted on sex reversal in aquatic animals using steroid extracts from sea cucumber viscera. Emilda (2015) and Saputra et al. (2018) reported that using the immersion technique to administer steroid extracts from sea cucumber viscera can enhance the percentage of male guppies by 65.13% and 88.9%, respectively. Riani, Sudrajat & Triajie (2010) reported that injecting testosterone hormone from sea cucumber viscera into giant freshwater prawns can produce 63.33% male prawns. The hormone is also effective in influencing zygotes and larvae to develop into male prawns without negatively impacting motility, fecundity, and hatching rates. This research provides crucial baseline information, indicating that sea cucumber viscera can serve as a natural steroid source for sex reversal in aquatic organisms.
Promising neuroprotective agent in debilitating central nervous system disorders
Triterpene glycoside or saponins are natural bioactive compounds found in sea cucumbers (Bahrami, Zhang & Franco, 2018). Numerous studies have demonstrated that the medicinal and health benefits of sea cucumbers are attributed to the presence of saponins, which are the most significant and primary secondary metabolites found in sea cucumbers (Zhao et al., 2018). Saponins have neuroprotective properties against a number of central nervous system disorders (Mitu et al., 2017).
Kleawyothatis et al. (2022) reported that the whole body (including viscera) and body wall extracts of sea cucumber (H. scabra) contain triterpene glycosides or saponins. These extracts have been shown to provide a neuroprotective effect against central nervous system disorders, specifically Alzheimer, in Caenorhabditis elegans models. The observed decrease in amyloid-β deposition and aggregation, along with the reduction in reactive oxygen species, resulted in lifespan extension of C. elegans Alzheimer’s disease models. These findings strongly suggest that sea cucumber extracts possess natural preventive and therapeutic properties for Alzheimer’s disease. Meanwhile, Bahrami, Zhang & Franco (2018) mentioned that sea cucumber viscera had substantially greater relative levels of saponins than the body and also contained some saponin congners. Although saponins are known to have significant neuroprotective properties (Mitu et al., 2017), further studies are needed to validate the role of saponins found in sea cucumber viscera in preventing and treating debilitating central nervous system disorders.
Conclusions
Sea cucumber viscera are excellent sources of nutrients and bioactive compounds, including saponins, sulphated polysaccharides, amino acids, fatty acids (especially EPA and DHA), vitamins, and minerals. These compounds can be developed into potential animal nutritional supplements and feed additives, utilized as sex reversal agents for aquatic animals, and incorporated into various industries, such as food, cosmeceutical, and biopharmaceutical. The potential utilization of sea cucumber viscera as a natural source of nutrients is promising, but the optimal extraction method has not yet been found, hindering their use on an industrial scale. Considering the abundance of various nutrients and bioactive compounds in sea cucumber viscera, further research should be conducted to optimize the extraction process and enable the industrial production of high-value sea cucumber viscera and support the development of sea cucumber aquaculture technology.
Supplemental Information
Funding Statement
This work was supported by the University Malaysia Terengganu and Hasanuddin University, Indonesia under the International Partnership Research Grant (UMT/IPRG/2021/55300). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yushinta Fujaya, Email: yushinta.fujaya@unhas.ac.id.
Khor Waiho, Email: waihokhor@gmail.com.
Additional Information and Declarations
Competing Interests
Khor Waiho is an Academic Editor for PeerJ.
Author Contributions
Muhammad Fatratullah Muhsin conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Yushinta Fujaya conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Andi Aliah Hidayani performed the experiments, prepared figures and/or tables, and approved the final draft.
Hanafiah Fazhan performed the experiments, prepared figures and/or tables, and approved the final draft.
Wan Adibah Wan Mahari performed the experiments, prepared figures and/or tables, and approved the final draft.
Su Shiung Lam performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Alexander Chong Shu-Chien analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Youji Wang analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Nor Afiqah-Aleng analyzed the data, prepared figures and/or tables, and approved the final draft.
Nita Rukminasari analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Khor Waiho conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review and hence did not utilize raw data.
References
- Abuzaytoun et al. (2022).Abuzaytoun R, Budge SM, Xia W, MacKinnon S. Unusual ether lipids and branched chain fatty acids in sea cucumber (Cucumaria frondose) viscera and their seasonal variation. Marine Drugs. 2022;20(7):435. doi: 10.3390/md20070435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achmad et al. (2020).Achmad H, Aflanie I, Putra AP, Noor F, Carmelita AB, Fauziah, Sukamna BI, Huldani An overview of the potential of sea cucumbers with antioxidant and antiviral contents as nutritional supplements. Systematic Review Pharmacy. 2020;11(6):761–770. doi: 10.31838/srp.2020.6.112. [DOI] [Google Scholar]
- Al Azad, Shaleh & Siddiquee (2017).Al Azad S, Shaleh SRM, Siddiquee S. Comparison of fatty acid and proximate composition between Holothuria edulis and Holothuria scabra collected from coastal water of Sabah, Malaysia. Advances in Bioscience and Biotechnology. 2017;8:91–103. doi: 10.4236/abb.2017.83007. [DOI] [Google Scholar]
- Al-Rashdi, Eechaut & Claereboudt (2012).Al-Rashdi KM, Eechaut I, Claereboudt MR. A manual on hatchery of sea cucumber Holothuria scabra in the sultanate of Oman. 2012. https://www.researchgate.net/publication/269808822_A_Manual_on_Hatchery_of_Sea_Cucumber_Holothuria_scabra_in_the_Sultanate_of_Oman p. 27.https://www.researchgate.net/publication/269808822_A_Manual_on_Hatchery_of_Sea_Cucumber_Holothuria_scabra_in_the_Sultanate_of_Oman Ministry of Agriculture and Fisheries Wealth, Aquaculture Centre, Muscat, Sultanate of Oman.
- Alemayehu, Geremew & Getahun (2018).Alemayehu TA, Geremew A, Getahun A. The role of functional feed additives in tilapia nutrition. Fisheries and Aquaculture Journal. 2018;9(2):1–6. doi: 10.4172/2150-3508.1000249. [DOI] [Google Scholar]
- Altamirano & Rodriguez (2022).Altamirano JP, Rodriguez JCJ. Hatchery production of sea cucumbers (sandfish Holothuria scabra) Tigbauan, Iloilo, Philippines: Aquaculture Department, Southeast Asian Fisheries Development Center; 2022. [Google Scholar]
- Andriamanamisata & Telesphore (2019).Andriamanamisata VLR, Telesphore AF. The nutritional values of two species of sea cucumbers (Holothuria scabra and Holothuria lessoni) from Managascas. African Journal of Food Science. 2019;13(11):281–286. doi: 10.5897/AJFS2019.1816. [DOI] [Google Scholar]
- Anisuzzaman et al. (2019).Anisuzzaman M, Jin F, Kabery K, Jeong U, Jung H, Lee S, Kang S. Lipid class and fatty acid compositions of dried sea cucumber Apostichopus japonicus. The Open Food Science Journal. 2019;11:79–86. doi: 10.2174/1874256401911010079. [DOI] [Google Scholar]
- Ardiansyah et al. (2020).Ardiansyah A, Rasyid A, Siahaan EA, Pangestuti R, Murniasih T. Nutritional value and heavy metals content of sea cucumber Holothuria scabra commercially harvested in Indonesia. Current Research in Nutrition and Food Science. 2020;8(3):765–773. doi: 10.12944/CRNFSJ.8.3.09. [DOI] [Google Scholar]
- Asha & Muthiah (2005).Asha PS, Muthiah P. Effects of temperature, salinity, and pH on larval growth, survival and development of sea cucumber Holothuria spinifera Theel. Aquaculture. 2005;250(3–4):823–829. doi: 10.1016/j.aquaculture.2005.04.075. [DOI] [Google Scholar]
- Ayangbenro & Babalola (2017).Ayangbenro AS, Babalola OO. A new strategy for heavy metal polluted environments: a review of microbial biosorbents. International Journal of Environmental Research and Public Health. 2017;14(1):94. doi: 10.3390/ijerph14010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin (2018).Aydin M. Biometry, density and the biomass of the commercial sea cucumber population of the Aegean Sea. Turkish Journal of Fisheries and Aquatic Sciences. 2018;19(6):463–474. doi: 10.4194/1303-2712-v19_6_02. [DOI] [Google Scholar]
- Aydin et al. (2011).Aydin M, Sevgili H, Turan B, Emre Y, Kose S. Proximate composition and fatty acid profile of three different fresh and dried commercial sea cucumber from Turkey. International Journal of Food Sciences and Technology. 2011;46(3):500–508. doi: 10.1111/j.1365-2621.2010.02512.x. [DOI] [Google Scholar]
- Babji et al. (2020).Babji AS, Lim SJ, Aliah D, Nadia MN, Chan CH, Tey CC. Sea cucumber viscera hydrolysate as a potential animal feed supplement. Conference Series: Earth and Environmental Science. 2020;478:1–7. doi: 10.1088/1755-1315/478/1/012067. [DOI] [Google Scholar]
- Bahrami, Zhang & Franco (2014).Bahrami Y, Zhang W, Franco CMM. Discovery of novel saponins from the viscera of the sea cucumber Holothuria lessoni. Marine Drugs. 2014;12(5):2633–2667. doi: 10.3390/md12052633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami, Zhang & Franco (2018).Bahrami Y, Zhang W, Franco CMM. Distribution of saponins in the sea cucumber Holothuria lessoni; the body wall versus the viscera, and their biological activities. Marine Drugs. 2018;16(11):423. doi: 10.3390/md16110423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzkar, Fariman & Taheri (2017).Barzkar N, Fariman GA, Taheri A. Proximate composition and mineral contents in the body wall of two species of sea cucumber from Oman Sea. Environment Science Pollution Research. 2017;24(23):18907–18911. doi: 10.1007/s11356-017-9379-5. [DOI] [PubMed] [Google Scholar]
- Bechtel, Oliveira & Smiley (2012).Bechtel PJ, Oliveira ACM, Smiley NDS. Chemical composition of the giant red sea cucumber, Parastichopus californicus, commercially harvested in Alaska. Food Science & Nutrition. 2012;1(1):63–73. doi: 10.1002/fsn3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel et al. (2013).Bechtel PJ, Oliveira ACM, Demir N, Smiley S. Chemical composition of the giant red sea cucumber, Parastichopus californicus, commercially harvested in Alaska. Food Science & Nutrition. 2013;1(1):63–73. doi: 10.1002/fsn3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hasan et al. (2021).Ben-Hasan A, Mitcheson YS, Cisneros-Mata MA, Jimenez EA, Daliri M, Cisneros-Montemayor AA, Nair RJ, Thankappan SA, Walters CJ, Christensen V. China’s fish maw demand and its implications for fisheries in source countries. Marine Policy. 2021;132:104696. doi: 10.1016/j.marpol.2021.104696. [DOI] [Google Scholar]
- Binod et al. (2017).Binod P, Sindhu R, Madhavan A, Abraham A, Mathew AK, Beevi US, Sukumaran RK, Singh SP, Pandey A. Recent developments in lglutaminase production and applications—an overview. Bioresource Technology. 2017;245:1766–1774. doi: 10.1016/j.biortech.2017.05.059. [DOI] [PubMed] [Google Scholar]
- Bordbar, Anwar & Saari (2011).Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumber for functional foods. Marine Drugs. 2011;9(10):1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Surayot & You (2017).Cao R-A, Surayot U, You S. Structural characterization of immunostimulating protein-sulfated fucan complex extracted from the body wall of a sea cucumber, Stichopus japonicus. International Journal of Biological Macromolecules. 2017;99:539–548. doi: 10.1016/j.ijbiomac.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Careaga, Muniain & Maier (2013).Careaga VP, Muniain C, Maier MS. Fatty acid composition of the edible sea cucumber Athyonidium chilensis. Natural Product Research. 2013;27(7):634–646. doi: 10.1080/14786419.2012.686909. [DOI] [PubMed] [Google Scholar]
- Carleton et al. (2013).Carleton C, Hambrey J, Govan H, Medley P, Kinch J. Effective management of sea cucumbers fisheries and the beche-de-mer trade in Melanesia. SPC Fisheries Newsletter. 2013;140:24–42. [Google Scholar]
- Chang-Lee, Price & Lampila (1989).Chang-Lee MV, Price RJ, Lampila LE. Effect of processing on proximate composition and mineral content of sea cucumbers (Parastichopus spp.) Journal of Food Science. 1989;54:567–568. doi: 10.1111/j.1365-2621.1989.tb04653.x. [DOI] [Google Scholar]
- Charan-Dixon et al. (2019).Charan-Dixon H, Goldstien SJ, Vanderhaven BJ, Halafihi T, Tuiano TL, Gaw S, Glover CN. Effects of traditional fishing techniques on internal organ regeneration, physiology, and biochemistry in tropical sea cucumber Stichopus horrens. Journal of Experimental Marin Biology and Ecology. 2019;510:15–22. doi: 10.1016/j.jembe.2018.09.007. [DOI] [Google Scholar]
- Conand (1990).Conand C. The fishery resources of pacific island countries. 1990. p. 143. Part 2: Holothurians; FAO Fisheries Technical Paper 272.2; Food and Agriculture Organization of the United Nations: Rome, Italy.
- Dong et al. (2011).Dong Y, Yu A, Wang Q, Dong S. Physiological responses in a variable environment: relationships between metabolism, hsp, and thermotolerance in an intertidal-subtidal species. PLOS ONE. 2011;6(10):e26446. doi: 10.1371/journal.pone.0026446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doungpai et al. (2022).Doungpai S, Siriwoharn T, Malila Y, Autsavapromporn N, Makkhun S, Yarnpakdee S, Jantanasakulwong K, Regenstein JM, Wangtueai S. Uv-b protective and antioxidant activities of protein hydrolysate from sea cucucmber (Holothuria scabra) using enzymatic hydrolysis. Fronttiers in Marine Science. 2022;9:892255. doi: 10.3389/fmars.2022.892255. [DOI] [Google Scholar]
- Elvevoll et al. (2022).Elvevoll EO, James D, Toppe J, Gamarro EG, Jensen I. Food safety risks posed by heavy metals and persistent organic pollutants (POPs) related to consumption of sea cucumbers. Foods. 2022;11(24):3992. doi: 10.3390/foods11243992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilda (2015).Emilda E. Utilization of steroid extract from sea cucumber viscera for sex reversal in gapi fish. Faktor Exacta. 2015;5(4):336–349. [Google Scholar]
- Eriksson et al. (2007).Eriksson H, Friedman K, Solofa A, Mulipola AT. A pilot study to investigate the survival of Stichopus horrens after viscera harvest in Samoa. SPC Beche de Mer Information Bulletin. 2007;26:2–4. [Google Scholar]
- Friedman et al. (2008).Friedman K, Purcell S, Bell J, Hair C. Sea cucumber fisheries: a manager’s toolbox. ACIAR Monograph. 2008;135:32. [Google Scholar]
- Gao et al. (2011).Gao QF, Wang Y, Dong S, Sun Z, Wang F. Absorption of different food sources by sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea): evidence from carbon stable isotope. Aquaculture. 2011;319:272–276. doi: 10.1016/j.aquaculture.2011.06.051. [DOI] [Google Scholar]
- Gocer, Olgunoglu & Olgunoglu (2018).Gocer M, Olgunoglu IA, Olgunoglu MP. A study on fatty acid profile and some major mineral contents of sea cucumber (Holothuria (platyperona) sanctori) from Mediterranean Sea (Turkey) Food Science and Quality Management. 2018;72 [Google Scholar]
- Gonzales-Duran et al. (2021).Gonzales-Duran E, Hernandez-Flores A, Headley MD, Canul JD. On the effects of temperature and pH on tropical and temperate holothurians. Conservation Physiology. 2021;9(1):coab092. doi: 10.1093/conphys/coab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales-Wanguemert et al. (2018).Gonzales-Wanguemert M, Roggatz CC, Rodrigues MJ, Barreira L, da silva MM, Custodio L. A new insight into the influence of habitat on the biochemical properties of three commercial sea cucumber species. International Aquatic Research. 2018;10:361–373. doi: 10.1007/s40071-018-0210-9. [DOI] [Google Scholar]
- Guo et al. (2015).Guo Y, Ding Y, Xu F, Liu B, Kou Z, Xiao W, Zhu J. Systems pharmacology-based drug discovery for marine resources: an example using sea cucumber (Holothurians) Journal of Ethnopharmacology. 2015;165:61–72. doi: 10.1016/j.jep.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Haider et al. (2015).Haider M, Sultana R, Jamil K, Tarat O, Afzal W. A study on proximate composition, amino acid profile, fatty acid profile, and some mineral contents in two species of sea cucumber. Journal of Animal and Plant Sciences. 2015;25(1):168–175. [Google Scholar]
- Hair et al. (2019).Hair C, Foale S, Kinch J, Frijlink S, Lindsay D, Southgate PC. Socioeconomic impacts of sea cucumber fishery in Papua New Guinea: is there an opportunity for mariculture? Ocean and Coastal Management. 2019;179:104826. doi: 10.1016/j.ocecoaman.2019.104826. [DOI] [Google Scholar]
- Han, Keesing & Liu (2016).Han Q, Keesing JK, Liu D. A review of sea cucumber aquaculture, ranching, and stock enhancement in China. Reviews in Fisheries Science & Aquaculture. 2016;24(4):326–341. doi: 10.1080/23308249.2016.1193472. [DOI] [Google Scholar]
- Hasan (2019).Hasan MH. Destruction of sea cucumber populations due to overfishing at Abu Shosoun area, Red Sea. The Journal of Basic and Applied Zoology. 2019;80:5. doi: 10.1186/s41936-019-0074-6. [DOI] [Google Scholar]
- He et al. (2017).He C, Wei H, Xiong H, Wu G, Wu J. Antioxidant and antifatigue activities of enzymatic protein hydrolysate from sea cucumber viscera. Journal of Food Science. 2017;38(21):201–206. doi: 10.7506/spkx1002-6630-201721032. [DOI] [Google Scholar]
- Hossain et al. (2022).Hossain Q, Yeo J, Dave D, Shahidi F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by-high-pressure processing (HPP) Antioxidants. 2022;11(2):337. doi: 10.3390/antiox11020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiram, Mohammed & Rao (2015).Janakiram NB, Mohammed A, Rao CV. Sea cucumbers metabolites as potent anti-cancer agents. Marine Drugs. 2015;13(5):2909–2923. doi: 10.3390/md13052909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao et al. (2011).Jiao G, Yu G, Zhang J, Ewart HS. Chemical structure and bioactivities of sulfated polysaccharides from marine algae. Marine Drugs. 2011;9(2):196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun (2009).Jun A. Challenging boom and bust market pressures: development of self-managed sea cucumber conservation in Rishiri Island, Hokkaido, Japan. Biosphere Conservation. 2009;9(2) doi: 10.20798/biospherecons.9.2_1. [DOI] [Google Scholar]
- Kamaruddin & Rehan (2015).Kamaruddin KR, Rehan MM. Morphological and molecular identification of Holothuria (merthensiothuria) leucospilota and Stichopus horrens from Pangkor Island, Malaysia. Tropical Life Science Research. 2015;26(1):87–99. [PMC free article] [PubMed] [Google Scholar]
- Karamac, Kosińska-Cagnazzo & Kulczyk (2016).Karamac M, Kosińska-Cagnazzo A, Kulczyk A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. International Journal of Molecular Sciences. 2016;17(7):1027. doi: 10.3390/ijms17071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya et al. (2004).Kariya Y, Mulloy B, Imai K, Tominaga A, Kaneko T, Asari A, Suzuki K, Masuda H, Kyogashima M, Ishii T. Isolation and partial characterization of fucan sulfates from the body wall of sea cucumber Stichopus japonicus and their ability to inhibit osteoclastogenesis. Carbohydrate Research. 2004;339(7):1339–1346. doi: 10.1016/j.carres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Kashenko (2000).Kashenko SD. Acclimation of the sea cucumber Apostichopus japonicus do decreased salinity at the blastula and gastrula stages: its effect on the desalination resistance of larvae at subsequent stages of development. Russian Journal of Marine Biology. 2000;26(6):422–426. doi: 10.1023/A:1009446705230. [DOI] [Google Scholar]
- Kim & Kim (2014).Kim J, Kim KH. Processing and characterization of salt-fermented fish (Jeotgal) using seafood by-products in Korea. In: Kim S, editor. Introduction to Seafood by-Products. Busan: Springer; 2014. [Google Scholar]
- Kinch et al. (2007).Kinch J, James M, Thomas E, Lauhi P, Gabiobu R. Socio-economic assessment of the Beche-de-mer- fishesies in the Western, Central and Manus Provinces, Papua New Guinea. 2007. p. 132. Report to the national fisheries authority, Port Moresby, Papua New Guinea.
- Kleawyothatis et al. (2022).Kleawyothatis W, Jattujan P, Chumphoochai K, Chalorak P, Sobhon P, Meemon K. Holothuria scabra extracts confer neuroprotective effect in C. elegans model of Alzheimer’s disease by attenuating amyloid-β aggregation and toxicity. Journal of Traditional and Complementary Medicine. 2022;13(1):93–104. doi: 10.1016/j/jtcme.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumara et al. (2013).Kumara P, Jayanatha J, Pushpakumara J, Bandara W, Dissanayake DCT. Artificial breeding and larval rearing of three tropical sea cucumber species-Holothuria scabra, Pseudocolochirus violaceus and Colochirus quadrangularis-in Sri Lanka. SPC Beche-de-Mer Infformation Bulletin. 2013;33:30–37. [Google Scholar]
- Kunili & Colakoglu (2019).Kunili IE, Colakoglu FA. Chemical and nutritional characteristics of Holothuria tubulosa (Gmelin, 1788); a seasonally comparative study. Journal of Aquatic Food Product Technology. 2019;28(7):716–728. doi: 10.1080/10498850.2019.1637383. [DOI] [Google Scholar]
- Kwon et al. (2018).Kwon T, Oh CT, Bak D, Kim JH, Seok J, Lee JH, Lim SH, Yoo KH, Kim BJ, Kim H. Effects on skin of Stichopus japonicus viscera extracts detected with saponin including Holothurin A: down-regulator of melanin synthesis and up-regulator of neocollagenesis mediated by ERK signaling pathway. Journal of Ethnopharmacology. 2018;226:73–81. doi: 10.1016/j.jep.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2012).Lee M, Kim Y, Moon HS, Kim K, Kim G, Cho H, Yoon NY, Sim KB, Park H, Lee D, Lim C, Yoon H, Han S. Comparison on proximate composition and nutritional profile of red and black sea cucumber (Apostichopus japonicus) from Ulleungdo (Island) and Dokdo (Island), Korea. Food Science and Biotechnology. 2012;21(5):1285–1291. doi: 10.1007/s10068-012-0169-z. [DOI] [Google Scholar]
- Lewerissa, Uneputty & Waliulu (2021).Lewerissa YA, Uneputty PA, Waliulu AMS. Spatial distribution of sea cucumber and their management effort in seagrass ecosystem in Buntal Island, Western Seram Regency. IOP Conference Series: Earth and Environmental Science. 2021;805:12007. doi: 10.1088/1755-1315/805/1/012007. [DOI] [Google Scholar]
- Li et al. (2021).Li Y, Li M, Xu B, Li Z, Qi Y, Song Z, Zhao Q, Du B, Yang Y. The current status and future perspective in combination of the processing technologies of sulfated polysaccharides from sea cucumbers: a comprehensive review. Journal of Functional Foods. 2021;87:104744. doi: 10.1016/j.jff.2021.104744. [DOI] [Google Scholar]
- Liang et al. (2022).Liang Q, Ahmed F, Zhang M, Sperou N, Franco CMM, Feng Q, Zhang W. In vivo and clinical studies of sea cucumber-derived bioactives for human health and nutrition from 2012–2021. Frontiers in Marine Science. 2022;9:917857. doi: 10.3389/fmars.2022.917857. [DOI] [Google Scholar]
- Liu (2015).Liu J. Chapter 5. Spatial distribution, population structures, management, and conservation. In: Yang H, Hamel JF, Mercier A, editors. The Sea Cucumber Apostichopus Japonicus. Cambridge: History, Biology, and Aquaculture, Academic Press; 2015. pp. 77–86. [Google Scholar]
- Liu et al. (2021).Liu Y, Dave D, Trenholm S, Ramakrisnhnan VV, Murphy W. Effect of drying on nutritional composition of Atlantic Sea cucumber (Cucumaria frondose) viscera derived from newfoundland fisheries. Processes. 2021;9(4):703. doi: 10.3390/pr9040703. [DOI] [Google Scholar]
- Liu, Xue & Li (2022).Liu H, Xue C, Li Z. Diversity, distribution, and biology of sea cucumber. In: Xue C, editor. Advances in Sea Cucumber Processing Technology and Product Development. Advance in Marine Bioprocesses and Bioproducts. Cham: Spinger; 2022. [Google Scholar]
- Liu et al. (2017).Liu F, Zamora L, Jeffs A, Quek SY. Biochemical composition of the Australasian Sea cucumber, Australostichopus mollis, from a nutritional point of view. Nutrire. 2017;42:1–12. doi: 10.1186/s41110-017-0036-z. [DOI] [Google Scholar]
- Liubana, Surbakti & Tubo (2022).Liubana DV, Surbakti JA, Tubo CZ. Cultured sand sea cucumber growth different water exchange system. Indonesian Aquaculture Journal. 2022;21(2):178–185. doi: 10.19027/jai.21.2.178-185. [DOI] [Google Scholar]
- Louw & Burgener (2020).Louw S, Burgener M. A rapid assessment of the sea cucumber trade from Africa to Asia. 2020. https://www.traffic.org/site/assets/files/13496/sea-cucumbers-trade-vfinal.pdf p. 20.https://www.traffic.org/site/assets/files/13496/sea-cucumbers-trade-vfinal.pdf TRAFFIC International, Cambridge, United Kingdom.
- Lu et al. (2010).Lu Y, Zhang BY, Dong Q, Wang BL, Sun XB. The effects of Stichopus japonicus acid mucopolysaccharide on the apoptosis of the human hepatocellular carcinoma cell line HepG2. The American Journal of Medical Science. 2010;339(2):141–144. doi: 10.1097/MAJ.0b013e3181c20d01. [DOI] [PubMed] [Google Scholar]
- Mamelona, Louis & Pelletier (2010).Mamelona J, Louis RS, Pelletier E. Proximate composition and nutritional profile of by-products from green urchin and Atlantic Sea cucumber processing plants. Journal of Food Science & Technology. 2010;45(10):2119–2126. doi: 10.1111/j.1365-2621.2010.02381.x. [DOI] [Google Scholar]
- Mitu et al. (2017).Mitu AA, Bose U, Suwansa-ard S, Turner LH, Zhao M, Elizur A, Ogbourne SM, Shaw PN, Cummins SF. Evidence for a saponin biosynthesis pathway in the body wall of the commercially significant sea cucumber Holothuria scabra. Marine Drugs. 2017;15(11):349. doi: 10.3390/md15110349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishanthan et al. (2018).Nishanthan G, Kumara PADA, de Cross MDST, Prasada DVP, Dissanayake DCT. Effect of processing on proximate and fatty acid compositions of six commercial sea cucumber species of Sri Lanka. Journal of Food Science and Technology. 2018;55(5):1933–1941. doi: 10.1007/s13197-018-3111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishanthan et al. (2021).Nishanthan G, Wickramasinghe I, Navaratne SB, Dissanayake DCT. Ready-to-prepare soup mix enriched with sea cucumbers: production, sensory attributes and nutritional composition. Journal of Food Science and Technology. 2021;58(6):2078–2088. doi: 10.1007/s13197-020-04716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiewo et al. (2010).Ochiewo J, Torre-Castro M, Muthama C, Munyi F, Nthuta JM. Socio-economic features of sea cucumber fisheries in Southern Coast of Kenya. Ocean & Coastal Management. 2010;53(4):192–202. doi: 10.1016/j.ocecoaman.2010.01.010. [DOI] [Google Scholar]
- Oedjoe (2017).Oedjoe MDR. Composition of nutritional content of sea cucumbers (Holothuroidea) in Mania Waters, Sabu Raijua Regency, East Nusa Tenggara. Journal of Aquaculture Research and Development. 2017;8(7):1–3. doi: 10.4172/2155-9546.1000502. [DOI] [Google Scholar]
- Oh et al. (2017).Oh G, Ko S, Lee DH, Heo S, Jung W. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries and Aquatic Sciences. 2017;20(28):1–17. doi: 10.1186/s41240-017-0071-y. [DOI] [Google Scholar]
- Oktaviani, Mulyani & Rochima (2015).Oktaviani D, Mulyani Y, Rochima E. Aktivitas antioksidan dan antibakteri ekstrak jeroan teripang Holothuria atra dari perairan Pulau Biawak kabupaten Indramayu [Antioxidant and antibacterial extract of the viscera of sea cucumber Holothuria atra from the waters of Pulau Biawak Indramayu regency] Jurnal Perikanan Kelautan. 2015;4(2):1–6. [Google Scholar]
- Omran (2013).Omran NESES. Nutritional value of some Egyptian sea cucumbers. African Journal of Biotechnology. 2013;12(35):5466–5472. doi: 10.5897/AJB2013.13020. [DOI] [Google Scholar]
- Page et al. (2021).Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoofmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whitinf P, Moher D. The PRISMA, 2020 statement: an updated guideline for reporting systematic reviews. Systematic Reviews. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangestuti & Arifin (2018).Pangestuti R, Arifin Z. Medicinal and health benefit effects of functional sea cucumbers. Journal of Traditional and Complementary Medicine. 2018;8(3):341–351. doi: 10.1016/j.jtcme.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrat et al. (2022).Pierrat J, Bedier A, Eecvkhaut I, Magalon H, Frouin P. Sophistication in a seemingly simple creature: a review of wild holothurian nutrition in marine ecosystems. Biological Reviews. 2022;97(1):273–298. doi: 10.1111/brv.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piferrer & Lim (1997).Piferrer F, Lim LC. Application of sex reversal technology in ornamental fish culture. Aquarium Sciences and Conservation. 1997;1(2):113–118. doi: 10.1023/A:1018391702814. [DOI] [Google Scholar]
- Plaganyi et al. (2020).Plaganyi EE, Murphy N, Skewes T, Dutra LXC, Dowling N, Fischer M. Development of a data-poor harvest strategy for a sea cucumber fishery. Fisheries Research. 2020;230:105635. doi: 10.1016/j.fishres.2020.105635. [DOI] [Google Scholar]
- Prasada (2020).Prasada DVP. Assessing the potential for closure rules in the Sri Lanka sea cucumber fishery: empirical models of practices and preferences of fisheries. Marine Policy. 2020;120(3):104130. doi: 10.1016/j.marpol.2020.104130. [DOI] [Google Scholar]
- Purcell et al. (2016a).Purcell SW, Conand C, Uthicke S, Byrne M. Ecological roles of exploited sea cucumbers. Oceanography and Marine Biology: an Annual Review. 2016a;54:367–386. doi: 10.1201/9781315368597-8. [DOI] [Google Scholar]
- Purcell, Williamson & Ngaluafe (2018).Purcell SW, Williamson DH, Ngaluafe P. Chinese market prices of beche-de-mer: implications for fisheries and aquaculture. Marine Policy. 2018;91:58–65. doi: 10.1016/j.marpol.2018.02.005. [DOI] [Google Scholar]
- Ragasa, Osei-Mensah & Amewu (2022).Ragasa C, Osei-Mensah YO, Amewu S. Impact of fish feed formulation training on feed use and farmer’s income: evidence from Ghana. Aquaculture. 2022;558:738378. doi: 10.1016/j.aquaculture.2022.738378. [DOI] [Google Scholar]
- Rahantoknam (2015).Rahantoknam SPT. Maturity gonad sea cucumber Holothuria scabra under the month cycle. IOP Conference Series: Earth and Environment Science. 2015;89:1–5. doi: 10.1088/1755-1315/89/1/012015. [DOI] [Google Scholar]
- Rahman & Yusoff (2017).Rahman MA, Yusoff FM. Sea cucumber fisheries: market potential, trace, utilization and challenges for expanding the production in the Southeast Asia. International Journal of Advances in Chemical Engineering & Biological Sciences. 2017;4(1):26–30. doi: 10.15242/IJACEBS.ER0117033. [DOI] [Google Scholar]
- Ram et al. (2017).Ram R, Chand RV, Forrest A, Southgate PC. Effect of processing method on quality, texture, collagen and amino acids composition of sandfish (Holothuria scabra) LWT—Food Science and Technology. 2017;86:261–269. doi: 10.1016/j.lwt.2017.08.003. [DOI] [Google Scholar]
- Ramalho et al. (2020).Ramalho A, Leblanc N, Fortin M, Marette A, Tchernof A, Jacques H. Characterization of a coproduct from the sea cucumber Cucumaria frondosa and its effects on visceral adipocyte size in male wistar rats. Marine drugs. 2020;18(11):530. doi: 10.3390/md18110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasyid (2017).Rasyid A. Nutritional value and heavy metals contents of the dried sea cucumber Stichopus vastus from Salemo Island, Indonesia. Journal of Science and Tropical Marine Technology. 2017;9(2):739–746. doi: 10.29244/jitkt.v9i2.19306. [DOI] [Google Scholar]
- Rasyid et al. (2020).Rasyid A, Murniasih T, Putra MY, Pangestuti R, Harahap IA, Untari F, Sembiring SBM. Evaluation of nutritional value of sea cucumber Holothuria scabra cultured in Bali, Indonesia. AACL Bioflux. 2020;13(4):2083–2093. [Google Scholar]
- Riani, Sudrajat & Triajie (2010).Riani E, Sudrajat AO, Triajie H. Effectiveness of sea cucumber extract that has been formulated to prawn masculinize. Bionatura-Journal of Life and Physical Sciences. 2010;12(3):142–152. [Google Scholar]
- Ridhowati et al. (2018).Ridhowati S, Chasanah E, Syah D, Zakaria F. A study on the nutrient substances of sea cucumber Stichopus variegatus flour using vacuum oven. International Food Research Journal. 2018;25(4):1419–1426. [Google Scholar]
- Ridzwan et al. (2014).Ridzwan BH, Hanita MH, Nurzafirah M, Norshuhadaa MPS, Hanis ZF. Free fatty acid composition in lipid extracts of several sea cucumbers species from Malaysian. International Journal of Biosciences, Biochemistry and Bioinformatics. 2014;4(3):204–207. doi: 10.7763/IJBBB.2014.V4.340. [DOI] [Google Scholar]
- Salarzadeh et al. (2012).Salarzadeh N, Afkhami M, Bastami KD, Ehsanpour M, Khazaali A, Mokhleei A. Proximate composition of two sea cucumber species Holothuria parva and Holothuria arenicola in Persian Gulf. Annals of Biological Research. 2012;3(3):1305–1311. [Google Scholar]
- Samyn, Massin & Vandenspiegel (2019).Samyn Y, Massin C, Vandenspiegel D. The sea cucumber Holothurialineata Ludwing, 1875 (Holothuroidea, Aspidochirotida, Holothuriidea) re-described from the newly found type. Zookeys. 2019;836:81–91. doi: 10.3897/zookeys.836.29932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos et al. (2015).Santos R, Dias S, Pinteus S, Silva J, Alves C, Tecelao C, Pedrosa R, Pombo A. Sea cucumber Holothuria forskali, a new resource for aquaculture? Reproductive biology and netraceutical approach. Aquaculture Research. 2015;47(7):2307–2323. doi: 10.1111/are.12683. [DOI] [Google Scholar]
- Saputra et al. (2018).Saputra A, Wulandari A, Ernawati, Yusuf MA, Erisandy I, Hidayani AA. Masculinization of guppy fish (Poecilia reticulata Peters, 1859) with extract of sea cucumber (Holothuria scabra) Journal of Ichthyology Indonesia. 2018;18(2):127–137. doi: 10.32491/jii.v18i2.427. [DOI] [Google Scholar]
- Scannella et al. (2022).Scannella D, Bono G, Lorenzo MD, Maio FD, Falsone F, Gancitano V, Germana G, Geraci ML, Lauria V, Mancuso M, Quattrocchi F, Sardo G, Titone A, Vitale S, Fiorentino F, Massi D. How does climate change affect a fishable resource? The case of the royal sea cucumber (Parastichopus regalis) in the central Mediterranean Sea. Frontiers in Marine Science. 2022;9:934556. doi: 10.3389/fmars.2022.934556. [DOI] [Google Scholar]
- Southeast Asian Fisheries Development Center (SEAFDEC) (2022).Southeast Asian Fisheries Development Center (SEAFDEC) The southeast Asian state of fisheries and aquaculture. 2022. http://repository.seafdec.org/handle/20.500.12066/6752 http://repository.seafdec.org/handle/20.500.12066/6752
- Senadheera, Dave & Shahidi (2021).Senadheera TRL, Dave D, Shahidi F. Antioxidant potential and physicochemical properties of protein hydrolysate from body parts of North Atlantic sea cucumber (Cucumaria frondosa) Food Production, Processing, and Nutrition. 2021;3:3. doi: 10.1186/s43014-020-00049-3. [DOI] [Google Scholar]
- Senadheera et al. (2023).Senadheera TRL, Hossain A, Dave D, Shahidi F. Functional and physiochemical properties of protein isolates from different body parts of North Atlantic sea cucumber (Cucumaria frondosa) Food Bioscience. 2023;52:102511. doi: 10.1016/j.fbio.2023.102511. [DOI] [Google Scholar]
- Shah, Wang & Ma (2020).Shah AM, Wang Z, Ma J. Glutamine metabolism and its role in immunity, a comprehensive review. Animals. 2020;10(2):326. doi: 10.3390/ani10020326. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shepon et al. (2022).Shepon A, Makov T, Hamilton HA, Muller DB, Gephart JA, Henriksson PJG, Troell M, Golden CD. Sustainable optimization of global aquatic omega-3 supply chain could substantially narrow the nutrient gap. Resources, Conservation & Recycling. 2022;181:106260. doi: 10.1016/j.resconrec.2022.106260. [DOI] [Google Scholar]
- Shi et al. (2004).Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y. Saponins from edible legumes: chemistry, processing, and health benefits. Journal of Medical Food. 2004;7(1):67–78. doi: 10.1089/109662004322984734. [DOI] [PubMed] [Google Scholar]
- Shojaei, Ebrahimi & Nazemi (2020).Shojaei RF, Ebrahimi S, Nazemi M. Determination of the nutritional value of processed sea cucumber of Stichopus herrmanni species from Qeshm Island (Persian Gulf) Journal of Innovation in Food Science and Technology. 2020;12(2):31–37. [Google Scholar]
- Slater & Chen (2015).Slater M, Chen J. Sea cucumber biology and ecology. In: Brown NP, Eddy SD, editors. Echinoderm Aquaculture. New Jersey: Willey-Blackwell; 2015. pp. 47–56. [Google Scholar]
- Sroyraya et al. (2017).Sroyraya M, Hanna PJ, Siangcham T, Tinikul R, Jattujan P, Poomtong T, Sobhon P. Nutritional components of the sea cucumber Holothuria scabra. Functional Foods in Health and Disease. 2017;7(3):168–181. doi: 10.12944/CRNFSJ.8.3.09. [DOI] [Google Scholar]
- Stiani et al. (2021).Stiani SN, Rudiana T, Setiawan Y, Setyowati E, Ansori S. Formulation and characterization of serum collagen of sea cucumber extract Stichopus horrens as an antioxidant. International Journal of Applied Pharmaceutics. 2021;13(4):176–182. doi: 10.22159/ijap.2021.v13s4.43854. [DOI] [Google Scholar]
- Sumarto & Karnila (2022).Sumarto, Karnila R. Bioactive components of meat powder and viscera-gonad Holothuria scabra from Terung Island Waters, Batam, Indonesia. Conference Series: Earth and Environmental Science. 2022;1118:1–17. doi: 10.1088/1755-1315/1118/1/012032. [DOI] [Google Scholar]
- Susanti, Sukmawardani & Musfiroh (2016).Susanti NN, Sukmawardani Y, Musfiroh I. Analysis contents of potassium and calcium in mackerel fish and cork fish. Indonesian Journal of Pharmaceutical Science and Technology. 2016;3(1):26–30. doi: 10.15416/ijpst.v3i1.7913. [DOI] [Google Scholar]
- Swanson, Block & Mousa (2012).Swanson D, Block R, Mousa SA. Omege-3 fatty acids EPA and DHA: health benefits throughout life. Advances in Nutrition: an International Review Journal. 2012;3(1):1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa (1955).Tanikawa E. Studies on the protein of the meat of sea cucumber (Stichopus japonicus), Hokaido: Memoar of the Facualety of Fisheries of Hokaido University. 1955. http://hdl.handle.net/2115/21818 http://hdl.handle.net/2115/21818
- Vergara & Rodriguez (2016).Vergara W, Rodriguez A. Nutritional composition of sea cucumber Isostichopus sp. Natural Resources. 2016;7:130–137. doi: 10.4236/nr.2016.73013. [DOI] [Google Scholar]
- Wan Mahari et al. (2022).Wan Mahari WA, Kee SH, Foong SY, Amelia TSM, Bhubalan K, Man M, Yang Y, Ong HC, Vithanage M, Lam SS, Sonne C. Generating alternative fuel and bioplastics from medical plastic waste and waste frying oil using microwave co-pyrolysis combined with microbial fermentation. Renewable and Sustainable Energy Reviews. 2022;153:111790. doi: 10.1016/j.rser.2021.111790. [DOI] [Google Scholar]
- Wang et al. (2022).Wang Y, Song Y, Chang Y, Liu Y, Chen G, Xue C. Dynamic changes of peptidome and release of polysaccharide in sea cucumber (Apostichopus japonicus) hydrolysates depending on enzymatic hydrolysis approaches. Food Science and Human Wellness. 2022;11(5):1331–1341. doi: 10.1016/j.fshw.2022.04.025. [DOI] [Google Scholar]
- Wei, Jian & Mao (2011).Wei L, Jian Z, Mao W. Purification and physicochemical analysis of polysaccharides from intestinal tract of Apostichopus japonicus. Journal of Food Science. 2011;32(17):118–122. doi: 10.7506/spkx1002-6630-201117023. [DOI] [Google Scholar]
- Widianingsih, Hartati & Endrawati (2014).Widianingsih, Hartati R, Endrawati H. Application of fission technology in sea cucumber cultivation. Info. 2014;16(2):59–71. [Google Scholar]
- Widianingsih et al. (2016).Widianingsih W, Zaenuri M, Anggoro S, Kusumaningrum HPS. Nutritional value of sea cucumber [Paracudina australis (Semper, 1868)] Aquatic Procedia. 2016;7:271–276. doi: 10.1016/j.aqpro.2016.07.038. [DOI] [Google Scholar]
- Wijesekara, Pangestuti & Kim (2011).Wijesekara I, Pangestuti R, Kim S. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydrate Polymers. 2011;84(1):12–21. doi: 10.1016/j.carbpol.2010.10.062. [DOI] [Google Scholar]
- Wolfe & Byrne (2022).Wolfe K, Byrne M. Overview of the Great Barrier Reef sea cucumber fishery with focus on vulnerable and endangered species. Biological Conservation. 2022;266:109451. doi: 10.1016/j.biocon.2022.109451. [DOI] [Google Scholar]
- Xin, Jing & Jian (2016).Xin L, Jing L, Jian Z. Purification and antitumor activity in vitro of polysaccharides from Apostichopus japonicus spawn. Journal of Food Science. 2016;90(1):47–55. doi: 10.7506/spkx1002-6630-201623018. [DOI] [Google Scholar]
- Yaghmour & Whittington-Jones (2018).Yaghmour F, Whittington-Jones B. First record of Holothuria (Metriatyla) scabra Jaegar, 1833 (Echinodermata: Holothuroidea) from the coastal waters of the United Arab Emirates. PeerJ. 2018;6:e5555. doi: 10.7717/peerj.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyavi et al. (2012).Yahyavi M, Afkhami M, Javadi A, Ehsanpour M, Khazaali A, Khoshnood R, Mokhlesi A. Fatty acid composition in two sea cucumber species, Holothuria scabra and Holothuria leucospilata from Qeshm Island (Persian Gulf) African Journal of Biotechnology. 2012;11(12):2862–2868. doi: 10.5897/AJB11.3529. [DOI] [Google Scholar]
- Yang, Hamel & Mercier (2015).Yang H, Hamel JF, Mercier A. The sea cucumber Aphotichopus japonicus history, biology and aquaculture. Cambridge: Academic Press; 2015. p. 478. [Google Scholar]
- Yang et al. (2020).Yang D, Lin F, Huang Y, Ye J, Xiao M. Separation, purification, structural analysis and ummune-enhancing activity of sulfated polysaccharide isolated from sea cucumber viscera. International Journal of Biological Macromolecules. 2020;155:1003–1018. doi: 10.1016/j.ijbiomac.2019.11.064. [DOI] [PubMed] [Google Scholar]
- Zacarias-Soto & Olvera-Novoa (2015).Zacarias-Soto M, Olvera-Novoa MA. Effect of different diets on biochemical composition of the four-sided sea cucumber, Isostichopus badionotus, under culture conditions. Journal of the World Aquaculture Society. 2015;46(1):45–52. doi: 10.1111/jwas.12163. [DOI] [Google Scholar]
- Zamora & Jeffs (2013).Zamora L, Jeffs A. Feeding, metabolism and growth in response to temperature in juveniles of the Australian Sea cucumber, Australostichopus mollis. Aquaculture. 2013;358:92–97. doi: 10.1016/j.aquaculture.2012.06.024. [DOI] [Google Scholar]
- Zamora et al. (2018).Zamora LN, Yuan X, Caton AG, Slater MJ. Role of deposit-feeding sea cucumbers in integrated multitrophic aquaculture: progress, problem, potential and future challenges. Review in Aquaculture. 2018;10(1):57–74. doi: 10.1111/raq.12147. [DOI] [Google Scholar]
- Zhang & Chang (2014).Zhang Y, Chang H. Research progress on the structural components and functional of sea cucumber viscera and their application. Science and Technology of Food Industry. 2014;13:382–386. [Google Scholar]
- Zhang et al. (2019).Zhang X, Han L, Sheng W, Li X, Zhang S, Guo J, Lin H, Liu K. Two novel non-holostane type glysides from the viscera of sea cucumber Apostichopus japonicus. Journal of Asian Natural Products Research. 2019;22(4):329–337. doi: 10.1080/10286020.2019.1576643. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang X, Li X, Zhang S, He Q, Hou H, Dang L, Guo J, Chen Y, Yu T, Peng D, Han L, Liu K. LC-MS/MS identification of novel saponins from the viscera of sea cucumber Apostichopus japonicus. Chemistry of Natural Compounds. 2018;54(4):721–725. doi: 10.1007/s10600-018-2454-4. [DOI] [Google Scholar]
- Zhao et al. (2018).Zhao Y, Xue C, Zhang T, Wang Y. Saponins from sea cucumber and their biological activities. Journal of Agriculture and Food Chemistry. 2018;66:7222–7237. doi: 10.1021/acs.jafc.8b01770. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2019).Zheng W, Zhou L, Lin L, Cai Y, Sun H, Zhao L, Gao N, Yin R, Zhao J. Phycicochemical characteristics and anticoagulant activities of the polysaccharides from sea cucumber Pattalus mollis. Marine Drugs. 2019;17(4):198. doi: 10.3390/md17040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Wang & Jiang (2012).Zhou X, Wang C, Jiang A. Antioxidant peptides isolated from sea cucumber Stichopus japonicus. European Food Research and Technology. 2012;234:441–447. doi: 10.1007/s00217-011-1610-x. [DOI] [Google Scholar]
- Zhou, Zhang & Li (2018).Zhou X, Zhang X, Li W. Effects of temperature and dissolved oxygen on the survival, activity and the adaptation strategy of metabolism in sea cucumber (Apostichopus japonicus) Journal of Fisheries of China. 2018;42(8):1209–1219. doi: 10.11964/jfc.20171111043. [DOI] [Google Scholar]
- Zhu et al. (2018).Zhu Z, Zhu B, Sun Y, Ai C, Wang L, Wen C, Yang J, Song S, Liu X. Sulfated polysaccharide from sea cucumber and its depolymerized derivate prevent obesity in associated with modification of gut microbiota in high-fat diet-fed mice. Molecular Nutrition and Food Research. 2018;62(23):e1800446. doi: 10.1002/mnfr.201800446. [DOI] [PubMed] [Google Scholar]
- Zmemlia et al. (2020).Zmemlia N, Bejaoui S, Khemiri I, Bouriga N, Louiz I, El-Bok S, Ben-Attia M, Souli A. Biochemical composition and antioxidant potential of the edible Mediterranean sea cucumber Holothuria tubulosa. Grasas y Aceites. 2020;71(3):1–8. doi: 10.3989/gya.0452191. [DOI] [Google Scholar]
- Zohdi et al. (2011).Zohdi RM, Zakaria ZAB, Yusof N, Mustapha NM, Abdullah MNH. Sea cucumber (Stichopus hermanii) based hydrogel to treat burn wounds in rats. Journal of Biomedical Materials Research Part B. 2011;98(1):30–37. doi: 10.1002/jbm.b.31828. [DOI] [PubMed] [Google Scholar]
Further Reading
- Abedin et al. (2012).Abedin MZ, Karim AA, Ahmed F, Latiff AA, Gan Y, Ghazali FC, Sarker MZI. Isolation and characterization of pepsin-solubilized collagen from the integument of sea cucumber (Stichopus vastus) Journal of the Science of Food and Agriculture. 2012;93(5):1083–1088. doi: 10.1002/jsfa.5854. [DOI] [PubMed] [Google Scholar]
- Afkhami et al. (2012).Afkhami M, Ehsanpour M, Khazaali A, Dabbagh A, Maziar Y. New observation of a sea cucumber, Holothuria (Thymiosycia) impatiens, from Larak Island (Persian Gulf, Iran) Marine Biodiversity Records. 2012;5(3):E62. doi: 10.1017/S1755267212000425. [DOI] [Google Scholar]
- Ahmed, Thandar & Ali (2020).Ahmed Q, Thandar AS, Ali QM. Holothuria (Lessonothuria) insignis Ludwig, 1875 (formally resurrected from synonymy of H. pardalis Selenka, 1867) and Holothuria (Lessonothuria) lineata Ludwig, 1875-new additions to the sea cucumber fauna of Pakistan, with a key to the subgenus Lessonothuria Deichmann (Echinodermata: Holothuroidea) Zootaxa. 2020;4767(2):307–318. doi: 10.11646/zootaxa.4767.2.6. [DOI] [PubMed] [Google Scholar]
- Amiri, Hosseini & Islami (2013).Amiri M, Hosseini H, Islami HR. Identification and distribution of sea cucumbers in the Chabahar Bay, Iran. VI International Conferences “Water & Fish”; 2013. pp. 487–490. [Google Scholar]
- Bruckner & Johnson (2003).Bruckner AW, Johnson KA. Conservation strategies for sea cucumber: can a CITES Appendix II listing promote sustainable international trade? SPC Beche-de-mer Information Bulletin. 2003;18:24–33. [Google Scholar]
- Canada, Resueno & Angara (2020).Canada MCB, Resueno MA, Angara EV. Species distribution, diversity, and abundance of sea cucumber in tropical intertidal zones of aurora, Philippines. Journal of Ecology. 2020;10(12):1–10. doi: 10.4236/oje.2020.1012047. [DOI] [Google Scholar]
- Cannon & Silver (1986).Cannon LRG, Silver H. Sea cucumber of Northern Australia. 1986. https://catalogue.nla.gov.au/catalog/2031103 p. 60.https://catalogue.nla.gov.au/catalog/2031103 Queensland Museum, South Brisbane, Australia.
- Chen et al. (2021).Chen X, Sun Y, Zhao H, Hu J, Chen B, Li H, Huang W. Complete mitochondrial genome of a tropical sea cucumber, Stichopus chloronotus. Mitochondrial DNA B Resource. 2021;6(10):2788–2790. doi: 10.1080/23802359.2021.1967218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark & Rowe (1971).Clark AM, Rowe FW. Monograph of swallow water Indo-West Pacific Echinoderms. London, UK: Trustees of the British Museum (Natural History); 1971. p. 238. [Google Scholar]
- Conand, Purcell & Gamboa (2013).Conand C, Purcell S, Gamboa R. Thelenota anax. 2013. https://www.iucnredlist.org/species/pdf/1615023 https://www.iucnredlist.org/species/pdf/1615023 The IUCN Red List of Threatened Species 2013: e.T180324A1615023.
- Dissanayake & Athukorala (2011).Dissanayake DCT, Athukorala S. Abundance, distribution and some biological aspects of Holothuria edulis off the northwest coast of Sri Lanka. SPC Beche-de-mer Information Bulletin. 2011;31:39–44. [Google Scholar]
- Dolorosa et al. (2017).Dolorosa RG, Salazar CL, Delfin MTV, Paduga JR, Balisco RAT. Sea cucumber fisheries in Rasa Island Wildlife Sanctuary, Narra, Palawan, Philippines. SPC Beche-de-mer Information Bulletin. 2017;37:9–20. [Google Scholar]
- Dong, Xue & Du (2008).Dong P, Xue C, Du Q. Separation of two main triterpene glycosides from sea cucumber Pearsonothuria graeffei by high-speed countercurrent chromatography. Acta Chromatographica. 2008;20(2):269–276. doi: 10.1556/achrom.20.2008.2.11. [DOI] [Google Scholar]
- Eriksson, Byrner & Torre-Castro (2012).Eriksson H, Byrner M, Torre-Castro MDL. Sea cucumber (Aspidochirotida) community, distribution and habitat utilization on the reefs of Mayotte, Western Indian Ocean. Marine Ecology Progress Series. 2012;452:159–170. doi: 10.3354/meps09665. [DOI] [Google Scholar]
- Ghanbari et al. (2012).Ghanbari R, Ebrahimpour A, Abdul-Hamid A, Ismail A, Saari N. Actinopyga lecanora hydrolysate as natural antibacterial agents. International Journal of Molecular Science. 2012;13(12):16796–16811. doi: 10.3390/ijms131216796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel et al. (2001).Hamel J, Conand C, Pawson DL, Mercier A. The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): its biology and exploitation as beche-de-mer. Advanced in Marine Biology. 2001;41:129–223. doi: 10.1016/S0065-2881(01)41003-0. [DOI] [Google Scholar]
- Hamel & Mercier (1996).Hamel J, Mercier A. Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea) Canadian Journal of Fisheries and Aquatic Science. 1996;53(2):253–271. doi: 10.1139/f95-186. [DOI] [Google Scholar]
- Herath et al. (2020).Herath HMTB, Hewage HHP, Nirooparaj B, Senanayake SAMAIK. First record of Lampert’s sea cucumber (Synaptula lamperti) in Northern Coast, Sri Lanka. International Conferences of Multidisciplinary Research; 2020. pp. 375–378. [Google Scholar]
- Hossain, Dave & Shahidi (2020).Hossain A, Dave D, Shahidi F. Northern sea cucumber (Cucumaria frondose): a potential candidate for functional food, nutraceutical, and pharmaceutical sector. Marine Drugs. 2020;18(5):274. doi: 10.3390/md18050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idressbabu & Sureshkumar (2017).Idressbabu KK, Sureshkumar S. Distribution pattern and community structure of sea cucumbers (Class: Holothuroidea) in different biogeographic regions of the selected Island of Lakshadweep Archipelago, India. Indian Journal of Geo Marine Sciences. 2017;46(3):569–575. [Google Scholar]
- Khazaali et al. (2016).Khazaali A, Kunzmann A, Bastami KD, Baniamam M. Baseline of polycyclic aromatic hydrocarbons in the surface sediment and sea cucumbers (Holothuria leucospilota and Stichopus hermanni) in the northern parts of Persian Gulf. Marine Pollution Bulletin. 2016;110(1):539–545. doi: 10.1016/j.marpolbul.2016.05.039. [DOI] [PubMed] [Google Scholar]
- Lane (1999).Lane DJW. Distribution and abundance of Thelenota rubralineata in the western Pacific: some conservation issues. SPC Beche-de-mer Information Bulletin. 1999;11:19–21. [Google Scholar]
- Lane & Vandenspiegel (2003).Lane DJW, Vandenspiegel D. A guide to sea stars and other Echinoderms of Singapore. Singapore: Singapore Science Centre; 2003. p. 187. [Google Scholar]
- Lee & Shin (2019).Lee T, Shin S. Complete mitochondrial genome of sea cucumber, Holothuria (Stauropora) pervicax (Holothuroidea, Holothuriida, Holothuriidae), from Jeju Island, Korea. Mitochondrial DNA Part B. 2019;4(1):1047–1048. doi: 10.1080/23802359.2019.1584058. [DOI] [Google Scholar]
- Liu & Shen (2021).Liu H, Shen M. Characterization and phylogenetic analysis of the complete mitochondrial genome of prickly redfish Thelenota ananas (Jaegar, 1833) Mitochondrial DNA Part B. 2021;6(8):2275–2277. doi: 10.1080/23802359.2021.1920509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massin (1996).Massin C. Result of the rumphius biohistorical expedition to Ambon (1990) part 4. The Holothurioidea (Echinodermata) collected at Ambon during the rumphius biohistorical expedition. Zoologische Verhandelingen. 1996;307:1–53. [Google Scholar]
- Massin et al. (2002).Massin, Zulfigar Y, Hwai ATS, Boss SZR. The genus Stichopus (Echinodermata: Holothuroidea) from the Johore marine park (Malaysia) with the description of two new species. Biologie. 2002;72:73–99. [Google Scholar]
- Mercier, Battaglene & Hamel (2000).Mercier A, Battaglene SC, Hamel J. Periodic movement, recruitment and size-related distribution of the sea cucumber Holothuria scabra in Solomon Island. Hydrobiologia. 2000;440:81–100. doi: 10.1023/A:1004121818691. [DOI] [Google Scholar]
- Mezali, Thandar & Khodja (2021).Mezali K, Thandar AS, Khodja I. On the taxonomic status of Holothuria (Holothuria) tubulosa (s.s) from the Algerian coast with the description of a new Mediterranean species, Holothuria (Holothuria) algeriesis n. sp. (Echinodermata: Holothuroidea: Holothuriidae) Zootaza. 2021;4981(1):89–106. doi: 10.11646/zootaxa.4981.1.4. [DOI] [PubMed] [Google Scholar]
- Minami et al. (2018).Minami K, Sawada H, Masuda R, Takaahashi K, Shirawaka H, Yamashita Y. Stage-specific distribution of Japanese sea cucumber Apaostichopus japonicus in Maizuru Bay, Sea of Japan, in relation to environmental factors. Fisheries Science. 2018;84:251–259. doi: 10.1007/s12562-017-1174-1. [DOI] [Google Scholar]
- Mosher (1980).Mosher C. Distribution of Holothuria Arenicola Semper in the Bahamas with observation on the habitat, behavior, and feeding activity (Echinodermata: Holothuroidea) Bulletin of Marine Science. 1980;30(1):1–12. [Google Scholar]
- O’Loughlin, Barmos & VandenSpiegel (2011).O’Loughlin PM, Barmos S, VandenSpiegel D. The paracaudinid sea cucumbers of Australia and New Zealand (Echinodermata: Holothuroidea: Molpadida: Caudinidae) Memoirs of Museum Victoria. 2011;68(1):37–65. doi: 10.24199/j.mmv.2011.68.03. [DOI] [Google Scholar]
- Ongkers et al. (2018).Ongkers OTS, Pattinasarany M, Mamesah JAB, Uneputty PRA, Pattikawa JA. Size distribution and growth pattern of Holothuria atra and Holothuria scabra in the coastal waters of Morella, Central Maluku Indonesia. International Journal of Fisheries and Aquatic Studies. 2018;6(5):301–305. [Google Scholar]
- Purcell et al. (2016b).Purcell SW, Piddocke TP, Dalton SJ, Wang Y. Movement and growth of the coral reef holothuroids Bohadschia argus and Thelenota ananas. Marine Ecology Progress Series. 2016b;551:201–214. doi: 10.3354/meps11720. [DOI] [Google Scholar]
- Purcell, Samyn & Conand (2012).Purcell SW, Samyn T, Conand C. Commercially important sea cucumbers of the world. 2012. https://www.fao.org/3/i1918e/i1918e.pdf https://www.fao.org/3/i1918e/i1918e.pdf FAO Species Catalogue for Fishery Proposes No.6.
- Rakaj et al. (2017).Rakaj A, Fianchini A, Boncagni P, Lovatelli A, Scardi M, Cataudella S. Spawning and rearing of Holothuria tubulosa: a new candidate for aquaculture in the Mediterranean region. Aquaculture Research. 2017;49(1):557–568. doi: 10.1111/are.13487. [DOI] [Google Scholar]
- Reichenbach (1999).Reichenbach N. Ecology and fishery biology of Holothuria fuscolgiva in the Maldives, Indian Ocean (Echinodermata: Holothuroidea) Bulletin of Marine Science. 1999;64(1):103–113. [Google Scholar]
- Sicuro & Levine (2011).Sicuro B, Levine J. Sea cucumber in the Mediterranean: a potential species for aquaculture in the Mediterranean. Review in Fisheries Science. 2011;19(3):299–304. doi: 10.1080/10641262.2011.598249. [DOI] [Google Scholar]
- Siddique & Ayub (2015).Siddique S, Ayub Z. Population dynamics and reproduction of Holothuria arenicola (Holothuroidea: Echinodermata) in coastal waters of Pakistan, North Arabian Sea. Journal of the Marine Biological Association of the United Kingdom. 2015;95(6):1–10. doi: 10.1017/S0025315415000041. [DOI] [Google Scholar]
- Skewes et al. (2010).Skewes TD, Murphy NE, McLeod I, Dovers E, Burridge C, Rochester W. Torres strait hand collectables, 2009 survey: sea cucumber. 2010. p. 70. CSIRO, Cleveland. [DOI]
- Stout (2021).Stout C. Endangered species act status review report: black teatfish (Holothuria nobilis) 2021. p. 53. Report to National Marine Fisheries Service, Office of Protected Resources, Silver Spring, MD.
- Trianni & Bryan (2004).Trianni MS, Bryan PG. Survey and estimates of commercially viable populations of the sea cucumber Actinopyga mauritiana (Echinodermata: Holothuroidea), on Tinian Island, commonwealth of the Northern Mariana Islands. Pacific Science. 2004;58(1):91–98. doi: 10.1353/psc.2004.0009. [DOI] [Google Scholar]
- Veronika et al. (2018).Veronika K, Edrisinghe U, Sivashanthini K, Athauda ARSB. Length-weight relationships of four different sea cucumber species in North-East coastal region of Sri Lanka. Tropical Agricultural Research. 2018;29(2):212–217. doi: 10.4038/tar.v29i2.8290. [DOI] [Google Scholar]
- Viyakarn et al. (2020).Viyakarn V, Chavanich S, Heery E, Raksasab C. Distribution of sea cucumber, Holothuria atra, on the reefs in the upper Gulf of Thailand and the effect of their population densities on sediment microalga productivity. Estuarine, Coastal and Shelf Science. 2020;235:1–6. doi: 10.1016/j.ecss.2019.106514. [DOI] [Google Scholar]
- Widianingsih et al. (2019).Widianingsih W, Hartati R, Zainuri M, Anggoro S, Kusumaningrum HP, Mahendrajaya RT. Morphological ossicles of sea cucumber Paracaudina australis from Kenjeran Waters, Surabaya, Indonesia. IOP Conference Series: Earth and Environmental Science. 2019;246:1–5. doi: 10.1088/1755-1315/246/1/012019. [DOI] [Google Scholar]
- Wiedemeyer (1994).Wiedemeyer WL. Biology of small juveniles of the tropical holothurian Actinopyga echinites: growth, mortality, and habitat preferences. Marine Biology. 1994;120:81–93. doi: 10.1007/BF00381944. [DOI] [Google Scholar]
- Woo et al. (2013).Woo SP, Yasin Z, Ismail SH, Tan SH. The distribution and diversity of sea cucumber in the coral reefs of the South China Sea, Sulu Sea, and Sulawesi Sea. Deep Sea Research Part II: Topical Studies in Oceanography. 2013;96:13–18. doi: 10.1016/j.dsr2.2013.04.020. [DOI] [Google Scholar]
- Yamada et al. (2002).Yamada K, Sasaki K, Harada Y, Isobe R, Higuchi R. Constituents of Holothuroidea, 12.1) Isolation and structure of glucocerebrosides from the sea cucumber Holothuria pervicax. Chemical and Pharmaceutical Bulletin. 2002;50(11):1467–1470. doi: 10.1248/cpb.50.1467. [DOI] [PubMed] [Google Scholar]
- Yamana, Hamano & Goshima (2009).Yamana Y, Hamano T, Goshima S. Seasonal distribution pattern of adult sea cucumber Apostichopus japonicus (Stichodidea) in Yoshimi Bay, western Yamaguchi Prefecture, Japan. Fisheries Science. 2009;75(3):585–591. [Google Scholar]
- Yu et al. (2013).Yu Z, Hu C, Zhou Y, Li H, Peng P. Survival and growth of the sea cucumber Holothuria leucospilota Brandt: a comparison between suspended and bottom cultures in a subtropical fish farm during summer. Aquaculture Research. 2013;44:114–124. doi: 10.1111/j.1365-2109.2011.03016.x. [DOI] [Google Scholar]
- Zhong et al. (2021).Zhong S, Qiao Y, Zhao L, Huang G, Liu Y, Huang L. Characterization and phylogenetic analysis of the complete mitochondrial genome of Actinopyga lecanora (Jaegar, 1833) (Holothuriida: Holothuriidae) Mitochondrial DNA B Resource. 2021;6(10):2801–2802. doi: 10.1080/23802359.2021.1970641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfigar et al. (2008).Zulfigar Y, Kwang SY, Shau-Hwai AT, Shirayaman Y. Field guide to the Echinoderms (sea cucumbers and sea stars) of Malaysia. First Edition. Kyoto, Japan: Kyoto University Press; 2008. pp. 9–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review and hence did not utilize raw data.