Abstract
Introduction
Expression and certain SNPs of interferon lambda 3 and 4 (IFNL3 and 4) have been associated with variable outcomes in COVID-19 patients in different regions, suggesting population-specific differences in the disease outcome. This study examined the association of INFL3 and INFL4 SNPs (rs12979860 and rs368234815, respectively) and nasopharyngeal expression with COVID-19 disease severity in Pakistani patients.
Methods
For this study, 117 retrospectively collected nasopharyngeal swab samples were used from individuals with mild and severe COVID-19 disease. qPCR assays were used to determine the viral loads and mRNA expression of IFNL3 and 4 through the Ct and delta Ct methods, respectively. Due to funding limitations, only one SNP each in INFL3 and INFL4 (found to be most significant through literature search) was analyzed using tetra-arm PCR and RFLP-PCR strategies, respectively. The Mann–Whitney U-test was applied to evaluate the statistical differences in the expression of IFNL3/4 genes in the mild and severe groups, while for SNPs, a Chi-square test was employed. A multivariate Cox regression test was performed to assess the relationship of different variables with COVID-19 severity.
Results
Comparative analysis of SNPs between mild and severe groups showed only the difference in SNP of the IFNL4 gene to be statistically significant (p = 0.001). Similarly, nasopharyngeal expression of IFNL3 and IFNL4 genes, respectively, was found to be 3.48-fold less and 3.48-fold higher in the severe group as compared to the mild group. Multivariate analysis revealed SNP in the IFNL4 gene and age to have a significant association with COVID-19 severity.
Conclusion
Despite the small sample size, IFNL4 gene SNP and patient age were associated with COVID-19 severity. Age, IFNL3/IFNL4 mRNA expression in the nasopharyngeal milieu, and the presence of SNP in the IFNL4 (rs368234815) gene in COVID-19 patients may be biomarkers for infection severity and help improve SARS-CoV-2 infection management.
Keywords: COVID-19 severity, single nucleotide polymorphisms, interferon lambda, IFNL4, IFNL3
Introduction
Infection from SARS-CoV-2 may lead to the development of severe pneumonia and its sequelae.1 Fever, dry cough, tiredness, etc., are the classical clinical features observed in patients with this infection, and these symptoms are very similar to the SARS-CoV infection previously discovered.1
The overexpression of pro-inflammatory cytokines (eg interleukins-6 and 12, interferon- γ, interleukin 1B, TNF-α, IP10, MIP1A, GCSF, and MCP1) in serum reportedly has a strong correlation with COVID-19 disease severity, including multiple organ systems failure among patients with SARS-CoV-2 infection.2,3 Interferon lambda (type III interferon) plays an important inhibitory role as part of mucosal immunity against viruses.4 The interferon lambda is expressed in myeloid as well as epithelial cells and is considered to work similarly to type I interferons. There is evidence that interferon lambda is involved not only in innate immunity but also in adaptive immunity, eg, the transduction route of interferon lambda is similar to that of type I interferons.4,5 These cytokines directly enhance the antiviral capability of the mucosal barrier by acting on the epithelial cells and immune cells.6 Furthermore, interferon lambda has been shown to inhibit the SARS-CoV-2 spread, thereby, limiting the transmission of the virus and lowering the risk of damage to the various organ systems.7 In a subset analysis, higher levels of IFNL3 were associated with the milder forms of COVID-19.8,9 Similarly, IFNL4 has been shown to have anti-viral activity against MERS-CoV and, therefore, may also play an important role in conferring protection against SARS-CoV-2 infections.10–12
The response emanating from the interferons is affected by the host as well as viral factors.13 These factors primarily include the viral load and the age of the patient. For instance, a low viral load suggests that the interferon response is adequate, and therefore, the viral clearance is effective and timely. Similarly, a high viral load indicates that there might be a delay in the production of the interferons resulting in inflammation-mediated damage to the lungs.14
The single nucleotide polymorphisms (SNPs) in IFNL3 and IFNL4 are distributed differentially on a global level and often reveal differences that are observed in the clinical outcomes against certain viral infections in different ethnicities.15,16 For example, IFNL3 and IFNL4 levels can be affected by the presence of different SNPs (INFL4 rs368234815 TT/∆G and IFNL3 rs1297860 C/T) of INFL3 and INFL4 in COVID-19 patients.17 IFNL4 SNPs, in particular, have also been associated with viral clearance and/or disease severity. For example, a study conducted on the Spanish population revealed a correlation between the IFNL4 polymorphism and the COVID-19 disease severity, suggesting that variations in the IFN4 gene may contribute to an individual’s susceptibility to the disease by interfering with the expression of IFNL4 and its antiviral properties.18 The frequency and distribution of SNPs in IFN lambda genes can vary between different populations, which often results in different disease outcomes in different ethnic groups. For instance, SNP rs12979860 associated with hepatitis C virus clearance is highest in East Asians (93%) and lowest in Africans (23%); therefore, such differences show the susceptibility profile of the populations and might also affect the response to the treatment in between populations.19
In Pakistan, more than 1.57 million COVID-19 cases and 30,000 COVID-19-related deaths have been reported by the time of writing of this manuscript.20 In our recent study, we reported a strong correlation between the nasopharyngeal expression of the cytokines (IL-1, IL-2, IL-4, IL-6, IL-10, IFN-γ, TGF-β1, and TNF-α) and severe COVID-19 disease.21 However, the role/association of IFNL3 and IFNL4 has not been explored in the Pakistani population. Therefore, in this study, we aimed to determine the association of IFNL3 and IFNL4 polymorphism and nasopharyngeal expression with COVID-19 disease severity in Pakistani patients.
Methodology
Study Design and Sample Collection
This was an analytical cross-sectional study, which is based on 117 retrospectively collected nasopharyngeal samples from patients with confirmed infection of the SARS-CoV-2 virus by RT-PCR. The collected samples were stored in a virus transport medium. As per the COVID-19 disease severity classification system, only the nasopharyngeal samples from patients with mild and severe COVID-19 disease were included in this study.21,22 For the mild group, the samples were collected within three days of the onset of symptoms, while for the severe group, the samples were collected within three days from the time of admission. These samples were collected from the biorepositories of Aga Khan University and the Cancer Foundation Hospital, Karachi, Pakistan.21 The samples and data about the age, gender, and disease severity were collected after obtaining written informed consent from the patients. The study was approved by the Ethics Review Committee of The Aga Khan University & Hospital (2022-5456-20388) and complies with the Declaration of Helsinki.
RNA Extraction, cDNA Synthesis, and qPCR for Estimation of Viral Loads and Cytokines Gene Expression
From each nasopharyngeal sample, viral RNA was extracted using the QIAamp viral RNA kit (Qiagen, Germany) as per the manufacturer’s instructions.21 The viral RNA samples were stored at −80°C till further use. To synthesize complementary DNA (cDNA) from each sample, 1 μg RNA in a 20 μL reaction was made using One Script Plus cDNA Synthesis Kit (CAT # G236; ABM) following the instructions provided by the manufacturer.23 The prepared cDNA samples were kept on ice or at −20°C until further work.
To determine the viral load COVID-19 genesig® Real-Time PCR assay (Primer design) was used. A 20μL triplex reaction mix containing 3ul of eluted RNA, and 7μL of the master mix along with 0.5ul of desired primers and 9ul of nuclease-free water was subjected to the following thermocycling conditions: 55°C for 10 min, 95°C for 2 min followed by 45 cycles of 95°C for 10s, 60°C for the 60s. Ct values of Internal control and Target (ORF1ab) genes were measured on Hex and FAM channels, respectively, on Rotor Gene™ 3000 (Qiagen, USA). Viral load was plotted as Ct values.
To determine the expression of IFNL3 and IFNL4 using the synthesized cDNA template, the following primer sets were used: IFN3-FOR GAGAGCAAGAGGAGGGAAGGAA, IFN3-Rev: GTGTGCCATTAGCCAGTCAGAT, IFN4-FOR: CGGCCTGCCTTGAGCTG, IFN4-REV: GGGTTTGTGACGCCTCTTCT. Beta Actin gene was used as an internal control. Each reaction mix contained 2μL of cDNA template with 4ul of BlasTaq 2X qPCR Mastermix (Cat # G891; ABM) and 0.5μL of their respective reverse and forward primer. The following thermocycling conditions were used for each reaction mix: 10 minutes to 95°C, and then 40 cycles of 95°C lasting for 10 seconds, and 60°C for 60 seconds. The delta Ct method was used to evaluate the expression of both genes in mild/severe groups, while the 2−ΔΔCt method was used to determine the fold change.24
SNP Genotyping
Due to funding limitations, only one single nucleotide polymorphism (SNP) each in INFL3 and INFL4 (found to be most significant through literature search) was analyzed. For the detection of SNPs in the interferon lambda 3 gene (rs12979860), the Tetra arms PCR was performed in two rounds using HotStart DNA Polymerase Master Mix (ABM Cat. No. G595). In the first round, the cDNA templates were amplified using the following primer pairs: forward outer primer: GGAGCTCCCCGAAGGCGT, reverse outer primer: CGAGTGTCTGGGCCGCAG, with the following conditions: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 60 seconds, followed by 72°C for 5 minutes. For the second round, amplified PCR products of round one were used as a template with the following primers, forward inner primer: GAGCTCCCCGAAGGCGC, reverse inner primer: GCTGCCAAGGCGCTGAGG with the same condition as above. The PCR products of the second round were visualized on 3% agarose gel showing amplified DNA of the size 433bp and 326bp.
To observe SNP presence in the interferon lambda 4 gene (rs368234815), a Restriction Fragment Length Polymorphism (RFLP) was performed using HotStart DNA Polymerase Master Mix (ABM Cat. No. G595) and the following primer set: forward primer: GACGCAGGACCCCTTGGGACAGGA, reverse primer: TCTGGGCCGCAGTGGCCGCGAGG. The RFLP PCR conditions were as follows: 94°C for 5 minutes, 40 cycles of 94°C for 20 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, followed by 72°C for 5 minutes. For RFLP analysis, PCR products were digested with 10 units of MspAI restriction endonuclease (ThermoFisher Scientific) for more than 2 hours in a thermocycler at 37°C. The digested fragments were visualized on 3% agarose gel showing bands at 175bp and 50bp.
Statistical Analysis
Shapiro–Wilk test was employed to test for checking the normality of the data. Based on data normality, the descriptive statistics for quantitative variables such as age, viral load, IFNL3, and IFNL4 expression were reported as mean ± SD or median (IQR). An independent t-test/Mann Whitney U-test was used to compare the quantitative variables between mild and severe cases of COVID-19 as appropriate. The categorical variables, ie, SNPs of IFNL3 and IFNL4, and gender were reported as frequency and percentages and were assessed by Chi-squared test or Fisher’s exact test as appropriate.
Unadjusted and adjusted prevalence ratios (PR) with their 95% Confidence intervals (CI) were reported by using the Cox Proportional algorithm. Variables exhibiting a p-value of 0.25 or less were included in the multivariate model, while the variables in multivariate analysis with a p-value <0.05 were considered significant. Plausible interactions were assessed to analyze the association between the expression and SNPs of the IFNL3 (rs12979860) and IFNL4 genes (rs368234815) with the disease severity.25 The interaction terms were tested at a 10% level of significance. All analyses were performed using STATA software v16.
Results
Patient Characteristics
Out of 117 COVID-19-positive patients confirmed by RT-PCR test for SARS-CoV-2, 70 and 47 patients, respectively, exhibited mild and severe symptoms as per the WHO COVID-19 guidelines.21,26,27 The median age of the patients in the mild group was significantly lower (45 years) as compared to patients with severe disease (60 years; p < 0.001). The mild group consisted of 27 females (38.57%) and 43 males (61.43%), while the severe group comprised 15 females (31.91%) and 32 males (68.09%); the patient’s gender showed no significant association (p = 0.462) with the severity of the COVID-19 disease (Supplementary Table 1).
The median viral Ct values in the mild and severe groups were 28 and 28.33, respectively (IQR mild: 9; IQR severe: 4.87). No statistical difference (p = 0.6905) was found when the association between the viral load and the severity of the disease was analyzed.
Analysis of Expression of Interferon Lambda 3 and Interferon Lambda 4 Genes
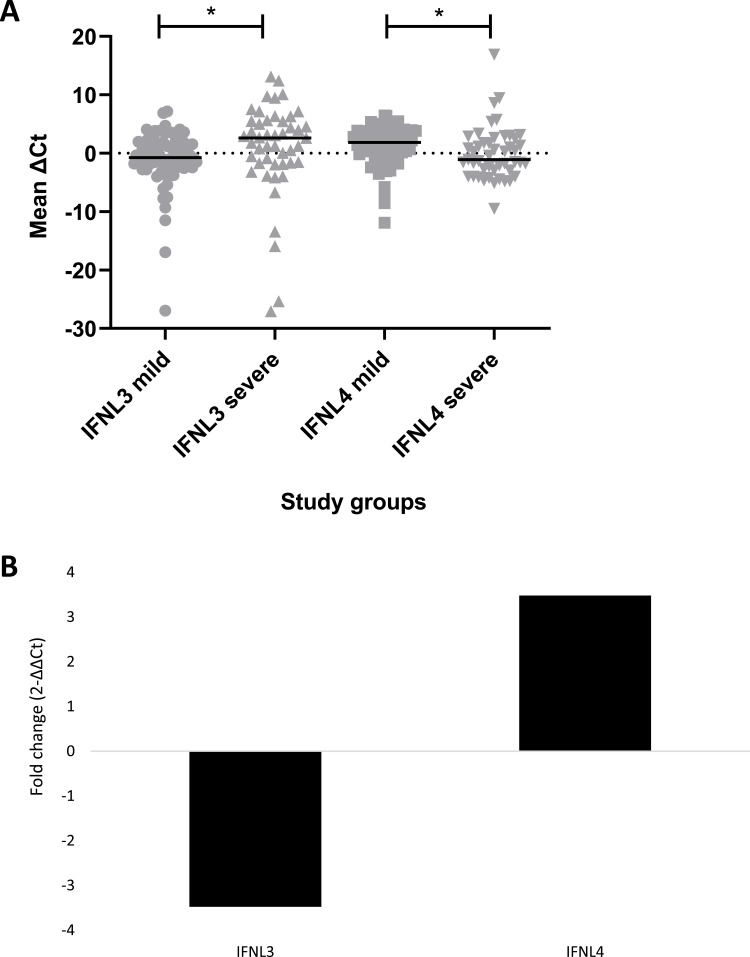
Comparison of mean expression of IFNL3 and IFNL4 between the mild and severe groups showed a significant difference (p = 0.0069) in the mRNA expression (Figure 1A), where the expression of IFNL3 was found to be 3.48-fold lower, while the expression of IFNL4 was found to be 3.48-fold higher in the severe group as compared to the mild group (Figure 1B).
Figure 1.
Gene expression analysis in mild versus severe group.
Notes: (A) Scatter plot showing individual (grey color symbols) and mean ΔCt values (black line) of interferon lambda 3 (IFNL3) and interferon lambda 4 (IFNL4) genes in severe and mild groups, where * indicates significant p-values. (B) Log2 normalized fold change (2−ΔΔCt) in IFNL3 and IFNL4 cytokine expression in severe groups relative to the mild group.
Single Nucleotide Polymorphisms in Interferon Lambda 3 (rs12979860) and Interferon Lambda 4 (rs368234815)
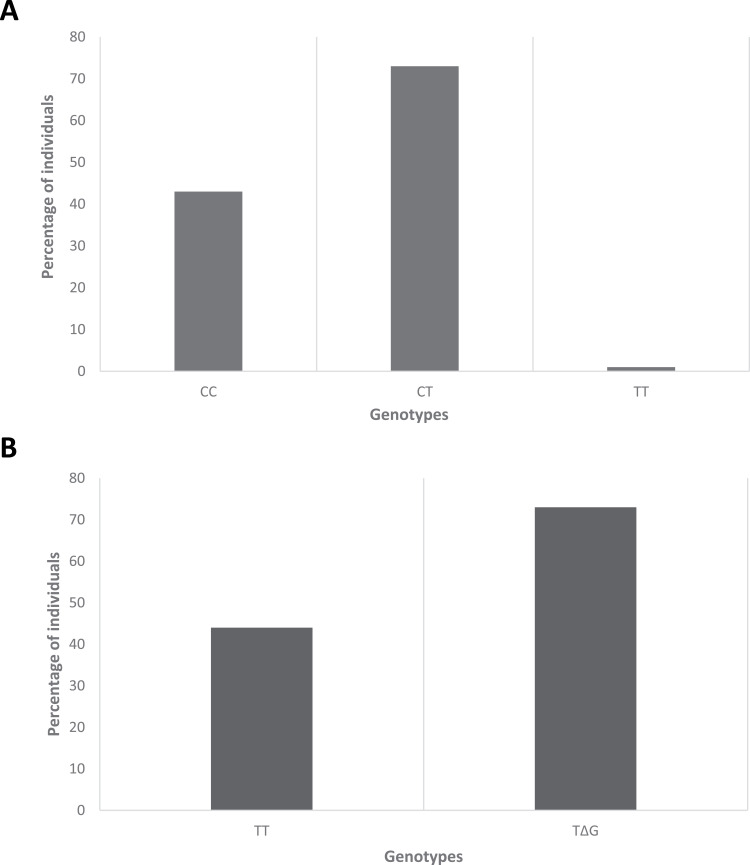
The descriptive statistics of SNP (rs12979860) in IFNL3 showed that 43 (36.75%) individuals had CC genotype, 1 (0.85%) had TT genotype, and 73 (62.39%) had CT genotype (Figure 2A). Similarly, the descriptive statistics of SNP (rs368234815) in IFNL4 showed that 44 (37.61%) individuals had TT/TT genotype, while 73 (62.39%) patients had TT/ΔG genotype (Figure 2B). Tetra ARMs-PCR and RFLP PCR product gel images were obtained in 3% agarose gel (Supplementary Figure 1A and B).
Figure 2.
Distribution of single nucleotide polymorphisms in IFNL3 and IFNL4 genes.
Notes: Distribution of single nucleotide polymorphisms (SNPs) of (A) interferon lambda 3 and (B) interferon lambda 4 genes among COVID-19 patients. The Y-axis shows the percentage of individuals with SNPs, while the X-axis shows the genotypes.
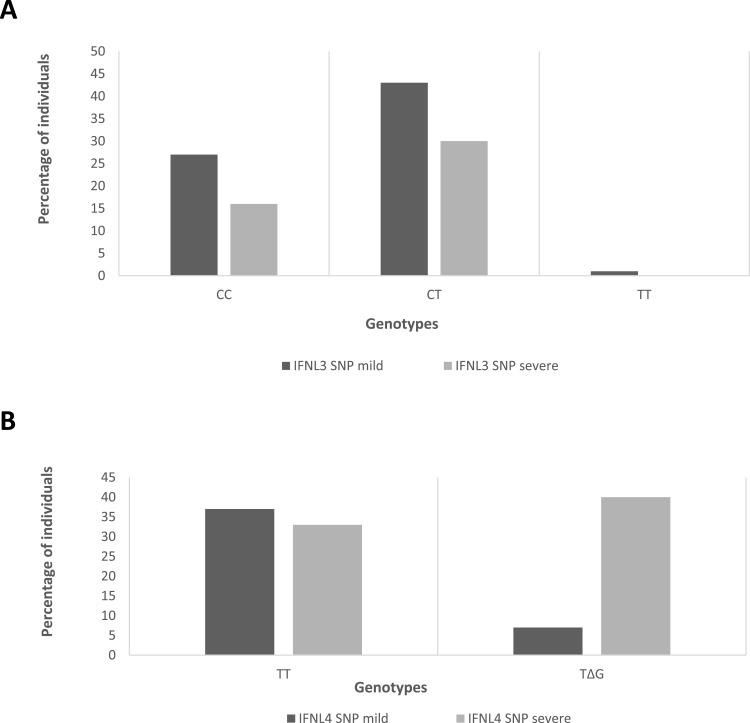
Comparative analysis of IFNL3 gene SNP (rs12979860) in the mild group showed that 27 (38.57%) individuals had the CC genotype, while 43 (61.43%) had the CT genotype. In the severe group, 16 (34.04%) individuals had the CC genotype, 1 (2.12%) had the TT genotype, and 30 (63.83%) had the CT genotype (Figure 3A). No statistically significant (p = 0.793) association was observed between the IFNL3 SNP and the severity of the disease (Table 1).
Figure 3.
Distribution of single nucleotide polymorphisms in IFNL3 and IFNL4 genes in mild and severe groups.
Notes: Distribution of single nucleotide polymorphisms (SNPs) of (A) interferon lambda 3 and (B) interferon lambda 4 genes among mild and severe cases of COVID-19 patients. The Y-axis shows the percentage of individuals with SNPs, while the X-axis shows the genotypes.
Table 1.
Comparison of Single Nucleotide Polymorphisms
| Variable | Mild | Severe | p-value |
|---|---|---|---|
| N (%) | N (%) | ||
| Single Nucleotide Polymorphisms in IFNL3 | |||
| CC/CC alleles | 27 (38.57) | 17 (36.17) | |
| CC/TT alleles | 43 (61.43) | 30 (63.83) | 0.793 |
| Single Nucleotide Polymorphisms in IFNL4 | |||
| TT/TT alleles | 37 (52.86) | 7 (14.89) | |
| TT/ ΔG alleles | 33 (47.14) | 40 (85.11) | <0.001** |
Notes: The table shows a comparison of SNPs in interferon lambda 3 (IFNL3) and interferon lambda 4 (IFNL4) among participants with mild versus severe SARS-CoV-2 Infections, where ** indicates a p-value <0.05, calculated by Chi-square test.
The analysis of IFNL4 SNP (rs368234815) showed that in the mild group, 37 (52.86%) individuals had TT/TT genotype, while 33 (47.14%) had TT/ΔG genotype. In the severe group, only 7 (14.89%) individuals had the TT/TT genotype, while 40 (85.11%) had the TT/ΔG genotype (Figure 3B). We found a significant association (p = 0.001) between the IFNL4 SNPs and the severity of the COVID-19 disease (Table 1).
Univariate & Multivariate Analyses
In the univariate analysis, where the severity of the disease was kept as the outcome variable, only age and IFNL4 SNP (rs368234815) were found to be significant (p-value <0.25). The univariate analysis also suggested that with every one-year increase in age, the chances of getting severe COVID-19 infection increased by 2%. Similarly, the univariate analysis also showed the probability of severe COVID-19 infection to be 3.44 times higher among patients with polymorphic genotypes as compared to those with wild-type alleles (95% confidence interval: 1.54 and 7.69) (Supplementary Table 2A).
Multivariate analysis of the variables also showed that the probability of severe COVID-19 infection was 3.34 times higher among individuals with IFNL4 SNPs as compared to those with wild-type alleles (95% CI: 1.49–7.47). Moreover, the model suggested that with every 1-year increase in age, the probability of severe COVID-19 infection was increased by 2% when adjusted for the SNPs in the IFNL4 gene (Supplementary Table 2B).
Discussion
In this study, we aimed to analyze the nasopharyngeal expression of IFNL3 and IFNL4 genes as well as examine the association of SNPs in these two genes with COVID-19 disease severity in the Pakistani population.
Comparison of expression of IFNL3 and IFNL4 between the mild and severe groups showed a statistically significant 3.48-fold less expression of the IFNL3 gene and 3.48-fold higher expression of IFNL4 in patients with severe COVID-19 infection as compared to the patients with mild COVID-19 infection. This finding is consistent with an earlier study, which reported that the mild cases of COVID-19 infection were found to have a higher expression of the IFNL3 gene, while the patients who had poor prognosis often had a reduced IFNL3 gene expression profile.28 However, it has also been shown that excessive production of interferon lambda in the lungs, in response to the RNA of viruses, often results in damage to the respiratory epithelium and increases the susceptibility of the host toward the acquisition of secondary bacterial infections.29,30 These findings may be important since the interferon lambdas have potent antiviral activity; however, SARS-CoV-2 mediated dysregulated induction of interferon lambda may lead to hyperinflammation and the severe form of COVID-19 disease.7,31
In COVID-19 patients, INF lambda is induced via activating RIG-I/MDA5 genes, and INF lambda 3 is activated by TLR7 and NF-kB pathways. The exact role of INF lambda in COVID-19 disease has yet to be established. On the one hand, the studies suggest that INF lambda has anti-SARS-CoV2 effects under in vitro conditions,32 while, on the other hand, the INF lambda reportedly adversely affects the repair process in the lungs following the COVID-19-associated tissue damage.30 Similarly, an early INF lambda response is associated with mild COVID-19 disease and the delay in INF lambda induction is associated with severe COVID-19 disease, as it allows for the virus to undergo a quicker replication process, which leads to the detection of viral RNA by pattern recognition receptors, resulting in the dysregulated expression of proinflammatory cytokines which may lead in extensive tissue damage.33 In addition, it has been reported that the INF lambda activity associated with mucosal lining is impaired in severe COVID-19 patients.34 The evidence indicates that there is substantial variability amongst patients related to INF response in COVID19 patients.
In our study, the IFNL3 SNP rs12979860 was not found to have an association with COVID-19 infection severity. However, a study conducted in Egypt showed that this variant was found to be associated with severe SARS-CoV-2 infections,35 suggesting differences in population immunogenetics may play a role in antiviral response. Previously, it was believed that CC substitution (rs12979860) in IFNL3 and TT/TT substitution (rs368234815) in IFNL4 genes conferred a higher resistance towards infection as compared to the other gene alleles.36 It has also been demonstrated in another study that people with IFNL3 TT genotype (rs12979860) and IFNL4 ∆G/∆G genotypes (rs368234815) lead to a lower viral clearance.17,33,37,38
We found the SNPs in the IFNL4 gene (rs368234815) to be statistically significantly different in the mild versus severe group, which was confirmed both in the univariate and multivariate analysis, where the unadjusted prevalence ratio (3.43) and adjusted prevalence ratio (3.33) were found to be in close agreement (Supplementary Tables 2A and B). This points to the possibility that people who have SNPs (rs368234815) in the IFNL4 gene may be more prone to getting severe COVID-19 disease in the Pakistani population, further creating the possibility that IFNL4 SNPs may serve as an indicator for severe infection. Careful monitoring/management of the patients with this SNP, from the beginning of the infection, might improve the clinical outcomes for the patients. This result is also consistent with the findings of a study conducted in Italy, which also concluded that SNPs (rs368234815) TT/ΔG in IFNL4 also lead to decreased viral clearance in COVID-19 infections.17 This finding is further confirmed by an Iranian study, which concluded that the severity of COVID-19 infection is associated with INFL4 SNP (rs368234815).36 These findings seem to provide the rationale for the beneficial use of JAK inhibitors such as Tofacitinib in severe COVID-19 patients since INFL4 activates the JAK-STAT signaling pathways which triggers hyperinflammation resulting in increased tissue damage and pneumonia in COVID-19 patients.39,40
We also found that the age of the patients was significantly associated with SARS-CoV-2 infection, which has been consistently reported in the literature.41 The severity of COVID-19 infection depends on the age as younger age groups might have mechanisms that are often protective, whereas since older patients tend to rely more on the activation of the memory T cells for recognition, which therefore might result in the decompensated immune response with damage to the tissues.42
In addition, this study early measurement (at the time of admission/diagnosis) of mRNA expression of IFNL3 and INFL4 in the nasopharyngeal milieu may act as a non-invasive method to predict the severity of the disease and may complement the more widely used methods in clinical laboratory, such as assessment of coagulation profile (LDH, D-dimers), biochemical and inflammatory markers (creatinine, CRP, ferritin), etc.43,44
We identify certain limitations of this study. Firstly, despite the low sample size for genetic studies,SNPs in the IFNL4 gene and the age of the patients were found to have a significant association with COVID-19 severity. Secondly, the information about co-morbid conditions in the patients was not available; therefore, such information was not taken into consideration as other risk factors that might affect the clinical outcome of COVID-19 disease. Thirdly, in this study, the gene expression of cytokines was conducted on the nasopharyngeal swab samples, and the expression in serum was not analyzed, due to unavailability. Finally, since only one (most important) SNPs in IFNL3 and IFNL4 genes were examined, due to funding limitations, there might be other genetic SNPs associated with INFL3 and INFL4 genes that might be associated with the severity of the disease; however, SNPs in the IFNL4 gene were found to have a significant association with COVID-19 severity.
In conclusion, the differences in nasopharyngeal expression of interferon lambda 3 (downregulated) and interferon lambda 4 (upregulated) in patients with a higher severity index of the disease may suggest a crucial role of the gene expression of these cytokines in determining the severity of COVID-19 disease. Furthermore, we found a statistically significant association between INFL4 SNPs and the severity of the disease, indicating a possible genetic predisposition to acquire the severe form of the disease in these patients, thereby, suggesting a possible role of these SNPs with the severity of the disease in this patient population.
Acknowledgment
We are grateful to Dr Abid Jamal, Cancer Foundation Hospital, Karachi, Pakistan, for providing access to the samples.
Funding Statement
The funding support for this project was provided by the Aga Khan University, Department of Biological and Biomedical Sciences, The Akbarali Habib Bandeali Endowment funds awarded to Syed Hani Abidi, and Nazarbayev University (grant # NU 021220CRP0822). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peiris JSM, Chu C-M, Cheng V-C-C, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong C, Lam C, Wu A, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye L, Schnepf D, Staeheli P. Interferon-λ orchestrates innate and adaptive mucosal immune responses. Nat Rev Immunol. 2019;19(10):614–625. doi: 10.1038/s41577-019-0182-z [DOI] [PubMed] [Google Scholar]
- 5.Kelly A, Fahey R, Fletcher JM, et al. CD141+ myeloid dendritic cells are enriched in healthy human liver. J Hepatol. 2014;60(1):135–142. doi: 10.1016/j.jhep.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 6.Boulant S, Forero A, Santer DM, Broggi A. Type III interferons: emerging roles beyond antiviral barrier defense. Front Immunol. 2022;13:1030812. doi: 10.3389/fimmu.2022.1030812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreakos E, Tsiodras S. COVID‐19: lambda interferon against viral load and hyperinflammation. EMBO Mol Med. 2020;12(6):e12465. doi: 10.15252/emmm.202012465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanoni I. Interfering with SARS-CoV-2: are interferons friends or foes in COVID-19? Curr Opin Virol. 2021;50:119–127. doi: 10.1016/j.coviro.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rand U, Kupke SY, Shkarlet H, et al. Antiviral activity of influenza A virus defective interfering particles against SARS-CoV-2 replication in vitro through stimulation of innate immunity. Cells. 2021;10(7):1756. doi: 10.3390/cells10071756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien TR, Thomas DL, Jackson SS, Prokunina-Olsson L, Donnelly RP, Hartmann R. Weak induction of interferon expression by SARS-CoV-2 supports clinical trials of interferon lambda to treat early COVID-19. Clin Infect Dis. 2020;71(6):1410–1412. doi: 10.1093/cid/ciaa453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamming OJ, Terczyńska‐Dyla E, Vieyres G, et al. Interferon lambda 4 signals via the IFNλ receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J. 2013;32(23):3055–3065. doi: 10.1038/emboj.2013.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorrentino L, Silvestri V, Oliveto G, et al. Distribution of interferon lambda 4 single nucleotide polymorphism rs11322783 genotypes in patients with COVID-19. Microorganisms. 2022;10(2):363. doi: 10.3390/microorganisms10020363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Lewis‐Antes A, Huang J, Balan M, Kotenko S. Regulation of apoptosis by type III interferons. Cell Prolif. 2008;41(6):960–979. doi: 10.1111/j.1365-2184.2008.00558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakadia BM, He F, Souho T, et al. Prevention and treatment of COVID-19: focus on interferons, chloroquine/hydroxychloroquine, azithromycin, and vaccine. Biomed Pharmacother. 2021;133:111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly C, Klenerman P, Barnes E. Interferon lambdas: the next cytokine storm. Gut. 2011;60(9):1284–1293. doi: 10.1136/gut.2010.222976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuura K, Tanaka Y. Host genetic variants influencing the clinical course of hepatitis C virus infection. J Med Virol. 2016;88(2):185–195. doi: 10.1002/jmv.24334 [DOI] [PubMed] [Google Scholar]
- 17.Amodio E, Pipitone RM, Grimaudo S, et al. SARS-CoV-2 viral load, IFNλ polymorphisms and the course of COVID-19: an observational study. J Clin Med. 2020;9(10):3315. doi: 10.3390/jcm9103315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saponi-Cortes JMR, Rivas MD, Calle F, et al. IFNL4 genetic variant can predispose to COVID-19. Sci Rep. 2021;11(1):21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45(2):164–171. doi: 10.1038/ng.2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COVID-19 dashboard [Internet]; 2022. Available from: https://covid19.who.int/. Accessed October 3, 2023.
- 21.Ghulam U, Nazim F, Farooqui N, et al. Analysis of differential gene expression of pro-inflammatory cytokines in the nasopharyngeal milieu of mild & severe COVID-19 cases. PLoS One. 2022;17(12):e0279270. doi: 10.1371/journal.pone.0279270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son K-B, Lee T-J, Hwang S-S. Disease severity classification and COVID-19 outcomes, Republic of Korea. Bull World Health Organ. 2021;99(1):62. doi: 10.2471/BLT.20.257758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed MA, Anwar MF, Ahmed K, et al. Baseline MMP expression in periapical granuloma and its relationship with periapical wound healing after surgical endodontic treatment. BMC Oral Health. 2021;21(1):1–9. doi: 10.1186/s12903-021-01904-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 25.O’Brien TR, Prokunina-Olsson L, Donnelly RP. IFN-λ4: the paradoxical new member of the interferon lambda family. J Interferon Cytokine Res. 2014;34(11):829–838. doi: 10.1089/jir.2013.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Therapeutics and COVID-19: living guideline, 20 November 2020. World Health Organization; 2020. [Google Scholar]
- 27.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sposito B, Broggi A, Pandolfi L, et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell. 2021;184(19):4953–68. e16. doi: 10.1016/j.cell.2021.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broggi A, Ghosh S, Sposito B, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020;369(6504):706–712. doi: 10.1126/science.abc3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369(6504):712–717. doi: 10.1126/science.abc2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akamatsu MA, de Castro JT, Takano CY, Ho PL. Off balance: interferons in COVID-19 lung infections. EBioMedicine. 2021;73:103642. doi: 10.1016/j.ebiom.2021.103642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardoso NP, Mansilla FC, Benedetti E, et al. Bovine interferon lambda is a potent antiviral against SARS-CoV-2 infection in vitro. Front Veter Sci. 2020;7:603622. doi: 10.3389/fvets.2020.603622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowery SA, Sariol A, Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: implications for COVID-19. Cell Host Microbe. 2021;29(7):1052–1062. doi: 10.1016/j.chom.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scagnolari C, Pierangeli A, Frasca F, et al. Differential induction of type I and III interferon genes in the upper respiratory tract of patients with coronavirus disease 2019 (COVID-19). Virus Res. 2021;295:198283. doi: 10.1016/j.virusres.2020.198283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agwa SH, Kamel MM, Elghazaly H, et al. Association between interferon-lambda-3 rs12979860, tll1 rs17047200 and ddr1 rs4618569 variant polymorphisms with the course and outcome of SARS-CoV-2 patients. Genes. 2021;12(6):830. doi: 10.3390/genes12060830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahimi P, Tarharoudi R, Rahimpour A, et al. The association between interferon lambda 3 and 4 gene single-nucleotide polymorphisms and the recovery of COVID-19 patients. Virol J. 2021;18(1):1–7. doi: 10.1186/s12985-021-01692-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahbazi M, Amri Maleh P, Bagherzadeh M, et al. Linkage of lambda interferons in protection against severe COVID-19. J Interferon Cytokine Res. 2021;41(4):149–152. doi: 10.1089/jir.2020.0187 [DOI] [PubMed] [Google Scholar]
- 38.Sorrentino L, Fracella M, Frasca F, et al. Alterations in the expression of IFN lambda, IFN gamma and toll-like receptors in severe COVID-19 patients. Microorganisms. 2023;11(3):689. doi: 10.3390/microorganisms11030689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385(5):406–415. doi: 10.1056/NEJMoa2101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh PK, Lalwani LK, Govindagoudar MB, et al. Tofacitinib associated with reduced intubation rates in the management of severe COVID-19 pneumonia: a preliminary experience. Indian J Crit Care Med. 2021;25(10):1108. doi: 10.5005/jp-journals-10071-23964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim M-H, Salloum S, Wang JY, et al. Type I, II, and III interferon signatures correspond to coronavirus disease 2019 severity. J Infect Dis. 2021;224(5):777–782. doi: 10.1093/infdis/jiab288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27(1):28–33. doi: 10.1038/s41591-020-01202-8 [DOI] [PubMed] [Google Scholar]
- 43.Sarkar A, Sanyal S, Majumdar A, et al. Development of lab score system for predicting COVID-19 patient severity: a retrospective analysis. PLoS One. 2022;17(9):e0273006. doi: 10.1371/journal.pone.0273006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pujani M, Raychaudhuri S, Singh M, et al. An analysis of hematological, coagulation and biochemical markers in COVID-19 disease and their association with clinical severity and mortality: an Indian outlook. Am J Blood Res. 2021;11(6):580. [PMC free article] [PubMed] [Google Scholar]