Abstract
Background
An estimated 260,000 children under the age of 15 years acquired HIV infection in 2012. As much as 42% of mother‐to‐child transmission is related to breastfeeding. Antiretroviral prophylaxis for mothers or infants has the potential to prevent mother‐to‐child transmission of HIV through breast milk.
Objectives
To determine which antiretroviral prophylactic regimens are efficacious and safe for reducing mother‐to‐child transmission of HIV through breastfeeding and thereby avert child morbidity and mortality.
Search methods
Using Cochrane Collaboration search methods in conjunction with appropriate search terms, we identified relevant studies from January 1, 1994 to January 14, 2014 by searching databases including Cochrane CENTRAL, EMBASE and PubMed, LILACS, and Web of Science/Web of Social Science.
Selection criteria
Randomized controlled trials in which HIV‐infected mothers breastfed their infants, and in which the mothers used antiretroviral prophylaxis while breastfeeding their children or their children received antiretroviral prophylaxis for at least four weeks while breastfeeding, were included.
Data collection and analysis
Abstracts of all trials identified were examined independently by two authors. We identified 15,922 references and examined 81 in detail. Data were abstracted independently using a standardized form.
Main results
Seven RCTs were included in the review.
One trial compared triple antiretroviral prophylaxis during pregnancy and breastfeeding with short antiretroviral prophylaxis to given to the mother to prevent mother‐to‐child transmission of HIV. At 12 months, the risks of HIV transmission, and of HIV transmission or death, were lower, but there was no difference in infant mortality alone in the triple arm versus the short arm. Using the GRADE methodology, evidence quality for outcomes in this trial was generally low to moderate.
One trial compared six months of breastfeeding using zidovudine, lamivudine, and lopinavir/ritonavir versus zidovudine, lamivudine, and abacavir from 26‐34 weeks gestation. At six months, there was no difference in risk of infant HIV infection, infant death, or infant HIV infection or death between the two groups. Evidence quality for outcomes in this trial was generally very low to low.
One trial of single dose nevirapine versus six weeks of infant zidovudine found the risk of HIV infection at 12 weeks to be greater in the zidovudine arm than in the single dose nevirapine arm. Evidence quality for outcomes in this trial was generally very low.
One multi‐country trial compared single dose nevirapine and six weeks of infant nevirapine. After 12 months, infants in the extended nevirapine group had a lower risk of infant mortality compared with the control. There was no difference in the risk of HIV infection or death or in HIV transmission alone in the extended nevirapine group compared with the control. Evidence quality for outcomes in this trial was generally low to moderate.
One trial compared single dose nevirapine plus one week zidovudine; the control regimen plus nevirapine up to 14 weeks; or the control regimen with dual prophylaxis up to 14 weeks. At 24 months, the extended nevirapine regimen group had a lower risk of HIV transmission and of HIV transmission or death vs. the control. There was no difference in infant mortality alone. Compared with controls, the dual prophylaxis group had a lower risk of HIV transmission and of HIV transmission or death, but no difference in infant mortality alone. There was no difference in these outcomes between the two intervention arms. Evidence quality for outcomes in this trial was generally moderate to high.
One trial compared six weeks of nevirapine with six months of nevirapine. Among infants of mothers not using highly active antiretroviral therapy, there was no difference in risk of HIV infection among the six month nevirapine group versus the six week nevirapine group. Evidence quality for outcomes in this trial was generally low to moderate.
One trial compared a maternal triple‐drug antiretroviral regimen, infant nevirapine, or neither intervention. Infants in the maternal prophylaxis arm were at lower risk for HIV, and HIV infection or death when compared with the control group. There was no difference in the risk of infant mortality alone. Infants with extended prophylaxis had a lower risk of HIV infection and of HIV infection or death versus the control group infants. There was no difference in the risk of infant mortality alone in the extended infant nevirapine group versus the control. There was no difference in HIV infection, infant mortality, and HIV infection or death between the maternal and extended infant prophylaxis groups. Evidence quality for outcomes in this trial was generally low to moderate.
Authors' conclusions
Antiretroviral prophylaxis, whether used by the HIV‐infected mother or the HIV‐exposed infant while breastfeeding, is efficacious in preventing mother‐to‐child transmission of HIV. Further research is needed regarding maternal resistance and response to subsequent antiretroviral therapy after maternal prophylaxis. An ongoing trial (IMPAACT 1077BF) compares the efficacy and safety of maternal triple antiretroviral prophylaxis versus daily infant nevirapine for prevention of mother‐to‐child transmission through breastfeeding.
Plain language summary
Using antiretroviral medication to prevent transmission of HIV from mother‐to‐child during breastfeeding
Worldwide, the primary cause of human immunodeficiency virus (HIV) infection in children is mother‐to‐child transmission (MTCT). MTCT of HIV can occur during pregnancy, around the time of delivery, or through breastfeeding. Great strides have been made in reducing MTCT during pregnancy and around the time of delivery. However, without intervention, a significant proportion of children born to HIV–infected mothers acquire HIV through breastfeeding.
Where affordable, feasible, acceptable, sustainable, and safe (AFASS) alternatives to breast milk are available, it is recommended that HIV‐infected mothers do not breastfeed. However, for a substantial number of HIV‐infected women in the developing world, complete avoidance of breastfeeding is not AFASS. These mothers are counseled to practice exclusive breastfeeding (giving a child only breast milk and no additional food, water, or other fluids). Provision of antiretrovirals (ARVs) either to the mother or to the child during breastfeeding represent potential interventions to reduce the risk of HIV transmission to breastfeeding children. This review explores the available evidence regarding the efficacy and safety of ARV prophylaxis regimens to reduce breast milk transmission of HIV.
Summary of findings
Summary of findings for the main comparison. An extended NVP regimen administered to infants for 14 weeks compared to sdNVP plus ZDV (1week) for prevention of breastfeeding transmission.
| An extended NVP regimen administered to infants for 14 weeks compared to sdNVP plus ZDV (1week) for prevention of breastfeeding transmission | ||||||
| Patient or population: Breastfeeding infants of HIV‐infected mothers Settings: Malawi (PEPI trial) Intervention: An extended NVP regimen administered to infants for 14 weeks1 Comparison: sdNVP plus ZDV (1week)2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SdNVP plus ZDV (1week) | An extended NVP regimen administered to infants for 14 weeks | |||||
| HIV Transmission at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 98 per 10003 | 56 per 1000 (41 to 75)3 | HR 0.56 (0.41 to 0.76)4 | 2019 (1 study) | ⊕⊕⊕⊝ moderate5,6,7,8 | |
| Infant Mortality at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 71 per 10003 | 51 per 1000 (36 to 74)3 | HR 0.72 (0.5 to 1.05)9 | 2019 (1 study) | ⊕⊕⊕⊝ moderate5,6,8 | |
| HIV Transmission or Death at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 168 per 10003,10 | 120 per 1000 (97 to 148)3,10 | HR 0.69 (0.55 to 0.87)4 | 2019 (1 study) | ⊕⊕⊕⊕ high5,6,7,10 | |
| HIV Transmission at 24 months among those uninfected at birth11 Follow‐up: 24 months | 156 per 100012 | 97 per 1000 (75 to 124)12 | HR 0.60 (0.46 to 0.78) | 1129 (1 study) | ⊕⊕⊕⊝ moderate8 | |
| Infant Mortality at 24 months among those uninfected at birth Follow‐up: 24 months | 165 per 100012 | 125 per 1000 (93 to 167)12 | HR 0.74 (0.54 to 1.01) | 1266 (1 study) | ⊕⊕⊕⊝ moderate8 | |
| HIV Transmission or Death at 24 months among those uninfected at birth11 Follow‐up: 24 months | 242 per 100012 | 179 per 1000 (149 to 215)12 | HR 0.71 (0.58 to 0.87) | 1129 (1 study) | ⊕⊕⊕⊝ moderate8 | |
| Infants with possible related Severe Adverse Events ‐ 24 months11 Follow‐up: 24 months | 43 per 1000 | 45 per 1000 (30 to 68) | RR 1.06 (0.7 to 1.59) | 2019 (1 study) | ⊕⊝⊝⊝ very low8 | |
| Infants with Probably Related Severe Adverse Events11 Follow‐up: 24 months | 2 per 1000 | 6 per 1000 (1 to 29) | RR 2.96 (0.6 to 14.64) | 2019 (1 study) | ⊕⊝⊝⊝ very low8 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Extended NVP prophylaxis included oral dose of nevirapine (2mg/kg) once daily during week 2, then 4mg/kg once daily during weeks 3‐14. 2 All infants received single oral dose of nevirapine (2mg/kg) plus oral zidovudine (4mg/kg) for 1 week. All mothers received intrapartum single dose nevirapine (except late presenters whose HIV infection was not identified until after they gave birth). 3 "Primary Analysis" denominator used. The primary analysis denominator includes all infants who were randomized to study arms with the exception of those infants who were determined to be HIV infected at birth and those with indeterminate results at birth. 4 Relative estimate of effect reported is hazard ratio presented in manuscript. Authors state that hazard ratios were adjusted for study group and other covariates that were considered to have biological or epidemiological importance. 5 This study was not blinded, however this outcome was not deemed subject to bias associated with lack of blinding. 6 As a single study, no comparison study is available to evaluate inconsistency. 7 As reviewed in Read JS. Diagnosis of HIV‐1 Infection in Children Younger Than 18 Months in the United States. Am Acad Ped 2007:120(6); e1547‐e1562, the sensitivity of DNA PCR for diagnosis of HIV in infants before 30 days is generally significantly less than 100%. For this reason, a baseline window of 4‐6 weeks is recommended to ensure that all in utero and intrapartum transmissions are captured. This study did not use 4‐6 weeks as baseline and as such may include some intrapartum transmissions. While there may be misclassification of timing of infection, there is no reason to believe that it would be differential across study arms. 8 Small number of events 9 Hazard Ratio was estimated using a log‐rank analysis. 10 Raw data for infant "HIV transmission or death" at 9 months was not provided by the study authors, so numerator was calculated as follows: [infant mortality at 9m + HIV transmission at 9m]. It is possible that some infants that died by 9 months may have tested positive for HIV prior to death and may have been double counted in the numerator. However the Hazard Ratio reported here from text already accounts for this. 11 Numerators from Taha manuscript 12 Calculated from rate of event times number at risk at 24 months
Summary of findings 2. An extended NVP plus ZDV regimen administered to infants for 14 weeks compared to sdNVP plus ZDV (1 week) for prevention of breastfeeding transmission.
| An extended NVP plus ZDV regimen administered to infants for 14 weeks compared to sdNVP plus ZDV (1 week) for prevention of breastfeeding transmission | ||||||
| Patient or population: Breastfeeding infants of HIV‐infected mothers Settings: Malawi (PEPI trial) Intervention: An extended NVP plus ZDV regimen administered to infants for 14 weeks1 Comparison: sdNVP plus ZDV (1 week) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SdNVP plus ZDV (1 week) | An extended NVP plus ZDV regimen administered to infants for 14 weeks | |||||
| HIV Transmission at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 98 per 1000 | 65 per 1000 (48 to 87) | HR 0.65 (0.48 to 0.88) | 2000 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| Infant Mortality at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 71 per 1000 | 47 per 1000 (33 to 68) | HR 0.66 (0.46 to 0.96) | 2000 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| HIV Transmission or Death at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 168 per 1000 | 124 per 1000 (100 to 153) | HR 0.72 (0.57 to 0.9) | 2000 (1 study) | ⊕⊕⊕⊕ high | |
| HIV Transmission at 24 months among those uninfected at birth Follow‐up: 24 months | 156 per 10003 | 104 per 1000 (81 to 134)3 | HR 0.65 (0.5 to 0.85) | 1123 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| Infant Mortality at 24 months among those uninfected at birth Follow‐up: 24 months | 165 per 10003 | 123 per 1000 (91 to 165)3 | HR 0.73 (0.53 to 1) | 1207 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| HIV Transmission or Death at 24 months among those uninfected at birth Follow‐up: 24 months | 242 per 10003 | 183 per 1000 (153 to 221)3 | HR 0.73 (0.6 to 0.9) | 1123 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| Infants with any Severe Adverse Events Follow‐up: 24 months | 43 per 1000 | 75 per 1000 (52 to 108) | RR 1.75 (1.22 to 2.53) | 2000 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Infants with Probably Related Severe Adverse Events Follow‐up: 24 months | 2 per 1000 | 6 per 1000 (1 to 30) | RR 3.02 (0.61 to 14.92) | 2000 (1 study) | ⊕⊝⊝⊝ very low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Extended NVP prophylaxis included oral dose of nevirapine (2mg/kg) once daily during week 2, then 4mg/kg once daily during weeks 3‐14. Extended ZDV prophylaxis included oral zidovudine (4mg/kg) twice daily during weeks 2‐5, 4mg/kg three times daily during weeks 6‐8, and 6mg/kg three times daily during weeks 9‐14. 2 Small number of events. 3 Calculated from rate of event times number at risk at 24 months
Summary of findings 3. An extended NVP plus ZDV dual regimen administered to infants for 14 weeks compared to an extended NVP regimen administered to infants for 14 weeks.
| An extended NVP plus ZDV dual regimen administered to infants for 14 weeks compared to an extended NVP regimen administered to infants for 14 weeks | ||||||
| Patient or population: Breastfeeding infants of HIV‐infected mothers1 Settings: Malawi (PEPI trial) Intervention: An extended NVP plus ZDV dual regimen administered to infants for 14 weeks2 Comparison: An extended NVP regimen administered to infants for 14 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| An extended NVP plus ZDV dual regimen administered to infants for 14 weeks | ||||||
| HIV Transmission at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 50 per 1000 | 61 per 1000 (42 to 89) | HR 1.23 (0.83 to 1.81) | 2013 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Infant Mortality at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 54 per 1000 | 49 per 1000 (33 to 73) | HR 0.91 (0.61 to 1.36) | 2013 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| HIV Transmission at 24 months among those uninfected at birth Follow‐up: 24 months | 108 per 10004 | 112 per 1000 (78 to 159)4 | HR 1.04 (0.71 to 1.51) | 1122 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| HIV Transmission or Death at 9 months among those whose HIV diagnostic testing was negative within 48 hours of birth Follow‐up: 9 months | 104 per 1000 | 113 per 1000 (86 to 147) | HR 1.09 (0.82 to 1.44) | 2013 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Infant Mortality at 24 months among those uninfected at birth Follow‐up: 24 months | 128 per 10004 | 117 per 1000 (80 to 169)4 | HR 0.91 (0.61 to 1.36) | 1273 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| HIV Transmission or Death at 24 months among those uninfected at birth Follow‐up: 24 months | 199 per 1000 | 199 per 1000 (153 to 257) | HR 1.00 (0.75 to 1.34) | 1122 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Infants with any Severe Adverse Events Follow‐up: 24 | 45 per 1000 | 75 per 1000 (53 to 107) | RR 1.66 (1.16 to 2.37) | 2013 (1 study) | ⊕⊕⊝⊝ low3 | |
| Infants with Probably Related Severe Adverse Events Follow‐up: 24 months | 6 per 1000 | 6 per 1000 (2 to 19) | RR 1.02 (0.33 to 3.15) | 2013 (1 study) | ⊕⊝⊝⊝ very low3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The aim of the trial was to determine whether extended prophylaxis of infants with nevirapine or with nevirapine plus zidovudine until the age of 14 weeks would decrease the rate of HIV‐1 infection, as compared with single‐dose nevirapine combined with 1 week of zidovudine. The trial was not designed to compare the 2 extended dose regimens. 2 Extended NVP prophylaxis included oral dose of nevirapine (2mg/kg) once daily during week 2, then 4mg/kg once daily during weeks 3‐14. Extended ZDV prophylaxis included oral zidovudine (4mg/kg) twice daily during weeks 2‐5, 4mg/kg three times daily during weeks 6‐8, and 6mg/kg three times daily during weeks 9‐14. 3 Small number of events. 4 Calculated from rate of event times number at risk at 24 months
Summary of findings 4. An extended NVP regimen administered to infants for 6 weeks compared to sdNVP for prevention of breastfeeding transmission.
| an extended NVP regimen administered to infants for 6 weeks compared to sdNVP for prevention of breastfeeding transmission | ||||||
| Patient or population: Breastfeeding infants of HIV‐infected mothers Settings: Ethiopia, India, Uganda (SWEN trial) Intervention: An extended NVP regimen administered to infants for 6 weeks1 Comparison: sdNVP2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SdNVP | An extended NVP regimen administered to infants for 6 weeks | |||||
| HIV Transmission at 6 months among those whose HIV diagnostic testing was negative within 7 days of birth Follow‐up: 6 months | 88 per 1000 | 69 per 1000 (50 to 94) | RR 0.78 (0.57 to 1.07) | 1887 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Infant Mortality at 6 months among those whose HIV diagnostic testing was negative within 7 days of birth Follow‐up: 6 months | 38 per 1000 | 18 per 1000 (10 to 32) | RR 0.47 (0.26 to 0.84) | 1887 (1 study) | ⊕⊕⊝⊝ low3 | |
| HIV Transmission or Death at 6 months among those whose HIV diagnostic testing was negative within 7 days of birth Follow‐up: 6 months | 115 per 1000 | 83 per 1000 (62 to 109) | RR 0.72 (0.54 to 0.95) | 1887 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Genotypic Resistance to NVP among Ugandan infants HIV‐infected at 6 weeks ViroSeq | 500 per 1000 | 840 per 1000 (545 to 1000) | RR 1.68 (1.09 to 2.6) | 49 (1 study) | ⊕⊕⊝⊝ low3 | |
| Persistance of Genotypic Resistance to NVP at 6 months among Ugandan infants found to be resistant at 6 weeks ViroSeq | 167 per 1000 | 730 per 1000 (175 to 1000) | RR 4.38 (1.05 to 18.28) | 13 (1 study) | ⊕⊕⊝⊝ low3 | |
| Genotypic Resistance to NVP among Indian infants HIV‐infected in utero or through peripartum/early‐breastfeeding transmission Standard Population Sequencing | 379 per 1000 | 918 per 1000 (558 to 1000) | RR 2.42 (1.47 to 3.97) | 41 (1 study) | ⊕⊕⊝⊝ low3 | |
| Genotypic Resistance to NVP among Indian infants HIV‐infected through late breastfeeding Standard Population Sequencing | 150 per 1000 | 154 per 1000 (30 to 799) | RR 1.03 (0.2 to 5.33) | 33 (1 study) | ⊕⊕⊝⊝ low3 | |
| HIV Transmission at 12 months among those whose HIV diagnostic testing was negative within 7 days of birth Follow‐up: 12 months | 104 per 1000 | 93 per 1000 (70 to 122) | RR 0.89 (0.67 to 1.17)4,5 | 1813 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Infant Mortality at 12 months among those whose HIV diagnostic testing was negative within 7 days of birth Follow‐up: 12 months | 50 per 1000 | 26 per 1000 (16 to 42)5 | RR 0.53 (0.32 to 0.85)5 | 2350 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| HIV Transmission or Death at 12 months among those whose HIV diagnostic testing was negative within 7 days of birth Follow‐up: 12 months | 135 per 1000 | 107 per 1000 (82 to 136) | RR 0.79 (0.61 to 1.01)4,5 | 1883 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The extended dose nevirapine regimen was initiated at day 8. The extended regimen consisted of 5mg daily NVP from days 8‐42 2 All mothers received 200mg NVP in labor and all infants received 2mg/kg NVP after birth. 3 Small number of events 4 Confidence interval crosses the default threshold of 1. 5 Estimates are inverse variance‐weighted means of country‐specific risks and risk ratios given in the published text.
Summary of findings 5. An extended ZDV regimen administered to breastfeeding infants for 6 months for prevention of breastfeeding transmission.
| an extended ZDV regimen administered to breastfeeding infants for 6 months for prevention of breastfeeding transmission | ||||||
| Patient or population: breastfeeding infants of HIV‐infected mothers Settings: Botswana (Mashi trial) Intervention: an extended ZDV regimen administered to breastfeeding infants for 6 months1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | An extended ZDV regimen administered to breastfeeding infants for 6 months | |||||
| HIV Transmission at 7 months among those whose HIV diagnostic testing was negative at one month after birth Follow‐up: 7 months | 5 per 10002,3 | 25 per 1000 (12 to 53)2,3 | HR 4.77 (2.22 to 10.24)4 | 1123 (1 study) | ⊕⊝⊝⊝ very low5,6,7,8 | |
| Infant Mortality at 18 months Follow‐up: 18 months | 105 per 10009 | 78 per 1000 (53 to 113)9 | HR 0.73 (0.49 to 1.08)4 | 1179 (1 study) | ⊕⊝⊝⊝ very low5,6,7,8,10 | |
| HIV Transmission or Death at 18 months among those whose HIV diagnostic testing was negative at one month after birth Follow‐up: 18 months | 91 per 10003,11 | 109 per 1000 (74 to 157)3,11 | HR 1.21 (0.81 to 1.8)4 | 1123 (1 study) | ⊕⊝⊝⊝ very low5,6,7,8,10 | |
| Infants with Grade 3/4 Signs or Symptoms Follow‐up: 7 months | 169 per 10009 | 125 per 1000 (95 to 166)9 | RR 0.74 (0.56 to 0.98) | 1179 (1 study) | ⊕⊝⊝⊝ very low6,7,8,12,13 | |
| Infants with Grade 3/4 Laboratory Abnormalities Follow‐up: 7 months | 142 per 10009 | 242 per 1000 (189 to 308)9 | RR 1.70 (1.33 to 2.17) | 1179 (1 study) | ⊕⊝⊝⊝ very low5,6,7,8,13 | |
| Infant with Toxicity Events leading to cessation of ZDV | 17 per 10009 | 94 per 1000 (48 to 182)9 | RR 5.53 (2.85 to 10.74) | 1179 (1 study) | ⊕⊝⊝⊝ very low6,7,8,12,14 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In all infants, infant zidovudine syrup was administered twice a day at 4mg/kg from birth until 1 month of age. In the breastfeeding arm, infant zidovudine was administered three times a day at 4mg/kg from 1‐2 months of age, and three times a day at 6mg/kg from 2‐6 months of age (while still breastfeeding). 2 Numerator obtained by taking cumulative number of infants with HIV infection at 7 months minus those that were infected at 1 month 3 Denominator calculated by taking number of live births in each group minus the number of infants at with HIV infection at 1month 4 Hazard Ratio was estimated using a log‐rank analysis. 5 This study was not blinded, however this outcome was not deemed subject to bias associated with lack of blinding. 6 As a single study, no comparison study is available to evaluate inconsistency. 7 The control arm of this study randomized women to exclusively formula feed, with ZDV prophylaxis for 1 month. The control arm did not include breastfeeding infants, so the intervention (extended ZDV prophylaxis during breastfeeding) can not be compared to breastfeeding without ARV prophylaxis. 8 Small number of events. 9 Denominator represents number of live births 10 Confidence interval crosses the default threshold of 1. 11 Numerator obtained by taking cumulative number of infants who had died or become HIV‐infected at 18 months minus those that were HIV‐infected at 1 month 12 This study was not blinded and this outcome was deemed possibly subject to bias associated with lack of blinding. 13 The occurrence of any grade 3 (severe) or worse laboratory toxicities and clinical adverse events within infants’ first 7 months of life differed by group. The rates of grade 3 or higher signs or symptoms (17.6% vs 13.1%; P=.03) and of hospitalization (20.3% vs 15.6%; p=0.04) by 7 months were significantly higher among infants in the formula‐ fed group than in the breastfed plus zidovudine group. The rate of grade 3 or higher laboratory abnormality associated with zidovudine toxicity was significantly higher in the breastfed plus zidovudine group than in the formula‐fed group (24.7% vs 14.8%; p<0.001). 14 There were significantly higher rates of infants with toxicity events leading to cessation of ZDV in the breastfed plus zidovudine group that in the formula‐fed group (9.2% vs 1.7%; p=0.001).
Summary of findings 6. An extended ZDV/3TC/LPV/r regimen administered to mothers compared to an extended ZDV/3TC/ABC administered to mothers for prevention of breastfeeding transmission.
| an extended ZDV/3TC/LPV/r regimen administered to mothers compared to an extended ZDV/3TC/ABC administered to mothers for prevention of breastfeeding transmission | ||||||
| Patient or population: HIV‐infected mothers and their breastfeeding infants Settings: Botswana (Mma Bana trial) Intervention: An extended ZDV/3TC/LPV/r regimen administered to mothers1 Comparison: An extended ZDV/3TC/ABC administered to mothers2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| An extended ZDV/3TC/ABC administered to mothers | An extended ZDV/3TC/LPV‐r regimen administered to mothers | |||||
| HIV transmission at 6 months among those whose HIV diagnostic testing was negative within 96 hours of birth Follow‐up: 6 months | 7 per 10003 | 2 per 1000 (0 to 31)3 | RR 0.21 (0.01 to 4.3) | 548 (1 study) | ⊕⊕⊝⊝ low4,5,6,7,8 | |
| Infant Mortality at 6 months Follow‐up: 6 months | 25 per 10009 | 26 per 1000 (9 to 73)9 | RR 1.05 (0.37 to 2.95) | 553 (1 study) | ⊕⊕⊝⊝ low4,5,7,8 | |
| HIV Transmission or Death at 6 months among those whose HIV diagnostic testing was negative within 96 hours of birth Follow‐up: 6 months | 32 per 10003,10 | 26 per 1000 (10 to 69)3,10 | RR 0.81 (0.3 to 2.14) | 548 (1 study) | ⊕⊝⊝⊝ very low4,5,6,7,8,10 | |
| Infants with Grade 3/4 Severe Adverse Events | 410 per 10009 | 463 per 1000 (381 to 562)9 | RR 1.13 (0.93 to 1.37) | 553 (1 study) | ⊕⊕⊝⊝ low5,8,11 | |
| Maternal Mortality at 6 months Follow‐up: 6 months | 4 per 100012 | 1 per 1000 (0 to 30)12 | RR 0.35 (0.01 to 8.44) | 560 (1 study) | ⊕⊕⊝⊝ low4,5,7,8 | |
| Mothers with any Grade 3/4 Severe Adverse Event | 147 per 100012 | 116 per 1000 (75 to 178)12 | RR 0.79 (0.51 to 1.21) | 560 (1 study) | ⊕⊕⊝⊝ low5,7,8,11 | |
| Mothers with Severe Adverse Events Requiring Treatment Modification | 25 per 100012 | 22 per 1000 (7 to 64)12 | RR 0.89 (0.3 to 2.61) | 560 (1 study) | ⊕⊝⊝⊝ very low5,7,8,11 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The extended ZDV/3TC/LPV/r regimen was administered to mothers from 26‐34 weeks gestation through planned weaning by 6 months postpartum. 2 The extended ZDV/3TC/ABC regimen was administered to mothers from 26‐34 weeks gestation through planned weaning by 6 months postpartum. 3 Denominator= Number of live births‐HIV transmissions in utero. 4 This study was not blinded, however this outcome was not deemed subject to bias associated with lack of blinding. 5 As a single study, no comparison study is available to evaluate inconsistency. 6 As reviewed in Read JS. Diagnosis of HIV‐1 Infection in Children Younger Than 18 Months in the United States. Am Acad Ped 2007:120(6); e1547‐e1562, the sensitivity of DNA PCR for diagnosis of HIV in infants before 30 days is generally significantly less than 100%. For this reason, a baseline window of 4‐6 weeks is recommended to ensure that all in utero and intrapartum transmissions are captured. This study did not use 4‐6 weeks as baseline and as such may include some intrapartum transmissions. While there may be misclassification of timing of infection, there is no reason to believe that it would be differential across study arms. 7 Small number of events. 8 Confidence interval crosses the default threshold of 1. 9 Denominator= Number of live births. 10 The composite measure of HIV transmission or Death at 6 months among those uninfected at birth was not provided by the study authors, and was calculated as follows: Numerator= [Infant mortality at 6m + (HIV transmission at 6 months‐HIV transmission in utero)]. 11 This study was not blinded and this outcome was deemed possibly subject to bias associated with lack of blinding. 12 Denominator = Number of women enrolled
Summary of findings 7. An extended ZDV/3TC/LPV/r regimen administered to mothers compared to short course ZDV (intrapartum ZDV/3TC/sdNVP) for preventing breastfeeding transmission.
| an extended ZDV/3TC/LPV/r regimen administered to mothers compared to short course ZDV (intrapartum ZDV/3TC/sdNVP) for preventing breastfeeding transmission | ||||||
| Patient or population: HIV‐infected mothers and their breastfeeding infants Settings: Burkina Faso, Kenya, South Africa (Kesho Bora trial) Intervention: An extended ZDV/3TC/LPV/r regimen administered to mothers1 Comparison: Short course ZDV (intrapartum ZDV/3TC/sdNVP)1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short course ZDV (intrapartum ZDV/3TC/sdNVP) | An extended ZDV/3TC/LPV/r regimen administered to mothers | |||||
| HIV transmission at 6 months among those whose diagnostic testing was negative at 6 weeks after birth Follow‐up: 6 months | 43 per 10002 | 16 per 1000 (6 to 44)2 | HR 0.36 (0.13 to 1.02) | 597 (1 study) | ⊕⊕⊝⊝ low3,4,5,6 | |
| HIV transmission at 12 months among those whose diagnostic testing was negative at 6 weeks after birth Follow‐up: 12 months | 56 per 10002 | 23 per 1000 (9 to 54)2 | HR 0.40 (0.16 to 0.96) | 597 (1 study) | ⊕⊕⊝⊝ low3,4,7,8 | |
| Infant Mortality at 6 months Follow‐up: 6 months | 41 per 10009 | 36 per 1000 (15 to 85)9 | HR 0.88 (0.36 to 2.11) | 624 (1 study) | ⊕⊕⊝⊝ low3,4,5,6 | |
| Infant Mortality at 12 months Follow‐up: 12 months | 79 per 10009 | 74 per 1000 (39 to 141)9 | HR 0.94 (0.48 to 1.85) | 624 (1 study) | ⊕⊕⊝⊝ low3,4,5,6,8 | |
| HIV transmission or Death at 6 months among those whose HIV diagnostic testing was negative at 6 weeks after birth Follow‐up: 6 months | 73 per 100010 | 45 per 1000 (22 to 89)10 | HR 0.61 (0.3 to 1.24) | 599 (1 study) | ⊕⊕⊝⊝ low3,4,5,6 | |
| HIV transmission or Death at 12 months among those whose HIV diagnostic testing was negative at 6 weeks after birth Follow‐up: 12 months | 109 per 10002 | 58 per 1000 (32 to 102)2 | HR 0.52 (0.28 to 0.93) | 599 (1 study) | ⊕⊕⊕⊝ moderate3,4,7,8 | |
| Maternal Grade 3/4 Severe Adverse Events | 117 per 100011 | 139 per 1000 (97 to 198)11 | RR 1.19 (0.83 to 1.7) | 824 (1 study) | ⊕⊝⊝⊝ very low4,5,6,12,13 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Data are derived from author correspondence. 2 Denominator = Number of breastfed infants at risk at birth as reported by authors for 'HIV infections' ‐ number infected at 6 weeks. 3 This study was not blinded, however this outcome was not deemed subject to bias associated with lack of blinding. 4 As a single study, no comparison study is available to evaluate inconsistency. 5 Small number of events. 6 Confidence interval crosses the default threshold of 1. 7 Very small number of events (n<100) and confidence interval approaches the default threshold of 1. 8 According to the authors, "the last baby was born in November 2008. The database used for the presented analyses was 31March09. At that date, 28% of the participants had not yet completed 12‐month follow‐up." 9 Denominator = Number of breastfed infants at risk at birth as reported by authors for 'deaths'. 10 Denominator = Number of breastfed infants at risk at birth as reported by authors for 'survival' ‐ number infected at 6 weeks. 11 Denominator= Number of women randomized to treatment arm. 12 Data for this outcome on the subset of mothers who ever breastfed was not reported by study authors. Numbers reported here are for the entire sample of women in the study. 13 Per ACTG 1992 Protocol Management Handbook, events of grade 3 or higher classify as "severe".
Summary of findings 8. An extended antiretroviral regimen administered to mothers for 28 weeks compared to 1 week of antiretroviral prophylaxis for transmission during breastfeeding.
| an extended antiretroviral regimen administered to mothers for 28 weeks compared to 1 week of antiretroviral prophylaxis for transmission during breastfeeding | ||||||
| Patient or population: HIV‐infected mothers and their breastfeeding infants Settings: Lilongwe, Malawi (BAN trial) Intervention: An extended antiretroviral regimen administered to mothers for 28 weeks1 Comparison: One week of antiretroviral prophylaxis2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 1 week of antiretroviral prophylaxis | An extended antiretroviral regimen administered to mothers for 28 weeks | |||||
| HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 51 per 10003 | 26 per 1000 (15 to 45)3 | RR 0.52 (0.3 to 0.89) | 1435 (1 study) | ⊕⊕⊕⊝ moderate4,5,6,7 | |
| Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 21 per 1000 | 14 per 1000 (6 to 30) | RR 0.67 (0.31 to 1.45) | 1517 (1 study) | ⊕⊕⊝⊝ low4,5,6,8,9 | |
| HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks Follow‐up: 28 weeks | 62 per 10003 | 38 per 1000 (23 to 59)3 | RR 0.61 (0.38 to 0.96) | 1435 (1 study) | ⊕⊕⊕⊝ moderate4,5,6,7,10 | |
| Infants with Severe Adverse Events | 126 per 100011 | 140 per 1000 (108 to 1000)11 | RR 1.11 (0.86 to 8.45) | 1517 (1 study) | ⊕⊕⊝⊝ low5,6,8,12 | |
| Mothers with Severe Adverse Events | 21 per 1000 | 46 per 1000 (25 to 84) | RR 2.19 (1.2 to 4) | 1517 (1 study) | ⊕⊕⊝⊝ low7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Mothers initially received Combivir twice daily and NVP once daily for 2 weeks and twice‐daily thereafter until 28 weeks. NVP was replaced with twice‐daily nelfinavir, then twice‐daily lopinavir plus ritonavir because of FDA black‐box warnings (NVP) and availability, safety, and potency concerns (nelfinavir). 2 Mothers in labor and newborns received sdNVP. Mother received Combivir twice‐daily for 7 days. Infants received twice‐daily zidovudine and lamivudine. 3 Demonitor: initially randomized pairs minus infections at 2 weeks 4 This study was not blinded, however this outcome was not deemed subject to bias associated with lack of blinding. 5 As a single study, no comparison study is available to evaluate inconsistency. 6 As reviewed in Read JS. Diagnosis of HIV‐1 Infection in Children Younger Than 18 Months in the United States. Am Acad Ped 2007:120(6); e1547‐e1562, the sensitivity of DNA PCR for diagnosis of HIV in infants before 30 days is generally significantly less than 100%. For this reason, a baseline window of 4‐6 weeks is recommended to ensure that all in utero and intrapartum transmissions are captured. This study did not use 4‐6 weeks as baseline and as such may include some intrapartum transmissions. While there may be misclassification of timing of infection, there is no reason to believe that it would be differential across study arms. 7 Small number of events. 8 Confidence interval crosses the default threshold of 1.0. 9 Very small number of events 10 Confidence interval approaches default threshold of 1.0. 11 Numerator: total percentage of SAEs times initially randomized pairs, as reported in Supplementary Appendix Table 5. 12 This study was not blinded and this outcome was deemed possibly subject to bias due to lack of blinding.
Summary of findings 9. An extended NVP regimen administered to infants for 28 weeks compared to 1 week of antiretroviral prophylaxis for preventing transmission during breastfeeding.
| An extended NVP regimen administered to infants for 28 weeks compared to 1 week of antiretroviral prophylaxis for preventing transmission during breastfeeding | ||||||
| Patient or population: Breastfeeding infants of HIV‐infected mothers Settings: Lilongwe, Malawi (BAN trial) Intervention: An extended NVP regimen administered to infants for 28 weeks1 Comparison: One week of antiretroviral prophylaxis2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 1 week of antiretroviral prophylaxis | An extended NVP regimen administered to infants for 28 weeks | |||||
| HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 51 per 10003 | 15 per 1000 (8 to 28)3 | RR 0.29 (0.15 to 0.56) | 1447 (1 study) | ⊕⊕⊝⊝ low4,5,6,7 | |
| Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 21 per 1000 | 13 per 1000 (6 to 28) | RR 0.62 (0.28 to 1.35) | 1520 (1 study) | ⊕⊕⊝⊝ low4,5,6,7,8 | |
| HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks | 62 per 10003 | 23 per 1000 (14 to 40)3 | RR 0.38 (0.22 to 0.65) | 1447 (1 study) | ⊕⊕⊕⊝ moderate4,5,6,9 | |
| Infants with Severe Adverse Events | 126 per 1000 | 157 per 1000 (122 to 202) | RR 1.25 (0.97 to 1.61) | 1520 (1 study) | ⊕⊕⊝⊝ low9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Infants received a daily dose of NVP, increasing from 10mg to 30mg over the 28 week period. 2 Mothers in labor and newborns received sdNVP. Mother received Combivir twice‐daily for 7 days. Infants received twice‐daily zidovudine and lamivudine. 3 Denominator: initially randomized pairs minus infections at 2 weeks 4 This study was not blinded, however this outcome was not deemed subject to bias associated with lack of blinding. 5 As a single study, no comparison study is available to evaluate inconsistency. 6 As reviewed in Read JS. Diagnosis of HIV‐1 Infection in Children Younger Than 18 Months in the United States. Am Acad Ped 2007:120(6); e1547‐e1562, the sensitivity of DNA PCR for diagnosis of HIV in infants before 30 days is generally significantly less than 100%. For this reason, a baseline window of 4‐6 weeks is recommended to ensure that all in utero and intrapartum transmissions are captured. This study did not use 4‐6 weeks as baseline and as such may include some intrapartum transmissions. While there may be misclassification of timing of infection, there is no reason to believe that it would be differential across study arms. 7 Very small number of events 8 Confidence interval crosses the default threshold of 1.0. 9 Small number of events.
Summary of findings 10. A once‐daily NVP regimen administered for 6 months compared to a once‐daily NVP regimen administered for 6 weeks for prevention of breastfeeding transmission of HIV.
| A once‐daily NVP regimen administered for 6 months compared to a once‐daily NVP regimen administered for 6 weeks for prevention of breastfeeding transmission of HIV | ||||||
| Patient or population: Breastfeeding infants of HIV‐infected mothers Settings: South Africa, Tanzania, Uganda, Zimbabwe (HPTN 046 trial) Intervention: A once‐daily NVP regimen administered for 6 months1 Comparison: A once‐daily NVP regimen administered for 6 weeks2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| A once‐daily NVP regimen administered for 6 weeks | A once‐daily NVP regmined administered for 6 months | |||||
| HIV transmission at 12 months among infants whose HIV diagnostic testing was negative within 6 weeks of birth and whose mothers were not on HAART | 39 per 10003 | 25 per 1000 (13 to 49)4 | HR 0.65 (0.33 to 1.28) | 1078 (1 study5) | ⊕⊕⊝⊝ low6 | |
| HIV transmission at 12 months among all infants whose HIV diagnostic testing was negative within 6 weeks of birth | 29 per 10007 | 19 per 1000 (10 to 37)7 | HR 0.66 (0.34 to 1.27) | 1512 (1 study5) | ⊕⊕⊝⊝ low6 | |
| Infant mortality at 12 months among all those whose HIV diagnostic testing was negative within 6 weeks of birth | 34 per 10007 | 26 per 1000 (14 to 46)7 | HR 0.75 (0.41 to 1.35) | 1522 (1 study5) | ⊕⊕⊝⊝ low6 | |
| HIV transmission or death at 12 months among all infants whose HIV diagnostic testing was negative within 6 weeks of birth | 60 per 10007 | 40 per 1000 (26 to 63)7 | HR 0.66 (0.42 to 1.05) | 1522 (1 study5) | ⊕⊕⊕⊝ moderate6 | |
| Infants with Severe Adverse Events | 829 per 10008 | 829 per 1000 (788 to 871)8 | RR 1.00 (0.95 to 1.05) | 1519 (1 study5) | ⊕⊕⊕⊕ high | |
| HIV transmission at 6 months among infants whose HIV diagnostic testing was negative within 6 weeks of birth and whose mothers were not on HAART Follow‐up: 6 months | 33 per 1000 | 14 per 1000 (6 to 30) | HR 0.41 (0.18 to 0.9) | 1078 (1 study) | ⊕⊕⊝⊝ low6 | |
| HIV transmission at 6 months among all infants whose HIV diagnostic testing was negative within 6 weeks of birth Follow‐up: 6 months | 24 per 1000 | 11 per 1000 (5 to 24) | HR 0.46 (0.21 to 0.99) | 1512 (1 study) | ⊕⊕⊝⊝ low6 | |
| Infant mortality at 6 months among all those whose HIV diagnostic testing was negative within 6 weeks of birth Follow‐up: 6 months | 10 per 1000 | 5 per 1000 (2 to 10) | HR 0.44 (0.21 to 0.95) | 1522 (1 study) | ⊕⊕⊝⊝ low6 | |
| HIV transmission or death at 6 months among all infants whose HIV diagnostic testing was negative within 6 weeks of birth Follow‐up: 6 months | 31 per 1000 | 22 per 1000 (12 to 41) | HR 0.70 (0.38 to 1.31) | 1522 (1 study) | ⊕⊕⊝⊝ low6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomly allocated infants started maskedstudy drug and continued a once‐daily dosing regimenuntil 6 months of age or until cessation of breastfeeding,whichever came first. The nevirapine dose was increasedwith age, ranging from 20 mg once‐daily after 6–8 weeksof age to 28 mg once‐daily after 5–6 months of age. 2 All infants received once‐daily open‐label nevirapine (10 mg/mL oral suspension) for the first 6 weeks of life, after which they were randomized. 3 Denominator is 539 non antiretroviral therapy plus 2 non‐HAART antiretroviral therapy. 4 Denominator is 537 no antiretroviral therapy plus 0 non‐HAART antiretroviral therapy 5 Randomized according to computer‐generatedpermutated block algorithms by site with random blocksizes. Because maternal receipt of anti retroviral drugs canaffect postnatal transmission, infants were stratified bymaternal antiretroviral treatment status at randomisation 6 Few events. 7 "Primary analysis" denominator 8 Infants with at least one adverse event
Summary of findings 11. An extended antiretroviral regimen administered to mothers for 28 weeks compared to an extended NVP regimen administered to infants for 28 weeks for preventing transmission during breastfeeding.
| An extended antiretroviral regimen administered to mothers for 28 weeks compared to an extended NVP regimen administered to infants for 28 weeks for preventing transmission during breastfeeding | ||||||
| Patient or population: HIV‐infected mothers and their breastfeeding infants Settings: Lilongwe, Malawi (BAN trial) Intervention: An extended antiretroviral regimen administered to mothers for 28 weeks1 Comparison: An extended NVP regimen administered to infants for 28 weeks2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| An extended NVP regimen administered to infants for 28 weeks | An extended antiretroviral regimen administered to mothers for 28 weeks | |||||
| HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 15 per 10003 | 26 per 1000 (13 to 53)3 | RR 1.78 (0.88 to 3.59) | 1618 (1 study) | ⊕⊕⊕⊝ moderate4,5,6,7,8 | |
| Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 13 per 1000 | 14 per 1000 (6 to 32) | RR 1.09 (0.48 to 2.47) | 1701 (1 study) | ⊕⊕⊕⊝ moderate4,5,6,7,8 | |
| HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks | 23 per 10003 | 37 per 1000 (21 to 66)3 | RR 1.60 (0.91 to 2.82) | 1618 (1 study) | ⊕⊕⊕⊝ moderate4,5,6,7,9 | |
| Infants with Severe Adverse Events | 157 per 100010 | 140 per 1000 (112 to 176)10 | RR 0.89 (0.71 to 1.12) | 1701 (1 study) | ⊕⊕⊝⊝ low5,6,7,11 | |
| Mothers with Severe Adverse Events | 16 per 1000 | 46 per 1000 (25 to 84) | RR 2.80 (1.53 to 5.11) | 1701 (1 study) | ⊕⊕⊝⊝ low5,6,9,11 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Mothers initially received Combivir twice daily and NVP once daily for 2 weeks and twice‐daily thereafter until 28 weeks. NVP was replaced with twice‐daily nelfinavir, then twice‐daily lopinavir plus ritonavir because of FDA black‐box warnings (NVP) and availability, safety, and potency concerns (nelfinavir). 2 Infants received a daily dose of NVP, increasing from 10mg to 30mg over the 28 week period. 3 Denominator: initially randomized pairs minus infections at 2 weeks 4 This study was not blinded, however this outcome was not deemed subject to bias associated with lack of blinding. 5 As a single study, no comparison study is available to evaluate inconsistency. 6 As reviewed in Read JS. Diagnosis of HIV‐1 Infection in Children Younger Than 18 Months in the United States. Am Acad Ped 2007:120(6); e1547‐e1562, the sensitivity of DNA PCR for diagnosis of HIV in infants before 30 days is generally significantly less than 100%. For this reason, a baseline window of 4‐6 weeks is recommended to ensure that all in utero and intrapartum transmissions are captured. This study did not use 4‐6 weeks as baseline and as such may include some intrapartum transmissions. While there may be misclassification of timing of infection, there is no reason to believe that it would be differential across study arms. 7 Confidence interval crosses the default threshold of 1.0. 8 Very small number of events 9 Small number of events. 10 Numerator: total percentage of SAEs times initially randomized pairs, as reported in Supplementary Appendix Table 5. 11 This study was not blinded and this outcome was deemed possibly subject to bias due to lack of blinding.
Background
By the end of 2012, approximately 35 million people worldwide were living with HIV or had the acquired immunodeficiency syndrome (AIDS). An estimated 260,000 children under the age of 15 years acquired HIV in 2012. However, ARV prophylaxis prevented the acquisition of HIV by an estimated 670,000 children in low and middle‐income countries from 2009 to 2012 (UNAIDS 2013).
MTCT of HIV can occur during three different time periods: antepartum, intrapartum, and postnatally through breastfeeding (De Cock 2000). In settings where breastfeeding is the norm, a significant proportion of MTCT of HIV occurs during breastfeeding. In an individual patient data meta‐analysis of over 3,000 mother‐infant pairs in sub‐Saharan Africa, up to 42% of MTCT of HIV was attributable to breastfeeding (BHITS 2004). Postpartum mothers appear to be at increased risk of incident infection, and thus transmission to the breastfeeding child (De Schacht 2014).
Identification of risk factors for breast milk transmission of HIV has led to the development of interventions to prevent such transmission (Read 2003). Because a longer exposure to breast milk from an HIV‐infected woman is associated with a greater risk of transmission, interventions to shorten the duration of exposure to breast milk have been evaluated: complete avoidance of breastfeeding and early weaning. Mixed breastfeeding, giving infants under the age of 6 months both breast milk and other liquids and/or foods, is associated with a greater risk of transmission. Therefore, avoidance of mixed breastfeeding (and encouragement of exclusive breastfeeding) has been considered to prevent breast milk transmission. Because greater maternal infectivity (e.g., higher maternal viral load in peripheral blood and in breast milk) is associated with a greater risk of breast milk transmission, maternal use of antiretrovirals during breastfeeding has been evaluated as an intervention to reduce transmission to the infant. Finally, improvement in infant defenses against infection with HIV (e.g., ARV prophylaxis to breastfeeding infants) has been assessed. In this review, we focus on the use of antiretroviral prophylaxis for breastfeeding HIV‐infected mothers or their infants to prevent MTCT of HIV through breast milk.
This review was one of a series of analyses prepared at the request of the World Health Organization (WHO) to inform the development of the 2010 prevention of mother‐to‐child transmission (PMTCT) guidelines. The 2010 guidelines (WHO 2010) reflect the findings in this review. Since the publication of the guidelines, this review has been updated with data published after 2009. However, the conclusions have not changed. WHO has subsequently released consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, including for PMTCT (WHO 2013).
Objectives
The objective of the review is to assess the efficacy and safety of ARV prophylaxis that can be used by HIV‐infected women or given to their infants during breastfeeding to prevent MTCT of HIV.
The specific research questions are:
Mothers: What ARV prophylaxis regimen (and of what duration) should be given to HIV‐infected women to prevent MTCT of HIV through breastfeeding?
Infants: What ARV prophylaxis regimen (and of what duration) should be given to HIV‐exposed infants to prevent MTCT of HIV through breastfeeding?
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
HIV‐infected, breastfeeding women and their infants.
Types of interventions
Any ARV prophylaxis for breastfeeding mothers during breastfeeding.
Any infant ARV prophylaxis during breastfeeding lasting more than four weeks.
Types of outcome measures
Distinct outcomes related to HIV transmission, mortality, and drug safety were assessed for maternal and infant ARV regimens.
Primary outcomes
Maternal regimens
HIV‐free survival at six months and any other future time point among their children who were HIV‐uninfected at 4‐6 weeks of age.
HIV acquisition by 12 weeks, six months, 12 months, and 18 months among their children who were HIV‐uninfected at 4‐6 weeks of age.
Maternal severe adverse events (SAEs) including hepatotoxicity in women given NVP with CD4+ counts of 250‐350 cells/mm3 and >350 cells/mm3; renal toxicity with tenofovir, and all other SAEs.
Infant regimens
Mortality at six months, one year, two years and any other future time point among children who were HIV‐uninfected at 4‐6 weeks.
HIV‐free survival at six months and any other future time point among children who were HIV‐uninfected at 4‐6 weeks of age.
Infant acquired antiretroviral resistance.
Infant SAEs (e.g., anemia, neutropenia, other).
Secondary outcomes
Maternal regimens
Maternal mortality at one year, two years, and beyond.
Maternal response to subsequent antiretroviral therapy (ART).
Maternal antiretroviral resistance.
Maternal adherence.
Child mortality at one and two years and any future time point.
Child response to subsequent ART: clinical, virological, immunological.
Infant regimens
HIV acquisition by 12 weeks, six months, 12 months, and 18 months among children who were HIV‐uninfected at 4‐6 weeks of age.
Infant response to subsequent ART: clinical, virological, immunological.
Search methods for identification of studies
We used the Cochrane HIV/AIDS Group's methods, and searches were performed with the assistance of the Cochrane HIV/AIDS Group. We devised a comprehensive, exhaustive search strategy in order to identify all relevant studies in all languages, published or unpublished (including those in press or in progress).
Electronic searches
Journal and trial databases
Electronic searches were undertaken using the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL)
EMBASE
Literatura Latino‐Americana e do Caribe em Ciencias da Saude (LILACS)
MEDLINE (via PubMed)
Web of Science/Web of Social Science.
With regard to the electronic literature search, the optimal, sensitive search strategy developed by The Cochrane Collaboration and detailed in the Cochrane Reviewers’ Handbook (Higgins 2008) was used, in conjunction with search terms identified in Table 12, to identify relevant studies from January 1, 1994 to June 17, 2009. Additional searches were later undertaken through January 14, 2014. See Table 12 for our strategies in searching Cochrane CENTRAL, EMBASE and PubMed, LILACS, and Web of Science/Web of Social Science. The search strategy was iterative. There were no restrictions on language.
1. Searches.
| PMTCT searches |
| Publication Date from 1994/01/01 to 2014/01/14 |
|
PubMed: HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunodeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) AND randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR observational [tw] OR cohort studies [mh] OR case‐control studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR controll* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) AND (MOTHER‐TO‐CHILD TRANSMISSION) OR (MOTHER TO CHILD TRANSMISSION) OR (ADULT TO CHILD TRANSMISSION) OR (ADULT‐TO‐CHILD TRANSMISSION) OR (MATERNAL TO CHILD TRANSMISSION) OR (MATERNAL‐TO‐CHILD TRANSMISSION) OR (VERTICAL TRANSMISSION) OR (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR PMTCT OR (Infectious Disease Transmission, Vertical/prevention and control[Mesh] AND HIV Infections/prevention and control[Mesh]) OR (mother[tw] AND HIV Infections/prevention and control[Mesh]) OR ((infant[tw] OR baby[tw]) AND HIV Infections/prevention and control[Mesh]) |
|
EMBASE: (((('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp)) OR (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp))) OR ((('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp)) OR (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp)))) OR ((((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp) OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp)) OR (('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp) OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp))) OR ((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp) OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp)) OR (('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp) OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp))))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) AND 'mother‐to‐child transmission' OR 'mother to child transmission' OR 'adult‐to‐child transmission' OR 'adult to child transmission' OR 'maternal‐to‐child transmission' OR 'maternal to child transmission' OR ('vertical transmission'/exp OR 'vertical transmission'/exp) OR ('vertical disease transmission' OR mtct OR pmtct OR 'perinatal transmission') AND [embase]/lim AND [1994‐2014]/py |
|
WEB OF SCIENCE, WEB OF SOCIAL SCIENCE: (TS=HIV OR TS=HIV/AIDS OR TS=AIDS) AND (TS=Mother‐to‐child transmission OR TS=vertical transmission OR TS=mother OR TS=infant OR TS=baby OR TS=perinatal OR TS=postnatal OR TS=prenatal OR TS=antenatal) AND Document Type=(Article OR Meeting Abstract OR Meeting Summary OR Meeting‐Abstract OR Proceedings Paper) Databases=SCI‐EXPANDED, SSCI Timespan=1994‐2014 |
|
LILACS: (HIV OR VIH OR AIDS OR SIDA OR HIV/AIDS) AND (mother‐to‐child OR PMTCT OR MTCT OR mother OR baby OR infant OR vertical) |
|
COCHRANE “CENTRAL”: (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (VIRAL SEXUALLY TRANSMITTED DISEASES) AND (MOTHER‐TO‐CHILD TRANSMISSION) OR (MOTHER TO CHILD TRANSMISSION) OR (ADULT‐TO‐CHILD TRANSMISSION) OR (ADULT TO CHILD TRANSMISSION) OR (MATERNAL‐TO‐CHILD TRANSMISSION) OR (MATERNAL TO CHILD TRANSMISSION) OR (MTCT OR PMTCT) OR (PERINATAL TRANSMISSION) OR (VERTICAL TRANSMISSION) OR (VERTICAL DISEASE TRANSMISSION) |
Conference abstracts
Abstracts from numerous relevant conferences, including the International AIDS Society (IAS) conferences (2007, 2009, 2011, 2013) and the annual Conference on Retroviruses and Opportunistic Infections (CROI) (2008‐2013) were searched.
We searched the World Health Organization’s (WHO) International Clinical Trials Registry Platform (ICTRP) in an effort to identify ongoing trials.
Searching other resources
Contacting researchers in the field
Experts in the field of HIV prevention were contacted to locate any further studies or relevant conference proceedings not included in the databases to ensure that unpublished studies were included.
Reference lists
We checked the reference lists of all studies identified by the above methods and examined the bibliographies of any systematic reviews, meta‐analyses, or current guidelines we identified during the search process.
Data collection and analysis
Our data collection and analysis methodology was based on the guidelines set forth in the Cochrane Handbooks of Systematic Reviews (Higgins 2008). The titles, abstracts and descriptor terms of all downloaded material from the electronic searches were read and irrelevant reports discarded to create a pool of potentially eligible studies. Two authors independently inspected each citation (JM and JR, or JM and AB) to determine whether the full article should be acquired. If there was uncertainty, the full article was obtained.
Selection of studies
Two authors independently applied the exclusion criteria. Studies were reviewed for relevance based on study design, types of participants, exposures and outcome measures. Finally, where resolution was not possible because further information was necessary, attempts were made to contact authors to provide further clarification of data. The method of conflict resolution was by consensus.
Data extraction and management
JM, JR, and AB independently extracted the data using the standardized data extraction form. For each included study, the following characteristics were extracted:
Study details: citation, start and end dates, location, study design, and study description.
Assessment of risk of bias: study design, sequence generation, allocation concealment, blinding, loss to follow‐up, incomplete outcome data and other potential bias.
Participant details: study population eligibility (inclusion and exclusion) criteria, ages, population size, attrition rate, details of HIV diagnosis and disease and any clinical, immunologic or virologic staging or lab information.
Interventions details: drug dose, duration of therapy, comparison group.
Outcome measures: HIV infection status of the child, overall survival, and HIV‐free survival. When infant prophylaxis was administered, infant acquired drug resistance was also assessed. When maternal prophylaxis was administered, maternal mortality and severe adverse events were assessed.
Assessment of risk of bias in included studies
Using the Cochrane Collaboration's tool (Higgins 2008) for assessing risk of bias, we summarized the risk of bias in each study into a table.
For trials, the Cochrane tool assesses risk of bias in individual studies across six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential biases.
Sequence generation
Adequate: investigators described a random component in the sequence generation process, such as the use of random number table, coin tossing, card or envelope shuffling, etc.
Inadequate: investigators described a non‐random component in the sequence generation process, such as the use of odd or even date of birth, algorithm based on the day or date of birth, hospital, or clinic record number.
Unclear: insufficient information to permit judgment of the sequence generation process.
Allocation concealment
Adequate: participants and the investigators enrolling participants cannot foresee assignment (e.g., central allocation; or sequentially numbered, opaque, sealed envelopes).
Inadequate: participants and investigators enrolling participants can foresee upcoming assignment (e.g., an open random allocation schedule, a list of random numbers); or envelopes were unsealed or non‐opaque or not sequentially numbered.
Unclear: insufficient information to permit judgment of the allocation concealment or the method not described.
Blinding
Adequate: blinding of the participants, key study personnel, and outcome assessor, and unlikely that the blinding could have been broken. No blinding in the situation where non‐blinding is not likely to introduce bias.
Inadequate: no blinding or incomplete blinding when the outcome is likely to be influenced by lack of blinding.
Unclear: insufficient information to permit judgment of adequacy or otherwise of the blinding.
Incomplete outcome data
Adequate: no missing outcome data, reasons for missing outcome data unlikely to be related to true outcome, or missing outcome data balanced in number across groups.
Inadequate: reason for missing outcome data likely to be related to true outcome, with either imbalance in number across groups or reasons for missing data.
Unclear: insufficient reporting of attrition or exclusions.
Selective reporting
Adequate: a protocol is available which clearly states the primary outcome as the same as in the final trial report.
Inadequate: the primary outcome differs between the protocol and final trial report.
Unclear: no trial protocol is available or there is insufficient reporting to determine if selective reporting is present.
Other forms of bias
Adequate: there is no evidence of bias from other sources.
Inadequate: there is potential bias present from other sources (e.g., early stopping of trial, fraudulent activity, extreme baseline imbalance, or bias related to specific study design).
Unclear: insufficient information to permit judgment of adequacy or otherwise of other forms of bias.
1.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
2.

Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
Quality of evidence
We assessed the quality of evidence with the GRADE approach (Guyatt 2008), defining the quality of evidence for each outcome as “the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest” (Higgins 2008). The quality rating across studies has four levels: high, moderate, low or very low. RCTs are initially categorized as high quality but can be downgraded; similarly, other types of controlled trials and observational studies are initially categorized as low quality but can be upgraded. Factors that decrease the quality of evidence include limitations in design, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision of results or high probability of publication bias. Factors that can increase the quality level of a body of evidence include a large magnitude of effect, if all plausible confounding would lead to an underestimation of effect and if there is a dose‐response gradient.
Measures of treatment effect
We used Review Manager 5 provided by the Cochrane Collaboration for statistical analysis and GRADEpro software (GRADEpro 2008) to produce GRADE Summary of Findings tables and GRADE evidence profiles.
We used hazard ratios (HRs) or risk ratios (RRs) as appropriate, with 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis was the individual infant.
Dealing with missing data
There were no missing data; we did not contact study authors.
Assessment of reporting biases
Using comprehensive search strategies, we minimized the likelihood of reporting biases. Our search included published and unpublished scientific articles, and covered many types of databases. It also included foreign language publications.
Data synthesis
Since there was significant heterogeneity among the trials in terms of ARVs provided, populations, and durations of prophylaxis, we did not pool data across studies or estimate summary effect sizes.
Results
Description of studies
Results of the search
We conducted an initial search which returned 13,945 citations on July 15, 2009. Of these, 4018 duplicates and 6592 irrelevant citations were removed. After screening the remaining 3335 abstracts, we identified 50 potentially relevant published manuscripts. In reviewing the 50 complete full‐text articles, we identified seven studies as potentially eligible for inclusion. After closer scrutiny, three randomized trials were determined to meet our inclusion criteria (Figure 3).
3.

Study flow diagram.
We undertook subsequent searches to bring the review up to date through January 14, 2014. This returned 1977 citations. Of these, 387 duplicates and 1035 irrelevant citations were removed. Thus, 507 citations remained to be screened. After screening these citations, we identified 31 potentially relevant articles. After reviewing the full text of these articles, four trials met the inclusion criteria.
We identified 24 related conference abstracts and found six to be potentially relevant. Of those, two met the inclusion criteria, but were omitted as the main study report was already accounted for among the included manuscripts.
Therefore, a total 15,922 citations were identified and 11,517 remained after removing duplicates. Of these 7627 were irrelevant and removed from consideration. The 3881 records screened yielded 81 potentially eligible articles, for which the full text was obtained. Seventy were excluded, and 11 manuscripts (representing seven trials) were included in the review (Figure 3).
Included studies
Studies included in this review were those that addressed maternal antiretroviral prophylaxis during breastfeeding (without infant extended prophylaxis), infant prophylaxis during breastfeeding, and both maternal and infant prophylaxis breastfeeding:
Maternal prophylaxis‐only studies: Kesho Bora 2011, and Shapiro 2010;
Infant prophylaxis‐only studies: Gray 2005, SWEN 2008, Kumwenda 2008, and Coovadia 2012;
Both maternal and infant prophylaxis study: Chasela 2010.
Details of each trial are given in the table “Characteristics of included studies” and are noted below:
Trials of maternal prophylaxis only:
Kesho Bora 2011: The Kesho Bora Study Group conducted a randomized controlled trial of triple‐ARV prophylaxis during pregnancy and breastfeeding (Triple‐ARV arm) compared to short ARV prophylaxis to prevent MTCT of HIV (Short arm) among women with CD4+ counts between 200‐500 cells/mm3, and two prospective cohort studies (of HIV‐infected women with CD4+ counts below 200 cells/mm3 and with CD4+ counts above 500 cells/mm3). A total of 1140 HIV‐infected pregnant women were enrolled in the Kesho Bora Study at five sites in Burkina Faso, Kenya and South Africa. Of these, 852 women had CD4+ counts between 200‐500 cells/mm3 and were enrolled in the randomized trial. Of these, 824 women were randomized to the two trial arms and they subsequently delivered a total of 805 live born singleton or first‐born infants. Of these 805 infants, 402 were randomized to the Triple‐ARV arm and 403 to the Short arm.
Mothers of infants in the Triple‐ARV arm received an extended prophylactic regimen of zidovudine (ZDV), lamivudine (3TC), and ritonavir‐boosted lopinavir (LPV/r) to a maximum of six and a half months post partum. Mother of infants in the Short arm received ZDV until delivery plus single dose nevirapine (sdNVP) (the protocol was changed during the trial to one week post‐partum ZDV and 3TC). All infants received sdNVP and/or one week of ZDV.
The primary objectives of this trial were to assess the efficacy and safety of the Triple‐ARV regimen versus the Short regimen for prevention of MTCT of HIV during pregnancy and breastfeeding. The primary endpoints were HIV‐free infant survival at six weeks and 12 months, HIV‐free survival at 12 months among infants who were ever breastfed, AIDS‐free survival in mothers at 18 months, and serious adverse events in mothers and infants. The analysis was by intention to treat. Not all women breastfed in the study. All women choosing to breastfeed were counseled to breastfeed exclusively for five and a half months, followed by weaning over a two week period. Results presented refer to the subset of infants (n = 307 in the Triple‐ARV group and 317 in the Short group) who ever breastfed.
At six months, there was no difference in HIV transmission (HR 0.36, 95% CI 0.13 to 1.02), in infant mortality (HR 0.88, 95% CI 0.36 to 2.11), or in infant mortality or transmission (HR 0.61, 95% CI 0.3 to 1.24) in the Triple‐ARV group versus the Short group.
At 12 months, the risks of HIV transmission (HR 0.40, 95% CI 0.16 to 0.96) and of HIV transmission or death (HR 0.52, 95% CI 0.28 to 0.93) were lower among infants in the Triple‐ARV arm versus the Short arm. There was no difference in infant mortality alone (HR 0.94, 95% CI 0.48 to 1.85), and no difference in maternal adverse events (RR 0.84, 95% CI 0.59 to 1.21) in the Triple‐ARV arm versus the Short arm.
Shapiro 2010: The Mma Bana trial assessed the efficacy through six months of breastfeeding of ZDV, 3TC, and LPV/r (Arm 1) versus ZDV, 3TC, and abacavir (ABC) (Arm 2) from 26‐34 weeks gestation among HIV‐infected women with CD4+ counts of 200 cells/mm3 or more. Women with CD4+ counts < 200 cells/mm3 or with an AIDS‐defining illness received treatment with ZDV, 3TC, and NVP beginning at 18‐34 weeks gestation. HIV‐infected women in the four clinical sites in Botswana were eligible if they had a hemoglobin level of at least 8.0 g/dL, an absolute neutrophil count of over 1000 cells/mm3, and alanine and aspartate aminotransferase levels no more than 2.5 times the upper limit of the normal range. Those who planned to exclusively formula feed were not eligible. Infants received sdNPV and 4 weeks of ZDV. In total, 285 were randomized to Arm 1, 275 were randomized to Arm 2, and 170 were assigned to the observation arm.
The primary objectives of this trial were to determine whether the maternal ARV regimens differed in terms of virologic suppression during pregnancy and breastfeeding, pregnancy outcomes, and adverse events among the enrolled women and their infants. All regimens were continued through planned weaning by 6 months postpartum.
At six months, there was no difference between Arm 1 and Arm 2 in the risk of infant HIV infection (RR 0.21, 95% CI 0.1 to 4.3), infant mortality (RR 1.05, 95% CI 0.37 to 2.14), or infant mortality and death (RR 0.81, 95% 0.3 to 2.14).
There was also no difference at six months between Arm 1 and Arm 2 in infant severe adverse events (RR 1.13, 95% CI 0.93 to 1.37), maternal mortality (RR 0.35, 95% CI 0.01 to 8.44), and maternal severe adverse events (RR 0.79, 95% CI 0.51 to 1.21).
Trials of infant prophylaxis only:
Gray 2005: In a trial conducted in three hospitals in South Africa, infants of HIV‐infected mothers were randomized to single dose NVP (sdNVP) or six weeks of ZDV every 12 hours. There was no restriction on maternal CD4+ count for eligibility. Full term infants, over 1,200 grams, not requiring ventilation and without congenital anomalies were eligible for the trial. Mothers were given subsidized formula if they chose not to breastfeed, or counseled to breastfeed exclusively for three to six months. Women with CD4+ cell counts <200‐106 cells/mm3 were given trimethoprim/sulfamethoxazole. The majority of women did not breastfeed their infants. Seventy‐eight infants (or 16.08%) in the NVP group, and 68 infants (13.44%) in the ZDV group were exclusively breastfed. The primary objective of this trial was to compare HIV infection rates at week 12 among infants without evidence of HIV infection at birth who received either sdNVP alone or else ZDV for six weeks.
Among infants without evidence of HIV infection within 10 days after birth who were breastfed, the risk of HIV infection at 12 weeks was greater in the ZDV arm than in the sdNVP arm (HR 2.35, 95% CI 1.07 to 5.17).
SWEN 2008: The Six Week Extended‐Dose Nevirapine (SWEN) Study Team conducted three separate, but coordinated trials in Ethiopia, India, and Uganda. There was no CD4+ eligibility criterion, but women in Ethiopia were required to have hemoglobin >75 g/L, creatinine <1·5 mg/dL, and alanine aminotransferase concentrations 5 times the upper limit of normal. In Arm 1 of the trials, 200 mg NVP was provided to a total of 1047 women in labor and 2 mg/kg to infants shortly after birth (control regimen). In Arm 2 of the trials, 997 women and their infants received the control regimen and the infants also received 5 mg NVP daily starting at seven days through six weeks of life. The primary endpoint was HIV infection at six months of age in infants who were HIV polymerase chain reaction (PCR) negative at birth. The risk of HIV infection and death at six weeks and six months of age in infants who were HIV‐uninfected at birth was estimated.
At six weeks of age, there was no difference in infection in infants in the extended dose group (RR 0.78, 95% CI 0.57 to 1.07) compared with the control group.
At six months of age, infants in the extended NVP group had a lower risk of infant mortality (RR 0.47, 95% CI 0.26 to 0.87) and HIV infection or death (RR 0.73, 95% CI 0.85 to 1.09) compared with the control group. There was no difference in the risk of HIV transmission alone (RR 0.80, 95% CI 0.58 to 1.1) or the risk of severe adverse events in infants (RR 0.93, 95% CI 0.85 to 1.09) in the extended NVP group versus the control.
There was an increased risk of genotypic resistance to NVP among Ugandan infants infected by six weeks (RR 1.68, 95% CI 1.09 to 2.6) and of persistent genotypic resistance among the same group (RR 4.38, 95% CI 1.05 to 18.28) in the extended NVP group versus the control group.
Among Indian infants, there was an increased risk of genotypic NVP resistance in the early breastfeeding (RR 2.42, 95% CI 1.47 to 3.79), but no difference in the late breastfeeding period (RR 1.03, 95% CI 0.2 to 5.33) in the extended NVP group compared with the control group.
At 12 months:
After 12 months, infants in the extended NVP group had a lower risk of infant mortality (RR 0.53, 95% CI 0.32 to 0.85), compared with the control group. There was no difference in the risk of HIV infection or death (RR 0.71, 95% CI 0.69 to 1.01), HIV transmission alone (RR 0.89, 95% CI 0.67 to 1.17), or of severe adverse events in infants (RR 0.93, 95% CI 0.85 to 1.02) in the extended NVP group, compared with the control group.
Kumwenda 2008: In the Post‐Exposure Prophylaxis of Infants (PEPI) trial, infants were randomly assigned at birth to one of three groups: a control regimen of sdNVP (2 mg/kg) plus one week of ZDV (4 mg/kg twice daily); the control regimen plus extended prophylaxis with NVP (2 mg/kg one daily during week 2, then 4 mg/kg once daily during weeks 3‐14) (extended NVP group); or the control regimen with NVP/ZDV (the same regimen as in the extended NVP group plus ZDV 4 mg/kg twice daily during weeks 2‐5, 4 mg/kg three times daily during weeks 6‐8, and 6 mg/kg three times daily during weeks 9‐14) (extended dual prophylaxis group) until the age of 14 weeks. HIV‐infected women who intended to breastfeed could enroll without regard to CD4+ count.
Enrolled infants were born to HIV‐infected women in Malawi who intended to breastfeed. A total of 1,016 infants were randomized to the extended NVP group, 1,089 infants to the extended dual prophylaxis group, and 1,003 infants to the control group. Women received intrapartum NVP prior to the randomization of the infants. The primary objectives of this trial were to determine whether extended ARV prophylaxis to infants until the age of 14 weeks would decrease the rate of HIV infection compared to the control regimen.
After nine months of follow‐up:
Compared with the control group, the extended NVP group had a lower risk of HIV transmission (HR 0.56, 95% CI 0.41 to 0.76) and HIV transmission or death (HR 0.69, 95% CI 0.55 to 0.87). There was a decreased risk of infant mortality alone (HR 0.72, 95% CI 0.55 to 0.87) and no difference in severe adverse events (RR 1.1, 95% CI 0.96 to 1.26) or adverse events probably attributable to the study drug (RR 2.96, 95% CI 0.6 to 14.64) in the extended NVP group compared with the control group.
Compared with the control group, the extended dual prophylaxis group had a lower risk of HIV transmission (HR 0.65, 95% CI 0.48 to 0.88), infant mortality (HR 0.66, 95% CI 0.46 to 0.96), and HIV transmission or death (HR 0.72, 95% CI 0.57 to 0.9). There was no statistically significant difference in severe adverse events (RR 1.08, 95% CI 0.94 to 1.24) or adverse events probably attributable to the study drug (RR 2.52, 95% CI 0.49 to 12.93) in the extended dual prophylaxis group compared with the control group.
There was no statistically significant difference in the risk of HIV transmission (HR 1.23, 95% CI 0.83 to 1.18) or infant mortality (HR 0.91, 95% CI 0.61 to 1.36) among infants receiving the extended dual prophylaxis regimen compared with the extended NVP‐only regimen. The risk of HIV transmission or death was lower among the extended dual prophylaxis group (HR 0.73, 95% CI 0.60 to 0.90), compared with the extended NVP group.
After 24 months of follow‐up:
Compared with the control group, the extended NVP regimen group had a lower risk of HIV transmission (HR 0.60, 95% CI 0.46 to 0.78) and of HIV transmission or death (HR 0.71, 95% CI 0.58 to 0.87). There was no statistically significant difference in infant mortality alone (HR 0.74, 95% CI 0.54 to 1.01), no statistically significant difference in severe adverse events (RR 1.6, 95% CI 0.70 to 1.59), and no statistically significant difference in adverse events probably attributable to the study drug (RR 2.96, 95% CI 0.6 to 14.64) in the extended NVP group compared with the control group.
Compared with the control group, the extended dual prophylaxis group had a lower risk of HIV transmission (HR 0.65, 95% CI 0.50 to 0.85), but no statistically significant difference in infant mortality (HR 0.73, 95% CI 0.53 to 1.00). The risk of HIV transmission or death was lower in the extended dual prophylaxis group compared with the control (HR 0.73, 95% CI 0.60 to 0.90). There was an increased risk of possibly related severe adverse events (RR 1.75, 95% CI 1.22 to 2.53), but adverse events probably attributable to the study drug showed no difference in risk (RR 3.02, 95% CI 0.61 to 14.92) in the extended dual prophylaxis group compared with the control group.
There was no statistically significant difference in the risk of HIV transmission (HR 1.04, 95% CI 0.71 to 1.51), infant mortality (HR 0.91, 95% CI 0.61 to 1.36), or HIV transmission or death (HR 1.00, 95% CI 0.75 to 1.34) among infants receiving the extended dual prophylaxis regimen compared with the extended NVP regimen. There was an increased risk of possibly related severe adverse events (RR 1.66, 95% CI 1.16 to 2.37), but no statistically significant difference in adverse events probably attributable to the ARV intervention (RR 1.02, 95% CI 0.33 to 3.15) in the extended dual prophylaxis group versus the extended NVP group.
Coovadia 2012: Coovadia and colleagues randomized 1527 infants of mothers visiting antenatal clinics in South Africa, Tanzania, Uganda, and Zimbabwe to either once daily NVP for six weeks or once daily NVP for six months (or until breastfeeding cessation) to assess the efficacy of the 6‐month regimen versus the 6‐week regimen. There was no CD4+ eligibility criterion. The primary outcome was HIV infection at six months.
At six months:
There was a decreased risk of HIV infection among the six month NVP group whose mothers were not on highly active antiretroviral therapy (HAART) versus the six week NVP group (HR 0.41, 95% CI 0.18 to 0.9).
Among infants of all mothers, the risk of HIV infection, death, and HIV infection or death was not different in the six month NVP group compared with the six week NVP group (HR 0.70, 95% CI 0.38 to 1.31).
At 12 months:
Among infants of mothers not using HAART, there was no statistically significant difference in risk of HIV infection among the six month NVP group versus the six week NVP group (HR 0.65, 95% CI 0.33 to 1.28).
Among infants of all mothers, the risk of HIV infection, death, and HIV infection or death was lower in the six month NVP group compared with the six week NVP group (HR 0.66, 95% CI 0.42 to 1.05).
Trial of maternal or infant prophylaxis:
Chasela 2010: In the BAN trial in Malawi, 2369 breast‐feeding mother‐infant pairs were randomized at delivery to either a maternal triple‐drug ARV regimen from 2‐28 weeks (n = 849), infant NVP from 2‐28 weeks (n = 852) or neither intervention (n =668). Only mothers with CD4+ counts over 250 cells/mm3 were eligible. All mother‐infant pairs received the following: mothers – a single dose of oral NVP at labor, ZDV 300 mg/3TC 150 mg orally every 12 hours from the onset of labor to seven days after delivery; infants – a single dose of oral NVP as well as ZDV (2 mg/kg) and 3TC (4 mg/kg) twice daily for seven days. Women randomized to the maternal intervention received NVP 200 mg once daily for two weeks postpartum and twice daily thereafter until 28 weeks. The protocol was subsequently revised to replace NVP with nelfinavir 1250 mg for 146 women, and the protocol was later revised again to replace nelfinavir with LPV/r (400 mg/100 mg). Infants randomized to the infant intervention received increasing dosages of NVP (10 mg daily for the first 2 weeks and increasing to 30 mg daily for weeks 19‐28). All women were counseled to breastfeed exclusively, then wean rapidly between 24 and 28 weeks postnatally. In the BAN trial in Malawi, 2369 breast‐feeding mother‐infant pairs were randomized to either a maternal triple‐drug ARV regimen from 2‐28 weeks (n = 849), infant NVP from 2‐28 weeks (n = 852) or neither intervention (n =668). All mother‐infant pairs received the following: mothers – a single dose of oral NVP at labor, ZDV 300 mg/3TC 150 mg orally every 12 hours from the onset of labor to seven days after delivery; infants – a single dose of oral NVP as well as ZDV (2 mg/kg) and 3TC (4 mg/kg) twice daily for seven days. Women randomized to the maternal intervention received NVP 200 mg one daily for two weeks and twice daily thereafter until 28 weeks. The protocol was subsequently revised to replace NVP with nelfinavir 1250 mg for 146 women, and the protocol was later revised again to replace nelfinavir with LPV/r (400 mg/100 mg). Infants randomized to the infant intervention received increasing dosages of NVP (10 mg daily for the first 2 weeks and increasing to 30 mg daily for weeks 19‐28). All women were counseled to breastfeed exclusively, then wean rapidly between 24 and 28 weeks postnatally.
The primary objective of this trial was to evaluate the efficacy of the maternal triple‐ARV prophylaxis regimen and the infant extended NVP prophylaxis. The primary outcome was infant HIV infection at 28 weeks among infants who where uninfected at two weeks and among all infants who underwent randomization. The secondary outcome was HIV‐free survival by 28 weeks among infants who were uninfected at two weeks and among all infants who underwent randomization.
Infants of mothers receiving the maternal prophylaxis regimen from 2‐28 weeks were at lower risk for HIV infection (RR 0.52, 95% CI 0.3 to 0.89), and HIV infection or death (RR 0.61, 95% CI 0.38 to 0.96) than infants in the control group.
There was no statistically significant difference in the risk of infant mortality alone (RR 0.67, 95% CI 0.31 to 1.45) when comparing the maternal prophylaxis group to the control group. Among mother‐infant pairs enrolled in the maternal prophylaxis group, there was no statistically significant difference in severe adverse events among infants (RR 1.11, 95% CI 0.86 to 8.45), but there was an increased risk of severe adverse events among mothers (RR 2.19, 95% CI 1.2 to 4.0) compared with those enrolled in the control group.
Infants enrolled in the infant extended prophylaxis group were at lower risk for HIV infection (RR 0.29, 95% CI 0.15 to 0.56), and HIV infection or death (RR 0.38, 95% CI 0.22 to 0.65) when compared with infants in the control group. There was no difference in the risk of infant mortality alone (RR 0.67, 95% CI 0.31 to 1.45) in the extended infant NVP group versus the control group.
When comparing the maternal prophylaxis group with the infant extended prophylaxis group, there was no statistically significant difference in HIV infection (RR 1.78, 95% CI 0.88 to 3.59), infant mortality (RR 1.78, 95% CI 0.48 to 2.47), and HIV infection or death (RR 1.60, 95% CI 0.91 to 2.82), although the trial was not powered to detect differences between the two groups. However, when comparing the maternal prophylaxis group with the infant extended prophylaxis group, there was an increased risk of severe adverse events in the mother (RR 2.8 95%, CI 1.53 to 5.11), but no statistically significant difference in infant severe adverse events (RR 0.89, 95% CI 0.71 to 1.12).
Excluded studies
We assessed the full text of 81 articles and later excluded 70 articles.
Risk of bias in included studies
Using the Cochrane Collaboration's tool (Higgins 2008) for assessing risk of bias, we summarized the risk of bias in each study into a table. The tool assesses risk of bias in the following areas: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential biases. There is a low risk of bias in the included studies. The most likely source of bias is the lack of blinding. However, this is unlikely to have affected the study outcomes. All trials except Coovadia 2012 were open‐label trials. Figure 1 and Figure 2 graphically show the assessment, and the Risk of Bias tables in Characteristics of included studies give further details about our assessment of each study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11
Extended ARV prophylaxis given to either mother or infant during breastfeeding is associated with decreased risk of HIV transmission and infant mortality.
Maternal prophylaxis only
One trial (Kesho Bora 2011) demonstrated an association at 12 months (but not at six months) between the use of maternal triple‐ARV prophylaxis during breastfeeding (compared with a short ARV prophylaxis regimen) and a decreased risk of HIV transmission, infant mortality, and HIV transmission or death.
Chasela 2010: The maternal ARV regimen was associated with a lower risk of HIV transmission and of HIV transmission or death in infants, compared with an infant ARV regimen.
Shapiro 2010 showed no difference between an NRTI‐only regimen and regimen containing a protease inhibitor. Both reduced the acquisition of HIV by infants.
Infant prophylaxis only
Three trials (Chasela 2010, Kumwenda 2008, SWEN 2008) showed an association between an extended ARV prophylaxis given to infants during breastfeeding and decreased risk of HIV transmission.
One trial, Coovadia 2012, assessed the relative efficacy of a six week infant prophylactic regimen versus a six month regimen. This study indicated a relationship between the six month infant prophylaxis and lower risk of HIV infection and infant mortality after six months among children born to mothers not using HAART. However, the larger group of children (born to mothers using or not using HAART) showed no difference in HIV infection at six months and infant mortality risk at either six or 12 months. Additionally, HIV infection among the six month infant NVP regimen (for children born to mothers not using HAART) was not significantly different from the six week regimen after 12 months of follow‐up.
Maternal or infant prophylaxis
The BAN trial (Chasela 2010), evaluated maternal and infant regimens, but the trial was not powered to detect a difference in efficacy between maternal and infant prophylaxis (and no difference was observed).
Maternal adverse events:
BAN: The maternal regimen in the BAN trial was associated with an increased risk of severe adverse events among mothers (RR 2.19, 95% CI 1.2 to 4). In the Kesho Bora trial, there was no difference in the risk of maternal adverse events with an extended maternal regimen.
Resistance:
Data for resistance are scarce. In the SWEN 2008 trial, Ugandan infants receiving infant NVP for six weeks who seroconverted in the first six weeks had an increased risk of genotypic resistance. In the same trial, infants in the Indian site who received the NVP regimen did not show an increased risk of resistance if they seroconverted in the six month period.
Discussion
Interventions to prevent MTCT of HIV are reducing the number of children who acquire HIV in low and middle income countries (UNAIDS 2013). However, how best to prevent HIV transmission through breastfeeding in areas where complete avoidance of breastfeeding is not AFASS remains an extremely important issue.
Seven RCTs were included in this review of ARV prophylaxis for the prevention of MTCT during breastfeeding. The data from these seven trials support the provision of ARV prophylaxis during breastfeeding to prevent MTCT of HIV through breast milk. Extended ARV prophylaxis, given to either mother or infant during breastfeeding, is associated with a decreased risk of infant HIV infection and of infant HIV infection or death. The relatively higher rate of transmission of HIV in the Triple‐ARV arm of the Kesho Bora trial, compared with other studies, could be related to the relatively low level of virologic suppression.
The longer the duration of extended NVP dosing of infants, the longer the duration of protection against MTCT of HIV. However, once prophylaxis is discontinued, cumulative infant HIV infection rates continue to increase – suggesting that infant (and presumably maternal) ARV prophylaxis to prevent breast milk transmission of HIV must continue as long as breastfeeding continues. Extended infant prophylaxis with NVP alone appears to be better than dual prophylaxis with NVP and ZDV in light of a greater risk of serious adverse events with dual prophylaxis. Infants who receive NVP prophylaxis but who later become HIV‐infected are more likely to have NVP resistance, and this has implications for their ARV treatment options.
More data are needed to characterize maternal resistance and maternal response to subsequent ART after maternal prophylaxis. Whether maternal or infant prophylaxis during breastfeeding is better cannot yet be determined. Only one trial to date included both maternal and infant ARV prophylaxis arms, and it was not powered for a direct comparison of the two. A comparison of the efficacy and safety of maternal versus infant ARV prophylaxis approaches for prevention of MTCT through breastfeeding will be made with data collected in an ongoing clinical trial (IMPAACT 1077BF). In addition, more data are needed regarding the effect of adherence. The different MTCT rates in Mma Bana, where virologic suppression was very high, versus Kesho Bora, where suppression rates were lower, suggests that adherence plays a role in the effectiveness of prophylactic regimens. Timing of initiation of maternal prophylaxis (antepartum versus postpartum) may also affect results. Meanwhile, some countries are proceeding to initiate lifelong ART among pregnant women determined to be HIV‐infected, i.e. in essence, making a decision to utilize maternal ARV prophylaxis of breast milk transmission of HIV.
Overall completeness and applicability of evidence
The trials included in this review offer robust evidence of the efficacy of maternal and infant ARV prophylaxis for prevention of MTCT of HIV. However, not all of the outcomes proposed in this review could be examined in the included studies.
For maternal regimens:
Three trials provided data on maternal severe adverse events, infant HIV free survival, infant HIV acquisition, and infant mortality (Chasela 2010, Kesho Bora 2011, Shapiro 2010)
Two trials reported on infant severe adverse events (Chasela 2010, Shapiro 2010)
One trial reported on maternal mortality (Shapiro 2010)
None of the trials included provided information on maternal adherence, maternal resistance, maternal response to subsequent ART, or infant response to subsequent ART.
For infant regimens:
Six trials reported HIV‐free survival at least one time point (Chasela 2010, Coovadia 2012, Kesho Bora 2011, Gray 2005, Kumwenda 2008, SWEN 2008)
Four trials provided infant mortality and adverse event data (Chasela 2010, Coovadia 2012, Kumwenda 2008, SWEN 2008)
One trial addressed infant acquired drug resistance (SWEN 2008).
None of the trials reported infant response to subsequent ART.
Further research is needed on maternal resistance and response to subsequent ART after maternal prophylaxis, as well as maternal mortality. For both maternal and infant regimens, infant response to subsequent ART requires more evidence.
Lyons 2005 found non‐nucleoside reverse transcriptase inhibitor (NNRTI) resistance among women after discontinuing a prophylactic three‐drug regimen. An observational study, though, found limited resistance among mothers after prophylaxis (Palombi 2012). Souda (Souda 2013) found no major resistance mutations among women in the BAN trial after stopping ARVs after a median of 266 days. The Mma Bana trial found an absolute increased mortality among women who discontinued ARVs after pregnancy,though it was not statistically significant (Shapiro 2013).
In addition to the resistance results from SWEN 2008, a separate analysis of samples from PEPI found an association between postpartum maternal HAART and multi‐class resistance among infants who did become HIV‐infected during the postnatal period (Fogel 2011). Other studies support this finding (Persaud 2011, Lidstrom 2010).
Quality of the evidence
There is high‐quality evidence from six randomized controlled trials that maternal and infant ARV prophylaxis during breastfeeding are efficacious in preventing MTCT of HIV and/or infant mortality. One trial contributed low quality evidence that a six‐week extended ZDV regimen was less efficacious than sdNVP in preventing MTCT of HIV. See Table 4.
Maternal prophylaxis
Kesho Bora 2011 showed, with moderate evidence, a decreased risk of HIV transmission or death, at six months and 12 months, where triple ARV prophylaxis was used compared with a maternal short course regimen. Similarly, the evidence for decreased transmission alone is also of moderate quality for six and 12 month time intervals. The evidence regarding the lack of association between maternal SAEs and each of the two regimens is of very low quality.
From Shapiro 2010, there was low quality evidence of no difference in HIV transmission or death and moderate quality of evidence of no difference in HIV transmission between six months of ZDV, 3TC, and LPV/r compared with ZDV, 3TC, and ABC. There was low quality of evidence for no difference in both maternal SAEs and maternal mortality between the groups.
In Chasela 2010, the evidence for increased maternal SAEs in the extended ARV group versus the one week of prophylaxis group was low.
Infant prophylaxis
The evidence that extended NVP regimens of six and 14 weeks, compared with sdNVP, (SWEN 2008 and Kumwenda 2008, respectively) are efficacious in reducing HIV transmission or death and infant mortality at six week, is of high quality.
The evidence of the association between extended NVP and increased risk of acquired resistance at six months, compared with sdNVP, is of moderate to high quality (SWEN 2008). At 12 months, evidence for no association for HIV transmission, infant mortality, and HIV transmission or death is of moderate quality. Evidence for no association for infant adverse events is of high quality.
The efficacy of an extended NVP/ZDV regimen versus sdNVP has high quality evidence (Kumwenda 2008). Moderate quality evidence indicates no difference in HIV transmission and infant mortality between the extended NVP group and extended dual prophylaxis. Low quality evidence shows no significant differences in infant severe adverse events and 'probably related' SAEs between these two arms.
Chasela 2010 had high quality evidence for decreased transmission, and moderate quality evidence for reduced infant mortality and transmission or death with an extended ARV regimen versus one week of prophylaxis. The evidence for increased SAEs among infants was low.
Gray 2005 had low quality evidence for increased transmission at 12 weeks among infants who received six weeks of prophylaxis versus sdNVP.
Authors' conclusions
Implications for practice.
As previously noted, HIV policies in some countries have evolved to incorporate testing of pregnant women for HIV and then, if a woman is HIV‐infected, to initiate lifelong ART. Therefore, in essence, HIV‐infected women who initiated ART during pregnancy and who continue such therapy postpartum while breastfeeding are utilizing an intervention (maternal ARV prophylaxis) to prevent breast milk transmission of the virus to their infants. In settings where this is the case, the utility of infant ARV prophylaxis while breastfeeding is unclear and few clinicians are likely to implement this. In settings where HIV‐infected, postpartum women are breastfeeding but have not initiated ART themselves, the question or whether to utilize maternal or infant ARV prophylaxis to prevent breast milk transmission of HIV is a difficult one without a clear answer from available data. Therefore, in such settings, many factors will likely be considered by the clinician, including the availability of ARVs for adult patients (including postpartum women) or for infants (i.e., in suitable formulations for use by infants). Additionally, clinicians may consider the potential programmatic simplicity and health benefit to mothers of lifelong ART after the initiation of ARV prophylaxis during pregnancy.
Implications for research.
A number of questions remain following completion of an analysis of data from multiple clinical trials involving maternal and infant ARV prophylaxis of MTCT of HIV through breastfeeding. How NVP resistance among children exposed to maternal or infant prophylaxis during breastfeeding affects later ARV treatment options among those children who become infected despite prophylaxis requires further study. Similarly, the extent to which women who use ARV prophylaxis for prevention of MTCT of HIV through breastfeeding develop ARV resistance, and how such resistance affects their response to later ART needs further evaluation. An important component of the IMPAACT 1077BF clinical trial is a direct comparison of the efficacy and safety of the two ARV prophylaxis approaches (maternal versus infant) for prevention of MTCT through breastfeeding.
Acknowledgements
We thank the entire Cochrane HIV/AIDS Group for their support.
Data and analyses
Comparison 1. PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week).
1.1. Analysis.

Comparison 1 PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 1 HIV Transmission at 9 months among those whose HIV diagnostic testing was negative within 48 hrs of birth.
1.2. Analysis.

Comparison 1 PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 2 Infant Mortality at 9 Months Among Those Whose HIV Diagnostic Testing was Negative Within 48 Hours of Birth.
1.3. Analysis.

Comparison 1 PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 3 HIV Transmission or Death at 9 Months Among Those Whose HIV Diagnostic Testing was Negative Within 48 Hours of Birth.
1.4. Analysis.

Comparison 1 PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 4 HIV Transmission at 24 Months Among Those Uninfected At Birth.
1.5. Analysis.

Comparison 1 PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 5 Infant Mortality at 24 Months Among Those Uninfected At Birth.
1.6. Analysis.

Comparison 1 PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 6 HIV Transmission or Death at 24 Months Among Those Uninfected At Birth.
1.7. Analysis.

Comparison 1 PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 7 Infants With Possible Related Severe Adverse Events‐24 Months.
1.8. Analysis.

Comparison 1 PEPI‐Malawi (extended NVP to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 8 Infants With Probably Related Severe Adverse Events.
Comparison 2. PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week).
2.1. Analysis.

Comparison 2 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 1 HIV Transmission at 9 months among those whose HIV diagnostic testing was negative within 48 hrs of birth.
2.2. Analysis.

Comparison 2 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 2 Infant Mortality at 9 Months Among Those Whose HIV Diagnostic Testing was Negative Within 48 Hours of Birth.
2.3. Analysis.

Comparison 2 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 3 HIV Transmission or Death at 9 Months Among Those Whose HIV Diagnostic Testing was Negative Within 48 Hours of Birth.
2.4. Analysis.

Comparison 2 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 4 HIV Transmission at 24 Months Among Those Uninfected At Birth.
2.5. Analysis.

Comparison 2 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 5 Infant Mortality at 24 Months Among Those Uninfected At Birth.
2.6. Analysis.

Comparison 2 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 6 HIV Transmission or Death at 24 Months Among Those Uninfected At Birth.
2.7. Analysis.

Comparison 2 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 7 Infants With Possible Related Severe Adverse Events‐24 Months.
2.8. Analysis.

Comparison 2 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. sdNVP + ZDV for 1 week), Outcome 8 Infants With Probably Related Severe Adverse Events.
Comparison 3. PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks).
3.1. Analysis.

Comparison 3 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks), Outcome 1 HIV Transmission at 9 months among those whose HIV diagnostic testing was negative within 48 hrs of birth.
3.2. Analysis.

Comparison 3 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks), Outcome 2 Infant Mortality at 9 Months Among Those Whose HIV Diagnostic Testing was Negative Within 48 Hours of Birth.
3.3. Analysis.

Comparison 3 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks), Outcome 3 HIV Transmission or Death at 9 Months Among Those Whose HIV Diagnostic Testing was Negative Within 48 Hours of Birth.
3.4. Analysis.

Comparison 3 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks), Outcome 4 HIV Transmission at 24 Months Among Those Uninfected At Birth.
3.5. Analysis.

Comparison 3 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks), Outcome 5 Infant Mortality at 24 Months Among Those Uninfected At Birth.
3.6. Analysis.

Comparison 3 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks), Outcome 6 HIV Transmission or Death at 24 Months Among Those Uninfected At Birth.
3.7. Analysis.

Comparison 3 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks), Outcome 7 Infants With Possible Related Severe Adverse Events‐24 Months.
3.8. Analysis.

Comparison 3 PEPI‐Malawi (extended NVP + ZDV to infant for 14 weeks vs. extended NVP for 14 weeks), Outcome 8 Infants With Probably Related Severe Adverse Events.
Comparison 4. SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP).
4.1. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 1 HIV Transmission at 6 Months Among Those Whose HIV Diagnostic Testing Was Negative Within 7 days of Birth.
4.2. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 2 Mortality at 6 months among those whose HIV diagnostic testing was negative within 7 days of birth.
4.3. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 3 HIV Transmission or Death at 6 months among those whose HIV diagnostic testing was negative within 7 days of birth.
4.4. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 4 Infants with Grade 3/4 Adverse Events by 6 months.
4.5. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 5 Genotypic Resistance to NVP among Ugandan infants HIV‐infected at 6 weeks.
4.6. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 6 Persistance of Genotypic Resistance to NVP at 6 months among Ugandan infants found to be resistant at 6 weeks.
4.7. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 7 Genotypic Resistance to NVP among Indian infants HIV‐infected in utero or through peripartum/early‐breastfeeding transmission.
4.8. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 8 Genotypic Resistance to NVP among Indian infants HIV‐infected through late breastfeeding.
4.9. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 9 HIV Transmission at 12 months among those whose HIV diagnostic testing was negative within 7 days of birth.
4.10. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 10 Infant Mortality at 12 months among those whose HIV diagnostic testing was negative within 7 days of birth.
4.11. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 11 HIV Transmission or Death at 12 months among those whose HIV diagnostic testing was negative within 7 days of birth.
4.12. Analysis.

Comparison 4 SWEN‐Ethiopia, India, Uganda (extended NVP to infant for 6 weeks vs. sdNVP), Outcome 12 Infants with Grade 3/4 Adverse Events by 12 months.
Comparison 5. MASHI‐Botswana (extended ZDV to infant for 6 months vs. formula feeding plus ZDV for 1 month).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV Transmission at 7 months among those whose HIV diagnostic testing was negative at one month after birth | 0 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Infant Mortality at 18 months | 0 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 HIV Transmission or Death at 18 months among those whose HIV diagnostic testing was negative at one month after birth | 0 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Infants with Grade 3/4 Signs or Symptoms | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Infants with Grade 3/4 Laboratory Abnormalities | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Infant with Toxicity Events leading to cessation of ZDV | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. PEP‐South Africa (extended ZDV to infants for 6 weeks vs. sdNVP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV Transmission at 12 weeks among those whose HIV diagnostic testing was negative within 10 days of birth | 1 | Hazard Ratio (Random, 95% CI) | 2.35 [1.07, 5.17] |
6.1. Analysis.

Comparison 6 PEP‐South Africa (extended ZDV to infants for 6 weeks vs. sdNVP), Outcome 1 HIV Transmission at 12 weeks among those whose HIV diagnostic testing was negative within 10 days of birth.
Comparison 7. Mma Bana‐Botswana (extended ZDV/3TC/LPV/r vs. extended ZDV/3TC/ABC to mothers through 6 months postpartum).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV transmission at 6 months among those whose HIV diagnostic testing was negative within 96 hours of birth | 1 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.30] |
| 2 Infant Mortality at 6 months | 1 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.37, 2.95] |
| 3 HIV Transmission or Death at 6 months among those whose HIV diagnostic testing was negative within 96 hours of birth | 1 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.30, 2.14] |
| 4 Infants with Grade 3/4 Severe Adverse Events | 1 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.93, 1.37] |
| 5 Maternal Mortality at 6 months | 1 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.44] |
| 6 Mothers with any Grade 3/4 Severe Adverse Event | 1 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.21] |
| 7 Mothers with Severe Adverse Events Requiring Treatment Modification | 1 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.30, 2.61] |
7.1. Analysis.

Comparison 7 Mma Bana‐Botswana (extended ZDV/3TC/LPV/r vs. extended ZDV/3TC/ABC to mothers through 6 months postpartum), Outcome 1 HIV transmission at 6 months among those whose HIV diagnostic testing was negative within 96 hours of birth.
7.2. Analysis.

Comparison 7 Mma Bana‐Botswana (extended ZDV/3TC/LPV/r vs. extended ZDV/3TC/ABC to mothers through 6 months postpartum), Outcome 2 Infant Mortality at 6 months.
7.3. Analysis.

Comparison 7 Mma Bana‐Botswana (extended ZDV/3TC/LPV/r vs. extended ZDV/3TC/ABC to mothers through 6 months postpartum), Outcome 3 HIV Transmission or Death at 6 months among those whose HIV diagnostic testing was negative within 96 hours of birth.
7.4. Analysis.
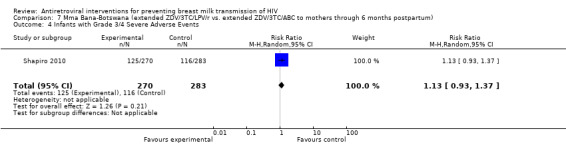
Comparison 7 Mma Bana‐Botswana (extended ZDV/3TC/LPV/r vs. extended ZDV/3TC/ABC to mothers through 6 months postpartum), Outcome 4 Infants with Grade 3/4 Severe Adverse Events.
7.5. Analysis.

Comparison 7 Mma Bana‐Botswana (extended ZDV/3TC/LPV/r vs. extended ZDV/3TC/ABC to mothers through 6 months postpartum), Outcome 5 Maternal Mortality at 6 months.
7.6. Analysis.

Comparison 7 Mma Bana‐Botswana (extended ZDV/3TC/LPV/r vs. extended ZDV/3TC/ABC to mothers through 6 months postpartum), Outcome 6 Mothers with any Grade 3/4 Severe Adverse Event.
7.7. Analysis.

Comparison 7 Mma Bana‐Botswana (extended ZDV/3TC/LPV/r vs. extended ZDV/3TC/ABC to mothers through 6 months postpartum), Outcome 7 Mothers with Severe Adverse Events Requiring Treatment Modification.
Comparison 8. Kesho Bora‐Burkina Faso, Kenya, South Africa (extended ZDV/3TC/LPV/r to mothers through 6.5 months postpartum vs. short course ZDV with intrapartum ZDV/3TC/sdNVP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV transmission at 6 months among those whose diagnostic testing was negative at 6 weeks after birth | 1 | Hazard Ratio (Random, 95% CI) | 0.36 [0.13, 1.02] | |
| 2 HIV transmission at 12 months among those whose diagnostic testing was negative at 6 weeks after birth | 1 | Hazard Ratio (Random, 95% CI) | 0.40 [0.16, 0.98] | |
| 3 Infant Mortality at 6 months | 1 | Hazard Ratio (Random, 95% CI) | 0.88 [0.36, 2.13] | |
| 4 Infant Mortality at 12 months | 1 | Hazard Ratio (Random, 95% CI) | 0.94 [0.48, 1.84] | |
| 5 HIV transmission or Death at 6 months among those whose HIV diagnostic testing was negative at 6 weeks after birth | 1 | Hazard Ratio (Random, 95% CI) | 0.61 [0.30, 1.24] | |
| 6 HIV transmission or Death at 12 months among those whose HIV diagnostic testing was negative at 6 weeks after birth | 1 | Hazard Ratio (Random, 95% CI) | 0.52 [0.29, 0.95] | |
| 7 Maternal Grade 3/4 Severe Adverse Events | 1 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.83, 1.70] |
8.1. Analysis.

Comparison 8 Kesho Bora‐Burkina Faso, Kenya, South Africa (extended ZDV/3TC/LPV/r to mothers through 6.5 months postpartum vs. short course ZDV with intrapartum ZDV/3TC/sdNVP), Outcome 1 HIV transmission at 6 months among those whose diagnostic testing was negative at 6 weeks after birth.
8.2. Analysis.

Comparison 8 Kesho Bora‐Burkina Faso, Kenya, South Africa (extended ZDV/3TC/LPV/r to mothers through 6.5 months postpartum vs. short course ZDV with intrapartum ZDV/3TC/sdNVP), Outcome 2 HIV transmission at 12 months among those whose diagnostic testing was negative at 6 weeks after birth.
8.3. Analysis.

Comparison 8 Kesho Bora‐Burkina Faso, Kenya, South Africa (extended ZDV/3TC/LPV/r to mothers through 6.5 months postpartum vs. short course ZDV with intrapartum ZDV/3TC/sdNVP), Outcome 3 Infant Mortality at 6 months.
8.4. Analysis.

Comparison 8 Kesho Bora‐Burkina Faso, Kenya, South Africa (extended ZDV/3TC/LPV/r to mothers through 6.5 months postpartum vs. short course ZDV with intrapartum ZDV/3TC/sdNVP), Outcome 4 Infant Mortality at 12 months.
8.5. Analysis.

Comparison 8 Kesho Bora‐Burkina Faso, Kenya, South Africa (extended ZDV/3TC/LPV/r to mothers through 6.5 months postpartum vs. short course ZDV with intrapartum ZDV/3TC/sdNVP), Outcome 5 HIV transmission or Death at 6 months among those whose HIV diagnostic testing was negative at 6 weeks after birth.
8.6. Analysis.

Comparison 8 Kesho Bora‐Burkina Faso, Kenya, South Africa (extended ZDV/3TC/LPV/r to mothers through 6.5 months postpartum vs. short course ZDV with intrapartum ZDV/3TC/sdNVP), Outcome 6 HIV transmission or Death at 12 months among those whose HIV diagnostic testing was negative at 6 weeks after birth.
8.7. Analysis.

Comparison 8 Kesho Bora‐Burkina Faso, Kenya, South Africa (extended ZDV/3TC/LPV/r to mothers through 6.5 months postpartum vs. short course ZDV with intrapartum ZDV/3TC/sdNVP), Outcome 7 Maternal Grade 3/4 Severe Adverse Events.
Comparison 9. BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to 1 week of antiretroviral prophylaxis for transmission during breastfeeding).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 1 | 1435 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.89] |
| 2 Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 1 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.31, 1.45] |
| 3 HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks | 1 | 1435 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.38, 0.96] |
| 4 Infants with Severe Adverse Events | 1 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.86, 1.45] |
| 5 Mothers with Severe Adverse Events | 1 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.20, 4.00] |
9.1. Analysis.

Comparison 9 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to 1 week of antiretroviral prophylaxis for transmission during breastfeeding), Outcome 1 HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth.
9.2. Analysis.

Comparison 9 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to 1 week of antiretroviral prophylaxis for transmission during breastfeeding), Outcome 2 Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth.
9.3. Analysis.

Comparison 9 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to 1 week of antiretroviral prophylaxis for transmission during breastfeeding), Outcome 3 HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks.
9.4. Analysis.

Comparison 9 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to 1 week of antiretroviral prophylaxis for transmission during breastfeeding), Outcome 4 Infants with Severe Adverse Events.
9.5. Analysis.

Comparison 9 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to 1 week of antiretroviral prophylaxis for transmission during breastfeeding), Outcome 5 Mothers with Severe Adverse Events.
Comparison 10. BAN Lilongwe (an extended NVP regimen administered to infants for 28).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 1 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.15, 0.56] |
| 2 Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 1 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.28, 1.35] |
| 3 HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks | 1 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.22, 0.65] |
| 4 Infants with Severe Adverse Events | 1 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.97, 1.61] |
10.1. Analysis.

Comparison 10 BAN Lilongwe (an extended NVP regimen administered to infants for 28), Outcome 1 HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth.
10.2. Analysis.

Comparison 10 BAN Lilongwe (an extended NVP regimen administered to infants for 28), Outcome 2 Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth.
10.3. Analysis.

Comparison 10 BAN Lilongwe (an extended NVP regimen administered to infants for 28), Outcome 3 HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks.
10.4. Analysis.

Comparison 10 BAN Lilongwe (an extended NVP regimen administered to infants for 28), Outcome 4 Infants with Severe Adverse Events.
Comparison 11. BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to an extended NVP regimen administered to infants for 28 weeks for preventing transmission during breastfeeding).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 1 | 1618 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.88, 3.59] |
| 2 Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth | 1 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.49, 2.47] |
| 3 HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks | 1 | 1618 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.91, 2.82] |
| 4 Infants with Severe Adverse Events | 1 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.71, 1.12] |
| 5 Mothers with Severe Adverse Events | 1 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [1.53, 5.11] |
11.1. Analysis.

Comparison 11 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to an extended NVP regimen administered to infants for 28 weeks for preventing transmission during breastfeeding), Outcome 1 HIV transmission at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth.
11.2. Analysis.

Comparison 11 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to an extended NVP regimen administered to infants for 28 weeks for preventing transmission during breastfeeding), Outcome 2 Infant mortality at 28 weeks among those whose diagnostic testing was negative at 2 weeks after birth.
11.3. Analysis.

Comparison 11 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to an extended NVP regimen administered to infants for 28 weeks for preventing transmission during breastfeeding), Outcome 3 HIV transmission or death at 28 weeks among those whose diagnostic test was negative at 2 weeks.
11.4. Analysis.

Comparison 11 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to an extended NVP regimen administered to infants for 28 weeks for preventing transmission during breastfeeding), Outcome 4 Infants with Severe Adverse Events.
11.5. Analysis.

Comparison 11 BAN Lilongwe (an extended antiretroviral regimen administered to mothers for 28 weeks compared to an extended NVP regimen administered to infants for 28 weeks for preventing transmission during breastfeeding), Outcome 5 Mothers with Severe Adverse Events.
Comparison 12. HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission).
12.1. Analysis.

Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 1 HIV transmission at 12 months among infants whose HIV diagnostic testing was negative within 6 weeks of birth and whose mothers were not on HAART.
12.2. Analysis.

Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 2 HIV transmission at 12 months among all infants whose HIV diagnostic testing was negative within 6 weeks of birth.
12.3. Analysis.
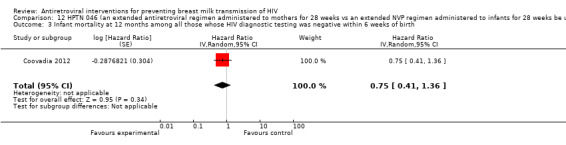
Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 3 Infant mortality at 12 months among all those whose HIV diagnostic testing was negative within 6 weeks of birth.
12.4. Analysis.

Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 4 HIV transmission or death at 12 months among all infants whose HIV diagnostic testing was negative within 6 weeks of birth.
12.5. Analysis.

Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 5 Infants with Severe Adverse Events.
12.6. Analysis.

Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 6 HIV transmission at 6 months among infants whose HIV diagnostic testing was negative within 6 weeks of birth and whose mothers were not on HAART.
12.7. Analysis.

Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 7 HIV transmission at 6 months among all infants whose HIV diagnostic testing was negative within 6 weeks of birth.
12.8. Analysis.

Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 8 Infant mortality at 6 months among all those whose HIV diagnostic testing was negative within 6 weeks of birth.
12.9. Analysis.

Comparison 12 HPTN 046 (an extended antiretroviral regimen administered to mothers for 28 weeks vs an extended NVP regimen administered to infants for 28 weeks be used for preventing transmission), Outcome 9 HIV transmission or death at 6 months among all infants whose HIV diagnostic testing was negative within 6 weeks of birth.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chasela 2010.
| Methods | Randomised Controlled Trial | |
| Participants | HIV‐infected, breastfeeding mothers and their infants were recruited at antenatal clinics in Lilongwe, Malawi for the BAN study of extended mother or infant ARV prophylaxis to reduce MTCT of HIV. Primary eligibility criteria included maternal age of at least 14 years, a gestational age of 30 weeks or less, a CD4+ lymphocyte count of at least 250 cells/mm3, a hemoglobin level of at least 7 g/dL, alanine aminotransferase level of no more than 2.5 times the upper limit of the normal range, and no serious pregnancy complication. Women who had a serious infection or who reported previous ARV use were excluded. After delivery, secondary eligibility criteria were applied. Mother infant‐pairs were randomized if the infant birth weight was over 2000 g, no infant or maternal condition would prevent safe use of the drug, mothers agreed to a 7‐day mother and infant ARV regimen, and the pairs enrolled within 36 hours of delivery. 3109 mother‐infant pairs met the initial eligibility criteria. 2369 of those mother‐infant pairs met the secondary criteria and underwent randomization. |
|
| Interventions | HIV‐infected, breastfeeding mothers and their infants were recruited at antenatal clinics in Lilongwe, Malawi for the BAN study of extended mother or infant ARV prophylaxis to reduce MTCT of HIV. Primary eligibility criteria included maternal age of at least 14 years, a gestational age of 30 weeks or less, a CD4+ lymphocyte count of at least 250 cells/mm3, a hemoglobin level of at least 7 g/dL, alanine aminotransferase level of no more than 2.5 times the upper limit of the normal range, and no serious pregnancy complication. Women who had a serious infection or who reported previous ARV use were excluded. After delivery, secondary eligibility criteria were applied. Mother infant‐pairs were randomized if the infant birth weight was over 2000 g, no infant or maternal condition would prevent safe use of the drug, mothers agreed to a 7‐day mother and infant ARV regimen, and the pairs enrolled within 36 hours of delivery. 3109 mother‐infant pairs met the initial eligibility criteria. 2369 of those mother‐infant pairs met the secondary criteria and underwent randomization. |
|
| Outcomes | The primary outcome was infant HIV infection at 28 weeks among infants who where uninfected at two weeks and among all infants who underwent randomization. The secondary outcome was HIV free survival by 28 weeks among infants who were uninfected at two weeks and among all infants who underwent randomization. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block method |
| Allocation concealment (selection bias) | Unclear risk | From protocol: Treatment assignment will occur after all possible eligibility criteria have been met up through the time immediately following delivery. By not revealing each mother‐infant pair’s assignment until after delivery, treatment assignment will occur independently of any other considerations. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 10% loss to follow up, with a small number of events |
| Selective reporting (reporting bias) | Low risk | No problems detected |
| Other bias | Low risk | No problems detected |
Coovadia 2012.
| Methods | Randomised controlled trial | |
| Participants | HIV‐infected women were recruited from antenatal clinics in South Africa, Tanzania, Uganda, and Zimbabwe. "Primary eligibility criteria included maternal age (≥18 years), infant HIV DNA PCR negative from a specimen obtained within 7 days of birth, and birth weight of 2000 grams or more. We excluded women and infants if either had a serious medical disorder that would interfere with study participation. Women receiving antiretroviral drugs for HIV treatment or for PMTCT were eligible." "Infants who developed HIV infections were taken off study drug and referred for additional care and treatment." Infants were stratified by maternal ARV status when randomized. "Infants had to be HIV DNA PCR negative on a specimen obtained within 21 days before randomization and still breastfeeding, with the mother’s intent to continue breastfeeding." |
|
| Interventions | All enrolled infants received once‐daily open‐label NVP (10 mg/mL oral suspension) for the first 6 weeks of life. "Randomly allocated infants started masked study drug and continued a once‐daily dosing regimen until six months of age or until cessation of breastfeeding, whichever came first. The NVP dose was increased with age, ranging from 20 mg once‐daily after 6–8 weeks of age to 28 mg once‐daily after 5–6 months of age. Women were counseled to exclusively breastfeed for 6 months." "Infant study visits were undertaken within 7 days postpartum, at 2, 5, 6, and 8 weeks, and at 3, 6, 9, 12, and 18 months. |
|
| Outcomes | "The primary efficacy endpoint was HIV infection at age 6 months in infants who were uninfected at age 6 weeks in each study group. Primary safety endpoints were frequency and severity of adverse reactions in randomly allocated infants until age 6 months in each group for all infants who received at least one dose of study intervention. Secondary endpoints included HIV‐free survival, relative rates of HIV infection, and infant survival rates (mortality irrespective of HIV infection) in the two study groups." |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated permuted block method |
| Allocation concealment (selection bias) | Low risk | "An independent contractor in the USA provided identical, sealed, individual study drug kits, which were prepared and labelled centrally according to the random allocation assignment generated by the HPTN Statistical and Data Management Centre (Seattle, WA, USA)." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study staff and participants were masked to the study drug (nevirapine or placebo) assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No problems apparent |
| Selective reporting (reporting bias) | Low risk | No problems apparent |
| Other bias | Low risk | No problems apparent |
Gray 2005.
| Methods | Randomized, two‐arm, open‐label trial of NVP compared to ZDV ‐ administered to infants born to HIV‐infected women who did not receive antepartum or intrapartum ARV prophylaxis. | |
| Participants | Women delivering in three hospitals in South Africa: [Chris Hani Baragwanath Hospital (Soweto), Coronation Hospital (Johannesburg), and Mowbray Hospital (Cape Town)], who did not have prior knowledge of their HIV infection status were offered postpartum voluntary counseling and rapid on‐site testing within 24 hours of delivery. Eligible women testing HIV positive were offered enrollment. Informed consent and randomization occurred within 24 hours of delivery. Eligibility Criteria: Eligible women testing positive for HIV were offered enrollment. Exclusion Criteria: Exclusion criteria for the infants of these women: preterm weighing < 1200 grams; requiring ventilation; unable to take oral medication or with congenital anomalies. 12,000 women delivered between October 2000 and September 2002 (women without HIV results). Of these 5634 (49.5%) accepted counseling and testing. 1530 (17.2%) were found to be HIV‐infected and 1051 infants (68.7%) were randomized into the study. (533 in ZDV arm and 518 in NVP arm). Mother‐infant pairs were followed up for 6 months or until 1 month after cessation of breastfeeding. Characteristics of women at baseline: At enrollment, maternal median CD4+ count was 467 cells/mm³; median plasma viral load was 21,800 copies/mL. Median age was 25 years. |
|
| Interventions | The objective of the trial was to evaluate the efficacy of sdNVP to the infant versus 6 weeks of ZDV to the infant in terms of infant HIV infection status at 6 weeks and 12 weeks among infants not infected at birth (<10 days after birth). Two groups: sdNVP to the infant, and six weeks of ZDV to the infant. (Ineligible infants or infants of mothers unwilling to participate were offered off‐study sdNVP.) Arm 1: sdNVP (2 mg/kg) within 24 hours of delivery. Arm 2: ZDV (4 mg/kg every 12 hours for 6 weeks). |
|
| Outcomes | HIV infection status at 6 weeks and 12 weeks among breastfeeding‐exposed, HIV‐uninfected infants within 10 days of birth. | |
| Notes | The majority of women did NOT breastfeed their infants. At baseline, exclusive formula feeding was reported in 82.06% (NVP arm) and 86.17% (ZDV arm). Exclusive breastfeeding was reported in 16.08% (NVP arm) versus 13.44% (ZDV arm). Mixed feeding was reported in 1.86% (NVP arm) and 0.40% (ZDV arm). Denominators included above incorporate breastfeeding exposure reported at any study visits (not only baseline visit). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was by computer‐generated random allocations. Enrolled infants were sequentially assigned the next study number. |
| Allocation concealment (selection bias) | Low risk | Allocation to the study arm was provided to study nurses in sequentially number non‐transparent sealed envelopes. The envelopes were only opened after informed consent was obtained. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No problems apparent |
| Selective reporting (reporting bias) | Low risk | No problems apparent |
| Other bias | Low risk | No problems apparent |
Kesho Bora 2011.
| Methods | Randomized Controlled Trial | |
| Participants | HIV‐infected pregnant women in five sites in Burkina Faso, Kenya and South Africa were enrolled in the Kesho Bora clinical trial of triple‐ARV prophylaxis during pregnancy and breastfeeding compared to short‐course ARV prophylaxis to prevent MTCT of HIV. Enrollment began in June 2005 and continued until August 2008. Eligibility criteria for RCT arms: HIV‐infected pregnant women with 200≤CD4≤500 cells/mm3 and WHO HIV Stage <4. 1140 HIV‐infected pregnant women were enrolled in the Kesho Bora Study, of whom 855 had CD4+ counts between 200‐500 cells/mm3 and were enrolled in the randomized trial. Of these, 824 were randomized to the two trial arms with a total of 805 live births. Of the live births, 402 were in the triple‐ARV arm and 403 were in the short‐course ARV arm. Characteristics of women at baseline: At enrollment, maternal median age was 27.4 years in the triple‐ARV arm and 27.4 years in the short‐course arm. Median CD4+ count (cells/mm³) was 336 in the Triple‐ARV arm and 339 cells/mm³ in the Short ARV arm. Proportion of women with at least primary education was 85.7% in the Triple‐ARV arm and 84.4% in the Short arm. |
|
| Interventions | The objective of the trial was to compare efficacy and safety of Triple‐ARV and Short MTCT prophylaxis. Triple‐ARV arm: ZDV, 3TC, and LPV/r (administered to mother from 28‐36 weeks pregnancy until 6 months postpartum) Short arm: ZDV (administered to mother from 28‐36 weeks pregnancy until labor) plus sdNVP at labor onset. Beginning in September 2007, ZDV/3TC was also administered during labor and through 1 week after delivery. All infants received sdNVP. Beginning in September 2007, all infants also received 1 week of ZDV. The objective of the trial was to compare efficacy and safety of triple‐ARV and short‐course MTCT prophylaxis. Triple Arm: zidovudine, lamivudine, and lopinavir‐ritonavir (administered to mother from 28‐36 weeks pregnancy until 6 months postpartum) Short Arm: Zidovudine (administered to mother from 28‐36 weeks pregnancy until labour) plus single‐dose nevirapine at labour onset. Beginning in September 2007, zidovudine/lamivudine was also administered during labour and through 1 week after delivery. All infants received single dose nevirapine. Beginning in September 2007, all infants also received 1 week zidovudine. |
|
| Outcomes | The primary outcomes of the study were HIV‐free infant survival at 6 weeks and 12 months; HIV‐free survival at 12 months in infants who were ever breastfed; severe adverse events in mothers and infants; and AIDS‐free survival of mothers at 18 months postpartum. Analysis was by intention to treat. | |
| Notes | Not all women breastfed in the study. All women choosing to breastfeed were counseled to breastfeed exclusively for 5 1/2 months, followed by weaning over a 2 week period. Results presented in GRADE refer to the subset of infants who were ever breastfed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequence generated by a computer" |
| Allocation concealment (selection bias) | Low risk | "Assignments allocated in sequentially numbered, opaque, sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and providers were not blinded to treatment, but outcomes were assessed is a blinded manner and lab‐based data were assessed by masked investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No problems apparent |
| Selective reporting (reporting bias) | Low risk | No problems apparent |
| Other bias | Low risk | No problems apparent |
Kumwenda 2008.
| Methods | Randomized, controlled, open‐label, phase 3 clinical trial. | |
| Participants | Pregnant women presenting for antenatal or delivery services (at Queen Elizabeth Central Hospital or at one of five other health centers in Blantyre, Malawi) were offered HIV counseling and testing. All eligible women provided written informed consent at enrollment. Infants were randomly assigned at birth to receive one of three regimens. Eligibility Criteria: HIV infection; at least 18 years of age (women < 18 years of age could be enrolled if they consented and a guardian gave permission); pregnant or had given birth within the previous 24 hours at one of the study clinics; resident of the study area; willing to return for postnatal follow‐up visits for up to 2 years; intended to breastfeed. Exclusion Criteria: The study excluded infants with life threatening conditions requiring immediate care. 46,186 women screened for HIV infection. A total of 3216 women and 3276 infants enrolled. The data reported refer to mother‐infant pairs enrolled from April 20, 2004 to August 7, 2007. Infants were randomized at birth. 1088 infants were assigned to the control group, 1099 infants were assigned to the extended NVP group, and 1089 infants were assigned to the extended dual prophylaxis group. Of these, 1003, 1016, and 997, respectively, were included in the primary analysis. Of these, 788, 800, and 801 reached the HIV infection end point or had no HIV infection and had completed or were participating in active follow‐up at the end of the study. (3016 infants were included in the primary analysis.) Characteristics of women at baseline: Median age = 26 years; 2/3 with ≤ 8th grade education; median hemoglobin = 11 g/dl, ANC > 8000, CD4+ (cells/mm³) median = ˜4000. |
|
| Interventions | The aim of the trial was to determine whether extended prophylaxis of infants with NVP or with NVP plus ZDV until the age of 14 weeks would decrease the rate of HIV infection, as compared with sdNVP combined with 1 week of ZDV. Arm 1: (Control regimen) Infants received single oral dose of NVP (2mg/kg of body weight) plus oral ZDV (4mg/kg) for 1 week. Mothers received intrapartum sdNVP (except late presenters whose HIV infection was not identified until after they gave birth). Arm 2: Control regimen + extended daily dose of NVP for 14 weeks. Extended NVP prophylaxis included oral dose of NVP (2mg/kg) once daily during week 2, then 4mg/kg once daily during weeks 3‐14. Arm 3: Control regimen + extended daily dose of NVP and ZDV for 14 weeks. Extended NVP prophylaxis as described above. Extended ZDV prophylaxis included oral ZDV (4mg/kg) twice daily during weeks 2 through 5, 4mg/kg three times daily during weeks 6 through 8, and 6mg/kg three times daily during weeks 9‐14. |
|
| Outcomes | Primary endpoint was rate of HIV infection at 9 months among live born infants negative for HIV infection at birth (within first 48 hours). Other primary endpoints were HIV‐free survival during follow‐up and safety. HIV transmission: Total numbers of infants with positive results on HIV DNA PCR at 9 months were 98 in the control group, 51 in the extended‐nevirapine group, and 61 in the extended dual‐prophylaxis group. After excluding infants infected at birth (6.5% in control group, 7.1% in both extended prophylaxis groups), the rate of HIV infection among 9 month old infants was examined: 10.6% in control group, 5.2% in extended nevirapine group (p < 0.001 for comparison with control group), and 6.4% in extended dual prophylaxis group (p = 0.002 for comparison with control group). Estimated protective efficacy of the extended‐nevirapine regimen was 51% (CI: 20‐66) at 9 months. Estimated protective efficacy of the extended‐dual prophylaxis regimen was 40% (CI: 16‐57) at 9 months. There were no significant differences between the two extended‐prophylaxis groups at any time point. Hazard ratio for extended‐nevirapine vs. control: Unadjusted 0.59 (95%CI: 0.44‐0.80, p<0.001), Adjusted 0.56 (95%CI: 0.41‐0.76, p<0.001) Hazard ratio for extended‐dual prophylaxis vs. control: Unadjusted 0.66 (95%CI: 0.49‐0.88, p=0.005), Adjusted 0.65 (95%CI: 0.48‐0.88, p=0.006) Primary endpoint was rate of HIV infection at 9 months among live born infants negative for HIV infection at birth (within first 48 hours). Other primary endpoints were HIV‐free survival during follow‐up and safety. HIV transmission: Total numbers of infants with positive results on HIV DNA PCR at 9 months Mortality: The number of infants that had died by the age of 9 months. HIV‐free survival Serious adverse events possibly related to study drug Serious adverse events Mortality: The number of infants that had died by the age of 9 months included 71 in the control group, 55 in the extended‐nevirapine group, and 51 in the extended dual‐prophylaxis group. At 9 months, mortality was 8.9% (CI: 7.1‐11.1) in the control group, 6.8% (CI: 5.2‐8.7) in the extended‐NVP group, and 6.4% (CI: 4.8‐8.2) in the extended dual prophylaxis group. HIV‐free survival: No statistically significant differences in mortality according to randomization arm. However, HIV‐free survival was better in both extended prophylaxis arms through the age of 9 months, and in the extended nevirapine arm through the age of 15 months. Hazard ratio for extended‐nevirapine vs. control: Unadjusted 0.72 (95%CI: 0.58‐0.90, p=0.004), Adjusted 0.69 (95%CI: 0.55‐0.87, p=0.001). Hazard ratio for extended‐dual prophylaxis vs. control: Unadjusted 0.76 (95%CI: 0.61‐0.95, p=0.02), Adjusted 0.72 (95%CI: 0.57‐0.90, p=0.004) Safety: There were no statistically significant differences among the three study groups for any adverse event. There were significantly more infants with serious adverse events possibly related to study drug in the extended dual prophylaxis group than in either the extended nevirapine group or the control group (p=0.02 for all comparisons). (The most common serious adverse event in the extended dual‐prophylaxis group was neutropenia.) The numbers of events that were deemed to be probably related to a study drug were low and did not differ among the study groups (p=0.42 for all comparisons). |
|
| Notes | The frequency of reported breastfeeding was high up to the age of 6 months, ranging from nearly all infants at 1 week to approximately 90% at 6 months in all three study groups. Between the ages of 6 and 9 months, there was a substantial reduction in the frequency of breastfeeding , with rates at 9 months of 32.0% in control group, 26.9% in extended‐NVP group, and 29.2% in extended dual‐prophylaxis group (p=0.16 for all comparisons). By the age of 15 months, the rate of breastfeeding had declined to 19.4% in the control group, 14.4% in the extended NVP, and 18.1% in the extended dual group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization procedures employed permutated block algorithms of size 9 and 12 stratified by study clinic and a computer generated randomization list assigned infants to the appropriate treatment arms using a 1:1:1 allocation ratio." (from Kumwenda 2008 Supplement) |
| Allocation concealment (selection bias) | Low risk | "Randomization numbers were placed in sequentially labelled and sealed envelopes." (from Kumwenda 2008 Supplement) |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was an open‐label trial and was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No problems apparent |
| Selective reporting (reporting bias) | Low risk | No problems apparent |
| Other bias | Low risk | No problems apparent |
Shapiro 2010.
| Methods | Randomized controlled trial | |
| Participants | HIV‐infected pregnant women in Botswana from four clinical sites who intended to breastfeed their infant. 730 women were enrolled: 560 randomized, 170 observational. | |
| Interventions | HIV‐infected pregnant women with CD4 ≥200 cells/mm3 were randomized to lopinavir‐ritonavir (LPV/r)/lamivudine (3TC)/zidovudine (ZDV) (Arm 1) vs. abacavir (ABC)/3TC/ZDV (Arm 2) from 26‐34 weeks gestation. Arm 1: ZDV/3TC/LPV/r administered to mothers. Lopinavir/ritonavir (400 mg/100 mg) and zidovudine/lamivudine (300 mg/150 mg) taken orally twice daily from 26 to 34 weeks gestation through six months postpartum. Arm 2: ZDV/3TC/ABC administered to mothers. abacavir 300mg, lamivudine 150mg, zidovudine 300mg (co‐formulated as Trizivir®) one tablet taken twice daily. HIV‐infected pregnant women with CD4+ ≥200 cells/mm3 were randomized to a three drug regimen consisting of ZDV, 3TC, and LPV/r (Arm 1) vs. ZDV, 3TC, and ABC/3TC/ZDV (Arm 2) from 26‐34 weeks gestation. Arm 1: LPV/r (400 mg/100 mg) and ZDV/3TC (300 mg/150 mg) taken orally twice daily from 26 to 34 weeks gestation through six months postpartum. Arm 2: ABC 300mg, 3TC 150mg, ZDV 300mg (co‐formulated as Trizivir®) one tablet taken twice daily. An observational group composed of women with CD4+ < 200 cells/mm3 received ZDV, 3TC, and NVP from 18‐34 weeks gestation. All regimens were continued through planned weaning by six months postpartum. from 18‐34 weeks gestation. All regimens were continued through planned weaning by six months postpartum. |
|
| Outcomes | HIV transmission at six months among those uninfected at birth (PCR negative within 96 hours) Infant mortality at six months among live born infants Infant HIV‐free survival at six months among those uninfected at birth Infant severe adverse events. Maternal mortality at 6 months. Maternal severe adverse events. Severe adverse events requiring treatment modification |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the random assignments were computer generated using permuted blocks procedure stratified by site." |
| Allocation concealment (selection bias) | Unclear risk | Central randomization (see protocol) |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The study was not blinded. Instead, it is more reflective of the actual reality (including pill burden) when using each regimen. Additional placebo formulations would have added to the pill burden and made adherence more difficult to assess in a real‐world setting." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No problems apparent |
| Selective reporting (reporting bias) | Low risk | No problems apparent |
| Other bias | Low risk | No problems apparent |
SWEN 2008.
| Methods | Combined analysis of data from three separate (but coordinated) randomized clinical trials in Uganda, India, and Ethiopia. | |
| Participants | Pregnant women who presented to antenatal and delivery facilities in three cities (three enrollment sites in Addis Ababa, Ethiopia; one enrollment site each in Pune, India and Kampala, Uganda), who were identified as HIV‐infected, were offered the local standard of care for prevention of MTCT of HIV and provided infant feeding counseling (consistent with WHO/UNICEF guidelines). Women who indicated an intention to breastfeed their infants and who provided informed consent were eligible for study enrollment. Enrollment occurred from February 2001 ‐ October 2006 in Ethiopia, August 2002‐March 2007 in India, and July 2004 ‐ January 2007 in Uganda. Enrollment was antepartum in Ethiopia and Uganda, and both antepartum and postpartum in India. Eligibility Criteria: · Ethiopia: ≥ 18 years of age or consent of guardian, ≥ 32 weeks' gestation; hemoglobin > 75 g/L, creatinine < 1.5 mg/dL, alanine aminotransferase concentration < 5 X the upper limit of normal. · · India: same as for Ethiopia, except ≥18 years of age only, ≥ 32 weeks' gestation and within 24 hours of delivery for the postpartum enrollments. · Uganda was the same as for Ethiopia, except ≥ 18 years of age only, 32‐36 weeks gestation. Exclusion Criteria: · Ethiopia: ART in addition to sdNVP for prevention of transmission, fetal or obstetrical complications, hypersensitivity to benzodiazepines. · · India: similar to those in Ethiopia, except mothers on HAART or receiving ART in addition to sdNVP were not excluded. · Uganda: similar to those in Ethiopia. Infant study drug administration criteria: · Ethiopia: Weight ≥ 2000 grams, alanine aminotransferase ≤ 5 X upper limit of normal, no acute illness, no life threatening illness, not known to be HIV‐infected, ability to tolerate oral medications. · · India: Same as Ethiopia, except hemoglobin > 75 g/L, alanine aminotransferase < 5 times upper limit of normal, serum creatinine < 1.0 mg/dL. · Uganda: Same as Ethiopia. Of 6075 HIV‐infected women, 2067 mothers in the randomized trial delivered, and there were 2037 randomized, live born infants (including sets of twins) and 2024 randomized infants with at least one specimen tested before 6 months. Of these 2024 infants, 1047 were assigned to sdNVP (986 in the primary analysis data set and 928 with endpoint status available at 6 months) and 977 assigned to extended dose NVP (901 in the primary analysis data set and 831 with endpoint status available at 6 months). Characteristics of women at baseline: The median age of the enrolled women was 25 years, with the median CD4+ count at enrollment of 394‐397 cells/mm³ and the median viral load at enrollment of 16,457‐17,400 copies/mL. | |
| Interventions | The trials were designed to assess the efficacy of daily NVP given to breastfed infants through 6 weeks of age for prevention of HIV transmission through breastfeeding. HIV‐infected women were randomly assigned to receive either sdNVP or 6 week extended dose NVP. Arm 1: (control) sdNVP (NVP 200 mg to women in labor and NVP 2 mg/kg to newborns after birth) Arm 2: 6 week extended dose NVP (NVP 200 mg to women in labor and NVP 2 mg/kg to neonates after birth plus NVP 5 mg daily from days 8‐42 for the infant). The extended dose NVP regimen was initiated at day 8. |
|
| Outcomes | The primary endpoint was HIV infection at six months of age in infants who were HIV PCR negative at birth (within seven days of birth). Six week data also given. HIV transmission at 6 weeks and 6 months. Mortality at 6 weeks and 6 months. HIV‐free survival among infants at 6 weeks and 6 months. |
|
| Notes | Moorthy manuscript presents NVP resistance data in cohort of Indian HIV‐infected infants from SWEN. Church manuscript presents NVP resistance data in cohort of Ugandan HIV‐infected infants from SWEN. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Ethiopia: block randomization (block sizes four and six) assigned by central data coordinating centre. Initially the randomization was antepartum, but later it was postpartum. India: same as Ethiopia, except all infants randomized postpartum and block size of six used. Uganda: Same as Ethiopia, except all infants were randomized antepartum and block size of eight and 16 used until later in the study, when block sizes of 10 and 20 were used. |
| Allocation concealment (selection bias) | Low risk | Randomization list provided to study pharmacists only. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was not blinded, however some procedures were implemented to have some degree of masking of study staff and caregivers. No one except study pharmacists had access to randomization assignments. Opaque dropper bottles and syringes were used for administration of study drugs. Infants in both study arms received 1 mL multivitamins from day 8 to day 42. Study products were only handled by study pharmacists before giving to the caregivers. Infants received two separate syringes or dropper bottles for administration of nevirapine and multivitamins (for those randomized to extended nevirapine dosing) and multivitamins in both dispensing tools (for those randomized to single‐dose nevirapine). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses were by modified intention to treat ‐ thus excluding infants with missing specimens, those with indeterminate HIV infection status, and those with confirmed HIV infection at birth. Therefore, there were some missing data: some infants had no specimens before 6 months of age for HIV diagnostic testing. Of those with specimens, some had missing birth specimens. "Since many infant deaths occurred at home or outside of the study clinics, information about the cause of these deaths was limited to verbal autopsy interviews of mothers and other family members. Thus, HIV status at the time of death was not available for infants who could have become HIV positivi e between their last study visit and their death. 38 (72%) of the deaths occurred among infant for whom their last HIV screening test was negative by HIV PCR. However, the mean time from this last study visit until death was 13.8 (SD 17.7) days, decreasing the likelihood that we failed to identify HIV‐infected infants before their death. We assessed the distribution of mortality by sex, because of an imbalance between study groups at baseline, but there was no significant difference between males and females in terms of the distribution of overall mortality (data not shown). We found no differences between groups in other factors that might be associated with infant mortality, including maternal age, maternal CD4 cell count, maternal viral load (available in India and Uganda), maternal education level, parity, gravidity, and gestational age at enrollment (data not shown)." |
| Selective reporting (reporting bias) | Low risk | No problems apparent |
| Other bias | Low risk | No problems apparent |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Becquet 2009 | review article |
| Becquet 2011 | review article |
| Bland 2010 | no ARVs given |
| Bobat 1997 | breastfeeding only |
| Chavula 2012 | additional data for Chasela 2010 |
| Chen 2010 | no relevant outcomes |
| Cherisch 2005 | review article |
| Chiappini 2009 | no breastfeeding |
| Chimienti 2010 | review article |
| Chung 2008 | outcome is viral load |
| Coovadia 2007 | breastfeeding only |
| Coovadia 2008 | review article |
| Coutsoudis 1999 | breastfeeding and Vitamin A supplementation only |
| Coutsoudis 2000 | breastfeeding only |
| Coutsoudis 2001 | breastfeeding and Vitamin A supplementation only |
| Engebretsen 2010 | review article |
| Fao 2012 | Trial already included |
| Fawzi 2002 | breastfeeding and Vitamin A supplementation only |
| Fogel 2011 | additional data from Kumwenda 2008 |
| Fowler 2002 | review article |
| Giuliano 2007 | ARV to one month only, outcome is viral load |
| Gray 2006 | no breastfeeding component |
| Heidari 2011 | systematic review |
| Homsy 2010 | no comparison group |
| Horvath 2009 | review article |
| Ikechebelu 2011 | prospective descriptive study |
| Iliff 2005 | breastfeeding only |
| John 2001 | review article |
| Kafulafula 2010 | no relevant outcomes |
| Kiarie 2004 | breastfeeding only |
| Kilewo 2009 | no comparison group |
| Kourtis 2007 | review article |
| Kuhn 2007 | breastfeeding only |
| Lambert 2003 | no breastfeeding component |
| Lehman 2009 | outcome is maternal resistance |
| Marazzi 2006 | all women offered formula milk. No report of proportion breastfeeding in article. |
| Marazzi 2009 | No comparison group |
| Marazzi 2011 | Retrospective |
| Mbori‐Ngacha 2001 | breastfeeding only |
| McIntyre 2006 | review article |
| McIntyre 2010 | review article |
| Meda 2011 | duplicate |
| Miotti 1999 | breastfeeding only |
| Mirochnick 2009 | PK |
| Mmiro 2009 | no outcomes of interest |
| Mofenson 2003 | review article |
| Mofenson 2008 | commentary only |
| Nduati 2000 | breastfeeding only |
| Nduati 2001 | breastfeeding only |
| Rabie 2001 | HIV infection status only assessed at 1 month, mothers encouraged to formula feed. Details on ZDV regimen unclear |
| Ramlal 2013 | Trial already included |
| Rousseau 2003 | outcome is breastmilk viral load |
| Rousseau 2004 | outcome is breastmilk viral load |
| Sartorius 2013 | Trial already included |
| Shapiro 2005 | outcome is breastmilk viral load |
| Shapiro 2006 | infants received ZDV for 1 month, however study evaluating maternal sdNVP. Outcome MTCT assessed at 1 month only. |
| Shetty 2003 | PK |
| Siegfried 2011 | review |
| SIMBA 2003 | comparison group |
| Taha 2009 | duplicate |
| Tess 1998 | breastfeeding only |
| Thior 2006 | quality of evidence not sufficient |
| Thomas 2011 | no comparison group |
| Volmink 2007 | review article |
| Wind‐Rotolo 2006 | review article |
Contributions of authors
JM, JR, TH, and AB screened articles and extracted data. HH conducted searches and designed the data abstraction form. AB drafted the review, and JM and JR contributed substantially to the manuscript. AA conducted initial analyses and provided ongoing analysis support.
Sources of support
Internal sources
Global Health Sciences, University of California, San Francisco, USA.
External sources
-
World Health Organization, Switzerland.
The World Health Organization (WHO) commissioned the first iteration of this review in 2009 to inform WHO’s 2010 PMTCT guidelines. That version of the review was never published. While we incorporate our work from the earlier review, we conducted the current updated and expanded review without external funding from any source.
Declarations of interest
The authors declare no conflicts of interest.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the U.S. Department of Health and Human Services or the National Institutes of Health.
New
References
References to studies included in this review
Chasela 2010 {published data only}
- Chasela C, Hudgens M, Jamieson D, Kayira D, Hosseinipour M, Kourtis AP, et al. for the BAN Study Group. Maternal or infant antiretroviral drugs to reduce HIV‐1 transmission. New England Journal of Medicine 2010;362(24):2271‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coovadia 2012 {published data only}
- Coovadia HM, Brown ER, Fowler MG, Chipato T, Moodley D, Manji K, et al. for the HPTN 046 protocol team. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV‐1 infection for prevention of postnatal HIV‐1 transmission (HPTN 046): a randomized, double‐blind, placebo‐controlled trial. Lancet January 2012;279:221‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2005 {published data only}
- Gray GE, Urban M, Chersich MF, Bolton C, Niekerk R, Violari A, et al. for the PEP Study Group. A randomized trial of two postexposure prophylaxis regimens to reduce mother‐to‐child HIV‐1 transmission in infants of untreated mothers. AIDS 2005;19(12):1289‐97. [DOI] [PubMed] [Google Scholar]
Kesho Bora 2011 {published data only}
- The Kesho Bora Study Group, Vincenzi I. Triple‐antiretrovira compared with zidovudine and single‐dose nevirapine prophylaxis during pregnancy and breastfeeding for the prevention of mother‐to‐child transmission of HIV‐1 (Kesho Bora study): a randomized controlled trial. Lancet 2011;11(3):171‐80. [DOI] [PubMed] [Google Scholar]
Kumwenda 2008 {published data only}
- Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, et al. Extended antiretroviral prophylaxis to reduce breast‐milk HIV‐1 transmission. New England Journal of Medicine 2008;359(2):119‐129. [DOI] [PubMed] [Google Scholar]
- Taha TE, Qing L, Hoover DR, Mipando L, Nkanaunena K, Thigpen MC, et al. Postexposure prophylaxis of breastfeeding HIV‐exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI‐Malawi Trial. Journal of Acquired Immune Deficiency Syndromes 2011;57(4):319‐325. [DOI] [PubMed] [Google Scholar]
Shapiro 2010 {published data only}
- Shapiro R, Hughes M, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast‐feeding in Botswana. New England Journal of Medicine 2010;362:2282‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
SWEN 2008 {published data only}
- Church J, Omer S, Guay L, Huang W, Lidstrom J, Musoke P, et al. Analysis of Nevirapine (NVP) Resistance in Ugandan Infants Who Were HIV Infected Despite Receiving Single‐Dose (SD) NVP versus SD NVP Plus Daily NVP Up to 6 Weeks of Age to Prevent HIV Vertical Transmission. Journal of Infectious Diseases 2008, 198:1075‐82. [DOI] [PMC free article] [PubMed]
- Moorthy A, Gupta A, Bhosale R, Tripathy S, Sastry J, Kulkarni S, et al. Effects of Single, Extended‐Dose Nevirapine Prophylaxis in Subtype C HIV‐Infected Infants. PLoS ONE 2009. 4(1):e4096. [DOI] [PMC free article] [PubMed]
- Omer SB for the Six Week Extended Dose Nevirapine (SWEN) Study Team. Twelve‐month follow‐up of Six Week Extended Dose Nevirapine randomized controlled trials: differential impact of extended‐dose nevirapine on mother‐to‐child transmission and infant death by maternal CD4 cell count. AIDS 2011;25:767‐776. [DOI] [PubMed] [Google Scholar]
- SWEN Study Team. Extended‐dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomized controlled trials. Lancet 2008;372(9635):300‐313. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Becquet 2009 {published data only}
- Becquet R, Ekouevi DK, Arrive E, Stringer JSA, Meda N, Chaix ML, et al. Universal Antiretroviral Therapy for Pregnant and Breast‐Feeding HIV‐1‐Infected Women: Towards the Elimination of Mother‐to‐Child Transmission of HIV‐1 in Resource‐Limited Settings. Clinical Infectious Diseases. 2009 49:12, 1936‐1945. [DOI] [PMC free article] [PubMed]
Becquet 2011 {published data only}
Bland 2010 {published data only}
- Bland RM, Coovadia HM, Coutsoudis A, Rollins NC, Newell ML. Cohort profile: Mamanengane or the Africa centre vertical transmission study. International Journal of Epidemiology. 2010 39:2, 351‐360. [DOI] [PMC free article] [PubMed]
Bobat 1997 {published data only}
- Bobat R, Moodley D, Coutsoudis A, Coovadia H. Breastfeeding by HIV‐1‐infected women and outcome in their infants: a cohort study from Durban, South Africa. AIDS 2007;11(13):1627‐33. [DOI] [PubMed] [Google Scholar]
Chavula 2012 {published data only}
- Chavula C, Long D, Mzembe E, Kayira D, Chasela C, Hudgens MG, et al. Stopping the control arm in response to the DSMB: mother's choice of HIV prophylaxis during breastfeeding in the BAN Study. Contemp Clin Trials. 2012 33:1, 55‐59. [DOI] [PMC free article] [PubMed]
Chen 2010 {published data only}
- Chen YQ, Young A, Brown ER, Chasela CS, Fiscus SA, Hoffman IF, et al. Population attributable fractions for late postnatal mother‐to‐child transmission of HIV‐1 in Sub‐Saharan Africa. J Acquir Immune Defic Syndr. 2010 54:3, 311‐316. [DOI] [PMC free article] [PubMed]
Cherisch 2005 {published data only}
- Chersich MF, Gray GE. Progress and Emerging Challenges in Preventing Mother‐to‐Child Transmission. Current Infectious Disease Reports. 2005 Sep;7(5):393‐400.. [DOI] [PubMed] [Google Scholar]
Chiappini 2009 {published data only}
Chimienti 2010 {published data only}
- Chimienti SN. More progress on preventing HIV infection in infants. Three trials this year pointed to the benefits of extending maternal antiretroviral prophylaxis throughout the breast‐feeding period. J Watch AIDS Clin Care. 2010 22:1, 6‐7. [PubMed]
Chung 2008 {published data only}
- Chung MH, Kiarie JN, Richardson BA, Lehman DA, Overbaugh J, Kinuthia J, Njiri F, John‐Stewart GC. Highly active antiretroviral therapy versus zidovudine/nevirapine effects on early breast milk HIV type‐1 Rna: a phase II randomized clinical trial. Antivir Ther 2008;13(6):799‐807. [PMC free article] [PubMed] [Google Scholar]
Coovadia 2007 {published data only}
- Coovadia H, Kindra G. Breastfeeding to prevent HIV transmission in infants: balancing pros and cons. Current Opinion in Infectious Diseases 8 Feb;21(1):11‐15. [DOI] [PubMed] [Google Scholar]
Coovadia 2008 {published data only}
- Coovadia H, Kindra G. Breastfeeding to prevent HIV transmission in infants: balancing pros and cons. . Current Opinion in Infectious Diseases. 2008 Feb;21(1):11‐5. [DOI] [PubMed] [Google Scholar]
Coutsoudis 1999 {published data only}
- Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant‐feeding patterns on early mother‐to‐child transmission of HIV‐1 in Durban, South Africa: a prospective cohort study. Lancet 1999 Aug 7;354(9177):471‐6. [DOI] [PubMed] [Google Scholar]
Coutsoudis 2000 {published data only}
- Coutsoudis A. Influence of infant‐feeding patterns on early mother‐to‐child transmission of HIV‐1 in Durban, South Africa: a prospective cohort study. Journal of Human Lactation 2000 Feb;16(1):73‐7. [PubMed] [Google Scholar]
Coutsoudis 2001 {published data only}
- Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM, South African Vitamin A Study Group. Method of feeding and transmission of HIV‐1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS 2001 Feb 16;15(3):379‐87. [DOI] [PubMed] [Google Scholar]
Engebretsen 2010 {published data only}
- Engebretsen IM, Tylleskar T. [HIV, breast feeding and antiretroviral agents]. Tidsskr Nor Laegeforen 2010;130:520‐522. [DOI] [PubMed] [Google Scholar]
Fao 2012 {published data only}
- Fao P, Ky‐Zerbo O, Gouem C, Somda P, Hien H, Ouedraogo PE, et al. Maternal HIV‐1 Disease Progression 18‐24 Months Postdelivery According to Antiretroviral Prophylaxis Regimen (Triple‐Antiretroviral Prophylaxis During Pregnancy and Breastfeeding vs Zidovudine/Single‐Dose Nevirapine Prophylaxis): The Kesho Bora Randomized Controlled Trial. Clinical Infectious Diseases. 2012:55, 3(449‐460). [DOI] [PMC free article] [PubMed]
Fawzi 2002 {published data only}
- Fawzi W, Msamanga G, Spiegelman D, Renjifo B, Bang H, Kapiga S, et al. Transmission of HIV‐1 through breastfeeding among women in Dar es Salaam, Tanzania. Journal of Acquired Immune Deficiency Syndromes 2002 Nov 1;31(3):331‐8. [DOI] [PubMed] [Google Scholar]
Fogel 2011 {published data only}
- Fogel J, Li Q, Taha TE, Hoover DR, Kumwenda NI, Mofenson LM, et al. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV‐infected infants. Clinical Infectious Diseases. 2011 52:8, 1069‐1076. [DOI] [PMC free article] [PubMed]
Fowler 2002 {published data only}
- Fowler MG, Newell ML. Breast‐feeding and HIV‐1 transmission in resource‐limited settings. Journal of Acquired Immune Deficiency Syndromes. 2002 Jun 1;30(2):230‐9. [DOI] [PubMed] [Google Scholar]
Giuliano 2007 {published data only}
- Giuliano M, Guidotti G, Andreotti M, Pirillo MF, Villani P, Liotta G, et al. Triple antiretroviral prophylaxis administered during pregnancy and after delivery significantly reduces breast milk viral load ‐ A study within the drug resource enhancement against AIDS and malnutrition program. Journal of Acquired Immune Deficiency Syndromes 2007 Mar 1;44(3):286‐91. [DOI] [PubMed] [Google Scholar]
Gray 2006 {published data only}
- Gray G, Violari A, McIntyre J, Jivkov B, Schnittman S, Reynolds L, Ledeine JM. Antiviral Activity of Nucleoside Analogues during short‐course monotherapy or dual therapy: Its role in preventing HIV infection in infants. J Acquir Immune Defic Syndr 2006 Jun;42(2):169‐76. [DOI] [PubMed] [Google Scholar]
Heidari 2011 {published data only}
Homsy 2010 {published data only}
- Homsy J, Moore D, Barasa A, Were W, Likicho C, Waiswa B, et al. Breastfeeding, mother‐to‐child HIV transmission, and mortality among infants born to HIV‐infected women on highly active antiretroviral therapy in rural Uganda. Journal of Acquired Immune Deficiency Syndromes. 2010 53:1, 28‐35. [DOI] [PubMed] [Google Scholar]
Horvath 2009 {published data only}
- Horvath T, Madi BC, Iuppa IM, Kennedy GE, Rutherford G, Read JS. Interventions for preventing late postnatal mother‐to‐child transmission of HIV. Cochrane Database Systematic Reviews. 2009 Jan 21;(1):CD006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ikechebelu 2011 {published data only}
- Ikechebelu JI, Ugboaja JO, Kalu SO, Ugochukwu EF. The outcome of prevention of mother to child transmission (PMTCT) of HIV infection programme in Nnewi, southeast Nigeria. Niger J Med. 2011 20:4, 421‐425. [PubMed]
Iliff 2005 {published data only}
- Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, Moulton LH, Ward BJ, Humphrey JH, ZVITAMBO study group. Early exclusive breastfeeding reduces the risk of postnatal HIV‐1 transmission and increases HIV‐free survival. AIDS 2005 Apr 29;19(7):699‐708. [DOI] [PubMed] [Google Scholar]
John 2001 {published data only}
- John GC, Richardson BA, Nduati RW, Mbori‐Ngacha D, Kreiss JK. Timing of breast milk HIV‐1 transmission: a meta‐analysis. East African Medical Journal. 2001 Feb;78(2):75‐9. [DOI] [PubMed] [Google Scholar]
Kafulafula 2010 {published data only}
- Kafulafula G, Hoover DR, Taha TE, Thigpen M, Li Q, Fowler MG, et al. Frequency of Gastroenteritis and Gastroenteritis‐Associated Mortality With Early Weaning in HIV‐1‐Uninfected Children Born to HIV‐Infected Women in Malawi. Journal of Acquired Immune Deficiency Syndromes 2010;53:1:6‐13. [DOI] [PubMed] [Google Scholar]
Kiarie 2004 {published data only}
- Kiarie JN, Richardson BA, Mbori‐Ngacha D, Nduati RW, John‐Stewart GC. Infant feeding practices of women in a perinatal HIV‐1 Prevention Study in Nairobi, Kenya. Journal of Acquired Immune Deficiency Syndromes 2004 Jan 1;35(1):75‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilewo 2009 {published data only}
- Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, Lipyoga R, Mhalu F, Biberfeld G. Prevention of mother‐to‐child transmission of HIV‐1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: The Mitra Plus study. Journal of Acquired Immune Deficiency Syndromes 2009;52:3:406‐416. [DOI] [PubMed] [Google Scholar]
Kourtis 2007 {published data only}
- Kourtis AP, Jamieson DJ, Vincenzi I, Taylor A, Thigpen MC, Dao H, et al. Prevention of human immunodeficiency virus‐1 transmission to the infant through breastfeeding: new developments. American Journal of Obstetrics Gynecology. 2007 Sep;197(3 Suppl):S113‐22. [DOI] [PubMed] [Google Scholar]
Kuhn 2007 {published data only}
- Kuhn L, Sinkala M, Kankasa C, Semrau K, Kasonde P, Scott N, Mwiya M, Vwalika C, Walter J, Tsai WY, Aldrovandi GM, Thea DM. High uptake of exclusive breastfeeding and reduced early post‐natal HIV transmission. PLoS One 2007 Dec 26;2(12):e1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert 2003 {published data only}
- Lambert JS, Nogueira SA, Abreu T, Machado ES, Costa TP, Bondarovsky M, et al. A pilot study to evaluate the safety and feasibility of the administration of ZDV/3TC fixed dose combination to HIV infected pregnant women and their infants in Rio de Janeiro, Brazil. Sexually Transmitted Infections 2003 Dec;79(6):448‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehman 2009 {published data only}
- Lehman DA, Chung MH, Mabuka JM, John‐Stewart GC, Kiarie J, Kinuthia J, et al. Lower Risk of Resistance After Short‐Course HAART Compared with Zidovudine/Single‐Dose Nevirapine Used for Prevention of HIV‐1 Mother‐to‐Child Transmission. Journal of Acquired Immune Deficiency Syndromes 2009 Aug 15;51(5):522‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marazzi 2006 {published data only}
- Marazzi MC, Germano P, Liotta G, Guidotti G, Loureiro S, Cruz Gomes A, et al. Safety of nevirapine‐containing antiretroviral triple therapy regimens to prevent vertical transmission in an African cohort of HIV‐1 infected pregnant women. HIV Medicine 6 Jul;7(5):338‐44. [DOI] [PubMed] [Google Scholar]
Marazzi 2009 {published data only}
- Marazzi MC, Nielsen‐Saines K, Buonomo E, Scarcella P, GermaNoP, Majid NA, et al. Increased infant human immunodeficiency virus‐type one free survival at one year of age in sub‐saharan Africa with maternal use of highly active antiretroviral therapy during breast‐feeding. Pediatric Infectious Disease Journal 2009;28:483‐487. [DOI] [PubMed] [Google Scholar]
Marazzi 2011 {published data only}
- Marazzi MC, Palombi L, Nielsen‐Saines K, Haswell J, Zimba I, Magid NA, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother‐to‐child transmission of HIV‐1 correlates with favorable pregnancy outcomes. AIDS 2011;25:1611‐1618. [DOI] [PubMed] [Google Scholar]
Mbori‐Ngacha 2001 {published data only}
- Mbori‐Ngacha D, Nduati R, John G, Reilly M, Richardson B, Mwatha A, et al. Morbidity and mortality in breastfed and formula‐fed infants of HIV‐1‐infected women: A randomized clinical trial. JAMA 2001 Nov 21;286(19):2413‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntyre 2006 {published data only}
- McIntyre J. Strategies to prevent mother‐to‐child transmission of HIV. Current Opinion Infectious Diseases. 2006 Feb;19(1):33‐8. [DOI] [PubMed] [Google Scholar]
McIntyre 2010 {published data only}
- McIntyre J. Use of antiretrovirals during pregnancy, breastfeeding in low‐income, middle‐income countries. Current Opinion in HIV AIDS. 2010 5:1. 48‐53. [DOI] [PMC free article] [PubMed]
Meda 2011 {published data only}
- Meda N, Fao P, Ky‐Zerbo O, Gouem C, Somda P, Hien H, et al. Triple antiretroviral compared with zidovudine and single‐dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother‐to‐child transmission of HIV‐1 (Kesho Bora study): a randomized controlled trial. Lancet Infectious Diseases 2011;11:171‐180. [DOI] [PubMed] [Google Scholar]
Miotti 1999 {published data only}
- Miotti PG, Taha TE, Kumwenda NI, Broadhead R, Mtimavalye LA, Hoeven L, et al. HIV transmission through breastfeeding: a study in Malawi. JAMA 1999 Aug 25;282(8):744‐9. [DOI] [PubMed] [Google Scholar]
Mirochnick 2009 {published data only}
- Mirochnick M, Thomas T, Capparelli E, Zeh C, Holland D, Masaba R, et al. Antiretroviral Concentrations in Breast‐Feeding Infants of Mothers Receiving Highly Active Antiretroviral Therapy. Antimicrobial Agents and Chemotherapy 2009 Mar;53(3):1170‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mmiro 2009 {published data only}
- Mmiro FA, Aizire J, Mwatha AK, Eshleman SH, Donnell D, Fowler MG, et al. Predictors of early and late mother‐to‐child transmission of HIV in a breastfeeding population: HIV Network for Prevention Trials 012 experience, Kampala, Uganda. Journal of Acquired Immune Deficiency Syndromes 2009;52:32‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mofenson 2003 {published data only}
- Mofenson LM. Advances in the prevention of vertical transmission of human immunodeficiency virus. Seminars in Pediatric Infectious Diseases. 2003 Oct;14(4):295‐308.. [DOI] [PubMed] [Google Scholar]
Mofenson 2008 {published data only}
- Mofenson LM. Antiretroviral prophylaxis to reduce breast‐milk HIV‐1 transmission oh HIV Type 1: New Data but Still Question. Journal of Acquired Immune Deficiency Syndromes 2008 Jul 1;48(3):237‐40. [DOI] [PubMed] [Google Scholar]
Nduati 2000 {published data only}
- Nduati R, John G, Mbori‐Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV‐1: a randomized clinical trial. JAMA 2000 Mar 1;283(9):1167‐74. [DOI] [PubMed] [Google Scholar]
Nduati 2001 {published data only}
- Nduati R, Richardson BA, John G, Mbori‐Ngacha D, Mwatha A, Ndinya‐Achola J, et al. Effect of breastfeeding on mortality among HIV‐1 infected women: a randomized trial. Lancet. 2001 May 26;357(9269):1651‐5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabie 2001 {published data only}
- Rabie H, Pieper CH, Robson B, Cotton MF. Postnatal Zidovudine in Prevention of Vertical HIV‐1 Transmission. Journal of Tropical Pediatrics 2001 Aug;47(4):215‐9. [DOI] [PubMed] [Google Scholar]
Ramlal 2013 {published data only}
- Ramlal RT, Tembo M, Soko A, Chigwenembe M, Tohill BC, Kayira D, King CC, Chasela C, Jamieson D, Horst C, Bentley ME, Adair LS, BAN Study Team. Patterns of body composition among HIV‐infected, pregnant Malawians and the effects of famine season. Matern Child Health J. 2013 Feb;17(2):265‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rousseau 2003 {published data only}
- Rousseau CM, Nduati RW, Richardson BA, Steele MS, John‐Stewart GC, Mbori‐Ngacha DA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. The Journal of Infectious Diseases 2003 Mar 1;187(5):741‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rousseau 2004 {published data only}
- Rousseau CM, Nduati RW, Richardson BA, John‐Stewart GC, Mbori‐Ngacha DA, Kreiss JK, et al. Association of levels of HIV‐1‐infected breast milk cells and risk of mother‐to‐child transmission. Journal of Infectious Diseases. 2004 Nov 15;190(10):1880‐8. Epub 2004 Oct 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sartorius 2013 {published data only}
- Sartorius BK, Chersich MF, Mwaura M, Meda N, Temmerman M, Newell ML, et al. Maternal anaemia and duration of zidovudine in antiretroviral regimens for preventing mother‐to‐child transmission: a randomized trial in three African countries. BMC Infect Dis. 2013:13, 1(522). [DOI] [PMC free article] [PubMed]
Shapiro 2005 {published data only}
- Shapiro RL, Holland DT, Capparelli E, Lockman S, Thior I, Wester C, Stevens L, Peter T, Essex M, Connor JD, Mirochnick M. Antiretroviral concentrations in breast‐feeding infants of women in Botswana receiving antiretroviral treatment. Journal of Infectious Diseases 2005 Sep 1;192(5):720‐7. [DOI] [PubMed] [Google Scholar]
Shapiro 2006 {published data only}
- Shapiro RL, Thior I, Gilbert PB, Lockman S, Wester C, Smeaton LM, Stevens L, Heymann SJ, Ndung'u T, Gaseitsiwe S, Novitsky V, Makhema J, Lagakos S, Essex M. Maternal single‐dose nevirapine versus placebo as part of an antiretroviral strategy to prevent mother‐to‐child HIV transmission in Botswana. AIDS 2006 Jun 12;20(9):1281‐8. [DOI] [PubMed] [Google Scholar]
Shetty 2003 {published data only}
- Shetty AK, Coovadia HM, Mirochnick MM, Maldonado Y, Mofenson LM, Eshleman SH, et al. for the HIVNET 023 Study Team. Safety and trough concentrations of nevirapine prophylaxis given daily, twice weekly, or weekly in breast‐feeding infants from birth to 6 months. Journal of Acquired Immune Deficiency Syndromes 2003 Dec 15;34(5):482‐90. [DOI] [PubMed] [Google Scholar]
Siegfried 2011 {published data only}
- Siegfried N, Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database Systematic Reviews 2011;7. [DOI] [PubMed] [Google Scholar]
SIMBA 2003 {published data only}
- Vyankandondera J, Luchters S, Hassink E, et al. Reducing risk of HIV‐1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (SIMBA study) [abstract# LB7]. Paper presented at: the 2nd International AIDS Society Conference on HIV Pathogenesis and Treatment. 2003.
Taha 2009 {published data only}
- Taha TE, Kumwenda J, Cole SR, Hoover DR, Kafulafula G, Fowler MG, Thigpen MC, Li Q, Kumwenda NI, Mofenson L. Postnatal HIV‐1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. The Journal of infectious diseases 2009;200:1490‐1497. [DOI] [PubMed] [Google Scholar]
Tess 1998 {published data only}
- Tess BH, Rodrigues LC, Newell ML, Dunn DT, Lago TD. Infant feeding and risk of mother‐to‐child transmission of HIV‐1 in Sao Paulo State, Brazil. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1998 Oct 1;19(2):189‐94. [DOI] [PubMed] [Google Scholar]
Thior 2006 {published data only}
- Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, Gilbert PB, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother‐to‐child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296(7):794‐805. [DOI] [PubMed] [Google Scholar]
Thomas 2011 {published data only}
- Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, OtieNoJ, Jamieson D, Thigpen MC, Bulterys M, Slutsker L, Cock KM, Amornkul PN, Greenberg AE, Fowler MG. Triple‐antiretroviral prophylaxis to prevent mother‐to‐child HIV transmission through breastfeeding‐the Kisumu Breastfeeding Study, Kenya: A clinical trial. PLoS Medicine 2011;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volmink 2007 {published data only}
- Volmink J, Siegfried NL, Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database Systematic Reviews. 2007 Jan 24;(1):CD003510. [DOI] [PubMed] [Google Scholar]
Wind‐Rotolo 2006 {published data only}
- Wind‐Rotolo M, Doherty MC. Viral latency and reservoirs: relevance for mother‐to‐child transmission and resistance. Current Opinion in HIV AIDS. 2006 Mar;1(2):167‐73. [DOI] [PubMed] [Google Scholar]
Additional references
De Cock 2000
- Fao P, Ky‐Zerbo O, Gouem C, Somda P, Hien H, Ouedraogo PE, et al. Maternal HIV‐1 Disease Progression 18‐24 Months Postdelivery According to Antiretroviral Prophylaxis Regimen (Triple‐Antiretroviral Prophylaxis During Pregnancy and Breastfeeding vs Zidovudine/Single‐Dose Nevirapine Prophylaxis): The Kesho Bora Randomized Controlled Trial. Clinical Infectious Diseases. 2012:55, 3(449‐460). [DOI] [PMC free article] [PubMed]
De Schacht 2014
- Schacht C, Mabunda N, Ferreira OC, Ismael N, Calú N, Santos I, Hoffman HJ, Alons C, Guay L, Jani IV. High HIV incidence in the postpartum period sustains vertical transmission in settings with generalized epidemics: a cohort study in Southern Mozambique. Journal of the International AIDS Society 2014 Mar 5;17:18808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Wiley and Sons, September 2008. [Google Scholar]
Kindra 2011
- Kindra G, Coutsoudis A, Esposito F, Esterhuizen T. Breastfeeding in HIV Exposed Infants Significantly Improves Child Health: A Prospective Study. Maternal and Child Health Journal 2011 Apr 20;16(3):632‐40. [DOI] [PubMed] [Google Scholar]
Lidstrom 2010
- Lidström J, Li Q, Hoover DR, Kafulafula G, Mofenson LM, Fowler MG, Thigpen MC, Kumwenda N, Taha TE, Eshleman SH. Addition of extended zidovudine to extended nevirapine prophylaxis reduces nevirapine resistance in infants who were HIV‐infected in utero. AIDS. 2010 Jan 28, 24(3):381‐6. [DOI] [PMC free article] [PubMed]
Palombi 2012
- Palombi L, Pirillo MF, Andreotti M, Liotta G, Erba F, Sagno JB, et al. Antiretroviral prophylaxis for breastfeeding transmission in Malawi: drug concentrations, virological efficacy and safety. Antiviral Therapy. 2012;17(8):1511‐9. [DOI] [PubMed] [Google Scholar]
Persaud 2011
- Persaud D, Bedri A, Ziemniak C, Moorthy A, Gudetta B, Abashawl A, Mengistu Y, Omer SB, Isehak A, Kumbi S, Adamu R, Lulseged S, Ashworth R, Hassen E, Ruff A, Ethiopian SWEN Study Team. Slower clearance of nevirapine resistant virus in infants failing extended nevirapine prophylaxis for prevention of mother‐to‐child HIV transmission. AIDS Research and Human Retroviruses. 2011 Aug, 27(8):823‐9. [DOI] [PMC free article] [PubMed]
Rankin 2005
- Rankin W, Brennan S, Schell E, Laviwah J, Rankin SH. The Stigma of Being HIV‐Positive in Africa. PLoS Medicine August 2005;2(8):247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Read 2003
- Read JS. Human milk, breastfeeding, and transmission of human immunodeficiency virus type 1 in the United States. Pediatrics 2003;112(5):1196‐1205. [DOI] [PubMed] [Google Scholar]
Shapiro 2013
- Shapiro RL, Kitch D, Ogwu A, Hughes MD, Lockman S, Powis K, Souda S, Moffat C, Moyo S, McIntosh K, Widenfelt E, Zwerski S, Mazhani L, Makhema J, Essex M. HIV transmission and 24‐month survival in a randomized trial of HAART to prevent MTCT during pregnancy and breastfeeding in Botswana.. AIDS 2013 Jul 31;27(12):1911‐20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Souda 2013
- Souda S, Gaseitsiwe S, Georgette N, Powis K, Moremedi D, Iketleng T, Leidner J, Moffat C, Ogwu A, Lockman S, Moyo S, Mmalane M, Musonda R, Makhema J, Essex M, Shapiro R. No clinically significant drug‐resistance mutations in HIV‐1 subtype C‐infected women after discontinuation of NRTI‐based or PI‐based HAART for PMTCT in Botswana. Journal of Acquired Immune Deficiency Syndromes 2013 Aug 15;63(5):572‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS 2013
- Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS Report on the Global AIDS Epidemic 2013. 2013.
Volmink 2007
- Volmink J, Siegfried NL, derMerwe L, Brocklehurst P. Antiretroviralsfor reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database of Systematic Reviews 2007;1. [DOI] [PubMed] [Google Scholar]
WHO 2010
- WHO. Antiretroviral drugs for the treating pregnant women and preventing HIV infections in children. Geneva 2010. [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection (2013). Available from: http://www.who.int/hiv/pub/guidelines/arv2013/en/ [accessed 28 September 2014]. [PubMed]
Zeh 2011
- Zeh C, Weidle PJ, Nafisa L, Lwamba HM, Okonji J, Anyango E, Bondo P, Masaba R, Fowler MG, Nkengasong JN, Thigpen MC, Thomas T. HIV‐1 drug resistance emergence among breastfeeding infants born to HIV‐infected mothers during a single‐arm trial of triple‐antiretroviral prophylaxis for prevention of mother‐to‐child transmission: a secondary analysis. PLoS Med 2011;8:e1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]


