Abstract
MicroRNAs (miRNAs) are a group of small non-coding RNAs that affect gene expression. The role of miRNAs in different types of cancers has been published and it was shown that several miRNAs are inappropriately expressed in different cancers. Among the mechanisms that can cause this lack of proper expression are epigenetics, chromosomal changes, polymorphisms or defects in processing proteins. Recent research shows that phytochemicals, including epigallocatechin-3-gallate (EGCG), exert important epigenetic-based anticancer effects such as pro-apoptotic or anti proliferative through miRNA gene silencing. Given that EGCG is able to modulate a variety of cancer-related process i.e., angiogenesis, proliferation, metastasis and apoptosis via targeting various miRNAs such as let-7, miR-16, and miR-210. The discovery of new miRNAs and the differences observed in their expression when exposed to EGCG provides evidence that targeting these miRNAs may be beneficial as a form of treatment. In this review, we aim to provide an overview, based on current knowledge, on how phytochemicals, including epigallocatechin-3-gallate, can be considered as potential miRNAs modulator to improve efficacy of current cancer treatments.
Keywords: Cancer, Epigallocatechin-3-gallate, Epigenetic, MicroRNAs, Molecular pathways
Introduction
The World Health Organization's Global Cancer Report suggests that, in addition to breast and lung cancer posing a considerable risk to human life and health, we may also be seeing an upswing in the number of gastric, colorectal, liver, and prostate cancer cases. It is predicted that by 2040, the global burden of cancer will be 50% higher than it is today, with 30 million new cases annually [1–3]. Although there are various treatments available for cancer, the results are far from satisfactory [4]. Chemotherapy drugs such as cisplatin, doxorubicin and even radiotherapy are frequently used in the management of many cancer cases, often causing negative side effects and severe toxicity. Radiotherapy furthermore has the risk of leading to impairments in memory, learning, and cognitive abilities, and may result in decreased brain functioning later in life [5]. In addition to the risk of developing a secondary tumor due to chemotherapy, there are also potential side effects resulting from damage to healthy tissues. In response to this, plant-derived compounds, such as alkaloids, terpenoids, phenols, flavonoids, and more, have proven to be effective in preventing and treating certain cancers, such as breast cancer lung cancer, and ovarian cancer. These compounds influence the inflammatory processes, cause cell apoptosis, inhibit the invasion and metastasis of cancer cells, and reduce the immune system's tolerance of tumors [6].
The beneficial effects of green tea are due to the presence of polyphenol compounds in it. Epigallocatechin and epigallocatechin 3-gallate are the most important and abundant polyphenols in green tea that many studies have shown to be responsible for various biological effects such as anti-methylation of DNA, anti-apoptotic, anti- inflammatory, anti-oxidant, anti-angiogenesis and anti-metastatic by modulating signaling pathways. Epigenetic effects of green tea by modulating of microRNAs involved in numerous signaling pathway has created a glimmer of hope in chemoprevention and treatment of several cancers.
MicroRNAs are small non-coding RNA molecules, ranging from 18 to 25 nucleotides in length, which are known to be involved in the regulation of gene expression. They participate in various cellular signaling pathways, such as those related to cell growth, proliferation, differentiation, survival, and apoptosis. EGCG has been demonstrated to be effective in controlling various diseases including cancer, by altering the expression of microRNAs (miRNAs). A study demonstrated that EGCG is known to influence miRNA expression in cancer, with the miRNA Let-7 being specifically up-regulated by the laminin receptor upon the compound's activation [7]. EGCG increases the production of miR-210, which has a regulating effect on the transcription factor HIF-1α, resulting in decreased progression of lung cancer [8]. Research has shown that EGCG modulating miR-16 can lead to a lower expression of the Bcl-2 protein that has an apoptotic effect in hepatocellular carcinoma. Additionally, EGCG modulation of miRNAs such as miR-15 has been linked with decreased cancerous growth and invasion in breast cancer [9]. A significant proportion of breast cancer cases with expressed hormones receptors are inhibited by miRNAs that have been modulated by EGCG [10]. Analyses of miRNA levels from cancer cells and those treated with EGCG provide a greater understanding of the involvement of miRNA in the development of cancer. A next-generation sequencing study that compared A549 lung cancer cells with A549 lung cancer cells treated with EGCG revealed that EGCG can regulate a variety of carcinogenic pathways through changes in the miRNA expression levels [11]. Herein, we highlighted potential role of EGCG against several cancers by modulating of microRNAs in various cancers in this literature review.
Structure of EGCG
EGCG consists of three aromatic rings (A, B, and D) which are connected by a pyran-ring (C; Fig. 1). This particular structure is thought to be the cause of EGCG's health-promoting properties. It has been suggested that its antioxidant activity is due in part to processes such as the transfer of hydrogen atoms or the transfer of single electrons, which take place among the hydroxyl groups located on the B and/or D rings [12]. Additionally, in vitro studies have indicated that both the B and D rings are linked to a reduction of proteasome function [13]. A ring of EGCG plays a part in restraining Heat-Shock Protein 90 (HSP90) [14]. The hydroxyl group on the B ring at the 5′ position has been found to impede the growth of Helicobacter pylori in the stomach [15].
Fig. 1.
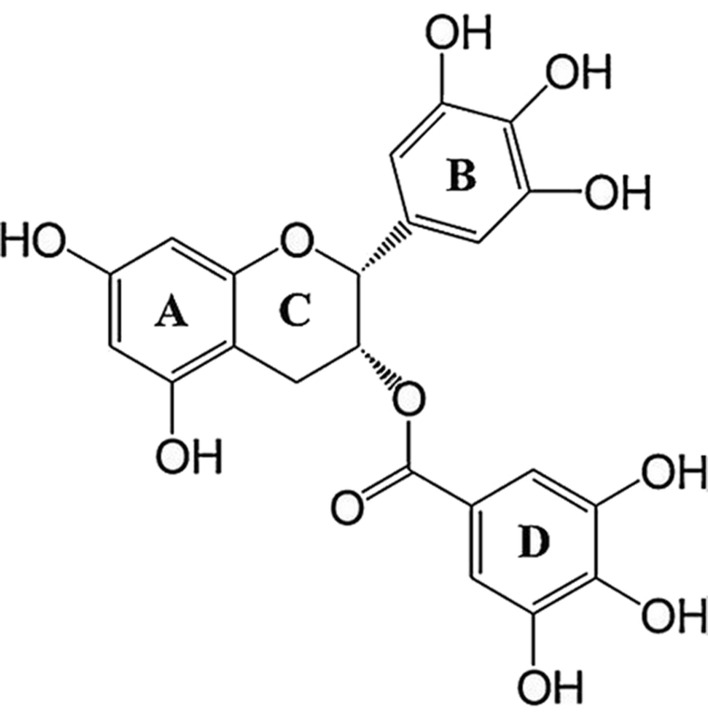
The structure of EGCG
EGCG and cancer
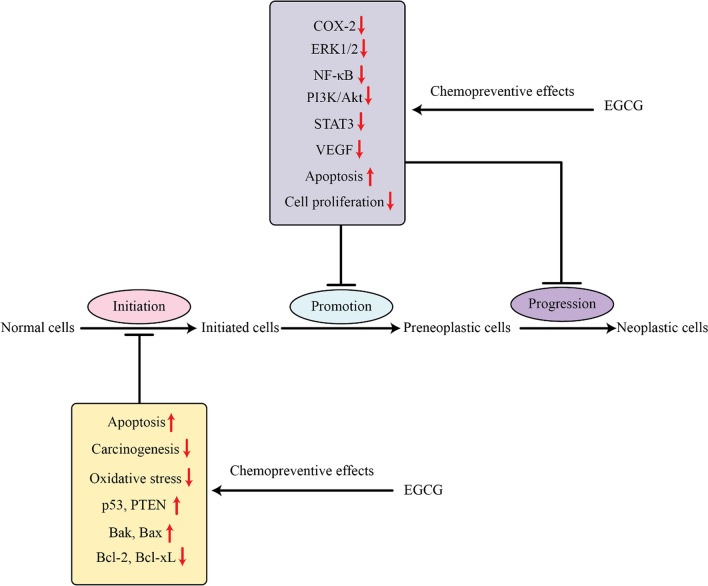
Research has been conducted to evaluate the potential of EGCG to act as a chemoprevention agent on breast tumors, showing excellent antitumor effects despite issues of bioavailability being a potential cause of conflicting and uneven results [9]. EGCG has been shown to have an effect on the viability and self-repair ability of triplenegative breast cancer (TNBC) cells, making them responsive to estrogen by activating ER-α. But the exact mechanism by which EGCG works on TNBC cells is still uncertain. Recent studies have uncovered that WISP-2/ CCN5 can have an impact on the survivability, expression of ER-α, and stem cell-like qualities found in TNBC and additional forms of cancer [16]. EGCG enhances the activity of CCN5 by improving its bioavailability and enhancing its anti-tumor properties. The action of EGCG against CCN5 results in the decrease of cell viability, diminishment of sphere-forming ability and suppression of tumor growth in vivo in TNBC cells [17, 18]. EGCG plays a crucial role in the advancement of cutting-edge epigenetic-based therapy [19]. The expression of genes associated with angiogenesis was assessed to determine the anti-angiogenic effects of EGCG treatment in cancer cells. EGCG treatment was observed to reduce the regulation of genes driving invasion, adhesion, and proliferation, as well as the expression of genes identified to have antagonistic effects [20]. EGCG demonstrated a major role in suppressing melanoma cell survival and prevented NF-κB activity, which consequently led to a decrease in IL-1β production in melanoma cells [21]. ECGC was found to inhibit tyrosine phosphorylation of the focal adhesion kinase and significantly reduce the activity of MMP-9. In addition, studies on animals showed that EGCG alone was able to reduce lung metastases [22]. Despite the presence of cancerous cells, EGCG was found to be effective at halting their invasion. This inhibition was possibly related to an increase in the level of E-cadherin which would regulate the process [23]. EGCG has been demonstrated to possess anti-tumor properties through its influence on different signalling pathways [24]. EGCG has been linked to the prevention of cancer development through its inhibition of the interleukin signaling pathways, TNF and COX. This inhibition is observed to enhance the activation of the proteins PTEN/p21 and p53, control cell death via Bcl-2/Bax, and disruption of transcription factors and regulatory molecules implicated in tumor growth [25]. The action of EGCG in cancer prevention is achieved by altering genes and associated signaling molecules, leading to the prevention of the beginning, promotion, and advancement of cancer (Fig. 2). NF-κB plays an important role in tumor development by regulating genes connected to the main features of tumors, such as survival, proliferation, metastasis, and inflammation [26]. EGCG suppresses NF-κB signaling by preventing phosphorylation and therefore destruction of IκBα, which stops the nuclear translocation of the proteins p50 and p65 [27, 28]. ECGC reduces the NF-κB pathway to inhibit the growth and decrease the ability of cells to invade [26, 28, 29]. EGCG has the ability to reduce the proliferation and invasiveness of breast tumors by blocking the Wnt pathway with the help of HBP1 gene. It does this by causing deregulation of the Wnt pathway which results in increased expression of G1 regulators, c-MYC, and cyclin D1 genes. This effect also leads to a reduction in the potential of the tumor to be invasive and migratory [30]. EGCG inhibits NFκ-B and HIF-1 activation, as well as VEGF expression, inhibiting the growth of tumors and the development of breast cancer. Treatment with EGCG resulted in a decreased cancer weight relative to the control and a lower expression of VEGF [31, 32]. The anti-angiogenic and anti-proliferative properties of EGCG make it a potentially valuable therapeutic treatment for breast cancer and head and neck squamous cell carcinomas (HNSCC). It does so by limiting the activation of STAT3 and NFκ-B in cancerous cells, which prevents the production of VEGF [33]. EGCG was demonstrated to reduce VEGF-induced DNA synthesis, cell proliferation, and the self-activation process of VEGFR-1 and -2 [34]. Although tea polyphenols have a good safety profile and are considered safe when consumed in high quantities (600–1800 mg/day), there have been reports of potential toxicity related to one of the substances within that group, EGCG [35]. Schmidt and their colleagues established that EGCG plays an integral role in the toxic effects of green tea extracts on hepatocytes [36]. Additionally, supplementation of EGCG was demonstrated to worsen the harm inflicted on beta cells in diabetic rats due to high-glucose levels [37]. EGCG induces a reduction in both the number of islet cells and the amount of insulin-producing beta cells by creating reactive oxygen species (ROS) at small concentrations in the plasma [37]. There have been a lot of research done on the potentially hazardous effects of EGCG causing liver failure [38, 39].
Fig. 2.
EGCG has been shown to influence the initiation, promotion, and progression of cancer by modifying multiple processes or genes. It is therefore thought to potentially play a role in the prevention and inhibition of certain cancers
Table 1 presents a compilation of research focusing on the impacts of EGCG on various types of cancer.
Table 1.
The effects of EGCG in different cancers
| Cancer | Dose (s) | Mechanism | Model (In vitro/ In vivo) | Cell line | Refs. |
|---|---|---|---|---|---|
| Glioma | 82 and 134 μg/mL | Decrease the guidance of axon process and different metabolic-related pathways | In vitro | 1321N1 | [40] |
| Ovarian cancer | 5–80 μg/mL |
-Increasing the activity of Bax and caspase-3 -Decreasing the activity of Bcl-2 |
In vitro | NIH-OVCAR-3, SKOV3, and CAOV-3 | [41] |
| Gastric cancer | 25, 50, 100, 200, 400, 800 μg/ml | Decrease HBV infection | In vitro | HepG2.2.15 | [42] |
| Gastric cancer | 12.5, 25, 50, 100, 200 μM | Increase autophagy | In vitro | HepG2, HepG2.2.15 | [43] |
| Gastric cancer | 0–100 μg/ml | Decrease proliferation and increase apoptosis | In vitro | HepG2 | [44] |
| Gastric cancer | 0–150 μM | Increase autophagy | In vitro | HepG2 | [45] |
| Breast cancer | 5 -20 μg/mL | Increase the control of caspase-9, caspase-3, PARP | In vitro | MCF-7 | [9] |
| Breast Cancer | 5 μM | The suppression of N-cadherin | In vitro | HCC1806, MDA-MB-231, MDA-MB-157, MCF-7, | [46] |
| Endometrial cancer | 20- 60 μM | Reduce the activity of Akt/ PI3K/mTOR/HIF-1α pathway to inhibit control of HIF-1α/VEGFA | In vitro | AN3CA, PHES, THP-1, RL95-2, | [47] |
| Breast cancer | 10–320 μM | Increased control of caspase-9, caspase-8, caspase-3 | In vitro | 4 T1 | [48] |
| Breast cancer | 20–120 μmol/L | Decreasing the activity of the p53 /Bcl-2 pathway | In vitro | MCF-7 | [49] |
| Breast cancer | 0–80 μM | Reduce control of the PI3K /Akt pathway | In vitro | T47D | [50] |
| Breast cancer | 40 nmol | Focus on pathways that either promote vascular growth or programmed cell death | In vitro | Hs578T | [51] |
| Breast cancer | 25- 100 mg/L | Reduce the control of VEGF and HIF-1α | In vitro | MCF-7 | [52] |
| Breast cancer | 10- 50 ug/mL | Decrease control of HIF-1α, NF-κB | In vitro | MCF-7, E0771 and MDA-MB- 231 | [31] |
| Ovarian cancer | 20–100 μg/ mL | Reduce control of AQP5, NF-κB, IκB-α and p65 | In vitro | SKOV3 | [27] |
| Endometrial cancer | 100 μM | The blocking of MAPK and Akt pathways | In vitro | Ishikawa cells | [53] |
| Breast cancer | 5–20 μM | Reducing the activity of the ERK/NF-κB/PI3K pathway | In vitro | MCF-7 | [54] |
| Breast cancer | 25–100 μM | The Wnt pathway and its target gene c-MYC can be suppressed | In vitro | MDA-MB-231 | [30] |
| Ovarian cancer | 20–40 μmol/L | Lessen the control of ETAR-influenced processes | In vitro | HEY and OVCA 433 | [55] |
| Ovarian cancer | 25- 100 μM | The expression of p21 can be increased, while the expression of PCNA and Bcl-xL can be reduced, and Bax can be elevated | In vitro | SKOV-3, OVCAR-3, PA-1 | [56] |
| Ovarian cancer | 50 mg/kg | Prevents the development of cancer by controlling the activity of PTEN/mTOR/Akt pathway | In vivo | – | [41] |
| Glioma | 87 mg/kg | Induce apoptosis | In vivo, In vitro | C6 | [57] |
| Breast cancer | 300 μg | DNMT2 methylation activity is inhibited | In vivo | – | [58] |
| Breast cancer | 50–100 mg/ kg | Suppresses cancer VEGF expression | In vivo | – | [31] |
| Endometrial cancer | 50 mg/kg | Preventing cancer angiogenesis and growth | In vivo | – | [59] |
| Endometrial cancer | 65 mg/kg | The suppression of VEGF has been demonstrated | In vivo | – | [60] |
| Ovarian cancer | 12.4 g/L | Reducing the amount of ETAR and ET-1 in cancer cells has been shown to impede their growth | In vivo | – | [55] |
EGCG and epigenetic modification
Cancer is affected by both genetic and epigenetic processes. Epigenetics can modify the gene expression without changing the genetic code, and the epigenetic mechanisms implicated in this alteration are DNA methylation and histone acetylation. These alterations lead to the formation of malignant cells. DNA methylation is the most studied epigenetic modification in human cells and is regulated by DNMTs. When DNA is hypermethylated by DNMTs, it can prevent binding of transcription factors to promoters and activate silencing proteins, resulting in gene silencing. The natural compound EGCG can interact with the catalytic site of DNMTs and inhibit its activity [61]. EGCG is capable of reversing methylation-associated decreases in the expression of the tumor suppressor the retinoic acid receptor β, p16INK4a, the DNA mismatch repair gene human mutL homolog 1 and O6-methylguanine methyltransferase, in esophageal cells, and subsequently attenuates cell growth and colony formation [62]. EGCG has been found to prevent cell growth and increase apoptosis in renal carcinoma cells, likely due to the fact that it boosts tissue factor pathway inhibitor-2 (TFPI-2) production. Since higher levels of TFPI-2 are correlated with lower levels of malignancy, this could explain why EGCG might have this effect [63]. Compared to the process of methylation, increasing histone acetylation leads to an open chromatin structure that triggers transcriptional activation. In skin cancer cells, scientists have discovered that EGCG can increase the level of acetylation on histone H3 and H4, thereby raising the expression of tumor-suppressor genes, including p16INK4a and Cip1/p21 [64]. EGCG has been shown to decrease androgen receptor acetylation, ultimately leading to the repressing of androgen-mediated transcription and cell growth in prostate carcinoma cells [65]. Recently, Ko et al. [66] indicated that EGCG could reduce Smad signalling through inhibiting acetylation in lung carcinoma cells. However, the influence of EGCG on acetylation is debatable and varies depending on the cell type and environment.
Biogenesis and the role of miRNAs
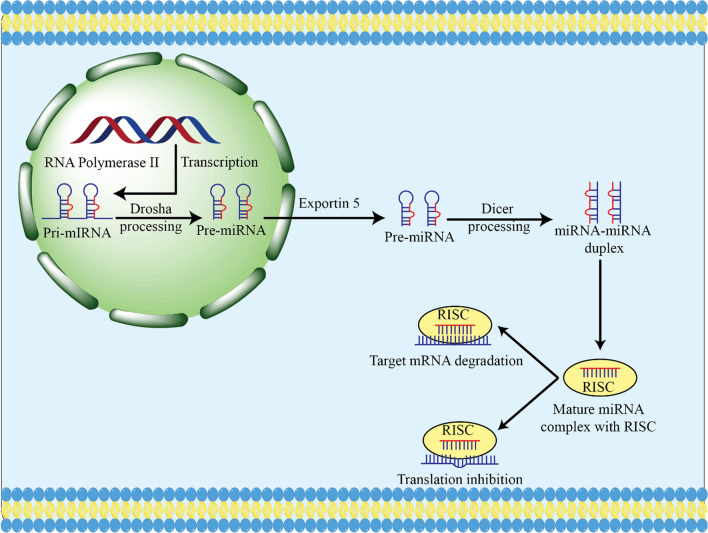
The majority of genes responsible for encoding miRNAs are situated in intergenic locations, while a few are in intragenic regions. Generally, miRNA transcription is done utilizing RNA polymerase II; however, there is a subsample of miRNAs located next to repetitive sequences such as Alu that are transcribed using RNA polymerase III (Fig. 3). The end product of transcription for both enzymes is the same, which is known as primary microRNA, which is denoted by a cap and a polyadenylated tail [67, 68]. The initial processing of miRNA takes place in the nucleus, wherein the pri-microRNA is cut by Drosha and its associated protein, DGCR8, at the hairpin region. This yields a stem-loop structure of roughly 70 nucleotides, known as pre-miR, which is then transported to the cytoplasm and further processed by exportin. After pre-miRNA is generated in the cytoplasm, Dicer launches the subsequent stage of processing. This enzyme leads to a double stranded molecule made of 18–25 nucleotides that consists of complimentary trailer and leader strands. The strand attaching to the Argonature proteins are destroyed, while its counterpart is then accumulated by the RNA-induced silencing complex (RISC) [69]. This evidence suggests that the 5-ends of both strands of the duplex are relatively unstable, and thus are more likely to be cleaved. However, the overall stability of the duplex has shown to remain intact, indicating that neither strand will be completely broken down [69].
Fig. 3.
A diagrammatic summary of the standard process of miRNA creation
The role of miRNAs in different cancers
The first studies linking miRNAs to cancer were done in 2002 on chronic lymphocytic leukemia patients. Calin and colleagues observed that in about 65 percent of these patients, the clusters of miR-15a and miR-1-16 genes are negatively regulated or deleted [70]. In fact, their tumor suppressor role was proposed. Subsequently, the same group prepared a map of a significant percentage of miRs and they saw that 52.5 percent of them are located in areas adjacent to cancer or fragile. In the year 2005, the first report stating the lack of proper regulation of 29 miRNAs in breast cancer was published. MiRNAs expression patterns are highly regulated. These molecules have important roles in proliferation, apoptosis and differentiation. Several studies have shown that the expression profile of miRNAs in normal tissues is different from tumor tissues and it is also different between types of tumors. MicroRNAs are one of the main regulators of programmed death in the tumorigenic process, and the survival of cancer cells is controlled by manipulating these microRNAs. On the other hand, cancer cells maintain their immortality by maintaining telomeres through the upregulation of telomerase. MiRNAs that are not functioning properly can lead to an increase in telomerase activity in tumors [71]. A number of miRNA have been found to be involved in epithelial-mesenchymal transition (EMT), which is a fundamental element of the process of invasion and metastasis of tumors [72]. It is remarkable to observe that a large proportion of microRNAs are positioned at regions of instability within the genome, many of which are connected to various forms of cancer. MicroRNAs play an important role in regulating anti-cancer immune responses via modulating of antigen processing and presentation, HLA-G expression, NKG2D ligands, PD-L1 level and metabolism in cancer cells [73]. Many body of MiRNAs have been identified that can induce angiogenesis via modulating different signaling pathways such as HIPK2, PML/Smad 1/5/8, PI3K/AKT, SRCIN1, TSP-1 and KLF2/KLF4/VEGF.
MiRNAs act as oncogenes
Studies have shown that miRNAs can have both oncogenic and tumor suppressor roles and they are called Oncomir and Ts-mir respectively. Oncomirs are observed in a range of tissue cancers and tend to occur in places of a genome with deletions, duplications, or genetic mutations. For example, 155 miRNAs are the only miRNAs that alone can induce tumorigenesis. Studies have shown that transgenic mice with increased expression of 155 miRNAs are prone to lymphoma [74]. There is enhanced expression of miRNA-155 found in a lot of different B-cell malignancies such as Hodgkin's lymphoma, a type of intense CLL and some Burkitt lymphoma. An oncogenic miRNA which is an earlier discovery is miRNA-17- 92 cluster which is located in chromosomal locus 13q31.3 in humans and it is formed from one polycistronic transcript, made up of seven microRNAs which contain miRNA-18a, miRNA-19b-1, miRNA-92-1, miRNA-17-5p, miRNA-19a, miRNA-17-3p, and miRNA-20a [75]. expression of miRNA-17-9 cluster increase in different types of cancers including lymphomas and lung cancer and many other cancers [75].
MiRNAs act as tumor suppressor
Let-7 is a prominent miRNA whose expression is notably decreased in malignant cells. It has the notable capability to act as a tumor suppressor, repressing oncogenes or genes that prevent cell differentiation or cause cell death. Low expression of let-7 has been seen in lung tumors in comparison to normal lung tissue. Furthermore, its compulsion to express has been seen to inhibit the development of cancer cells in vivo as well as in vitro. Additionally, a reduction in the expression of let-7 is connected with patients having shorter post-surgery life spans in a variety of cancer types [76].
Natural product compounds and miRNAs in cancer
Natural product compounds and their derivatives have been employed in the treatment of various illnesses like diabetes, neurological issues, gastrointestinal diseases, obesity, and cancer. These kinds of compounds have been praised due to their unique molecular structures, possible biological activity, and reduced harm to healthy cells, making them a valuable tool in the fight against cancer by targeting multiple cellular pathways [77]. The significant variety of natural products, coupled with their intricate mechanisms, poses a hurdle to their use in different contexts [78, 79]. Natural products have an advantage in treating tumorigenesis because their multifunctionality mechanism allows them to interact with multiple cellular signaling pathways that are involved in the multi-stage process [80, 81]. It is believed that utilizing natural products to treat cancer is more effective compared to single chemotherapy treatments because they can target multiple areas at the same time. This is why single chemotherapy is often not successful in treating cancer and why natural products may be better suited for the task [82].
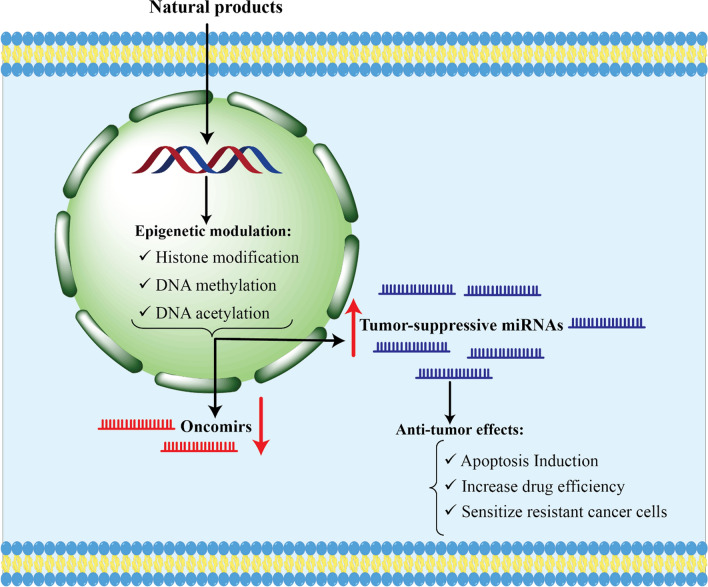
Recent evidence has shown that natural products can act against cancer growth by altering the epigenetics of cancer cells, in addition to displaying other possible biological activities [83]. Natural compounds have the capability of preventing cancer by interacting with a variety of misaligned cell signaling pathways [84]. Likewise, miRNAs influence a large number of different biological processes, including tumor growth, advancement, and cellular death processes [85]. It has been suggested that natural products have the capacity to modulate miRNAs, which could ultimately lead to therapeutic treatments for cancer and a variety of other diseases. Evidence points to the fact that both natural compounds and miRNAs affect many cellular targets, as shown in Fig. 4. The results of the research suggested that natural products can have anti-cancer properties by altering miRNA profiles, yet the exact mechanism remains unknown.
Fig. 4.
A visual representation of how natural product compounds can alter miRNAs in cancer cells
Curcumin has been found to reduce tumor cell invasion and inhibit EMT in breast cancer cells by regulating expression of miR-34a, resulting in the suppression of the CD24 Slug, and Axl, genes which are responsible for the promotion of EMT [86]. In addition, curcumin inhibits the proliferation and movement of thymic carcinoma cells, and disrupts the signaling pathways of Notch and mTOR by decreasing the expression of miR-27a [87]. Despite this, combining the MAPK inhibitor PD98059 resulted in a marked increase in the anti-tumor effects [88]. Resveratrol has an effect on multiple tumor suppressor microRNAs (miR-200c-3p, miR-409-3p, miR-542-3p, miR-122-5p, miR-125b,) to regulate the cell cycle and apoptosis of breast cancer cells. This increases the amount of Bcl-2, XIAP, and CDKs thus suppressing tumor development and inducing cell death [89].
Quercetin has been found to increase the expression of miR-16 and HOXA10, which in turn diminishes the viability of the oral cancer cells as well as the invasion, migration, and activity of MMP-2 and MMP-9 [90]. Additionally, quercetin has been found to have an anti-cancer effect in OSCC by increasing the level of miR-22. It is believed that by raising miR-22 with the assistance of quercetin, levels of Wnt1/β-catenin signaling are diminished, both in lab and animal testings, resulting in a reduced tumor mass [91].
Epigallocatechin-3-gallate and the modulation of miRNAs in different cancers
Green tea produced from the leaves of the Camellia sinensis plant is a very popular drink around the world. Green tea polyphenols have preventive effects due to their anticancer properties in many cancers, including breast cancer [49], hepatocellular carcinoma (HCC) [92], prostate cancer [93], gastric and colorectal cancer [94], Lung cancer [95], and are also useful in the treatment of diabetes, Parkinson's, stroke, Alzheimer's and obesity. Important polyphenols of green tea are Epicatechin (EC), (Epigallocatechin) (EGC), (Epicatechin-3-gallate) (ECG) (Epigallocatechin-3-gallate) (EGCG). J Qin's study with the effect of EGCG on the T24 bladder cancer cell line resulted in beneficial results, including the inhibition of cancer cell growth by the effect of EGCG[96]. Jun Ma and his colleagues evaluated cell survival and death in the treatment of AGS gastric cancer cells with EGCG (a component of green tea) and stated that EGCG contained in green tea extract promotes apoptosis and inhibition of proliferation in AGS gastric cancer cells [97]. In another study, Nishikawa performed EGCG treatment on hepatocellular carcinoma cell line (HCC) and reported that EGCG inhibited the growth of these cancer cells and also decreased the expression of Bcl_2 and Bcl_XL genes as its concentration increased [98]. Green tea' catechins anticancer effects may be caused by a number of different methods, including inhibition protein synthesis, lipogenesis, cell growth cell motility and invasion, telomerase, angiogenesis, induction apoptosis and cell death. It seems that the chemotherapeutic effects of catechins take place through the regulation of microRNA expression profile. According to a study, treatment of different cervical carcinoma cells to EGCG showed that can inhibit cell growth and induce apoptosis via modulating various miRNAs expression [99]. The expression of hsa-miR-221, hsa-miR-222, hsa-miR-21, hsa-miR-146b and hsa-miR-204 involved in thyroid cancer progression, differentiation Thyroid-specific genes in the anaplastic thyroid carcinoma (ATC) cell lines (8505C cell line and SW1736 cell line) was assessed after treatments with EGCG in another study. EGCG increased all the mRNAs related to differentiation Thyroid-specific genes in 8505C cells and reduced in SW1736 cells. In SW1736, EGCG had no effect on cell growth ability, while it had a reducing effect on the formation of colonies in 8505C cells.in this study, only hsa-miR-221 downregulated by EGCG and had no effect on the expression of other miRNAs involved in tumor tumorigenesis [100]. Appari et al. [101] attempted to improve promising pancreatic cancer therapy by single or combined Sulforaphane, quercetin and catechins. In MIA-PaCa2 cells, single bioactives significantly reduced the colony-forming capacity and effect of ECG or CG was superior to EGCG. The combination quercetin/sulforaphane and sulforaphane/EGCG had more strong effect in inhibition colony formation than single bioactives. Besides, in MIA-PaCa2 and BxPc-3 cells, the effect of the combination of natural bioactives in reducing spheroid formation, viability and apoptosis was stronger compared to single doses. Reduced expression of miR-let-7 and increased expression of K-ras in different cancers is well proven. Therefore, in this study, effect of treatment of combination or single natural bioactives on expression of miR-let-7 and K-ras assessed in MIA-PaCa2, BxPC-3, primary PDA cells, PacaDD-183 and CRL 1097 non-malignant pancreatic ductal cells. Single or combination treatments had strong effects on up-regulation of miR-let-7 and down-regulation of K-ras in all cells expect non-malignant CRL 1097 cells [101]. Next-Generation Sequencing (NGS) study using MDA-MB-231 cells and EGCG-treated MDA-MB-231 cells showed that EGCG can harmonize different breast cancer-related pathways by modifying 873 known and 47 novel miRNAs expressions. This study, potential role of EGCG in up- and down-regulation of tumor suppressor and oncogenic miRNAs, respectively is well confirm [102]. In another study, Green Tea’ EGC and EGCG alone significantly altered the key molecules in neuroblastoma SH-SY5Y and SK-N-DZ Cells that have role in extrinsic and intrinsic apoptotic pathways and induced morphological features of apoptosis in these cells [103]. miR-7-1 expression in both cell lines increased via treatment with EGC or EGCG. This microRNA is a potent tumor suppressor that cells committed to entry apoptosis process [103]. Table 2 lists various studies on the effects of EGCG on microRNAs in various cancers.
Table 2.
The effects of EGCG on microRNAs in various cancers
| microRNA | Study type | Model | Target | Effects | Refs. |
|---|---|---|---|---|---|
| miR-21, miR-16 | In vitro | Breast cancer | Down | Controlling NF-kB | [104] |
| hsa-miR-204, hsa-miR-222, hsa-miR-21, hsa-miR-146b, hsa-miR-221 | In vitro | Thyroid cancer | Up | Improving the apoptosis | [100] |
| miR-106b, miR-93, miR-92 | In vitro | Malignant neuroblastoma | Down | Improving the apoptosis | [105] |
| miR-99a, miR-34a, miR-7-1 | In vitro | Malignant neuroblastoma | Up | Improving the apoptosis | [105] |
| miR-27, miR-21 | In vitro | Breast cancer | Dow | Changes in the miRNA control of possible cancer-causing genes and tumor-suppressing genes | [10] |
| miR-16 | In vivo | Breast cancer | Up | NF-κB pathway | [106] |
| miR-485 | In vivo | Non-small cell lung cancer | Up | Control miR-485/CD44 axis | [107] |
| hsa-mir-485-5p | In vivo | Non-small cell lung cancer | Up | Control the hsa-mir-485-5p/RXRα axis | [108] |
| miR483-3p | In vivo | Hepatocellular carcinoma cells | Down | Hypermethylation of the miR483-3p promoter region | [109] |
| miR-155-5p | In vitro | Colorectal Cancer | Up | Suppressing GRP78/NF-κB/miR-155-5p/MDR1 Pathway | [110] |
| miR-1226-3p, miR-185-3p, miR-642a-5p, miR-3116, miR-31-5p, | In vitro | Bladder cancer | Up | Modulation Hippo signaling pathway | [111] |
|
hsa-miR-1915 hsa-miR-29b-1-5p hsa-miR-210 hsa-miR-1225-5p hsa-miR-1202 hsa-miR-1246 hsa-miR-1973 hsa-miR-3162 hsa-miR-4281 hsa-miR-3656 hsa-miR-3665 hsa-miR-1207-5p hsa-miR-3196 hsa-miR-34a hsa-miR-2861 |
In vitro | Nasopharyngeal carcinoma | Up | Apoptosis, Cell cycle, Cell proliferation | [112] |
| miR-296 | In vitro | Nasopharyngeal Carcinoma | Up | Suppressing migratory properties of anoikis-resistant cells | [113] |
| miR-34a, let-7a | In vitro | Hepatocellular carcinoma | Up | Improving cytotoxicity and inducing the apoptotic pathway | [114] |
| miR-195 | In vitro | Prostate cancer | Up | Affect on EMT | [115] |
| miR-181a | In vitro | Prostate Cancer | Up | Prompting the cellular apoptosis | [116] |
| miRNA-21 | In vivo | Prostate cancer | Down | Suppressing cells growth and Antagonist of AR signaling and | [117] |
| miRNA-330 | In vivo | Prostate cancer | Up | Suppressing cells growth and antagonist of AR signaling and | [117] |
| miR-200c, miR-145, miR-34a, | In vitro | Colorectal cancer | Up | Suppressing Notch and Bmi1, Ezh2, and Suz12 | [118] |
| miR-210 | In vitro | Lung cancer | Up | Anti-proliferation | [8] |
| miR-30e-3p | In vitro | Malignant melanoma | Up | Controlling circ_MITF/miR-30e-3p/HDAC2 axis | [119] |
| microRNA-let-7b | In vitro | Malignant melanoma | Up | Down-modulstion of high mobility group A2 (HMGA2) | [120] |
| miR-204 | In vitro | Oral squamous cell carcinomas | Down | miR204-mediated inhibition of Sox4 and Slug | [121] |
| miR-25 | In vitro | Breast cancer | Down | Anti-proliferation and pro-apoptosis | [9] |
| let-7 | In vitro | Lung cancer | Up | Let-7 signaling pathway | [122] |
| hsa-miR-98-5p | In vitro | Non-small cell lung cancer | Down | Elevating of p53 | [123] |
| miR-133a/b | In vivo | Prostatic hyperplasia | Down | [124] | |
| miR-203, miR-125b | In vitro | Cervical carcinoma | Down | Induce cell cycle arrest and apoptosis | [99] |
| miR-29a, miR-210, miR-29, miR-203, miR-125b, | In vitro | Cervical carcinoma | Up | Induce cell cycle arrest and apoptosis | [99] |
Acknowledgements
Medical Science Research Project Plan of Hebei province (NO. 20190873). Youth Found Project of Hebei Education Department (NO. QN2018031). 2020 Scientific Research Project of Hebei North University (NO. YB2020017).
Author contributions
CW, WB, ZS, NY, AZ, SG, and ZA contributed to the conception, design, and drafting of the manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Meiling Bai, Email: blxiaobai2022@126.com.
Zatollah Asemi, Email: asemi_r@yahoo.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20(5):338–349. doi: 10.1038/s41571-023-00747-0. [DOI] [PubMed] [Google Scholar]
- 3.Maresso KC, Basen-Engquist K, Hawk E. Cancer epidemiology, prevention, and survivorship. In: Hagberg C, Gottumukkala V, Buggy D, editors. Perioperative care of the cancer patient. Amsterdam: Elsevier; 2023. pp. 3–14. [Google Scholar]
- 4.Colditz GA, Dart H. Cancer: epidemiology and associations between diet and cancer. Encyclopedia of human nutrition. 4. Amsterdam: Elsevier; 2023. pp. 146–153. [Google Scholar]
- 5.Ongnok B, Chattipakorn N, Chattipakorn SC. Doxorubicin and cisplatin induced cognitive impairment: the possible mechanisms and interventions. Exp Neurol. 2020;324:113118. doi: 10.1016/j.expneurol.2019.113118. [DOI] [PubMed] [Google Scholar]
- 6.Cao K, Tait SW. Apoptosis and cancer: force awakens, phantom menace, or both? Int Rev Cell Mol Biol. 2018;337:135–152. doi: 10.1016/bs.ircmb.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Yamada S, Tsukamoto S, Huang Y, Makio A, Kumazoe M, Yamashita S, et al. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci Rep. 2016;6:19225. doi: 10.1038/srep19225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis. 2011;32(12):1881–1889. doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zan L, Chen Q, Zhang L, Li X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered. 2019;10(1):374–382. doi: 10.1080/21655979.2019.1657327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fix LN, Shah M, Efferth T, Farwell MA, Zhang B. MicroRNA expression profile of MCF-7 human breast cancer cells and the effect of green tea polyphenon-60. Cancer Genomics Proteomics. 2010;7(5):261–277. [PubMed] [Google Scholar]
- 11.Bhardwaj V, Mandal AKA. Next-generation sequencing reveals the role of epigallocatechin-3-gallate in regulating putative novel and known microRNAs which target the MAPK pathway in non-small-cell lung cancer A549 cells. Molecules (Basel, Switzerland) 2019;24(2):368. doi: 10.3390/molecules24020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501(1):65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landis-Piwowar KR, Kuhn DJ, Wan SB, Chen D, Chan TH, Dou QP. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (-)-EGCG analogs and their prodrugs. Int J Mol Med. 2005;15(4):735–742. [PubMed] [Google Scholar]
- 14.Khandelwal A, Hall JA, Blagg BS. Synthesis and structure–activity relationships of EGCG analogues, a recently identified Hsp90 inhibitor. J Org Chem. 2013;78(16):7859–7884. doi: 10.1021/jo401027r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsubara S, Shibata H, Ishikawa F, Yokokura T, Takahashi M, Sugimura T, et al. Suppression of helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun. 2003;310(3):715–719. doi: 10.1016/j.bbrc.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 16.Bimonte S, Cascella M, Barbieri A, Arra C, Cuomo A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: an update of the current state of knowledge. Infect Agent Cancer. 2020;15:2. doi: 10.1186/s13027-020-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Haque I, Ray P, Ghosh A, Dutta D, Quadir M, et al. CCN5 activation by free or encapsulated EGCG is required to render triple-negative breast cancer cell viability and tumor progression. Pharmacol Res Perspect. 2021;9(2):e00753. doi: 10.1002/prp2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deepak Singh D, Han I, Choi EH, Yadav DK. CRISPR/Cas9 based genome editing for targeted transcriptional control in triple-negative breast cancer. Comput Struct Biotechnol J. 2021;19:2384–2397. doi: 10.1016/j.csbj.2021.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan MA, Hussain A, Sundaram MK, Alalami U, Gunasekera D, Ramesh L, et al. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol Rep. 2015;33(4):1976–1984. doi: 10.3892/or.2015.3802. [DOI] [PubMed] [Google Scholar]
- 20.Tudoran O, Soritau O, Balacescu O, Balacescu L, Braicu C, Rus M, et al. Early transcriptional pattern of angiogenesis induced by EGCG treatment in cervical tumour cells. J Cell Mol Med. 2012;16(3):520–530. doi: 10.1111/j.1582-4934.2011.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis LZ, Liu W, Luo Y, Okamoto M, Qu D, Dunn JH, et al. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem Biophys Res Commun. 2011;414(3):551–556. doi: 10.1016/j.bbrc.2011.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JD, Chen SH, Lin CL, Tsai SH, Liang YC. Inhibition of melanoma growth and metastasis by combination with (-)-epigallocatechin-3-gallate and dacarbazine in mice. J Cell Biochem. 2001;83(4):631–642. doi: 10.1002/jcb.1261. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Lin Y, Liu H, Li J. Inhibition of invasion and up-regulation of E-cadherin expression in human malignant melanoma cell line A375 by (-)-epigallocatechin-3-gallate. J Huazhong Univ Sci Technolog Med Sci. 2008;28(3):356–359. doi: 10.1007/s11596-008-0330-3. [DOI] [PubMed] [Google Scholar]
- 24.Yang CS, Wang H. Cancer preventive activities of tea catechins. Molecules. 2016;21(12):1679. doi: 10.3390/molecules21121679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Z, Zhang Z, Han Y, Wang J, Wang Y, Chen X, et al. A review on anti-cancer effect of green tea catechins. J Funct Foods. 2020;74:104172. [Google Scholar]
- 26.Zhang L, Xie J, Gan R, Wu Z, Luo H, Chen X, et al. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J Cancer. 2019;10(26):6543–6556. doi: 10.7150/jca.34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan C, Yang J, Shen L, Chen X. Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch Gynecol Obstet. 2012;285(2):459–467. doi: 10.1007/s00404-011-1942-6. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Lei Z, Huang Z, Zhang X, Zhou Y, Luo Z, et al. Epigallocatechin-3-gallate(EGCG) suppresses melanoma cell growth and metastasis by targeting TRAF6 activity. Oncotarget. 2016;7(48):79557–79571. doi: 10.18632/oncotarget.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharifi-Rad M, Pezzani R, Redaelli M, Zorzan M, Imran M, Ahmed Khalil A, et al. Preclinical pharmacological activities of epigallocatechin-3-gallate in signaling pathways: an update on cancer. Molecules. 2020;25(3):467. doi: 10.3390/molecules25030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, et al. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281(16):10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 31.Gu JW, Makey KL, Tucker KB, Chinchar E, Mao X, Pei I, et al. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc Cell. 2013;5(1):9. doi: 10.1186/2045-824X-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh DD, Verma R, Tripathi SK, Sahu R, Trivedi P, Yadav DK. Breast cancer transcriptional regulatory network reprogramming by using the CRISPR/Cas9 system: an oncogenetics perspective. Curr Top Med Chem. 2021;21(31):2800–2813. doi: 10.2174/1568026621666210902120754. [DOI] [PubMed] [Google Scholar]
- 33.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2(6):350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 34.Neuhaus T, Pabst S, Stier S, Weber AA, Schrör K, Sachinidis A, et al. Inhibition of the vascular-endothelial growth factor-induced intracellular signaling and mitogenesis of human endothelial cells by epigallocatechin-3 gallate. Eur J Pharmacol. 2004;483(2–3):223–227. doi: 10.1016/j.ejphar.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Min KJ, Kwon TK. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr Med Res. 2014;3(1):16–24. doi: 10.1016/j.imr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt M, Schmitz HJ, Baumgart A, Guédon D, Netsch MI, Kreuter MH, et al. Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture. Food Chem Toxicol. 2005;43(2):307–314. doi: 10.1016/j.fct.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Yun SY, Kim SP, Song DK. Effects of (-)-epigallocatechin-3-gallate on pancreatic beta-cell damage in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;541(1–2):115–121. doi: 10.1016/j.ejphar.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 38.Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Ann Intern Med. 2006;144(1):68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- 39.Gloro R, Hourmand-Ollivier I, Mosquet B, Mosquet L, Rousselot P, Salamé E, et al. Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol. 2005;17(10):1135–1137. doi: 10.1097/00042737-200510000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Abdul Rahman A, Wan Ngah WZ, Jamal R, Makpol S, Harun R, Mokhtar N. Inhibitory mechanism of combined hydroxychavicol with epigallocatechin-3-gallate against glioma cancer cell lines: a transcriptomic analysis. Front Pharmacol. 2022;13:844199. doi: 10.3389/fphar.2022.844199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin J, Fu M, Wang J, Huang F, Liu H, Huangfu M, et al. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin-3-gallate in ovarian cancer. Oncol Rep. 2020;43(6):1885–1896. doi: 10.3892/or.2020.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pang JY, Zhao KJ, Wang JB, Ma ZJ, Xiao XH. Green tea polyphenol, epigallocatechin-3-gallate, possesses the antiviral activity necessary to fight against the hepatitis B virus replication in vitro. J Zhejiang Univ Sci B. 2014;15(6):533–539. doi: 10.1631/jzus.B1300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong L, Hu J, Shu W, Gao B, Xiong S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015;6(5):e1770. doi: 10.1038/cddis.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabry D, Abdelaleem OO, El Amin Ali AM, Mohammed RA, Abdel-Hameed ND, Hassouna A, et al. Anti-proliferative and anti-apoptotic potential effects of epigallocatechin-3-gallate and/or metformin on hepatocellular carcinoma cells: in vitro study. Mol Biol Rep. 2019;46(2):2039–2047. doi: 10.1007/s11033-019-04653-6. [DOI] [PubMed] [Google Scholar]
- 45.Zhao L, Liu S, Xu J, Li W, Duan G, Wang H, et al. A new molecular mechanism underlying the EGCG-mediated autophagic modulation of AFP in HepG2 cells. Cell Death Dis. 2017;8(11):e3160. doi: 10.1038/cddis.2017.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis KA, Jordan HR, Tollefsbol TO. Effects of SAHA and EGCG on growth potentiation of triple-negative breast cancer cells. Cancers (Basel) 2018;11(1):23. doi: 10.3390/cancers11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Man GCW, Chan TH, Kwong J, Wang CC. A prodrug of green tea polyphenol (-)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018;412:10–20. doi: 10.1016/j.canlet.2017.09.054. [DOI] [PubMed] [Google Scholar]
- 48.Wei R, Mao L, Xu P, Zheng X, Hackman RM, Mackenzie GG, et al. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018;9(11):5682–5696. doi: 10.1039/c8fo01397g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang CY, Han Z, Li X, Xie HH, Zhu SS. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol Lett. 2017;14(3):3623–3627. doi: 10.3892/ol.2017.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moradzadeh M, Hosseini A, Erfanian S, Rezaei H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol Rep. 2017;69(5):924–928. doi: 10.1016/j.pharep.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Braicu C, Pileczki V, Pop L, Petric RC, Chira S, Pointiere E, et al. Dual targeted therapy with p53 siRNA and epigallocatechingallate in a triple negative breast cancer cell model. PLoS ONE. 2015;10(4):e0120936. doi: 10.1371/journal.pone.0120936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo HQ, Xu M, Zhong WT, Cui ZY, Liu FM, Zhou KY, et al. EGCG decreases the expression of HIF-1α and VEGF and cell growth in MCF-7 breast cancer cells. J buon. 2014;19(2):435–439. [PubMed] [Google Scholar]
- 53.Park SB, Bae JW, Kim JM, Lee SG, Han M. Antiproliferative and apoptotic effect of epigallocatechin-3-gallate on Ishikawa cells is accompanied by sex steroid receptor downregulation. Int J Mol Med. 2012;30(5):1211–1218. doi: 10.3892/ijmm.2012.1104. [DOI] [PubMed] [Google Scholar]
- 54.Sen T, Moulik S, Dutta A, Choudhury PR, Banerji A, Das S, et al. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009;84(7–8):194–204. doi: 10.1016/j.lfs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Spinella F, Rosanò L, Di Castro V, Decandia S, Albini A, Nicotra MR, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits the endothelin axis and downstream signaling pathways in ovarian carcinoma. Mol Cancer Ther. 2006;5(6):1483–1492. doi: 10.1158/1535-7163.MCT-06-0053. [DOI] [PubMed] [Google Scholar]
- 56.Huh SW, Bae SM, Kim YW, Lee JM, Namkoong SE, Lee IP, et al. Anticancer effects of (-)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol Oncol. 2004;94(3):760–768. doi: 10.1016/j.ygyno.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 57.Kuduvalli SS, Daisy PS, Vaithy A, Purushothaman M, Ramachandran Muralidharan A, Agiesh KB, et al. A combination of metformin and epigallocatechin gallate potentiates glioma chemotherapy in vivo. Front Pharmacol. 2023;14:1096614. doi: 10.3389/fphar.2023.1096614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalil H, Tazi M, Caution K, Ahmed A, Kanneganti A, Assani K, et al. Aging is associated with hypermethylation of autophagy genes in macrophages. Epigenetics. 2016;11(5):381–388. doi: 10.1080/15592294.2016.1144007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang CC, Xu H, Man GC, Zhang T, Chu KO, Chu CY, et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis. 2013;16(1):59–69. doi: 10.1007/s10456-012-9299-4. [DOI] [PubMed] [Google Scholar]
- 60.Laschke MW, Schwender C, Scheuer C, Vollmar B, Menger MD. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod. 2008;23(10):2308–2318. doi: 10.1093/humrep/den245. [DOI] [PubMed] [Google Scholar]
- 61.Lee WJ, Shim J-Y, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005;68(4):1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 62.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Can Res. 2003;63(22):7563–7570. [PubMed] [Google Scholar]
- 63.Gu B, Ding Q, Xia G, Fang Z. EGCG inhibits growth and induces apoptosis in renal cell carcinoma through TFPI-2 overexpression. Oncol Rep. 2009;21(3):635–640. [PubMed] [Google Scholar]
- 64.Nandakumar V, Vaid M, Katiyar SK. (−)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p 16 INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32(4):537–544. doi: 10.1093/carcin/bgq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee Y-H, Kwak J, Choi H-K, Choi K-C, Kim S, Lee J, et al. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int J Mol Med. 2012;30(1):69–74. doi: 10.3892/ijmm.2012.966. [DOI] [PubMed] [Google Scholar]
- 66.Ko H, So Y, Jeon H, Jeong M-H, Choi H-K, Ryu S-H, et al. TGF-β1-induced epithelial–mesenchymal transition and acetylation of Smad2 and Smad3 are negatively regulated by EGCG in human A549 lung cancer cells. Cancer Lett. 2013;335(1):205–213. doi: 10.1016/j.canlet.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 67.Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochem Biophys Acta. 2010;1803(11):1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 68.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 69.Sotillo E, Thomas-Tikhonenko A. Shielding the messenger (RNA): microRNA-based anticancer therapies. Pharmacol Ther. 2011;131(1):18–32. doi: 10.1016/j.pharmthera.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Negrini M, Nicoloso MS, Calin GA. MicroRNAs and cancer—new paradigms in molecular oncology. Curr Opin Cell Biol. 2009;21(3):470–479. doi: 10.1016/j.ceb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Abdelgawad A, Mosbah A, Eissa LA. Expression of microRNA-155 and human telomerase reverse transcriptase in patients with bladder cancer. Egypt J Basic Appl Sci. 2020;7(1):315–322. [Google Scholar]
- 72.Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285(2):116–126. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 73.Yi M, Xu L, Jiao Y, Luo S, Li A, Wu K. The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol. 2020;13(1):1–14. doi: 10.1186/s13045-020-00848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hata A, Lieberman J. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci Signal. 2015;8(368):re3. doi: 10.1126/scisignal.2005825. [DOI] [PubMed] [Google Scholar]
- 75.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20(12):1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10(2):111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 77.Efferth T, Saeed ME, Kadioglu O, Seo E-J, Shirooie S, Mbaveng AT, et al. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol Adv. 2020;38:107342. doi: 10.1016/j.biotechadv.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discovery. 2015;14(2):111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 79.Smanski MJ, Zhou H, Claesen J, Shen B, Fischbach MA, Voigt CA. Synthetic biology to access and expand nature's chemical diversity. Nat Rev Microbiol. 2016;14(3):135–149. doi: 10.1038/nrmicro.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alnuqaydan AM, Rah B, Almutary AG, Chauhan SS. Synergistic antitumor effect of 5-fluorouracil and withaferin-A induces endoplasmic reticulum stress-mediated autophagy and apoptosis in colorectal cancer cells. Am J Cancer Res. 2020;10(3):799. [PMC free article] [PubMed] [Google Scholar]
- 81.Rah B, Nayak D, Rasool R, Chakraborty S, Katoch A, Amin H, et al. Reprogramming of molecular switching events in upr driven er stress: Scope for development of anticancer therapeutics. Curr Mol Med. 2016;16(8):690–701. doi: 10.2174/1566524016666160829152658. [DOI] [PubMed] [Google Scholar]
- 82.Singh I, Amin H, Rah B, Goswami A. Targeting EGFR and IGF 1R: a promising combination therapy for metastatic cancer. Front Biosci (Schol Ed) 2013;5:231–246. doi: 10.2741/s369. [DOI] [PubMed] [Google Scholar]
- 83.Li S, Kuo H-CD, Yin R, Wu R, Liu X, Wang L, et al. Epigenetics/epigenomics of triterpenoids in cancer prevention and in health. Biochem Pharmacol. 2020;175:113890. doi: 10.1016/j.bcp.2020.113890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan H, Reale M, Ullah H, Sureda A, Tejada S, Wang Y, et al. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: updates and future directions. Biotechnol Adv. 2020;38:107385. doi: 10.1016/j.biotechadv.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Yuan M, Zhang X, Zhang J, Wang K, Zhang Y, Shang W, et al. DC-SIGN–LEF1/TCF1–miR-185 feedback loop promotes colorectal cancer invasion and metastasis. Cell Death Differ. 2020;27(1):379–395. doi: 10.1038/s41418-019-0361-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gallardo M, Kemmerling U, Aguayo F, Bleak TC, Muñoz JP, Calaf GM. Curcumin rescues breast cells from epithelial-mesenchymal transition and invasion induced by anti-miR-34a. Int J Oncol. 2020;56(2):480–493. doi: 10.3892/ijo.2019.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han Z, Zhang J, Zhang K, Zhao Y. Curcumin inhibits cell viability, migration, and invasion of thymic carcinoma cells via downregulation of microRNA-27a. Phytother Res. 2020;34(7):1629–1637. doi: 10.1002/ptr.6629. [DOI] [PubMed] [Google Scholar]
- 88.Qiang Z, Meng L, Yi C, Yu L, Chen W, Sha W. Curcumin regulates the miR-21/PTEN/Akt pathway and acts in synergy with PD98059 to induce apoptosis of human gastric cancer MGC-803 cells. J Int Med Res. 2019;47(3):1288–1297. doi: 10.1177/0300060518822213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Venkatadri R, Muni T, Iyer A, Yakisich J, Azad N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell death Dis. 2016;7(2):e2104. doi: 10.1038/cddis.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao J, Fang Z, Zha Z, Sun Q, Wang H, Sun M, et al. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur J Pharmacol. 2019;847:11–18. doi: 10.1016/j.ejphar.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Zhang C, Hao Y, Sun Y, Liu P. Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/β-catenin axis. J Pharmacol Sci. 2019;140(2):128–136. doi: 10.1016/j.jphs.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Yang H, Wang M, Sun H, Zhu S, Jin J. Synergetic effect of EP1 receptor antagonist and (-)-Epigallocatechin-3-gallate in hepatocellular carcinoma. Pharmacology. 2019;104(5–6):267–275. doi: 10.1159/000502076. [DOI] [PubMed] [Google Scholar]
- 93.Duhon D, Bigelow RL, Coleman DT, Steffan JJ, Yu C, Langston W, et al. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol Carcinog. 2010;49(8):739–749. doi: 10.1002/mc.20649. [DOI] [PubMed] [Google Scholar]
- 94.Cerezo-Guisado MI, Zur R, Lorenzo MJ, Risco A, Martín-Serrano MA, Alvarez-Barrientos A, et al. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem Toxicol. 2015;84:125–132. doi: 10.1016/j.fct.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 95.Shi J, Liu F, Zhang W, Liu X, Lin B, Tang X. Epigallocatechin-3-gallate inhibits nicotine-induced migration and invasion by the suppression of angiogenesis and epithelial-mesenchymal transition in non-small cell lung cancer cells. Oncol Rep. 2015;33(6):2972–2980. doi: 10.3892/or.2015.3889. [DOI] [PubMed] [Google Scholar]
- 96.Qin J, Wang Y, Bai Y, Yang K, Mao Q, Lin Y, et al. Epigallocatechin-3-gallate inhibits bladder cancer cell invasion via suppression of NF-κB-mediated matrix metalloproteinase-9 expression. Mol Med Rep. 2012;6(5):1040–1044. doi: 10.3892/mmr.2012.1054. [DOI] [PubMed] [Google Scholar]
- 97.Ma J, Shi M, Li G, Wang N, Wei J, Wang T, et al. Regulation of Id1 expression by epigallocatechin-3-gallate and its effect on the proliferation and apoptosis of poorly differentiated AGS gastric cancer cells. Int J Oncol. 2013;43(4):1052–1058. doi: 10.3892/ijo.2013.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishikawa T, Nakajima T, Moriguchi M, Jo M, Sekoguchi S, Ishii M, et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J Hepatol. 2006;44(6):1074–1082. doi: 10.1016/j.jhep.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 99.Zhu Y, Huang Y, Liu M, Yan Q, Zhao W, Yang P, et al. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp Ther Med. 2019;17(3):1742–1748. doi: 10.3892/etm.2018.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Allegri L, Rosignolo F, Mio C, Filetti S, Baldan F, Damante G. Effects of nutraceuticals on anaplastic thyroid cancer cells. J Cancer Res Clin Oncol. 2018;144:285–294. doi: 10.1007/s00432-017-2555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Appari M, Babu KR, Kaczorowski A, Gross W, Herr I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int J Oncol. 2014;45(4):1391–1400. doi: 10.3892/ijo.2014.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Banerjee S, Mandal AKA. Role of epigallocatechin-3-gallate in the regulation of known and novel microRNAs in breast carcinoma cells. Front Genet. 2022;13:995046. doi: 10.3389/fgene.2022.995046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chakrabarti M, Ai W, Banik NL, Ray SK. Overexpression of miR-7-1 increases efficacy of green tea polyphenols for induction of apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-DZ cells. Neurochem Res. 2013;38(2):420–432. doi: 10.1007/s11064-012-0936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmed F, Ijaz B, Ahmad Z, Farooq N, Sarwar MB, Husnain T. Modification of miRNA Expression through plant extracts and compounds against breast cancer: Mechanism and translational significance. Phytomedicine. 2020;68:153168. doi: 10.1016/j.phymed.2020.153168. [DOI] [PubMed] [Google Scholar]
- 105.Chakrabarti M, Ai W, Banik NL, Ray SK. Overexpression of miR-7-1 increases efficacy of green tea polyphenols for induction of apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-DZ cells. Neurochem Res. 2013;38:420–432. doi: 10.1007/s11064-012-0936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jang J-Y, Lee J-K, Jeon Y-K, Kim C-W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13(1):1–12. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang P, Xu C, Chen L, Chen A, Wu X, Zhou M, et al. EGCG inhibits CSC-like properties through targeting miR-485/CD44 axis in A549-cisplatin resistant cells. Mol Carcinog. 2018;57(12):1835–1844. doi: 10.1002/mc.22901. [DOI] [PubMed] [Google Scholar]
- 108.Jiang P, Xu C, Chen L, Chen A, Wu X, Zhou M, et al. Epigallocatechin-3-gallate inhibited cancer stem cell–like properties by targeting hsa-mir-485-5p/RXRα in lung cancer. J Cell Biochem. 2018;119(10):8623–8635. doi: 10.1002/jcb.27117. [DOI] [PubMed] [Google Scholar]
- 109.Kang Q, Tong Y, Gowd V, Wang M, Chen F, Cheng K-W. Oral administration of EGCG solution equivalent to daily achievable dosages of regular tea drinkers effectively suppresses miR483-3p induced metastasis of hepatocellular carcinoma cells in mice. Food Funct. 2021;12(8):3381–3392. doi: 10.1039/d1fo00664a. [DOI] [PubMed] [Google Scholar]
- 110.La X, Zhang L, Li Z, Li H, Yang Y. (−)-Epigallocatechin Gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway. J Agric Food Chem. 2019;67(9):2510–2518. doi: 10.1021/acs.jafc.8b06665. [DOI] [PubMed] [Google Scholar]
- 111.Lee H-Y, Chen Y-J, Chang W-A, Li W-M, Ke H-L, Wu W-J, et al. Effects of Epigallocatechin Gallate (EGCG) on urinary bladder urothelial carcinoma-next-generation sequencing and bioinformatics approaches. Medicina. 2019;55(12):768. doi: 10.3390/medicina55120768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li B-B, Huang G-L, Li H-H, Kong X, He Z-W. Epigallocatechin-3-gallate modulates microrna expression profiles in human nasopharyngeal carcinoma CNE2 cells. Chin Med J. 2017;130(01):93–99. doi: 10.4103/0366-6999.196586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin C-H, Wang H-H, Chen T-H, Chiang M-C, Hung P-H, Chen Y-J. Involvement of microrna-296 in the inhibitory effect of epigallocatechin gallate against the migratory properties of anoikis-resistant nasopharyngeal carcinoma cells. Cancers. 2020;12(4):973. doi: 10.3390/cancers12040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mostafa SM, Gamal-Eldeen AM, Maksoud NAE, Fahmi AA. Epigallocatechin gallate-capped gold nanoparticles enhanced the tumor suppressors let-7a and miR-34a in hepatocellular carcinoma cells. Anais da Academia Brasileira de Ciencias. 2020 doi: 10.1590/0001-3765202020200574. [DOI] [PubMed] [Google Scholar]
- 115.Fatemeh S, Katayoun DA. Efficacy of green tea extract on PC3 prostate cancer cells through upregulation of miR-195 expression and suppression of epithelial to mesenchymal transition. J Tradit Chin Med. 2022;42(5):681. doi: 10.19852/j.cnki.jtcm.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Safari F, Azad NR, Ezdiny AA, Pakizehkar S, Koohpar ZK, Ranji N. Antitumor activities of green tea by up-regulation of miR-181a expression in LNCaP cells using 3D cell culture model. Avicenna J Med Biotechnol. 2022;14(1):89. doi: 10.18502/ajmb.v14i1.8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25(4):1198. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toden S, Tran H-M, Tovar-Camargo OA, Okugawa Y, Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. 2016;7(13):16158. doi: 10.18632/oncotarget.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu K, Wei Y, Yu Y, Shan M, Tang Y, Sun Y. Green tea polyphenols inhibit malignant melanoma progression via regulating circ_MITF/miR-30e-3p/HDAC2 axis. Biotechnol Appl Biochem. 2022;69(2):808–821. doi: 10.1002/bab.2153. [DOI] [PubMed] [Google Scholar]
- 120.Yamada S, Tsukamoto S, Huang Y, Makio A, Kumazoe M, Yamashita S, et al. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci Rep. 2016;6(1):19225. doi: 10.1038/srep19225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu C-C, Chen P-N, Peng C-Y, Yu C-H, Chou M-Y. Suppression of miR-204 enables oral squamous cell carcinomas to promote cancer stemness, EMT traits, and lymph node metastasis. Oncotarget. 2016;7(15):20180. doi: 10.18632/oncotarget.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhong Z, Dong Z, Yang L, Chen X, Gong Z. Inhibition of proliferation of human lung cancer cells by green tea catechins is mediated by upregulation of let-7. Exp Ther Med. 2012;4(2):267–272. doi: 10.3892/etm.2012.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou D-H, Wang X, Feng Q. EGCG enhances the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC A549 cells. Nutr Cancer. 2014;66(4):636–644. doi: 10.1080/01635581.2014.894101. [DOI] [PubMed] [Google Scholar]
- 124.Zhou J, Lei Y, Chen J, Zhou X. Potential ameliorative effects of epigallocatechin-3-gallate against testosterone-induced benign prostatic hyperplasia and fibrosis in rats. Int Immunopharmacol. 2018;64:162–169. doi: 10.1016/j.intimp.2018.08.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.