Abstract
Background/Aims
Transjugular intrahepatic portosystemic shunts (TIPS) in patients with hepatocellular carcinoma (HCC) may improve access to curative therapies, treat portal hypertension (PH)‐related complications without worsening liver function, and increase overall survival. Data on the efficacy and safety of TIPS to treat PH complications in HCC patients, as well as the HCC treatment response, were evaluated.
Methods
Studies reporting efficacy in controlling bleeding/ascites or response to HCC therapy, safety, and survival in patients with HCC and TIPS were searched systematically on PubMed and Embase. An extraction of articles using predefined data fields and quality indicators was used.
Results
We selected 19 studies and found 937 patients treated for ascites/bleeding and 177 evaluating HCC treatment response. Over half were under 5 cm and solitary lesions, and most studies included tumours with portal vein thrombosis. Regarding PH studies, TIPS resolved bleeding/ascites in >60% of patients, more effective for bleeding. There were no lethal complications reported and procedural bleeding occurred in <5%. Hepatic encephalopathy occurred in 15%–30% within three months. In the HCC treatment‐response studies, major complication rates were low with no mortality. In the studies that evaluated the response to transarterial chemoembolization, complete response rate of patients with TIPS varied from 16% to 75%. Liver transplantation rate varied from 8% to 80%, with >40% rate in half of the studies.
Conclusions
In the published studies, TIPS is effective in treating PH complications in patients with HCC. Prospective studies on TIPS placement in patients with HCC are urgently needed to evaluate the efficacy and safety of TIPS in this setting.
Keywords: cirrhosis, HCC, hepatocellular carcinoma, liver cancer, portal hypertension, TIPS
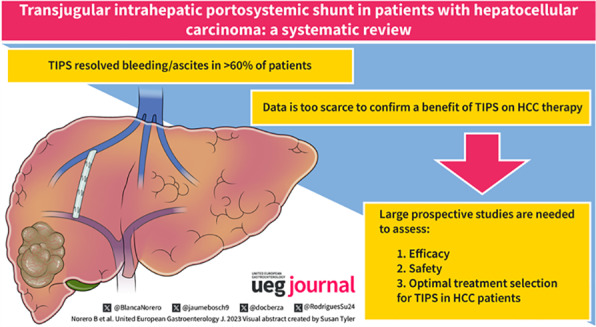
Key summary.
Summarise the established knowledge on this subject
Treatment of hepatocellular carcinoma (HCC) is limited in patients with portal hypertension‐related complications.
Although transjugular intrahepatic portosystemic shunt (TIPS) may be an effective treatment for ascites and bleeding and provide patients with HCC access to therapies, the current data is too scarce and inconsistent to recommend, globally, its use. Patients should be discussed on a case‐by‐case basis.
What are the significant and/or new findings of this study?
This review demonstrates that TIPS in HCC is effective in treating PH with low procedural bleeding, but large prospective studies are required in order to better assess efficacy, safety and optimal patient selection for TIPS in the setting of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) and portal hypertension (PH)‐related decompensation, mainly variceal bleeding (VB) and ascites, are the most common complications of liver cirrhosis and frequently coexist. In early HCCs, around 31%–45% of patients have clinically significant PH, defined by hepatic venous pressure gradient or clinical and noninvasive criteria, whilst its prevalence is likely higher but is unknown in advanced stages of HCC. 1 , 2 , 3 TIPS is an established therapy for severe cases of PH. Pre‐emptive TIPS placed within the first 72 h of admission for acute VB improves survival in patients with cirrhosis Child‐Pugh B > 7 and active bleeding and Child C < 14, and TIPS can increase survival rates in selected patients with recurrent or refractory ascites. 4 , 5 , 6 , 7 , 8
PH‐related complications can occur in patients with HCC, and HCC, per se, represents an independent risk factor for rebleeding after an index episode. 9 , 10 Short‐term mortality in patients with VB and HCC depends on bleeding, and TIPS could be a potential life‐saving procedure in these cases. However, in the past, the risks of post‐TIPS liver failure and tumour dissemination, as well as the risk of limiting further local therapeutic options, contraindicated TIPS in patients with HCC. 11
Transjugular intrahepatic portosystemic shunts diverts portal venous flow, leading to concerns of liver failure and tumour dissemination in patients undergoing transarterial chemoembolization (TACE), 11 but this has not been observed in other studies. Therefore, traditionally, HCC, particularly outside Milan criteria, has been considered an exclusion criterion in most randomized controlled trials and a relative contraindication, in clinical practice. Recently, diagnostic and therapeutic options for HCC have significantly increased, leading to an increase in overall survival ,OS, particularly in the late stages. Bleeding is a frequent cause of death in these patients 10 and ascites limits access to curative locoregional therapy, suggesting that an optimal treatment of PH might improve outcomes in patients with HCC. In some non‐controlled retrospective cohorts, TIPS placement has shown to be safe and effective in patients with HCC.
Nevertheless, it is unestablished whether TIPS in patients with HCC can improve survival, and which patients would benefit the most. The aim of this systemic review is to synthesize the evidence on the efficacy and safety of TIPS in HCC patients to treat PH complications and as a bridge to other therapies and highlight the current gaps in knowledge.
METHODS
We performed a systematic review of the published studies in this field; the study search and selection, and data extraction were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses recommendations. 12 Ethics approval was waived due to the nature of the study.
Definitions
Studies including patients with HCC who were submitted to TIPS were included if sufficient data regarding the main characteristics of patients, cirrhosis stage, HCC, and outcomes were provided. There was no minimum follow‐up period defined for the outcomes. Sufficient baseline data was defined as follows: 1) at least 10 patients with HCC and TIPS; 2) available demographic and disease stage data (age, gender, aetiology, Child‐Pugh or MELD score). Two types of studies were found; first those that assessed the use of TIPS to treat complications of PH (mostly VB and ascites) in patients with HCC and second those that assessed the effect of TIPS on patients' response to treatment for HCC (local or systemic therapy). The PH treatment studies will be referred to as “PH studies” and the latter studies as “HCC treatment response” studies. For the PH studies, sufficient outcome data was defined as follows: 1) rate of VB ascites resolution; 2) OS rate; and 3) overall complication rate. For the HCC treatment response studies, sufficient outcome data was defined as follows: 1) treatment type and response rate and 2) OS rate. The main outcomes assessed for the PH studies were technical feasibility, major procedural complication rate, efficacy in treating ascites or VB, and survival. The main outcomes assessed for the HCC treatment response studies were treatment response (RECIST criteria), major procedural complication rate, and survival. Regarding efficacy in treating VB and ascites, the reporting is inconsistent. Some studies defined this as absence of clinically detectable ascites with or without diuretic therapy or no need for further paracentesis and/or no further VB episodes after TIPS, 13 , 14 whereas others establish a specific time point for recurrence of gastroesophageal VB and ascites/hydrothorax, such as in 12 months. 15
We used the following definitions. “Technical feasibility”: successful access to the portal vein, and placement of TIPS. “Major procedural complication”: serious or potentially life‐threatening event because of endovascular treatment, for example, intraperitoneal bleeding or after locoregional therapy such as postembolization syndrome. “Technical follow‐up complications”: shunt‐related complications such as stenosis, dysfunction or thrombosis of the stent. Stent dysfunction was reported as per authors' criteria. In most cases, TIPS was revised, if either changes in Doppler ultrasound or recurrent bleeding and/or ascites, and in some cases, it was undefined.
Search strategy and inclusion and exclusion criteria
Two electronic databases were searched: PubMed and Embase for articles in the English language that were published until 31 August 2022 (last search run). Randomised‐controlled trials and prospective and retrospective cohort studies with original data and full text published were included. Search terms were (“hepatocellular carcinoma” or “HCC”) and (“transjugular intrahepatic portosystemic shunt” or “TIPS”) and (“cirrhosis” or “liver cirrhosis”). Search results were merged.
Exclusion criteria were case reports or studies with fewer than 10 patients and studies lacking the minimal data required for the analysis (see “data extraction”). Studies lacking data on efficacy and/or treatment response (for PH and HCC treatment response studies, respectively), complication rates and survival, in addition to providing baseline characteristics of the patients (age, sex, Child‐Pugh or MELD score) were excluded. Abstracts were independently reviewed by two investigators (BN and SGR). In case of discrepancy regarding the eligibility for the analysis, a consensus was reached by another author (AB), and in the case of cohort overlap, the most recent publication was selected.
Data extraction
After selecting the studies, the authors separated them into PH and treatment response studies. General characteristics were collected: first author, year published, study design, age, sex, Child‐Pugh Score, MELD Score, presence of portal vein thrombosis (PVT), alpha fetoprotein value, size, number and location of HCC, Barcelona Clinic Liver Cancer (BCLC) stage, whether HCC within Milan criteria, and TIPS indication and type of stent. Regarding outcome data extraction, in the PH studies, the following were extracted: efficacy in the treatment of PH, portal pressure gradient (PPG) change, major procedural and technical complications, further decompensation, survival/liver transplantation, and response to HCC therapy. For studies in the treatment response group, in addition to the baseline characteristics, the following data was obtained: the type of treatment used, systemic or local, and number of sessions, local response, major procedural complications, recurrence, and bridge to transplantation/survival.
Quality assessment from individual studies
Quality assessment was performed using the Newcastle‐Ottawa Scale. 16 Studies were considered of high quality if ≥8 points (89), moderate quality, if 6–7 points, and low quality, if ≤5 points (Supplementary Tables 1 and 2). Ethical approval was not sought because of the study design.
RESULTS
Results of the study search are depicted in Figure 1. Among the final 34 excluded full texts, three studies were considered irrelevant due to insufficient outcome data. Of the 19 included studies, 11 dealt with HCC patients who received TIPS to treat PH‐related complications, and the remaining eight evaluated the effect of TIPS on HCC treatment response. As clarified in the Methods section, the results of these two groups are presented separately.
FIGURE 1.
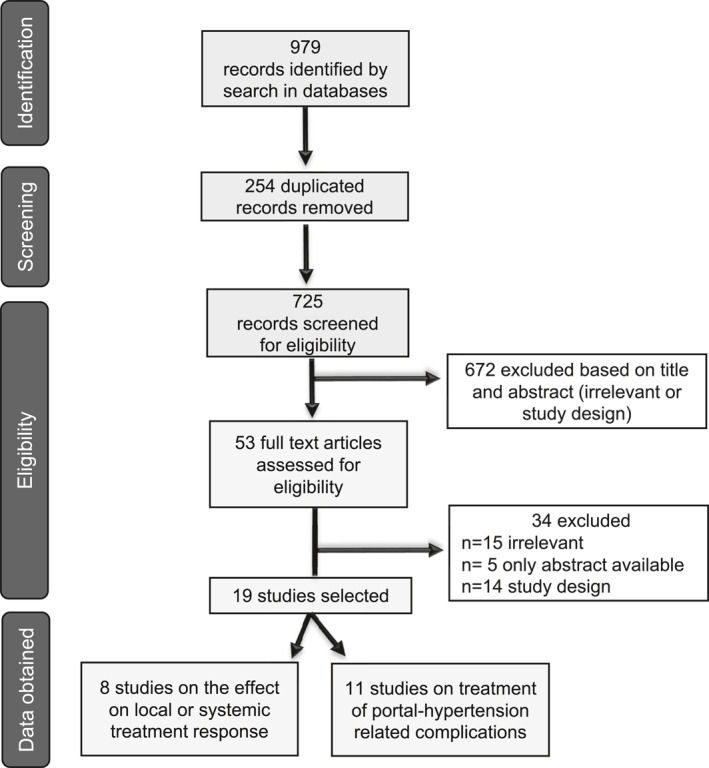
Flowchart of studies in the systematic review following PRISMA guideline and recommendation.
Indication for TIPS: Treatment of portal hypertension‐related complications
Study selection and characteristics of included studies
All 11 studies (Table 1) were retrospective of low to medium quality (Supplementary Table 1) and in total included 937 patients, 870 of which with TIPS placement after the diagnosis of HCC to treat the complications. 13 , 14 , 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Most studies originated from China and the majority had hepatitis B virus‐associated cirrhosis and/or alcohol‐related disease. Patients were mostly male (range: 57.9%–100%) and the mean age ranged from 46.3 to 63.5 years. Regarding Child‐Pugh class, with the exception of one small study that evaluated patients in the palliative setting, 17 the majority of patients were Child‐Pugh A (19.0%–61.5%) and B (34.4%–72.5%), with the latter group being the most frequent. In four studies, including 178 patients, the patients were in an advanced stage of HCC with PVT, considered tumoral thrombus. Seven out of 10 studies provided data regarding the number of lesions. Most were solitary, ranging from 33.8% to 77.8%. Six studies provided the average size or percentage of patients with lesions under 3 or 5 cm, in which 57.4%–97.1% were under 5 cm. Transjugular intrahepatic portosystemic shunts was indicated for VB (50%–100%) and/or ascites (7.7%–35%) One study specifically evaluated TIPS in patients with refractory ascites without bleeding. 25
TABLE 1.
Baseline characteristics: Treatment of portal hypertension.
| Author | Year | Country | Design | Number of patients | Age (years) | Male sex (%) | Aetiology (%) | Child‐Pugh class (%) | MELD | AFP (ng/mL) | PVTT (%) | Degree PVT (%) | Size HCC | Within Milan criteria (%) | Location | Solitary (%) | BCLC (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jiang Z‐B | 2004 | China | Retro | 14 (10) a | 56.3 (28–75) | 92.9 | NA | C 100 | NA | NA | 100 | 57.1 complete | NA | None | NA | NA | D 100 |
| 42.9 partial | |||||||||||||||||
| Liu L | 2014 | China | Retro | 58 | 51.7 (28–72) | 86.2 | 98.2 HBV | A 19 | NA | NA | 100 |
|
8.87 cm (2.6–16) | NA | NA | 1–2 lesions 65.5 | C/D 60/40 |
| B 59 | |||||||||||||||||
| C 22 | |||||||||||||||||
| Zhao J‐B | 2014 | China | Retro | 11 | 54.3 ± 12.7 | 100 | 100 HBV | A 54.5 | NA | NA | 100 | 100 complete | NA | NA | 45% RL | NA | NA |
| B 45.5 | 27% LL | ||||||||||||||||
| C 0 | 27% both | ||||||||||||||||
| Qiu B | 2015 | China | Retro | 261 (209) a | 48.3 ± 12.5 | 57.9 | 86 HBV | A 39.7 | NA | NA | 14.8 | NA | <3 cm: 24.8% | NA | NA | 70.8 | NA |
| 5.7 HCV | B 34.4 | 3–5 cm: 49% | |||||||||||||||
| 1 both | C 25.8 | >5 cm: 25.8% | |||||||||||||||
| Bettinger D | 2015 | Germany | Retro | 40 | 63.5 (40–83) | 87.5 | 62.5 alcohol; 27.5 HCV | A 7.5 | 13 (7–22) | 6.7 (0.7–7125.0) | 32.5 | NA | 3.7 ± 1.8 | 40 | 37.5% centrally | 55 | NA |
| B 72.5 | |||||||||||||||||
| C 20.0 | |||||||||||||||||
| Qiu B | 2017 | China/USA | Retro | 95 (91) a | 49.1 ± 11.5 | 59.0 | NA | A 17 | NA | NA | 100 | <3 cm: 25.3% | NA | NA | 73.6 | NA | |
| B 39 | 3–5 cm: 54.8% | ||||||||||||||||
| C 35 | >5 cm: 19.9% | ||||||||||||||||
| Luo S‐H | 2019 | China | Retro | 217 (212) a | 46.32 ± 12.43 | 54.2 |
|
A 25 | 10.21 ± 5.25 | 468.53 ± 34.27 | 0 | NA | NA | NA | NA | NA |
|
| B 60.8 | |||||||||||||||||
| C 16 | |||||||||||||||||
| Yan H | 2020 | China | Retro | 68 | 58 (46–63) | 89.7 | 97.1 HBV | A 10.3 |
|
>400 29.4% | 66.2 | NA | <5 cm: 57.4% | 22.1 | NA | 33.8 |
|
| B 72.1 | |||||||||||||||||
| C (17.6) | |||||||||||||||||
| Luo J | 2021 | China | Retro | 34 | 54.9 ± 8.5 | 91.2 | 91.2 HBV | 7 (6–8) | 11.5 (9.75–13.25) | >100 14.7% | 0 | NA | <3 cm 97.1% | 100 | 47.1%: Subcapsular; 23.5% Hilar; 5.9% Peri‐diaphragm; 11.8% Peri‐vascular | 64.7 | NA |
| A 41.2 | |||||||||||||||||
| B 52.9 | |||||||||||||||||
| C 5.9 | |||||||||||||||||
| Dong H | 2021 | China | Retro | 13 | 58 (52.5–62.5) | 61.5 | 92.3 HBV | A 61.5 | NA | NA | 0 | NA | NA | NA | RL 100% | NA |
|
| B 38.5 | |||||||||||||||||
| C 0 | |||||||||||||||||
| Tsauo J | 2021 | China | Retro | 126 (124) a | 54.1 ± 10.2 | 87.3 | 81.7 HBV | 7.2 ± 1.5 | 11.4 ± 3.3 | NA | 23.8 | 3.2 Vp1 b | 4.2 cm ± 3.0 | NA |
|
77.8 | A 38.1 |
| 7.9 HCV | 4.8 Vp2 b | B 11.9 | |||||||||||||||
| 3.2 alcohol | 15.9 Vp3 b | C 42.1 | |||||||||||||||
| 3.2 HBV + HCV; 4.0 other | 4.0 Vp4 b | D 7.9 |
Abbreviations: AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer; BL, bilobar; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LL, left lobe; MELD, model for end‐stage liver disease; NA, not available; PVTT, portal vein tumour thrombosis; Retro, retrospective; RL, right lobe.
Number of patients in which TIPS were placed.
Liver Cancer Study Group of Japan (LCSGJ), distinguished four grades of portal vein tumour thrombosis.
Outcomes: Feasibility, efficacy and survival
A summary of the outcomes reported is provided in Table 2.
TABLE 2.
Outcomes: Treatment of portal hypertension.
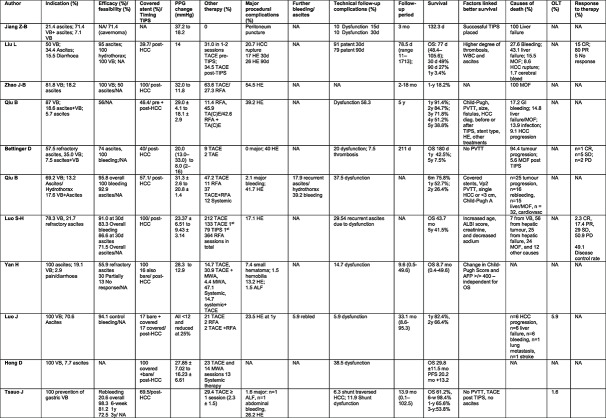
|
Abbreviations: ALF, acute liver failure; CR, complete response; d, days; GI, gastrointestinal; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; mo, months; MOF, multiorgan failure; MWA, microwave ablation; NA, not available; OLT, orthotopic liver transplantation; OS, overall survival; PD, progressive disease; PFS, progression free survival; PPG, portal pressure gradient; PR partial response; RFA, radiofrequency ablation; SD, stable disease; TA(C)E, transarterial (chemo)embolization; TIPS, transjugular intrahepatic portosystemic shunt; VB, variceal bleeding; w, weeks; WBC, white blood cell count; y, years.
It is impossible to evaluate the feasibility of TIPS placement in the setting of HCC, as most studies simply report the data on patients in whom TIPS was placed and provide no information on the number of attempted/failed cases.
The absolute decrease in PPG varied from a reduction in 10–20 mmHg. However, only four studies reported average decrease in gradient to a level ˂12 mmHg. 13 , 14 , 17 , 20 Regarding PH‐related complications, 56%–100% (in most studies >70%) of patients did not rebleed, and ascites resolved in 50%–95%.
Aside from TIPS for the PH‐related complications, 30%–60% of patients received sessions of local (TACE and/or ablation) or systemic HCC‐directed therapy. The OS varied greatly between 2.6 and 43.7 months. This depended on the stage of liver disease and HCC. In studies including exclusively patients without PV macrovascular invasion, 14 , 23 the OS ranged between 30 and 44 months. Better liver function, that is, lower Child‐Pugh Score, absence of macrovascular invasion, solitary or smaller HCCs (<3 cm) and ALBI score were among the factors linked to better survival. Death was attributed predominantly to liver failure and tumour progression, and liver transplantation rate, was reported in only two studies from 1.6% to 5.9%. 14 , 24
Complications
No lethal complications were reported. Bleeding rate was low (mostly <5%) and the rate of post‐TIPS hepatic encephalopathy (HE) ranged from 13% to 55%, with most studies reporting an incidence between 15% and 30% within the first 3 months post‐TIPS. Stent dysfunction in the follow‐up was described within the first year, in most studies, and ranged 6%–38%, with one study reporting dysfunction in 58%. 15 In three studies with higher dysfunction rates (≥20%), the stents were bare and covered (vs. 100% covered), 13 , 19 , 23 or in an advanced HCC setting. 14
Indication: Effect of transjugular intrahepatic portosystemic shunts on treatment response
Study selection and characteristics of included studies
All eight (Table 3) were retrospective of low to medium quality (Supplementary Table 2) and in total included 177 patients. 11 , 26 , 27 , 28 , 29 , 30 , 31 , 32 Transarterial chemoembolization was the most frequent treatment, but some patients were treated with ablation, 28 , 29 , 30 radioembolization 28 , 32 and in one case stereotactic body radiotherapy. 30 Most studies originate from the United States. The aetiology in American studies was mostly alcohol or hepatitis C and hepatitis B in Asian countries. Patients were mostly males (range: 59%–90%) aged 51–73 years. Over 50% of the cases were in Child‐Pugh B. MELD score was reported in six studies, from 7 to 25 points.
TABLE 3.
Baseline characteristics: Treatment response.
| Author | Year | Country | Design | Number of patients | Age | Male sex (%) | Aetiology (%) | Child‐Pugh (%) | MELD | AFP (ng/mL) | PVTT (%) | Size HCC | Within Milan criteria (%) | Location (%) | Solitary (%) | BCLC (%) | Indication (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kang JW | 2012 | Korea | Retro | 20 | 56.6 + 10.7 | 75 |
|
|
NA | Median 67.1 | 15 | 3.3 cm mean | NA |
|
65 | NA |
|
| Kuo Y‐C | 2013 | USA | Retro | 10 | 59 (51–72) | 90 |
|
14 (10–18) | NA | NA | 2.7 (1.2–4.8) | NA | NA | 80 |
|
NA | |
| Wang Z | 2014 | China | Retro | 19 | 54 (36–70) | 89.5 | 94.7 HBV 5.3 HCV |
|
13.37 ± 2.57 (10–18) | NA | NA |
|
NA | NA | 63.2 |
|
|
| Padia SA | 2015 | USA | Retro |
|
58 (42–73) | 76 |
|
|
12 (8–25) | >50: 11% | 79 | 23 (11–55) | NA | Unilobar 79 | 69 |
|
|
| Park JK | 2015 | USA | Retro | 19 | 62.2 (51–73) | 79 |
|
|
NA | NA | NA | 2.6 cm mean | NA | NA | NA | NA | NA |
| Miura JT | 2015 | USA | Retro | 16 | 60.5 (52.5–67.5) | 75 |
|
|
12.5 (7.5–13) | NA | NA |
|
NA | NA | 56.2 |
|
|
| Ruohoniemi DM | 2020 | USA | Retro | 25 | 60.0 (57–63.5) | 76 | 72 HCV | NA | 13 (11–15) | NA | 8 | 2.5 (1.8–3.2) | NA | Unilobar 84 | 76 | NA | NA |
| 13.2 + 2.7 | |||||||||||||||||
| Gordon AC | 2021 | USA | Retro | 39 | 63.5 (61.5–66.6) | 59 |
|
|
18 (16.4–19.4) | <200 84.6% | 0 | 2.9 cm (2.4–3.4) | NA | BL 20.5 | 66.7 |
|
|
Abbreviations: AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer; BL, bilobar; BSC, best supportive care; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; LL, left lobe; MELD, model for end‐stage liver disease; NA, not available; PVTT, portal vein tumour thrombosis; Retro, retrospective; RL, right lobe; VB, variceal bleeding.
Number of patients in which TIPS were placed.
Macrovascular invasion was reported in one study only for three patients 26 and PVT in 6/29 (21%) and 2/25 (8%) in two studies. 28 , 31 Most had a solitary HCC (56%–80%), with a mean size 1.2–8.8 cm. All had HCC treatment after TIPS placement, except in one, 26 where some underwent sessions of TACE before TIPS. Indications for TIPS were VB (43%–100%) and ascites (29%–56%). Regarding BCLC stage, patients were distributed heterogeneously: stage A 18.8%–50%, stage B 7.7%–36.8%, stage C 0%–52% and stage D 0%–26.3%.
Outcomes: Complications, survival and response to hepatocellular carcinoma therapy
Major complication rates were low with no mortality (Table 4).
TABLE 4.
Outcomes: Treatment response.
| Author | Therapy | Number of sessions/timing | Response (%) | Major procedural complications (%) | Recurrence (%) | Further therapy | OLT (%) | Follow‐up period | Survival | Causes of death |
|---|---|---|---|---|---|---|---|---|---|---|
| Kang JW | All TACE |
|
|
35 postembolization syndrome; 5 major complication (fever, worsened ascites) | NA | NA | NA | 32.6 months |
|
|
| Kuo Y‐C | All TACE | 1.4 (1–3) |
|
70 ≥ 1 hepatobiliary SAE (data extracted from other source) |
|
2 patients ethanol injection | 80 | NA | 3 years 60% | NA |
| Wang Z | 54 TACE sessions | (Mean, 2.84 sessions; range, 1–7 sessions) |
|
|
NA | NA | 10.5 | 20.9 months (4.1–50.1) |
|
|
| Padia SA |
|
NA |
|
NA | NA | NA |
|
|
|
NA |
| Park JK | RFA | 25 |
|
NA | NA | NA | 58 |
|
|
|
| Miura JT |
|
|
|
|
NA | NA | 18.8 | 11.5 months |
|
NA |
| Ruohoniemi DM |
|
|
|
1 SAE (CTCAE grade >2) | NA | 40% systemic therapy | 8 | NA | OS 72 months | NA |
| Gordon AC | Y90 RE | Median total RE:1 (1.1–1.8) |
|
Grade 3: 5% | NA | NA | 53.8 | NA | OS: 31.6 months (censored for OLT) | NA |
Abbreviations: ALF, acute liver failure; CR, complete response; d, days; DEE‐TACE, drug‐eluting embolic‐TACE; GI, gastrointestinal; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; mo, months; MOF, multiorgan failure; MWA, microwave ablation; NA, not available; OLT, orthotopic liver transplantation; OS, overall survival; PD, progressive disease; PFS, progression free survival; PPG, portal pressure gradient; PR partial response; RE, radioembolization; RFA, radiofrequency ablation; SAE, serious adverse event; SBRT, stereotactic body radiotherapy; SD, stable disease; TA(C)E, transarterial (chemo)embolization; TIPS, transjugular intrahepatic portosystemic shunt; VB, variceal bleeding; w, weeks; WBC, white blood cell count; y, years.
In patients with TIPS who received locoregional treatment for HCC, evaluating the response to TACE, 11 , 26 , 27 , 28 complete response was reported in 16%–75%, partial 6.3%–20%, stable 10%–26% and progressive 5%–42%.
Data on recurrence was scarce. The median follow‐up periods varied from 12 to 33 months. Overall survival ranged between 23 and 75 months, with 1‐year and 3‐year rates of 85%–100% and 30%–67%, respectively. Seven studies reported liver transplantation rates from 8% to 80%, half reporting transplantation ≥40% of cases.
DISCUSSION
Transjugular intrahepatic portosystemic shunts is recognized as an effective and minimally invasive technique that improves survival in selected patients with cirrhosis and complications of PH. 7 , 33 HCC has been traditionally considered a relative contraindication, particularly if centrally located 34 mostly for fear of tumour spread, lung dissemination and liver failure, and concerns regarding the risks of portal flow diversion in HCC patients requiring TACE. 11
As a result, most patients with HCC were denied TIPS, even with complications of PH, which have worse outcomes in HCC patients. 9 , 10 However, this was linked with inadequate treatment for the prevention of recurrent variceal hemorrhage. 10 Since TIPS has been proved life‐saving when used early in high‐risk patients, understanding the current status on TIPS for PH complications in HCC is a compelling question. We conducted this systematic review to assess data in the context of HCC and TIPS. As expected, the largest number of published studies reported TIPS to treat complications of PH, which are common in HCC. One important limitation is the lack of reporting on TIPS feasibility. This is clinically very relevant, in the setting of HCC, and authors do not report the limiting factors in TIPS placement, such as anatomical or technical restrictions. Furthermore, in the specific setting of PVT, authors refer to “tumoral thrombus” but do not define how the diagnosis was established between bland and tumoral thrombus. Overall, in >70% of patients, TIPS successfully reduced portal pressure and controlled VB and/or ascites. In studies with smaller HCCs, bleeding stopped in over 90% of cases, and ascites improved in over 70%. 13 , 14 , 19 , 22 , 23 , 24 Even so, important technical details such as the method and timing of pressure gradient measurement, and the type and size of stents used, are largely missing. In studies, excluding palliative stage of HCC, the OS rate at 1‐year after TIPS ranged from 43% to 91%, which is similar to reported survival after TIPS without HCC. 5 , 8 , 35 , 36 The authors do not provide comparative data on the treatment of PH complications and survival in patients not submitted to TIPS, thus hampering analysis of outcomes with TIPS. According to published data, patients with acute VB and HCC, of all stages, have a higher 6‐week mortality rate, 26.4%. 37 Future prospective studies/trials involving TIPS in HCC patients require a direct comparison with the standard of care to establish efficacy.
Interestingly, the rate of major complications and post‐TIPS HE is also similar to that observed in patients without HCC and occurring in 20%–40% of patients. 38 A high incidence of stent dysfunction was noted, particularly in advanced HCC and PVT, in whom the rate of dysfunction was ≥50%. In the era of covered stents, as the standard of care, dysfunction is relatively infrequent in patients without HCC, about 24% at 2 years. 39 This contrasts sharply with the HCC setting between 12% and 59%. Nevertheless, the definition of stent dysfunction varied throughout the studies. We highlight the importance of consistent definitions when reporting outcome efficacy and procedural complications.
Kuo et al 11 suggested TIPS patients required more TACE sessions to achieve an objective response with a similar time to progression. Another study suggested that TIPS patients who submitted to TACE experienced more hepatotoxicity, although the transplantation rate within 1 year after TACE was 2.5 times higher in TIPS patients. 40 Even if this data has to be interpreted with caution, TIPS should not be considered a contraindication to superselective TACE.
This review highlights the incomplete and inconsistent evidence in this field, and calls for urgent prospective and well‐designed studies in patients with HCC and PH complications. Most studies were retrospective and included a heterogeneous population, with some belonging to the palliative care setting. They did not stratify by tumour size and number, degree of liver failure and presence of PVT. Furthermore, >80% of the patients were Asian mainly with hepatitis B, and whether these findings can be generalized to other ethnicities and aetiologies is unclear. Regarding the studies assessing loco‐regional treatment response in patients with TIPS and HCC, it is unclear whether current systemic treatment combinations, with improved OS compared to sorafenib, 41 may or not reduce the need for TIPS to gain access to locoregional treatment, underlining the complexity and the lack of current data. Furthermore, tumour recurrence data in patients with HCC and TIPS is scant, and was stated in only one out of eight studies 11 .
Transjugular intrahepatic portosystemic shunts insertion, in selected cases of patients with HCC, may be effective to control PH‐related complications and allow for bridging to loco‐regional and systemic therapy, eventually improving survival. On the other hand, and as expected, some studies 17 , 18 , 20 have shown that, in patients in an advanced stage (BCLC C and D), TIPS did not bring any survival benefit. Notwithstanding, data on tumoral characteristics, such as size, location and presence of PVT, is scarce and inconsistent and significantly hampers granularity and definitive conclusions. Another relevant aspect is that the studies did not use consistent endpoints, nor timeframes, for VB and ascites treatment response, such as those established by Baveno V (5‐week treatment failure and 6‐week mortality rate). 42 Harmonization of outcomes after TIPS is essential to construct prospective studies and trials in this setting.
It is still unclear whether TIPS in HCC patients could allow curative treatments in selected cases with good liver function, but with PH‐related complications. Moreover, whether TIPS is an option to treat PH complications in selected patients, not transplant candidates, also remains open. Current evidence supports TIPS in patients with clear indications, that is, VB and recurrent/refractory ascites. In the setting of HCC, TIPS placement should be assessed case‐by‐case, until large, cooperative, prospective studies provide data on efficacy, safety and optimal patient selection.
CONFLICT OF INTEREST STATEMENT
Jaume Bosch disclosures: consultant to Astra‐Zeneca, BioVie, Boehringer Ingelheim, NovoNordisk, Resolution Therapeutics. Blanca Norero, Jaume Bosch, Annalisa Berzigotti, Susana G. Rodrigues have no conflicts of interest regarding this manuscript to declare.
Supporting information
Supplementary Information S1
ACKNOWLEDGEMENT
Open access funding provided by BIBLIOSAN.
Norero B, Bosch J, Berzigotti A, Rodrigues SG. Transjugular intrahepatic portosystemic shunt in patients with hepatocellular carcinoma: a systematic review. United European Gastroenterol J. 2023;11(8):733–744. 10.1002/ueg2.12454
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Liu J, Zhang H, Xia Y, Yang T, Gao Y, Li J, et al. Impact of clinically significant portal hypertension on outcomes after partial hepatectomy for hepatocellular carcinoma: a systematic review and meta‐analysis. HPB Oxf. 2019;21(1):1–13. 10.1016/j.hpb.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 2. Xia F, Huang Z, Zhang Q, Ndhlovu E, Chen X, Zhang B, et al. Clinically significant portal hypertension (CSPH) on early‐stage HCC following hepatectomy: what's the impact? Eur J Surg Oncol. 2023;49(4):771–779. 10.1016/j.ejso.2022.11.005 [DOI] [PubMed] [Google Scholar]
- 3. Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75(4):960–974. 10.1016/j.jhep.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 4. García‐Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–2379. 10.1056/nejmoa0910102 [DOI] [PubMed] [Google Scholar]
- 5. Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant‐free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152(1):157–163. 10.1053/j.gastro.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 6. Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4(8):587–598. 10.1016/s2468-1253(19)30090-1 [DOI] [PubMed] [Google Scholar]
- 7. de Franchis R, Bosch J, Garcia‐Tsao G, Reiberger T, Ripoll C, Abraldes JG, et al. Baveno VII ‐ renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–974. 10.1016/j.jhep.2021.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicoară‐Farcău O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, et al. Effects of early placement of transjugular portosystemic shunts in patients with high‐risk acute variceal bleeding: a meta‐analysis of individual patient data. Gastroenterology. 2021;160(1):193–205.e10. 10.1053/j.gastro.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 9. Lim J, Kim HI, Kim E, Kim J, An J, Chang S, et al. Variceal bleeding is aggravated by portal venous invasion of hepatocellular carcinoma: a matched nested case‐control study. BMC Cancer. 2021;21(1):11. 10.1186/s12885-020-07708-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ripoll C, Genescà J, Araujo IK, Graupera I, Augustin S, Tejedor M, et al. Rebleeding prophylaxis improves outcomes in patients with hepatocellular carcinoma. A multicenter case‐control study. Hepatology. 2013;58(6):2079–2088. 10.1002/hep.26629 [DOI] [PubMed] [Google Scholar]
- 11. Kuo YC, Kohi MP, Naeger DM, Tong RT, Kolli KP, Taylor AG, et al. Efficacy of TACE in TIPS patients: comparison of treatment response to chemoembolization for hepatocellular carcinoma in patients with and without a transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol. 2013;36(5):1336–1343. 10.1007/s00270-013-0698-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71. 10.1016/j.ijsu.2021.105906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bettinger D, Knüppel E, Euringer W, Spangenberg HC, Rössle M, Thimme R, et al. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPSS) in 40 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2015;41(1):126–136. 10.1111/apt.12994 [DOI] [PubMed] [Google Scholar]
- 14. Luo SH, Chu JG, Huang H, Yao KC. Safety and efficacy of transjugular intrahepatic portosystemic shunt combined with palliative treatment in patients with hepatocellular carcinoma. World J Clin Cases. 2019;7(13):1599–1610. 10.12998/wjcc.v7.i13.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qiu B, Li K, Dong X, Liu FQ. Transjugular intrahepatic portosystemic shunt for portal hypertension in hepatocellular carcinoma with portal vein tumor thrombus. Cardiovasc Intervent Radiol. 2017;40(9):1372–1382. 10.1007/s00270-017-1655-8 [DOI] [PubMed] [Google Scholar]
- 16. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 17. Jiang ZB, Shan H, Shen XY, Huang MS, Li ZR, Zhu KS, et al. Transjugular intrahepatic portosystemic shunt for palliative treatment of portal hypertension secondary to portal vein tumor thrombosis. World J Gastroenterol. 2004;10(13):1881–1884. 10.3748/wjg.v10.i13.1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu L, Zhao Y, Qi X, Cai G, He C, Guo W, et al. Transjugular intrahepatic portosystemic shunt for symptomatic portal hypertension in hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol Res. 2014;44(6):621–630. 10.1111/hepr.12162 [DOI] [PubMed] [Google Scholar]
- 19. Qiu B, Zhao MF, Yue ZD, Zhao HW, Wang L, Fan ZH, et al. Combined transjugular intrahepatic portosystemic shunt and other interventions for hepatocellular carcinoma with portal hypertension. World J Gastroenterol. 2015;21(43):12439–12447. 10.3748/wjg.v21.i43.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao JB, Feng C, Zhu QH, He XF, Li YH, Chen Y. Transjugular intrahepatic portosystemic shunt with covered stents for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2014;20(6):1602–1607. 10.3748/wjg.v20.i6.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan H, Wang G, Zhu W, Feng K, Zhu W, Wu X, et al. Feasibility and clinical value of TIPS combined with subsequent antitumor treatment in HCC patients with refractory ascites. Transl Oncol. 2020;13(12):100864. 10.1016/j.tranon.2020.100864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo J, Li M, Wu C, Zhu D, Wang H, Huang M, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy for prevention of variceal rebleeding in patients with hepatocellular carcinoma meeting the Milan criteria. Eur J Gastroenterol Hepatol. 2021;33(3):436–442. 10.1097/meg.0000000000001750 [DOI] [PubMed] [Google Scholar]
- 23. Dong H, Zhang C, Li Z, Yang H, Wang Y, Liu J, et al. Trans‐jugular intrahepatic portosystemic shunt in patients with hepatic cellular carcinoma: a preliminary study. J Cancer Res Ther. 2021;17(3):784–789. [DOI] [PubMed] [Google Scholar]
- 24. Tsauo J, Tie J, Xue H, Zhao JB, Li JJ, Fang ZT, et al. Transjugular intrahepatic portosystemic shunt creation for the prevention of gastric variceal rebleeding in patients with hepatocellular carcinoma: a multicenter retrospective study. J Vasc Interv Radiol. 2021;32(7):963–969. 10.1016/j.jvir.2021.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Yan H, Qiu Z, Xiang Z, Feng K, Huang M, Gao F. TIPS improves outcomes in patients with HCC and symptomatic portal hypertension: a multi‐institution experience. Cancer Imag. 2022;22(1):13. 10.1186/s40644-022-00451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang JW, Kim JH, Ko GY, Gwon DI, Yoon HK, Sung KB. Transarterial chemoembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt. Acta Radiol. 2012;53(5):545–550. 10.1258/ar.2012.110476 [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Zhang H, Zhao H, Wang X, Tsauo J, Luo X, et al. Repeated transcatheter arterial chemoembolization is safe for hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Diagn Interv Radiol. 2014;20(6):487–491. 10.5152/dir.2014.13493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Padia SA, Chewning RH, Kogut MJ, Ingraham CR, Johnson GE, Bhattacharya R, et al. Outcomes of locoregional tumor therapy for patients with hepatocellular carcinoma and transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol. 2015;38(4):913–921. 10.1007/s00270-014-1009-8 [DOI] [PubMed] [Google Scholar]
- 29. Park JK, Al‐Tariq QZ, Zaw TM, Raman SS, Lu DS. Radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol. 2015;38(5):1211–1217. 10.1007/s00270-015-1050-2 [DOI] [PubMed] [Google Scholar]
- 30. Miura JT, Rilling WS, White SB, Hieb RA, Tutton SM, Patel PJ, et al. Safety and efficacy of transarterial chemoembolization in patients with transjugular intrahepatic portosystemic shunts. HPB Oxf. 2015;17(8):707–712. 10.1111/hpb.12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruohoniemi DM, Taslakian B, Aaltonen EA, Hickey R, Patel A, Horn JC, et al. Comparative analysis of safety and efficacy of transarterial chemoembolization for the treatment of hepatocellular carcinoma in patients with and without pre‐existing transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2020;31(3):409–415. 10.1016/j.jvir.2019.11.020 [DOI] [PubMed] [Google Scholar]
- 32. Gordon AC, Gupta AN, Gabr A, Thornburg BG, Kulik LM, Ganger DR, et al. Safety and efficacy of segmental yttrium‐90 radioembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol. 2021;32(2):211–219. 10.1016/j.jvir.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 33. García‐Pagán JC, Saffo S, Mandorfer M, Garcia‐Tsao G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep. 2020;2(4):100122. 10.1016/j.jhepr.2020.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41(2):386–400. 10.1002/hep.20559 [DOI] [PubMed] [Google Scholar]
- 35. Wang CY, Li S. Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: a single center 14 years experience from China. Medicine (Baltim). 2019;98(4):e14070. 10.1097/md.0000000000014070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Will V, Rodrigues SG, Berzigotti A. Current treatment options of refractory ascites in liver cirrhosis ‐ a systematic review and meta‐analysis. Dig Liver Dis. 2022;54(8):1007–1014. 10.1016/j.dld.2021.12.007 [DOI] [PubMed] [Google Scholar]
- 37. Lee YR, Park SY, Tak WY. Treatment outcomes and prognostic factors of acute variceal bleeding in patients with hepatocellular carcinoma. Gut Liver. 2020;14(4):500–508. 10.5009/gnl19155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rössle M. TIPS: 25 years later. J Hepatol. 2013;59(5):1081–1093. 10.1016/j.jhep.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 39. Bureau C, Garcia Pagan JC, Layrargues GP, Metivier S, Bellot P, Perreault P, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long‐term results of a randomized multicentre study. Liver Int. 2007;27(6):742–747. 10.1111/j.1478-3231.2007.01522.x [DOI] [PubMed] [Google Scholar]
- 40. Kohi MP, Fidelman N, Naeger DM, LaBerge JM, Gordon RL, Kerlan RK, Jr . Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: do two rights make a wrong? J Vasc Interv Radiol. 2013;24(1):68–73. 10.1016/j.jvir.2012.08.032 [DOI] [PubMed] [Google Scholar]
- 41. Fulgenzi CAM, Scheiner B, Korolewicz J, Stikas CV, Gennari A, Vincenzi B, et al. Efficacy and safety of frontline systemic therapy for advanced HCC: a network meta‐analysis of landmark phase III trials. JHEP Rep. 2023;5(5):100702. 10.1016/j.jhepr.2023.100702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762–768. 10.1016/j.jhep.2010.06.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information S1
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


