Abstract
Objectives
Ethnically diverse family carers of people living with dementia (hereafter carers and people with dementia) experience more psychological distress than other carers. To reduce this inequality, culturally adapted, multilingual, evidence-based practical assistance is needed. This paper details the Draw-Care study protocol including a randomised control trial (RCT) to test the effectiveness of a digital intervention comprising a multilingual website, virtual assistant, animated films, and information, on the lives of carers and people with dementia in Australia.
Methods
The Draw-Care intervention will be evaluated in a 12-week active waitlist parallel design RCT with 194 carers from Arabic, Cantonese, Greek, Hindi, Italian, Mandarin, Spanish, Tamil, and Vietnamese-speaking language groups. Our intervention was based on the World Health Organization's (WHO) iSupport Lite online carer support messages and was co-designed with carers, people with dementia, service providers, and clinicians. Culturally adapted multilingual digital resources were created in nine languages and English.
Results
In Phase I (2022), six co-design workshops with stakeholders and interviews with people with dementia informed the development of the intervention which will be trialled and evaluated in Phases II and III (2023 and 2024).
Conclusions
Digital media content is a novel approach to providing cost-effective access to health care information. This study protocol details the three study phases including the RCT of a co-designed, culturally adapted, multilingual, digital intervention for carers and people with dementia to advance the evidence in dementia and digital healthcare research and help meet the needs of carers and people with dementia in Australia and globally.
Keywords: animation, Australia, ethnic minority, carer, dementia, digital health, iSupport Lite
Introduction
Australia, like other high-income countries, is undergoing a demographic transition wherein the population is ageing and becoming more multicultural. 1 About 30% of Australians aged 65 and over were born overseas, mainly in non-English speaking countries, and >24% speak a language other than English at home. 2 By 2056 the proportion of older Australians from an ethnically diverse background will increase with the highest growth projected for Asia-born populations (>200% growth). 3 Concomitant with the rising prevalence of dementia, there will be a rise in the number of non-English speaking people living with it.
In many ethnically diverse families, early signs of dementia are conflated with normal ageing, and later symptoms with madness. Causality is attributed to family conflict or neglect.4,5 Such beliefs breed denial, fear, and misunderstanding, and exacerbate inequalities between ethnically diverse groups’ and the general population's rates of timely diagnosis, referral to specialist care, medication use, and participation in clinical trials.6,7 While culturally appropriate home and community-based services can help address these inequalities, too few services, difficulty in identifying them, reluctance to engage with services that are not perceived as culturally relevant and competent, prohibitive costs, and long wait-time delay in care and service utilisation.8–11 The result is higher rates of distress and care burden reported among ethnically diverse carers. Research shows that such carers experienced more than twice the rate (OR = 2.45) of psychological distress compared to their Australian-born counterparts. 12 Many also experienced a loss of household productivity and income due to unpaid care. 13
Supporting people with dementia and their carers by delivering high-quality, culturally appropriate, and sustainable models of care at home is imperative. Recognising this need and the interruption of healthcare services due to the COVID-19 pandemic, the World Health Organization (WHO) developed iSupport Lite, short practical support messages for carers of people with dementia to reduce mental and physical health problems associated with care and to improve their quality of life. 14 iSupport Lite is enhanced with comic-graphic art images within six key themes titled: (a) Reach Out for Support, (b) Take Care of Yourself, (c) Ensure Continuity of Care, (d) Respond to Change, (e) Be Flexible and (f) Communicate Effectively. 14 Comics, in this context, are not comical or intended to make light of dementia. Comics are used as a way to express ideas with images combined with plain and simple text for the purpose of telling a story and to communicate health messages clearly. 15
Harnessing the potential of digital technologies to deliver healthcare resources in a culturally appropriate, practical, and user-friendly way, we have collaboratively conceived the Draw–Care intervention, based on iSupport Lite. There is a growing use of digital interventions to improve dementia care. 16 The novelty of Draw-Care is its focus on ethnically diverse carers. Funded by the Australian Medical Research Future Fund, the intervention comprises six co-designed short-animated films, six digital information sheets, a website with a virtual assistant or ‘helper’ with multilingual capability in nine languages plus English. Arabic, Cantonese, Greek, Hindi, Italian, Mandarin, Spanish, Tamil, and Vietnamese are the top nine languages spoken at home other than English, by established and emerging ethnically diverse communities aged 65 + in Australia. 2 Three sequential phases comprise the Draw-Care study. Phase I the co-design and development of the intervention has been completed. A brief retrospective description is included in the present paper for context. Details are published elsewhere. 17 Phase II comprises the RCT to test the intervention and Phase III comprises evaluation of the intervention's clinical and cost-effectiveness in reducing carer burden. This paper details the overarching study protocol, rationale, and significance of the three phases.
Methods
Theoretical approach
The Draw-Care study is guided by the stress-health model, 18 whereby the goal is to change the nature of specific stressors, their appraisal, and/or the carers’ response. Using this model, our intervention seeks to create a change through cognitive reframing, problem-solving, and emotional regulation. 19 We will do this by ensuring the intervention focuses on imparting knowledge and skills on dementia care, problem-solving, and stress management.
Phase I (year 1): co-design, development, and user-testing of the Draw-Care intervention
In the study's first year, the Draw-Care intervention was developed. iSupport Lite online resources were culturally adapted with carers, clinicians, service providers, and people with dementia. A co-design approach emphasises the importance of lived experience, collaboration, and participation of all stakeholders in the research. 20 A series of six co-design workshops were undertaken to collaboratively draw upon lived experiences of carers and service providers and incorporate them to culturally adapt one iSupport Lite theme in each workshop: (a) Reaching out to others for help; (b) Caring for myself; (c) Ensuring that the person with dementia continues to receive care; (d) Responding to changes in the person with dementia; (e) Providing everyday care to the person with dementia; and (f) Communicating information to the person with dementia.
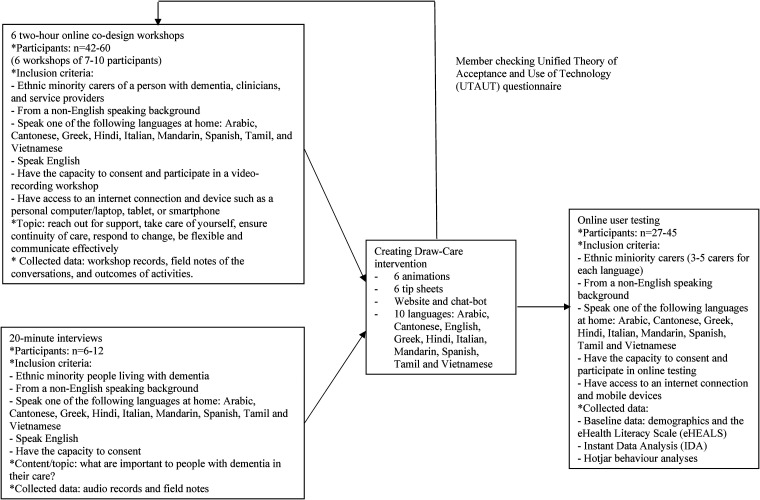
The data captured from workshops informed the content and cultural adaptation of six short, animated films and six practical information or tip sheets summarising the key points of each corresponding animation. These resources were translated into nine languages plus English i.e., Arabic, Cantonese, Greek, Hindi, Italian, Mandarin, Spanish, Tamil, and Vietnamese. To deliver these resources to end users i.e., family carers, the concept for a purpose-built multilingual website with helper function was also co-designed with stakeholders. Figure 1 shows an overview of Phase I and the study inclusion criteria.
Figure 1.
Flow diagram of Phase I co-design and development of the Draw-Care resources.
The Draw-Care intervention was member-checked with all stakeholders to ensure accuracy and trustworthiness. A short survey guided by the Unified Theory of Acceptance and Use of Technology (UTAUT) was used to examine the potential users’ acceptability and intention to use the Draw-Care intervention. 21 Feedback was incorporated and followed by website user-testing with a further 29 family carers to refine the intervention ahead of study Phase II.
Sampling and recruitment
Convenience, purposeful and snowball sampling was used to enrol participants into the co-design workshops through existing research contacts and networks with carers, people with dementia, clinicians, service providers and organisations. Contacts and networks were advised of the study and invited to participate directly, make referrals, and/or promote the workshops via organisational newsletters, social media, and their broader networks. The rationale for the estimated target sample sizes in Phase I was based on balancing sufficient diversity with study feasibility, including ensuring national and linguistic representation. A heterogeneous mix of participants were enrolled and all spoke one of the nine corresponding languages at home (n = 21).
All participants attended a minimum of one workshop and 12 participants attended two or more. Each workshop comprised between 5 to 8 participants. 17 Collectively, 40 attendees participated across the six workshops which reflects a participation rate of between 66% and 95% based on the estimated target sample size range (n = 42–60).
Three people with dementia were enrolled and interviewed separately to the online workshops. Based on the estimated target sample size range (n = 6–12), the participation rate was low, between 25% and 50%. Our aim was to enrol 1 or 2 people from each language group but in practice, this was not feasible. The challenges of including people with dementia from ethnically diverse communities are discussed elsewhere (unpublished – under review).
Co-design participants were invited to member-check the intervention. User-testing sessions of the website were conducted with a cohort of family carers, including former carers if they no longer cared for a person with dementia. For example, if the person had moved into a care facility or had died. Feedback from member-checking and user-testing was consolidated, discussed, and implemented to refine the intervention for Phase II.
Method: co-design workshops and interviews
Two-hour online workshops were facilitated using the nominal group technique used in health and education research, given its strength in democratically eliciting ideas. 22 Before each workshop, researchers provided participants with the iSupport Lite messages in an online format, the proposed images for Draw-Care animations, and a baseline script to be co-designed during the workshop. Participants first worked alone to craft their feedback, and then shared and discussed their ideas.
Separately, we conducted 20-minute interviews – either online or in-person – with people with dementia and/or mild cognitive impairment either self-reported or reported by their carer. During these interviews, researchers showed participants the iSupport Lite messages and images and asked them which messages they considered important for their care needs. Interviews were structured to meet the needs of the person with dementia, including using an interpreter where necessary and/or having a family member present.
The co-designed Draw-Care intervention suite of resources that were developed from workshops and interviews i.e. six multilingual short animations and six information sheets hosted on the multilingual website with ‘helper’ function was member-checked.
The website was also user-tested by instant data analysis (IDA) to identify and rank usability issues. 23 Participants completed two online questionnaires before the allocated user-test session with the researcher to collect demographic characteristics and assess digital health literacy using the eHealth Literacy Scale (eHEALS) 24 and were emailed a unique website log-in code. During the 30-minute sessions, the user experience was documented by the researcher. Each participant completed short tasks including screen sharing while navigating the site and concurrently thinking aloud. Tasks included whether the participant could access the website with the log-in code, select the preferred language option on the site, select a short film to view, and locate information and the rating scale with and without the helper function. Hotjar data analytics was used to capture interactive heatmaps of how users engaged with the website. 25
Analysis
Co-design workshops and interviews were audio-recorded, semi-transcribed, and thematically analysed. User-test sessions were analysed using IDA, i.e. initially brainstorming at the end of each day of testing to identify usability issues observed and by reviewing recordings of the heatmaps. Issues were ranked as either critical (unable to complete a task), severe (significant delay or frustration in task completion), or cosmetic (minor issues). After all user-test sessions were completed, issues were inductively separated out and aggregated into larger themes using affinity mapping. Ideas and insights were organised into overarching themes. 23 The major usability issues of the intervention including the website were identified with suggestions for improvement. Demographic data, eHEALS scores and technology-specific scores were analysed to explore relationships between e-health literacy levels of participants, technology usage, and other demographic characteristics. Quantitative data were analysed using SPSS V28. 26 Refinements and revisions to the relevant resources were made before commencement of the RCT.
Phase II (year 2): randomised controlled trial of the Draw-Care intervention
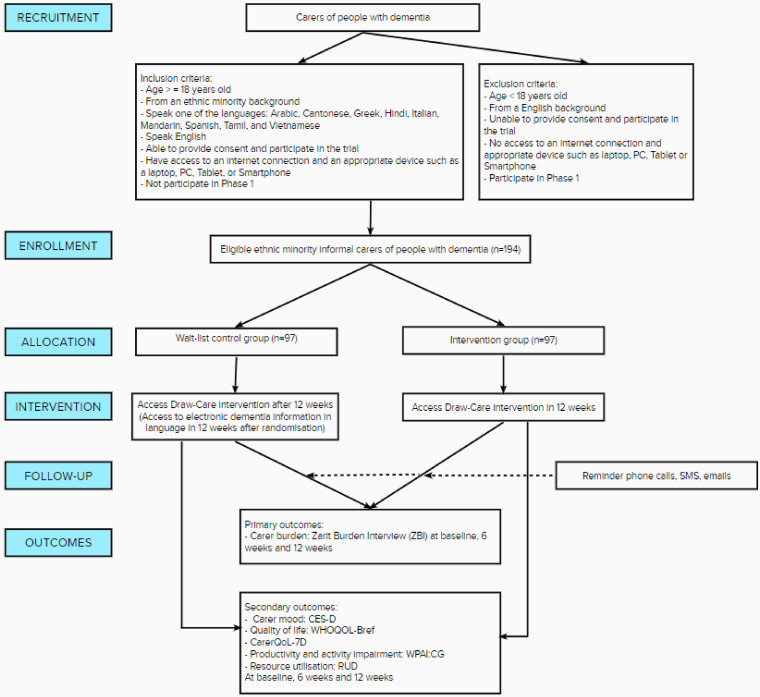
A parallel, 12-week waitlist randomised control trial (RCT) will be conducted to test the Draw-Care intervention with 194 carers. The design and eligibility criteria are shown in Figure 2 and Appendices 1–3.
Figure 2.
Flow diagram of Phase II the randomised controlled trial of the Draw-Care Intervention.
CarerQoL-7D: Carer Quality Of Life Seven important burden Dimensions; CES-D: Center for Epidemiologic Studies Depression Scale; RUD: Resource Utilization in Dementia; WHOQOL-Bref: World Health Organization Quality of Life; WPAI:CG: Work Productivity and Activity Impairment questionnaire as adapted for caregiving; ZBI: Zarit Burden Interview
Recruitment and sampling
We aim to recruit a balanced sample of carers from each of the nine language backgrounds. Family carers of people with dementia will be eligible to participate if they provided direct care for at least two months in the preceding twelve months. We will recruit carers using the National Ageing Research Institute (NARI) Culturally and Linguistically Diverse Research Engagement Network, comprising 1000+ ethnically diverse community groups and services across Australia. We will also recruit carers through our partners including Dementia Australia and the Federation of Ethnic Communities Council of Australia.
Randomisation and blinding
Interested individuals will be screened according to the study criteria. To minimise performance bias, a benign deception will be applied. That is, eligible participants will be informed that there are two components to the intervention they will receive but the order in which they receive them will be randomised. Upon providing consent and completion of baseline assessments (t0) participants will be block randomised by an external clinical trials service to ensure equal allocation of participants into either the intervention group or the waitlist control group (1:1 ratio). The service will be blinded to the study arm and will randomise participants into either intervention A or intervention B using the participant's identification code assigned by the researchers and by the language group. Participants randomised to the intervention group will receive a website link and a log-in code to access the site ad libitum for the 12-week test period. Participants randomised to the active waitlist control group will receive an electronic dementia information comic (e-comic) in their preferred language. The control group will be provided access to the Draw-Care intervention after the 12-week test period. Likewise, the intervention group will be provided with the e-comic after the 12-week test period. During the test period, participants in either group will also be asked not to discuss the intervention they receive with anyone to minimise confounding.
Outcome measures
The primary outcome measure is carer burden according to the Zarit Burden Interview (ZBI). 27 Secondary outcome measures are as follows: the carer's depressive symptoms as measured by the Center for Epidemiological Studies-Depression screening tool (CES-D), 28 quality of life of the carer and the person with dementia as measured by the World Health Organization Quality of Life–Bref (WHOQOL-Bref) 29 and of a sub-group of carers using the Care-related Quality of Life instrument (CarerQoL-7D), 30 and productivity and activity impairment as measured by the Work Productivity and Activity Impairment Questionnaire as adapted for caregiving (WPAI:CG) 31 and Resource Utilization in Dementia (RUD). 32
Participants will complete survey tools in English or a validated translated version. CarerQoL is validated in English, Italian, and Spanish. Therefore, these measures will be collected in these language groups to evaluate concordance between directly elicited CarerQoL utilities and EQ-5D-5L utilities mapped from WHOQOL-Bref data. 33 Outcome measurements will be collected using REDCap at baseline (t0) and 6- and 12-week (t1 and t2) time points.
Statistical analysis
The ZBI was used for the power calculation. Based on the normative data from carers of people with dementia, 27 we expect change scores between the baseline and 12 weeks to reflect an effect size (Cohen d) of 0.40 between the two groups (active control/intervention). Assuming an alpha of 0.05 and a statistical power (1-beta) of 0.80 in a one-tailed test, we will need 78 respondents in each of the conditions, resulting in 156 participants. Assuming 25% attrition, the total sample is therefore 194 participants. Sample-size calculations were carried out with Stata V16. To minimise drop-out and encourage research compliance, all participants will receive a monthly ‘check-in’ via an email or a phone text message from the researchers and a follow-up reminder call to complete assessments at the relevant time points.
To evaluate the primary outcome, we will use mixed effects of generalised linear regression in Stata V16. Random effects will account for repeated measures from participating carers. The time point and intervention/control group will be specified as fixed. Other independent variables to be considered include age, sex, ethnicity, location, country of birth, English proficiency, and relationship to the person with dementia.
Fidelity/adherence data will be derived from analytics, which will examine the participant's hours of viewing, completeness of viewing of animations, frequency/timing of viewing, and interactions with the helper. Acceptability of the intervention is pre-specified as >70% of participants rating the intervention ‘completely acceptable’.
Qualitative analysis
A sub-set of study participants enrolled into the RCT (n = 45–55) will be contacted with their consent and interviewed to explore differences between ethnic groups and potential barriers and facilitators to uptake of the intervention. Interviewees will include those who did not complete the intervention and/or found it unacceptable. A minimum of five participants from each of the nine language groups will be interviewed with an interpreter where necessary. Interviews will be audio-recorded, transcribed, and thematically analysed in NVivo.
Phase III (year 3): economic evaluation
We will conduct a trial-based economic evaluation of the cost-effectiveness of the intervention from a societal perspective, as compared to an enhanced control condition approximating usual care. In line with the main analysis, the primary outcome of the trial-based analysis will be carer burden as measured by the ZBI and the secondary outcome measure will be quality-adjusted life-years (QALYs) 34 in carers and people with dementia. Calculations will be based on the three time points (t0, t1, and t2) using EQ-5D-5L utilities mapped from WHOQOL-Bref data. 33 Supplementary analyses will be conducted in the sub-group for which we have CarerQoL 30 utilities, calculating patient QALYs using mapped EQ-5D-5L utilities and calculating carer QALYs using CarerQoL utilities.
Treatment effects with respect to the ZBI will be estimated as per the main effectiveness analysis. Treatment effects with respect to total QALYs for up to 12-week follow-up will be estimated using one-part generalised linear models (GLMs), controlling for carers and patients i.e., person with dementia, WHOQoL-Bref scores at the baseline and specifying appropriate variance and link functions. 35 Direct costs of delivering the intervention and control conditions will be calculated based on administrative records and fidelity/adherence data i.e., RUD. 32 Health service utilisation for the carer and person with dementia within the trial period will be calculated based on the carer self-report. Productivity gains/losses will be calculated using the friction cost approach 36 based on WPAI:CG data for each carer at the baseline, and 6 and 12 weeks. Base-case analyses will exclude productivity gains/losses due to the risk of double-counting. 34 We will conduct supplementary analyses to evaluate the potential for bias due to separate inclusion/exclusion of productivity gains/losses. Given the likely structure of our data and the advice of Buntin and Zaslavsky, 37 treatment effects with respect to the total cost will be estimated using a one-part GLM model with a gamma variance function and a log link (rather than transformed OLS or two-part models); controlling for patient and carer characteristics at the baseline. Results will be expressed as the (i) cost per point improvement on the ZBI at the trial end and (ii) cost per QALY gained. We will summarise sampling error and parameter uncertainty using the bootstrap acceptability method to calculate confidence intervals and generate cost-effectiveness acceptability curves. 35
Data storage and transfer
The data management plan has been approved by the Human Research Ethics Committee (HREC) of Curtin University, Australia (Approval number: BRIJNB-VC10288). Participants’ data collected across the three study phases including those via Redcap will be stored in password-protected databases. Behavioural analyses will be stored in a Hotjar database and can be exported and stored electronically at the NARI. All recordings, transcriptions, NVivo files, Stata files, publication or report drafts, and data spreadsheets will be stored electronically at the NARI, Curtin University, and Monash University respectively in password-protected folders. Only de-identified data will be reported. The research team will consider options for long-term preservation and curation of datasets beyond the study at a later date. All digital data will be transferred to Curtin University's R Drive and NARI N drives. All hard and soft copy data collected will be securely stored by Curtin University and the NARI for 7 years after the research is published and then archived. Project outputs such as the intervention will be made available to all nations where the relevant languages are spoken, including India and China, which are projected to have the highest prevalence of dementia in the future. 38
Project dissemination
We will disseminate data in peer-reviewed scholarly journal articles, presentations and conferences, and articles in media and health publications consistent with an open access policy. All data and publications will be deposited to the Curtin Research Data Collection and made publicly available within 12 months from the completion of the study or initial publication.
Data availability
All aggregate and/or de-identified data – e.g. transcripts and anonymised survey data, RCT data, and data analytics – will be available from the corresponding author upon request. Only aggregate and/or de-identified data will be published.
Results
Draw-Care brings together multidisciplinary expertise and subject matter experts from anthropology, public health, psychology, psychiatry, geriatrics, film and media studies, digital health and informatics, biostatistics, health economics, and social work. Data derived from co-design workshops with stakeholders and qualitative interviews with people with dementia in Phase I (Year 1) were used to develop the intervention which will be tested in Phase II and evaluated in Phase III (Years 2 and 3 respectively). Recruitment of 194 carers for the RCT will commence in Year 2 and will be followed by an evaluation of the clinical and cost-effectiveness of the intervention in Year 3. The Draw-Care study will conclude in December 2024.
Discussion
It is well documented that considerable gaps exist in meeting the care needs of ethnically diverse families caring for a person with dementia. 11 In this paper, we detail the Draw-Care study protocol comprising three sequential phases including the co-design, testing, and evaluation of a culturally adapted multilingual digital health intervention developed to allay some of these needs.
There is growing potential for digital health interventions delivered by digital technologies – including websites, as a means of providing effective, cost-effective, and safe options to improve health and healthcare. This potential may in part be limited by the current evidence base regarding what digital interventions work for whom and under what circumstances. 39 As such, a key step in building this evidence base is not only evaluating the clinical and cost-effectiveness of a digital intervention through a RCT design, but also ensuring that is feasible and acceptable to the target group using co-design. Study protocols, such as ours, that detail the multiple phases in the development of complex digital health interventions and subsequent evaluation advance the science by providing details of our approach thus enabling useful synthesis and comparison of data generated by different studies. 40
For example, a recent review of digital health interventions with potential to support family carers managing the needs of a person living with a chronic disease found these to be robust in providing support and improving psychological health, self-efficacy, caring skills, quality of life and problem-coping abilities. However, digital health interventions are lacking for carers from diverse backgrounds and more interventions must be tailored with cultural and linguistic sensitivity. 41 As the prevalence of dementia continues to grow, the need to support carers and people with dementia using technology is also warranted and well-designed RCTs are critical to examine and evaluate the available interventions. 42
The Draw-Care study will establish to what degree a co-designed, culturally adapted, multilingual digital health intervention is meeting the needs of ethnically diverse family carers of a person living with dementia.
Strengths and limitations
Limitations include the inability to culturally validate all secondary outcomes (e.g. CarerQoL) and a relatively short follow-up period. Exclusion of non-digitally literate carers and/or those with limited internet access and/or those without a smartphone, tablet or computer may result in excluding older family carers, many of whom have been described as having low digital literacy. 43 However, this study uses a theory-driven co-designed intervention, validating surrogates such as carer burden and QoL and health outcomes (mental health) in multiple languages. The study mobilises a digital intervention that bypasses the potential for COVID-19 restrictions to data collection and uses proven implementation techniques to sustain uptake and adherence, and a cost-effectiveness analysis to evaluate and inform decisions regarding wider roll-out of the intervention. Our existing networks will support meeting the target sample of carers to test the intervention in Phase II and enable dissemination of the results following the evaluation in Phase III.
Conclusions
If our findings demonstrate that our intervention is effective according to the measures detailed, then the Draw-Care study offers a unique blueprint for effective development and evaluation of further co-designed digital media interventions targeted and tailored to ethnically diverse families and people with dementia. With the importance and prevalence of digital media in society today, this method represents a novel and impactful approach to provide better and broader access and quality of healthcare in a cost-effective fashion.
Supplemental Material
Supplemental material, sj-doc-1-dhj-10.1177_20552076231205733 for Improving the lives of ethnically diverse family carers and people living with dementia using digital media resources – Protocol for the Draw-Care randomised controlled trial by Antonia Thodis, Thu-Ha Dang, Josefine Antoniades and Andrew S. Gilbert, Tuan Nguyen, Danijela Hlis, Mary Gurgone, Briony Dow, Claudia Cooper, Lily-Dongxia Xiao, Nilmini Wickramasinghe, Nalika Ulapane, Mathew Varghese, Santosh Loganathan, Joanne Enticott, Duncan Mortimer, Bianca Brijnath in DIGITAL HEALTH
Supplemental material, sj-doc-2-dhj-10.1177_20552076231205733 for Improving the lives of ethnically diverse family carers and people living with dementia using digital media resources – Protocol for the Draw-Care randomised controlled trial by Antonia Thodis, Thu-Ha Dang, Josefine Antoniades and Andrew S. Gilbert, Tuan Nguyen, Danijela Hlis, Mary Gurgone, Briony Dow, Claudia Cooper, Lily-Dongxia Xiao, Nilmini Wickramasinghe, Nalika Ulapane, Mathew Varghese, Santosh Loganathan, Joanne Enticott, Duncan Mortimer, Bianca Brijnath in DIGITAL HEALTH
Supplemental material, sj-docx-3-dhj-10.1177_20552076231205733 for Improving the lives of ethnically diverse family carers and people living with dementia using digital media resources – Protocol for the Draw-Care randomised controlled trial by Antonia Thodis, Thu-Ha Dang, Josefine Antoniades and Andrew S. Gilbert, Tuan Nguyen, Danijela Hlis, Mary Gurgone, Briony Dow, Claudia Cooper, Lily-Dongxia Xiao, Nilmini Wickramasinghe, Nalika Ulapane, Mathew Varghese, Santosh Loganathan, Joanne Enticott, Duncan Mortimer, Bianca Brijnath in DIGITAL HEALTH
Acknowledgements
The project received support from the Department of Mental Health and Substance Use, World Health Organization, the Dementia Australia, and the Federation of Ethnic Communities Council of Australia. The authors thank the co-design workshop participants in Phase I for sharing their lived experiences in a cultural context to adapt the stories depicted in the animated films and information sheets. We acknowledge the expertise of our technical collaborators in translation, animation, and website design.
Abbreviations
- CarerQoL-7D
Care-related Quality of Life instrument
- CES-D
Center for Epidemiological Studies-Depression
- eHEALS
eHealth Literacy Scale
- GLM
Generalised Linear Models
- HREC
Human Research Ethics Committee
- MRFF
Medical Research Future Fund
- NARI
The National Ageing Research Institute
- PICF
Participant Information and Consent Forms
- QALY
Quality-Adjusted Life-Years
- RCT
Randomised Controlled Trial
- RUD
Resource Utilization in Dementia
- UTAUT
Unified Theory of Acceptance and Use of Technology
- WHO
World Health Organization
- WHOQOL-Bref
World Health Organization Quality of Life–Bref
- WPAI:CG
Work Productivity and Activity Impairment Questionnaire for Caregiving
- ZBI
Zarit Burden Interview
Footnotes
Contributorship: BB searched the literature, and conceived and led the study. All authors were involved in the protocol development. JA, THD and AT gained the ethical approval. AT and THD conducted the participant recruitment and qualitative data analyses in Phase I. BB, AT and THD wrote the first draft, and edited the subsequent versions including the final version of the manuscript for submission. All authors reviewed and approved the final version of the manuscript for submission.
Conflict of interest: Bianca Brijnath is the Principal Investigator. Antonia Thodis is the Project Manager. Thu-Ha Dang is the Research Assistant. Josefine Antoniades, Claudia Cooper, Thu-Ha Dang, Briony Dow, Joanne Enticott, Andrew S Gilbert, Mary Gurgone, Danijela Hlis, Santosh Loganathan, Duncan Mortimer, Tuan Nguyen, Antonia Thodis, Nalika Ulapane, Mathew Varghese, Nilmini Wickramasinghe and Lily-Dongxia Xiao are members of the Executive Committee. The following organisations have contributed either financial or in-kind support to the Draw-Care project: Medical Research Future Fund (MRFF), Department of Mental Health and Substance Use, WHO, Dementia Australia, Federation of Ethnic Communities Council of Australia (FECCA) and NARI. Bianca Brijnath, Josefine Antoniades, Andrew S Gilbert, and Tuan Nguyen have received other grant funding from the MRFF and the NHMRC. All authors have no other relationships or activities or interests to disclose related to the content of this submission.
Ethical approval: The Draw-Care study has been approved by the HREC of Curtin University, Australia (HRE2022-0004) and the NARI Research Governance Office. The trial has been prospectively registered on ANZCTR with trial registration numbers ACTRN12622000382774 and ACTRN12622000358741.
Funding: The author(s) disclosed the receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Medical Research Future Fund (MRFF), Australian Government Department of Health [grant number APP2008065].
The study sponsor and funders do not have any role in the study's design, data collection, management, analysis, interpretation of data or writing reports or publications.
Guarantor: BB has received the grant from the MRFF.
ORCID iD: Thu-Ha Dang https://orcid.org/0000-0001-9105-6920
Supplemental material: Supplementary material for this article is available online.
References
- 1.Department of economic and social affairs PD. International migrant stock 2015. New York: United Nations, 2015. [Google Scholar]
- 2.Australian bureau of statistics . Census of population and housing: Cultural diversity data summary, 2021. TableBuilder, 2022.
- 3.Wilson T, McDonald P, Temple JB, et al. The changing country of birth composition of Australia’s older population. Genus 2020; 76: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brijnath B. Unforgotten: love and the culture of dementia care in India. New York: Berghahn Books, 2014. [Google Scholar]
- 5.Brijnath B, Gilbert AS, Kent M, et al. Beyond crisis: enacted sense-making among ethnic minority carers of people with dementia in Australia. Dementia (London, England) 2021; 20: 1910–1924. [DOI] [PubMed] [Google Scholar]
- 6.Cooper C, Tandy AR, Balamurali TB, et al. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry 2010; 18: 193–203. [DOI] [PubMed] [Google Scholar]
- 7.Low LF, Barcenilla-Wong AL, Brijnath B. Including ethnic and cultural diversity in dementia research. Med J Aust 2019; 211: 345–346 e341. [DOI] [PubMed] [Google Scholar]
- 8.Mukadam N, Cooper C, Livingston G. A systematic review of ethnicity and pathways to care in dementia. Int J Geriatr Psych 2011; 26: 12–20. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen TR, Nielsen DS, Waldemar G. Barriers to post-diagnostic care and support in minority ethnic communities: a survey of Danish primary care dementia coordinators. Dementia 2020; 19(8): 2702–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roche M, Higgs P, Aworinde J, et al. A review of qualitative research of perception and experiences of dementia among adults from black, African, and Caribbean background: what and whom are we researching? Gerontologist 2021; 61: e195–e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert AS, Antoniades J, Croy S, et al. The experience of structural burden for culturally and linguistically diverse family carers of people living with dementia in Australia. Health Soc Care Community 2022; 30(6): e4492–e4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Temple JB, Dow B. The unmet support needs of carers of older Australians: prevalence and mental health. Int Psychogeriatr 2018; 30(12): 1849–1860. [DOI] [PubMed]
- 13.Prince M, Wimo A, Guerchet M, et al. World Alzheimer Report 2015. London: Global Observatory for Ageing and Dementia Care, Health Service and Population Research Department, 2015. [Google Scholar]
- 14.WHO. i-Support for dementia, https://www.who.int/publications/i/item/9789241515863 (2021, accessed 15 July 2021).
- 15.Rakower J, Hallyburton A. Disease information through comics: a graphic option for health education. J Med Humanit 2022; 43: 475–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra RK, Park C, Momin AS, et al. Care4AD: a technology-driven platform for care coordination and management: acceptability study in dementia. Gerontology 2023; 69: 227–238. [DOI] [PubMed] [Google Scholar]
- 17.Dang TH, Thodis A, Ulapane N, et al. It’s too nice’: adapting iSupport Lite for multicultural family carers of a person with dementia. Clinical Gerontologist 2023; 11: 1–14. [DOI] [PubMed] [Google Scholar]
- 18.Schulz R. Family caregiving of persons with dementia: Prevalence, health effects, and support strategies. Am J Geriatr Psychiatry 2004; 12: 240–249. [PubMed] [Google Scholar]
- 19.Schulz R. Handbook on dementia caregiving: evidence-based interventions for family caregivers. New York, New York: Springer Publishing Company, 2000. [Google Scholar]
- 20.Borgstrom E, Barclay S. Experience-based design, co-design and experience-based co-design in palliative and end-of-life care. BMJ Support Palliat Care 2019; 9: 60–66. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesh V, Morris MG, Davis GB, et al. User acceptance of information technology: toward a unified view. MIS Q 2003; 27: 425–478. [Google Scholar]
- 22.McMillan SS, Kelly F, Sav A, et al. Using the nominal group technique: how to analyse across multiple groups. Health Serv Outcomes Res Methodol 2014; 14: 92–108. [Google Scholar]
- 23.Joe J, Chaudhuri S, Le T, et al. The use of think-aloud and instant data analysis in evaluation research: exemplar and lessons learned. J Biomed Inform 2015; 56: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman CD, Skinner HA. eHEALS: the eHealth literacy scale. J Med Internet Res 2006; 8: e27–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotjar. Hotjar, https://www.hotjar.com/ (2023, accessed 7 September 2023).
- 26.IBM Corporation. IBM SPSS Statistics , https://www.ibm.com/au-en/products/spss-statistics (2022, accessed 13 June 2022).
- 27.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980; 20: 649–655. [DOI] [PubMed] [Google Scholar]
- 28.Lewinsohn PM, Seeley JR, Allen NB, et al. Center for epidemiologic studies depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997; 12: 277–287. [DOI] [PubMed] [Google Scholar]
- 29.WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med 1998; 28: 551–558. [DOI] [PubMed] [Google Scholar]
- 30.Brouwer WBF, van Exel NJA, van Gorp B, et al. The CarerQol instrument: A new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res 2006; 15: 1005–1021. [DOI] [PubMed] [Google Scholar]
- 31.Giovannetti ERP, Wolff JLP, Frick KDP, et al. Construct validity of the work productivity and activity impairment questionnaire across informal caregivers of chronically ill older patients. Value Health 2009; 12: 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wimo A, Gustavsson A, Jönsson L, et al. Application of Resource Utilization in Dementia (RUD) instrument in a global setting. Alzheimer's Dementia: J Alzheimer's Assoc 2013; 9: 429–435.e417. [DOI] [PubMed] [Google Scholar]
- 33.Wee HL, Yeo KK, Chong KJ, et al. Mean rank, equipercentile, and regression mapping of World Health Organization quality of life brief (WHOQOL-BREF) to EuroQoL 5 dimensions 5 levels (EQ-5D-5L) utilities. Med Decis Making 2018; 38: 319–333. 2018/03/28. [DOI] [PubMed] [Google Scholar]
- 34.Shiroiwa TP, Fukuda TP, Ikeda SMDP, et al. QALY And productivity loss: empirical evidence for “double counting”. Value Health 2013; 16: 581–587. [DOI] [PubMed] [Google Scholar]
- 35.Glick HA, Doshi JA, Sonnad SS, et al. Economic Evaluation in Clinical Trials. Oxford: Oxford: Oxford University Press, Incorporated, 2014. [Google Scholar]
- 36.Brouwer WBF, Koopmanschap MA. The friction-cost method: replacement for nothing and leisure for free? Pharmacoeconomics 2005; 23: 105–111. [DOI] [PubMed] [Google Scholar]
- 37.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation?: Comparing methods of modeling Medicare expenditures. J Health Econ 2004; 23: 525–542. [DOI] [PubMed] [Google Scholar]
- 38.Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022; 7: e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray E, Hekler EB, Andersson G, et al. Evaluating digital health interventions: key questions and approaches. Am J Prev Med 2016; 51: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer LC, Kölligan V, Wieland N, et al. Development and evaluation of a digital health intervention for substance use reduction in young refugees with problematic use of alcohol and/or cannabis-study protocol for a single-armed feasibility trial. Front Public Health 2021; 9: 557431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhai S, Chu F, Tan M, et al. Digital health interventions to support family caregivers: an updated systematic review. Digit Health 2023; 9: 20552076231171967–20552076231171967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knapp M, Shehaj X, Wong G. Digital interventions for people with dementia and carers: effective, cost-effective and equitable? Neurodegener Dis Manag 2022; 12: 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.FECCA. Digital access and equity for multicultural communities. Federation of Ethnic Communities Councils of Australia. 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-dhj-10.1177_20552076231205733 for Improving the lives of ethnically diverse family carers and people living with dementia using digital media resources – Protocol for the Draw-Care randomised controlled trial by Antonia Thodis, Thu-Ha Dang, Josefine Antoniades and Andrew S. Gilbert, Tuan Nguyen, Danijela Hlis, Mary Gurgone, Briony Dow, Claudia Cooper, Lily-Dongxia Xiao, Nilmini Wickramasinghe, Nalika Ulapane, Mathew Varghese, Santosh Loganathan, Joanne Enticott, Duncan Mortimer, Bianca Brijnath in DIGITAL HEALTH
Supplemental material, sj-doc-2-dhj-10.1177_20552076231205733 for Improving the lives of ethnically diverse family carers and people living with dementia using digital media resources – Protocol for the Draw-Care randomised controlled trial by Antonia Thodis, Thu-Ha Dang, Josefine Antoniades and Andrew S. Gilbert, Tuan Nguyen, Danijela Hlis, Mary Gurgone, Briony Dow, Claudia Cooper, Lily-Dongxia Xiao, Nilmini Wickramasinghe, Nalika Ulapane, Mathew Varghese, Santosh Loganathan, Joanne Enticott, Duncan Mortimer, Bianca Brijnath in DIGITAL HEALTH
Supplemental material, sj-docx-3-dhj-10.1177_20552076231205733 for Improving the lives of ethnically diverse family carers and people living with dementia using digital media resources – Protocol for the Draw-Care randomised controlled trial by Antonia Thodis, Thu-Ha Dang, Josefine Antoniades and Andrew S. Gilbert, Tuan Nguyen, Danijela Hlis, Mary Gurgone, Briony Dow, Claudia Cooper, Lily-Dongxia Xiao, Nilmini Wickramasinghe, Nalika Ulapane, Mathew Varghese, Santosh Loganathan, Joanne Enticott, Duncan Mortimer, Bianca Brijnath in DIGITAL HEALTH
Data Availability Statement
All aggregate and/or de-identified data – e.g. transcripts and anonymised survey data, RCT data, and data analytics – will be available from the corresponding author upon request. Only aggregate and/or de-identified data will be published.