Abstract
The mammalian G protein-coupled bile acid receptor 1 (TGR5) is involved in the inflammatory response. However, the functions of TGR5 in the immune response of fish remain unclear. In this study, the full-length sequence of tgr5 from hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) was cloned, and the function of TGR5 in the immune response was explored. The results showed that the ORF of tgr5 gene in hybrid grouper was 1029 bp and encoded 342 amino acids. Activation of TGR5 by INT-777 significantly decreased the activities and mRNA expression of TNFα and IL1β, whereas inhibition of TGR5 by SBI-115 showed the opposite effect. SBI-115 treatment significantly increased the expression of phosphorylated inhibitor κB α (p-IKBα) protein. After the INT-777 treatment, the concentration of protein kinase C (PKC) and expression of the p38 mitogen-activated protein kinases (p38a), p38b and p38c, were significantly decreased in vivo. INT-777 agonist significantly decreased the expression of phosphorylated phosphoinositide 3-kinase (p-PI3K) protein and the ratio of phosphorylated and nonphosphorylated serine/threonine-protein kinase (p-AKT/AKT). In conclusion, activation of hepatic TGR5 inhibited the PKC/P38 MAPK, PI3K/AKT, NFκB signaling pathway and improved hepatic immune responses of hybrid grouper in vivo and in vitro.
Keywords: fish, inflammatory responses; NFκB; PI3K/AKT; TGR5
The purpose of this study was to explore the roles of hepatic TGR5 in triggering immune responses and ultimately help develop management strategies to control hepatic inflammation and mortality in cultured fish. Understanding the functions of hepatic TGR5 may help scientists develop management strategies to reduce the liver inflammation in hybrid grouper or other fish.
Introduction
G protein-coupled bile acid receptor 1 (TGR5, GPBAR1), as the first G protein-coupled receptor (GPCR) that was discovered in 2002, is also named as membrane-type bile acids (BAs) receptor (Keitel et al., 2020; Xu et al., 2022a). The wide range of hydrophobic endogenous ligands capable of activating TGR5 includes all known BAs and many neurosteroids, including pregnanolone and estradiol (Sato et al., 2008; Martin et al., 2013). Upon binding to the ligand, TGR5 can activate multiple effector pathways to convert extracellular signals into intracellular downstream cascades (Xiong et al., 2018; Xu et al., 2022e). For example, activation of TGR5 regulates the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), serine/threonine-protein kinase (AKT), phosphoinositide 3-kinase (PI3K), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK) pathways to participate in energy metabolism, inflammatory responses, carcinogenesis, and liver regeneration in animals (Anwer, 2012; Reich et al., 2016; Chao-Fan et al., 2018; Keitel et al., 2020). Previous studies that have been conducted focus on the analysis of TGR5 expression, localization, and function in mammals (Keitel et al., 2020). However, to date, there have been no studies on the role of TGR5 in aquatic animals, with the exception of zebrafish (Danio rerio) (Xiong et al., 2021).
Chronic inflammation is known to trigger a variety of diseases and TGR5 may be a key and potential target for intervention in some inflammation-induced diseases (Wang et al., 2018b; Xiong et al., 2021; Zhang et al., 2022). For example, activation of TGR5 can differentially suppress inflammatory responses in the lung, liver, stomach, and intestines (Yoneno et al., 2013; Guo et al., 2016a), and TGR5 was able to exhibit strong anti-inflammatory effects in Kupffer cells and macrophages, including significant inhibition of proinflammatory cytokine expression and secretion (Wang et al., 2011; Guo et al., 2016b). Many different downstream signaling pathways have been proposed to contribute to the anti-inflammatory properties of TGR5, and they seem to focus on inhibiting the NFκB pathway (Guo et al., 2016a). Activated NFκB enters the nucleus, which in turn promotes the transcription of proinflammatory cytokines and the ongoing cycle of inflammation, including tumour necrosis factor-α (TNFα), interleukin 1β (IL1β), and IL6 (Xiong et al., 2021). Although the anti-inflammatory effects of TGR5 have been demonstrated in a variety of mammalian models and cell lines (Perino and Schoonjans, 2015), little is known about the relationship between TGR5 and the immune response of fish in vivo or in vitro.
As a natural ligand for TGR5, treatment with BAs reduced PKC activity, then inhibited the P38 MAPK signaling pathway, which in turn inhibited the P53 and NFκB signaling pathways, leading to reduced apoptosis and a reduced inflammatory response in mice (Anwer, 2012; Pan et al., 2016). In addition, BAs produce different effects by activating different isoforms of PI3K (Anwer, 2012; Chen et al., 2015), while TGR5 mediates monocyte adhesion and macrophage migration by activating the AKT signaling (Kida et al., 2013). Simultaneously, studies on mammals reported that NFκB could be regulated by the PI3K/AKT signaling (An et al., 2018; Ji et al., 2020). Therefore, previous studies raise the possibility that TGR5 may regulate the NFκB signaling, PKC/P38 MAPK, and PI3K/AKT pathway in fish.
The hybrid grouper, Epinephelus fuscoguttatus ♂ × Epinephelus lanceolatus ♀, is a mariculture fish popular with Southeast Asia and China and is farmed at high densities along the region’s coastlines (Xiu-ping et al., 2018; Hong-yu et al., 2021). However, in recent years, various factors, including nutritional imbalance, environmental degradation, and poor management, have in turn compromised the immune system of grouper, thus posing a serious threat to the profitability and sustainability of its commercial culture (Liang et al., 2013; Zhu et al., 2015; Xu et al., 2022b). For example, grouper increase susceptibility to Vibrio under high-density culture patterns (Shapawi et al., 2019). In addition, high-fat diets can induce hepatic inflammatory responses in vivo and in vitro by exacerbating lipid accumulation (Zou et al., 2019) or impairing bile acids metabolism (Xu et al., 2022c). In a previous study, we found the addition of BAs activated the TGR5 signaling pathway and improved the liver health of hybrid grouper, while increasing the mRNA expression of proinflammatory factors and reducing the anti-inflammatory factors (Xu et al., 2022c). Thus, we proposed that TGR5 signaling might play a role in the immunoregulation in hybrid grouper. Based on the research basis and reports in the literature, we hypothesized that TGR5 may regulate the hepatic immune response in hybrid grouper via NFκB, PI3K/AKT, and PKC/P38 MAPK signaling pathways. Thus, in this study, we characterized the tgr5 gene of hybrid grouper, investigated the phylogenetic relationships and expression patterns of tgr5, and explored the functions of TGR5 in the immune response in fish by agonist and antagonist treatments in vivo and in vitro. The purpose of this study was to explore the roles of hepatic TGR5 in triggering immune responses and ultimately help develop management strategies to control hepatic inflammation and mortality in cultured fish.
Materials and Methods
Reagents and animals
Animal handling and experimental procedures were carried out in accordance with the “Care and Use of Laboratory Animals in China” by the Animal Ethical and Welfare Committee of the Chinese Society for Laboratory Animals. This study was approved by the Animal Ethical and Welfare Committee of Guangdong Ocean University. The license to conduct animal experiments was GDOU-AEWC-20180063.
The SBI-115 (TGR5 antagonist, CAS: 882366-16-7) and INT-777 (TGR5 agonist, CAS: 1199796-29-6) were purchased from the MedChemExpress (Monmouth Junction, USA). The MEM medium was purchased from Procell Co. (Wuhan, China). The fetal bovine serum (FBS) was purchased from Gibco Invitrogen (UK). The Penicillin and streptomycin were purchased from Sigma–Aldrich (USA). The kit for the total protein extraction (A045-4) was purchased from Nanjing Jian Cheng Bioengineering Institute (Nanjing, China). The ELISA kits for TNFα (ml025930-2), IL1β (ml036463-2), PKC (ml026738), and P53 (ml027638) were purchased from Shanghai Enzyme-linked Biotechnology (Shanghai, China). The primary antibodies against PI3K p85 (4292S), phosphor-PI3K class III (Ser249, 13857S), AKT (9272S), phosphor-AKT (Ser473, 9272S), IKKα (IκB kinase α, 61294S), IKKβ (8943S), phosphor-IKKα/β (Ser176/180, 2697S), IKBα (inhibitor κB α, 4814S), phosphor-IKBα (2859S), GAPDH (2118S) were purchased from the Cell Signaling Technology (MA, USA), whereas primary antibodiy against TGR5 (NBP2-23669SS) was purchased from Novus Co. (China).
Hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) (weight 11.31 ± 0.12 g, before sex differentiation, n = 500) were purchased obtained all at one time from a hatchery in Zhanjiang (China) and domesticated in concrete pond for 1 week to acclimatize them to the culture environment. During domestication and experiment period, groupers were fed a commercial feed (50% crude protein and 10% crude fat, Haitong, China, mainly composed of imported Antarctic krill powder, white fish powder, squid powder, deep-sea squid oil, and a variety of imported vitamins, minerals, etc.), and approximately, 70% of the water in the tank was replaced daily. Culture water temperature, dissolved oxygen concentration, ammonia, and nitrate levels were maintained at appropriate levels of 28–30 °C, >7 mg/L and <0.03 mg/L, respectively. At the end of the experimental period, fish from each tank were anaesthetized with MS-222 (Sigma–Aldrich) at a concentration of 100 mg L−1 and were euthanized to take sample by a trauma to the head and dissection.
Experimental design and sample collection
Experiment 1: The cloning of tgr5 and patterns of mRNA expression
The healthy groupers (80 fish) were random selected from the domesticated concrete pond to conduct this experiment. These fish were divided into eight plastic tanks (10 fish/tank, 300 L). These tanks were randomly named to the one of eight different time points (0, 6, 12, 24, 48, 72, 120, and 192 h). All fish were injected intraperitoneally with 0.2 ml of Vibrio parahaemolyticus solution at a concentration of 7.41 × 108 CFU/ml. The LD50 for V. parahaemolyticus was based on the results of our team’s pre-experiments (He et al., 2020). Tissue samples were subsequently collected from four fish at eight different time points (0, 6, 12, 24, 48, 72, 120, and 192 h) postinjection, including trunk kidney, spleen, gills, brain, head kidney, liver, white muscle, heart, foregut, midgut, and hindgut. Samples were rapidly frozen in liquid nitrogen immediately after collecting and stored at −80 °C for long periods of time for subsequent analytical experiments.
Experiment 2: Incubation with the agonist or antagonist of TGR5 in primary hepatocytes of hybrid grouper
The healthy groupers (another about 20 fish) were random selected from the domesticated concrete pond to conduct this experiment. Primary hepatocytes were isolated from the liver of hybrid grouper by referring to the previous method and making slight adjustments (Zou et al., 2019). The steps were as follows: 1) the fish were thoroughly disinfected with alcohol; 2) the livers were carefully removed and thoroughly washed twice with MEM medium supplemented with streptomycin (200 μg/ml) and penicillin (200 IU/ml); 3) the livers were cut with a scalpel and scissors, moderately digested with trypsin and terminated with MEM medium containing fetal bovine serum; 4) the cell suspensions were filtered and collected, followed by centrifugation 3 times, including 2 times (100 × g, 5 min) and 1 time (50 × g, 5 min); 5) resuspended the hepatocytes with MEM medium containing 15% fetal bovine serum, penicillin (100 IU/ml) and streptomycin (100 μg/ml) and count them with a haemocytometer. When cell viability exceeded 92%, the cell suspension was inoculated onto cell culture flasks (106 cells/mL). Cells were subsequently digested with trypsin when cell fusion reached 80%–90% and transferred to two new 75 cm2 cell flasks. This experiment was divided into three treatments. 1) VCN group: hepatocytes without any treatment; 2) VSBI group: hepatocytes incubated with 1 μM SBI-115; 3) V777 group: hepatocytes incubated with 10 μM INT-777. According to the results of our previous trials (Xu et al., 2022e), the concentrations of SBI-115 and INT-777 incubation were selected. Sampling was conducted after 48 h of incubation.
Experiment 3: Intraperitoneal injection of agonist or antagonist of TGR5
The healthy groupers (another 54 fish) were random selected from the domesticated concrete pond to conduct this experiment. These fish were randomly allocated to nine plastic 500 L culture tanks (six fish/tank), and randomly assigned to three experimental groups: 1) IPBS group: fish were injected with PBS; 2) ISBI group: fish were injected with 1 mg of SBI-115 per kg of fish body weight; 3) I777 group: fish were injected with 1 mg kg−1 of INT-777. All fish specimens were intraperitoneally injected with 0.1 ml of a solution corresponding to their experimental group. Forty-eight hours after the injection, four fish were randomly selected from each tank, and liver samples were collected and subsequently rapidly frozen in liquid nitrogen and stored at −80 °C. The injection periods and concentrations of SBI-115 and INT-777 were based on the results of our pre-experiments (Xu et al., 2022a).
The clone and sequence analyses of the tgr5 gene in hybrid grouper
Total RNA was extracted from sample livers and hepatocytes according to the method in our previous study (Xu et al., 2022d). RNA concentration was determined by UV colorimetry (Nanodrop Thermo Fisher Scientific, USA), and RNA quality was determined by 1.5% agarose gel electrophoresis. To amplify the partial cDNA of tgr5, primers were designed based on a transcript sequence obtained from hybrid grouper (Xu et al., 2022d). The full-length cDNA sequences of tgr5 were amplified by 3ʹ-RACE and 5ʹ-RACE with the SMARTer RACE cDNA Amplification Kit (Clontech, USA) according to the instructions. PCR products of tgr5 amplified with full-length primers were sequenced to verify the results of RACE cloning. The above primers are listed in Table 1.
Table 1.
Primers used for the amplification of tgr5 gene of hybrid grouper
| Gene | Primer sequence (5’–3’) | Usage |
|---|---|---|
| tgr5-PF | ATGATGGACTGCAACGACTCC | Partial PCR |
| tgr5-PR | GGTACCCAGCATGCCAGAAA | |
| tgr5 5’ | AGTTGGTCGTGTTGTG | RACE-PCR |
| ATGATGACAAGGTTGGCC | ||
| tgr5 3’ | ACATTTGTCGCCTCCATCGCTCAG | |
| CAGAGGCTGAACCTGCGGTACACT | ||
| tgr5-FF | GGAGGAAGTCGGCAGATT | Full-length PCR |
| tgr5-FR | CGGGTATCAACCACCATCAT |
Sequence of tgr5 was analyzed by the methods of previous studies (Zhi-xin et al., 2013; Chao et al., 2017; Shao-hong et al., 2019). The similarity of sequence, sequences of putative amino acid, and localization of nuclear signal were predicted by the BLAST, NCBI’s ORF Finder, and PSORTII online tools, respectively. The physicochemical properties of sequences and signal peptide were predicted by the Expasy’s ProtParam and SignalP v3.0 software. The secondary and tertiary structure of protein domains basing on amino acid sequences were predicted by the SMART, SWISS-MODEL, and NetPhos online tools, respectively. Amino acid sequences of TGR5 from other vertebrates’ animals were aligned and used to reconstruct phylogenies with the help of the neighbor-joining method implemented in MEGA7.0.
The qPCR analyses
The total RNA concentration of all tested samples was diluted to 800 ng/μl and then reverse transcribed using an Evo M-MLV reagent Kit with gDNA Eraser (Accurate Biotechnology (Hunan) Co., Ltd). The qPCR was performed using SYBR Green Pro Taq HS (Accurate Biotechnology (Hunan) Co., Ltd) and quantified on LightCycler 480 (Roche Diagnostics GmbH, USA). The amplification efficiency of the primers was determined after six steps of 10-fold dilutions of the cDNA template. The results showed that the amplification efficiency ranged from 91.82% to 101.83%. The expression of the following genes was tested in the present study: tgr5; three proinflammatory cytokines (tnfα, il1β, and il6); PKC/P38 MAPK pathway genes [p38 mitogen-activated protein kinase a (p38a), p38b, p38c, and p53]; PI3K/AKT pathway genes [phosphatidylinositol 3-kinase regulatory subunit 5 (pi3k-rs5), 3-phosphoinositide dependent kinase-1 (pdk), akt, tuberous 1 (tsc1), and tsc2]; and NFκB pathway genes (ikkα1, ikkα2, ikkβ, ikbα1, ikbα2, p65) (Table 2). As the references genes, 18s rRNA (F: AGCAACTTTAGTATACGCTATTG; R: CCTGAGAAACGGCTACCACATC) and β-actin (F: TACGAGCTGCCTGACGGACA; R: GGCTGTGATCTCCTTCTGC), were used to calculate the relative expression of genes using the 2−ΔΔCT method (Xu et al., 2022a).
Table 2.
Primers designed for qPCR
| Target | Sequences forward | Sequences reverse | Primer efficiency (%) |
|---|---|---|---|
| tgr5 | ATGCCATCACCATACCGCTG | CCAGGCGATGCCTAAGATGA | 99.39 |
| tnfα | GCTGCGGCTCGAAGACAAT | CAGACGGTGCGGATGGAGT | 96.35 |
| il6 | GCACTACAAAATCTCCTCACTTCCA | GACCAGGAGGCCACCGTAGTA | 97.67 |
| il1β | CCAGCGTTGAGGGCAGAA | ATCGTCTCCAGATGTAAGGTT | 92.45 |
| p38a | AACTGGATGCACTACAACATGACA | TCCTGCTTATGAGGGAGGCTGGGG | 94.76 |
| p38b | ATCCTGGACTTTGGTTTGGCACGG | GAGATTTTCATCAAGAGCTCGGGC | 101.67 |
| p38c | CAGAGACCTCAAGCCAAGTAATGT | TCGATGTAGTCAGTTCCAGGAAAG | 91.82 |
| p53 | CGCAACAGGCTTCAATCGT | GAAGCATCAGAGGCGAAGA | 94.73 |
| pi3k-rs5 | GCCGAGGAGGAAGAGGATGTAGAC | GAGGAGATGGTGGAGAAGGTGGAG | 96.65 |
| 3-pdk1 | GGCAGCCATTACTGGAGCTTCTC | TGCGAGCAGGAACAGATGACAAC | 93.69 |
| tsc1 | GGACACCAAGGTCCAGAGCA | CGTCTCGGTCTGAAGCGTCT | 96.33 |
| tsc2 | TACGGAGACGACGGAGAGTTCAC | GGCATCAGTGTGGCTATGTGGAAG | 104.26 |
| akt | GGCAGGATGTGGTACAGAAGAAGC | TGTCTGGAGGAGTGAGTGTGATGG | 100.56 |
| ikka1 | GCAGAGGCAGCAGTGATG | CGCTCATTCTCGTCCAGTAAC | 98.22 |
| ikka2 | ACTGTTGGAGCCGATGGA | TGTGGACGACCTTCATACTCA | 94.92 |
| ikkb | GCCTTGGAGCCTCATGGACT | CGGTTTGGACGAAGCGGATG | 93.30 |
| ikbα1 | ACGCAGAACAGCCAGCAGCACAT | CGTGAAGCCGCCGTAGTTCAAGC | 94.69 |
| ikbα2 | CTCACCTACGGTCGCACCAA | GTCAGGCAACTCCCTCAGGT | 98.35 |
| p65 | AACCTCACCGAGCCCATTA | TTGTCACTCAGCCTGTATTCATCT | 101.83 |
The enzyme activities and western blot analyses
The concentrations of TNFα, IL1β, PKC, and P53 were determined by the method of ELISA kits (Chen et al., 2016; Wang et al., 2018a). Reagent configuration, sample pretreatment, and assay steps were performed according to the operating instructions, and the accuracy of the standard curves made from the standard solutions was to be above 99.9% (Wang et al., 2017; Li et al., 2022).
Western blot analysis followed the protocols detailed in our recent study (Xu et al., 2022c, 2022d). Briefly, hepatocytes or liver samples were lysed in RIPA buffer. After measuring and adjusting the protein concentration, they are denatured in a boiling water bath, added to a 10% SDS-PAGE with 32 mg of protein, electrophoretically separated, transferred to a PVDF membrane and then closed for a period of time with 5% bovine serum albumin (Zhang et al., 2018). The membranes were then incubated overnight at 4 °C with primary antibodies, including PI3K (1: 1000), p-PI3K (1: 1000), AKT (1: 1000), p-AKT (1: 1000), IKKα (1: 500), IKKβ (1: 500), p-IKKα/β (1: 500), IKBα (1: 1000), p-IKBα (1: 1000), TGR5 (1: 500), GAPDH (1: 1000). Then, probes were performed with HRP-conjugated anti-rabbit secondary antibodies. Finally, target protein bands were visualized by enhanced ECL reagents (Billerica, MA, USA) and quantified by ImageJ software (version 1.42, National Institutes of Health). The original files of WB image were presented in Supplementary Figure S1–S22.
Statistical analysis
Shapiro–Wilk test and Levene test were used for normality and homogeneity test of results, respectively. One-way ANOVA was used for evaluation, and Duncan’s multiple range test was used for significance analysis. SPSS 23.0 (IBM, Armonk, NY, USA) was used for the above analysis. The final results were expressed as mean ± standard deviation (SD), where the threshold of statistical significance was < 0.05.
Results
Identification of the tgr5 gene in hybrid grouper
As shown in GenBank Accession No. OK572534 and Figure 1, the full-length of hybrid grouper tgr5 is 2525 bp in length and contains a 1029 bp open reading frame (ORF) encoding 342 amino acids. The N-terminal amino acid of tgr5 sequence was methionine, and the predicted eukaryotic localization showed that tgr5 was a membrane protein, mainly distributed on the cell membrane (Supplementary Figure S23). The secondary structure of TGR5 protein consisted of 47.37% alpha helix, 38.60% random coil, and 14.04% extended strand (Supplementary Table S1). The protein domains show that TGR5 contains seven transmembrane (7 TM) domains (at N-terminal positions 19–41, 53–75, 85–107, 128–150, 197–219, 252–274, and 289–311) and two low-complexity domains (at positions 92–102 and 129–141; Figure 1 and Supplementary Figure S23). This was also confirmed by the predicted three-dimensional model (Supplementary Figure S23). The predicted glucorylation sites were not found in the sequence, and the predicted numbers of phosphorylation sites for Ser, Thr, and Tyr were 17, 9, and 4, respectively (Supplementary Figure S23).
Figure 1.
Nucleotide and amino acid sequences of tgr5 in hybrid grouper. The start codon (ATG) is bolded. The stop codon (TGA) is marked with an asterisk. The predicted seven transmembrane domains (transmembrane regions at positions 19–41, 53–75, 85–107, 128–150, 197–219, 252–274, and 289–311 of the N-terminus, respectively) are grayed. The two low-complexity regions (at positions 92–102 and 129–141) are underlined. The predicted phosphorylation sites of Ser, Thr, and Tyr are marked by a square box, triangle box, and rounded box, respectively.
As shown in Supplementary Table S2, the multiple alignments analyses showed that the TGR5 amino acid sequence of hybrid grouper had high similarity to orthologues in other fish. The similarity of TGR5 orthologues was more than 56% in teleost, but below 34% in mammals and birds. In particular, the similarity was high with Perciformes orthologues: 92.98% with Sander lucioperca, 92.11% with Notothenia coriiceps and 91.52% with Perca flavescens. The phylogenetic tree inferred using TGR5 orthologues was divided into two main clades: one containing all of the teleost and amphibian sequences, and the other containing mammalian and avian sequences (Figure 2). Within the teleost clade, the TGR5 of hybrid grouper clustered within the monophyletic Perciformes cluster, with S. lucioperca and P. flavescens as the closest relatives included.
Figure 2.
The Neighbor-Joining phylogenetic dendrogram of 33 vertebrate TGR5 amino acid sequences. TGR5 of hybrid grouper sequence is highlighted by a diamond-shaped sign. Numbers on nodes indicate statistical support, estimated using 1,000 bootstraps.
Modulation of tgr5 expression in response to V. parahaemolyticus stimulation
In healthy hybrid grouper, the tgr5 was expressed in a variety of tissues (Figure 3A). Compared to muscle (set as the reference point: 1×), expression was highest in spleen (10.45×), followed by in midgut (9.69×) and brain (4.88×); whereas lowest in hindgut (0.38×). In response to V. parahaemolyticus, the expression of tgr5 was decreased significantly in the spleen and midgut at all time-points (all P < 0.05, Figure 3B and C). Meanwhile, in the liver, it increased significantly at 6 and 12 h, then decreased significantly at 48, 72, and 120 h, and returned to the initial level (0 h) at 192 h (Figure 3D). In the head kidney, it decreased significantly at most time points (except at 72 h), and returned to the initial level (0 h) at 192 h (Figure 3E).
Figure 3.
The mRNA expression of tgr5 in various tissues of a healthy hybrid grouper (A), and at different time-points in tissues after V. parahaemolyticus stimulation (B–E). (A) Data in relation to the muscle tissue; (B–E) data in relation to the 0 h time-point. Values are presented as means with plus error bars (standard deviation, SD), where significant (P < 0.05) differences between groups are indicated by different letters.
Regulatory effects of TGR5 on hepatic immune responses in vivo
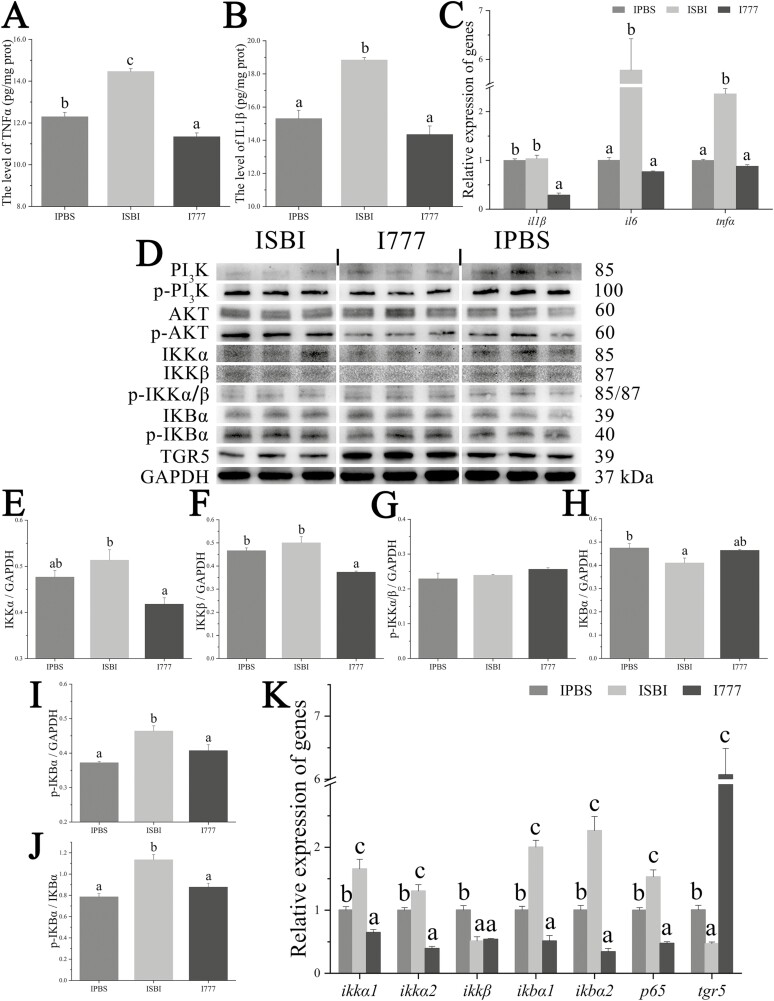
The hepatic level of TNFα was significantly increased in the ISBI group (14.46 ± 0.14 pg/mg prot) and significantly decreased in the I777 group (11.34 ± 0.18 pg/mg prot), compared to the IPBS group (12.29 ± 0.21 pg/mg prot, Figure 4A). The hepatic level of IL1β was significantly increased in the ISBI (18.84 ± 0.15 pg/mg prot), compared to the IPBS (15.30 ± 0.51 pg/mg prot) and I777 (14.34 ± 0.52 pg/mg prot) groups (Figure 4B). The ISBI treatment significantly upregulated the expression of il6 and tnfα, whereas the I777 treatment significantly downregulated the il1β, compared to the IPBS treatment (Figure 4C).
Figure 4.
The alterations of hepatic inflammation (A–C) and NFκB signaling (D–K) induced by an agonist or antagonist of TGR5 in vivo. IPBS group: fish injected with PBS; I777 group: fish injected with INT-777; ISBI group: fish injected with SBI-115. Values are presented as means with SD, where significant (P < 0.05) differences between groups are indicated by different letters.
Regulatory effects of TGR5 on the NFκB signaling pathway in vivo
Compared to the IPBS group, the ISBI treatment significantly decreased the expression of TGR5 and IKBα proteins, and significantly increased the expression of p-IKBα protein and the ratio of p-IKBα/IKBα (Figure 4D–J). The I777 treatment significantly increased the expression of TGR5, and significantly decreased the expression of the IKKβ protein. Compared to the IPBS group, the ISBI treatment significantly upregulated the expression of ikkα1, ikkα2, ikbα1, ikbα2, and p65, and significantly downregulated the expression of tgr5 and ikkβ (Figure 4K). The I777 treatment significantly upregulated the expression of tgr5, and significantly downregulated the expression of ikkα1, ikkα2, ikkβ, ikbα1, ikbα2, and p65.
Regulatory effects of TGR5 on the PKC/P38 MAPK signaling pathway in vivo
The hepatic level of PKC was significantly increased in the ISBI group (13.44 ± 0.17 mU/g prot) and significantly decreased in the I777 group (9.76 ± 0.25 mU/g prot), both compared to the IPBS group (11.20 ± 0.19 mU/g prot, Figure 5A). The hepatic level of P53 was significantly increased in the ISBI group (18.14 ± 0.10 pg/mg prot) and significantly decreased in the I777 group (14.94 ± 0.23 pg/mg prot), both compared to the IPBS group (17.14 ± 0.23 pg/mg prot, Figure 5B). The ISBI treatment significantly upregulated the expression of p38c, whereas the I777 treatment significantly downregulated the expression of p38a, p38b, and p38c (Figure 5C).
Figure 5.
The alterations of PKC/P38 MAPK signaling (A–C) and PI3K/AKT signaling (D–J) induced by an agonist or antagonist of TGR5 in vivo. IPBS group: fish injected with PBS; I777 group: fish injected with INT-777; ISBI group: fish injected with SBI-115. Values are presented as means with SD, where significant (P < 0.05) differences between groups are indicated by different letters.
Regulatory effects of TGR5 on the PI3K/AKT signaling pathway in vivo
Compared to the IPBS group, the ISBI treatment significantly decreased the expression of PI3K protein, and it significantly increased the expression of the p-AKT protein, and the ratio of p-PI3K/PI3K (Figure 5D–I). The I777 treatment significantly increased the expression of AKT protein, and significantly decreased the expression of p-PI3K and PI3K proteins, and the ratio of p-AKT/AKT. Compared to the IPBS group, the ISBI treatment significantly decreased the expression of pdk, tsc1, and tsc2, and it significantly increased the expression of pi3k-rs5 and akt. The I777 treatment significantly increased the expression of pdk and tsc1, and it significantly decreased the expression of akt (Figure 5J).
Regulatory effects of TGR5 on hepatic immune responses in vitro
The hepatic level of TNFα was significantly increased in the VSBI group (0.65 ± 0.02 pg/mg prot) compared to the V777 group (0.52 ± 0.02 pg/mg prot) and VCN group (0.58 ± 0.02 pg/mg prot, Figure 6A). The hepatic level of IL1β was significantly increased in the VSBI group (1.93 ± 0.04 pg/mg prot) and significantly decreased in the V777 group (1.42 ± 0.04 pg/mg prot), compared to the VCN group (1.58 ± 0.02 pg/mg prot, Figure 6B). The VSBI treatment significantly upregulated the expression of il1β, il6, and tnfα, whereas the V777 treatment significantly downregulated the il6 and tnfα, compared to the VCN treatment (Figure 6C).
Figure 6.
The alterations of hepatic inflammation (A–C) and NFκB signaling (D–K) induced by an agonist or antagonist of TGR5 in vitro. VCN group: hepatocytes without any treatment; V777 group: hepatocytes incubated with INT-777; VSBI group: hepatocytes incubated with SBI-115. Values are presented as means with SD, where significant (P < 0.05) differences between groups are indicated by different letters.
Regulatory effects of TGR5 on the NFκB signaling pathway in vitro
Compared to the VCN group, the VSBI treatment significantly increased the expression of IKKα, IKKβ, p-IKKα/β, IKBα, p-IKBα proteins (Figure 6D–J). The V777 treatment significantly decreased the expression of the IKKβ protein, and it significantly increased the expression of TGR5, IKKα, IKBα and p-IKBα proteins. Compared to the VCN group, the VSBI treatment significantly downregulated the expression of tgr5, and it significantly upregulated the expression of ikkα1, ikkα2, ikkβ, ikbα1, ikbα2, and p65 (Figure 6K). The V777 treatment significantly upregulated the expression of tgr5, and it significantly downregulated the expression of ikkα1 and ikkβ.
Regulatory effects of TGR5 on the PKC/P38 MAPK signaling pathway in vitro
The hepatic level of PKC was significantly decreased in the V777 group (0.74 ± 0.02 mU/g prot), compared to the VSBI (0.96 ± 0.02 mU/g prot) and VCN groups (0.95 ± 0.03 mU/g prot, Figure 7A). The hepatic level of P53 was significantly increased in the VSBI group (1.88 ± 0.06 pg/mg prot), compared to the V777 (1.11 ± 0.04 pg/mg prot) and VCN groups (1.24 ± 0.02 pg/mg prot, Figure 7B). The VSBI treatment significantly upregulated the expression of p38a, p38b, p38c, and p53, whereas the V777 treatment significantly downregulated the expression of p38b (Figure 7C).
Figure 7.
The alterations of PKC/P38 MAPK signaling (A–C) and PI3K/AKT signaling (D–J) induced by an agonist or antagonist of TGR5 in vitro. VCN group: hepatocytes without any treatment; V777 group: hepatocytes incubated with INT-777; VSBI group: hepatocytes incubated with SBI-115. Values are presented as means with SD, where significant (P < 0.05) differences between groups are indicated by different letters.
Regulatory effects of TGR5 on the PI3K/AKT signaling pathway in vitro
Compared to the VCN group, the VSBI treatment significantly decreased the expression of PI3K proteins, and it significantly increased the ratio of p-PI3K/PI3K (Figure 7D–I). The V777 treatment significantly decreased the expression of p-PI3K and p-AKT proteins, and the ratio of p-AKT/AKT. Compared to the VCN group, the VSBI treatment significantly decreased the expression of tsc2, and it significantly increased the expression of pi3k-rs5, pdk, and akt. The V777 treatment significantly increased the expression of tsc1 and tsc2, and it significantly decreased the expression of pi3k-rs5 and akt (Figure 7J).
Discussion
Sequence analysis of the tgr5 gene in hybrid grouper
The human TGR5 gene has a coding region size of 993 bp, corresponding to 330 amino acids (Godoy et al., 2013), and the rat and mouse tgr5 genes have a coding region size of 990 bp, producing a protein of 329 amino acids (Keitel et al., 2020). However, the ORF of the tgr5 gene in hybrid grouper is 1029 bp and encodes a predicted protein of 342 amino acids. Furthermore, in mammals, the protein sequence of human, bovine, rabbit, rat, and mouse TGR5 is highly conserved, with approximately 82%–91% amino acid identity (Keitel et al., 2020). In bony fish, the homologous similarity of TGR5 was more than 56%. The low conserveness rate of TGR5 in bony fish (compared to mammals) may be due to the high taxonomic and ecological diversity of aquatic animals (Clements et al., 2014). However, tgr5 of hybrid grouper is very similar to that in Perciformes (both over 91%).
Structural predictions of the protein indicated that the TGR5 proteins of all species contains seven TM structural domains, with only slight differences in amino acid lengths between the domains of different species (Xiong et al., 2021). In addition, TGR5 mainly located on the plasma membrane at the subcellular level. Similarly, in the present study, the plastid protein TGR5 from the hybrid grouper contained seven TM domains. BAs link TMs 3 and 6 to activate receptors, which is common in agonists of GPCR, while additional TMs are resolved to enhance its efficacy (Gertzen et al., 2015). For example, one of the TGR5 agonists, INT-777 (α-ethyl-23(S)-methyl-cholic acid), a semi-synthetic BA derived from cholic acid (Pellicciari et al., 2009), is the most potent TGR5 agonist against zebrafish and mammals (Xiong et al., 2021). Despite the wide range of ligands recognized by TGR5, only one antagonist, SBI-115, has been developed (Masyuk et al., 2017). In this study, we further explored the function of TGR5 in hybrid grouper by using INT-777 to activate TGR5 expression and SBI-115 to inhibit TGR5 expression.
High expression of tgr5 has been detected in some mammalian tissues, such as lung, stomach, small intestine, liver, and especially placenta and spleen (Guo et al., 2016a; Keitel et al., 2020). Consistent with this part, in this experiment, highly expressed tgr5 was found in the spleen, midgut, and brain of hybrid grouper. In addition, the expression level of tgr5 in mouse liver tissue was the lowest. The expression level of that in rat liver tissue is average, and the expression level of that in human liver tissue is the highest (Keitel and Häussinger, 2018). In hybrid grouper, the expression level of tgr5 in liver was intermediate. In response to V. parahaemolyticus stimulation, the mRNA level of tgr5 in liver was significantly upregulated at the initial injection stage and then gradually returned to normal (initial) levels. These observations suggested that tgr5 might play an important role in the immune response induced by V. parahaemolyticus infection in the liver of hybrid grouper.
TGR5 signaling pathway reduced the immune response in vivo and in vitro
TGR5 agonists have the potential to ameliorate inflammation in human cell models, as well as in other animal models, including Crohn’s disease and ulcerative colitis (Yoneno et al., 2013; Perino and Schoonjans, 2015). In vivo studies showed that activation of TGR5 recruited cAMP response element-binding protein enrichment to the IL10 promoter in the intestine and liver of mice, which specifically activated the transcriptional promoter of the IL10 gene (Xie et al., 2013; Chiang and Ferrell, 2020), which in turn reduced the levels of pro-inflammatory cytokines, including TNFα, IL1β, IL6, and IL18 (Guo et al., 2015, 2016b; Perino and Schoonjans, 2015; Gui et al., 2019). Similarly, in the present study, the concentrations of TNFα and IL1β decreased significantly in vivo and in vitro after TGR5 was activated by INT-777. In mammalian studies, mice with targeted deletion of TGR5 exhibited severe liver injury and activated immune responses (Keitel et al., 2020). In a model of LPS-induced inflammation, mice with the Tgr5-null mutation exhibited severe liver necrosis and inflammation (Wang et al., 2011). At the same time, the cytokine content in Tgr5−/− macrophages increased, which further confirmed the role of TGR5 in regulating macrophage immune response (Eggink et al., 2014). Similarly, in this study, the inhibition of TGR5 by SBI-115 increased the concentrations and mRNA levels of proinflammatory cytokines in vivo or in vitro. In general, the TGR5 signaling pathway controls the immune response involved in the production of proinflammatory cytokines in hybrid grouper.
TGR5 inhibited the NFκB signaling pathway in vivo and in vitro
In LPS-stimulated mouse macrophages, activation of Tgr5 inhibits nuclear translocation of NFκB, which exerts anti-inflammatory effects, including the production of proinflammatory cytokines and mediators (Pols et al., 2011). Therefore, the next step in this study was to explore the relationship between TGR5 and the NFκB signaling pathway. NFκB is a transcription factor associated with a variety of cellular processes, such as inflammation, proliferation, apoptosis, and development (Guo et al., 2016a; Papademetrio et al., 2016). NFκB contains five components: p50, RelB, p52, RelA (p65), and c-Rel (Sun et al., 2013). NFκB remain in a nonactivated state in plasma by binding to IKB family members, including BCL3, p100, p105, IKBα, IKBβ, IKBγ, and IKBε (DiDonato et al., 2012). Specifically, IKK regulates the phosphorylation of IKBα or IKBβ to activate NFκB and its downstream target genes (Guo et al., 2016a).
In healthy organisms, NFκB signaling is tightly controlled by multiple negative feedback mechanisms (Guo et al., 2015). However, TGR5 activation inhibits IKK activity and IKB phosphorylation, further suppressing IKB and NFκB p65 activity in the cytoplasm, leading to a decrease in NFκB transcriptional activity in vitro (macrophages, HepG2 cells and gastric cells) (Wang et al., 2011; Guo et al., 2015; Iracheta-Vellve et al., 2018; Lyu et al., 2019; Keitel et al., 2020) and in vivo (Hedvat et al., 2009). In this study, activation of TGR5 by INT-777 significantly repressed the expression of IKKβ protein in vivo, and significantly downregulated the expression of ikkα1, ikkα2, ikkβ, ikbα1, ikbα2, and p65. These results suggested that TGR5 activation inhibited the NFκB pathway in hybrid grouper in vivo. In addition, although the I777 treatment significantly decreased the expression of IKKβ protein and ikkα1 and ikkβ genes, it significantly increased the expression of IKKα, IKBα, and p-IKBα proteins, indicating that activation of TGR5 affected the activation of NFκB pathway in vitro. So, the regulation of the NFκB pathway by TGR5 activation was different in vivo and in vitro, and further studies are needed to clarify this issue.
The inhibition of TGR5 by SBI-115 significantly increased the expression of p-IKB protein and significantly upregulated the expression of ikkα1, ikkα2, ikbα1, ikbα2, and p65 both in vivo and in vitro, indicating that inhibition of TGR5 activated the NFκB pathway of hybrid grouper. Furthermore, we noted that in the present study, activation of TGR5 inhibited specific NFκB target gene sets, but not all target genes, in hybrid grouper. This phenomenon was also observed for TGR5 in a liver inflammation experiment in mice (Wang et al., 2011). These results are in agreement that TGR5 negatively regulated inflammatory response in the liver and stomach by antagonizing NFκB activity, thereby inhibiting NFκB-mediated production of proinflammatory cytokine (Wang et al., 2011; Guo et al., 2015). Our results suggested that activation of TGR5 inhibited the NFκB signaling pathway in hybrid grouper.
TGR5 repressed the PKC/P38 MAPK signaling pathway in vivo and in vitro
PKC is a member of the serine/threonine protein kinase family with at least 12 isoforms and is involved in the regulation of various cellular functions. For example, PKC regulates the body’s immune response by activating P38 MAPK and NFκB (Jia et al., 2018; Keitel et al., 2020). In the present study, the hepatic PKC level was significantly increased in the ISBI group in vivo, and significantly reduced in the INT-777 treatment both in vivo and in vitro, suggesting that TGR5 expression negatively regulated PKC activity in hybrid grouper. MAPKs regulate a variety of cellular functions, including proliferation, immunity, apoptosis, and differentiation, by conducting and processing intracellular and extracellular stimulus signals (Anwer, 2012). In general, there are four main types of MAPK cascades in cells, including P38 MAPK, JNK, ERK1/2, and ERK5 cascades (Morrison, 2012). In the present study, activation of TGR5 significantly downregulated the expression of p38a, p38b, and p38c in vivo or in vitro, whereas the inhibition of TGR5 significantly upregulated the expression of these genes, suggesting that TGR5 expression negatively regulated the P38 MAPK activity in hybrid grouper. This was further confirmed by the reduced expression of P53 (both the protein and gene) in the INT-777 treatment, as decreased P38 MAPK activity induces suppression of P53 activity (Anwer, 2012). On one hand, decreased PKC activity downregulated the expression of IKKβ and IKKγ (not IKKα) in Kupffer cells in rats (Anwer, 2012); but on the other hand, TGR5 activation decreased the expression of IL1β, IL6, and TNFα by activating P38 MAPK in mouse macrophages (Wenyu et al., 2012). In addition, agonists of TGR5 inhibited the expression of the IL1β and TNFα through the PKC-JNK/P38-P53 signaling pathway in NR8383 cells (Qi et al., 2021). Combined with our observations that TGR5 expression negatively regulated PKC and P38 MAPK activities, we propose that TGR5 expression inhibited the PKC/P38 MAPK in hybrid grouper.
TGR5 repressed the PI3K/AKT signaling pathway in vivo and in vitro
PI3Ks are a class of lipid kinases (classes I, II, and III) that phosphorylate phosphatidylinositol (PI) at position 3 of the inositol ring, a step known as D3 phosphorylation. The resulting phosphorylated PI (PIP) interacts with phosphocreatinine dependent kinases (PDK) and regulates the activity of downstream kinases, such as AKT. In the present study, INT-777 treatment significantly decreased the expression of p-PI3K protein, whereas SBI-115 treatment significantly increased the ratio of p-PI3K/PI3K both in vivo and in vitro. Since the TGR5-PI3K-mediated pathway play an important role in protecting against liver injury (Stepanov et al., 2013) and PI3K-p110β can also be activated by GPCR (Guillermet-Guibert et al., 2008), our results suggested that TGR5 expression negatively regulated the PI3K activity in hybrid grouper. On the plasma membrane, the interaction between the PH structural domain of AKT and phosphatidylinositol trisphosphate (PIP3) leads to the subsequent modification and activation of AKT at position 308 of threonine (Stepanov et al., 2013; Guo et al., 2016a). In the current study, the activation of TGR5 significantly reduced the ratio of p-AKT/AKT both in vivo and in vitro. Meanwhile, TGR5 agonist treatment significantly enhanced the phosphorylation of AKT in bovine aortic endothelial cells (Kida et al., 2013), while the phosphorylation of AKT in mouse TGR5-deficient peritoneal macrophages was significantly decreased (Xiong et al., 2018). These observations indicated that TGR5 expression negatively regulated the AKT activity in hybrid grouper. In general, we suggested that TGR5 expression negatively regulated the PI3K/AKT signaling pathway in hybrid grouper.
Limitations
Compared with the SBI-115 treatment, IL1β level, IKBα, and p-IKBα protein expression, il6 and IL1β gene expression, p-PI3K/PI3K ratio and other changes were less significant after INT-777 treatment in vivo or in vitro. We hypothesized that the nonsignificant changes in these inflammatory factors and other indicators might be related to the health status of the fish. If the fish immune system is in an inflammatory mode induced by LPS or other factors, these changes in inflammatory status may be more pronounced in response to TGR5 activation. However, this is still hypothetical and more research is needed to clarify the issue.
Conclusion
In conclusion, this study revealed the following results: 1) characterized the tgr5 gene of hybrid grouper, which contains seven trans-membrane structural domains; 2) tgr5 gene was highly expressed in the spleen, midgut, and brain; 3) the activation of TGR5 reduced hepatic immune response and inhibited the NFκB signaling pathway in hybrid grouper; 4) the activation of TGR5 inhibited the PKC/P38 MAPK and PI3K/AKT pathways. Understanding the functions of hepatic TGR5 may help scientists develop management strategies to reduce the liver inflammation in hybrid grouper or other fish.
Supplementary Material
Glossary
Abbreviations:
- 18s
18s rRNA
- 3-pdk1
3-phosphoinositide dependent kinase-1
- 7 TM
seven transmembrane
- akt
serine/threonine-protein kinase
- BAs
bile acids
- GPCR
G protein-coupled receptor
- I777
fish were injected with 1 mg kg−1 of INT-777
- IKBα
inhibitor κB α
- ikkα
IκB kinase α
- ikkβ
IκB kinase β
- il1β
interleukin 1β
- il6
interleukin 6
- IPBS
fish were injected with PBS
- ISBI
fish were injected with 1 mg of SBI-115 per kg of fish body weight
- MAPK
mitogen-activated protein kinase
- MEM
minimum essential medium
- NFκB p65
nuclear factor kappa B p65
- ORF
open reading frame
- p38a
p38 mitogen-activated protein kinase a
- p38b
p38 mitogen-activated protein kinase b
- p38c
p38 mitogen-activated protein kinase c
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- p-IKBα
phosphorylated-inhibitor κB α
- p-IKKα
phosphorylated-IκB kinase α
- p-IKKβ
phosphorylated-IκB kinase β
- tgr5
G protein-coupled bile acid receptor 1
- tnfα
tumor necrosis factor-alpha
- tsc1
tuberous 1
- tsc2
tuberous 2
- V777
hepatocytes incubated with 10 μM INT-777
- VCN
hepatocytes without any treatment
- Vibrio parahaemolyticus
V. parahaemolyticus
- VSBI
hepatocytes incubated with 1 μM SBI-115
Contributor Information
Jia Xu, Laboratory of Aquatic Animal Nutrition and Feed, Fisheries College, Guangdong Ocean University, Zhanjiang, China; Guangxi Key Laboratory of Marine Environmental Science, Guangxi Academy of Marine Sciences, Guangxi Academy of Sciences, Nanning, China.
Junming Cao, Laboratory of Aquatic Animal Nutrition and Feed, Fisheries College, Guangdong Ocean University, Zhanjiang, China; Key Laboratory of Aquatic, Livestock and Poultry Feed Science and Technology in South China, Ministry of Agriculture, Zhanjiang, China; Aquatic Animals Precision Nutrition and High Efficiency Feed Engineering Research Center of Guangdong Province, Zhanjiang, China.
Beiping Tan, Laboratory of Aquatic Animal Nutrition and Feed, Fisheries College, Guangdong Ocean University, Zhanjiang, China; Aquatic Animals Precision Nutrition and High Efficiency Feed Engineering Research Center of Guangdong Province, Zhanjiang, China.
Shiwei Xie, Laboratory of Aquatic Animal Nutrition and Feed, Fisheries College, Guangdong Ocean University, Zhanjiang, China; Key Laboratory of Aquatic, Livestock and Poultry Feed Science and Technology in South China, Ministry of Agriculture, Zhanjiang, China; Aquatic Animals Precision Nutrition and High Efficiency Feed Engineering Research Center of Guangdong Province, Zhanjiang, China.
Acknowledgments
This work was supported by the National Key R&D Program of China (2019YFD0900200), the Science and Technology Project of Zhanjiang (2020A05003), the China Agriculture Research System of MOF and MARA (CARS-47), the Natural Science Foundation of Guangdong Province (2018A030313154 & 2020A1515011129), and the National Natural Science Foundation of China (31772864).
Author Contributions
Conceptualization: Jia Xu: Conceptualization, Data Curation, Methodology, Formal analysis, Investigation, Software, Validation, Writing-Original Draft; Junming Cao: Methodology, Resources, Validation, Funding acquisition; Beiping Tan: Project administration, Conceptualization, Investigation, Supervision, Writing-Review & Editing, Funding acquisition; Shiwei Xie: Conceptualization, Data Curation, Resources, Software, Visualization, Supervision. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare no real or perceived conflict of interests.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
The experimental procedures were approved by the Animal Ethical and Welfare Committee of Guangdong Ocean University (Guangdong, China), processing ID: GDOU-AEWC-20180063.
Literature Cited
- An, W., Li W., Tan B., Yang Q., Dong X., Liu H., Zhang S., Yang Y., and Zhang H... 2018. Optimum calcium and phosphorus supplemental levels in diets of large size Litopenaeus vannamei. J. Guangdong Ocean Univer. 38:8–19. doi: 10.3969/j.issn.1673-9159.2018.04.002 [DOI] [Google Scholar]
- Anwer, M. S. 2012. Intracellular signaling by bile acids. J. Biosci (Rajshari). 20:1–23. doi: 10.3329/jbs.v20i0.17647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Fan, Z., Xin-Ming J., Cheng-Ze M., Lü Y., Peng, Z.. 2018. Present marine environment situation investigation in the nearshore area of Guanhe estuary in spring. J. Guangdong Ocean Univer. doi: 10.3969/j.issn.1673-9159.2018.05.005 [DOI]
- Chao, L., Zhe Z., Qing-heng W., Rong-lian H., Yue-wen D., and Jun-hui L... 2017. Molecular characterization and expression analysis of Pm-ApoL2 gene from Pinctada fucata martensii. J. Guangdong Ocean Univer. 37:7. doi: 10.3969/j.issn.1673-9159.2017.03.001 [DOI] [Google Scholar]
- Chen, Q., Liu H., Tan B., Dong X., Chi S., Yang Q., and Zhang S... 2016. Effects of dietary cholesterol level on growth performance, blood biochemical parameters and lipid metabolism of juvenile cobia (Rachycentron canadum). J. Guangdong Ocean Univer. 36:35–43. doi: 10.3969/j.issn.1673-9159.2016.01.007 [DOI] [Google Scholar]
- Chen, Y. Y., Chang-Ling L. I., and Huang X. H... 2015. Effects of microcystin on activities of immune enzymes in the White shrimp Litopenaeus Vannamei. J. Guangdong Ocean Univer 35:35–39. doi: 10.3969/j.issn.1673-9159.2015.06.007 [DOI] [Google Scholar]
- Chiang, J. Y. L., and Ferrell J. M... 2020. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 318:G554–G573. doi: 10.1152/ajpgi.00223.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements, K. D., Angert, E. R., Montgomery, W. L., Choat, J. H.. 2014. Intestinal microbiota in fishes: what’s known and what’s not. In: Wiley Online Library. [DOI] [PubMed] [Google Scholar]
- DiDonato, J. A., Mercurio F., and Karin M... 2012. NF-κB and the link between inflammation and cancer. Immunol. Rev. 246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x [DOI] [PubMed] [Google Scholar]
- Eggink, H. M., Soeters M. R., and Pols T. W... 2014. TGR5 ligands as potential therapeutics in inflammatory diseases. Int. J. Interferon Cytokine Mediator Res. 6:27–38. doi: 10.2147/IJICMR.S40102 [DOI] [Google Scholar]
- Gertzen, C. G., Spomer L., Smits S. H., Häussinger D., Keitel V., and Gohlke H... 2015. Mutational mapping of the transmembrane binding site of the G-protein coupled receptor TGR5 and binding mode prediction of TGR5 agonists. Eur. J. Med. Chem. 104:57–72. doi: 10.1016/j.ejmech.2015.09.024 [DOI] [PubMed] [Google Scholar]
- Godoy, P., Hewitt N. J., Albrecht U., Andersen M. E., Ansari N., Bhattacharya S., Bode J. G., Bolleyn J., Borner C., and Boettger J... 2013. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 87:1315–1530. doi: 10.1007/s00204-013-1078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui, L., Mai H., Chi S., Zhou W., Li Y., and Tan B... 2019. Effects of yeast culture on growth performance, hematological parameters, immunity and disease resistance of Litopenaeus vannamei. J. Guangdong Ocean Univer. 39:30–37. doi: 10.3969/j.issn.1673-9159.2019.03.005 [DOI] [Google Scholar]
- Guillermet-Guibert, J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A. J., Okkenhaug K., and Vanhaesebroeck B... 2008. The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110γ. Proc. Natl. Acad. Sci. USA. 105:8292–8297. doi: 10.1073/pnas.0707761105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C., Chen W. D., and Wang Y. D... 2016a. TGR5, not only a metabolic regulator. Front. Physiol. 7:646. doi: 10.3389/fphys.2016.00646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C., Qi H., Yu Y., Zhang Q., Su J., Yu D., Huang W., Chen W. D., and Wang Y. D... 2015. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) inhibits gastric inflammation through antagonizing NF-kappaB signaling pathway. Front. Pharmacol. 6:287. doi: 10.3389/fphar.2015.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C., Xie S., Chi Z., Zhang J., Liu Y., Zhang L., Zheng M., Zhang X., Xia D., Ke Y.,. et al. 2016b. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 45:802–816. doi: 10.1016/j.immuni.2016.09.008 [DOI] [PubMed] [Google Scholar]
- He, Y., Ye G., Chi S., Tan B., Dong X., Yang Q., Liu H., and Zhang S... 2020. Integrative transcriptomic and small RNA sequencing reveals immune-related miRNA-mRNA regulation network for soybean meal-induced enteritis in hybrid grouper, Epinephelus fuscoguttatusfemale symbol x Epinephelus lanceolatusmale symbol. Front. Immunol. 11:1502. doi: 10.3389/fimmu.2020.01502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedvat, M., Huszar D., Herrmann A., Gozgit J. M., Schroeder A., Sheehy A., Buettner R., Proia D., Kowolik C. M., Xin H.,. et al. 2009. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 16:487–497. doi: 10.1016/j.ccr.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-yu, L., Li-xian L., Stephen A., Ze T., Wei F., Bei-ping T., Xiao-hui D., Shu-yan C., Qi-hui Y., Shuang Z.,. et al. 2021. Effects of dietary yeast culture supplementation on growth, intestinal morphology, immunity, and disease resistance in Epinephelus fuscoguttatus ♀×Epinephelus lanceolatu ♂. J. Guangdong Ocean Univer. 41:11. doi: 10.3969/j.issn.1673-9159.2021.03.001 [DOI] [Google Scholar]
- Iracheta-Vellve, A., Calenda C. D., Petrasek J., Ambade A., Kodys K., Adorini L., and Szabo G... 2018. FXR and TGR5 agonists ameliorate liver injury, steatosis, and inflammation after binge or prolonged alcohol feeding in mice. Hepatol. Commun. 2:1379–1391. doi: 10.1002/hep4.1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, K., Liang H., Ren M., Ge X., Mi H., Pan L., and Yu H... 2020. The immunoreaction and antioxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala) involves the PI3K/Akt/Nrf2 and NF-kappaB signal pathways in response to dietary methionine levels. Fish Shellfish Immunol. 105:126–134. doi: 10.1016/j.fsi.2020.07.005 [DOI] [PubMed] [Google Scholar]
- Jia, W., Xie G., and Jia W... 2018. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15:111–128. doi: 10.1038/nrgastro.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel, V., Gertzen C. G., Spomer L., Gohlke H., and Häussinger D... 2020. TGR5 (GPBAR1) in the liver. Liver Biol. Pathobiol. 24:286–298. doi: 10.1002/9781119436812.ch24 [DOI] [Google Scholar]
- Keitel, V., and Häussinger, D.. 2018. Role of TGR5 (GPBAR1) in liver disease. In: Seminars in liver disease (Vol. 38, No. 04, pp. 333–339). doi: 10.1055/s-0038-1669940 [DOI] [PubMed] [Google Scholar]
- Kida, T., Tsubosaka Y., Hori M., Ozaki H., and Murata T... 2013. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 33:1663–1669. doi: 10.1161/ATVBAHA.113.301565 [DOI] [PubMed] [Google Scholar]
- Li, W., Li L., Liu H., Tan B., Dong X., Yang Q., Chi S., Zhang S., and Xie R... 2022. Effects of Clostridium butyricum on growth, antioxidant capacity and non-specific immunology of Litopenaeus vannamei fed with concentrated cottonseed protein replacement of fishmeal. J. Guangdong Ocean Univer. 42:29–37. doi: 10.3969/j.issn.1673-9159.2022.02.004 [DOI] [PubMed] [Google Scholar]
- Liang, H., Huang D., Wu Y., Wang C., and Zhong W... 2013. Effects of temperature and salinity on survival and food intake of grouper hybrid (Epinephelus lanceolatus♂× E. fuscoguttatus♀). J. Guangdong Ocean Univer. 33:22–26. doi:CNKI:SUN:QDHB.0.2019-01-017 [Google Scholar]
- Lyu, D., Tang N., Wang J., Zhang Y., Chang J., Liu Z., and Liu H... 2019. TGR5 agonist INT-777 mitigates inflammatory response in human endometriotic stromal cells: a therapeutic implication for endometriosis. Int. Immunopharmacol. 71:93–99. doi: 10.1016/j.intimp.2019.02.044 [DOI] [PubMed] [Google Scholar]
- Martin, R. E., Bissantz C., Gavelle O., Kuratli C., Dehmlow H., Richter H. G., Obst Sander U., Erickson S. D., Kim K., and Pietranico-Cole S. L... 2013. 2-Phenoxy-nicotinamides are potent agonists at the bile acid receptor GPBAR1 (TGR5). ChemMedChem. 8:569–576. doi: 10.1002/cmdc.201390010 [DOI] [Google Scholar]
- Masyuk, T. V., Masyuk A. I., Lorenzo Pisarello M., Howard B. N., Huang B. Q., Lee P. Y., Fung X., Sergienko E., Ardecky R. J., and Chung T. D... 2017. TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Gαs signaling. Hepatology 66:1197–1218. doi: 10.1002/hep.29284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, D. K. 2012. MAP kinase pathways. Cold Spring Harbor Perspect. Biol. 4:a011254. doi: 10.1101/cshperspect.a011254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, J., Han Y., Huo Y., Su P., and Jiang Z... 2016. Effects of dietary alginate oligosaccharide on intestinal morphology, activities of digestive enzymes and apparent digestibility of turbot (Scophthalmus maximus l). J Guangdong Ocean Univer. 36:39–44. doi: 10.3969/j.issn.1673-9159.2016.03.007 [DOI] [Google Scholar]
- Papademetrio, D. L., Lompardía S. L., Simunovich T., Costantino S., Mihalez C. Y., Cavaliere V., and Álvarez E... 2016. Inhibition of survival pathways MAPK and NF-kB triggers apoptosis in pancreatic ductal adenocarcinoma cells via suppression of autophagy. Targeted Oncol 11:183–195. doi: 10.1007/s11523-015-0388-3 [DOI] [PubMed] [Google Scholar]
- Pellicciari, R., Gioiello A., Macchiarulo A., Thomas C., Rosatelli E., Natalini B., Sardella R., Pruzanski M., Roda A., Pastorini E.,. et al. 2009. Discovery of 6α-ethyl-23 (S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 52:7958–7961. doi: 10.1021/jm901390p [DOI] [PubMed] [Google Scholar]
- Perino, A., and Schoonjans K... 2015. TGR5 and immunometabolism: insights from physiology and pharmacology. Trends Pharmacol. Sci. 36:847–857. doi: 10.1016/j.tips.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Pols, T. W., Nomura M., Harach T., Lo Sasso G., Oosterveer M. H., Thomas C., Rizzo G., Gioiello A., Adorini L., Pellicciari R.,. et al. 2011. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 14:747–757. doi: 10.1016/j.cmet.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, Y., Shi L., Duan G., Ma Y., and Li P... 2021. Taurochenodeoxycholic acid increases cAMP content via specially interacting with bile acid receptor TGR5. Molecules 26:7066. doi: 10.3390/molecules26237066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich, M., Deutschmann K., Sommerfeld A., Klindt C., Kluge S., Kubitz R., Ullmer C., Knoefel W. T., Herebian D., Mayatepek E.,. et al. 2016. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut 65:487–501. doi: 10.1136/gutjnl-2015-309458 [DOI] [PubMed] [Google Scholar]
- Sato, H., Macchiarulo A., Thomas C., Gioiello A., Une M., Hofmann A. F., Saladin R., Schoonjans K., Pellicciari R., and Auwerx J... 2008. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure − activity relationships, and molecular modeling studies. J. Med. Chem. 51:1831–1841. doi: 10.1021/jm7015864 [DOI] [PubMed] [Google Scholar]
- Shao-hong, M., Yu-chong H., Ji-chang J., and Shuang-hu C... 2019. Cloning and prokaryotic expression of PspF gene from Vibrio harveyi. J. Guangdong Ocean Univer. 39:1–7. doi: 10.3969/j.issn.1673-9159.2019.05.001 [DOI] [Google Scholar]
- Shapawi, R., Abdullah F. C., Senoo S., and Mustafa S... 2019. Nutrition, growth and resilience of tiger grouper (Epinephelus fuscoguttatus)×giant Grouper (Epinephelus lanceolatus) hybrid - a review. Rev. Aquacult. 11. doi: 10.1111/raq.12292 [DOI] [Google Scholar]
- Stepanov, V., Stankov K., and Mikov M... 2013. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J. Recept. Signal Transduction 33:213–223. doi: 10.3109/10799893.2013.802805 [DOI] [PubMed] [Google Scholar]
- Sun, S.-C., Chang J.-H., and Jin J... 2013. Regulation of nuclear factor-κB in autoimmunity. Trends Immunol. 34:282–289. doi: 10.1016/j.it.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A., Yang Q., Tan B., Xiao W., Jia J., Dong X., and Zhang S... 2018a. Effects of enzymolytic soybean meal on growth performance, serum biochemical indices, non-specific immunity and disease resistance of juvenile Litopenaeus vannamei. J. Guangdong Ocean Univer. 38, 14–21. [Google Scholar]
- Wang, G., Sun Y., Niu F., He F., Mo W., Zhu X., Cao J., and Huang Y... 2017. Effects of exogenous enzyme supplementation on digestive enzyme activity, apparent digestibility and fecal nitrogen and phosphorus content of juvenile yellow catfish. J Guangdong Ocean Univer. 37:19–25. doi: 10.3969/j.issn.1673-9159.2017.06.004 [DOI] [Google Scholar]
- Wang, L. J., Li J. Y., Xie L., Chen F., and Zheng M. L... 2018b. Analysis of hydrological characteristics for Huguangyan Maar lake with Cruise data in winter and spring.
- Wang, Y. D., Chen W. D., Yu D., Forman B. M., and Huang W... 2011. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology 54:1421–1432. doi: 10.1002/hep.24525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenyu, Z., Wendong H., and Guiyu L... 2012. Tgr5 induces il-1β, tnf-α and il-6 mRNA transcription by p38 mapk pathway in mouse macrophages. J. Third Military Medical Univer 34:07. doi: 10.16016/j.1000-5404.2012.07.009 [DOI] [Google Scholar]
- Xie, J., Qiu D., Liu C., Zhu W., and Zeng L... 2013. Effcets of Vibrio alginolyticus peptidoglycan on astaxanthin level, immune indicators and protection in Litopenaeus vannamei. J. Guangdong Ocean Univer. 33:50–55. doi: 10.3969/j.issn.1673-9159.2018.04.003 [DOI] [Google Scholar]
- Xiong, F., Cao L., Wu X. M., and Chang M. X.. 2021. The function of zebrafish gpbar1 in antiviral response and lipid metabolism. Dev. Comp. Immunol. 116:103955. doi: 10.1016/j.dci.2020.103955 [DOI] [PubMed] [Google Scholar]
- Xiong, Q., Huang H., Wang N., Chen R., Chen N., Han H., Wang Q., Siwko S., Liu M., Qian M.,. et al. 2018. Metabolite-sensing G protein coupled receptor TGR5 protects host from viral infection through amplifying type I interferon responses. Front. Immunol. 9:2289. doi: 10.3389/fimmu.2018.02289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu-ping, F., Xiao-ming Q., Chao-hua Z., Jian-ping C., and Qian-feng Z... 2018. Nutritional and volatile flavor components of dorsal and ventral muscle from hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). J. Guangdong Ocean Univer. 38:8. doi: 10.3969/j.issn.1673-9159.2018.01.006 [DOI] [Google Scholar]
- Xu, J., He G., Chen L., Xie S., Chi S., Zhang S., Cao J., and Tan B... 2022a. Farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (TGR5) signaling pathways improved the hepatic lipid metabolism in hybrid grouper. Aquacult. Rep. 22:100997. doi: 10.1016/j.aqrep.2021.100997 [DOI] [Google Scholar]
- Xu, J., Li X., Yao X., Xie S., Chi S., Zhang S., Cao J., and Tan B... 2022b. Protective effects of bile acids against hepatic lipid accumulation in hybrid grouper fed a high-lipid diet. Front Nutr 9:813249. doi: 10.3389/fnut.2022.813249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Xie S., Chi S., Zhang S., Cao J., and Tan B... 2022c. Protective effects of taurocholic acid on excessive hepatic lipid accumulation via regulation of bile acid metabolism in grouper. Food Funct. 13:3050–3062. doi: 10.1039/d1fo04085e [DOI] [PubMed] [Google Scholar]
- Xu, J., Xie S., Chi S., Zhang S., Cao J., and Tan B... 2022d. Short-term dietary antibiotics altered the intestinal microbiota and improved the lipid metabolism in hybrid grouper fed medium and high-lipid diets. Aquaculture 547:737453. doi: 10.1016/j.aquaculture.2021.737453 [DOI] [Google Scholar]
- Xu, J., Yao X., Li X., Xie S., Chi S., Zhang S., Cao J., and Tan B... 2022e. Bile acids reduced the lipid deposition in fatty degenerated hepatocytes of pearl gentian grouper (Epinephelus fuscoguttatus ♂ × E. lanceolatus ♀) in vitro. Front. Mar. Sci. 9:861–877. doi: 10.3389/fmars.2022.861117 [DOI] [Google Scholar]
- Yoneno, K., Hisamatsu T., Shimamura K., Kamada N., Ichikawa R., Kitazume M. T., Mori M., Uo M., Namikawa Y., Matsuoka K.,. et al. 2013. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology 139:19–29. doi: 10.1111/imm.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Pu X., Yang Q., Tan B., Dong X., Chi S., Liu H., and Zhang S... 2018. Effects of replacing fish meal with high-protein cottonseed meal on growth performance, non-specificiImmune index and disease resistance for Litopenaeus vannamei. J. Guangdong Ocean Univer. 38:20–26. doi: 10.3969/j.issn.1673-9159.2018.04.003 [DOI] [Google Scholar]
- Zhang, W., Tan B., Pang A., Deng J., Yang Q., and Zhang H... 2022. Screening of potential biomarkers for soybean meal induced enteritis in pearl gentian grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). J. Guangdong Ocean Univer. 42:1–12. doi: 10.3969/j.issn.1673-9159.2018.04.003 [DOI] [Google Scholar]
- Zhi-xin, W., Hai-ying L., Xiao-dong D., Rong-lian H., Yue-wen D., Qing-heng W., and Yu J... 2013. Cloning and Express Characters of HSP60 gene from Pinctada martensii. J. Guangdong Ocean Univer. 33:14–23. doi: 10.3969/j.issn.1673-9159.2013.03.001 [DOI] [Google Scholar]
- Zhu, W., Qiu D., Gan Z., Lu Y., and Jian J... 2015. Antivirus effects of Vibrio alginolyticus peptidoglycan on Litopenaeus vannamei against white spot syndrome virus. J. Guangdong Ocean Univer. 35:40–46. doi: 10.3969/j.issn.1673-9159.2015.06.008 [DOI] [Google Scholar]
- Zou, C., Su N., Wu J., Xu M., Sun Z., Liu Q., Chen L., Zhou Y., Wang A., and Ye C... 2019. Dietary Radix Bupleuri extracts improves hepatic lipid accumulation and immune response of hybrid grouper (Epinephelus lanceolatusmale symbol x Epinephelus fuscoguttatusfemale symbol). Fish Shellfish Immunol. 88:496–507. doi: 10.1016/j.fsi.2019.02.052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.