Abstract
Aims
The strength of the relationship of triglyceride-rich lipoproteins (TRL) with risk of coronary heart disease (CHD) compared with low-density lipoprotein (LDL) is yet to be resolved.
Methods and results
Single-nucleotide polymorphisms (SNPs) associated with TRL/remnant cholesterol (TRL/remnant-C) and LDL cholesterol (LDL-C) were identified in the UK Biobank population. In a multivariable Mendelian randomization analysis, TRL/remnant-C was strongly and independently associated with CHD in a model adjusted for apolipoprotein B (apoB). Likewise, in a multivariable model, TRL/remnant-C and LDL-C also exhibited independent associations with CHD with odds ratios per 1 mmol/L higher cholesterol of 2.59 [95% confidence interval (CI): 1.99–3.36] and 1.37 [95% CI: 1.27–1.48], respectively. To examine the per-particle atherogenicity of TRL/remnants and LDL, SNPs were categorized into two clusters with differing effects on TRL/remnant-C and LDL-C. Cluster 1 contained SNPs in genes related to receptor-mediated lipoprotein removal that affected LDL-C more than TRL/remnant-C, whereas cluster 2 contained SNPs in genes related to lipolysis that had a much greater effect on TRL/remnant-C. The CHD odds ratio per standard deviation (Sd) higher apoB for cluster 2 (with the higher TRL/remnant to LDL ratio) was 1.76 (95% CI: 1.58–1.96), which was significantly greater than the CHD odds ratio per Sd higher apoB in cluster 1 [1.33 (95% CI: 1.26–1.40)]. A concordant result was obtained by using polygenic scores for each cluster to relate apoB to CHD risk.
Conclusion
Distinct SNP clusters appear to impact differentially on remnant particles and LDL. Our findings are consistent with TRL/remnants having a substantially greater atherogenicity per particle than LDL.
Keywords: Apolipoprotein B, Mendelian randomization, Remnants, Cardiovascular disease, Genetics, Single-nucleotide polymorphisms, LDL cholesterol, Triglyceride, UK Biobank
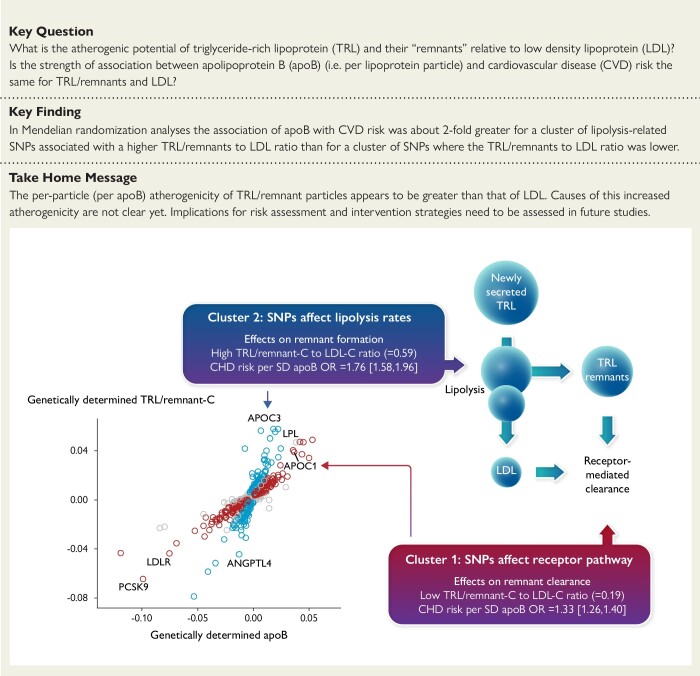
Structured Graphical Abstract
Structured Graphical Abstract.
Cluster-based SNP analysis showing higher per particle atherogenicity for triglyceride-rich lipoproteins/remnants than for low density lipoproteins.
See the editorial comment for this article ‘Triglyceride-rich remnant lipoproteins are more atherogenic than LDL per particle: is this important?’, by A. Tybjærg-Hansen et al., https://doi.org/10.1093/eurheartj/ehad419.
Introduction
Genetic studies reveal that the association between triglyceride-rich lipoproteins (TRL) and coronary heart disease (CHD) is likely causal,1,2 although the features of these lipoproteins that promote development of atherosclerosis are not yet clear.2 Most attention to date has focused on the cholesterol content of TRL and their ‘remnants’, the products of partial lipolysis of apolipoprotein (apo)B48-containing chylomicrons and apoB100-containing very-low-density lipoproteins (VLDL).1–5 In analogy with the pathogenic mechanisms linked to low-density lipoproteins (LDL), remnant lipoproteins can penetrate the sub-endothelial space in artery walls and bind to proteoglycans, thereby initiating cholesterol deposition and foam cell formation.2,6
Questions arise as to the strength of the relationship of TRL/remnants to CHD relative to the benchmark of LDL,7 and differing conclusions have been reached on this issue. On the one hand, some studies have shown that the CHD risk associated with a unit change in plasma apoB linked to variation in genes known to affect triglyceride (TG), and by extrapolation TRL, was quantitatively similar to the risk of the same change in apoB due to variation in genes affecting LDL.8 This observation led to the concept that the atherogenicity of TRL and LDL was broadly the same, and risk was a function of the number of apoB-containing particles in the circulation.9,10 Other studies have provided evidence that the risk linked to a given increase in TRL/remnant cholesterol (TRL/remnant-C) is substantially greater than that associated with the same increase in LDL cholesterol (LDL-C).1,11–13 Resolving these discordant findings is important since it impacts on risk assessment, on the design and interpretation of intervention trials, and ultimately on therapeutic strategy.
In the present investigation, rather than undertake Mendelian randomization (MR) studies based on instrumental variables derived using single-nucleotide polymorphisms (SNPs) from a single or few genes thought to alter the risk factor (exposure) of interest, we adopted a more agnostic, polygenic approach where all informative SNPs identified by genome-wide association studies (GWAS) of the risk factors (TRL/remnant-C and LDL-C) were included in the analysis.14 With this methodology, we evaluated the relationship of TRL/remnants to CHD risk in a large, well-characterized population—the UK Biobank. Our primary aim was to investigate the relative atherogenicity of TRL/remnants in relation to LDL. We also examined the nature of the genes influencing TRL/remnant-C levels and identified clusters of SNPs with differing effects on TRL/remnant-C relative to LDL-C.
Methods
Study population
This investigation utilized individual-level data from the UK Biobank population (over 502 000 UK residents of mainly European ancestry).15 Genetic instruments were derived using data from subjects who had the required plasma lipoprotein levels available and were not on lipid-lowering therapy at baseline (see Supplementary data online, Figure S1). Assessment of the association of genetically predicted lipid variables with CHD risk was carried out using the expanded group of subjects which included those on lipid-lowering treatment.
Lipid measurements
Low-density lipoprotein cholesterol was measured directly (Beckman Coulter, Brea, CA) (data field 30 780). Non–high-density lipoprotein (HDL) cholesterol was determined as the difference between plasma cholesterol (data field 30 690) and HDL cholesterol (data field 30 760).15,16 TRL/remnant-C was derived by subtracting direct LDL-C from non-HDL-C, and since it was based on measured parameters, it was deemed an indirectly ‘measured’ concentration (note the term ‘TRL/remnant’ is used throughout to recognize the fact that there is no clear definition of remnant particles that allows them to be identified separately from other TRL; they are part of a continuum).2 All other analytes were measured by standard laboratory methods (see online showcase of UK Biobank methods: https://biobank.ctsu.ox.ac.uk).
Genetic analyses
Genotyping with the UK BiLEVE Axiom or UK Biobank Axiom arrays provided an evaluation of 805 426 SNPs spanning the entire genome (see Supplementary data online, Figure S1).
Genome-wide association study
A GWAS adjusted for age, sex, and genetic principal components 1–5 was performed to identify SNPs associated with TRL/remnant-C and/or LDL-C. Single-nucleotide polymorphisms meeting the significance threshold of <5 × 10−8 were pruned for linkage disequilibrium (r2 < 0.1 with a window size of 20Mb) and minor allele frequency (threshold >0.01). If two SNPs were in linkage disequilibrium, the SNP with the largest combined effect size [square root of (LDL-C effect size squared plus TRL/remnant-C effect size squared)] was selected. The list was further filtered for association (Bonferroni–Holms adjusted P < 0.05) with lipoprotein(a), which excluded 28 SNPs. The process yielded a final set of 1125 SNPs (see Supplementary data online, Figure S1).
Definition of gene clusters
The SNPs identified by GWAS were assigned to clusters based on their effects on TRL/remnant-C (representing the concentration of TRL/remnant particles) relative to total apoB (representing the concentration of all apoB-containing lipoproteins). A frequency distribution of effect size ratio—genetically predicted difference in TRL/remnant-C in mmol/L divided by the genetically predicted difference in apoB in g/L for the minor compared with major allele—was generated, and two broad peaks were observed. Single-nucleotide polymorphisms in the first peak were assigned to cluster 1, and SNPs in the second peak were assigned to cluster 2. Single-nucleotide polymorphisms in the nadir between the peaks were unallocated (see Supplementary data online, Table S1 for list of SNPs having largest effect sizes in each cluster).
Generation of polygenic scores
The SNPs allocated to clusters 1 and 2 were used to create a polygenic score (PGS) for each cluster. Single-nucleotide polymorphisms were included in the PGSs based on their conditional association with apoB, using a ridge regression procedure. The final PGSs for each subject—one for cluster 1 and a second for cluster 2—were calculated as the sum of the number of apoB raising alleles present, weighted by their conditional additive contribution to explained variance in plasma apoB. For each cluster, the cohort was then divided into deciles of PGS and mean level of apoB was determined per decile.
Coronary heart disease outcomes
These are defined in Supplementary data online, Table S2. Estimation of odds ratios for CHD using MR models was based on the combination of prevalent and incident events (myocardial infarction and coronary revascularization). Evaluation of the relationship between apoB and CHD using the PGS for clusters 1 and 2 was based on incident events occurring during the ∼12-year follow-up period.
Statistical methods
Multivariable MR analyses based on the inverse variance–weighted (IVW) method (which assumes all variants are ‘valid’ instrumental variables; that is, the SNP effect on CHD outcome is solely through its effect on the exposure/risk factor17) were undertaken to determine genetic relationships between lipoprotein variables and the association of these variables with CHD outcomes. Beta coefficients (effect sizes) were derived using exposure data from subjects who had all required lipid measurements and were not on lipid-lowering treatment. Odds ratios for CHD outcomes were determined per unit change (1.0 mmol/L for lipids or 1.0 g/L for apoB) and per population standard deviation (Sd) in the variable of interest.
Polygenic scores were formulated as described above and used to provide within the cohort a cluster-based assessment of the relationships between variation in apoB and TRL/remnant-C and between apoB and CHD event rate. The hazard ratio (HR) for CHD events was estimated for each decile of the cluster 1 PGS and of the cluster 2 PGS by Cox proportional hazards modeling. To allow direct comparison of their association with CHD risk, the two PGS were scaled to units of apoB by linear regression and a single Cox proportional hazards model (adjusted for age, sex, and body mass index) constructed which included the cluster 1 PGS and cluster 2 PGS as continuous variables. The model was used to estimate a HR per Sd change in apoB for each SNP cluster.
Sensitivity/replication analyses
To explore the impact of choice of linkage disequilibrium threshold, further pruning of the GWAS SNP set was undertaken at r2 < 0.01 and r2 < 0.001, and odds ratios were estimated for key lipoprotein variables. The possible impact of outliers and pleiotropic effects (SNP variants influencing the outcome independent of any effect on the exposure) was examined using the MR-IVW with robust weighting, the MR-Egger,18 and the MR-Lasso methods19 (see Supplementary data online, File, for discussion of these statistical methods).17
The CARDIoGRAMplusC4D data set (http://www.cardiogramplusc4d.org/data-downloads/) was used in a replication analysis where, for SNPs in common (1049 of the 1125), the beta coefficients for lipid traits estimated using the UK Biobank were applied to SNP-CHD outcome data from this independent cohort (see Supplementary data online, File, for detailed methods). All statistical analyses were performed using R version 4.0.4. MR-analyses were performed using the R-package ‘MendelianRandomization’ v0.5.0.20
Results
The UK Biobank cohort comprises 502 460 men and women with a mean age of 56.5 years old at enrolment. Based on available measured lipid values, TRL/remnant-C could be derived for 350 110 participants not on lipid-lowering treatment. In these subjects, TRL/remnant-C correlated strongly with TG (r2 = 0.63, P < 0.0001) and moderately with LDL-C (r2 = 0.37, P < 0.0001) (see Supplementary data online, Figure S2). Assessment of the association of genetically predicted lipoprotein variation with CHD risk was undertaken in 487 202 subjects in whom the total number of events, prevalent plus incident, was 29 183. Association of apoB by PGS decile with incident CHD was examined in 478 811 subjects free of CHD at baseline who had a total of 20 792 events (see Supplementary data online, Table S2).
Association of genetically predicted triglyceride-rich lipoproteins/remnant cholesterol with coronary heart disease risk and its independence from low-density lipoprotein cholesterol and apolipoprotein B
Multivariable MR analyses revealed that TRL/remnant-C was associated with CHD risk independently of both apoB and LDL-C (Table 1). Furthermore, TRL/remnant-C was associated with a significantly greater CHD risk per 1.0 mmol/L difference than LDL-C with odds ratios of 2.59 (95% CI: 1.99–3.36) and 1.37 (95% CI: 1.27–1.48), respectively.
Table 1.
Multivariable Mendelian randomization modelsa of apolipoprotein B plus lipid variables and risk of an CHD event
| Multivariable MR models | CHD causal effect estimate | P value | |
|---|---|---|---|
| OR per unit change (95% CI)b | OR per Sd change (95% CI)c | ||
| Model 1 | |||
| ApoB | 2.99 (2.32–3.85) | 1.29 (1.22–1.37) | 2.0 × 10−17 |
| TRL/remnant-C | 2.47 (1.90–3.21) | 1.34 (1.23–1.46) | 1.9 × 10−11 |
| Model 2 | |||
| LDL-C | 1.37 (1.27–1.48) | 1.29 (1.22–1.38) | 4.0 × 10−16 |
| TRL/remnant-C | 2.59 (1.99–3.36) | 1.36 (1.25–1.48) | 1.2 × 10−12 |
ApoB, apolipoprotein B; CI, confidence interval; GWAS, genome-wide association study; LDL-C, low-density lipoprotein cholesterol; MR, Mendelian randomization; OR, odds ratio; Sd, standard deviation; SNP, single-nucleotide polymorphism; remnant-C, remnant cholesterol; TG, triglycerides; TRL, triglyceride-rich lipoprotein.
Multivariable randomization models used the 1125 SNPs identified by GWAS and the inverse variance–weighted method for odds ratio calculation. The potential impact of SNP pleiotropic effects was tested as set out in Supplementary data online, Table S5. The subject cohort used in this analysis involved all subjects in whom TRL/remnant-C could be determined.
OR per 1.0 g/L change in apoB or per 1.0 mmol/L change in TG, LDL-C, and TRL/remnant-C.
OR per population Sd change in respective variable (apoB Sd, 0.23 g/L; TRL/remnant-C Sd, 0.30 mmol/L; LDL-C Sd, 0.82 mmol/L).
To test if these findings were replicated in a separate data set, we used the SNPs and corresponding beta coefficients for lipid traits estimated in the UK Biobank and applied them to outcome data from the CARDIoGRAMplusC4D cohort. As shown in Supplementary data online, Table S3, the finding that TRL/remnant-C remained a significant predictor of CHD risk in a model adjusted for apoB, or in a model that included LDL-C, was validated in this cohort.
Identifying two single-nucleotide polymorphism clusters with differing effects on triglyceride-rich lipoproteins/remnant cholesterol and low-density lipoprotein cholesterol
The aim of the following analyses was to investigate the per-particle atherogenicity (change in CHD risk per unit change in particle number) of TRL/remnants relative to LDL.
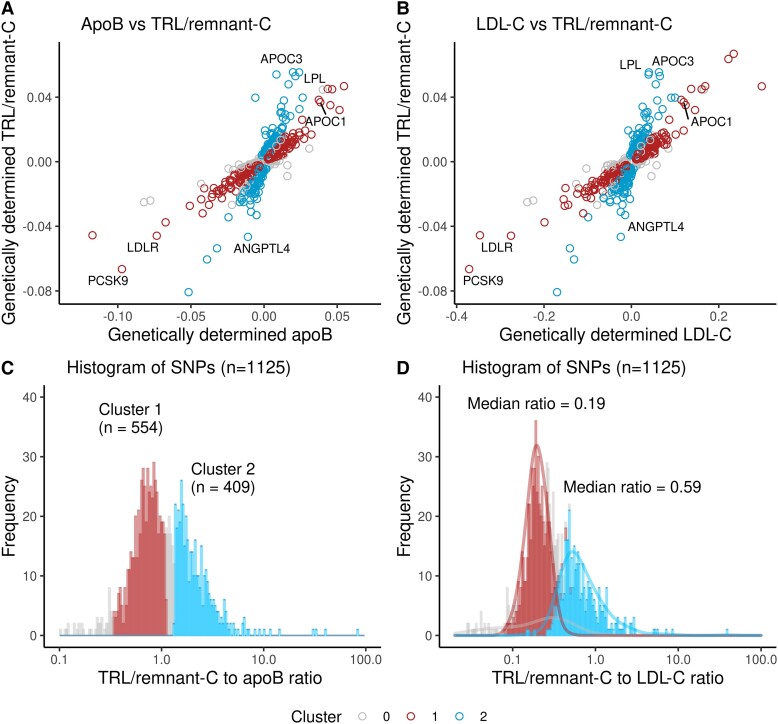
Our approach was first to build on the observation in Table 1 that TRL/remnant-C—a marker of the abundance of TRL/remnant particles—was a strong predictor of risk statistically independent of apoB level which reflects the concentration of all apoB-containing lipoproteins. Figure 1A plots for each SNP identified in the GWAS its effect size (beta coefficient) on TRL/remnant-C relative to its effect size on apoB. The SNPs appeared to fall into two groups; one (marked blue) had a much greater effect on TRL/remnant-C per unit difference in apoB than the other (marked red). When the frequency distribution of SNP effect sizes for TRL/remnant-C vs. apoB was plotted, it was found to be approximately bi-modal (Figure 1C). Accordingly, SNPs were divided formally into two ‘clusters’; those SNPs (n = 554) with a lower effect size ratio for TRL/remnant-C relative to apoB—between 0.33 and 1.1 (approximate boundaries of the first peak)—were allocated to cluster 1, while SNPs (n = 409) with a higher effect size ratio, above 1.33 (lower boundary of the second peak), were allocated to cluster 2. Most unallocated SNPs (n = 162) had a ratio that was between the limits used to define the clusters (Figure 1C).
Figure 1.
Identification of genes influencing TRL/remnant cholesterol. Plotting the beta coefficients (beta coefficients can be interpreted as the amount that a SNP, per effect-allele, increases or decreases the biomarker in question. Hence, it can be interpreted as a genetically predicted change in apoB, LDL-C, and TRL/remnant-C. For LDL-C and TRL/remnant-C, the effect, per allele, is measured in mmol/L cholesterol, and for apoB, the effect is measured in g/L) for TRL/remnant-C vs. apoB or LDL-C (for the 1125 SNPs identified in the GWAS) revealed the presence of two clusters. In one cluster, SNPs (marked blue) were characterized by having a larger effect size on TRL/remnant-C relative to apoB (A) or to LDL-C (B), while in the other cluster (marked red), SNPs had a smaller effect on TRL/remnant-C relative to apoB or to LDL-C. (C) Histogram of the TRL/remnant-C to apoB beta coefficient ratio for the 1125 SNPs. The two clusters were defined formally based on range limits for the ratio of TRL/remnant-C to apoB. Single-nucleotide polymorphisms were allocated to cluster 1 if they had a ratio between 0.33 and 1.1 and to cluster 2 if the ratio was >1.33. (D) Distribution of SNP effect sizes on TRL/remnant-C relative to LDL-C in the two defined clusters.
Second, we plotted the SNP effect sizes for TRL/remnant-C vs. LDL-C (Figure 1B) and these showed the same pattern as in Figure 1A. This is not surprising since most apoB is carried in LDL and LDL-C exhibited a constant effect size per unit difference in apoB across the SNP set. (see Supplementary data online, Figure S3). Thus, the clusters identified in Figure 1C had different effect size ratios for TRL/remnant-C relative to LDL-C (Figure 1D). Cluster 2 had a median genetically predicted TRL/remnant-C to LDL-C ratio of 0.59, while for cluster 1, this ratio was 0.19. The clusters could now be used to ascertain if the per-particle atherogenicity of TRL/remnants differed from that of LDL. Since each of these lipoprotein particles contains one apoB protein, we would be able to test if the strength of the association of apoB with CHD risk was the same or different depending on the proportion of TRL/remnant particles vs. LDL.
Supplementary data online, Table S1, illustrates the outcome of this categorization by giving the effect sizes and TRL/remnant-C to apoB ratio for the 40 SNPs with the largest effect size allocated to clusters 1 and 2. It was noteworthy that SNPs in cluster 1 included variants in the genes for proprotein convertase subtilisin/kexin 9 (PCSK9), the LDL receptor (LDLR), and apoB (APOB) (as annotated in Figure 1). In contrast, SNPs in cluster 2 included variants in genes for angiopoietin-like protein 4 (ANGPTL4), apoCIII (APOC3), and lipoprotein lipase (LPL). The functional distinction between the SNP clusters was evident also in their effects on TG vs. apoB (see Supplementary data online, Figure S3). Cluster 1 SNPs were not associated strongly with genetically predicted differences in TG, whereas cluster 2 SNPs had a substantial effect on TG.
Association of apolipoprotein B with coronary heart disease risk in single-nucleotide polymorphism clusters 1 and 2
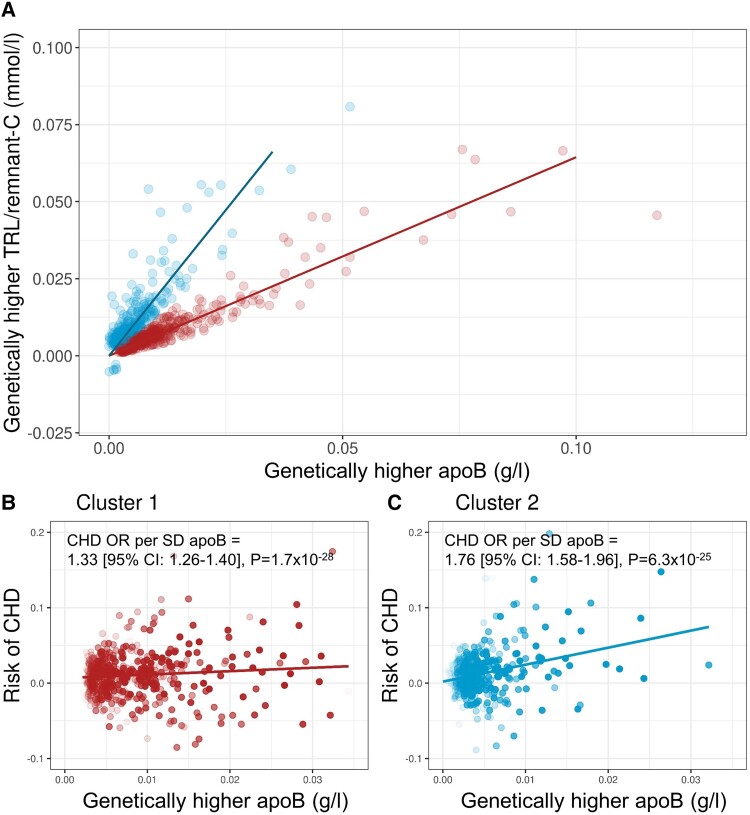
To assess the association of apoB with CHD risk in each cluster, the exposure allele was defined as the variant that raised apoB. Figure 2A gives the gradient of association of TRL/remnant-C with apoB for each SNP cluster. Figure 2B and C shows for each cluster the relationship of genetically predicted difference in apoB to risk of an CHD event. The odds ratio for a CHD outcome per population Sd higher apoB was 1.76 (95% CI: 1.58–1.96) in cluster 2 vs. 1.33 (95% CI: 1.26–1.40) in cluster 1. These results were also replicated in the CARDIoGRAMplusC4D cohort with odds ratios at 1.68 (95% CI: 1.52–1.85) for cluster 2 and 1.44 (95% CI: 1.36–1.52) for cluster 1 (see Supplementary data online, Table S3).
Figure 2.
Association of apoB with TRL-C and CHD risk in clusters 1 and 2. (A) Association of TRL/remnant-C with apoB in each SNP cluster with the exposure allele defined as the variant raising apoB. ApoB has units of g/L, and TRL/remnant-C has units of mmol/L. Panels (B) and (C) show, for clusters 1 and 2, respectively, each SNPs’ effect on apoB and on CHD (prevalent + incident) outcome [note that the x-axis for cluster 1 in (B) has been truncated to allow better visual comparison with the apoB range for cluster 2 in (C)]. Data points in (B) and (C) are colored as less translucent the lower the P value for apoB. Mendelian randomization modeling (inverse variance–weighted method) was used to determine the odds ratio (OR) for CHD risk per Sd change in apoB (0.23 g/L) for each cluster in the cohort of subjects off or on lipid-lowering treatment.
Association of apolipoprotein B with coronary heart disease risk by cluster-specific polygenic scores
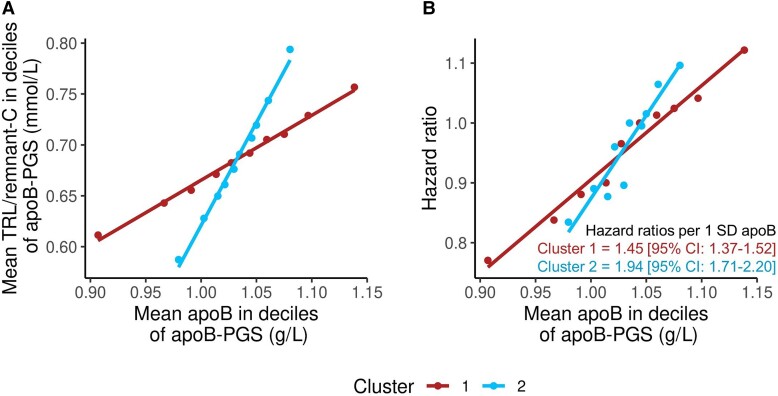
The cohort was divided into deciles of cluster 1 PGS and cluster 2 PGS, and for each decile, TRL/remnant-C and incident CHD event rate were related to apoB level (Figure 3). The PGS for clusters 1 and 2 showed a clear differentiation with respect to change in TRL/remnant-C relative to apoB across the deciles of the scores (Figure 3A). A difference in gradient between clusters in the quantitative relationship of apoB to risk of a CHD event was also evident (Figure 3B). In a Cox proportional hazards model which included the two PGS, expressed per Sd higher apoB, the HR for cluster 2 PGS at 1.94 (95% CI: 1.71–2.20) was significantly greater than that for cluster 1 PGS at 1.45 (95% CI: 1.37–1.52).
Figure 3.
Association of polygenic scores for clusters 1 and 2 with TRL/remnant-C and CHD risk. Subjects without a history of CHD were divided into deciles according to the cluster-specific PGS; for each PGS, the relationship of apoB to TRL/remnant-C was examined (A). For illustrative purposes, hazard ratios (HRs) for CHD events (using decile 5 as referent for each PGS) were estimated for each decile of cluster 1 PGS and cluster 2 PGS (B). To compare the gradient of association of apoB with CHD risk between clusters, a single Cox model was constructed that included the scaled cluster 1 PGS and cluster 2 PGS as continuous variables. The derived HRs per 1 Sd change in apoB are given in (B).
Sensitivity analyses
The impact of varying the significance threshold for linkage disequilibrium pruning was examined by re-deriving the GWAS SNP set using r2 criteria of <0.01 and <0.001. It can be seen in Supplementary data online, Table S4, that the odds ratios for the association of TRL/remnant-C and LDL-C with CHD risk were in close agreement regardless of the r2 threshold chosen.
The possibility that SNP pleiotropic effects and/or invalid instrumental variables had influenced the results of the multivariable MR analyses presented above was examined as detailed in Supplementary data online, Table S5. A range of statistical methods that evaluate, and are tolerant of, potential bias was used to generate causal risk estimates. The close agreement in odds ratio estimates across all methods indicated that it was unlikely that the results seen using the inverse variance–weighted approach was substantially biased.
Discussion
The principal findings of the present study are clear and of importance in understanding the role of lipoproteins in atherosclerosis. First, TRL/remnant-C was a strong predictor of CHD risk independent of apoB and LDL-C. Second, two major SNP clusters were identified that had differing genetic effects on TRL/remnant-C relative to LDL-C. Third, the increment in CHD risk per particle (per apoB) was approximately two-fold greater in the SNP cluster with the larger effect on TRL/remnants. These observations indicate that TRL/remnant particles have a substantially greater atherogenicity than LDL (Structured Graphical Abstract).
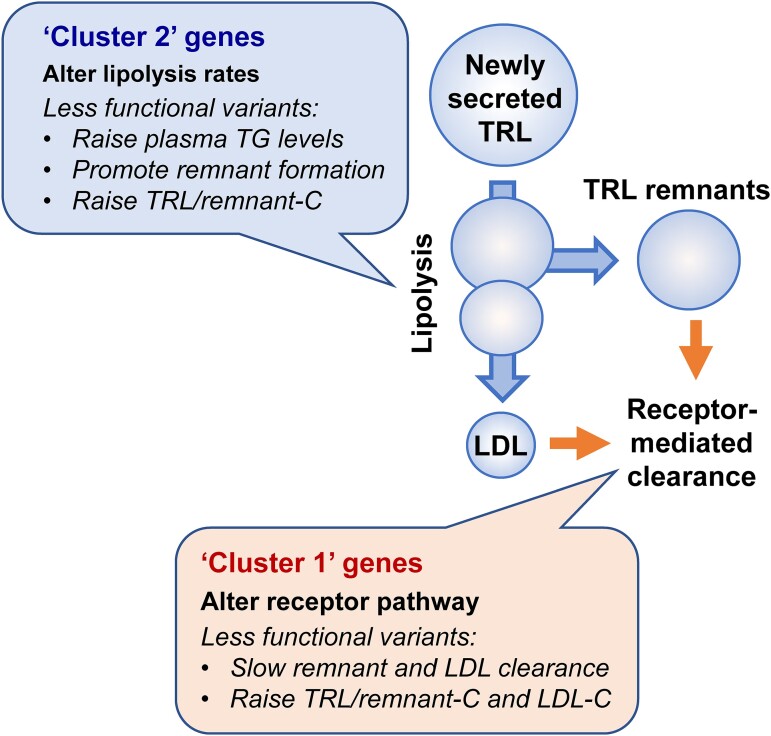
Our cluster-based analysis also revealed genetic aspects of the regulation of TRL/remnant levels. While assignment of SNPs to each cluster was based empirically on their effects on TRL/remnants relative to plasma apoB, the underlying reason for their categorization can be understood in light of the impact of the associated genetic loci on the metabolism of apoB-containing lipoproteins (Figure 4). Cluster 1 included SNPs linked to genes known to alter the activity of lipoprotein receptor pathways (such as PCSK9, APOB, and LDLR), and for these variants, change in TRL/remnant-C was accompanied by substantial changes in LDL-C and plasma apoB levels. As depicted in Figure 4, these SNPs influence the efficiency of receptor pathways (the LDL receptor per se or the ligand apoB) and likely alter, in concert, remnant lipoprotein and LDL clearance.21 The effect of cluster 1 SNPs on TG was modest, possibly because the associated genes do not impact on the levels of TG-rich, newly secreted TRL particles entering the circulation. Cluster 2 included SNPs in genes linked to variation in TRL lipolysis (such as LPL, ANGPTL4, and APOC3), and changes in TRL/remnant-C of a similar magnitude to those seen in cluster 1 were accompanied by much smaller changes in LDL-C and apoB. These SNPs affecting the lipolysis pathway likely influence the rate of remnant formation.2,21
Figure 4.
Impact of variation in genes influencing lipolysis and those influencing lipoprotein receptors on TRL/remnant and LDL metabolism. In this schematic, the putative differential effects of variants of reduced functionality are depicted in boxes. Triglyceride-rich lipoproteins remnants are defined as lipoprotein particles that have undergone partial lipolysis.2,21,22 SNPs in genes that cause a reduction in the efficiency of lipolysis (‘cluster 2’ SNPs) potentially increase the rate of TRL/remnant particle formation but have smaller effects on LDL. Since TRL/remnants contain apoB, there is a modest increment in plasma total apoB levels. Metabolic studies have established that VLDL- and chylomicron-remnants are cleared from the circulation by the LDL receptor and possibly other receptors binding to apoB on the particle surface and facilitating endocytosis and degradation.2,21 Cluster 1 SNPs which reduce the activity of the LDL receptor or alter the ligand, apoB, affect remnant clearance and cause increases in the concentration of both TRL/remnant and LDL particles with a consequent substantial rise in plasma apoB.
Previous studies reached differing conclusions regarding the potential atherogenicity of TRL/remnants relative to LDL. A combined cohort analysis8 showed that SNPs linked to the LPL gene with effects on TG, and SNPs linked to the LDLR gene with effects on LDL-C, had the same impact on CHD risk when the associated change in apoB was equalized. Further, it was found that genetic variants linked to plasma TG had no predictive value in models that included apoB. These observations led to the conclusion that the primary biomarker of risk was the number of apoB-containing particles, be they TRL or LDL, and that each particle had a similar atherogenic potential. In contrast, extensive reports from the Copenhagen General Population Study1,11–13,23 and other cohorts12 indicated that TRL cholesterol or remnant cholesterol was associated with a higher CHD risk per mmol/L increase than LDL-C, and since remnants have a higher cholesterol/apoB ratio than LDL,2 this implies a greater per-particle atherogenicity for the former as compared with the latter.
The results of the present investigation based on the UK Biobank are in accordance with these findings in the Danish population; that is, per 1 mmol/L, TRL/remnant-C was associated with a higher relative risk for CHD than LDL-C. Our findings are also in line with the recent observation that genetic variants associated with a higher non-HDL-C/apoB ratio (linked in turn to SNPs affecting TG) are associated with increased CHD risk compared with variants associated with a lower non-HDL-C/apoB ratio.24 The apparent discordancy between the present and earlier8 results regarding the risk associated with apoB in TRL/remnants vs. LDL may be attributable, at least in part, to the choice of SNPs used as genetic instrumental variables and plasma lipid exposures (TG vs. TRL/remnant-C). In adopting a polygenic approach to MR, our aim was to reflect better the complexity of metabolic pathways that determine TRL/remnant concentrations. Indeed, TRL/remnant levels are the net result of multiple factors regulating the rates of formation and removal of these particles of which the action of lipoprotein lipase is just one key element among others.21 Further, the use of variants linked to the LDL receptor gene as an instrumental variable for LDL-C is compromised by the revelation from metabolic studies that the LDL receptor is involved also in TRL/remnant clearance.2,21
The key question that arises from the present and earlier investigations involves the mechanistic basis of the enhanced atherogenicity of a remnant particle.22,25 It may be because remnant particles contain more cholesterol per apoB. However, since the ‘atherogenicity’ of TRL/remnant-C is also higher than that of LDL-C, this implies that the per-particle impact on atherosclerotic processes must be even greater than can be accounted for by the cholesterol content alone. The high atherogenic potential may be related to apoproteins present on the remnant’s surface that enhance interaction with proteoglycans (apoE and apoC-III)25 or the presence of lysophospholipids, partially digested glycerides, or minor lipids such as ceramide that are cytotoxic or stimulate inflammatory mechanisms in the artery wall. Observations such as those reported here highlight the need for further evaluation of the role of TRL/remnant particles in atherogenesis. In this regard, it is of interest to note recent novel insights into the interaction of TRL particles with endothelial cells leading to intracellular lipid accumulation.26
The present investigation has limitations. The main one is that the core analysis was performed using a single, large cohort of mainly white European ancestry. However, our key results were replicated when the derived SNP exposure estimates were applied to the CARDIoGRAMplusC4D data set. There was also broad agreement between the present findings and those from the Copenhagen General Population Study.1,11–13,23 Our ‘measurement’ of TRL/remnant-C was indirect, and there will be accumulated analytical errors in the values obtained. No measurement of TRL/remnant apoB was available, and so, the association of apoB with risk in TRL/remnants vs. LDL was inferred by analysis of clusters of SNPs that differentially affected the levels of TRL/remnants vs. those of LDL. Finally, in using a polygenic approach, we cannot eliminate the possibility that pleiotropic effects confounded the results, although the likelihood that this was a major issue is diminished in light of the multiple statistical methods used to validate the results of the MR analyses.
In conclusion, we have shown that the strength of association of apoB with CHD risk is not uniform; rather, it depends on which particle the apoB resides; TRL/remnant particles appear to have an inherent atherogenicity that is substantially greater than that of LDL. The implications of the present investigation and earlier findings4,27 are (i) that use of non-HDL-C as a single, aggregate marker of risk may not provide as accurate prediction of CHD risk as first thought since its component parts—TRL/remnant-C and LDL-C—appear to have differing quantitative associations with risk and (ii) that interventions targeted to regulate TRL/remnant lipoproteins may prove beneficial, especially when addressing the residual TRL/remnant-associated risk in statin-treated patients. While we found that TRL/remnant-C was associated with a higher risk per 1 mmol/L increment compared with LDL-C, the odds ratios per Sd for these two lipid variables were similar. Since TRL/remnant-C concentrations were on average ∼five-fold lower than LDL-C, the latter will contribute quantitatively more to CHD risk than TRL/remnants on a population basis, but the relative impact will change as TG levels rise. Finally, there is a pressing need not only to develop more refined methods for assessment of remnant concentrations but also to improve the understanding of the molecular basis of the atherogenicity of this lipoprotein species.
Supplementary Material
Acknowledgements
We thank Dr Carl Bonander for statistical advice on causal inference and general helpful discussions. This research has been conducted using the UK Biobank Resource under application number [53308]. Our thanks are due also to Prof. M John Chapman for his review of the manuscript and for his constructive and insightful comments.
Contributor Information
Elias Björnson, Department of Molecular and Clinical Medicine, University of Gothenburg, Sahlgrenska University Hospital, 41345 Gothenburg, Sweden.
Martin Adiels, Department of Molecular and Clinical Medicine, University of Gothenburg, Sahlgrenska University Hospital, 41345 Gothenburg, Sweden; School of Public Health and Community Medicine, University of Gothenburg, Medicinaregatan 18A, 41390 Gothenburg, Sweden.
Marja-Riitta Taskinen, Research Program for Clinical and Molecular Metabolism, University of Helsinki, Biomedicum 1, Haartmanninkatu 8, 00290 Helsinki, Finland.
Stephen Burgess, MRC Biostatistics Unit, University of Cambridge, Robinson Way, Cambridge, CB2 0SR, UK; Cardiovascular Epidemiology Unit, Victor Phillip Dahdaleh Heart and Lung Research Institute, University of Cambridge, Papworth Road, Cambridge, CB2 0BD, UK.
Aidin Rawshani, Department of Molecular and Clinical Medicine, University of Gothenburg, Sahlgrenska University Hospital, 41345 Gothenburg, Sweden.
Jan Borén, Department of Molecular and Clinical Medicine, University of Gothenburg, Sahlgrenska University Hospital, 41345 Gothenburg, Sweden.
Chris J Packard, Institute of Cardiovascular and Medical Sciences, University of Glasgow, 126 University Place, G12 8TA Glasgow, UK.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
MRT declares grants from the Novo Nordisk Foundation, Finnish Foundation for Cardiovascular Research, Sigrid Juselius Foundation and Amgen. Consulting fees from Novartis, Novo Nordisk, Akcea and Amgen. Honoraria from Novartis, Akcea, Amgen, Novo Nordisk and Mylan. Support for attending meetings from Novartis, Amgen and Akcea. Participation in Advisory Boards for Novartis, Akcea, Amgen, Chiesi Pharma, Eli Lilly and Novo Nordisk. SB declares support from Wellcome Trust/Royal Society, UK Research and Innovation (UKRI) Medical Research Council and National Institute for Health Research Cambridge Biomedical Research Centre. JB declares grants from the NovoNordisk Foundation, Swedish Heart-Lung Foundation, Swedish Research Council, Knut and Alice Wallenberg Foundation and Sahlgrenska Hospital ALF. Consulting fees from Novartis, Novo Nordisk, Akcea and Amgen. Honoraria from Novartis, Akcea, Amgen, Novo Nordisk and Pfizer. Participation in Advisory Boards for Novartis, Akcea and Amgen. CP declares a grant from Pfizer. Consulting fees from Amgen, Amarin, MSD, Dalcor and Novartis. Honoraria from Daiichi-Sankyo, Novartis and Amarin.
Data Availability
Data analysed in this study was made available through the UK Biobank. Data is available upon application to UK Biobank https://www.ukbiobank.ac.uk.
Funding
The work in this paper was supported by grants from Swedish Research Council, Swedish Heart Lung Foundation, from the Swedish state under the agreement between the Swedish government and the county councils; the ALF-agreement (ALFGBG-965404), Sigrid Juselius Foundation, Helsinki, Finland, and the Finnish Foundation for Cardiovascular Research. SB is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (204623/Z/16/Z). This research was funded by United Kingdom Research and Innovation Medical Research Council (MC_UU_00002/7) and supported by the National Institute for Health Research Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care. For the purpose of open access, the author has applied a CC-BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Ethical Approval
This research has been conducted using the UK Biobank Resource under application number [53308].
Pre-registered Clinical Trial Number
Not applicable.
References
- 1. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res 2016;118:547–563. 10.1161/CIRCRESAHA.115.306249 [DOI] [PubMed] [Google Scholar]
- 2. Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, et al. . Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J 2021;42:4791–4806. 10.1093/eurheartj/ehab551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dallinga-Thie GM, Kroon J, Boren J, Chapman MJ. Triglyceride-rich lipoproteins and remnants: targets for therapy? Curr Cardiol Rep 2016;18:67. 10.1007/s11886-016-0745-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varbo A, Nordestgaard BG. Remnant lipoproteins. Curr Opin Lipidol 2017;28:300–307. 10.1097/MOL.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 5. Boren J, Taskinen MR, Bjornson E, Packard CJ. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat Rev Cardiol 2022;19:577–592. 10.1038/s41569-022-00676-y [DOI] [PubMed] [Google Scholar]
- 6. Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832–1844. 10.1161/CIRCULATIONAHA.106.676890 [DOI] [PubMed] [Google Scholar]
- 7. Salinas CAA, Chapman MJ. Remnant lipoproteins: are they equal to or more atherogenic than LDL? Curr Opin Lipidol 2020;31:132–139. 10.1097/MOL.0000000000000682 [DOI] [PubMed] [Google Scholar]
- 8. Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, et al. . Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 2019;321:364–373. 10.1001/jama.2018.20045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, et al. . Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol 2019;4:1287–1295. 10.1001/jamacardio.2019.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marston NA, Giugliano RP, Melloni GEM, Park JG, Morrill V, Blazing MA, et al. . Association of apolipoprotein B-containing lipoproteins and risk of myocardial infarction in individuals with and without atherosclerosis: distinguishing between particle concentration, type, and content. JAMA Cardiol 2022;7:250–256. 10.1001/jamacardio.2021.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 12. Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A, et al. . Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J 2021;42:4324–4332. 10.1093/eurheartj/ehab432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varbo A, Nordestgaard BG. Directly measured vs. calculated remnant cholesterol identifies additional overlooked individuals in the general population at higher risk of myocardial infarction. Eur Heart J 2021;42:4833–4843. 10.1093/eurheartj/ehab293 [DOI] [PubMed] [Google Scholar]
- 14. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. . Guidelines for performing Mendelian randomization investigations. Wellcome Open Res 2019;4:186. 10.12688/wellcomeopenres.15555.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. . UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welsh C, Celis-Morales CA, Brown R, Mackay DF, Lewsey J, Mark PB, et al. . Comparison of conventional lipoprotein tests and apolipoproteins in the prediction of cardiovascular disease. Circulation 2019;140:542–552. 10.1161/CIRCULATIONAHA.119.041149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun 2020;11:376. 10.1038/s41467-019-14156-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 2017;32:377–389. 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rees JMB, Wood AM, Dudbridge F, Burgess S. Robust methods in Mendelian randomization via penalization of heterogeneous causal estimates. PLoS One 2019;14:e0222362. 10.1371/journal.pone.0222362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Broadbent JR, Foley CN, Grant AJ, Mason AM, Staley JR, Burgess S. Mendelianrandomization v0.5.0: updates to an R package for performing Mendelian randomization analyses using summarized data. Wellcome Open Res 2020;5:252. 10.12688/wellcomeopenres.16374.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Packard CJ, Boren J, Taskinen MR. Causes and consequences of hypertriglyceridemia. Front Endocrinol (Lausanne) 2020;11:252. 10.3389/fendo.2020.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. . Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345–1361. 10.1093/eurheartj/ehr112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 2013;61:427–436. 10.1016/j.jacc.2012.08.1026 [DOI] [PubMed] [Google Scholar]
- 24. Helgadottir A, Thorleifsson G, Snaebjarnarson A, Stefansdottir L, Sveinbjornsson G, Tragante V, et al. . Cholesterol not particle concentration mediates the atherogenic risk conferred by apolipoprotein B particles: a Mendelian randomization analysis. Eur J Prev Cardiol 2022;29:2374–2385. 10.1093/eurjpc/zwac219 [DOI] [PubMed] [Google Scholar]
- 25. Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. . Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020;41:2313–2330. 10.1093/eurheartj/ehz962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cabodevilla AG, Tang S, Lee S, Mullick AE, Aleman JO, Hussain MM, et al. . Eruptive xanthoma model reveals endothelial cells internalize and metabolize chylomicrons, leading to extravascular triglyceride accumulation. J Clin Invest 2021;131:e145800. 10.1172/JCI145800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lotta LA, Stewart ID, Sharp SJ, Day FR, Burgess S, Luan J, et al. . Association of genetically enhanced lipoprotein lipase-mediated lipolysis and low-density lipoprotein cholesterol-lowering alleles with risk of coronary disease and type 2 diabetes. JAMA Cardiol 2018;3:957–966. 10.1001/jamacardio.2018.2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data analysed in this study was made available through the UK Biobank. Data is available upon application to UK Biobank https://www.ukbiobank.ac.uk.