As all lifeforms must display sensitivity to surrounding chemicals, the mammalian olfactory bulb (OB) and its peripheral fibers have evolved to sample the air. However, this structural blueprint may enable transmission of disease triggers into the rhinencephalon (nose-brain). For example, the OB and olfactory peduncle may be affected with Lewy pathology in early stages of dementia with Lewy bodies (DLB) and Parkinson’s disease (PD), particularly in cases with amygdalar predominance [1, 2, 5, 6]. The olfactory peduncle (i.e., foot) connects the OB with the forebrain and encompasses the anterior olfactory nuclei (AON) and tenia tectae [7]. The OB and olfactory peduncle are rostral components of le grand lobe limbique, defined by Broca as the limbus (border) of the cortex (Figure 1 in [13]). However, the modern view of the limbic system centers on the amygdala, a nuclear complex operating in support of emotional regulation.
Figure 1: Properties of limbic α-synucleinopathy in humans, rats, and mice.
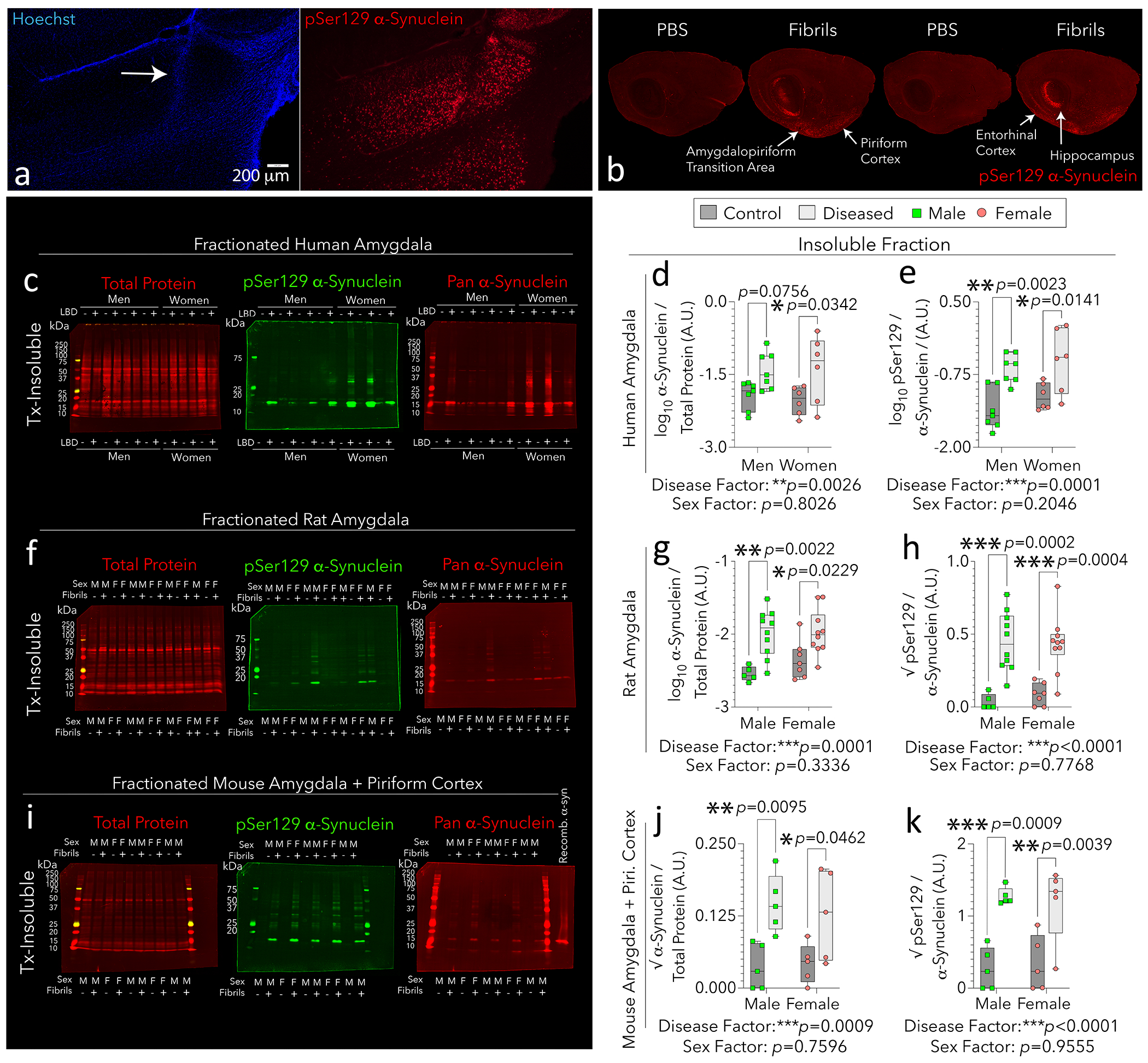
Sprague-Dawley outbred rats were bilaterally infused with preformed fibrils (n = 20; 15 μg in 3 μL) or an equivalent volume of phosphate-buffered saline (PBS; n = 12) in the OB/AON at 4 months of age (a-b). Four months later, hemibrains were immersion-fixed in formalin, sectioned in the sagittal plane, and immunostained with clone EP1536Y against pSer129 α-synuclein via TSA amplification as in [4]. Arrow in a points to tissue disturbance along the needle track, stained with the pan-nuclear Hoechst reagent. Panel b was imaged at 5-micron resolution on an Odyssey M (extended in Fig. S1). The other hemisphere was reserved for biochemical assays (f-h). Infusion sites in additional animals are included in Fig. S1. Insoluble fractions were extracted from postmortem limbic tissues of humans with (n = 13) or without (n = 13) diagnoses of Lewy body disorders (c-e), or from limbic tissues of rats and mice infused in the OB/AON with PBS (control) or preformed fibrils (f-k). For panels i-k, outbred mice were bilaterally infused in the OB/AON with preformed fibrils (5 μg in 1 μL; n = 10) or an equivalent volume of PBS (n = 10) at 9–11 months of age. Six weeks post-infusion, amygdala and piriform cortical tissues were collapsed for ultracentrifugation and detergent-based fractionation. Recombinant α-synuclein monomers were loaded onto the last lane in panel i as a negative control for the phospho-specific EP1536Y antibody. LBD = Lewy body disorder; A.U. = arbitrary units. Triton X-100 = Tx. Each subject is illustrated as a colored dot as the statistical unit. Two-tailed, multiplicity-adjusted p values per two-way ANOVA/Bonferroni are shown, and factor effects are listed. For details on methods and subject numbers, please consult the Supplemental Files. Gaussian/heteroscedastic raw data that failed assumptions of parametric testing are also in the Supplemental Files.
Alongside others working on OB-seeded α-synucleinopathy [15], we demonstrated that parts of the mouse rhinencephalon develop pathologic inclusions after preformed fibrils are infused into the bulbar extension of the AON (OB/AON) [3, 4, 9–11]. The inclusions harbor phosphorylated α-synuclein (pSer129) with occasional ubiquitin, and stain with the Proteostat aggregate dye [9, 10]. The affected brain regions extend substantial efferents towards the OB/AON and encompass the piriform and hippocampal cortices, cortical nuclei of the amygdala, deep layers of the entorhinal cortex, and occasionally, the ventral tegmental area and accumbens of the mesolimbic pathway. This limbic-centered pattern remains evident after tyramide-signal amplification (Fig. S1–S3 in [4]).
Many behaviors controlled by limbic structures display biological sex differences, including smell, anxiety, and spatial/location memory [8, 17, 19]. Hence, we evaluated the impact of sex on the expression, phosphorylation, and insolubility of α-synuclein within limbic tissues of humans with Lewy body disorders or unaffected controls (n = 26; Table S3) and outbred mice and rats infused in the OB/AON with fibrils or vehicle (n = 77; Fig. 1a; S1a–b). As shown in young and aged mice [4, 10], the most heavily affected regions in rats form a crescent along ventrolateral boundaries of the cerebral allocortex (Fig. 1b; S1c).
Lewy body disease and its experimental correlate induced no changes in net expression of α-synuclein in bulbar tissues (Fig. S2b, e, h). However, women expressed more bulbar α-synuclein than did men (Fig. S2b). Lewy body disease and fibril infusions increased the fraction of α-synuclein phosphorylated at Ser129 in women and female rats (Fig. S2c, f). Stated differently, the impact of disease was masked in whole-fraction tissues in men and male rats. Only in mice did both sexes display experimental disease-induced α-synuclein phosphorylation in whole tissue extracts (Fig. S2i). Previously, we noted denser inclusions in some limbic structures of female mice after OB/AON fibril infusions [10], but AI-driven analyses reveal that female mice form larger limbic inclusions than do males [4].
Given the centrality of the amygdala in limbic function, we evaluated nonionic detergent-insoluble α-synuclein in this structure. Lewy body disease and its experimental correlate raised insoluble α-synuclein levels in amygdalae of all three species, as well as the fraction of insoluble α-synuclein phosphorylated at Ser129 (Fig. 1c–k). The independent factor of disease—not sex—had main statistical effects on insoluble, α-synuclein. Monomeric α-synuclein typically migrates at ~17 kDa in denaturing gels [16], the band quantified in all figures. Other molecular masses were also evident, particularly in amygdalae from diseased women, and may reflect post-translational modifications (e.g., acetylation, truncation) and polymerization of α-synuclein. Bands of greater mass displayed high intragroup variation, perhaps due to distinct diagnoses methods across independent cohorts, and could be explored as a function of disease stage/subtype in the future.
Subtle sex differences were apparent in the soluble fraction of rat amygdalar tissues (Fig. S3f), and sex differences in whole-fraction rat tissues (. S2f; S6a–b) translated better to humans (Fig. S2c; S4d; S5a–d) than did tissues of mice (Fig. S2i; S4f; S7a–d). An intervariable interaction for disease x sex was only noted for whole-fraction human OBs (Fig. S5b). Thus, the rat fibril model offers some advantages over mice, in that females may display more α-synucleinopathy in these limbic regions, but biological sex does not explain most of the statistical variance in 17 kDa, insoluble α-synuclein.
Lack of consensus on sex-linked Lewy body disease outcomes has arisen, perhaps because of the paucity of work on early-stage, drug-naïve patients [14]. However, men with de novo PD display more synaptic dysfunction in the amygdala and hippocampus [18], and men are more likely to die with limbic Lewy pathology than women (Fig. 1B in [12]). Historically, variability in female data was touted as a reason to ovariectomize female rodents or avoid using females altogether. Nowadays, males and female data tend to be collapsed for understandable reasons (time/money/space), and modern work is often underpowered to distinguish sex-stratifications. However, we have observed that collapsed data hide effects that run in opposing directions for the sexes [11]. Therefore, stratifying measurements by sex is helpful in unmasking distinct properties of male vs. female brains, or in testing sex-skewed exposures to potential disease triggers, such as the inhalation and swallowing of industrial toxicants or microbiota.
Methods
All methods are in SI files.
Supplementary Material
Acknowledgements
Experiments/analyses/figures: KMM, ASJ, TNB, RNC, MA, RKL. Revisions/feedback: KCL, TNB. Writing: RKL, KMM. Design/supervision/mentoring: RKL. We thank Duquesne University, NeuroBiobank (HHSN-271–201300029C), and NIH (1R15NS130532–01, R21AG068608–01, 1R21NS11267, 1R21NS107960–01).
Data Availability
See SI files for raw data (also available upon emailing leakr@duq.edu).
References
- 1.Attems J, Toledo JB, Walker L, Gelpi E, Gentleman S, Halliday G, Hortobagyi T, Jellinger K, Kovacs GG, Lee EB et al. (2021) Neuropathological consensus criteria for the evaluation of Lewy pathology in postmortem brains: a multi-centre study. Acta Neuropathol 141: 159–172 Doi 10.1007/s00401-020-02255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R et al. (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117: 613–634 Doi 10.1007/s00401-009-0538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia TN, Clark RN, Needham PG, Miner KM, Jamenis AS, Eckhoff EA, Abraham N, Hu X, Wipf P, Luk KC et al. (2021) Heat Shock Protein 70 as a Sex-Skewed Regulator of alpha-Synucleinopathy. Neurotherapeutics 18: 2541–2564 Doi 10.1007/s13311-021-01114-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia TN, Jamenis AS, Abbas M, Clark RN, Miner KM, Chandwani MN, Kim RE, Hilinski W, O’Donnell LA, Luk KC et al. (2023) A 14-day pulse of PLX5622 modifies alpha-synucleinopathy in preformed fibril-infused aged mice of both sexes. Neurobiol Dis: 106196 Doi 10.1016/j.nbd.2023.106196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghammer P, Just MK, Horsager J, Skjaerbaek C, Raunio A, Kok EH, Savola S, Murayama S, Saito Y, Myllykangas L et al. (2022) A postmortem study suggests a revision of the dual-hit hypothesis of Parkinson’s disease. NPJ Parkinsons Dis 8: 166 Doi 10.1038/s41531-022-00436-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: 197–211 Doi 10.1016/s0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 7.Brunjes PC, Kay RB, Arrivillaga JP (2011) The mouse olfactory peduncle. J Comp Neurol 519: 2870–2886 Doi 10.1002/cne.22662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO (1998) Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry 55: 405–413 Doi 10.1001/archpsyc.55.5.405 [DOI] [PubMed] [Google Scholar]
- 9.Mason DM, Nouraei N, Pant DB, Miner KM, Hutchison DF, Luk KC, Stolz JF, Leak RK (2016) Transmission of alpha-synucleinopathy from olfactory structures deep into the temporal lobe. Mol Neurodegener 11: 49 Doi 10.1186/s13024-016-0113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason DM, Wang Y, Bhatia TN, Miner KM, Trbojevic SA, Stolz JF, Luk KC, Leak RK (2019) The center of olfactory bulb-seeded alpha-synucleinopathy is the limbic system and the ensuing pathology is higher in male than in female mice. Brain Pathol 29: 741–770 Doi 10.1111/bpa.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miner KM, Jamenis AS, Bhatia TN, Clark RN, Rajasundaram D, Sauvaigo S, Mason DM, Posimo JM, Abraham N, DeMarco BA et al. (2022) alpha-synucleinopathy exerts sex-dimorphic effects on the multipurpose DNA repair/redox protein APE1 in mice and humans. Prog Neurobiol 216: 102307 Doi 10.1016/j.pneurobio.2022.102307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson PT, Schmitt FA, Jicha GA, Kryscio RJ, Abner EL, Smith CD, Van Eldik LJ, Markesbery WR (2010) Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol 257: 1875–1881 Doi 10.1007/s00415-010-5630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pessoa L, Hof PR (2015) From Paul Broca’s great limbic lobe to the limbic system. J Comp Neurol 523: 2495–2500 Doi 10.1002/cne.23840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picillo M, Fasano A (2015) How much does sex matter in Parkinson disease? Neurology 84: 2102–2104 Doi 10.1212/WNL.0000000000001621 [DOI] [PubMed] [Google Scholar]
- 15.Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VM, Brundin P (2016) Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med 213: 1759–1778 Doi 10.1084/jem.20160368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharon R, Goldberg MS, Bar-Josef I, Betensky RA, Shen J, Selkoe DJ (2001) alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc Natl Acad Sci U S A 98: 9110–9115 Doi 10.1073/pnas.171300598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorokowski P, Karwowski M, Misiak M, Marczak MK, Dziekan M, Hummel T, Sorokowska A (2019) Sex Differences in Human Olfaction: A Meta-Analysis. Front Psychol 10: 242 Doi 10.3389/fpsyg.2019.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tremblay C, Abbasi N, Zeighami Y, Yau Y, Dadar M, Rahayel S, Dagher A (2020) Sex effects on brain structure in de novo Parkinson’s disease: a multimodal neuroimaging study. Brain: Doi 10.1093/brain/awaa234 [DOI] [PubMed] [Google Scholar]
- 19.Voyer D, Voyer SD, Saint-Aubin J (2017) Sex differences in visual-spatial working memory: A meta-analysis. Psychon Bull Rev 24: 307–334 Doi 10.3758/s13423-016-1085-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See SI files for raw data (also available upon emailing leakr@duq.edu).


