Summary
Background:
Although rectal indomethacin (100mg) is effective in reducing the frequency and severity of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis in high-risk patients, the optimal dose is unknown and pancreatitis rates remain high despite its use. The aim of this study was to compare the efficacy of two dose regimens of rectal indomethacin on the frequency and severity of post-ERCP pancreatitis in high-risk patients.
Methods:
Eligible patients were those at high-risk for the development of post-ERCP pancreatitis, enrolled at six tertiary centers in a randomized double-blinded comparative effectiveness trial. Patients, study personnel, and treating physicians were blinded to study group assignment. The randomization schedule, stratified according to study center but without other restrictions, was computer-generated by an investigator uninvolved in the clinical care of any subjects, distributed to the other sites, and kept by personnel not directly involved with the study. This same personnel was responsible for packaging the drug and placebo in opaque envelopes. The primary endpoint was the development of post-ERCP pancreatitis. Analyses were conducted by intention-to-treat principle. The trial was registered with Clinicaltrials.gov (https://www.clinicaltrials.gov/): NCT01912716, and ended with complete enrollment.
Findings:
1037 eligible patients were randomized between 7/9/13–3/22/18. Pancreatitis occurred in 141 patients (13.6%), with no significant difference between the two groups (100mg vs 200mg) (76/515, 14.8% vs 65/522, 12.5%, P=0.32). There were 19 adverse events that were potentially attributable to the study drug. Clinically significant bleeding occurred in 14 of the 1037 patients (1.4%), 6/515 (1.2%) in the standard-dose group and 8/522 (1.5%) in the high-dose group (P = 0.79). Three patients developed acute kidney injury developed, all in the high-dose group (3/522, 0.6%). A non-ST elevation myocardial infarction occurred in the standard-dose group 2 days after ERCP. A transient ischemic attack occurred in the high-dose group 5 days after ERCP. All 19 adverse events, in addition to the 141 patients who developed post-ERCP pancreatitis, were considered to be serious adverse events as all required hospitalization. There were no allergic reactions or deaths at the 30-day follow-up.
There was no difference between the two groups regarding incidence of bleeding or renal failure. No allergic reactions or deaths occurred.
Interpretation:
Dose escalation to 200mg of rectal indomethacin does not confer any advantage over the standard 100mg regimen, with pancreatitis rates remaining high in high-risk patients. Current practice patterns should continue unchanged. Further research needs to consider the pharmacokinetics of NSAIDs to determine the optimal timing of their administration in prevention of post-ERCP pancreatitis.
Funding:
This work was supported by a Clinical Research Award obtained from the American College of Gastroenterology.
Introduction:
Pancreatitis is the most frequent and potentially devastating complication of endoscopic retrograde cholangiopancreatography (ERCP), accounting for substantial morbidity, occasional mortality, and increased health care costs.1–3 Multiple pharmacologic agents have been evaluated in the prevention of post-ERCP pancreatitis with limited success.4 Recently, rectal non-steroidal anti-inflammatory drugs – either indomethacin or diclofenac at a dose of 100mg in the peri-procedural period – have been adopted into widespread clinical use on the basis of high-quality randomized trials consistently showing an approximately 50% risk reduction in high-risk patients. The benefit in average–risk patients, however, remains a source of debate.5–20 Despite this advance, the rate of post-ERCP pancreatitis remains at or above 10% in high-risk cases despite pharmacoprevention and the placement of a prophylactic pancreatic stent – the only mechanical intervention to reduce risk.
When used as an analgesic or anti-inflammatory agent in the management of patients with arthritis, the accepted maximal daily dose of indomethacin is 200mg per day, in divided doses (product insert, Merck & Co., Kenilworth, NJ). This higher dose of indomethacin, which should lead to a higher peak serum concentration, might further lower post-ERCP pancreatitis rates. In addition, since the half-life of indomethacin is approximately 4.5 hours, a second dose of the drug might lead to a more sustained impact on the inflammatory cascade. We hypothesized that both of these dose modifications are important in pancreatitis prevention, and that a regimen consisting of a higher initial dose followed by a second dose (i.e. dose escalation) would be superior to the existing standard. Therefore, we conducted a randomized, double-blind trial comparing modified and standard dose regimens of rectal indomethacin for preventing post-ERCP pancreatitis in high-risk patients.
Methods:
Study design and participants
We enrolled patients at six tertiary medical centers in the United States in a randomized double-blinded comparative effectiveness trial of two dosing regimens of rectal indomethacin for preventing post-ERCP pancreatitis in high-risk cases. Approval was obtained from the human studies review committee at each participating institution. Selected subjects who did not meet any exclusion criteria were consented for the trial by a clinical research coordinator or one of the investigators, with this consent process occurring prior to ERCP in outpatient clinic or the procedure preparation area. At this time, the objectives of the study as well as the risks and benefits of enrolling were explained in detail to potential subjects. Consent from a legal authorized representative was not allowed per study protocol. Patient recruitment was not consecutive as certain factors beyond coordinator or investigator control limited this approach (eg. procedure performed too late in the day to allow for administration of the 4-hour drug or placebo, inability to verify creatinine or lipase level). The study was granted an exemption by the Food and Drug Administration for an Investigational New Drug application. An independent data and safety monitoring board (DSMB) reviewed blinded subject data biannually and conducted the a priori scheduled interim analyses. The study protocol is available online (see appendix p1–10).
The eligibility criteria were intended to select a group of patients at high risk (approximately 10%) for post-ERCP pancreatitis. These criteria were based on patient and procedure-related risk factors that have been previously shown to independently predict pancreatitis.21 Patients were eligible if they met one or more of the following major criteria: clinical suspicion of sphincter of Oddi dysfunction (defined as chronic abdominal pain or suspected biliary or pancreatic origin, accompanied by elevated serum liver tests or pancreatic enzymes and/or bile or pancreatic duct dilation on abdominal imaging), a history of post-ERCP pancreatitis, pancreatic sphincterotomy, precut (access) sphincterotomy, more than eight cannulation attempts (as determined by the endoscopist), pneumatic dilation of an intact biliary sphincter, or papillectomy. Patients were also eligible if they met two or more of the following minor criteria: age less than 50 years and female sex, a history of recurrent pancreatitis (≥2 episodes), three or more contrast injections into the pancreatic duct with at least one injection to the tail of the pancreas, pancreatic acinarization, or pancreatic duct brush cytology.
Patients were excluded from study participation if they met one or more of the following criteria: unwillingness or inability to consent for the study, age < 18 years (no upper age limit was exclusionary), intrauterine pregnancy, breastfeeding mother, standard contraindications to ERCP (eg. uncontrolled coagulopathy or hemodynamic instability), allergy/ hypersensitivity to aspirin or NSAIDs, received NSAIDS in prior 7 days (aspirin 325 mg or less OK), renal insufficiency (serum creatinine > 1.4 mg/dL), active or recent (within 4 weeks) gastrointestinal hemorrhage, acute pancreatitis (lipase peak > 3x upper limit of normal) within 72 hours, known chronic calcific pancreatitis, pancreatic head mass, procedure performed on major papilla/ventral pancreatic duct in a patient with pancreas divisum (dorsal duct not attempted or injected), ERCP for biliary stent removal or exchange without anticipated pancreatogram, subject with prior biliary sphincterotomy now scheduled for repeat biliary therapy without anticipated pancreatogram, anticipated inability to follow protocol, or known active cardiovascular or cerebrovascular disease.
Randomization and Masking
Eligible patients underwent randomization at the conclusion of the ERCP, because patients without a priori risk factors for pancreatitis could be included in the study on the basis of procedure-related factors that developed during the case. Immediately after the procedure, if the endoscopist and research coordinator determined that eligibility criteria had been satisfied, patients were randomly assigned to receive either two 50mg indomethacin suppositories and a placebo suppository (standard-dose group) or three 50mg indomethacin suppositories (high-dose group). Four hours after the ERCP, patients who were assigned to the high-dose group (having already received 150mg) received an additional 50mg indomethacin suppository whereas patients in the standard-dose group (having already received 100mg) received an additional placebo suppository. While the indomethacin and placebo suppositories were not identical, suppositories were administered by clinical nursing personnel uninvolved in the post-procedure care of patients or the adjudication of study outcomes.
An identical administration regimen in both groups and the exclusion of investigators and research coordinators (who were involved in the care of the patient and the assessment of outcomes) from placing the suppositories were intended to ensure that study subjects, treating physicians and study coordinators remained blinded to study group assignment. The success of study blinding, however, was not formally assessed.
The randomization schedule was generated centrally at the University of Michigan by an investigator uninvolved in the clinical care of any subjects (AKW) using the Stata 12.1 ralloc command (StataCorp, College Station, TX, USA) and was stratified according to study center. There were no additional restrictions to sequence generation. The randomization schedule was provided to the investigational drug service (Medical University of South Carolina and University of Michigan) or a research coordinator not directly involved in the trial (other sites) who then dispensed the assigned suppositories according to the randomization schedule upon being informed by the study coordinator that a patient had met eligibility criteria. This same personnel was responsible for packaging the drug and placebo in opaque envelopes. With this method of allocation, assignment to study group was concealed from study participants, treating physicians and coordinators without knowledge of the next assignment in the sequence.
Procedures
All ERCP procedures were performed under general anaesthesia or monitored anaesthesia care (deep sedation) according to standards established at each individual site. All procedure-related interventions, including method of cannulation, all therapeutics and the decision to place a prophylactic pancreatic stent, were left to the discretion of the attending endoscopist. Experience of each endoscopist ranged from an average of 200 to 800 ERCP procedures performed on an annual basis. The first dose was administered immediately after ERCP while the patient was still in the procedure room. The second dose was administered in the recovery area or in a clinic examination room (if the patient had been discharged and returned later for the second dose). The study protocol did not allow for dose reductions or interruptions. All study patients, regardless of randomization arm, received aggressive intravenous fluid administration peri- and post-procedure in an attempt to minimize pancreatitis risk, per institutional practice, unless contraindications were present (e.g., history of congestive cardiac failure, liver disease with ascites).
The indomethacin suppositories were purchased from one manufacturer: G&W Laboratories, Inc. (South Plainfield, NJ). With the exception of the Medical University of South Carolina site, which purchased its own indomethacin suppositories from G&W, all suppositories were purchased by the Indiana University clinical research team and then distributed directly to each participating site by IROKO Pharmaceuticals, LLC (Philadelphia, PA). Formal potency testing had previously confirmed that the vendor provided indomethacin suppositories that were pharmacodynamically equivalent.5
Most patients were observed in the recovery area after the procedure for 4 hours, at which point they received the second study dose of indomethacin or placebo. Patients in whom abdominal pain developed during this observation period which was unresponsive to oral analgesics were admitted to the hospital (or for current inpatients, kept in the hospital). A small fraction of clinically well patients were discharged from the recovery area after approximately 90 minutes and returned at the 4-hour mark to receive the second dose.
Decisions regarding the evaluation and treatment of adverse events after the procedure and in-hospital care were left to the discretion of the endoscopist and treating clinicians, all of whom were unaware of study group assignment. Among hospitalized patients, serum amylase and lipase levels were measured at least once approximately 24 hours after the procedure and subsequently at clinical discretion.
Patients who were discharged after an uneventful ERCP were contacted by telephone at 5±2 days to capture delayed occurrence of the primary endpoint. Patients were again contacted at 30±5 days to assess for delayed adverse events and to determine the severity of post-ERCP pancreatitis, which is defined in part by the length of hospitalization for pancreatitis.22 No specific follow-up laboratory monitoring was required unless clinical required by a treating physician. Patient demographics, risk factors, ERCP procedural elements, and follow-up data were recorded on standardized data collection forms by a study coordinator who was unaware of study group assignment. Assessment of the primary outcome was centrally reviewed the study coordinator and primary investigator at the Indiana University site, both of whom were blinded to treatment group allocation. Patients were free to withdraw from the study at any time after signing informed consent, with no criteria for investigator-initiated patient withdrawal. Analyses were performed by intent-to-treat principle. Patients were considered lost to follow-up for the 5-day or 30-day endpoints if repeated efforts to contact them and/or to obtain their medical records were unsuccessful when the trial ended.
Outcomes
The primary outcome of the study was the development of post-ERCP pancreatitis, which was defined according to consensus criteria: new onset (or worsening of baseline) pain in the upper abdomen, an elevation in pancreatic enzymes of at least three times the upper limit of the normal range approximately 24 hours after the procedure, and hospitalization for at least 2 nights.22 The secondary outcome was the development of moderate or severe post-ERCP pancreatitis. Moderate post-ERCP pancreatitis is defined as that requiring a 4–10 day hospitalization, while severe pancreatitis leads to hospitalization >10 days, or any of: intensive care unit stay, development of necrosis, pseudocyst, radiologic/surgical intervention, or death. Adjudication of outcome was made by the site research nurse, confirmed by the treating physician who were both blinded to study group allocation, and then forwarded to the central site for confirmation.
Adverse events were defined as reported previously.22,23 Adverse events that were potentially attributable to the study drug were reported to the local institutional review board and the DSMB. These reportable adverse events were gastrointestinal bleeding, acute kidney injury, allergic reaction, myocardial infarction, cerebrovascular accident, and death.
Statistical Analysis
Our prior large-scale randomized trial, which compared 100mg of rectal indomethacin to placebo among 602 high-risk patients, employed identical eligibility criteria and a similar protocol to this study.5 That trial revealed a rate of post-ERCP pancreatitis of 9.2% (27 of 295 patients) in the indomethacin group. Thus we estimated that 1036 patients (518 per study group) would provide a power of at least 80% to detect a 50% reduction in the incidence of post-ERCP pancreatitis, from 9.2% in the 100mg group to 4.6% in the 200mg group, on the basis of Fisher’s exact test, with a two-sided significance level of 0.05. This absolute reduction in incidence was felt to be clinically relevant and substantial enough to change existing clinical practice.
For the analysis of the primary outcome in this superiority trial, we used a two-tailed Fisher’s exact test to analyze the difference in the proportion of patients with post-ERCP pancreatitis in the two groups, with a final two-sided P value of less than 0.05 indicating statistical significance. The secondary outcome, the proportion of patients with moderate or severe post-ERCP pancreatitis in each study group, was similarly calculated. Both the primary and secondary analyses were conducted by intention-to-treat.
When information for the first 400 patients (37.7% of total enrollment) could be evaluated, an ad hoc rule was used to trigger a blinded interim analysis by the independent DSMB. If greater than 66% of the pancreatitis cases or bleeding cases were in a particular group, a formal comparison between groups would be performed with the use of a two-sided stopping boundary of 0.005. Similarly, the DSMB recommended a second interim analysis after 600 patients (56.6% of total enrollment) were evaluated to ensure the safety of subjects.
To generate hypotheses regarding patient groups that may particularly benefit from the high-dose indomethacin regimen, we performed exploratory subgroup analyses on the following pre-specified patient and procedural characteristics: age, sex, race, body mass index, suspicion of sphincter of Oddi dysfunction, prior post-ERCP pancreatitis, history of recurrent pancreatitis, difficult cannulation, performance of pancreatography, pre-cut (access) sphincterotomy, pancreatic sphincterotomy, pancreatic acinarization, biliary sphincterotomy, double wire cannulation technique, pancreatic stent placement, trainee involvement, cardioprotective aspirin use, inpatient vs. outpatient status, and participating medical center. All subgroup analyses were evaluated for interaction effects with indomethacin dose by testing for significance of a corresponding interaction term in a multiple logistic-regression model.24 Statistical analyses were conducted using STATA 14.2 (StataCorp, College Station, TX, USA). The sample size was estimated using STATA 12.1 during the design phase of the trial.
All authors had access to the study data and reviewed and approved the final manuscript. This trial was registered with Clinicaltrials.gov (https://www.clinicaltrials.gov/): NCT01912716).
Role of the Funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results:
Patients
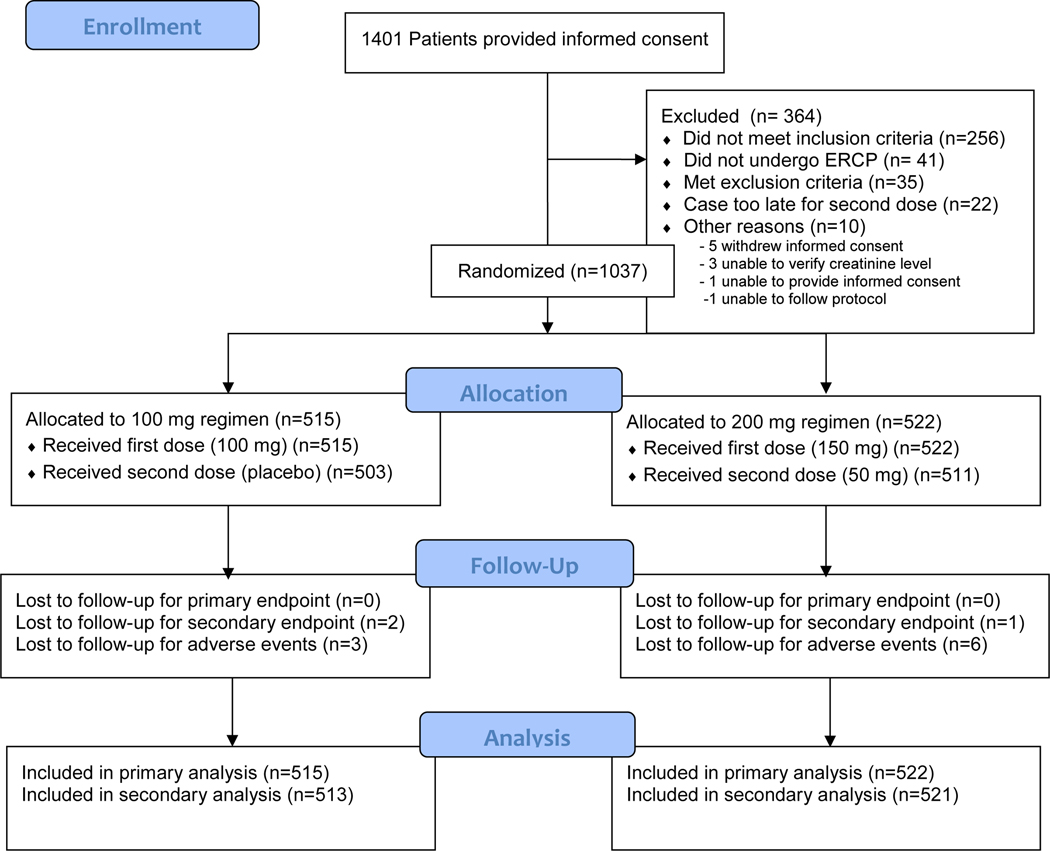
From July 9, 2013 through March 22, 2018, a total of 1037 eligible subjects were enrolled (Figure 1), all of whom had data available for analysis (see appendix p11). Three additional patients were enrolled who did not meet eligibility (inclusion) criteria, and one patient had the informed consent signed by her legal authorized representative. Data from these four patients were not included in the analyses after discussion with the study DSMB. Indications for ERCP included the following (some patients had more than one indication): suspected sphincter of Oddi dysfunction or post-sphincterotomy stenosis (559 patients), choledocholithiasis (57), abnormal liver function tests (132), recurrent acute pancreatitis (381), pancreas divisum (148), papillectomy (68), primary sclerosing cholangitis (8), bile leak (5), and a number of less frequent indications. In February 2015 and February 2016, interim analyses were performed by the DSMB to assess the outcomes of the first 400 and 600 patients, respectively. Both analyses failed to meet the predetermined stopping criteria, and recommendations were made to continue the study. Ultimately, 515 patients were randomized to receive the standard dose of rectal indomethacin (100mg) whereas 522 were randomized to receive the high dose (200mg). Every patient in both groups received the immediate post-procedural suppositories. Twelve (2.3%) of 515 patients in the standard-dose group and 11 (2.1%) of 522 patients in the high-dose group did not receive the 4-hour post-procedural suppository. Thus 11 patients in the high-dose group received only 150mg of indomethacin but were analyzed according to the intention-to-treat principle. None of these 23 patients who did not receive the second dose chose to do so due to drug-related toxicity, but most commonly chose to leave the recovery area before the 4-hour time period had elapsed.
Figure 1.
Enrollment and Outcomes
Follow-up of all patients for the primary endpoint was complete (Figure 1). All 1037 patients, therefore, were included in the analysis of the primary outcome, with a median follow-up of 5 days and interquartile range (IQR) of 1. Nine (0.9%) of 1037 patients were lost to follow-up at the 30-day visit and thus could not be fully assessed for delayed adverse events. Of these, 3 (0.3%) of the 1037 patients had developed post-ERCP pancreatitis and thus could not be included in the analysis of the secondary endpoint (severity of post-ERCP pancreatitis), leaving 1034 patients (99.7%) available for analysis. The median follow-up for analysis of the secondary outcome and delayed adverse events was 30 days with an IQR of 2. Baseline characteristics, including all major and minor inclusion criteria, were similar in the two study groups, except that patients in the high-dose group had a lower rate of pre-cut sphincterotomy (46/515, 8.8% vs. 71/522, 13.8%, Table 1).
Table 1:
Baseline Characteristics
| Characteristic | 100 mg regimen (N=515) | 200 mg regimen (N=522) |
|---|---|---|
| Age – years (SD) | 49.3 (15.2) | 50.4 (15) |
| Female sex – no. (%) | 392 (76.1) | 421(80.7) |
| Body mass index (SD) | 28.6 (7.03) | 29.2 (7.63) |
| Obese (BMI ≥ 30) – no (%) | 193 (37.5) | 198 (37.9) |
| Clinical suspicion of sphincter of Oddi dysfunction — no. (%) | 319 (61.8) | 331 (63.4) |
| History of post-ERCP pancreatitis — no. (%) | 77 (15) | 100 (19.2) |
| History of recurrent pancreatitis — no. (%) | 202 (39.2) | 210 (40.2) |
| Difficult cannulation (>8 attempts) — no. (%) | 148 (28.7) | 146 (28) |
| Precut sphincterotomy — no. (%) | 71 (13.8) | 46 (8.8) |
| Double-wire cannulation technique – no. (%) | 18 (3.5) | 18 (3.4) |
| Pancreatography | ||
| Patients — no. (%) | 446 (87) | 433 (83) |
| Mean no. of injections of the pancreatic duct (SD) | 2.12 (1.63) | 1.96 (1.66) |
| Pancreatic sphincterotomy — no. (%) | 245 (47.6) | 231 (44.3) |
| Placement of pancreatic stent — no. (%) | 400 (77.7) | 393 (75.3) |
| Papillectomy — no. (%) | 30 (5.8) | 32 (6.1) |
| Biliary sphincterotomy — no. (%) | 302 (58.8) | 290 (55.6) |
| Trainee involvement in ERCP — no. (%) | 84 (16.3) | 68 (13.0) |
Study Outcomes
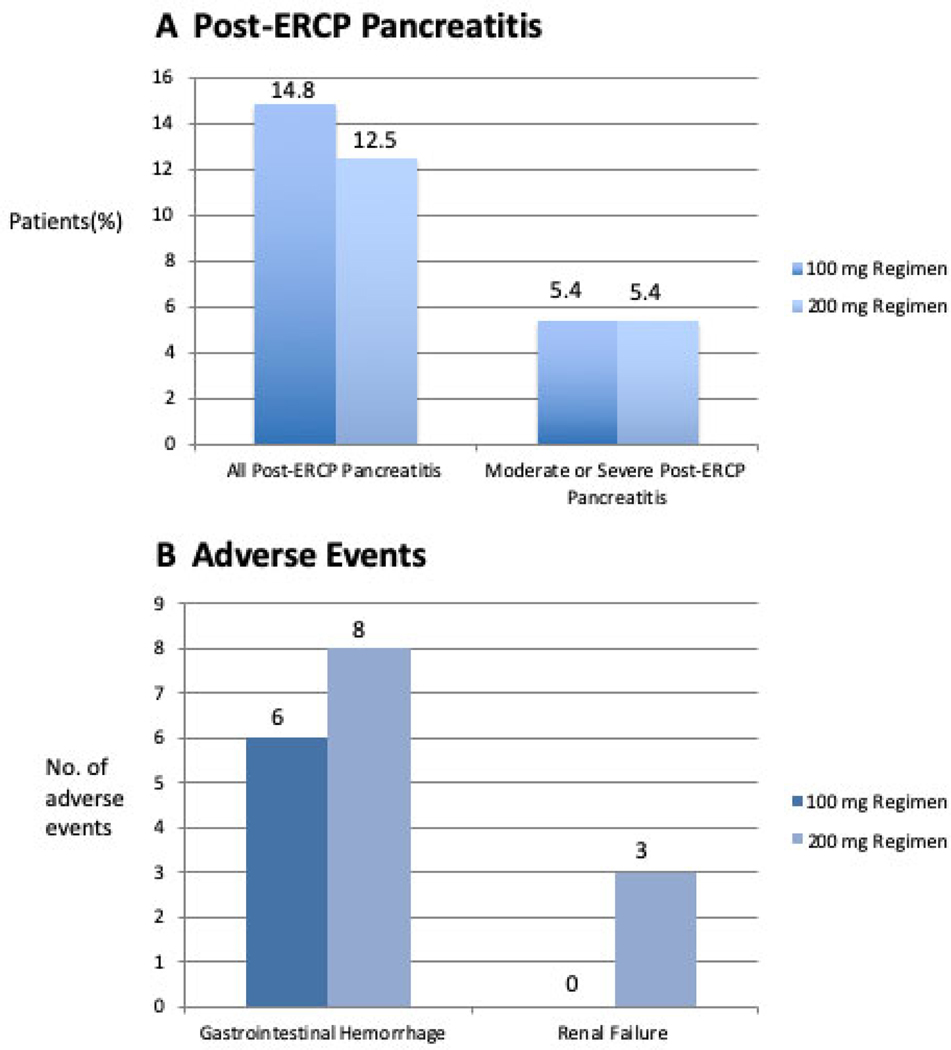
The primary outcome of post-ERCP pancreatitis occurred in 141 of 1037 patients (13.6%). Of these events, 76 of 515 (14.8%) occurred in the standard-dose group and 65 of 522 (12.5%) occurred in the high-dose group (P = 0.32; RR 1.19, 95%CI 0.8–71.61; Figure 2). The secondary outcome of moderate or severe post-ERCP pancreatitis occurred in 56 patients: 28 (5.4%) of 515 patients in the standard-dose group and 28 (5.4%) of 522 patients in the high-dose group (P = 1.000) (Figure 2). Four (0.8%) of 515 patients in the standard-dose group and 2 (0.4%) in the high-dose group experienced severe post-ERCP pancreatitis. Of the severe cases, 5 were hospitalized for 10 days or longer and 1 in the high-dose group developed a pancreatic fluid collection that required drainage. No cases of cardiopulmonary organ failure were observed. One patient did develop transient renal failure (described below, Adverse Events) which resolved within 48 hours.
Figure 2.
Incidence of (A): Post-ERCP Pancreatitis and (B): Adverse Events, in the two treatment groups
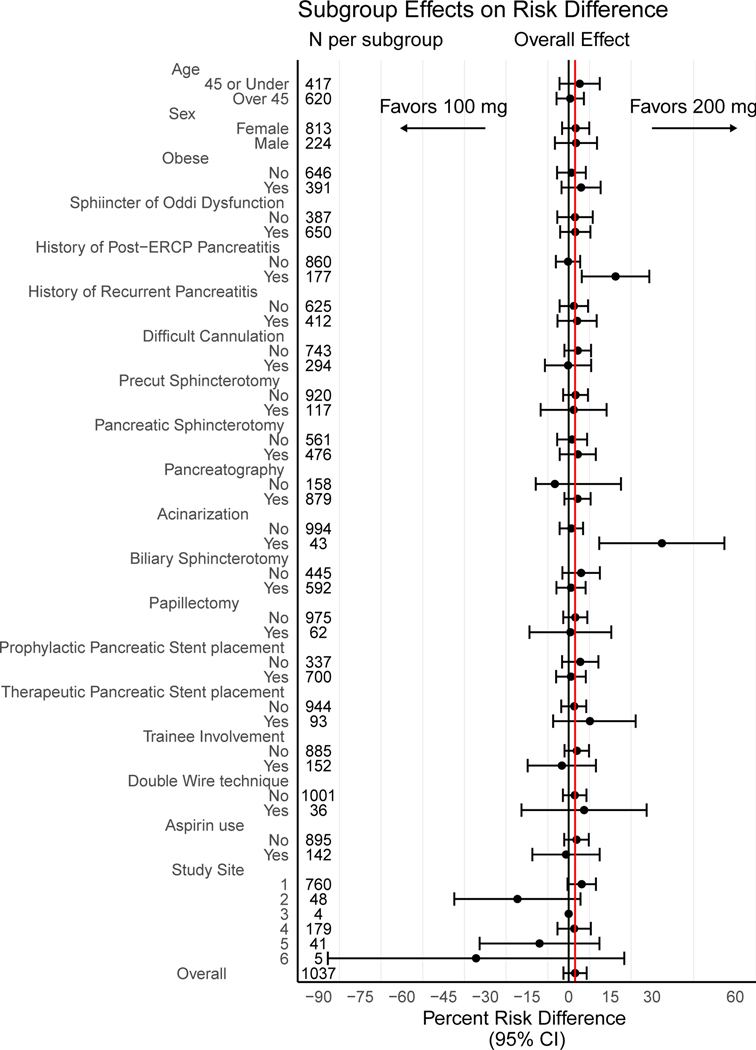
Exploratory Subgroup Analyses
With the exception of patients in whom pancreatic acinarization occurred or those with a prior history of post-ERCP pancreatitis, all trends observed in the subgroup analysis were not significant (P>0.05; Figure 3). In the majority of subgroups, a trend favoring the high-dose regimen at a magnitude similar to the overall effect was observed. We observed a lower event associated with the high-dose regimen in the subgroups of patients who experienced acinarization (RR 0.12, 95% CI 0.016–0.87) or had a history of post-ERCP pancreatitis (RR 0.44, 95% CI 0.24–0.80).
Figure 3.
Subgroup Effects on Risk Difference
Adverse Events
There were 19 adverse events that were potentially attributable to the study drug (Figure 2). Clinically significant bleeding occurred in 14 of the 1037 patients (1.4%), 6/515 (1.2%) in the standard-dose group and 8/522 (1.5%) in the high-dose group (P = 0.79). All 14 patients had received a sphincterotomy, 4 of whom also underwent papillectomy. Three of the bleeding events required blood transfusion of 2–4 units of packed red cells. All 14 patients underwent repeat upper endoscopic evaluation, with hemostatic maneuvers required in 8 patients. One patient who had presented with hematochezia within 24 hours of ERCP had a negative follow-up EGD/ERCP, and subsequent colonoscopy revealed large hemorrhoids. No patient required angiography or surgery for hemostasis. The median length of stay for these 14 patients was 3 days, with an IQR of 2. Three cases of acute kidney injury developed, all in the high-dose group (3/522, 0.6%). We considered acute kidney injury as the same degree of renal insufficiency which would have precluded study entry (serum creatinine > 1.4mg/dl). Of the three cases, one presented 20 days post-ERCP with dehydration, and the serum creatinine normalized with intravenous fluid resuscitation. Both the DSMB and IRB considered this not drug-related. In the second case, the primary in-patient surgical team had decreased the intravenous fluids to maintenance rate only 2 hours post-ERCP, serum creatinine was 1.46 mg/dl the following day, but returned to baseline the next day with additional intravenous fluids. This was deemed possibly related to the drug by the DSMB, but was not reported to the site IRB. The third case involved a patient who developed severe post-ERCP pancreatitis. The creatinine increased to 2.3 mg/dl by day 2 post-ERCP, but decreased to 1.1 mg/dl by day 3 and remained normal thereafter. This event was also reported to the DSMB but not the site IRB. A non-ST elevation myocardial infarction occurred in the standard-dose group 2 days after ERCP. A transient ischemic attack occurred in the high-dose group 5 days after ERCP. There were no allergic reactions or deaths at the 30-day follow-up.
Discussion:
Our findings demonstrated that a high-dose treatment regimen (200mg) of rectal indomethacin was not more efficacious than the standard 100mg regimen in lowering post-ERCP pancreatitis rates in patients at elevated risk for this complication. Furthermore, there was no difference in severity of post-ERCP pancreatitis between the standard and high-dose groups. While there was a trend towards benefit of the high-dose group in most subgroups analyzed, this did not reach statistical significance except in two situations: patients where acinarization occurred at ERCP or those with a prior history of post-ERCP pancreatitis. The explanation for this isolated benefit is purely speculative at this point, but additional ad hoc analyses are planned.
Indomethacin shares important pharmacological properties with other non-steroidal anti-inflammatory drugs (NSAIDs) and inhibits cyclooxygenase, phospholipase A2 and neutrophil-endothelial interactions, all believed to play a major role in the pathogenesis of pancreatitis.25–27 While diclofenac undergoes first-pass metabolism with only 50–60% of the drug reaching the systemic circulation as intact diclofenac, indomethacin is not subject to significant first-pass metabolism. The serum concentration of indomethacin peaks within 30–90 minutes after rectal administration, and bioavailability is complete.27,28 This peak concentration is sustained for up to two hours following administration and then decreases over four hours.29 In this study, our high-dose treatment regimen consisted of a higher indomethacin dose (150mg) immediately post-ERCP, followed by an additional 50mg dose four hours later. When designing the study protocol, we had assumed that a higher initial dose might lead to higher therapeutic drug levels, and a second dose might lead to a more sustained effect. It remains possible that the entire 200mg dose given immediately post-ERCP, earlier in the cascade of events which occur in pancreatitis (rather than the 150mg dose followed by the four-hour 50mg dose), may have led to a more beneficial effect than that observed here. Alternatively, it has been suggested that administration of an NSAID at the beginning of the ERCP or during cannulation rather than at the end of the procedure may further reduce pancreatitis rates,8,9 although this point remains controversial and additional randomized trials specifically addressing timing of administration would be of interest.11–14 Pharmacokinetic studies to determine the optimal regimen and inform future randomized trials are necessary.
The rate of post-ERCP pancreatitis observed in both the standard dose (76/515, 14.8%) and high-dose (65/522, 12.5%) groups exceeded that observed in our prior randomized trial of rectal indomethacin that employed nearly identical eligibility criteria.5 The EPISOD trial,30 which changed the way in which patients formerly suspected to have Type 3 sphincter of Oddi dysfunction are managed, was published ten months after our current study was begun, prompting a protocol change excluding these patients from study entry. However, the interpretation of post-ERCP pain and the decision to hospitalize a patient after the procedure, which are components of the definition of post-ERCP pancreatitis, are subjective and vary according to practice styles and institutional policies. Indeed, practitioners with a lower threshold to hospitalize patients after ERCP may observe a higher rate of post-procedure pancreatitis, and vice versa. Thus, between-study and between-center comparisons of post-ERCP pancreatitis rates must be interpreted with caution as they may reflect a different cohort of centers in the study or secular temporal changes in practice patterns.
More than 75% of patients (793/1037) in this clinical trial received a pancreatic duct stent. Of these, 88% (700/793) received a temporary, protective pancreatic duct stent on the basis of their increased risk of post-ERCP pancreatitis, with no difference in incidence of stent placement between the standard indomethacin dose and high-dose groups. Certain patients, however, did not receive temporary stents, either because the endoscopist did not believe this was indicated,31 or less frequently, because placement was not technically feasible (ansa loop ductal configuration prohibiting wire passage). Regardless of whether a protective stent was placed, there was no difference observed in pancreatitis rates between the standard indomethacin dose and high-dose regimens. Nearly 12% of patients (93/793) who received a pancreatic duct stent had a therapeutic stent placed, typically for management of chronic pancreatitis. Similarly, there was no difference in pancreatitis rates between the two indomethacin regimens, regardless of whether a therapeutic stent was placed. The results of our study are similar to a recent study from Taiwan.32 This single center, randomized trial of 162 patients also failed to demonstrate a benefit of double dose rectal indomethacin (200mg) compared to the standard single dose (4.8% vs 9.5%, P=0.24). However, these two studies clearly evaluated different patient populations. The Taiwanese study recruited consecutive patients undergoing ERCP for all indications, the majority of which (144/162) were performed for choledocholithiasis. Furthermore, when considering procedural characteristics as well, only 42/162 patients were considered to be at high-risk for post-ERCP pancreatitis, in contrast to our study.
There are a number of limitations in this study. While patients were recruited from six busy US academic centers, 760 of 1037 patients (73.3%) were recruited from one site, potentially limiting generalizability. We acknowledge that patients with chronic pancreatitis are typically considered to be at reduced risk of ERCP-induced pancreatitis. However, these patients qualified for study entry due to the presence of additional high-risk features.21 Furthermore, patients with calcific pancreatitis, a protective factor against post-ERCP pancreatitis, were excluded. This study was not designed a priori to determine the benefit of pancreatic duct stent placement vs. indomethacin alone vs. combination stent+indomethacin, as is currently being investigated elsewhere.31 Additionally, aggressive intravenous fluid administration, particularly lactated ringers, has recently been shown to have a protective effect against post-ERCP pancreatitis.33,34 While this recommendation was followed at each site according to institutional and physician practice, a set protocol with type and volume of fluid was not followed or systematically tracked across all sites, as this study began patient recruitment in 2013. Furthermore, established consensus criteria22 for the definition of post-ERCP pancreatitis were used in this study. These consensus criteria have been most commonly used in landmark trials on post-ERCP prevention, epidemiological studies and in ERCP guidelines.35 However, duration of hospitalization is a key component of these criteria, which may be confounded by patient pain tolerance and physician practice, among other factors. The revised Atlanta classification of acute pancreatitis consensus definitions stratify pancreatitis severity based on the presence of local and systemic adverse events, including duration of organ failure, rather than duration of hospitalization.36 Although not specific for post-ERCP pancreatitis, the revised Atlanta classification may provide an alternative for assessing the severity of this complication.35
NSAIDs are an attractive option in the prevention of post-ERCP pancreatitis, as they are easily administered, relatively inexpensive and have a favorable risk profile when given as a single dose. An initial concern with the current trial was the potential for increased adverse events with the 200mg dose. However, the observed risk of adverse events that were potentially attributable to indomethacin was similar in the standard and high-dose regimens. Specifically, there was no significant difference between the groups in the frequency or severity of bleeding events, consistent with prior reports that NSAIDs (in standard doses) do not increase the rate of post-sphincterotomy bleeding.3,37 As with our previous randomized trial,5 patients with contraindications to NSAIDs were excluded from study participation.
In summary, dose escalation to 200mg of rectal indomethacin does not appear to confer an advantage over the standard 100mg regimen. Post-ERCP pancreatitis rates continue to remain high in high-risk patients despite rectal indomethacin. Additional interventions are necessary to further reduce the risk of post-ERCP pancreatitis.
Supplementary Material
Research in context.
Evidence before this study
Pancreatitis is the most common and most-feared complication of ERCP. Rectal non-steroidal anti-inflammatory drugs – either indomethacin or diclofenac at a dose of 100mg in the peri-procedural period – have been adopted into widespread clinical use in an attempt to minimize the likelihood of this complication. A systematic literature review using the PubMed, Ovid and Cochrane Library electronic databases as well as ClinicalTrials.gov to facilitate identification of ongoing trials was performed, with a start date of January 1, 1990 and end date of June 30, 2019. The electronic search was performed using the search terms “post-ERCP complications”, “post-ERCP pancreatitis prevention”, NSAIDs and pancreatitis” AND “indomethacin and pancreatitis prevention”, without limitation to type of study (eg. randomized controlled trial) or English language. The pooled estimate from several meta-analyses demonstrates an approximately 50% risk reduction in high-risk patients. The rate of post-ERCP pancreatitis, however, remains at or above 10% in high-risk cases despite this intervention. The optimal dose of this phamacoprevention is unknown.
Added value of this study
Rectal NSAIDs have been shown to reduce the incidence of post-ERCP pancreatitis in high-risk patients. This primary objective of this study was to determine whether a more aggressive, high-dose indomethacin regimen (200mg) would further lower the risk of this complication. While this study demonstrates that the high-dose regimen does not appear to offer any advantage over the standard dose (100mg), the importance of the study is undeniable. NSAIDs have been the sole effective pharmacopreventive strategy identified to date to definitively reduce post-ERCP pancreatitis rates, and studies to define the optimal dose and timing of administration are needed to refine this intervention. This “negative” study will help guide the design of future trials aimed at reducing the incidence of this complication.
Implications of all the available evidence
Published guidelines from the American College of Gastroenterology and the American and European Societies for Gastrointestinal Endoscopy support the administration of rectal NSAIDs for the prevention of post-ERCP pancreatitis in high-risk patients. This study failed to demonstrate a clear benefit of a high-dose regimen of rectal indomethacin, and current practice patterns should continue unchanged. However, further research needs to consider the pharmacokinetics of NSAIDs to determine the optimal timing of their administration in prevention of post-ERCP pancreatitis. It remains possible that administration of a high-dose prior to the procedure, perhaps given as a single dose, may yet prove to be more effective than the standard 100mg dose given post-procedure.
Acknowledgment:
Dr. Fogel’s effort was partially funded by the National Institute of Health’s consortium for the study of Chronic Pancreatitis, Diabetes and Pancreatic Cancer (CPDPC, U01DK108323). This work was supported by a Clinical Research Award obtained from the American College of Gastroenterology. All of the funds provided by this Clinical Research Award were used to purchase some of the indomethacin suppositories which were used in this study.
Declaration of interests:
Dr. Easler reports personal fees from Boston Scientific Co., outside the submitted work. Dr. Elmunzer reports other from Takeda Pharmaceuticals, outside the submitted work. Dr. Guda reports personal fees from Boston Scientific Corporation, outside the submitted work. Dr. Lehman reports personal fees from Cook Medical, Bloomington, IN, personal fees from AscentX Medical, Inc., San Diego, CA, outside the submitted work. Dr. Pleskow reports grants from Boston Scientific, grants from Olympus, grants from Fujifilm, from NinePoint Medical, from CSA, outside the submitted work. Dr. Sherman reports other from Boston Scientific, other from Cook, other from Mi-Tech, outside the submitted work. The other authors declared no conflicts of interest.
Footnotes
Data Sharing Plan
All data collected during the study, including de-identified participant data and a data dictionary defining each field in the set, will be made available to others with publication. Additional related documents (study protocol, statistical analysis plan, informed consent form, case report forms, etc.) will also be made available. These data and forms may all be made available following communication with the lead research study coordinator, Suzette Schmidt, RN (suschmid@iu.edu). Prior to sharing these data (with professional colleagues or other investigators), the request will be reviewed by the corresponding author, Dr. Fogel. No specific access criteria are required (eg. who has initiated the request, with or without investigator support, nature of the proposal), but all proposals must be approved by Dr. Fogel, with a signed data access agreement in hand prior to release of the data.
Contributor Information
Evan L Fogel, Division of Gastoenterology and Hepatology, Indiana University, Indianapolis, IN.
Glen A. Lehman, Division of Gastoenterology and Hepatology, Indiana University, Indianapolis, IN.
Paul Tarnasky, University of Texas Southwestern, Digestive Health Associates of Texas, Dallas, TX.
Gregory A. Cote, Division of Gastroenterology, Medical University of South Carolina, Charleston, SC.
Suzette E. Schmidt, Department of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN.
Akbar K. Waljee, Division of Gastroenterology, University of Michigan, Ann Arbor, MI.
Peter D.R. Higgins, Division of Gastroenterology, University of Michigan, Ann Arbor, MI.
James L. Watkins, Division of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN.
Stuart Sherman, Division of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN.
Richard S.Y. Kwon, Division of Gastroenterology, University of Michigan, Ann Arbor, MI.
Grace H. Elta, Division of Gastroenterology, University of Michigan, Ann Arbor, MI.
Jeffrey J. Easler, Division of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN.
Douglas K. Pleskow, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA.
James M. Scheiman, Division of Gastroenterology, University of Virginia, Charlottesville, VA (work took place at University of Michigan, Ann Arbor, MI).
Ihab I. El Hajj, Division of Gastroenterology, University of Balamand, Beirut, Lebanon (work took place at Indiana University, Indianapolis, IN).
Nalini M. Guda, Division of Gastroenterology, University of Wisconsin, Milwaukee, WI (work took place at Aurora St.Luke’s Medical Center, Milwaukee, WI).
Mark A. Gromski, Division of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN.
Lee McHenry, Jr., Division of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN.
Seena Arol, Digestive Health Associates of Texas, Dallas, TX.
Sheryl Korsnes, Division of Gastroenterology, University of Michigan, Ann Arbor, MI.
Alejandro L. Suarez, Division of Gastroenterology, Yale University, New Haven, CT (much of this work took place at Medical University of South Carolina, Charleston, SC).
Rebecca Spitzer, Division of Gastroenterology, Medical University of South Carolina, Charleston, SC.
Marilyn MIller, Division of Gastroenterology, University of Wisconsin, Milwaukee, WI.
Maria Hofbauer, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA.
Badih Joseph Elmunzer, Division of Gastroenterology, Medical University of South Carolina, Charleston, SC.
References:
- 1.La Ferla G, Gordon S, Archibald M, Murray WR. Hyperamylasaemia and acute pancreatitis following endoscopic retrograde cholangiopancreatography. Pancreas 1986;1(2):160–163. [DOI] [PubMed] [Google Scholar]
- 2.Sherman S, Lehman GA. ERCP and endoscopic sphincterotomy-induced pancreatitis. Pancreas 1991;6(3):350–367. [DOI] [PubMed] [Google Scholar]
- 3.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996;335(13):909–918. [DOI] [PubMed] [Google Scholar]
- 4.Kubiliun NM, Adams MA, Akshintala VS, et al. Evaluation of Pharmacologic Prevention of Pancreatitis After Endoscopic Retrograde Cholangiopancreatography: A Systematic Review. Clin Gastroenterol Hepatol 2015;13(7):1231–1239. [DOI] [PubMed] [Google Scholar]
- 5.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 2012;366(15):1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levenick JM, Gordon SR, Fadden LL, et al. Rectal indomethacin does not prevent post-ERCP pancreatitis in consecutive patients. Gastroenterology 2016;150(4):911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiruvengadam NR, Forde KA, Ma GK, et al. Rectal indomethacin reduces pancreatitis in high- and low-risk patients undergoing endoscopic retrograde cholangiopancreatography. Gastroenterology 2016;151(2):288–297. [DOI] [PubMed] [Google Scholar]
- 8.Luo H, Zhao L, Leung J, et al. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet 2016;387(10035):2293–2301. [DOI] [PubMed] [Google Scholar]
- 9.Shen C, Shi Y, Liang T, Su P. Rectal NSAIDs in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in unselected patients: systematic review and meta-analysis. Dig Endosc 2017;29(3):281–290. [DOI] [PubMed] [Google Scholar]
- 10.Inamdar S, Han D, Passi M, Seipal DV, Trindade AJ. Rectal indomethacin is protective against post-ERCP pancreatitis in high-risk patients but not average-risk patients: a systematic review and meta-analysis. Gastrointest Endosc 2017;85(1):67–75. [DOI] [PubMed] [Google Scholar]
- 11.He X, Zheng W, Ding Y, Tang X, Si J, Sun LM. Rectal indomethacin is protective against pancreatitis after endoscopic retrograde cholangiopancreatography: systematic review and meta-analysis. Gastroenterol Res Pract 2018, May 9, 9784841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu LM, Zhao KJ, Lu B. Use of NSAIDs via the rectal route for the prevention of pancreatitis after ERCP in all-risk patients: an updated meta-analysis. Gastroenterol Res Pract 2018, Feb 8, 1027530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Li C, Huang Y, Jin H. Nonsteroidal anti-inflammatory drugs for endoscopic retrograde cholangiopancreatography postoperative pancreatitis prevention: a systematic review and meta-analysis. J Gastrointest Surg 2018;Sept 24. doi: 10.1007/s11605-018-3967-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Zhao Y, Li W, et al. Rectal nonsteroidal anti-inflammatory drugs administration is effective for the prevention of post-ERCP pancreatitis: an updated meta-analysis of randomized controlled trials. Pancreatology 2017;17(5):681–688. [DOI] [PubMed] [Google Scholar]
- 15.Freeman ML, Kozarek RA. Take 2 indomethacin (suppositories) and call me in the morning? The role of nonsteroidal anti-inflammatory drugs in protection against post-endoscopic retrograde cholangiopancreatography pancreatitis (editorial). Gastroenterology 2016;150(4):805–808. [DOI] [PubMed] [Google Scholar]
- 16.Akhter A, Pfau PR, Gopal DV. Rectal indomethacin to prevent post-ERCP pancreatitis (editorial). Gastroenterology 2016;151(3):568–569. [DOI] [PubMed] [Google Scholar]
- 17.Elmunzer BJ, Foster LD, Durkalski V. Should we still administer prophylactic rectal NSAIDs to average-risk patients undergoing ERCP? (editorial) Gastroenterology 2016;151(3):566–567. [DOI] [PubMed] [Google Scholar]
- 18.He XK, Sun LM. Does rectal indomethacin prevent post-ERCP pancreatitis in average-risk patients? (editorial) Gastrointest Endosc 2017;85(3):687. [DOI] [PubMed] [Google Scholar]
- 19.Barkin JA, Souto EO, Barkin JS. Rectal indomethacin should be used routinely in all patients for prevention of post-ERCP pancreatitis. Gastrointest Endosc 2017;85(3):687–688. [DOI] [PubMed] [Google Scholar]
- 20.Akshintala VS, Singh VK, Reddy DN. Rectal indomethacin for post-ERCP pancreatitis prophylaxis in average risk patients: too early to terminate and too early to conclude. Gastroenterology 2016;151(3):567–568. [DOI] [PubMed] [Google Scholar]
- 21.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001;54(4):425–434. [DOI] [PubMed] [Google Scholar]
- 22.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37(3):383–393. [DOI] [PubMed] [Google Scholar]
- 23.Anderson MA, Fisher L, Jain R, et al. Complications of ERCP. Gastrointest Endosc 2012;75(3):467–473. [DOI] [PubMed] [Google Scholar]
- 24.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;Aug 12,11:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross V, Leser HG, Heinisch A, Schölmerich J. Inflammatory mediators and cytokines-new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterol 1993;40(6):522–530. [PubMed] [Google Scholar]
- 26.Makela A, Kuusi T, Schroeder T. Inhibition of serum phospholipase A2 in acute pancreatitis by pharamacologic agents in vitro. Scand J Clin Lab Invest 1997;57(5):401–408. [DOI] [PubMed] [Google Scholar]
- 27.Davies NM, Anderson KE. Clinical phamacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet 1997;33(3):184–213. [DOI] [PubMed] [Google Scholar]
- 28.Tammaro S, Caruso R, Pallone F, Monteleone G. Post-endoscopic retrograde cholangio-pancreatography pancreatitis: is time for a new preventive approach? World J Gastroenterol 2012;18(34):4635–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen KM, Grenabo L. Bioavailability of indomethacin after intramuscular injection and rectal administration of solution and suppositories. Acta Pharmacol Toxicol (Copenh) 1985;57:322–327. [DOI] [PubMed] [Google Scholar]
- 30.Cotton PB, Durkalski V, Romagnuolo J, et al. Effect of endoscopic sphincterotomy for suspected sphincter of Oddi dysfunction on pain-related disability following cholecystectomy. The EPISOD randomized clinical trial. JAMA 2014;311(20):2101–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmunzer BJ, Serrano J, Chak A, et al. Rectal indomethacin alone versus indomethacin and prophylactic pancreatic stent placement for preventing pancreatitis after ERCP: study protocol for a randomized controlled trial. Trials 2016;17(1):120. doi: 10.1186/s13063-016-1251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai JH, Hung CY, Chu CH, et al. A randomized trial comparing the efficacy of single-dose and double-dose administration of rectal indomethacin in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis. Medicine 2019;98(20):e15742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandrasekhara V, Khashab MA, Muthusamy VR, et al. Adverse events associated with ERCP. Gastrointest Endosc 2017;85(1):32–47. [DOI] [PubMed] [Google Scholar]
- 34.Zhang ZF, Duan ZJ, Wang LX, Zhao G, Deng WG. Aggressive hydration with lactated ringer solution in prevention of postendoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis of randomized controlled trials. J Clin Gastroenterol 2017;51(3):e17–e26. [DOI] [PubMed] [Google Scholar]
- 35.Smeets XJNM, Bouhouch N, Buxbaum J, et al. The revised Atlanta criteria more accurately reflect severity of post-ERCP pancreatitis compared to the consensus criteria. United European Gastroenterol J 2019;7(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102–111. [DOI] [PubMed] [Google Scholar]
- 37.Nelson DB, Freeman ML. Major hemorrhage from endoscopic sphincterotomy: risk factor analysis. J Clin Gastroenterol 1994;19(4):283–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.