Abstract
Cardiovascular disease is the leading cause of death in women and men globally, with most due to atherosclerotic cardiovascular disease (ASCVD). Despite progress during the last 30 years, ASCVD mortality is now increasing, with the fastest relative increase in middle-aged women. Missed or delayed diagnosis and undertreatment do not fully explain this burden of disease. Sex-specific factors, such as hypertensive disorders of pregnancy, premature menopause (especially primary ovarian insufficiency), and polycystic ovary syndrome are also relevant, with good evidence that these are associated with greater cardiovascular risk. This position statement from the European Atherosclerosis Society focuses on these factors, as well as sex-specific effects on lipids, including lipoprotein(a), over the life course in women which impact ASCVD risk. Women are also disproportionately impacted (in relative terms) by diabetes, chronic kidney disease, and auto-immune inflammatory disease. All these effects are compounded by sociocultural components related to gender. This panel stresses the need to identify and treat modifiable cardiovascular risk factors earlier in women, especially for those at risk due to sex-specific conditions, to reduce the unacceptably high burden of ASCVD in women.
Keywords: Women, Atherosclerotic cardiovascular disease, Sex-specific risk, Lipids, Cholesterol, Triglycerides, Lipoprotein(a)
Graphical Abstract
Graphical Abstract.
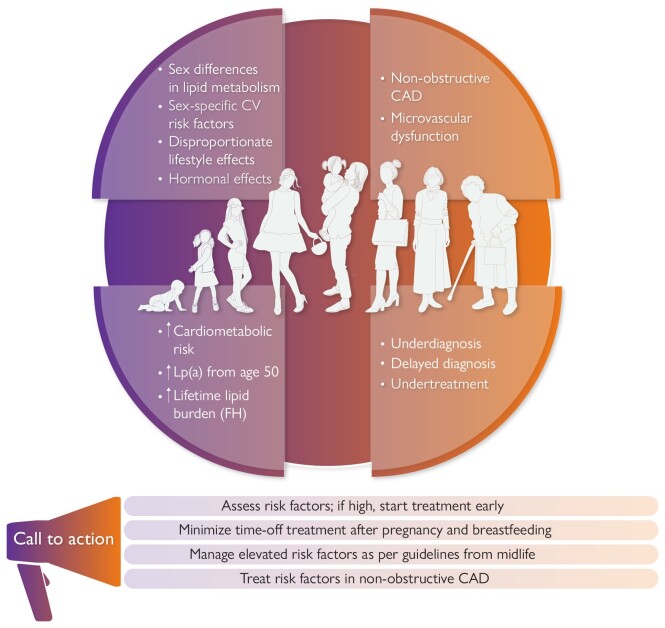
Key messages from this EAS position statement. In addition to sex-specific risk factors, women are disproportionately impacted by some lifestyle factors which increase cardiometabolic risk, and atherosclerotic cardiovascular disease presentation differs from that in men. Action is needed to change the perception of risk, assess and treat elevated risk factors early, and address gaps in the management and understanding of cardiovascular risk in women. CAD, coronary artery disease; CV, cardiovascular; FH, familial hypercholesterolaemia; Lp(a), lipoprotein(a).
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in women and men, and its incidence continues to increase as the pandemics of obesity and cardiometabolic disease escalate.1–3 Among adults <65 years, men have higher absolute ASCVD event rates than women, but in Europe and the USA, the fastest relative increase in ASCVD mortality is in middle-aged women (45–64 years).1,2 Thus, a focus on ASCVD risk in women is important.
Missed or delayed diagnosis and undertreatment of ASCVD are key contributors,4,5 with evidence that women are less likely than men to receive guideline-recommended preventive therapies.6–10 Familial hypercholesterolaemia (FH) exemplifies this. Global data11 show that women are diagnosed later and undertreated, and with time-off lipid-lowering therapy (LLT) during pregnancy and breast feeding, have greater cumulative cholesterol exposure than men with FH,12 possibly explaining why the relative impact of FH on cardiovascular risk is higher in women than men.13,14 The effects of traditional and risk-enhancing factors also differ in women vs. men.15,16 Sex-specific factors such as pregnancy-related complications, polycystic ovary syndrome (PCOS), and premature menopause also adversely influence cardiometabolic risk factors and impact atherosclerosis progression.17–19 Evaluating women for cardiovascular risk, ideally from midlife,20 would improve early identification of those with elevated modifiable risk factors or sex-specific risk factors, and prompt early initiation of guideline-recommended treatment.
This European Atherosclerosis Society (EAS) position statement is a ‘call to action’ for improving ASCVD prevention strategies in women, with a focus on sex differences in lipids over the life course. The panel acknowledges that while ‘female’ refers to an individual’s biological sex, and ‘woman’ refers to an individual’s gender identity, historically these terms have been used interchangeably in the literature. Therefore, this statement uses the term ‘women’ for consistency.
Do cardiovascular risk factors differ in women?
While both sexes share many of the traditional cardiovascular risk factors, the impact of these may differ in women vs. men.21 For example, although more prevalent in men,22 diabetes confers a greater relative (although not necessarily absolute) increase in cardiovascular risk in women vs. men of all ages.23–27 In part this may relate to greater adiposity and more cardiovascular risk factors in women than men at the time of diagnosis,27–29 as well as sex-specific risk factors for diabetes (e.g. PCOS and gestational diabetes).27 Women are also typically less physically active and have a higher body mass index (BMI) than men,30 which is known to associate with ASCVD risk.31
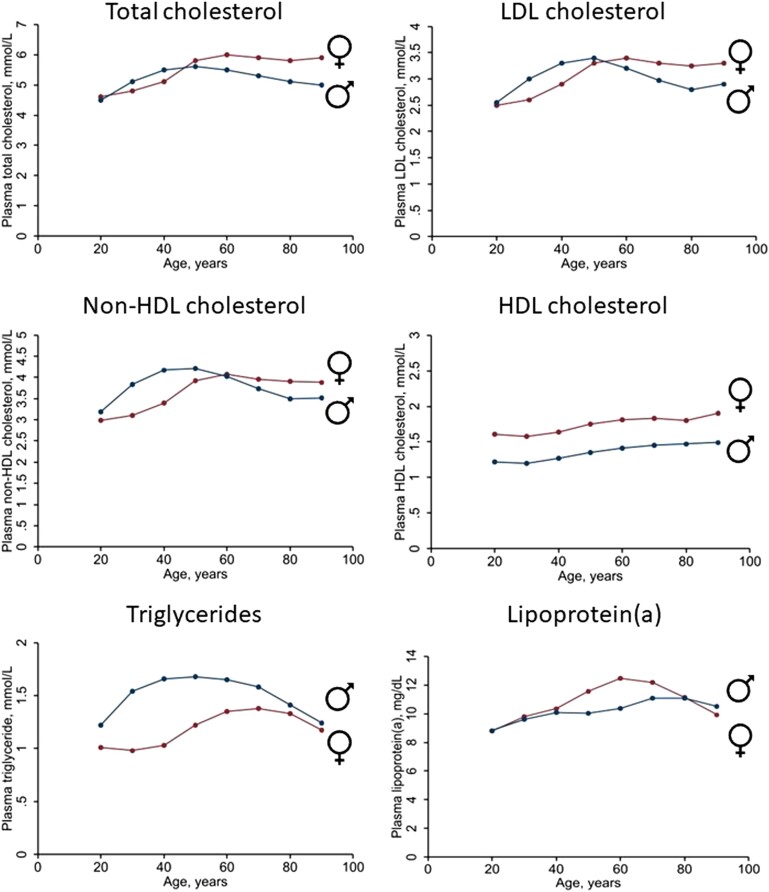
Disentangling the effects of declining oestradiol levels at menopause from ageing is difficult and much debated. Most of the large longitudinal studies with measurements before, during, and after menopause transition show changes in cardiovascular risk factors including weight gain, visceral adiposity, adverse effects on lipids (Figure 1),32 and increases in inflammatory markers and blood pressure, especially systolic blood pressure.33–37 Whether these changes also associate with increased risk for ASCVD is more contentious. Two longitudinal studies (249 and 890 subjects)38,39 reported progression of carotid intima-media thickness (CIMT) related to the menopause, independent of baseline age, although another study (up to 3892 subjects) showed no association between menopausal transition and CIMT progression.37 This latter study did, however, suggest that increasing adiposity and blood glucose with menopausal transition may impact diabetes risk.37 Added to this, premature menopause was shown to be associated with an increased relative risk of incident ASCVD compared with similarly-aged women without premature menopause, especially in those with premature ovarian insufficiency with menopause before the age of 40 years.18,40,41 Women with PCOS have an increased relative risk of cerebrovascular events but not of ASCVD events.19
Figure 1.
Mean non-fasting plasma levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, non-high-density lipoprotein (HDL) cholesterol, HDL cholesterol, triglycerides (TGs), and lipoprotein(a) [Lp(a)] based on data from 59,278 women and 48,314 men in the Copenhagen General Population Study. Plasma levels of total cholesterol, HDL cholesterol, and TG were measured using standard hospital assays (Konelab, ThermoFisher Scientific, Waltham, Massachusetts, USA). The LDL cholesterol was calculated using the Friedewald equation if TG was ≤4 mmol/L (≤354 mg/dL) or measured directly. Non-HDL cholesterol was calculated as total cholesterol minus HDL cholesterol. The Lp(a) was analysed with different assays over time, all calibrated corresponding to values using the Denka assay on fresh samples. Samples were either fresh or stored at −80°C before measurement. Tests for interaction were performed by inclusion of two-factor interaction terms between age and sex in the linear regression model on TG and plasma Lp(a) using a likelihood ratio test between models excluding and including the interaction term. P-value for interaction between age and sex on plasma TG = 3 × 10−207; on plasma Lp(a) = 5 × 10−8. To convert cholesterol from mmol/L to mg/dL multiply by 38.7. To convert TG from mmol/L to mg/dL multiply by 88.6. To convert Lp(a) in mg/dL to nmol/L: Lp(a), nmol/L = 2.18 × Lp(a), mg/dL − 3.8332
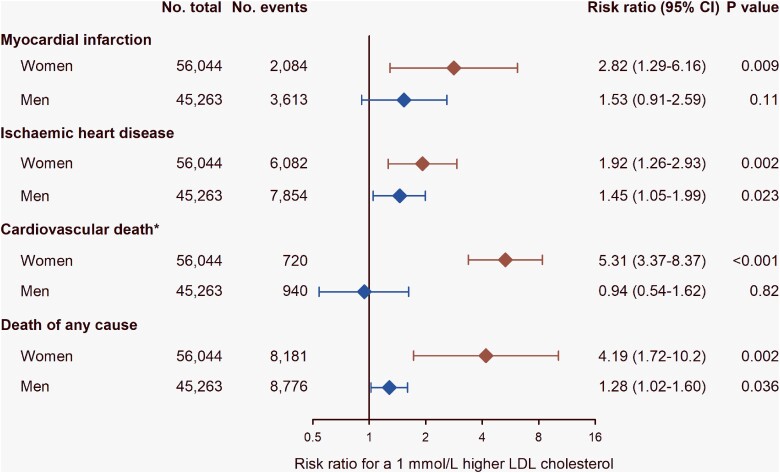
It has been suggested that low-density lipoprotein cholesterol (LDL-C) is less important as a determinant of ASCVD risk in women vs. men given their lower risk of ASCVD, specifically myocardial infarction (MI), reported in some observational studies.42 However, in the World Health Organization CVD Risk Chart Working Group report, both sexes had similar risk for fatal and non-fatal MI, coronary heart disease (CHD), and stroke per 1 mmol/L increase in total cholesterol.43 Data from the Copenhagen City Heart Study and the Copenhagen General Population Study also showed comparable causal genetic effects of LDL-C on risk for MI and ischaemic heart disease (IHD) in both sexes (Figure 2).44,45 These findings therefore support a similar causal effect of LDL-C on cardiovascular disease in women and men.46
Figure 2.
Causal effect of a 1 mmol/L (38.7 mg/dL) higher low-density lipoprotein cholesterol (LDL-C) level on risk for myocardial infarction, ischaemic heart disease, cardiovascular death*, and death from any cause in women and men in the general population, the Copenhagen City Heart Study, and the Copenhagen General Population Study. Data were estimated using Mendelian randomization with a weighted allele score of LDLR (rs267607213, rs121908025, and rs138947766), PCSK9 (rs11591147, rs148195424, rs562556, and rs505151), and HMGCR (rs17238484) using instrumental variable analysis.44,45 The strength of the genetic instrument (i.e. the strength of the association of the genotypes with LDL-C) was similar in women and men (F-statistics 70.8 in women and 67.8 in men). *Cardiovascular death was defined as the primary cause of death. No., number; CI, confidence interval
Sex-specific factors in women merit consideration.47,48 In US guidelines, factors such as pre-eclampsia and early menopause are regarded as ‘risk-enhancing’ with recommendation for statin therapy in women otherwise at borderline or intermediate risk.49 The 2021 European Society of Cardiology (ESC) Prevention guideline recommends screening for hypertension and diabetes in women with a history of pregnancy-induced hypertension, PCOS, and gestational diabetes.16 Women are also disproportionately at risk of chronic kidney disease, itself a risk factor for ASCVD,16 which presents earlier than in men.50 Auto-immune inflammatory diseases,15 which impact women more than men increasing risk for premature ASCVD51,52 independent of traditional risk factors,53–59 are also considered ‘risk-enhancing’ factors by guidelines (Table 1).16,49,60
Table 1.
Cardiovascular risk in women: what to consider?
|
|
|
|
|
Gender
Sociocultural components of gender additionally impact ASCVD risk. Compared with men, women are less likely to seek healthcare that they need. This is particularly true for those with more traditional roles,61 who may prioritize family, household, and caregiver responsibilities over their own health.62 Psychosocial stress is also more evident among women than men, a reflection of higher prevalence of low education attainment, depression, and anxiety contributing to ASCVD risk.63–65 This is especially the case for women of non-Caucasian ethnicity,66 who are less likely to be aware of ASCVD as a cause of death67 and to seek care.68
Key points.
The impact of several lifestyle-related risk factors is disproportionately greater in women than in men. Adverse changes in weight, lipids, blood pressure, and glucose metabolism with menopause transition highlight potential accelerating cardiovascular risk.
Female-specific risk factors, such as pregnancy-associated disorders, should be considered to promote earlier ASCVD risk factor assessment.
Gender-related sociocultural contributors also disproportionately influence cardiovascular health in women.
Does cardiovascular risk prediction differ in women?
As the first manifestation of ASCVD is more likely to be CHD in men but stroke in women,69,70 recent amendments to risk scores in guidelines to include cardiovascular outcomes and fatal and non-fatal events16,71 better reflect the total burden of clinical ASCVD in women. Despite this, current cardiovascular risk prediction models based on traditional risk factors relate to 10-year rather than lifetime risk and are biased by underdiagnosis of events in women thereby underestimating risk.72–76 Even women with a large burden of subclinical atherosclerosis are more likely to be categorized as low risk.77 Given that all prediction models are based on existing data and mostly use retrospective event rates to predict future events, underdiagnosis of events in past studies results in a lower event rate and thus underestimation of risk in models based on these data.72,78
Women generally experience ASCVD events at a later age and have lower event rates than men,79 but given longer life expectancy,80 have a similar lifetime cardiovascular risk.69 Evolution of risk factors over the lifetime also differs between the sexes.81,82 Furthermore, women-specific risk factors are rarely incorporated when developing cardiovascular risk prediction models, given limited supportive evidence.83,84 Thus, the concepts of lifetime cardiovascular risk and treatment benefit are promising approaches to tailoring ASCVD prevention in women.16 Recent findings from the UK Biobank identifying sex differences in genetic loci for CMIT, and correlations with BMI and glucometabolic traits,85,86 highlight potential for leveraging ‘big data’ to provide incremental value for ASCVD prediction and prevention in women.
Key points.
Cardiovascular risk prediction based on traditional risk factors over a 10-year span underestimates risk in women.
Lifetime cardiovascular risk and treatment benefit may be preferable approaches to tailoring cardiovascular disease prevention in women.
Are there sex-related differences in the pathogenesis of atherosclerosis?
A key difference between men and women relates to the levels and ratios of the sex hormones 17β oestradiol, progesterone, and testosterone. Although no randomized controlled trials have unequivocally proved an effect of these sex hormones on ASCVD risk, experimental studies have shown that all three affect biological processes relevant to atherosclerosis.87 Oestrogens decrease atherosclerotic plaque burden in models of atherosclerosis,88,89 and oestradiol can increase endothelial nitric oxide production in vitro,90,91 resulting in increased vasodilation and improved endothelial cell function in mouse models,92 in isolated human arterioles,93 and in cis- and transgender human females treated with oestradiol.94,95 Oestrogens also affect inflammatory pathways, as they can reduce the up-regulation of cytokine-induced E-selectin, vascular cell and intercellular adhesion molecules in endothelial cells,96 reduce leucocyte recruitment97 and interleukin-6 expression98 in (atherosclerotic) mice, and have been shown to prevent vascular smooth muscle cell (SMC) proliferation and extracellular matrix deposition,99 all key processes that drive atherogenesis.
Oestradiol is also involved in maintaining lipid homeostasis. With lipid loading, oestrogens can modulate reverse cholesterol transport mechanisms, resulting in lower LDL-C and higher high-density lipoprotein cholesterol (HDL-C) levels, and prevent excessive lipid uptake by macrophages.100 However, while oestradiol seems to have favourable effects on many pathogenic mechanisms important in atherosclerosis, the cellular and molecular basis of these phenomena, and the crosstalk of these phenomena are largely unknown.
The experimental data fit well with the observation that after menopause, when oestrogen levels decrease, post-menopausal women show a less-favourable lipid profile than in pre-menopausal women, have less efficient vasodilation, and suppress inflammation less efficiently. This suggests that the increase in ASCVD in post-menopausal women may be caused by more complex mechanisms than just oestrogen depletion, or other unspecified effects of oestrogens on ASCVD. The results of clinical trials of post-menopausal hormone replacement therapy (HRT) are inconclusive for the net effect on primary prevention of ASCVD in post-menopausal women, although systematic review of trials and cohort studies did suggest an increase in stroke risk.101 The Estrogen in Prevention of Atherosclerosis Trial (EPAT) did, however, suggest an oestrogen-dependent reduction in atherosclerosis progression.102
The other main difference between female and male sex are the X and Y chromosomes, which contain many (X) vs. few (Y) genes. An experimental mouse model of atherosclerosis showed that the X-chromosome adversely impacted lipid metabolism, promoting increased absorption and availability of dietary fat, leading to increased atherosclerosis.103 However, human data relating to the impact of the X-chromosome on cardiovascular disease are limited.
Other pathways contribute to differences in ASCVD between women and men. Genome wide association studies identified sex-specific single nucleotide polymorphisms, notably rs16986953 (close to APOB) and rs7865618 (CDKN2B-AS1), associated with cardiovascular disease solely in men. Integrative systems biology approaches revealed clear differences in gene networks between the sexes.104,105 Female plaque contained more networks associated with SMC phenotypic modulation and endothelial mesenchymal transition, whereas male plaques exhibited pathways associated with immunoreactivity.105 Consistent with this, carotid endarterectomy specimens from women showed less inflammatory infiltrates, smaller necrotic cores, and enhanced SMC and collagen content.106 Imaging studies revealed fewer atherosclerotic plaques with a smaller intima-media thickness and necrotic core, fewer cholesterol crystals, and less calcification, as well as a lower frequency of intraplaque haemorrhage or plaque rupture in women than in men.106 Thus, despite the paucity of human data, emerging experimental evidence implicates sex as an important player in the pathogenesis of atherosclerosis. Further study is needed to understand mechanisms that drive these differences.
Does sex impact atherothrombosis risk?
Thrombosis often underlies the transformation of a silent atherosclerotic plaque into an acute ischaemic syndrome.107,108 Over 30 years ago, studies suggested an impaired platelet response to aspirin in women, although conclusive evidence for effects on the underlying processes in thrombus formation is still lacking.109,110 While there is support for higher platelet activity, platelet counts and on-treatment platelet reactivity and thus greater propensity for thrombosis in women than in men,111 these differences are small and unlikely to confer a worse clinical prognosis.112
It is plausible that female sex hormones (notably oestrogen) regulate procoagulant protein levels, platelet function, and vessel wall biology and composition, which may translate to sex-based differences in thrombosis.113 Differences in platelet and coagulation activities may, at least partly, explain observations that women are more likely to be aspirin-resistant, to receive distinct benefit from aspirin therapy in primary prevention, and to present with different patterns of venous thrombosis and stroke.113 Women also have a higher tendency for a hypercoagulable state, although the underlying mechanism is uncertain. Oestrogen activated platelets and enhanced aggregation and haemostatic activity in a study using platelets showing differential sex-dependent signalling and cellular activation.114 Another important consideration is bleeding complications, which are more prevalent in women than in men.111,114,115 Further investigation of sex-based mechanisms regulating thrombosis is merited.
Key points.
Sex (sex hormones and sex chromosomes) influences the pathogenesis of atherothrombosis, but understanding of the underlying mechanisms is limited.
Sex-based mechanisms may contribute to differing susceptibility to bleeding complications from antiplatelet and anticoagulant therapy in women and men.
Does atherosclerotic cardiovascular disease presentation differ in women?
Ischaemic heart disease
Compared with men, women have smaller coronary arteries with smaller plaques,116 and a higher burden of microvascular dysfunction with more ischaemia with non-obstructive coronary arteries (INOCA), especially in the 45–65 year age group.117–119 As symptoms are more diverse and vague than in men, even with obstructive coronary artery disease,120–122 MI is often silent or missed.121,123,124
Women have similar atheroma burden as men,125 often with concealed atheroma. Both high-risk plaque and non-obstructive left main disease are stronger predictors of major adverse cardiovascular events (MACE) in women than in men, even after adjustment for the presence of stenosis.126,127 Given the limitations of conventional angiography in women with INOCA,46 computed tomography angiography is useful to exclude obstructive disease, and to identify plaque burden and low attenuation plaque, a powerful predictor of MI risk.128,129 Stress positron emission tomography or stress magnetic resonance imaging can aid diagnosis of coronary microvascular dysfunction. Coronary artery calcium (CAC), although less prevalent in women than men, is associated with a 30% higher risk for cardiovascular death.130 A CAC score >100 or ≥75th age/sex percentile identifies women at elevated risk of MACE; a CAC score >300 was associated with similar event rates as a stable secondary prevention population,131 supporting guideline recommendations for treating a CAC score >300 in primary prevention similar to secondary prevention. Greater lesion size and higher plaque density contribute to higher cardiovascular mortality in women than men with extensive calcified disease.130 Irrespective of the pathophysiology, women with acute coronary syndrome tend to have poorer outcomes than men,132 reflecting increased comorbidities, and delays and underuse of guideline-recommended treatment.133,134
Stroke
Stroke is the third leading cause of death and disability in women.135 Lifetime prevalence is higher and outcome poorer than in men due to older age at onset.136 As for IHD, women with stroke often present with atypical symptoms, increasing the risk of missed or delayed diagnosis.137,138 Unlike men, stroke is more likely to be cardioembolic due to a higher prevalence of atrial fibrillation and less often due to large vessel disease caused by atherosclerosis.136 Some traditional risk factors for stroke such as hypertension, metabolic syndrome, and obesity are more prevalent in women. Other risk factors are only present in women (pre-eclampsia, gestational diabetes, and oral contraceptive use) or increase the risk of stroke more in women than in men (migraine with aura and diabetes).136 Finally, an adverse lipid profile is one of the most important preventable causes of stroke in women.139 Despite evidence that women gain the same benefit as men from statin treatment, they are less likely to be prescribed treatment or attain desired cholesterol levels.6–10,140
Peripheral artery disease
Although peripheral artery disease (PAD) is at least as prevalent in women as in men,141,142 women are often asymptomatic and therefore less likely to be diagnosed and treated. In the Women’s Health and Aging study, only one in six women with PAD were aware of their condition, and two-thirds of those with PAD did not recognize their symptoms.143,144 Even among symptomatic PAD patients, women are more likely to experience atypical limb symptoms rather than intermittent claudication,145 with greater and faster reduction in functional status. Consequently, women with PAD are more likely to present with advanced, multilevel lower extremity disease146–149 and are less likely to be treated effectively with antithrombotic medications, and lipid- and blood pressure-lowering therapy than men with PAD.150–153 While risk for MACE and mortality is similar,154,155 women with PAD are at higher risk for above knee amputation than men.156
Key points.
Symptoms of ASCVD in women are underappreciated and underrecognized.
Women have a higher burden of microvascular dysfunction than men.
For all presentations of ASCVD in women, delayed or missed diagnosis is common and contributes to undertreatment.
Cardiovascular risk factors in women: focus on lipids
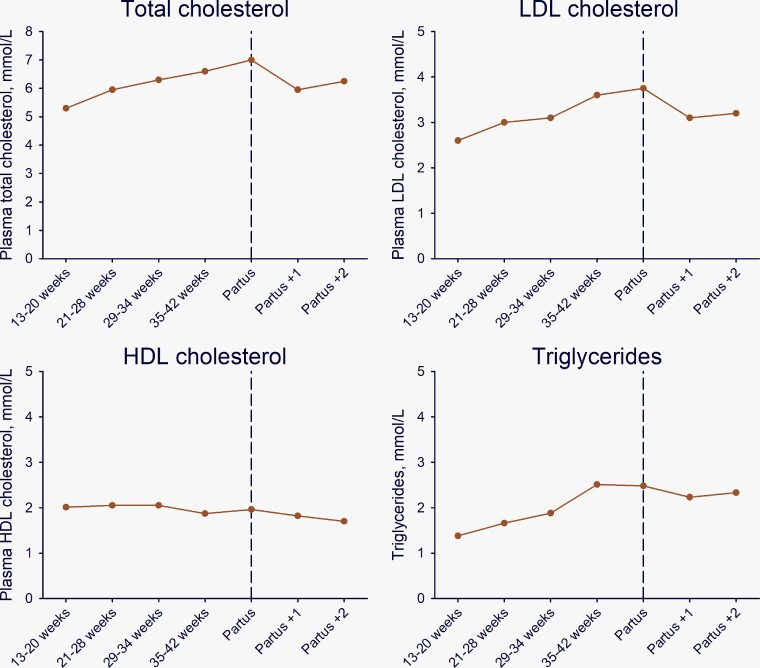
There is limited information on how female sex influences major lipids, including LDL-C, triglyceride-rich lipoproteins (TRLs), and lipoprotein(a) [Lp(a)]. Cumulative exposure differs (Figure 1), with higher lipid levels from birth in girls than boys,157–159 persisting during adolescence.160,161 Lipids also vary during the menstrual cycle (highest at ovulation),162 a possible consideration in lipid testing. Increases during pregnancy in levels of total cholesterol and LDL-C (∼30%) and triglycerides (TGs) (∼50% at 35–42 weeks) (Figure 3)163 are important in women with higher pre-pregnancy levels.13,14,163 Breastfeeding favourably modulates hyperlipidaemia,164,165 and when continued for >12 months over the lifetime, is associated with lower risk of ASCVD.166–168 After menopause transition, women experience a worsening in the lipid profile (Figure 1) with increases in total cholesterol, LDL-C, and TG levels potentially contributing to accelerating ASCVD risk.169,170
Figure 3.
Mean plasma levels of total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol, and triglycerides as a function of pregnancy week, at delivery (partus), and one and two weeks after delivery. Data are medians calculated from the 2.5 and 97.5 percentiles from Klajnbard et al.163 To convert total, LDL, and HDL cholesterol from mmol/L to mg/dL multiply by 38.7. To convert triglycerides from mmol/L to mg/dL multiply by 88.6
Familial hypercholesterolaemia
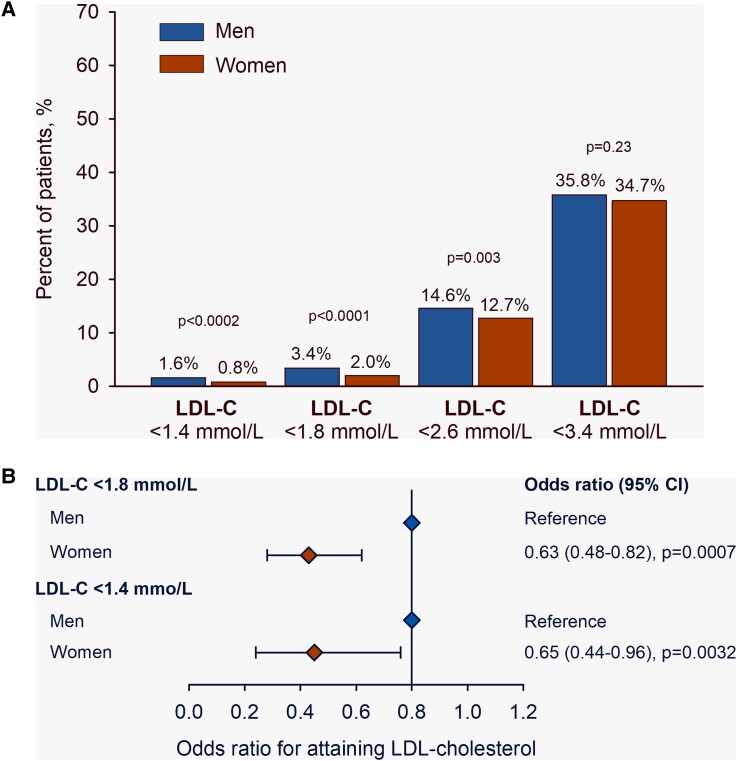
Familial hypercholesterolaemia is characterized by elevated LDL-C levels compared with the general population.171 Women with FH appear to be at risk of higher cholesterol burden than men with FH for a number of reasons, including higher LDL-C levels from an early age,172 later diagnosis (on average, by ∼2.5 years), and underuse of maximal statin doses or combination LLT.11 Attainment of LDL-C goal is consequently lower (Figure 4), and ∼40% of women do not attain levels <1.8 mmol/L (<70 mg/dL) (odds ratio .63, 95% CI .48–.82; P = .0007).11 These disparities in FH care impact ASCVD risk, with registry data showing the highest excess CHD risk among younger women with FH.171,173
Figure 4.
Attainment of low-density lipoprotein cholesterol (LDL-C) targets among men and women with familial hypercholesterolaemia on lipid-lowering therapy (statin, ezetimibe, and/or a PCSK9 inhibitor). Panel A shows the percentage of patients on treatment and below different LDL-C thresholds. Panel B shows the likelihood of attaining an LDL-C below different thresholds according to sex, using men as the reference. The odds ratio was adjusted by age, baseline comorbidities (hypertension, diabetes, smoking, and body mass index), high-density lipoprotein cholesterol, logtriglycerides, lipid-lowering therapy, and index case status. CI, confidence interval. Reproduced with permission11
Adding to this, discontinuation of LLT before and during pregnancy and breastfeeding60 increases LDL-C burden in women with FH. In a recent study of FH subjects with serial lipid measurement over 12 years, a theoretical threshold (area under the curve) for LDL-C burden indicative of higher MI risk (125 mmol/L-years or 5000 mg/dL-years)174 was attained earlier by women than men.12 All FH women had attained this LDL-C threshold by age 33 years, seven years earlier than for FH men.12 Although updated US Food and Drug Administration (FDA) guidance allows for more flexible options for shared decision making in highest-risk women during pregnancy,175 the FDA also acknowledges the lack of data on the efficacy, risks, and benefits of statin therapy during pregnancy and the need for more research, both into safety for the foetus and adverse effect on high-risk women without effective LLT for this time. While limited evidence suggests no higher risk of preterm delivery, low birth weight, or congenital malformation in infants of FH mothers than in the general population with most pregnancies (85%) successfully carried to full-term,176,177 further study is needed. The European Medicines Agency has so far not responded to the statement of the FDA. For now, this panel does not recommend continuing statin therapy during pregnancy and breastfeeding.
Taken together, childbearing (planning, pregnancy, and breastfeeding) represents a considerable loss of LLT (by ∼20% at ∼30 years) in women with FH.178 This panel stresses the need for close monitoring of FH women during pregnancy and breastfeeding, to minimize their time-off statin therapy. Whether LDL-C goals should be lowered in FH women to compensate for lost treatment merits consideration.
Key points.
Female sex influences lipids during transitions (pregnancy, breastfeeding, and menopause). After menopause, women experience a worsening in the lipid profile, potentially contributing to accelerating ASCVD risk.
The LDL-C burden associated with FH is higher among women than men due to delayed diagnosis, underuse of maximal statin doses with lower LDL-C goal attainment, and discontinuation of statin therapy before and during pregnancy and breastfeeding.
This panel recommends action to minimize time-off statin therapy for FH women after pregnancy and breastfeeding.
Triglyceride-rich lipoproteins
High levels of plasma TGs are a marker for high levels of TRLs, and as these are metabolized by cells, the cholesterol component—remnant cholesterol or TRL cholesterol—is more important for atherosclerosis.179 High plasma TGs also associate with vascular and systemic inflammation.180,181 Mendelian randomization studies suggested that the observed associations between TGs and remnant cholesterol may be causal182–187; however, with the lack of robust evidence that lowering TGs or remnant cholesterol reduces ASCVD,179,180,188–191 current guidelines do not provide treatment goals.60
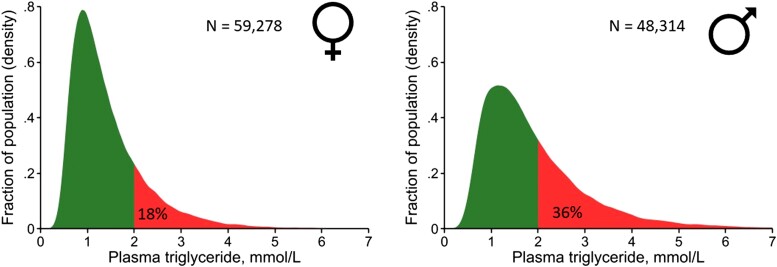
As discussed, TGs increase from childhood until ∼70 years in women and ∼60 years in men (Figure 1).192 From 20–80 years, women consistently have lower TG levels than men, partly explained by higher alcohol intake in men,182 contributing to a lower prevalence with TGs >2 mmol/L (Figure 5). Risk of ASCVD and mortality increases similarly in women and men as TG and remnant cholesterol levels increase.182–184,193
Figure 5.
Density distribution of plasma levels of triglycerides in 59,278 women and 48,314 men from the Copenhagen General Population Study. Triglycerides were measured in the non-fasting state and analysed on fresh samples using standard hospital assays. To convert triglycerides from mmol/L to mg/dL multiply by 88.6
Although there is no solid evidence that TG concentration is a better predictor of ASCVD in women than in men, large prospective studies provide insights. The Copenhagen City Heart Study showed a higher risk of MI (HR 1.20, 95% CI 1.05–1.37) and total mortality (HR 1.18, 95% CI 1.10–1.27) in women vs. men per 1 mmol/L increase in non-fasting TG (after multifactorial adjustment for age, total cholesterol, BMI, hypertension, diabetes, smoking, alcohol consumption, physical inactivity, lipid-lowering treatment, post-menopausal status, and HRT).182 A similar trend per 1 mmol/L increase in remnant cholesterol was reported for PAD [HR 1.6, 95% CI 1.3–1.9 in women and 1.2, 95% CI 1.1–1.3 in men (P for interaction sex per remnant cholesterol on risk of PAD = .01)].185 The Emerging Risk Factors Collaboration showed interaction between sex and fasting TG on risk of CHD (P for interaction = .02) with a slightly higher risk per 1 standard deviation (SD) increase in TGs in women (HR 1.06, 95% CI .96–1.16) than men (HR .97, 95% CI .91–1.03) but no interactions between sex and HDL-C or non-HDL-C on risk194. In the Women’s Health Study in >28,000 subjects,195 a 1 SD increase in LDL-C was associated with 38% higher CHD risk before age 55 years, but 1 SD increase in non-HDL-C, TGs, and remnant cholesterol increased CHD risk by 67%, 114%, and 66%, respectively. Thus, as for men, elevated TRLs are important to ASCVD risk in women, with lifestyle intervention a priority for management.
Key points.
Triglyceride levels are a marker for TRL; remnant cholesterol contained in TRL is important for atherosclerosis.
The association of increasing TG levels and ASCVD risk is similar in men and women.
While there is currently no solid evidence that TG concentration is a better predictor of ASCVD in women than in men, evidence suggests that TRLs are important risk factors for premature CHD in women.
Lipoprotein(a)
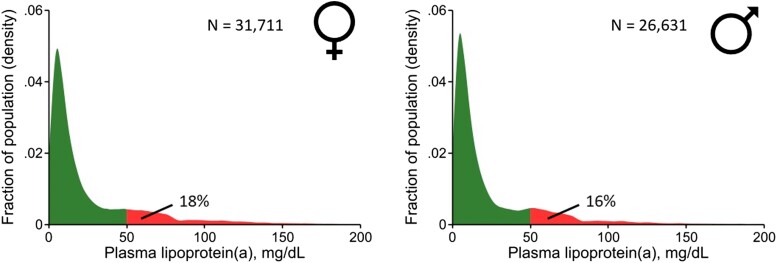
There is clear evidence for the causality of Lp(a) in ASCVD and aortic valve stenosis.196–206 Plasma levels of Lp(a) show similar distribution in men and women, varying with ethnicity207,208 (Figure 6). The Lp(a) concentration increases in women around 50 years coinciding with the onset of menopause209–212 (Figure 1), possibly due to hormonal changes and/or ageing. Indeed, Lp(a) concentration is 12%–20% lower in women on HRT vs. controls,213–215 and approximately doubles in pregnancy.216,217 In the Copenhagen General Population Study in >70,000 individuals, Lp(a) levels were generally similar in men and women aged 20–49 years, but on average 17% higher in women from age 50218(Figure 1). While both sexes show similar associations between high Lp(a) (>40 mg/dL or >83 nmol/L) and cardiovascular morbidity and mortality after age 50 years, higher levels in women (Figure 1) suggest that Lp(a) at this age is a relatively more common cardiovascular risk factor than in men. These findings therefore challenge current recommendations that only one Lp(a) measurement is adequate to capture the lifetime concentration of Lp(a) in women.60,219
Figure 6.
Density distribution of plasma levels of lipoprotein(a) [Lp(a)] in 31,711 women and 26,631 men from the Copenhagen General Population Study. The Lp(a) concentration was measured in the non-fasting state and analysed with different assays over time, but all assays were calibrated corresponding to values using the Denka assay on fresh samples. The majority of measurements was performed on fresh samples using this assay. Samples stored prior to measurements were kept at −80°C before measurement. To convert Lp(a) in mg/dL to nmol/L: Lp(a), nmol/L = 2.18 × Lp(a), mg/dL − 3.8332
Key points.
In women, Lp(a) concentration increases during pregnancy, and from the onset of menopause (circa 50 years).
High Lp(a) levels are more common in women than men after 50 years, which may impact ASCVD risk. This might suggest that guideline recommendations to measure Lp(a) once are inadequate in women.
Call to action for women and atherosclerotic cardiovascular disease
Although ASCVD is the leading cause of death in women, their cardiovascular health is often neglected. Underappreciation of women’s ASCVD risk, missed or delayed diagnosis, and undertreatment are important contributors. Despite clear evidence that statin therapy is similarly efficacious in both sexes,220 women at high risk for ASCVD are less likely than men to be prescribed any statin therapy or to receive a statin at guideline-recommended intensity,7 and more likely to refuse or discontinue statin treatment due to perceived side effects.7,221,222 Clearly, action is needed to overcome these inequities.
As discussed in this EAS statement, there are also other important considerations. Women are disproportionately impacted by some lifestyle factors, and sociocultural components related to gender impact risk. Hormonal and chromosomal effects also influence ASCVD progression, although gaps remain in understanding the underlying mechanisms (Graphical abstract). Lipids influence ASCVD risk in women during life course events (pregnancy, breastfeeding, and menopause). During menopause transition, LDL-C levels increase36 and elevated Lp(a) is more common than in men. In women with FH, discontinuation of statin therapy with pregnancy and breastfeeding contributes to greater cumulative cholesterol exposure compared with men with FH.
This panel stresses the importance of early assessment of cardiovascular risk and early treatment of dyslipidaemia in women (Table 2 and Graphical abstract). Further studies to understand the effects of female sex hormones on atherosclerosis development and progression are needed. Targeted action to address these gaps is a priority to reduce the unacceptably high burden of ASCVD in women.
Table 2.
Key recommendations
|
Acknowledgements
This European Atherosclerosis Society panel was co-chaired by Jeanine E. Roeters van Lennep (JERV) and Lale S. Tokgözoğlu (LST).
Contributor Information
Jeanine E Roeters van Lennep, Department of Internal Medicine, Cardiovascular Institute, Erasmus Medical Center, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
Lale S Tokgözoğlu, Department of Cardiology, Hacettepe University Faculty of Medicine, Ankara, Turkey.
Lina Badimon, Cardiovascular Science Program-ICCC, IR-Hospital de la Santa Creu I Santa Pau, Ciber CV, Autonomous University of Barcelona, Barcelona, Spain.
Sandra M Dumanski, Department of Medicine, Cumming School of Medicine, University of Calgary, Libin Cardiovascular Institute, and O’Brien Institute for Public Health, Calgary, Canada.
Martha Gulati, Barbra Streisand Women’s Heart Center, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, USA.
Connie N Hess, Division of Cardiology, Department of Medicine, University of Colorado School of Medicine, Aurora and CPC Clinical Research Aurora, CO, USA.
Kirsten B Holven, Department of Nutrition, Institute of Basic Medical Sciences, Faculty of Medicine, University of Oslo, and National Advisory Unit on Familial Hypercholesterolemia, Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway.
Maryam Kavousi, Department of Epidemiology, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Meral Kayıkçıoğlu, Department of Cardiology, Faculty of Medicine, Ege University, Izmir, Turkey.
Esther Lutgens, Cardiovascular Medicine and Immunology, Mayo Clinic, Rochester, MN, USA.
Erin D Michos, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Eva Prescott, Department of Cardiology, Bispebjerg University Hospital, Bispebjerg Bakke 23, 2400 Copenhagen, Denmark.
Jane K Stock, European Atherosclerosis Society, Mässans Gata 10, SE-412 51 Gothenburg, Sweden.
Anne Tybjaerg-Hansen, Department of Clinical Biochemistry, Copenhagen University Hospital-Rigshospitalet, The Copenhagen General Population Study, Copenhagen University Hospital-Herlev and Gentofte Hospital, and Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Marieke J H Wermer, Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurology at University Medical Center Groningen, Groningen, The Netherlands.
Marianne Benn, Department of Clinical Biochemistry, Copenhagen University Hospital-Rigshospitalet, The Copenhagen General Population Study, Copenhagen University Hospital-Herlev and Gentofte Hospital, and Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Declarations
Disclosure of Interest
Potential conflicts of interest outside the submitted work (participation in trials; receipt of fellowships, or grants for travel, research, or staffing support; and/or personal honoraria for consultancy or lectures/speaker’s bureau) are summarized as follows: Ab-Biotics (L.B.), Abbott (L.S.T.), Abdi Ibrahim (M.K.), Amarin-PACE (L.B.), Akcea (A.T.H.), Amgen (C.N.H., K.B.H., A.T.H., L.S.T., and E.D.M.), American College of Surgeons (C.N.H.), Amryt Pharma (M.K. and J.E.R.V.), AstraZeneca (L.B., E.D.M., A.T.H., and L.S.T.), Bayer (C.N.H., E.D.M., and L.S.T.), Boehringer Ingelheim (E.D.M.), Canadian Institutes of Health (S.M.D.), Daiichi Sankyo (L.S.T.), Draupnir Bio (A.T.H.), Edwards (E.D.M.), Esperion (E.D.M.), European Union (Covirna, Transbioline, Cardiateam, and Era-CVD; L.B.), Health Institute Carlos III Spain (L.B.), International Aspirin Foundation (L.B.), International Atherosclerosis Society (L.B.), Ionis (L.B.), Janssen (C.N.H. and L.S.T.), Kidney Foundation (S.M.D.), LIB Therapeutics (M.K.), Medtronic (E.D.M.), Ministry of Research Spain (L.B.), MSD (L.S.T.), Mylan (L.S.T.), NHLBI (M.G.), Novartis (M.K., E.L., E.D.M., J.E.R.V., A.T.H., and L.S.T.), Novo-Nordisk (L.B., M.K., E.L., E.D.M., and L.S.T.), Novonordisk (L.B.), Pfizer (L.B., E.D.M., and L.S.T.), Recordati (L.S.T.), Regeneron (A.T.H.), Sanofi (L.B., K.B.H., J.E.R.V., A.T.H., and L.S.T.), Sanovel (M.K.), Servier (L.S.T.), Silence Therapeutics (A.T.H.), and US Department of Defence (M.G.). J.K.S. reports stock in AstraZeneca and GSK.
L.B. has patents planned, issued, or pending for Gly-ApoJ, Ivestatin, and DJ-1F (unrelated to work), and is a member European Society of Cardiology (ESC)-Nominating Committee. S.M.D. is a member of the Canadian Women’s Heart Health Alliance—and the Women’s Health Research Cluster. C.N.H. receives royalties as co-author on UptoDate section on Investigational Therapies in Treatment of Peripheral Artery Disease. M.G. is President of the American Society for Preventive Cardiology. K.B.H. and J.E.R.V. are members of the scientific board of FH Europe. M.K. is chair of FH Turkey. E.L. is Chair of the ESC Working Group on Atherosclerosis and Vascular Biology, and a member of the programme committee for ATVB and AHA. J.E.R.V. is a member advisory board LEEFH, and the executive board of the EAS. L.S.T. is Past President of the EAS and the Past President of the Turkish Society of Cardiology. Membership of a Data Safety Monitoring Board is reported by C.N.H. (LIMIT trial) and M.J.H.W. (Trident trial). M.B., M.K., and E.P. have no disclosures.
Data Availability
No data were generated or analysed for this manuscript.
Funding
All authors declare no funding for this contribution.
References
- 1. Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022;43:716–99. 10.1093/eurheartj/ehab892 [DOI] [PubMed] [Google Scholar]
- 2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 2022;145:e153–639. 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3. Curtin SC. Trends in cancer and heart disease death rates among adults aged 45–64: United States, 1999–2017. Natl Vital Stat Rep 2019;68:1–9. [PubMed] [Google Scholar]
- 4. Bairey Merz CN, Andersen H, Sprague E, Burns A, Keida M, Walsh MN, et al. Knowledge, attitudes, and beliefs regarding cardiovascular disease in women: the Women’s Heart Alliance. J Am Coll Cardiol 2017;70:123–32. 10.1016/j.jacc.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 5. Cushman M, Shay CM, Howard VJ, Jimenez MC, Lewey J, McSweeney JC, et al. Ten-year differences in women’s awareness related to coronary heart disease: results of the 2019 American Heart Association national survey: a special report from the American Heart Association. Circulation 2021;143:e239–48. 10.1161/CIR.0000000000000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redfors B, Angeras O, Ramunddal T, Petursson P, Haraldsson I, Dworeck C, et al. Trends in gender differences in cardiac care and outcome after acute myocardial infarction in Western Sweden: a report from the Swedish web system for enhancement of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). J Am Heart Assoc 2015;4:e001995. 10.1161/JAHA.115.001995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nanna MG, Wang TY, Xiang Q, Goldberg AC, Robinson JG, Roger VL, et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes 2019;12:e005562. 10.1161/CIRCOUTCOMES.118.005562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters SAE, Colantonio LD, Zhao H, Bittner V, Dai Y, Farkouh ME, et al. Sex differences in high-intensity statin use following myocardial infarction in the United States. J Am Coll Cardiol 2018;71:1729–37. 10.1016/j.jacc.2018.02.032 [DOI] [PubMed] [Google Scholar]
- 9. Udell JA, Fonarow GC, Maddox TM, Cannon CP, Frank Peacock W, Laskey WK, et al. Sustained sex-based treatment differences in acute coronary syndrome care: insights from the American Heart Association Get With The Guidelines Coronary Artery Disease Registry. Clin Cardiol 2018;41:758–68. 10.1002/clc.22938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benson RA, Okoth K, Keerthy D, Gokhale K, Adderley NJ, Nirantharakumar K, et al. Analysis of the relationship between sex and prescriptions for guideline-recommended therapy in peripheral arterial disease, in relation to 1-year all-cause mortality: a primary care cohort study. BMJ Open 2022;12:e055952. 10.1136/bmjopen-2021-055952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC) . Global perspective of familial hypercholesterolaemia: a cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021;398:1713–25. 10.1016/S0140-6736(21)01122-3 [DOI] [PubMed] [Google Scholar]
- 12. Johansen AK, Bogsrud MP, Christensen JJ, Rundblad A, Narverud I, Ulven S, et al. Young women with familial hypercholesterolemia have higher LDL-cholesterol burden than men: novel data using repeated measurements during 12-years follow-up. Atheroscler Plus 2023;51:28–34. 10.1016/j.athplu.2023.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graham DF, Raal FJ. Management of familial hypercholesterolemia in pregnancy. Curr Opin Lipidol 2021;32:370–7. 10.1097/MOL.0000000000000790 [DOI] [PubMed] [Google Scholar]
- 14. Dathan-Stumpf A, Vogel M, Jank A, Thiery J, Kiess W, Stepan H. Reference intervals of serum lipids in the second and third trimesters of pregnancy in a Caucasian cohort: the LIFE Child study. Arch Gynecol Obstet 2019;300:1531–9. 10.1007/s00404-019-05342-2 [DOI] [PubMed] [Google Scholar]
- 15. Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis 2004;10:2005–11. 10.3201/eid1011.040367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 17. Maas A, Rosano G, Cifkova R, Chieffo A, van Dijken D, Hamoda H, et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J 2021;42:967–84. 10.1093/eurheartj/ehaa1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roeters van Lennep JE, Heida KY, Bots ML, Hoek A. , collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:178–86. 10.1177/204748731455600 [DOI] [PubMed] [Google Scholar]
- 19. Wekker V, van Dammen L, Koning A, Heida KY, Painter RC, Limpens J, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update 2020;26:942–60. 10.1093/humupd/dmaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shifren JL, Gass ML. The North American Menopause Society recommendations for clinical care of midlife women. Menopause 2014;21:1038–62. 10.1097/GME.0000000000000319 [DOI] [PubMed] [Google Scholar]
- 21. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet 2004;364:937–52. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 22. [(accessed 4 May 2023).]. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020 2021. Available from:
- 23. Angoulvant D, Ducluzeau PH, Renoult-Pierre P, Fauchier G, Herbert J, Semaan C, et al. Impact of gender on relative rates of cardiovascular events in patients with diabetes. Diabetes Metab 2021;47:101226. 10.1016/j.diabet.2021.101226 [DOI] [PubMed] [Google Scholar]
- 24. Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 2019;62:1550–60. 10.1007/s00125-019-4926-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prospective Studies Collaboration and Asia Pacific Cohort Studies Collaboration . Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol 2018;6:538–46. 10.1016/S2213-8587(18)30079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014;57:1542–51. 10.1007/s00125-014-3260-6 [DOI] [PubMed] [Google Scholar]
- 27. Broni EK, Ndumele CE, Echouffo-Tcheugui JB, Kalyani RR, Bennett WL, Michos ED. The diabetes-cardiovascular connection in women: understanding the known risks, outcomes, and implications for care. Curr Diab Rep 2022;22:11–25. 10.1007/s11892-021-01444-x [DOI] [PubMed] [Google Scholar]
- 28. Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 2011;54:3003–6. 10.1007/s00125-011-2313-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wannamethee SG, Papacosta O, Lawlor DA, Whincup PH, Lowe GD, Ebrahim S, et al. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012;55:80–7. 10.1007/s00125-011-2284-4 [DOI] [PubMed] [Google Scholar]
- 30. Walli-Attaei M, Joseph P, Rosengren A, Chow CK, Rangarajan S, Lear SA, et al. Variations between women and men in risk factors, treatments, cardiovascular disease incidence, and death in 27 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020;396:97–109. 10.1016/S0140-6736(20)30543-2 [DOI] [PubMed] [Google Scholar]
- 31. Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Langlois MR, Nordestgaard BG, Langsted A, Chapman MJ, Aakre KM, Baum H, et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from EAS and EFLM. Clin Chem Lab Med 2020;58:496–517. 10.1515/cclm-2019-1253 [DOI] [PubMed] [Google Scholar]
- 33. Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009;54:2366–73. 10.1016/j.jacc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lau ES, Michos ED. Blood pressure trajectories through the menopause transition: different paths, same journey. Circ Res 2022;130:323–5. 10.1161/CIRCRESAHA.122.320664 [DOI] [PubMed] [Google Scholar]
- 35. Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Thurston RC, et al. Trajectories of blood pressure in midlife women: does menopause matter? Circ Res 2022;130:312–22. 10.1161/CIRCRESAHA.121.319424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khoudary SRE, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation 2020;142:e506–32. 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 37. Clayton GL, Soares AG, Kilpi F, Fraser A, Welsh P, Sattar N, et al. Cardiovascular health in the menopause transition: a longitudinal study of up to 3892 women with up to four repeated measures of risk factors. BMC Med 2022;20:299. 10.1186/s12916-022-02454-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause 2013;20:8–14. 10.1097/gme.0b013e3182611787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matthews KA, Chen X, Barinas-Mitchell E, Brooks MM, Derby CA, Harlow S, et al. Age at menopause in relationship to lipid changes and subclinical carotid disease across 20 years: study of women’s health across the nation. J Am Heart Assoc 2021;10:e021362. 10.1161/JAHA.121.021362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA 2019;322:2411–21. 10.1001/jama.2019.19191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Honigberg MC, Zekavat SM, Niroula A, Griffin GK, Bick AG, Pirruccello JP, et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation 2021;143:410–23. 10.1161/CIRCULATIONAHA.120.051775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cupido AJ, Asselbergs FW, Schmidt AF, Hovingh GK. Low-density lipoprotein cholesterol attributable cardiovascular disease risk is sex specific. J Am Heart Assoc 2022;11:e024248. 10.1161/JAHA.121.024248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. WHO CVD Risk Chart Working Group . World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–45. doi: 10.1016/S2214-109X(19)30318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjaerg-Hansen A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017;357:j1648. 10.1136/bmj.j1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Low LDL cholesterol by PCSK9 variation reduces cardiovascular mortality. J Am Coll Cardiol 2019;73:3102–14. 10.1016/j.jacc.2019.03.517 [DOI] [PubMed] [Google Scholar]
- 46. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, et al. The Lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet 2021;397:2385–438. 10.1016/S0140-6736(21)00684-X [DOI] [PubMed] [Google Scholar]
- 47. Elder P, Sharma G, Gulati M, Michos ED. Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am J Prev Cardiol 2020;2:100028. 10.1016/j.ajpc.2020.100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agarwala A, Michos ED, Samad Z, Ballantyne CM, Virani SS. The use of sex-specific factors in the assessment of women’s cardiovascular risk. Circulation 2020;141:592–9. 10.1161/CIRCULATIONAHA.119.043429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019;140:e596–646. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmed SB, Dumanski SM. Do sex and gender matter in kidney and cardiovascular disease? Am J Kidney Dis 2021;78:177–9. 10.1053/j.ajkd.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 51. Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med 2003;349:2407–15. 10.1056/NEJMoa035611 [DOI] [PubMed] [Google Scholar]
- 52. Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum 2005;52:3045–53. 10.1002/art.21288 [DOI] [PubMed] [Google Scholar]
- 53. del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737–45. < 2737::AID-ART460>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 54. Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7. 10.1002/art.24092 [DOI] [PubMed] [Google Scholar]
- 55. Aviña-Zubieta JA, To F, Vostretsova K, De Vera M, Sayre EC, Esdaile JM. Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population-based study. Arthritis Care Res (Hoboken). 2017;69:849–856. 10.1002/acr.23018 [DOI] [PubMed] [Google Scholar]
- 56. Hansildaar R, Vedder D, Baniaamam M, Tausche AK, Gerritsen M, Nurmohamed MT. Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol 2021;3:e58–70. 10.1016/S2665-9913(20)30221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giannelou M, Mavragani CP. Cardiovascular disease in systemic lupus erythematosus: a comprehensive update. J Autoimmun 2017;82:1–12. 10.1016/j.jaut.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 58. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR Recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. 10.1136/annrheumdis-2016-209775 [DOI] [PubMed] [Google Scholar]
- 59. Conrad N, Verbeke G, Molenberghs G, Goetschalckx L, Callender T, Cambridge G, et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 2022;400:733–43. 10.1016/S0140-6736(22)01349-6 [DOI] [PubMed] [Google Scholar]
- 60. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 61. Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, et al. Sex versus gender-related characteristics: which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol 2016;67:127–35. 10.1016/j.jacc.2015.10.067 [DOI] [PubMed] [Google Scholar]
- 62. Colella TJF, Hardy M, Hart D, Price JAD, Sarfi H, Mullen KA, et al. The Canadian Women’s Heart Health Alliance atlas on the epidemiology, diagnosis, and management of cardiovascular disease in women—chapter 3: patient perspectives. CJC Open 2021;3:229–35. 10.1016/j.cjco.2020.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Connelly PJ, Azizi Z, Alipour P, Delles C, Pilote L, Raparelli V. The importance of gender to understand sex differences in cardiovascular disease. Can J Cardiol 2021;37:699–710. 10.1016/j.cjca.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 64. Fabreau GE, Leung AA, Southern DA, Knudtson ML, McWilliams JM, Ayanian JZ, et al. Sex, socioeconomic status, access to cardiac catheterization, and outcomes for acute coronary syndromes in the context of universal healthcare coverage. Circ Cardiovasc Qual Outcomes 2014;7:540–9. 10.1161/CIRCOUTCOMES.114.001021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Backholer K, Peters SAE, Bots SH, Peeters A, Huxley RR, Woodward M. Sex differences in the relationship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epidemiol Community Health 2017;71:550–7. 10.1136/jech-2016-207890 [DOI] [PubMed] [Google Scholar]
- 66. Norris CM, Yip CYY, Nerenberg KA, Clavel MA, Pacheco C, Foulds HJA, et al. State of the science in women’s cardiovascular disease: a Canadian perspective on the influence of sex and gender. J Am Heart Assoc 2020;9:e015634. 10.1161/JAHA.119.015634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Daponte-Codina A, Knox EC, Mateo-Rodriguez I, Seims A, Regitz-Zagrosek V, Maas A, et al. Gender and social inequalities in awareness of coronary artery disease in European countries. Int J Environ Res Public Health 2022;19:1388. 10.3390/ijerph19031388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Samulowitz A, Gremyr I, Eriksson E, Hensing G. “Brave Men” and “Emotional Women”: a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res Manag 2018;2018:6358624. 10.1155/2018/6358624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leening MJ, Ferket BS, Steyerberg EW, Kavousi M, Deckers JW, Nieboer D, et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 2014;349:g5992. 10.1136/bmj.g5992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res 2022;130:512–28. 10.1161/CIRCRESAHA.121.319915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. SCORE2 working group and ESC Cardiovascular risk collaboration . SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J 2021;42:2439–54. 10.1093/eurheartj/ehab309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wenzl FA, Kraler S, Ambler G, Weston C, Herzog SA, Raber L, et al. Sex-specific evaluation and redevelopment of the GRACE score in non-ST-segment elevation acute coronary syndromes in populations from the UK and Switzerland: a multinational analysis with external cohort validation. Lancet 2022;400:744–56. 10.1016/S0140-6736(22)01483-0 [DOI] [PubMed] [Google Scholar]
- 73. Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350:g7873. 10.1136/bmj.g7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cook NR, Paynter NP, Eaton CB, Manson JE, Martin LW, Robinson JG, et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic women’s health initiative. Circulation 2012;125:1748–56. S1–11. 10.1161/CIRCULATIONAHA.111.075929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med 2007;167:2437–42. 10.1001/archinte.167.22.2437 [DOI] [PubMed] [Google Scholar]
- 76. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds risk score. JAMA 2007;297:611–9. 10.1001/jama.297.6.611 [DOI] [PubMed] [Google Scholar]
- 77. Kavousi M, Desai CS, Ayers C, Blumenthal RS, Budoff MJ, Mahabadi AA, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA 2016;316:2126–34. 10.1001/jama.2016.17020 [DOI] [PubMed] [Google Scholar]
- 78. Mortensen MB, Tybjaerg-Hansen A, Nordestgaard BG. Statin eligibility for primary prevention of cardiovascular disease according to 2021 European prevention guidelines compared with other international guidelines. JAMA Cardiol 2022;7:836–43. 10.1001/jamacardio.2022.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Roth GA, Mensah GA, Fuster V. The global burden of cardiovascular diseases and risks: a compass for global action. J Am Coll Cardiol 2020;76:2980–1. 10.1016/j.jacc.2020.11.021 [DOI] [PubMed] [Google Scholar]
- 80. Austad SN. Sex differences in health and aging: a dialog between the brain and gonad? Geroscience 2019;41:267–73. 10.1007/s11357-019-00081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 2020;5:19–26. 10.1001/jamacardio.2019.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, et al. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation 2021;143:761–3. 10.1161/CIRCULATIONAHA.120.049360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Baart SJ, Dam V, Scheres LJJ, Damen J, Spijker R, Schuit E, et al. Cardiovascular risk prediction models for women in the general population: a systematic review. PLoS One 2019;14:e0210329. 10.1371/journal.pone.0210329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J 2019;40:1113–20. 10.1093/eurheartj/ehy863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yeung MW, Wang S, van de Vegte YJ, Borisov O, van Setten J, Snieder H, et al. Twenty-five novel loci for carotid intima-media thickness: a genome-wide association study in >45 000 individuals and meta-analysis of >100 000 individuals. Arterioscler Thromb Vasc Biol 2022;42:484–501. 10.1161/ATVBAHA.121.317007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Strawbridge RJ, Ward J, Bailey MES, Cullen B, Ferguson A, Graham N, et al. Carotid intima-media thickness: novel loci, sex-specific effects, and genetic correlations with obesity and glucometabolic traits in UK Biobank. Arterioscler Thromb Vasc Biol 2020;40:446–61. 10.1161/ATVBAHA.119.313226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vaura F, Palmu J, Aittokallio J, Kauko A, Niiranen T. Genetic, molecular, and cellular determinants of sex-specific cardiovascular traits. Circ Res 2022;130:611–31. 10.1161/CIRCRESAHA.121.319891 [DOI] [PubMed] [Google Scholar]
- 88. Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest 2001;107:333–40. 10.1172/JCI11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A 1996;93:10022–7. 10.1073/pnas.93.19.10022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schulz E, Anter E, Zou MH, Keaney JF Jr. Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation 2005;111:3473–80. 10.1161/CIRCULATIONAHA.105.546812 [DOI] [PubMed] [Google Scholar]
- 91. Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res 2016;119:375–96. 10.1161/CIRCRESAHA.116.306531 [DOI] [PubMed] [Google Scholar]
- 92. Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, et al. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ Res 2002;90:413–9. 10.1161/hh0402.105096 [DOI] [PubMed] [Google Scholar]
- 93. SenthilKumar G, Katunaric B, Bordas-Murphy H, Young M, Doren EL, Schulz ME, et al. 17beta-estradiol promotes sex-specific dysfunction in isolated human arterioles. Am J Physiol Heart Circ Physiol 2023;324:H330–7. 10.1152/ajpheart.00708.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, et al. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol 2008;28:348–52. 10.1161/ATVBAHA.107.158634 [DOI] [PubMed] [Google Scholar]
- 95. New G, Timmins KL, Duffy SJ, Tran BT, O’Brien RC, Harper RW, et al. Long-term estrogen therapy improves vascular function in male to female transsexuals. J Am Coll Cardiol 1997;29:1437–44. 10.1016/S0735-1097(97)00080-6 [DOI] [PubMed] [Google Scholar]
- 96. Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest 1996;98:36–42. 10.1172/JCI118774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Alvarez A, Hermenegildo C, Issekutz AC, Esplugues JV, Sanz MJ. Estrogens inhibit angiotensin II-induced leukocyte-endothelial cell interactions in vivo via rapid endothelial nitric oxide synthase and cyclooxygenase activation. Circ Res 2002;91:1142–50. 10.1161/01.RES.0000046018.23605.3E [DOI] [PubMed] [Google Scholar]
- 98. Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM. Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17beta-estradiol. Arterioscler Thromb Vasc Biol 1998;18:1498–505. 10.1161/01.ATV.18.9.1498 [DOI] [PubMed] [Google Scholar]
- 99. Ueda K, Lu Q, Baur W, Aronovitz MJ, Karas RH. Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol 2013;33:1837–43. 10.1161/ATVBAHA.112.300752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fernandez-Suarez ME, Escola-Gil JC, Pastor O, Davalos A, Blanco-Vaca F, Lasuncion MA, et al. Clinically used selective estrogen receptor modulators affect different steps of macrophage-specific reverse cholesterol transport. Sci Rep 2016;6:32105. 10.1038/srep32105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gartlehner G, Patel SV, Reddy S, Rains C, Schwimmer M, Kahwati L. Hormone therapy for the primary prevention of chronic conditions in postmenopausal persons: updated evidence report and systematic review for the US preventive services task force. JAMA 2022;328:1747–65. 10.1001/jama.2022.18324 [DOI] [PubMed] [Google Scholar]
- 102. Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001;135:939–53. 10.7326/0003-4819-135-11-200112040-00005 [DOI] [PubMed] [Google Scholar]
- 103. AlSiraj Y, Chen X, Thatcher SE, Temel RE, Cai L, Blalock E, et al. XX sex chromosome complement promotes atherosclerosis in mice. Nat Commun 2019;10:2631. 10.1038/s41467-019-10462-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pasterkamp G, den Ruijter HM, Giannarelli C. False utopia of one unifying description of the vulnerable atherosclerotic plaque: a call for recalibration that appreciates the diversity of mechanisms leading to atherosclerotic disease. Arterioscler Thromb Vasc Biol 2022;42:e86–95. 10.1161/ATVBAHA.121.316693 [DOI] [PubMed] [Google Scholar]
- 105. Hartman RJG, Owsiany K, Ma L, Koplev S, Hao K, Slenders L, et al. Sex-stratified gene regulatory networks reveal female key driver genes of atherosclerosis involved in smooth muscle cell phenotype switching. Circulation 2021;143:713–26. 10.1161/CIRCULATIONAHA.120.051231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hellings WE, Pasterkamp G, Verhoeven BA, De Kleijn DP, De Vries JP, Seldenrijk KA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg 2007;45:289–96. discussion 96–7. 10.1016/j.jvs.2006.09.051 [DOI] [PubMed] [Google Scholar]
- 107. Badimon L, Padro T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care 2012;1:60–74. 10.1177/2048872612441582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 2014;276:618–32. 10.1111/joim.12296 [DOI] [PubMed] [Google Scholar]
- 109. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers 2019;5:56. 10.1038/s41572-019-0106-z [DOI] [PubMed] [Google Scholar]
- 110. Ramaiola I, Padro T, Pena E, Juan-Babot O, Cubedo J, Martin-Yuste V, et al. Changes in thrombus composition and profilin-1 release in acute myocardial infarction. Eur Heart J 2015;36:965–75. 10.1093/eurheartj/ehu356 [DOI] [PubMed] [Google Scholar]
- 111. Patti G, De Caterina R, Abbate R, Andreotti F, Biasucci LM, Calabro P, et al. Platelet function and long-term antiplatelet therapy in women: is there a gender-specificity? A ‘state-of-the-art’ paper. Eur Heart J 2014;35:2213–23. 10.1093/eurheartj/ehu279 [DOI] [PubMed] [Google Scholar]
- 112. Breet NJ, Sluman MA, van Berkel MA, van Werkum JW, Bouman HJ, Harmsze AM, et al. Effect of gender difference on platelet reactivity. Neth Heart J 2011;19:451–7. 10.1007/s12471-011-0189-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bailey AL, Scantlebury DC, Smyth SS. Thrombosis and antithrombotic therapy in women. Arterioscler Thromb Vasc Biol 2009;29:284–8. 10.1161/ATVBAHA.108.179788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Coleman JR, Moore EE, Kelher MR, Samuels JM, Cohen MJ, Sauaia A, et al. Female platelets have distinct functional activity compared with male platelets: implications in transfusion practice and treatment of trauma-induced coagulopathy. J Trauma Acute Care Surg 2019;87:1052–60. 10.1097/TA.0000000000002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chichareon P, Modolo R, Kerkmeijer L, Tomaniak M, Kogame N, Takahashi K, et al. Association of sex with outcomes in patients undergoing percutaneous coronary intervention: a subgroup analysis of the GLOBAL LEADERS randomized clinical trial. JAMA Cardiol 2020;5:212–9. 10.1001/jamacardio.2019.4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wentzel JJ, Papafaklis MI, Antoniadis AP, Takahashi S, Cefalo NV, Cormier M, et al. Sex-related differences in plaque characteristics and endothelial shear stress related plaque-progression in human coronary arteries. Atherosclerosis 2022;342:9–18. 10.1016/j.atherosclerosis.2021.12.013 [DOI] [PubMed] [Google Scholar]
- 117. Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, et al. Association of sex with severity of cronary artery disease, ischemia, and symptom burden in patients with moderate or severe ischemia: secondary analysis of the ISCHEMIA randomized clinical trial. JAMA Cardiol 2020;5:773–86. 10.1001/jamacardio.2020.0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored women’s ischemia syndrome evaluation. Circulation 2006;114:894–904. 10.1161/CIRCULATIONAHA.105.609990 [DOI] [PubMed] [Google Scholar]
- 119. Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008;117:1787–801. 10.1161/CIRCULATIONAHA.107.726562 [DOI] [PubMed] [Google Scholar]
- 120. Ferry AV, Anand A, Strachan FE, Mooney L, Stewart SD, Marshall L, et al. Presenting symptoms in men and women diagnosed with myocardial infarction using sex-specific criteria. J Am Heart Assoc 2019;8:e012307. 10.1161/JAHA.119.012307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lichtman JH, Leifheit EC, Safdar B, Bao H, Krumholz HM, Lorenze NP, et al. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: evidence from the VIRGO study (variation in recovery: role of gender on outcomes of young AMI patients). Circulation 2018;137:781–90. 10.1161/CIRCULATIONAHA.117.031650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bogsrud MP, Graesdal A, Retterstol K, Holven KB. A physically fit woman in her thirties with exertional dyspnoea. Tidsskr Nor Laegeforen 2017;137:456–8. 10.4045/tidsskr.16.0538 [DOI] [PubMed] [Google Scholar]
- 123. de Torbal A, Boersma E, Kors JA, van Herpen G, Deckers JW, van der Kuip DA, et al. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam study. Eur Heart J 2006;27:729–36. 10.1093/eurheartj/ehi707 [DOI] [PubMed] [Google Scholar]
- 124. van der Ende MY, Juarez-Orozco LE, Waardenburg I, Lipsic E, Schurer RAJ, van der Werf HW, et al. Sex-based differences in unrecognized myocardial infarction. J Am Heart Assoc 2020;9:e015519. 10.1161/JAHA.119.015519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol 2010;23:511–9. 10.1111/j.1540-8183.2010.00598.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018;3:144–52. 10.1001/jamacardio.2017.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xie JX, Eshtehardi P, Varghese T, Goyal A, Mehta PK, Kang W, et al. Prognostic significance of nonobstructive left main coronary artery disease in women versus men: long-term outcomes from the CONFIRM (coronary CT angiography evaluation for clinical outcomes: an international multicenter) registry. Circ Cardiovasc Imaging 2017;10:e006246. 10.1161/CIRCIMAGING.117.006246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pagidipati NJ, Hemal K, Coles A, Mark DB, Dolor RJ, Pellikka PA, et al. Sex differences in functional and CT angiography testing in patients with suspected coronary artery disease. J Am Coll Cardiol 2016;67:2607–16. 10.1016/j.jacc.2016.03.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mansour M, Radaideh Q, Alaiwah MN, Alnimer Y, Devabhaktuni SR, Dhar G, et al. Major adverse cardiac events in symptomatic women with non-obstructive CAD on coronary CTA: pooled analysis from PROMISE and SCOT-HEART. Int J Cardiovasc Imaging 2022;38:683–93. 10.1007/s10554-021-02429-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC consortium. Eur Heart J 2018;39:3727–35. 10.1093/eurheartj/ehy534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kalra DK, Heo R, Valenti V, Nakazato R, Min JK. Role of computed tomography for diagnosis and risk stratification of patients with suspected or known coronary artery disease. Arterioscler Thromb Vasc Biol 2014;34:1144–54. 10.1161/ATVBAHA.113.302074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kilickap M, Erol MK, Kayikcioglu M, Kocayigit I, Gitmez M, Can V, et al. Short and midterm outcomes in patients with acute myocardial infarction: results of the nationwide TURKMI registry. Angiology 2021;72:339–47. 10.1177/0003319720975302 [DOI] [PubMed] [Google Scholar]
- 133. Trutter L, Bigeh A, Pecci C, Muzaffar M, Gulati M. Diagnostic and management dilemmas in women presenting with acute coronary syndromes. Curr Cardiol Rep 2020;22:163. 10.1007/s11886-020-01410-1 [DOI] [PubMed] [Google Scholar]
- 134. Bachelet BC, Hyun K, D’Souza M, Chow CK, Redfern J, Brieger DB. Sex differences in the management and outcomes of non-ST-elevation acute coronary syndromes. Med J Aust 2022;216:153–5. 10.5694/mja2.51220 [DOI] [PubMed] [Google Scholar]
- 135. [(accessed 4 May 2023).]. https://www.paho.org/en/enlace/leading-causes-death-and-disability Pan American Health Organization. Leading causes of death, and disability 2019/Available from:
- 136. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1545–88. 10.1161/01.str.0000442009.06663.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ali M, van Os HJA, van der Weerd N, Schoones JW, Heymans MW, Kruyt ND, et al. Sex differences in presentation of stroke: a systematic review and meta-analysis. Stroke 2022;53:345–54. 10.1161/STROKEAHA.120.034040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yu AYX, Penn AM, Lesperance ML, Croteau NS, Balshaw RF, Votova K, et al. Sex differences in presentation and outcome after an acute transient or minor neurologic event. JAMA Neurol 2019;76:962–8. 10.1001/jamaneurol.2019.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case–control study. Lancet 2016;388:761–75. 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 140. Goldstein LB, Amarenco P, Lamonte M, Gilbert S, Messig M, Callahan A, et al. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study. Stroke 2008;39:2444–8. 10.1161/STROKEAHA.107.513747 [DOI] [PubMed] [Google Scholar]
- 141. Higgins JP, Higgins JA. Epidemiology of peripheral arterial disease in women. J Epidemiol 2003;13:1–14. 10.2188/jea.13.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Srivaratharajah K, Abramson BL . Women and peripheral arterial disease: a review of sex differences in epidemiology, clinical manifestations, and outcomes. Can J Cardiol 2018;34:356–61. 10.1016/j.cjca.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 143. McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women’s health and aging study. Circulation 2000;101:1007–12. 10.1161/01.CIR.101.9.1007 [DOI] [PubMed] [Google Scholar]
- 144. McDermott MM, Ferrucci L, Simonsick EM, Balfour J, Fried L, Ling S, et al. The ankle brachial index and change in lower extremity functioning over time: the women’s health and aging study. J Am Geriatr Soc 2002;50:238–46. 10.1046/j.1532-5415.2002.50054.x [DOI] [PubMed] [Google Scholar]
- 145. McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med 1999;159:387–92. 10.1001/archinte.159.4.387 [DOI] [PubMed] [Google Scholar]
- 146. Vouyouka AG, Egorova NN, Salloum A, Kleinman L, Marin M, Faries PL, et al. Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. J Vasc Surg 2010;52:1196–202. 10.1016/j.jvs.2010.05.106 [DOI] [PubMed] [Google Scholar]
- 147. Ramkumar N, Suckow BD, Brown JR, Sedrakyan A, Cronenwett JL, Goodney PP. Sex-based assessment of patient presentation, lesion characteristics, and treatment modalities in patients undergoing peripheral vascular intervention. Circ Cardiovasc Interv 2018;11:e005749. 10.1161/CIRCINTERVENTIONS.117.005749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. McCoach CE, Armstrong EJ, Singh S, Javed U, Anderson D, Yeo KK, et al. Gender-related variation in the clinical presentation and outcomes of critical limb ischemia. Vasc Med 2013;18:19–26. 10.1177/1358863X13475836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jackson EA, Munir K, Schreiber T, Rubin JR, Cuff R, Gallagher KA, et al. Impact of sex on morbidity and mortality rates after lower extremity interventions for peripheral arterial disease: observations from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Am Coll Cardiol 2014;63:2525–30. 10.1016/j.jacc.2014.03.036 [DOI] [PubMed] [Google Scholar]
- 150. Altin SE, Castro-Dominguez YS, Kennedy KF, Orion KC, Lanksy AJ, Abbott JD, et al. Predictors of underutilization of medical therapy in patients undergoing endovascular revascularization for peripheral artery disease. JACC Cardiovasc Interv 2020;13:2911–8. 10.1016/j.jcin.2020.08.036 [DOI] [PubMed] [Google Scholar]
- 151. Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Wahlberg E. Risk factor profiles and use of cardiovascular drug prevention in women and men with peripheral arterial disease. Eur J Cardiovasc Prev Rehabil 2009;16:39–46. 10.1097/HJR.0b013e32831c1383 [DOI] [PubMed] [Google Scholar]
- 152. Hess CN, Cannon CP, Beckman JA, Goodney PP, Patel MR, Hiatt WR, et al. Effectiveness of blood lipid management in patients with peripheral artery disease. J Am Coll Cardiol 2021;77:3016–27. 10.1016/j.jacc.2021.04.060 [DOI] [PubMed] [Google Scholar]
- 153. Cacoub PP, Abola MT, Baumgartner I, Bhatt DL, Creager MA, Liau CS, et al. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the reduction of atherothrombosis for continued health (REACH) registry. Atherosclerosis 2009;204:e86–92. 10.1016/j.atherosclerosis.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 154. Steg PG, Bhatt DL, Wilson PW, D’Agostino R Sr., Ohman EM, Rother J, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297:1197–206. 10.1001/jama.297.11.1197 [DOI] [PubMed] [Google Scholar]
- 155. Dreyer RP, van Zitteren M, Beltrame JF, Fitridge R, Denollet J, Vriens PW, et al. Gender differences in health status and adverse outcomes among patients with peripheral arterial disease. J Am Heart Assoc 2014;4:e000863. 10.1161/JAHA.114.000863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lefebvre KM, Chevan J. The persistence of gender and racial disparities in vascular lower extremity amputation: an examination of HCUP-NIS data (2002–2011). Vasc Med 2015;20:51–9. 10.1177/1358863X14565373 [DOI] [PubMed] [Google Scholar]
- 157. Oyri LKL, Bogsrud MP, Christensen JJ, Ulven SM, Brantsaeter AL, Retterstol K, et al. Novel associations between parental and newborn cord blood metabolic profiles in the Norwegian mother, father and child cohort study. BMC Med 2021;19:91. 10.1186/s12916-021-01959-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bansal N, Cruickshank JK, McElduff P, Durrington PN. Cord blood lipoproteins and prenatal influences. Curr Opin Lipidol 2005;16:400–8. 10.1097/01.mol.0000174154.61307.16 [DOI] [PubMed] [Google Scholar]
- 159. Morrison KM, Anand SS, Yusuf S, Atkinson SA, Schulze KM, Rao-Melacini P, et al. Maternal and pregnancy related predictors of cardiometabolic traits in newborns. PLoS One 2013;8:e55815. 10.1371/journal.pone.0055815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Balder JW, Lansberg PJ, Hof MH, Wiegman A, Hutten BA, Kuivenhoven JA. Pediatric lipid reference values in the general population: the Dutch lifelines cohort study. J Clin Lipidol 2018;12:1208–16. 10.1016/j.jacl.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 161. Dathan-Stumpf A, Vogel M, Hiemisch A, Thiery J, Burkhardt R, Kratzsch J, et al. Pediatric reference data of serum lipids and prevalence of dyslipidemia: results from a population-based cohort in Germany. Clin Biochem 2016;49:740–9. 10.1016/j.clinbiochem.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 162. Mumford SL, Dasharathy S, Pollack AZ, Schisterman EF. Variations in lipid levels according to menstrual cycle phase: clinical implications. Clin Lipidol 2011;6:225–34. 10.2217/clp.11.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Klajnbard A, Szecsi PB, Colov NP, Andersen MR, Jorgensen M, Bjorngaard B, et al. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clin Chem Lab Med 2010;48:237–48. 10.1515/CCLM.2010.033 [DOI] [PubMed] [Google Scholar]
- 164. Zhang Z, Lai M, Piro AL, Alexeeff SE, Allalou A, Rost HL, et al. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: the SWIFT study. BMC Med 2021;19:241. 10.1186/s12916-021-02095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Knopp RH, Walden CE, Wahl PW, Bergelin R, Chapman M, Irvine S, et al. Effect of postpartum lactation on lipoprotein lipids and apoproteins. J Clin Endocrinol Metab 1985;60:542–7. 10.1210/jcem-60-3-542 [DOI] [PubMed] [Google Scholar]
- 166. Stuebe AM, Michels KB, Willett WC, Manson JE, Rexrode K, Rich-Edwards JW. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol 2009;200:138.e1–8. 10.1016/j.ajog.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Schwarz EB, Ray RM, Stuebe AM, Allison MA, Ness RB, Freiberg MS, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol 2009;113:974–82. 10.1097/01.AOG.0000346884.67796.ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Peters SAE, Yang L, Guo Y, Chen Y, Bian Z, Du J, et al. Breastfeeding and the risk of maternal cardiovascular disease: a prospective study of 300 000 Chinese women. J Am Heart Assoc 2017;6:e006081. 10.1161/JAHA.117.006081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ambikairajah A, Walsh E, Cherbuin N. Lipid profile differences during menopause: a review with meta-analysis. Menopause 2019;26:1327–33. 10.1097/GME.0000000000001403 [DOI] [PubMed] [Google Scholar]
- 170. Raguindin PF, Cardona I, Muka T, Lambrinoudaki I, Gebhard C, Franco OH, et al. Does reproductive stage impact cardiovascular disease risk factors? Results from a population-based cohort in Lausanne (CoLaus study). Clin Endocrinol (Oxf) 2022;97:568–80. 10.1111/cen.14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Iyen B, Qureshi N, Weng S, Roderick P, Kai J, Capps N, et al. Sex differences in cardiovascular morbidity associated with familial hypercholesterolaemia: a retrospective cohort study of the UK Simon Broome register linked to national hospital records. Atherosclerosis 2020;315:131–7. 10.1016/j.atherosclerosis.2020.10.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Holven KB, Narverud I, van Lennep JR, Versmissen J, Oyri LKL, Galema-Boers A, et al. Sex differences in cholesterol levels from birth to 19 years of age may lead to increased cholesterol burden in females with FH. J Clin Lipidol 2018;12:748–755.e2. 10.1016/j.jacl.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 173. Mundal L, Sarancic M, Ose L, Iversen PO, Borgan JK, Veierod MB, et al. Mortality among patients with familial hypercholesterolemia: a registry-based study in Norway, 1992–2010. J Am Heart Assoc 2014;3:e001236. 10.1161/JAHA.114.001236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol 2018;72:1141–56. 10.1016/j.jacc.2018.06.046 [DOI] [PubMed] [Google Scholar]
- 175. [(accessed 4 May 2023).]. https://www.fda.gov/safety/medical-product-safety-information/statins-drug-safety-communication-fda-requests-removal-strongest-warning-against-using-cholesterol US Food and Drug Administration. Statins: Drug Safety Communication - FDA Requests Removal of Strongest Warning Against Using Cholesterol-lowering Statins During Pregnancy 2021. Available from:
- 176. Toleikyte I, Retterstol K, Leren TP, Iversen PO. Pregnancy outcomes in familial hypercholesterolemia: a registry-based study. Circulation 2011;124:1606–14. 10.1161/CIRCULATIONAHA.110.990929 [DOI] [PubMed] [Google Scholar]
- 177. Botha TC, Pilcher GJ, Wolmarans K, Blom DJ, Raal FJ. Statins and other lipid-lowering therapy and pregnancy outcomes in homozygous familial hypercholesterolaemia: a retrospective review of 39 pregnancies. Atherosclerosis 2018;277:502–7. 10.1016/j.atherosclerosis.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 178. Klevmoen M, Bogsrud MP, Retterstol K, Svilaas T, Vesterbekkmo EK, Hovland A, et al. Loss of statin treatment years during pregnancy and breastfeeding periods in women with familial hypercholesterolemia. Atherosclerosis 2021;335:8–15. 10.1016/j.atherosclerosis.2021.09.003 [DOI] [PubMed] [Google Scholar]
- 179. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res 2016;118:547–63. 10.1161/CIRCRESAHA.115.306249 [DOI] [PubMed] [Google Scholar]
- 180. Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J 2021;42:4791–806. 10.1093/eurheartj/ehab551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Varbo A, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013;128:1298–309. 10.1161/CIRCULATIONAHA.113.003008 [DOI] [PubMed] [Google Scholar]
- 182. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308. 10.1001/jama.298.3.299 [DOI] [PubMed] [Google Scholar]
- 183. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 2008;300:2142–52. 10.1001/jama.2008.621 [DOI] [PubMed] [Google Scholar]
- 184. Varbo A, Nordestgaard BG. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol 2019;85:550–9. 10.1002/ana.25432 [DOI] [PubMed] [Google Scholar]
- 185. Wadstrom BN, Wulff AB, Pedersen KM, Jensen GB, Nordestgaard BG. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort-based study. Eur Heart J 2022;43:3258–69. 10.1093/eurheartj/ehab705 [DOI] [PubMed] [Google Scholar]
- 186. Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. 10.1056/NEJMoa1308027 [DOI] [PubMed] [Google Scholar]
- 187. Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J 2013;34:1826–33. 10.1093/eurheartj/ehs431 [DOI] [PubMed] [Google Scholar]
- 188. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 189. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020;324:2268–80. 10.1001/jama.2020.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Doi T, Langsted A, Nordestgaard BG. A possible explanation for the contrasting results of REDUCE-IT vs. STRENGTH: cohort study mimicking trial designs. Eur Heart J 2021;42:4807–17. 10.1093/eurheartj/ehab555 [DOI] [PubMed] [Google Scholar]
- 191. Pradhan A D, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med 2022;387:1923–34. 10.1056/NEJMoa2210645 [DOI] [PubMed] [Google Scholar]
- 192. Jacobs DR J, Woo JG, Sinaiko AR, Daniels SR, Ikonen J, Juonala M, et al. Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med 2022;386:1877–88. 10.1056/NEJMoa2109191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol 2020;75:2122–35. 10.1016/j.jacc.2020.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. doi: 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol 2021;6:437–47. 10.1001/jamacardio.2020.7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol 2014;63:470–7. 10.1016/j.jacc.2013.09.038 [DOI] [PubMed] [Google Scholar]
- 197. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–12. 10.1056/NEJMoa1109034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J 2022;43:3925–46. 10.1093/eurheartj/ehac361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331–9. 10.1001/jama.2009.801 [DOI] [PubMed] [Google Scholar]
- 200. Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased eisk of heart failure in the general population. JACC Heart Fail 2016;4:78–87. 10.1016/j.jchf.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 201. Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J 2019;40:2760–70. 10.1093/eurheartj/ehy902 [DOI] [PubMed] [Google Scholar]
- 202. Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol 2019;74:54–66. 10.1016/j.jacc.2019.03.524 [DOI] [PubMed] [Google Scholar]
- 203. Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 2009;361:2518–28. 10.1056/NEJMoa0902604 [DOI] [PubMed] [Google Scholar]
- 204. Emerging Risk Factors C, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–23. doi: 10.1001/jama.2009.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. O’Donoghue ML, Morrow DA, Tsimikas S, Sloan S, Ren AF, Hoffman EB, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol 2014;63:520–7. 10.1016/j.jacc.2013.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein(a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: a population-based study. Arterioscler Thromb Vasc Biol 2020;40:255–66. 10.1161/ATVBAHA.119.312951 [DOI] [PubMed] [Google Scholar]
- 207. Pare G, Caku A, McQueen M, Anand SS, Enas E, Clarke R, et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation 2019;139:1472–82. 10.1161/CIRCULATIONAHA.118.034311 [DOI] [PubMed] [Google Scholar]
- 208. Patel AP, Wang M, Pirruccello JP, Ellinor PT, Ng K, Kathiresan S, et al. Lp(a) (lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease: new insights from a large national Biobank. Arterioscler Thromb Vasc Biol 2021;41:465–74. 10.1161/ATVBAHA.120.315291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Kim CJ, Kim TH, Ryu WS, Ryoo UH. Influence of menopause on high density lipoprotein-cholesterol and lipids. J Korean Med Sci 2000;15:380–6. 10.3346/jkms.2000.15.4.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Slunga L, Asplund K, Johnson O, Dahlen GH. Lipoprotein (a) in a randomly selected 25–64 year old population: the Northern Sweden Monica study. J Clin Epidemiol 1993;46:617–24. 10.1016/0895-4356(93)90034-X [DOI] [PubMed] [Google Scholar]
- 211. Ushioda M, Makita K, Takamatsu K, Horiguchi F, Aoki D. Serum lipoprotein(a) dynamics before/after menopause and long-term effects of hormone replacement therapy on lipoprotein(a) levels in middle-aged and older Japanese women. Horm Metab Res 2006;38:581–6. 10.1055/s-2006-950504 [DOI] [PubMed] [Google Scholar]
- 212. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the study of women’s health across the nation. Am J Epidemiol 2009;169:1352–61. 10.1093/aje/kwp043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol 2008;52:124–31. 10.1016/j.jacc.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Shlipak MG, Simon JA, Vittinghoff E, Lin F, Barrett-Connor E, Knopp RH, et al. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA 2000;283:1845–52. 10.1001/jama.283.14.1845 [DOI] [PubMed] [Google Scholar]
- 215. Gregersen I, Hoibraaten E, Holven KB, Lovdahl L, Ueland T, Mowinckel MC, et al. Effect of hormone replacement therapy on atherogenic lipid profile in postmenopausal women. Thromb Res 2019;184:1–7. 10.1016/j.thromres.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 216. Zechner R, Desoye G, Schweditsch MO, Pfeiffer KP, Kostner GM. Fluctuations of plasma lipoprotein-A concentrations during pregnancy and post partum. Metab Clin Exp 1986;35:333–6. 10.1016/0026-0495(86)90150-2 [DOI] [PubMed] [Google Scholar]
- 217. Sattar N, Clark P, Greer IA, Shepherd J, Packard CJ. Lipoprotein (a) levels in normal pregnancy and in pregnancy complicated with pre-eclampsia. Atherosclerosis 2000;148:407–11. 10.1016/S0021-9150(99)00296-8 [DOI] [PubMed] [Google Scholar]
- 218. Simony SB, Mortensen MB, Langsted A, Afzal S, Kamstrup PR, Nordestgaard BG. Sex differences of lipoprotein(a) levels and associated risk of morbidity and mortality by age: the Copenhagen General Population Study. Atherosclerosis 2022;355:76–82. 10.1016/j.atherosclerosis.2022.06.1023 [DOI] [PubMed] [Google Scholar]
- 219. Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol 2021;37:1129–50. 10.1016/j.cjca.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 220. Cholesterol Treatment Trialists Collaboration, Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397–405. 10.1016/S0140-6736(14)61368-4 [DOI] [PubMed] [Google Scholar]
- 221. Navar AM, Peterson ED, Li S, Robinson JG, Roger VL, Goldberg AC, et al. Prevalence and management of symptoms associated with statin therapy in community practice: insights from the PALM (patient and provider assessment of lipid management) registry. Circ Cardiovasc Qual Outcomes 2018;11:e004249. 10.1161/CIRCOUTCOMES.117.004249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, et al. Patient-reported reasons for declining or discontinuing statin therapy: insights from the PALM registry. J Am Heart Assoc 2019;8:e011765. 10.1161/JAHA.118.011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for this manuscript.