Abstract
Background:
Although the population of older transplant recipients has increased dramatically, there are limited data describing the impact of immunosuppression regimen choice on outcomes in this recipient group.
Methods:
National data for U.S. Medicare-insured adult kidney recipients (N=67,362; 2005–2016) were examined to determine early immunosuppression regimen and associations with acute rejection, death-censored graft failure and mortality using multivariable regression analysis in younger (18–64 years) and older (>65 years) adults.
Results:
The use of anti-thymocyte globulin (TMG) or alemtuzumab (ALEM) induction with triple maintenance immunosuppression (reference) was less common in older compared with younger (36.9% vs 47.0%) recipients, as was TMG/ALEM + steroid avoidance (19.2% vs 20.1%) and mTORi-based (6.7% vs 7.7%) treatments. Conversely, older patients were more likely to receive IL2-receptor antibody (IL2rAb) + triple maintenance (21.1% vs 14.7%), IL2rAb + steroid avoidance (4.1% vs 1.8%), and cyclosporine-based (8.3% vs 6.6%) immunosuppression. Compared to older recipients treated with TMG/ALEM + triple maintenance (reference regimen), those managed with TMG/ALEM + steroid avoidance (adjusted odds ratio (aOR), 0.440.520.61) and IL2rAb + steroid-avoidance (aOR, 0.390.550.79) had lower risk of acute rejection. Older patients experienced more death censored graft failure when managed with Tac+ antimetabolite avoidance (adjusted hazard (aHR), 1.411.782.25), mTORi-based (aHR, 1.702.142.71), and cyclosporine-based (aHR, 1.411.782.25) regimens, versus the reference regimen. mTORi-based and cyclosporine-based regimens were associated with increased mortality in both older and younger patients.
Conclusions:
Lower-intensity immunosuppression regimens (e.g. steroid-sparing) appear beneficial for older kidney transplant recipients, while mTORi and cyclosporine-based maintenance immunosuppression are associated with higher risk of adverse outcomes.
INTRODUCTION
Kidney transplantation is the preferred treatment option for patients with kidney failure, providing superior clinical outcomes at lower healthcare costs than chronic dialysis.1 In recent years, the number of elderly patients with kidney failure has risen substantially worldwide, due to a global increase in the aging population, improved survival on dialysis,2–5 and increased referral of older patients for transplantation.6–8 The growing number of elderly kidney transplant candidates is correlated with a rise in the number and proportional representation of elderly kidney transplant recipients.9,10 In the United States, the percentage of kidney transplant recipients age >65 years increased from 14% in 1999 to 24% in 2018.2,9,11Similar trends have been observed in other countries.12,13
Older recipients appear to have improved short-term death-censored graft survival as compared with younger patients, perhaps due to immunosenescence and decreased acute rejection risk.14,15 While the incidence of acute rejection tends to fall with age, the risk of allograft loss following a rejection event is significantly increased in elderly recipients.5,16 Older recipients also carry a higher risk of chronic allograft loss resulting from a variety of age-related immune and non-immune factors.17,18 Age-related immunologic and non-immunologic changes increase the susceptibility of elderly recipients to post-transplant cardiovascular disease, infection, and malignancy, contributing to significant morbidity and mortality.17 In aggregate, the increase risk of death among older recipients results in lower 5-year kidney allograft survival rates compared to younger recipients. 9,14,15
Immunosuppressive (ISx) management in older kidney transplant recipients is complex, given increased risk of infection and malignancy after transplant in this population,16,19–23 reduced immunogenicity, 24,25 and greater likelihood of receiving a higher-risk donor organ. 11 General guidelines suggest that tacrolimus (Tac), mycophenolate mofetil (MMF), and corticosteroids be used as first-line maintenance ISx agents following kidney transplantation, which remains the most commonly prescribed ISx protocol across all age groups. 2,24,26 However, age-related immune dysfunction and associated co-morbidities make older recipients more susceptible to complications associated with ISx agents.17,27 Thus, practitioners have considered tailoring ISx protocols for older kidney transplant recipients, such as favoring steroid avoidance/withdrawal, antimetabolite avoidance, and mammalian target of rapamycin inhibitor (mTORi)-based regimens (with CNI avoidance or minimization protocols).2,5,17,28–31Robust data to guide these decisions is lacking as most reports are the result of small, single-center observational studies with limited follow-up. 2,5 These trials lack sufficient sample size to adjust for pertinent donor and recipient characteristics beyond recipient age.
Given the paucity of trial data on preferred ISx regimen in the older kidney transplant recipients, we examined the impact of early ISx regimen selection (induction and maintenance over the first 6 months) on outcomes in a national cohort of U.S. transplant recipients with sufficient sample size to adjust for potential confounding effects including donor characteristics. Our goal was to examine associations of ISx regimen selection with graft and patient survival among older (age ≥65 years) and younger (age 18–64) recipients. By providing data from a large, robust national cohort, we hope to inform ISx regimen selection for at risk older adults and plan future study to better tailor management older kidney transplant recipients.
METHODS
Data Source and Sampling
Study data were drawn from U.S. Renal Data System (USRDS) records, which integrate Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS) records with Medicare billing claims. The primary study sample comprised adult (age ≥18 years) recipients of kidney-only transplants in the U.S. from 2005 to 2016 with Medicare as the primary payer for the first 6 months post-transplant, and ISx data available during this period. Because our primary exposure was based on pharmacy claims for ISx, we also required Medicare-reimbursed fills for ISx in the first 6 months after transplantation. Younger and older adults were defined by age 18–64 versus ≥65 years, respectively, consistent with Medicare payments guidelines which provide coverage based on age 65 and geriatrics/gerontology standards.32 This study was deemed to be Human Subjects Exempt by the Institutional Review Board of Saint Louis University.
Definition of Immunosuppression Regimens
Use of induction agents was defined by OPTN reporting. Early maintenance ISx regimen was defined using Medicare pharmacy claims for ISx agents submitted within the first 6 months after transplant, reimbursed through Part B or Part D benefits. Patients were classified based on induction and maintenance ISx regimens into 7 study regimens, similar to previous methods: 33
Triple maintenance, after T-cell depleting induction: Anti-thymocyte globulin (TMG)/ alemtuzumab (ALEM) + Tac + MPA (mycophenolic acid: MMF or mycophenolate sodium)/azathioprine (AZA) + prednisone (Pred)
Triple maintenance, after IL2-receptor antibody (IL2rAb) induction: IL2rAb + Tac + MPA/AZA + Pred
Steroid avoidance/withdrawal, after T-cell depleting induction: TMG/ALEM + Tac + MPA/AZA, without Pred
Steroid avoidance/withdrawal, after IL2rAb induction: IL2rAb + Tac + MPA/AZA, without Pred
Antimetabolite avoidance: Tac alone or Tac + Pred
mTORi-based regimens
Cyclosporine (CsA)-based regimens
Triple maintenance therapy with T-cell depleting induction, was considered the reference regimen as it was the most frequently used regimen during the study period. MPA included mycophenolate mofetil and mycophenolate sodium. IL2rAb included the two agents available in the U.S. in the study period, basiliximab and daclizumab. Groups 5 to 7 were defined independent of induction. Patients in groups 1 to 5 did not receive mTORi or CsA. mTORi-based ISx was classified before CsA-based to enable assignment of mutually exclusive regimens, as per previous methods.34 mTORi- and CsA-based regimens were not further sub-classified due to low frequencies of patients treated with these regimens. Specific data on fill patterns, regimen weaning, compliance and drug levels are not provided in this database.
Covariate and Outcome Measures
Transplant recipient clinical and demographic characteristics, as well as characteristics of the donated organ and other transplant factors, were defined by the OPTN Transplant Candidate Registration and Transplant Recipient Registration forms (Table 1).
Table 1.
Distributions of early immunosuppression regimen use among kidney transplant recipients according to baseline clinical traits (N=67,362)
| TMG/ALEM+Triple Therapy (Reference) (N=30,134) | IL2rAb+Triple Therapy (N=10,836) | TMG/ALEM+ Tac+MPA/AZA,No Pred (N=13,055) | IL2rAb+ Tac+MPA/AZA, No Pred (N=1,553) | Tac, Tac+Pred (N=2,034) | mTORi-based (N=5,043) | CsA-based (N=4,707) | |
|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | |
| Age, years | |||||||
| 18 to 64 | 47.0 | 14.7 | 19.2 | 1.8 | 3.1 | 7.7 | 6.6 |
| ≥65 | 36.9 | 21.1 | 20.1 | 4.1 | 2.8 | 6.7 | 8.3 |
| Sex | |||||||
| Male | 42.3 | 17.2 | 20.1 | 2.4 | 3.1 | 7.9 | 7.0 |
| Female | 48.4 | 14.5 | 18.2 | 2.1 | 3.0 | 6.9 | 6.9 |
| Race | |||||||
| White | 39.5 | 17.9 | 20.1 | 3.0 | 3.4 | 8.2 | 7.9 |
| African American | 53.4 | 12.5 | 17.8 | 1.3 | 2.7 | 7.3 | 5.1 |
| Hispanic | 45.2 | 16.2 | 20.8 | 2.2 | 2.4 | 6.4 | 6.8 |
| Other | 40.3 | 19.8 | 17.5 | 2.7 | 3.2 | 6.3 | 10.3 |
| Employment status | |||||||
| Working | 43.4 | 15.9 | 21.5 | 2.4 | 3.7 | 7.2 | 5.9 |
| Not working | 45.1 | 16.4 | 18.8 | 2.4 | 2.8 | 7.7 | 6.9 |
| Body mass index, kg/m2 | |||||||
| <18.5 | 47.5 | 17.4 | 13.7 | 2.8 | 3.8 | 7.8 | 7.1 |
| 18.5 to 24.9 | 45.3 | 16.9 | 17.8 | 2.4 | 3.1 | 7.5 | 7.0 |
| 25.0 to 29.9 | 43.9 | 16.9 | 19.0 | 2.6 | 3.0 | 7.9 | 6.8 |
| ≥30.0 | 45.6 | 14.9 | 20.7 | 2.0 | 2.8 | 7.0 | 7.0 |
| Comorbid conditions | |||||||
| Hypertension | 43.5 | 16.0 | 19.5 | 2.4 | 3.0 | 8.4 | 7.3 |
| Diabetes mellitus | 41.6 | 16.7 | 20.9 | 2.7 | 3.1 | 7.3 | 7.7 |
| Coronary artery disease | 36.2 | 16.7 | 19.6 | 3.7 | 3.4 | 10.6 | 9.8 |
| Cerebral vascular disease | 34.1 | 16.1 | 24.2 | 2.6 | 1.5 | 10.9 | 10.7 |
| Peripheral vascular disease | 42.0 | 17.7 | 20.2 | 3.0 | 2.3 | 7.0 | 7.8 |
| COPD | 33.6 | 14.0 | 21.4 | 4.8 | 3.3 | 11.3 | 11.6 |
| Hepatitis C positive | 43.9 | 18.2 | 14.7 | 2.9 | 3.2 | 6.7 | 10.4 |
| Cause of ESKD | |||||||
| Hypertension | 46.6 | 15.2 | 19.3 | 2.0 | 2.7 | 8.0 | 6.3 |
| Diabetes mellitus | 41.2 | 16.8 | 21.6 | 2.8 | 3.0 | 7.1 | 7.6 |
| Glomerulonephritis | 48.0 | 15.3 | 17.6 | 2.0 | 3.0 | 7.3 | 6.7 |
| Polycystic kidney disease | 41.9 | 16.0 | 21.5 | 2.1 | 3.4 | 8.3 | 6.7 |
| Other | 43.5 | 18.4 | 16.5 | 2.7 | 3.8 | 7.4 | 7.8 |
| Duration of dialysis, months | |||||||
| None (pre-emptive) | 28.9 | 22.7 | 22.4 | 4.5 | 4.3 | 7.7 | 9.4 |
| >0 to 24 | 35.1 | 20.8 | 20.6 | 3.7 | 3.6 | 8.3 | 7.9 |
| 25 to 60 | 42.9 | 16.0 | 21.3 | 2.2 | 3.1 | 7.9 | 6.7 |
| >60 | 53.1 | 13.1 | 16.7 | 1.5 | 2.5 | 6.7 | 6.5 |
| Most current PRA level (%) | |||||||
| <10 | 39.0 | 18.6 | 21.6 | 2.7 | 3.2 | 7.6 | 7.4 |
| 10 to 79 | 55.2 | 11.9 | 15.7 | 1.4 | 2.8 | 6.9 | 6.1 |
| ≥80 | 70.2 | 6.0 | 11.0 | 0.5 | 2.2 | 5.2 | 4.9 |
| HLA mismatches | |||||||
| Zero A, B, DR | 39.9 | 17.8 | 17.2 | 5.2 | 3.3 | 7.6 | 8.9 |
| Zero DR | 45.1 | 15.0 | 20.5 | 2.2 | 3.1 | 7.0 | 7.0 |
| Other | 45.1 | 16.1 | 19.4 | 2.1 | 3.0 | 7.5 | 6.8 |
| Cold ischemia time, hours | |||||||
| ≤12 | 42.1 | 19.0 | 18.5 | 2.7 | 3.0 | 7.9 | 6.9 |
| 13 to 24 | 48.4 | 14.9 | 18.1 | 1.8 | 2.9 | 7.2 | 6.7 |
| 25 to 36 | 48.3 | 13.0 | 20.6 | 1.5 | 2.9 | 7.0 | 6.7 |
| >37 | 43.3 | 7.9 | 32.7 | 1.2 | 2.9 | 7.1 | 4.9 |
| Previous organ transplant | |||||||
| Yes | 59.2 | 10.0 | 11.5 | 1.3 | 3.3 | 7.8 | 7.1 |
| No | 42.2 | 17.2 | 20.8 | 2.5 | 3.0 | 7.4 | 7.0 |
| Donor Type | |||||||
| Living donor | 34.6 | 20.5 | 22.3 | 3.9 | 3.2 | 7.7 | 7.8 |
| Deceased, KPDI <20 | 45.7 | 16.8 | 17.7 | 2.1 | 3.9 | 6.7 | 7.1 |
| Deceased, KDPI 20 to 85 | 49.0 | 14.2 | 18.5 | 1.7 | 2.7 | 7.3 | 6.7 |
| Deceased, KDPI >85 | 44.0 | 14.5 | 20.4 | 2.3 | 2.9 | 9.7 | 6.4 |
| Transplant era | |||||||
| 2005 to 2008 | 33.8 | 15.5 | 19.7 | 3.1 | 4.6 | 11.7 | 11.7 |
| 2009 to 2012 | 45.2 | 16.3 | 20.4 | 2.5 | 2.8 | 6.5 | 6.3 |
| 2013 to 2016 | 53.4 | 16.4 | 18.0 | 1.4 | 1.9 | 5.1 | 3.9 |
Percentages are row percentages. P<0.05 for comparison of distributions of ISx regimen across all traits, due to large sample size
“Other race” includes Asian, Native American, Pacific Islander, and multi-racial.
Abbreviations: ALEM, alemtuzumab; AZA, azathioprine; COPD, chronic obstructive pulmonary disease; CsA, cyclosporine A; ESKD, end-stage kidney disease; HLA, human leukocyte antigen; IL2, interleukin-2; KDPI, kidney donor profile index; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitor; No Pred, no prednisone use post-transplant; Pred, prednisone use documented post-transplant; Tac, tacrolimus; TMG, anti-thymocyte globulin; Triple Therapy, Tac+MPA/AZA+Pred; PRA, panel reactive antibody.
Graft failure was defined as return to maintenance dialysis or retransplant. Patient death was defined by transplant center reports to OPTN and supplemented with the Social Security Death Master File. All-cause graft failure included graft loss due to death. The OPTN queries centers for information on acute rejection according to periods covered by specific reporting forms (0 to 6 months, 7 to 12 months, then annually), but dates of acute rejection within reporting periods are not collected. We defined acute rejection from OPTN records according to center reports occurring in a reporting period, as per prior methods.35–37 Rejection was analyzed through 3 years given declining incidence and completion of rejection reporting beyond 3 years.
Statistical Analyses
Clinical characteristics of the study sample were described as proportions. Continuous variables were categorized into clinically relevant strata. Missing categorical covariate data were grouped with the absence of a characteristic when such categories were relevant, or into a category distinct from the reference group. Distributions of ISx regimen use according to baseline characteristics were compared by the Chi-square test.
The analysis was stratified based on age group (18–64 versus ≥65 years). Given ISx ascertainment during the first 6-months after transplant, origin time for outcomes analyses began at 6-months post-transplant. Death-censored graft survival, patient survival, and all-cause graft survival over time after 6-months post-transplant were estimated using Kaplan-Meier method. The adjusted association of ISx regimen with graft failure and mortality (adjusted hazard ratio, 95%LCLaHR95%UCL) after transplant was assessed using multivariable Cox proportional hazards analysis, adjusted for recipient, donor, and transplant factors (Table 1). At-risk time was censored at 5 years post-transplant or the end of study (December 31, 2016). Logistic regression was used to assess the adjusted odds ratio (aOR) of any acute rejection event by 3 years after transplant. Outcome models were also stratified by quintile of propensity for assignment to each ISx regimen compared with the reference regimen in binomial logistic regression, as per previous methods.37
The primary comparisons examined outcomes associated with each regimen compared to the reference regimen, within older and younger adults. We also assessed for interactions between ISx regimens and age groups by testing interaction terms in the model. A p-value <0.05 was considered statistically significant. Data management and analysis were performed using SAS version 9.4 (SAS institute Inc., Cary, NC, USA).
RESULTS
Clinical characteristics
Of 193,984 kidney transplant recipients in the study period, 67,362 had Medicare claims data for study ISx regimens within the first 6 months after transplant. Compared to recipients without available data for inclusion, the study sample of Medicare beneficences included a higher representation of older (22.7% vs. 14.9%) and patients who were non-white (54.2% vs 45.6%) and unemployed (73.8% vs 53.4%) (Table S1). Medicare beneficiaries also had longer periods of pretransplant dialysis (>60 months: 39.9% vs. 24.4%) with correspondingly fewer living donor transplant (22.8% vs 41.4%).
Recipient characteristics were associated with the ISx regimen selection (Table 1). Use of some regimens was less common in older compared to younger recipients, including TMG/ALEM + triple maintenance (36.9% vs 47.0%), TMG/ALEM + steroid avoidance (19.2% 20.1%), antimetabolite avoidance (2.8% vs 3.1%), and mTORi-based, 6.7% vs 7.7%. Conversely, IL2rAb + triple maintenance (21.1% vs 14.7%), IL2rAb + steroid avoidance (4.1% vs 1.8%), and CSA-based, 8.3% vs 6.6% were more common in older compared to younger recipients. Compared to white patients, African American patients more commonly received TMG/ALEM + triple maintenance (53.4% vs 39.5%) and less commonly received IL2rAb + triple maintenance (12.5% vs 17.9%) or steroid-free regimens (17.8% vs 20.1%). Use of TMG/ALEM + triple maintenance was substantially more common among highly sensitized patients with panel reactive antibody (PRA) ≥80% compared to those with PRA <10% (70.2% vs 39.0%) while use of IL2rAb + triple maintenance was less common (6.0% vs 18.6%). Use of TMG/ALEM + triple maintenance was also more common among retransplant recipients while the use of CsA and mTORi based regimens decreased in more recent years of study. Similar patterns in the distribution of ISx regimen use by baseline traits among both younger and older patients (Tables S2A and S2B).
Acute rejection
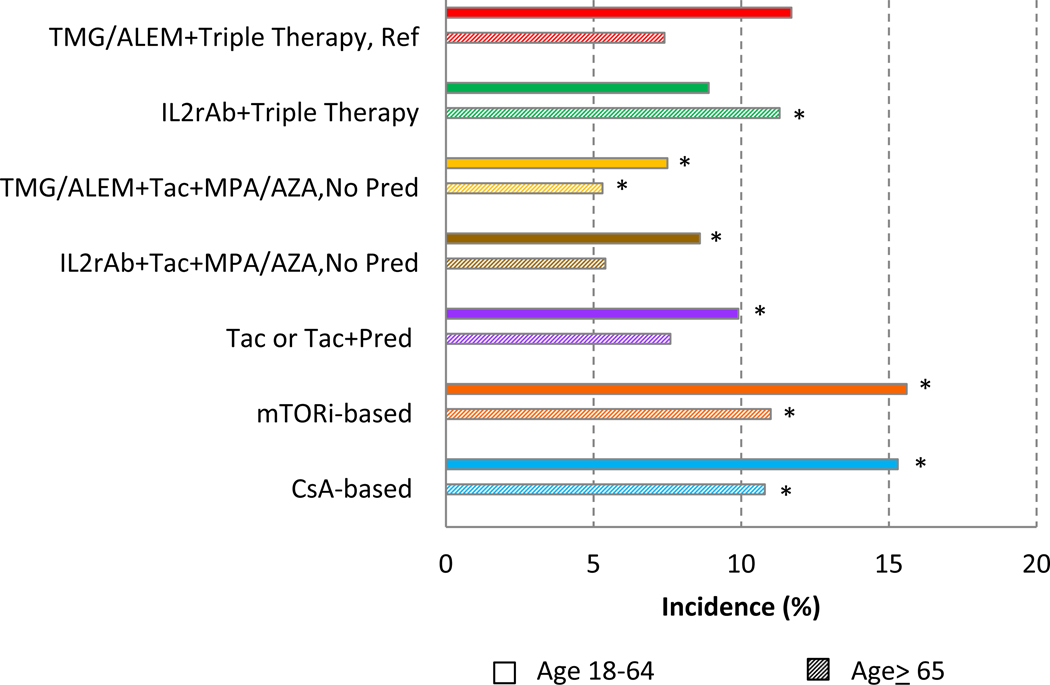
Compared with younger patients, older recipients experienced a lower risk of acute rejection >6 months to 3 years for all regimens except IL2rAb + triple therapy (Figure 1). Among older recipients, the unadjusted incidence of acute rejection was significantly higher in those who received mTORi-based (11.0%) and CsA-based (10.8%) regimens, and significantly lower in those who received TMG/ALEM + Tac + MPA/AZA (5.3%) than those on the maintenance regimen (7.4%). Similar patterns were noted among younger adults, when comparing the unadjusted incidence of acute rejection patients who received TMG/ALEM + triple maintenance (11.7%), to those who received TMG/ALEM + Tac + MPA/AZA (7.5%), IL2rAb+Tac+MPA/AZA (8.6%), Tac, Tac+Pred (9.9%), mTORi-based (15.6%) and CsA-based (15.3%) ISx regimens (Figure 1)
Figure 1.
Acute rejection incidence >6 months to 3 years after kidney transplant, by early ISx regimen and recipient age. * P<0.05 for comparison to reference regimen, within each age group. ALEM, alemtuzumab; AZA, azathioprine; CsA, cyclosporine; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitor; Pred, prednisone; Tac, tacrolimus; TMG, anti-thymocyte globulin
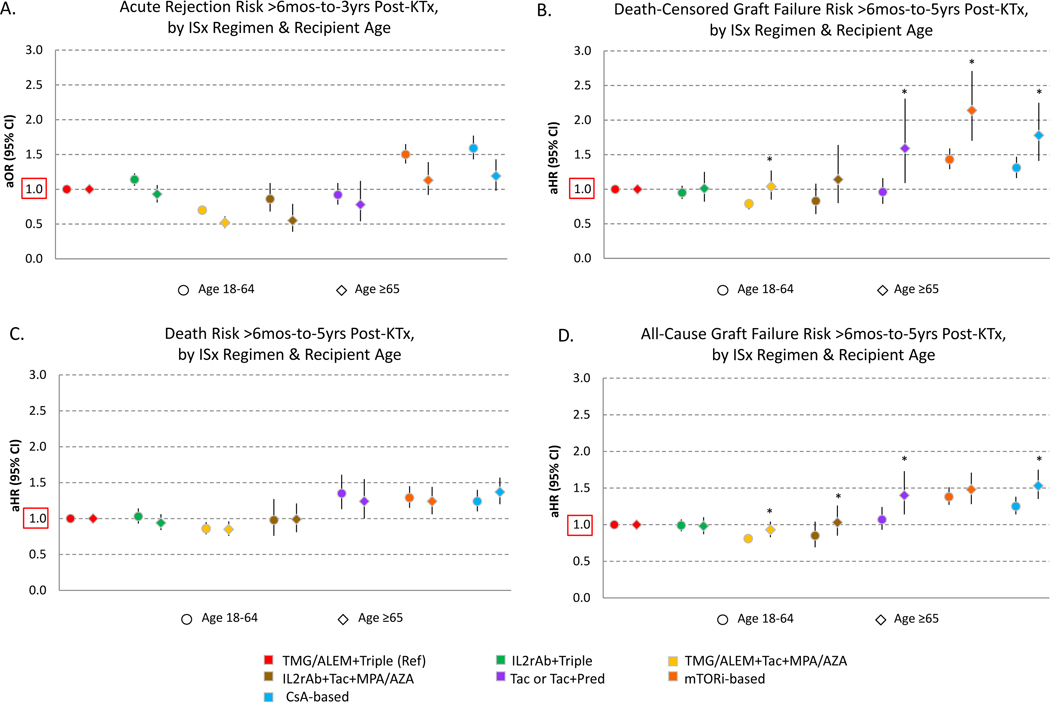
Compared to the reference regimen, the incidence of acute rejection varied significantly by ISx after multivariate adjustment (Figure 2A). Patients aged ≥65 years receiving TMG/ALEM + steroid avoidance (aOR 0.440.520.61) and IL2rAb + steroid-avoidance (aOR 0.390.550.79) experienced lower adjusted risk of acute rejection, while CsA-based ISx was associated with increased risk (aOR 0.981.191.43). Similarly, among recipients aged 18–64, TMG/ALEM + steroid avoidance (aOR 0.640.700.76) was associated with a lower risk of acute rejection, while IL2rAb + triple ISx (aOR 1.051.141.23), mTORi-based (aOR 1.371.501.65) and CsA-based (aOR 1.431.591.77) regimens were also associated with increased risk of acute rejection.
Figure 2.
Relative risks of acute rejection (A), death censored graft failure (B), death (C), and all cause graft failure (2D) according to early ISx regimen and recipient age. Confidence intervals designate comparison of each regimen to the reference regimen, within age groups. *P<0.05 for test of interaction of age group and regimen effects.
Death-censored graft failure
Unadjusted death-censored graft failure >6 months to 5 years after kidney transplant among patients aged ≥65 years who received TMG/ALEM + triple ISx (8.2%) was similar to patients treated with IL2r + triple ISx (7.4%) and TMG/ALEM + Tac/MMF (8.3%), but lower than those treated with Tac + antimetabolite-avoidance (11.7%), mTORi-based (16.3%) or CsA-based (12.4%) regimens (Figure 3A). Conversely, recipients aged 18–64 who received the reference regimen had higher death censored graft failure (13.9%) rates than patients who received IL2rAb + triple ISx (12.5%), TMG/ALEM + steroid avoidance (10.6%) or IL2rAb + steroid-avoidance (10.8%), but lower than in those who received mTORi-based (19.3%) or CsA-based (16.3%) regimens.
Figure 3.
Death-censored draft failure (A) and death (B) incidence >6 months to 5 years after transplant, according to early ISx regimen and recipient age.
After covariate and propensity adjustment, compared with older recipients on the reference regimen, Tac + antimetabolite avoidance (aHR, 1.091.592.31), mTORi-based (aHR, 1.702.142.71), and CsA-based (aHR, 1.411.782.25) regimens were significantly associated with higher adjusted death-censored graft failure risk (Figure 2B). Among recipients aged 18–64 years, TMG/ALEM + steroid-avoidance (aHR, 0.710.790.86) was associated with significantly lower risk of death-censored graft failure compared to the reference regimen, whereas mTORi-based (aHR, 1.291.431.59) and CsA-based regimens (aHR, 1.161.311.47) were associated with significantly increased death-censored graft failure risk. Interaction testing demonstrated that these risks varied significantly by age for TMG/ALEM + steroid-avoidance, Tac + antimetabolite avoidance, mTORi-based and CsA-based regimens
Patient mortality
Among adults age 18–64, compared to patient mortality >6 months to 5 years among those who received the reference regimen of TMG/ALEM + triple ISx (11.2%), unadjusted mortality was significantly higher in those who received Tac + antimetabolite avoidance (14.0%), mTORi-based (14.2%) or CsA-based (13.9%) regimens (Figure 3B). Among patients aged ≥65, compared to mortality in those who received the reference regimen (24.0%), unadjusted mortality was significantly higher in patients who received mTORi-based (29.3%) and CsA-based (31.6%) regimens, and lower in those who received IL2rAb + triple maintenance (21.9%) and TMG/ALEM + steroid avoidance (21.0%). Distribution of causes of death varied by ISx regimen, but patterns were generally similar across age groups excepted as shown (Figure S1A).
After covariate and propensity adjustment, in older recipients, Tac + antimetabolite avoidance (aHR, 1.001.241.55), mTORi-based (aHR, 1.061.241.44), and CsA-based (aHR,1.201.371.57) regimens were associated with significantly higher mortality than the reference regimen (Figure 2C). Similarly, in recipients aged 18–64 years, TMG/ALEM + steroid avoidance (aHR, 0.780.860.95) was associated with significantly lower mortality compared to the reference regimen, whereas Tac + antimetabolite avoidance (aHR, 1.131.351.61), mTORi-based (aHR, 1.151.291.45) and CsA-based regimens (aHR,1.101.241.40) were associated with significantly higher mortality. (Figure 3). There were no significant interactions of age and regimen for patient mortality.
Graft failure
Among older adults, compared to the reference regimen, Tac + antimetabolite avoidance (aHR, 1.141.401.73), mTORi-based (aHR, 1.281.481.71) and CsA-based (aHR, 1.351.531.75) regimens were significantly associated with all-cause graft failure (Figure 2D). In younger patients, compared to the reference regimen of TMG/ALEM + triple maintenance, adjusted all-cause graft failure risk was significantly lower in those who received TMG/ALEM + steroid avoidance (aHR, 0.750.810.87), but significantly higher in those who received mTORi-based (aHR, 1.271.381.51) and CsA-based (aHR, 1.141.251.38) regimens. These effects differed significantly by age for TMG/ALEM + steroid avoidance, IL2rAb + steroid-avoidance, Tac + antimetabolite avoidance, and CsA-based regimens. Cause of graft failure differed by regimen and recipient age. Graft loss due to rejection was a more common cause of graft loss in older patients, while recurrent disease occurred more frequently in younger patients (Figure S1B).
DISCUSSION
We examined associations of early posttransplant kidney transplant ISx regimen with graft and patient survival in a large national cohort of older and younger adults, and observed several key findings. First, there has been a significant shift in ISx regimen away from CsA- and mTOR-based regimens to Tac-based regimens accompanied by greater use of T-cell depleting antibodies. This shift occurred across age groups. Second, while older adults are less likely than younger patients to be maintained on triple therapy with T-cell depleting induction, many older patients are still exposed to potent ISx regimens despite growing evidence of immunosenescence and higher risks of infection and malignancy in older transplant patients. Finally, lower intensity ISx regimens (steroid-sparing or IL2rAb induction with triple therapy) have statistically equivalent graft outcomes to T-cell depleting antibodies with triple ISx in older patients and, in the case of steroid-sparing regimens, lower risks of acute rejection and death.
In the older transplant recipient population, immunosenescence is considered a shift from naïve T-cells toward relatively more memory T-cells, leading to reduced immune reactivity.18,38–40 While older recipient age appears protective against acute rejection,5,16 age-related immune dysfunction and associated co-morbidities make the older recipients more susceptible to complications from ISx agents such as infections and cancer.5,17,27,20,22,41,42 These complications can result in death, and lower intensity ISx may be protective, as observed in this study. Amongst older kidney transplant recipients, the dominant cause of allograft loss is death with functioning graft.41,43 Compared to kidney transplant recipients aged 18–29 years, previous studies have shown those aged >65 had a 7-fold risk of death with function due to greater burdens of cardiovascular mortality.13,44 Therefore, tailoring of early ISx regimens to minimize the risks of accelerated cardiovascular disease should be considered, particularly those that eliminate corticosteroids.2,45
Although corticosteroids have traditionally been a mainstay of maintenance ISx in kidney transplant recipients, the elderly are more susceptible than younger patients to corticosteroid-related side-effects including infections, post-transplant diabetes, fractures, myopathy.45,46 Previous systematic reviews have demonstrated that early steroid withdrawal/avoidance is well tolerated in low-risk kidney allograft recipients treated with modern potent ISx; however, data evaluating this strategy in older recipients were lacking due to lack of data sets with sufficient sample sizes.47–50 Recently, using the OPTN/UNOS database, Harris et al. evaluated the outcomes of kidney transplant recipients aged >65 with steroid avoidance/early withdrawal (based on steroid use at discharge), and found comparable patient survival and death-censored graft survival at 3 years.51 Our current study of Medicare-insured U.S. kidney transplant recipients has the benefit of observing actual medication fill patterns. We report significantly reduced mortality among recipients (both older and younger groups) managed with steroid avoidance/withdrawal after T-cell depleting induction. These data support the move to further personalize the ISx regimen according to recipient and donor characteristics and limit exposure to more intense ISx regimens.
Although supporting evidence is limited, mTORi-based regimens (either de novo or conversion) have been suggested as an option to reduce CNI exposure, preserve renal function, and improve survival.52 The use of mTORi has also been associated with a lower incidence of post-transplant malignancy in the clinical trials53–55 and further supported by an analysis of UNOS database (with a 60% reduction in the risk of any post-transplant malignancy and a 55% reduction in the risk of solid malignancy).56 The TRANSFORM study was conducted to compare the outcomes of 2,037 kidney transplant recipients randomized to standard dose CNI + MMF versus reduced CNI + mTORi (everolimus).57 Compared to standard dose CNI + MMF, the reduced CNI + mTORi group had comparable allograft function with a significantly lower incidence of viral infections at 1-year post-transplant. The mean age of patients was 49.3 years, and follow-up time was only at 1-year post-transplant.57 Notably, the use of mTORi was associated with an increased risk of impaired wound healing, interstitial lung disease, and lipid abnormalities.52
However, it is not clear whether mTORi trial data generalize to the older adult population who may be more at risk of complications from antimetabolites and mTORis. The SENATOR trial, conducted by two German centers in the European Senior Program, reported increased adverse events in older patients transitioned from CNIs to everolimus.58 In this trial, kidney transplant recipients aged >65 years receiving their first kidney from an older deceased donor aged (>65 years) were randomized to standard therapy with CNI and MMF or conversion from standard therapy to MMF + everolimus with basiliximab at week 7. Only 77 (37.2%) of 207 enrolled patients were randomized, with the majority of patients excluded due to abnormal lab values or acute rejection (17% of trial participants) prior to randomization. Among the patients who were converted, those who remained on everolimus had comparable kidney function at 6-month post-transplant. However, there was a higher rate of discontinuation of trial assigned regimen (27.8% vs. 0%) and acute rejection (21.9% vs. 10%) among patients randomized to everolimus.58 In the current study, older patients treated initially with mTORi-based regimens experienced a 2-fold greater odds of death-censored graft failure and higher risk adjusted mortality. These data suggest that mTORi treatment should be undertaken cautiously in older patients.
The addition of MMF allows CNI and steroid doses to be decreased or withdrawn, with a positive impact on long-term allograft outcome. Earlier studies showed concern regarding increased adverse events with MMF among older transplant recipients, especially opportunistic infection, graft loss and mortality,59,60 and thus antimetabolite avoidance regimen has been proposed as potential regimen for older recipients.5,11,17,27,31,60 However, there is conflicting data on the effect of MMF on infectious complications in older kidney transplant recipients,59–61and a subsequent study demonstrated the benefits and safety of MMF in older recipients ≥55 years.61 Nevertheless, limitations of this study included small sample size, differences in length of follow-up evaluation between each study group.61 In our study, we demonstrated that antimetabolite avoidance was associated with higher risks of graft failure and mortality among older recipients in comparison to steroid avoidance and triple therapy regimens, suggesting that the benefit of elimination are offset by complications.
The field has also moved away from CsA-based ISx. CsA-based regimens decreased from 11.7% of patients to 3.9% between 2005 and 2016. CsA is generally believed to be less potent than Tac resulting in inferior outcomes after transplant. 62 In this analysis, CsA-based regimens were associated with higher rates of death-censored graft failure and mortality for younger and older patients. These findings may reflect altered CNI pharmacokinetics in older patients. In a prospective study of nearly 2,500 patients, CNI levels were 50% higher in patients ≥65 years old, potentially due to changes in CYP3A4 with aging.63 This enhanced CNI level is compounded by greater variation in CsA levels in older adults, particularly with generic formulations, which may contribute to the observed adverse outcomes. Consequently, older patients without conditions that preclude the use of Tac (e.g. severe neurologic side effects) appear to benefit from early Tac-based therapy.
While a national linkage of transplant registry and pharmacy claims offers the opportunity to study the impact of early ISx regimen on outcomes with sufficient sample size, diversity of management approaches, and follow-up to generate meaningful conclusions, there are inherent limitations in this type of analysis. First, this is a retrospective analysis of Medicare beneficiaries, and results may not generalize to kidney transplant recipients with private insurance.64 Given the focus on elderly patients, these Medicare data are likely an accurate reflection of outcome in this age group as the proportion of older adults was enriched in this sample. Second, exact ISx exposure, weaning plans, drug levels, and side effects are not available using these data. However, the assignment to the ISx regimen is likely to be accurate because, unlike center reported data in UNOS, these data represent paid claims in the Medicare system. A pharmacy data confirms that prescriptions were filled, medication adherence may vary across different regimens, and therapeutic drug level monitoring was not available. As the pharmacokinetics of CNIs change with older age and maintenance therapy with CNIs among older transplant recipients potentially needs more frequent monitoring and adjustments, it is possible that observed outcome differences were due to medication management as well as selection.65 While we assume lower exposure in patients on only two maintenance medications, this is not validated with objective measures. Third, it is possible that the choice of ISx regimen might have been affected by uncaptured risk factors in the database such as prior history of malignancy, biopsy data, other donor characteristics, intolerance of standard medications, or inability to afford these medications. For example, mTORi-based regimens may have been selected for recipients who received allografts with higher chronic Banff scores and higher vascular intimal thickening on biopsy data to avoid CNI toxicity. Conversely, patients with new-onset diabetes mellitus after transplant may have been switched to CSA to avoid this use. Fourth, early ISx choice is highly influenced by center practices.34 Consequently, center practice associated with higher graft failure may be associated with the use of non-standard ISx. The USRDS database does not provide center identifiers and, therefore, we could not independently assess the impact of center performance patterns on patient outcomes with different ISx regimens.
In summary, ISx selection after kidney transplant should be personalized based on donor and recipient risk factors. Older patients represent a growing but high-risk population of kidney transplant recipients. Our results suggest that reduced exposure to ISx may be beneficial. Alternative regimens, including CsA- and mTORi-based maintenance therapy, were associated with higher risk rates of adverse outcomes. Further study is needed to characterize the impact of alternations of pharmacokinetics associated with aging on transplant outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The data reported here have been supplied by the United States Renal Data System (USRDS). This work was funded by a grant from National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) R01DK120518. K.L.L. is supported by the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Funding:
This work was funded by a grant from National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) R01DK120518. K.L.L. is supported by the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation.
Abbreviations
- aHR
adjusted hazard ratio
- ALEM
alemtuzumab
- aOR
adjusted odds ratio
- AZA
azathioprine
- CNI
calcineurin inhibitor
- CsA
cyclosporine
- DCGF
death-censored graft failure
- IL2rAb
interleukin-2-receptor antibody
- ISx
immunosuppression
- KDPI
kidney donor profile index
- KTx
kidney transplant
- MMF
mycophenolate mofetil
- MPA
mycophenolic acid
- mTORi
mammalian target of rapamycin inhibitor
- OPTN
Organ Procurement and Transplantation Network
- PRA
panel reactive antibody
- Pred
prednisone
- Tac
tacrolimus
- TMG
anti-thymocyte globulin
- UNOS
United Network of Organ Sharing
- USRDS
U.S. Renal Data System
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose.
Authors Roles:
KLL and MAS participated in study design, acquisition of data and regulatory approvals, interpretation and writing of the manuscript. WC, NNL and DA participated in study design, interpretation, and writing of the manuscript. HX participated in data analysis and manuscript preparation. MMD, DLS, SB, JBA, GPH, YC, HBR and BLK participated in study design, interpretation, and manuscript review/critical editing as part of our pharmacoepidemiologic consortium.
REFERENCES
- 1.Axelrod DA, Schnitzler MA, Xiao H, et al. An economic assessment of contemporary kidney transplant practice. Am J Transplant. 2018;18(5):1168–1176. [DOI] [PubMed] [Google Scholar]
- 2.Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation. 2007;84(3):285–291. [DOI] [PubMed] [Google Scholar]
- 3.Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saran R, Robinson B, Abbott K. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 73. Doi: 10.1053/j.ajkd.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoll GA. Kidney transplantation in the older adult. Am J Kidney Dis. 2013;61(5):790–797. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DW, Herzig K, Purdie D, et al. A comparison of the effects of dialysis and renal transplantation on the survival of older uremic patients. Transplantation. 2000;69(5):794–799. [DOI] [PubMed] [Google Scholar]
- 7.Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16(6):1859–1865. [DOI] [PubMed] [Google Scholar]
- 8.Jay CL, Washburn K, Dean PG, et al. Survival benefit in older patients associated with earlier transplant with high KDPI kidneys. Transplantation. 2017;101(4):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 Annual data report: Kidney. Am J Transplant. 2020;20 Suppl s1:20–130. Doi: 10.1111/ajt.15672. [DOI] [PubMed] [Google Scholar]
- 10.Alhamad T, Axelrod D, Lentine KL. The Epidemiology, outcomes, and costs of contemporary kidney transplantation. Ch. 34 in ‘Chronic Kidney Disease, Dialysis, and Transplantation: A Companion to Brenner and Rector’s the Kidney’ 4th ed. Edition. Eds. Himmelfarb J. & Ikizler TA: Elsevier; 2019. [Google Scholar]
- 11.Gill J, Sampaio M, Gill JS, et al. Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States. Clin J Am Soc Nephrol. 2011;6(5):1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam NN, Kim SJ, Knoll GA, et al. The risk of cardiovascular disease is not increasing over time despite aging and higher comorbidity burden of kidney transplant recipients. Transplantation. 2017;101(3):588–596. [DOI] [PubMed] [Google Scholar]
- 13.Dreyer GJ, de Fijter JW. Transplanting the elderly: mandatory age- and minimal histocompatibility matching. Front Immunol. 2020;11:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier-Kriesche HU, Ojo AO, Cibrik DM, et al. Relationship of recipient age and development of chronic allograft failure. Transplantation. 2000;70(2):306–310. [DOI] [PubMed] [Google Scholar]
- 15.Lufft V, Kliem V, Tusch G, et al. Renal transplantation in older adults: is graft survival affected by age? A case control study. Transplantation. 2000;69(5):790–794. [DOI] [PubMed] [Google Scholar]
- 16.Meier-Kriesche HU, Ojo A, Hanson J, et al. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 2000;69(5):885–889. [DOI] [PubMed] [Google Scholar]
- 17.Saxena R, Yu X, Giraldo M, et al. Renal transplantation in the elderly. Int Urol Nephrol. 2009;41(1):195–210. [DOI] [PubMed] [Google Scholar]
- 18.Martins PN, Pratschke J, Pascher A, et al. Age and immune response in organ transplantation. Transplantation. 2005;79(2):127–132. [DOI] [PubMed] [Google Scholar]
- 19.Karim A, Farrugia D, Cheshire J, et al. Recipient age and risk for mortality after kidney transplantation in England. Transplantation. 2014;97(8):832–838. [DOI] [PubMed] [Google Scholar]
- 20.Kauffman HM, McBride MA, Cors CS, et al. Early mortality rates in older kidney recipients with comorbid risk factors. Transplantation. 2007;83(4):404–410. [DOI] [PubMed] [Google Scholar]
- 21.Faravardeh A, Eickhoff M, Jackson S, et al. Predictors of graft failure and death in elderly kidney transplant recipients. Transplantation. 2013;96(12):1089–1096. [DOI] [PubMed] [Google Scholar]
- 22.Meier-Kriesche HU, Ojo AO, Hanson JA, et al. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59(4):1539–1543. [DOI] [PubMed] [Google Scholar]
- 23.Trouillhet I, Benito N, Cervera C, et al. Influence of age in renal transplant infections: cases and controls study. Transplantation. 2005;80(7):989–992. [DOI] [PubMed] [Google Scholar]
- 24.Group KDIGOTW. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1. Doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 25.Kälble T, Lucan M, Nicita G, et al. EAU guidelines on renal transplantation. European urology. 2005;47(2):156–166. [DOI] [PubMed] [Google Scholar]
- 26.Meier-Kriesche HU, Li S, Gruessner RW, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6(5 Pt 2):1111–1131. [DOI] [PubMed] [Google Scholar]
- 27.Maggiore U, Abramowicz D, Budde K. Renal transplantation in the elderly. Transplant Rev (Orlando). 2015;29(4):191–192. [DOI] [PubMed] [Google Scholar]
- 28.Arbogast H, Hückelheim H, Schneeberger H, et al. A calcineurin antagonist-free induction/maintenance strategy for immunosuppression in elderly recipients of renal allografts from elderly cadaver donors: long-term results from a prospective single centre trial. Clin Transplant. 2005;19(3):309–315. [DOI] [PubMed] [Google Scholar]
- 29.Emparan C, Wolters H, Laukötter M, Senninger N. Long-term results of calcineurin-free protocols with basiliximab induction in “old-to-old” programs. Transplant Proc. 2004;36(9):2646–2648. [DOI] [PubMed] [Google Scholar]
- 30.Segoloni GP, Messina M, Squiccimarro G, et al. Preferential allocation of marginal kidney allografts to elderly recipients combined with modified immunosuppression gives good results. Transplantation. 2005;80(7):953–958. [DOI] [PubMed] [Google Scholar]
- 31.Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Seminars in nephrology. 2009;29(6):621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAdams-DeMarco MA, Van Pilsum Rasmussen SE, Chu NM, et al. Perceptions and practices regarding frailty in kidney transplantation: results of a national survey. Transplantation. 2020;104(2):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dharnidharka VR, Schnitzler MA, Chen J, et al. Differential risks for adverse outcomes 3 years after kidney transplantation based on initial immunosuppression regimen: a national study. Transpl Int. 2016;29(11):1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Axelrod DA, Naik AS, Schnitzler MA, et al. National variation in use of immunosuppression for kidney transplantation: a call for evidence-based regimen selection. Am J Transplant. 2016;16(8):2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lentine KL, Gheorghian A, Axelrod D, et al. The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation. 2012;94(4):369–376. [DOI] [PubMed] [Google Scholar]
- 36.Gheorghian A, Schnitzler MA, Axelrod DA, et al. The implications of acute rejection and reduced allograft function on healthcare expenditures in contemporary US kidney transplantation. Transplantation. 2012;94(3):241–249. [DOI] [PubMed] [Google Scholar]
- 37.Dharnidharka VR, Schnitzler MA, Chen J, et al. Differential risks for adverse outcomes 3 years after kidney transplantation based on initial immunosuppression regimen: a national study. Transplant International. 2016;29(11):1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKay D, Jameson J. Kidney transplantation and the ageing immune system. Nat Rev Nephrol. 2012;8(12):700–708. [DOI] [PubMed] [Google Scholar]
- 39.Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2017;8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2(11):659–666. [DOI] [PubMed] [Google Scholar]
- 41.Lemoine M, Titeca Beauport D, Lobbedez T, et al. Risk factors for early graft failure and death after kidney transplantation in recipients older than 70 years. Kidney Int Rep. 2019;4(5):656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527–535. [DOI] [PubMed] [Google Scholar]
- 43.Huang E, Poommipanit N, Sampaio MS, et al. Intermediate-term outcomes associated with kidney transplantation in recipients 80 years and older: an analysis of the OPTN/UNOS database. Transplantation. 2010;90(9):974–979. [DOI] [PubMed] [Google Scholar]
- 44.Ojo AO, Hanson JA, Wolfe RA, et al. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57(1):307–313. [DOI] [PubMed] [Google Scholar]
- 45.Camilleri B, Pararajasingam R, Buttigieg J, et al. Immunosuppression strategies in elderly renal transplant recipients. Transplant Rev (Orlando). 2020;34(2):100529. [DOI] [PubMed] [Google Scholar]
- 46.Shah T, Kasravi A, Huang E, et al. Risk factors for development of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2006;82(12):1673–1676. [DOI] [PubMed] [Google Scholar]
- 47.Pascual J, Royuela A, Galeano C, et al. Very early steroid withdrawal or complete avoidance for kidney transplant recipients: a systematic review. Nephrol Dial Transplant. 2012;27(2):825–832. [DOI] [PubMed] [Google Scholar]
- 48.Pascual J, Galeano C, Royuela A, et al. A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation. 2010;90(4):343–349. [DOI] [PubMed] [Google Scholar]
- 49.Pascual J, Zamora J, Galeano C, et al. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2009(1):CD005632. [DOI] [PubMed] [Google Scholar]
- 50.Haller MC, Royuela A, Nagler EV, et al. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2016(8):CD005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris M, Irish W, Pura J, et al. Comparative effectiveness of triple immunosuppressive therapy versus steroid avoidance or early withdrawal among kidney transplant recipients over age 65.: abstract# 2918. Transplantation. 2014;98:144–145. [Google Scholar]
- 52.Kreis H, Oberbauer R, Campistol JM, et al. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15(3):809–817. [DOI] [PubMed] [Google Scholar]
- 53.Kahan BD, Yakupoglu YK, Schoenberg L, et al. Low incidence of malignancy among sirolimus/cyclosporine-treated renal transplant recipients. Transplantation. 2005;80(6):749–758. [DOI] [PubMed] [Google Scholar]
- 54.Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. The New England journal of medicine. 2005;352(13):1317–1323. [DOI] [PubMed] [Google Scholar]
- 55.Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18(4):446–449. [DOI] [PubMed] [Google Scholar]
- 56.Kauffman HM, Cherikh WS, Cheng Y, et al. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80(7):883–889. [DOI] [PubMed] [Google Scholar]
- 57.Berger SP, Sommerer C, Witzke O, et al. Two-year outcomes in de novo renal transplant recipients receiving everolimus-facilitated calcineurin inhibitor reduction regimen from the TRANSFORM study. Am J Transplant. 2019;19(11):3018–3034. [DOI] [PubMed] [Google Scholar]
- 58.Brakemeier S, Arns W, Lehner F, et al. Everolimus in de novo kidney transplant recipients participating in the Eurotransplant senior program: Results of a prospective randomized multicenter study (SENATOR). PLoS One. 2019;14(9):e0222730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson DW, Nicol DL, Preston JM, et al. Use of mycophenolate mofetil in immunosuppressive protocols in elderly renal transplant recipients. Transplantation. 2003;76(3):619. [DOI] [PubMed] [Google Scholar]
- 60.Meier-Kriesche HU, Friedman G, Jacobs M, et al. Infectious complications in geriatric renal transplant patients: comparison of two immunosuppressive protocols. Transplantation. 1999;68(10):1496–1502. [DOI] [PubMed] [Google Scholar]
- 61.Sureshkumar KK, Nghiem DD. Use of mycophenolate mofetil in immunosuppressive protocols in elderly renal transplant recipients. Transplantation. 2003;76(2):441–442. [DOI] [PubMed] [Google Scholar]
- 62.Webster A, Woodroffe RC, Taylor RS, et al. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005(4):CD003961. [DOI] [PubMed] [Google Scholar]
- 63.Jacobson PA, Schladt D, Oetting WS, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12(12):3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.How good are the data? USRDS data validation special study. Am J Kidney Dis. 1992;20(5 Suppl 2):68–83. [PubMed] [Google Scholar]
- 65.Staatz CE, Tett SE. Pharmacokinetic considerations relating to tacrolimus dosing in the elderly. Drugs Aging. 2005;22(7):541–557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.