Abstract
Background
This is an updated version of the original Cochrane review published in Issue 3, 2005 of The Cochrane Library. For many years antidepressant drugs have been used to manage neuropathic pain, and are often the first choice treatment. It is not clear, however, which antidepressant is more effective, what role the newer antidepressants can play in treating neuropathic pain, and what adverse effects are experienced by patients.
Objectives
To determine the analgesic effectiveness and safety of antidepressant drugs in neuropathic pain.
Search methods
Randomised controlled trials (RCTs) of antidepressants in neuropathic pain were identified in MEDLINE (1966 to Oct 2005); EMBASE (1980 to Oct 2005); the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, Issue 3, 2005; and the Cochrane Pain, Palliative and Supportive Care Trials Register (May 2002). Additional reports were identified from the reference list of the retrieved papers, and by contacting investigators.
Selection criteria
RCTs reporting the analgesic effects of antidepressant drugs in adult patients, with subjective assessment of pain of neuropathic origin. Studies that included patients with chronic headache and migraine were excluded.
Data collection and analysis
Two review authors agreed the included studies, extracted data, and assessed methodological quality independently. In this update a total of sixty one trials of 20 antidepressants were considered eligible (3293 participants) for inclusion (including 11 additional studies (778 participants)) Relative Risk (RR) and Number‐Needed‐to‐Treat (NNTs) were calculated from dichotomous data for effectiveness and adverse effects.
Main results
Sixty one RCTs were included in total. Tricyclic antidepressants (TCAs) are effective and have an NNT of 3.6 (95% CI 3 to 4.5) RR 2.1 (95% CI 1.8 to 2.5) for the achievement of at least moderate pain relief. There is limited evidence for the effectiveness of the newer SSRIs but no studies of SNRIs were found. Venlafaxine (three studies) has an NNT of 3.1 (95% CI 2.2 to 5.1) RR 2.2 (95% CI 1.5 to 3.1). There were insufficient data to assess effectiveness for other antidepressants such as St Johns Wort and L‐tryptophan. For diabetic neuropathy the NNT for effectiveness was 1.3 (95% CI 1.2 to 1.5) RR 12.4 (95% CI 5.2 to 29.2) (five studies); for postherpetic neuralgia 2.7 (95% CI 2 to 4.1), RR 2.2 (95% CI 1.6 to 3.1) (four studies). There was evidence that TCAs are not effective in HIV‐related neuropathies. The number needed to harm (NNH) for major adverse effects defined as an event leading to withdrawal from a study was 28 (95% CI 17.6 to 68.9) for amitriptyline and 16.2 (95% CI 8 to 436) for venlafaxine. The NNH for minor adverse effects was 6 (95% CI 4.2 to 10.7) for amitriptyline and 9.6 (95% CI 3.5 to 13) for venlafaxine.
Authors' conclusions
This update has provided additional confirmation on the effectiveness of antidepressants for neuropathic pain and has provided new information on another antidepressant ‐ venlafaxine. There is still limited evidence for the role of SSRIs. Whether antidepressants prevent the development of neuropathic pain (pre‐emptive use) is still unclear. Both TCAs and venlafaxine have NNTs of approximately three. This means that for approximately every three patients with neuropathic pain who are treated with either of these antidepressants, one will get at least moderate pain relief. There is evidence to suggest that other antidepressants may be effective but numbers of participants are insufficient to calculate robust NNTs. SSRIs are generally better tolerated by patients and more high quality studies are required.
Plain language summary
Antidepressants for treating neuropathic pain
A number of medicines used to treat depression (antidepressants) are effective in treating pain associated with nerve damage (neuropathic pain). At least one third of patients with neuropathic pain who took traditional antidepressants (such as amitriptyline) obtained moderate pain relief or better. There is also evidence that Venlafaxine, a newer antidepressant, has similar effectiveness to traditional antidepressants. However, approximately one fifth of those who take these medicines for pain discontinue the therapy due to adverse effects. There is very limited evidence that some other newer antidepressants, known as SSRIs, may be effective but more studies are needed to confirm this. Neuropathic pain can be treated with antidepressants and the effect is independent of any effect on depression.
Background
This is an updated version of the original Cochrane review published in Issue 3, 2005 of The Cochrane Library. Further updating was started in mid 2009 with plans to split this large review into several smaller ones. Work has began on Amitriptyline for neuropathic pain. Amitriptyline is widely used to treat this condition. Neuropathic pain refers to a group of painful disorders characterised by pain due to dysfunction or disease of the nervous system at a peripheral level, a central level, or both. It is a complex entity with many symptoms and signs that fluctuate in number and intensity over time. The three common components of neuropathic pain are steady and neuralgic pain; paroxysmal spontaneous attacks; and hypersensitivity (Woolf 1999).
Neuropathic pain can be very disabling, severe and intractable, causing distress and suffering for individuals, including dysaesthesia and paraesthesia. Sensory deficits, such as partial or complex loss of sensation, are also commonly seen. In addition, there are significant psychological and social consequences linked to chronic neuropathic pain, which contribute to a reduction in quality of life.
Neuropathic pain is quite common in general medical practice. The prevalence of trigeminal neuralgia is 2.1 to 4.7 persons per 100,000 of the population, and of painful diabetic neuropathy occurs in 11% to 16% of Type 1 diabetics as well as Type II diabetics and postherpetic neuralgia is found in approximately 34 per 100,000 of the population (McQuay 2007). Treatment of neuropathic pain is not easy. Patients with neuropathic pain do not always respond to standard analgesics such as non‐steroidal anti‐inflammatory drugs (NSAIDs) and to some extent neuropathic pain is resistant to opiates. The pharmacologic agents best studied and longest used for the treatment of neuropathic pain are antidepressants and anticonvulsants (Woolf 1999). The clinical impression is that both drug classes are useful for neuropathic pain but there are unanswered questions, such as, 'Which drug class ‐ antidepressants or anticonvulsants ‐ should be the first‐line choice?'; 'Is one antidepressant drug superior to another?'; 'Is there any difference in response to antidepressants in different neuropathic syndromes?'.
Previous systematic reviews of anticonvulsants (antiepileptic drugs) for the treatment of chronic pain found a number of controlled trials showing analgesic effectiveness (McQuay 1995; Tremont‐Lukats 2000; Wiffen 2001). Carbamazepine has been shown to be an effective treatment for trigeminal neuralgia. Gabapentin is effective in post‐herpetic neuralgia and diabetic neuropathy but may not be superior to carbamazepine in terms of analgesic effectiveness. Five review articles are known; two are reviews (Magni 1991; Onghena 1992), and three are systematic reviews (Collins 2000; Max 1995; McQuay 1995). In the most recent of these, a systematic review of antidepressants and anticonvulsants for diabetic neuropathy and post herpetic neuralgia, both drug classes were shown to be equally effective (Collins 2000).
The mechanisms of action of antidepressant drugs in the treatment of neuropathic pain remain uncertain. Analgesia is often achieved at lower dosage and faster (usually within a few days) than the onset of any antidepressant effect which can take up to six weeks. In addition, there is no correlation between the effect of antidepressants on mood and pain. Furthermore, antidepressants produce analgesia in patients with and without depression (Onghena 1992).
Two main groups of antidepressants are in common use. The older tricyclic antidepressants (TCAs) such as amitriptyline, imipramine and many others, and a newer group of selective serotonin reuptake inhibitors (SSRIs). The clinical impression was that TCAs are more effective in treating neuropathic pain. However, SSRIs are gaining acceptance for pain relief (McCleane 2003).
For the purpose of this review, antidepressants have been classed into groups as follows:
TCAs such as amitriptyline and imipramine (the so‐called tetracyclics e.g. mianserin and maprotiline are also included in this group;
newer antidepressants including SSRIs such as paroxetine; serotonin and noradrenaline reuptake inhibitors (SNRIs), venlafaxine and reversible inhibitors of monoamine oxidase type A (RIMAs) such as moclobemide;
herbal treatments (St John's Wort);
any other antidepressants to include mono‐amine oxidase inhibitors (MAOIs), bupropion and L‐tryptophan. It is not implied that these are in any way similar.
TCAs exhibit more significant adverse effects, which limit clinical use, particularly in older people. The most serious adverse effects of TCAs occur within the cardiovascular system, such as postural hypotension, heart block and arrhythmias. The most common adverse effects are sedation and anticholinergic effects (such as dry mouth, constipation and urinary retention) (BNF 2006). SSRIs are better tolerated, they are free of cardiovascular side effects, are less sedative and have fewer anticholinergic effects than TCAs (Feighner 1999; Glassman 1993; Glassman 1998; Peretti 2000).
Objectives
To determine the analgesic effectiveness and adverse effects of antidepressant drugs in the treatment of neuropathic pain.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of antidepressants in the treatment of neuropathic pain. All identified trials, published and unpublished, were eligible. There was no language restriction. In the case of cross‐over studies, first period results were used (if available) in order to exclude any carry over effect. Abstracts and reviews were excluded. Studies could have taken place in any care setting (in‐patient, outpatient, day‐care, community). Studies with fewer than ten participants were excluded as small studies yield unreliable results (Moore 1998).
Types of participants
Adult female and male patients (over 18 years of age) with any neuropathic pain were included. Migraine and headache studies were not included as these are considered in another Cochrane review (Jackson 2004).
Types of interventions
Studies examining the use of any antidepressant drugs were considered. Studies assessing lithium were not sought and not included.
Trials compared:
antidepressant with placebo;
antidepressant with any other active control drug;
antidepressant with another antidepressant;
antidepressant with any other intervention.
The antidepressant could be administered by any route, in any dose and for any duration.
A list of antidepressants was compiled using Martindale 2004, the British National Formulary (BNF 2006), and for the first version of this review, the Monthly Index of Medical Specialities (MIMS 2002). Drug therapies considered in this review were as follows.
Tricyclic antidepressants:
amineptine, amitriptyline, amoxapine, butriptyline, clomipramine, desipramine, dibenzepin, dosulepin, dothiepin, doxepin, mipramine, lofepramine, maprotiline, mianserin, nortriptyline, protriptyline, opipramol, quinupramine, trazodone, and trimipramine.
MAOIs (monoamine oxidase inhibitors):
iproniazid, isocarboxazid, nialamide, phenelzine, and tranylcypromine.
SSRIs (selective serotonin reuptake inhibitors):
citalopram, fluoxetine, fluvoxamine maleate, lofepramine, paroxetine, and sertraline.
SNRIs (serotonin and noradrenaline reuptake inhibitors):
milnacipran, reboxetine, sibutramine, and viloxazine.
RIMAs (reversible inhibitors of monoamine oxidase type A):
benactyzine, brofaromine, moclobemide, and toloxatone.
Newer antidepressants:
nefazodone, mirtazepine and venlafaxine.
Other:
bupropion, etoperidone, flupenthixol, fluphenazine, hypericum (St John's Wort), mirtazepine, nefazodone, reboxetine, tianeptine, and tryptophan.
Types of outcome measures
Measures of effectiveness
Clinical outcomes included patient‐reported global improvement or pain relief, or both, measured on any scale. Effectiveness measures after the longest duration of treatment were used.
Overall quality of life measures
Data from any general commonly used quality of life scale was considered.
Adverse effects measures
minor adverse effects (all adverse effects noted in patient reports)
major adverse effects defined as leading to withdrawal from treatment
Sleep parameters
Self‐reported experience of sleep quality or satisfaction with the following was assessed:
sleep,
total sleep duration,
number of nocturnal awakenings, and
total nocturnal awakening time.
Depression measures
Data from any general commonly used depression scale were considered.
Search methods for identification of studies
Electronic searches
The following databases were searched to identify all relevant studies:
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 1, 2004, subsequent search ran on Issue 3, 2005);
Cochrane Pain Palliative and Supportive Care (PaPaS) Trials Register (December 2003, subsequent search ran on October 2005);
MEDLINE (1966 to December 2005, subsequent search October 2005);
EMBASE (1980 to December 2005, subsequent search October 2005).
A combination of free text and controlled vocabulary (e.g. MeSH) search terms were applied. Searches were restricted to human subjects. Please see Appendix 1 for search strategy.
Searching other resources
Reference search
Additional studies were sought from:
reference lists of identified studies,
chapters in standard pain textbooks,
published meta‐analyses and narrative reviews in The Cochrane Library (Cochrane Database of Systematic Reviews)
Personal contact
Four authors of identified randomised trials were contacted for information about other published and unpublished studies; three responded (Graff‐Radford 2000; Langohr 1982; Shlay 1998).
Data collection and analysis
Study assessment
Trial reports that appeared potentially relevant were identified using the search strategy described above. Using the full text of each study, trials were assessed for inclusion in the review by two review authors (TS and PW) according to defined inclusion criteria. Data were extracted using forms designed for this purpose. Reasons for excluding trials from the review are documented in the 'Characteristics of Excluded Studies' table. Disagreement was resolved by discussion.
Study quality
The quality of the included studies was assessed where possible in terms of the adequacy of concealment of allocation. This was done using the criteria defined in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006) where grade A is adequate concealment; grade B is uncertain allocation concealment; grade C is clearly inadequate concealment, grade D is not used).
In addition, the Oxford Quality Scale (Jadad 1996) was used to assess the methodological quality of the included studies. This scale covers three dimensions of study quality: randomisation, blinding and study withdrawals. The maximum possible score is five.
The three item scale is as follows:
Randomisation
Was the study described as randomised? (1 = yes; 0 = no).
Was the method of randomisation well described and appropriate? (1= yes; 0 = no); deduct one point if inappropriate.
Blinding
Was the study described as double‐blind ? (1 = yes; 0 = no).
Was the double blinding well described and appropriate? (1 = yes; 0 = no); deduct one point if inappropriate.
Description of study withdrawals and dropouts
Were withdrawals and dropouts described? (1 = yes; 0 = no).
Analysis
For cross‐over trials data were used from the first arm (if available) and also any data relating to patient preference were recorded. Analysis was carried out using an intention‐to‐treat model. Analyses were planned using a fixed‐effect model unless significant heterogeneity was found. The data were extracted from included trials and, where appropriate, entered into RevMan Analyses 1.1.2 in RevMan 4.2.10. An Excel template developed locally was used to calculate Numbers‐Needed‐to‐Treat (NNT) and numbers needed to harm (NNH)
The following data items were extracted: participants: age range, gender, type of neuropathic disorder, setting; intervention: antidepressant, dose, duration, route of administration; control: placebo, other active treatment, other intervention; outcomes: pain relief, global improvement, depression score, quality of sleep, quality of life, adverse events and effects; design: methods of randomisation, study design (parallel, cross‐over), and whether specifically designed to measure pain.
Statistical considerations
Where appropriate, data from included studies were combined using RevMan Analyses 1.1.2 in RevMan 4.2.10.
No continuous data were suitable for analysis. For dichotomous variables, the Relative Benefit (expressed as Relative Risk (RR) in RevMan Analyses) with 95% confidence interval (CI) were calculated for individual studies. Data from all similar studies were pooled using fixed‐effect RR and 95% CIs. Results were also reported as Number‐Needed‐to‐Treat (NNTs) for pain relief and global improvement and Number‐Needed‐to‐Harm (NNHs) for mild and serious adverse drug reactions (Cook 1995).
Sub‐group/sensitivity analyses:
Where data were available, sub‐group analyses was carried out according to:
neuropathic disorder,
antidepressant,
different classes of antidepressant and individual drug (TCAs, SSRIs).
Results
Description of studies
This update identified 13 new studies of which three were excluded (Aragona 2005; Beaumont 1980; Vidal 2004). One study awaiting assessment in the previous version is now included (Ciaramella 2000). The eleven new included studies reported on a total of 778 participants (Bowsher 1997; Ciaramella 2000; Forssell 2004; Lampl 2002; Mercadante 2002; Raja 2002; Reuben 2004; Robinson 2004; Rowbotham 2004; Sindrup 2003; Yucel 2004). Three of the new studies investigated antidepressant drugs as pre‐emptive treatments to prevent development of neuropathic pain (Bowsher 1997; Lampl 2002; Reuben 2004).
Study selection
In total one hundred and fifteen reports were identified. Forty‐nine were excluded (see "Characteristics of excluded studies" table). Five studies were identified as secondary publications and are listed in the included references under the primary publication.
In this update 11 additional studies were identified which provides a new total of 61 RCTs of 20 different antidepressants (3293 participants) for inclusion in the review. However, data from six of these studies were not included in the quantitative analysis because:
two studies had fewer than ten patients in the final analyses (Brady 1987; Simpson 2001);
one study included patients with musculoskeletal pain (Sharav 1987); and
two studies investigated the efficacy of a combination of anticonvulsants and antidepressants (Gerson 1977; Simpson 2001);
one study did not differentiate between neuropathic and non neuropathic pain (Ciaramella 2000.
Study design
Twenty six studies had a parallel design and 35 were crossover studies.
Eight out of 39 crossover studies reported first treatment period data. In three studies with pooled data of first and second treatment periods there was neither a washout period nor were analyses of any carry‐over effect performed (Gomez‐Perez 1985; Lascelles 1966; Panerai 1990). In four other studies with or without washout period, some carry‐over effect was reported (Kvinesdal 1984; Max 1987; Max 1988; Sindrup 1990b).
Outcomes
Pain was patient‐reported in 41 studies, but in 17 studies it was not clear how pain was assessed. In two studies, the investigators' assessment of the patients' pain was reported, and the authors reported no significant difference in the level of pain relief reported by investigators and patients (Langohr 1982; Pilowsky 1982).
The number of patients with global improvement of pain relief was available in 40 studies; in 21 studies only mean (continuous) data were available.
Study methods
One study was single blind (Carasso 1979), three were open (Ciaramella 2000; Dallocchio 2000; Gerson 1977) and in a further three studies blinding was not clear (Hampf 1989; Max 1992a; Max 1992b). The remaining studies were reported as double blind. Forty five studies were placebo controlled, one of these (Robinson 2004) used an 'active' placebo ‐ benztropine.
Antidepressants
Studies were found for the following antidepressants:
nine TCA drugs (amitriptyline, clomipramine, desipramine, dothiepin, doxepin, imipramine, mianserin, maprotiline, nortriptyline);
five SSRIs/SNRIs (citalopram, fluvoxamine, fluoxetine, paroxetine, sertraline);
five other antidepressant drugs (bupropion, L‐tryptophan, phenelzine, venlafaxine and trazodone);
one study of St John's Wort.
Patient conditions
The underlying conditions studied were as follows:
Diabetic neuropathy: 17 studies;
Postherpetic neuralgia: 11 studies;
Postherpetic and trigeminal neuralgia: one study;
Central pain: five studies;
Atypical facial pain: five studies;
Burning mouth pain: two studies;
HIV‐related neuropathy: two studies;
Neuropathic cancer pain : one study;
Post‐treatment/surgery neuropathic pain in breast cancer patients: three studies;
Post amputation pain: one study;
Painful polyneuropathy: one study;
Mixed neuropathic pain: 12 studies.
Details of these eligible reports are provided in 'Characteristics of included studies' table.
Risk of bias in included studies
Each report was scored independently for quality by two of the review authors (TS & PW) using the three‐item Oxford Quality Scale (Jadad 1996). The quality scores for individual trials are reported in the notes section of 'Characteristics of included studies' table.
The median quality score for the 45 placebo‐controlled studies was four (range one to five), and for the active‐control studies two (range one to four). Twenty one studies reported continuous data. In 16 studies the data was not evaluable mainly because investigators failed to report a standard deviation of means. Details are in Additional Table 1.
1. Continuous data studies.
| Study ID | Condition | Medicines used | Continuous data |
| Cardenas 2002 | Spinal cord injury | Amitriptyline versus benztropine (active placebo) | No significant difference between acive and placebo |
| Gomez Perez 1996 | Diabetic Neuropathy | Nortriptyline‐Fluphenazine versus carbamazepine | Mean percent change but no standard deviations (SD) |
| Graff Radford 2000 | Post herpetic neuralgia | Amitiptyline and Fluphenazine | VAS change scores‐ see Metaview |
| Hampf 1989 | Mixed pain but only neuropathic pain analysed for this review | Distigmine and Amitriptyline | VAS change scores but no SDs |
| Harrison 1997 | Chronic Idiopathic Facial Pain | Fluoxetine | No evaluable data |
| Kalso 1995 | Post mastectomy pain | Amitriptyline | Pre and post VAS with Median and range scores. |
| Max 1987 | Diabetic Neuropathy | Amitriptyline versus benztropine (active placebo) | No evaluable data |
| Max 1992a | Diabetic neuropathy | Desipramine, amitriptyline and fluoxetine | Mean change scores with standard error. See metaview |
| McCleane 2000a | Neuropathic pain | Topical doxepin, topical capsaicin, and combination of both | Change scores with 95% CI |
| McCleane 2000b | Neuropathic pain | Topical doxepin | Mean plus SD for change in pain scores. See metaview |
| Panerai 1990 | Central pain | Clomipramine, nortriptyline | No evaluable data |
| Sharav 1987 | Chronic facial pain | Amitriptyline | Change VAS data with range |
| Sindrup 1990a | Diabetic neuropathy | Paroxetine, imipramine | No evaluable data |
| Sindrup 1990b | Diabetic neuropathy | Clomipramine, desipramine | No evaluable data |
| Sindrup 1992a | Diabetic neuropathy | Citalopram | No mean scores or SD |
| Sindrup 1992b | Diabetic neuropathy | Mianserin | No mean scores or SD |
| Tasmuth 2002 | Neuropathic pain following treatment for breast cancer | Venlafaxine | Medians for pain relief with ranges |
| Ventafridda 1987 | Neuropathic cancer pain | Amitriptyline, trazodone | No evaluable data. |
| Rowbotham 2004 | Diabetic Neuropathy | Venlafaxine | Means but no SD |
Effects of interventions
Sixty one studies (66 reports) with a total of 3293 participants (of which 2218 received antidepressant medicines) were included in this review.
Tricyclic antidepressants
Thirty‐one placebo‐controlled trials studied TCAs in various kinds of neuropathies (Bowsher 1997; Cardenas 2002; Feinmann 1984; Gomez‐Perez 1985; Graff‐Radford 2000; Kalso 1996, Kieburtz 1998; Kishore‐Kumar 1990; Kvinesdal 1984; Lampl 2002; Leijon 1989; Max 1987; Max 1988; Max 1991; McCleane 2000a; McCleane 2000b; Mercadante 2002; Panerai 1990; Pilowsky 1982; Raja 2002; Robinson 2004; Rowbotham 2004; Sharav 1987; Shlay 1998; Sindrup 1989; Sindrup 1990a; Sindrup 1990b; Sindrup 1992b; Sindrup 2003; Turkington 1980; Vrethem 1997; Watson 1982).
In 17 studies with global improvement or pain relief measurements (at least moderate improvement) (Bowsher 1997; Feinmann 1984; Gomez‐Perez 1985; Kieburtz 1998; Kishore‐Kumar 1990; Kvinesdal 1984; Lampl 2002; Leijon 1989; Max 1988; Max 1991; Pilowsky 1982; Shlay 1998; Sindrup 1989; Sindrup 2003; Turkington 1980; Vrethem 1997; Watson 1982) the overall effectiveness risk benefit showed a significant effect for TCAs compared to placebo: RR 2.1 (95% CI 1.8 to 2.5). Active treatment was significantly better than placebo in 13 studies out of 17 including studies of diabetic neuropathy, postherpetic neuralgia, atypical facial pain, neuropathy of traumatic, surgical or infectious origin, central pain, and polyneuropathy. In two studies with HIV‐related neuropathy no difference was found between active treatment and placebo (Kieburtz 1998; Shlay 1998), neither was a difference found in one study with chronic intractable pain without specific organic cause (Pilowsky 1982), nor in a study of prevention of post stroke pain (Lampl 2002).
Overall the NNTs for effectiveness using moderate pain relief or better were 3.6 (96% CI 3 to 4.5). NNTs for individual agents were:
amitriptyline for a range of doses up to 150 mg daily (ten studies, 588 patients), NNT 3.1 (95% CI 2.5 to 4.2);
desipramine (two studies, 100 patients), NNT 2.6 (95% CI 1.9 to 4.5)
imipramine (three studies, 114 patients), NNT 2.2 (95% CI 1.7 to 3.22)
Mean data only were available in 13 studies, giving 14 comparisons between TCAs and placebo (Cardenas 2002; Graff‐Radford 2000; Kalso 1996; Max 1987; McCleane 2000a; McCleane 2000b; Mercadante 2002; Panerai 1990; Raja 2002; Robinson 2004; Sindrup 1990a; Sindrup 1990b; Sindrup 1992b). Only three comparisons out of 14 failed to demonstrate superiority of TCAs over placebo using a vote counting method.
Eighty‐four patients with spinal cord injury were randomised to amitriptyline or placebo. After six weeks' treatment no difference showed between the groups (Cardenas 2002). In 26 patients with diabetic neuropathy, clomipramine was demonstrated to be more effective than placebo, but for desipramine there was no difference from placebo (Sindrup 1990b). Similarly imipramine was effective in the treatment of 22 patients with diabetic neuropathy, but no effect was found with mianserin (Sindrup 1992b).
Imipramine was shown to be effective in a study of diabetic neuropathy by Sindrup 1990a. Both clomipramine and nortriptyline were more effective than placebo in treatment of central pain (Panerai 1990). Three studies found amitriptyline to be superior to placebo in treatment of diabetic neuropathy (Max 1987), postherpetic neuralgia (Graff‐Radford 2000), and postoperative neuropathic pain in breast cancer patients (Kalso 1996). Doxepin cream was more efficient than placebo cream in two studies of different neuropathic pain syndromes (McCleane 2000a; McCleane 2000b). A one week treatment with amitriptyline did not improve analgesia in 16 morphine treated patients with advanced cancer, except for so‐called 'worst pain', (a term used by the investigators) which was significantly improved by amitriptyline (Mercadante 2002). In 39 patients with pain after amputation amitriptyline was not superior to placebo (Robinson 2004). Tricyclic antidepressants nortriptyline and desipramine were superior to placebo in treatment of postherpetic neuralgia in 76 patients (Raja 2002).
One study included patients with musculoskeletal pain but there were no evaluable data (Sharav 1987).
Tricyclic antidepressant versus another tricyclic antidepressant
Twelve studies compared two different TCA treatments:
amitriptyline versus clomipramine (Carasso 1979);
amitriptyline versus imipramine (Turkington 1980);
amitriptyline versus maprotiline (Vrethem 1997; Watson 1992);
amitriptyline versus nortriptyline (Watson 1998);
amitriptyline versus desipramine (Max 1992a);
clomipramine versus nortriptyline (Panerai 1990);
clomipramine versus desipramine (Sindrup 1990b);
imipramine versus mianserin (Sindrup 1992b);
desipramine versus nortriptyline (Raja 2002);
amitriptyline versus desipramine (Rowbotham 2004);
amitriptyline versus trazodone (Ventafridda 1987).
In six studies which reported global improvement or pain relief measurements (Carasso 1979; Rowbotham 2004; Turkington 1980; Vrethem 1997; Watson 1992; Watson 1998) no significant differences were found in overall effectiveness: RR for amitriptyline versus other TCAs was 1.1 (95% CI 0.9 to 1.3).
Only mean data were reported in six studies (Max 1992a; Panerai 1990; Raja 2002; Sindrup 1990b; Sindrup 1992b).
No difference was reported between amitriptyline and desipramine in the treatment of 54 patients with diabetic neuropathy (Max 1992a);
Clomipramine was reported to be more effective than nortriptyline in 39 patients with central pain (Panerai 1990);
In the treatment of diabetic neuropathy clomipramine tended to be more efficacious than desipramine (Sindrup 1990b);
Imipramine more effective than mianserin (Sindrup 1992b);
One study compared amitriptyline 75 mg to trazodone 225 mg in different neuropathic pain disorders, and the authors reported no difference between the study groups in final pain scores (Ventafridda 1987);
No difference was reported between desipramine and nortriptyline in treatment of 59 patients with postherpetic neuralgia (Raja 2002).
Tricyclic antidepressant versus. other active treatment
Thirteen studies compared TCAs to other active treatments, including tramadol (Göbel 1997), aspirin (Langohr 1982), capsaicin cream (Biesbroeck 1995; McCleane 2000a), mexiletine (an antiarrythmic) (Kieburtz 1998), lorazepam (an anxiolytic and benzodiazepine) (Max 1988), fluphenazine (a neuroleptic) (Graff‐Radford 2000), distigmine (an anticholinesterase) (Hampf 1989), gabapentin (an anticonvulsant) (Dallocchio 2000; Morello 1999), carbamazepine (an anticonvulsant) (Gomez‐Perez 1996; Leijon 1989) and opioids, morphine and methadone (Raja 2002).
Global improvement or pain relief was measured in five studies (Biesbroeck 1995; Göbel 1997; Kieburtz 1998; Langohr 1982; Max 1988). In the study of Göbel et al. tramadol 600 mg was at least as effective as clomipramine 100 mg in treatment of postherpetic neuralgia; 6/11 patients had complete or satisfactory pain relief during clomipramine treatment as compared to 9/10 during tramadol treatment, RR 0.6 (95% CI 0.3 to 1.1) (Göbel 1997).
Clomipramine 150 mg was not significantly better than aspirin 500 mg in the treatment of neuropathic pain of traumatic, infectious or surgical origin, 10/19 patients reported pain relief on clomipramine as compared to 4/20 on aspirin, RR 2.63 (not significant) (Langohr 1982). Both mexiletine and amitriptyline were reported as ineffective in the treatment of HIV‐related neuropathy in 145 patients (Kieburtz 1998).
Amitriptyline 150 mg was more effective than lorazepam 6 mg in the treatment of postherpetic neuralgia,16/34 patients had at least moderate improvement on amitriptyline as compared to 6/40 on lorazepam, RR 3.1 (95% CI 1.4 to 7.1) (Max 1988).
Capsaicin cream was compared to TCAs in two studies: Biesbroeck 1995 demonstrated that capsaicin cream was as effective as amitriptyline 125 mg taken orally in patients with diabetic neuropathy, 79/108 patients stated at least as good pain relief on amitriptyline as compared to 75/104 on capsaicin, RR 1.01 (not significant). In another study of 200 patients with different neuropathic syndromes only mean data were reported, but the authors reported that doxepin cream, capsaicin cream and combination of capsaicin and doxepin creams were all equally effective, but no pain relief was achieved with a placebo cream (McCleane 2000a).
Also in studies by Graff‐Radford 2000 and Hampf 1989 only mean data were reported. Amitriptyline 200 mg was more effective than fluphenazine 3 mg in patients with postherpetic neuralgia (Graff‐Radford 2000). No differences were found between amitriptyline, distigmine (an anticholinesterase) and a combination therapy of amitriptyline and distigmine in 50 patients with different neuropathic disorders, all treatments were equally effective (Hampf 1989). The Raja 2002 study of opioids, morphine and methadone, resulted in somewhat greater pain relief than TCAs, desipramine and nortriptyline, in patients with postherpetic neuralgia.
There were four small studies comparing antidepressants to anticonvulsants: three studies of diabetic neuropathy (Dallocchio 2000; Gomez‐Perez 1996; Morello 1999), and one of post stroke pain (Leijon 1989). Overall no significant difference in pain relief was found, the overall effectiveness RR was 1.3 (95% CI 0.9 to 1.8) for three studies including number of patients with pain relief or global improvement (Dallocchio 2000; Leijon 1989; Morello 1999). In a study of post stroke pain, amitriptyline was not superior to carbamazepine (Leijon 1989). In one study only mean data were reported and the authors could not show any differences between the two treatments, however, it was stated that all patients responded (Gomez‐Perez 1996).
One study of 29 patients with postherpetic neuralgia compared combination of clomipramine and carbamazepine treatment to transcutaneous electrical nerve stimulation (TENS), and demonstrated marked pain relief in 8/9 patients with drug combination and in 2/3 patients in the TENS group (Gerson 1977). However, twelve additional patients crossed over due to lack of efficacy (four from drug to TENS group and eight from TENS to drug group).
Selective Serotonin Re‐uptake Inhibitors (SSRIs)
Four studies compared SSRI to placebo (Harrison 1997; Max 1992b; Sindrup 1990a; Sindrup 1992a). Generally only mean data were reported. Max 1992b reported on global ratings but both randomised and non randomised participants were reported together. In all four studies SSRIs were superior to placebo. Fluoxetine 20 mg and 40 mg were stated to be more effective than placebo in 98 patients with idiopathic facial pain (Harrison 1997) and in treatment of 54 patients with diabetic neuropathy (Max 1992b); paroxetine 40 mg (Sindrup 1990a) and citalopram 40 mg (Sindrup 1992a) were also reported to be effective in treatment of diabetic neuropathy in two small studies. See more detailed data of the studies in the 'Characteristics of Included Studies' table. Insufficient reporting of the data in these studies prevents the calculation of NNTs.
One study that compared paroxetine 20 mg or sertraline 50 mg to amisulpride 50 mg (antipsychotic) in patients with burning mouth syndrome did not demonstrate any differences between the two SSRIs (Maina 2002). RR for effectiveness was 0.96 (not significant), 16/23 patients responded to paroxetine and 13/18 to sertraline. No difference was found in efficacy of SSRIs and amisulpride, RR 1.01 (not significant). Twenty‐nine patients out of 41 responded to SSRIs compared to 19/27 who responded to amisulpride.
Only two controlled studies compared SSRIs to TCAs. In the first placebo controlled study paroxetine 40 mg was compared to imipramine in doses of up to 350 mg/day in 26 patients with diabetic neuropathy (Sindrup 1990a). Only mean data were reported. Both drugs reduced the neuropathy score more effectively than placebo, but paroxetine was reported to be 'somewhat less effective' than imipramine. In spite of this, the visual analogue scale (VAS) pain scores shown graphically are similar for paroxetine and imipramine. In the other study fluoxetine was compared to amitriptyline and desipramine in 47 patients with postherpetic neuralgia. Clinically meaningful pain relief (moderate or better) was significantly more likely with desipramine (12/15) than with amitriptyline (9/17) or fluoxetine (5/15) (Rowbotham 2004).
Ciaramella 2000 compared fluoxetine with fluvoxamine in patients with chronic pain and depression. While fluvoxamine was slightly better than fluoxetine it is not possible to evaluate the data as both neuropathic and non neuropathic pain patients were included.
Serotonin and noradrenaline reuptake inhibitors
No studies were found for SNRI antidepressants
Venlafaxine
Venlafaxine was investigated in six studies (Forssell 2004; Reuben 2004; Simpson 2001; Sindrup 2003; Tasmuth 2002; Yucel 2004).
In three studies with global improvement or pain relief measurements the overall effectiveness showed a significant effect for venlafaxine compared to placebo, RR 2.2 (95 % CI 1.5 to 3.1). NNT was 3.1 (95% CI 2.2 to 5.1) (Reuben 2004; Sindrup 2003; Yucel 2004). Doses used were 75 mg, 150 mg and 225 mg.
In three studies only mean data were reported (Forssell 2004; Simpson 2001; Tasmuth 2002). Some pain relief was reported with venlafaxine 75 mg in the treatment of postoperative neuropathic pain in 15 breast cancer patients (Tasmuth 2002). The effectiveness of venlafaxine in addition to gabapentin was studied in diabetic neuropathy, but only seven patients were included in the analyses (Simpson 2001). No significant pain relief was reported with venlafaxine (dose up to 75 mg) in a placebo controlled study of 30 patients with atypical facial pain (Forssell 2004).
In one placebo controlled study venlafaxine 225 mg was compared to imipramine 150 mg in 40 patients with polyneuropathy. At least moderate pain relief was demonstrated by venlafaxine in 8/30, imipramine 14/29 and 2/29 in placebo arm. Both venlafaxine and imipramine were effective in pain relief. No statistically significant difference was seen between the two antidepressants (Sindrup 2003).
Other antidepressant drugs
These are considered together as a group for simplicity of reporting. There is no implication that they are similar in action or effect. Other types of antidepressants were compared to placebo in six studies (Brady 1987; Davidoff 1987; Lascelles 1966; Semenchuk 2001; Sindrup 2001; Tammiala‐Salonen 99).
Antidepressants were superior to placebo in three out of five studies: 15/20 patients with atypical facial pain responded to phenelzine, and 7/20 on placebo (Lascelles 1966); on bupropion pain relief was reported in 30/41 patients with different neuropathic pain syndromes as compared to 4/41 on placebo (Semenchuk 2001); and St Johns Wort was slightly better than placebo in treatment of polyneuropathy, 9/47 patients had complete or a good response with St Johns Wort and 2/47 on placebo (Sindrup 2001). There was no significant difference between trazodone and placebo in two studies; pain relief was demonstrated in 4/9 patients with traumatic myelopathy during trazodone treatment compared to 3/9 during placebo (Davidoff 1987); and in another study of burning mouth syndrome 8/11 patients benefited from trazodone and 13/17 from placebo (Tammiala‐Salonen 99).
In a small study L‐tryptophan was studied in eight patients with different neuropathic pain syndromes (Brady 1987). Only mean data was available for this study.
Diabetic neuropathy
Thirteen placebo controlled studies investigated the effect of antidepressants in treatment of diabetic neuropathy (Gomez‐Perez 1985; Kvinesdal 1984; Max 1987; Max 1991; Max 1992b; Rowbotham 2004; Simpson 2001; Sindrup 1989; Sindrup 1990a; Sindrup 1990b; Sindrup 1992a; Sindrup 1992b; Turkington 1980). In five small studies with global improvement or pain relief measurements the overall effectiveness risk ratio showed a significant effect for tri‐or tetracyclic antidepressants compared to placebo, RR was 12.4 (95% CI 5.3 to 29), in ITT analyses RR was 12.4 (95% CI 5.2 to 29) (Gomez‐Perez 1985; Kvinesdal 1984; Max 1991; Sindrup 1989; Turkington 1980). Antidepressants effectively relieved pain in all five studies. The overall NNT for effectiveness compared with placebo was 1.3 (95% CI 1.2 to 1.5).
Seven additional studies published only mean data (Max 1987; Max 1992b; Simpson 2001; Sindrup 1990a; Sindrup 1990b; Sindrup 1992a; Sindrup 1992b). Tricyclic antidepressant drugs amitriptyline, clomipramine and imipramine were demonstrated to relieve pain significantly in four studies (Max 1987; Sindrup 1990a; Sindrup 1990b; Sindrup 1992b), but desipramine and mianserin failed to be any different from placebo in two studies (Sindrup 1990b; Sindrup 1992b). SSRIs fluoxetine, citalopram and paroxetine were shown to be effective in three studies (Max 1992b; Sindrup 1990a; Sindrup 1992a). In a study by Simpson et al. only seven patients were included in their analyses so were not considered in this analysis (Simpson 2001).
Postherpetic neuralgia
Six placebo‐controlled studies were found in postherpetic neuralgia. All demonstrated superiority of antidepressants over placebo. Four of them included data of global improvement or pain relief with risk ratio of effectiveness NNT 2.7 (95% CI 2 to 4), RR 2.3 (95% CI 1.7 to 3.2) (Bowsher 1997; Kishore‐Kumar 1990; Max 1988; Watson 1982). The overall NNT for effectiveness compared with placebo was 2.7 (95% CI 2 to 4.1), in ITT analyses 2.2 (95% CI 1.6 to 3.1). In two studies only mean data were available: in the first study according to the authors amitriptyline was superior to placebo in the treatment of postherpetic neuralgia in 50 patients (Graff‐Radford 2000). In the other study of 71 patients TCAs, desipramine and nortriptyline, reduced pain more effectively than placebo (Raja 2002).
In addition, one study compared amitriptyline, desipramine and fluoxetine in 47 patients (Graff‐Radford 2000) with postherpetic neuralgia. Clinically meaningful pain relief was seen in 9/17 amitriptyline, 12/15 desipramine and 5/15 fluoxetine treated patients. All three drugs reduced pain, with desipramine providing satisfactory pain relief in 80% of those treated.
Central pain
Five placebo‐controlled studies were available in central pain: in the study of post stroke pain 10/15 patients had partial or complete pain relief on amitriptyline as compared to 1/15 on placebo (Leijon 1989); in 4/9 patients with traumatic myelopathy trazodone 150 mg was effective, while placebo was effective in 3/9 patients (Davidoff 1987); no effect of amitriptyline was demonstrated in study of 84 patients with spinal cord injury (Cardenas 2002); clomipramine and nortriptyline were shown to be more effective than placebo in treatment of 39 patients with central pain of different aetiology (Panerai 1990). Amitriptyline was not different from placebo in prevention of post stroke pain in 39 patients with thalamic stroke (Lampl 2002).
HIV‐related neuropathy
No effect was seen in two large placebo‐controlled studies (395 participants) of TCAs in the treatment of HIV‐related neuropathy (Kieburtz 1998; Shlay 1998). Kieburtz 1998 compared amitriptyline, mexiletine and placebo. Improvement in the amitriptyline and mexiletine arms were not significantly different from placebo. Shlay 1998 enrolled participants into a three arm study: standardised acupuncture and amitriptyline versus placebo, standardised acupuncture versus control points and amitriptyline versus placebo. Neither acupuncture or amitriptyline was more effective than placebo in relieving the pain of HIV related peripheral neuropathy.
Atypical facial pain
Four placebo‐controlled studies were identified in atypical facial pain (Feinmann 1984; Forssell 2004, Harrison 1997; Lascelles 1966). Relative risk of effectiveness for two of these studies including data of pain relief or global improvement was RR 1.6 (95% CI 1.2 to 2.3) and the NNT for effectiveness compared to placebo 3.4 (95% CI 2.2 to 7.7) (Feinmann 1984; Lascelles 1966). ITT analyses were performed in both studies: 34/48 patients on dothiepin 150 mg experienced pain relief as compared to 21/45 on placebo (Feinmann 1984), and in another study 15/20 patients reported pain relief on phenelzine 45 mg as compared to 7/20 on placebo (Lascelles 1966). In two studies mean data were reported (Forssell 2004; Harrison 1997). In a study of 97 patients fluoxetine 20 mg was reported by authors to be more effective than placebo (Harrison 1997). Venlafaxine 75 mg was not statistically significantly superior to placebo in 30 patients (Forssell 2004).
In the study by Sharav et al. patients with musculoskeletal pain were included (Sharav 1987) so further analysis was not possible.
Burning mouth syndrome
Only one placebo‐controlled study was found in burning mouth pain. In that study trazodone 200 mg was shown to be ineffective (Tammiala‐Salonen 99).
Postoperative neuropathic pain after breast cancer treatments
Three small placebo‐controlled studies of postoperative neuropathic pain after breast cancer surgery and radiotherapy were found (Kalso 1996; Reuben 2004; Tasmuth 2002). In one study chronic pain was significantly reduced by venlafaxine 75 mg (14/48 patients in venlafaxine arm; 34/47 in placebo arm). Also analgesic use was significantly lower in the venlafaxine group 8/48 versus 26/47 in the placebo group (Reuben 2004). Only mean data were available in two studies. In the first study amitriptyline 100 mg was reported to relieve pain significantly in 20 breast cancer patients (Kalso 1996); in the second study some pain relief was reported with venlafaxine 75 mg in 15 breast cancer patients (Tasmuth 2002).
Pre‐emptive use of antidepressants
Five studies investigated pre‐emptive use of antidepressants in neuropathic pain. Three small placebo‐controlled studies of postoperative neuropathic pain after breast cancer surgery and radiotherapy were found (Kalso 1996; Reuben 2004; Tasmuth 2002). In all three studies at least some preventive effect was demonstrated with antidepressants, either amitriptyline or venlafaxine, in prevention of postmastectomy pain. In one study low dose of amitriptyline (25 mg) was shown to be superior to placebo in prevention of postherpetic neuralgia in 80 patients (Bowsher 1997). In another small placebo controlled study of 39 thalamic stroke patients amitriptyline 75 mg was not superior to placebo in prevention of post stroke pain (Lampl 2000).
Adverse effects and drug‐related study withdrawal
Across all studies, 453 participants dropped out of active groups for a variety of reasons including adverse effects (13% of participants receiving antidepressants). Number‐needed‐to‐harm (NNHs) were calculated for different antidepressants irrespective of the condition treated. These were calculated for minor harm, which included symptoms such as drowsiness, dizziness, dry mouth, constipation, nausea, urinary retention, sweating, headache, blurred vision, palpitations, irritability and ataxia. Studies with active control were not included in analyses of NNH for minor harm.
Major harm was defined as any effect leading to withdrawal from the study. The NNH for major harm for tri‐and tetracyclic antidepressants was 28 (95% CI 17 to 68) for amitriptyline (RR 2.2, 95% CI 1.3 to 3.6) and 16 (95% CI 8 to 436) for venlafaxine (RR 2.5; not significant). For other antidepressants no statistically significant difference was found as compared to placebo. The NNHs for minor harm for TCAs was 6 (95% CI 4.2 to 10.7) for amitriptyline (RR 1.3, 95% CI 1.1 to 1.4) and 9 (95% CI 3.5 to 13) for venlafaxine (RR 1.20; not significant).
Sleep
Sleep was investigated in eight studies (Biesbroeck 1995; Cardenas 2002; Kalso 1996; Mercadante 2002; Raja 2002; Semenchuk 2001; Turkington 1980; Vrethem 1997). In three studies no effect of antidepressants on sleep was found (Biesbroeck 1995; Cardenas 2002; Mercadante 2002), however, in two of them active placebo (benztropine and diazepam) was used (Biesbroeck 1995; Cardenas 2002). In three other studies there was significantly less sleep disturbance with TCAs than with placebo (Kalso 1996; Raja 2002; Turkington 1980). Sleep was improved with bupropion 300 mg as compared to placebo (Semenchuk 2001), and was somewhat improved also with amitriptyline 75 mg as compared to maprotiline 75 mg and placebo (Vrethem 1997).
Depression
Depression was studied in 18 studies (Cardenas 2002; Ciaramella 2000; Feinmann 1984; Forssell 2004; Gerson 1977; Graff‐Radford 2000; Harrison 1997; Lascelles 1966; Leijon 1989; Maina 2002; Mercadante 2002; Pilowsky 1982; Raja 2002; Robinson 2004; Tasmuth 2002; Turkington 1980; Vrethem 1997; Watson 1992). In 12 studies no effect of antidepressants on depression was demonstrated (Cardenas 2002; Feinmann 1984; Forssell 2004; Graff‐Radford 2000; Leijon 1989; Maina 2002; Mercadante 2002; Pilowsky 1982; Raja 2002; Robinson 2004; Tasmuth 2002; Watson 1992). In a study of patients with atypical facial pain, depression was improved in 15/20 patients with phenelzine 45 mg treatment as compared to 7/20 on placebo (Lascelles 1966). In patients with diabetic neuropathy depression scores decreased significantly with imipramine 100 mg and amitriptyline 100 mg treatments, but not with placebo (Turkington 1980). Amitriptyline 75 mg also decreased depression symptom scores more effectively than maprotiline 75 mg in patients with polyneuropathy (Vrethem 1997). In patients with postherpetic neuralgia there was no difference in superiority with a combination of carbamazepine and clomipramine compared to treatment with transcutaneous electrical nerve stimulation (TENS) with respect to mental outlook (Gerson 1977). Harrison 1997 showed that SSRIs improved pain in non‐depressed facial pain patients and that mood scores were unaffected by the antidepressant administered. Ciaramella 2000 reported that pain relief was independent of any effect on depression in a study of depressed patients.
There appears to be no correlation between depression and pain relief. Watson 1992 stated 'most patients are not depressed and obtain pain relief without a change in rating scales for depression, indicating that the drugs have an independent analgesic action'. This statement is supported by the results of this review.
Continuous Data analysis
A comment was made during the peer review process for the first edition of this review that continuous data had not been analysed. On careful examination only two studies (Max 1992a; McCleane 2000b) provided data that could be entered into analyses and for Max 1992a this required calculation of standard deviations from standard errors. The numbers in these studies are too small to draw meaningful conclusions.
Discussion
The additional trials now included have strengthened the evidence for the use of TCAs and provided new information of the use of venlafaxine. There is limited evidence that antidepressants do not have a pre‐emptive effect in preventing the development of neuropathic pain. This review of 61 RCTs provides robust evidence for the effectiveness of antidepressants in treating neuropathic pain. Tricyclic antidepressants have an NNT of 3.6 (95% CI 3 to 4.5); RR 2.1 (95% CI 1.8 to 2.5) and venlafaxine of 3.1 (2.2 to 5.1); RR 2.2 (95 % CI 1.5 to 3.1). This means that for every three or four patients with neuropathic pain who are treated with these antidepressants, one will get at least moderate pain relief. There is evidence to suggest that other antidepressants may be effective but numbers of participants are insufficient to calculate robust NNTs.
There is limited evidence to suggest that the newer SSRI antidepressants may be effective. These medicines are generally better tolerated by patients and more high quality studies are required. Studies of other antidepressants such as St Johns Wort and L‐tryptophan were too small for any firm conclusions to be made.
In terms of specific conditions, TCAs were shown to be effective in diabetic neuropathy and post herpetic neuralgia. There is some indication of effectiveness in central pain and atypical facial pain but few trials and small participant numbers prevent firm recommendations.
There is a lack of evidence for any effect in burning mouth syndrome. There is evidence that TCAs are ineffective in HIV related neuropathies.
Adverse effects with TCAs can be significant and lead to withdrawal from treatment. In this review, 20% of participants receiving antidepressants withdrew because of intolerable adverse effects. The adverse effects of TCAs are well documented from the clinical experience of treating depression. These include troublesome effects such as drowsiness, dry mouth, blurred vision, constipation and urinary retention. The daily dose administered as a single night time dose often helps patients cope with the drowsiness element. Severe adverse effects include arrhythmias and heart block. Caution is needed in patients who have a history of cardiac disease, or are elderly, or both.
It is well recognised that pain has an emotional component. This review demonstrates in a limited way that analgesia is independent of any effect that these drugs are having on depression. This supports the clinical impression of pain specialists and published work (Onghena 1992).
The quality of the reporting limited the ability to combine data: many reports gave insufficient information, used a variety of different outcome measurements, and variable dosing. The quality of reporting in recent trials remains disappointing, in particular insufficient details are provided to enable effectiveness to be assessed. This is marked by an on‐going preference to only report mean pain data rather than by reporting the number of participants responding.
Authors' conclusions
Implications for practice.
Antidepressants are effective for the treatment of neuropathic pain. The best evidence of pain relief is for TCAs and amitriptyline in particular which has an NNT of 3.1 (95% CI 2.5 to 4.2). There is some new data on venlafaxine in the treatment of neuropathic pain (three studies) which shows NNTs quite similar to TCAs (NNT for venlafaxine 3 (95% CI 2.2 to 5.1). Only limited data exist for the effectiveness of SSRIs. For patients who get relief from TCAs but find the adverse effects a problem, the very limited data on SSRIs suggests that a trial of SSRIs in those individuals may yield benefit.
The effect of antidepressants was mainly demonstrated in treatment of diabetic neuropathy and postherpetic neuralgia. A limited number of studies were available in the other neuropathic pain syndromes such as central pain, atypical facial pain and postoperative pain after breast cancer treatments. No effect of antidepressants was demonstrated in one small study of burning mouth syndrome and in two studies of HIV related neuropathic pain.
The clinical impression is that antidepressants are effective. Effectiveness is usually seen in a few days if there is a response. It also seems to confirm clinical practice, that if one antidepressant is not effective or not tolerated then another may be effective. It would seem prudent, based on this evidence, to initiate treatment with amitriptyline and to switch to an alternative TCAs or venlafaxine if some pain relief is achieved but side effects are troublesome. Limited evidence suggests that antidepressants do not have a pre‐emptive effect.
Implications for research.
Further research is needed with regard to the effect of newer types of antidepressants, such as SSRIs or SNRIs in the treatment of neuropathic pain, this is important because these drugs generally are better tolerated than TCAs. There is some evidence suggesting that these medicines may be of benefit in neuropathic pain. Head to head trials with TCAs are required to demonstrate effectiveness. The on‐going trend to report only mean data in RCTs does restrict analysis. Reporting of responder numbers in each arm of a trial would significantly add to the usefulness of those trials in clinical practice.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2014 | Review declared as stable | This review has been split into individual antidepressants for neuropathic pain. See Published notes |
History
Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 27 June 2012 | Amended | Contact details updated. |
| 24 September 2010 | Amended | Contact details updated. |
| 4 September 2009 | Amended | This review is being updated by being split into smaller components. The first will be a review of the effectiveness of amitriptyline which is widely used to treat neuropathic pain. |
| 20 August 2008 | Amended | Converted to new review format. |
| 19 August 2007 | Amended | This update identified 13 new studies of which three were excluded (Aragona 2005; Beaumont 1980; Vidal 2004). One study awaiting assessment in the previous version is now included (Ciaramella 2000). The eleven new included studies reported on a total of 778 participants (Bowsher 1997; Ciaramella 2000; Forssell 2004; Lampl 2002; Mercadante 2002; Raja 2002; Reuben 2004; Robinson 2004; Rowbotham 2004; Sindrup 2003; Yucel 2004). Three of the new studies investigated antidepressant drugs as pre‐emptive treatments to prevent development of neuropathic pain (Bowsher 1997; Lampl 2002; Reuben 2004). We have added a table listing the studies with continuous data (Additional Table 01). This update has provided additional confirmation on the effectiveness of antidepressants for neuropathic pain and has provided new information on another antidepressant ‐ venlafaxine and previous readers of the review are advised to re‐read. |
Notes
September 2009: This review is being updated by being split into smaller components. The first will be a review of the effectiveness of amitriptyline which is widely used to treat neuropathic pain.
January 2014: The following reviews and protocols have been published, or are in development:
Amitriptyline for neuropathic pain and fibromyalgia in adults
Milnacipran for neuropathic pain and fibromyalgia in adults
Venlafaxine for neuropathic pain
Imipramine for neuropathic pain in adults
Desipramine for neuropathic pain in adults
Acknowledgements
Frances Fairman devised the original data extraction form, was involved in study selection for the first publication, helped with the search strategy, obtained papers, and contributed to final drafts. She was not involved in this update. Anne Eisinga at the UK Cochrane Centre helped with data extraction of an Italian language paper.
Appendices
Appendix 1. Search strategy
1. For the intervention, antidepressants
Free text searches included individual drug names, and the general term 'antidepressant'.
amesergide OR amineptine OR amitriptyline OR amoxapine OR benactyzine OR brofaromine OR bupropion OR butriptyline OR cianopramine OR citalopram OR clomipramine OR clorgyline OR clovoxamine OR demexiptiline OR desipramine OR dibenzepin OR dimetacrine tartrate OR dosulepin OR dothiepin OR doxepin OR etoperidone OR femoxetine OR fezolamine fumarate OR fluoxetine OR flupenthixol OR fluphenazine OR fluvoxamine maleate OR ifoxetine OR imipramine OR iprindole OR iproniazid phosphate OR isocarboxazid OR levoprotiline OR lofepramine OR l‐tryptophan OR maprotiline OR medifoxamide OR melitracen OR metapramine fumarate OR mianserin OR milnacipran OR minaprine OR mirtazepine OR moclobemide OR nefazodone OR nialamide OR nomifensine maleate OR nortriptyline OR opipramol OR oxaflozane OR oxaprotiline OR oxitriptan OR paroxetine OR phenelzine sulphate OR pirlindole OR propizepine OR protriptyline OR quinupramine OR reboxetine OR rolipram OR rubidium chloride OR sertraline OR setiptiline OR sibutramine OR sulpiride OR teniloxazine OR tianeptine sodium OR tofenacin OR toloxatone OR tranylcypromine sulphate OR trazodone OR trimipramine OR tryptophan OR venlafaxine OR viloxazine OR viqualine OR zimeldine OR antidepressant.
2. For the condition, pain:
Free text searches and MeSH for 'pain' and 'disease condition' (such as post herpetic neuralgia, trigeminal neuralgia, central pain, diabetic neuropathy and atypical facial pain)
3. For the trial design
Free text searches and MeSH for 'trial design' (such as randomised, randomized, controlled, placebo controlled, prospective)
The results of searches 1 and 2 and 3 were combined using the term "AND": (Pain OR disease condition) AND (antidepressant OR generic names of the drugs listed above) AND (randomised OR randomized OR controlled OR prospective OR placebo).
Data and analyses
Comparison 1. Global improvement ‐ number of patients with moderate pain relief or better.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amitriptyline versus placebo | 10 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [1.35, 3.69] |
| 2 Desipramine vs placebo | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.75 [2.19, 15.08] |
| 3 Imipramine vs placebo | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.0 [3.97, 90.84] |
| 4 Other antidepressants vs placebo | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Tricyclics versus anticonvulsants | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Venlafaxine vs placebo | 3 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.50, 3.11] |
1.1. Analysis.
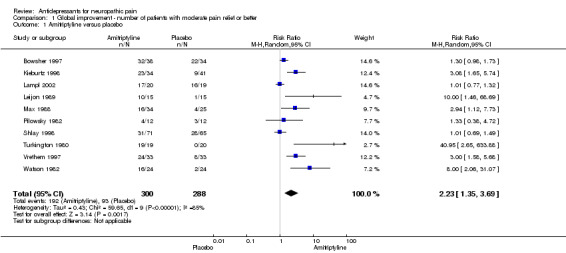
Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 1 Amitriptyline versus placebo.
1.2. Analysis.
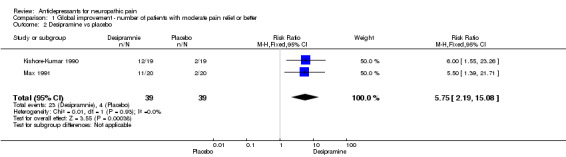
Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 2 Desipramine vs placebo.
1.3. Analysis.
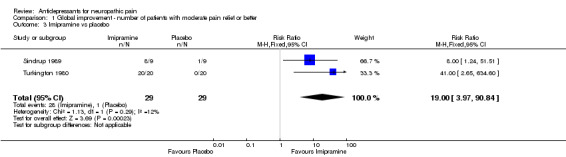
Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 3 Imipramine vs placebo.
1.4. Analysis.
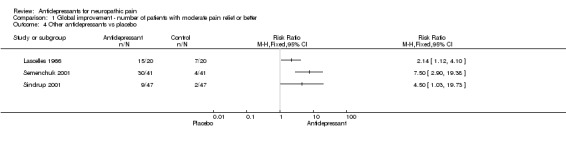
Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 4 Other antidepressants vs placebo.
1.5. Analysis.
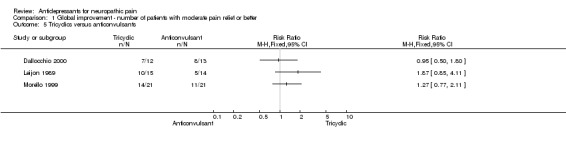
Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 5 Tricyclics versus anticonvulsants.
1.6. Analysis.
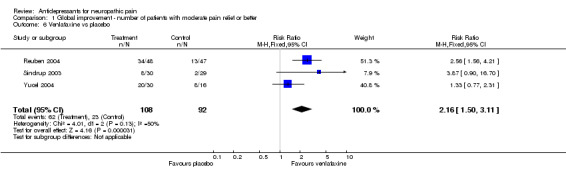
Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 6 Venlafaxine vs placebo.
Comparison 2. Diabetic neuropathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressant vs placebo. Number of patients with moderate pain relief or better | 5 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.41 [5.27, 29.21] |
| 2 Changes in pain intensity: Desipramine vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Changes in pain intensity: Amitriptyline vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Changes in pain intensity: Fluoxetine vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
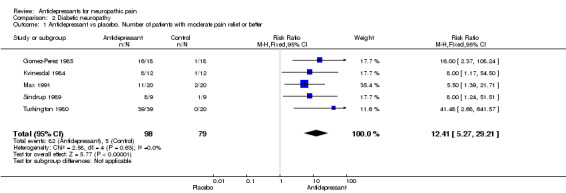
Comparison 2 Diabetic neuropathy, Outcome 1 Antidepressant vs placebo. Number of patients with moderate pain relief or better.
2.2. Analysis.
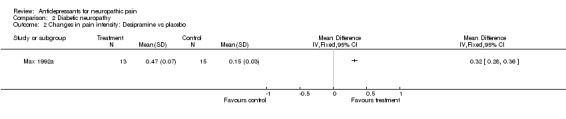
Comparison 2 Diabetic neuropathy, Outcome 2 Changes in pain intensity: Desipramine vs placebo.
2.3. Analysis.
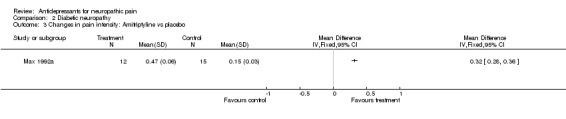
Comparison 2 Diabetic neuropathy, Outcome 3 Changes in pain intensity: Amitriptyline vs placebo.
2.4. Analysis.
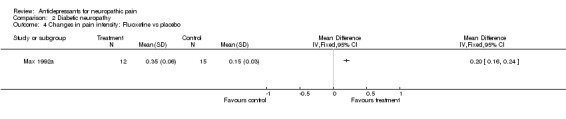
Comparison 2 Diabetic neuropathy, Outcome 4 Changes in pain intensity: Fluoxetine vs placebo.
Comparison 3. Postherpetic neuralgia‐ number of patients with moderate pain relief or better.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressant vs placebo | 4 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.70, 3.19] |
3.1. Analysis.
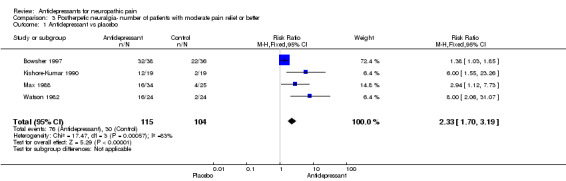
Comparison 3 Postherpetic neuralgia‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo.
Comparison 4. Central pain‐ number of patients with moderate pain relief or better.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressant vs placebo | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [1.31, 9.33] |
4.1. Analysis.
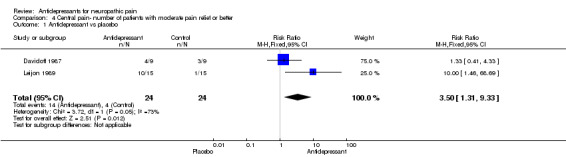
Comparison 4 Central pain‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo.
Comparison 5. Atypical facial pain‐ number of patients with moderate pain relief or better.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antidepressant vs placebo | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.22, 2.29] |
5.1. Analysis.
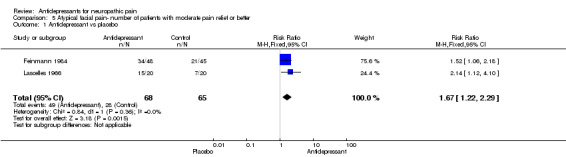
Comparison 5 Atypical facial pain‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo.
Comparison 7. Topical Doxepin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change scores ‐ doxepin vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
7.1. Analysis.
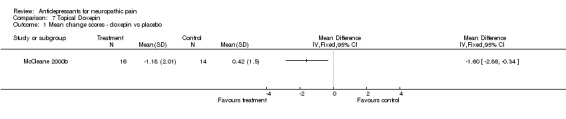
Comparison 7 Topical Doxepin, Outcome 1 Mean change scores ‐ doxepin vs placebo.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Biesbroeck 1995.
| Methods | Double blind double dummy parallel design study, eight weeks four week titration to max tolerated dose of amitriptyline then four week stable dose Randomisation method not stated Inclusion criteria: age 21 to 85 years, duration of symptoms at least four months |
|
| Participants | Painful diabetic neuropathy of three to five years. 235 participants (212 final number). Age range 21 to 85 years. Baseline pain score in amitriptyline group VAS 64.5 and in capsaicin cream group VAS 61.7 | |
| Interventions | Amitriptyline dose escalation from 25 mg to 125 mg daily orally + active placebo in first two weeks (methyl nicotinate). Capsaicin cream topically 4 x daily + active placebo (benzatropine dose escalation from 0.25 mg to 1.25 mg, and for first two weeks diazepam 2 mg to 6 mg | |
| Outcomes | Pain patients reported. 6‐item global improvement, VAS, pain relief by VAS (from no relief to complete relief) At least better on amitriptyline 79/108 (complete response 11, much better 35, better 33, no change 23, worse 5, much worse 1), on capsaicin cream 75/104 (complete response 8, much better 31, better 36, no change 23, worse 4, much worse 2) VAS decreased on amitriptyline 29.1 (+/‐ 3.0), on capsaicin cream 26.1 (+/‐ 2.9) Pain relief on amitriptyline 57.0 (+/‐ 3.6) and on capsaicin cream 55.1 (+/‐3.5) Sleep improved on amitriptyline in 64/108 patients and on capsaicin cream in 59/104 patients |
|
| Notes | Dropouts: 9/117
on amitriptyline, 14/118 on capsaicin cream Reason for withdrawal not stated QS = 4 (R2, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bowsher 1997.
| Methods | Double blind placebo controlled study for 90 days. Follow up between six to eight months | |
| Participants | Pre‐emptive treatment of PHN 80 participants age 60 or older | |
| Interventions | Amitriptyline 25 mg at night or placebo for 90 days | |
| Outcomes | Complete pain relief, duration of pain, AEs Pain free at six months: 32/38 amitriptyline, 22/34 placebo |
|
| Notes | Dropouts: eight ‐ either non compliant or lost to follow up QS = 5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Brady 1987.
| Methods | Double blind placebo controlled crossover design four weeks. Two four weeks treatment period, no washout. No analyses of carry over effect. Patients with discogenic pain were excluded Patients used high carbohydrate, low protein and low fat diet during the study. Randomisation methods not stated |
|
| Participants | Ten participants (eight final number). Any neuropathic pain: five with atypical facial pain, two with postherpetic neuralgia, one with trigeminal neuralgia and two with discogenic pain. Mean age 47.3 years (range 26 to 81), four males and four female patients | |
| Interventions | L‐tryptophan 4000 mg, or placebo daily orally | |
| Outcomes | Global improvement, pain rating index (PRI), present pain intensity (PPI), Beck depression inventory (BDI), Hamilton depression rating scale (HDRS), Hamilton anxiety scale (HAS) PRI: less pain in all patients during the active treatment than placebo. PPI pain less intensive on L‐tryptophan 5/8, equal 2/8, less intensive on placebo 2/8 Pain scores on L‐tryptophan PRI 21.4, PI 2.9, on placebo PRI 31.0, 3.4 Depression scores on L‐tryptophan BDI 11.8, HDRS 9.2 and HAS 9.2; on placebo BDI 13.5, HDRS 12.8 and HAS 10.4 |
|
| Notes | No dropouts, 2/10 patients with discogenic pain excluded from the review No withdrawals due to side‐effects QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Carasso 1979.
| Methods | Parallel group single blind study, three months 67 patients included, 31 patients with tension headache were excluded from analysis. Numbers of patients are conflicting (36 randomised, results of 39 patients). Randomisation method not stated |
|
| Participants | 36 participants. Trigeminal neuralgia in 17 patients and postherpetic neuralgia in 19 patients. Age range 35 to 70, 15 male and 21 female | |
| Interventions | Amitriptyline dose escalation from 30 mg to 110 mg, or clomipramine from 20 mg to 75 mg daily orally | |
| Outcomes | Pain patients reported, five‐item global improvement, patients global satisfaction with treatment (yes/no) At least moderate improvement on amitriptyline 8/39 (marked improvement 4, moderate 4, slight 4, no change 7, worse 0), on clomipramine 10/39 (marked improvement 4, moderate 6, slight 5, no change 4, worse 1) Patients with trigeminal neuralgia: at least moderate improvement on amitriptyline 3/9 (marked improvement 2, moderate 1, slight 2, no change 4, worse 0); on clomipramine 7/9 (marked improvement 3, moderate 4, slight 5, no change 1, worse 0) Patients with postherpetic neuralgia: at least moderate improvement on amitriptyline 5/10 (marked improvement 2, moderate 3, slight 2, no change 3, worse 0,); on clomipramine 3/11 (marked improvement 1, moderate 2, slight 4, no change 3, worse 1) 10 patients satisfied on amitriptyline, and 13 on clomipramine Three patients with trigeminal neuralgia satisfied on amitriptyline, 8 on clomipramine; 7 patients with postherpetic neuralgia satisfied on amitriptyline, 5 on clomipramine |
|
| Notes | No dropouts QS = 1 (R1, DB0, W0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cardenas 2002.
| Methods | Double blind placebo controlled parallel design, six weeks Inclusion criteria: age 18 to 65 years, duration of pain at least three months |
|
| Participants | Central pain: spinal cord injury. 84 participants (84 final number). Age range 21 to 64, 67 male and 17 female patients Pain score in amitriptyline group NRS 5.5 (1.8) and MPQ 17.5 (9.8), in placebo group NRS 5.0 (1.7) and MPQ 15.7 (7.4). Depression score in amitriptyline group 17.1 (9.7) and in placebo group 13.3 (8.6) |
|
| Interventions | Amitriptyline dose escalation form 10 mg to125 mg daily orally, median dose 50 mg / day; or active placebo benztropine 0.5 mg daily orally | |
| Outcomes | Pain patients reported, NRS (0‐10) and VRS (MPQ). 20‐item depression scale CES‐D Pain on amitriptyline NRS 4.5 (1.9) and MPQ 14.6 (9.7); on placebo NRS 4.0 ( 2.0) and MPQ 12.8 (8.0) Depression on amitriptyline 13.4 (10.9), on placebo 11.2 (8.6) On amitriptyline no patients reported poor sleep, on placebo three patients |
|
| Notes | Dropouts: 8/44 on amitriptyline (7 SE, 1 failure to return week two medication), 3/40 on placebo (2 adverse events, 1 hospitalisation for an unrelated problem) SE: 43/44 on amitriptyline, 36/40 on placebo 7/44 withdrawn on amitriptyline (one constipation, three urinary retention and/or autonomic dysreflexia, three other systemic symptoms), 2/40 withdrawn on placebo (one constipation, one urinary retention and constipation) QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ciaramella 2000.
| Methods | Randomised parallel group study. Not blinded, two months with assessment at 14, 28 and 56 days | |
| Participants | 53 participants. Age 46 years(SD 12 years). Patients with depression and chronic pain. 14 complained of low back pain, 11 fibromyalgia, 5 PHN, 4 facial pain and 6 migraine | |
| Interventions | Fluoxetine 10 mg daily for two weeks then 20 mg daily or Fluvoxamine 50 mg daily then 100 mg daily | |
| Outcomes | Italian pain questionnaire, Pain rating index rank co‐efficient, Hamilton rating scale for depression Results: Both groups showed reduction in pain intensity. Fluvoxamine greater than fluoxetine (sig diff). Pain relief independent of any impact on depression |
|
| Notes | Analysis per protocol (20 per group). Can't differentiate between those with neuropathic pain and non neuropathic. No evaluable data In first three days, 8/28 withdrew on fluvoxmine, 5/25 withdrew on fluoxetine due to nausea, somnolence and headache QS = 2 (R1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Dallocchio 2000.
| Methods | Open label parallel design, 12 weeks (four week titration to max tolerated dose then eight week stable dose). Randomisation method not stated. Inclusion criteria: age over 65 years, duration of pain at least six months | |
| Participants | Diabetic neuropathy of 8 to 48 months 25 participants (25 final number). Age range 61 to 83 years, 11 male and 14 female patients. Pain score in amitriptyline group 2.8 (0.8), in gabapentin group 2.9 (0.8) Duration of pain significantly longer in gabapentin group than in amitriptyline group |
|
| Interventions | Amitriptyline dose escalation from 10 mg to 90 mg daily orally, median dose 53 mg (16 mg); or gabapentin dose escalation from 400 mg to 2400 mg daily orally, median dose 1785 mg (351 mg) | |
| Outcomes | Pain relief (pain score one or less), VRS (0 to 4) 7/12 on amitriptyline reported pain relief, 8/13 on gabapentin. VRS 1.5 (0.8) on amitriptyline, 1.0 (0.7) on gabapentin |
|
| Notes | No dropouts SE: 11/12 on amitriptyline, 4/13 on gabapentin; no withdrawals due to SE QS = 2 (R1, DB0, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Davidoff 1987.
| Methods | Double blind placebo controlled parallel design, eight weeks (one dose escalation, thereafter stable dose). Randomisation method not stated Inclusion criteria: age at least 18 years, duration of symptoms at least one month |
|
| Participants | Traumatic myelopathy. 18 participants (18 final number). mean age 39 years, 16 male and 2 female patients Pain score in trazodone group by PRI 33.2 (6.9), by NWC 12.0 (1.7), by PPI 2.9 (0.6), by SPI day 58.2 (9.4), by SPI week 63.8 (7.0), by PAD 55.1 (4.6); in placebo group by PRI 31.2 (6.4), by NWC 12.3 (1.5), by PPI 2.1 (0.3), by SPI day 56.6 (8.7), by SPI week 62.6 (8.8), by PAD 55.8 (4.4) |
|
| Interventions | Trazodone 150 mg or placebo daily orally | |
| Outcomes | Global assessment of efficacy (yes/no), MPQ: pain rating index (PRI), number of words (NWC), present pain intensity (PPI), Sternback pain intensity (0 to 100) day and week (SPI), Zung pain and distress index (PAD) Global improvement on trazodone 4/9 and on placebo 3/9 Pain on trazodone by PRI 33.5 (2.4), by NWC 14.0 (1.0), by PPI 2.6 (0.2), by SPI day 61.7 (6.8), by SPI week 73.9 (4.7), by PAD 67.2 (3.8); in placebo group by PRI 32.1 (3.5), by NWC 13.2 (1.5), by PPI 1.7 (0.2), by SPI day 63.4 (8.4), by SPI week 68.3 (6.9), by PAD 53.0 (3.2) |
|
| Notes | Dropouts 6/18; 5/9 on trazodone, 1/9 on placebo Reasons for dropouts not stated SE: 4/9 on trazodone and 1/9 on placebo In placebo group there were more patients with sensory complete spinal cord injuries (four patients in placebo group, one in trazodone) QS = 2 (R1, DB1, W0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Feinmann 1984.
| Methods | Double blind placebo controlled parallel design, nine weeks, dothiepin versus dothiepin + nocturnal bite guards vs placebo vs placebo + nocturnal bite guards (analysed in two groups: dothiepin +/‐ bite guards and placebo +/‐ bite guards), 12 months follow‐up. Randomisation method not stated. Inclusion criteria: age 16 to 65 years | |
| Participants | Psychogenic facial pain of median 3.4 years (3 months to 30 years), 50 patients with facial arthro myalgia and 43 with atypical facial pain. 93 participants (93 final number). Age range 19 to 65, 20 male and 73 female patients Pain score in dothiepin group 2.2 (0.6), in placebo group 2.2 (0.6). Number of psychiatric cases 26/48 in dothiepin group and 27/45 in placebo group |
|
| Interventions | Dothiepin dose escalation from 25 mg to 150 mg daily orally +/‐ nocturnal bite guard, mean dose 130 mg; or placebo daily orally +/‐ nocturnal bite guard | |
| Outcomes | Pain relief (yes or no), number of patients reduced analgesic use. Number of psychiatric cases Pain relief in 34/48 patients on dothiepin, 21/45 on placebo Reduction in analgesic use 40/48 patients on dothiepin, 19/45 on placebo Number of psychiatric cases 7/48 on dothiepin, 10/45 on placebo |
|
| Notes | Dropouts: 1/48 on dothiepin (SE), 1/45 on placebo (SE) SE: 1/48 withdrawn on dothiepin (epilepsy), 1/45 on placebo (loss of consciousness). No effect of bite guard QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Forssell 2004.
| Methods | Double blind placebo controlled crossover design 2 x 4 weeks. Two four week treatment periods, with two week washout Follow up for 12 weeks |
|
| Participants | Atypical facial pain. Pain at least three on 11 point scale. 30 participants. Median age 52 (range 38 to 66) | |
| Interventions | Venlafaxine 37.5 mg vs placebo. Doses up to Venlafaxine 75 mg daily. NSAIDs and paracetamol allowed | |
| Outcomes | Pt reported VASPI, VRS, VASPR, anxiety, Beck depression, AEs and use of escape medication No significant difference between Venlafaxine and placebo for reduction in PI. > use of rescue meds in placebo group |
|
| Notes | 10 dropouts. 8 due to AEs: 6 venlafaxine (nausea 5, fatigue 1), 2 placebo (rash 1, dizziness 1) 2 non compliant QS = 5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Gerson 1977.
| Methods | Open label placebo controlled parallel design, two weeks, carbamazepine + clomipramine vs transcutaneous electrical nerve stimulation. Randomisation method not stated. Inclusion criteria: duration of pain at least three months | |
| Participants | Postherpetic neuralgia, 29 participants (12 final number) Pain score in drug group 59.0 (9.2), in TENS group 27.0 |
|
| Interventions | Clomipramine dose escalation from 10 mg to 75 mg daily orally and carbamazepine dose from 150 mg to 1000 mg daily orally; or transcutaneous electrical nerve stimulation (TENS) | |
| Outcomes | Global improvement, pain intensity VAS change, mental outlook‐VAS Marked pain relief in drug group 8/9 patients, in placebo 2/3 VAS degreased in drug group 42.3 (9.8), in TENS group 8.3 Improvement in mental outlook in drug group 29 (from 34 to 5), in TENS group 6 (from 17 to 11) |
|
| Notes | Dropouts 17/29; in drug group dropouts and four crossed over to the other treatment group; in TENS group two dropouts and eight crossed over Side‐effects not reported QS = 2 (R1, DB 0, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Gomez‐Perez 1985.
| Methods | Double blind placebo controlled crossover study, 30 days. Two 30 days periods, no washout. No analyses of carry over effect Dose escalation during the first week Randomisation method not stated |
|
| Participants | Diabetic neuropathy. 24 participants (18 final number). Mean age 55 (range 30 to 73), 9 male and 9 female patients | |
| Interventions | Nortriptyline dose escalation from 30 mg to 60 mg and fluphenazine from 1.5 mg to 3 mg daily orally, or placebo daily orally | |
| Outcomes | Pain patients reported, pain relief 50% or more, VAS change from baseline Pain relief on active treatment 16/18 , on placebo 1/18 Pain decreased 63.97 % on active treatment, 22.11 % on placebo |
|
| Notes | Dropouts 6/24 (one ketoacidosis,
2 lack of compliance,
3 lost to follow‐up) No withdrawals due to SE QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Gomez‐Perez 1996.
| Methods | Double blind double dummy crossover design study, 30 days. Two 30 day treatment periods (15 days titration to max dose then 15 days stable dose), two to four weeks washout, during which the symptoms returned to baseline level. During the washout period patient received placebos of both therapies Randomisation method not stated. First period results also available, but number of patients inadequate Inclusion criteria: duration of pain at least six months |
|
| Participants | Diabetic neuropathy of 2.15 years. 16 participants (14 final number). Mean age 47 years | |
| Interventions | Nortriptyline dose escalation from 10 mg to 60 mg and fluphenazine from 0.5 mg to 3 mg daily orally; or carbamazepine dose escalation from 100 mg to 600 mg daily orally | |
| Outcomes | Pain (VAS) change from baseline Pain decreased 66.6 % on nortriptyline + fluphenazine; 49.0 % on carbamazepine |
|
| Notes | Dropouts: 2/16 (one upper GI bleeding ‐ alcohol gastritis related, one lack of adherence to the medication) SE: 8/16 on nortriptyline + fluphenazine; 3/16 on carbamazepine. 1/16 withdrawn due to alcohol related gastric bleeding on nortriptyline + fluphenazine QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Graff‐Radford 2000.
| Methods | Double blind placebo controlled parallel design, eight weeks Randomisation method not stated Inclusion criteria: duration of pain at least six months |
|
| Participants | Postherpetic neuralgia of 33.4 (29.5) months. 50 participants (49 final number). Mean age 72.9 (10.1), 27 male and 22 female patients. Pain score VAS 55.22 (16.34) and MPQ 23.22 (13.23). Pain score per group: in amitriptyline VAS 55.9 (19.58) and MPQ 22.54 (13.95), in amitriptyline + fluphenazine VAS 47.6 (13.43) and MPQ 27.25 (17.71), in fluphenazine VAS 65.4 (10.87) and MPQ 21.75 (10.18), and in placebo group VAS 53.92 (17.05) and MPQ 21.46 (10.89) | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 200 mg, or amitriptyline from 12.5 mg to 200 mg + fluphenazine from 1 mg to 3 mg, or fluphenazine from 1 mg to 3 mg, or active placebo (glycopyrrolate or cellulose) daily orally | |
| Outcomes | Pain patients reported, VAS and MPQ. Beck Depression Inventory (BDI) Pain by VAS on amitriptyline 26.6 (SD 16.77), on amitriptyline + fluphenazine 35.41 (SD 24.53), on fluphenazine 53.9 (SD 27.79), on placebo 48.53 (SD 24.99) Pain by MPQ on amitriptyline 17.36 (SD 10.92), on amitriptyline + fluphenazine 23.50 (SD 13.52), on fluphenazine 19.83 (SD 8.83), on placebo 17.83 (SD 13.94) Depression by BDI on amitriptyline 11.1 (SD 7.5), on amitriptyline + fluphenazine 7.2 (SD 6.03), on fluphenazine 14.2 (SD 6.5), on placebo 14.0 (SD 14.3). Fluphenazine made no difference either alone or enhancing amitriptyline |
|
| Notes | Dropouts: 1/12 on amitriptyline (SE), 0/12 on amitriptyline + fluphenazine, 0/13 on fluphenazine, 0/13 on placebo SE: one withdrawn on amitriptyline due to sedation Results of other depression scales also available QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Göbel 1997.
| Methods | Parallel study design, six weeks (only three patients used levomepromazine). Inclusion criteria: age at least 65 years |
|
| Participants | Postherpetic neuralgia. 35 participants (22 final number). Pain score in clomipramine group 4.1 (0.8) and in tramadol group 3.6 (0.7) | |
| Interventions | Clomipramine 100 mg +/‐ levomepromazine 100 mg, or tramadol 600 mg daily orally | |
| Outcomes | 5‐item global improvement, 5‐item VRS At least satisfactory global improvement 6/11 on clomipramine, 9/10 on tramadol Pain on clomipramine 2.3, on tramadol 2.2 |
|
| Notes | Dropouts: 7/18 on clomipramine, 7/17 on tramadol SE: 83.3 % on clomipramine, 76.5 % on tramadol. Withdrawn due to side effects QS = 2 (R1, DB0, W1) Pain results only in figures |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hampf 1989.
| Methods | Parallel study design, five weeks (two weeks dose escalation of amitriptyline, one week of distigmine, thereafter stable dose). Patients were randomly allocated to three treatment groups, in addition fourth group of patients who have already taken amitriptyline were included in the study Patients in group four are excluded from the review as well as patients with low back pain and multiple sclerosis Randomisation methods not stated |
|
| Participants | Any neuropathic pain. Duration of symptoms from four months to 13 years. Age range from 30 to 75 years. 65 participants (24 final number). Pain score in amitriptyline group 7.0, in distigmine group 6.8, and in placebo group 7.6 | |
| Interventions | Amitriptyline dose escalation from 25 to 75 mg; distigmine from 5 mg to 10 mg; or combination of amitriptyline and distigmine daily orally | |
| Outcomes | Pain intensity measured by VAS VAS on amitriptyline 4.9, on distigmine 4.5 and on combination therapy 4.2 |
|
| Notes | Dropouts 41/65; 15/65 reason not stated, 14/65 in group four, 12/65 low back pain or multiple sclerosis.
Side‐effects not reported QS = 1 (R1, DB0, W0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Harrison 1997.
| Methods | Double blind placebo controlled parallel design, 13 weeks. 178 patients included, 89 had also cognitive behavioral therapy, results of which was analysed separately (excluded) Randomisation methods not stated. Inclusion criteria: age from > 16 to 65 years, duration of pain at least three months |
|
| Participants | Idiopathic facial pain. 98 participants (63 final number) Pain score 3.7 in fluoxetine group, 3.3 in placebo group |
|
| Interventions | Fluoxetine 20 mg or placebo daily orally | |
| Outcomes | Pain patient reported, MPI (multidimensional pain inventory) Pain severity on fluoxetine 2.3, on placebo 2.7; change from baseline ‐1.4 on fluoxetine, ‐0.6 on placebo |
|
| Notes | Dropouts: 12/44 on fluoxetine, 14/45 on placebo Reason for withdrawal not stated Result presented only in figures QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Kalso 1996.
| Methods | Double blind placebo controlled crossover design four weeks. Two four weeks treatment period and two weeks washout. No analyses of carry over effect | |
| Participants | Postoperative pain after breast cancer treatment in ipsilateral arm and scar area. 20 participants (13 final number). Mean age 56 years (range 39‐72), all females. Arm pain in 11, scar pain in 10 patients. Baseline pain score in arm MPQ words 8 (2 to 13), MPQ score 275 (49 to 654), VAS 5 (1.7 to 7.1) and VRS 4 (2 to 7); pain score in scar MPQ words 8 (5 to 15), MPQ score 326 (154 to 618), VAS 3.3 (1.4 to 6.2) and VRS 3 (2 to 6) Two patients were depressed, 8 patients in arm group had sleep disturbance and 6 in scar group Effect on daily life in arm group three (1 to 5), in scar group two (0 to 3) |
|
| Interventions | Amitriptyline dose escalation from 5 mg to 100 mg daily orally (13 patients escalated up to 100 mg, two patients up to 50 mg), or placebo daily orally. | |
| Outcomes | Pain patients reported, VAS, VRS ( 0‐7), MPQ (number of words and score), pain relief (VRS 5‐item),
depressed (0 to 3),
disturbed sleep,
effect on daily life (0 to 4) Arm pain relief on amitriptyline 3 (2 to 5), on placebo 2 (1 to 4). Arm pain on amitriptyline by MPQ words 4 (0‐11), MPQ score 205 (0 to 404), VAS 0.5 (0 to 3.0) and VRS 1.8 (1 to 4); on placebo MPQ words 5 (0 to 12), MPQ score 165 (0 to 582), VAS 5.0 (0 to 9.4) and VRS 3.0 (1 to 8) Scar pain relief on amitriptyline 3 (2 to 5), on placebo 1.5 (1 to 4). Scar pain on amitriptyline by MPQ words 2 (0 to 7) , MPQ score 58 (0 to 305), VAS 0.2 (0 to 4.3) and VRS 1.9 (1 to 5); on placebo MPQ words 6 (2 to 13), MPQ score 235 (59 to 661), VAS 3.1 (0.7 to 5.5) and VRS 2.7 (1 to 6) Sleep disturbance in arm group on amitriptyline 1/13 and on placebo 6/13; in scar group on amitriptyline 0/13 and on placebo 6/13 patients. Effect on daily life in arm group one (0 to 4) on amitriptyline, 2 (0 to 4) on placebo; in scar group 0.5 (0 to 1) on amitriptyline and 1.4 (0 to 4) on placebo. |
|
| Notes | Dropouts 7/20
(four side effects, two dose escalation only up to 50 mg, one poor compliance) SE: 4/20 withdrawn due to SE (tiredness) QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kieburtz 1998.
| Methods | Double blind placebo controlled parallel design study, nine weeks (four week dose escalation to max tolerated dose then stable dose), follow up time 10 weeks | |
| Participants | HIV related painful neuropathy. 145 participants (121 to 128 final number) Mean age 41 years, 139 male and 6 female patients Pain score 1.02 (0.05) in amitriptyline group, 1.06 (0.04) in mexiletine group, 1.13 (0.04) in placebo group |
|
| Interventions | Amitriptyline dose escalation 25 mg to 100 mg + inactive placebo, or mexiletine dose escalation from 150 mg to 600 mg + active placebo (benztropine 0.125 mg ‐ 0.500 mg), or inactive + active placebo daily orally | |
| Outcomes | Pain patient reported, pain relief (0 to 6), Gracely verbal scale (VRS) 0 to 1.75, analgesic consumption Complete pain relief on amitriptyline in 3/34 patients, a lot 13/34, moderate 7/34, slight 6/34, no pain relief 4/34, pain worse in 1/34 patient; on mexiletine complete relief 4/37, a lot 11/37, moderate 7/34, slight 5/37, no 8/37, worse 2/37; on placebo complete relief 1/41, a lot 8/41, moderate 15/41, slight 6/41, no 8/41, worse 3/41 Chance in Gracely scale on amitriptyline +0.31, on mexiletine +0.23, on placebo +0.20 Analgesic consumption on amitriptyline decreased in 7/41 patients, no change in 22/41 and increased in 12/41; on mexiletine decreased 7/44, no change 23/44, increased 14/44; on placebo decreased 10/43, no change 20/43, increased 13/43 |
|
| Notes | Dropouts: 14/47 on amitriptyline (3 toxicity, 4 investigations or patients request , 4 miscellaneous, 2 lost to follow up, 1 did not receive treatment); 14/48 on mexiletine (4 toxicity, 3 investigators or patients requests, 6 miscellaneous, 1
lost to follow up), 13/50 on placebo (1 toxicity, 2 investigators or patients requests, 8 miscellaneous, 1 lost to follow up, 1 did not receive treatment) SE: 3 withdrew on amitriptyline, 4 on mexiletine, 1 on placebo QS = 5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kishore‐Kumar 1990.
| Methods | Double blind placebo controlled crossover design, six weeks. Two six weeks treatment periods (four weeks titration to max dose then two weeks stable dose), no washout. No carry over effect Randomisation method not stated Inclusion criteria: duration of symptoms at least three months |
|
| Participants | Postherpetic neuralgia of 28.5 months (3 months to 8 years). 26 participants (19 final number). Mean age 62 years (range 38 to 79 years), 17 male and 9 female patients | |
| Interventions | Desipramine dose escalation 12.5 mg to 250 mg daily orally, mean dose 167 mg (13 mg); or active placebo (benzatropine 0.5 mg to 1 mg and lactose) daily orally (19 patients took 1 mg, 3 patients 0.5 mg) | |
| Outcomes | Pain patients reported, 6‐item global improvement At least moderate improvement 12/19 on desipramine (complete improvement 1, a lot 7, moderate 4, slight 2, no change 4, worse one), 2/19 on placebo (complete improvement 0, a lot 1, moderate 1, slight 0, no change 9, worse 8) |
|
| Notes | Dropouts 7/26 (SE or intercurrent medical illnesses) SE 19/19 on desipramine, 15/19 on placebo. Withdrawn due to SE 5/19 on desipramine (1 syncope, 1 palpitation and left bundle branch block, 1 chest pain, 1 fever, 1 vertigo); 3/19 on placebo (1 vertigo and nausea, 1one skin rash, 1 feeling of unsteadiness) Pain results illustrated only in figures QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kvinesdal 1984.
| Methods | Double blind placebo controlled crossover design, five weeks (one week titration of dose then four weeks stable dose). Randomisation method not stated. Data biased, carry over effect. First period analyses also available, but inadequate number of patients | |
| Participants | Diabetic neuropathy over two years. 15 participants (12 final number). Mean age 55 (range 30 to 75), five male and seven female patients | |
| Interventions | Imipramine dose escalation 50 mg to 100 mg, or placebo daily orally | |
| Outcomes | Symptoms patients reported, 3‐item global improvement of neuropathic symptoms (including pain) On imipramine symptoms improved 8/12, no change 4/12, worse 0/12; on placebo improved 1/12, no change 11/12, worse 0/12 |
|
| Notes | Dropouts 3/15 (2 poor compliance, 1 SE) SE: withdrawn 1 on desipramine (dizziness) Pain not analysed separately, included in neuropathic score QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lampl 2002.
| Methods | Double blind placebo controlled parallel design study for one year | |
| Participants | Prophylaxis of central post stroke pain after thalamic stroke. 39 participants age 36 to 68 | |
| Interventions | Amitriptyline extended release, 10 to 75 mg daily or placebo for one year | |
| Outcomes | Time to event (pain), Pain intensity, type, site and distribution. Presence/absence of allodynia. AES Average time to pain: placebo 318 days (SE 23) amitriptyline 324 days (SE 24) Number experiencing pain 3/20 amitriptyline, 4/19 placebo | |
| Notes | Two moderate AEs in amitriptyline group requiring dose reduction. two withdrew due to protocol violations QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Langohr 1982.
| Methods | Double blind crossover design, two weeks. Two 2 weeks treatment periods, one week washout (three days titration of dose). First period analyses Randomisation assured from author |
|
| Participants | Neuropathy of traumatic, infectious or surgical origin. 48 participants (39 final number) | |
| Interventions | Clomipramine dose escalation from 50 mg to 150 mg, or aspirin dose escalation to 1500 mg daily orally | |
| Outcomes | Pain physicians reported, 4‐item global improvement On clomipramine complete improvement 1/19, good 9/19, partial relief 4/19, no change 5/19; on aspirin complete improvement 1/20, good 3/20, partial relief 5/20, no change 11/20 |
|
| Notes | Dropouts: 5/24 on clomipramine, 4/24 on aspirin Reason for withdrawal not stated. SE 37% on clomipramine, 17% on placebo Patients and physicians reported similar results QS = 2 (R1, DB1, W0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lascelles 1966.
| Methods | Double blind placebo controlled crossover design, four weeks. Two four week treatment periods, no washout. No analyses of carry over effect. First period analyses | |
| Participants | Atypical facial pain. 40 participants (40 final number) | |
| Interventions | Phenelzine 45 mg or placebo daily orally | |
| Outcomes | 4‐item global improvement.
Hamilton depression rating scale On phenelzine markedly improved 6/20, improved 9/20, no change 5/20, worse 0/20; on placebo markedly improved 1/20, improved 6/20, no change 9/20, worse 4/20 On phenelzine depression improved 15/20, no change 5/20, worse 0/20; on placebo improved 5/20, no change 14/20,worse 17/20 |
|
| Notes | No dropouts No withdrawal due to SE QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Leijon 1989.
| Methods | Double blind placebo controlled crossover design, four weeks Three four week periods, two one week washout periods (final doses reached on day six for amitriptyline and on day 18 for carbamazepine) Randomisation method not stated |
|
| Participants | Central post stroke pain of 54 months (range 11 to 154). 15 participants (15 final number). Mean age 66 years (range 53 to 74), 12 male and 3 female patients. Pain score in amitriptyline group 4.7 (1.3), in carbamazepine group 4.6 (1.2), in placebo group 5.5 (1.5) Depression score 2.9 (range 0 to 6.5) |
|
| Interventions | Amitriptyline dose escalation from 25 mg to 75 mg (75 mg for all patients); or carbamazepine dose escalation from 200 mg to 800 mg (10 patients 800 mg, 2 patients 600 mg, 1 400 mg and 1 200 mg); or placebo daily orally | |
| Outcomes | Pain patients reported, 5‐item global improvement, 10‐step VRS 10‐item comprehensive psychopathological rating scale (CPRS) At least improved 10/15 on amitriptyline (complete improvement 0, much improved 5, improved 5, no change 3, worse 2), 5/14 on carbamazepine (complete improvement 1, much improved 1, improved 3, no change 9, worse 0), 1/15 on placebo (complete improvement 1, much improved 0, improved 0, no change 12, worse 2) Pain on amitriptyline 4.2 (1.6), on carbamazepine 4.2 (1.7), on placebo 5.3 (2.0) Depression on amitriptyline 2.2 (range 0 to 8), on carbamazepine 3.0 (range 0 to 7), on placebo 2.6 (range 0 to 6). |
|
| Notes | Dropouts 1/15 on carbamazepine (drug interaction) SE: 14/15 on amitriptyline, 13/15 on carbamazepine, 7/15 on placebo. No patients were withdrawn due to SE QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Maina 2002.
| Methods | Double blind parallel design, eight weeks. Randomisation method not stated | |
| Participants | Burning mouth syndrome of 1.4 years. 76 participants (68 final number). Mean age 63.5, 16 male and 60 female patients Pain score 7.2 (1.2) in amisulpride group, 7.0 (1.2) in paroxetine group, 7.2 (1.0) in sertraline group HAM for depression 10.5 ( 2.4) and HAM for anxiety 15.5 (8.2) in amisulpride group, HAM‐D 10.3 (2.4) and HAM‐A 15.9 (7.7) in paroxetine group, HAM‐D 10.9 (2.6) and HAM‐A 16.1 (7.1) in sertraline group |
|
| Interventions | Amisulpride 50 mg, or paroxetine 20 mg, or sertraline 50 mg daily orally | |
| Outcomes | Pain patients reported, global improvement (global improvement score <3 and VAS reduced >50 %), VAS Hamilton rating scale for depression (HAM‐D) and for anxiety (HAM‐A) Global improvement 19/27 on amisulpride, 16/23 on paroxetine, 13/18 on sertraline VAS 3.2 (1.7) on amisulpride, 3.2 (2.1) on paroxetine, 2.8 (2.4) on sertraline HAM‐D 7.2 ( 3.0) and HAM‐A 10.4 (7.0) on amisulpride, HAM‐D 7.2 (2.7) and HAM‐A 11.1 (6.1) on paroxetine, HAM‐D 7.4 (1.8) and HAM‐A 11.6 (7.4) on sertraline |
|
| Notes | Dropouts: 0/27 on amisulpride, 3/26 on paroxetine (1 lack of compliance, 1 side effects, 1 lack of efficacy), 5/23 on sertraline (1 lack of compliance, 1 concurrent medication, 2 side effects, 1 lack of efficacy) SE: withdrawn 0/27 on amisulpride, 1/26 on paroxetine, 2/23 on sertraline QS = 2 (R1, DB0, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Max 1987.
| Methods | Double blind placebo controlled crossover design, six weeks. Two six week periods, no washout (3 weeks titration of dose then 3 weeks stable dose). Carry over effect. First period analyses. Randomisation method not stated | |
| Participants | Diabetic neuropathy of 2 years. 37 participants (29 final number). Mean age 57 years, 17 male and 12 female patients Pain score in amitriptyline group 0.91, in placebo group 1.2 14 depressed and 15 non depressed |
|
| Interventions | Amitriptyline dose escalation from 25 mg to 150 mg daily orally, mean dose 116 mg (for first period), or active placebo benztropine 1 mg daily orally + diazepam 5 mg for days 1to 18 | |
| Outcomes | Pain patients reported, VRS (13‐item word list) Pain on amitriptyline 0.45, on placebo 0.89 |
|
| Notes | Dropouts: 8/37
(5 side effects, 1 failure to keep diary, 1 lack of effect, 1 unstable angina) SE: 28/37 on amitriptyline, 25/37 on placebo; withdrawn on amitriptyline 3/37 (2 dizziness, 1 syncope), on placebo 3/37 (1 dizziness, 1 abdominal pain, 1 forgetfulness and increased pain) Pain results illustrated in figures only QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Max 1988.
| Methods | Double blind placebo controlled crossover design, six weeks. Two six week periods, one week washout (three week titration to max tolerated dose then stable dose) Randomisation groups: placebo followed by amitriptyline, placebo followed by lorazepam, amitriptyline followed by lorazepam, and lorazepam followed by amitriptyline Randomisation methods not stated. A significant drug‐time interaction Inclusion criteria: duration of symptoms at least 3 months |
|
| Participants | Postherpetic neuralgia of 19 months (range 3 months ‐ 25 years). 62 participants (41 final number). Mean age 72 (range 25 to 86), 31 male and 27 female patients, 15 depressed and 43 non depressed | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 150 mg, mean dose 65 mg; or lorazepam from 0.5 mg to 6 mg, mean dose 2.4 mg; or placebo (lactose 250 mg ‐ 1500 mg) daily orally | |
| Outcomes | Pain patients reported, 6‐item global improvement At least moderate improvement 16/34 on amitriptyline (complete improvement 1, a lot 12, moderate 3, slight 10, no change 6, worse 2), 6/40 on lorazepam (complete improvement 0, a lot 3, moderate 3, slight 7, no change 20, worse 7), 4/25 on placebo (complete improvement 0, a lot 2, moderate 2, slight 4, no change 11, worse 6) |
|
| Notes | Dropouts 21/62
(14 drug reactions, 3 no pain relief, 2 onset of more severe pain not related to neuropathy, 1 acute bereavement, 1 medication error, 1 no reason given) SE: 55/62 on amitriptyline, 62/62 on lorazepam, 45/62 on placebo. Withdrawn due to SE 5 on amitriptyline (1 rash, 1 palpitation, 1 dizziness, 1 sedation, 1 urinary retention), 6 on lorazepam (4 acute depression, 1 ataxia, 1 nightmares), 3 on placebo (1 dizziness, 1 disorientation, 1 rash) Results illustrated only in figures QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Max 1991.
| Methods | Double blind placebo controlled crossover design, six weeks. Two six week periods, no washout, no carry over effect (four week titration to max tolerated dose then stable dose) Randomisation methods not stated. Inclusion criteria: duration of symptoms at least three months |
|
| Participants | Diabetic neuropathy of 24 months (range 5 to 120). 24 participants (20 final number). Mean age 62 years (range 21 to 71), 15 male and 9 female patients. 4 depressed and 16 non depressed by Hamilton; 7 depressed and 13 non depressed by psychiatrist's | |
| Interventions | Desipramine dose escalation from 12.5 mg 250 mg, mean dose 201 mg (87.5 to 250 mg), or active placebo (benztropine 0.5 mg ‐ 1 mg and lactose) daily orally | |
| Outcomes | Pain patients reported, 6‐item global improvement At least moderate improvement on desipramine 11/20 (complete improvement 0, a lot 4, moderate 7, slight 2, no change 5, worse 2), on placebo 2/20 (complete improvement 0, a lot 1, moderate 1, slight 3, no change 5, worse 10) |
|
| Notes | Dropouts 4/24; on desipramine 2 (SE) , on placebo 2 (1 angina pectoris, 1 lack of effect) SE: 18/20 on desipramine, 17/20 on placebo. Withdrawn due to SE 2/20 on desipramine (1 seizure, 1 insomnia), 0/20 on placebo Results illustrated only in figures QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Max 1992a.
| Methods | Crossover design, six weeks. Two six weeks periods, two week washout, no carry over effect (four week titration to max tolerated dose then stable dose). Patients were randomised to two different studies (Max 1992a and b). 49 randomised + 5 additional patients. 5 additional patients were excluded from the review, only first period results available Randomisation methods not stated, blinding not clear Inclusion criteria: duration of symptoms at least three months |
|
| Participants | Diabetic neuropathy of 3 years (range 0.5‐12). 54 participants (25 final number in first period analyses: 12 in amitriptyline group and 13 on desipramine group). Mean age 58 years (range 20 to 84), 33 male and 21 female patients | |
| Interventions | Amitriptyline escalation from 12.5 mg to 150 mg, mean dose 105 mg (37 mg), or desipramine from 12.5 mg to 150 mg, mean dose 111 mg (39 mg) daily orally | |
| Outcomes | Pain patients reported, VRS Pain score decreased on amitriptyline 0.47 (0.09), on desipramine 0.45 (0.12) |
|
| Notes | Dropouts 16/54 (14 side effects, 2 not specified). SE: on amitriptyline 31/38, on desipramine 29/38. Withdrawn due to SE 7/38 on amitriptyline (2 confusion, 1 orthostatic hypotension, 1 fatigue, 1 malaise, 1 hypomania, 1 rash), 7/38 on desipramine (3 rash, 1 orthostatic hypotension, 1 fever, 1 tremor, 1 left bundle branch block). QS = 1 (R1, DB0, W0 ) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Max 1992b.
| Methods | Crossover design, six weeks. Two six week periods, two week washout, no carry over effect (four week titration to max tolerated dose then stable dose). Patients were randomised to two different studies (Max 1992a and b). 37 randomised + 17 additional non‐randomised patients. 17 non randomised patients were excluded from analyses, only first period results available Randomisation methods not stated, blinding not clear Inclusion criteria: duration of symptoms at least three months. |
|
| Participants | Diabetic neuropathy of 4 years (range 0.5 to 12). 54 participants (27 final number in first period analyses: 12 on in fluoxetine group and 15 in placebo group). Mean age 58 (range 25 to 84), 31 male and 23 female patients | |
| Interventions | Fluoxetine dose escalation from 20 mg to 40 mg daily orally (40 mg for all patients, except one); or active placebo benztropine from 0.125 mg to 1.5 mg daily orally, mean dose 1.3 (0.2 mg) | |
| Outcomes | Pain patients reported, VRS Pain score decreased on fluoxetine 0.35 (0.11), on placebo 0.15 (0.07) |
|
| Notes | Dropouts 8/54(5 SE, others not reported) SE: 29/46 on fluoxetine, 31/46 on placebo Withdrawn due to SE 3/46 on fluoxetine (1 orthostatic hypotension, 1 headache, 1 rash), 2/46 on placebo (1 fatigue, 1 chest pain) QS = 1 (R1, DB0, W0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
McCleane 2000a.
| Methods | Double blind parallel group four week study. Randomisation method not stated | |
| Participants | Any neuropathic pain of 62.7 months. 200 participants (151 final number). Mean age 46 years, 63 male and 88 female patients. Pain score in doxepin group 7.29, in capsaicin group 7.11, in doxepin + capsaicin group 7.47, in placebo group 7.13 | |
| Interventions | 3.3 % doxepin hydrochloride x 3 / day topically, or 0.025 capsaicin cream x 3 / day, or 3.3% doxepin + 0.025% capsaicin x 3 / day, or placebo (aqueous cream) x 3 / day | |
| Outcomes | Pain patients reported, VAS, patients wish to continue therapy Number of patiens wished to continue doxepin 17/41, capsaicin 13/41, doxepin + capsaicin 9/33, placebo 1/36 VAS decreased on doxepin 0.9 (95% CI 0.34‐1.46), on capsaicin 1.12 (0.44‐1.8), on doxepin + capsaicin 1.07 (0.39‐1.75), no change on placebo |
|
| Notes | Dropouts 49/200 Reason for withdrawal not stated. SE: 11 on doxepin (4 drowsiness, 1 skin rash, 2 itch, 4 burning discomfort), 27 on capsaicin (burning discomfort), 25 on doxepin + capsaicin (2 drowsiness, 1 headache, 22 burning discomfort) Duration of pain was significantly longer in the combination group. QS = 4 (R2, DB2, W0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
McCleane 2000b.
| Methods | Double blind parallel group four week study of topical doxepin | |
| Participants | Any neuropathic pain of 69 months (range 3 to 324). 40 participants (30 final number). Mean age 52 years (range 27 to 80) Pain score in doxepin group 6.22 (2.51), in placebo group 6.49 (1.98) |
|
| Interventions | 5% doxepin hydrochloride x 2/day topically, or placebo (aqueous cream) | |
| Outcomes | Pain patients reported, VAS VAS on doxepin 5.04 (2.61), on placebo 6.91 (2.15). VAS decreased on doxepin 1.18 (2.01), on placebo VAS increased 0.42 (1.5) |
|
| Notes | Dropouts 10/30; 4/20 on doxepin, 6/20 on placebo Reason for withdrawal not stated QS = 4 (R2, DB2, W0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Mercadante 2002.
| Methods | Double blind placebo controlled crossover design, two week no washout, carry over effect not analysed | |
| Participants | Neuropathic cancer pain range 4 to 7 on 11 point scale. 16 advanced cancer patients on systemic morphine therapy. Age 55 to 78 | |
| Interventions | Amitriptyline up to 50 mg at night for patients < 65 yrs, Amitriptyline up to 30 mg at night for patients > 65 yrs. All patients used Morphine | |
| Outcomes | Opioid consumption, global pain intensity. No significant difference in global pain intensity, least pain intensity or for opioid consumption. Significant difference for worst pain | |
| Notes | No washout so likely to be significant carry over for in first phase Amitriptyline group AEs reported as drowsiness, confusion, dry mouth QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Morello 1999.
| Methods | Double blind double dummy crossover design six weeks, two six week periods, one week washout, no carry over effect (one week dose titration then stabile dose). First period results also available. Inclusion criteria: age at least 18 years, duration of symptoms at least three months | |
| Participants | Diabetic neuropathy of 5.7 (4.2) years. 25 participants (21 final number). Mean age 60.4 (10.8) years, 24 male and one female patients | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 75 mg orally, mean dose 59 mg; or gabapentin from 300 mg to 1800 mg orally, mean dose 1565 mg | |
| Outcomes | Pain patients reported, 6‐item global improvement , 13 words VRS At least moderate improvement on amitriptyline 14/21 (complete improvement 1, a lot 4, moderate 9, slight 4, no change 3, worse 0), on gabapentin 11/21 (complete improvement 1, a lot 5, moderate 5, slight 3, no change 6, worse 1) Pain decreased 0.44 (0.089) in 9 patients on amitriptyline, 0.31 (0.064) in 10 patients on gabapentin during the first study period |
|
| Notes | Dropouts 4/25, on amitriptyline 2 (1 protocol violation and 1 SE), on gabapentin 2 (1 SE and 1 SE + protocol violation) Early crossover from amitriptyline to gabapentin 1/13 (SE), from gabapentin to amitriptyline 2/12 (SE and lack of effect) SE: 17/21 on amitriptyline, 18/21 on gabapentin. Withdrawn due to SE 2/21 on amitriptyline, 3/21 on gabapentin QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Panerai 1990.
| Methods | Double blind placebo controlled crossover design, three weeks (one week dose titration then stable dose) Three week periods, no washout, carry over effect not analysed Randomisation method not stated Inclusion criteria: age from 18 to 80 years, duration of symptoms at least six months |
|
| Participants | Central pain: phantom or stump pain 28 patients, posttraumatic nerve lesions 7, postherpetic neuralgia 4. 39 participants (24 final number). Mean age 49 years, 22 male and 17 female patients. Mean duration of pain 20.6 months. Pain score in clomipramine group 49.1 (17.13), in nortriptyline group 45.9 (16.6), in placebo group 37.1 (13.13) In clomipramine group non‐depressed 3 (HAM score < 7), borderline depressed 1 (HAM 8‐13), moderate or severely depressed 4 (HAM > 13); in nortriptyline group non‐depressed 6, borderline depressed 1 and moderate or severely depressed 3; in placebo group non‐depressed 4, borderline depressed 1 and moderate or severely depressed 1 |
|
| Interventions | Clomipramine dose escalation from 25 mg to 100 mg, or nortriptyline from 25 mg to 100 mg, or placebo daily orally | |
| Outcomes | Pain patients reported, VAS. HAM depression score VAS on clomipramine 12 (7), on nortriptyline 28 (16), on placebo 36.5 (16) In depressed patiens VAS on clomipramine 15 (2.5), on nortriptyline 24 (21), on placebo 30 (SD 17); in non‐depressed patients VAS on clomipramine 11 (8), on nortriptyline 32 (8), on placebo 41 (6) |
|
| Notes | Dropouts 15/39; on clomipramine 1 (poor efficacy), on nortriptyline 7 (5 poor efficacy, 2 poor tolerability), on placebo 7 (6‐poor efficacy, 1 poor tolerability) SE: 23/39 on clomipramine, 22/39 on nortriptyline, 10/39 on placebo Withdrawn due to SE 2/39 on nortriptyline, 1/39 on placebo Results illustrated in figures QS = 2 (R1, DB0, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pilowsky 1982.
| Methods | Double blind placebo controlled crossover design, six weeks (two week dose titration then stable dose). Two six week periods, no washout, carry over effect not analysed Randomisation method not stated |
|
| Participants | Chronic intractable pain without specific organic cause. 52 participants (21 final number). 27 male and 27 female patients. Pain score 54.84 (21.78), depression score 48.82 (9.86) | |
| Interventions | Amitriptyline dose escalation from 50 mg to 150 mg daily orally, or placebo | |
| Outcomes | Global improvement clinicians reported, VAS, Zung depression questionnaire Partial or complete pain relief 4/12 on amitriptyline, 3/12 on placebo VAS on amitriptyline 50.62, on placebo 53.03 Depression score 50.24 on amitriptyline, 49.38 on placebo |
|
| Notes | Dropouts 20/52 (10 on amitriptyline and 10 on placebo, mainly related to side effects) Side effects reported in scores Clinicians and patients reported global improvement did not differ significantly QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Raja 2002.
| Methods | Double blind placebo controlled crossover design, three treatment periods of eight weeks. (four week dose titration two week maintenance, two to three week taper off),one week washout, carry over effect not analysed Randomisation method not stated |
|
| Participants | PHN with pain of at least three months after resolution of lesions. 76 participants. Median age 73 yrs (range 32 to 90) | |
| Interventions | Morphine up to 240 mg daily , nortriptyline up to 160 mg daily or placebo in 2 or 3 divided doses. Drugs in same class also offered (methadone or desipramine) | |
| Outcomes | Pt reported 11 point PI and PR, cognitive function, sleep, mood,. AEs and treatment preference Mean dose for morphine 91 mg (15 mg to 225 mg). Reduction in pain scores greater on Morphine: 2.2 (95%CI 1.6 to 2.7), nortriptyline 1.2 (95%CI 0.7 to 1.7), For 33% reduction in pain; 20/38 morphine, 9/27 nortriptyline and 7/43 placebo Treatment preference : opioids 54%, TCA 30%, Placebo 16% |
|
| Notes | 50 completed 2 periods and 44 completed 3 periods. 20 dropouts on opioids, 6 TCA, 1 placebo QS = 5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Reuben 2004.
| Methods | Double blind placebo controlled parallel group study, two weeks treatment. Follow up for six months | |
| Participants | Pre‐emptive treatment of post mastectomy pain syndrome. 100 participants age 38 to 54 yrs | |
| Interventions | Venlafaxine 75mg SR at night for two weeks or placebo starting night prior to surgery. Post op PCA used | |
| Outcomes | VASPI , pain scores at four hours, one month and at six months. Pain at rest, movement, arm and chest wall pain. sensory tests. analgesic consumption.
Pain scores on movement , axilla pain and chest wall pain lower in venlafaxine group at six months Axilla pain: 29/48 had pain in venlafaxine group, placebo 24/47 at six months Chronic pain: 14/48 had pain in venlafaxine group, placebo 34/47 at six months |
|
| Notes | 94 completed , no withdrawals for AEs QS = 4 (R2, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Robinson 2004.
| Methods | Randomised double blind placebo controlled parallel group study, six weeks treatment | |
| Participants | 39 participants age 22 to 65 years, Amputation related pain of > 6 months. Average pain at least 2 on 11 point scale | |
| Interventions | Amitriptyline 10 mg / day up to 125 mg/day. Active placebo (benztropine 0.5 mg) dose not escalated | |
| Outcomes | Pt reported average PI on 11 pt scale. SF McGill, unmodified BPI, depression scale. Functional ability assessment, satisfaction with life Amitriptyline was not different from placebo for phantom limb pain or residual limb pain. No sig diff in depression scores between amitriptyline and placebo |
|
| Notes | Two withdrew in amitriptyline group due to AEs. Dry mouth, dizziness commonly reported QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rowbotham 2004.
| Methods | Multicentre randomised double blind placebo controlled parallel group study with dose escalation over first two weeks | |
| Participants | 245 participants with pain full diabetic neuropathy of at least moderate severity for three months or longer and metabolically stable (Type 1 or type 2 diabetes) | |
| Interventions | Placebo, venlafaxine 75 mg or venlafaxine 150 to 225 mg daily for six weeks followed by two week tapered dose | |
| Outcomes | VASPI, VASPR, clinical global impressions‐severity (CGI‐s) and CGI‐I (improvement)‐ both clinician assessed Patients global rating of pain relief Results: >50% pain relief: 46/82 Ven 150/225, 27/80 placebo (derived data) NNT 4.5 (95%CI 2.7‐ 13.5) Mean scores for PR reported but no SD so cannot be evaluated |
|
| Notes | Withdrawals: totals 12/81 placebo, 12/81 Ven 75, 18/82 Ven150/225
withdrawals due to AEs: 3/81 placebo, 6/81 Ven75, 8/82 Ven 150/225. NNH not significant QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Semenchuk 2001.
| Methods | Double blind placebo controlled crossover design, six weeks (one week dose titration then stable dose). Two six week periods, no washout, no carry over effect Randomisation method not stated Inclusion criteria: age at least 18 years, duration of symptoms at least three months |
|
| Participants | Any neuropathic pain of four years. 41 participants (41 final number) Mean age 60 years (range 23 to 88), 19 male and 22 female patients. Pain score 5.7 (0.26) |
|
| Interventions | Bupropion dose escalation from 150 mg to 300 mg daily orally, or placebo | |
| Outcomes | Pain patients reported, 5‐item global improvement, Wisconsin Brief Pain Inventory (0 to 10). Sleep and mood (from 0 no problems to 10 major problems). Pain at least improved 30/41 on bupropion (complete improvement 1, much improved 14, improved 15, no change 8, worse 3), 4/41 on placebo (complete improvement 0, much improved 2, improved 2, no change 23, worse 14) Pain on bupropion 3.99 (0.41), on placebo 5.78 (0.32) Mood on bupropion 2.85 (0.44), on placebo 4.46 (0.41) Sleep on bupropion 2.93 (0.48), on placebo 4.15 (0.48) |
|
| Notes | Dropouts 4/41;
on bupropion 4 (2 SE and 2 unrelated medical problems); on placebo 1 (SE) SE: 22/41 on bupropion, 8/41 on placebo. Withdrawn due to SE: 2/41 on bupropion (1 dizziness, 1 nausea and vomiting), 1/41 on placebo (nausea and vomiting) QS = 4 (R1, DB2, W10 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sharav 1987.
| Methods | Double blinded placebo controlled crossover study four weeks. Randomly allocated to one of three groups: low dose amitriptyline versus placebo, high dose amitriptyline versus placebo, or high dose versus low dose Patients in group high vs low amitriptyline are excluded from the review. Two four week treatment periods and two weeks washout Randomisation method not stated Inclusion criteria: age at least 18 years, duration of pain at least six months |
|
| Participants | Chronic facial pain including both musculoskeletal and neurogenic origin. 32 participants (19 final number) Mean age 41.5 years, 6 male and 22 female patients |
|
| Interventions | Amitriptyline low dose escalation from 10 mg to 30 mg daily orally, mean dose 23.6 mg; high dose from 50 to 150 mg, mean dose 129.4 mg; or placebo | |
| Outcomes | Change in pain intensity (VAS) and MPQ, pain relief‐VAS, Hamilton depression inventory (HDI) Change in pain intensity on amitriptyline 29, on placebo 5; change in MPQ on amitriptyline 11 and on placebo 4; pain relief on amitriptyline 32 and on placebo 19 |
|
| Notes | Dropouts 19/32 (2 use of other drugs, 2 failure to complete the study, 9 low versus high amitriptyline comparison) Side effects not reported Results from figures QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Shlay 1998.
| Methods | Double blind parallel group study, 14 weeks.
22 weeks follow‐up Original study design: amitriptyline + standardised acupuncture regimen (SAR) vs amitriptyline + control points vs placebo + SAR vs placebo + control points (125 patients). Later additional 114 patients were randomised between SAR and control points and 11 patients between amitriptyline and placebo. From these patients 136 were able to comparison between amitriptyline +/‐ SAR or control points and placebo +/‐ SAR or control points Inclusion criteria: age at least 13 years (assured from author no patient were younger than 19 years) |
|
| Participants | HIV associated peripheral neuropathy. 136 participants (101 final number). Mean age in amitriptyline group 40.1 (7.1) years, in placebo group 39.9 (5.9), 124 male and 12 female patients. Pain score in amitriptyline group 1.10 (0.3), in placebo group 1.13 (0.3) | |
| Interventions | Amitriptyline dose escalation from 25 mg to 75 mg daily orally, mean dose 178 mg, +/‐ SAR or control points; or placebo +/‐ SAR or control points | |
| Outcomes | 6‐item global improvement, Gracely verbal scale (0.0 to 1.75), 39‐item QOL assessment tool At least moderate pain relief 31/61 on amitriptyline (complete improvement 3, a lot 6, moderate 22, slight 14, none 11, worse 5), 28/60 on placebo (complete improvement 3, a lot 10, moderate 15, slight 13, none 11, worse 8) Gracely score decreased 0.26 on amitriptyline, 0.30 on placebo Mean change in QOL 7.1 on amitriptyline, 0.6 on placebo |
|
| Notes | Dropouts 35/136; 22/71 on amitriptyline, 13/65 on placebo Reasons for dropout not stated QS = 3 (R1, DB2, W0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Simpson 2001.
| Methods | Double blind placebo controlled parallel group study, eight weeks Gabapentin non‐responding patients were randomised to gabapentin + venlafaxine or gabapentin + placebo (dose escalation during the first three weeks, thereafter stabile dose Randomisation methods not stated |
|
| Participants | Diabetic neuropathy. 11 participants (7 final number) Pain score in venlafaxine group 6.4, in placebo group 6.5 |
|
| Interventions | Gabapentin dose escalation from 300 to 3600 mg + venlafaxine dose escalation from 37.5 mg to 150 mg daily orally; or maximal tolerated dose of gabapentin + placebo | |
| Outcomes | Global improvement, pain score (0 to 10) Much or moderate pain relief on venlafaxine 3/4 patients , on placebo 1/3 patients Pain score on venlafaxine 4.4, on placebo 6.1 |
|
| Notes | Dropouts 4/11; 2/6 on venlafaxine (1 treatment failure, 1 side‐effects); 2 on placebo (treatment failure) Withdrawn due to side‐effects 1/6 on venlafaxine QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Sindrup 1989.
| Methods | Double blind placebo controlled crossover design, three weeks (dose finding before randomisation according to plasma levels). Two three week periods, no washout, no carry over effect Randomisation method not stated Inclusion criteria: duration of symptoms at least one year |
|
| Participants | Diabetic neuropathy. 13 participants (9 final number) Mean age 49, 4 male and 5 female patients |
|
| Interventions | Imipramine dose escalation from 125 mg to 200 mg daily orally, mean dose 178 mg; or placebo | |
| Outcomes | Pain patients reported, 6‐item neuropathic scale including pain, global improvement in neuropathic score 8/9 patients preferred imipramine, 1/9 preferred placebo Neuropathic score lower on imipramine 8/9 patients, on placebo 0/9, no difference in one patient Mean neuropathic score 2.2 on imipramine, 5 on placebo |
|
| Notes | Dropouts 4/13; 1/13 on imipramine (SE), 2/13 on placebo (SE), 1/13 group is not known (acute myocardial infarction) SE: withdrawn due to SE 1/9 on imipramine (dizziness), 2/9 on placebo (dizziness) Pain not analysed separately,included in 6‐item neuropathic score QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sindrup 1990a.
| Methods | Double blind placebo controlled crossover design, two weeks (imipramine dose finding before randomisation according to plasma levels). Three two week periods, two to four weeks washout for slow metabolizers when needed, no washout for extensive metabolizers, no analyses of carry over effect Randomisation method not stated Inclusion criteria: duration of symptoms at least one year |
|
| Participants | Diabetic neuropathy of 4.75 years (range 1 to 12 years) 26 participants (20 final number). Mean age 46.9 years (range 28 to 75), 10 male and 10 female patients |
|
| Interventions | Paroxetine 40 mg; or imipramine from 25 mg to 350 mg (mean dose 197.5 mg); or placebo daily orally | |
| Outcomes | Pain patients reported, 5‐item pain score (0 to 2) Pain score on paroxetine 0.49, on imipramine 0.52, on placebo 1.47 |
|
| Notes | Dropouts 7/26 (four SE, two need of analgesia not related to neuropathy,
one compliance problem) SE: five patients withdrawn on imipramine QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sindrup 1990b.
| Methods | Double blind placebo controlled crossover design, two weeks Three two week periods, at least one week washout for extensive metabolisers and at least three weeks for poor metabolisers. Some residual effect after clomipramine. Randomisation method not stated |
|
| Participants | Diabetic neuropathy of 3.5 years (range 1 to 20). 26 participants (19 final number). Mean age 54.7 years (range 29 to 78), 9 male and 10 female patients | |
| Interventions | Clomipramine 50 mg for poor metabolisers and 75 mg for extensive metabolisers; or desipramine 50 mg for poor and 200 mg for extensive metabolisers; or placebo daily orally | |
| Outcomes | 5‐item VRS (0 to 2) Pain on clomipramine 0.99 (range 0 to 2.0), on desipramine 1.02 (range 0 to 2.0), on placebo 1.5 (range 0.5 to 2) |
|
| Notes | Dropouts 7/26; on clomipramine 4 (3 SE, 1 lack of effect), on desipramine 3 (SE) SE: withdrawn on clomipramine 3/26 (nausea, tiredness, dizziness, confusion), on desipramine 3/26 (1 nausea, 1 tiredness, 1 dizziness) QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sindrup 1992a.
| Methods | Double blind placebo controlled crossover design, three weeks. Two three week periods, one week washout, no carry over effect. First period results also available, but number of patients inadequate Randomisation method not stated |
|
| Participants | Diabetic neuropathy of four years (range 1 to 17). 18 participants (15 final number). Mean age 56 years (range 31 to 66), 12 male and 3 female patients Neuropathic score in citalopram group 6.0, in placebo group 6.2 |
|
| Interventions | Citalopram 40 mg daily orally, or placebo | |
| Outcomes | Symptoms patients reported, 6‐item neuropathic score (0 to 2 each) Neuropathic score on citalopram 4.5, on placebo 7.0 |
|
| Notes | Dropouts 3/18; (1 SE, 1 poor control of diabetes, 1 measurable level of citalopram during both treatment periods) SE: withdrawn 2/18 on citalopram (1 nausea and vomiting, 1 gastric upset and diarrhoea) Pain not reported separately, only neuropathic score available QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sindrup 1992b.
| Methods | Double blind placebo controlled crossover design, two weeks Three two week periods, one to three weeks washout, no carry over effect. First period results available, but number of patients inadequate Randomisation method not stated |
|
| Participants | Diabetic neuropathy of 3.7 years (range 1 to 11). 22 participants (18 final number). mean age 55.8 years (range 29 to 80), 9 male and 9 female patients | |
| Interventions | Mianserin 60 mg; or imipramine from 125 mg to 250 mg; or placebo daily orally | |
| Outcomes | Symptoms patients reported, 6‐item neuropathic score (0 to 2 each item) Neuropathic score on mianserin 5.5, on imipramine 4.0, on placebo 5.0 |
|
| Notes | Dropouts: 4/22; 1 on mianserin (personal reasons), 1 on imipramine (SE), 2 on placebo (1 SE and 1 persona reasons) SE: withdrawn 1/22 on imipramine, 1/1 on placebo Pain not analysed separately, only neuropathic score available QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sindrup 2001.
| Methods | Double blind placebo controlled crossover design, five weeks. Two five week periods, at least one week washout, no carry over effect Inclusion criteria: age at least 20 years, duration of symptoms at least six months |
|
| Participants | Polyneuropathy (diabetic 18, non‐diabetic 29). 54 participants (47 final number) Mean age 58 years (range 30 to 82), 31 male and 16 female patients Pain score 14 (25 to 75 % CI: 9 to 19), mean consumption of paracetamol 500 mg six tablets / week (25 to 75 % CI: 0 to 22) |
|
| Interventions | St.John's wort (total hypericin) 2700 mcg daily orally, or placebo | |
| Outcomes | Pain patients reported, 6‐item global improvement, sum pain score (0 to 40), paracetamol weekly consumption (number of 500 mg tablets), overall period reference On St.John's complete or good improvement 6/47, moderate 3/47, slight 4/47, no change 22/47, worse 12/47: on placebo complete or good improvement 0/47, moderate 2/47, slight 7/47, no change 25/47, worse 13/47 Pain on St.John's 14 (25 to 75 % CI: 7 to 21), on placebo 15 (9 to 19) Paracetamol consumption on St.John's 4 (25 to 75 % CI: 0 to 21), on placebo 5 (0 to18) Overall period preference 25 for St.John's, 16 for placebo, 6 no difference |
|
| Notes | Dropouts 7/54;
on St John's 2 (1 SE and 1 lost to follow‐up), on placebo 4 (1 SE, 3 needed pain treatment), 1 inconsistent pain rating SE: 13/54 on St. Johns, 15/54 on placebo. Withdrawn due to SE 1/54 on St. johns, 1/54 on placebo QS = 5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Sindrup 2003.
| Methods | Double blind placebo controlled crossover design, three way crossover, 3 x 4 week periods, one week washout | |
| Participants | Painfull polyneuropathy of > 6 months duration. 40 participants mean age 56, range 31 to 69 yrs | |
| Interventions | Venlafaxine 225 mg, imipramine 150 mg or placebo | |
| Outcomes | Patient rated pain paroxysms, constant pain, touch and pressure evoked pain; all on 11 point VAS. Global impression of pain relief 6 point (none to complete 5 pt or worse). AEs, rescue medication NNTs for moderate or better pain relief Venlafaxine 5.2 (2.7 to 5.9), imipramine 2.7 (1.8 to 5.5). For moderate or better pain relief: 2/33 placebo, 8/33 venlafaxine, 14/33 imipramine |
|
| Notes | 33 completed all 3 arms. 7 withdrew due to AEs. (2 placebo, 4 venlafaxine, 1 imipramine) one lost to follow up QS = 5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Tammiala‐Salonen 99.
| Methods | Double blind placebo controlled parallel group design, eight weeks (dose titration during the first five days). Pain more intensive in trazodone group at baseline. Inclusion criteria: duration of symptoms at least six months | |
| Participants | Burning mouth pain from six months to 20 years. 37 participants (28 final number). Mean age 58.6 years (range 39 to 71), all females. Pain score in trazodone group 59.2 by VAS and 8.2 by MPQ; in placebo group VAS 46.6 and MPQ 7.5. 17 patients depressed |
|
| Interventions | Trazodone dose escalation from 100 mg to 200 mg daily orally, or placebo | |
| Outcomes | Pain patients reported, 3‐item global improvement, VAS, VRS (MPQ) On trazodone pain improved 8/11, no change 2/11, worse 1/11; on placebo improved 13/17, no change 4/17, worse 1/17 VAS on trazodone 45.3, on placebo 34.3 Benefit in relation to side‐effects: on trazodone effective 6/11, neutral 4/11, inconvenient 1/11; on placebo effective 13/16, neutral 3/16 |
|
| Notes | Dropouts 9/37;
7/ 18 on trazodone (SE), 2/19 on placebo (SE) SE: 16/18 on trazodone, 11/19 on placebo Withdrawn due to SE 7/18 on trazodone, 2/19 on placebo QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Tasmuth 2002.
| Methods | Double blind placebo controlled crossover design, four weeks (four weeks dose titration to max tolerated dose). Two four week periods, two weeks washout, no carry over effect | |
| Participants | Postoperative neuropathic pain in breast cancer patients. 15 participants (13 final number). Mean age 55 years (range 37 to 72), all females. Pain score by VRS (0 to 7) 3 (range 3 to 4), depression score 10 (range 1 to 28) | |
| Interventions | Venlafaxine dose escalation from 18.75 mg to 75 mg daily orally, (11 had 75 mg); or placebo | |
| Outcomes | Pain patients reported, pain relief (VRS 0 to 4), pain intensity VRS (0 to 7). Beck's Depression Inventory (0 to 63) Pain relief on venlafaxine 2 (range 0 to 4), on placebo 0 (range 0 to 4) Pain intensity on venlafaxine 1 (range 0 to 3), on placebo 2 (range 0 to 4) Depression score on venlafaxine 7 (range 1 to 39), on placebo 7 (range 1 to 11) |
|
| Notes | Dropouts 2/15 (1 SE and 1 no compliance) SE: withdrawn 1/13 on venlafaxine (nausea, sweating, headache) QS = 4 (R2, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Turkington 1980.
| Methods | Double blind placebo controlled parallel group study, three months Randomisation methods not stated Inclusion criteria: age from 20 to 59 years |
|
| Participants | Diabetic neuropathy. 59 participants (59 final number). Age range 20 to 59 years, 27 male and 32 female patients. All patients had pain at baseline Depression score 8.4 (0.6) in imipramine group, 7.8(0.4) in amitriptyline group, in placebo group 8.0 (0.6) Sleep was disturbed in all patients at baseline |
|
| Interventions | Imipramine 100 mg, or amitriptyline 100 mg, or placebo daily orally | |
| Outcomes | Number of patients with painful legs.
Kupfer‐Detre depression form 1 Pain free legs on imipramine 20/20, on amitriptyline 19/19, on placebo 0/20 Depression on imipramine 3.9 (0.3), on amitriptyline 3.7 (0.4), on placebo 8.2 (0.6) Sleep disturbance on imipramine 0/20, on amitriptyline 0/19, on placebo 20/20 |
|
| Notes | No dropouts SE: no withdrawals due to SE QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ventafridda 1987.
| Methods | Double blind parallel group study, 15 days (dose escalation during three days) Randomisation not stated |
|
| Participants | Any neuropathy (27 cancer related peripheral nerve lesions, 9 non‐cancer related nerve lesions, 6 postherpetic neuralgia, 3 other). 45 participants (31 final number). Age range 34 to 79 years. Pain score in amitriptyline group 66, in trazodone group 46 | |
| Interventions | Amitriptyline dose escalation from 25 mg to 75 mg, or trazodone from 75 mg to 225 mg daily orally | |
| Outcomes | Pain score 0 to 240 (intensity and duration of daily pain) Pain score 26 on amitriptyline, on trazodone 31. Pain score decreased on amitriptyline 40, on trazodone 15 |
|
| Notes | Dropouts 14/45; 4/22 on amitriptyline (2 death, 2 lack of compliance), 10/23 on trazodone (6 SE, 1 no effect, 3 lack of compliance) SE: withdrawn 0/22 on amitriptyline, 6/23 on trazodone Results illustrated only in figures QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Vrethem 1997.
| Methods | Double blind placebo controlled crossover design, four weeks (one week dose titration then stable dose) Three 3 week periods, one week washout, no carry over effect Randomisation method not stated Inclusion criteria: duration of symptoms at least six months |
|
| Participants | Polyneuropathy (19 diabetic, 18 non‐diabetic). 37 participants (33 final number). Mean pain duration 48 months, 17 male and 19 female patients Pain score for diabetics 5.0 (1.4) and for non‐diabetic 4.1 (1.9). Depression score for diabetic 2.8 (range 0 to18.0) and non‐diabetic 2.9 (range 0 to 22.5) |
|
| Interventions | Amitriptyline dose escalation from 25 mg to 75 mg, or maprotiline from 25 mg to 75 mg, or placebo daily orally | |
| Outcomes | Pain patients reported, 5‐item global improvement, more than 20 % pain decrease Comprehensive Psychopathological Rating Scale Number of patients with improved sleep On amitriptyline pain completely improved 1/33, much improved 11/33, improved 10/33, no change 10/33, worse 1/33; on maprotiline completely improved 1/33, much improved 3/33, improved 10/33, no change 17/33, worse 2/33; on placebo completely improved 0/33, much improved 1/33, improved 7/33, no change 22/33, worse 3/33 Pain reduced at least 20% on amitriptyline 20/33, on maprotiline 15/33, on placebo 7/33 Depression score in diabetic patients on amitriptyline 1.2 (range 0‐12.5), on maprotiline 2.4 (range 0‐145), on placebo 2.3 (range 0‐12.5); in non‐diabetic patients on amitriptyline 1.1 (range 0‐10.5), on maprotiline 1.5 (range 0‐11.5), on placebo 1.6 (range 0‐11). Sleep improved on amitriptyline 11/33, on maprotiline 5/33, on placebo 4/33. |
|
| Notes | Dropouts 7/37 (5 SE, 1 depression, 1 early drop out) SE: 21/33 on amitriptyline, on 21/33 on maprotiline, 6/33 on placebo. Withdrawn due to SE 3 on amitriptyline (1 severe thirst, 1 urinary retention, 1 hyperglycaemia), 2 on maprotiline (1 sedation and vertigo, 1 urticaria) QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Watson 1982.
| Methods | Double blind placebo controlled crossover design, three weeks Two three week periods, one to two weeks washout, no analyses of carry over effect Randomisation method not stated |
|
| Participants | Postherpetic neuralgia of 3.8 years (range 4 months to 9 years). 24 participants (24 final number). Mean age 66 years (range 49 to 81), 8 male and 16 female patients. 9 patients depressed | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 25 mg daily orally, dose range 25 mg ‐ 137.5 mg; or placebo | |
| Outcomes | 4‐item global improvement At least good on amitriptyline 16/24 (excellent improvement 3, good 13, no change 2, poor 6), on placebo 2/24 (excellent improvement 0, good 1, no change 21, poor 2) |
|
| Notes | Dropouts 6/24; 1 on amitriptyline (SE), 5 on placebo (SE, pain, depression)
SE: 16/24 on amitriptyline, 13/24 on placebo QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Watson 1992.
| Methods | Double blind crossover design, five weeks. Two five week periods, 2 weeks washout, no carry over effect Randomisation method not stated Inclusion criteria: duration of symptoms at least three months |
|
| Participants | Postherpetic neuralgia of 14 months (range 4 months to 7 years). 35 participants (32 final number). Mean age 71 years (range 55 to 85), 18 male and 17 female patients 11 depressed. Pain score in amitriptyline group: steady pain 61.6, jabbing pain 58.3 and skin pain 71.1; in maprotiline group steady pain 56.3, jabbing pain 41.9 and skin pain 59.4 |
|
| Interventions | Amitriptyline dose escalation from 12.5 mg to 25 mg + placebo daily orally, median dose 100 mg (range 37.5 to 150 mg); or maprotiline from 12.5 mg to 25 mg + placebo daily orally, median dose 100 mg (range 50 to 150 mg) | |
| Outcomes | Pain patients reported, 4‐item global improvement, 4‐item scale for effectiveness (including pain relief, side effects, sleep and satisfaction), VAS. The Bock Depression Inventory On amitriptyline no pain 3/32, mild 12/32, moderate 7/32, no change 10/32; on maprotiline no pain 3/32, mild 9/32, moderate 9/32, no change 11/32 Effectiveness on amitriptyline excellent 4/32, good 10/32, slight 10/32, no change 8/32; on maprotiline excellent 3/32, good 3/32, slight 12/32, no change 14/32 Amitriptyline better than maprotiline in 11/32 patients, maprotiline better than amitriptyline in 9/32 patients, no difference in 12/32 patients On amitriptyline steady pain VAS 41.4, jabbing pain 23.7 and skin pain 42.7; on maprotiline steady pain 17.7, jabbing pain 11.4 and skin pain 25.6 9/32 depressed on amitriptyline, 12/32 on maprotiline |
|
| Notes | Dropouts 3/35; on amitriptyline 2 (1 SE and 1 pain didn't return after washout period), on maprotiline 1 (pain didn't return after washout period) SE: 20/35 on amitriptyline, 28/35 on maprotiline. Withdrawn 3 on amitriptyline (dry mouth and constipation, dizziness, sedation, lethargy, mouth ulceration or nausea), 3 on maprotiline (1 dry mouth and nausea, 1 nausea and vomiting, 1 restless legs) QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Watson 1998.
| Methods | Double blind crossover design, five weeks. Two five week periods, 2 weeks washout, no carry over effect Inclusion criteria: duration of symptoms at least three months |
|
| Participants | Postherpetic neuralgia of 13 months. 33 participants (31 final number) | |
| Interventions | Amitriptyline dose escalation from 10 mg to 20 mg daily orally, mean dose 68.48 mg (range 10‐140 mg); or nortriptyline 10 mg to 20 mg daily orally, mean dose 85.13 mg (range 10 to 160 mg) | |
| Outcomes | Pain patients reported, satisfied or unsatisfied (pain relief and side‐effects) On amitriptyline satisfied 17/31 and unsatisfied 14/31; on nortriptyline satisfied 15/31 and unsatisfied 16/32 |
|
| Notes | Dropouts 2/33 (SE) SE: 31/33 on amitriptyline, 31/33 on nortriptyline Withdrawn 1 on amitriptyline (slurred speech, urinary retention), 1 on nortriptyline (increased pain, bad dreams, fever, perspiration, epigastric pain) QS = 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Yucel 2004.
| Methods | Double blind placebo controlled trial for eight weeks | |
| Participants | 60 participants aged 33 to 69 years. Neuropathic pain for longer than six months of at least four on 11 pt VASPI Patients subjected to experimentally induced pain |
|
| Interventions | Venlafaxine 75 mg /day, venlafaxine 150 mg/ or placebo for eight weeks . paracetamol 500 mg 3/4 time daily for rescue. Antidepressants or anticonvulsants nota allowed | |
| Outcomes | VASPI, Patient satisfaction, activities of daily , AEs, global impression of change. Experimentally induced pain scores not used for this review. Global impression of improvement 8/16 placebo, 13/16 venlafaxine 75 mg, 1/14 venlafaxine 150 mg. No significant difference between venlafaxine and placebo | |
| Notes | 5/60 withdrew: 1 placebo, 1 venlafaxine 75 mg, 3 venlafaxine 150 mg QS = 3 (R1, DB1, W1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
AEs‐adverse events BDI ‐ Beck depression inventory HAS‐ Hamilton anxiety scale HDRS ‐ Hamilton depression rating scale MPQ ‐ McGill Pain Questionairre NRS‐ numerical rating score PAD‐ Zung pain and distress index PCA‐ patient controlled analgesia PHN‐ post herpetic neuralgia PI‐ pain intensity PPI ‐ present pain intensity PR ‐ pain relief PRI ‐ pain rating index QOL ‐ quality of life QS ‐ quality score SD ‐ standard deviation SE ‐ side effects TCA ‐ tricyclic andidepressants. VAS‐ visual analogue scale VRS ‐ verbal rating scale yrs ‐ years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aragona 2005 | Somatoform pain disorder‐not neuropathic pain |
| Aronoff 1982 | Review/not a study |
| Battla 1981 | not RCT |
| Beaumont 1980 | Terminal pain not neuropathic pain |
| Blumer 1980 | not RCT |
| Blumer 1981 | not RCT, follow up toBlumer 1980 |
| Bogetto 1999 | RCT but pain not assessed |
| Brenne 1997 | Dose finding study |
| Davis 1977 | Not RCT |
| Eberhard 1988 | Dose finding not RCT |
| Edelbroek 1986 | Idiopathic pain study |
| Erzurumlu 1996 | Not RCT |
| Evans 1973 | Chronically ill patients, not neuropathic pain |
| Gade 1980 | Case report, not neuropathic pain |
| Gourlay 1986 | Chronic pain study |
| Hameroff 1982 | Spinal pain study |
| Hameroff 1984 | Spinal pain study |
| Hameroff 1985 | Dual publication of Hameroff 1984 |
| Johansson 1979 | Chronic pain study |
| Jørgensen 1984 | Depressive patients with somatic symptoms |
| Khurana 1983 | Not RCT |
| Kumar 1998 | Comparison between electrotherapy and amitriptyline, and sham treatment and amitriptyline |
| Loldrup 1989 | Chronic idiopathic pain study |
| McQuay 1992 | Chronic pain study, not neuropathic pain. |
| McQuay 1993 | Dose finding study, not neuropathic pain |
| Mendel 1986 | Inadequate number of patients (6) |
| Minotti 1998 | Chronic cancer pain study |
| Onghena 1993 | Chronic pain study |
| Pilowsky 1990 | Psychogenic pain study |
| Pilowsky 1995 | Comparison between cognitive‐behavioural therapy alone and with amitriptyline |
| Plesh 2000 | Not RCT |
| Raftery 1979 | Not RCT |
| Rawn 2000 | Appraisal of Morello 1999 |
| Semenchuk 2000 | Not RCT |
| Sindrup 1990c | Concentration‐response pharmacokinetic study |
| Sindrup 1991 | Concentration‐response pharmacokinetic study |
| Sindrup 1992c | No pain outcome |
| Standford 1992 | Case report |
| Stockstill 1989 | Chronic myofascial pain study |
| Takeda 1988 | Not RCT |
| Van Houdenhove 1992 | Chronic idiopathic pain, "masked" depression |
| Van Kempen 1992 | Somatoform pain study |
| Vidal 2004 | Review |
| von Knorring 1979 | Chronic pain study‐ not neuropathic pain |
| von Knorring 1980 | Chronic pain secondary publication of von Knorring 1979 |
| Watson 1985 | Not RCT |
| Young 1985 | Inadequate number of patients (6) |
| Zitman 1990 | Chronic pain study |
| Zitman 1991 | Somatoform pain study |
Contributions of authors
TS identified the studies, extracted information and data, undertook the analysis, and wrote the first draft. PW checked and updated searches, wrote the second draft and undertook some new data calculations Both review authors agreed the final draft. For this update the authors independently extracted data, met to agree data to be included and both wrote and agreed the final version.
Sources of support
Internal sources
UK Cochrane Centre, UK.
NHS Research and Development, UK.
External sources
Finnish Association of Pain Research, Finland.
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Biesbroeck 1995 {published data only}
- Biesbroeck R, Bril V, Hollander P, Kabadi U, Schwartz S, Singh SP, et al. A double‐blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Advance in Therapy 1995;12(2):111‐20. [PubMed] [Google Scholar]
Bowsher 1997 {published data only}
- Bowsher D. The effects of pre‐emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double‐blind, placebo‐controlled trial. Journal of Pain and Symptom Management 1997;13(6):327‐31. [DOI] [PubMed] [Google Scholar]
Brady 1987 {published data only}
- Brady JP, Cheatle MD, Ball WA. A trial of L‐tryptophan in chronic pain syndrome. Clinical Journal of Pain 1987;3:39‐43. [Google Scholar]
Carasso 1979 {published data only}
- Carasso RL, Yehuda S, Streifler M. Clomipramine and amitriptyline in the treatment of severe pain. International Journal of Neuroscience 1979;9:191‐4. [DOI] [PubMed] [Google Scholar]
Cardenas 2002 {published data only}
- Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain 2002;96:365‐73. [DOI] [PubMed] [Google Scholar]
Ciaramella 2000 {published data only}
- Ciaramella A, Grosso S, Poli P. Fluoxetine versus fluvoxamine for treatment of chronic pain [Fluoxetine versus fluvoxamina nel trattamento del dolore cronico]. Minerva Anestesiologica 2000;66:55‐61. [PubMed] [Google Scholar]
Dallocchio 2000 {published data only}
- Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs amitriptyline in painful diabetic neuropathy: an open label pilot study. Journal of Pain and Symptom Management 2000;20(4):280‐5. [DOI] [PubMed] [Google Scholar]
Davidoff 1987 {published data only}
- Davidoff G, Guarracini M, Roth E, Sliwa J, Yarkony G. Trazodone hydrochloride in the treatment of dysesthetic pain in traumatic myelopathy: a randomized, double‐blind, placebo‐controlled study. Pain 1987;29(2):151‐61. [DOI] [PubMed] [Google Scholar]
Feinmann 1984 {published data only}
- Feinmann C. Psychogenic facial pain: presentation and treatment. Journal of Psychosomatic Research 1983;27(5):404‐10. [DOI] [PubMed] [Google Scholar]
- Feinmann C, Harris M. Psychogenic facial pain: Part 1 The clinical presentation. British Dental Journal 1984;156:165‐8. [DOI] [PubMed] [Google Scholar]
- Feinmann C, Harris M. Psychogenic facial pain: Part 2 Management and Prognosis. British Dental Journal 1984;156:205‐8. [DOI] [PubMed] [Google Scholar]
- Feinmann C, Harris M, Crawley R. Psychogenic facial pain: presentation and treatment. BMJ 1984;288:436‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forssell 2004 {published data only}
- Forssell H, Tasmuth T, Tenovuo O, Hampf G, Kalso E. Venlafaxine in the treatment of atypical facial pain: a randomized controlled trial. Journal of Orofacial Pain 2004;18(2):131‐7. [PubMed] [Google Scholar]
Gerson 1977 {published data only}
- Gerson GR, Jones RB, Luscombe DK. Studies on the concomitant use of carbamazepine and clomipramine for the relief of post‐herpetic neuralgia. Postgraduate Medical Journal 1977;53 Suppl 4:104‐9. [PubMed] [Google Scholar]
Göbel 1997 {published data only}
- Göbel H, Stadler TH. Treatment of post‐herpes zoster pain with tramadol [Traitement des doulers post‐zostériennes par le tramodol]. Drugs 1997;53 Suppl 2:34‐9. [DOI] [PubMed] [Google Scholar]
Gomez‐Perez 1985 {published data only}
- Gomez‐Perez FJ, Rull JA, Dies H, Rodriquez‐Rivera JG, Gonzalez‐Barranco J, Lozano‐Castañeda O. Nortriptyline and fluphenazine in the symptomatic treatment of diabetic neuropathy. A double blind cross over study. Pain 1985;23:395‐400. [DOI] [PubMed] [Google Scholar]
Gomez‐Perez 1996 {published data only}
- Gomez Perez FJ, Choza R, Rios JM, et al. Nortriptyline‐fluphenazine versus carbamazepine in the symptomatic treatment of diabetic neuropathy. Archives of Medical Research 1996;27(4):525‐9. [PubMed] [Google Scholar]
Graff‐Radford 2000 {published data only}
- Graff‐Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. The Clinical Journal of Pain 2000;16:188‐92. [DOI] [PubMed] [Google Scholar]
Hampf 1989 {published data only}
- Hampf G, Bowsher D, Nurmikko T. Distigmine and amitriptyline in the treatment of chronic pain. Anesthesia Progress 1989;36(2):58‐62. [PMC free article] [PubMed] [Google Scholar]
Harrison 1997 {published data only}
- Harrison SD, Glover L, Feinmann C, Pearce SA, Harris M. A comparison of antidepressant medication alone and in conjunction with cognitive behavioural therapy for chronic idiopathic facial pain. Proceedings of the 8th world congress on pain ‐ Progress in pain research and management. Seattle: IASP Press, 1997; Vol. 8:663‐72.
Kalso 1996 {published data only}
- Kalso E, Tasmuth T, Neuvonen PJ. Amitriptyline effectively relieves neuropathic pain following treatment of breast cancer. Pain 1996;64(2):293‐302. [DOI] [PubMed] [Google Scholar]
Kieburtz 1998 {published data only}
- Kieburtz K, Simpson D, Yiannoutsos C, et al. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. Neurology 1998;51(6):1682‐8. [DOI] [PubMed] [Google Scholar]
Kishore‐Kumar 1990 {published data only}
- Kishore‐Kumar R, Max MB, Schafer SC, et al. Desipramine relieves postherpetic neuralgia. Clinical Pharmacology and Therapeutics 1990;47:305‐12. [DOI] [PubMed] [Google Scholar]
Kvinesdal 1984 {published data only}
- Kvinesdal B, Molin D, Froland A, Gram LA. Imipramine therapy of painful diabetic neuropathy. Journal of the American Medical Association 1984;251:1727‐30. [PubMed] [Google Scholar]
- Kvinesdal B, Molin J, Froland A, Gram LF. Imipramine in the treatment of painful diabetic neuropathy of the extremities [Imipramin ved behandling af smerter ved diabetisk ekstemitetsneuropati]. Ugeskrift for Laeger 1983;145(39):3018‐9. [PubMed] [Google Scholar]
Lampl 2002 {published data only}
- Lampl C, Yazdi K, Roper C. Amitriptyline in the prophylaxis of central poststroke pain. Preliminary results of 39 patients in a placebo‐controlled, long‐term study. Stroke 2002;33(12):3030‐2. [DOI] [PubMed] [Google Scholar]
Langohr 1982 {published data only}
- Langohr HD, Stöhr M, Petruch F. An open and double blind cross over study on the efficacy of Clomipramine (Anafranil) in patients with painful mono‐ and polyneuropathies. European Neurology 1982;21:309‐17. [DOI] [PubMed] [Google Scholar]
Lascelles 1966 {published data only}
- Lascelles RG. Atypical facial pain and depression. British Journal of Psychiatry 1966;112:651‐9. [DOI] [PubMed] [Google Scholar]
Leijon 1989 {published data only}
- Leijon G, Boivie J. Central post‐stroke pain‐a controlled trial of amitriptyline and carbamazepine. Pain 1989;36(1):27‐36. [DOI] [PubMed] [Google Scholar]
Maina 2002 {published data only}
- Maina G, Vitalucci A, Gandolfo S, Bogetto F. Comparative efficacy of SSRIs and amisulpride in burning mouth syndrome: a single‐blind study. Journal of Clinical Psychiatry 2002;63(1):38‐42. [DOI] [PubMed] [Google Scholar]
Max 1987 {published data only}
- Max MB, Culnane M, Schafer S, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987;37:589‐96. [DOI] [PubMed] [Google Scholar]
Max 1988 {published data only}
- Max MB, Schafer SC, Culnane M, Smoller B, Dubner R, Gracely RH. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology 1988;38:1427‐32. [DOI] [PubMed] [Google Scholar]
Max 1991 {published data only}
- Max MB, Kishore‐Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo‐controlled trial. Pain 1991;45:3‐9. [DOI] [PubMed] [Google Scholar]
Max 1992a {published data only}
- Max MB, Lynch SA, Muir J, Shoaf SF, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. New England Journal of Medicine 1992;326:1250‐6. [DOI] [PubMed] [Google Scholar]
Max 1992b {published data only}
- Max MB, Lynch SA, Muir J, Shoaf SF, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. New England Journal of Medicine 1992;326:1250‐6. [DOI] [PubMed] [Google Scholar]
McCleane 2000a {published data only}
- McCleane G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: a randomized, double‐blind, placebo‐controlled study. British Journal of Clinical Pharmacology 2000;49(6):574‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCleane 2000b {published data only}
- McCleane GJ. Topical doxepin hydrochloride reduces neuropathic pain: a randomized double‐blind, placebo controlled study. The Pain Clinic 2000;12:47‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercadante 2002 {published data only}
- Mercadante S, Arcuri E, Tirelli W, Villari P, Casuccio A. Amitriptyline in neuropathic cancer pain in patients on morphine therapy: a randomized placebo‐controlled, double‐blind crossover study. Tumori 2002;88(3):239‐42. [DOI] [PubMed] [Google Scholar]
Morello 1999 {published data only}
- Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double‐blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Archives of Internal Medicine 1999;159(16):1931‐7. [DOI] [PubMed] [Google Scholar]
- Rawn T, Papoushek C, Evans MF. Gabapentin or amitriptyline for painful diabetic neuropathy?. Canadian Family Physician 2000;46:2215‐7. [PMC free article] [PubMed] [Google Scholar]
Panerai 1990 {published data only}
- Panerai AE, Monza G, Movilia P, Bianchi M, Francucci BM, Tiengo M. A randomized, within‐patient, cross‐over, placebo‐controlled trial on the efficacy and tolerability of the tricyclic antidepressants chlorimipramine and nortriptyline in central pain. Acta Neurologica Scandanavica 1990;82(1):34‐8. [DOI] [PubMed] [Google Scholar]
Pilowsky 1982 {published data only}
- Pilowsky I, Hallett EC, Bassett DL, Thomas PG, Penhall RK. A controlled study of amitriptyline in the treatment of chronic pain. Pain 1982;14(2):169‐79. [DOI] [PubMed] [Google Scholar]
Raja 2002 {published data only}
- Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo‐controlled trial. Neurology 2002;59(7):1015‐21. [DOI] [PubMed] [Google Scholar]
Reuben 2004 {published data only}
- Reuben SS, Makari‐Judson G, Lurie SD. Evaluation of efficacy of the perioperative administration of venlafaxine XR in the prevention of postmastectomy pain syndrome. Journal of Pain and Symptom Management 2004;27(2):133‐9. [DOI] [PubMed] [Google Scholar]
Robinson 2004 {published data only}
- Robinson LR, Czerniecki JM, Ehde DM, Edwards WT, Judish DA, Goldberg ML, et al. Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Archives of Physical Medicine and Rehabilitation 2004;85(1):1‐6. [DOI] [PubMed] [Google Scholar]
Rowbotham 2004 {published data only}
- Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double blind, placebo‐controlled study. Pain 2004;110:697‐706. [DOI] [PubMed] [Google Scholar]
Semenchuk 2001 {published data only}
- Semenchuk MR, Sherman S, Davis B. Double‐blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology 2001;57(9):1583‐8. [DOI] [PubMed] [Google Scholar]
Sharav 1987 {published data only}
- Sharav Y, Singer E, Schmidt E, Dionne RA, Dubner R. The analgesic effect of amitriptyline on chronic facial pain. Pain 1987;31(2):199‐209. [DOI] [PubMed] [Google Scholar]
Shlay 1998 {published data only}
- Shlay J, Chaloner K, Max M, Flaws B, Reichelderfer P, Wentworth D, et al. Acupuncture and amitriptyline for pain due to HIV‐related peripheral neuropathy. JAMA 1998;280:1590‐5. [DOI] [PubMed] [Google Scholar]
Simpson 2001 {published data only}
- Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. Journal of Clinical Neuromuscular Disease 2001;3(2):53‐62. [DOI] [PubMed] [Google Scholar]
Sindrup 1989 {published data only}
- Sindrup SH, Ejlertsen B, Frøland A, Sindrup EH, Brøsen K, Gram LF. Imipramine treatment in diabetic neuropathy: relief of subjective symptoms without changes in peripheral and autonomic nerve function. European Journal of Clinical Pharmacology 1989;37:151‐3. [DOI] [PubMed] [Google Scholar]
Sindrup 1990a {published data only}
- Sindrup SH, Gram LF, Brosen K, Eshoj O, Mogensen EF. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain 1990;42:135‐44. [DOI] [PubMed] [Google Scholar]
Sindrup 1990b {published data only}
- Sindrup SH, Gram LF, Skjold T, Grodum E, Brosen K, Beck‐Nielsen H. Clomipramine vs. desipramine vs. placebo in the treatment of diabetic neuropathy symptoms. A double‐blind cross‐over study. British Journal of Clinical Pharmacology 1990;30:683‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sindrup 1992a {published data only}
- Sindrup SH, Bjerre U, Dejgaard A, Brosen K, Aaes‐Jorgensen T, Gram LF. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clinical Pharmacology & Therapeutics 1992;52(5):547‐52. [DOI] [PubMed] [Google Scholar]
Sindrup 1992b {published data only}
- Sindrup SH, Tuxen C, Gram LF, et al. Lack of effect of mianserin on the symptoms of diabetic neuropathy. European Journal of Clinical Pharmacology 1992;43:251‐5. [DOI] [PubMed] [Google Scholar]
Sindrup 2001 {published data only}
- Sindrup SH, Madsen C, Bach FW, Gram LF, Jensen TS. St. John's wort has no effect on pain in polyneuropathy. Pain 2001;91(3):361‐5. [DOI] [PubMed] [Google Scholar]
Sindrup 2003 {published data only}
- Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology 2003;60(8):1284‐9. [DOI] [PubMed] [Google Scholar]
Tammiala‐Salonen 99 {published data only}
- Tammiala‐Salonen T, Forssell H. Trazodone in burning mouth pain: a placebo‐controlled, double‐blind study. Journal of Orofacial Pain 1999;13(2):83‐8. [PubMed] [Google Scholar]
Tasmuth 2002 {published data only}
- Tasmuth T, Hartel B, Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. European Journal of Pain 2002;6(1):17‐24. [DOI] [PubMed] [Google Scholar]
Turkington 1980 {published data only}
- Turkington RW. Depression masquerading as diabetic neuropathy. JAMA 1980;243:1147‐50. [PubMed] [Google Scholar]
Ventafridda 1987 {published data only}
- Ventafridda V, Bonezzi C, Caraceni A, Conno F, Guarise G, Ramella G, et al. Antidepressants for cancer pain and other painful syndromes with deafferentation component: comparison of amitriptyline and trazodone. Italian Journal of Neurological Sciences 1987;8(6):579‐87. [DOI] [PubMed] [Google Scholar]
- Ventafridda V, Caraceni A, Saita L, Bonezzi C, Conno F, Guarise G, et al. Trazodone for deafferentation pain. Comparison with amitriptyline. Psychopharmacology 1988;95 Suppl:S44‐9. [DOI] [PubMed] [Google Scholar]
Vrethem 1997 {published data only}
- Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindstrom T, Thorell LH. A comparison a amitriptyline and maprotiline in the treatment of painful polyneuropathy in diabetics and nondiabetics. Clinical Journal of Pain 1997;13(4):313‐23. [DOI] [PubMed] [Google Scholar]
Watson 1982 {published data only}
- Watson CP, Evans RJ, Reed K, Merskey H, Goldsmith L, Warsh J. Amitriptyline versus placebo in postherpetic neuralgia. Neurology 1982;32:671‐3. [DOI] [PubMed] [Google Scholar]
Watson 1992 {published data only}
- Watson CP, Chipman M, Reed K, Evans RJ, Birkett N. Amitriptyline versus maprotiline in postherpetic neuralgia: a randomized,double‐blind, crossover trial. Pain 1992;48(1):29‐36. [DOI] [PubMed] [Google Scholar]
Watson 1998 {published data only}
- Watson CP, Vernich L, Chipman M, Reed K. Nortriptyline versus amitriptyline in postherpetic neuralgia: a randomized trial. Neurology 1998;51(4):1166‐71. [DOI] [PubMed] [Google Scholar]
Yucel 2004 {published data only}
- Yucel A, Ozyalcin S, Koknel Talu G, Kiziltan E, Yucel B, Andersen OK, et al. The effect of venlafaxine on ongoing and experimentally induced pain in neuropathic pain patients: a double blind, placebo controlled study. European Journal of Pain 2005;9(4):407‐16. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aragona 2005 {published data only}
- Aragona M, Bancheri L, Perinelli D, Tarsitani L, Pizzimenti A, Conte A, et al. Randomized double‐blind comparison of serotonergic (Citalopram) versus noradrenergic (Reboxetine) reuptake inhibitors in outpatients with somatoform, DSM‐IV‐TR pain disorder. European Journal of Pain 2005;9(1):33‐8. [DOI] [PubMed] [Google Scholar]
Aronoff 1982 {published data only}
- Aronoff GM, Evans WO. Doxepin as an adjunct in the treatment of chronic pain. Journal of Clinical Psychiatry 1982;43(8 part 2):42‐7. [PubMed] [Google Scholar]
Battla 1981 {published data only}
- Battla H, Silverblatt CW. Clinical trial of amitriptyline and fluphenazine in diabetic peripheral neuropathy. Southern Medical Journal 1981;74(4):417‐8. [DOI] [PubMed] [Google Scholar]
Beaumont 1980 {published data only}
- Beaumont G, Seldrup J. Comparative trial of clomipramine and placebo in the treatment of terminal pain. Journal of International Medical Research 1980;8 (supp):37‐9. [PubMed] [Google Scholar]
Blumer 1980 {published data only}
- Blumer D, Heilbronn M. Second‐year follow‐up study on systematic treatment of chronic pain with antidepressants. Henry Ford Hospital Medical Journal 1981;29(2):67‐8. [PubMed] [Google Scholar]
Blumer 1981 {published data only}
- Brenne E, Hagen K, Maehlum E, Husebo S. Treatment of chronic pain with amitriptyline. A double‐blind dosage study with determination of serum levels. Tidsskrift for den Norske Laegeforening 1997;117:3491‐4. [PubMed] [Google Scholar]
Bogetto 1999 {published data only}
- Bogetto F, Bonatto Revello R, Ferro G, Maina G, Ravizza L. Psychopharmacological treatment of burning mouth syndrome [Trattamento psicofarmacologico della Burning Mouth Syndrome]. Minerva Psichiatrica 1999;40:1‐10. [Google Scholar]
Brenne 1997 {published data only}
- Brenne E, Hagen K, Maehlum E, Husebo S. Treatment chronic pain with amitriptyline. A double‐blind dosage study with determination of serum levels [Behandling av pasienter med kroniske smerter med amitriptylin. Dobbeltblind doseringsundersokelse med serumkonsentrasjonsbestemmelse]. Tidsskr Nor Laegeforen 1997;117(24):3491‐4. [PubMed] [Google Scholar]
Davis 1977 {published data only}
- Davis JL, Lewis SB, Gerich JE, Kaplan RA, Schultz TA, Wallin JD. Peripheral diabetic neuropathy treated with amitriptyline and fluphenazine. JAMA 1977;238(21):2291‐2. [PubMed] [Google Scholar]
Eberhard 1988 {published data only}
- Eberhard G, vonKnorring L, Nilsson HL, Sundequist U, Bjorling G, Linder H, et al. A double‐blind randomised study of clomipramine versus maprotiline in patients with idiopathic pain syndromes. Neuropsychobiology 1988;19:25‐34. [DOI] [PubMed] [Google Scholar]
Edelbroek 1986 {published data only}
- Edelbroek PM, Linssen AC, Zitman FG, Rooymans HG, Wolff FA. Analgesic and antidepressive effects of low‐dose amitriptyline in relation to its metabolism in patients with chronic pain. Clinical Pharmacology & Therapeutics 1986;39(2):156‐62. [DOI] [PubMed] [Google Scholar]
Erzurumlu 1996 {published data only}
- Erzurumlu A, Dursun H, Gunduz S, Kalyon TA, Apracioglu O. The management of chronic pain at spinal cord injured patients. The comparison of effectiveness amitryptiline and carbamazepine combination and electroacupuncture application [Spinal Kord Yarali Hastalarda Kronik Agri Tedavisi]. Journal of Rheumatology and Medical Rehabilitation 1996;7(3):176‐80. [Google Scholar]
Evans 1973 {published data only}
- Evans W, Gensler F, Blackwell B, Galbrecht C. The effects of antidepressant drugs on pain relief and mood in the chronically ill. A double blind study. Psychosomatics 1973;14:214‐5. [DOI] [PubMed] [Google Scholar]
Gade 1980 {published data only}
- Gade GN, Hofeldt FD, Treece GL. Diabetic neuropathic cachexia. Beneficial response to combination therapy with amitriptyline and fluphenazine. JAMA 1980;243(11):1160‐1. [DOI] [PubMed] [Google Scholar]
Gourlay 1986 {published data only}
- Gourlay GK, Cherry DA, Cousins MJ, Love BL, Graham JR, McLachlan MO. A controlled study of a serotonin reuptake blocker, zimelidine in the treatment of chronic pain. Pain 1986;25:35‐52. [DOI] [PubMed] [Google Scholar]
Hameroff 1982 {published data only}
- Hameroff SR, Cork RC, Scherer K, Crago BR, Neuman C, Womble JR, et al. Doxepin effects on chronic pain, depression and plasma opioids. Journal of Clinical Psychiatry 1982;43(8 Pt2):22‐7. [PubMed] [Google Scholar]
Hameroff 1984 {published data only}
- Hameroff SR, Weiss JL, Lerman JC, Cork RC, Watts KS, Crago BR, et al. Doxepin's effects on chronic pain and depression: a controlled study. Journal of Clinical Psychiatry 1984;45(3 Pt2):47‐53. [PubMed] [Google Scholar]
Hameroff 1985 {published data only}
- Hameroff SR, Cork RC, Weiss JL, Crago BR, Davis TP. Doxepin effects on chronic pain and depression a controlled study. The Clinical Journal of Pain 1985;1:171‐6. [Google Scholar]
Johansson 1979 {published data only}
- Johansson F, Knorring L. A double‐blind controlled study of seratonin uptake inhibitor (Zimelidine) versus placebo in chronic pain patients. Pain 1979;7(1):69‐78. [DOI] [PubMed] [Google Scholar]
Jørgensen 1984 {published data only}
- Jørgensen B, Nørrelund N, Bech P, Jakobsen K. Pain and depressive symptoms in general practice [Smerter og depressive symptomer i almen praksis]. Ugeskrift for Laeger 1984;146(38):2868‐72. [PubMed] [Google Scholar]
Khurana 1983 {published data only}
- Khurana RC. Treatment of painful diabetic neuropathy with trazodone. JAMA 1983;250(11):1392. [DOI] [PubMed] [Google Scholar]
Kumar 1998 {published data only}
- Kumar D, Alvaro MS, Julka IS, Marshall HJ. Diabetic peripheral neuropathy: effectiveness of electrotherapy and amitriptyline for symptomatic relief. Diabetes Care 1998;21(8):1322‐5. [DOI] [PubMed] [Google Scholar]
Loldrup 1989 {published data only}
- Loldrup D, Langemark M, Hansen HJ, Olesen J, Bech P. Clomipramine and mianserin in chronic idiopathic pain syndrome. Psychopharmacology 1989;99:1‐7. [DOI] [PubMed] [Google Scholar]
McQuay 1992 {published data only}
- McQuay HJ, Carroll D, Glynn CJ. Low dose amitriptyline in the treatment of chronic pain. Anaesthesia 1992;47:646‐52. [DOI] [PubMed] [Google Scholar]
McQuay 1993 {published data only}
- McQuay HJ, Carroll D, Glynn CJ. Dose‐response for analgesic effect of amitriptyline in chronic pain. Anaesthesia 1993;48(4):281‐5. [DOI] [PubMed] [Google Scholar]
Mendel 1986 {published data only}
- Mendel CM, Klein RF, Chappell DA, Dere WH, Gertz BJ, Karam JH. A trial of amitriptypline and fluphenazine in the treatment of painful diabetic neuropathy. JAMA 1986;255(5):637‐9. [PubMed] [Google Scholar]
Minotti 1998 {published data only}
- Minotti V, Angelis V, Righetti E, et al. Double‐blind evaluation of short‐term analgesic efficacy of orally administered diclofenac, diclofenac plus codeine, and diclofenac plus imipramine in chronic cancer pain. Pain 1998;74(2):133‐7. [DOI] [PubMed] [Google Scholar]
Onghena 1993 {published data only}
- Onghena P, Cuyper H, Houdenhove B, Verstraeten D. Mianserin and chronic pain: a double‐blind placebo‐controlled process and outcome study. Acta Psychiatrica Scandanavica 1993;88(3):198‐204. [DOI] [PubMed] [Google Scholar]
Pilowsky 1990 {published data only}
- Pilowsky I, Barrow CG. A controlled study of psychotherapy and amitriptyline used individually and in combination in the treatment of chronic intractable, 'psychogenic' pain. Pain 1990;40(1):3‐19. [DOI] [PubMed] [Google Scholar]
Pilowsky 1995 {published data only}
- Pilowsky I, Spence N, Rounsefell B, Forsten C, Soda J. Out‐patient cognitive‐behavioural therapy with amitriptyline for chronic non‐malignant pain: a comparative study with 6‐month follow‐up. Pain 1995;60(1):49‐54. [DOI] [PubMed] [Google Scholar]
Plesh 2000 {published data only}
- Plesh O, Curtis D, Levine J, McCall Jnr D. Amitriptyline treatment of chronic pain in patients with temporomandibular disorders. Journal of Oral Rehabilitation 2000;27:834‐41. [DOI] [PubMed] [Google Scholar]
Raftery 1979 {published data only}
- Raftery H. The management of post herpetic pain using sodium valproate and amitriptyline. Journal of the Irish Medical Association 1979;72(9):399‐401. [PubMed] [Google Scholar]
Rawn 2000 {published data only}
- Rawn T, Papoushek C, Evans MF. Gabapentin or amitriptyline for painful diabetic neuropathy?. Canadian Family Physician 2000;46:2215‐7. [PMC free article] [PubMed] [Google Scholar]
Semenchuk 2000 {published data only}
- Semenchuk MR, Davis B. Efficacy of sustained‐release bupropion neuropathic pain: An open‐label study. The Clinical Journal of Pain 2000;16:6‐11. [DOI] [PubMed] [Google Scholar]
Sindrup 1990c {published data only}
- Sindrup SH, Gram LF, Skjold T, Frøland A, Beck‐Nielsen H. Concentration‐ response relationship in imipramine treatment of diabetic neuropathy symptoms. Clinical Pharmacology and Therapeutics 1990;47(4):509‐15. [DOI] [PubMed] [Google Scholar]
Sindrup 1991 {published data only}
- Sindrup SH, Grodum E, Gram LF, Beck‐Nielsen H. Concentration‐response relationship in paroxetine treatment of diabetic neuropathy symptoms: a patient‐blinded dose‐escalation study. Therapeutic Drug Monitoring 1991;13(5):408‐14. [DOI] [PubMed] [Google Scholar]
Sindrup 1992c {published data only}
- Sindrup SH, Bach FW, Gram LF. Plasma beta‐endorphin is not affected by treatment with imipramine or paroxetine in patients with diabetic neuropathy symptoms. Clinical Journal of Pain 1992;8(2):145‐8. [DOI] [PubMed] [Google Scholar]
Standford 1992 {published data only}
- Sandford PR, Lindblom LB, Haddox JD. Amitriptyline and carbamazepine in the treatment of dysesthetic pain in spinal cord injury. Archives of Physical Medicine and Rehabilitation 1992;73(3):300‐1. [PubMed] [Google Scholar]
Stockstill 1989 {published data only}
- Stockstill JW, McCall WD, Gross AJ, Piniewski B. The effect of L‐tryptophan supplementation and dietary instruction on chronic myofascial pain. Journal of the American Dental Association 1989;118(4):457‐60. [DOI] [PubMed] [Google Scholar]
Takeda 1988 {published data only}
- Takeda Y, Kobayashi H, Suzuki K, Sei Y, Nishio K, Ishizaki H. Analgesic effects of clomipramine (Anafranil) on postherpetic neuralgia. Skin Research 1988;30:33‐6. [Google Scholar]
Van Houdenhove 1992 {published data only}
- Houdenhove B, Verstraeten D, Onghena P, DeCuyper H. Chronic idiopathic pain, minanserin and 'masked depression'. Psychotherapy and Pyschosomatics 1992;58:45‐53. [DOI] [PubMed] [Google Scholar]
Van Kempen 1992 {published data only}
- Kempen GMJ, Zitman FG, Linssen ACG, Edelbroek PM. Biochemical measures in patients with a somatoform pain disorder, before, during and after treatment with amitriptyline with or without fulpentixol. Biological Psychiatry 1992;31:670‐80. [DOI] [PubMed] [Google Scholar]
Vidal 2004 {published data only}
- Vidal M, Martinez‐Fernandez E, Martinez‐ Vazquez de Castro J, et al. Effectiveness of gabapentine and amitriptyline in the diabetic neuropathy pain. Revista de la Sociedad Espanola del Dolor 2004;11(5):292‐305. [Google Scholar]
von Knorring 1979 {published data only}
- Knorring L, Johansson F. Visual evoked potentials as a predictor of reported side effects during a trial of zimelidine versus placebo in chronic pain patients. Psychiatry Research 1979;1(31):225‐30. [DOI] [PubMed] [Google Scholar]
von Knorring 1980 {published data only}
- Knorring L, Johansson F. Changes in the augmenter‐reducer tendency and in pain measures as a result of treatment with a serotonin‐reuptake‐inhibitor ‐ Zimelidine. Neuropsychobiology 1980;6:313‐8. [DOI] [PubMed] [Google Scholar]
Watson 1985 {published data only}
- Watson CPN, Evans RJ. A comparative trial of amitriptyline and zimelidine in post‐herpetic neuralgia. Pain 1985;23:387‐94. [DOI] [PubMed] [Google Scholar]
Young 1985 {published data only}
- Young RJ, Clarke BF. Pain relief in diabetic neuropathy: The effectiveness of imipramine and related drugs. Diabetic Medicine 1985;2:363‐6. [DOI] [PubMed] [Google Scholar]
Zitman 1990 {published data only}
- Zitman FG, Linssen ACG, Edelbrock PM, Stijnen T. Low dose amitriptyline in chronic pain: the gain is modest. Pain 1990;42:35‐42. [DOI] [PubMed] [Google Scholar]
Zitman 1991 {published data only}
- Zitman FG, Linssen AC, Edelbroek PM, Kempen GM. Does addition of low‐dose flupentixol enhance the analgetic effects of low‐dose amitriptyline in somatoform pain disorder?. Pain 1991;47(1):25‐30. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Paladini 1987 {published data only}
- Paladini VA, Batiigelli D, Antonaglia V, Benvegnu M, Longo A, et al. Doxepin in pain caused by peripheral neuropathy [La doxopine nel dolore da neuropatia periferica]. Minerva Anestesiol 1987;53:413‐8. [PubMed] [Google Scholar]
Additional references
BNF 2006
- British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary Number 51. http://www.bnf.org March 2006.
Collins 2000
- Collins SL, Moore RA, McQuay HJ, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. Journal of Pain and Symptom Management 2000;20(6):449‐58. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feighner 1999
- Feighner JP. Mechanism of action of antidepressant medications. Journal of Clinical Psychiatry 1999;60(Suppl 4):4‐11. [PubMed] [Google Scholar]
Glassman 1993
- Glassman AH, Preud'homme XA. Review of the cardiovascular effects of heterocyclic antidepressants. Journal of Clinical Psychiatry 1993;54(Suppl):16‐22. [PubMed] [Google Scholar]
Glassman 1998
- Glassman AH. Cardiovascular effects of antidepressant drugs: updated. Journal of Clinical Psychiatry 1998;59(Suppl 15):13‐8. [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. In: The Cochrane Library. John Wiley & Sons: Chichester, UK, Issue 4, 2006. [Google Scholar]
Jackson 2004
- Jackson JL, Berbano EP, DeZee KJ, Diemer MM, Mikita JA, O'Malley PG, et al. Tricyclic antidepressants for the prevention of migraine and tension‐type headache. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD005036.] [DOI] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Magni 1991
- Magni G. The use of antidepressants in the treatment of chronic pain: a review of the current evidence. Drugs 1991;42:730‐48. [DOI] [PubMed] [Google Scholar]
Martindale 2004
- Martindale. The Complete Drug Reference. 4th Edition. London: Pharmaceutical Press, 2004. [Google Scholar]
Max 1995
- Max MB. Thirteen consecutive well‐designed randomized trials show that antidepressants reduce pain in diabetic neuropathy and postherpetic neuralgia. Pain Forum 1995;4:348‐53. [Google Scholar]
McCleane 2003
- McCleane G. Pharmacological management of neuropathic pain. CNS Drugs 2003;14(14):1031‐43. [DOI] [PubMed] [Google Scholar]
McQuay 1995
- McQuay H, Carroll D, Jadad AR, Wiffen P, Moore A. Anticonvulsant drugs for management of pain: a systematic review. BMJ 1995;311:1047‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2007
- McQuay HJ, Smith LA, Moore RA. Chronic pain. Health Needs Assessment. Oxford: Radcliffe Publishing Ltd, 2007:519‐99. [Google Scholar]
MIMS 2002
- Anon. Monthly Index of Medical Specialities. London: Haymarket Medical Publications, May 2002. [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Onghena 1992
- Onghena P, Houdenhove B. Antidepressant‐induced analgesia in chronic non‐malignant pain: a meta‐analysis of 39 placebo‐controlled studies. Pain 1992;49:205‐20. [DOI] [PubMed] [Google Scholar]
Peretti 2000
- Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants versus selective serotonine reuptake inhibitors. Acta Psychiatrica Scandanavica 2000;403(Suppl):17‐25. [DOI] [PubMed] [Google Scholar]
Tremont‐Lukats 2000
- Tremont‐Lukats IW, Megeff C, Backonja MM. Anticonvulsants for neuropathic pain syndromes. Drugs 2000;60(5):1029‐52. [DOI] [PubMed] [Google Scholar]
Wiffen 2001
- Wiffen P, Collins S, McQuay H, Carroll D, Jadad A, Moore RA. Anticonvulsant drugs for acute and chronic pain. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001133.pub2] [DOI] [Google Scholar]
Woolf 1999
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 1999;353:1959‐64. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
McQuay 1996
- McQuay HJ, Tramer M, Nye BA, Carroll D, Wiffen PJ, Moore RA. A systematic review of antidepressants in neuropathic pain. Pain 1996;68:217‐27. [DOI] [PubMed] [Google Scholar]


