Key Points
Question
Does the choice between cefepime and piperacillin-tazobactam affect the risks of acute kidney injury or neurological dysfunction in adults hospitalized with acute infection?
Findings
Among 2511 adults hospitalized with acute infection, the highest stage of acute kidney injury or death was not significantly different between patients randomized to cefepime and those randomized to piperacillin-tazobactam. Patients randomized to cefepime experienced more neurological dysfunction, a secondary outcome.
Meaning
Among hospitalized adults, the risk of acute kidney injury did not differ between cefepime and piperacillin-tazobactam, but neurological dysfunction was more common with cefepime.
Abstract
Importance
Cefepime and piperacillin-tazobactam are commonly administered to hospitalized adults for empirical treatment of infection. Although piperacillin-tazobactam has been hypothesized to cause acute kidney injury and cefepime has been hypothesized to cause neurological dysfunction, their comparative safety has not been evaluated in a randomized clinical trial.
Objective
To determine whether the choice between cefepime and piperacillin-tazobactam affects the risks of acute kidney injury or neurological dysfunction.
Design, Setting, and Participants
The Antibiotic Choice on Renal Outcomes (ACORN) randomized clinical trial compared cefepime vs piperacillin-tazobactam in adults for whom a clinician initiated an order for antipseudomonal antibiotics within 12 hours of presentation to the hospital in the emergency department or medical intensive care unit at an academic medical center in the US between November 10, 2021, and October 7, 2022. The final date of follow-up was November 4, 2022.
Interventions
Patients were randomized in a 1:1 ratio to cefepime or piperacillin-tazobactam.
Main Outcomes and Measures
The primary outcome was the highest stage of acute kidney injury or death by day 14, measured on a 5-level ordinal scale ranging from no acute kidney injury to death. The 2 secondary outcomes were the incidence of major adverse kidney events at day 14 and the number of days alive and free of delirium and coma within 14 days.
Results
There were 2511 patients included in the primary analysis (median age, 58 years [IQR, 43-69 years]; 42.7% were female; 16.3% were Non-Hispanic Black; 5.4% were Hispanic; 94.7% were enrolled in the emergency department; and 77.2% were receiving vancomycin at enrollment). The highest stage of acute kidney injury or death was not significantly different between the cefepime group and the piperacillin-tazobactam group; there were 85 patients (n = 1214; 7.0%) in the cefepime group with stage 3 acute kidney injury and 92 (7.6%) who died vs 97 patients (n = 1297; 7.5%) in the piperacillin-tazobactam group with stage 3 acute kidney injury and 78 (6.0%) who died (odds ratio, 0.95 [95% CI, 0.80 to 1.13], P = .56). The incidence of major adverse kidney events at day 14 did not differ between groups (124 patients [10.2%] in the cefepime group vs 114 patients [8.8%] in the piperacillin-tazobactam group; absolute difference, 1.4% [95% CI, −1.0% to 3.8%]). Patients in the cefepime group experienced fewer days alive and free of delirium and coma within 14 days (mean [SD], 11.9 [4.6] days vs 12.2 [4.3] days in the piperacillin-tazobactam group; odds ratio, 0.79 [95% CI, 0.65 to 0.95]).
Conclusions and Relevance
Among hospitalized adults in this randomized clinical trial, treatment with piperacillin-tazobactam did not increase the incidence of acute kidney injury or death. Treatment with cefepime resulted in more neurological dysfunction.
Trial Registration
ClinicalTrials.gov Identifier: NCT05094154
This randomized clinical trial compares the use of cefepime vs piperacillin-tazobactam and the risk of acute kidney injury and neurological dysfunction in adults hospitalized with acute infection.
Introduction
Acutely ill adults presenting to a hospital with suspected infection frequently receive empirical antibiotics. For patients at risk for infection with resistant gram-negative bacteria, guidelines recommend the administration of antipseudomonal antibiotics such as cefepime or piperacillin-tazobactam.1
Because cefepime and piperacillin-tazobactam have similar activity against many gram-negative bacteria (efficacy), selection between the 2 is likely to depend on differences within their adverse effect profiles (safety). Some observational studies have reported an association between cefepime and neurotoxicity,2,3,4 ranging from agitation to coma.5 Some observational studies have reported an association between piperacillin-tazobactam and acute kidney injury (AKI), particularly with concurrent receipt of vancomycin.6,7,8 A randomized clinical trial comparing treatments for methicillin-resistant Staphylococcus aureus found that the addition of an antistaphylococcal β-lactam antibiotic to vancomycin increased the risk of AKI.9 To our knowledge, no randomized clinical trial has compared cefepime vs piperacillin-tazobactam; therefore, it is unknown whether the risks of AKI or neurological dysfunction differ between the 2 drugs.
To compare the safety of cefepime vs piperacillin-tazobactam in adults presenting to the hospital with suspected infection, we conducted the Antibiotic Choice on Renal Outcomes (ACORN) randomized clinical trial.
Methods
Trial Design and Oversight
Between November 10, 2021, and October 7, 2022, we conducted a pragmatic, open-label, parallel-group, randomized comparative safety trial of cefepime vs piperacillin-tazobactam in adult patients with suspected infection in the emergency department (ED) or medical intensive care unit (ICU) at Vanderbilt University Medical Center. The trial was initiated by the investigators, approved by the institutional review board with a waiver of informed consent, registered before enrollment commenced, and overseen by an independent data and safety monitoring board. The trial protocol and statistical analysis plan were published before enrollment concluded10 and appear in Supplement 1.
Patient Population
Adults (≥18 years of age) in the ED or medical ICU for whom a clinician initiated an order for cefepime or piperacillin-tazobactam within 12 hours of presentation to the hospital were eligible. Patients were excluded if they had an allergy to cephalosporins or penicillins, had received more than 1 dose of an antipseudomonal cephalosporin or penicillin within the previous 7 days (patients who had received other antipseudomonal antibiotics were eligible), were incarcerated, or if the treating clinician determined that 1 of the 2 drugs represented a better treatment option for that patient. An electronic health record tool screened all patients for eligibility and an automated alert within the electronic order entry system confirmed patient eligibility with clinicians.
Randomization
Using software within the electronic health record, patients were assigned via simple randomization without stratification in a 1:1 to ratio to receive cefepime or piperacillin-tazobactam (Figure 1). Trial group assignment was concealed until enrollment.
Figure 1. Flow of Participants Through the ACORN Trial.
ACORN indicates Antibiotic Choice on Renal Outcomes.
aA tool in the electronic health record screened all patients presenting to the study hospital for the presence of inclusion criteria (≥18 years of age; being treated in participating emergency department or intensive care unit; <12 hours since presentation to the hospital; and order initiated for antipseudomonal cephalosporin or penicillin).
bIncluded clinician preference, metronidazole shortage, and no reason recorded.
Treatments
A clinical decision support tool in the electronic health record facilitated placement of an order for the assigned antibiotic and recommended a dose and frequency based on the patient’s estimated glomerular filtration rate. Per institutional protocols, the standard administration of cefepime was a 2-g intravenous push over 5 minutes every 8 hours and piperacillin-tazobactam was administered as a 3.375-g bolus over 30 minutes for the initial administration followed by an extended infusion of 3.375 g every 8 hours infused over 4 hours for subsequent doses (additional information appears in the eMethods in Supplement 2). Cefepime was the only antipseudomonal cephalosporin and piperacillin-tazobactam was the only antipseudomonal penicillin available in the trial settings.
Treating clinicians determined the duration of antipseudomonal antibiotic therapy and whether to administer additional antibiotics, such as vancomycin or metronidazole. For the 7 days after enrollment, if treating clinicians discontinued the assigned antibiotic or ordered the unassigned antibiotic, an automated alert reminded them of the trial and recorded the reason for the change. The study ED and ICU were each staffed by a dedicated clinical pharmacist; non-ICU inpatient units were not. Clinical pharmacists remotely monitored drug levels for intravenous vancomycin but not for the β-lactam antibiotics.
Data Collection
Data were collected in routine care and electronically extracted from the electronic health record of the study institution. Data included information on demographics; diagnoses; preenrollment kidney function; medication administration; vital signs; laboratory values; microbiological cultures; organ support therapies; Sequential Organ Failure Assessment score11; Confusion Assessment Method for the ICU (CAM-ICU) score12; Richmond Agitation-Sedation Scale (RASS) score13; Glasgow Coma Scale score14; dates of admission, transfer, and discharge; and vital status at hospital discharge (eMethods in Supplement 2).
Race and ethnicity were self-reported by patients or reported by their surrogates as part of clinical care. Race and ethnicity were automatically extracted from the electronic health record using fixed categories to facilitate assessment of the representativeness of the trial population and the generalizability of the trial results. The frequency of assessments and laboratory measurements were determined by treating clinicians and preexisting clinical protocols. At the study institution, the RASS and Glasgow Coma Scale scores were recorded every 12 hours for all patients and the CAM-ICU score was recorded every 12 hours for patients admitted to the ICU.
Trial personnel adjudicated whether sepsis was present at enrollment using Sepsis-3 criteria,15 the presumed source of infection using previously published categories,16 whether kidney replacement therapy (KRT) had been received, and the indication for KRT.
Outcomes
The primary outcome was the highest stage of AKI or death arising between randomization and day 14, measured on a 5-level ordinal scale. The stages of AKI were defined using the Kidney Disease: Improving Global Outcomes criteria for creatinine level.17 Surviving patients who did not experience new or worsening AKI were assigned a value of 0, those who experienced stage 1 AKI (creatinine level that was 1.5-1.9 times the baseline level or increased by ≥0.3 mg/dL [≥26.5 μmol/L]) were assigned a value of 1, those who experienced stage 2 AKI (creatinine level that was 2.0-2.9 times the baseline level) were assigned a value of 2, and those who experienced stage 3 AKI (creatinine level that was ≥3.0 times the baseline level, AKI with a creatinine level ≥4.0 mg/dL [≥353.7 μmol/L], or receipt of new KRT) were assigned a value of 3. To account for the competing risk of death, patients who died were assigned a value of 4.
The value for baseline creatinine level was determined using a previously described hierarchical approach in which creatinine values obtained during the year before hospitalization were given priority over in-hospital measurements obtained before enrollment and an estimated value was used when no preenrollment measurements were available.18,19 For patients with AKI at enrollment, new AKI was defined as a creatinine level that was at least 0.3-mg/dL greater than the lowest prior creatinine level since enrollment. Patients who had received KRT before enrollment could not experience AKI and received a value of 0 if they survived and 4 if they died. All data were censored at hospital discharge. The primary outcome was selected to assess for an effect of piperacillin-tazobactam on the development of AKI using an international consensus definition over the same period used in prior observational studies.17,20
Two secondary outcomes were prespecified. The first was the proportion of patients who experienced a major adverse kidney event at day 14, which was the composite of death, receipt of new KRT, or persistent kidney dysfunction (final inpatient creatinine level that was ≥2 times the baseline level), and censored at hospital discharge or 14 days after enrollment, whichever occurred first. This outcome was recommended by the National Institute of Diabetes and Digestive and Kidney Diseases work group on clinical trials in AKI as a patient-centered outcome for phase 3 trials, and it is considered more clinically meaningful than outcomes based purely on laboratory values.18,21 The other secondary outcome was the number of days alive and free of delirium and coma within 14 days, which was defined as the number of calendar days on which the patient was alive and without a positive assessment on the CAM-ICU12 or a RASS13 score of −4 or −5, and censored at hospital discharge. This outcome was selected to assess the effect of cefepime on the development of neurological dysfunction using a common measure of neurological function among acutely ill adults22,23,24 (eMethods in Supplement 2).
Statistical Analysis
Details regarding the sample size estimation and reestimation have been reported previously.10 The trial was initially designed to enroll 2050 patients to provide 80% statistical power to detect an odds ratio (OR) of 0.65 in the primary analysis (eMethods in Supplement 2). Because the association between piperacillin-tazobactam and AKI has been hypothesized to be conditioned on concurrent receipt of vancomycin6,7,8 and the timing of the trial’s interim analysis, 75% of patients in the trial population were receiving vancomycin at enrollment; therefore, the data and safety monitoring board recommended increasing the sample size from 2050 to 2500 patients. Assuming a 2-sided α of .05 and a distribution of the primary outcome with approximately 70% of patients experiencing no AKI, 10% experiencing stage 1 AKI, 7% experiencing stage 2 AKI, 7% experiencing stage 3 AKI, and 6% experiencing death, we calculated that enrollment of 2500 patients would provide 92% statistical power to detect an OR of 0.75 in the primary analysis. An OR of 0.75 would equate to an absolute between-group difference of 5% in patients who experienced AKI of any stage or death.
The primary analysis population included all randomized patients who received at least 1 dose of either cefepime or piperacillin-tazobactam, analyzed in the group to which they were assigned. The primary analysis compared trial groups using an unadjusted proportional odds regression model with between-group differences expressed using an unadjusted OR and 95% CI, for which values less than 1.0 indicate a lower odds of AKI or death with cefepime compared with piperacillin-tazobactam. Major adverse kidney events were compared between groups using an unadjusted logistic regression model. Days alive and free of delirium and coma were compared between groups using an unadjusted proportional odds regression model.
As a secondary analysis, an adjusted analysis of the primary outcome was performed using a multivariable proportional odds regression model with prespecified baseline covariates including age, sex, baseline creatinine level, prior receipt of KRT, receipt of vasopressors, receipt of mechanical ventilation, Sequential Organ Failure Assessment score, presumed source of infection, and location at enrollment. Similar variables were used for the adjusted analyses of the secondary outcomes.
Prespecified sensitivity analyses included (1) an analysis of all enrolled patients, including those who never received cefepime or piperacillin-tazobactam; (2) an analysis restricted to patients who received treatment with cefepime or piperacillin-tazobactam for more than 48 hours (greater exposure to the antibiotics under study); (3) an analysis removing race as a variable in the equation to calculate creatinine for those without measurement of creatinine level prior to the current illness; (4) an analysis restricted to patients with a measured creatinine level prior to the current hospitalization; and (5) an analysis using only the preillness creatinine level as the baseline creatinine level.
In accordance with published guidelines,25 we examined whether the prespecified baseline variables modified the effect of trial group assignment on the primary and secondary outcomes using models with independent variables for each trial group, the proposed effect modifier, and the interaction between the 2 (eMethods in Supplement 2). The baseline variables prespecified as potential effect modifiers included presence of sepsis, source of infection, receipt of vancomycin on the day of enrollment, chronic kidney disease, presence and stage of AKI at enrollment, and admitting service (medical or surgical).
When data were missing for the secondary or exploratory outcomes, a complete case analysis was performed, excluding cases in which data were missing for the analyzed outcome. Missing data were not imputed for these outcomes (eMethods in Supplement 2).
The data and safety monitoring board reviewed a single planned interim analysis after enrollment of the first 1025 patients, with a stopping boundary of P ≤ .001 for the between-group difference in the primary outcome. For the final analysis of the primary outcome, a 2-sided P value of less than .05 was considered to indicate statistical significance.26 Between-group differences in the secondary and exploratory outcomes are reported as point estimates and 95% CIs. The widths of the 95% CIs for the secondary analyses were not adjusted for multiplicity and should not be used to infer definitive between-group differences in the treatment effects. All analyses were performed with R version 4.2.2 (R Foundation for Statistical Computing).
Results
Trial Population
Among 3806 patients who met inclusion criteria, 1172 (30.8%) were excluded. Of the 2634 patients (69.2%) enrolled, 4 (0.2%) were incarcerated and excluded from subsequent data collection and analysis and 119 (4.5%) did not receive a dose of cefepime or piperacillin-tazobactam during the 7 days after enrollment and were not included in the primary analysis. A total of 2511 patients (95.3%) were included in the primary analysis (Figure 1). The median age was 58 years (IQR, 43-69 years), 2378 patients (94.7%) were enrolled in the ED, and the median time between presentation to the hospital and enrollment was 1.2 hours (IQR, 0.4-3.5 hours). At the time of enrollment, 1362 patients (54.2%) had sepsis and the most common suspected sources of infection were intra-abdominal and pulmonary. A total of 1214 patients (48.3%) in the cefepime group and 1297 patients (51.7%) in the piperacillin-tazobactam group were included in the primary analysis (Table 1 and eTables 1-6 in Supplement 2). The final date of follow-up was November 4, 2022.
Table 1. Patient Characteristics at Baseline.
| Patient characteristicsa | Cefepime (n = 1214) | Piperacillin-tazobactam (n = 1297) |
|---|---|---|
| Age, median (IQR), y | 57 (42 to 68) | 59 (44 to 69) [n = 1296] |
| Sex, No. (%) | ||
| Female | 523 (43.1) | 548/1296 (42.3) |
| Male | 691 (56.9) | 748/1296 (57.7) |
| Race and ethnicity, No./total (%) | ||
| Hispanic | 59/1186 (5.0) | 73/1264 (5.8) |
| Non-Hispanic Black | 190/1186 (16.0) | 209/1264 (16.5) |
| Non-Hispanic White | 913/1186 (77.0) | 950/1264 (75.2) |
| Other raceb | 24/1186 (2.0) | 32/1264 (2.5) |
| Hours from hospital presentation to enrollment, median (IQR) | 1.3 (0.5 to 3.7) | 1.1 (0.4 to 3.2) |
| Location at enrollment, No. (%) | ||
| Emergency department | 1135 (93.5) | 1243 (95.8) |
| Intensive care unit | 79 (6.5) | 54 (4.2) |
| Sepsis, No. (%)c | 658 (54.2) | 704 (54.3) |
| Suspected source of infection at enrollment, No. (%)d | ||
| Intra-abdominal | 319 (26.3) | 293 (22.6) |
| Lung | 257 (21.2) | 300 (23.1) |
| Skin and soft tissue | 201 (16.6) | 245 (18.9) |
| Genitourinary | 100 (8.2) | 144 (11.1) |
| Other | 104 (8.6) | 97 (7.5) |
| Unknown | 233 (19.2) | 218 (16.8) |
| Sequential Organ Failure Assessment score, median (IQR)e | 2 (0 to 5) | 2 (0 to 4) |
| Type of treatment, No. (%) | ||
| Mechanical ventilation | 110 (9.1) | 95 (7.3) |
| Vancomycin on day of enrollment | 942 (77.6) | 997 (76.9) |
| Charlson Comorbidity Index, median (IQR)f | 4 (2 to 7) [n = 1191] | 4 (2 to 6) [n = 1276] |
| Chronic kidney disease, No./total (%)g | 243/1191 (20.4) | 259/1276 (20.3) |
| Assessment at enrollment, No. (%)h | ||
| No acute kidney injury | 623 (51.3) | 652 (50.3) |
| Stage 1 acute kidney injury | 231 (19.0) | 311 (24.0) |
| Stage 2 acute kidney injury | 134 (11.0) | 123 (9.5) |
| Stage 3 acute kidney injury | 148 (12.2) | 144 (11.1) |
| Prior receipt of kidney replacement therapy (ineligible for acute kidney injury) | 78 (6.4) | 67 (5.2) |
| Creatinine level, median (IQR), mg/dLi | ||
| Lowest in prior 12 mo (between 365 d and 12 h before enrollment) | 0.7 (0.6 to 0.8) [n = 1136] | 0.8 (0.6 to 0.9) [n = 1229] |
| At enrollment | 1.0 (0.8 to 1.6) [n = 1136] | 1.0 (0.8 to 1.5) [n = 1229] |
| Richmond Agitation-Sedation Scale score, median (IQR)j | 0 (−1 to 0) [n = 1158] | 0 [n = 1211] |
| Coma, No. (%)j | 84 (6.9) | 77 (5.9) |
| Delirium, No. (%)k | 62 (5.1) | 51 (3.9) |
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Additional baseline characteristics appear in eTable 1 in Supplement 2.
American Indian, Asian, Multiple, and Pacific Islander.
Defined according to the Sepsis-3 criteria.15
Collected for 21 categories and condensed into these 6. “Other” includes bone and joint, central nervous system, intravascular catheter, primary bloodstream, or other. “Unknown” was used when clinicians were uncertain about the suspected source of infection at enrollment.
Composed of scores from 6 organ systems and graded from 0 to 4 according to the degree of organ dysfunction or failure. Scores range from 0 (no evidence of organ dysfunction or failure) to 24 (evidence of severe organ dysfunction or failure) and were calculated using the data collected during the 6 hours prior to enrollment or 12 hours after enrollment.
Based on the International Classification of Diseases, 10th Revision (ICD-10) diagnosis codes. Each comorbidity category has an associated weight (from 1-6) based on the adjusted risk of mortality or resource use. The sum of all the weights results in a single comorbidity score that ranges from 0 (no comorbidities) to 37 (higher probability of death).
Defined as the presence of 1 or more of the ICD-10 diagnoses codes associated with chronic kidney disease.
Acute kidney injury stages are defined according to the Kidney Disease: Improving Global Outcomes criteria17 for creatinine level.
Patients had not received kidney replacement therapy prior to enrollment.
Scale assesses the level of alertness and agitated behavior; range, –5 (unarousable) to 0 (alert and calm) to 4 (combative). Coma defined as −4 or −5 on the Richmond Scale in the 12 hours before or 6 hours after enrollment.
Defined as “positive” in the 12 hours before or 6 hours after enrollment. Among patients not experiencing coma, a value of “negative” suggests delirium is not present.
Antibiotic Therapy
In the 14 days after enrollment, 1154 of 1214 patients (95.1%) in the cefepime group received at least 1 dose of cefepime and 1276 of 1297 patients (98.4%) in the piperacillin-tazobactam group received at least 1 dose of piperacillin-tazobactam. Patients received the assigned antibiotic for a median of 3 days (IQR, 1-4 days) in each group (Figure 2 and eFigures 1-2 and eTables 7-8 in Supplement 2). In the 14 days after enrollment, 228 of 1214 patients (18.8%) in the cefepime group received at least 1 dose of piperacillin-tazobactam and 223 of 1297 patients (17.2%) in the piperacillin-tazobactam group received at least 1 dose of cefepime. A total of 85 patients (7.0%) in the cefepime group and 92 patients (7.1%) in the piperacillin-tazobactam group received other extended-spectrum gram-negative antibiotics, most commonly an aminoglycoside or carbapenem.
Figure 2. Receipt of Antibiotics by Group.

Patients may have received both cefepime and piperacillin-tazobactam on a study day when switching from one antibiotic to the other; 32 patients (1.3%) received both antibiotics on more than 1 consecutive study day.
A total of 942 patients (77.6%) in the cefepime group and 997 patients (76.9%) in the piperacillin-tazobactam group were receiving intravenous vancomycin at the time of enrollment. In the first 14 days, at least 1 dose of vancomycin was received by 1004 patients (82.7%) in the cefepime group and 1049 patients (80.9%) in the piperacillin-tazobactam group. Patients received vancomycin for a median duration of 2 days (IQR, 1-4 days).
Primary Outcome
The highest stage of AKI or death by day 14 did not significantly differ between the cefepime group and the piperacillin-tazobactam group (OR, 0.95 [95% CI, 0.80 to 1.13], P = .56; Table 2 and eTable 9 and eFigures 3-6 in Supplement 2). Of the 2511 patients included in the primary outcome analysis, 75.0% in the cefepime group (910 of 1214) vs 73.4% in the piperacillin-tazobactam group (952 of 1297) did not die or experience AKI of any stage by day 14; 7.1% (n = 86) vs 7.7% (n = 100), respectively, experienced stage 1 AKI; 3.4% (n = 41) vs 5.4% (n = 70) experienced stage 2 AKI; 7.0% (n = 85) vs 7.5% (n = 97) experienced stage 3 AKI; and 7.6% (n = 92) vs 6.0% (n = 78) died.
Table 2. Primary, Secondary, and Exploratory Outcomes.
| Cefepime (n = 1214) |
Piperacillin-tazobactam (n = 1297) |
Between-group difference expressed as RD or OR (95% CI)a | |
|---|---|---|---|
| Primary outcome | |||
| Acute kidney injury or death by day 14, No. (%) | OR, 0.95 (0.80 to 1.13) | ||
| No stage (survived) | 910 (75.0) | 952 (73.4) | |
| Stage 1 (survived) | 86 (7.1) | 100 (7.7) | |
| Stage 2 (survived) | 41 (3.4) | 70 (5.4) | |
| Stage 3 (survived) | 85 (7.0) | 97 (7.5) | |
| Stage 4 (died) | 92 (7.6) | 78 (6.0) | |
| Secondary outcomes | |||
| Major adverse kidney events at day 14, No. (%)b | 124 (10.2) | 114 (8.8) | RD, 1.4 (−1.0 to 3.8) |
| Death, No. (%) | 92 (7.6) | 78 (6.0) | RD, 1.6 (−0.5 to 3.6) |
| New kidney replacement therapy, No./total (%) | 37/1136 (3.3) | 28/1230 (2.3) | RD, 1.0 (−0.4 to 2.4) |
| Final creatinine level ≥2 times the baseline level, No./total (%) | 15/1136 (1.3) | 29/1230 (2.4) | RD, −1.0 (−2.2 to 0.1) |
| Delirium- and coma-free days within 14 dc | |||
| Median (IQR) | 14 (14 to 14) | 14 (14 to 14) | OR, 0.79 (0.65 to 0.95) |
| Mean (SD) | 11.9 (4.6) | 12.2 (4.3) | |
| Delirium, No. (%)d | 200 (16.5) | 181 (14.0) | RD, 2.5 (−0.4 to 5.4) |
| Coma, No. (%)d | 164 (13.5) | 162 (12.5) | RD, 1.0 (−1.7 to 3.7) |
| Delirium or coma, No. (%)d | 252 (20.8) | 225 (17.3) | RD, 3.4 (0.3 to 6.6) |
| Exploratory outcomes | |||
| Major adverse kidney events at day 28, No. (%)b | 135 (11.1) | 132 (10.2) | RD, 0.9 (−1.6 to 3.4) |
| Death, No. (%) | 104 (8.6) | 106 (8.2) | RD, 0.4 (−1.9 to 2.6) |
| New kidney replacement therapy, No./total (%) | 44/1136 (3.9) | 28/1230 (2.3) | RD, 1.6 (0.1 to 3.1) |
| Final creatinine level ≥2 times the baseline level, No./total (%) | 14/1136 (1.2) | 26/1230 (2.1) | RD, −0.9 (−2.0 to 0.2) |
| Delirium- and coma-free days within 28 dc | |||
| Median (IQR) | 28 (28 to 28) | 28 (28 to 28) | OR, 0.80 (0.66 to 0.97) |
| Mean (SD) | 24.4 (8.6) | 24.8 (8.2) | |
| Kidney replacement therapy–free days within 28 dc | |||
| Median (IQR) | 28 (28 to 28) | 28 (28 to 28) | OR, 0.78 (0.62 to 0.98) |
| Mean (SD) | 24.4 (9.1) | 25.0 (8.5) | |
| Vasopressor-free days within 28 dc | |||
| Median (IQR) | 28 (28 to 28) | 28 (28 to 28) | OR, 0.96 (0.80 to 1.16) |
| Mean (SD) | 24.8 (8.3) | 25.1 (7.9) | |
| Ventilator-free days within 28 dc | |||
| Median (IQR) | 28 (28 to 28) | 28 (28 to 28) | OR, 0.84 (0.68 to 1.03) |
| Mean (SD) | 24.8 (8.4) | 25.0 (8.2) | |
| Intensive care unit–free days within 28 dc | |||
| Median (IQR) | 28 (25 to 28) | 28 (26 to 28) | OR, 0.92 (0.77 to 1.09) |
| Mean (SD) | 23.9 (8.6) | 24.2 (8.4) | |
| Hospital-free days within 28 dc | |||
| Median (IQR) | 22 (15 to 24) | 22 (15 to 24) | OR, 0.99 (0.86 to 1.13) |
| Mean (SD) | 18.1 (8.6) | 18.3 (8.5) | |
| Allergic reaction to study antibiotic, No. (%)e | 16 (1.3) | 15 (1.2) | RD, 0.2 (−0.8 to 1.1) |
The absolute risk difference (RD) or odds ratio (OR). The ORs were generated by a proportional odds model.
Defined as a composite of death, receipt of new kidney replacement therapy, or final creatinine level that was at least 2 times the baseline level, with all events censored at hospital discharge or at the study day of assessment, whichever occurred first. The effect of study group on major adverse kidney events was the conditional effect. Data on the receipt of new kidney replacement therapy and final creatinine level that was at least 2 times the baseline level are provided for the 2366 patients not known to have received kidney replacement therapy before enrollment.
An OR greater than 1.0 indicates a better outcome (eg, more days alive and free from mechanical ventilation) with cefepime compared with piperacillin-tazobactam.
Post hoc outcome that was included to characterize components of the prespecified secondary outcome of delirium- and coma-free days.
Reported by the clinician via the trial’s electronic health record alert.
The results were similar in the adjusted analyses and in all the prespecified sensitivity analyses, including among the intention-to-treat population of all enrolled patients and the analysis limited to the 1798 patients who received at least 48 hours of antipseudomonal antibiotic therapy (eTables 10-11 in Supplement 2). The results were also similar in all the post hoc analyses, including an analysis limited to patients who received at least 72 hours of antipseudomonal antibiotic therapy, an analysis limited to patients who received at least 96 hours of antipseudomonal antibiotic therapy, and an analysis excluding patients who were receiving KRT or who had AKI at enrollment (eTables 11-13 in Supplement 2). The results of the prespecified subgroup analyses appear in Figure 3 and eTable 14 in Supplement 2.
Figure 3. Effect Modification of the Primary and Secondary Outcomes.
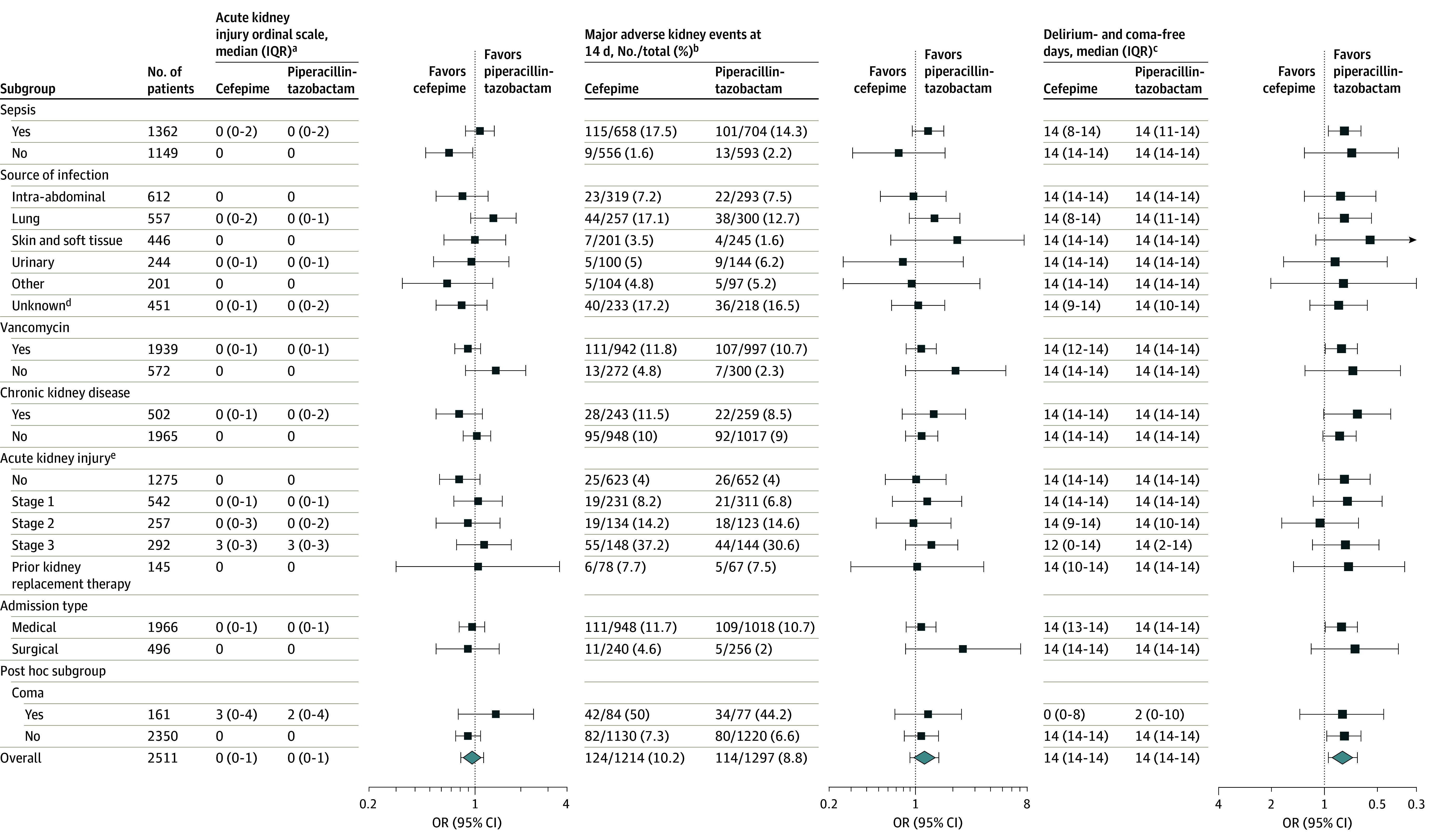
The results of tests for interaction appear in eTables 14, 21, and 22 in Supplement 2.
aThe primary outcome was the highest stage or death by day 14 (score range, 0 [alive without acute kidney injury] to 4 [dead]). An odds ratio (OR) <1.0 indicates a better outcome with cefepime.
bDefined as a composite of death, receipt of new kidney replacement therapy, or final creatinine level that was at least 2 times the baseline level. An OR <1.0 indicates a better outcome with cefepime.
cThe number of days from randomization to day 14. An OR >1.0 indicates a better outcome with cefepime.
dClinician uncertain about the suspected source of infection at enrollment.
ePresence or absence at enrollment.
Secondary Outcomes
A total of 124 patients (10.2%) in the cefepime group and 114 patients (8.8%) in the piperacillin-tazobactam group experienced a major adverse kidney event at day 14 (absolute difference, 1.4% [95% CI, −1.0% to 3.8%]; eTable 15 in Supplement 2). Patients in the cefepime group experienced fewer days alive and free of delirium and coma within 14 days compared with patients in the piperacillin-tazobactam group (mean, 11.9 [SD, 4.6] days vs 12.2 [SD, 4.3] days, respectively; OR, 0.79 [95% CI, 0.65 to 0.95]), consistent with a greater burden of delirium, coma, or death in the cefepime group compared with the piperacillin-tazobactam group.
A total of 252 patients (20.8%) in the cefepime group experienced coma or delirium between enrollment and day 14 compared with 225 patients (17.3%) in the piperacillin-tazobactam group (absolute difference, 3.4% [95% CI, 0.3%-6.6%]). The results were similar in the post hoc analyses of days alive and free of delirium and coma within 14 days, adjusting for receipt of sedation at enrollment (OR, 0.78 [95% CI, 0.62-0.99]), baseline delirium and coma (OR, 0.80 [95% CI, 0.63-1.01]), and using alternative definitions of delirium and coma (eTables 16-20 in Supplement 2). The subgroup analyses of the secondary outcomes appear in Figure 3 and eTables 21-22 in Supplement 2.
Exploratory Outcomes
The trial groups did not differ with regard to the highest stage of AKI or death at 7 days (OR, 0.99 [95% CI, 0.82 to 1.19]) or major adverse kidney events at 7 days (absolute difference, 1.8% [95% CI, −0.4% to 3.9%]). Death by day 28 occurred in 104 patients (8.6%) in the cefepime group and 106 patients (8.2%) in the piperacillin-tazobactam group (absolute difference, 0.4% [95% CI, −1.9% to 2.6%]). Kidney replacement therapy was initiated by day 28 in 44 patients (3.9%) in the cefepime group and 28 patients (2.3%) in the piperacillin-tazobactam group (absolute difference, 1.6% [95% CI, 0.1% to 3.1%]). Indications for KRT were similar between groups (eTable 23 in Supplement 2). Additional exploratory outcomes, allergic drug reactions, and adverse events appear in Table 2 and eTables 24-28 in Supplement 2.
Discussion
Among adults presenting to the hospital with suspected infection in this pragmatic trial, the highest stage of AKI or death did not differ between patients randomized to cefepime or piperacillin-tazobactam. Patients randomized to cefepime experienced more neurological dysfunction, as measured by the number of days alive and free of delirium and coma.
Prior preclinical and observational studies of the relationship between piperacillin-tazobactam and AKI have yielded conflicting results. Preclinical studies found piperacillin-tazobactam decreased the secretion of creatinine into the urine and increased creatinine levels in the blood by inhibiting organic anion transporters 1 and 3 on kidney tubular cells without affecting glomerular filtration rate as measured by cystatin C.27,28 Piperacillin-tazobactam was protective against vancomycin-induced AKI in animal models.29,30
In contrast, some observational studies reported an association between piperacillin-tazobactam and AKI in hospitalized adults, particularly with concurrent receipt of vancomycin, prompting the US Food and Drug Administration to add the following statement to the package insert for piperacillin-tazobactam: “co-administration of [piperacillin-tazobactam] with vancomycin may increase the incidence of acute kidney injury.”31 The potential for indication bias and unmeasured confounding in prior observational studies was addressed by the randomized clinical trial design of the current study.
Piperacillin-tazobactam did not appear to affect the risk of AKI at any of the measured time points, overall, or among the 1939 patients receiving vancomycin at enrollment. We included patients with preexisting AKI at enrollment because these patients are at high risk of worsening kidney injury and death, outcomes that could be affected by the choice of antibiotics. The results were similar, however, in the analyses in which these patients were excluded.
Cefepime crosses the blood-brain barrier,32 exhibits concentration-dependent inhibition of γ-amino butyric acid receptors,33 and has been reported in case series and cohort studies to be associated with neurotoxicity, including coma, delirium, encephalopathy, and seizures.34 Neurotoxicity with cefepime has been reported to occur more commonly among patients with impaired kidney function and conditions that disrupt the blood-brain barrier, such as sepsis.34,35,36 In the current trial, patients in the cefepime group experienced fewer days alive and free of delirium and coma than patients in the piperacillin-tazobactam group. Available data from preclinical studies, observational studies, and the current randomized clinical trial suggest that cefepime may modestly increase the risk of neurological dysfunction in hospitalized adults.
This trial has several strengths. A large randomized clinical trial embedded within real-world clinical care through the electronic health record represents a rigorous approach to comparing the safety of 2 commonly used, effective treatment options.37 The trial design included randomization to balance baseline characteristics, concealment of group assignment until enrollment to prevent selection bias, and enrollment occurring at a median of 1 hour after hospital presentation to minimize exposure to antibiotics prior to enrollment. The sample size permitted precise estimates of treatment effect, overall, and among the 1939 patients receiving concurrent vancomycin at enrollment.
Limitations
This trial has several limitations. First, conducting a trial at a single academic center may limit generalizability. Second, patients and clinicians were not blinded to group assignment, which could have affected clinical assessments like RASS and CAM-ICU or the frequency of laboratory measurements like creatinine, although the number of clinical and laboratory assessments appeared to be similar between groups.
Third, almost 1 in 5 patients in each group received a least 1 dose of the unassigned antibiotic within the first 14 days, which decreases the separation between groups and increases the risk of failing to detect a true between-group difference in the outcomes (type II error). Fourth, a preillness creatinine measure was not available for all patients, although creatinine level was available prior to or at enrollment for 99% of patients and the results were similar in the analyses restricted to patients with available measurements.
Fifth, outcome assessment was censored at hospital discharge. Sixth, patients in the trial received a median of only 3 days of treatment with cefepime or piperacillin-tazobactam; however, the outcomes were similar among the 1798 patients who received longer courses of therapy. Seventh, the current trial collected data on delirium and coma, but not other types of neurological dysfunction that might be attributed to cefepime, such as agitation, myoclonus, and seizures.
Eighth, unlike trials evaluating the effect of 1 treatment on a single efficacy outcome, the current trial compared 2 treatments with regard to 2 safety outcomes (kidney injury and neurological dysfunction), which may increase the risk of type I error but for which guidance on addressing multiplicity is lacking.38 Ninth, in the setting of this trial, piperacillin-tazobactam was administered as an extended infusion. These results may be less informative in settings that use a different approach.
Conclusion
Among hospitalized adults in this randomized clinical trial, treatment with piperacillin-tazobactam did not increase the incidence of AKI or death. Treatment with cefepime resulted in more neurological dysfunction.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial protocol and statistical analysis plan
List of members of the Vanderbilt Center for Learning Healthcare
List of members of the Pragmatic Critical Care Research Group
eMethods
eTable 1. Patient Characteristics at Baseline
eTable 2. Charlson Comorbidity Index
eTable 3. Baseline Vasopressor Use
eTable 4. Baseline Continuous Analgosedation
eTable 5. Baseline Receipt of Medications That May Impact Kidney and Neurologic Function
eTable 6. Baseline Laboratory Measures and Vital Signs
eTable 7. Summary of Antibiotic Receipt within 14 Days
eTable 8. Details of Antibiotic Receipt within 14 Days
eTable 9. First Daily Value of Creatinine and Blood Urea Nitrogen through 14 days
eTable 10. Multivariable Modeling of the Primary Outcome
eTable 11. Sensitivity Analyses of the Primary Outcome
eTable 12. Proportion of patients who experienced AKI or death within specified time frame (post-hoc)
eTable 13. Time to acute kidney injury (post-hoc)
eTable 14. Effect Modification of the Primary Outcome
eTable 15. Multivariable Model for Major Adverse Kidney Events at 14 days
eTable 16. Multivariable Model for Days Alive and Free of Delirium and Coma within 14 days
eTable 17. Post-hoc Multivariable Analysis of Days Alive and Free of Delirium and Coma within 14 Days Including Receipt of Continuous Sedation at Baseline
eTable 18. Post-hoc Multivariable Analysis of Days Alive and Free of Delirium and Coma within 14 Days Adjusting for Delirium and Coma at Baseline
eTable 19. Post-hoc Sensitivity Analyses of Days Alive and Free of Delirium and Coma within 14 days
eTable 20. Worst Daily Value of GCS, RASS, and CAM through 14 days
eTable 21. Effect Modification of major adverse kidney events at day 14
eTable 22. Effect Modification of delirium and coma-free days within 14 days
eTable 23. Indications for New Kidney Replacement Therapy in 14 and 28 Days
eTable 24. Exploratory Kidney Outcomes
eTable 25. Exploratory Neurologic Outcomes
eTable 26. Exploratory Clinical Outcomes
eTable 27. Non-allergic Drug Reactions
eTable 28. Adverse Event Reporting
eFigure 1. Cumulative number of days on which patients received assigned antibiotic between enrollment and day 14
eFigure 2. Antibiotic receipt by group
eFigure 3. Primary outcome by group
eFigure 4. Cumulative incidence of death or new kidney replacement therapy
eFigure 5. Competing risk analysis of acute kidney injury or death
eFigure 6. Competing risk analysis of coma or delirium or death
eReferences
Nonauthor collaborators
Data sharing statement
References
- 1.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247. doi: 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abanades S, Nolla J, Rodríguez-Campello A, Pedro C, Valls A, Farré M. Reversible coma secondary to cefepime neurotoxicity. Ann Pharmacother. 2004;38(4):606-608. doi: 10.1345/aph.1D322 [DOI] [PubMed] [Google Scholar]
- 3.Balderia PG, Chandorkar A, Kim Y, Patnaik S, Sloan J, Newman GC. Dosing cefepime for renal function does not completely prevent neurotoxicity in a patient with kidney transplant. J Patient Saf. 2018;14(2):e33-e34. doi: 10.1097/PTS.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 4.Drago L, De Vecchi E. The safety of cefepime in the treatment of infection. Expert Opin Drug Saf. 2008;7(4):377-387. doi: 10.1517/14740338.7.4.377 [DOI] [PubMed] [Google Scholar]
- 5.Appa AA, Jain R, Rakita RM, Hakimian S, Pottinger PS. Characterizing cefepime neurotoxicity: a systematic review. Open Forum Infect Dis. 2017;4(4):ofx170. doi: 10.1093/ofid/ofx170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellos I, Karageorgiou V, Pergialiotis V, Perrea DN. Acute kidney injury following the concurrent administration of antipseudomonal β-lactams and vancomycin: a network meta-analysis. Clin Microbiol Infect. 2020;26(6):696-705. doi: 10.1016/j.cmi.2020.03.019 [DOI] [PubMed] [Google Scholar]
- 7.Blevins AM, Lashinsky JN, McCammon C, Kollef M, Micek S, Juang P. Incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam, cefepime, or meropenem. Antimicrob Agents Chemother. 2019;63(5):e02658-18. doi: 10.1128/AAC.02658-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Callaghan K, Hay K, Lavana J, McNamara JF. Acute kidney injury with combination vancomycin and piperacillin-tazobactam therapy in the ICU: a retrospective cohort study. Int J Antimicrob Agents. 2020;56(1):106010. doi: 10.1016/j.ijantimicag.2020.106010 [DOI] [PubMed] [Google Scholar]
- 9.Tong SYC, Lye DC, Yahav D, et al. ; Australasian Society for Infectious Diseases Clinical Research Network . Effect of vancomycin or daptomycin with vs without an antistaphylococcal β-lactam on mortality, bacteremia, relapse, or treatment failure in patients with MRSA bacteremia: a randomized clinical trial. JAMA. 2020;323(6):527-537. doi: 10.1001/jama.2020.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian ET, Casey JD, Wright A, et al. ; Vanderbilt Learning Healthcare System Platform Investigators . Protocol and statistical analysis plan for the Antibiotic Choice On ReNal outcomes (ACORN) randomised clinical trial. BMJ Open. 2023;13(3):e066995. doi: 10.1136/bmjopen-2022-066995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762-774. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. doi: 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 13.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. doi: 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 14.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81-84. doi: 10.1016/S0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 15.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A; Co-operative Antimicrobial Therapy of Septic Shock Database Research Group . Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189(10):1204-1213. doi: 10.1164/rccm.201310-1875OC [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138. doi: 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 18.Semler MW, Rice TW, Shaw AD, et al. Identification of major adverse kidney events within the electronic health record. J Med Syst. 2016;40(7):167. doi: 10.1007/s10916-016-0528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semler MW, Self WH, Wanderer JP, et al. ; SMART Investigators and the Pragmatic Critical Care Research Group . Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colantuoni E, Scharfstein DO, Wang C, et al. Statistical methods to compare functional outcomes in randomized controlled trials with high mortality. BMJ. 2018;360:j5748. doi: 10.1136/bmj.j5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palevsky PM, Molitoris BA, Okusa MD, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol. 2012;7(5):844-850. doi: 10.2215/CJN.12791211 [DOI] [PubMed] [Google Scholar]
- 22.Andersen-Ranberg NC, Poulsen LM, Perner A, et al. ; AID-ICU Trial Group . Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387(26):2425-2435. doi: 10.1056/NEJMoa2211868 [DOI] [PubMed] [Google Scholar]
- 23.Girard TD, Exline MC, Carson SS, et al. ; MIND-USA Investigators . Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516. doi: 10.1056/NEJMoa1808217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian ET, Wang L, Stollings JL, Casey JD, Rice TW, Semler MW. Piperacillin-tazobactam versus anti-pseudomonal cephalosporins and renal and neurologic outcomes in critically ill adults: a secondary analysis of the SMART Trial. J Intensive Care Med. Published online June 26, 2023. doi: 10.1177/08850666231184177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schandelmaier S, Briel M, Varadhan R, et al. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192(32):E901-E906. doi: 10.1503/cmaj.200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient, I: introduction and design. Br J Cancer. 1976;34(6):585-612. doi: 10.1038/bjc.1976.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen S, Wang C, Duan Y, et al. OAT1 and OAT3 also mediate the drug-drug interaction between piperacillin and tazobactam. Int J Pharm. 2018;537(1-2):172-182. doi: 10.1016/j.ijpharm.2017.12.037 [DOI] [PubMed] [Google Scholar]
- 28.Miano TA, Hennessy S, Yang W, et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48(9):1144-1155. doi: 10.1007/s00134-022-06811-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang J, Pais GM, Valdez K, Marianski S, Barreto EF, Scheetz MH. Glomerular function and urinary biomarker changes between vancomycin and vancomycin plus piperacillin-tazobactam in a translational rat model. Antimicrob Agents Chemother. 2022;66(3):e0213221. doi: 10.1128/aac.02132-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pais GM, Liu J, Avedissian SN, et al. Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother. 2020;75(5):1228-1236. doi: 10.1093/jac/dkz563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfizer Injectables . ZOSYN (piperacillin and tazobactam) for injection. Published May 2017. Accessed June 7, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050684s88s89s90_050750s37s38s39lbl.pdf
- 32.Durand-Maugard C, Lemaire-Hurtel AS, Gras-Champel V, et al. Blood and CSF monitoring of cefepime-induced neurotoxicity: nine case reports. J Antimicrob Chemother. 2012;67(5):1297-1299. doi: 10.1093/jac/dks012 [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto M, Uchida I, Mashimo T, et al. Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins. Neuropharmacology. 2003;45(3):304-314. doi: 10.1016/S0028-3908(03)00188-6 [DOI] [PubMed] [Google Scholar]
- 34.Payne LE, Gagnon DJ, Riker RR, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017;21(1):276. doi: 10.1186/s13054-017-1856-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boschung-Pasquier L, Atkinson A, Kastner LK, et al. Cefepime neurotoxicity: thresholds and risk factors: a retrospective cohort study. Clin Microbiol Infect. 2020;26(3):333-339. doi: 10.1016/j.cmi.2019.06.028 [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ. Cefepime-induced neurotoxicity. J Neurocritical Care. 2019;12(2):74-84. doi: 10.18700/jnc.190109 [DOI] [PubMed] [Google Scholar]
- 37.Mani N, Murray J, Gulick RM, et al. Novel clinical trial designs for the development of new antiretroviral agents. AIDS. 2012;26(8):899-907. doi: 10.1097/QAD.0b013e3283519371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US Department of Health and Human Services . Multiple endpoints in clinical trials guidance for industry. Published January 2017. Accessed June 7, 2023. https://www.fda.gov/media/162416/download
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
List of members of the Vanderbilt Center for Learning Healthcare
List of members of the Pragmatic Critical Care Research Group
eMethods
eTable 1. Patient Characteristics at Baseline
eTable 2. Charlson Comorbidity Index
eTable 3. Baseline Vasopressor Use
eTable 4. Baseline Continuous Analgosedation
eTable 5. Baseline Receipt of Medications That May Impact Kidney and Neurologic Function
eTable 6. Baseline Laboratory Measures and Vital Signs
eTable 7. Summary of Antibiotic Receipt within 14 Days
eTable 8. Details of Antibiotic Receipt within 14 Days
eTable 9. First Daily Value of Creatinine and Blood Urea Nitrogen through 14 days
eTable 10. Multivariable Modeling of the Primary Outcome
eTable 11. Sensitivity Analyses of the Primary Outcome
eTable 12. Proportion of patients who experienced AKI or death within specified time frame (post-hoc)
eTable 13. Time to acute kidney injury (post-hoc)
eTable 14. Effect Modification of the Primary Outcome
eTable 15. Multivariable Model for Major Adverse Kidney Events at 14 days
eTable 16. Multivariable Model for Days Alive and Free of Delirium and Coma within 14 days
eTable 17. Post-hoc Multivariable Analysis of Days Alive and Free of Delirium and Coma within 14 Days Including Receipt of Continuous Sedation at Baseline
eTable 18. Post-hoc Multivariable Analysis of Days Alive and Free of Delirium and Coma within 14 Days Adjusting for Delirium and Coma at Baseline
eTable 19. Post-hoc Sensitivity Analyses of Days Alive and Free of Delirium and Coma within 14 days
eTable 20. Worst Daily Value of GCS, RASS, and CAM through 14 days
eTable 21. Effect Modification of major adverse kidney events at day 14
eTable 22. Effect Modification of delirium and coma-free days within 14 days
eTable 23. Indications for New Kidney Replacement Therapy in 14 and 28 Days
eTable 24. Exploratory Kidney Outcomes
eTable 25. Exploratory Neurologic Outcomes
eTable 26. Exploratory Clinical Outcomes
eTable 27. Non-allergic Drug Reactions
eTable 28. Adverse Event Reporting
eFigure 1. Cumulative number of days on which patients received assigned antibiotic between enrollment and day 14
eFigure 2. Antibiotic receipt by group
eFigure 3. Primary outcome by group
eFigure 4. Cumulative incidence of death or new kidney replacement therapy
eFigure 5. Competing risk analysis of acute kidney injury or death
eFigure 6. Competing risk analysis of coma or delirium or death
eReferences
Nonauthor collaborators
Data sharing statement



