Abstract
Background
Neurofibromatosis type 2 (NF2)-related schwannomatosis is an autosomal dominant tumor-predisposition syndrome characterized by bilateral vestibular schwannomas (VS). In patients with VS associated with NF2, vascular endothelial growth factor A inhibitor, bevacizumab, is a systemic treatment option. The aim of this study is to retrospectively evaluate NF2 patient responses to bevacizumab on VS growth and symptom progression.
Methods
This is a retrospective analysis of patients seen at the Mayo Clinic Rochester Multidisciplinary NF2 Clinic.
Results
Out of 76 patients with NF2 evaluated between 2020 and 2022, we identified 19 that received treatment with bevacizumab. Thirteen of these patients discontinued bevacizumab after median treatment duration of 12.2 months. The remaining 6 patients are currently receiving bevacizumab treatment for a median duration of 9.4 months as of March, 2023. Fifteen patients had evaluable brain MRI data, which demonstrated partial responses in 5 patients, stable disease in 8, and progression in 2. Within 6 months of bevacizumab discontinuation, 5 patients had rebound growth of their VS greater than 20% from their previous tumor volume, while 3 did not. Three patients with rebound growth went on to have surgery or irradiation for VS management.
Conclusions
Our single-institution experience confirms prior studies that bevacizumab can control progression of VS and symptoms associated with VS growth. However, we note that there is the potential for rapid VS growth following bevacizumab discontinuation, for which we propose heightened surveillance imaging and symptom monitoring for at least 6 months upon stopping anti-VEGF therapy.
Keywords: bevacizumab, MRI, neurofibromatosis 2, vestibular schwannoma
Key Points.
Bevacizumab slows vestibular schwannomas (VS) growth with a trend toward hearing preservation.
Most patients discontinue bevacizumab due to toxicity rather than VS progression.
A subset of patients who stop bevacizumab experience rebound VS growth.
Importance of the Study.
Bevacizumab has been established as a therapy for neurofibromatosis type 2 (NF2) patients which can slow vestibular schwannomas (VS) growth and symptom progression and delay more aggressive management with irradiation or surgery. Here we retrospectively review our Mayo Clinic NF2 experience and confirm these results. Further, we show volumetric data which identifies a subset of patients who, after bevacizumab discontinuation, experience rapid growth of their VS within a brief timeframe of 1–6 months. This demonstrates a need for monitoring in the post-bevacizumab time period and highlights a focus for future research.
Neurofibromatosis type 2 (NF2)-related schwannomatosis is an autosomal dominant tumor-predisposition syndrome characterized by multiple tumor types including vestibular schwannomas (VS), meningiomas, and ependymomas.1,2 Bilateral VS represents the predominant cause of morbidity (progressive hearing loss and brainstem compression) in patients with NF2.1,2 VS can grow throughout the patient’s life, potentially leading to progressive hearing loss, facial weakness, and brainstem compression that can cause additional cranial neuropathies, cerebellar tonsillar herniation, and obstructive hydrocephalus.3 Management options for NF2-related VS are typically predicated on tumor size, hearing status, associated symptoms, patient age, and comorbidities, with observation, irradiation, and microsurgery representing the mainstays of treatment.4,5 Observation with serial magnetic resonance imaging (MRI) brain examinations and audiological monitoring is reasonable for incidentally discovered, asymptomatic tumors. Management options for progressive, symptomatic tumors often involve microsurgical resection,4the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab,6 and irradiation.7,8 Treatment approach can vary dependent on the specific patient characteristics and institutional practice with options including initial surgical management9,10 and irradiation.11,12 Surgery is associated with good tumor control, hearing preservation, and slower VS growth13 with rare complications such as persistent facial paralysis or loss of residual acoustic hearing, among other less common complications.14–16 Radiotherapy, delivered in single or multiple fractions, is also associated with good tumor control, but patients may still experience progressive hearing loss, other cranial nerve dysfunction, and tumor progression despite irradiation.17 Additionally, there is an elevated risk of secondary tumor development and malignancy in this patient population who already have a defect in the NF2 tumor suppressor gene.18 Systemic therapy with bevacizumab is an emerging strategy to delay surgical resection or irradiation and the possible comorbidities of tumor treatment and/or progression.19 Retrospective case series and several prospective single-arm clinical trials have shown the benefit of bevacizumab for controlling VS progression with improvements in objective hearing and decreases in VS volume reported.6,20–24
In this single-institution retrospective study, we examine our experience within the Mayo Clinic Rochester Multidisciplinary NF2 Clinic for VS volume changes on bevacizumab, tolerability, changes in audiological capacity, and treatment course following bevacizumab discontinuation. Here we continue to show the benefits of bevacizumab therapy for the control of VS growth in the majority of our treated patients. However, we also identify rapid VS growth in some patients previously responsive to bevacizumab shortly after discontinuation of therapy.
Materials and Methods
This study was examined by the local Institutional Review Board which approved the study and granted a waiver of consent for retrospective analysis. We searched for patients with a definitive diagnosis of NF2 seen at the Mayo Clinic Rochester Multidisciplinary NF2 Clinic between 2020 and 2022 using an institutional data retrieval platform and obtained detailed patients. The institutional NF2 clinic is comprised of physicians, registered nurses, advanced practice providers, and research staff representing departments of clinical genomics, otolaryngology, neurosurgery, neurology, medical oncology, radiation oncology, radiology, and physical medicine and rehabilitation. The clinic provides multidisciplinary review and guidance to patients and families with NF2 and associated tumors. For each participant, data was retrieved and recorded from the clinic’s medical records including patient demographics, disease characteristics, treatment, procedures, and evaluations related to NF2 treatment, and response. No exclusions were made based on patient gender, race, age, NF2 presentation/phenotype, or treatment; however, patients without a history of bevacizumab treatment were excluded from detailed analysis.
Radiologic Evaluation
Three-dimensional volumetric data for VS were measured using manual segmentation with Visage PACS (Visage imaging, version 7.1.18) software under the direct supervision of a board-certified neuroradiologist. Time points used for VS evaluation included (1) nearest available MRI prior to bevacizumab initiation, (2) MRI with the most favorable response or longest documented stability in size after bevacizumab initiation, (3) nearest available MRI following bevacizumab discontinuation, and (4) MRI at a goal timepoint of 3–6 months following bevacizumab discontinuation. Volumes were measured in mm3 on the dedicated postGadolinium axial thin-section images through the posterior fossa. These were co-registered with thin section heavily T2-weighted images in each case to more accurately identify and exclude other cranial nerve schwannomas, posterior fossa meningiomas, and post-surgical enhancement, when present. Imaging assessment was performed by a board-certified neuroradiologist with more than 15 years of imaging experience. Disease response was evaluated with partial response (PR) as greater than or equal to 25% volume decrease in at least one of the two bilateral VS, stable disease (SD) as volume change in bilateral VS of less than 25%, and progressive disease (PD) as a volume increase of greater than or equal to 25% in at least one of the two bilateral VS.
Audiological Evaluation
All audiologic data were collected at Mayo Clinic by staff audiologists. Measurements were taken using over-the-hear headphones or inserted earphones, and each ear was considered independently for this study. Data collected included pure-tone thresholds from 250 Hz to 8000 Hz, pure-tone average (PTA) using pure-tone thresholds at 500, 1000, 200, and 3000 Hz, and word recognition scores (WRS) using standard calibrated audiometry.25
Audiological data were collected for three time points with respect to bevacizumab treatment: immediately pretreatment, end-of-treatment/most recent if still using bevacizumab, and one-year posttreatment for applicable patients.
Evaluation of Clinical Outcomes and Statistical Analysis
Relevant clinical and demographic data were collected from the electronic medical record (EMR) and clinical documentation from the Mayo Clinic NF2 clinic and saved on a secure server. Treating clinician notes were used to identify milestones in the patients’ clinical courses and treatments.
Continuous features were summarized with medians and ranges and categorical features were summarized with frequencies and percentages. Comparisons of audiological outcomes at different time points were evaluated using a Kruskal–Wallis test. Statistical analyses were performed using SPSS version 25 (IBM Corporation, Armonk, New York). All tests were two-sided and P-values < .05 were considered statistically significant.
Results
Patient Characteristics
Seventy-six patients with NF2 actively followed by Mayo Clinic Rochester Multidisciplinary NF2 Clinic who were seen in the last 3 years were screened for participation (Supplementary Figure 1). Nineteen patients who had prior bevacizumab use with subsequent discontinuation (n = 13) or ongoing bevacizumab use as of April, 2023 (n = 6) were included. All patients who received bevacizumab at the Mayo Clinic were appropriately monitored before each infusion. Among the 19 patients included in this study, the median age at NF2 diagnosis was 19 years (range 0.5–61 years; Table 1) and 7 were female (37%). The majority of patients were White (n = 14, 75%) with 2 Asian patients (10%), 2 Pacific Islander (5%), and 2 patients of other/mixed heritage (10%). Genetic testing for NF2 mutations was available for 11 of the 19 patients (58%). Genetic severity score, a scoring system used since 2010 that categorizes patients into severity groups with 1 predicting “mild” symptom burden and 3 “severe,26” was available for 18 patients, with the most common phenotype severity being 2 (n = 10, 56%), followed by 3 (n = 6, 33%) and 1 (n = 2, 11%). Eighteen patients had bilateral VS, one patient (ID 18) only had a left VS. One patient (ID 11) had surgery for bilateral VS, 7 patients had resection of 1 VS (37%), and 6 patients had SRS (32%) prior to initiation of bevacizumab. All patients who received surgery or irradiation at the Mayo Clinic for VS control were at risk for VS-associated symptom progression. A majority of patients had either surgical or irradiation therapy for their VS prior to initiation of bevacizumab (n = 12, 63%).
Table 1.
Patient Characteristics
| Patient ID # | Sex | Age at NF2 Dx | NF2 mutation | Phenotype Severity | Tumor Burden | VS Surgery Prior to Bev | VS RT Prior to Bev | VS Surgery PostBev | VS RT PostBev | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 40 | N/A | 2 | BL VS | L: Yes | R: No | L: No | R: Yes | L: No | R: No | L: No | R: No |
| 2 | M | 37–47* | Nonsense mutation, p.Arg466Ter, Exon 13 | 2 | BL VS, L petrous ridge mening., r cerebellar mening, r lateral temporal convexity mening | L: No | R: No | L: No | R: No | L: No | R: No | L: No | R: No |
| 3 | M | 0.5 | Nonsense mutation, c.1021C > T, Exon 11 | 3 | BL VS, multiple mening | L: No | R: No | L: No | R: No | L: Yes | R: No | L: No | R: Yes |
| 4 | F | 15 | Deletion, c.855delT | 3 | BL VS, L temporal bone mening, L foramen magnum tumor, R lateral ventricular tumor | L: No | R: No | L: Yes | R: No | L: No | R: Yes | L: No | R: Yes |
| 5 | M | 61 | N/A | 1 | BL VS | L: No | R: Yes | L: Yes | R: No | L: No | R: No | L: No | R: No |
| 6 | F | 35 | (-) Genetic Testing | 2 | BL VS | L: Yes | R: No | L: No | R: No | L: No | R: No | L: No | R: No |
| 7 | M | 18 | Deletion, c.102delG | 2 | BL VS, R C3 schwannoma, T8 intramedullary lesion, T11 schwannoma, multifocal cauda equina most prominent R L5 | L: No | R: Yes | L: No | R: No | L: No | R: No | L: Yes | R: No |
| 8 | M | 15 | Deletion, c.1_11del | 3 | BL VS, multiple meningiomas, L mastoid schwannoma, multiple spinal tumors | L: No | R: No | L: No | R: No | L: No | R: No | L: No | R: No |
| 9 | M | 17 | Substitution, c.364-2A > G | 3 | BL VS, cranial mening, spinal and peripheral disease | L: No | R: No | L: No | R: No | L: No | R: Yes | L: No | R: No |
| 10 | F | 13 | Mosaic NF2 mutation | 2 | BL VS, spinal schwannomas, cavernous sinus schwannoma, T3 intramedullary tumor | L: No | R: No | L: No | R: No | L: No | R: No | L: No | R: Yes |
| 11 | F | 10 | N/A | 3 | BL VS, multiple mening, multiple head/neck schwannomas, multiple spinal tumors, multiple peripheral tumors | L: Yes | R: Yes | L: No | R: No | L: Yes | R: No | L: No | R: No |
| 12 | F | 14 | N/A | 2 | BL VS, C2-3 ependymoma | L: No | R: Yes | L: No | R: No | L: No | R: No | L: No | R: No |
| 13 | F | 53 | N/A | 1 | BL VS | L: No | R: No | L: No | R: No | L: No | R: Yes | L: No | R: No |
| 14 | M | 14 | Deletion, het truncating mutation in NF2 | 2 | BL VS, BL 5th CN tumors, multiple mening and spinal tumors | L: No | R: No | L: No | R: No | L: No | R: No | L: No | R: No |
| 15 | M | 19 | Deletion, NF2 locus that encompasses NIPSNAP1 and the first exon of NF2 | 3 | BL VS, R LCN schwannoma, R brachial plexus lesion, multiple spinal/cutaneous | L: No | R: No | L: No | R: Yes | L: No | R: No | L: No | R: No |
| 16 | F | 26 | N/A | 2 | BL VS, parasag. Mening, R tentorial tumor, multiple spine lesions (cervical, thoracic) and proximal cauda equina | L: No | R: No | L: Yes | R: No | L: No | R: No | L: No | R: No |
| 17 | M | 35 | Deletion, c.1633delG | 2 | BL VS, L Meckel’s cave schwannoma, spinal schwannomas/ependymomas | L: No | R: No | L: Yes | R: No | L: No | R: No | L: No | R: No |
| 18 | M | 32 | N/A | N/A | BL VS, T7-8 mening | L: No | R: Yes | L: No | R: No | L: No | R: No | L: No | R: No |
| 19 | M | 35 | N/A | 2 | L facial schw, R VS, L trigeminal schw, multiple C3/C7/cauda equina tumors, R occipital mass | L: No | R: Yes | L: No | R: No | L: No | R: No | L: No | R: No |
NF2: neurofibromatosis type 2-related schwannomatosis; VS: vestibular schwannoma; Bev: bevacizumab; RT: Radiation therapy; BL: bilateral; multiple: ≥3 discreet areas; mening.: meningioma; L: left; R: right; parasag: parasagittal; schw: schwannoma. Het: heterogenous.
* Specific age given by patient as an estimate, no outside records available to corroborate exact age at diagnosis. Age of “42” used for the purposes of relevant calculations.
Bevacizumab Treatment
The median duration of initial bevacizumab treatment for the 13 patients therapy was 12.2 months (range 2–88 months; Table 2). The 6 patients currently on bevacizumab as of April 2023 have a median treatment duration of 9.4 months (range 3.7–48.3 months). Bevacizumab was given at doses ranging between 5 and 10 mg/kg at intervals ranging from 2–3 weeks (Supplementary Table S1). While bevacizumab was initially well tolerated, treatment had to be eventually initially stopped due to toxicity for 10 patients. Specific toxicities that required treatment discontinuation included hypertension (n = 5), proteinuria (n = 3), and fatigue (n = 2). In one case treatment was stopped due to noncompliance. Only two patients (15%) discontinued bevacizumab due to disease progression at 23.9 and 88 months after initiation of bevacizumab. Bevacizumab was resumed in 5 of the 13 patients initially treated with bevacizumab (patient IDs 3, 5, 6, 9, and 11), 4 of whom remain on bevacizumab therapy as of April 2023 (IDs 3, 6, 9, and 11). For these patients who resumed bevacizumab, all posttherapy MRI data was collected before bevacizumab was re-initiated.
Table 2.
Patient Tumor Volumes and Response to Bevacizumab
| ID # | Bev Treatment Duration (m) | Reason Bev DC’d | Pretreatment V (mm3) | Follow-Up Tumor V | % Change Pre and F/u V | Tumor Response | V nearest to Bev DC | V > 3 m PostBev | % Change Pre and PostBev DCȣ |
|---|---|---|---|---|---|---|---|---|---|
| 1L | 39.6 | Proteinuria | 1198.6 | 1172 | −2.2 | Partial | 1127.8 | 2200 | 95.1 |
| 1R | 1848.1 | 1195.7 | −35.3 | 1232.7 | 1320.1 | 7.1 | |||
| 2L | 13.3 | Hypertension | 55.5 | 78.3 | 41.1 | Progression | 65.6 | 52.73 | -19.6 |
| 2R | 156.6 | 153.6 | −1.9 | 136.9 | 95.58 | -30.2 | |||
| 3L | 23.9 | Progression | 1185 | 2255 | 90.3 | Progression | 3182.2 | 3858.49 | 21.3 |
| 3R | 1935 | 2834.5 | 46.5 | 3381 | 3644.3 | 7.8 | |||
| 4L | 12.2 | Proteinuria | * | * | – | Stable | * | * | - |
| 4R | 1699.1 | 1708 | 0.5 | 2242.3 | 2212 | 31.3 | |||
| 5L | 19 | Proteinuria | 7220 | 5584 | −22.7 | Stable | N/A | 5391.5 | -3.4 |
| 5R | Prior Rsxn | Prior Rsxn | – | Prior Rsxn | Prior Rsxn | - | |||
| 6L | 12.9 | Hypertension | Prior Rsxn | Prior Rsxn | – | Stable | Prior Rsxn | Prior Rsxn | - |
| 6R | 1050 | 1033 | −1.6 | 1149 | 1323.05 | 15.1 | |||
| 7L | 3.3 | Hypertension | 1279.7 | N/A | – | Cannot determine | N/A | 1582.33 | - |
| 7R | 308.8 | N/A | – | N/A | 380.86 | - | |||
| 8L | 9 | Fatigue | 397.4 | 386.84 | −2.7 | Stable | 397.5 | N/A | 2.8 |
| 8R | 321.1 | 311.11 | −3.1 | 336.25 | N/A | 8.1 | |||
| 9L | 11.9 | Compliance | 6272.1 | 5317.38 | −15.2 | Stable | 5424.5 | 6552.25 | 20.8 |
| 9R | 7357.5 | 7994.75 | 8.7 | 9307.62 | 11038 | 18.6 | |||
| 10L | 11.1 | Hypertension | 7.2 | 8.4 | 16.7 | Stable | 27 | 33 | 22.2 |
| 10R | 53.8 | 62.4 | 16 | 50 | 57 | 14 | |||
| 11L | 2 | Fatigue** | N/A | N/A | – | Cannot determine | N/A | N/A | - |
| 11R | N/A | N/A | – | N/A | N/A | - | |||
| 12L | 88 | Progression | N/A | 7790.1 | – | Stableǂ | 9147 | 14000 | 53.1 |
| 12R | N/A | 971.5 | – | 939.7 | 292.4 | -68.9 | |||
| 13L | 11.1 | Hypertension | N/A | N/A | – | Cannot determine | N/A | N/A | - |
| 13R | N/A | N/A | – | N/A | N/A | - | |||
| 14L | 3.7 | - | 2028.7 | 1477.1 | −27.2 | Partial | Ongoing Rx with Bev§ | Ongoing Rx with Bev § | Ongoing Rx with Bev § |
| 14R | 11100 | 7165.6 | −35.4 | ||||||
| 15L | 48.3 | - | 1522.6 | 1560.2 | 2.5 | Partial | |||
| 15R | 4004.8 | 1792.7 | −55.2 | ||||||
| 16L | 6.2 | - | 737 | 627.8 | −14.8 | Partial | |||
| 16R | 151.2 | 108.2 | −28.4 | ||||||
| 17L | 12.5 | - | 5853.9 | 4330.2 | −26 | Partial | |||
| 17R | 3041.4 | 2503.4 | −17.7 | ||||||
| 18L | 34.5 | - | 2490.7 | 2141.6 | −14 | Stable | |||
| 18R | – | – | – | ||||||
| 19L | 6 | – | 925.1 | 900.4 | −2.7 | Stable | |||
| 19R | 634.3 | 667.2 | 5.2 |
Bev: bevacizumab; m: month; DC: discontinue; V: volume; F/u: follow-up; L: left; R: right; Rsxn: resection; Rx: treatment.
ȣPercent change in volume of VS at first evaluable MRI ≥ 1 month following bevacizumab discontinuation normalized to most recent MRI while patient was either on bevacizumab therapy or ≤1 month out from bevacizumab discontinuation.
* Unable to assess volume due to superimposed meningioma.
** Clinical note cited “poor tolerability” to bevacizumab.
ǂ Response assessed via physician clinical note.
§ As of April 2023.
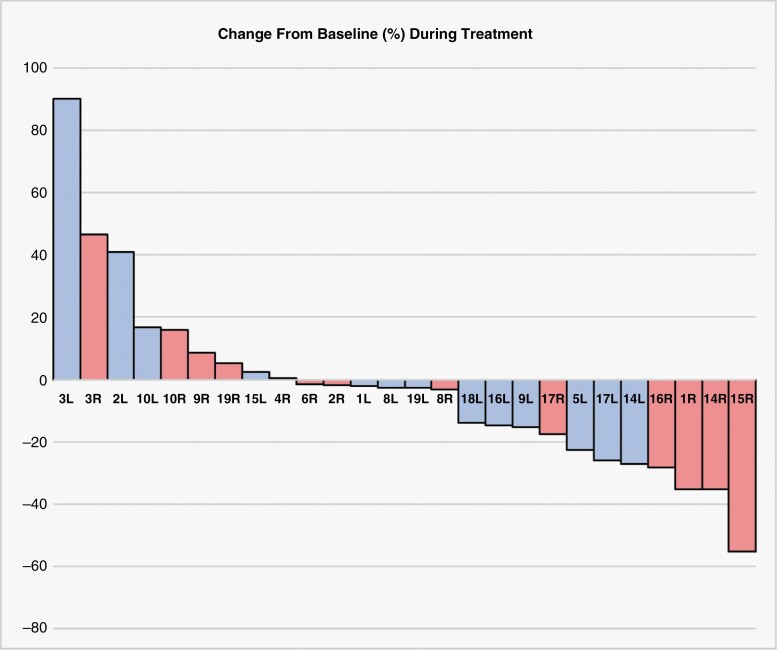
Of the 19 patients included in our study, 15 had sufficient brain MRI data available to evaluate VS volumetric change during bevacizumab treatment. Overall, we observed partial response in 5 patients, stable disease (SD) in 8, and progressive disease (PD) per criteria defined in the “Materials and Methods” section (Table 2). Response assessment could not be completed for 1 patient as pretreatment MRI was not available for volumetric analysis. Our measurement of best response or longest stability on bevacizumab demonstrated that most NF2 patients had stable disease or partial response on bevacizumab (Figure 1).
Figure 1.
Percent change in VS volume during bevacizumab therapy. Volumetric data was assessed from evaluable brain MRI at the timepoint closest to bevacizumab initiation and compared to brain MRI imaging which showed the best response or longest clinical stability. Percent volume change is normalized to the brain MRI at the timepoint closest to bevacizumab initiation. Patient ID number and laterality of the specific VS are shown.
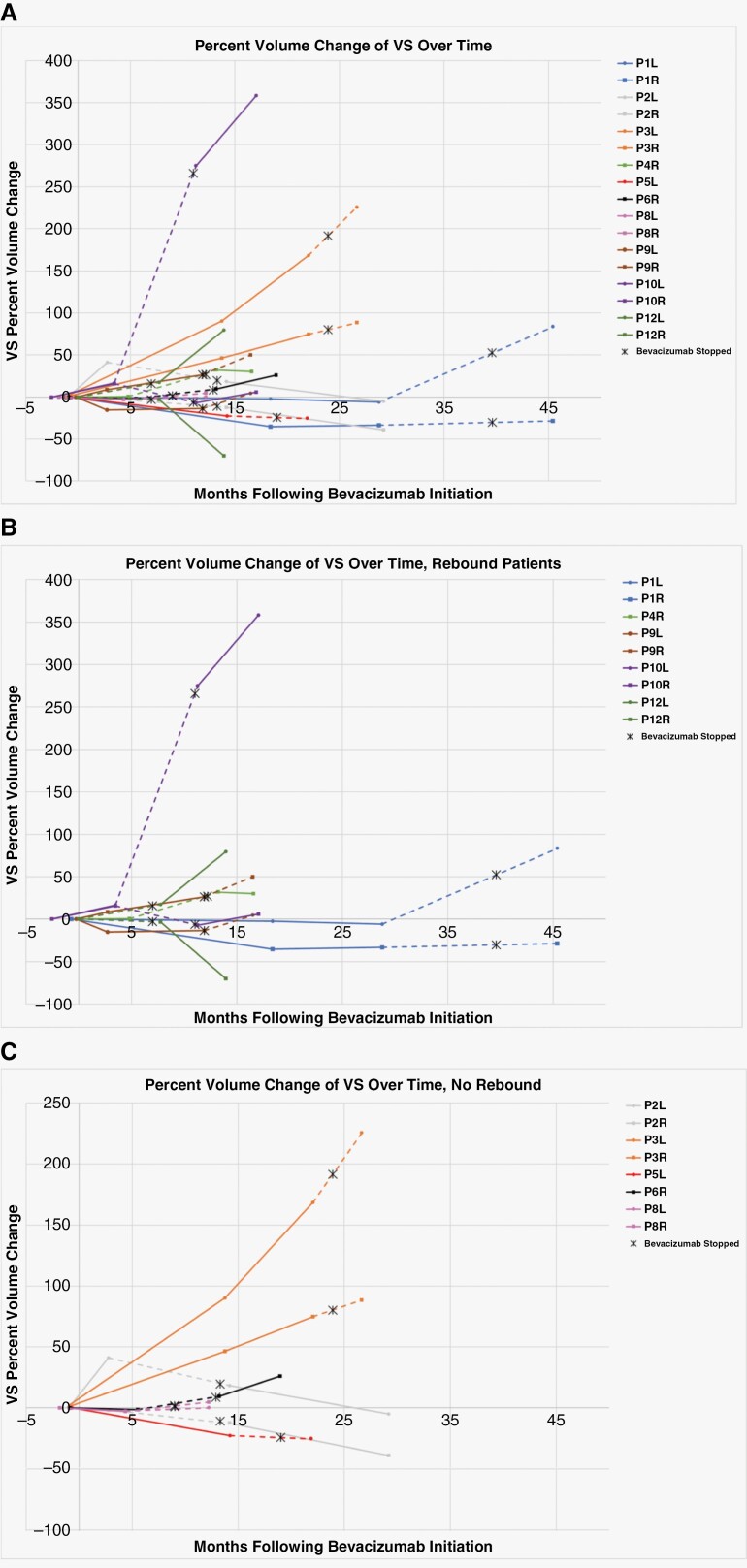
Ten of the 13 patients who discontinued bevacizumab had additional MRI data available to evaluate for VS volume changes after treatment termination. For evaluation of VS volume changes after bevacizumab discontinuation, percent change was normalized to VS volume at bevacizumab stop date or less than 1 month after stop date and compared to VS volume at next MRI at least 1 month after discontinuation. Following bevacizumab treatment termination, of the 8 bevacizumab responsive patients (7 SD, 1 PR), 5 (63%) patients had rapid growth of at least one of their bilateral VS defined as>20% volume increase, while the remaining 3 (37%) showed continued VS volume stability (<20% volume change). Imaging confirming this growth was obtained within a range of 0.9–5.8 months of bevacizumab discontinuation, with the exception of patient ID 2, who had next MRI imaging 15.7 months after bevacizumab discontinuation and has not had surgery, RT, or other therapy for VS since bevacizumab until April 2023. Overall, volume changes over the entirety of the specific patients’ courses were normalized to the pre-bevacizumab treatment volume and charted in Figure 2. Response over the patients’ disease course starting at bevacizumab initiation is shown in Figure 3. Time was measured in months, starting at time of bevacizumab initiation and with individual points representing a distinct VS volume assessment by brain MRI. We defined “rebound” growth as a greater than 20% volume increase in VS volume following bevacizumab discontinuation as discussed previously. Volume data from patient MRI’s was collected retrospectively, with variation in timepoints between bevacizumab discontinuation and follow-up MRI. As such, further MRI assessments are not available at exact timepoints. Specific raw data are available in the Supplementary Table S2.
Figure 2.
Percent change in VS volume during and after bevacizumab therapy. Volumetric data was assessed from evaluable brain MRI at the timepoint closest to bevacizumab initiation and compared to brain MRI imaging which showed the best response or longest clinical stability, the closest timepoint to bevacizumab discontinuation, and the following timepoint >1 month after bevacizumab discontinuation. The dotted line represents the time interval during which bevacizumab therapy was terminated. (A) All evaluable patient VS volume changes over time. (B) Patients with >20% growth in at least one VS following bevacizumab termination. (C) Non-responsive to bevacizumab patients (ID 2 and 3) and patients with VS volume stability after bevacizumab termination. *Patient ID 12 had a prolonged period of bevacizumab therapy (88 months). For clarity, the first brain MRI time point has been altered to 0 months, with additional MRI time points reflecting that change.
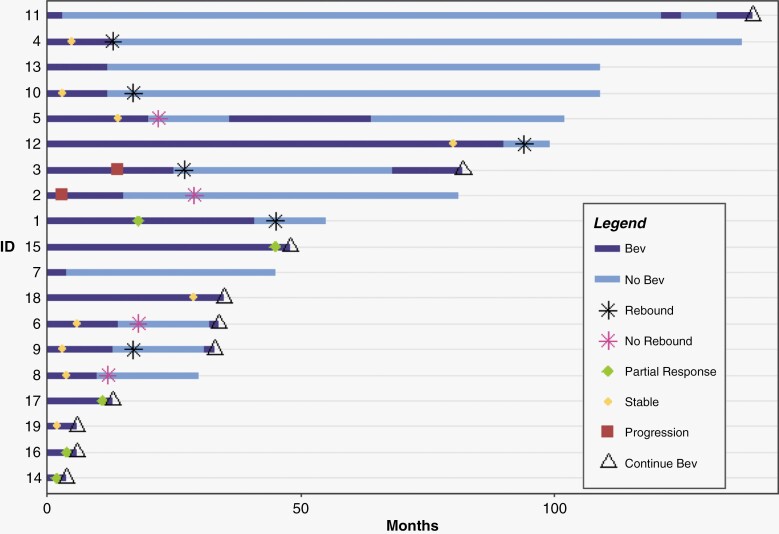
Figure 3.
Swimmer plot of patient treatment course following bevacizumab initiation. Bev: Bevacizumab. First bevacizumab treatment was assigned to time 0 months. All patients remain alive as of March 2023. Patient ID is consistent with patient ID in all other tables and figures. We define “rebound” as greater than 20% volume change in VS volume following bevacizumab discontinuation, partial response as greater than 25% volume reduction on volumetric assessment, stable disease as less than 25% volume change on volumetric assessment, and progressive disease as greater than 25% volume increase on volumetric assessment.
Following bevacizumab discontinuation, 2 patients (15%) underwent both surgery and radiotherapy (RT) for control of their VS, 3 (23%) had surgery alone, and 2 (15%) had RT alone. The remaining 6 (46%) patients who discontinued bevacizumab have had no additional therapy for VS control as of April 2023. Of the 5 patients with rebound VS growth, 1 patient (ID 4) required both surgery and RT for VS control, 1 (ID 9) required surgery, and 1 (ID 10) required RT.
Audiological Outcomes
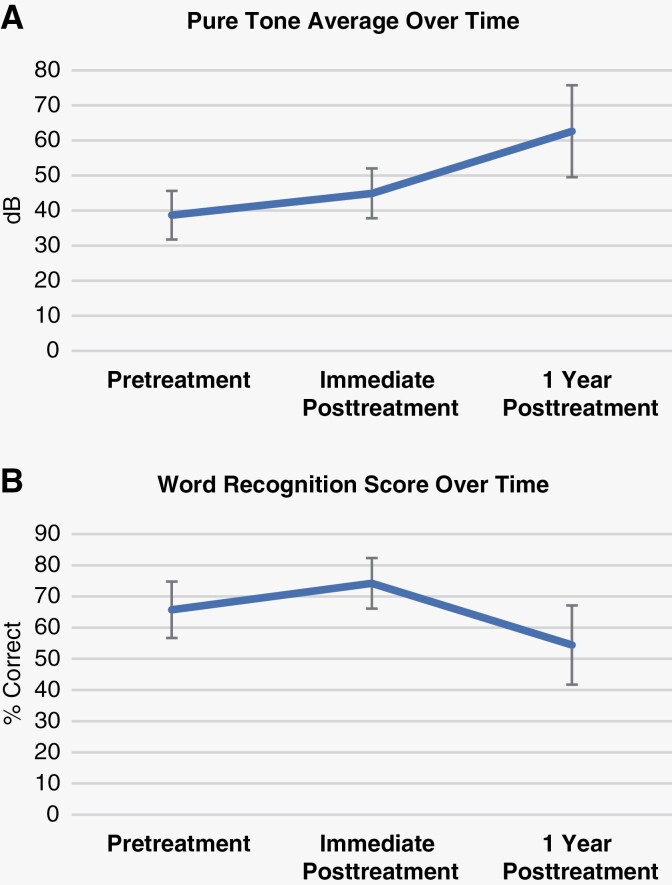
Audiological data were available for 11 patients (18 ears). The remaining 8 patients either did not have prior audiological testing or accessible audiological data. PTA and WRS over time are detailed in Supplementary Table S3, with averages for all evaluable patients and ears in Figure 4. Average PTA at the onset of therapy was 38.7 ± 29.3 decibels (dB), 44.9 ± 30.1 dB at end-of-treatment/most recent for those still on therapy, and 62.6 ± 39.3 dB 1 year after therapy. Notably, the PTA worsened at each time point; however, the interval of change appears to be greater from the end-of-therapy to 1 year after cessation of therapy. However, differences between time points are not significant (P = .330). Patient audiological data was collected retrospectively, with variation in timepoints between bevacizumab discontinuation and follow-up.
Figure 4.
Patient average PTA and WRS over the course of bevacizumab therapy. Timepoints of audiological data collection correspond to the closest evaluation prior to bevacizumab therapy, the closest evaluation following bevacizumab termination, and the closest evaluation at approximately 1 year following bevacizumab termination. (A) Average PTA at onset of therapy (“pretreatment”) was 38.7 ± 29.3 dB, 44.9 ± 30.1 dB at end-of-treatment/most recent for those still on therapy (“immediate postreatment”), and 62.6 ± 39.3 dB one year after therapy (“1 year posttreatment”). No significant difference between groups, P = .330. (B) Average WRS at onset of therapy (“pretreatment”) was 65.7 ± 38.4%, 74.2 ± 34.5% at end-of-treatment/most recent for those still on therapy (“immediate postreatment”), and 54.4 ± 38.1% 1 year after therapy (“1 year posttreatment”). No significant difference between groups, P = .371.
Regarding WRS, we note that average WRS at onset of therapy was 65.7 ± 38.4%, 74.2 ± 34.5% at end-of-treatment/most recent for those still on therapy, and 54.4 ± 38.1% 1 year after therapy. Interestingly, we note that average WRS improved slightly during therapy, but this dropped after cessation thereof. However, again, individual scores were quite variable, and this trend is not significant (P = 0.371).
Discussion
VSs represent a significant source of morbidity for patients with NF2, associated with progressive hearing loss, tinnitus, facial weakness, and brainstem compression. The goal of VS treatment centers around preservation of functional status for as long as possible. Bevacizumab has emerged as a systemic therapy that can delay the need for interventions while maintaining neurologic function. In a multi-institutional, uncontrolled phase II study with 14 patients, bevacizumab 7.5 mg/kg administered every 3 weeks was associated with hearing improvement in 36% of patients and no further hearing decline in all patients for the duration of the 12-month study period.21 In a phase II study of 22 patients with NF2-associated VS treated with intravenous bevacizumab, 10 mg/kg every 2 weeks for 6 months, 41% of patients had a hearing response and 32% had a radiographic response.27 In that earing response was defined using a 95% critical difference table and radiographic response was defined as a greater than or equal to 20% decrease in tumor volume. In a subsequent meta-analysis outcomes were reported for 161 NF2 patients with 196 assessable VS.28 Partial radiographic regression was reported among 41% and hearing was improved in 20% of the patients over a median 16-month treatment duration.17 In this retrospective, single center study, we sought to confirm this published data on NF2-related VS response to bevacizumab therapy in our own patient cohort and evaluate posttreatment outcomes.
Among our cohort with available volumetric data, radiographic response was noted in 33% of patients. Thirty-six percent experienced improvement in their PTA and 36% also had improvements in their WRS, both metrics in at least one ear. While our findings did not reach statistical significance, this is likely due to the small number of patients as these trends are in keeping with previous clinical trial results, underscoring the effectiveness of bevacizumab therapy in preserving neurologic function and providing at least transient hearing improvement among a subset of NF2 patients. Additionally, patients with progressive VS prior to treatment had stable disease following discontinuation, further demonstrating effectiveness of systemic therapy in delaying other interventions. These patterns may be related to specific NF2 mutations. Given our limited number of patients, all with unique NF2-generating mutations, further research is necessary to evaluate whether there is a relationship between bevacizumab response and specific NF2 mutations.
However, long-term toxicities were not uncommon, with 10 patients (52% of the entire cohort) requiring discontinuation of bevacizumab at a median 12 months of treatment. Adverse events in our patient cohort were in line with prior reports on bevacizumab use for NF2-related VS and other tumors. No novel toxicities were uncovered. Alternative dosing strategies including dose reduction or increased interval between doses may mitigate these toxicities without compromising efficacy and should be considered for further study.
Importantly, our study explored tumor outcomes immediately following bevacizumab discontinuation. Whether treatment was stopped due to toxicity or disease progression, we demonstrated that a subset of patients had rapid tumor growth with a 20% or greater increase in volume immediately following termination of bevacizumab treatment. A similar phenomenon was previously reported in patients with high-grade glioma, in which patients experienced rapid tumor growth and clinical decline following withdrawal of bevacizumab.29 This has also been observed in murine models of high-grade glioma30 and it has been hypothesized that, while patients on bevacizumab have reduced tumor vasculature, they retain vascular scaffolding including the basement membrane and accompanying pericytes which allows rapid vascular regrowth on anti-VEGF withdrawal.
Our study has several limitations, notably that this is a single center, retrospective study with limited follow-up. Our study also specifically focuses on patients who have received continuous bevacizumab therapy, rather than intermittent therapy. Furthermore, our patient data is not always complete, preventing consistent analysis of MRI or audiological data at all requisite time points for all patients. Finally, our imaging collection time points were nearest available MRI (1) prior to bevacizumab initiation, (2) at most favorable response or longest documented stability in size, (3) following bevacizumab discontinuation, and (4) 3–6 months following bevacizumab discontinuation. Due to the retrospective nature of this study, not all imaging timepoints are perfectly consistent or available, which we have addressed by providing detailed timepoint information in Table 2 and Figures 2 and 3. This also does not exclude that patients may have had a PR prior to determination of SD, with only the latter listed given the limitations of our MRI data collection. Additionally, we acknowledge that we have a heterogeneous patient population that may impact our results, as previous studies have noted that younger patients do not respond as well to bevacizumab therapy and may have larger tumors.31 We have addressed this by including individual patient data, which may be used in further studies with larger patient populations.
Bevacizumab therapy to preserve hearing and decrease VS growth rate is well established3,6,20,21,27 and our data supports ongoing use of bevacizumab therapy for select NF2 patients. We see a trend toward hearing improvement or at least stability while patients are treated with bevacizumab with large reductions in WRS following bevacizumab discontinuation. Additionally, most patients treated within the Mayo Clinic Rochester Multidisciplinary NF2 Clinic had either clinical stability or partial radiographic response on anti-VEGF therapy, leading to temporal delays in surgery, and RT for control of VS. Bevacizumab therapy was also generally well tolerated within our patient cohort with no novel toxicities identified. However, in our clinical experience, we see 2 subsets of patients: (1) those who do not benefit from therapy and experience tumor growth despite anti-VEGF therapy and (2) patients who do experience benefit from bevacizumab therapy, but experience rebound growth following termination of treatment. There is a need for prospective studies to evaluate these populations and determine if we can predict response to bevacizumab therapy. There may also be a benefit in gradually tapering bevacizumab therapy, rather than sudden discontinuation, in preventing VS rebound growth, though this hypothesis requires prospective evaluation. Until that time, we propose heightened surveillance imaging and symptom monitoring for at least 6 months upon stopping anti-VEGF therapy to evaluate for patients who may experience rebound VS growth and symptom progression.
Supplementary Material
Contributor Information
M J Webb, Department of Hematology/Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Bryan J Neth, Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA; Department of Neuro-Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Lauren M Webb, Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA.
Jamie J Van Gompel, Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota, USA; Department of Otolaryngology, Mayo Clinic, Rochester, Minnesota, USA.
Michael J Link, Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota, USA; Department of Otolaryngology, Mayo Clinic, Rochester, Minnesota, USA.
Brian A Neff, Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota, USA; Department of Otolaryngology, Mayo Clinic, Rochester, Minnesota, USA.
Matthew L Carlson, Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota, USA; Department of Otolaryngology, Mayo Clinic, Rochester, Minnesota, USA.
Colin L Driscoll, Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota, USA; Department of Otolaryngology, Mayo Clinic, Rochester, Minnesota, USA.
Jim Dornhoffer, Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota, USA.
Michael W Ruff, Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA; Department of Neuro-Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Kelsey A Anderson, Department of Otolaryngology, Mayo Clinic, Rochester, Minnesota, USA.
Sani H Kizilbash, Department of Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Jian L Campian, Department of Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Joon H Uhm, Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA; Department of Neuro-Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Jack I Lane, Department of Radiology, Mayo Clinic, Rochester, Minnesota, USA.
John C Benson, Department of Radiology, Mayo Clinic, Rochester, Minnesota, USA.
Daniel J Blezek, Department of Radiology, Mayo Clinic, Rochester, Minnesota, USA.
Parv M Mehta, Department of Radiology, Mayo Clinic, Rochester, Minnesota, USA.
Girish Bathla, Department of Radiology, Mayo Clinic, Rochester, Minnesota, USA.
Ugur T Sener, Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA.
Funding
This publication was supported by the National Center for Advancing Translational Sciences (grant number UL1 TR002377). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Ugur Sener receives funding from the National Center for Advancing Translational Sciences (grant number UL1 TR002377).
Conflict of interest statement
None declared.
Authorship statement
M.J.W. and U.T.S. designed and directed the study. M.J.W., B.J.N., and J.D. created the figures. J.I.L., J.C.B., D.J.B., P.M.M., and G.B. contributed to volumetric analysis. M.J.W, L.M.W., B.J.N., and U.T.S. analyzed and interpreted the data. J.J.V.G., M.J.L., B.A.N., M.L.C., C.L.D., and J.D. provided expertise in Neurosurgery and Otolaryngology necessary for writing this manuscript. J.I.L., J.C.B., D.J.B., P.M.M., and G.B. provided expertise in radiographic techniques necessary for writing this manuscript. M.J.W., L.M.W., and U.T.S. wrote the paper. All authors contributed to the writing and/or editing of the manuscript.
Data Availability
All data are available upon reasonable request to the corresponding author, M.J.W.
References
- 1. Evans DG, Sainio M, Baser ME.. Neurofibromatosis type 2. J Med Genet. 2000; 37(12):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009; 373(9679):1974–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Plotkin SR, Merker VL, Muzikansky A, BarkerFG, 2nd, SlatteryW, 3rd. Natural history of vestibular schwannoma growth and hearing decline in newly diagnosed neurofibromatosis type 2 patients. Otol Neurotol. 2014; 35(1):e50–e56. [DOI] [PubMed] [Google Scholar]
- 4. Kaul V, Cosetti MK.. Management of vestibular schwannoma (including NF2): Facial nerve considerations. Otolaryngol Clin North Am. 2018; 51(6):1193–1212. [DOI] [PubMed] [Google Scholar]
- 5. Yao L, Alahmari M, Temel Y, Hovinga K.. Therapy of sporadic and NF2-related vestibular schwannoma. Cancers (Basel). 2020; 12(4):835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plotkin SR, Merker VL, Halpin C, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol. 2012; 33(6):1046–1052. [DOI] [PubMed] [Google Scholar]
- 7. Meijer OW, Vandertop WP, Baayen JC, Slotman BJ.. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study. Int J Radiat Oncol Biol Phys. 2003; 56(5):1390–1396. [DOI] [PubMed] [Google Scholar]
- 8. Yang I, Sughrue ME, Han SJ, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg. 2010; 112(4):851–859. [DOI] [PubMed] [Google Scholar]
- 9. Slattery WH, 3rd, Fisher LM, Hitselberger W, Friedman RA, Brackmann DE.. Hearing preservation surgery for neurofibromatosis Type 2-related vestibular schwannoma in pediatric patients. J Neurosurg. 2007; 106(4 Suppl):255–260. [DOI] [PubMed] [Google Scholar]
- 10. Jiramongkolchai P, Schwartz MS, Friedman RA.. Management of neurofibromatosis type 2-associated vestibular schwannomas. Otolaryngol Clin North Am. 2023; 56(3):533–541. [DOI] [PubMed] [Google Scholar]
- 11. Link MJ, Driscoll CL, Foote RL, Pollock BE.. Radiation therapy and radiosurgery for vestibular schwannomas: indications, techniques, and results. Otolaryngol Clin North Am. 2012; 45(2):353–366, viii. [DOI] [PubMed] [Google Scholar]
- 12. Mallory GW, Pollock BE, Foote RL, et al. Stereotactic radiosurgery for neurofibromatosis 2-associated vestibular schwannomas: toward dose optimization for tumor control and functional outcomes. Neurosurgery. 2014; 74(3):292–300; discussion 300–291. [DOI] [PubMed] [Google Scholar]
- 13. Gugel I, Zipfel J, Hartjen P, et al. Managing NF2-associated vestibular schwannomas in children and young adults: review of an institutional series regarding effects of surgery and bevacizumab on growth rates, tumor volume, and hearing quality. Childs Nerv Syst. 2020; 36(10):2471–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morton RP, Ackerman PD, Pisansky MT, et al. Prognostic factors for the incidence and recovery of delayed facial nerve palsy after vestibular schwannoma resection. J Neurosurg. 2011; 114(2):375–380. [DOI] [PubMed] [Google Scholar]
- 15. Ryzenman JM, Pensak ML, TewJM, Jr. Headache: a quality of life analysis in a cohort of 1,657 patients undergoing acoustic neuroma surgery, results from the acoustic neuroma association. Laryngoscope. 2005; 115(4):703–711. [DOI] [PubMed] [Google Scholar]
- 16. Sughrue ME, Yang I, Aranda D, et al. Beyond audiofacial morbidity after vestibular schwannoma surgery. J Neurosurg. 2011; 114(2):367–374. [DOI] [PubMed] [Google Scholar]
- 17. Chung LK, Nguyen TP, Sheppard JP, et al. A systematic review of radiosurgery versus surgery for neurofibromatosis type 2 vestibular schwannomas. World Neurosurg. 2018; 109:47–58. [DOI] [PubMed] [Google Scholar]
- 18. Evans DG, Halliday D, Obholzer R, et al. ; English Specialist NF2 Research Group. Radiation treatment of benign tumors in NF2-related-schwannomatosis: A national study of 266 irradiated patients showing a significant increase in malignancy/malignant progression. Neurooncol Adv. 2023; 5(1):vdad025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forde C, King AT, Rutherford SA, et al. Disease course of neurofibromatosis type 2: a 30-year follow-up study of 353 patients seen at a single institution. Neuro Oncol. 2021; 23(7):1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plotkin SR, Stemmer-Rachamimov AO, BarkerFG, 2nd, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009; 361(4):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blakeley JO, Ye X, Duda DG, et al. Efficacy and biomarker study of bevacizumab for hearing loss resulting from neurofibromatosis type 2-associated vestibular schwannomas. J Clin Oncol. 2016; 34(14):1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sponghini AP, Platini F, Rondonotti D, Soffietti R.. Bevacizumab treatment for vestibular schwannoma in a patient with neurofibromatosis type 2: hearing improvement and tumor shrinkage. Tumori. 2015; 101(6):e167–e170. [DOI] [PubMed] [Google Scholar]
- 23. Hochart A, Gaillard V, Baroncini M, et al. Bevacizumab decreases vestibular schwannomas growth rate in children and teenagers with neurofibromatosis type 2. J Neurooncol. 2015; 124(2):229–236. [DOI] [PubMed] [Google Scholar]
- 24. Mautner VF, Nguyen R, Kutta H, et al. Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol. 2010; 12(1):14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tillman TW, Carhart R.. An expanded test for speech discrimination utilizing CNC monosyllabic words. Northwestern University Auditory Test No. 6. SAM-TR-66-55. Tech Rep SAM-TR. 1966; 1:12. [DOI] [PubMed] [Google Scholar]
- 26. Halliday D, Emmanouil B, Pretorius P, et al. Genetic severity score predicts clinical phenotype in NF2. J Med Genet. 2017; 54(10):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plotkin SR, Duda DG, Muzikansky A, et al. Multicenter, prospective, phase II and biomarker study of high-dose bevacizumab as induction therapy in patients with neurofibromatosis type 2 and progressive vestibular schwannoma. J Clin Oncol. 2019; 37(35):3446–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu VM, Ravindran K, Graffeo CS, et al. Efficacy and safety of bevacizumab for vestibular schwannoma in neurofibromatosis type 2: a systematic review and meta-analysis of treatment outcomes. J Neurooncol. 2019; 144(2):239–248. [DOI] [PubMed] [Google Scholar]
- 29. Zuniga RM, Torcuator R, Jain R, et al. Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol. 2010; 99(2):237–242. [DOI] [PubMed] [Google Scholar]
- 30. Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006; 116(10):2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morris KA, Golding JF, Axon PR, et al. ; UK NF2 Research group. Bevacizumab in neurofibromatosis type 2 (NF2) related vestibular schwannomas: a nationally coordinated approach to delivery and prospective evaluation. Neurooncol Pract. 2016; 3(4):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon reasonable request to the corresponding author, M.J.W.