Abstract
Peripheral neuropathy (PN) and peripheral arterial disease (PAD) are life-limiting comorbidities among adults with lower-limb loss that may not be adequately addressed in current care models. The objective of this study was to evaluate underreporting of PN and PAD among adults with lower-limb loss. We conducted a secondary analysis of a cross-sectional dataset of community-dwelling adults with unilateral lower-limb loss seen in an outpatient Limb Loss Clinic (n = 196; mean age = 56.7 ± 14.4 years; 73.5% male). Individuals participated in standardized clinical examinations including Semmes-Weinstein monofilament testing to assess for PN and pedal pulse palpation to assess for PAD. Bivariate regression was performed to identify key variables for subsequent stepwise logistic regression to discern risk factors. Clinical examination results indicated 16.8% (n = 33) of participants had suspected PN alone, 15.8% (n = 31) had suspected PAD alone, and 23.0% (n = 45) had suspected PN and PAD. More than half of participants with clinical examination findings of PN or PAD failed to self-report the condition (57.7% and 86.8%, respectively). Among adults with lower-limb loss with suspected PN, participants with dysvascular amputations were at lower risk of underreporting (odds ratio [OR] = 0.2, 95% CI: 0.1-0.6). For those with suspected PAD, those who reported more medication prescriptions were at lower risk of underreporting (OR = 0.8, 95% CI: 0.7-1.0). Adults with lower-limb loss underreport PN and PAD per a medical history checklist, which may indicate underdiagnosis or lack of patient awareness. Routine assessment is highly recommended in this population and may be especially critical among individuals with non-dysvascular etiology.
Keywords: amputation, peripheral arterial disease, peripheral neuropathy, diagnostic screening, risk factor
What do we already know about this topic?
Comorbid conditions, such as peripheral arterial disease and neuropathy, are associated with poor health outcomes and increased mortality following lower-limb loss.
How does your research contribute to the field?
Our findings indicate that self-report medical history is unreliable for detecting peripheral arterial disease and peripheral neuropathy, which suggests either lack of patient awareness or underdiagnosis, of which both are concerning given these comorbidities increase the patient’s risk for a second amputation.
What are your research’s implications toward theory, practice, or policy?
Comorbidity status is a critical factor when developing a treatment plan post-amputation, so healthcare practitioners should incorporate comorbidity screening in their clinical examinations of patients with lower-limb loss rather than relying on patient-reported medical history.
Introduction
Self-management of chronic health conditions is closely linked to quality of life. 1 Self-management of one’s healthcare is encouraged with a patient-led, rather than practitioner-led, team model, where the patient is equipped with the knowledge and support to assume the primary leadership role. 2 For the patient to be actively involved in their care team, they must have comprehensive knowledge of their past health history and current health status, including comorbidities, medications, diagnostic tests, and treatments (eg, surgeries, hospitalizations), as well as knowledge of and ability to access healthcare resources and support services. Patients can be empowered as healthcare team leads, in part, through regular follow-ups and clinical assessment, which are associated with improved self-management of health conditions and healthier behaviors. 3 However, agreement between medical records and self-report medical history varies broadly (kappa = .20-.93) depending on the medical diagnosis and the patient population.4,5
Comorbidities are highly prevalent in adults with lower-limb loss (LLL), with reports of multiple comorbidities ranging from 28% to 62%.6,7 The prevalence of diabetes (35%-69%),6 -8 peripheral neuropathy (15%), 9 and vascular disease (48%-58%)8,10 are especially concerning, as these comorbidities significantly increase an individual’s risk of falls, morbidity, and mortality, as well as subsequent amputation.11,12
Among adults with LLL, comorbidity presence is primarily obtained from medical records and/or self-report. In other patient populations, agreement between medical records and self-reported medical history ranges from poor-to-excellent, depending on the medical diagnosis, patient population, and format for data acquisition.4,5 Conditions requiring active management, such as diabetes and visual impairment, are more reliably reported than other conditions (eg, hypertension, vascular disease), 4 and agreement between self-reported medical history and physician- or hospital-provided medical records is more discrepant among older adults, males, and minorities. 5
Underreporting of health conditions among adults with LLL remains largely unknown. Allied health professionals treating patients with LLL, including physical therapists and prosthetists, utilize self-reported medical histories to inform treatment plans; hence, it is critical to identify factors related to underreporting of life-limiting conditions among adults with LLL. If adults with LLL frequently underreport these conditions, essential clinical assessment measures, such as monofilament testing for peripheral neuropathy, should be prioritized to improve preventative care. Clinical assessment enables early identification of risk for life-limiting events, such as amputation, so that patients can receive necessary education and treatments.
To inform clinical assessment practices post-LLL, we aimed to estimate the percentage of individuals with LLL seen in outpatient clinics that are underreporting life-limiting comorbidities, that is, peripheral neuropathy (PN) and peripheral arterial disease (PAD), and to identify factors associated with underreporting. We hypothesized that some factors related to underreporting would be similar to those seen in other populations (ie, sex, race, age), 5 but that unique, amputation-specific factors would also be identified.
Material and Methods
Study Sample
We extracted data for this secondary analysis from a pre-existing dataset from an outpatient interdisciplinary Limb Loss Clinic held from September 2013 through December 2021 at the University of Delaware (UD). This project received UD Institutional Review Board for Human Subjects Research approval (project number: 531197). Prior to data acquisition via the standardized clinical examination, participants signed a written informed consent and Health Insurance Portability and Accountability Act disclosures form. Participants were considered for inclusion in the study if they were ≥18 years-old, community-dwelling, and had undergone major (ie, ankle disarticulation or more proximal) LLL prior to their Limb Loss Clinic evaluation. Participants with bilateral amputation (>toes), incomplete or missing medical histories, and those with missing data on ≥1 assessment measure (ie, monofilament testing, pedal pulse palpation) were excluded.
Self-Reported Information
Participants completed a standardized demographics questionnaire including age, sex, ethnicity, race, highest level of education, and living status. Participants also completed a standardized medical history checklist of common health conditions across 14 body systems (eg, cardiac, respiratory, endocrine-metabolic) and reported on history of cancer, as well as current medications. When applicable, lay terms were used to describe health conditions (eg, hypertension was listed as “high blood pressure”). Medical history for PN and PAD was considered positive if the participant reported that they currently had or had ever been diagnosed with the condition. Participants also reported on the presence of phantom limb sensation, defined as non-painful sensations in the area of the amputated limb; phantom limb pain, defined as painful sensations in the area of the amputated limb; and residual limb pain, defined as pain in the remaining portion of the amputated limb. We utilized the General Practice Physical Activity Questionnaire (GPPAQ) 13 to assess physical activity level. To ensure accuracy of reporting, we verified level of amputation, cause of amputation, and date of initial amputation with participants’ prosthetic medical records.
Clinical Assessment
The clinical team involved in data collection included a physiatrist, physical therapist, and prosthetist. These trained clinicians conducted all clinical assessments. The clinician determined presence of PN, a significant predictor of foot ulceration and future LLL, 14 using the Semmes-Weinstein monofilament test. On the sound limb, a 5.07/10g monofilament was applied to the plantar aspects of the great toe, third toe, first metatarsal head, third metatarsal head, and fifth metatarsal head, avoiding areas of callusing, for up to 3 trials per site. 15 During the assessment, participants closed their eyes to eliminate visual feedback; ordering of testing site was randomized and timing was varied to prevent false negative testing. We considered the inability to detect the monofilament on ≥1 site to be indicative of PN. 15 This clinical assessment tool has shown good reliability [interrater intraclass correlation coefficient (ICC)2, 1 = .75, intrarater ICC2, 1 = .85] 16 and diagnostic accuracy (sensitivity ≥ 90%, specificity 93%-100%) 15 when compared to the gold standard of neurological assessment (eg, nerve conduction study).
The clinician evaluated for PAD presence, which is a risk factor for falls and subsequent amputation,10,11 through sound limb pedal pulse palpation. We considered absence of the dorsalis pedis and posterior tibial pulses as indicative of PAD. 17 This clinical assessment measure has shown good diagnostic accuracy (sensitivity 73.0%, specificity 83%-98%)17,18 when compared to the gold standard of Doppler Ankle Brachial Index, and interrater agreement (weighted kappa = .65) 18 has previously been reported.
Data Analysis
We conducted analyses using SPSS Statistics 26 (IBM Corp., Armonk, New York, USA). Descriptive statistics were determined for the entire sample and calculated with respect to cause of amputation, that is, dysvascular versus non-dysvascular. Between-group differences were evaluated with independent sample proportion tests for prevalence data, Chi-squared analysis for categorical data (ie, race), Mann-Whitney U tests for nonparametric data (ie, time since amputation, number of medications prescribed), and independent sample t-tests for parametric data (ie, age).
We classified participants who had clinically present PN or PAD per the clinical examination but did not self-disclose the condition when completing the standardized medical history checklist as an “under-reporter” for that measure. To report prevalence of clinical presence of comorbidity and of under-reporting, Agresti-Coull confidence intervals were calculated. To identify demographic, amputation-specific, social, pain-related, and medical factors associated with PN and PAD underreporting, we conducted logistic regression analyses. We excluded participants who did not have PN or PAD per the clinical examination from regression analyses. We coded data as 0 = PN/PAD present per the clinical examination and reported on the standardized medical history checklist, 1 = PN/PAD present per the clinical examination but not reported on the standardized medical history checklist. Several variables (eg, race, education, pain, cause of amputation) were reduced to binary variables given frequency distributions and the number of variables.
To minimize the number of independent variables included in the 2 logistic regression models (ie, one for PN and one for PAD), we first used binary logistic regression models to evaluate associations between patient characteristics and underreporting for each comorbidity. Variables that significantly contributed to the dependent variable with a significance of P ≤ .200 were retained as independent characteristics for further consideration in the final logistic regression model. A relaxed P-value in the initial step reduces potential type-II errors, and is in accordance with previous research among individuals with limb loss.12,19 In the final logistic regression model, we implemented a conditional forward stepwise approach with 0.100 for entry and 0.200 for removal. Based on a previous exploratory analysis utilizing this approach, we set statistical significance for the final model at P ≤ .100. 19 All P-values are reported to aid in results interpretation. As this was a secondary analysis of an existing dataset, we did not conduct an a priori power analysis. 20
Results
Participant Characteristics
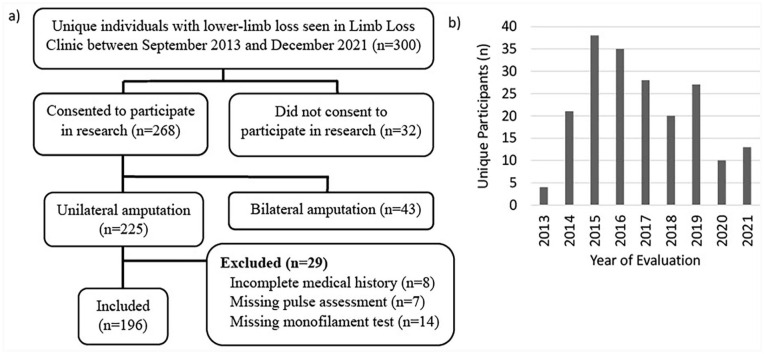
Clinicians saw a total of 300 unique individuals with LLL from September 2013 through December 2021, of whom 268 consented to data collection. Of these, 43 were excluded due to contralateral amputation (> toes), which precluded monofilament and pulse assessment. An additional 8 participants were excluded due to incomplete self-reported medical history checklists, 7 due to missing pulse data, and 14 due to missing monofilament data (Figure 1a). Hence, 196 participants were ultimately included in this secondary analysis. The number of unique participants seen each year ranged from 4 in 2013 to 38 in 2015, with a significant decrease in 2020 and 2021 due to the COVID-19 pandemic (Figure 1b).
Figure 1.
(a) Participant inclusion flow diagram for this study on underreporting of comorbidity (b) among 196 adults with unilateral lower-limb loss seeking assessment for prosthetic needs at an interdisciplinary outpatient clinic (2013-2021).
Table 1 presents participant characteristics. The most commonly affected body systems were vascular (63.3%), respiratory (59.2%), and neurological (53.6%). The most commonly reported comorbidities were hypertension (52.0%) and diabetes (43.4%). Prevalence of self-reported PN and PAD were 24.0% and 8.2%, respectively. Adults with a dysvascular cause of amputation were more likely to identify as a minority, and generally reported poorer health (eg, more prescription medications, greater likelihood of comorbidity of the cardiovascular, renal, neurological, and endocrine systems). Adults with non-dysvascular amputations were farther out from amputation, younger, and more active, but were more likely to report a higher level of amputation and to experience residual limb pain (Table 1).
Table 1.
Characteristics of 196 Adults With Unilateral Lower-Limb Loss Seeking Assessment for Prosthetic Needs (2013-2021).
| Participant characteristics | Total sample (n = 196) | Dysvascular cause (n = 94) | Non-dysvascular cause (n = 102) | P-value |
|---|---|---|---|---|
| Sociodemographics | ||||
| Sex, male | n = 196 | n = 94 | n = 102 | |
| 144 (73.5%) | 70 (74.5%) | 74 (72.5%) | .761 | |
| Age, years | n = 196 | n = 94 | n = 102 | |
| 56.7 ± 14.4 | 63.7 ± 11.6 | 50.2 ± 13.8 | <.001* | |
| Ethnicity, Hispanic/Latinx | n = 193 | n = 94 | n = 99 | |
| 10 (5.2%) | 2 (2.1%) | 8 (8.1%) | .062 | |
| Race | n = 192 | n = 94 | n = 98 | |
| White/Caucasian | 139 (72.4%) | 60 (63.8%) | 79 (80.6%) | .034* |
| Black/African American | 49 (25.5%) | 33 (35.1%) | 16 (16.3%) | |
| Asian | 1 (0.5%) | 0 (0.0%) | 1 (1.0%) | |
| Native American/Alaska Native | 1 (0.5%) | 0 (0.0%) | 1 (1.0%) | |
| Multiple races | 2 (1.0%) | 1 (1.1%) | 1 (1.0%) | |
| Education, > high school | n = 177 | n = 85 | n = 92 | |
| 116 (65.5%) | 51 (60.0%) | 65 (70.7%) | .172 | |
| Amputation-specific factors | n = 196 | n = 94 | n = 102 | |
| Level of amputation, transtibial | 119 (60.7%) | 67 (71.3%) | 52 (51.0%) | .004* |
| Time since initial amputation, years a | 2.4 (0.5, 9.1) | 1.4 (0.3, 3.7) | 6.9 (0.9, 28.1) | <.001* |
| Social characteristics | ||||
| Living status, alone | n = 195 | n = 94 | n = 101 | |
| 32 (16.4%) | 14 (14.9%) | 18 (17.8%) | .581 | |
| GPPAQ, inactive | n = 191 | n = 92 | n = 99 | |
| 105 (55.0%) | 61 (66.3%) | 44 (44.4%) | .002* | |
| Pain characteristics | ||||
| Phantom sensation, yes | n = 194 | n = 93 | n = 101 | |
| 137 (70.6%) | 70 (75.3%) | 67 (66.3%) | .172 | |
| Phantom limb pain, yes | n = 194 | n = 93 | n = 101 | |
| 113 (58.2%) | 58 (62.4%) | 55 (54.5%) | .264 | |
| Residual limb pain, yes | n = 193 | n = 92 | n = 101 | |
| 81 (42.0%) | 30 (32.6%) | 51 (50.5%) | .012* | |
| Medications | n = 191 | n = 94 | n = 97 | |
| Number of medications prescribed a | 5 (2, 8) | 6 (4, 10) | 2 (1, 5) | <.001* |
| Opioid use, yes | 57 (29.8%) | 27 (28.7%) | 30 (30.9%) | .739 |
| Gabapentinoid use, yes | 62 (32.5%) | 28 (29.8%) | 34 (35.1%) | .437 |
| Affected body systems per medical history b | n = 196 | n = 94 | n = 102 | |
| Cardiac | 58 (29.6%) | 41 (43.6%) | 17 (16.7%) | <.001* |
| Vascular | 124 (63.3%) | 79 (84.0%) | 45 (44.1%) | <.001* |
| Hematological | 27 (13.8%) | 17 (18.1%) | 10 (9.8%) | .093 |
| Respiratory | 116 (59.2%) | 59 (62.8%) | 57 (55.9%) | .327 |
| Ear, eye, nose, throat | 69 (35.2%) | 38 (40.4%) | 31 (30.4%) | .142 |
| Upper gastrointestinal | 24 (12.2%) | 10 (10.6%) | 14 (13.7%) | .510 |
| Lower gastrointestinal | 32 (16.3%) | 19 (20.2%) | 13 (12.7%) | .158 |
| Hepatic/pancreatic | 12 (6.1%) | 5 (5.3%) | 7 (6.9%) | .652 |
| Renal | 26 (13.3%) | 19 (20.2%) | 7 (6.9%) | .006* |
| Genitourinary | 15 (7.7%) | 9 (9.6%) | 6 (5.9%) | .331 |
| Musculoskeletal | 55 (28.1%) | 30 (31.9%) | 25 (24.5%) | .249 |
| Neurological | 105 (53.6%) | 63 (67.0%) | 42 (41.2%) | <.001* |
| Endocrine-metabolic | 91 (46.4%) | 66 (70.2%) | 25 (24.5%) | <.001* |
| Psychiatric/behavioral | 54 (27.6%) | 21 (22.3%) | 33 (32.4%) | .117 |
| Cancer | 30 (15.3%) | 11 (11.7%) | 19 (18.6%) | .179 |
Note. GPPAQ = General Practice Physical Activity Questionnaire.
Data presented as median (25th percentile, 75th percentile) rather than mean ± standard deviation or n (% of sample).
Data presented as n (% of sample) reporting presence or history of ≥1 health condition within the body system.
Indicates significant between-group differences based on cause of amputation (P < .050).
Clinical Screening
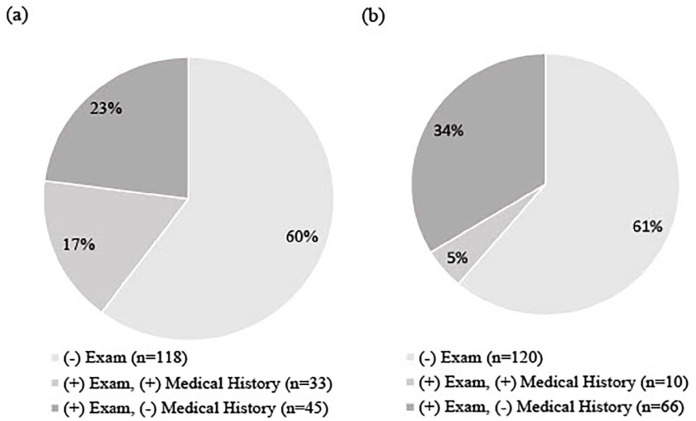
During the clinical examination, n = 33 (16.8% [95% CI = 12.2%, 22.7%]) of participants had clinical examination findings consistent with PN alone, n = 31 (15.8% [95% CI = 11.3%, 21.6%]) had clinical examination findings consistent with PAD alone, and n = 45 (23.0% [95% CI = 17.6%, 29.4%]) had findings consistent with both PN and PAD. Of the participants with PN (n = 78) per monofilament testing, 45 (57.7% [95% CI = 46.6, 68.0%]) failed to report a history of PN (Figure 2). Of the participants with PAD (n = 76) per pulse palpation, 66 (86.8% [95% CI = 77.3%, 92.9%]) failed to report a history of PAD (Figure 2).
Figure 2.
Self-reported presence versus clinical examination for (a) peripheral neuropathy (n = 196) and (b) peripheral arterial disease (n = 196) among adults with unilateral lower-limb loss seeking assessment for prosthetic needs (2013-2021).
Regression Analyses
Table 2 depicts participant characteristics evaluated for their ability to predict underreporting of PN (n = 78) and PAD (n = 76). Per bivariate regression analyses, 8 variables were significantly associated with PN underreporting (P ≤ .200) and considered in the final PN model (Table 3). Among adults with LLL, dysvascular cause of amputation was associated with a 5 (OR = 0.2 [0.1, 0.6]) times reduced odds of underreporting PN per multiple regression analysis (pseudo-R 2 = .15).
Table 2.
Results of Bivariate Analyses Exploring Factors Related to Underreporting of Peripheral Neuropathy and Peripheral Arterial Disease Among Adults With Unilateral Lower-Limb Loss Seeking Assessment for Prosthetic Needs (2013-2021).
| Variable | Peripheral neuropathy (n = 78) | Peripheral arterial disease (n = 76) | ||
|---|---|---|---|---|
| Odds ratio [95% CI] | P-value | Odds ratio [95% CI] | P-value | |
| Demographics | ||||
| Sex, male | 0.6 [0.2, 2.4] | .268 | 2.3 [0.6, 9.1] | .249 |
| Age, years | 1.0 [0.9, 1.0] | .137* | 1.0 [0.9, 1.1] | .787 |
| Ethnicity, Hispanic/Latinx | 0.7 [0.0, 12.1] | .824 | 0.3 [0.0, 3.4] | .320 |
| Race, minority** | 1.2 [0.5, 3.1] | .704 | 1.9 [0.4, 7.9] | .390 |
| Education, >high school | 0.8 [0.3, 2.2] | .703 | 1.2 [0.3, 4.7] | .769 |
| Amputation-specific factors | ||||
| Level of amputation, transtibial | 0.5 [0.2, 1.4] | .170* | 0.7 [0.2, 3.2] | .696 |
| Cause of amputation, dysvascular | 0.2 [0.1, 0.6] | .005* | 0.4 [0.0, 3.2] | .374 |
| Time since amputation, years | 1.1 [1.0, 1.1] | .085* | 1.1 [0.9, 1.4] | .458 |
| Social characteristics | ||||
| Living status, alone | 3.6 [0.9, 14.1] | .062* | 0.7 [0.1, 3.9] | .696 |
| GPPAQ, inactive | 0.6 [0.2, 1.7] | .354 | 1.1 [0.3, 4.8] | .880 |
| Pain characteristics | ||||
| Phantom sensation, yes | 0.9 [0.3, 2.4] | .778 | 0.4 [0.0, 3.1] | .355 |
| Phantom limb pain, yes | 0.8 [0.3, 2.2] | .726 | 0.2 [0.0, 1.3] | .083* |
| Residual limb pain, yes | 2.2 [0.8, 5.9] | .113* | 0.7 [0.2, 2.5] | .540 |
| Medical history | ||||
| Number of medications | 0.9 [0.8, 1.0] | .034* | 0.8 [0.7, 1.0] | .048* |
| Opioid use, yes | 0.6 [0.2, 1.6] | .283 | 0.4 [0.1, 1.5] | .155* |
| Gabapentinoid use, yes | 2.1 [0.8, 5.4] | .126* | 0.8 [0.2, 2.9] | .679 |
Note. CI = confidence interval; GPPAQ = General Practice Physical Activity Questionnaire.
Indicates variables included in multiple regression analysis (P ≤ .200).
Minority race defined as Native American/American Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander, and/or Black/African American.
Table 3.
Full Model Results Identifying Risk Factors for Underreporting of Peripheral Neuropathy and Peripheral Arterial Disease Among Adults With Unilateral Lower-Limb Loss Seeking Assessment for Prosthetic Needs (2013-2021).
| Variable | Odds ratio [95% CI] | P-value |
|---|---|---|
| Peripheral neuropathy (n = 78)* | ||
| Cause of amputation, dysvascular | 0.2 [0.1, 0.6] | .006 |
| Peripheral arterial disease (n = 76)** | ||
| Number of medications | 0.8 [0.7, 1.0] | .044 |
Note. CI = confidence interval; SE = standard error; df = degrees of freedom.
Variables included in the initial model: Age, level of amputation, time since amputation, living status, residual limb pain, number of medications, gabapentinoid use.
Variables included in the initial model: Phantom limb pain, opioid use.
Three variables were significantly associated with PAD underreporting (P ≤ .200) during bivariate regression analysis and considered in the final PAD model (Table 3). Among adults with LLL, each additional medication prescription was associated with 1.3 (OR = 0.8 [0.7, 1.0]) times lower odds of underreporting neuropathy per multiple regression analysis (pseudo-R 2 = .11).
Discussion
This study is among the first to investigate comorbidity reporting among adults with LLL who present to an outpatient clinic for prosthetic-related needs. Nearly 40% of participants had clinical examination findings consistent with PN and/or PAD, and in support of our hypothesis, over half of individuals who had a positive clinical examination failed to self-disclose the condition. This could be due to lack of patient awareness or involvement in their healthcare, or it could be due to under-diagnosis of these conditions. In support of the latter, prior research has shown physicians relying on interpretation of self-report symptoms without clinical assessment fail to diagnose two-thirds of mild-to-moderate cases of PN and one-third of severe PN cases. 21 With PAD, diagnosis often occurs following complaints or complications in the presence of disease rather than as part of a proactive evaluation plan. 22 Our findings, combined with prior evidence, highlight the need for obligatory assessment and education surrounding PN and PAD to improve patients’ ability to appropriately disclose and manage their comorbidities.
For PN, adults with dysvascular causes of LLL were significantly less likely to underreport the condition than their peers with non-dysvascular causes of amputation. As PN increases risk for dysvascular amputation, 23 adults who lost their limbs due to dysvascularity may have received more healthcare and education related to assessment for and management of these conditions in order to reduce subsequent amputation and mortality risk. 24 The related education and monitoring may explain the protective effect of dysvascular LLL.
Surprisingly, each additional medication reported was associated with 1.3 times lower odds of PAD underreporting. Unfortunately, medication use was self-reported and unable to be cross-referenced with other medical charts given access was limited to physical therapy and prosthetic medical records; thus, data may be subject to suboptimal medication recall. In support of this hypothesis, Marks et al 25 found that, when prompted, adults who were taking an average of 5.9 (SD = 3.1) medications, could name, on average, only 56% of their medications. As the adults in our study similarly self-reported 5 medications on average, it is possible that those who reported a large number of medications were utilizing a medication card or list. Use of such a memory aid may indicate greater self-awareness and management, as well as active involvement in their care team.
Given relationships among patient demographics and comorbidity reporting in the general population, 5 we anticipated increased age, sex, and minority race would be risk factors for underreporting. While age arose as a potential risk factor for underreporting of PN (Table 2), it was not significant in the final model (Table 3). This may be explained by the finding that adults with non-dysvascular LLL, who tend to be younger than peers with dysvascular amputations, 6 are more likely to underreport PN. Sex was not significantly associated with either PAD or PN, which may be due to the relatively low proportion of female participants (26.5%) in the study. Race was not significantly associated with either PAD or PN underreporting in this population, potentially due to the racial disparity in dysvascular amputation. 6 Adults who identify as African-American are at far greater risk of dysvascular amputation than adults who identify as white, even after controlling for age and sex. 26 Therefore, the additional education and monitoring routinely provided after dysvascular LLL may reduce the impact of demographic variables (eg, age, race) on underreporting. Alternatively, the study may not have a large enough sample size to detect small effects of demographic variables.
As previously noted, for a patient to be an active participant in their medical care, they require knowledge about their current health status. Findings of this study indicate that many individuals with LLL, especially those with non-dysvascular amputations, lack awareness of their comorbidities. While the risk factors identified have limited utility to guide clinicians in prediction of individuals with LLL likely to under-report PAD and PN, the high prevalence of under-reporting indicates need for widespread clinical assessment in this population. Factors related to comorbidity underreporting in the general population (eg, age, race) 5 may not be as significant in this population. This work supports future investigations of the impact of clinical assessment and education of comorbidities on patient outcomes following LLL.
Study Limitations
Causal relationships cannot be determined given the cross-sectional study design. While we utilized reliable clinical tools to assess for PN and PAD, medical diagnoses were not confirmed with advanced technology (eg, nerve conduction test, Doppler ankle brachial index). We did not have full access to patient medical records, as might be available within a large healthcare system with integrated electronic medical records, to determine if underreporting was due to lack of patient awareness or a result of failure to diagnose the condition. Further, while data collection for the clinic was standardized, several participants had missing or incomplete data sets (due to clinician prioritization of examinations given time constraints), resulting in study exclusion and a reduced sample size, which may have affected regression model results. While results should be interpreted with caution, this study is one of the largest among patients with lower-limb loss seen in an outpatient care setting, offering critical insight into this vulnerable population at risk for life-limiting comorbidities.
We acknowledge assessment of underreporting of the medical conditions without consideration for layman’s terms that may be more readily recognized by adults in non-medical fields (eg, “poor circulation” instead of arterial disease, “loss of feeling in the foot” rather than PN). Additionally, study findings may not be generalized to those with bilateral major LLL, those residing in assisted-care facilities, or minors. Generalizability might also be considered with respect to medical care access, as all participants resided within the greater Delaware region and were able to attend an outpatient ambulatory care appointment. Finally, while the sample size is large when compared to many studies among patients with LLL, representation of some minorities was low, requiring race dichotomization.
Conclusions
Among adults with LLL who present to an outpatient clinic for prosthetic needs, there is significant underreporting of PN and PAD. Few factors were identified that could be utilized to guide clinician decision-making regarding administration of clinical assessments of these conditions. Clinicians should assess for PN and PAD among adults with LLL who do not self-disclose these diagnoses, particularly among those with non-dysvascular amputation.
Footnotes
Authors’ Note: The material within has not been previously published, though some material within has been presented on a poster at the American Academy of Orthotists and Prosthetists Annual Meeting & Scientific Symposium in Atlanta, GA, in March of 2022.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ms. Samantha Stauffer is supported by a Graduate Education Fund provided by Independence Prosthetics-Orthotics, Inc., where she works as a Certified Prosthetist-Orthotist. Dr. Mayank Seth is supported by a Postdoctoral Research Fund provided by Independence Prosthetics-Orthotics, Inc. Dr. Emma Beisheim-Ryan was supported by the Promotion of Doctoral Students (PODS) I/II Scholarships from the Foundation for Physical Therapy Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions nor the authors’ affiliated institutions.
Ethics: Approval was obtained from the University of Delaware Institutional Review Board, project ID #531197. Written informed consent was obtained from all participants.
ORCID iDs: Samantha Jeanne Stauffer  https://orcid.org/0000-0002-3050-1071
https://orcid.org/0000-0002-3050-1071
Jaclyn Megan Sions  https://orcid.org/0000-0002-8151-1341
https://orcid.org/0000-0002-8151-1341
References
- 1. Devan H, Carman AB, Hendrick PA, Ribeiro DC, Hale LA. Perceptions of low back pain in people with lower limb amputation: a focus group study. Disabil Rehabil. 2015;37(10):873-883. doi: 10.3109/09638288.2014.946158 [DOI] [PubMed] [Google Scholar]
- 2. Del Piero LB, Williams RM, Mamiya K, Turner AP. The role of interprofessional teams in the biopsychosocial management of limb loss. Curr Phys Med Rehabil Rep. 2020;8(4):396-404. doi: 10.1007/s40141-020-00293-1 [DOI] [Google Scholar]
- 3. Kang C, Kawamura A, Noguchi H. Benefits of knowing own health status: effects of health check-ups on health behaviours and labour participation. Appl Econ Lett. 2021;28(11):926-931. doi: 10.1080/13504851.2020.1786001 [DOI] [Google Scholar]
- 4. Bergmann MM, Jacobs EJ, Hoffmann K, Boeing H. Agreement of self-reported medical history: comparison of an in-person interview with a self-administered questionnaire. Eur J Epidemiol. 2004;19(5):411-416. doi: 10.1023/B:EJEP.0000027350.85974.47 [DOI] [PubMed] [Google Scholar]
- 5. Merkin SS, Cavanaugh K, Longenecker JC, Fink NE, Levey AS, Powe NR. Agreement of self-reported comorbid conditions with medical and physician reports varied by disease among end-stage renal disease patients. J Clin Epidemiol. 2007;60(6):634-642. doi: 10.1016/j.jclinepi.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amtmann D, Morgan SJ, Kim J, Hafner BJ. Health-related profiles of people with lower limb loss. Arch Phys Med Rehabil. 2015;96(8):1474-1483. doi: 10.1016/j.apmr.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan SJ, Friedly JL, Amtmann D, Salem R, Hafner BJ. Cross-sectional assessment of factors related to pain intensity and pain interference in lower limb prosthesis users. Arch Phys Med Rehabil. 2017;98(1):105-113. doi: 10.1016/j.apmr.2016.09.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lefebvre KM, Metraux S. Disparities in level of amputation among minorities: implications for improved preventative care. J Natl Med Assoc. 2009;101(7):649-655. doi: 10.1016/S0027-9684(15)30973-1 [DOI] [PubMed] [Google Scholar]
- 9. Seth M, Beisheim EH, Pohlig RT, Horne JR, Sarlo FB, Sions JM. Time since lower-limb amputation: an important consideration in mobility outcomes. Am J Phys Med Rehabil. 2022;101(1):32-39. doi: 10.1097/PHM.0000000000001736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong CK, Chihuri ST. Impact of vascular disease, amputation level, and the mismatch between balance ability and balance confidence in a cross-sectional study of the likelihood of falls among people with limb loss: perception versus reality. Am J Phys Med Rehabil. 2019;98(2):130-135. doi: 10.1097/PHM.0000000000001034 [DOI] [PubMed] [Google Scholar]
- 11. Norvell DC, Czerniecki JM. Risks and risk factors for ipsilateral re-amputation in the first year following first major unilateral dysvascular amputation. Eur J Vasc Endovasc Surg. 2020;60(4):614-621. doi: 10.1016/j.ejvs.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller WC, Speechley M, Deathe B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch Phys Med Rehabil. 2001;82(8):1031-1037. doi: 10.1053/apmr.2001.24295 [DOI] [PubMed] [Google Scholar]
- 13. Ahmad S, Harris T, Limb E, et al. Evaluation of reliability and validity of the General Practice Physical Activity Questionnaire (GPPAQ) in 60–74 year old primary care patients. BMC Fam Pract. 2015;16(1):113. doi: 10.1186/s12875-015-0324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng Y, Schlösser FJ, Sumpio BE, Haven N. The Semmes Weinstein monofilament examination is a significant predictor of the risk of foot ulceration and amputation in patients with diabetes mellitus. J Vasc Surg. 2011;53(1):220-226.e5. doi: 10.1016/j.jvs.2010.06.100 [DOI] [PubMed] [Google Scholar]
- 15. Feng Y, Schlösser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50(3):675-682.e1. doi: 10.1016/j.jvs.2009.05.017 [DOI] [PubMed] [Google Scholar]
- 16. Snyder BA, Munter AD, Houston MN, Hoch JM, Hoch MC. Interrater and intrarater reliability of the semmes-weinstein monofilament 4-2-1 stepping algorithm. Muscle Nerve. 2016;53(6):918-924. doi: 10.1002/mus.24944 [DOI] [PubMed] [Google Scholar]
- 17. Armstrong DWJ, Tobin C, Matangi MF. The accuracy of the physical examination for the detection of lower extremity peripheral arterial disease. Can J Cardiol. 2010;26(10):e346-e350. doi: 10.1016/S0828-282X(10)70467-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herráiz-Adillo Á, Piñar-Serrano O, Mariana-Herráiz JÁ, Martínez-Vizcaíno V, Pozuelo-Carrascosa DP, Notario-Pacheco B. Physical examination to screen for peripheral artery disease in a defined primary care population: a diagnostic accuracy study. Int J Clin Pract. 2018;72(11):e13253. doi: 10.1111/ijcp.13253 [DOI] [PubMed] [Google Scholar]
- 19. Major MJ. Fall prevalence and contributors to the likelihood of falling in persons with upper limb loss. Phys Ther. 2019;99(4):377-387. doi: 10.1093/ptj/pzy156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dziak JJ, Dierker LC, Abar B. The interpretation of statistical power after the data have been gathered. Curr Psychol. 2020;39(3):870-877. doi: 10.1007/s12144-018-0018-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care. 2005;28(6):1480-1481. doi: 10.2337/diacare.28.6.1480 [DOI] [PubMed] [Google Scholar]
- 22. Lecouturier J, Scott J, Rousseau N, Stansby G, Sims A, Allen J. Peripheral arterial disease diagnosis and management in primary care: a qualitative study. BJGP Open. 2019;3(3):1-11. doi: 10.3399/bjgpopen19X101659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16(S1):S75-S83. doi: [DOI] [PubMed] [Google Scholar]
- 24. Fleury AM, Salih SA, Peel NM. Rehabilitation of the older vascular amputee: a review of the literature. Geriatr Gerontol Int. 2013;13(2):264-273. doi: 10.1111/ggi.12016 [DOI] [PubMed] [Google Scholar]
- 25. Marks JR, Schectman JM, Groninger H, Plews Ogan ML. The association of health literacy and socio-demographic factors with medication knowledge. Patient Educ Couns. 2010;78(3):372-376. doi: 10.1016/j.pec.2009.06.017 [DOI] [PubMed] [Google Scholar]
- 26. Dillingham TR, Pezzin LE, Mackenzie EJ. Racial differences in the incidence of limb loss secondary to peripheral vascular disease: a population-based study. Arch Phys Med Rehabil. 2002;83(9):1252-1257. doi: 10.1053/apmr.2002.34805 [DOI] [PubMed] [Google Scholar]