Abstract
To evaluate the potential for an interaction between clarithromycin and loratadine, healthy male volunteers (n = 24) received each of the following regimens according to a randomized crossover design: 500 mg of clarithromycin orally every 12 h (q12h) for 10 days, 10 mg of loratadine orally q24h for 10 days, and the combination of clarithromycin and loratadine. A washout interval of 14 days separated regimens. The addition of loratadine did not statistically significantly affect the steady-state pharmacokinetics of clarithromycin or its active metabolite, 14(R)-hydroxy-clarithromycin. However, the addition of clarithromycin statistically significantly altered the steady-state maximum observed plasma concentration and the area under the plasma concentration-time curve over a dosing interval for loratadine (+36 and +76%, respectively) and for descarboethoxyloratadine (DCL), the active metabolite of loratadine (+69 and +49%, respectively). Clarithromycin probably inhibits the oxidative metabolism of loratadine and DCL by the cytochrome P-450 3A subfamily. Electrocardiograms (n = 12) were obtained over 24-h periods at baseline and steady state (day 10). The mean maximum QTc interval and area under the QTc interval-time curve on day 10 were modestly increased (<3%) from baseline for all three regimens, but no QTc interval exceeded 439 ms for any subject. Elevated steady-state concentrations of loratadine and DCL do not appear to be associated with adverse cardiovascular effects related to prolongation of the QTc interval. Loratadine and clarithromycin were well tolerated, alone and in combination.
Clarithromycin is a macrolide antibiotic with a broad spectrum of activity in vitro against clinically important gram-positive and gram-negative aerobes and anaerobes. The activity of clarithromycin is enhanced by its extensive distribution into tissues and by the formation of a primary microbiologically active metabolite, 14(R)-hydroxy-clarithromycin. Clarithromycin and other macrolide antibiotics can inhibit oxidative metabolism by forming inactive complexes with cytochrome P-450 (8). This has led to pharmacokinetic interactions between macrolide antibiotics and several drugs eliminated by oxidative metabolism (7, 15). A clinically important drug interaction between the macrolides clarithromycin and erythromycin and the nonsedating H1-antagonist terfenadine has been reported elsewhere (12). Inhibition of terfenadine first-pass metabolism by drugs including macrolide antibiotics and ketoconazole results in increased concentrations in plasma of unmetabolized terfenadine, which is a risk factor for drug-induced torsades de pointes (18), a rare but potentially life-threatening ventricular tachyarrhythmia associated with prolongation of the QT interval (2, 14).
Loratadine is a long-acting tricyclic antihistamine with selective peripheral histamine H1-receptor antagonistic activity. Unlike terfenadine and astemizole, loratadine appears to be devoid of cardiovascular effects, such as prolongation of the QT interval (1, 10). Loratadine is thought to undergo extensive first-pass metabolism, resulting in the formation of descarboethoxyloratadine (DCL), which possesses antihistamine activity (11, 13). DCL is also extensively metabolized (11, 13).
Because of the likelihood that clarithromycin and loratadine will be coadministered and because of the clinically important drug interactions between some macrolide antibiotics and some nonsedating antihistamines, the current study was undertaken to investigate the potential for a drug interaction between clarithromycin and loratadine.
(Results from this study were presented as a paper at the Third International Conference on the Macrolides, Azalides and Streptogramins, Lisbon, Portugal, January 1996.)
MATERIALS AND METHODS
Subjects.
Subjects were eligible for inclusion in the study if they were male, between 18 and 40 years of age, and within 10% of ideal weight range and were nonusers of tobacco. Subjects were excluded if they had a history of major illness or abnormal results in electrocardiogram (ECG) or laboratory tests (including a screening electrocardiographic QTc interval greater than 420 ms) or had consumed alcohol within 72 h of the start of the study, taken any prescription or over-the-counter medication within 2 weeks of the start of the study, or taken terfenadine, astemizole, loratadine, or oral antifungal medication within 90 days of the start of the study.
Prior to admission, a medical history was taken, a physical examination was performed, and routine laboratory tests (serum electrolytes, blood urea nitrogen, serum creatinine, liver function, urinalysis, and a complete blood count with differential) were performed for each subject. The study protocol was Investigational Review Board approved, and each subject gave written informed consent.
Drug administration.
Subjects were randomly assigned to one of six different sequences of the following three regimens: the combination of 500 mg of clarithromycin every 12 h (q12h) and 10 mg of loratadine daily, 10 mg of loratadine daily, and 500 mg of clarithromycin q12h. Each regimen was administered for 10 consecutive days; a washout period of at least 14 days separated the regimens. All study medications were administered with approximately 180 ml of water, swallowed whole, and not chewed. Loratadine doses were administered at approximately 0800 h, and clarithromycin doses were administered 12 h apart at approximately 0800 h and 2000 h.
Confinement and diet.
Subjects were confined to the study area from approximately 36 h prior to the first dose of study drug until approximately 48 h after the final dose in each period. Morning drug administration was under fasting conditions (breakfast consumed approximately 2 h after dosing), while evening drug administration was under nonfasting conditions (dinner consumed approximately 1 h before dosing). Lunch was consumed at 1400 h, and a light snack was consumed at 2200 h. Throughout each study period, subjects were provided standardized meals which were the same for all subjects and identical on corresponding days of each period. Consumption of grapefruit or a grapefruit-containing beverage was prohibited for the duration of the study.
Sample collection and analysis.
Venous blood samples (approximately 14 ml) for drug analysis were collected at 0800 h (immediately prior to drug administration) on day 1 and day 7 through day 9 of each period. Blood samples were collected immediately prior to and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 h after the 0800-h dosing on day 10. All blood samples were collected into two 7-ml heparinized collection tubes. Plasma was separated by centrifugation within 60 min after collection, placed in labeled plastic tubes, and stored frozen at −20°C or colder until analysis.
Plasma samples were assayed for clarithromycin and 14(R)-hydroxy-clarithromycin at BAS Analytics (West Lafayette, Ind.) by a validated high-performance liquid chromatography assay with electrochemical detection (4). The coefficients of variation were 12.6% for clarithromycin and 7.4% for 14(R)-hydroxy-clarithromycin at the lowest quality control concentration (0.04 μg/ml). The lower limit of quantitation for clarithromycin and 14(R)-hydroxy-clarithromycin was 0.02 μg/ml.
Plasma samples were assayed for loratadine and DCL at Phoenix International Life Sciences Inc. (Montreal, Quebec, Canada) by a validated high-performance liquid chromatography assay with mass spectrometric detection. The coefficients of variation were 4.0% for loratadine and 2.2% for DCL at the lowest quality control concentration (0.3 ng/ml). The lower limit of quantitation was 0.1 ng/ml for both loratadine and DCL.
Electrocardiographic monitoring.
Twelve-lead ECGs were obtained for pharmacodynamic assessment immediately prior to and at 1, 2, 3, 4, 6, 8, 12, 14, 16, 20, and 24 h after the 0800-h dosing on day 10 and at the corresponding times on day −1. The profile of the day −1 ECGs served as the baseline for that period. ECGs were obtained with a Hewlett-Packard PageWriter XLi Cardiograph Model M1700A electrocardiograph in the 12-lead format at 25 mm/s. The machine was configured to provide PR, QRS, and QT intervals and to calculate the corrected QT interval (QTc) by Bazett’s formula. In addition, a 10-s rhythm strip depicting leads II, V2, and V4 was obtained at 50 mm/s. If a given ECG showed a greater than 40-ms increase from baseline, the QTc was overread with the average value from three ventricular complexes in the rhythm strip lead (either II, V2, or V4) which provided the longest QTc. All overreadings were performed by the same investigator, who was blinded as to the treatment regimen each subject received. If the overreading confirmed a change within 20 ms of the change indicated by the automated electrocardiography reading, then the electrocardiograph interpretation was considered the primary determination.
Additional ECGs were obtained on day 1 (1000 h), day 2 through day 9 (1000 h), and day 12 (0800 h). Only if these ECGs were normal were the subjects allowed to continue in the study. These ECGs were not used for pharmacodynamic analysis.
Safety monitoring.
All observed or volunteered adverse events were recorded after administration of each dose, and their time or onset, severity, duration, and possible relationship to study drug were noted. Hematology and clinical chemistry were monitored. Blood pressures and pulse rates were also monitored throughout the study.
Data analysis.
Pharmacokinetic analysis was performed on plasma concentration-time data obtained following the final dose (day 10) of each period. Estimates for pharmacokinetic parameters were obtained by using noncompartmental methods. The maximum plasma concentration (Cmax) and the time to reach the maximum plasma concentration (Tmax) were read directly from the plasma concentration-time data for each subject. The area under the plasma concentration-time curve (AUC) was calculated by conventional linear trapezoidal summation. For clarithromycin and 14(R)-hydroxy-clarithromycin, Cmax, Tmax, and AUC0–12 were determined for the 12-h dosing interval beginning with the 0800-h dosing on day 10. For loratadine and DCL, Cmax, Tmax, and AUC0–24 were determined for the 24-h dosing interval beginning with the 0800-h dosing on day 10.
Pharmacokinetic parameters (Tmax and the natural logarithms of Cmax and AUC) for each of the four analytes of interest were compared between regimens by analysis of variance (ANOVA) with Procedure GLM of SAS version 6.09 (SAS Institute, Inc., Cary, N.C.). For loratadine and DCL, sources of variation included in the ANOVAs were subject, period, and regimen. Due to possible unequal carryover effects for clarithromycin and 14(R)-hydroxy-clarithromycin pharmacokinetic parameters, ANOVAs were performed with subject, period, regimen, and regimen of the preceding period as the sources of variation. For each analyte, point estimates and 95% confidence intervals were constructed within the ANOVA framework for Cmax and AUC for the combination regimen relative to clarithromycin alone or loratadine alone. To address whether steady state was achieved by day 10, pairwise comparisons were performed on day 7 through day 11 trough concentrations with the one-sample t test.
From the ECG data from each subject, the maximum QTc interval and the area under the QTc interval-versus-time curve (AURC; computed by linear trapezoidal summation) were determined over the 24-h intervals beginning at 0800 h on day −1 and day 10 of each period. Maximum QTc interval, AURC, and change in these variables from day −1 to day 10 were statistically analyzed with a crossover ANOVA model with effects for subject, sequence, period, and regimen. All statistical tests were performed at a significance level of 0.05 and were two tailed.
The study size (24 subjects) was designed to provide greater than 99 and 85% power to detect differences of 720 and 480 ms · h for AURC, respectively, between any two regimens. These differences in AURC correspond to average QTc interval differences of 30 and 20 ms over a 24-h period. For these calculations, a standard deviation of 25 ms for the average QTc interval difference was assumed, based on data from a previous study of interaction between clarithromycin and terfenadine (17). Also, the study was designed to have at least 80% power for detecting a 50% increase from monotherapy to combination therapy in the central values for Cmax and AUC of loratadine, DCL, clarithromycin, and 14(R)-hydroxy-clarithromycin.
RESULTS
Subjects.
A total of 24 healthy adult male volunteers between the ages of 23 and 40 years (mean age, 34 years) who weighed between 135 and 192 lb (mean weight, 160 lb) participated in this study, which was conducted at the South Florida Bioavailability Clinic in Miami, Fla. Of the subjects, 12 were Hispanic, 10 were white, and 2 were black. One subject was released due to an adverse event during the washout interval following the first period: safety and electrocardiographic data from this subject were included in the analyses, but his pharmacokinetic data were excluded.
Pharmacokinetics.
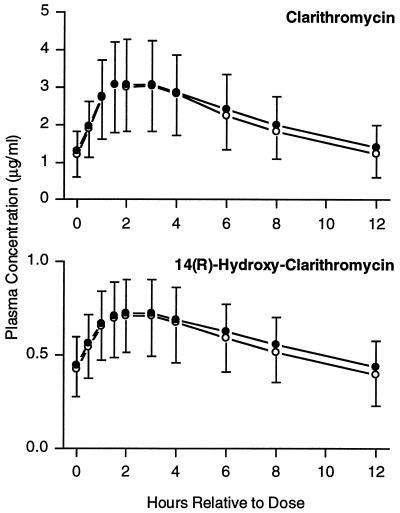
Mean steady-state plasma concentration-time profiles for clarithromycin and 14(R)-hydroxy-clarithromycin following administration of clarithromycin with loratadine were similar to those observed following administration of clarithromycin alone (Fig. 1). There were no statistically significant differences between regimens for Cmax, Tmax, or AUC0–12 for clarithromycin or 14(R)-hydroxy-clarithromycin (Table 1).
FIG. 1.
Mean steady-state clarithromycin and 14(R)-hydroxy-clarithromycin plasma concentration-time profiles following administration of clarithromycin alone or in combination with loratadine. Open circles, clarithromycin alone; filled circles, clarithromycin with loratadine. Error bars each represent 1 standard deviation.
TABLE 1.
Summary of pharmacokinetic parameters after dosing for 10 daysa
| Agent |
Cmax
|
Ratio of LSM (95% CI) |
Tmax (h)
|
AUC
|
Ratio of LSM (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| Single agent | Combination | Single agent | Combination | Single agent | Combination | |||
| Clarithromycin | 3.27 ± 1.24 | 3.26 ± 1.17 | 1.04 (0.78–1.38) | 2.9 ± 2.6 | 2.6 ± 2.2 | 26.0 ± 9.7 | 27.4 ± 9.7 | 1.07 (0.84–1.35) |
| 14(R)-Hydroxy-clarithromycin | 0.74 ± 0.22 | 0.76 ± 0.19 | 0.99 (0.81–1.20) | 2.8 ± 2.1 | 2.3 ± 0.8 | 6.81 ± 2.05 | 7.15 ± 1.75 | 1.00 (0.84–1.20) |
| Loratadine | 4.12 ± 4.45 | 7.25 ± 12.5b | 1.36 (1.17–1.60) | 1.3 ± 0.5 | 1.5 ± 0.6 | 14.6 ± 23.7 | 40.8 ± 116b | 1.76 (1.48–2.09) |
| DCL | 3.89 ± 2.41 | 6.41 ± 3.30b | 1.69 (1.53–1.86) | 2.9 ± 2.3 | 2.0 ± 0.8b | 52.7 ± 49.1 | 79.2 ± 77.1b | 1.49 (1.38–1.60) |
n = 23. Values, except for ratios and 95% confidence intervals (CI), are shown as means ± standard deviations. LSM, least-squares means obtained from ANOVA. Cmax values are expressed as micrograms per milliliter for clarithromycin and 14(R)-hydroxy-clarithromycin and as nanograms per milliliter for loratadine and DCL. AUC values are for 0 to 12 h, in micrograms · hour per milliliter, for clarithromycin and 14(R)-hydroxy-clarithromycin and for 0 to 24 h, in nanograms · hour per milliliter, for loratadine and DCL.
P ≤ 0.05 (ANOVA).
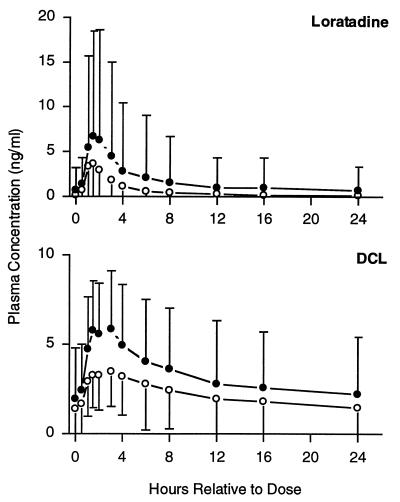
Mean loratadine and DCL plasma concentrations after administration of the combination regimen were greater throughout the dosing interval than that of loratadine administered alone (Fig. 2). Cmax and AUC0–24 values for loratadine and DCL were significantly greater after the combination regimen than after loratadine alone (Table 1). For loratadine, within-subject differences (combination versus monotherapy regimens) ranged from −33 to 227% for Cmax and from −18 to +423% for AUC0–24. For DCL, within-subject differences ranged from +22 to +161% for Cmax and from +1 to +103% for AUC0–24. Compared to monotherapy, the mean Tmax after the combination regimen was increased by 0.2 h for loratadine (not statistically significant) and decreased by 0.9 h for DCL (P = 0.049).
FIG. 2.
Mean steady-state loratadine and DCL plasma concentration-time profiles following administration of loratadine alone or in combination with clarithromycin. Open circles, loratadine alone; filled circles, loratadine with clarithromycin. Error bars each represent 1 standard deviation.
Statistical analysis of day 7 through day 11 trough plasma concentrations suggested that steady-state concentrations of clarithromycin, 14(R)-hydroxy-clarithromycin, and loratadine were achieved by day 10. For DCL, the increase in the mean concentrations from day 9 to day 10 was 0.01 ng/ml (P = 0.77), but then the increase from day 10 to day 11 was a statistically significant 0.14 ng/ml. There was also an isolated increase in clarithromycin and 14(R)-hydroxy-clarithromycin trough concentrations from day 10 to day 11, and there was confidence that there had been ample time for steady state to be reached for these compounds.
Pharmacodynamics and safety.
One subject was released due to an adverse event (atrial fibrillation) deemed not related to the study drug. The atrial fibrillation occurred during day 16 of the washout interval following the first period (loratadine alone) and spontaneously resolved within 2 h. Overall, the percentages of subjects with one or more adverse events were 13, 29, and 22% for the clarithromycin-with-loratadine, loratadine-alone, and clarithromycin-alone regimens, respectively. All adverse events were of mild intensity, with the exception of the moderate atrial fibrillation, and were transitory. No clinically significant laboratory test abnormalities that were considered to be related to study drug treatment were detected. There were no clinically relevant changes in blood pressures, pulse rates, or body temperatures in individuals during the study.
ECG findings are summarized in Table 2. The mean maximum QTc interval and AURC on day 10 were modestly increased (<3%) from baseline for all three regimens, but no QTc interval exceeded 439 ms for any subject. After 10 days of the combination regimen, maximum QTc intervals (range, 363 to 431 ms) were similar to baseline values (range, 371 to 439 ms): the individual changes in maximum QTc interval from baseline to day 10 ranged from −26 to +24 ms. The mean change from baseline in maximum QTc interval for the combination regimen (+4 ms) was not significantly different compared with that for loratadine alone (+3 ms) and was significantly less than that for the clarithromycin-alone regimen (+11 ms). AURC values at day 10 of the combination regimen (range, 8,538 to 9,979 ms · h) were also similar to those of baseline (range, 8,423 to 9,863 ms · h): the mean day 10 value of 9,314 ms · h corresponds to an average QTc interval of 388 ms. The change from baseline in AURC for the combination regimen (range, −130 to +609 ms · h) was significantly greater than that for loratadine alone (−281 to +407 ms · h) but was not significantly different compared with that for clarithromycin alone (−58 to +435 ms · h). Across all regimens, individual changes in average QTc intervals from baseline to day 10 ranged from −12 to +25 ms · h.
TABLE 2.
Summary of QTc and AURC measurements at baseline and after dosing for 10 daysa
| Agent (n) | Maximum QTc (ms)
|
AURC (ms · h)
|
||||
|---|---|---|---|---|---|---|
| Day −1 (baseline) | Day 10 | Change from baseline | Day −1 (baseline) | Day 10 | Change from baseline | |
| Clarithromycin (23) | 392 ± 17b | 403 ± 17 | +11 ± 9b | 9,047 ± 383 | 9,259 ± 380b | +212 ± 132 |
| Loratadine (24) | 393 ± 18b | 396 ± 17b | +3 ± 9 | 9,070 ± 359 | 9,115 ± 382b | +45 ± 179b |
| Clarithromycin and loratadine (23) | 400 ± 19 | 404 ± 17 | +4 ± 13 | 9,089 ± 414 | 9,314 ± 396 | +225 ± 181 |
Values are shown as means ± standard deviations.
Statistically significantly different (P ≤ 0.05 [ANOVA]) from the combination of clarithromycin and loratadine.
DISCUSSION
In this study, a pharmacokinetic interaction was observed between clarithromycin and loratadine, whereby clarithromycin increased concentrations in plasma of loratadine and DCL, the major active metabolite of loratadine. Pharmacokinetic parameters for clarithromycin or 14(R)-hydroxy-clarithromycin were unaffected by loratadine and were consistent with historical values for healthy adults (5).
Clarithromycin increased the steady-state Cmax and AUC0–24 central values for loratadine by 36 and 76%, respectively. Similarly, Brannan et al. reported that erythromycin increased the steady-state Cmax and AUC0–24 central values for loratadine by 53 and 40%, respectively (3). Since loratadine is well absorbed and extensively metabolized (13), these results suggest that clarithromycin and erythromycin inhibit loratadine metabolism. Clarithromycin (8, 9) and erythromycin (8) inhibit the metabolic activity of CYP3A, the cytochrome P-450 isoform subfamily predominantly involved in the metabolism of loratadine to DCL (19).
Clarithromycin increased the steady-state Cmax and AUC0–24 central values for DCL by 69 and 49%, respectively. In the Brannan et al. study, erythromycin increased the steady-state Cmax and AUC0–24 central values for DCL by 61 and 46%, respectively (3). The observed increase in DCL concentrations suggests that formation of DCL is not completely inhibited by concomitant administration of clarithromycin or erythromycin and that the metabolic clearance of DCL may be dependent on CYP3A. The former assertion is supported by the finding that, in the presence of ketoconazole or troleandomycin (inhibitors of CYP3A), loratadine is metabolized to DCL principally by the CYP2D6 isoform (19). The latter assertion is supported by the knowledge that DCL is highly metabolized (13) although the enzyme system(s) responsible has not yet been identified. In addition to increasing DCL plasma concentrations, clarithromycin reduced the Tmax of DCL from 2.9 to 2.0 h.
Although substantially elevated by concomitant macrolide administration, the mean Cmax and AUC0–24 values for loratadine and DCL observed in the erythromycin-loratadine interaction study (3) and the current study were below those that were well tolerated in studies involving larger doses of loratadine administered alone (1, 6, 16). For example, after administration of 40 mg of loratadine q24h for 10 days to healthy adult male subjects, mean Cmax and AUC0–24 values were 27.1 ng/ml and 96.0 ng · h/ml, respectively, for loratadine and 28.6 ng/ml and 420.7 ng · h/ml, respectively, for DCL (16). In widespread clinical studies involving daily doses of 10 to 40 mg, loratadine tolerability was comparable to that for placebo groups (6). Also, Brannan et al. reported that, despite the significant pharmacokinetic interaction observed between erythromycin and loratadine, therapeutic doses of these drugs taken in combination for 10 days were well tolerated, including an absence of effect on the QTc interval (3).
In the present study, although concentrations in plasma of loratadine and DCL were increased by concomitant clarithromycin administration, no corresponding electrocardiographic pharmacodynamic interaction was observed. Mean maximum QTc interval and AURC values on day 10 were slightly increased (<3%) from baseline for all three regimens. The increase in the maximum QTc interval or AURC from baseline after the combination regimen was not greater than that after clarithromycin alone. It has been suggested that clinically important electrocardiographic markers of proarrhythmic drug effect include >5% of treated subjects having QTc intervals of >500 ms and a change in the QTc interval for an individual subject of >70 ms (14). In the current study, no subject exhibited a QTc interval value of greater than 439 ms, and the greatest individual increase in average QTc interval was 25 ms.
In conclusion, concomitant administration of therapeutic doses of clarithromycin and loratadine was safe and well tolerated. Therefore, given the wide margin of safety associated with loratadine, the observed pharmacokinetic interaction between clarithromycin and loratadine is probably clinically unimportant.
ACKNOWLEDGMENTS
We thank Keith A. Erdman and Kristine M. Hopkins for their assistance in the analysis of the data and the preparation of the manuscript.
REFERENCES
- 1.Affrime M B, Lorber R, Danzig M, Cuss F, Brannan M D. Three month evaluation of electrocardiographic effects of loratadine in humans. J Allergy Clin Immunol. 1993;91:259. [Google Scholar]
- 2.Benedict C R. The QT interval and drug-associated torsades de pointes. Drug Invest. 1993;5:69–79. [Google Scholar]
- 3.Brannan M D, Reidenberg P, Radwanski E, Shneyer L, Lin C C, Cayen M N, Affrime M B. Loratadine administered concomitantly with erythromycin: pharmacokinetic and electrocardiographic evaluations. Clin Pharmacol Ther. 1995;58:269–278. doi: 10.1016/0009-9236(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 4.Chu S Y, Sennello L T, Sonders R C. Simultaneous determination of clarithromycin and 14(R)-hydroxyclarithromycin in plasma and urine using high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1991;571:199–208. doi: 10.1016/0378-4347(91)80446-j. [DOI] [PubMed] [Google Scholar]
- 5.Chu S Y, Wilson D S, Deaton R L, Mackenthun A V, Eason C N, Cavanaugh J H. Single- and multiple-dose pharmacokinetics of clarithromycin, a new macrolide antimicrobial. J Clin Pharmacol. 1993;33:719–726. doi: 10.1002/j.1552-4604.1993.tb05613.x. [DOI] [PubMed] [Google Scholar]
- 6.Clissold S P, Sorkin E M, Goa K L. Loratadine. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1989;37:42–57. doi: 10.2165/00003495-198937010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Descotes J, Andre P, Evreux J C. Pharmacokinetic drug interactions with macrolide antibiotics. J Antimicrob Chemother. 1985;15:659–664. doi: 10.1093/jac/15.6.659. [DOI] [PubMed] [Google Scholar]
- 8.Gascon M P, Dayer P. Comparative effects of macrolide antibiotics on liver monoxygenases. Clin Pharmacol Ther. 1991;49:158. [Google Scholar]
- 9.Gustavson L E, Kaiser J F, Edmonds A L, Locke C. Effect of omeprazole on concentrations of clarithromycin in plasma and gastric tissue at steady state. Antimicrob Agents Chemother. 1995;39:2078–2083. doi: 10.1128/aac.39.9.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hey J A, del Prado M, Egan R W, Sherwood J, Kreutner W. Loratadine produces antihistamine activity without adverse CNS, ECG or cardiovascular effects in guinea pigs. Comparative studies with terfenadine and sedating antihistamines. Int Arch Allergy Immunol. 1995;107:418–419. doi: 10.1159/000237062. [DOI] [PubMed] [Google Scholar]
- 11.Hilbert J, Radwanski E, Weglein R, Luc V, Perentesis G, Symchowicz S, Zampaglione N. Pharmacokinetics and dose proportionality of loratadine. J Clin Pharmacol. 1987;27:694–698. doi: 10.1002/j.1552-4604.1987.tb03090.x. [DOI] [PubMed] [Google Scholar]
- 12.Honig P K, Wortham D C, Zamani K, Cantilena L R. Comparison of the effect of the macrolide antibiotics erythromycin, clarithromycin and azithromycin on terfenadine steady-state pharmacokinetics and electrocardiographic parameters. Drug Invest. 1994;7:148–156. [Google Scholar]
- 13.Katchen B, Cramer J, Chung M, Gural R, Hilbert J, Luc V, Mortizen V, D’Souza R, Symchowicz S, Zampaglione N. Disposition of 14C-SCH 29851 in humans. Ann Allergy. 1985;55:393. [Google Scholar]
- 14.Morganroth J, Brozovich F V, McDonald J T, Jacobs R A. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol. 1991;67:774–776. doi: 10.1016/0002-9149(91)90541-r. [DOI] [PubMed] [Google Scholar]
- 15.Periti P, Mazzei T, Mini E, Novelli A. Pharmacokinetic drug interactions of macrolides. Clin Pharmacokinet. 1992;23:106–131. doi: 10.2165/00003088-199223020-00004. [DOI] [PubMed] [Google Scholar]
- 16.Radwanski E, Hilbert J, Symchowicz S, Zampaglione N. Loratadine: multiple-dose pharmacokinetics. J Clin Pharmacol. 1987;27:530–533. doi: 10.1002/j.1552-4604.1987.tb03061.x. [DOI] [PubMed] [Google Scholar]
- 17.Siepman N, Blahunka K S, Harris S I, Palmer R N. ECG changes observed following multiple dose co-administration of clarithromycin (C) and terfenadine (T) Pharm Res. 1993;10:S354. [Google Scholar]
- 18.Woosley R L, Chen Y, Freiman J P, Gillis R A. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269:1532–1536. [PubMed] [Google Scholar]
- 19.Yumibe N, Huie K, Chen K J, Snow M, Clement R P, Cayen M N. Identification of human liver cytochrome P450 enzymes that metabolize the nonsedating antihistamine loratadine. Formation of descarboethoxyloratadine by CYP3A4 and CYP2D6. Biochem Pharmacol. 1996;51:165–172. doi: 10.1016/0006-2952(95)02169-8. [DOI] [PubMed] [Google Scholar]